Abstract
IgA nephropathy (IgAN) is an important cause of ESKD for which there are no approved therapies. A challenge for evaluating treatments for IgAN is the usual long time course for progression to ESKD. The aim of this Kidney Health Initiative project was to identify surrogate end points that could serve as reliable predictors of a treatment’s effect on long-term kidney outcomes in IgAN and be used as a basis for approval. Proteinuria was identified as the most widely recognized and well studied risk factor for progression to ESKD in IgAN. The workgroup performed a critical review of the data on proteinuria reduction as a surrogate end point for a treatment’s effect on progression to ESKD in IgAN. Epidemiologic data indicate a strong and consistent relationship between the level and duration of proteinuria and loss of kidney function. Trial-level analyses of data from 13 controlled trials also show an association between treatment effects on percent reduction of proteinuria and treatment effects on a composite of time to doubling of serum creatinine, ESKD, or death. We conclude that data support the use of proteinuria reduction as a reasonably likely surrogate end point for a treatment’s effect on progression to ESKD in IgAN. In the United States, reasonably likely surrogate end points can be used as a basis for accelerated approval of therapies intended to treat serious or life-threatening conditions, such as IgAN. The clinical benefit of products approved under this program would need to be verified in a postmarketing confirmatory trial.
Keywords: IgA nephropathy; surrogate endpoint; clinical trials; Glomerulonephritis; IGA; creatinine; risk factors; proteinuria; kidney; Kidney Failure, Chronic
Introduction
IgA nephropathy (IgAN) is the most common form of GN in the world and an important cause of ESKD. Despite advances in our understanding of the pathogenesis of IgAN, there has been little progress in its treatment with no licensed or approved therapies. One of the key challenges in the evaluation of treatments for IgAN is its usually slowly progressive nature, with ESKD typically only developing after many years. Although a significant loss of kidney function has been accepted as a surrogate end point for progression to ESKD, clinical trials in CKD may still need to be relatively large and long to demonstrate a treatment effect on that end point. Hence, there has been interest in earlier end points that could serve as reliable predictors of a treatment’s effect on long-term kidney outcomes in IgAN.
In March of 2016, the Kidney Health Initiative, a public-private partnership between the American Society of Nephrology and the US Food and Drug Administration (FDA) (1), initiated a project to identify end points that could be used as a basis for IgAN therapy approval. To date, the most widely recognized and well studied risk factor for progression to ESKD in patients with IgAN is proteinuria. Although other biomarkers have been studied, none were considered to have been as consistently associated with adverse clinical outcomes in IgAN as sustained proteinuria. This led the workgroup to focus on whether there were sufficient data to support the use of proteinuria reduction as a surrogate end point for a treatment’s effect on progression to ESKD in patients with IgAN.
Surrogate End Points and United States Approval Pathways
Surrogate end points have been widely used to establish the effectiveness of therapies to slow the progression of kidney disease and treat its complications (e.g., a doubling of serum creatinine as a basis for approval of therapies intended to slow progression to ESKD [2]).
As indicated in the Biomarkers, EndpointS, and other Tools (BEST) Resource (3), surrogate end points are used in clinical trials as a substitute for a direct measure of how a patient feels, functions, or survives; they do not measure the clinical benefit of primary interest but are expected to predict that clinical benefit. In the United States, validated surrogate end points can be used as a basis for traditional approval of therapies, whereas “reasonably likely” surrogate end points can be used as a basis for accelerated approval of therapies intended to treat a serious or life-threatening condition such as IgAN (4). Just as the name implies, the accelerated approval program enables approval of a therapy earlier in its development pathway than traditional approval. However, because there is still some uncertainty about the efficacy on a clinical end point of products granted approval under this program, a postmarketing trial is typically required to verify the clinical benefit. Of note, the accelerated approval program is the only pathway in the United States that provides the FDA the mechanism to resolve issues related to effectiveness (i.e., verify the benefit) in the postmarketing setting.
Evidentiary Considerations Related to Surrogate End Points
From a drug development perspective, it is important to distinguish between “surrogate end points” and biomarkers. A biomarker is a defined characteristic that is measured as an indicator of normal biologic processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions (3,5). For example, biomarkers may be used in drug development to enrich a trial population with patients who are more likely to progress to an outcome of interest over the course of a trial or select a population that is more likely to respond to a particular intervention. Of note, biomarkers that perform well in identifying patients at risk of a particular outcome may not perform well when used as a surrogate end point to predict the effect of an intervention on that same clinical outcome in a trial. This can happen for a number of reasons—sometimes the biomarker is not on the causal pathway; drugs can also have off-target adverse effects that work through pathways that are independent of the biomarker. For further discussion of this issue and examples, see the referenced review (6).
The BEST glossary classifies surrogate end points as “validated,” “reasonably likely,” and “candidate” surrogate end points, on the basis of their level of clinical validation. Factors that are considered when assessing whether a surrogate end point is a validated surrogate end point include (1) the biologic plausibility of the relationship between the surrogate end point and clinical outcome of interest, (2) the strength and consistency of the epidemiologic data supporting the relationship, and (3) whether treatment effects on the surrogate end point have been shown to predict treatment effects on the clinical outcome of interest using different types of interventions. The evidence supporting a reasonably likely surrogate end point falls short of that needed to support its use as a validated surrogate end point and may include epidemiologic, therapeutic, pathophysiologic, or other evidence. General factors that should be considered when assessing whether a surrogate end point is reasonably likely to predict a meaningful clinical benefit are addressed in the FDA’s Guidance for Industry titled Expedited Programs for Serious Conditions—Drugs and Biologics (7). As noted in that guidance, an important consideration is how well a disease process is understood, and whether the disease process is complex and/or multiple causal pathways are thought to exist. In general, it can be difficult to determine whether an effect on a surrogate end point will translate into a clinical benefit when a disease process is complex and/or poorly understood.
Identifying Patients at High Risk of Progression
It is well recognized that the severity and course of disease vary considerably among patients with IgAN (8,9). Whereas some patients have an indolent disease with an excellent prognosis with supportive therapy alone, others experience loss of kidney function over years, and a minority may suffer a rapid progression to ESKD over weeks or months. When evaluating the effect of therapeutic interventions in IgAN, it is important that clinical trials enrich the study population with patients at greater risk of disease progression. It is also important to recognize risk factors of progression in interpreting and comparing the results of past clinical studies, on the basis of the patient populations they have included.
To date, the most well studied and consistent clinical risk factors of progression of IgAN are histopathology, decreased GFR, sustained proteinuria, and hypertension. Of these factors, decreased GFR and sustained proteinuria are most commonly used to enrich trials for patients at high risk of progression. Other risk factors for disease progression in IgAN include genetic and demographic factors, such as race and ethnicity, and laboratory findings.
