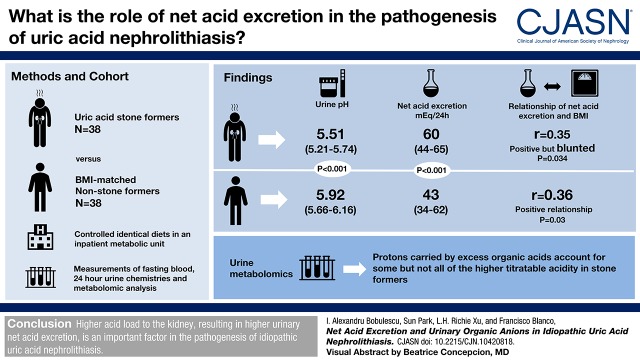
Visual Abstract
Keywords: uric acid, kidney stones, metabolic study, urine metabolomics, Body Mass Index, Metabolic Syndrome, Protons, Prevalence, Fasting, Buffers, Ammonium Compounds, Metabolomics, Citric Acid Cycle, Inpatients, obesity, Kidney Calculi, kidney, Anions, Amino Acids, lipids
Abstract
Background and objectives
Idiopathic uric acid nephrolithiasis, which is closely associated with obesity and the metabolic syndrome, is increasing in prevalence. Unduly acidic urine pH, the quintessential pathophysiologic feature of this disease, is in part explained by inadequate excretion of the principal urinary buffer ammonium. The role of net acid excretion in the pathogenesis of uric acid nephrolithiasis is incompletely understood.
Design, setting, participants, & measurements
We compared acid-base parameters of patients with idiopathic uric acid nephrolithiasis with matched control subjects under controlled diets in an inpatient metabolic unit. Measurements included fasting blood and 24-hour urine chemistries and 24-hour urine metabolomic analysis. Comparisons between groups included analysis of covariance models controlling for urine pH or body mass index.
Results
Subjects with idiopathic uric acid nephrolithiasis had lower urine pH (5.5 versus 5.9; P<0.001) and higher net acid excretion (60 versus 43 mEq/24 h; P<0.001), with the excess H+ carried by nonammonium buffers. In all subjects, there was a positive relationship of net acid excretion with higher body mass index in spite of strictly controlled equivalent dietary acid intake. This relationship was most evident among control subjects (r=0.36; P=0.03). It was attenuated in patients with idiopathic uric acid nephrolithiasis whose net acid excretion remained fixedly high and ammonium excretion remained low relative to net acid excretion, resulting in low urine pH over a wide body mass index range. Urinary metabolomics was performed to attempt to identify excess organic acids presented to the kidney in idiopathic uric acid nephrolithiasis. Among the tricarboxylic acid cycle intermediates and amino acid and lipid metabolites analyzed, 26 organic anions with acid dissociation constants values in the range of urine pH showed greater protonation. However, protons carried by the identified organic acids did not entirely account for the higher titratable acidity seen in idiopathic uric acid nephrolithiasis.
Conclusions
Higher acid load to the kidney, resulting in higher urinary net acid excretion, is an important factor in the pathogenesis of idiopathic uric acid nephrolithiasis.
Introduction
Uric acid nephrolithiasis made up 8%–10% of all kidney stones in the United States at the turn of the 21st century (1,2), and this proportion is increasing with time (3). Compounded by increasing overall prevalence of nephrolithiasis, uric acid nephrolithiasis is fast becoming a health care burden (4,5). The vast majority (>90%) of patients with uric acid stones have idiopathic uric acid nephrolithiasis (6), which is defined as uric acid stone formation in the absence of distinct pathogenic factors, such as low urine volume, and in the absence of congenital, acquired, or iatrogenic hyperuricosuria (7). Importantly, the proportion of uric acid stones is significantly higher in subsets of stone formers, such as patients with obesity or type 2 diabetes (2,7–9).
The single most important pathogenic factor responsible for uric acid precipitation in patients with idiopathic uric acid nephrolithiasis is an overly acidic urine, which promotes the protonation of urate (acid dissociation constant [pKa] 5.35 at 37°C) to the poorly soluble uric acid (6,10). Because patients with obesity or type 2 diabetes also tend to have overly acidic urine, regardless of kidney stone status, low urine pH is likely necessary but perhaps not sufficient for uric acid stone formation (7–11).
Previous studies have shown that overly acidic urine is attributable to inadequate excretion of the principal urinary buffer ammonia (NH3; which is protonated to ammonium [NH4+]), and it is likely compounded by higher net acid excretion, which stems in part from dietary intake (8–10). Although low NH4+ and high net acid excretion can theoretically be synergistic, the contribution of higher net acid excretion to the pathogenesis of idiopathic uric acid nephrolithiasis is much less well defined than that of inadequate urinary NH3/NH4+ excretion.
NH3 is the only major urinary buffer with a high pKa (>9) that effectively accepts H+ in the entire range of physiologic urine pH. Inadequate NH3/NH4+ production/excretion results in lower urine pH for any given net acid excretion. The higher free H+ then titrates the four major non-NH3 buffers (phosphate, urate, creatinine, and citrate), with protonation of urate contributing to precipitation of the sparingly soluble uric acid, thus setting the stage for lithogenesis. In addition, higher net acid excretion in idiopathic uric acid nephrolithiasis also involves excessive production and excretion of yet to be defined acids, where the conjugate anions may contribute in part to the greater titratable acid (defined as H+ carried by non-NH3 buffers). The identities of these anions are unknown.
