Abstract
Background
Age‐related macular degeneration (AMD) is the most common cause of uncorrectable severe vision loss in people aged 55 years and older in the developed world. Choroidal neovascularization (CNV) secondary to AMD accounts for most cases of AMD‐related severe vision loss. Intravitreous injection of anti‐vascular endothelial growth factor (anti‐VEGF) agents aims to block the growth of abnormal blood vessels in the eye to prevent vision loss and, in some instances, to improve vision.
Objectives
• To investigate ocular and systemic effects of, and quality of life associated with, intravitreous injection of three anti‐VEGF agents (pegaptanib, ranibizumab, and bevacizumab) versus no anti‐VEGF treatment for patients with neovascular AMD
• To compare the relative effects of one of these anti‐VEGF agents versus another when administered in comparable dosages and regimens
Search methods
To identify eligible studies for this review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Eyes and Vision Trials Register (searched January 31, 2018); MEDLINE Ovid (1946 to January 31, 2018); Embase Ovid (1947 to January 31, 2018); the Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to January 31, 2018); the International Standard Randomized Controlled Trials Number (ISRCTN) Registry (www.isrctn.com/editAdvancedSearch ‐ searched January 31, 2018); ClinicalTrials.gov (www.clinicaltrials.gov ‐ searched November 28, 2018); and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en ‐ searched January 31, 2018). We did not impose any date or language restrictions in electronic searches for trials.
Selection criteria
We included randomized controlled trials (RCTs) that evaluated pegaptanib, ranibizumab, or bevacizumab versus each other or versus a control treatment (e.g. sham treatment, photodynamic therapy), in which participants were followed for at least one year.
Data collection and analysis
Two review authors independently screened records, extracted data, and assessed risks of bias. We contacted trial authors for additional data. We compared outcomes using risk ratios (RRs) or mean differences (MDs). We used the standard methodological procedures expected by Cochrane.
Main results
We included 16 RCTs that had enrolled a total of 6347 participants with neovascular AMD (the number of participants per trial ranged from 23 to 1208) and identified one potentially relevant ongoing trial. Six trials compared anti‐VEGF treatment (pegaptanib, ranibizumab, or bevacizumab) versus control, and 10 trials compared bevacizumab versus ranibizumab. Pharmaceutical companies conducted or sponsored four trials but funded none of the studies that evaluated bevacizumab. Researchers conducted these trials at various centers across five continents (North and South America, Europe, Asia, and Australia). The overall certainty of the evidence was moderate to high, and most trials had an overall low risk of bias. All but one trial had been registered prospectively.
When compared with those who received control treatment, more participants who received intravitreous injection of any of the three anti‐VEGF agents had gained 15 letters or more of visual acuity (risk ratio [RR] 4.19, 95% confidence interval [CI] 2.32 to 7.55; moderate‐certainty evidence), had lost fewer than 15 letters of visual acuity (RR 1.40, 95% CI 1.27 to 1.55; high‐certainty evidence), and showed mean improvement in visual acuity (mean difference 6.7 letters, 95% CI 4.4 to 9.0 in one pegaptanib trial; mean difference 17.8 letters, 95% CI 16.0 to 19.7 in three ranibizumab trials; moderate‐certainty evidence) after one year of follow‐up. Participants treated with anti‐VEGF agents showed improvement in morphologic outcomes (e.g. size of CNV, central retinal thickness) compared with participants not treated with anti‐VEGF agents (moderate‐certainty evidence). No trial directly compared pegaptanib versus another anti‐VEGF agent and followed participants for one year; however, when compared with control treatments, ranibizumab and bevacizumab each yielded larger improvements in visual acuity outcomes than pegaptanib.
Visual acuity outcomes after bevacizumab and ranibizumab were similar when the same RCTs compared the same regimens with respect to gain of 15 or more letters of visual acuity (RR 0.95, 95% CI 0.81 to 1.12; high‐certainty evidence) and loss of fewer than 15 letters of visual acuity (RR 1.00, 95% CI 0.98 to 1.02; high‐certainty evidence); results showed similar mean improvement in visual acuity (mean difference [MD] ‐0.5 letters, 95% CI ‐1.5 to 0.5; high‐certainty evidence) after one year of follow‐up, despite the substantially lower cost of bevacizumab compared with ranibizumab. Reduction in central retinal thickness was less among bevacizumab‐treated participants than among ranibizumab‐treated participants after one year (MD ‐11.6 μm, 95% CI ‐21.6 to ‐1.7; high‐certainty evidence); however, this difference is within the range of measurement error, and we did not interpret it to be clinically meaningful.
Ocular inflammation and increased intraocular pressure (IOP) after intravitreal injection were the most frequently reported serious ocular adverse events. Researchers reported endophthalmitis in less than 1% of anti‐VEGF‐treated participants and in no cases among control groups. The occurrence of serious systemic adverse events was comparable across anti‐VEGF‐treated groups and control groups; however, the numbers of events and trial participants may have been insufficient to show a meaningful difference between groups (evidence of low‐ to moderate‐certainty). Investigators rarely measured and reported data on visual function, quality of life, or economic outcomes.
Authors' conclusions
Results of this review show the effectiveness of anti‐VEGF agents (pegaptanib, ranibizumab, and bevacizumab) in terms of maintaining visual acuity; studies show that ranibizumab and bevacizumab improved visual acuity in some eyes that received these agents and were equally effective. Available information on the adverse effects of each medication does not suggest a higher incidence of potentially vision‐threatening complications with intravitreous injection of anti‐VEGF agents compared with control interventions; however, clinical trial sample sizes were not sufficient to estimate differences in rare safety outcomes. Future Cochrane Reviews should incorporate research evaluating variable dosing regimens of anti‐VEGF agents, effects of long‐term use, use of combination therapies (e.g. anti‐VEGF treatment plus photodynamic therapy), and other methods of delivering these agents.
Plain language summary
Anti‐vascular endothelial growth factor for neovascular age‐related macular degeneration
What is the aim of this review? The aim of this Cochrane review was to compare treatment with anti‐vascular endothelial growth factor (anti‐VEGF) agents for neovascular age‐related macular degeneration (wet AMD). This review focuses on two questions: (1) whether using anti‐VEGF agents is better than not using them, and (2) which anti‐VEGF agent works best.
Key messages Anti‐VEGF agents were better than no anti‐VEGF agents or other types of treatment for patients with wet AMD. When studies compared anti‐VEGF agents, researchers found that ranibizumab and bevacizumab were similar in terms of vision‐related outcomes and numbers of adverse events among participants followed for at least one year. The major difference was cost, as bevacizumab was cheaper.
What was studied in this review? Wet AMD is a common cause of severe vision loss among people 55 years of age and older. The macula, located in the central retina in the back of the eye, is important for vision. Wet AMD occurs when abnormal growth of blood vessels in the back of the eye damages the macula. Wet AMD causes blurriness, darkness, or distortion in the center of the field of vision, thus reducing the individual's ability to read, drive, and see faces.
Injection into the eye of medicines like pegaptanib, ranibizumab, and bevacizumab can help block abnormal growth of blood vessels in the back of the eye. These drugs are known as anti‐VEGF agents. We conducted this review to compare benefits and risks of treatment with anti‐VEGF agents versus treatment without anti‐VEGF agents and to compare different types of anti‐VEGF agents.
What are the main results of the review? We found 16 studies that enrolled a total of 6347 people with wet AMD. Six studies compared anti‐VEGF agents against no anti‐VEGF agent, and ten studies compared bevacizumab versus ranibizumab. Drug companies conducted or sponsored four of the studies. Investigators conducted the 16 studies at various centers on five continents (North and South America, Europe, Asia, and Australia); they treated people and provided follow‐up for at least one year.
After one year, more people treated with any of the three anti‐VEGF agents (pegaptanib, ranibizumab, or bevacizumab) had improved vision, fewer had vision loss, and fewer were legally blind in the study eye when compared with people who did not receive anti‐VEGF agents. People treated with anti‐VEGF agents also showed structural improvements in the eye, which doctors use to monitor the disease and determine the need for more treatment. People who did not receive anti‐VEGF agents did not show the same kind of improvement.
Treatment with ranibizumab or bevacizumab yielded larger improvements in vision compared with treatment with pegaptanib in trials comparing anti‐VEGF treatment against treatment not using anti‐VEGF agents. Comparison of bevacizumab versus ranibizumab revealed no major differences with respect to any vision‐related outcomes. The major difference between the two agents was cost; bevacizumab was cheaper.
Inflammation and increased pressure in the eye were the most common unwanted effects caused by anti‐VEGF agents. Investigators reported endophthalmitis (infection in the inner part of the eye, which can cause blindness) in less than 1% of anti‐VEGF‐treated eyes and observed no cases among those not treated with anti‐VEGF agents. The occurrence of serious side effects, such as high blood pressure and internal bleeding, was low and was similar between anti‐VEGF‐treated groups and groups that did not receive anti‐VEGFs. The number of total side effects was very small, so it is impossible to tell which drug may have caused the most harmful effects.
How up‐to‐date is this review? Cochrane researchers searched for studies that had been published up to January 31, 2018.
Summary of findings
Background
Description of the condition
Introduction
Age‐related macular degeneration (AMD) is a progressive, degenerative disease of the retina that occurs with increasing frequency with advancing age. Two major types of AMD are known; these are commonly referred to as non‐neovascular ("dry") and neovascular ("wet") AMD. The non‐neovascular type is characterized by drusen (yellow spots under the retina), pigmentary changes (redistribution of melanin within the retinal pigment epithelium [RPE] under the retina and migration of melanin into the retina), and geographic atrophy (loss of the RPE and choriocapillaris).
This review is concerned with neovascular AMD and its treatment. The hallmark of neovascular AMD is choroidal neovascularization (CNV). Breaks in the RPE and in Bruch’s membrane allow naturally occurring vessels in the choroid to grow aberrantly into the subretinal space. These choroidal neovascular vessels typically leak and bleed, causing exudative or hemorrhagic retinal detachments. Without treatment, the process usually evolves into a fibrous scar, which replaces the outer layers of the retina, the RPE, and the choriocapillaris. The scarred retina has greatly diminished visual capacity.
Epidemiology
AMD is a leading cause of irreversible vision loss among the elderly in developed countries (Bourne 2014; Bunce 2006; Congdon 2004; Ghafour 1983; Hyman 1987; Leibowitz 1980; Tielsch 1994). Although the non‐neovascular type is much more common, the neovascular form of AMD is responsible for most cases of severe vision loss. The incidence of progression from non‐neovascular AMD to neovascular AMD is increased by the presence of numerous large and confluent drusen in the macula, as well as by the presence of pigment in the macula. Neovascular AMD occurs in only 10% of people with AMD, yet 80% of those with severe visual loss (worse than 20/200 Snellen acuity) have the neovascular form (Leibowitz 1980). Once neovascular disease develops in one eye, the risk of developing neovascular disease in the other eye of the same person is approximately 40% by five years (AREDS 2001; SST 20).
The overall prevalence of AMD, estimated in a meta‐analysis of studies from Australia, Europe, and the United States, was 1.47% (95% confidence interval [CI] 1.38% to 1.55%) (Friedman 2004); however, AMD increases in prevalence with advancing age, and incidence is low among individuals younger than 50 years of age. Thus, the burden of disease is greatest in regions where life expectancy is highest. Among those aged 80 years or older, the prevalence of neovascular AMD has been estimated to be 5.79% (95% CI 4.72% to 7.01%) in the UK (Owen 2003), and 8.18% (95% CI 7.07% to 9.29%) in the United States (Friedman 2004).
No consistent evidence indicates that modifiable factors such as lipid levels, blood pressure, light exposure, or alcohol intake put people at greater risk of AMD. One notable exception is smoking (Klein 2008; Mitchell 2002; Smith 1996). Elevated baseline levels of inflammatory biomarkers such as C‐reactive protein have been found to be associated with the development of early and late AMD in a large population‐based cohort (Boekhoorn 2007). Furthermore, several studies have shown gene‐environment interactions of complement factor H with smoking and C‐reactive protein (Deangelis 2007; Haddad 2006; Schaumberg 2007; Seddon 2006). High doses of vitamins C and E, beta‐carotene, and zinc provide a modest protective effect against progression to advanced AMD among individuals with extensive drusen or in initially unaffected fellow eyes with neovascular AMD (AREDS 2001; AREDS2 2013).
As the population continues to age, a higher prevalence of this disease is expected in the future, at least in certain populations. A population‐based survey estimated that AMD, as a contributing cause of blindness, had increased worldwide from 4.4% (95% CI 4.0 to 5.1) in 1990 to 6.6% (95% CI 5.9 to 7.9) in 2010 (Bourne 2014).
Presentation and diagnosis
Neovascular AMD may affect one eye or both eyes at the same time or sequentially. Symptoms of neovascular AMD include metamorphopsia (distortions while looking at objects), scotomata (black or gray spots), and blurry vision. Depending upon the location of the CNV and the quality of vision in the fellow eye, individuals with AMD may be unaware of a change in visual acuity or may note difficulty when performing normal activities that require good central vision, such as reading and writing, watching television, driving, and recognizing faces. When AMD affects only one eye, visual loss may go undetected until monocular testing is performed at a routine eye examination, or until chance occlusion of the better eye is noted. Frequently, people are unaware that their disturbed binocular vision is caused by changes in only one eye.
Neovascular AMD is diagnosed clinically with the help of imaging such as optical coherence tomography (OCT) and fluorescein angiography, which may be necessary to detect subtle exudation in some individuals who have experienced a recent change in visual acuity. At the onset of symptoms, fundus examination often reveals subretinal exudation of fluid, lipid, or blood. OCT, a non‐invasive imaging modality, shows cross‐sectional images of the retina, RPE, and choroid. Some studies have defined the characteristic appearance of different stages of the disease process on OCT (Ting 2002; Van Kerckhoven 2001). The most characteristic findings on OCT corresponding to a CNV lesion include areas of hyporeflectivity under the retina that, in turn, correspond to subretinal fluid, cystic hyporeflective changes consistent with macular edema, and attenuation of the photoreceptor/choriocapillaris layer. CNV can be seen in several characteristic patterns on fluorescein angiography. Classic CNV is defined as an area of early hyperfluorescence with increasing fluorescein leakage on late frames of the angiogram (MPSG 1991). Occult CNV occurs in two different patterns: fibrovascular pigment epithelial detachment and late fluorescein leakage from an undetermined source. Classic CNV typically has well‐demarcated borders, in contrast to the poorly demarcated borders usually seen in occult CNV.
Another test ‐ indocyanine green (ICG) angiography ‐ may facilitate evaluation of individuals with neovascular AMD, as it images the choroidal circulation better than fluorescein angiography and may show "hot" spots under the RPE that are amenable to treatment. ICG angiography is particularly useful in the diagnosis of polypoidal choroidal vasculopathy, a form of AMD that is most common among Asian populations.
Description of the intervention
Until the advent of anti‐vascular endothelial growth factor (VEGF) agents, treatments most frequently used for neovascular AMD included thermal laser photocoagulation and verteporfin photodynamic therapy (PDT). A Cochrane systematic review concluded that laser photocoagulation effectively slowed the progression of neovascularization in non‐subfoveal lesions compared with observation alone (Virgili 2007). A Cochrane review of verteporfin PDT concluded that PDT was effective in preventing clinically significant vision loss (Wormald 2007). However, neither laser photocoagulation nor PDT offered any significant chance for vision improvement.
Over the past two decades, researchers have developed new drugs for the treatment of patients with neovascular AMD. These drugs target a protein in the body known as vascular endothelial growth factor (VEGF), which stimulates the growth of abnormal blood vessels in neovascular AMD through a process called angiogenesis; the drugs block VEGF, leading to regression of abnormal blood vessels. Antiangiogenic therapy currently is the most commonly used treatment for neovascular AMD, particularly of subfoveal neovascular lesions.
An example of an anti‐VEGF antagonist is pegaptanib (Macugen, a trademark of Eyetech/Pfizer, Inc.). Pegaptanib is a chemically synthesized 28‐base ribonucleic acid molecule. It is an aptamer (foldable single‐strand nucleic acid) that has the ability to change its three‐dimensional structure to fit a target protein, in this case VEGF. By binding to VEGF, pegaptanib blocks and inactivates VEGF, thus halting the process of neovascularization. The US Food and Drug Administration (FDA) approved pegaptanib in December 2004 for the treatment of patients with neovascular AMD.
Ranibizumab, previously known as rhuFab‐VEGF (Lucentis, a trademark of Genentech, Inc.), is another example of an anti‐VEGF medication developed for ocular administration. It is a humanised antibody fragment capable of binding to the VEGF protein to prevent it from binding to its receptor, thus inhibiting angiogenic activity. Ranibizumab was the first treatment for neovascular AMD that offered a realistic hope for vision improvement; it was approved by the FDA in 2007.
Bevacizumab (Avastin, a trademark of Genentech, Inc.) is a humanized monoclonal antibody against VEGF. It is the larger parent molecule from which ranibizumab was derived. Bevacizumab currently is approved for the treatment of patients with conditions such as colorectal cancer, but it is widely used off‐label by ophthalmologists to treat neovascular AMD.
Aflibercept, previously known as VEGF Trap (Eylea, a trademark of Regeneron Pharmaceuticals, Inc.), is another anti‐VEGF agent; the molecule serves as a VEGF decoy to inhibit the growth of new blood vessels. The FDA approved aflibercept in 2011 for treatment of neovascular AMD. Conbercept, a drug similar to aflibercept, has been developed in China. Because the mechanism of action of these types of drugs is slightly different from that of the drugs listed above (pegaptanib, ranibizumab, and bevacizumab), and because they were introduced after the protocol for this review was developed, we have not evaluated aflibercept or conbercept in this review.
How the intervention might work
Angiogenesis is a complex process whereby interactions between stimulatory and inhibitory factors result in new blood vessel formation. These factors have been identified in CNV formation in animal models and human tissue (Aiello 1994; Kvanta 1996; Lopez 1996). Antiangiogenic therapies work by blocking stimulatory factors or by promoting inhibitory factors, thus disrupting the formation of new vessels. Agents that block the activity of VEGF (anti‐VEGFs), a polypeptide with mitogenic effects on endothelial blood vessels, form one type of anti‐angiogenic therapy. VEGF antagonists have been shown to inhibit CNV in animal models.
In the past, the primary goal of both laser photocoagulation and PDT was to prevent or delay further loss of visual acuity in the treated eye. With the development of agents to counteract VEGF, together known as anti‐VEGF agents, the primary goal of intravitreal injection of these agents is to retain or improve visual acuity. Currently, anti‐VEGF agents are administered most commonly via monthly intravitreous injections or as needed after three consecutive monthly injections.
Why it is important to do this review
Previous versions of this Cochrane review have documented the effectiveness of anti‐VEGF agents in halting the loss of visual acuity in a substantial fraction of treated eyes (Solomon 2014; Vedula 2008). Further, intravitreal injections with ranibizumab led to improved vision in about one‐third of eyes ‐ an improvement not previously observed with other AMD treatments (Solomon 2014; Solomon 2016). Since this Cochrane review was first published in 2008, numerous studies have been conducted to evaluate the safety and effectiveness of various anti‐VEGF agents, treatment regimens, and combination therapies for treatment of patients with neovascular AMD (Table 3). This review is restricted to primary randomized controlled trials (RCTs) of anti‐VEGF agents versus no anti‐VEGF treatment; and head‐to‐head (comparative effectiveness) RCTs of one anti‐VEGF agent versus another. Studies on dosage, different treatment strategies, and anti‐VEGF agents combined with other treatments are outside the scope of this review. The emphasis of this updated review is improvement in visual acuity with treatment.
1. Table of study acronyms.
| Acronym | Details |
| Included studies | |
| ABC | The Avastin® (Bevacizumab) in Choroidal Neovascularization Trial |
| ANCHOR | Anti‐VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age‐related Macular Degeneration |
| BRAMD | Comparison of Bevacizumab (Avastin®) and Ranibizumab (Lucentis®) in Exudative Age‐related Macular Degeneration |
| CATT | Comparison of Age‐related Macular Degeneration Treatment Trials |
| GEFAL | French Evaluation Group Avastin® Versus Lucentis® |
| IVAN | A Randomized Controlled Trial of Alternative Treatments to Inhibit VEGF in Age‐related Choroidal Neovascularisation |
| LUCAS | Lucentis® Compared to Avastin® Study |
| MANTA | A Randomized Observer and Subject Masked Trial Comparing the Visual Outcome After Treatment With Ranibizumab or Bevacizumab in Patients With Neovascular Age‐related Macular Degeneration Multicenter Anti‐VEGF Trial in Austria |
| MARINA | Minimally Classic/Occult Trial of the Anti‐VEGF Antibody Ranibizumab in the Treatment of Neovascular Age‐Related Macular Degeneration |
| PIER | A Phase IIIb, Multicenter, Randomized, Double‐Masked, Sham Injection‐Controlled Study of the Efficacy and Safety of Ranibizumab in Subjects With Subfoveal Choroidal Neovascularization With or Without Classic CNV Secondary to Age‐related Macular Degeneration |
| SAVE‐AMD | Safety of VEGF Inhibitors in Age‐Related Macular Degeneration |
| VISION | VEGF Inhibition Study in Ocular Neovascularization |
| Ongoing study | |
| VIBERA | Prevention of Vision Loss in Patients With Age‐Related Macular Degeneration by Intravitreal Injection of Bevacizumab and Ranibizumab |
Objectives
To investigate ocular and systemic effects of, and quality of life associated with, intravitreous injection of anti‐VEGF agents (pegaptanib, ranibizumab, and bevacizumab) versus no anti‐VEGF treatment for patients with neovascular AMD
To compare the relative effects of one of these anti‐VEGF agents versus another when administered in comparable dosages and regimens
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs in this review.
Types of participants
We included trials in which participants had neovascular AMD as defined by study investigators.
Types of interventions
We included studies that compared anti‐VEGF treatment versus another treatment, sham treatment, or no treatment. We did not include studies that compared different doses of one anti‐VEGF treatment against another, studies that included no control or comparator group, or studies that used anti‐VEGF agents in combination with other treatments. We did not include studies of aflibercept (VEGF Trap‐Eye/EYLEA solution) or studies that compared different treatment schedules (e.g. monthly vs as needed dosing), because other Cochrane reviews have evaluated these interventions (Li 2016; Sarwar 2016).
Types of outcome measures
Primary outcomes
The primary outcome for this review was based on best‐corrected visual acuity (BCVA) at one‐year follow‐up. All included RCTs randomized only one eye per participant (i.e. the study eye); therefore we defined the primary outcome for the comparison of treatments as the proportion of participants who gained 15 or more letters (three lines) of BCVA in the study eye when BCVA was measured on a visual acuity chart with a LogMAR scale.
Secondary outcomes
Visual acuity outcomes
Proportion of participants who gained 15 or more letters of BCVA in the study eye as measured at two‐year follow‐up
Proportion of participants who lost fewer than 15 letters of visual acuity at one year and at two years
Proportion of participants who lost fewer than 30 letters of visual acuity at one year and at two years
Proportion of participants for whom blindness was avoided in the study eye, defined as eyes with visual acuity better than 20/200 at one year and at two years
Proportion of participants maintaining visual acuity, defined as a gain of zero or more letters (i.e. no loss of BCVA from baseline) at one year and at two years
Mean change in visual acuity from baseline to one year and to two years
Other secondary outcomes
Contrast sensitivity, reading speed, or any other validated measure of visual function as measured in the included studies
Assessment of morphologic characteristics by fluorescein angiography or OCT, including mean change in size of CNV, mean change in size of total lesion, and mean change in central retinal thickness (CRT)
Quality of life measures, as assessed with any validated measurement scale
Economic data, such as comparative cost analyses
Ocular or systemic adverse outcomes
Follow‐up
We included only trials in which participants were followed for at least one year. We also included outcomes at two‐year follow‐up when these data were available.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomized controlled trials and controlled clinical trials. This search included no language or publication year restrictions. The date of the most recent search was January 31, 2018.
Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched January 31, 2018) (Appendix 1).
MEDLINE Ovid (1946 to January 31, 2018) (Appendix 2).
Embase Ovid (1980 to January 31, 2018) (Appendix 3).
Latin American and Caribbean Health Sciences Literature (LILACS) information database (1982 to January 31, 2018) (Appendix 4).
International Standard Randomized Controlled Trials Number (ISRCTN) registry (www.isrctn.com/editAdvancedSearch; searched January 31, 2018) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched January 31, 2018) (Appendix 6).
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched January 31, 2018) (Appendix 7).
Searching other resources
We reviewed the reference lists of included trial reports and related systematic reviews to identify additional potentially relevant trials.
We contacted pharmaceutical companies conducting or sponsoring studies of anti‐VEGF drugs to ask for information about any ongoing or completed clinical trials not published. One review author (SSV) handsearched abstracts from annual meetings of the Association for Research in Vision & Ophthalmology (ARVO) for the years 2006 and 2007 for ongoing trials (http://files.abstractsonline.com/SUPT/163/1807/PresentationTitle.htm; http://files.abstractsonline.com/SUPT/163/1601/Presentation_Title_PDF_wlinks.htm; accessed November 24, 2007). After 2007, Cochrane Eyes and Vision personnel handsearched conference abstracts reporting clinical trials; the trial records identified are included in CENTRAL. Another review author (KL) handsearched abstracts from the 2006 annual meeting of the European VitreoRetinal Society (http://www.evrs.eu/2006‐evrs‐congress‐cannes/; accessed November 27, 2012). If future updates of this review are performed, we will consider handsearching abstracts for the following conferences when they have not been searched by Cochrane Eyes and Vision: ARVO; Macula Society; Retina Society; subspecialty meetings at the American Academy of Ophthalmology meeting; American Society of Retinal Surgeons; and European VitreoRetinal Society.