Patients with decreased GFR at diagnosis of IgAN (e.g., eGFR<60 ml/min per 1.73 m2) are at higher risk of progression to ESKD (10,11). Although patients with severe kidney dysfunction (e.g., eGFR<30 ml/min per 1.73 m2) are at greatest risk of reaching ESKD, these patients have usually been excluded from clinical trials because of concern that the disease is too advanced to respond to immune-modulating therapy or that the risks of therapy may outweigh the potential benefits. In general, when selecting criteria for enrollment, it is important to consider patients for whom a drug may provide benefit on the basis of its mechanism of action and targeted effect. For example, somewhat different patient populations may benefit from treatments targeting inflammation versus those targeting fibrosis. Histopathology findings have also been incorporated in the patient selection criteria of some more recent clinical trials (e.g., clinicaltrials.gov identifier: NCT02808429, NCT02062684), reflecting the recent advances made in understanding histologic predictors of progression (12–16). However, when the interval between the biopsy and enrollment is long or treatment has been used, histopathology findings may well have changed and may no longer be reliable indicators of the level of disease “activity” at the time of trial enrollment (with the possible exception of exclusionary criteria on the basis of irreversible glomerular sclerosis or tubule-interstitial scarring). Overall, of the main risk factors of progression identified to date, the presence of significant proteinuria, especially if it is persistent despite antihypertensive and renin-angiotensin system blockade, is the most commonly used inclusion criterion for clinical trials in IgAN. The rationale for this is elaborated in the subsequent section.
Rationale for Focusing on Proteinuria as a Surrogate End Point
At the start of the project, the workgroup considered a number of candidate surrogate end points for clinical trials in IgAN, including proteinuria, biopsy findings, and other urine and serum biomarkers that are under active investigation in IgAN. Therapies for IgAN may be expected to result in the resolution of histopathologic signs of ongoing “active” GN (e.g., mesangial hypercellularity, endocapillary proliferation, glomerular crescents). Although small studies of repeat kidney biopsy specimens suggest that “active” lesions in IgAN may change with therapy (17–20), the association between short-term pathologic changes and long-term kidney survival is not well established. Hence, the workgroup did not think the available evidence was sufficient to support the use of changes in histopathologic findings from repeat kidney biopsy specimens as a surrogate end point in registration trials in IgAN. Further work is needed to determine the specific histopathologic changes that could be used to evaluate efficacy in such trials.
A number of novel urine and serum biomarkers have been suggested to reflect progression of kidney disease in IgAN. Examples include urinary chemokine CXCL1 (12); monocyte chemoattractant protein-1 and EGF (21); serum galactose-deficient IgA, TNF receptors 1 and 2 (22); and complement factor C3 (23–25). However, to date, evidence that changes in these biomarkers track with clinical measures of disease activity and/or response to therapy is weak and/or nonexistent. Indeed, in many studies, these biomarkers did not appear to add information about the risk of disease progression beyond that provided by proteinuria.
Given these data, the workgroup decided to focus on the evidence supporting proteinuria as a surrogate end point in clinical trials of IgAN. The sections that follow address the biologic plausibility that proteinuria is on the causal pathway to ESKD in IgAN, data from registries and observational studies on the association between proteinuria and kidney outcomes, and data from intervention trials on the relationship between treatment effects on proteinuria and treatment effects on outcomes.
Evidence Supporting Proteinuria as a Causal Factor in Disease Progression
Experimental data derived from in vitro studies indicate that proteinuria directly contributes to kidney injury and to the decline in kidney function across chronic proteinuric nephropathies (26). Urinary proteins from patients with nephrotic syndrome induce inflammatory and cytotoxic responses in tubular epithelial cells in vitro and this is thought to contribute to interstitial fibrosis (27). Furthermore, kidney tubulo-interstitial tissue from patients with IgAN can be distinguished from control tissue on the basis of the expression of in vitro–derived albumin-regulated genes (28). There also appears to be a proteinuria “dose” effect within IgAN (29,30). Although such data support the concept that proteinuria is damaging to the kidney, it has been difficult to reconcile the association of low levels of sustained proteinuria with loss of kidney function in patients with IgAN with the finding that similar low levels of proteinuria do not appear to be associated with loss of kidney function in other glomerular diseases (31). This difference suggests that factors other than level of proteinuria alone play a role. Although not evidence of causality, such in vitro data provide a possible explanation for the observed differences among various glomerulopathies in the relationship between level of proteinuria and loss of kidney function. Data from intervention trials, discussed below, also indicate that treatment effects on proteinuria correlate with effects on loss of GFR, providing further support for a possible causal relationship.
Epidemiologic Associations
Seven registry studies (n=3628) from across diverse regions, including North America (29), Europe (32,33), and Asia (30), have shown a significant association between changes in proteinuria and kidney outcome in univariable and multivariable analyses (Table 1). An independent association between baseline proteinuria and the risk of loss of GFR has been reported in some (34), but not other, studies (29,35). However, the association between the severity and duration of proteinuria and kidney function loss is consistent across studies.
Table 1.