In this study, we sought to determine net acid excretion in uric acid stone formers and matched nonstone-forming control subjects under tightly controlled metabolic conditions to eliminate exogenous confounders of net acid excretion, such as diet. We also examined the relationship between net acid excretion and body mass index (BMI). Finally, we conducted a pilot metabolomic analysis aimed at identifying new anions that may contribute to higher titratable acid and net acid excretion in idiopathic uric acid nephrolithiasis.
Materials and Methods
Study Population
This study enrolled adults >21 years old of either sex and any ethnicity. Patients with idiopathic uric acid nephrolithiasis confirmed by stone analysis were recruited from the University of Texas Southwestern–affiliated Mineral Metabolism Clinic and Urology Clinic. Nonstone-forming volunteers were recruited by campus advertising, and they were matched for age and sex with patients with idiopathic uric acid nephrolithiasis. Additional matching for BMI was conducted to control for the confounding effects of BMI on acid-base parameters. Exclusion criteria for nonstone formers were any history of confirmed or suspected kidney stone disease. Exclusion criteria for all groups were any history of CKD, proteinuria, eGFR<60 ml/min per 1.73 m2, recent or recurrent urinary tract infection, chronic liver disease, chronic alcohol use, chronic diarrheal illness, anemia, abnormal thyroid function, and pregnancy for women. Subjects receiving alkali treatment and/or allopurinol were instructed to discontinue these medications 2 weeks before the study. The study was approved by the Institutional Review Board at the University of Texas Southwestern, and written informed consent was obtained from each participant. In the first analysis presented, we compare net acid excretion and its determinants in patients with idiopathic uric acid nephrolithiasis and age-, sex-, and BMI-matched controls. In the second analysis presented, we compare urinary metabolome in patients with idiopathic uric acid nephrolithiasis and lean and obese controls.
Study Protocol
The study included a 3-day outpatient stabilization phase followed by a 2-day inpatient stabilization and evaluation phase as described previously (10), with modifications. During the outpatient phase, participants were maintained on a constant dietitian-formulated frozen metabolic diet (30% fat, 55% carbohydrate, 15% protein, low-acid ash content; providing 400 mg calcium, 800 mg phosphorus, 100 mEq sodium, and 2500–3000 ml water daily). The same diet was provided during the inpatient phase in the University of Texas Southwestern’s Clinical Research Unit. Participants were instructed to abstain from any other food or beverage intake during the 5 days of metabolic diet. During the last 2 days, 24-hour urine samples were collected for urine biochemistry. A 10-ml aliquot from the second 24-hour urine collection was frozen at −20°C for metabolomic analysis. Fasting venous blood samples were collected on days 5 and 6 for serum biochemistry.
Analytic Methods and Calculations
Urine electrolyte measurements were performed in the Clinical Laboratory Improvement Amendments–certified Mineral Metabolism Laboratory at the University of Texas Southwestern using a Cobas MIRA CC automated analyzer (Roche Diagnostics, Indianapolis, IN). Calcium was measured by atomic absorption spectrophotometry in an acidified urine aliquot to prevent precipitation. Uric acid was measured using the uricase method in an alkalinized urine aliquot to prevent precipitation. Creatinine was measured using the alkaline picrate method, citrate was measured using the citrate lyase method, NH4+ was measured using the glutamate dehydrogenase method, and sulfate was measured using ion chromatography (Dionex; Thermo Fisher Scientific, Waltham, MA). Urine pH was measured with a pH electrode. Titratable acid in urine was determined in a urine aliquot using an automated burette end point titration system (Radiometer, Copenhagen, Denmark) as the amount of OH− (in milliequivalents) required to bring the pH of the aliquot to 7.4. Bivalent and trivalent citrate species were calculated on the basis of measured citrate, measured urine pH, and the pKa of citrate2−/citrate3− of 5.6 (12). Repeating the analyses using a pKa of citrate2−/citrate3− of 6.4 (13) altered the absolute value of urine citrate in milliequivalents per day but did not alter the relative difference between uric acid stone formers and nonstone formers or the ratio of NH4+ to net acid excretion. HCO3− was calculated on the basis of measured urine pH and CO2 (14). Net acid excretion was calculated as 24-hour urine titratable acid plus NH4+ minus citrate and bicarbonate (all in milliequivalents). Net gastrointestinal alkali absorption was estimated as previously described (15). The supersaturation index (SI) of uric acid was calculated using JESS (16). Urine metabolomic analysis was performed using quantitative two-dimensional gas chromatography-time of flight mass spectrometry as previously described (17), with data obtained for 105 analytes. Of these, 26 measured organic anions had pKa values that allowed them to titrate H+ within the pH range of urine. Serum measurements were performed by Quest Diagnostics (Madison, NJ).
Titration of Artificial Urine
Titratable acidity of artificial urine testing was conducted using a laboratory-constructed system of an automated adjustable syringe pump holding a KOH solution, a continuous magnetic stirrer, and a pH electrode with real time recording all controlled and recorded by a laptop computer with a program developed by our group. pH and amount of OH− added were recorded and plotted automatically by the computer.
Statistical Analyses
Data are presented as median and interquartile range unless otherwise noted. We used the Kruskal–Wallis test to compare continuous variables between groups, the Wilcoxon rank sum test for pairwise comparisons, and the chi-squared test for categorical variables. Linear regression slopes were compared between uric acid stone formers and controls with analysis of covariance models controlling for urine pH or BMI. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
Patient Characteristics and Serum Biochemistry
In Table 1, baseline demographic and anthropometric characteristics and serum chemistry after equilibration on a fixed metabolic diet are shown for uric acid stone formers versus nonstone-forming volunteers matched for age, sex, and BMI. There were no differences between groups in serum electrolytes, pH, or bicarbonate. Serum uric acid, BUN, and creatinine were higher in patients with idiopathic uric acid nephrolithiasis. Significant differences between groups were noted for metabolic markers, including serum triglycerides and fasting glucose, but were not noted for total cholesterol, HDL cholesterol, or LDL cholesterol.
