Abstract
Despite increases in female contraceptive options, 40–45% of pregnancies across the world are still unplanned. While several effective female contraceptive methods have been developed, contraceptive choices for men are still limited to the male condom with its high failure rates and to vasectomies, which are invasive and not reliably reversible. Several studies have demonstrated a great interest among men and women for effective, reversible, and safe male contraceptive methods. Over the years, numerous studies have been performed to develop male hormonal and nonhormonal safe and effective contraceptives. A variety of new molecules are under development as oral or transdermal hormonal contraceptives for men demonstrating few side effects. In our overpopulated world, the development and commercialization of a male contraceptive method that will allow both men and women to take an active role in family planning is mandatory and further research on this topic is required.
Keywords: contraception, male contraception, male hormonal contraception
Introduction
Options available for male contraception are still limited to condoms characterized by a high failure rate with typical use1 and to vasectomy, which is invasive and not easily reversible.2 For these reasons, family planning continues to be the responsibility of women even though a large number of men would welcome the opportunity to use male contraceptive methods, recognizing that the possibility of sharing family planning should be an individual right rather than a responsibility.3
The availability of male hormonal contraceptives would give men the chance to have control over their own fertility and to share the responsibility for family planning. Among the different approaches to control male fertility, hormonal contraception is the closest to possible clinical application. However, despite the significant progress showing efficacy, feasibility, and acceptability of hormonal regimens, research in this field has thus far not led to a product approved for clinical use. The support of government agencies such as the World Health Organization (WHO), Contraceptive Research and Development (CONRAD), and the National Institute of Child Health and Human Develop-ment has led to important progress in this field and numerous studies have confirmed that the hormonal approach is feasible, relatively effective, and acceptable. However, progress in research continues to be slow and a marketable product is not on the horizon.
Action mechanism of male hormonal contraception
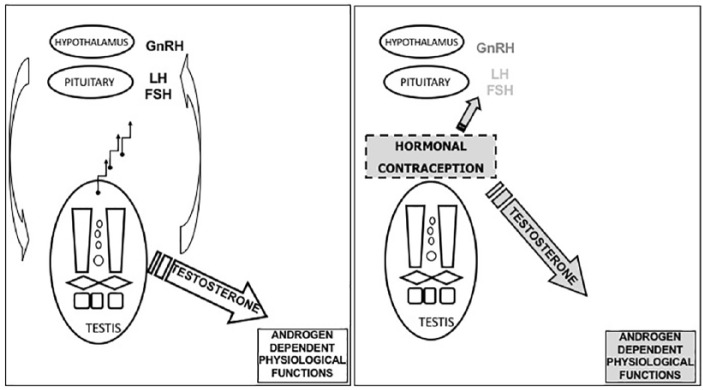
The basic mechanism by which hormones affect fertility in men is through the suppression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) with subsequent reversible inhibition of testicular function, namely spermatogenesis and testosterone production (Figure 1). Both the decrease of testosterone and suppression of FSH lead to a decrease of Sertoli cell function essential for germ-cell maturation. To maintain androgen-dependent physiological functions an androgen (usually testosterone) must be part of the contraceptive regimen.4,5
Figure 1.
Schematic representation of the male’s hypothalamic-pituitary-gonadal axis (left panel) and the hormonal contraception mechanism of action (right panel). FSH, follicle-stimulating hormone; GnRH, Gonadotropin Releasing Hormone; LH, luteinizing hormone.
Overview of all hormonal contraceptive regimens tested in men: background and efficacy studies
Over the last few decades several studies have been performed to evaluate the suppression of spermatogenesis provided by different hormonal contraceptive regimens. These studies varied in their size, molecules used, and duration of study, but they all demonstrated that testosterone is efficient in suppressing sperm concentrations. Subsequent trials demonstrated that testosterone alone is not as efficient as testosterone plus a progestin, both in the rate and extent of suppression of spermatogenesis.6 In addition to these studies, the feasibility of hormonal contraception for men was tested in a few contraceptive efficacy studies (which evaluated the ability of the formulation to prevent pregnancy) (Table 1). They showed that azoospermia or severe oligozoospermia (⩽ 1 million/ml) induced by hormone regimens provide excellent pregnancy protection comparable with female oral hormonal contraception.