Summary data on the association of proteinuria reduction with long-term kidney and patient outcomes from cohort studies of patients with IgAN
| Study | N | Outcome | Follow-Up (yr) | Baseline Characteristics | Measure of Proteinuria | Change in Proteinuria Significantly Associated with Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Race/Ethnicity | Proteinuria, g/d | eGFR, ml/min per 1.73 m2 | Unadjusted/Univariable Analysis | Adjusted/Multivariable Analysis | Variables Included in Adjusted Analysis | |||||
| Bartosik et al. (35) | 298 | Rate of creatinine clearance decrease | 6 | 65% white, 3% black, 17% Asian, 15% other | 2.3±2.3 | 76±35 | Proteinuria/24 h | Yes | Yes | MAP during follow-up, proteinuria at baseline, proteinuria during follow-up (Lee classification) |
| Donadio et al. (44), IgAN 1 | 91 | ESKD | Median 5.8 | ND | ND | ND | Proteinuria/24 h, slope of urine protein over prior year | Yes | Yes | Urine protein (1-yr measurement and slope); SCr (1-yr measurement and slope), hypertension at 1 yr and glomerular score at biopsy |
| Donadio et al. (44), IgAN 2 | 63 | ESKD | Median 1.6 | ND | ND | ND | Proteinuria/24 h, slope of urine protein over prior year | Yes | Yes | |
| Reich et al. (29) | 542 | Rate of creatinine clearance decrease | Mean 6.5 SD 4.9, range 1–26.3 | 50% white, 3% black, 23% Asian, 10% other, 14% unknown | 2.37±2.5 | 77±33 | Proteinuria/24 h. TA-P: average of the mean of every 6-mo measurements | Yes | Yes | Proteinuria (baseline and during follow-up), MAP (baseline and during follow-up), average number of BP medications during follow-up, exposure to RAS blockade |
| Berthoux et al. (45) | 332 | Death or dialysis | Mean 12.9 SD 9.5, median 11.3, range 0.01–56.0 | White (North African patients were excluded) | 0.97±1.44 | 74.7±28.3 | Protein/24 h. Reduction of proteinuria defined as proteinuria<1 g/d after >2 yr of FU | Yes | Yes | Hypertension, proteinuria≥1 g/d, and global optical score of ≥8, ARR scoring (number of these simplified dichotomous risk factors present at diagnosis) |
| Le et al. (30) | 1155 | ESKD | Median 5.4 IQR 4.1–7.2 | Chinese | 0.89 (IQR 0.51–1.59) | 89±33 | TA-P>1 or 0.5–1.0 or <0.5 g/d TA-P: area under the curve of proteinuria during follow-up divided by the years of total follow-up | Yes | Yes | TA-P, time-average microscopic hematuria, eGFR at biopsy and TA-MAP |
| Tesar et al. (32) | 1147 | Rate of kidney function decline and/or ESKD | Median 4.7 IQR 2.4–7.9 Minimum 1 | Majority white | 1.3 (IQR 0.6–2.6) | 73±30 | Proportion of individuals with an initial proteinuria >1 g/d that achieved proteinuria<1 g/d during follow-up. Proteinuria changes before, during, and after therapy | Yes | Yes (propensity score matched) | Propensity score on the basis of: age, sex, eGFR, proteinuria, any immunosuppression before the biopsy, pathology findings, TA-P, TA-BP, time-average number of BP medications, proportion of the follow-up under RAS blockade, use of fish oil. |
MAP, mean arterial pressure; IgAN, IgA nephropathy; ND, not done; SCr, serum creatinine; TA-P, time average proteinuria; RAS, renin angiotensin system; FU, follow-up; ARR score, absolute kidney risk score on the basis of the presence of hypertension, proteinuria, and severity of histologic changes; IQR, interquartile range; TA-MAP, time-average mean arterial pressure; TA-BP, time average blood pressure.
The severity and duration of sustained proteinuria has been commonly assessed by calculating the time-average proteinuria (TA-P), although the method used to calculate TA-P has varied among studies (29,30,36,37). In IgAN, sustained proteinuria, typically >1 g/d, has been strongly associated with poorer kidney outcomes. In an analysis of data from the Toronto GN Registry of 542 patients followed for a mean of 6.5 years (61% males, 50% whites, 23% Asians, with mean initial proteinuria 2.4 g/d, and creatinine clearance of 77 ml/min per 1.73 m2), TA-P (defined as an average of the mean of every 6-month period’s proteinuria measurements) was the most important predictor of kidney function decline and risk of ESKD (defined as GFR<15 ml/min per 1.73 m2, dialysis, or transplantation) independently of BP and use of renin-angiotensin blockers. If TA-P was maintained <1 g/d, the 10-year risk of ESKD was 5%, versus 20% with a TA-P of 1–2 g/d, 40% with a TA-P of 2–3 g/d, and 60% with >3 g/d (29). In this study, kidney survival was not significantly different between patients attaining a proteinuria<0.3 g/d compared with those with proteinuria between 0.3 and 1 g/d, whereas in the VALIGA study (1147 patients from Europe), patients with a TA-P<0.5 g/d had a lower risk of progression to a 50% reduction of GFR or ESKD as compared with patients with proteinuria of 0.5–0.9 g/d (33). Similar to the findings reported in these populations, a multivariable analysis of data from the Nanjing GN registry, comprising 1155 Chinese patients with IgAN (median baseline proteinuria 0.89 [interquartile range, 0.51–1.59] g/d, and mean eGFR 89±33 ml/min per 1.73 m2), TA-P (the area under the curve of proteinuria during follow-up divided by the years of total follow-up) was also the most important risk factor for progression to ESKD. In this study population, TA-P>1 g/d was associated with a hazard ratio of 9.4 (95% confidence interval [95% CI], 6.1 to 14.5) of reaching a 50% decline in GFR or ESKD relative to patients with <1 g/d, after adjustment for time-average mean arterial pressure and GFR at biopsy (30). The authors also reported that a TA-P<0.5 g/d was associated with a lower risk of a 50% decline in GFR or ESKD compared with those with TA-P of 0.5–1.0 g/d (compared with a TA-P<0.5, the hazard ratio was 9.1 [95% CI, 2.7 to 30.0; P<0.001] for TA-P of 0.5–1 g/d, and 46.5 [95% CI, 14.7 to 147.5; P<0.001] for TA-P of >1 g/d).
Intervention Trials
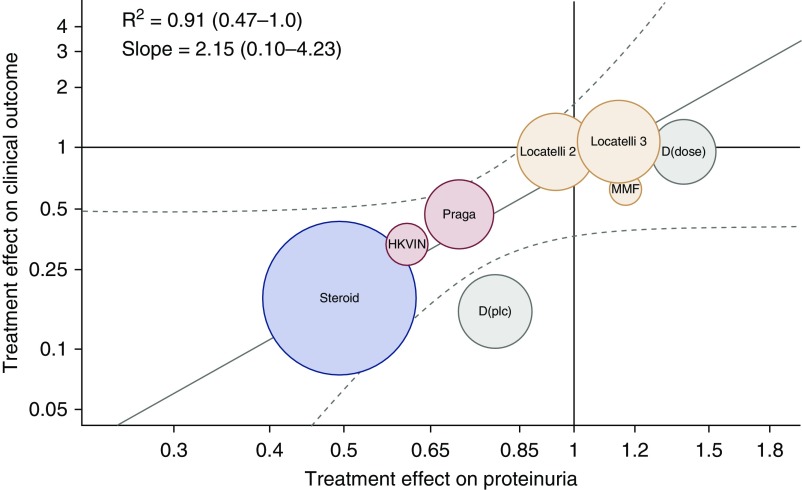
To date, the only attempt to formally evaluate proteinuria as a surrogate end point using data from intervention trials has been performed by Inker et al. (38). The authors performed a systematic search of the medical literature to identify randomized, controlled trials in adults with IgAN and obtained access to data from 11 studies investigating four intervention types (renin angiotensin system blockade, fish oil, steroids, or other immunosuppression agents) in a total of 830 subjects (included in Table 2). In addition to other analyses, the authors evaluated the relationship between the treatment effect on the change in proteinuria from baseline to approximately 9 months (measurements could be made between 7 and 12 months) and the treatment effect on the clinical end point of interest, defined as the composite of the time to the first occurrence of a doubling of serum creatinine level, ESKD, or death (38). This trial-level analysis showed an association between treatment effects on proteinuria and treatment effects on the clinical end point of interest in patients with IgAN (Figure 1).