Data collection and analysis
Selection of studies
Two review authors independently evaluated the titles and abstracts obtained through electronic searches. We classified each record as "definitely relevant," "possibly relevant," or "definitely not relevant"; a third review author resolved discrepancies. We obtained full‐text reports for all records assessed as "definitely relevant" or "possibly relevant." Two review authors independently assessed the full‐text reports and classified each study as "include," "exclude," "awaiting classification," or "ongoing"; a third review author resolved discrepancies. For trials identified by handsearching of conference abstracts, a second review author verified eligibility based on the stated criteria. We contacted study authors to clarify any details necessary for a complete assessment of relevance of the study. We documented studies excluded after review of the full‐text report and noted the reasons for exclusion.
Data extraction and management
Two review authors independently extracted study characteristics, including details of study methods, participants, interventions, outcomes, and funding sources, using data collection forms developed specifically for this purpose. We contacted trial authors for data on primary and secondary outcomes of individual trials when this information was not clearly available from published reports. We extracted data regarding visual acuity, adverse events, and other outcomes for the two trials forming part of the VISION 2004 study from documents available on the FDA website. We also extracted data from figures published in trial reports and communicated with study authors to verify extracted data. One review author entered data into Review Manager (Review Manager 5 2014), and a second review author verified the data entered.
Assessment of risk of bias in included studies
Two review authors assessed potential sources of bias in trials according to methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered the following parameters: random sequence generation and method of allocation concealment before randomization (selection bias), masking of participants and researchers (performance bias), masking of outcome assessors (detection bias), rates of loss to follow‐up and non‐compliance as well as failure to include in analyses all randomized participants (attrition bias), reporting bias, and other potential sources of bias. We judged each potential source of bias as conferring low risk, unclear risk, or high risk of bias in each trial and contacted authors of trial reports for additional information when study methods needed to assess bias domains were described unclearly or were not reported.
Measures of treatment effect
Data analysis was guided by Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). The primary outcome and many secondary outcomes for this review relied on measurements of best‐corrected visual acuity (BCVA) of the study eye. We analyzed BCVA, measured on LogMAR charts, as both dichotomous and continuous outcomes. We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. Dichotomous visual acuity outcomes included proportion of participants who gained 15 or more letters (same as a gain of three or more lines) of visual acuity; proportion of participants who lost fewer than 15 letters (fewer than three lines) of visual acuity; proportion of participants who lost fewer than 30 letters (fewer than six lines) of visual acuity; proportion of participants not blind in the study eye (defined as visual acuity better than 20/200); and proportion of participants who maintained baseline visual acuity (gain of zero or more letters) in the study eye. We calculated the mean difference (MD) between treatment groups for mean change in BCVA from baseline to follow‐up time as a continuous visual acuity outcome.
Secondary outcomes related to visual function and morphology of CNV also included both dichotomous and continuous outcomes. We calculated risk ratios with 95% confidence intervals for dichotomous outcomes, and mean differences with 95% confidence intervals for continuous outcomes. We reported contrast sensitivity outcomes, measured by Pelli‐Robson charts, both dichotomously (proportion of participants with a gain of nine or more letters, three levels of contrast, on the chart) and continuously (mean number of letters read correctly on the chart) depending on available data. We calculated mean differences with 95% confidence intervals for near visual acuity and reading speed outcomes when sufficient data were available.
Continuous morphologic outcomes included mean change in size of CNV, mean change in total size of the neovascular lesion, and mean change in CRT. We sought data for only one dichotomous morphologic outcome: resolution of subretinal or intraretinal fluid based on OCT evaluation.
We analyzed quality of life scores as continuous data. Because trials that reported quality of life outcomes included in meta‐analyses used the same scale, we did not calculate standardized mean differences.
We reported adverse events as risk ratios with 95% confidence intervals when sufficient data were available. Otherwise, we reported the numbers of participants who experienced adverse events in both narrative and tabular form.
Unit of analysis issues
The unit of analysis was the individual (one study eye per participant).
Dealing with missing data
We used multiple sources to identify relevant data for this review, such as journal publications, conference abstracts, FDA documents, and clinical trial registries. When data were unclear (e.g. numbers were extracted from graphs or were derived from percentages), we contacted study investigators for verification. When data were missing, we contacted study investigators for additional information. If we received no response within two weeks, we attempted to contact them again. When we received no response by six weeks after the first attempt, we used data as available.
For outcome data, we used data provided in trial reports or supplied by primary investigators. We noted the number of participants with missing data and statistical methods used in individual studies to analyze data (e.g. available case analysis, last observation carried forward). We did not impute missing outcome data for our analyses.
Assessment of heterogeneity
We assessed statistical heterogeneity based on the Chi² test, the I² statistic, and the overlap of confidence intervals in forest plots. We considered a Chi² P value < 0.10 to represent significant statistical heterogeneity, and an I² statistic of 60% or more to represent substantial statistical heterogeneity. We assessed clinical and methodological heterogeneity among studies by comparing study populations, interventions, and study methods.
Assessment of reporting biases
We assessed selective outcome reporting for each study by comparing outcomes specified in a protocol, research plan, or clinical trial registry with reported results. When protocols, research plans, or clinical trial registry records were not available, we assessed selective outcome reporting based on outcomes specified in the methods section of the study reports and on data collected as specified in the study design. In future updates of this review, when outcome data from 10 or more studies are included in a meta‐analysis, we will use a funnel plot to judge potential publication bias.
Data synthesis
We performed statistical analyses using Review Manager 5 2014. We did not combine studies in meta‐analysis when we identified clinical or methodological heterogeneity (e.g. different anti‐VEGF agents, different outcome time points); instead we analyzed data by type of anti‐VEGF agent and time point, or, when data were not sufficient for meta‐analysis, we provided a narrative summary. We used a random‐effects model for all analyses. When the I² statistic was 60% or greater, suggesting substantial statistical heterogeneity, we assessed the direction of treatment effects across studies and the overlap of confidence intervals to determine whether meta‐analysis was appropriate. We did not adjust estimates of treatment effects to account for the multiplicity of outcomes considered in this review.
Subgroup analysis and investigation of heterogeneity
In the first published version of this review, we conducted subgroup analyses of the primary outcome, as specified in the protocol, by stratifying data according to the angiographic subtype of CNV, using definitions adopted in the included trials (Vedula 2008). Because we changed the primary outcome to a gain of 15 or more letters of visual acuity for later versions of the review, and because available data were insufficient, we did not conduct these subgroup analyses; if data by angiographic subtype of CNV become available for inclusion in any future update to this review, we will include these subgroup analyses. For this review update, we combined in meta‐analysis outcome data from trials that had compared any anti‐VEGF agent versus a control other than an anti‐VEGF agent; we presented the effect estimates for individual anti‐VEGF agents (pegaptanib, ranibizumab, and bevacizumab) in subgroups.
Sensitivity analysis
In the first published version of this review, we conducted sensitivity analyses to examine potential bias caused by missing data for participants excluded after randomization or lost to follow‐up in analyses of the primary outcome. We analyzed the primary outcome while assuming that (1) participants lost to follow‐up had lost 15 or more letters of visual acuity (worst‐case analysis); and (2) participants lost to follow‐up did not lose 15 or more letters of visual acuity at one‐year follow‐up (best‐case analysis) (Vedula 2008). Because these analyses did not alter the conclusions of this review, and because studies added in subsequent updates were too small to affect estimates of effectiveness and safety, we did not conduct sensitivity analyses for this version of the review and do not believe they would be needed in a future update.
We planned to conduct sensitivity analyses to assess the impact of studies graded as having high risk of bias on any parameter, unpublished data only, or industry funding. After assessing the studies included and the data collected, we determined that these analyses were not needed because studies within each meta‐analysis did not differ on the basis of these factors.
"Summary of findings"
We prepared "Summary of findings" tables for each comparison assessed in this review. These tables include relative and absolute effects for the following outcomes of interest at one‐year follow‐up: (1) visual acuity gain of 15 or more letters, (2) visual acuity loss of fewer than 15 letters, (3) mean change in visual acuity (number of letters), (4) reduction in central retinal thickness, (5) quality of life scores, (6) serious systemic adverse events, and (7) serious ocular adverse events. We assessed the certainty of evidence for all outcomes by using the GRADE classification system (GRADEpro 2014).
Results
Description of studies
Results of the search
Electronic searches for the first published version of this review (conducted in August 2005, October 2006, June 2007, and February 2008) yielded a total of 1407 titles and abstracts (Vedula 2008). We selected 36 records for full‐text review and identified five trials described in 10 reports for inclusion in the review (ANCHOR 2006; EOP 1003; EOP 1004; FOCUS 2006; MARINA 2006). We excluded 16 studies (24 reports) and added two studies identified by handsearching of abstracts as awaiting classification. Table 3 lists acronyms used to refer to many studies in this review.
We identified two concurrent randomized trials that used individual participant data meta‐analyses under the acronym VISION (Gragoudas 2004) ‐ EOP 1003 (an international trial) and EOP 1004 (a North American trial). In the first published version of this review, we assessed the data from these two trials separately and analyzed them according to the original protocol of the review. We obtained data for primary and secondary outcomes for the two trials by accessing information available on the FDA website and by contacting study authors. For the 2014 update of this review (Solomon 2014), we considered the two trials as one study (VISION 2004), and we collected new data from published articles as available. We have summarized characteristics of the two individual trials in Appendix 8 and Appendix 9.
For the 2014 update, we refined the eligibility criteria to exclude studies in which researchers gave anti‐VEGF treatment in combination with other AMD treatments, and to include trials that compared two anti‐VEGF agents (i.e. head‐to‐head trials). A separate Cochrane review will cover combination therapies for AMD. Thus, in subsequent updates of the review, we did not include FOCUS 2006, which compared ranibizumab plus PDT versus PDT alone and was included in the first version of this review. Electronic searches in September 2008, April 2011, February 2013, and March 2014 yielded 4827 unique records from bibliographic databases, 403 clinical trial registrations, and 19 additional records identified by handsearching of conference abstracts. Of 153 reports from potentially relevant records, we included 12 RCTs (reported in 108 records) and excluded 39 studies (reported in 45 records). We excluded two additional studies from three records identified by handsearching and included the remaining 16 records identified by handsearching as additional reports on the included studies. We identified seven additional studies from the search of clinical trial registries ‐ one that was awaiting classification owing to insufficient information to determine eligibility, and six assessed as ongoing or completed with results not yet published.
We updated the electronic searches on January 31, 2018, and identified 2737 unique records (Figure 1). We excluded 2702 records after screening titles and abstracts, and 21 records after reviewing full‐text reports. Of the 35 records not excluded, four pertained to four newly included studies (BRAMD 2016; LUCAS 2015; SAVE‐AMD 2017; Scholler 2014), 14 were reports from studies already included, and three were from studies excluded earlier. We had classified one newly included study as ongoing in the 2014 version of this review. Two excluded studies had been labeled as ongoing in an earlier version but were terminated before enrollment (GALATIR 2014; RATE 2011). Overall, we identified and included 16 eligible studies and one ongoing study and excluded 65 studies after review of the full‐text reports. We have listed reasons for exclusion of each of the 65 studies in the Characteristics of excluded studies table and described the ongoing study in the Characteristics of ongoing studies section. All but one included study had been documented in a clinical trial register.
1.
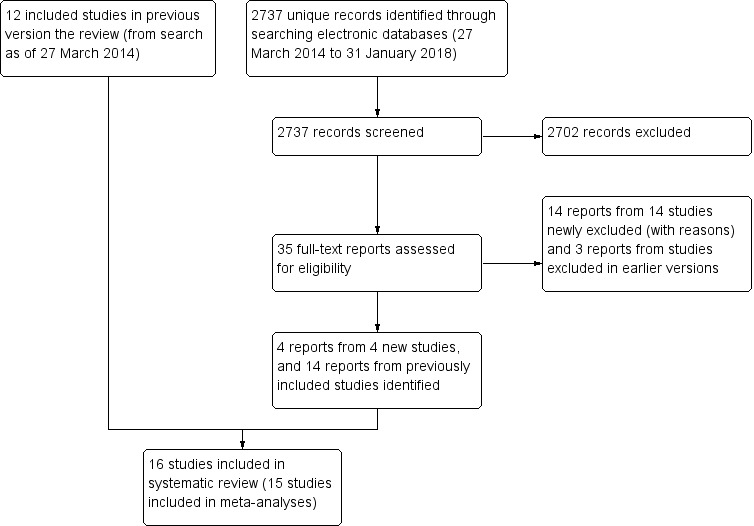
Study flow diagram.
Included studies
Types of participants
This review included a total of 6347 participants from 16 RCTs; the number of participants per trial ranged from 23 to 1208. In 14 of 16 trials, investigators reported that they had randomized one eye per participant; this was unclear in 2 of 16 trials (SAVE‐AMD 2017; Scholler 2014). Countries in which the trials were conducted spanned the globe: two studies were international (ANCHOR 2006; VISION 2004); four were conducted in the United States only (CATT 2011; MARINA 2006; PIER 2008; Subramanian 2010), three in Austria (MANTA 2013; Sacu 2009; Scholler 2014), two in the United Kingdom (ABC 2010; IVAN 2013), and one each in France (GEFAL 2013), India (Biswas 2011), the Netherlands (BRAMD 2016), Norway (LUCAS 2015), and Switzerland (SAVE‐AMD 2017). The 16 trials were similar in that all enrolled both men and women 50 years of age or older who had subfoveal CNV secondary to AMD; BRAMD 2016 also enrolled participants with juxtafoveal or extrafoveal CNV. The goal of SAVE‐AMD 2017 was to compare the effects of anti‐VEGF agents on neovascular and non‐neovascular AMD, with random assignment of participants in each cohort to ranibizumab or bevacizumab. Among the included trials, reports describe variation in types of eligible neovascular lesions (e.g. predominantly classic CNV, minimally classic CNV, occult CNV), lesion sizes, and baseline visual acuities of participants. A majority of participants in most trials were women, but one trial enrolled a greater number of men than women (Subramanian 2010).
All trials predefined visual acuity eligibility criteria for the study eye of each participant. Six studies specified the most common criterion: study eye BCVA of 20/40 to 20/320 (Snellen equivalent) in the study eye (ABC 2010; ANCHOR 2006; MANTA 2013; MARINA 2006; PIER 2008; VISION 2004). BCVA eligibility ranges included somewhat better visual acuity in CATT 2011 and LUCAS 2015 (20/25 to 20/320), GEFAL 2013 (20/32 to 20/320), IVAN 2013 (20/320 or better), and Scholler 2014 (20/40 to 20/320), but potentially worse visual acuity in Sacu 2009 (20/40 to 20/800) and Subramanian 2010 (20/400 or better). In Biswas 2011, participants with a BCVA between 35 and 70 Early Treatment Diabetic Retinopathy Study (ETDRS) letters were eligible, but study authors did not report the test distance. In BRAMD 2016, participants with a BCVA between 20 and 78 ETDRS letters were eligible.
Eight trials included only participants who had received no previous treatment for CNV or AMD (Biswas 2011; CATT 2011; IVAN 2013; LUCAS 2015; MANTA 2013; Sacu 2009; SAVE‐AMD 2017; Scholler 2014). The remaining trials included participants who had received previous therapy for AMD, with certain restrictions as to type of treatment (e.g. verteporfin PDT, intravitreal injections, surgery), location of treatment, and time interval since last treatment. Six trials enrolled participants with primary or recurrent CNV in the study eye (ANCHOR 2006; BRAMD 2016; MARINA 2006; PIER 2008; Subramanian 2010; VISION 2004), and one enrolled only participants with primary CNV (ABC 2010).
Among seven studies that reported the type of neovascular lesion, ANCHOR 2006 had the highest proportion of participants with predominantly classic CNV (410/423; 97%). In ABC 2010, 25% of 131 participants had predominantly classic CNV; the remaining 75% had minimally classic or occult CNV. In VISION 2004, 26% of 1208 participants had predominantly classic CNV, 36% had minimally classic CNV, and 38% had occult CNV. PIER 2008 reported proportions similar to VISION 2004, with 19% of 184 participants having predominantly classic CNV, 38% having minimally classic CNV, and 43% having occult CNV at baseline. In BRAMD 2016, 27% had predominantly classic CNV, 16% had minimally classic CNV, and 57% of 327 participants had occult CNV. Forty‐four per cent of 120 participants had occult CNV in Biswas 2011. MARINA 2006 was limited to participants with minimally classic or occult CNV and, thus, included the greatest proportion of participants with occult CNV (451/716; 63%).
Three studies that did not report neovascular lesion type described the subfoveal component of the CNV lesion in the study population. In CATT 2011 (1208 participants), 58% had CNV in the foveal center, 27% had fluid in the foveal center, 8% had hemorrhage in the foveal center, and 6% had other foveal center involvement. The distribution was similar in IVAN 2013 (628 participants), in which 54% of participants had CNV in the foveal center, 29% had hemorrhage in the foveal center, and 13% had other foveal center involvement. In LUCAS 2015 (431 participants with data), 69% had CNV in the foveal center, 80% had fluid in the foveal center, and 20% had hemorrhage in the foveal center. The three smallest studies (Sacu 2009; SAVE‐AMD 2017; Subramanian 2010), with 28, 23, and 28 participants, as well as GEFAL 2013 (501 participants) and MANTA 2013 (321 participants), did not describe the type of neovascularization nor the subfoveal component of the CNV lesion in the study population.
Six trials specified lesion size as an inclusion criterion. Five trials included participants with lesions of 12 disc areas (DAs) or smaller (1 DA = 2.54 mm², i.e. standard DA) (ABC 2010; BRAMD 2016; GEFAL 2013; MARINA 2006; PIER 2008), and one study set four DAs as the maximum lesion size (Sacu 2009).
We have summarized in the Characteristics of included studies table additional details about each trial included in this review.
Types of interventions
We have listed in Table 4 comparisons of interventions evaluated by trials included in this review, and we summarize them here. Among the 16 included trials, we focused on two main comparisons of interventions: (1) anti‐VEGF monotherapy versus control, and (2) one anti‐VEGF monotherapy versus a different anti‐VEGF monotherapy. Of six studies that compared anti‐VEGF monotherapy versus control, one study evaluated three doses of pegaptanib versus sham injection (VISION 2004), three studies compared two doses of ranibizumab versus sham injections or PDT (ANCHOR 2006; MARINA 2006; PIER 2008), and two studies compared bevacizumab with other treatments for AMD (ABC 2010; Sacu 2009). The remaining ten studies were head‐to‐head trials of bevacizumab versus ranibizumab (Biswas 2011; BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013; SAVE‐AMD 2017; Scholler 2014; Subramanian 2010).
2. Treatment groups in included trials.
|
Study Treatment period |
Intervention 1 | Intervention 2 | Intervention 3 | Intervention 4 |
| Pegaptanib vs control | ||||
|
VISION 2004 2 years; re‐randomized at end of first year |
0.3 mg pegaptanib every 6 weeks | 1.0 mg pegaptanib every 6 weeks | 3.0 mg pegaptanib every 6 weeks | Sham every 6 weeks |
| Ranibizumab vs control | ||||
|
ANCHOR 2006 2 years |
0.3 mg ranibizumab monthly plus sham verteporfin PDT | 0.5 mg ranibizumab monthly plus sham verteporfin PDT | Sham intravitreal injection plus verteporfin PDT | ‐ |
|
MARINA 2006 2 years |
0.3 mg ranibizumab monthly | 0.5 mg ranibizumab monthly | Sham intravitreal injection monthly | ‐ |
|
PIER 2008 2 years |
0.3 mg ranibizumab monthly for 3 months, then every 3 months | 0.5 mg ranibizumab monthly for 3 months, then every 3 months | Sham intravitreal injection monthly for 3 months, then every 3 months | ‐ |
| Bevacizumab vs control | ||||
|
ABC 2010 1 year |
1.25 mg bevacizumab given first 3 injections every 6 weeks, then as needed | Standard therapy (0.3 mg pegaptanib every 6 weeks, verteporfin PDT, or sham injection) | ‐ | ‐ |
|
Sacu 2009 1 year |
1.0 mg bevacizumab monthly for 3 months, then as needed | Verteporfin PDT plus same day 4 mg triamcinolone acetonide | ‐ | ‐ |
| Bevacizumab vs ranibizumab | ||||
|
CATT 2011 2 years; re‐randomized at end of first year |
1.25 mg bevacizumab monthly for 1 year; at 1 year, re‐randomization to ranibizumab monthly or variable dosing | 0.5 mg ranibizumab monthly for 1 year; at 1 year, re‐randomization to ranibizumab monthly or variable dosing | 1.25 mg bevacizumab as needed after first injection for 2 years | 0.5 mg ranibizumab as needed after first injection for 2 years |
|
IVAN 2013 2 years; ongoing |
1.25 mg bevacizumab monthly for 2 years | 0.5 mg ranibizumab monthly for 2 years | 1.25 mg bevacizumab monthly for 3 months, then as needed in 3‐month cycles | 0.5 mg ranibizumab monthly for 3 months, then as needed in 3‐month cycles |
| Biswas 2011 18 months | 1.25 mg bevacizumab monthly for 3 months, then as needed | 0.5 mg ranibizumab monthly for 3 months, then as needed | ‐ | ‐ |
| BRAMD 2016 1 year | 1.25 mg bevacizumab monthly for 1 year | 0.5 mg ranibizumab monthly for 1 year | ‐ | ‐ |
|
GEFAL 2013 1 year |
1.25 mg bevacizumab; maximum of 1 injection per month | 0.5 mg ranibizumab; maximum of 1 injection per month | ‐ | ‐ |
|
LUCAS 2015 1 year |
1.25 mg bevacizumab; treat and extend protocol | 0.5 mg ranibizumab; treat and extend protocol | ‐ | ‐ |
| MANTA 2013 1 year | 1.25 mg bevacizumab monthly for 3 months, then as needed | 0.5 mg ranibizumab monthly for 3 months, then as needed | ‐ | ‐ |
|
SAVE‐AMD 2017 1 year |
1.25 mg bevacizumab at day 1 and at week 4, then as needed | 0.5 mg ranibizumab at day 1 and at week 4, then as needed | ‐ | ‐ |
|
Scholler 2014 1 year |
1.25 mg bevacizumab for 3 months, at 30‐day intervals, then as needed | 0.5 mg ranibizumab for 3 months, at 30‐day intervals, then as needed | ‐ | ‐ |
|
Subramanian 2010 1 year |
0.05 mL bevacizumab monthly for 3 months, then as needed | 0.05 mL ranibizumab monthly for 3 months, then as needed | ‐ | ‐ |
PDT: photodynamic therapy.
Anti‐VEGF monotherapy versus control
VISION 2004 investigators compared sham injections versus intravitreous injections of pegaptanib at dosages of 0.3 mg, 1.0 mg, and 3.0 mg given every six weeks over a 48‐week period.
Three trials evaluated two different doses of ranibizumab (0.3 mg and 0.5 mg) (ANCHOR 2006; MARINA 2006; PIER 2008). Control groups and the injection schedule for ranibizumab differed among the three trials. MARINA 2006 compared monthly intravitreal injection of ranibizumab (for 12 months) with sham intravitreal injections. Participants assigned to receive sham intravitreal injections in MARINA 2006 were allowed verteporfin PDT whenever the CNV lesions in the eyes became predominantly classic CNV. ANCHOR 2006 compared monthly injections of ranibizumab combined with sham PDT (for 24 months) versus verteporfin PDT and sham intravitreal ranibizumab injections. PIER 2008 compared a regimen of monthly injection of ranibizumab for three months followed by an injection every three months versus sham intravitreal injections.
Two trials evaluated bevacizumab versus control. ABC 2010 compared a 1.25 mg dose of bevacizumab versus standard therapy, which was determined by clinical evaluation and included 0.3 mg pegaptanib, verteporfin PDT, or sham injection. Sacu 2009 (a small trial) compared a 1 mg dose of bevacizumab versus verteporfin PDT combined with intravitreal triamcinolone.
Bevacizumab versus ranibizumab
Ten trials compared bevacizumab for non‐inferiority versus ranibizumab. In addition to the primary comparison of the two agents, CATT 2011 and IVAN 2013 compared monthly injections of anti‐VEGF agents with an "as‐needed" regimen after three initial injections of the assigned agent. Biswas 2011, GEFAL 2013, MANTA 2013, and Subramanian 2010 used the latter treatment regimen (a 0.5 mg dose of ranibizumab and a 1.25 mg dose of bevacizumab) to compare the two anti‐VEGF agents. BRAMD 2016 used a monthly injection schedule, and LUCAS 2015 used a "treat‐and‐extend" protocol for both drugs. In Scholler 2014, investigators did not specify the hypothesis and treated participants with an "as‐needed" regimen after three initial injections of the assigned agent. In SAVE‐AMD 2017, researchers gave intravitreous injections after the initial two injections PRN for the remainder of the one‐year follow‐up period.
Types of outcome measures
Visual acuity
BCVA formed the basis of the primary outcome for all included studies except Scholler 2014. The primary outcome for this review ‐ the proportion of participants who gained 15 or more letters of BCVA at one‐year follow‐up ‐ was the primary outcome for the ABC 2010 included study and a secondary outcome for the remaining 15 studies. The proportion of participants losing fewer than 15 letters at one year was the primary outcome for the three earliest studies (ANCHOR 2006; MARINA 2006; VISION 2004), and it was a secondary outcome for 11 of the remaining 13 studies. The primary outcome was mean change in visual acuity at one year for eight studies (BRAMD 2016; CATT 2011; GEFAL 2013; LUCAS 2015; MANTA 2013; PIER 2008; Sacu 2009; Subramanian 2010), and the primary outcome was mean change in visual acuity at 18 months for one study (Biswas 2011). Five of the remaining studies reported mean change in visual acuity as a secondary outcome. The primary outcome for IVAN 2013 was best‐corrected distance visual acuity at two‐year follow‐up; we did not analyze mean BCVA (as opposed to mean change from baseline) as an outcome for this review.