Table 1.
Demographic and anthropometric characteristics and serum biochemistry for uric acid stone formers and age-, sex-, and body mass index–matched nonstone formers
| Variable | N | Uric Acid Stone Formers, n=38 | N | BMI-Matched Nonstone Formers, n=38 |
|---|---|---|---|---|
| Age, yr | 53 (46–62) | 55 (48–62) | ||
| Sex (women/men) | 12/26 | 12/26 | ||
| Race/ethnicitya | ||||
| Asian | 1 | 3% | 1 | 3% |
| Black | 4 | 11% | 11 | 29% |
| Hispanic | 3 | 8% | 3 | 8% |
| White | 30 | 79% | 23 | 61% |
| BMI, kg/m2 | 32.5 (29.1–36.5) | 31.6 (27.1–35.1) | ||
| Sodium, mmol/L | 138 (137–139) | 137 (136–139) | ||
| Potassium, mmol/L | 4.2 (4.0–4.4) | 4.3 (4.1–4.5) | ||
| Chloride, mmol/L | 105 (103–107) | 104 (103–106) | ||
| Bicarbonate, mmol/L | 25 (25–28) | 26 (25–27) | ||
| pH | 7.38 (7.36–7.39) | 7.39 (7.38–7.40) | ||
| Uric acid, mg/dl | 8.1 (7.1–9.2) | 6.8 (5.4–7.6)b | ||
| BUN, mg/dl | 15 (11–19) | 12 (10–14)b | ||
| Creatinine, mg/dl | 1.00 (0.86–1.40) | 0.88 (0.76–1.01)b | ||
| Triglycerides, mg/dl | 191 (132–291) | 156 (102–179)b | ||
| Total cholesterol, mg/dl | 200 (179–230) | 191 (174–217) | ||
| LDL cholesterol, mg/dl | 119 (104–151) | 113 (97–140) | ||
| HDL cholesterol, mg/dl | 40 (32–45) | 40 (38–47) | ||
| Free fatty acids, mmol/L | 0.38 (0.33–0.56) | 0.38 (0.35–0.47) | ||
| Fasting glucose, mg/dl | 100 (89–118) | 91 (84–97)b | ||
| Type 2 diabetes | 12 | 32% | 9 | 24% |
| HOMA-IR (in nondiabetic subjects) | 1.89 (1.00–2.31) | 1.13 (0.68–1.45) |
Data are presented as median (interquartile range) or percentage. BMI, body mass index; HOMA-IR, Homeostatic Model Assessment—Insulin Resistance.
Percentages for race/ethnicity may not add up to 100% because of rounding.
P<0.05 compared with uric acid stone formers.
The 24-Hour Urine Biochemistry
Urine biochemistry after equilibration on a fixed metabolic diet is shown in Table 2. There were no differences between patients with idiopathic uric acid nephrolithiasis and controls in 24-hour creatinine excretion or urine electrolytes, including sodium and sulfate, indicating adherence to the same metabolic diet. Overall, patients with idiopathic uric acid nephrolithiasis had significantly lower urine pH and markedly elevated uric acid SI (SI uric acid) without elevation in 24-hour urine uric acid excretion. Compared with control subjects, patients with idiopathic uric acid nephrolithiasis had higher titratable acid and net acid excretion and lower 24-hour citrate excretion. NH4+ excretion was not different between groups, but the fraction of net acid excretion excreted as NH4+ (NH4+/net acid excretion) was markedly reduced in idiopathic uric acid nephrolithiasis. The reduced NH3/NH4+ generation and excretion have previously been noted (10,18–20), but the sources of greater net acid excretion are not known.
Table 2.
Urine biochemistry for uric acid stone formers and age-, sex-, and body mass index–matched nonstone formers
| Variable | Uric Acid Stone Formers, n=38 | Nonstone Formers, n=38 |
|---|---|---|
| Total volume, L/24 h | 2.47 (2.27–3.00) | 2.70 (2.47–2.88) |
| pH | 5.51 (5.21–5.74) | 5.92 (5.66–6.16)a |
| Creatinine, mg/24 h | 1581 (1229–1863) | 1563 (1069–1916) |
| Sodium, mEq/24 h | 93 (63–116) | 94 (74–123) |
| Potassium, mEq/24 h | 36 (31–41) | 40 (33–51)a |
| Calcium, mg/24 h | 123 (79–149) | 128 (72–169) |
| Citrate, mEq/24 hb | 5.5 (2.7–8.3) | 8.4 (6.1–11.4)a |
| Uric acid, mg/24 h | 514 (330–634) | 557 (460–666) |
| NH4+, mEq/24 h | 31 (22–36) | 31 (26–40) |
| Titratable acid, mEq/24 hc | 31 (27–40) | 24 (18–37)a |
| NAE, mEq/24 hc | 60 (44–65) | 43 (34–62)a |
| NH4+/NAE, mEq/mEqc | 0.54 (0.44–0.64) | 0.70 (0.59–0.85)a |
| Sulfate, mEq/24 h | 37 (31–42) | 34 (30–41) |
| NAE/sulfate, mEq/mEqc | 1.45 (1.30–1.74) | 1.30 (0.97–1.87)a |
| Phosphorus, mg/24 h | 697 (580–804) | 578 (472–749)a |
| Net gastrointestinal alkali absorption, mEq/24 h | 14 (5–25) | 26 (13–32)a |
| SI uric acid | 1.37 (1.01–1.60) | 1.02 (0.46–1.45)a |
| Creatinine clearance, ml/min | 103 (78–125) | 117 (94–141)a |
| Chloride, mEq/24 h | 85 (61–100) | 96 (74–114) |
| Oxalate, mg/24 h | 27 (23–31) | 26 (22–30) |
Data are presented as median (interquartile range). NH4+, ammonium; NAE, net acid excretion; SI, saturation index.