Table 2.
Summary data on the association of proteinuria reduction with long-term kidney and patient outcomes from randomized clinical trials in IgA nephropathy
| Study | N | Primary Outcome | Follow-Up (yr) | Baseline Characteristics | Measure of Proteinuria | Change in Proteinuria Significantly Associated with Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Race/Ethnicity | Proteinuria g/d | eGFR ml/min per 1.73 m2 | Unadjusted/Univariable Analysis | Adjusted/Multivariable Analysis | Variables Included in Adjusted Analysis | |||||
| Donadio et al. (46) | 106 | Increase ≥50% in serum creatinine | Mean 6.4, median 6.8 | 97% white | Fish oil 2.55±1.71, placebo 3.22±3.21 | Creatinine clearance fish oil 82±30, placebo 81±27 | Proteinuria/24 h | ND | ND | |
| Donadio et al. (47) | 73 | Slope in serum creatinine | Minimum 2 | 92% white | Low-dose fish oil 1.79 (0.76, 2.57), high-dose fish oil 1.52 (0.70, 3.60) | Serum creatinine low-dose fish oil 2.1 (0.7), high-dose fish oil 2.3 (0.7) | Proteinuria/24 h | ND | ND | |
| Katafuchi et al. (48) | 90 | Change in urine protein excretion from baseline | Mean 5.4 | Asian (Japan) | Steroid 2.2±2, control 1.1±0.9 | Creatinine clearance steroid 90.8±27.3, control 90.5±26.1 | UPCR | No | ND | |
| Maes et al. (49) | 34 | Decrease ≥25% in inulin clearance during the 3-yr treatment period | All for 3 yr | >90% white | MMF 1.9±0.3, placebo 1.3±0.4 | Inulin clearance MMF 73±5, placebo 69±7 | Proteinuria/24 h | ND | ND | |
| Frisch et al. (50) | 32 | 50% increase in baseline serum creatinine | Mean 1.3, range 0.1–2.5 | 72% white, 16% Asian, 12% Hispanic | MMF 2.7±1.6, placebo2.7±1.4 | Creatinine clearance MMF 57±28.6, placebo 75±42.3 | Proteinuria/24 h | No | ND | |
| Li et al. (43) | 109 | Time to doubling of baseline serum creatinine level or ESKD | All for 2 yr | Chinese (Hong Kong) | Placebo 2.3±1.7, valsartan 1.8±1.2 | Placebo 87±36, valsartan 87±36 | Proteinuria/24 h | Yes | Yes | Average BP, treatment, proteinuria |
| Lv et al. (37) | 63 | 50% increase in serum creatinine | Mean 2.3, SD 0.6, range 1.3–4 | Chinese | Cilazapril 2.0±0.8, cilazapril and steroid 2.5±0.9 | MDRD cilazapril 101.5, cilazapril and steroid 101.2 | Proteinuria/24 h TA-P was defined as the average of the mean of every 6-mo period of proteinuria measurements | Yes | Yes | “Sex, age, kidney function, hypertension, proteinuria, TA-MAP, TA-P, and treatment” |
| Manno et al. (51) | 97 | Combination of doubling of baseline serum creatinine or ESKD | Median 5, range 3–9 | ND (Italian study) | Ramipril 1.5 (1.4–2.3), prednisolone and ramipril 1.7 (1.2–2.5) | MDRD ramipril 97.5±27.7, prednisolone and ramipril 100.4±26.1 | Proteinuria/24 h | Yes during the first 2 yr of follow-up | ND | |
| Tang et al. (52) | 40 | ESKD and doubling of serum creatinine | 6 yr for all participants | 100% Chinese | MMF 1.8±0.21, control 1.87±0.28 | MMF 52.5±4.40, control 50.0±4.51 | Proteinuria/24 h and ACR changes from baseline to each time point | Yes | Yes | “Age, gender, BP, and histologic score” |
| Pozzi et al. (53) | 207 | 50% increase in serum creatinine | Median 4.9, IQR 3.0–6.4 | Does not state, 27 Swiss and Italian nephrology centers | 2.0 (IQR 1.5–3.0) | MDRD 66 (48–87) | 24 h collection. “Proteinuria over time.” Absolute reduction. | Yes | Yes | Age, sex, systolic BP, antihypertensive medications, RAS blocker, complete remission of proteinuria, treatment group |
| Pozzi et al. (54) | 46 | 50% increase in plasma creatinine from baseline | Median 4.5, IQR 2.9–6.1 | Does not state | 2.4 (IQR 1.5–3.8) | MDRD 25 (20–31), C-G 34 (25–41) | Proteinuria/24 h. Absolute reduction from baseline, 6 mo, 12 mo, then yearly to end of follow-up | Yes | Yes | Sex, proteinuria, antihypertensive medications, RAS blocker, treatment group |
| Rauen et al. (STOP-IgAN) (39) | 162 | UPCR<0.2 g/g and decrease in EPI eGFR of <5 ml/min per 1.73 m2 from baseline eGFR, decrease in the EPI eGFR of at least 15 ml/min per 1.73 m2 from baseline eGFR | 0.5 yr run-in 3-yr for trial | Does not state, 32 German nephrology centers | Supportive 1.6±0.7 g/d, supportive plus immunosuppression 1.8±0.8 g/d | EPI supportive 57.4±24.9, supportive plus immunosuppression 61.1±29.0 | Proteinuria/24 h during the run-in phase, switch to UPCR during the randomized, controlled trial phase | ND | No (RCT) | |
| Lv et al. 2017 (TESTING) (40) | 262 | 40% decrease in eGFR, ESKD, and death due to kidney failure | Median 2.1 yr, no IQR stated for whole study | 95.8% Chinese, 3.1% white, 1.1% Southeast Asian | Methylprednisolone 2.55±2.45, placebo 2.23±1.11 | EPI methylprednisolone 60.0±24.8, placebo 58.6±25.2 | Proteinuria reduction (TA-P). Proteinuria remission defined as <200 mg/24 h and partial proteinuria remission defined as <50% of baseline and <1 g/24 h. | Yes (RCT) | No (RCT) | |
ND, not done; UPCR, urine protein-to-creatinine ratio; MMF, mycophenolate mofetil; MDRD, modification of diet in renal disease equation for estimating GFR; TA-P, time average proteinuria; TA-MAP, time-average mean arterial pressure; ACR, albumin-to-creatinine ratio; IQR, interquartile range; C-G, Cockroft-Gault equation for estimating creatinine clearance; RAS, renin angiotensin system; STOP-IgA, STOP-IgAN trial; EPI, epidemiology collaboration (CKD-EPI) equation for estimating GFR; RCT, randomized, controlled trial.
Figure 1.