Table 1.
Efficacy studies.
| Regimen | Enrolled subjects |
Sperm concentration threshold (million/ml) |
Subjects reaching threshold | Subjects entering efficacy |
Subjects completing efficacy | Pregnancy rate N (%/couple-year) |
|---|---|---|---|---|---|---|
| TE 200 mg/week7 | 271 | azoospermia | 157 | 157 | 119 | 1 (0.8) |
| TE 200 mg/week8 | 399 | < 3 (reduced from < 5) | 349 | 268 | 209 | 4 (1.4) |
| TU 1000 (loading) + 500 mg/4 week9 | 308 | < 3 | 299 | 296 | 280 | 1 (2.3)* |
| Depot MPA 300 mg/12 week testosterone pellets 800 mg/24–16 week10 |
55 | < 1 | 53 | 51 | 30 | 0 (0) |
| TU 1000 (loading) + 500 mg/4 week11 | 1045 | ⩽ 1 | 855 | 855 | 733 | 9 (1.1) |
| TU 1000 mg + NETE 200 mg/8 week12 | 320 | ⩽ 1 | 274 | 266 | 111$ | 4 (1.57) |
One pregnancy was attributed to sperm rebound.
Trial terminated before the planned end of the study.
MPA, medroxyprogesterone acetate; NETE, norethisterone enanthate; TE, testosterone enanthate; TU, testosterone undecanoate.
Hormonal contraceptive injectable regimes using testosterone only
Testosterone enanthate
The US National Institutes of Health began male hormonal contraceptive clinical trials in the 1970s using short-acting testosterone formulations such as testosterone enanthate (TE). These studies demonstrated in healthy male volunteers that intramuscular administration of TE suppresses sperm concentration to very low levels.13
TE was also used in two large WHO-supported efficacy studies confirming that testosterone can induce profound suppression of spermatogenesis (to azoospermia or severe oligozoospermia) in men.7,8 In 1990, in the first multicenter study, 271 healthy volunteers received 200 mg TE weekly and 157 (65% at 6 months) became azoospermic with a mean time of 120 days. The mean time of recovery of spermatogenesis after stopping testosterone injections was 3.7 months.7 The study demonstrated that azoospermia induced by 200 mg TE injections was able to provide highly effective, sustained, and reversible contraception: among the 157 azoospermic men who entered the 12-month efficacy phase there was only one pregnancy (0.8 conceptions per 100 person-years).7
In the second WHO study, 399 men were enrolled and 357 completed the suppression phase with weekly intramuscular injections of 200 mg TE, with 8 (2.2%) failing to reach the oligozoospermic threshold (⩽ 3 million/ml).8 A total of 42 men discontinued before the end for personal reasons or dislike of the injection schedule, pregnancy during the suppression phase (n = 7), or for medical reasons (n = 6). Men from Asian centers reached azoospermia sooner than men from other centers (91 days versus 112 days). Nonsuppression to the oligozoospermic threshold (⩽ 3 million/ml) occurred in 8 out of 242 men from nonAsian centers compared with none of the 115 men from the Asian centers. In this study, efficacy was tested in men with different levels of oligospermia. Four pregnancies were reported during 29.5 person-years in oligozoospermic men (sperm count 0.1–3 × 10 (6)/ml) and no pregnancy was reported during 230.4 person-years in azoospermic men.8
Side effects included discomfort at the injection sites, acne, psychological changes, weight gain, polycythemia, and abnormal lipids.7,8
Testosterone undecanoate
Long-acting intramuscular testosterone undecanoate (TU) was studied as a potential hormonal male contraceptive agent in a large Chinese phase II contraceptive efficacy study. In this trial TU was used in 308 healthy men9: during the 6-month suppression phase, an initial loading dose of 1000 mg TU, followed by 500 mg TU every month was administered until the achievement of azoospermia or severe oligozoospermia (< 3 million/sperm ml). The 12-month treatment period included a 6-month suppression phase followed by a 6-month efficacy phase and a 12-month recovery period. The threshold for entering the efficacy phase was defined as azoospermia (< 3 million/sperm ml). Only 9 men did not achieve the required level within the 6-month suppression phase whereas 296 men entered the efficacy phase.9 During the efficacy phase, 500 mg TU was administered at monthly intervals for 6 months. A total of 296 men used the hormonal method for contraception. During the efficacy phase, one pregnancy occurred, and it was attributed to sperm rebound. In this phase the continuation rate was 95/100 couple-years. Overall the total failure rate of the methods was 5.2% and total efficacy was 94.8%. Spermatogenesis returned to the normal range within the recovery period without any serious adverse events occurring during the study.9 Side effects included tenderness or discomfort at the injection sites, acne, and self-reported changes in sexual desire.9