P<0.05 compared with uric acid stone formers.
Citrate excretion rate in milliequivalents per 24 hours was calculated according to the pH of each urine sample.
Titratable acidity was not measured in one stone former, leading to the inability to calculate NAE.
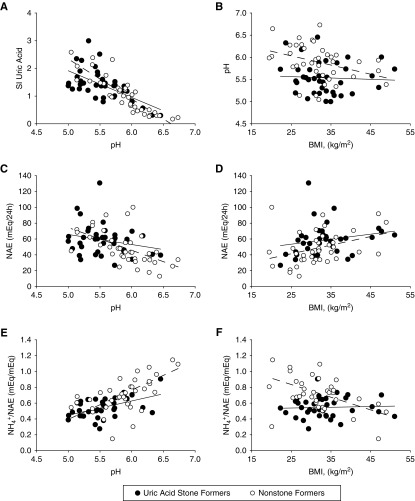
Net acid excretion, NH4+/net acid excretion, and pH from all study participants, stratified by stone status, are plotted against BMI and urine pH in Figure 1. Despite scatter and overlap, uric acid stone formers (black circles in Figure 1) and nonstone formers (white circles in Figure 1) segregated into two groups, consistent with overall differences summarized in Table 2. There was a strong negative correlation between 24-hour urine pH and SI uric acid (Figure 1A) in both uric acid stone formers (r=−0.64; P<0.001) and controls (r=−0.85; P<0.001), with no significant difference between the slopes of the lines. There was a stronger negative correlation between 24-hour urine pH and net acid excretion in nonstone formers (r=−0.55; P<0.001) than in uric acid stone formers (r=−0.23; P=0.17), with no difference between the slopes of the two lines (Figure 1B). Both uric acid stone formers (r=0.51; P=0.001) and controls (r=0.60; P<0.001) had strong positive correlations between 24-hour urine pH and NH4+/net acid excretion, with the slope of the line in uric acid stone formers blunted compared with controls, although the difference was not statistically significant (Figure 1C).
Figure 1.
Uric acid stone formers and nonstone formers segregate into two groups based on 24-hour urine biochemistry after equilibration on a fixed metabolic diet. (A) Correlation between 24-hour urine pH and uric acid saturation index (SI). (B) Urine pH decreased with greater BMI in nonstone formers, but remained low in uric acid stone formers regardless of BMI. (C) Correlation between 24-hour urine pH and net acid excretion (NAE). (D) NAE increased with BMI in nonstone formers, but this relationship was blunted in uric acid stone formers. (E) Correlation between 24-hour urine pH and urine ammonium excretion as proportion of NAE. (F) The proportion of NAE excreted as ammonium decreased with BMI in nonstone formers, but not in uric acid stone formers.
In contrast, when urine pH, net acid excretion, and NH4+/net acid excretion were plotted against BMI (Figure 1, D–F), trend lines had different slopes. Urine pH decreased with greater BMI in nonstone formers (r=−0.35; P=0.03), but pH remained low in patients with idiopathic uric acid nephrolithiasis regardless of BMI (r=−0.06; P=0.74) (Figure 1D). Net acid excretion increased (Figure 1E) (r=0.36; P=0.03) and NH4+/net acid excretion decreased (Figure 1F) (r=−0.45; P<0.01) with BMI in nonstone formers, but these relationships were blunted and nonsignificant in uric acid stone formers. From these plots, it is apparent that the differences in urine pH, net acid excretion, and NH4+/net acid excretion between the two groups were attenuated at higher BMI values.
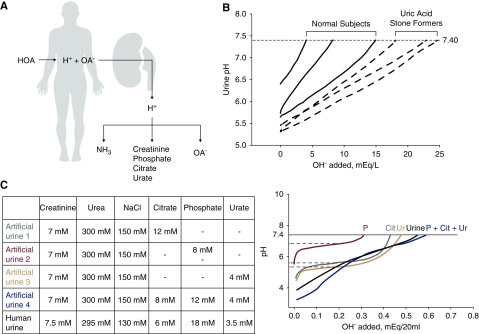
The significantly higher titratable acid in patients with idiopathic uric acid nephrolithiasis was partially explained by the lower urine pH and to a certain extent, the higher phosphate providing a buffer, whereas urate and creatinine were not different. There are potentially other buffers contributing to the higher titratable acid. To better understand this higher titratable acid, we examined the pH titration profile of urine from patients with idiopathic uric acid nephrolithiasis versus control participants. If the source of higher net acid excretion is from organic acids that are not metabolized, the conjugate anion of these acids should be present in the urine (Figure 2A). The rationale of this study is that, if there are abundant additional anions in the urine of patients with idiopathic uric acid nephrolithiasis from organic acids, additional “shoulders” may be evident in the pH titration profile, and the pKa of the unidentified buffer(s) may shed light on the identity of the anion. Figure 2B shows a typical comparison of pH titration profiles of patients with idiopathic uric acid nephrolithiasis versus control subjects. The lower starting urine pH and the higher amount of OH− required to reach pH 7.4 were evident in stone formers. The titration profiles of artificial urine with more than one major buffer or normal human urine showed completely smooth transitions, with no identifiable flat portions (Figure 2C). This indicates that urinary H+ buffering is carried out not by a few major buffers but instead, by multiple buffers with wide overlapping ranges of pKa values, and each one has a small contribution so that individual buffers are not “detectable” in titration curves. Therefore, it is unlikely that the “extra” organic acids in urine can be accounted for by a single species of organic anions.