Trial-level assessment of the validity of proteinuria as a surrogate end point. Circles are the observed treatment effects on the clinical outcome (vertical axis) and change in urine protein (horizontal axis) for each study or study group. The sizes of the circles are the inverse to the SEM of the treatment effect on the clinical end point, which is related to the number of events of interest. Colors indicate intervention. Red, renin-angiotensin system blockade; yellow, fish oil; green, immunosuppression; purple, steroids. Treatment effects on the clinical outcome are expressed as hazard ratios. Treatment effect on urine protein was computed as change in log urine protein (follow-up−baseline) in the treatment versus control groups. The treatment effect estimate was exponentiated to obtain the geometric mean ratio of the change in urine protein for the treatment versus control arm. A number <1 indicates a larger reduction in proteinuria in the treatment than in the control group. The brown regression line is the regression line from the Bayesian analyses summarizing the prediction of the true treatment effects on the clinical outcome from the true treatment effects on change in urine protein. Gray lines indicate the confidence band around the regression line. Overall, the slope is 2.15 with a 95% Bayesian credible interval range from 0.10 to 4.32 with R2 of 0.91, 95% Bayesian credible interval range from 0.47 to 1.0, indicating that for a given treatment effect on urine protein excretion, the treatment effect on the clinical outcome is expected to be double the treatment effect on urine protein excretion when the respective treatment effects are expressed on the log hazard ratio and log geometric mean scales. The Bayesian credible interval around the slope was wide but did not cross zero, suggesting that there is a significant positive relationship between treatment effects on urine protein and on the clinical end point. D(dose), Donadio (dose); D(plc), Donadio (placebo); HKVIN, Hong Kong Study Using Valsartan in IgA Nephropathy; MMF, mycophenolate mofetil. Reprinted from ref. 38, with pending permission.
After the publication of this analysis, the results of two international, randomized, double-blind, placebo-controlled trials in IgAN—the Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING) (37) and the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) (39) trials—were published. Accordingly, the data from these trials were added to the trial data presented by Inker et al. (38). Two updated trial-level analyses were performed, one in which STOP-IgAN (39) and TESTING (40) were added as two separate new trials and a second analysis in which the results of TESTING were combined with those of the other trials of corticosteroids (see Supplemental Material for methodology). The results of the updated trial-level analyses are shown in Figure 2. The results of the STOP-IgAN and TESTING trials, including the treatment effect on proteinuria and on the composite end point of time to the first occurrence of a doubling of serum creatinine level, ESKD, or death, are summarized in Table 3. Both trial-level analyses continue to show an association between treatment effects on proteinuria and treatment effects on the clinical end point of interest in patients with IgAN.
Figure 2.
Trial-level assessment of the validity of proteinuria as a surrogate end point with the addition of the data from the STOP-IgAN and TESTING trials. (A) With the results of Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) and Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING) added as two separate trials. (B) With the results of TESTING combined with those of other trials of corticosteroids. Circles are the observed treatment effects on the clinical outcome (vertical axis) and change in urine protein (horizontal axis) for each study or study group as for Figure 1. The sizes of the circles are the inverse to the SEM of the treatment effect on the clinical end point, which is related to the number of events of interest. Colors indicate intervention. Red, renin-angiotensin system blockade; yellow, fish oil; green, immunosuppression; dark blue, steroids; light blue, TESTING trial; orange, STOP-IgAN trial. D(dose), Donadio (dose); D(plc), Donadio (placebo); HKVIN, Hong Kong Study Using Valsartan in IgA Nephropathy (43); MMF, mycophenolate mofetil; STOP, STOP-IgAN trial (39); TESTING, TESTING trial (40).
Table 3.
Summary of patient characteristics and treatment effect in the STOP-IgAN and TESTING trials
| Trial | Proteinuria | Composite End Point | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| N | Mean at Baseline (g/d) | Ratio of Proteinuria at 12-mo to Baseline | N | Mean at Baseline (g/d) | Ratio of Proteinuria at 12-mo to Baseline | Proteinuria Treatment Effect (95% CI) | Number of Events | Hazard Ratio (95% CI) | |
| STOP-IgAN (39) | 76 | 0.97 | 0.41 (−58.0%) | 67 | 1.06 | 0.73 (−27.0%) | 0.57 (0.45 to 0.73) | 12 | 0.57 (0.17 to 1.88) |
| TESTING (40) | 93 | 2.76 | 0.44 (−55.7%) | 87 | 2.24 | 0.98 (−1.7%) | 0.45 (0.34 to 0.59) | 23 | 0.48 (0.20 to 1.13) |
The table presents a summary of the data for the patients in the treatment and control groups for whom paired proteinuria measurements were available at baseline and at 12 mo, thus allowing calculation of the change in proteinuria with treatment. Composite end point: time to the first occurrence of a doubling of serum creatinine level, ESKD, or death. 95% CI, 95% confidence interval; STOP-IgAN, Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy; TESTING, Therapeutic Evaluation of Steroids in IgA Nephropathy Global.
Conclusions
Although several biomarkers of disease have been explored in IgAN, to date, the most widely recognized and well studied risk factor for progression to ESKD is proteinuria. For this reason, and because of consistent evidence linking sustained proteinuria with adverse clinical outcomes in this disease, the workgroup focused on the data supporting proteinuria as a surrogate end point in IgAN. As summarized in Table 4, epidemiologic data indicate a strong and consistent relationship between the level and duration of proteinuria and loss of kidney function in patients with IgAN. Trial-level analyses of data from 13 randomized, controlled trials also show an association between treatment effects on proteinuria and treatment effects on a composite of the time to the first occurrence of a doubling of serum creatinine level, ESKD, or death. The geometric mean of proteinuria at baseline was 1.8 g/d with two thirds of patients included in these trials having a value in the range 1.0–3.2 g/d; the geometric mean for eGFR at baseline was 63 ml/min per m2 with two thirds of patients having a value in the range 47–86 L/min per m2; and the age range across studies was 31.6±11.5–46.4±13.4 years (no study included children<14 years old). These data support the use of proteinuria reduction as a reasonably likely surrogate end point for a treatment’s effect on the loss of kidney function and progression to ESKD in future trials enrolling a similar population. The clinical benefit of products approved under this program should be verified in a postmarketing confirmatory trial.
Table 4.