Another subsequent large multicenter, phase III, WHO-supported, contraceptive efficacy clinical trial was conducted in China enrolling 1045 couples who received an initial loading dose of 1000 mg TU followed by 500 mg every month for up to 6 months during the suppression phase and then for 24 months of the efficacy phase.11 A total of 43 participants (4.8%) did not achieve azoospermia or severe oligozoospermia within the 6-month suppression phase. Of the 855 men entering the efficacy phase, 122 discontinued early and 733 completed the 24-month efficacy phase. A total of 10 participants presented sperm rebound during the 24-month efficacy phase, with a secondary method failure rate of 1.3%. Nine pregnancies were reported in 1554.1 person-years of exposure in the 24-month efficacy phase for a cumulative contraceptive failure rate of 1.1 per 100 couple-years. Six pregnancies were retrospectively attributed to sperm rebound and three attributed to men whose sperm concentration was no greater than 1 × 106/ml. The combined method failure rate was 6.1%, of which 4.8% had inadequate suppression and 1.3% had postsuppression sperm rebound. The cumulative contraceptive failure rates were 1.0% and 1.1% at the end of months 12 and 24, respectively, based on 1554.1 person-years of exposure in the 24-month efficacy phase. No serious adverse events were reported during the study period. A total of 18 participants discontinued early and the most frequent complaint was tenderness or discomfort at the injection sites, acne, changes in mood or behavior, and facial swelling or skin rash.11 Spermatogenesis returned to the normal fertile reference range in all but two participants.11 This study showed that in the couples who completed the efficacy phase, contraceptive protection was excellent.14
Hormonal contraceptive injectable regimes using testosterone combined with other molecules
Testosterone plus progestin
Testosterone administration demonstrated contraceptive efficacy but testosterone alone is not as efficient as testosterone plus a progestin in the rate and extension of spermatogenesis suppression. These two steroids have synergic and additive effects on the hypothalamus-pituitary axis resulting in more rapid and profound gonadotropins and sperm suppression compared with each compound administered alone.
Adding a progestin also allows the reduction of the testosterone dose therefore reducing possible side effects related to supraphysiological doses of testosterone, therefore improving the safety of the regimen.15
Various pilot studies using testosterone injections (esters or testosterone implants) plus different progestins have confirmed the efficacy of these formulations in sperm suppression. Various progestins including depot medroxyprogesterone acetate (MPA), levonorgestrel pills and implants, desogestrel pills, etonogestrel implants, oral cyproterone acetate, and injectable norethisterone enanthate (NETE) have been studied.
The combination of TU with etonogestrel (active metabolite of desogestrel) was studied in 2008 in a multicenter trial involving 354 men.16 This trial showed that the combination of etonogestrel subcutaneous implants with 750 mg or 1000 mg TU every 10–12 weeks was associated with sperm suppression to 1 million/ml. This regimen was well tolerated and provided an effective and reversible suppression of spermatogenesis.16
In a small trial the efficacy of a regimen using testosterone pellets (four 200 mg implants, every 4 or 6 months) and 300 mg depot MPA injected every 3 months demonstrated high contraceptive efficacy (no pregnancies occurred in 426 person-months (35.5 person-years); 95% confidence interval [CI] for contraceptive failure rate: 0–8%/annum) with satisfactory short-term safety and recovery of spermatogenesis.10 Recovery was complete in all but one man with an incidental testicular disorder. Side effects included problems with pellets, symptoms of androgen deficiency, and mood fluctuations.