Some included studies reported other visual acuity outcomes relevant to this review. Five studies reported loss of fewer than 30 letters of visual acuity (ABC 2010; ANCHOR 2006; MARINA 2006; Subramanian 2010; VISION 2004); eight studies reported BCVA better than 20/200 (ANCHOR 2006; CATT 2011; GEFAL 2013; IVAN 2013; MARINA 2006; PIER 2008; Subramanian 2010; VISION 2004); and four studies reported maintenance of visual acuity (defined as a gain of zero or more letters) (ANCHOR 2006; Sacu 2009; Subramanian 2010; VISION 2004). Investigators in included studies reported several other visual acuity outcomes that we did not consider in this review.
All studies measured visual acuity on a LogMAR scale, typically using ETDRS charts. Each line on the ETDRS chart consists of five letters; thus, a change of 15 letters approximates a three‐line change (0.3 LogMAR change) in visual acuity. Researchers reported the outcome for visual acuity of 20/200 or better as the Snellen equivalent.
Visual function
Five studies assessed visual function outcomes. ABC 2010 specified contrast sensitivity and reading ability as secondary outcomes. IVAN 2013 specified contrast sensitivity, near visual acuity, and reading index outcomes as secondary outcomes. We identified one conference abstract that reported contrast sensitivity outcomes for ANCHOR 2006, MARINA 2006, and PIER 2008.
Eleven studies did not report visual function outcomes (Biswas 2011; BRAMD 2016; CATT 2011; GEFAL 2013; LUCAS 2015; MANTA 2013; Sacu 2009; SAVE‐AMD 2017; Scholler 2014; Subramanian 2010; VISION 2004).
Morphologic outcomes
All studies included at least one measure related to morphologic characteristics of neovascular lesions in study eyes. In many cases, publications or conference abstracts did not provide sufficient data for informative analysis of these outcomes. When possible, we used data provided by primary investigators, or we asked primary investigators to confirm data extracted from graphs in study reports. We have not reported data derived from graphs included in study reports unless we received confirmation of the data from study investigators.
All studies except SAVE‐AMD 2017 used fluorescein angiography to monitor lesion activity; that study used OCT to monitor lesion status. Six studies also used fundus photography (ANCHOR 2006; GEFAL 2013; LUCAS 2015; MARINA 2006; PIER 2008; VISION 2004), and two studies used ICG angiography (GEFAL 2013; Sacu 2009). Six studies evaluated mean change in CNV size by fluorescein angiography (ABC 2010; ANCHOR 2006; GEFAL 2013; MARINA 2006; PIER 2008; VISION 2004), and eight studies used fluorescein angiography to evaluate mean change in the size of neovascular lesions (ABC 2010; ANCHOR 2006; CATT 2011; IVAN 2013; MARINA 2006; PIER 2008; Scholler 2014; VISION 2004).
The earliest study included in the review did not use OCT for assessment of subretinal characteristics of eyes with neovascular AMD (VISION 2004). The next three studies, which were conducted chronologically (ANCHOR 2006; MARINA 2006; PIER 2008), used OCT to assess a subset of study participants. The 12 most recently reported studies used OCT for all study participants and specified at least one OCT measure as a primary or secondary outcome (ABC 2010; Biswas 2011; BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013; Sacu 2009; SAVE‐AMD 2017; Scholler 2014; Subramanian 2010). All studies that used OCT assessed mean change in central retinal thickness (CRT) from baseline. We considered central macular thickness, central foveal thickness, and center point thickness as interchangeable terms for CRT.
Individual studies reported other morphologic outcomes, such as area of CNV leakage and subretinal fluid, but we did not include these outcomes in this review.
Quality of life outcomes
Four studies evaluated vision‐specific quality of life using the 25‐item National Eye Institute‐Visual Functioning Questionnaire (NEI‐VFQ) (ANCHOR 2006; MARINA 2006; PIER 2008; VISION 2004). The NEI‐VFQ, which was administered by an interviewer, relies on patient‐reported responses to specific visual function questions to calculate overall and subscale scores, which can range from 0 to 100, with higher values representing better visual function.
In one study (IVAN 2013), participants completed the EuroQoL Group health‐related quality of life assessment (EQ‐5D). The EQ‐5D converts participant responses to specific health questions using scales of 1 to 3, on which 1 represents no health problems, 2 represents moderate health problems, and 3 represents extreme health problems. Investigators then summarize scores for each of the five subscale domains (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression) into a single index score ranging from ‐0.59 to 1.00, with 1.00 representing no health problems. Both the NEI‐VFQ and the EQ‐5D are validated tools that can be used to assess quality of life outcomes.
The remaining studies either did not measure or have not reported quality of life outcomes (ABC 2010; Biswas 2011; BRAMD 2016; CATT 2011; GEFAL 2013; LUCAS 2015; MANTA 2013; Sacu 2009; SAVE‐AMD 2017; Scholler 2014; Subramanian 2010).
Economic outcomes
Two studies included economic‐related outcomes as prespecified secondary outcomes. CATT 2011 evaluated annual costs associated with each treatment group. IVAN 2013 evaluated cumulative resource use and costs for each treatment group.
Adverse events
Seven studies reported individual ocular and non‐ocular adverse events up to one‐year follow‐up (ABC 2010; GEFAL 2013; LUCAS 2015; MANTA 2013; Sacu 2009; Scholler 2014; Subramanian 2010), one study up to 18‐month follow‐up (Biswas 2011), five studies up to five‐year follow‐up (ANCHOR 2006; CATT 2011; IVAN 2013; MARINA 2006; PIER 2008), and one study up to seven‐year follow‐up (VISION 2004). BRAMD 2016 investigators did not report ocular adverse events but reported major systemic adverse events. SAVE‐AMD 2017 investigators reported that there was "no serious ocular adverse event (e.g. endophthalmitis, retina detachment, and lens damage)" during the 12‐month follow‐up period.
Excluded studies
We excluded 65 studies after completing full‐text assessments: 23 studies were not RCTs; 13 followed participants for less than one year; nine were dose‐response studies that included no control or comparator arm; six compared combination therapies in which treatment groups received the same anti‐VEGF therapy; two did not administer anti‐VEGF agents via intravitreous injection; one did not include participants with neovascular AMD; ten evaluated agents that were not eligible for this review (aflibercept, brolucizumab, and pazopanib eye drops); one did not report any outcome targeted for this review; and two potentially relevant studies were terminated before enrollment.
Risk of bias in included studies
We have provided assessments of risks of bias for each included study at the end of each respective Characteristics of included studies table. When we needed unpublished information to assess the risk of bias for any given parameter, we contacted primary investigators for additional information. We have documented these instances together with investigators' responses in the Characteristics of included studies table. Figure 2 summarizes "Risk of bias" assessments for all 16 studies.
2.
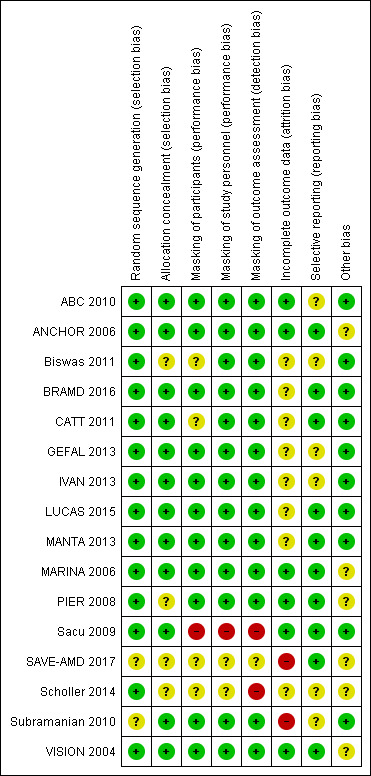
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Overall, the included studies were at low risk of selection bias. Reports from 14 of the 16 studies described methods of random sequence generation that we judged to confer low risk of bias; SAVE‐AMD 2017 investigators and Subramanian 2010 did not describe the methods used in sufficient detail for us to assess risk of bias in this domain. Six studies used dynamic randomization, the method used most commonly for random sequence generation (ABC 2010; ANCHOR 2006; BRAMD 2016; MARINA 2006; PIER 2008; VISION 2004). Four studies used permuted block randomization designs (CATT 2011; IVAN 2013; LUCAS 2015; MANTA 2013), three used random number tables or lists (Biswas 2011; GEFAL 2013; Scholler 2014), and one reported only use of a computer‐randomized schema (Sacu 2009).
Investigators in 12 of the 16 trials reported adequate allocation concealment. For Biswas 2011, the report was unclear as to whether the randomization sequence, determined by random numbers tables generated before study enrollment, was concealed or was made available to study investigators. PIER 2008 did not describe how assignments were allocated, and we were unable to make an assessment by using only available information. Eight studies employed a third party or a central co‐ordinating center (ABC 2010; ANCHOR 2006; GEFAL 2013; LUCAS 2015; MANTA 2013; MARINA 2006; Sacu 2009; Subramanian 2010), and four studies used a computer‐based portal for allocation concealment (BRAMD 2016; CATT 2011; IVAN 2013; VISION 2004).
Communication with investigators from Biswas 2011, PIER 2008,SAVE‐AMD 2017, and Subramanian 2010 yielded no additional information about methods used to assess risks of selection bias (email communication).
Masking (performance bias and detection bias)
We judged most of the included studies to be at low risk of performance bias and detection bias. Only one study was an open‐label study that employed no form of masking (Sacu 2009). CATT 2011 initially masked participants to the drug (not to the injection protocol), but participants may have become aware of treatment assignments through billing records. Biswas 2011, SAVE‐AMD 2017, and Scholler 2014 did not report whether study participants were masked. Biswas 2011 and CATT 2011 masked personnel and outcome assessors. The remaining 11 studies masked study participants, personnel (other than personnel directly administering treatment), and outcome assessors; thus, we assessed these studies as being at low risk of performance bias and detection bias. Investigators in studies that compared intravitreous injections versus no injections most commonly used sham injections when participants were not assigned or did not require an injection. In head‐to‐head studies of ranibizumab versus bevacizumab, researchers masked participants to their assigned treatment group. To minimize detection bias, study investigators who were involved in assessing outcomes were separate from treating physicians and were masked to treatment groups, with the exception of Sacu 2009, which provided no masking. SAVE‐AMD 2017 and Scholler 2014 provided no information on masking of study participants, study personnel, or outcome assessors.
Incomplete outcome data
In all 16 trials, few participants missed the follow‐up examination specified as the primary time for assessing the study's primary outcome or were not treated in accord with the randomized treatment assignment. In nine trials, rates of loss to follow‐up at primary follow‐up visits were less than 15%; BRAMD 2016, GEFAL 2013, LUCAS 2015, MANTA 2013, SAVE‐AMD 2017, and Subramanian 2010 had 16% to 25% of participants with missing outcome data. Losses to follow‐up were evenly balanced between treatment groups in the included studies.
Eight trials included in this review analyzed the data using methods designed to overcome, in part, loss of information due to missed follow‐up examinations. Seven of these eight trials used the last observation carried forward method to impute missing data (ABC 2010; ANCHOR 2006; BRAMD 2016; MANTA 2013; MARINA 2006; PIER 2008; VISION 2004), and the eighth trial did not report the method used to impute data for one participant with missing data (Sacu 2009). The remaining eight trials reported available case data and included in the analysis only participants with data: 87% in Biswas 2011, 91.5% in CATT 2011, 81% in GEFAL 2013, 89% in IVAN 2013, 84% in LUCAS 2015, 81% in SAVE‐AMD 2017, 83% in Scholler 2014, and 79% in Subramanian 2010. Investigators in all trials reported that they had analyzed data for participants by assigned treatment arms. Analyses using simple imputation methods or available case data assume that participants are lost to follow‐up at random; bias may be introduced when this assumption is not true, with greater risk of bias associated with higher rates of missing data.
Selective reporting
With the exception of Biswas 2011, we identified design articles, protocols, or clinical trial registrations for 15 of the included studies. We judged 11 of these 15 trials to be free of reporting bias on the basis of consistency between study outcomes defined in protocols and clinical trial registrations and those reported in study results papers. Researchers did not specify quality of life outcomes, and we identified no report on quality of life findings from Subramanian 2010. We found no data on reading ability outcomes, which ABC 2010 specified as secondary outcomes. Published articles on one‐year and two‐year results did not report findings for three outcomes specified in the protocol for IVAN 2013: treatment satisfaction, survival free from treatment failure, and exploratory (serum) analysis. Differences in outcomes between trial registration and published one‐year results of GEFAL 2013 included the following: differences in details of outcome specifications (e.g. efficacy of treatments vs proportion of participants with a gain of 15 or more letters of visual acuity); outcomes specified in the trial register but not reported in publications; and an outcome that was not mentioned in the trial registration document.
Other potential sources of bias
We considered various other aspects of trial design and reporting, trial sponsorship, and financial interests of investigators as other potential sources of bias.
Pharmaceutical companies marketing the study drugs under investigation sponsored ANCHOR 2006, MARINA 2006, PIER 2008, and VISION 2004, and submitted data from these trials to the FDA to obtain approval for ranibizumab and pegaptanib. In addition, pharmaceutical company sponsors had important roles in trial design, analysis, and reporting. Some investigators from other trials reported that they received trial agents or financial support from pharmaceutical companies; however, because the companies did not directly sponsor these trials, we did not judge them to be at risk of bias for this domain (CATT 2011; GEFAL 2013; IVAN 2013; Scholler 2014). We observed no other potential sources of bias for the remaining eight studies.
Effects of interventions
Summary of findings for the main comparison. Summary of findings: anti‐VEGF treatment versus control.
| Anti‐VEGF treatment versus control for neovascular age‐related macular degeneration | ||||||
|
Participant or population: people with neovascular age‐related macular degeneration Settings: clinical centers Intervention: intravitreal injections of anti‐VEGF agents (pegaptanib, ranibizumab, or bevacizumab) Control: standard therapy at the time of the trial (sham injections, verteporfin photodynamic therapy with or without triamcinolone acetonide, or intravitreal injections of pegaptanib) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anti‐VEGF treatment | |||||
| Gain of 15 or more letters visual acuity at 1 year | 43 per 1000 | 179 per 1000 (99 to 322) | RR 4.19 (2.32 to 7.55) | 2667 (6) | ⊕⊕⊕⊝ Moderatea | |
| Loss of fewer than 15 letters visual acuity at 1 year | 599 per 1000 | 838 per 1000 (760 to 928) |
RR 1.40 (1.27 to 1.55) |
2667 (6) | ⊕⊕⊕⊕ High | |
| Mean change in visual acuity at 1 year (number of letters) | Mean change across control groups ranged from a loss of 10 to 16 letters | See comment | See comment | 2508 (4) | ⊕⊕⊕⊝ Moderateb | Owing to substantial statistical heterogeneity between pegaptanib and ranibizumab subgroups, we did not combine data across subgroups Mean change in visual acuity in pegaptanib groups was on average 6.72 more letters gained (95% CI 4.43 letters to 9.01 letters); MD 6.72 (95% CI 4.43 to 9.01) Mean change in visual acuity in ranibizumab groups was on average 17.80 more letters gained (95% CI 15.95 letters to 19.65 letters); MD 17.80 (95% CI 15.95 to 19.65) Mean change from baseline in visual acuity was 7.0 letters in bevacizumab group and ‐9.4 letters in control group in 1 study. The second study reported that participants in bevacizumab group gained 8 letters on average and participants in control group lost 3 letters on average |
| Reduction in central retinal thickness at 1 year | See comment | See comment | See comment | See comment | See comment | We were unable to find data on central retinal thickness in reports from the only trial comparing pegaptanib with control and from any of the 3 included trials comparing ranibizumab with control Mean change was ‐91 μm in bevacizumab group and ‐55 μm in control group in one study, and ‐113 μm in bevacizumab group and ‐72 μm in control group in the other study |
| Mean change in vision‐related quality of life | Mean change across control groups in vision‐related quality of life scores ranged from ‐3 to 2 points | Mean change across control groups in vision‐related quality of life scores ranged from 5 to 7 points | MD 6.69 (3.38 to 9.99) | 1134 (2) | ⊕⊕⊕⊝ Moderatea | Use of the NEI‐VFQ questionnaire with a 10‐point difference considered clinically meaningful |
| Serious systemic adverse events at 1 year | Range of 5 to 83 per 1000 for various systemic adverse events | Range of 0 to 55 per 1000 for various systemic adverse events | Range of RR 0.17 (0.01 to 4.24) to 2.08 (0.23 to 18.45) | 2667 (6) | ⊕⊕⊕⊝ Moderatec | |
| Serious ocular adverse events at 1 year | Range of 0 to 68 per 1000 for various ocular adverse events | Range of 3 to 118 per 1000 for various ocular adverse events | Range of RR 0.52 (0.03 to 8.25) to 2.71 (1.36 to 5.42) | 2667 (6) | ⊕⊕⊕⊝ Moderatec | |
| *The basis for the assumed risk is estimated by the proportion with the event in the control group. The corresponding risk (and its 95% CI) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Anti‐VEGF: anti‐vascular endothelial growth factor; CI: confidence interval; MD: mean difference; NEI‐VFQ: National Eye Institute‐Visual Functioning Questionnaire; RR: risk ratio. | ||||||
| Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded (‐1) owing to imprecision in the confidence interval. bDowngraded (‐1) owing to inconsistency in effect between types of anti‐VEGF agents. cAdverse events downgraded to moderate quality as not all eligible trials reported all types of adverse events, and numbers were small (< 1%) for many specific adverse events.
Summary of findings 2. Summary of findings: bevacizumab versus ranibizumab.
| Bevacizumab versus ranibizumab for neovascular age‐related macular degeneration | ||||||
|
Participant or population: people with neovascular age‐related macular degeneration Settings: clinical centers Intervention: intravitreal injections of bevacizumab Comparison: intravitreal injections of ranibizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ranibizumab | Bevacizumab | |||||
| Gain of 15 or more letters visual acuity at 1 year | 252 per 1000 | 239 per 1000 (204 to 282) | RR 0.95 (0.81 to 1.12) | 3144 (8) | ⊕⊕⊕⊕ High | |
| Loss of fewer than 15 letters visual acuity at 1 year | 944 per 1000 | 944 per 1000 (926 to 963) | RR 1.00 (0.98 to 1.02) | 3144 (8) | ⊕⊕⊕⊕ High | |
| Mean change in visual acuity at 1 year (number of letters) | Mean change across ranibizumab groups ranged from gains of 3 to 8 letters | Mean change in visual acuity in bevacizumab groups was on average 0.58 fewer letters gained (95% CI 1.55 fewer letters to 0.40 more letters) | MD ‐0.6 (‐1.6 to 0.4) | 3190 (9) | ⊕⊕⊕⊕ High | |
| Reduction in central retinal thickness at 1 year | Mean reduction in central retinal thickness across ranibizumab groups ranged from 30 to 182 μm | Mean reduction in central retinal thickness in bevacizumab groups was on average 11.61 μm less (95% CI 21.55 less to 1.66 less) | MD ‐11.6 (‐21.6 to ‐1.7) | 2693 (6) | ⊕⊕⊕⊕ High | Three additional trials reported no differences between groups for this outcome; however, these data were not reported in formats that could be included in meta‐analysis |
| No problems in quality of life domains at 1 year | Range of 591 per 1000 to 861 per 1000 across 5 quality of life domains | Range of 608 per 1000 to 828 per 1000 across 5 quality of life domains | Range of RR 0.96 (0.90 to 1.04) to 1.02 (0.89 to 1.17) | 548 (1) | ⊕⊕⊕⊝ Moderatea | Quality of life domains included mobility, self‐care, usual activities, pain/discomfort, anxiety/depression |
| Serious systemic adverse events at 1 yearb | 156 per 1000 with at least 1 serious systemic adverse event | 179 per 1000 (154 to 209) | RR 1.15 (0.99 to 1.34) | 3365 (6) | ⊕⊕⊕⊝ Moderatea | |
| Serious ocular adverse events at 1 year | < 5 per 1000 | < 5 per 1000 | Range of RR 0.51 (0.05 to 5.62) to 7.05 (0.36 to 136.28) | Range 1670 to 2280 (2 to 3) | ⊕⊕⊕⊝ Moderatea | Studies reported different ocular adverse events. One study reported only that there was no difference between treatment arms |
| *The basis for the assumed risk is estimated by the proportion with the event in the ranibizumab group. The corresponding risk (and its 95% CI) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||||
| Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aQuality of life and adverse event outcomes downgraded to moderate quality as not all eligible trials reported these outcomes, and numbers of some adverse events were small (< 1%). bA Cochrane review on systemic safety of bevacizumab versus ranibizumab includes more complete data for this finding (Moja 2014). Please refer to Moja 2014 for the most complete information on systemic safety for bevacizumab versus ranibizumab.
We conducted meta‐analyses of results through comparisons of treatments by combining different doses and regimens of the same drug evaluated in individual trials (Table 4). Forest plots presented in this review for visual acuity outcomes show that effect estimates to the right of the vertical line of the forest plots (i.e. risk ratios > 1 and mean differences > 0) favor the test treatment defined for the comparison.
Anti‐VEGF monotherapy versus control
Six studies that had enrolled 2690 participants compared an anti‐VEGF monotherapy versus no anti‐VEGF treatment. Overall, we rated the risk of bias as low among the six studies. Pharmaceutical companies funded four studies (ANCHOR 2006; MARINA 2006; PIER 2008; VISION 2004). All six studies followed participants for at least one year after enrollment.
One study, comprising two individual RCTs, compared three doses of intravitreal pegaptanib (0.3 mg, 1.0 mg, and 3.0 mg) versus a sham injection control (VISION 2004). The study, which was conducted at 117 international centers, enrolled 1208 adult participants (50 years of age or older) with subfoveal CNV lesions secondary to AMD. The pegaptanib groups included 904 participants, and the sham injection group included 304. At one‐year follow‐up, follow‐up analyses included 1186 (98%) participants, and investigators re‐randomized according to their original treatment assignment 1053 (87%) who remained in the study. Researchers re‐randomized participants in the pegaptanib groups to continue current treatment or to discontinue treatment and participants in the sham group to continue with sham injections, discontinue sham injections, or receive one of the three study doses of pegaptanib. Study follow‐up continued for one year after re‐randomization, and study authors analyzed participants in three cohorts: those who continued with their original assignments, those who discontinued treatment, and those who received sham injections during the first year then pegaptanib during the second year. In total, two‐year analysis included 1053 (87%) participants; however, we could not analyze the two‐year data because they reflect changes from year 1 to year 2 rather than from baseline to year 2.
Three studies comprising a total of 1323 participants compared two doses of intravitreal ranibizumab (0.3 mg and 0.5 mg) versus sham or control treatment. In ANCHOR 2006, 280 participants received ranibizumab and 143 received verteporfin PDT therapy. Study personnel administered injections monthly and administered verteporfin PDT therapy on day 0 and as needed at visits at months 3, 6, 9, and 12. In MARINA 2006, 478 participants received ranibizumab and 238 received sham injections, all of which were administered on a monthly basis. In PIER 2008, 121 participants received ranibizumab and 63 received sham injections monthly for the first three months, then every three months. During the second year of PIER 2008, participants in the 0.3 mg ranibizumab and sham‐treated groups crossed over to receive 0.5 mg ranibizumab. At one‐year follow up, investigators excluded from analyses two participants ‐ one in ANCHOR 2006 and one in PIER 2008. Analysis included remaining study participants, and researchers imputed missing data by using the last observation carried forward method.
Two studies of 159 total participants compared intravitreal bevacizumab injections with control treatment. In ABC 2010, 131 participants received 1.25 mg intravitreal bevacizumab (65 participants) or standard therapy consisting of pegaptanib injections (38 participants), verteporfin PDT (16 participants), or sham injections (12 participants). In Sacu 2009, 28 participants received 1.0 mg intravitreal bevacizumab (14 participants) or verteporfin PDT with 4 mg intravitreal triamcinolone acetonide (14 participants). In both studies, investigators administered intravitreal bevacizumab as needed after the first three scheduled injections.
(1) Visual acuity
(a) Gain of 15 or more letters of visual acuity
At one year, more participants in the anti‐VEGF group than in the control group had a gain of 15 or more letters of visual acuity. The risk ratio for combined anti‐VEGF versus control groups was 4.19 (95% CI 2.32 to 7.55), that is, eyes treated with an anti‐VEGF agent 4 times as often gained 15 or more letters of vision than control eyes. Assessment of effect by type of anti‐VEGF agent revealed that the direction of effect consistently favored anti‐VEGF treatment (Analysis 1.1; Figure 3). We graded the certainty of evidence for the outcome as moderate, after downgrading for imprecision (‐1).
1.1. Analysis.
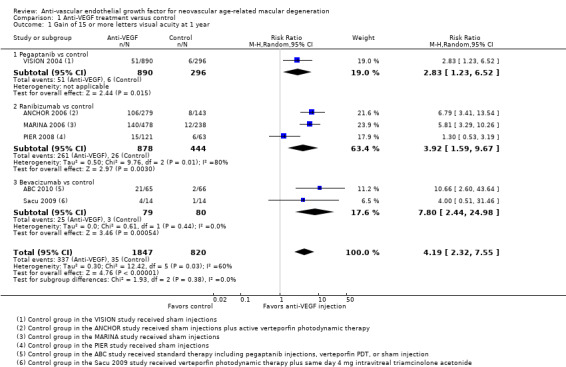
Comparison 1 Anti‐VEGF treatment versus control, Outcome 1 Gain of 15 or more letters visual acuity at 1 year.
3.
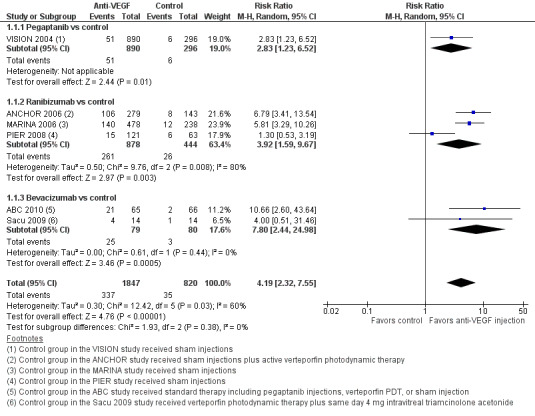
Forest plot of comparison: 2 Ranibizumab versus control, outcome: 2.1 Gain of 15 or more letters visual acuity at 1 year.