Summary of findings
| Finding | Summary |
|---|---|
| Proteinuria as causal factor in IgAN disease progression | There is no direct disease-specific evidence establishing a causal role of proteinuria (as routinely measured) in the pathogenesis of progressive kidney dysfunction in IgAN. |
| Epidemiologic association | Studies to date have explored the relationship between proteinuria and kidney outcomes using a variety of measures: |
| • Measures on the basis of time-average calculations of proteinuria (which incorporate both the severity and duration of proteinuria). | |
| • Measures on the basis of reduction of proteinuria to below a threshold (e.g., <0.5 or <1 g/d) as measured by time-average proteinuria or sustained over time. | |
| • Relative percent reduction of proteinuria along a continuum on the basis of the measurement of proteinuria at a certain time point. | |
| • Data from these studies show an association between lower time-average proteinuria (e.g., to <1 g/d) and achieving a sustained proteinuria to <1 g/d and lower rate of loss of kidney function or reaching a kidney end point (50% decline in GFR or ESKD). | |
| Data from randomized interventional trials | Trial-level analysis of data from randomized, controlled trials also show an association between treatment effects on proteinuria and treatment effects on a composite of the time to the first occurrence of a doubling of serum creatinine level, ESKD, or death in patients with an eGFR>25 ml/min per 1.73 m2 and proteinuria levels >0.6 g/d. |
IgAN, IgA nephropathy.
There are, however, knowledge gaps warranting further investigation:
The relationship between treatment effects on proteinuria and treatment effects on patient and kidney outcome is best supported for the proteinuria and eGFR levels described in the previous paragraph. Whether this association holds at lower baseline levels of GFR or proteinuria is unclear and warrants further study.
Analyses indicate that the reduction of proteinuria must be sustained to confer protection against progressive loss of GFR. The minimal duration of proteinuria reduction that confers a protective effect is not known and should be investigated in future studies.
Some data suggest that the sustained reduction of proteinuria to a level <0.5 g/d is associated with the best kidney outcome. Even with this near complete normalization of proteinuria, the minimal magnitude and duration of proteinuria reduction that confers a protective effect is currently unknown and should be investigated in future studies.
A “complete remission” may be defined as a treatment response associated with a low rate of persistent or recurrent disease activity and excellent long-term kidney and patient survival. What constitutes complete remission in IgAN is not well defined (41,42), and may include criteria other than proteinuria alone. Future studies should explore this issue.
It is also important to emphasize that this paper addresses proteinuria as a surrogate end point in settings in which it is likely to perform well and where there is uncertainty about its performance. It should not be interpreted as suggesting that a broader population of patients cannot or should not be studied in drug development programs, that other biomarkers should not be explored as potential surrogate end points, or that other end points should not be used or considered.
Given the current state of the data on proteinuria as a surrogate in IgAN, how should the nephrology community move forward with defining a proteinuria-based end point for registration trials of treatments of IgAN? Although specifying a set definition of proteinuria response that should be used by all development programs may be viewed as attractive by some, the available data do not support such an approach. Trial-level analyses indicate a graded relationship between a treatment’s effect on proteinuria (as measured by the percent reduction in proteinuria) and the treatment effect on the loss of kidney function and progression to ESKD. This suggests that, for the purpose of accelerated approval, the end point could be defined as the percent reduction in proteinuria from baseline (as opposed to defining a “response” as achieving a proteinuria level below some threshold). Because the benefit of the product would need to be confirmed in a postmarketing trial, the magnitude of effect on proteinuria in the premarket trial would need to be sufficient to provide confidence that the treatment effect could be confirmed in a feasible postmarketing trial. We believe that this provides a feasible pathway for product development in this area, bearing in mind the current state of the data on proteinuria as a surrogate in this disease and with the understanding that, as data accrue, the approach to using proteinuria as a surrogate end point will likely evolve.
Disclosures
A.T. and P.H.N. cochaired the workgroup. K.J.C. and L.A.I. conducted the repeat trial-level meta-analysis. J.F. and V.P. provided data from Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy and Therapeutic Evaluation of Steroids in IgA Nephropathy Global, respectively. S.B.-S., R.W.M., and J.I.S. extracted and tabulated data from the epidemiologic studies and clinical trials. All authors reviewed the data, discussed the findings and conclusions, and contributed to and reviewed the manuscript. P.H.N. is currently employed by University of Minnesota. He has received research funding from National Institutes of Health (NIH) and FDA, and as site PI for clinical trials (ChemoCentryx, Otsuka, Aurinia, InflaRx, Omeros), has received honoraria from ASN (BRCU) and is a scientific advisor or member of ASN (PGE Committee Chair, Education Committee). A.T. is currently employed by the FDA. The following are members of the workgroup: J.B. is currently employed by University of Leicester Consultancy Agreements. He has received research funding from Calliditas, GlaxoSmithKline, Novartis, has received honoraria from AstraZeneca and is scientific advisor or Member of Editorial Board of Kidney International, CJASN & Clinical Science. He is site PI for clinical trials for Calliditas, Retrophin, Novartis EMD Serono, Akebia, AstraZeneca, Otsuka, Astellas, and Bayer. K.C. is currently employed by KJC Statistics Limited and serves as a consultant for GSK Pharmaceuticals, Otsuka Pharmaceuticals, Retrophin, Omeros. He is a member of Kidney Health Initiative. D.C.C. is currently employed by Toronto General Hospital and serves as a consultant for Chemocentryx, Dimerix, Calliditas, Analyn. He has received research funding from Genetech, has received honoraria from Mallincrodt and is a scientific advisor or member of the Kidney International and NephCure. J.F. is currently employed by RWTH University of Aachen and serves as a consultant for Amgen, Calliditas, Chugai, Fresenius, Omeros, Vifor. He has received honoraria from Amgen, Boehringer, Fresenius, Vifor and is a scientific advisor or member of the Chugai Speakers Bureau. He is site PI for clinical trials for Calliditas, Retrophin, and Omeros. B.S.G. is currently employed by Covance Clinical Research Organizatiom. She is a scientific advisor or member of the Kidney Health Initiative Board of Directors, National Kidney Disease CKD Registry, Scientific Advisory Board, NKF/FDA/EMA Renal Workshop, and Stakeholder Committee. L.A.I. is currently employed by Tufts Medical Center in Boston and serves as a consultant for Tricidia and Omeros. She has received research funding from the NIH, NKF, Retrophin, and Reata, and is site PI for clinical trials by Reata and Omeros. Patents and Inventions include Provisional patent [Coresh, Inker and Levey] filed 8/15/2014, PCT/US2015/044567. The technology is not licensed in whole or in part to any company. Tufts Medical Center, John Hopkins University and Metabolon Inc. have a collaboration agreement to develop a product to estimate GFR from a panel of markers. Her other interests and relationships memberships from National Kidney Disease Education Program, American Society of Nephrology, and NKF. A.T.K. is currently employed by Allena Pharmaceuticals, Inc. She has ownership interest with Keryx Biopharmaceuticals, Inc. and is a member of the Oxalosis Hyperoxaluria Foundation Working Group. A.W.M. is currently employed by JAMCO and serves as a consultant for Retrophin, Inc. V.P. is currently employed by The George Institute for Global Health. He has received research funding from Australian National Health and Medical Research Council (Senior Research Fellowship and Program Grant). He has received honoraria from Boehringer Ingelheim and Astra Zeneca. He is a scientific advisor or member for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Pfizer. He has also served on advisory boards and/or has spoken at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier and Vitae. H.N.R. is a member of the editorial board of the Kidney International and serves as a scientific advisor for Omeros. She has received honoraria from ASN (ASN Early program, ASN Highlights). B.H.R. is currently employed by the Ohio State University Wexner Medical Center. He is a consultant for Alexion, Aurinia, Biogen, Biomarin, Bristol Myers Squibb, Calliditas, Chemocentryx, EMD-Serono, Genentech, Janssen, Lilly, Mallinckrodt, MedImmune, Morphosys, Novartis, Omeros, Pfizer, Pharmalink, Ra Pharmaceuticals, Rigel, Retrophin, and Takeda. He has received research funding from EMD-Serono and has received honoraria from Lilly, Genentech, Mallinckrodt, Chemocentryx, Roche, Pharmalink, Alexion, Aurinia, Biogen, Biomarin, Bristol Myers Squibb, Calliditas, RA and Retrophin. He is site PI for clinical trials for AstraZeneca, Chemocentryx, EMD Serono, Hoffman-LaRoche, Human Genome Sciences, Retrophin, Rigel, the RILITE Foundation, and the NIH/NIDDK. He serves as scientific advisor or is a member of Kidney International, Kidney international Reports, Nephrology Dialysis and transplantation and Lupus Foundation of America. His other Interests/relationships are work with ASN educational courses and the Central Society.