In 2001 the combination of the injectable depot preparation of TU 1000 mg plus NETE 200 mg injected every 6 weeks in 14 subjects was shown to induce profound suppression of spermatogenesis and the absence of serious side effects.17 In a subsequent study, the injection interval was increased to 8 weeks and 9 out of 10 subjects still achieved azoospermia and all were severely oligozoospermic (< 1 million/ml) by the end of the 48-week study period.18 A further increase of the injection interval to 12 weeks led to a decrease in sperm suppression.
After the promising results of these small studies, WHO and CONRAD decided to use this regimen in an efficacy study. The appeal of this regimen was that both TU and NETE are dissolved in castor oil and thus in principle they can be included in one formulation that is injected every 8 weeks providing an acceptable hormonal contraceptive regimen. A large multinational phase II efficacy trial was carried out to determine the safety and efficacy of TU 1000 mg combined with NETE 200 mg for sperm suppression and contraceptive efficacy. Participants received injections every 2 months during the suppression and efficacy phases. Within 24 weeks, 274 of the 320 initial participants suppressed to a sperm concentration less than or equal to 1 million/ml.12 During the efficacy phase of up to 56 weeks, among 266 male participants, 4 pregnancies occurred in the partners (rate of 1.57 per 100 continuing users [95% CI: 0.59–4.14]). During the 52-week recovery phase, 94.8 per 100 continuing users (95% CI: 91.5–97.1) recovered to a sperm concentration of at least 15 million/ml or to a total sperm count of at least of 39 million per ejaculate.12
Reported side effects included acne, increased libido, injection site pain, myalgia, and mood alterations; 6% of men discontinued due to a side effect. The reported frequency of moderate to severe mood changes, occurring in some but not all sites, lead to an external safety review committee recommending stopping further injections before the planned end of the study.12
Testosterone plus Gonadotropin Releasing Hormone (GnRH) antagonists
GnRH antagonists act by competitive binding to receptors and reduce both LH and FSH to undetectable levels. GnRH antagonists are very effective in suppressing spermatogenesis. Short-term studies have shown that the suppression of spermatogenesis by GnRH antagonists plus testosterone is profound.19,20 However, GnRH antagonists are expensive and the majority of them still require frequent subcutaneous injections. New long-acting depot formulations of GnRH antagonists, such as degarelix, are under investigation.21 Those new long-acting formulations may represent an option for male contraception but remain to be demonstrated. Therefore, until newly developed GnRH antagonists are marketed, these regimens do not represent a realistic option for male contraception.
Hormonal contraceptive transdermal regimes using testosterone and Nestorone: gel-gel combination
Nestorone (segesterone acetate) (NES) is a 19-norprogesterone-derived progestin characterized by the absence of androgenic, estrogenic, or glucocorticoid effects.22 It provides inhibition of gonadotropins through a negative feedback mechanism but it also inhibits local testosterone production directly in the testis.
Testosterone gel can be used together with NES gel. This combination used daily in healthy men has shown effective gonadotropin suppression.23
A total of 56 subjects were randomized to receive one of these treatments: testosterone gel at a dose of 10 g, testosterone plus NES at a dose of 8 mg, or testosterone plus NES at a dose of 12 mg. Sperm suppression below 1 million/ml or less was significantly more probable in men treated with testosterone plus NES 8 mg (89%, p < 0.0001) and testosterone plus NES 12 mg (88%, p = 0.0002) compared with men treated with testosterone (23%).24 Adverse effects were minimal in all groups with 21% of subjects presenting mild or moderate acne.
Testosterone and NES can be combined into a single gel with reduced volume thus simplifying application and improving adherence. A phase IIb efficacy trial of a testosterone plus NES gel has been initiated enrolling 400 couples. If the men achieve sperm concentrations of ⩽ 1 million/ml, the couples will enter a 52-week efficacy phase.