At two years, data were available from only the three ranibizumab trials. The proportion of participants who were treated with ranibizumab and had gained 15 or more letters at two years was nearly six times the proportion of those treated with control who gained 15 or more letters (RR 5.77, 95% CI 3.38 to 9.84; Analysis 1.2; Figure 4). We graded the certainty of evidence for the two‐year outcome also as moderate, again downgrading for imprecision (‐1).
1.2. Analysis.
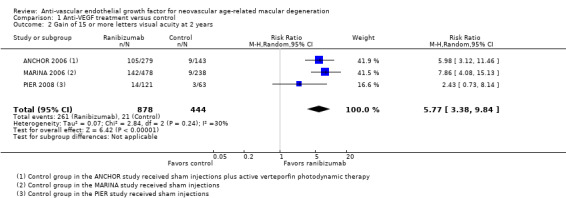
Comparison 1 Anti‐VEGF treatment versus control, Outcome 2 Gain of 15 or more letters visual acuity at 2 years.
4.
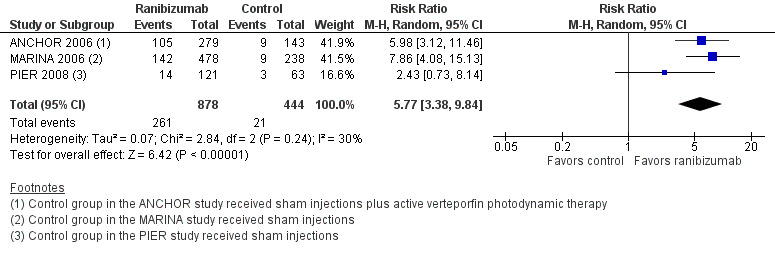
Forest plot of comparison: 2 Ranibizumab versus control, outcome: 2.2 Gain of 15 or more letters visual acuity at 2 years.
(b) Loss of fewer than 15 letters of visual acuity
At one year, a greater proportion of participants treated with an anti‐VEGF agent lost fewer than 15 letters of visual acuity in the study eye compared with those treated with control. Participants were estimated to be 1.40 times more likely not to lose 15 or more letters of visual acuity when treated with an anti‐VEGF agent than with sham or control therapy (RR 1.40, 95% CI 1.27 to 1.55; Analysis 1.3). Although we observed statistical heterogeneity among the trials (I² = 62%), effect estimates of individual trials were in the same direction, and confidence intervals of individual trials overlapped one another. We graded the certainty of evidence for this outcome as high, upon finding no reason to downgrade.
1.3. Analysis.
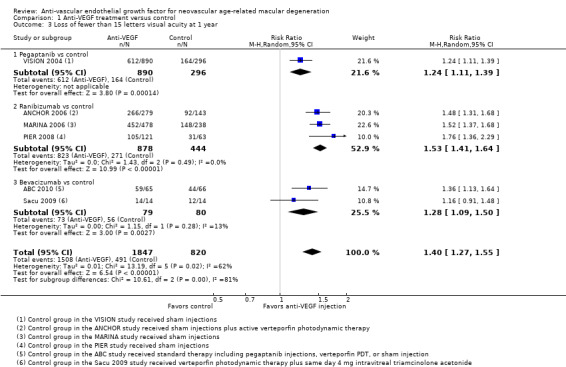
Comparison 1 Anti‐VEGF treatment versus control, Outcome 3 Loss of fewer than 15 letters visual acuity at 1 year.
At two years, data were available for only the three ranibizumab trials. The beneficial effect of ranibizumab for this outcome persisted at a similar magnitude when compared with control therapy. Sixty per cent more participants treated with ranibizumab lost fewer than 15 letters of visual acuity at two‐year follow‐up as participants in control groups (RR 1.62, 95% CI 1.32 to 1.98; Analysis 1.4). We observed substantial statistical heterogeneity in the analysis comparing ranibizumab with control (I² = 78%; P value for Chi² test of homogeneity = 0.01); however, confidence intervals among individual studies overlapped, and effect estimates were in the same direction. Heterogeneity may have been attributable to the fact that the control group in ANCHOR 2006 received an active treatment (verteporfin PDT therapy) but control eyes in MARINA 2006 and PIER 2008 received sham injections. We graded the certainty of evidence for this outcome as high, as we found no reason to downgrade.
1.4. Analysis.

Comparison 1 Anti‐VEGF treatment versus control, Outcome 4 Loss of fewer than 15 letters visual acuity at 2 years.
(c) Loss of fewer than 30 letters of visual acuity
At one year, four trials reported this outcome (ABC 2010; ANCHOR 2006; MARINA 2006; VISION 2004), and two trials did not report this outcome (PIER 2008; Sacu 2009). The risk ratio for combined anti‐VEGF groups versus the control group was 1.12 (95% CI 1.06 to 1.19), indicating that 12% (95% CI 6% to 19%) fewer eyes treated with an anti‐VEGF agent than eyes treated with control lost 30 or more letters of visual acuity (Analysis 1.5). Estimates from the included studies revealed statistical heterogeneity (I² = 73%); however, effect estimates of individual trials showed the same direction, and confidence intervals of individual trials overlapped one another, except for ABC 2010. We graded the certainty of evidence for this outcome as high, with no reason to downgrade.
1.5. Analysis.
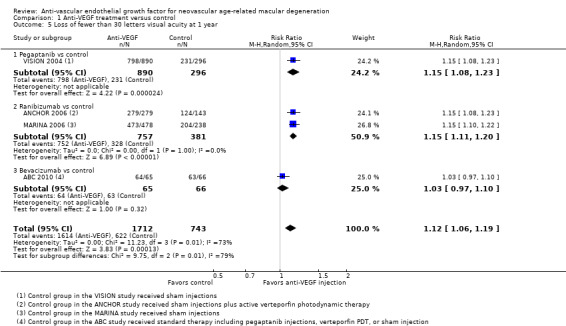
Comparison 1 Anti‐VEGF treatment versus control, Outcome 5 Loss of fewer than 30 letters visual acuity at 1 year.
The ranibizumab treatment effect for this outcome persisted through two years, and fewer participants treated with ranibizumab in two trials lost 30 or more letters (16/757; 2%) than participants given control treatment (77/381; 20%). When comparing ranibizumab groups versus controls, we estimated a 22% benefit of ranibizumab with respect to loss of fewer than 30 letters of visual acuity after two years (RR 1.22, 95% CI 1.15 to 1.29; Analysis 1.6). We graded the certainty of evidence for this outcome as high, upon finding no reason to downgrade. No study reported this outcome for two years for bevacizumab or pegaptanib.
1.6. Analysis.
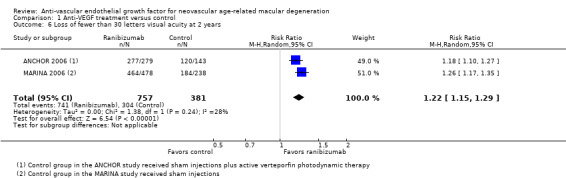
Comparison 1 Anti‐VEGF treatment versus control, Outcome 6 Loss of fewer than 30 letters visual acuity at 2 years.
(d) Prevention of blindness in the study eye (visual acuity better than 20/200)
At one year, four trials reported this outcome (ANCHOR 2006; MARINA 2006; PIER 2008; VISION 2004). Treatment with pegaptanib or ranibizumab resulted in fewer blind study eyes at one‐year follow‐up; the summary effect estimate (risk ratio) for visual acuity better than 20/200 was 1.58 (95% CI 1.34 to 1.86) for the two anti‐VEGF agents compared with control (Analysis 1.7). Although point estimates and confidence intervals of individual studies showed some degree of overlap, we noted a substantial amount of statistical heterogeneity for this outcome (I² = 73%). Neither bevacizumab trial reported data sufficient for analysis; Sacu 2009 did not report blindness, and authors of the ABC 2010 trial reports noted that more participants in the bevacizumab group than in the control group had visual acuity of 20/200 or better at one year. At two years, based on comparison of the combined ranibizumab groups with control intervention groups, we again estimated that a substantially greater proportion of study eyes of participants in the ranibizumab groups had visual acuity better than 20/200 than those in the control group (RR 1.73, 95% CI 1.52 to 1.98; Analysis 1.8). We graded the certainty of evidence for this outcome as high both at one year and at two years, finding no reason to downgrade.
1.7. Analysis.
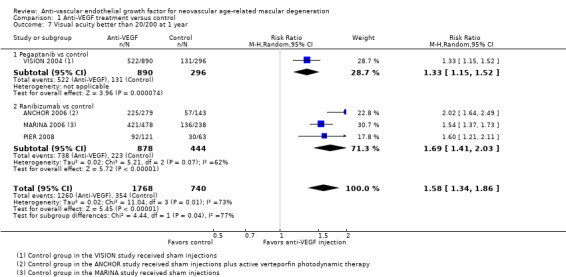
Comparison 1 Anti‐VEGF treatment versus control, Outcome 7 Visual acuity better than 20/200 at 1 year.
1.8. Analysis.
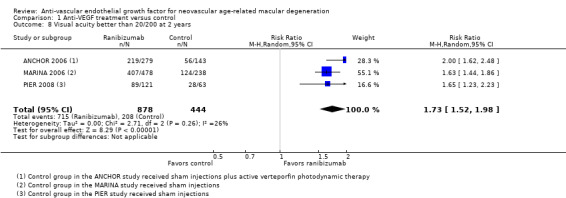
Comparison 1 Anti‐VEGF treatment versus control, Outcome 8 Visual acuity better than 20/200 at 2 years.
(e) Maintenance of visual acuity
At one year, data on maintenance of visual acuity were available from only three trials that had compared anti‐VEGF treatment with a control intervention (ANCHOR 2006; Sacu 2009; VISION 2004); three trials did not report this outcome (ABC 2010; MARINA 2006; PIER 2008). Twice as many participants treated with an anti‐VEGF agent maintained visual acuity in the study eye (i.e. visual acuity at follow‐up was the same as or better than at baseline) compared with participants in the control group; the risk ratio was 1.98 (95% CI 1.31 to 3.00; Analysis 1.9). At two years, the corresponding effect estimate from ANCHOR 2006 was 2.71 (95% CI 2.08 to 3.54; Analysis 1.10). We graded the certainty of evidence for this outcome at both time points as high, upon finding no reason to downgrade.
1.9. Analysis.
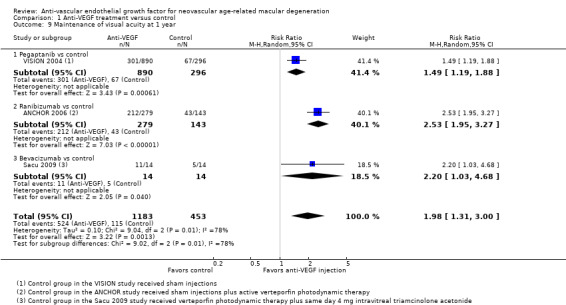
Comparison 1 Anti‐VEGF treatment versus control, Outcome 9 Maintenance of visual acuity at 1 year.
1.10. Analysis.
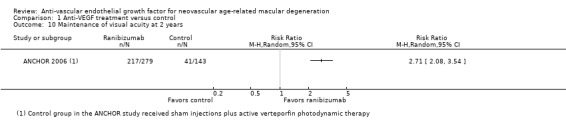
Comparison 1 Anti‐VEGF treatment versus control, Outcome 10 Maintenance of visual acuity at 2 years.
(f) Mean change in visual acuity
At one year, data for mean change in visual acuity from baseline were available for analysis from four trials that had compared anti‐VEGF treatment with a control intervention (ANCHOR 2006; MARINA 2006; PIER 2008; VISION 2004). Owing to significant heterogeneity (I² = 98%), we did not pool data from pegaptanib and ranibizumab trials. The mean difference in mean change in visual acuity from baseline between the pegaptanib group and the sham group was 6.7 letters (95% CI 4.4 to 9.0) when measured on an ETDRS chart placed at 2 meters; thus, eyes treated with pegaptanib lost on average 6.7 letters fewer than sham‐treated eyes (Analysis 1.11). On the logMAR scale, 0.10 logMAR unit corresponds to one line (five letters) on the visual acuity chart. Thus, the mean difference between pegaptanib and sham groups was equivalent to 0.13 logMAR units, that is, the mean change in visual acuity was better in the pegaptanib groups than in the sham group by 0.13 logMAR unit. At one year, participants treated with ranibizumab also read more letters on ETDRS charts placed at 4 m than participants treated with control. Participants treated with ranibizumab were able to read 18 more letters at one‐year follow‐up (mean difference [MD] 17.8, 95% CI 16.0 to 19.6; Analysis 1.11). Available data from the bevacizumab studies were insufficient for analysis of the difference in mean changes in visual acuity between treatment groups. In ABC 2010, the mean change from baseline in visual acuity was 7.0 letters in the bevacizumab group and ‐9.4 letters in the control group at one‐year follow‐up. This equates to a mean difference of 16.4 letters (more than three lines of visual acuity); however, we were unable to compute the standard error (SE) of the mean using the information available. Sacu 2009 reported a statistically significant difference between groups at one year, when participants in the bevacizumab group had gained eight letters on average and participants in the control group had lost three letters on average. We graded the certainty of evidence for this outcome as moderate, after downgrading for inconsistency (‐1).
1.11. Analysis.
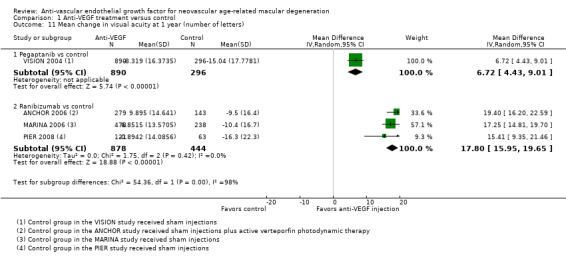
Comparison 1 Anti‐VEGF treatment versus control, Outcome 11 Mean change in visual acuity at 1 year (number of letters).
At two years, participants treated with ranibizumab were able to read 20 more letters (0.4 logMAR unit) compared with participants given control treatment (MD 20.1, 95% CI 18.1 to 22.2; Analysis 1.12). We graded the certainty of evidence for this outcome as high, finding no reason to downgrade.
1.12. Analysis.
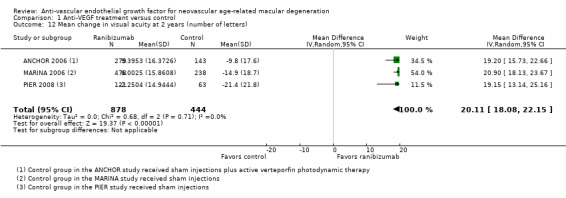
Comparison 1 Anti‐VEGF treatment versus control, Outcome 12 Mean change in visual acuity at 2 years (number of letters).
(2) Visual function
Sacu 2009 and VISION 2004 did not report visual function outcomes. The ranibizumab trials did not specify visual function outcomes as outcomes of interest (ANCHOR 2006; MARINA 2006; PIER 2008); however, we identified one conference abstract that discussed contrast sensitivity outcomes among participants from these trials (see Korobelnik 2006 under ANCHOR 2006). Investigators reported no between‐group comparisons for contrast sensitivity as measured by Pelli‐Robson charts, but the abstract author reported that participants in the ranibizumab groups had statistically significant increases of two to four letters (i.e. approximately one contrast level) after one year. Participants in the control groups lost an average of three letters (i.e. one contrast level) at one year. The mean difference when ranibizumab was compared with control was six letters (i.e. two contrast levels on the Pelli‐Robson chart), as determined on the basis of data extracted from the abstract.
ABC 2010 reported outcomes for contrast sensitivity measured with Pelli‐Robson charts. At one year, the difference observed between bevacizumab and control groups in terms of a gain of 15 or more letters (i.e. five levels of contrast) of contrast sensitivity was uncertain (RR 2.03, 95% CI 0.39 to 10.71); however, a greater proportion of participants in the bevacizumab group (23/65) compared with the control group (10/66) gained six or more letters (i.e. two levels of contrast) of contrast sensitivity (RR 2.34, 95% CI 1.21 to 4.51). Also, five times as many participants in the control group lost six or more letters (two contrast levels) of contrast sensitivity compared with participants in the bevacizumab group (RR 0.22, 95% CI 0.07 to 0.72). We graded the certainty of evidence for visual function as low, after downgrading for imprecision (‐1) and inconsistency (‐1). Although the published protocol for ABC 2010 also listed reading ability (including maximum reading speed, critical print size, and reading acuity) measured with Minnesota Reading Charts as a secondary outcome for the study, we found no report of results for this outcome.
(3) Morphologic outcomes
(a) Mean change in size of CNV
Available information was insufficient for analysis of the mean change in size of CNV in VISION 2004; however, study investigators provided data that allowed us to evaluate mean CNV size at one‐year follow‐up. Given that baseline CNV sizes were comparable among all study participants, the difference in mean size of CNV between study groups at one year was used to estimate the treatment effect. Researchers measured total CNV sizes as numbers of standard disc areas (DAs). Pegaptanib treatment, across all doses studied, resulted in a lower final mean CNV size compared with that of the sham group at the one‐year follow‐up examination (MD 0.92 DAs, 95% CI 0.42 to 1.42; Analysis 1.13). We considered a difference in the size of CNV of one or more DAs as a clinically meaningful difference. We graded the certainty of evidence for this outcome as moderate, after downgrading for imprecision (‐1).
1.13. Analysis.

Comparison 1 Anti‐VEGF treatment versus control, Outcome 13 Reduction in size of CNV at 1 year (mean number of disc areas).
ABC 2010 reported the median change in the size of CNV. At 54 weeks, the size of CNV regressed by 0.88 mm² (interquartile range [IQR], reduction of 4.08 mm² to increase of 0.40 mm²) in the bevacizumab‐treated group compared with 0.27 mm² (IQR, reduction of 2.58 mm² to increase of 1.24 mm²) in the control group.
We were unable to identify and extract any data on mean change in size of the CNV from any of the three included trials comparing ranibizumab with control interventions (ANCHOR 2006,MARINA 2006,PIER 2008), and from one of the trials comparing bevacizumab with control interventions (Sacu 2009).
(b) Mean change in size of lesion
Available information was insufficient to estimate mean change in size of the total subfoveal lesion with pegaptanib; however, study investigators provided data that allowed us to analyze mean size of the lesion at one‐year follow‐up. Pegaptanib treatment resulted in smaller mean lesion size at one‐year follow‐up compared with sham treatment (MD 0.86 DAs, 95% CI 0.35 to 1.37; Analysis 1.14). We graded the certainty of evidence for this outcome as moderate, after downgrading for imprecision (‐1).
1.14. Analysis.
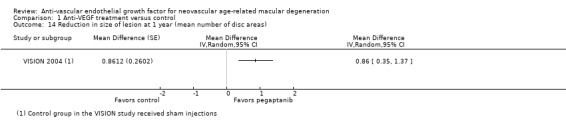
Comparison 1 Anti‐VEGF treatment versus control, Outcome 14 Reduction in size of lesion at 1 year (mean number of disc areas).
Data for the mean change in size of the total subfoveal lesion were available from two of the three included trials that had compared ranibizumab with control interventions (ANCHOR 2006; PIER 2008). The mean reduction in lesion size was greater by 2.34 DAs (95% CI 1.88 to 2.81) among participants treated with ranibizumab compared with participants treated with control interventions after one year (Analysis 1.15). At two years, this effect persisted in ANCHOR 2006 (MD 2.44, 95% CI 1.87 to 3.00) but not in PIER 2008 (MD 0.59, 95% CI ‐0.55 to 1.73; Analysis 1.16). Owing to substantial statistical heterogeneity (I² = 88%) and differences between control groups in the two trials during the second year of follow‐up, we did not combine these study findings in a meta‐analysis. We graded the certainty of evidence for the outcome as moderate, after downgrading for inconsistency (‐1).
1.15. Analysis.
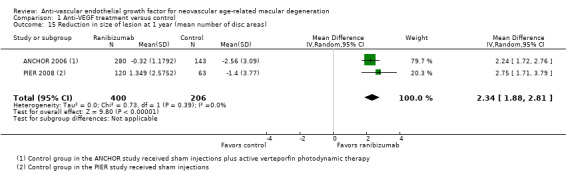
Comparison 1 Anti‐VEGF treatment versus control, Outcome 15 Reduction in size of lesion at 1 year (mean number of disc areas).
1.16. Analysis.
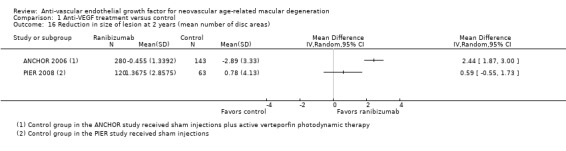
Comparison 1 Anti‐VEGF treatment versus control, Outcome 16 Reduction in size of lesion at 2 years (mean number of disc areas).
ABC 2010 reported median change in subfoveal lesion size. At 54 weeks, the size of the total subfoveal lesion regressed by 0.03 mm² (IQR, reduction of 1.88 mm² to increase of 2.63 mm²) in the bevacizumab‐treated group and increased by 2.33 mm² (IQR, reduction of 0.06 mm² to increase of 6.44 mm²) in the control group.
MARINA 2006 and Sacu 2009 did not report this outcome.
(c) Mean change in CRT
VISION 2004 did not use OCT and did not measure CRT outcomes. We were unable to find data on CRT in reports from any of the three included trials that had compared ranibizumab with control interventions (ANCHOR 2006; MARINA 2006; PIER 2008).
In ABC 2010, the mean change in CRT after 54 weeks was ‐91 μm in the bevacizumab group and ‐55 μm in the control group (P = 0.08). In Sacu 2009, the mean change in CRT by 12 months was ‐113 μm in the bevacizumab group and ‐72 μm in the control group (P = 0.8; analysis of variance [ANOVA]). Investigators reported no measures of variability for these outcomes, precluding meta‐analysis.
(4) Quality of life outcomes
One of the two trials from VISION 2004 (EOP 1004) and the three ranibizumab trials measured vision‐related quality of life using the NEI‐VFQ questionnaire (ANCHOR 2006; MARINA 2006; PIER 2008). Two trials provided sufficient data for inclusion in a meta‐analysis (ANCHOR 2006; MARINA 2006). Investigators in both studies considered a 10‐point change in scores as clinically meaningful. We did not extract the limited data available from PIER 2008 because investigators presented data in the full‐text articles only as graphs, and information provided in the conference abstracts was insufficient for inclusion in our analysis. Correspondence with trial investigators yielded no additional data. VISION 2004 reported that treatment with pegaptanib was associated with better scores on the NEI‐VFQ questionnaire, specifically for distance vision and role limitation domains. However, investigators did not report standard deviations for scores; thus we could not include these data in our meta‐analysis.
At one year, overall vision‐related quality of life improved more often among participants in ranibizumab groups than among those in control groups (MD 6.7, 95% CI 3.4 to 10.0). The mean difference was greater in MARINA 2006 (MD 8.2, 95% CI 6.0 to 10.4) than in ANCHOR 2006 (MD 4.8, 95% CI 1.7 to 7.9). This difference between the two trials may have occurred because participants in the control group in ANCHOR 2006 received an active treatment (verteporfin PDT therapy). Three subscale domains of the NEI‐VFQ questionnaire in which participants in ranibizumab groups showed approximately a 10‐point greater improvement at one‐year follow‐up compared with participants in control groups were distance vision activities, vision‐related dependency, and driving ability (Analysis 1.17). The I² statistic for subscale analyses ranged from 0 to 91%, possibly due to different effects among control groups. We graded the certainty of evidence for quality of life outcomes at one year as moderate, after downgrading for imprecision (‐1).
1.17. Analysis.
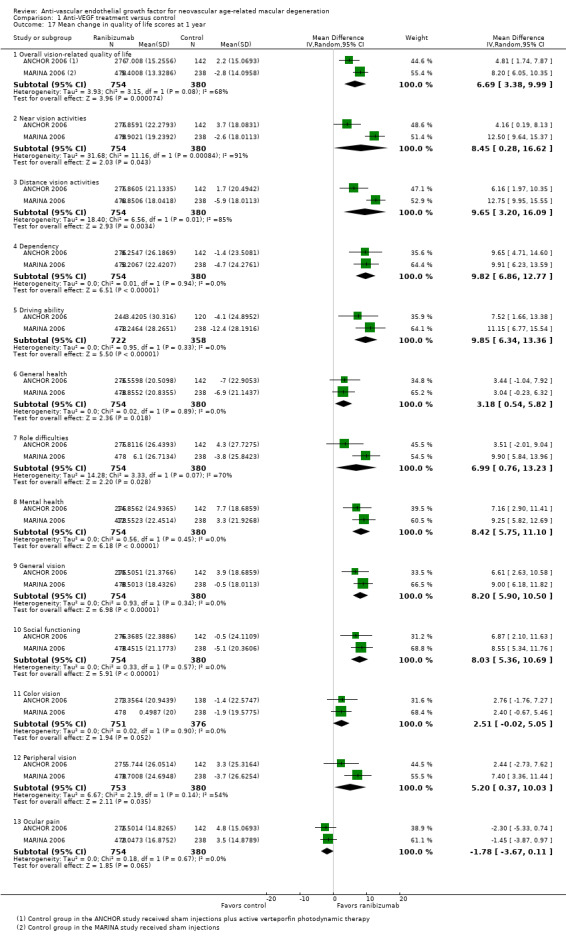
Comparison 1 Anti‐VEGF treatment versus control, Outcome 17 Mean change in quality of life scores at 1 year.