Supplementary Material
Acknowledgments
We would like to acknowledge Dr. Hsien Ming (Jim) Hung, Food and Drug Administration (FDA), for his input on statistical issues.
This work was supported by the Kidney Health Initiative (KHI), a public-private partnership between the American Society of Nephrology, the US FDA, and >90 member organizations and companies to enhance patient safety and foster innovation in kidney disease. KHI funds were used to defray costs incurred during the conduct of the project, including project management support, which was expertly provided by American Society of Nephrology staff members Melissa West, Meaghan Allain, and Elle Silverman. There was no honorarium or other financial support provided to KHI workgroup members. The authors of this paper had final review authority and are fully responsible for its content. KHI makes every effort to avoid actual, potential, or perceived conflicts of interest that may arise as a result of industry relationships or personal interests among the members of the workgroup. More information on KHI, the workgroup, or the conflict of interest policy can be found at www.kidneyhealthinitiative.org.
The views and opinions expressed in this publication are those of the authors and do not necessarily reflect the official policies of any KHI member organization, the US Department of Veterans Affairs, or the US Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08600718/-/DCSupplemental.
Methods for updated meta-analysis of the effect of treatment on proteinuria reduction and preservation of GFR from randomized, controlled trials in IgAN.
References
- 1.Archdeacon P, Shaffer RN, Winkelmayer WC, Falk RJ, Roy-Chaudhury P: Fostering innovation, advancing patient safety: The kidney health initiative. Clin J Am Soc Nephrol 8: 1609–1617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson A: Proteinuria as a surrogate end point--more data are needed. Nat Rev Nephrol 8: 306–309, 2012 [DOI] [PubMed] [Google Scholar]
- 3.FDA-NIH Biomarker Working Group: BEST (Biomarkers, EndpointS, and other Tools) resource [Internet], Silver Spring, MD, Food and Drug Administration (US), 2016. Available at: http://www.ncbi.nlm.nih.gov/books/NBK326791/. Accessed February 24, 2018 [PubMed]
- 4.Hastings MC, Bursac Z, Julian BA, Villa Baca E, Featherston J, Woodford SY, Bailey L, Wyatt RJ: Life expectancy for patients from the Southeastern United States with IgA nephropathy. Kidney Int Rep 3: 99–104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration, Center for Drug Evaluation and Research (CDER). CDER biomarker qualification program [Internet]. Available at: https://www.fda.gov/drugs/developmentapprovalprocess/drugdevelopmenttoolsqualificationprogram/biomarkerqualificationprogram/default.htm
- 6.Fleming TR, DeMets DL: Surrogate end points in clinical trials: Are we being misled? Ann Intern Med 125: 605–613, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration : Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), FDA: Guidance for industry: Expedited programs for serious conditions – drugs and biologics [Internet], 2014. Available at: https://www.fda.gov/downloads/Drugs/Guidances/UCM358301.pdf
- 8.D’Amico G: Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24: 179–196, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lv J, Zhang H, Zhou Y, Li G, Zou W, Wang H: Natural history of immunoglobulin A nephropathy and predictive factors of prognosis: A long-term follow up of 204 cases in China. Nephrology (Carlton) 13: 242–246, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Arroyo AH, Bomback AS, Butler B, Radhakrishnan J, Herlitz L, Stokes MB, D’Agati V, Markowitz GS, Appel GB, Canetta PA: Predictors of outcome for severe IgA Nephropathy in a multi-ethnic U.S. cohort. Clin Nephrol 84: 145–155, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y: A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant 24: 3068–3074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hisano S, Joh K, Katafuchi R, Shimizu A, Hashiguchi N, Kawamura T, Matsuo S; IgA Nephropathy Study Group in Japan : Reproducibility for pathological prognostic parameters of the Oxford classification of IgA nephropathy: A Japanese cohort study of the Ministry of Health, Labor and Welfare. Clin Exp Nephrol 21: 92–96, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H: Validation study of oxford classification of IgA nephropathy: The significance of extracapillary proliferation. Clin J Am Soc Nephrol 6: 2806–2813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu Z-H, Roberts ISD, Yuzawa Y, Zhang H, Feehally J; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants : Oxford classification of IgA nephropathy 2016: An update from the IgA nephropathy classification working group. Kidney Int 91: 1014–1021, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Luo M-N, Yao C-W, Xu B-H, Xu Y-Z, Liu WJ, Feng Y-M, Tao J-L, Liu H-F: Continuation of immunosuppressive treatment may be necessary in IgA nephropathy patients with remission of proteinuria: Evaluation by repeat renal biopsy. Exp Ther Med 7: 553–559, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X-H, Liang S-S, Chen H-M, Le W-B, Jiang S, Zeng C-H, Zhou M-L, Zhang H-T, Liu Z-H: Reversal of active glomerular lesions after immunosuppressive therapy in patients with IgA nephropathy: A repeat-biopsy based observation. J Nephrol 28: 441–449, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Hou J-H, Le W-B, Chen N, Wang W-M, Liu Z-S, Liu D, Chen J-H, Tian J, Fu P, Hu Z-X, Zeng C-H, Liang S-S, Zhou M-L, Zhang H-T, Liu Z-H: Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: A randomized controlled trial. Am J Kidney Dis 69: 788–795, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Beckwith H, Medjeral-Thomas N, Galliford J, Griffith M, Levy J, Lightstone L, Palmer A, Roufosse C, Pusey C, Cook HT, Cairns T: Mycophenolate mofetil therapy in immunoglobulin A nephropathy: Histological changes after treatment. Nephrol Dial Transplant 32: i123–i128, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Torres DD, Rossini M, Manno C, Mattace-Raso F, D’Altri C, Ranieri E, Pontrelli P, Grandaliano G, Gesualdo L, Schena FP: The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int 73: 327–333, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Oh YJ, An JN, Kim CT, Yang SH, Lee H, Kim DK, Joo KW, Paik JH, Kang S-W, Park JT, Lim CS, Kim YS, Lee JP: Circulating tumor necrosis factor α receptors predict the outcomes of human IgA nephropathy: A prospective cohort study. PLoS