Oral formulations: the ‘male pill’
Up to now, studied male hormonal contraceptives are designed to be administered through injections or implants which may be uncomfortable so many men prefer other administration methods such as oral or transdermal self-administration.25 The oral delivery of testosterone has been challenging with the problem of hepatoxicity with methyltestosterone or for the necessity of multiple doses per day in the case of TU.26
New androgens are currently under development as potential oral male hormonal contraceptives. Dimethandrolone undecanoate (DMAU) is a derivative of 19-nortestosterone with both androgenic and progestational activity in preclinical studies.27 Recently, it has been studied in 82 healthy men for 28 days: a single dose of up to 400 mg daily was safe, well-tolerated, and demonstrated its ability to suppress serum testosterone, LH, and FSH to levels consistent with effective contraception.28 Limitations of oral DMAU are the need for concomitant food administration for effective absorption29 and androgenic side effects (i.e. weight gain, increased hematocrit, and reduction in HDL-cholesterol).
Another new molecule is 11-beta-methyl-19-nortes-tosterone 17-beta-dodecylcarbonate (11-ßMNTDC), a derivative of 19-nortestosterone. In preclinical data, it showed serum gonadotropin suppression,30 and one study in men demonstrated that given in oral doses of 100–800 mg with food it is well tolerated and able to suppress testosterone.31
Side effects and risks
In trials which tested testosterone-only regimens, the most common side effects were related to high-dose testosterone administration: common side effects were acne, altered libido, night sweats, increased weight, and mood changes. The combination of testosterone with a progestin allowed a reduction of testosterone dose minimizing androgenic side effects.15 When the testosterone dose was decreased and combined with a progestin, side effects were correlated with the type of progestin used. Progestins derived from nortestosterone, which retain their androgenic activity, more often caused androgen-related adverse side effects such as weight gain, acne, or decreased HDL- cholesterol. Although side effects were reported in all studies, a placebo-controlled study that compared treatment with etonogesterel combined with TU versus placebo proved very interesting. In this study, active treatment was more frequently associated with mood and libido alterations, acne, weight gain, and night sweats.16 Interestingly, although not surprisingly, it should be acknowledged that adverse side events reported by 93% of men on active treatment were also reported by 81% of men on placebo treatment. In this placebo-controlled trial, treatment was associated with a decrease of total cholesterol, HDL-, and LDL-cholesterol, with an increase in the total cholesterol/HDL- cholesterol ratio.16 This placebo-controlled trial was methodologically precise, however a different regimen and longer administration time may lead to different adverse side events. For this reason, postmarketing monitoring will be mandatory. Also, the clinical significance of these changes, that is, the HDL decrease, in terms of increased cardiovascular risk remains unclear. No significant changes in prostate volumes were detected in studies where the prostate was monitored by ultrasound and digital rectal examination. PSA levels did not change throughout the study periods in any study.
Pregnancy and fetal outcomes during and after male hormonal contraceptive treatment are similar to those of the general population.32 Spontaneous miscarriage rates (6–11%) were comparable to the general population (8–20%). The congenital malformation rate was 0.9–1.8% (0·0–6·3), which is consistent with the congenital malformation rate in spontaneous and ART pregnancies (4%), but the power of the analysis was not sufficient to exclude the possibility that congenital malformation rates may be increased by male hormonal contraceptives.32
Factors influencing suppression and reversibility
In the Chinese population, testosterone alone induced azoospermia and thereby effective contraceptive protection in most subjects.7–9,11 In Caucasian men testosterone alone cannot guarantee effective contraception as it produces azoospermia in only two-thirds of volunteers and azoospermia or severe oligospermia in about 95% of subjects.8 To achieve uniform azoospermia or severe oligospermia in Caucasian men, testosterone must be combined with a progestin. Progestin co-administration almost doubles the rate and extent of sperm suppression.6 In addition to ethnicity, the dose of testosterone may also modulate the extent of suppression as a higher total administered dose may be related to a higher proportion of incomplete suppression,6,33,34 while higher baseline endogenous testosterone is also associated with slower suppression.6
Even after drug and dose optimization, male hormonal contraceptive regimens can have some variability on the extent of sperm suppression depending on body mass index age, and initial sperm count but the effect of these cofactors is still to be fully understood.