At two years, overall vision‐related quality of life improved more often among participants in ranibizumab groups than among those in control groups (MD 8.6, 95% CI 3.3 to 14.0). Similar to one‐year results, the mean difference was greater in MARINA 2006 (MD 11.2, 95% CI 8.8 to 13.5) than in ANCHOR 2006 (MD 5.7, 95% CI 2.0 to 9.4). Subscale domains of the NEI‐VFQ questionnaire in which participants in ranibizumab groups showed greater improvement at two‐year follow‐up compared with those in control groups were consistent with those identified at one year (Analysis 1.18). The I² statistic for subscale analyses ranged from 0 to 87%, reflecting greater comparative differences between treatment and sham control groups in MARINA 2006 than between treatment and active control groups in ANCHOR 2006. For six subscales, mean differences differed by approximately 10 or more points between ranibizumab and control groups: near vision activities, distance vision activities, vision‐related dependency, driving ability, mental health, and general vision (Analysis 1.18). We graded the certainty of evidence for quality of life outcomes at two years as moderate, after downgrading for imprecision (‐1).
1.18. Analysis.
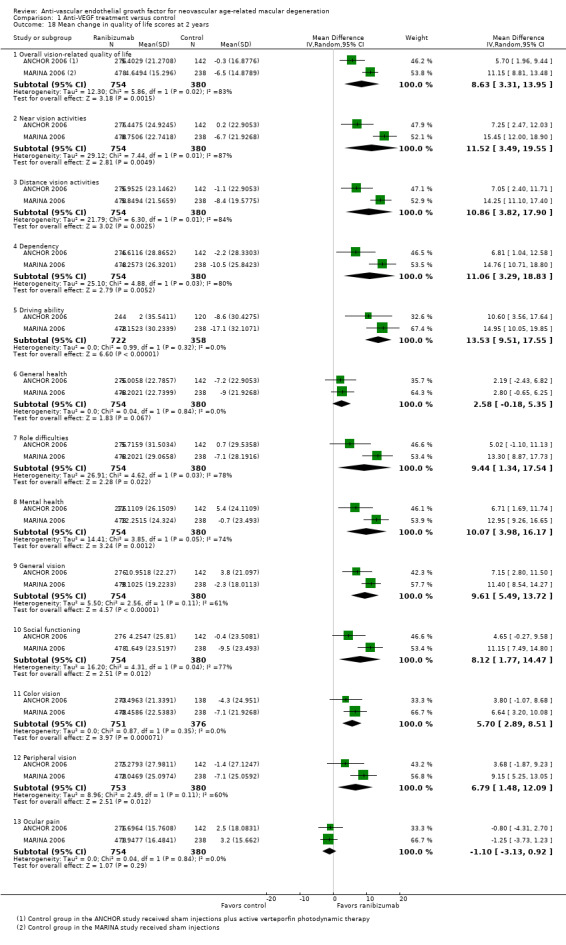
Comparison 1 Anti‐VEGF treatment versus control, Outcome 18 Mean change in quality of life scores at 2 years.
ABC 2010 and Sacu 2009 did not assess quality of life outcomes.
(5) Economic outcomes
We found no report of economic outcomes from ABC 2010, Sacu 2009, or VISION 2004. We found no data on economic outcomes when ANCHOR 2006, MARINA 2006, and PIER 2008 directly compared ranibizumab with controls. Data from MARINA 2006 yielded estimates of the cost of treatment with ranibizumab at USD 27,004 for the first year and USD 26,417 for the second year; investigators did not report data for the control group (Brown 2008).
(6) Adverse events
We have reported adverse events separately by type of anti‐VEGF agent because investigators of different trials reported different types of adverse events.
(a) Pegaptanib versus control
VISION 2004 reported ocular and systemic adverse events. Participants in the pegaptanib groups experienced an ocular adverse event nearly four times as often (RR 3.84, 95% CI 0.91 to 16.20) and were estimated to be 1.25 times as likely to have a serious systemic adverse event (RR 1.25, 95% CI 0.93 to 1.70) as participants in the control group. We have presented results for the most frequent adverse events in Table 5. Although uncommon, 12 eyes treated with pegaptanib injections for one year developed endophthalmitis compared with no cases in control eyes. Because of the small number of events, risk estimates for individual adverse events are imprecise. We graded the certainty of evidence for ocular and systemic adverse events as moderate, after downgrading for imprecision (‐1).
3. Adverse events up to 1 year: pegaptanib versus control.
| Ocular adverse eventa | 0.3 mg pegaptanib n = 295 | 1.0 mg pegaptanib n = 301 | 3.0 mg pegaptanib n = 296 | All doses pegaptanib n = 892 | Control n = 298 | RR [95% CI] All doses vs control |
| Any eye disorder | 9 (3%) | 4 (1%) | 10 (3%) | 23 (3%) | 2 (< 1%) | 3.84 [0.91 to 16.20] |
| Endophthalmitis | 6 (2%) | 3 (1%) | 3 (1%) | 12 (1%) | 0 | 8.37 [0.50 to 140.95] |
| Retinal detachment | 1 (< 1%) | 2 (< 1%) | 2 (< 1%) | 5 (< 1%) | 0 | 3.68 [0.20 to 66.41] |
| Traumatic cataract | 1 (< 1%) | 2 (< 1%) | 2 (< 1%) | 5 (< 1%) | 0 | 3.68 [0.20 to 66.41] |
| Retinal hemorrhage | 1 (< 1%) | 0 | 1 (< 1%) | 2 (< 1%) | 0 | 1.67 [0.08 to 34.77] |
| Vitreous hemorrhage | 0 | 0 | 1 (< 1%) | 1 (< 1%) | 0 | 1.00 [0.04 to 24.59] |
| Uveitis | 0 | 0 | 1 (< 1%) | 1 (< 1%) | 0 | 1.00 [0.04 to 24.59] |
| Elevated intraocular pressure | 1 (< 1%) | 0 | 0 | 1 (< 1%) | 0 | 1.00 [0.04 to 24.59] |
| Papilledema | 0 | 0 | 0 | 0 | 1 (< 1%) | 0.11 [0.00 to 2.73] |
| Non‐ocular adverse eventa | 0.3 mg pegaptanib n = 295 | 1.0 mg pegaptanib n = 301 | 3.0 mg pegaptanib n = 296 | All doses pegaptanib n = 892 | Control n = 298 | RR [95% CI] All doses vs control |
| At least 1 serious adverse event | 55 (19%) | 50 (17%) | 64 (22%) | 169 (19%) | 45 (15%) | 1.25 [0.93 to 1.70] |
| Cardiac disorders | 11 (4%) | 4 (1%) | 10 (3%) | 25 (3%) | 14 (5%) | 0.60 [0.31 to 1.13] |
| Neoplasms (benign, malignant, unspecified) | 11 (4%) | 7 (2%) | 8 (3%) | 26 (3%) | 12 (4%) | 0.72 [0.37 to 1.42] |
| Injury and procedural complications, such as fractures (also includes traumatic cataracts) | 10 (3%) | 9 (3%) | 8 (3%) | 27 (3%) | 3 (1%) | 3.01 [0.92 to 9.84] |
| Nervous system disorders | 10 (3%) | 5 (2%) | 10 (3%) | 25 (3%) | 7 (2%) | 1.19 [0.52 to 2.73] |
| Infections and infestations | 2 (<1%) | 7 (2%) | 11 (4%) | 20 (2%) | 5 (2%) | 1.34 [0.51 to 3.53] |
| Gastrointestinal disorders | 3 (1%) | 6 (2%) | 5 (2%) | 14 (2%) | 4 (1%) | 1.17 [0.39 to 3.52] |
| Respiratory, thoracic, mediastinal disorders | 2 (< 1%) | 5 (2%) | 5 (2%) | 12 (1%) | 4 (1%) | 1.00 [0.33 to 3.08] |
| Musculoskeletal and connective tissue | 1 (< 1%) | 5 (2%) | 3 (1%) | 9 (1%) | 2 (<1%) | 1.50 [0.33 to 6.92] |
| Renal and urinary disorders | 2 (< 1%) | 3 (1%) | 2 (<1%) | 7 (<1%) | 3 (1%) | 0.78 [0.20 to 3.00] |
| Vascular disorders | 3 (1%) | 2 (< 1%) | 2 (< 1%) | 7 (< 1%) | 3 (1%) | 0.78 [0.20 to 3.00] |
CI: confidence interval.
RR: risk ratio.
aMost frequent serious adverse events experienced by 1190 participants in the VISION 2004 study.
(b) Ranibizumab versus control
Of the three ranibizumab trials (ANCHOR 2006; MARINA 2006; PIER 2008), ANCHOR 2006 and PIER 2008 reported ocular and systemic adverse events at one‐year follow‐up (Table 6), and all three studies at two‐year follow up (Table 7). At both one‐year and two‐year follow‐up, small numbers of participants had experienced ocular adverse events, such as endophthalmitis, uveitis, retinal detachment, and retinal or vitreous hemorrhage, and non‐ocular adverse events, such as myocardial infarction, stroke or cerebral infarction, ischemic cardiomyopathy, and death (< 1% of total participants). Because of the small number of events, risk estimates for these adverse events are imprecise.
4. Adverse events up to 1 year: ranibizumab versus control.
| Ocular adverse eventa | 0.3 mg ranibizumab n = 196 | 0.5 mg ranibizumab n = 201 | All doses ranibizumab n = 397 | Control n = 206 | RR [95% CI] All doses vs control |
| Endophthalmitis | 0 | 2 (< 1%) | 2 (< 1%) | 0 | 2.60 [0.13 to 53.92] |
| Retinal detachment | 1 (< 1%) | 0 | 1 (< 1%) | 1 (< 1%) | 0.52 [0.03 to 8.25] |
| Traumatic cataract | 18 (9%) | 22 (11%) | 40 (10%) | 14 (7%) | 1.48 [0.83 to 2.66] |
| Retinal hemorrhage | 2 (1%) | 0 | 2 (< 1%) | 2 (< 1%) | 0.52 [0.07 to 3.66] |
| Vitreous hemorrhage | 1 (< 1%) | 0 | 1 (< 1%) | 0 | 1.56 [0.06 to 38.13] |
| Uveitis | 0 | 1 (< 1%) | 1 (< 1%) | 0 | 1.56 [0.06 to 38.13] |
| Elevated intraocular pressure (≥ 30 mmHg increase) | 13 (7%) | 17 (8%) | 30 (8%) | 7 (3%) | 2.22 [0.99 to 4.98] |
| Ocular inflammation (trace to 4+) | 21 (11%) | 26 (13%) | 47 (12%) | 9 (4%) | 2.71 [1.36 to 5.42] |
| Non‐ocular adverse eventa | 0.3 mg ranibizumab n = 196 | 0.5 mg ranibizumab n = 201 | All doses ranibizumab n = 397 | Control n = 206 | RR [95% CI] All doses vs control |
| Death | 3 (2%) | 2 (<1%) | 5 (1%) | 2 (< 1%) | 1.30 [0.25 to 6.63] |
| Myocardial infarction | 1 (< 1%) | 3 (1%) | 4 (1%) | 1 (< 1%) | 2.08 [0.23 to 18.45] |
| Stroke or cerebral infarction | 1 (< 1%) | 1 (< 1%) | 2 (< 1%) | 1 (< 1%) | 1.04 [0.09 to 11.38] |
| Ischemic cardiomyopathy | 0 | 0 | 0 | 1 (< 1%) | 0.17 [0.01 to 4.24] |
| Treatment‐emergent hypertension | 7 (4%) | 15 (7%) | 22 (6%) | 17 (8%) | 0.67 [0.36 to 1.24] |
| Non‐ocular hemorrhage | 9 (5%) | 13 (6%) | 22 (6%) | 6 (3%) | 1.90 [0.78 to 4.62] |
CI: confidence interval.
RR: risk ratio.
aAdverse events experienced by 420 participants in ANCHOR 2006 and by 183 participants in PIER 2008. Adverse events at 1‐year follow‐up not reported in MARINA 2006.
5. Adverse events up to 2 years: ranibizumab versus control.
| Ocular adverse eventa | 0.3 mg ranibizumab n = 434 | 0.5 mg ranibizumab n = 440 | All doses ranibizumab n = 874 | Control n = 441 | RR [95% CI] All doses vs control |
| Endophthalmitis | 2 (< 1%) | 6 (1%) | 8 (< 1%) | 0 | 8.59 [0.50 to 148.44] |
| Retinal detachment | 2 (< 1%) | 0 | 2 (< 1%) | 2 (< 1%) | 0.50 [0.07 to 3.57] |
| Traumatic cataract | 65 (15%) | 76 (17%) | 141 (16%) | 57 (13%) | 1.25 [0.94 to 1.66] |
| Retinal hemorrhage | 1 (< 1%) | 0 | 1 (< 1%) | 1 (< 1%) | 0.50 [0.03 to 8.05] |
| Vitreous hemorrhage | 3 (< 1%) | 1 (< 1%) | 4 (< 1%) | 2 (< 1%) | 1.01 [0.19 to 5.49] |
| Uveitis | 3 (< 1%) | 4 (< 1%) | 7 (<1%) | 0 | 7.58 [0.43 to 132.36] |
| Elevated intraocular pressure (≥ 30 mmHg increase)b | 45 (15%) | 61 (20%) | 106 (18%) | 11 (4%) | 4.81 [2.63 to 8.81] |
| Ocular inflammation (1+ to 4+) | 32 (7%) | 30 (7%) | 62 (7%) | 8 (2%) | 3.91 [1.89 to 8.09] |
| Non‐ocular adverse eventa | 0.3 mg ranibizumab n = 434 | 0.5 mg ranibizumab n = 440 | All doses ranibizumab n = 874 | Control n = 441 | RR [95% CI] All doses vs control |
| Death | 12 (3%) | 9 (2%) | 21 (2%) | 13 (3%) | 0.82 [0.41 to 1.61] |
| Myocardial infarction | 7 (2%) | 8 (2%) | 15 (2%) | 7 (2%) | 1.08 [0.44 to 2.63] |
| Stroke or cerebral infarction | 6 (1%) | 6 (1%) | 12 (1%) | 5 (1%) | 1.21 [0.43 to 3.42] |
| Ischemic cardiomyopathy | 0 | 0 | 0 | 1 (< 1%) | 0.17 [0.01 to 4.12] |
| Treatment‐emergent hypertension | 60 (14%) | 69 (16%) | 129 (15%) | 68 (15%) | 0.96 [0.73 to 1.25] |
| Non‐ocular hemorrhage | 38 (9%) | 40 (9%) | 78 (9%) | 24 (5%) | 1.64 [1.05 to 2.55] |
CI: confidence interval.
RR: risk ratio.
aAdverse events experienced by 420 participants in ANCHOR 2006; 713 participants in MARINA 2006; and 182 participants in PIER 2008. bAdverse events for elevated intraocular pressure not reported in ANCHOR 2006 at 2‐year follow‐up (n = 297 in 0.3 mg ranibizumab group, n = 300 in 0.5 mg ranibizumab group, and n = 298 in 0.3 mg control group).
With respect to ocular adverse events, cataract developed in study eyes treated with ranibizumab more often than in eyes treated with control at both one‐year (RR 1.48, 95% CI 0.83 to 2.66) and two‐year follow‐up (RR 1.25, 95% CI 0.94 to 1.66). Elevated intraocular pressure (IOP), defined as an increase of 30 mmHg or more, was observed more often in eyes in the ranibizumab groups than in eyes in the control groups by both one‐year (RR 2.22, 95% CI 0.99 to 4.98) and two‐year follow‐up (RR 4.81, 95% CI 2.63 to 8.81). Ocular inflammation, graded from trace (1+) to 4+, also occurred more often in eyes in the ranibizumab groups than in eyes in the control groups at both one‐year (RR 2.71, 95% CI 1.36 to 5.42) and two‐year follow‐up (RR 3.91, 95% CI 1.89 to 8.09). Two eyes during the first year of ranibizumab injections and six more eyes during the second year developed endophthalmitis compared with no cases in control eyes. We graded the certainty of evidence for ocular adverse events as moderate, after downgrading, because not all eligible trials reported all types of adverse events and because numbers were small (< 1%) for many specific adverse events (‐1).
With respect to non‐ocular adverse events, participants in ranibizumab groups less often experienced treatment‐emergent hypertension than participants in control groups at one‐year follow‐up (RR 0.67, 95% CI 0.36 to 1.24); however, at two‐year follow‐up, risk was similar in the ranibizumab and control groups (RR 0.96, 95% CI 0.73 to 1.25). Non‐ocular hemorrhage occurred more often among participants in ranibizumab groups than among those in control groups at both one‐year (RR 1.90, 95% CI 0.78 to 4.62) and two‐year follow‐up (RR 1.64, 95% CI 1.05 to 2.55). We graded the certainty of evidence for systemic adverse events as moderate, after downgrading, because not all eligible trials reported all types of adverse events and because numbers were small (< 1%) for many specific adverse events (‐1).
(c) Bevacizumab versus control
ABC 2010 reported serious ocular and non‐ocular adverse events among 65 bevacizumab‐treated participants and 66 control participants. Serious ocular events that affected at least one study participant included uveitis (two bevacizumab participants; one control participant), rhegmatogenous retinal detachment (no bevacizumab participant; one control participant), vitreous hemorrhage (one bevacizumab participant; no control participant), and ocular inflammation (eight bevacizumab participants; four control participants). Investigators reported no instances of presumed endophthalmitis, retinal tear, or lens damage in either group. Three participants experienced a non‐ocular adverse event: myocardial infarction (bevacizumab group), death due to vascular cause (bevacizumab group), or non‐ocular hemorrhage reported as serious (control group).
Sacu 2009 reported no occurrence of severe ocular or systemic events during the study period. We graded the certainty of evidence for ocular and systemic adverse events for bevacizumab as low; we downgraded for imprecision (‐2).
Bevacizumab versus ranibizumab
Ten trials directly compared intravitreous injections of bevacizumab with ranibizumab. These studies included a total of 3657 participants. The largest study randomized 1208 participants in a 2 × 2 factorial design (two drugs administered in two injection schedules) to receive 1.25 mg intravitreal bevacizumab or 0.5 mg intravitreal ranibizumab on a monthly or as‐needed basis (CATT 2011). Participants in the as‐needed injection groups received the first three injections monthly, followed by an injection whenever treatment was needed, as determined by monthly examinations. After one year of treatment, participants in the groups treated monthly were re‐randomized to continue treatment on a monthly basis or to change to treatment as needed. Participants in the as‐needed groups remained on their original assignments, and all participants were followed for another year. IVAN 2013, with 628 participants, included four treatment groups similar to those in the CATT study: 1.25 mg intravitreously injected bevacizumab monthly, 0.5 mg intravitreously injected ranibizumab monthly, 1.25 mg intravitreously injected bevacizumab as needed, and 0.5 mg intravitreously injected ranibizumab as needed. Participants in as‐needed dosing groups received the first three injections monthly, followed by three consecutive monthly treatments whenever treatment was needed. The treatment period lasted two years. Six of the eight smaller studies randomized participants to receive 1.25 mg intravitreal bevacizumab or 0.5 mg intravitreal ranibizumab on a monthly basis (BRAMD 2016), or on an as‐needed basis (GEFAL 2013; LUCAS 2015; MANTA 2013; Subramanian 2010), for one year or 18 months (Biswas 2011). We combined 18‐month data from Biswas 2011 with 12‐month data from the other trials. Two small studies randomized participants to an as‐needed treatment regimen following two and three injections, respectively, given at monthly intervals (SAVE‐AMD 2017; Scholler 2014).
For data analyses in this section, we combined groups given the same drug type regardless of injection regimen. Thus the bevacizumab and ranibizumab groups included both monthly and as‐needed injection schedules. Risk of bias in most domains was low among these studies, and none were funded by pharmaceutical companies. At one‐year follow‐up, investigators provided data for the primary outcome for 3164 (88%) of 3657 participants.
(1) Visual acuity
(a) Gain of 15 or more letters of visual acuity
At one‐year follow‐up, investigators provided analyzable data for the primary outcome of this review for 3144 (86%) of 3657 participants. Overall, the proportion of participants who gained 15 or more letters of visual acuity at one year was not statistically significantly different between bevacizumab‐ and ranibizumab‐treated groups (RR 0.95, 95% CI 0.81 to 1.12; Analysis 2.1; Figure 5). Individual confidence intervals for seven of the eight trials crossed unity, and all confidence intervals overlapped. We graded the certainty of evidence for this outcome as high, with no reason to downgrade.
2.1. Analysis.
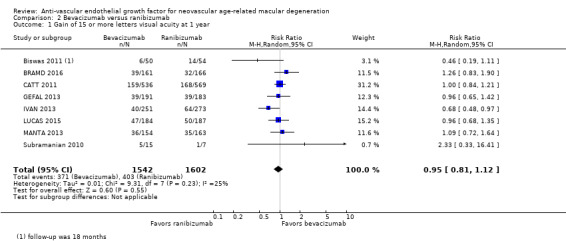
Comparison 2 Bevacizumab versus ranibizumab, Outcome 1 Gain of 15 or more letters visual acuity at 1 year.
5.
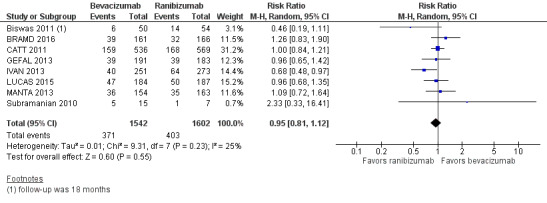
Forest plot of comparison: 4 Bevacizumab versus ranibizumab, outcome: 4.1 Gain of 15 or more letters visual acuity at 1 year.
At two years, data were available for 1030 (85%) of 1208 participants in CATT 2011, and for 517 (82%) of 628 participants in IVAN 2013. Results were consistent with one‐year outcomes in terms of the effect estimate and confidence intervals when we compared the proportion of participants who gained 15 or more letters of visual acuity between ranibizumab‐ and bevacizumab‐treated groups (RR 0.84, 95% CI 0.64 to 1.11; Analysis 2.2; Figure 6). When we analyzed only the 778 participants who remained in their originally randomized groups in CATT 2011 (i.e. excluding participants who were switched to a different treatment regimen after one year), summary results were unchanged (RR 0.84, 95% CI 0.64 to 1.11). The I² statistics for these analyses were at or about 50%, indicating a difference in magnitude but not in direction of the treatment effect estimated based on data from CATT 2011 and IVAN 2013. We graded the certainty of evidence for this outcome as high, finding no reason to downgrade.
2.2. Analysis.
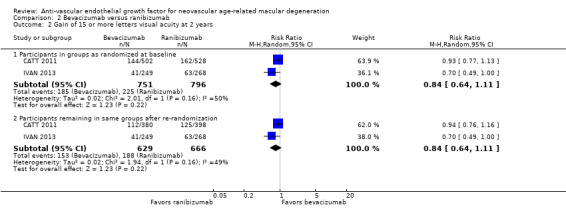
Comparison 2 Bevacizumab versus ranibizumab, Outcome 2 Gain of 15 or more letters visual acuity at 2 years.
6.

Forest plot of comparison: 4 Bevacizumab versus ranibizumab, outcome: 4.2 Gain of 15 or more letters visual acuity at 2 years.
(b) Loss of fewer than 15 letters of visual acuity
At one‐year follow‐up, the overall estimated effect (risk ratio) for loss of fewer than 15 letters of visual acuity was 1.00 (95% CI 0.98 to 1.02) when we compared participants treated with bevacizumab and those treated with ranibizumab (Analysis 2.3). Confidence intervals for all eight individual studies also crossed the line of equality. These results suggest no meaningful clinical or statistical difference between the two anti‐VEGF agents in terms of loss of fewer than 15 letters of visual acuity after one year of treatment. We graded the certainty of evidence for this outcome as high, with no reason to downgrade.
2.3. Analysis.
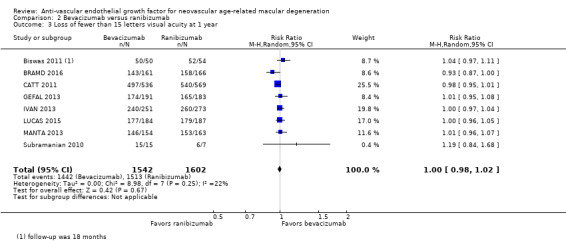
Comparison 2 Bevacizumab versus ranibizumab, Outcome 3 Loss of fewer than 15 letters visual acuity at 1 year.
At two‐year follow‐up, the relative treatment effect between the two drugs was almost identical to the relative effect at one year when researchers analyzed participants on the basis of their original randomization (RR 0.97, 95% CI 0.94 to 1.00) or those who remained in their originally randomized groups (RR 0.98, 95% CI 0.94 to 1.01; Analysis 2.4). We also graded the certainty of evidence for this outcome as high.
2.4. Analysis.
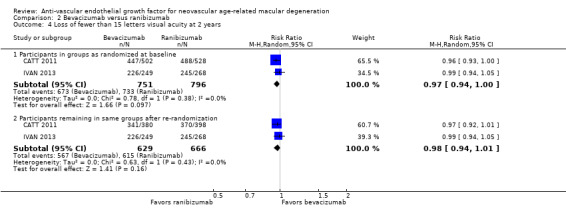
Comparison 2 Bevacizumab versus ranibizumab, Outcome 4 Loss of fewer than 15 letters visual acuity at 2 years.
(c) Loss of fewer than 30 letters of visual acuity
No participant in Subramanian 2010 lost 30 or more letters of visual acuity during the one‐year study period. In Scholler 2014, one participant in each group was reported to have lost fewer than 30 letters of visual acuity (RR 1.3, 95% CI 0.09 to 19.53). The other trials did not report this outcome, likely because of the low risk of losing 15 or more letters of BCVA..