One 10: e0132826, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SJ, Koo HM, Lim BJ, Oh HJ, Yoo DE, Shin DH, Lee MJ, Doh FM, Park JT, Yoo T-H, Kang S-W, Choi KH, Jeong HJ, Han SH: Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One 7: e40495, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizerska-Wasiak M, Małdyk J, Rybi-Szumińska A, Wasilewska A, Miklaszewska M, Pietrzyk J, Firszt-Adamczyk A, Stankiewicz R, Bieniaś B, Zajączkowska M, Gadomska-Prokop K, Grenda R, Pukajło-Marczyk A, Zwolińska D, Szczepańska M, Turczyn A, Roszkowska-Blaim M: Relationship between serum IgA/C3 ratio and severity of histological lesions using the Oxford classification in children with IgA nephropathy. Pediatr Nephrol 30: 1113–1120, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki Y, Maeda R, Ohara S, Suyama K, Hosoya M: Serum IgA/C3 and glomerular C3 staining predict severity of IgA nephropathy. Pediatr Int (Roma) 60: 162–167, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Cravedi P, Remuzzi G: Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol 76: 516–523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z, Wen Q, Zhou S-F, Yu X-Q: Differential chemokine expression in tubular cells in response to urinary proteins from patients with nephrotic syndrome. Cytokine 42: 222–233, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Reich HN, Tritchler D, Cattran DC, Herzenberg AM, Eichinger F, Boucherot A, Henger A, Berthier CC, Nair V, Cohen CD, Scholey JW, Kretzler M: A molecular signature of proteinuria in glomerulonephritis. PLoS One 5: e13451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, Liu Z: Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 27: 1479–1485, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Cattran DC, Reich HN, Beanlands HJ, Miller JA, Scholey JW, Troyanov S: Genes, Gender and Glomerulonephritis Group: The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant 23: 2247–2253, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Tesar V, Troyanov S, Bellur S, Verhave JC, Cook HT, Feehally J, Roberts ISD, Cattran D, Coppo R; VALIGA study of the ERA-EDTA Immunonephrology Working Group : Corticosteroids in IgA nephropathy: A retrospective analysis from the VALIGA study. J Am Soc Nephrol 26: 2248–2258, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts ISD, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A; VALIGA study of the ERA-EDTA Immunonephrology Working Group : Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radford MG Jr., Donadio JV Jr., Bergstralh EJ, Grande JP: Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 8: 199–207, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Nam KH, Kie JH, Lee MJ, Chang T-I, Kang EW, Kim DW, Lim BJ, Park JT, Kwon YE, Kim YL, Park KS, An SY, Oh HJ, Yoo T-H, Kang S-W, Choi KH, Jeong HJ, Han D-S, Han SH: Optimal proteinuria target for renoprotection in patients with IgA nephropathy. PLoS One 9: e101935, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Inker LA, Mondal H, Greene T, Masaschi T, Locatelli F, Schena FP, Katafuchi R, Appel GB, Maes BD, Li PK, Praga M, Del Vecchio L, Andrulli S, Manno C, Gutierrez E, Mercer A, Carroll KJ, Schmid CH, Levey AS: Early change in urine protein as a surrogate end point in studies of IgA nephropathy: An individual-patient meta-analysis. Am J Kidney Dis 68: 392–401, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JFE, Hilgers R-D, Floege J; STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu Z-H, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang H-Y, Perkovic V; TESTING Study Group : Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA 318: 432–442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sevillano AM, Gutiérrez E, Yuste C, Cavero T, Mérida E, Rodríguez P, García A, Morales E, Fernández C, Martínez MA, Moreno JA, Praga M: Remission of hematuria improves renal survival in IgA nephropathy. J Am Soc Nephrol 28: 3089–3099, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka K, Moriyama T, Iwasaki C, Takei T, Nitta K: Effect of hematuria on the outcome of IgA nephropathy with mild proteinuria. Clin Exp Nephrol 19: 815–821, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Li PK-T, Leung CB, Chow KM, Cheng YL, Fung SK-S, Mak SK, Tang AW-C, Wong TY-H, Yung CY, Yung JC-U, Yu AW-Y, Szeto CC; HKVIN Study Group : Hong Kong study using valsartan in IgA nephropathy (HKVIN): A double-blind, randomized, placebo-controlled study. Am J Kidney Dis 47: 751–760, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM: Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant 17: 1197–1203, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donadio JV Jr., Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC; Mayo Nephrology Collaborative Group : The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. J Am Soc Nephrol 10: 1772–1777, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Donadio JV Jr., Larson TS, Bergstralh EJ, Grande JP: A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol 12: 791–799, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Katafuchi R, Ikeda K, Mizumasa T, Tanaka H, Ando T, Yanase T, Masutani K, Kubo M, Fujimi S: Controlled, prospective trial of steroid treatment in IgA nephropathy: A limitation of low-dose prednisolone therapy. Am J Kidney Dis 41: 972–983, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, Van Damme B, Vanrenterghem YFC: Mycophenolate mofetil in IgA nephropathy: Results of a 3-year prospective placebo-controlled randomized study. Kidney Int 65: 1842–1849, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Frisch G, Lin J, Rosenstock J, Markowitz G, D’Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A, Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol Dial Transplant 20: 2139–2145, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Tang SCW, Tang AWC, Wong SSH, Leung JCK, Ho YW, Lai KN: Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int 77: 543–549, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Pozzi C, Andrulli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, Vogt B, De Cristofaro V, Allegri L, Cirami L, Procaccini AD, Locatelli F: Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 21: 1783–1790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, Pecchini P, Rustichelli R, Finocchiaro P, Del Vecchio L, Locatelli F: IgA nephropathy with severe chronic renal failure: A randomized controlled trial of corticosteroids and azathioprine. J Nephrol 26: 86–93, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.