Figure 2.
Titration of human and artificial urine suggests that proton titration in the urine is accomplished by multiple buffers with wide overlapping ranges of acid dissociation constant (pKa) values. (A) Rationale for search for organic anions. High acid addition to the body results in high net acid excretion by the kidney. The H+ is carried by the conventional urinary buffers—ammonia (NH3), creatinine, phosphate, citrate, and urate. Inadequate ammoniagenesis/excretion leads to obligatory buffering by (B) 20 ml of spot urine from three normal subjects, and three subjects with idiopathic uric acid nephrolithiasis were titrated with dropwise addition of 10 μl 0.1 N NaOH until urine pH was 7.40. (C) Urinary titration was performed on artificial urine with the compositions shown in the table in the upper panel and compared with human urine. Titration curves are shown in the lower panel. Although ΔpH/NaOH added can identify values approximated by the acid dissociation constant (pKa) values of single buffers, such as phosphate, citrate, and urate, when all three buffers are added to artificial urine, the titration curve exhibits a smooth and almost constant slope, resembling real human urine. HOA, organic acid; OA-, organic anion.
Urine Metabolomics
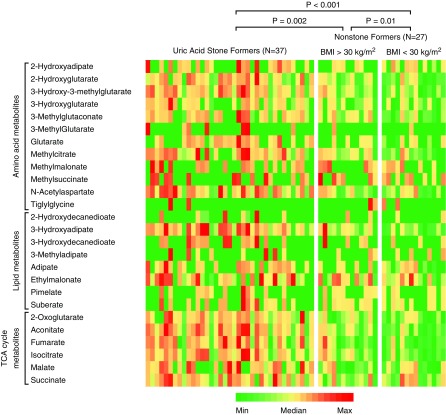
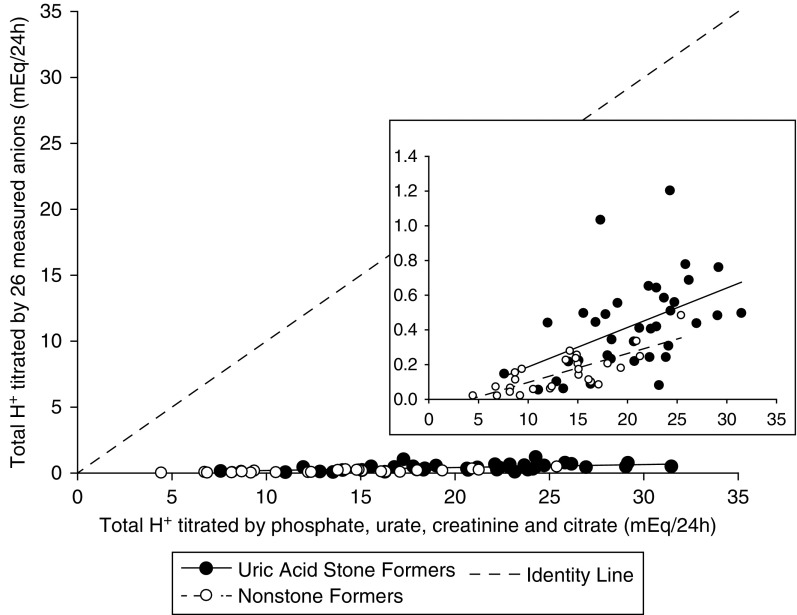
Baseline demographic and anthropometric characteristics and serum biochemistry of the cohort of 37 patients with idiopathic uric acid nephrolithiasis and 27 nonstone-forming controls for whom urinary metabolomics data are available are shown in Table 3. To assess for the potential confounding effects of obesity on urine metabolomics, we divided nonstone formers into obese (BMI>30 kg/m2) and nonobese (BMI<30 kg/m2) subgroups. Urine biochemistry for the metabolomics subcohort after equilibration on a fixed metabolic diet is shown in Table 4. Patients with idiopathic uric acid nephrolithiasis had significantly lower urine pH than the two other groups, whereas nonobese controls had significantly lower titratable acid and higher NH4+/net acid excretion compared with the two other groups. All measured organic anions and their relative excretion rates are shown in Supplemental Figure 1. Twenty-six of the organic anions measured have pKa values that allow them to titrate H+ within the pH range of urine. The excretion rates for these anions were in the micromole to millimole range per 24 hours, and they were not significantly different between groups. However, differences emerged when we looked at the amount of H+ titrated by these anions. Figure 3 shows the relative amounts of H+ titrated by each of these 26 urinary anions calculated for each subject using the actual pH of the urine sample from which the metabolomic analysis was performed. The differences between groups for H+ titrated by individual anions were most evident for tricarboxylic acid cycle metabolites, with the most H+ titrated in idiopathic uric acid nephrolithiasis followed in order by obese and nonobese controls. The cumulative H+ titrated by the 26 anions in each study subject strongly correlated with, but represented only a small proportion (a few percent) of, the total H+ titrated in the same subject by the four major non-NH4+ urinary buffers (phosphate, urate, creatinine, and citrate) (Figure 4).