(d) Prevention of blindness in the study eye (visual acuity better than 20/200)
Four trials reported the proportion of participants with visual acuity better than 20/200 as an outcome (CATT 2011; GEFAL 2013; IVAN 2013; Subramanian 2010), but six trials did not (Biswas 2011; BRAMD 2016; LUCAS 2015; MANTA 2013; SAVE‐AMD 2017; Scholler 2014).
At one‐year follow‐up, the proportion of participants with visual acuity of the study eye better than 20/200 was neither clinically nor statistically significantly different when we compared participants treated with bevacizumab and those treated with ranibizumab (RR 0.98, 95% CI 0.96 to 1.01; Analysis 2.5). Results showed no statistically significant heterogeneity among studies (I² = 0%), and all confidence intervals for these four individual studies crossed the line of unity. We graded the certainty of evidence for this outcome as high, finding no reason to downgrade.
2.5. Analysis.
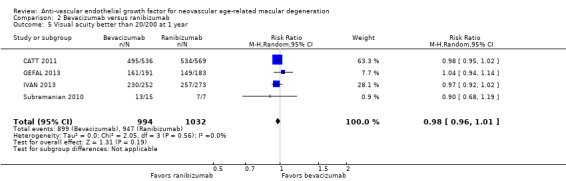
Comparison 2 Bevacizumab versus ranibizumab, Outcome 5 Visual acuity better than 20/200 at 1 year.
At two years, results were consistent with one‐year outcomes, in that CATT 2011 and IVAN 2013 investigators observed no significant difference in the proportion of participants with visual acuity of the study eye better than 20/200 between bevacizumab‐ and ranibizumab‐treated groups (RR 1.00, 95% CI 0.95 to 1.06; Analysis 2.6). When we analyzed only the 778 participants in CATT 2011 who remained in their originally randomized groups, we found similar results (RR 1.01, 95% CI 0.95 to 1.06). Evidence shows moderate statistical heterogeneity between CATT 2011 and IVAN 2013 for these analyses (I² > 40%), possibly due to the precision of individual study effect estimates. We graded certainty of evidence for this outcome as high, finding no reason to downgrade.
2.6. Analysis.
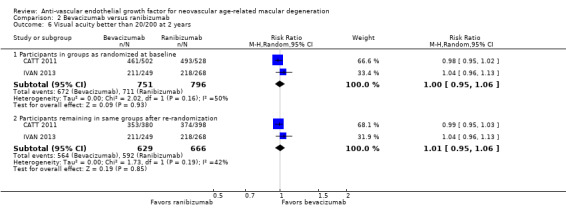
Comparison 2 Bevacizumab versus ranibizumab, Outcome 6 Visual acuity better than 20/200 at 2 years.
(e) Maintenance of visual acuity
BRAMD 2016, CATT 2011, GEFAL 2013, IVAN 2013, LUCAS 2015, MANTA 2013, SAVE‐AMD 2017, and Scholler 2014 did not report maintenance of visual acuity. In Subramanian 2010, 10 of 15 (67%) participants maintained baseline visual acuity after one year of treatment with bevacizumab and 6 of 7 (86%) participants maintained baseline visual acuity after one year of treatment with ranibizumab (RR 0.78, 95% CI 0.49 to 1.24). We graded the certainty of evidence for this outcome as moderate, after downgrading for imprecision (‐1).
Biswas 2011 reported different cut‐points for change in visual acuity at 18‐month follow‐up. In the bevacizumab group, 16 (32%) participants gained more than five letters, 30 (60%) did not change more than five letters, and four (8%) lost more than five letters of visual acuity. In the ranibizumab group, 18 (33%) participants gained more than five letters, 30 (56%) did not change more than five letters, and six (11%) lost more than five letters of visual acuity.
(f) Mean change in visual acuity
At one year, the mean difference in mean change in visual acuity between bevacizumab and ranibizumab groups was less than one ETDRS letter (MD ‐0.5, 95% CI ‐1.5 to 0.4; Analysis 2.7). Confidence intervals for all nine individual studies crossed the line of no difference. The I² statistic was 0%. The SAVE‐AMD 2017 investigators reported only that they found no difference in mean BCVA between eyes treated with bevacizumab or ranibusumab as combined improvement of 4.1 lines (20 letters).
2.7. Analysis.
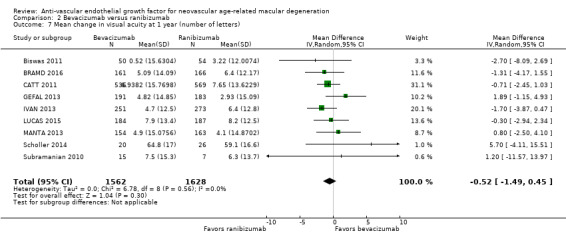
Comparison 2 Bevacizumab versus ranibizumab, Outcome 7 Mean change in visual acuity at 1 year (number of letters).
IVAN 2013 reported data for mean change from baseline in visual acuity at two years; CATT 2011 reported data for only the 778 participants who remained in their originally randomized groups. The mean difference between bevacizumab and ranibizumab groups was less than two ETDRS letters (MD ‐1.2, 95% CI ‐2.8 to 0.5; Analysis 2.8). We graded the certainty of evidence for this outcome as high, with no reason to downgrade.
2.8. Analysis.
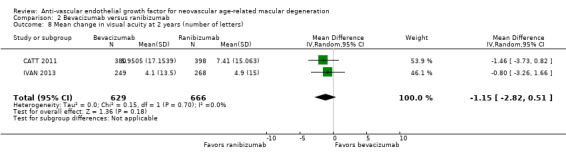
Comparison 2 Bevacizumab versus ranibizumab, Outcome 8 Mean change in visual acuity at 2 years (number of letters).
(2) Visual function
Only one of the ten trials that compared bevacizumab with ranibizumab reported visual function outcomes (IVAN 2013). At one year, participants in the ranibizumab and bevacizumab groups were comparable with regard to mean letters of contrast sensitivity (adjusted MD 0.2 letters, that is, less than one 3‐letter segment on the Pelli‐Robson chart, 95% CI ‐0.5 to 0.9) and reading index (MD ‐5.53, 95% CI ‐14.59 to 3.54). Participants in the ranibizumab group had slightly better (8%) near LogMAR visual acuity than those in the bevacizumab group (adjusted geometric mean ratio 0.92, 95% CI 0.84 to 1.00; P = 0.058).
At two‐year follow‐up, results for visual function outcomes were similar to those obtained at one year. Participants in the ranibizumab and bevacizumab groups were comparable with regard to mean letters of contrast sensitivity (adjusted MD 0.21, 95% CI ‐0.62 to 1.04) and reading index (MD ‐1.34, 95% CI ‐8.29 to 5.61). Participants in the ranibizumab group had slightly better (6%) near LogMAR visual acuity than those in the bevacizumab group (adjusted geometric mean ratio 0.94, 95% CI 0.85 to 1.04). We graded the certainty of evidence for these outcomes at one and two years as high, finding no reason to downgrade.
(3) Morphologic outcomes
(a) Mean change in size of CNV
One study reported mean change in size of CNV from baseline (GEFAL 2013). At one year, results showed no differences between bevacizumab (156 participants) and ranibizumab (144 participants) groups (MD 0.00 DAs, 95% CI ‐0.32 to 0.32). We graded the certainty of evidence for this outcome as high, upon finding no reason to downgrade.
(b) Mean change in size of lesion
Two of the eight studies reported the outcome of change in total lesion size. We considered a difference of one or more DAs as a clinically meaningful difference.
In CATT 2011, the mean change in lesion size was similar in the bevacizumab (479 participants) and ranibizumab (509 participants) groups at one year (MD 0.20 optic DAs, 95% CI ‐0.09 to 0.49). Among the 778 participants who remained in their originally randomized groups through two years, participants in the bevacizumab groups (341 participants) showed larger increases in lesion size compared with those in the ranibizumab groups (360 participants) (MD 1.37 mm², 95% CI 0.39 to 2.36).
In IVAN 2013, the median change in lesion size after one year of treatment was similar in the bevacizumab (median ‐1.79 DAs, IQR ‐5.18 to 0.00) and ranibizumab (median ‐1.92 DAs, IQR ‐4.81 to ‐0.01) groups. After two years, the median change in lesion size was ‐1.86 DAs (IQR ‐5.51 to 0.16) in the bevacizumab group and ‐0.96 DAs (IQR ‐4.29 to 0.39) in the ranibizumab group.
Biswas 2011, BRAMD 2016, GEFAL 2013, LUCAS 2015, MANTA 2013, and Subramanian 2010 did not report mean change in lesion size in DAs. MANTA 2013 reported that "no significant difference was observed in terms of lesion size between the two groups (P = 0.55)." We graded the certainty of evidence for mean change in lesion size as moderate, upon downgrading for lack of analyzable data from two trials reporting this outcome (‐1).
(c) Mean change in CRT
Nine of the ten trials reported on mean change in CRT at one year. Participants treated with bevacizumab showed less reduction in CRT compared with those treated with ranibizumab in six trials (MD ‐11.6 μm, 95% CI ‐21.6 to ‐1.7; Analysis 2.9). This difference is not considered to be clinically meaningful, as it falls within the typical range of measurement error. Subramanian 2010 reported a mean change of ‐50 μm in the bevacizumab group and ‐91 μm in the ranibizumab group at one year. Neither MANTA 2013 nor SAVE‐AMD 2017 nor Scholler 2014 investigators reported mean change in CRT by treatment group. MANTA 2013 investigators stated that "differences were not significant between the groups (P = 0.81)." SAVE‐AMD 2017 investigators reported for the combined treatment groups that CRT changed from 401.5 μm at baseline to 310 μm after one year, and that "no significant difference was observed between both [sic] drugs." Similarly, Scholler 2014 reported that "The mean CRT did not differ significantly between groups (p=0.088 after 12 months)."
2.9. Analysis.
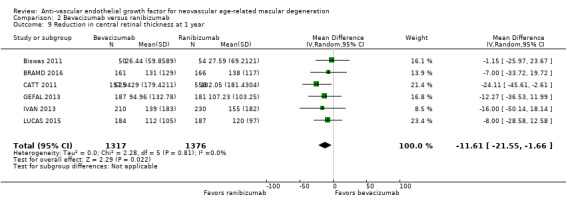
Comparison 2 Bevacizumab versus ranibizumab, Outcome 9 Reduction in central retinal thickness at 1 year.
At two years, the finding of no or a trivial difference persisted only among study participants who remained in their originally randomized groups in CATT 2011 and IVAN 2013. Participants in the bevacizumab groups showed only slightly less reduction in CRT compared with those in the ranibizumab groups (MD ‐12.4 μm, 95% CI ‐33.8 to ‐9.0; Analysis 2.10). We graded the certainty of evidence for this outcome at both one and two years as high, with no reason to downgrade.
2.10. Analysis.
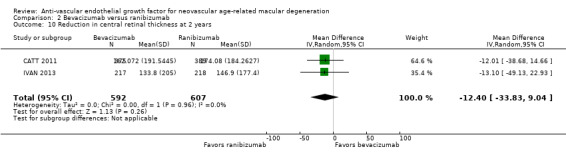
Comparison 2 Bevacizumab versus ranibizumab, Outcome 10 Reduction in central retinal thickness at 2 years.
(4) Quality of life outcomes
IVAN 2013 evaluated quality of life among trial participants using the EQ‐5D.
At one‐year follow‐up, the median (IQR) EQ‐5D summary score was the same for bevacizumab‐ and ranibizumab‐treated groups (median 0.85, IQR 0.73 to 1.00). The number of participants who reported "no health problems" for each of the five subscale domains was similar between groups (Analysis 2.11).
2.11. Analysis.

Comparison 2 Bevacizumab versus ranibizumab, Outcome 11 No problems in quality of life domain at 1 year.
At two‐year follow‐up, the median (IQR) EQ‐5D summary score was the same as at one‐year follow‐up (median 0.85, IQR 0.73 to 1.00) in bevacizumab‐ and ranibizumab‐treated groups. The number of participants who reported "no health problems" for each of the five subscale domains was similar in the two groups (Analysis 2.12).
2.12. Analysis.
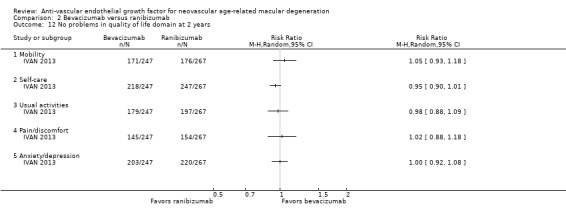
Comparison 2 Bevacizumab versus ranibizumab, Outcome 12 No problems in quality of life domain at 2 years.
The remaining nine studies did not report quality of life outcomes (Biswas 2011; BRAMD 2016; CATT 2011; GEFAL 2013; LUCAS 2015; MANTA 2013; SAVE‐AMD 2017; Scholler 2014; Subramanian 2010). We graded the certainty of evidence for quality of life as moderate, after downgrading for absence of data from other trials (‐1).
(5) Economic outcomes
Three studies included economic‐related outcomes as prespecified secondary outcomes. CATT 2011 evaluated annual costs associated with each treatment group in USD. IVAN 2013 evaluated cumulative resource use and costs for each treatment group in GBP. GEFAL 2013 prespecified medicoeconomic outcomes as secondary outcomes of interest; however, GEFAL 2013 investigators had published no results for economic outcomes by the time this review was prepared.
In the first year of treatment in CATT 2011, the average annual cost of treatment per participant was USD 490 in the bevacizumab groups (USD 595 when treated monthly and USD 385 when treated as needed) compared with USD 18,590 (USD 23,400 when treated monthly and USD 13,800 when treated as needed) in the ranibizumab groups. For the 778 participants who remained in their originally randomized groups, the average cost of two years of treatment was USD 860 per participant in the bevacizumab groups (USD 1170 when treated monthly and USD 705 when treated as needed) and USD 31,805 per participant in the ranibizumab groups (USD 44,800 when treated monthly and USD 25,200 when treated as needed).
In IVAN 2013, the average total cost of treatment per participant for the first year was GBP 1580 in the bevacizumab groups (GBP 1654 when treated monthly and GBP 1509 when treated as needed) compared with GBP 8035 in the ranibizumab groups (GBP 9656 when treated monthly and GBP 6398 when treated as needed). These values corresponded to approximately USD 2500 and USD 12,700 for the bevacizumab and ranibizumab groups, respectively (based on an average exchange rate of 1.58 for years 2010 to 2011). The mean difference was GBP 8001 (SE 113) when monthly treatment with ranibizumab versus bevacizumab was compared, and GBP 4889 (SE 184) when as‐needed treatment with ranibizumab versus bevacizumab was compared. IVAN 2013 reported economic outcomes at two‐year follow‐up.
(6) Adverse events
Although all ten trials provided information related to adverse events, data reported varied by study regarding the types and specificity of adverse events.
At one year, investigators from four trials reported no serious ocular events (Biswas 2011; MANTA 2013; SAVE‐AMD 2017; Subramanian 2010). Minor adverse events reported from three of these trials included subconjunctival hemorrhage, increased IOP, transient postinjection pain, and mild ocular inflammation; investigators did not report the numbers of participants who experienced these adverse events. No case of endophthalmitis or retinal detachment was reported from the four trials. In CATT 2011, GEFAL 2013, IVAN 2013, and LUCAS 2015, less than 1% of participants had endophthalmitis, retinal detachment, retinal pigment epithelial tear, traumatic cataract, or uveitis (Table 8). Scholler 2014 reported two eyes with subretinal bleeding, both treated with ranibizumab. BRAMD 2016 did not mention ocular adverse events. As a result of the small numbers of events, risk estimates for these adverse events are imprecise. We graded the certainty of evidence for ocular events as moderate, after downgrading for imprecision (‐1).
6. Adverse events up to 1 year: bevacizumab versus ranibizumab.
| Serious ocular adverse event | Studies reporting adverse events1 | Bevacizumab | Ranibizumab | RR [95% CI] Bevacizumab vs ranibizumab | ||
| Number with event | Total participants | Number with event | Total participants | |||
| Endophthalmitisa | CATT 2011; GEFAL 2013; LUCAS 2015 | 5 (< 1%) | 1052 | 3 (< 1%) | 1059 | 1.68 [0.40 to 7.00] |
| Retinal detachment | CATT 2011; GEFAL 2013 | 3 (< 1%) | 832 | 0 | 838 | 7.05 [0.36 to 136.28] |
| Retinal pigment epithelial tear | CATT 2011; IVAN 2013; LUCAS 2015 | 4 (< 1%) | 1102 | 3 (< 1%) | 1134 | 1.37 [0.31 to 6.12] |
| Traumatic cataract | CATT 2011; GEFAL 2013; IVAN 2013 | 1 (< 1%) | 1128 | 2 (< 1%) | 1152 | 0.51 [0.05 to 5.62] |
| Severe uveitis | CATT 2011; IVAN 2013 | 4 (< 1%) | 882 | 1 (< 1%) | 913 | 4.14 [0.46 to 36.97] |
| Non‐ocular adverse event | Studies reporting adverse events2 | Bevacizumab | Ranibizumab | RR [95% CI] Bevacizumab vs ranibizumab | ||
| Number with event | Total participants | Number with event | Total participants | |||
| At least 1 serious adverse event | BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 298 (18%) | 1663 | 265 (16%) | 1702 | 1.15 [0.99 to 1.34] |
| Death | BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 30 (2%) | 1663 | 28 (2%) | 1702 | 1.10 [0.66 to 1.83] |
| Myocardial infarction | CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 8 (< 1%) | 1502 | 16 (1%) | 1536 | 0.51 [0.22 to 1.19] |
| Stroke or cerebral infarction | CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 7 (< 1%) | 1502 | 11 (< 1%) | 1536 | 0.65 [0.25 to 1.67] |
| Transient ischemic attack | CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015 | 6 (< 1%) | 1348 | 4 (< 1%) | 1373 | 1.53 [0.43 to 5.40] |
| Venous thrombotic event | CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015 | 8 (< 1%) | 1348 | 4 (< 1%) | 1373 | 2.04 [0.61 to 6.75] |
| Cardiac disorders | BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 46 (3%) | 1663 | 56 (3%) | 1702 | 0.84 [0.57 to 1.23] |
| Gastrointestinal disorders | BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 31 (2%) | 1663 | 18 (1%) | 1702 | 1.76 [0.99 to 3.14] |
| Infections | BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 50 (3%) | 1663 | 36 (2%) | 1702 | 1.42 [0.93 to 2.17] |
| Injury and procedural complications | BRAMD 2016CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 36 (2%) | 1663 | 29 (2%) | 1702 | 1.27 [0.78 to 2.06] |
| Neoplasms (benign, malignant, unspecified) | BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 32 (2%) | 1663 | 33 (2%) | 1702 | 0.99 [0.61 to 1.61] |
| Nervous system disorders | BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 29 (2%) | 1663 | 26 (2%) | 1702 | 1.14 [0.68 to 1.93] |
| Surgical or medical procedure | BRAMD 2016CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013 | 40 (2%) | 1663 | 29 (2%) | 1702 | 1.41 [0.88 to 2.27] |
CI: confidence interval. RR: risk ratio.
aIncludes endophthalmitis and pseudo‐endophthalmitis.
1CATT 2011 (n = 586 in bevacizumab group; n = 599 in ranibizumab group); GEFAL 2013 (n = 246 in bevacizumab group; n = 239 in ranibizumab group); IVAN 2013 (n = 296 in bevacizumab group; n = 314 in ranibizumab group); LUCAS 2015 (n = 220 in bevacizumab group; n = 221 in ranibizumab group). 2BRAMD 2016 (n = 161 in bevacizumab group; n = 166 in ranibizumab group); CATT 2011 (n = 586 in bevacizumab group; n = 599 in ranibizumab group); GEFAL 2013 (n = 246 in bevacizumab group; n = 239 in ranibizumab group); IVAN 2013 (n = 296 in bevacizumab group; n = 314 in ranibizumab group); LUCAS 2015 (n = 220 in bevacizumab group; n = 221 in ranibizumab group); MANTA 2013 (n = 154 in bevacizumab group; n = 163 in ranibizumab group).
Biswas 2011 did not assess systemic adverse events. At one year, Subramanian 2010 reported no serious systemic adverse events. Scholler 2014 reported a transient ischemic attack in one patient but did not specify the treatment assignment for participants. SAVE‐AMD 2017 investigators reported three thromboembolitic events but did not report the anti‐VEGF agent given to those participants, nor whether cases were in the neovascular AMD or non‐neovascular AMD cohort. The remaining six trials reported that 18% of participants in the bevacizumab groups versus 16% in the ranibizumab groups experienced at least one serious adverse event (RR 1.15, 95% CI 0.99 to 1.34) (BRAMD 2016; CATT 2011; GEFAL 2013; IVAN 2013; LUCAS 2015; MANTA 2013). Mortality from any cause was approximately 2% in the bevacizumab and ranibizumab groups during the first year of follow‐up (RR 1.10, 95% CI 0.66 to 1.83). Less than 1% of participants experienced myocardial infarction, stroke or cerebral infarction, transient ischemic attack, or a venous thrombotic event (Table 8). Rates were comparable between bevacizumab and ranibizumab groups with respect to cardiac disorders (RR 0.84, 95% CI 0.57 to 1.23), neoplasms (RR 0.99, 95% CI 0.61 to 1.61), and nervous system disorders (RR 1.14, 95% CI 0.68 to 1.93). Investigators reported more gastrointestinal disorders (RR 1.76, 95% CI 0.99 to 3.14), infections (RR 1.42, 95% CI 0.93 to 2.17), injuries and procedural complications (RR 1.27, 95% CI 0.78 to 2.06), and surgical or medical procedures (RR 1.41, 95% CI 0.88 to 2.27) in the bevacizumab groups than in the ranibizumab groups at one year. We graded the certainty of evidence for systemic adverse events as moderate, after downgrading for imprecision (‐1).
At two years, data for ocular and systemic adverse events were available for CATT 2011 and IVAN 2013. Less than 1% of participants were reported to have had endophthalmitis, retinal detachment, retinal pigment epithelial tear, traumatic cataract, or uveitis (Table 9). As a result of the small numbers of events, risk estimates for these adverse events are imprecise. In the bevacizumab groups, 36% of participants had at least one serious adverse event compared with 30% in the ranibizumab groups (RR 1.20, 95% CI 1.05 to 1.37). Mortality from any cause was 6% and 5% in the bevacizumab and ranibizumab groups, respectively (RR 1.12, 95% CI 0.76 to 1.65). In all, 2% or fewer participants experienced myocardial infarction, stroke or cerebral infarction, a venous thrombotic event, or a transient ischemic attack (Table 9). As with one‐year outcomes, investigators reported more gastrointestinal disorders (RR 2.74, 95% CI 1.49 to 5.02), infections (RR 1.37, 95% CI 0.96 to 1.95), and injuries and procedural complications (RR 1.33, 95% CI 0.86 to 2.05) in the bevacizumab groups than in the ranibizumab groups, and reported more cardiac disorders in the bevacizumab groups than in the ranibizumab groups at two years (RR 1.25, 95% CI 0.92 to 1.71). Rates were comparable between bevacizumab and ranibizumab groups with respect to neoplasms (RR 0.98, 95% CI 0.63 to 1.53), nervous system disorders (RR 1.06, 95% CI 0.70 to 1.60), and surgical or medical procedures (RR 0.91, 95% CI 0.44 to 1.84). We graded the certainty of evidence for ocular and systemic adverse events at two years as moderate, after downgrading for imprecision (‐1).
7. Adverse events up to 2 years: bevacizumab versus ranibizumab.
| Ocular adverse event (CATT trial)a | Bevacizumab n = 586 | Ranibizumab n = 599 | RR [95% CI] Bevacizumab vs ranibizumab |
| Endophthalmitis | 7 (1%) | 4 (< 1%) | 1.79 [0.53 to 6.08] |
| Ocular adverse event (IVAN trial)b | Bevacizumab n = 296 | Ranibizumab n = 314 | RR [95% CI] Bevacizumab vs ranibizumab |
| Traumatic cataract | 1 (< 1%) | 1 (< 1%) | 1.06 [0.07 to 16.88] |
| Severe uveitis | 1 (< 1%) | 0 | 3.18 [0.13 to 77.80] |
| Retinal detachment | 0 | 1 (< 1%) | 0.35 [0.01 to 8.64] |
| Retinal pigment epithelial tear | 1 (< 1%) | 3 (< 1%) | 0.35 [0.04 to 3.38] |
| Non‐ocular adverse eventc | Bevacizumab n = 882 | Ranibizumab n = 913 | RR [95% CI] Bevacizumab vs ranibizumab |
| At least 1 serious adverse event | 314 (36%) | 271 (30%) | 1.20 [1.05 to 1.37] |
| Death | 51 (6%) | 47 (5%) | 1.12 [0.76 to 1.65] |
| Myocardial infarction | 11 (1%) | 13 (1%) | 0.88 [0.39 to 1.94] |
| Stroke or cerebral infarction | 11 (1%) | 14 (2%) | 0.81 [0.37 to 1.78] |
| Venous thrombotic event | 14 (2%) | 6 (< 1%) | 2.42 [0.93 to 6.26] |
| Transient ischemic attack | 1 (< 1%) | 1 (< 1%) | 1.04 [0.06 to 16.52] |
| Cardiac disorders | 81 (9%) | 67 (7%) | 1.25 [0.92 to 1.71] |
| Gastrointestinal disorders | 37 (4%) | 14 (2%) | 2.74 [1.49 to 5.02] |
| Infections | 66 (7%) | 50 (5%) | 1.37 [0.96 to 1.95] |
| Injury and procedural complications | 45 (5%) | 35 (4%) | 1.33 [0.86 to 2.05] |
| Neoplasms (benign, malignant, unspecified) | 36 (4%) | 38 (4%) | 0.98 [0.63 to 1.53] |
| Nervous system disorders | 44 (5%) | 43 (5%) | 1.06 [0.70 to 1.60] |
| Surgical or medical procedure | 14 (5%) | 16 (5%) | 0.91 [0.44 to 1.84] |
CI: confidence interval.