Hormonal male contraceptive regimens have shown full reversibility within a predictable time course. Nonrecovery has only been reported twice in men diagnosed with other causes of sterility, such as epididymitis and myotonic dystrophy.10,11 Different covariables can affect the rate but not the extent of recovery.35 In 1549 men who underwent 1283.5 man-years of treatment and 705 man-years of recovery, higher rates of recovery were detected with older age, Asian origin, shorter treatment duration, shorter-acting testosterone preparations, higher sperm concentrations at baseline, faster suppression of spermatogenesis, and lower blood concentrations of LH at baseline.35
In the longest study of 855 men treated with androgen for up to 30 months, the median time to recovery of sperm output to at least 20 million/ml was 7.6 months, longer than the median recovery time of 3.4 months previously calculated.11 In another study of 354 men administered with combined androgen-progestin therapy for 42–44 weeks the median recovery time was 3.7 months and all men had recovered after 16 months.16 In the most recent study of 266 men treated for up to 54 weeks with NETE and TU, the cumulative rate of recovery to a sperm concentration of 15 million/ml or total sperm count of 39 million per ejaculate was 94.8 per 100 continuing users (95% CI: 91.5–97.1) in a 52-week recovery phase.12
Reversibility has also been evaluated in volunteers with subnormal semen parameters according to WHO parameters. Volunteers received injections of 1000 mg TU at weeks 0, 6, 14, and 24, followed by a 24-week recovery and follow-up period. A total of 23 men with normal semen parameters and 18 with sperm counts below 20 million completed the trial. All sperm counts, in both normal and subnormal volunteers, returned to the starting range confirming the same safe pattern in both groups.36
A limitation in the assessment of reversibility of male hormonal contraception is that the longest study had treatment duration of 30 months and further data would only be available with postmarketing follow up.
Acceptability
The ideal male contraceptive method should be independent of the sexual act and without short- or long-term side effects and interference with libido. It should be effective quickly, fully reversible, have no impact on eventual offspring, and be easily accepted by both partners.37,38
A large number of men surveyed internationally are interested and would welcome the opportunity to use male contraceptive methods. Several studies have been performed in different countries to evaluate the level of acceptability of possible male contraceptive methods. In these studies 44–83% of men interviewed welcomed male hormonal methods. Zhang and colleagues reported that acceptability of an injectable monthly regimen was good in 308 interviewed men, although frequency of injections, monthly semen analysis, and the need to use another contraceptive method during the period of sperm suppression were reported as inconveniences of the method potentially limiting continuation and satisfaction rates.39 In two different studies performed in Italy and China, men participating in clinical trials on a potential hormonal injectable contraceptive found the method acceptable.37,40 In the Italian study 79% of men indicated that they would use the method if available and 74% of subjects reported that their partner would appreciate it.40
The transdermal route can improve the acceptance rate of the hormonal contraceptive method in some men over injectable regimens. Of 79 studied subjects 56% were satisfied or extremely satisfied with this gel-gel method and 51% would recommend it to others.24
Conclusion
Nowadays, despite increases in female contraceptive options,41 40–45% of pregnancies across the world are still unplanned and several studies have confirmed that there is great interest among men and women for effective, reversible, and safe male contraceptive methods. Numerous studies have been performed to develop male hormonal and nonhormonal safe and effective contraceptives, however progress in research in the last decade has been slow and commercialization is not on the horizon. A variety of new molecules are still under development as oral or transdermal hormonal contraceptives for men demonstrating few side effects. The goal for the future is the development and commercialization of a male contraceptive method that will allow both men and women to take an active role in family planning.
Footnotes
Author’s Note: Giulia Gava: contribution to the conception and the design of the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published.
Maria Cristina Meriggiola: contribution to the conception and the design of the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published.
Ethical approval: Ethical approval was not required for this review.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iD: Giulia Gava  https://orcid.org/0000-0001-9232-312X
https://orcid.org/0000-0001-9232-312X
Maria Cristina Meriggiola  https://orcid.org/0000-0003-0061-9834
https://orcid.org/0000-0003-0061-9834
Contributor Information
Giulia Gava, Gynecology and Physiopathology of Human Reproduction, S. Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna, Italy.