Table 3.
Demographic and anthropometric characteristics and serum biochemistry of the metabolomics cohort, with stratification by stone status and body mass index
| Variable | Uric Acid Stone Formers, n=37 | Nonstone Formers | |
|---|---|---|---|
| BMI>30 kg/m2, n=13 | BMI<30 kg/m2, n=14 | ||
| Age, yr | 59 (50–65) | 55 (49–57) | 51 (47–57) |
| Sex (women/men) | 15/22 | 5/8 | 6/8 |
| Race/ethnicity | |||
| Asian | 1 (3%) | 0 (0%) | 0 (0%) |
| Black | 6 (16%) | 3 (23%) | 6 (43%) |
| Hispanic | 4 (11%) | 1 (8%) | 0 (0%) |
| White | 26 (70%) | 9 (69%) | 8 (57%) |
| BMI, kg/m2 | 37.5 (32.0–43.3) | 40.7 (32.2–47.0) | 24.5 (21.1–24.9)a,b |
| Sodium, mmol/L | 137 (137–139) | 137 (136–138) | 138 (137–138) |
| Potassium, mmol/L | 4.2 (4.1–4.5) | 4.3 (4.2–4.5) | 4.3 (4.2–4.5) |
| Chloride, mmol/L | 103 (102–105) | 103 (103–105) | 104 (103–105) |
| Bicarbonate, mmol/L | 25 (24–26) | 25 (24–26) | 28 (25–28) |
| pH | 7.38 (7.36–7.40) | 7.42 (7.40–7.43) | 7.38 (7.36–7.39) |
| Uric acid, mg/dl | 8.0 (7.2–8.9) | 7.0 (5.2–8.1) | 5.4 (5.0–6.3)a |
| BUN, mg/dl | 18 (15–20) | 14 (11–17) | 13 (12–14)a |
| Creatinine, mg/dl | 1.06 (0.81–1.41) | 0.77 (0.67–0.91)a | 0.84 (0.65–0.97) |
| Triglycerides, mg/dl | 206 (133–287) | 150 (98–172)a | 116 (84–137)a |
| Total cholesterol, mg/dl | 198 (181–236) | 183 (170–203) | 194 (179–208) |
| LDL cholesterol, mg/dl | 120 (92–153) | 110 (98–129) | 112 (85–124) |
| HDL cholesterol, mg/dl | 37 (30–45) | 41 (37–50)a | 58 (49–66)a,b |
| Free fatty acids, mmol/L | 0.47 (0.36–0.64) | 0.46 (0.37–0.52) | 0.37 (0.23–0.39)a,b |
| Fasting glucose, mg/dl | 102 (89–117) | 95 (92–100) | 84 (82–89)a,b |
Data are presented as median (interquartile range) or number of subjects (percentage). BMI, body mass index.
P<0.05 versus uric acid stone formers.
P<0.05 versus nonstone formers with BMI>30 kg/m2 by the Wilcoxon rank sum test.
Table 4.
Urine biochemistry of the metabolomics cohort, with stratification by stone status and body mass index
| Variable | Uric Acid Stone Formers, n=37 | Nonstone Formers | |
|---|---|---|---|
| BMI>30, n=13 | BMI<30, n=14 | ||
| pH | 5.47 (5.25–5.88) | 5.95 (5.75–6.16)a | 6.23 (6.02–6.44)a |
| Creatinine, mg/24 h | 1445 (1272–1757) | 1623 (1082–1904) | 1243 (908–1374)a |
| Creatinine/kg, mg/24 h per 1 kg | 13.8 (12.1–17.4) | 12.3 (9.9–15.7) | 18.3 (15.3–20.0)a,b |
| Sodium, mEq/24 h | 98 (84–117) | 91 (74–116) | 118 (73–143) |
| Potassium, mEq/24 h | 40 (36–49) | 51 (42–54) | 42 (35–52) |
| Calcium, mg/24 h | 88 (68–138) | 111 (80–141) | 159 (70–187) |
| Citrate, mEq/24 hc | 6.5 (3.6–10.7) | 7.5 (5.9–8.7) | 8.8 (7.4–11.0) |
| Uric acid, mg/24 h | 581 (384–682) | 662 (575–788) | 483 (396–540)b |
| NH4+, mEq/24 h | 30 (21–34) | 31 (24–35) | 27 (25–31) |
| Titratable acid, mEq/24 h | 37 (30–45) | 37 (21–47) | 19 (13–24)a,b |
| NAE, mEq/24 h | 56 (44–65) | 55 (34–71) | 34 (21–45)a |
| NH4+/NAE, mEq/mEq | 0.52 (0.41–0.58) | 0.59 (0.49–0.74) | 0.81 (0.66–1.05)a,b |
| Sulfate, mEq/24 h | 34 (29–40) | 34 (31–39) | 30 (27–31)a,b |
| NAE/sulfate, mEq/mEq | 1.50 (1.35–1.99) | 1.40 (1.04–1.96) | 1.04 (0.75–1.47)a |
| NAE/body weight, mEq/kg | 0.52 (0.41–0.60) | 0.48 (0.36–0.64) | 0.52 (0.34–0.66) |
| Phosphorus, mg/24 h | 705 (573–798) | 765 (543–803) | 488 (409–695)a,b |
| Net gastrointestinal alkali absorption, mEq/24 h | 20 (7–26) | 21 (13–30) | 32 (18–44) |
| SI uric acid | 1.47 (1.08–1.87) | 1.05 (0.50–1.40) | 0.36 (0.26–0.59)b |
| Creatinine clearance, ml/min | 99 (72–133) | 128 (103–151) | 100 (91–114) |
| Chloride, mEq/24 h | 96 (82–112) | 96 (83–114) | 112 (71–139) |
| Oxalate, mg/24 h | 29 (25–33) | 28 (25–33) | 24 (22–26)a,b |
| Organic acids, mmol/24 h | 1.26 (1.03–1.49) | 1.25 (0.98–1.44) | 1.10 (0.99–1.24) |
| Organic acids, mEq titrated | 0.41 (0.22–0.50) | 0.18 (0.11–0.25)a | 0.09 (0.06–0.18)a |
| PO4, Cit, Cr, UA, mEq titrated | 20.7 (15.5–24.1) | 15.1 (13.8–19.3)a | 9.9 (8.7–14.8)a,b |
Data are presented as median (interquartile range). BMI, body mass index; NH4+, ammonium; NAE, net acid excretion; SI, saturation index; PO4, phosphate; Cit, citrate; Cr, creatinine; UA, urate.