RR: risk ratio.
aAdverse events for endophthalmitis not reported in IVAN 2013; data for CATT 2011 only. bAdverse events for traumatic cataract, uveitis, retinal detachment, retinal pigment epithelial tear, transient ischemic attack, and surgical or medical procedure not reported in CATT 2011; data for IVAN 2013 study only. cAdverse events experienced by 1185 participants in CATT 2011 and by 610 participants in IVAN 2013.
Discussion
Summary of main results
Most of the 16 trials included in this systematic review were of good methodological quality. The six trials of anti‐vascular endothelial growth factor (VEGF) monotherapy versus control demonstrated the beneficial effect of anti‐VEGF therapy on visual acuity in the management of neovascular age‐related macular degeneration (AMD). Participants treated with any anti‐VEGF agent featured in these trials ‐ pegaptanib (one trial), ranibizumab (three trials), or bevacizumab (two trials) ‐ more often gained or maintained visual acuity at one year and less often lost visual acuity compared with participants who received no anti‐VEGF agent. Stability of visual acuity at one year was achieved more often in an anti‐VEGF treatment group than in a control group not treated with anti‐VEGF agents. The safety profile of anti‐VEGFs, as reported in the included studies, was acceptable.
Functional outcomes (e.g. visual acuity) correlated with quality of life outcomes, when reported, and anatomic outcomes (e.g. lesion size, retinal thickness).across trials. Participants treated with pegaptanib showed a decrease in the size of the choroidal neovascular complex with less leakage observed on fluorescein angiography than in participants treated with sham injections. Bevacizumab‐ and ranibizumab‐treated participants experienced reductions in central retinal thickness (CRT) as measured by optical coherence tomography (OCT) compared with control participants.
Investigators more often reported improvement in vision‐specific quality of life in anti‐VEGF‐treated groups than in control groups, as well as improved scores on the National Eye Institute‐Visual Functioning Questionnaire (NEI‐VFQ) scale with both pegaptanib and ranibizumab compared with control. Cost utility analysis, based on data from one trial and standardized utilities of degree of visual loss, compared ranibizumab with pegaptanib and revealed that ranibizumab was associated with better quality of life when compared with pegaptanib (Brown 2008). Data on visual function (e.g. contrast sensitivity) and treatment costs were sparse in these trials.
We found only one small trial that performed a head‐to‐head comparison of pegaptanib (n = 18) versus bevacizumab (n = 13), which we excluded because participants were followed for only six months (Schmid‐Kubista 2011). Investigators reported improved best‐corrected visual acuity (BCVA) with bevacizumab compared to pegaptanib. Ten head‐to‐head trials compared bevacizumab versus ranibizumab. At one‐year and two‐year follow‐up, visual acuity outcomes were comparable for bevacizumab and ranibizumab, clinically and statistically, although confidence intervals for some outcomes reported by individual studies indicated some uncertainty regarding true effects. In terms of other measures of visual function, one trial showed better near LogMAR visual acuity among participants in the ranibizumab group than among those in the bevacizumab group at one‐year follow‐up; this effect had diminished at two‐year follow‐up. At one‐year and two‐year follow‐up, researchers noted no clinically meaningful differences in reduction of CRT between bevacizumab‐treated and ranibizumab‐treated participants. Participant responses to quality of life questionnaires were comparable between treatment groups. Researchers reported a small number of ocular adverse events for both bevacizumab and ranibizumab groups (< 1%) across all trials. However, endophthalmitis rates were higher with injection of anti‐VEGF agents than with intravitreal surgery, except when estimates were based on numbers of injections given rather than on numbers of eyes treated. Individuals with AMD and their ophthalmologists must be aware of this small but serious risk. At both one‐year and two‐year follow‐up, fewer participants in the ranibizumab groups experienced any serious systemic adverse events compared with those in the bevacizumab groups.
Overall completeness and applicability of evidence
We conducted this review to investigate effects on vision and quality of life associated with intravitreous injections of anti‐VEGF agents for treatment of patients with neovascular AMD versus sham treatment or a different anti‐VEGF agent administered at comparable dosages and regimens. In this review, we included only randomized controlled trials (RCTs), each with a minimum follow‐up of one year. The primary outcome for this review was the proportion of participants who gained 15 or more letters of BCVA by one‐year follow‐up examination. Secondary outcomes included other visual acuity outcomes at one‐year and two‐year follow‐up, visual function outcomes, morphologic characteristics assessed by fluorescein angiography or OCT, ocular and systemic adverse outcomes, cost outcomes, and quality of life. We used multiple sources to identify relevant data for this review ‐ not only journal publications, but also conference abstracts, FDA documents, and descriptions in clinical trial registries, when available. When data were unclear or were missing, we contacted study investigators to request clarification or information.
This review ultimately included representative and applicable outcomes data from 6347 participants enrolled in 16 trials conducted in various countries that included both men and women aged 50 years or older with subfoveal choroidal neovascularization (CNV) secondary to AMD. Approximately half of the included trials reported the type of neovascular lesion, and all lesion types (predominantly classic CNV, minimally classic CNV, and occult CNV only) were represented among these trials. All studies included at least one measure related to morphologic characteristics of study eyes, with fluorescein angiography used in 15 studies and OCT used in all but the earliest of these RCTs.
The earliest RCTs of anti‐VEGF agents incorporated in this review individually and collectively established a new paradigm for management of neovascular AMD, particularly for lesions under or near the fovea, and validated administration of intravitreal anti‐VEGF therapy in affected individuals with clinical profiles similar to those of participants enrolled in these trials. Reported outcomes related to visual acuity gains, stability of visual acuity at one year, decreased risk of significant visual acuity loss, and low rates of ocular and systemic adverse events are mirrored in real‐life clinical encounters when anti‐VEGF agents are used to manage neovascular AMD in the retina specialist's office (Carneiro 2012; Gillies 2014; Holz 2013; Rasmussen 2014). As observed in the clinical trials incorporated into this review, morphologic changes in the CNV lesion complex, with regard to decreased size on fluorescein angiography, decreased leakage on fluorescein angiography, and decreased CRT on OCT, also have been observed to occur in clinical practice among individuals given intravitreous anti‐VEGF therapy for neovascular AMD (Carneiro 2012).
With completion and reporting of ten head‐to‐head trials of bevacizumab versus ranibizumab, and the finding of little or no difference in outcomes between the two drugs, the primary consideration for the ophthalmologist and the individual with AMD has been the choice of anti‐VEGF agent. Issues include costs, availability, and quality control of the preparation of bevacizumab for intravitreal injection. Issues as yet unresolved include optimal frequency with which anti‐VEGF agents should be injected into affected eyes, length of calendar time over which anti‐VEGF agents must be injected to maintain the benefits seen with two‐year outcomes, and long‐term ocular and systemic effects of these treatments. These issues have been or will be addressed in other systematic reviews prepared by Cochrane reviewers and others.
Quality of the evidence
In addition to inclusion of only RCTs in this review, two review authors assessed potential sources of bias in these trials according to methods established by Cochrane. We assessed the certainty of evidence for most outcomes in this review as moderate to high on the basis of consistency of findings across trials and proper trial design. Parameters considered in risk of bias assessment included selection bias, performance bias, detection bias, attrition bias, and reporting bias; we graded each potential source of bias as low risk, unclear risk, or high risk. Overall, we found the included studies to be at low risk for most categories of bias. In most trials, few participants missed the primary outcome examination or were not treated per protocol assignment. In nine trials, proportions of participants lost to follow‐up at primary follow‐up visits were less than 15%, despite the age of participants and the death of some. Although not the best method that can be used to account for missing data, seven trials used the last observation carried forward method to impute missing data. We identified design publications, protocols, or clinical trial registration for 15 of the 16 included studies. We judged nine of these 15 trials to be free of reporting bias on the basis of consistency between study outcomes as defined in the protocols and clinical trial registers and reported in study publications to date.
In retrospect, we should not have included ABC 2010 in this review because the "standard care" control arm included pegaptanib, which was approved for intravitreous injection by the Naational Health Service at the time the trial commenced. We elected to retain ABC 2010 in the current updated review for consistency with earlier versions of this systematic review.
We considered various other aspects of trial design, reporting, and financial support as potential sources of bias. Pharmaceutical companies that marketed the study drugs under investigation sponsored four of 16 trials ‐ one study of pegaptanib and three studies that compared ranibizumab with control. In addition, pharmaceutical company sponsors had important roles in design, analysis, and reporting of these trials, and some investigators reported that they had financial relationships with the company that manufactured the study drug.
Potential biases in the review process
For this review, we conducted broad electronic searches for studies and imposed no date or language restrictions to minimize potential biases in the study selection process. We followed standard Cochrane review methods. The outcomes evaluated in the review were those commonly specified for RCTs examining treatments for neovascular AMD.
Agreements and disagreements with other studies or reviews
Whether assessed by systematic, comprehensive reviews, such as this one, or by more traditional, clinical reviews, anti‐VEGF compounds for treatment of patients with neovascular AMD appear to be efficacious and safe (Ip 2008; Mitchell 2011; Schmucker 2010; Schmucker 2012). Beneficial effects of pegaptanib, ranibizumab, and bevacizumab are evident in terms of the proportion of participants with stabilization or small losses of BCVA. Ranibizumab and bevacizumab additionally resulted in a greater proportion of participants with improved BCVA after one and two years of injections. In independent studies and comprehensive reviews; effects on visual acuity have been consistent with morphologic changes in the size and composition of the CNV lesion complex, as well as with the observed change in CRT on OCT following treatment with these agents. In general, considerations of costs were limited in the trials included in this systematic review; additional analyses indicating a favorable cost/utility ratio for anti‐VEGF agents versus control or no treatment were cited in research using RCTs and observational data (Cohen 2008; Earnshaw 2007; Fletcher 2008; Hernandez‐Pastor 2008; Javitt 2008; Wolowacz 2007). Economic analyses have documented the lower cost of bevacizumab compared with ranibizumab in achieving the same benefits (Raftery 2007; Stein 2014). A separate Cochrane review that specifically evaluated the systemic safety of bevacizumab versus ranibizumab also found no clinically or statistically significant differences between intravitreal injection of the two drugs after two years of follow‐up with respect to death or overall serious systemic adverse events (Moja 2014).
Authors' conclusions
Implications for practice.
The results of this review indicate the effectiveness of three anti‐VEGF agents (pegaptanib, ranibizumab, and bevacizumab) in terms of stability or improvement in visual acuity after one year and two years of treatment. Ranibizumab and bevacizumab have resulted in improved visual acuity in a sizable fraction of treated eyes. The beneficial effects of these anti‐VEGF agents with respect to visual acuity are consistent with their effects on changes in lesion size as evaluated on fluorescein angiography and by OCT. Available information on adverse effects of each medication does not suggest a substantially higher incidence of potentially vision‐threatening complications with intravitreal injection compared with control interventions; however, this review may not be sufficiently powered to detect rare safety outcomes. We found no trials that had compared pegaptanib directly with either ranibizumab or bevacizumab for 12 months or longer.
Since anti‐VEGF agents were introduced into clinical practice, ophthalmologists have observed among their patients with neovascular AMD that individual patients respond differently to different agents, and that tolerance for individual anti‐VEGF agents develops in some patients with prolonged use. Thus, access to multiple agents may offer the patient the best chance for a good visual acuity outcome. However, we did not address these issues in this review and, thus, cannot make an evidence‐based recommendation.
At the time of preparation of this review, bevacizumab remains an off‐label therapy for patients with neovascular AMD. The manufacturer (Genentech) that produces both bevacizumab and ranibizumab has not submitted bevacizumab for approval as treatment for AMD. Bevacizumab is a significantly less expensive treatment option, so perhaps it would compete with the company's more costly and FDA‐approved ophthalmic anti‐VEGF agent, ranibizumab. Thus, trials comparing functional, anatomic, vision‐specific quality of life, as well as cost utility outcomes, between bevacizumab and ranibizumab ultimately may have no effect on the treatment of individuals with neovascular AMD if off‐label therapy with bevacizumab were proscribed. The US Centers for Medicare and Medicaid (CMS) and other national health agencies currently cover the cost of bevacizumab for ophthalmic use in hospital outpatient settings; however, other national health agencies do not include off‐label use of bevacizumab in their coverage (CMS 2014; Cohen 2014).
Implications for research.
Use of anti‐VEGF agents for treatment of AMD has become part of standard clinical practice; however, certain issues regarding their use remain. Several factors encourage evaluation of the efficacy of alternative and less‐frequent dosing regimens with anti‐VEGF compounds. These include lingering concerns about ocular and systemic toxicity, convenience for individuals with AMD and their physicians associated with fewer intravitreal injections, and costs of treatment. Research to evaluate long‐term use of anti‐VEGF agents should consider both effects of the drugs on vision and long‐term effects of multiple injections over time. It remains unclear how one can best evaluate these effects, as RCTs to identify rare events during long follow‐up periods are difficult to conduct and finance. Some of the RCTs included in this review have provided data for up to seven years of follow‐up (Rofagha 2013); however, these follow‐up data are observational, were not included in the trial protocols, and include only a subset of originally enrolled participants. CATT 2011 received funding from the US National Eye Institute to continue follow‐up of participants enrolled in that trial so investigators could document long‐term positive and negative effects of intravitreous injections of ranibizumab and bevacizumab; five year outcomes have been reported for the trial cohort.
Use of anti‐VEGF agents in combination with other neovascular AMD treatments, such as verteporfin photodynamic therapy (PDT) or intravitreal steroids, is an important and active area of research because acceptance of anti‐VEGF therapy may make it unethical to conduct trials without providing this treatment to all participants with neovascular AMD. The goal of combination treatments would be to improve vision and quality of life even further than is achievable with anti‐VEGF agents alone, and perhaps to reduce the number of intravitreal injections needed. Research also is needed to evaluate methods of delivering these agents other than intravitreally. Vehicles already under development include implants and refillable reservoirs (de Juan 2013).
Based on our review, only one or more very large randomized trials with findings that differ substantially from those reported by trials already completed would be required to modify or reverse our conclusions that (1) intravitreous injection of pegaptanib, ranibizumab, and bevacizumab has beneficial effects on best‐corrected visual acuity in eyes with neovascular age‐related macular degeneration, and (2) ranibizumab and bevacizumab have equivalent safety and effectiveness in such eyes. Thus, we do not anticipate a need for future updates to this systematic review. Future reviews are expected to address the effects of newer agents and other approaches for treating eyes with neovascular age‐related macular degeneration.
What's new
| Date | Event | Description |
|---|---|---|
| 31 January 2018 | New citation required but conclusions have not changed | Four new trials included that met the inclusion criteria (BRAMD 2016; LUCAS 2015; SAVE‐AMD 2017; Scholler 2014) |
| 31 January 2018 | New search has been performed | Searches updated |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 15 September 2014 | Amended | Updated reference for Cochrane review was Moja 2014 |
| 27 August 2014 | New citation required and conclusions have changed | Issue 8, 2014 ‐ We changed the primary outcome and added new studies and analyses |
| 27 August 2014 | New search has been performed | Issue 8, 2014 ‐ Updated searches yielded 8 new trials that met the inclusion criteria (ABC 2010; Biswas 2011; CATT 2011; GEFAL 2013; IVAN 2013; MANTA 2013; Sacu 2009; Subramanian 2010) |
| 23 June 2008 | Amended | We converted the review to new review format |
| 20 February 2008 | New citation required and conclusions have changed | We made substantive amendments |
Acknowledgements
We are grateful to the editorial team of the Cochrane Eyes and Vision Group (CEVG) for comments on and evaluation of this review. Iris Gordon and Karen Blackhall, Trials Search Co‐ordinators for CEVG, designed and conducted the electronic searches. Usha Reddy, MD, and Harold Woodcome Jr, MD, contributed to the protocol for this review. We thank Gianni Virgili, Richard Wormald, Jennifer Evans, Anupa Shah, Tasanee Smith, and Marie Diener‐West for their comments on the review or the review update. Sara Money assisted with retrieval and review of records for the 2018 updated review. Our CEVG colleagues, particularly Jimmy Le, Renee Wilson, and Tianjing Li, advised us regarding various aspects of updating this review.
We also are grateful to Dr. Brown (ANCHOR 2006), Dr. Deppenschmidt at Genentech (ANCHOR 2006; MARINA 2006;PIER 2008), Dr. Feinsod at Eyetech Pharmaceuticals (VISION 2004), Dr. Regillo (PIER 2008), Dr. Rosenfeld (MARINA 2006;PIER 2008;SAILOR 2009), Dr. Sacu (Sacu 2009), and Dr. Subramanian for their kind assistance in providing additional information on trial methods and results for the purposes of this review (Subramanian 2010).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Degeneration] explode all trees #2 MeSH descriptor: [Retinal Degeneration] explode all trees #3 MeSH descriptor: [Retinal Neovascularization] explode all trees #4 MeSH descriptor: [Choroidal Neovascularization] explode all trees #5 MeSH descriptor: [Macula Lutea] explode all trees #6 maculopath* #7 (macula* or retina* or choroid*) near/3 degenerat* #8 (macula* or retina* or choroid*) near/3 neovascul* #9 macula* near/2 lutea #10 AMD or AMRD or CNV #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #13 MeSH descriptor: [Angiogenesis Inducing Agents] explode all trees #14 MeSH descriptor: [Endothelial Growth Factors] explode all trees #15 MeSH descriptor: [Vascular Endothelial Growth Factors] explode all trees #16 anti near/2 VEGF* #17 anti near/1 angiogen* #18 endothelial near/2 growth near/2 factor* #19 (macugen* or pegaptanib* or lucentis* or rhufab* or ranibizumab* or bevacizumab* or avastin* or aflibercept* or conbercept*) #20 VEGF TRAP* #21 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 #22 #11 and #21
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp macular degeneration/ 14. exp retinal degeneration/ 15. exp retinal neovascularization/ 16. exp choroidal neovascularization/ 17. exp macula lutea/ 18. maculopath$.tw. 19. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 20. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 21. (macula$ adj2 lutea).tw. 22. (AMD or ARMD or CNV).tw. 23. or/13‐22 24. exp angiogenesis inhibitors/ 25. angiogenesis inducing agents/ 26. endothelial growth factors/ 27. exp vascular endothelial growth factors/ 28. (anti adj2 VEGF$).tw. 29. (endothelial adj2 growth adj2 factor$).tw. 30. (anti adj1 angiogen$).tw. 31. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$ or conbercept$).tw. 32. VEGF TRAP$.tw. 33. or/24‐32 34. 23 and 33 35. 12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retina macula degeneration/ 34. exp retinal degeneration/ 35. exp subretinal neovascularization/ 36. maculopath$.tw. 37. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 38. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 39. (macula$ adj2 lutea).tw. 40. (AMD or ARMD or CNV).tw. 41. or/33‐40 42. angiogenesis/ 43. exp angiogenesis inhibitors/ 44. angiogenic factor/ 45. endothelial cell growth factor/ 46. monoclonal antibody/ 47. vasculotropin/ 48. (anti adj2 VEGF$).tw. 49. (endothelial adj2 growth adj2 factor$).tw. 50. (anti adj1 angiogen$).tw. 51. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$ or conbercept$).tw. 52. VEGF TRAP$.tw. 53. or/42‐52 54. 41 and 53 55. 32 and 54
Appendix 4. LILACS search strategy
Macular Degeneration OR AMD OR ARMD [Words] and Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR conbercept
Appendix 5. ISRCTN search strategy
(Macular Degeneration OR AMD OR ARMD) AND (Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR conbercept)
Appendix 6. ClinicalTrials.gov search strategy
(Macular Degeneration OR AMD OR ARMD) AND (Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR conbercept)
Appendix 7. ICTRP search strategy
Macular Degeneration OR AMD OR ARMD = Condition AND Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR conbercept= Intervention
Appendix 8. EOP 1003 study data
| Methods | Method of randomization: stochastic treatment allocation algorithm based on the variance method Method of allocation concealment: centralized randomization where the study co‐ordinator was instructed the code of the medication for the patient after determining her eligibility. The medication packet was not opened until just before administering the injection Masking: Participants: yes Care providers: examiner: yes; injector: no Outcome assessors: yes Number randomized: 144 to 0.3 mg pegaptanib, 146 to 1 mg pegaptanib, 143 to 3 mg pegaptanib, and 145 to placebo Exclusions after randomization: none Number analyzed: 144 in 0.3 mg pegaptanib group, 146 in 1 mg pegaptanib group, 143 in 3 mg pegaptanib group, and 145 in placebo group for the primary outcome alone Losses to follow‐up: 11 in placebo group, 12 in 0.3 mg pegaptanib group, 17 in 1 mg pegaptanib group, and 20 in 3 mg pegaptanib group discontinued therapy during the trial Intention‐to‐treat analysis: reported an intention‐to‐treat analysis only for the primary outcome Unit of analysis: individuals Reported power calculations: yes |
| Participants | Country: USA, Canada Age: mean age was 78, 76.5, 77.1, and 76.7 years in 0.3 mg pegaptanib, 1 mg pegaptanib, 3 mg pegaptanib, and placebo groups, respectively Gender: 56%, 53%, 69%, and 57% in 0.3 mg pegaptanib, 1 mg pegaptanib, 3 mg pegaptanib, and placebo groups, respectively, were female Inclusion criteria: age greater than or equal to 50 years; subfoveal choroidal neovascularization (CNV) secondary to age‐related macular degeneration; best‐corrected visual acuity of 20/40 to 20/320 in the treated eye and greater than 20/800 in the fellow eye; CNV lesion may be predominantly classic, minimally classic, occult with no classic; size of lesion < 12 disc areas (including blood, scar/atrophy, neovascularization); no greater than 50% of lesion could be due to subretinal hemorrhage and 50% of lesion had to be due to CNV; for occult lesions, lesions had to be subretinal and no greater than 50% of total lesion area, or presence of lipid or loss of 15 letters or more of visual acuity during previous 12 weeks; patients were eligible even if they received 1 photodynamic treatment if it was at least 8 to 12 weeks before enrollment; intraocular pressure < 23 mmHg; adequate pupil dilation; clear media Exclusion criteria: atrophy exceeding 20% of total lesion or subfoveal scarring; previous thermal laser; therapy with another investigational drug; likelihood of requiring cataract removal within 2 years; other potential causes of CNV including high myopia, ocular histoplasmosis, angioid streaks, choroidal rupture, multi‐focal choroiditis, any intraocular surgery within 3 months or extrafoveal/juxtafoveal laser within 2 weeks of study entry or posterior vitrectomy or scleral buckle or presence of intraretinal tears or rips; concomitant presence of diabetic retinopathy, severe cardiac disease, myocardial infarction within 6 months, ventricular tachycardia requiring treatment, unstable angina, evidence of peripheral vascular disease, stroke within 12 months, acute or chronic periocular infection, previous therapeutic radiation to eye/head/neck; any treatment with any investigational agent within past 30 days; serious allergies to fluorescein dye or indocyanine green or components of pegaptanib Equivalence of baseline characteristics: treatment groups were similar with respect to age, gender, race, smoking status, angiographic subtypes, prior treatment status with photodynamic therapy, and Early Treatment Diabetic Retinopathy Study visual acuity scores |
| Interventions | Treatment: intravitreal injection of pegaptanib at dosages of 0.3 mg, 1.0 mg, or 3.0 mg given every 6 weeks over period of 48 weeks Control: sham injection with patients treated identically with the exception of scleral penetration with the needle Length of follow‐up: 54 weeks |
| Outcomes | Primary outcome: proportion of participants losing fewer than 15 letters of visual acuity between baseline and week 54 Other outcomes reported: gain of 3 or more lines of visual acuity, maintenance of visual acuity or gain of 0 lines of visual acuity, mean visual acuity, legal blindness, loss of 30 letters or more of visual acuity, size of lesion, and total CNV size Reported quality of life indicators: yes Intervals at which outcome assessed: every 6 weeks before treatment, with main assessment analyzed after 54 weeks |
| Notes | Funding: Eyetech Pharmaceuticals and Pfizer NCT00321997 |
Appendix 9. EOP 1004 study data
| Methods | Method of randomization: stochastic treatment allocation algorithm based on the variance method Method of allocation concealment: centralized randomization where the study co‐ordinator was instructed the code of the medication for the patient after determining her eligibility. The medication packet was not opened until just before administering the injection Masking: Participants: yes Care providers: examiner: yes; injector: no Outcome assessors: yes Number randomized: 151 to 0.3 mg pegaptanib, 155 to 1 mg pegaptanib, 153 to 3 mg pegaptanib, and 153 to placebo Exclusions after randomization: none Number analyzed: 151 in 0.3 mg pegaptanib group, 155 in 1 mg pegaptanib group, 153 in 3 mg pegaptanib group, and 153 in placebo group for the primary outcome alone Losses to follow‐up: 12 in placebo group, 11 in 0.3 mg pegaptanib group, 13 in 1 mg pegaptanib group, and 17 in 3 mg pegaptanib group discontinued therapy during the trial Intention‐to‐treat analysis: yes; except don't know why 18 patients were excluded after randomization Unit of analysis: individuals Reported power calculations: yes |