Maria Cristina Meriggiola, Gynecology and Physiopathology of Human Reproduction, S. Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Via Massarenti, 9, 40138 Bologna, Italy.
References
- 1. Sundaram A, Vaughan B, Kost K, et al. Contraceptive failure in the United States: estimates from the 2006–2010 National Survey of Family Growth. Perspect Sex Reprod Health 2017; 49: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel AP, Smith RP. Vasectomy reversal: a clinical update. Asian J Androl 2016; 18: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wigginton B, Harris ML, Loxton D, et al. Who takes responsibility for contraception, according to young Australian women? Sex Reprod Healthc 2018; 15: 2–9. [DOI] [PubMed] [Google Scholar]
- 4. Ilani N, Swerdloff RS, Wang C. Male hormonal contraception: potential risks and benefits. Rev Endocr Metab Disord 2011; 12: 107–117. [DOI] [PubMed] [Google Scholar]
- 5. Amory JK. Progress and prospects in male hormonal contraception. Curr Opin Endocrinol Diabetes Obes 2008; 15: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu PY, McLachlan RI. Male hormonal contraception: so near and yet so far. J Clin Endocrinol Metab 2008; 93: 2474–2476. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet 1990; 336: 955–959. [PubMed] [Google Scholar]
- 8. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril 1996; 65: 821–829. [PubMed] [Google Scholar]
- 9. Gu Y-Q, Wang X-H, Xu D, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab 2003; 88: 562–568. [DOI] [PubMed] [Google Scholar]
- 10. Turner L, Conway AJ, Jimenez M, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab 2003; 88: 4659–4667. [DOI] [PubMed] [Google Scholar]
- 11. Gu Y, Liang X, Wu W, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab 2009; 94: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 12. Behre HM, Zitzmann M, Anderson RA, et al. Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab 2016; 101: 4779–4788. [DOI] [PubMed] [Google Scholar]
- 13. Steinberger E, Smith KD. Testosterone enanthate a possible reversible male contraceptive. Contraception 1977; 16: 261–268. [DOI] [PubMed] [Google Scholar]
- 14. Trussell J. Contraceptive failure in the United States. Contraception 2011; 83: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meriggiola MC, Farley TMM, Mbizvo MT. A review of androgen-progestin regimens for male contraception. J Androl 2003; 24: 466–483. [DOI] [PubMed] [Google Scholar]
- 16. Mommers E, Kersemaekers WM, Elliesen J, et al. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab 2008; 93: 2572–2580. [DOI] [PubMed] [Google Scholar]
- 17. Kamischke A, Venherm S, Plöger D, et al. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab 2001; 86: 303–309. [DOI] [PubMed] [Google Scholar]
- 18. Meriggiola MC, Costantino A, Saad F, et al. Norethisterone enanthate plus testosterone undecanoate for male contraception: effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metab 2005; 90: 2005–2014. [DOI] [PubMed] [Google Scholar]
- 19. Pavlou SN, Brewer K, Farley MG, et al. Combined administration of a gonadotropin-releasing hormone antagonist and testosterone in men induces reversible azoospermia without loss of libido. J Clin Endocrinol Metab 1991; 73: 1360–1369. [DOI] [PubMed] [Google Scholar]
- 20. Bagatell CJ, Matsumoto AM, Christensen RB, et al. Comparison of a gonadotropin releasing-hormone antagonist plus testosterone (T) versus T alone as potential male contraceptive regimens. J Clin Endocrinol Metab 1993; 77: 427–432. [DOI] [PubMed] [Google Scholar]
- 21. Tornøe CW, Agersø H, Nielsen HA, et al. Population pharmacokinetic modeling of a subcutaneous depot for GnRH antagonist degarelix. Pharm Res 2004; 21: 574–584. [DOI] [PubMed] [Google Scholar]
- 22. Sitruk-Ware R, Nath A. The use of newer progestins for contraception. Contraception 2010; 82: 410–417. [DOI] [PubMed] [Google Scholar]