P<0.05 versus uric acid stone formers.
P<0.05 versus nonstone formers with BMI>30 kg/m2 by the Wilcoxon rank sum test.
Citrate excretion rate in milliequivalents per 24 hours was calculated according to the pH of each urine sample.
Figure 3.
Compared with nonstone formers, uric acid stone formers have higher relative amounts of H+ titrated by each of the 26 measured urinary anions that have acid dissociation constant values that allow them to titrate H+ within the pH range of urine. Each row represents one anion (with color coding within each row), and each column represents one study participant. Statistical comparison between groups was performed using the Kruskall–Wallis one-way ANOVA using the cumulative H+ titrated by all 26 anions in each study subject. BMI, body mass index; TCA, tricarboxylic acid.
Figure 4.
The cumulative H+ titrated by 26 measured urinary anions with acid dissociation constant (pKa) values within the pH range of urine strongly correlated with, but represented only a small proportion of, the total H+ titrated by the four major nonammonium urinary buffers. The cumulative H+ titrated by the 26 measured urinary anions that have pKa values that allow them to titrate H+ within the pH range of urine is plotted against the total H+ titrated in the same subjects by the four major nonammonium urinary buffers (phosphate, urate, creatinine, and citrate). The inset shows that the sum of these measured urinary anions is consistently higher in idiopathic uric acid nephrolithiasis versus nonstone formers.
Discussion
This is a comprehensive study investigating the pathophysiology of idiopathic uric acid nephrolithiasis in patients and nonstone-forming controls after carefully controlled dietary equilibration that included an inpatient phase on a monitored and fixed metabolic diet. We previously showed that patients with idiopathic uric acid nephrolithiasis excrete less of their urinary net acid as NH4+, resulting in unduly acidic urine pH in the absence of clinically apparent systemic acid-base disturbance (10). Superimposed on and compounding the defect in ammoniagenesis/excretion is the higher net acid excretion despite the controlled diet in idiopathic uric acid nephrolithiasis, but the origin of this extra acid is unknown.
There are several key findings from this dataset. First, there are no distinct heightened “peaks” of urine buffer capacity. Polyprotic buffers, such as phosphate and citrate, with multiple pKa values already render the titration curve continuous (Figure 2C). The four traditional “abundant” buffers (Figure 2A) are on the basis of their concentrations. In real urine, H+ acceptors distribute continuously throughout the entire range of urine pH.
Second, increased kidney net acid excretion is a fundamental biochemical feature of idiopathic uric acid nephrolithiasis independent of diet, and it is in part responsible for the observed lower NH4+-to-net acid excretion ratio, because the denominator in this fraction increases more than the numerator in patients with idiopathic uric acid nephrolithiasis compared with nonstone formers. Net acid excretion increases with higher BMI independent of diet in nonstone formers, whereas in idiopathic uric acid nephrolithiasis, net acid excretion remains elevated even at lower BMI values. Although these findings are consistent with the previously identified role of inappropriately low NH3/NH4+ production/excretion in idiopathic uric acid nephrolithiasis (10,11,18,21), they unequivocally show that increased net acid excretion is a primary sine qua non defect that challenges NH4+ excretion, unmasking and accentuating the deficiency.
Third, although higher BMI in nonstone formers correlates with lower urine pH and NH4+/net acid excretion, the slope of this relationship is relatively flat in idiopathic uric acid nephrolithiasis, suggesting that there is probably a “fixed” defect in this condition. It is likely that obesity-induced urine biochemical changes increase uric acid stone risk in some patients with idiopathic uric acid nephrolithiasis—the severely obese in particular. Prevalent type 2 diabetes per se may confer additional contributions to uric acid stone risk in these patients as previously described (11). Importantly, BMI showed much weaker correlations with pH, net acid excretion, and NH4+/net acid excretion among patients with idiopathic uric acid nephrolithiasis in our study (Figure 1), suggesting that factors other than obesity also contribute to the urine biochemical changes that are pathogenic for idiopathic uric acid nephrolithiasis, particularly in patients who have lower BMI. These factors remain to be determined.
Fourth, our pilot metabolomic analysis looked at 26 urinary anions that can act as H+ carriers at human urine pH and that are not routinely measured in clinical or even research studies. The excretion rates of these anions range from micromoles to millimoles per 24 hours, and they are not significantly different between the two groups. However, when analyzed cumulatively, these 26 urinary anions titrate significantly more H+ in patients with idiopathic uric acid nephrolithiasis driven mainly by the lower urine pH, thus accounting for a portion of increased titratable acid in these patients. Two points are noteworthy. First, some of these organic anions are metabolizable, and therefore, they are potentially bases, akin to citrate; these metabolizable anions are genuine urinary buffers, but they do not indicate true net acid excretion. Second, the organic anions identified herein only accounted quantitatively for a small portion of the excess titration acid in idiopathic uric acid nephrolithiasis.