- 23. Ilani N, Roth MY, Amory JK, et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab 2012; 97: 3476–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roth MY, Ilani N, Wang C, et al. Characteristics associated with suppression of spermatogenesis in a male hormonal contraceptive trial using testosterone and Nestorone(®) gels. Andrology 2013; 1: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin CW, Anderson RA, Cheng L, et al. Potential impact of hormonal male contraception: cross-cultural implications for development of novel preparations. Hum Reprod Oxf Engl 2000; 15: 637–645. [DOI] [PubMed] [Google Scholar]
- 26. Meriggiola MC, Bremner WJ, Costantino A, et al. An oral regimen of cyproterone acetate and testosterone undecanoate for spermatogenic suppression in men. Fertil Steril 1997; 68: 844–850. [DOI] [PubMed] [Google Scholar]
- 27. Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity. Endocrinology 2006; 147: 3016–3026. [DOI] [PubMed] [Google Scholar]
- 28. Thirumalai A, Ceponis J, Amory JK, et al. Effects of 28 days of oral dimethandrolone undecanoate in healthy men: a prototype male pill. J Clin Endocrinol Metab 2019; 104: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayoub R, Page ST, Swerdloff RS, et al. Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): a potential oral, male contraceptive. Andrology 2017; 5: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Attardi BJ, Marck BT, Matsumoto AM, et al. Long-term effects of dimethandrolone 17β-undecanoate and 11β-methyl-19-nortestosterone 17β-dodecylcarbonate on body composition, bone mineral density, serum gonadotropins, and androgenic/anabolic activity in castrated male rats. J Androl 2011; 32: 183–192. [DOI] [PubMed] [Google Scholar]
- 31. Wu S, Yuen F, Swerdloff RS, et al. Safety and pharmacokinetics of single dose novel oral androgen 11β-methyl-19-nortestosterone-17β-dodecylcarbonate in men. J Clin Endocrinol Metab 2019; 104: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piotrowska K, Wang C, Swerdloff RS, et al. Male hormonal contraception: hope and promise. Lancet Diabetes Endocrinol 2017; 5: 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meriggiola MC, Costantino A, Bremner WJ, et al. Higher testosterone dose impairs sperm suppression induced by a combined androgen-progestin regimen. J Androl 2002; 23: 684–690. [PubMed] [Google Scholar]
- 34. Michel E, Bents H, Akhtar FB, et al. Failure of high-dose sustained release luteinizing hormone releasing hormone agonist (buserelin) plus oral testosterone to suppress male fertility. Clin Endocrinol (Oxf) 1985; 23: 663–675. [DOI] [PubMed] [Google Scholar]
- 35. Liu PY, Swerdloff RS, Christenson PD, et al. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet Lond Engl 2006; 367: 1412–1420. [DOI] [PubMed] [Google Scholar]
- 36. Nieschlag E. Clinical trials in male hormonal contraception. Contraception 2010; 82: 457–470. [DOI] [PubMed] [Google Scholar]
- 37. Wang C, Swerdloff RS. Hormonal approaches to male contraception. Curr Opin Urol 2010; 20: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nieschlag E. The struggle for male hormonal contraception. Best Pract Res Clin Endocrinol Metab 2011; 25: 369–375. [DOI] [PubMed] [Google Scholar]
- 39. Zhang L, Shah IH, Liu Y, et al. The acceptability of an injectable, once-a-month male contraceptive in China. Contraception 2006; 73: 548–553. [DOI] [PubMed] [Google Scholar]
- 40. Meriggiola MC, Cerpolini S, Bremner WJ, et al. Acceptability of an injectable male contraceptive regimen of norethisterone enanthate and testosterone undecanoate for men. Hum Reprod Oxf Engl 2006; 21: 2033–2040. [DOI] [PubMed] [Google Scholar]
- 41. Bearak J, Popinchalk A, Alkema L, et al. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: estimates from a Bayesian hierarchical model. Lancet Glob Health 2018; 6: e380–e389. [DOI] [PMC free article] [PubMed] [Google Scholar]