There are some experimental limitations and many unanswered questions. The variance of organic anion excretion is extremely high even on a fixed diet. Small differences between patients with idiopathic uric acid nephrolithiasis and controls can be hidden underneath the biologic noise. The list of metabolites included in this metabolomic analysis is not exhaustive, and it is possible that other organic acid species not included in our analysis may be elevated in idiopathic uric acid nephrolithiasis. The source of elevated organic acid remains elusive and may include both endogenous production by the body and exogenous sources, such as absorption of microbially derived organic acids from the gut lumen. Estimated net gastrointestinal absorption was lower in uric acid stone formers, raising the possibility of intestinal alkali loss, although in other studies, we found a much lower stool pH in uric acid stone formers (not shown), which would not be compatible with reduced alkali absorption. A final limitation is that only venous acid-base was measured, and arterial values were not obtained.
In conclusion, increased H+ load to the kidney is as important for the pathogenesis of idiopathic uric acid nephrolithiasis as inadequate NH4+ excretion. The identities and sources of the excess acid are diverse and need to be resolved.
Disclosures
I.A.B. and N.M.M. received research funding from Takeda Pharmaceutical Company Ltd via the Investigator-Initiated Sponsored Research mechanism for research unrelated to this article. There was no funding or involvement from Takeda Pharmaceutical Company Ltd or any other commercial entity in any aspect of this article, including no involvement in study design, conduct, analysis, manuscript preparation, or decision to publish.
Supplementary Material
Acknowledgments
The authors are grateful for the assistance of the research nursing and technical staff at the Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, the Mineral Metabolism Laboratory, and the Clinical and Translational Research Center at the University of Texas Southwestern. We thank Drs. Lawrence Sweetman and Erland Arning (Center of Metabolomics, Baylor Institute of Metabolic Disease, Dallas, TX) for assistance with urine metabolomics service.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK113377 (to I.A.B.), R01-DK081423 (to K.S. and O.W.M.), and R01-DK091392 (to O.W.M.); NIDDK O’Brien Kidney Research Center Grant P30-DK07938; and Endowed Professors Collaborative Research Support Grant (to K.S. and O.W.M.) from the Charles and Jane Pak Foundation. The University of Texas Southwestern Clinical Research Unit is supported in part by National Institutes of Health Grant UL1-TR001105.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10420818/-/DCSupplemental.
Supplemental Figure 1. There are no differences between non-stone formers and stone formers in the relative amounts of organic acids when reported as mmol/mol creatinine.
References
- 1.Mandel NS, Mandel GS: Urinary tract stone disease in the United States veteran population. II. Geographical analysis of variations in composition. J Urol 142: 1516–1521, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Lieske JC, de la Vega LS, Gettman MT, Slezak JM, Bergstralh EJ, Melton LJ 3rd, Leibson CL: Diabetes mellitus and the risk of urinary tract stones: A population-based case-control study. Am J Kidney Dis 48: 897–904, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Xu LHR, Adams-Huet B, Poindexter JR, Maalouf NM, Moe OW, Sakhaee K: Temporal changes in kidney stone composition and in risk factors predisposing to stone formation. J Urol 197: 1465–1471, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC: Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 63: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Scales CD Jr ., Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH: Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int 60: 757–761, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Maalouf NM, Cameron MA, Moe OW, Sakhaee K: Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens 13: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K: Urine composition in type 2 diabetes: Predisposition to uric acid nephrolithiasis. J Am Soc Nephrol 17: 1422–1428, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K: Low urine pH: A novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2: 883–888, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Sakhaee K, Adams-Huet B, Moe OW, Pak CY: Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int 62: 971–979, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Maalouf NM, Cameron MA, Moe OW, Sakhaee K: Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol 5: 1277–1281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caudarella R, Vescini F, Buffa A, Stefoni S: Citrate and mineral metabolism: Kidney stones and bone disease. Front Biosci 8: s1084–s1106, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hamm LL: Renal handling of citrate. Kidney Int 38: 728–735, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Lennon EJ, Lemann J Jr., Litzow JR: The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 45: 1601–1607, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh MS: A new method for estimating G-I absorption of alkali. Kidney Int 36: 915–917, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Rodgers A, Allie-Hamdulay S, Jackson G: Therapeutic action of citrate in urolithiasis explained by chemical speciation: Increase in pH is the determinant factor. Nephrol Dial Transplant 21: 361–369, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Sweetman L, Ashcraft P, Bennett-Firmin J: Quantitative organic acids in urine by two dimensional gas chromatography-time of flight mass spectrometry (GCxGC-TOFMS). Methods Mol Biol 1378: 183–197, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW: Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol 294: F1315–F1322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobulescu IA, Maalouf NM, Capolongo G, Adams-Huet B, Rosenthal TR, Moe OW, Sakhaee K: Renal ammonium excretion after an acute acid load: Blunted response in uric acid stone formers but not in patients with type 2 diabetes. Am J Physiol Renal Physiol 305: F1498–F1503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron M, Maalouf NM, Poindexter J, Adams-Huet B, Sakhaee K, Moe OW: The diurnal variation in urine acidification differs between normal individuals and uric acid stone formers. Kidney Int 81: 1123–1130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW: Reduction of renal triglyceride accumulation: Effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol 297: F1419–F1426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.