| Participants | Country: USA, Canada, Europe, Israel, Australia, South America Age: mean age was 74.9, 74.5, 75.4, and 74.9 years in 0.3 mg pegaptanib, 1 mg pegaptanib, 3 mg pegaptanib, and placebo groups, respectively Gender: 54%, 56%, 61%, and 63% in 0.3 mg pegaptanib, 1 mg pegaptanib, 3 mg pegaptanib, and placebo groups, respectively, were female Inclusion criteria: age greater than or equal to 50 years; subfoveal choroidal neovascularization (CNV) secondary to age‐related macular degeneration; best‐corrected visual acuity of 20/40 to 20/320 in the treated eye and greater than 20/800 in the fellow eye; CNV lesion may be predominantly classic, minimally classic, occult with no classic; size of lesion < 12 disc areas (including blood, scar/atrophy, neovascularization); no greater than 50% of lesion could be due to subretinal hemorrhage and 50% of lesion had to be due to CNV; for occult lesions, lesions had to be subretinal and no greater than 50% of total lesion area, or presence of lipid or loss of 15 letters or more of visual acuity during previous 12 weeks; patients were eligible even if they received 1 photodynamic treatment if it was at least 8 to 12 weeks before enrollment; intraocular pressure < 23 mmHg; adequate pupil dilation; clear media Exclusion criteria: atrophy exceeding 20% of total lesion or subfoveal scarring; previous thermal laser; therapy with another investigational drug; likelihood of requiring cataract removal within 2 years; other potential causes of CNV including high myopia, ocular histoplasmosis, angioid streaks, choroidal rupture, multifocal choroiditis, any intraocular surgery within 3 months or extrafoveal/juxtafoveal laser within 2 weeks of study entry, or posterior vitrectomy or scleral buckle or presence of intraretinal tears or rips; concomitant presence of diabetic retinopathy, severe cardiac disease, myocardial infarction within 6 months, ventricular tachycardia requiring treatment, unstable angina, evidence of peripheral vascular disease, stroke within 12 months, acute or chronic periocular infection, previous therapeutic radiation to eye/head/neck; any treatment with any investigational agent within last 30 days; serious allergies to fluorescein dye or indocyanine green or components of pegaptanib Equivalence of baseline characteristics: treatment groups were similar with respect to age, gender, race, smoking status, angiographic subtypes, prior treatment status with photodynamic therapy, and Early Treatment Diabetic Retinopathy Study visual acuity scores |
| Interventions | Treatment: intravitreal injection of pegaptanib at dosages of 0.3 mg, 1.0 mg, or 3.0 mg given every 6 weeks over period of 48 weeks Control: sham injection with participants treated identically with the exception of scleral penetration with the needle Length of follow‐up: 54 weeks |
| Outcomes | Primary outcome: proportion of participants losing fewer than 15 letters of visual acuity between baseline and week 54 Other outcomes reported: gain of 3 or more lines of visual acuity, maintenance of visual acuity or gain of 0 lines of visual acuity, mean visual acuity, legal blindness, loss of 30 letters or more of visual acuity, size of lesion, and total CNV size Reported quality of life indicators: yes Intervals at which outcome assessed: every 6 weeks before treatment, with main assessment analyzed after 54 weeks |
| Notes | Funding: Eyetech Pharmaceuticals and Pfizer NCT00021736 |
Data and analyses
Comparison 1. Anti‐VEGF treatment versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gain of 15 or more letters visual acuity at 1 year | 6 | 2667 | Risk Ratio (M‐H, Random, 95% CI) | 4.19 [2.32, 7.55] |
| 1.1 Pegaptanib vs control | 1 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [1.23, 6.52] |
| 1.2 Ranibizumab vs control | 3 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [1.59, 9.67] |
| 1.3 Bevacizumab vs control | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 7.80 [2.44, 24.98] |
| 2 Gain of 15 or more letters visual acuity at 2 years | 3 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 5.77 [3.38, 9.84] |
| 3 Loss of fewer than 15 letters visual acuity at 1 year | 6 | 2667 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.27, 1.55] |
| 3.1 Pegaptanib vs control | 1 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.11, 1.39] |
| 3.2 Ranibizumab vs control | 3 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.41, 1.64] |
| 3.3 Bevacizumab vs control | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.09, 1.50] |
| 4 Loss of fewer than 15 letters visual acuity at 2 years | 3 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.32, 1.98] |
| 5 Loss of fewer than 30 letters visual acuity at 1 year | 4 | 2455 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.06, 1.19] |
| 5.1 Pegaptanib vs control | 1 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.08, 1.23] |
| 5.2 Ranibizumab vs control | 2 | 1138 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.11, 1.20] |
| 5.3 Bevacizumab vs control | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.10] |
| 6 Loss of fewer than 30 letters visual acuity at 2 years | 2 | 1138 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.15, 1.29] |
| 7 Visual acuity better than 20/200 at 1 year | 4 | 2508 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.34, 1.86] |
| 7.1 Pegaptanib vs control | 1 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.15, 1.52] |
| 7.2 Ranibizumab vs control | 3 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.41, 2.03] |
| 8 Visual acuity better than 20/200 at 2 years | 3 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.52, 1.98] |
| 9 Maintenance of visual acuity at 1 year | 3 | 1636 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.31, 3.00] |
| 9.1 Pegaptanib vs control | 1 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.19, 1.88] |
| 9.2 Ranibizumab vs control | 1 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.95, 3.27] |
| 9.3 Bevacizumab vs control | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 2.2 [1.03, 4.68] |
| 10 Maintenance of visual acuity at 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Mean change in visual acuity at 1 year (number of letters) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Pegaptanib vs control | 1 | 1186 | Mean Difference (IV, Random, 95% CI) | 6.72 [4.43, 9.01] |
| 11.2 Ranibizumab vs control | 3 | 1322 | Mean Difference (IV, Random, 95% CI) | 17.80 [15.95, 19.65] |
| 12 Mean change in visual acuity at 2 years (number of letters) | 3 | 1322 | Mean Difference (IV, Random, 95% CI) | 20.11 [18.08, 22.15] |
| 13 Reduction in size of CNV at 1 year (mean number of disc areas) | 1 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 14 Reduction in size of lesion at 1 year (mean number of disc areas) | 1 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 15 Reduction in size of lesion at 1 year (mean number of disc areas) | 2 | 606 | Mean Difference (IV, Random, 95% CI) | 2.34 [1.88, 2.81] |
| 16 Reduction in size of lesion at 2 years (mean number of disc areas) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Mean change in quality of life scores at 1 year | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Overall vision‐related quality of life | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 6.69 [3.38, 9.99] |
| 17.2 Near vision activities | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 8.45 [0.28, 16.62] |
| 17.3 Distance vision activities | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 9.65 [3.20, 16.09] |
| 17.4 Dependency | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 9.82 [6.86, 12.77] |
| 17.5 Driving ability | 2 | 1080 | Mean Difference (IV, Random, 95% CI) | 9.85 [6.34, 13.36] |
| 17.6 General health | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 3.18 [0.54, 5.82] |
| 17.7 Role difficulties | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 6.99 [0.76, 13.23] |
| 17.8 Mental health | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 8.42 [5.75, 11.10] |
| 17.9 General vision | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 8.20 [5.90, 10.50] |
| 17.10 Social functioning | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 8.03 [5.36, 10.69] |
| 17.11 Color vision | 2 | 1127 | Mean Difference (IV, Random, 95% CI) | 2.51 [‐0.02, 5.05] |
| 17.12 Peripheral vision | 2 | 1133 | Mean Difference (IV, Random, 95% CI) | 5.20 [0.37, 10.03] |
| 17.13 Ocular pain | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐3.67, 0.11] |
| 18 Mean change in quality of life scores at 2 years | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Overall vision‐related quality of life | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 8.63 [3.31, 13.95] |
| 18.2 Near vision activities | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 11.52 [3.49, 19.55] |
| 18.3 Distance vision activities | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 10.86 [3.82, 17.90] |
| 18.4 Dependency | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 11.06 [3.29, 18.83] |
| 18.5 Driving ability | 2 | 1080 | Mean Difference (IV, Random, 95% CI) | 13.53 [9.51, 17.55] |
| 18.6 General health | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐0.18, 5.35] |
| 18.7 Role difficulties | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 9.44 [1.34, 17.54] |
| 18.8 Mental health | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 10.07 [3.98, 16.17] |
| 18.9 General vision | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 9.61 [5.49, 13.72] |
| 18.10 Social functioning | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | 8.12 [1.77, 14.47] |
| 18.11 Color vision | 2 | 1127 | Mean Difference (IV, Random, 95% CI) | 5.70 [2.89, 8.51] |
| 18.12 Peripheral vision | 2 | 1133 | Mean Difference (IV, Random, 95% CI) | 6.79 [1.48, 12.09] |
| 18.13 Ocular pain | 2 | 1134 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐3.13, 0.92] |
Comparison 2. Bevacizumab versus ranibizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gain of 15 or more letters visual acuity at 1 year | 8 | 3144 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.12] |
| 2 Gain of 15 or more letters visual acuity at 2 years | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Participants in groups as randomized at baseline | 2 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.11] |
| 2.2 Participants remaining in same groups after re‐randomization | 2 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.11] |
| 3 Loss of fewer than 15 letters visual acuity at 1 year | 8 | 3144 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.02] |
| 4 Loss of fewer than 15 letters visual acuity at 2 years | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Participants in groups as randomized at baseline | 2 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 4.2 Participants remaining in same groups after re‐randomization | 2 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.01] |
| 5 Visual acuity better than 20/200 at 1 year | 4 | 2026 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.01] |
| 6 Visual acuity better than 20/200 at 2 years | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Participants in groups as randomized at baseline | 2 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.06] |
| 6.2 Participants remaining in same groups after re‐randomization | 2 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.06] |
| 7 Mean change in visual acuity at 1 year (number of letters) | 9 | 3190 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.49, 0.45] |
| 8 Mean change in visual acuity at 2 years (number of letters) | 2 | 1295 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.82, 0.51] |
| 9 Reduction in central retinal thickness at 1 year | 6 | 2693 | Mean Difference (IV, Random, 95% CI) | ‐11.61 [‐21.55, ‐1.66] |
| 10 Reduction in central retinal thickness at 2 years | 2 | 1199 | Mean Difference (IV, Random, 95% CI) | ‐12.40 [‐33.83, 9.04] |
| 11 No problems in quality of life domain at 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.1 Mobility | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Self‐care | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Usual activities | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Pain/discomfort | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Anxiety/depression | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 No problems in quality of life domain at 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.1 Mobility | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Self‐care | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Usual activities | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Pain/discomfort | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.5 Anxiety/depression | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Loss of fewer than 30 letters visual acuity at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
2.13. Analysis.
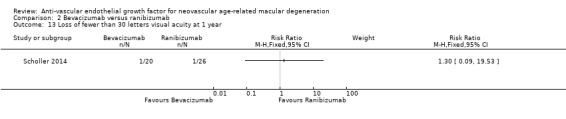
Comparison 2 Bevacizumab versus ranibizumab, Outcome 13 Loss of fewer than 30 letters visual acuity at 1 year.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ABC 2010.
| Methods |
Number randomized (total and per group): 131 participants randomly assigned to study treatment: 65 to intravitreal bevacizumab and 66 to "standard treatment." Standard treatment included intravitreal pegaptanib injections (n = 38), PDT with verteporfin (n = 16), or sham injection (n = 12) Exclusions after randomization: none Number analyzed (total and per group): 131 total participants: 65 bevacizumab and 66 standard treatment Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: bevacizumab group: 1 participant died; standard treatment group: 3 participants withdrew from the trial and chose to have alternative treatment, and 1 participant withdrew owing to pain of treatment Compliance: limited information given: "more than 90% of patients in each group (overall 96%) were receiving treatment at the last treatment visit (48 weeks) and were followed up to week 54" Intention‐to‐treat analysis: yes; using last observation carried forward for 1 participant in bevacizumab group and 4 in standard treatment group Reported power calculation: yes; sample of 130 participants to provide power of 82% to detect or rule out a difference of 25% to 67% in outcome rates at P < 0.05 Study design comment: "standard treatment" was not uniform; it was decided for each participant before randomization based on eligibility for NHS coverage of treatments at the time |
|
| Participants |
Country: UK (London, England) Age: mean in bevacizumab group was 79 years and in standard treatment group was 81 years Gender (per cent): 80/131 (61%) women and 51/131 (39%) men Inclusion criteria: age 50 years or older; primary subfoveal CNV lesion in study eye secondary to AMD; occult CNV lesions required evidence of "disease progression," based on deteriorating VA, subretinal or intraretinal blood, or increase in lesion size; evidence of central macular thickening assessed by OCT; lesion in study eye with total size < 12 optic disc areas for minimally classic or occult lesions; area of fibrosis < 25% of total lesion area; area of subretinal blood < 50% of total lesion area; no more than 5400 microns in greater linear dimension for predominantly classic lesions; BCVA of 20/40 to 20/320 on ETDRS chart; no permanent structural damage to central fovea Exclusion criteria: surgery or other treatment in study eye; participation in any other clinical trial of anti‐angiogenic agents or (within previous month) of investigational drugs; primarily hemorrhagic lesion; coexisting ocular disease; premenopausal women not using adequate contraception; current treatment for active systemic infection; history of cardiac event (myocardial infarction, unstable angina) or cerebrovascular event in preceding 6 months; history of allergy to fluorescein; inability to obtain fundus photographs or fluorescein angiograms of sufficient quality to be analyzed and graded; inability to comply with study or follow‐up procedures Equivalence of baseline characteristics: yes Diagnoses in participants: 3/4 (75% of bevacizumab group and 76% of standard treatment group) had "minimally classic‐occult" CNV; remainder of participants had predominantly classic CNV |
|
| Interventions |
Intervention 1: bevacizumab: 3 initial injections every 6 weeks (1.25 mg in 0.05 mL per injection). "After the first three injections, investigators masked to treatment allocation used standardized criteria to decide whether to give further injections... Patients could therefore receive between three and nine injections over a total of 54 weeks." PRN after first 3 injections
Intervention 2: standard treatment group: 1 of 3 treatment options decided for each participant before randomization based on eligibility for NHS coverage of treatments
Follow‐up Planned length: 54 weeks Actual length: 96% followed to week 54 Frequency of assessments for retreatment: 6‐week intervals |
|
| Outcomes |
Primary outcome, as defined: proportion of participants gaining 15 or more letters of BCVA at 1 year (54 weeks), as measured on an ETDRS chart
Secondary outcomes, as defined: proportions of participants gaining 10 or more letters of BCVA at 6 months and 1 year (54 weeks), and proportions of participants gaining 5 or more letters of BCVA at 6 months and 1 year (54 weeks), as measured on an ETDRS chart; proportion with stable vision (defined as loss of < 15 letters); mean change in VA at 12 months; mean change in macular thickness from baseline to 6‐ and 12‐month examinations; contrast sensitivity (Pelli‐Robson charts), unspecified outcome definition and time; reading ability (maximum reading speed, critical print size, and reading acuity) using Minnesota Reading cards; unspecified outcome definition and time
Adverse events Intervals at which outcomes assessed: 1 week (safety visit); 6, 12, 18, 24, 30, 36, 42, 48 weeks (treatment or assessment for treatment); 1 year (54 weeks) |
|
| Notes |
Full study name: The Avastin (bevacizumab) for Choroidal Neovascularization (ABC) Trial Trial registration: ISRCTN83325075 Funding sources: special trustees of Moorfields Eye Hospital; Department of Health, through an award by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology; additional support from the National Eye Research Centre, Bristol Declarations of interest: "The authors who work at Moorfields Eye Hospital have no financial gain from this endeavour, and no patents or patent applications with regard to bevacizumab are owned by the authors or Moorfields Pharmaceuticals"; "The pharmaceutical division at Moorfields (Moorfields Pharmaceuticals) is involved in the repackaging of bevacizumab for intraocular use for sale to other institutions"; various authors reported being on advisory boards for Novartis, Pfizer, GSK, MSD, and/or Allergan; receiving research grants for investigator sponsored trials, money, travel grants, and/or lecture fees from Novartis; and/or being a shareholder of a software company that has business links with Novartis and Pfizer Study period: August 2006 to November 2008 (enrollment August 2006 to November 2007) Reported subgroup analyses: by type of neovascular lesion (minimally classic/occult; predominantly classic); type of standard treatment Contacting study investigators: trial authors contacted; no additional information provided for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to treatment groups by minimisation ‐ a dynamic process" |
| Allocation concealment (selection bias) | Low risk | "The trial manager telephoned the clinical trials unit to obtain a treatment allocation" |
| Masking of participants (performance bias) | Low risk | "To maintain masking, patients randomized to bevacizumab received sham treatments if they did not require intravitreal treatment at that visit" Participants also received placebo PDT therapy if in the bevacizumab group; "care was taken to ensure that the intravenous infusion pump and line were covered as the active verteporfin solution is green while the placebo infusion is a clear solution" |
| Masking of study personnel (performance bias) | Low risk | Treating physicians were not masked; however, "investigators masked to treatment allocation used standardised criteria to decide whether to give further injections" in the bevacizumab group |
| Masking of outcome assessment (detection bias) | Low risk | "We assured outcome assessors were masked to treatment allocation by the use of a standard operating procedure that kept the outcome assessors out of contact with treating physicians and unable to obtain access to the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants in the standard treatment group and 1 participant in the bevacizumab group were without 54‐week VA outcome data. Intent‐to‐treat analysis was followed using last observation carried forward for missing data |
| Selective reporting (reporting bias) | Unclear risk | Study outcomes were published in a design and methods paper. We identified published results for these outcomes, with the exception of outcomes related to reading ability (maximum reading speed, critical print size, and reading acuity) |
| Other bias | Low risk | The standard therapy group did not receive the same intervention (PDT, pegaptanib injection, or sham injection) |
ANCHOR 2006.
| Methods |
Number randomized (total and per group): 423 participants randomly assigned to study treatment: 140 to 0.3 mg ranibizumab, 140 to 0.5 mg ranibizumab, and 143 to verteporfin PDT Exclusions after randomization: 3 participants in the 0.3 mg ranibizumab group did not receive treatment after randomization: 1 because of participant's decision and 2 based on physician's decision Number analyzed (total and per group): 422 total participants: 140 in 0.3 mg ranibizumab group, 139 in 0.5 mg ranibizumab group, and 143 in verteporfin PDT group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 10 in 0.3 mg ranibizumab group, 5 in 0.5 mg ranibizumab group, and 10 in verteporfin PDT group; reasons included death, adverse events, loss to follow‐up, participant's decision, physician's decision, and participant non‐compliance Compliance: limited information given: "more than 90% of patients in each group (91.5% overall) were receiving treatment at 12 months" Intention‐to‐treat analysis: yes; using last observation carried forward for missing data Reported power calculation: yes; sample of 426 participants to provide power of 96% to detect or rule out differences in proportions of participants losing fewer than 15 letters at 12 months, assuming 67% of participants in the PDT control arm and 84% in the ranibizumab arms will have that outcome (α ≤ 0.05) Study design comment: randomization stratified by study center and baseline visual acuity |
|
| Participants |
Country: USA, France, Germany, Hungary, Czech Republic, and Australia (83 study centers) Age: mean (range) was 77 years (54 to 97) in 0.3 mg ranibizumab group, 76 years (54 to 93) in 0.5 mg ranibizumab group, and 78 years (53 to 95) in verteporfin PDT group Gender (per cent): 211/423 (50%) women and 212/423 (50%) men Inclusion criteria: age 50 years or older; subfoveal CNV lesion secondary to AMD determined independently on the basis of fluorescein angiography and fundus photography to be predominantly classic in composition and suitable for treatment with verteporfin PDT; ≤ 5400 microns in greater linear dimension; BCVA of 20/40 to 20/320 Snellen using equivalent ETDRS charts; no permanent structural damage to central fovea; participants with juxtafoveal or extrafoveal photocoagulation in the study eye more than 1 month before day 0 and prior verteporfin PDT in the non‐study eye more than 7 days before study day 0 were included Exclusion criteria: surgery or other treatment in study eye; treatment with verteporfin PDT in the non‐study eye less than 7 days preceding study day 0; participation in any other clinical trial of anti‐angiogenic agents or (within previous month) of investigational drugs; subretinal hemorrhage in study eye 50% or more of lesion area; subfoveal fibrosis or atrophy in study eye; coexisting ocular disease; premenopausal women not using adequate contraception; current treatment for active systemic infection; history of other disease, metabolic dysfunction, or physical examination or laboratory finding giving reasonable suspicion of a condition that contraindicates use of an investigational drug or that might affect interpretation of results of the study or might place the participant at high risk for complications; history of allergy to fluorescein; inability to obtain fundus photographs or fluorescein angiograms of sufficient quality to be analyzed and graded; inability to comply with study or follow‐up procedures Equivalence of baseline characteristics: a slightly higher percentage of participants in 0.3 mg ranibizumab group were aged 75 to 84 years (60%, compared with 45.7% in 0.5 mg group and 51.7% in verteporfin PDT group) Diagnoses in participants: 410/423 (97%) had predominantly classic CNV (> 95% of each treatment group); 12/423 (3%) had minimally classic CNV; and 1/423 (0.2%) had occult with no classic CNV |
|
| Interventions |
Intervention 1: 0.3 mg ranibizumab monthly intravitreal injections plus sham verteporfin PDT (intravenous infusion of saline followed by laser irradiation of macula), need for retreatment based on assessment of fluorescein angiograms at 3‐month intervals
Intervention 2: 0.5 mg ranibizumab monthly intravitreal injections plus sham verteporfin PDT when needed for retreatment, as above
Intervention 3: sham intravitreal injection plus active verteporfin PDT (laser irradiation of macula following intravenous administration of verteporfin)
Ranibizumab was injected into the study eye at monthly intervals (ranging from 23 to 37 days) for a total of 12 injections in the first year beginning on day 0. Verteporfin or sham verteporfin PDT was administered on day 0 and then if needed on the basis of investigators' evaluation of angiography at month 3, 6, 9, or 12
Follow‐up Planned length: 2 years Actual length: 2 years Frequency of assessments for retreatment: 3‐month intervals for PDT and sham PDT |
|
| Outcomes | Primary outcome, as defined: proportion of participants losing fewer than 15 letters from baseline visual acuity in the study eye at 12 months Secondary outcomes reported: proportion of participants gaining 15 or more letters from baseline; proportion of participants with a Snellen equivalent of 20/40 or better; proportion of participants with a Snellen equivalent of 20/200 or worse; mean change from baseline (letters over time); mean change from baseline to month 12 in size of the classic CNV component and total area of leakage from CNV Exploratory efficacy endpoints: loss of 30 or more letters of visual acuity, mean changes in area of CNV and area of the entire lesion Safety assessments: IOP measurement before and 50 to 70 minutes after each study treatment, ocular and non‐ocular adverse events, changes and abnormalities in clinical laboratory parameters and vital signs, and immunoreactivity to ranibizumab Quality of life indicators Intervals at which outcomes were assessed: "at regularly scheduled study visits"; at 12 and 24 months; angiography evaluation was performed at months 3, 6, 9, and 12 | |
| Notes |
Full study name: Anti‐VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age‐Related Macular Degeneration (ANCHOR) Trial Trial registration: NCT00061594 Funding sources: Genentech, USA, and Novartis Pharma, Switzerland Declarations of interest: several authors reported that they received consulting fees from Genentech, Eyetech, Novartis, Allergan, Alcon, Thea, Alimera, Oxigene, Genzyme, iScience, ISTA, Regeneron, Theragenics, VisionCare, and/or Jerini; lecture fees from Genentech, Eyetech, Novartis, Allergan, Pfizer, Alcon, Thea, and/or Jerini; grant support from Alcon, Acuity Pharmaceuticals, Allergan, Alimera, Eyetech, Pfizer Novartis, Genentech, Eli Lilly, Oxigene, or the Diabetic Retinopathy Clinical Research network; and/or that they had an equity interest in Pfizer or were full‐time employees of Genentech, held an equity interest in the company, and had received stock options Study period: May 2003 to September 2006 Reported subgroup analyses: analyses of visual acuity outcome by baseline age, visual acuity, and CNV lesion type reported and specified as retrospective analyses in Kaiser 2007 (referenced under ANCHOR 2006) Contacting study investigators: trial authors were contacted and contributed information for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A dynamic randomization method was used, stratified by study center and visual acuity scores on day 0 (< 45 letters vs ≥ 45 letters) "Dynamic randomization, a generalization of the hierarchical method proposed by Signorini, et al (1993)" (email communication with Genentech, dated 24 October 2007) |
| Allocation concealment (selection bias) | Low risk | "A centralized IVRS was used to conduct the randomization. Participants, study site personnel, and Sponsors’ personnel were masked to the treatment assignment throughout the study, except for the injecting physician, designated unmasked site personnel, and Sponsors’ drug accountability monitors" (email communication with Genentech, dated 24 October 2007) |
| Masking of participants (performance bias) | Low risk | "To maintain masking, patients who had received saline as well as those who had received verteporfin were instructed to follow exposure‐to‐light‐precautions after PDT administration according to the verteporfin package insert" "An empty, needle‐less syringe was used for sham injections, with pressure applied to the anesthetized and prepared eye at the site of a typical intravitreal injection. Pre‐ and post‐injection procedures (described previously) were identical for ranibizumab and sham injections" |
| Masking of study personnel (performance bias) | Low risk | "The 'injecting' ophthalmologist administering the study treatments was unmasked. All other study site personnel (except those assisting with study treatment administration), patients, and central reading center personnel were masked to treatment assignment" |
| Masking of outcome assessment (detection bias) | Low risk | "Double masking of treatment assignment necessitated at least two investigators per study site: an unmasked 'injecting' ophthalmologist to administer the study treatments and a masked 'evaluating' ophthalmologist to perform study assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Efficacy analyses were performed on an intent‐to‐treat basis (including all randomized patients and according to the treatment group to which they were assigned) using a last‐observation‐carried‐forward method to impute missing data (primary analysis) and using observed data (exploratory sensitivity analysis)" |
| Selective reporting (reporting bias) | Low risk | We did not have access to the protocol. However, primary and secondary outcomes reported to the FDA were reported in the publication with no changes |
| Other bias | Unclear risk | Sponsored by Genentech and Novartis Pharma |
Biswas 2011.
| Methods |
Number randomized (total and per group): 120 participants randomly assigned to study treatment: 60 in bevacizumab group and 60 in ranibizumab group Exclusions after randomization: none Number analyzed (total and per group): 104 total participants who completed 18 months of follow‐up: 50 in bevacizumab group and 54 in ranibizumab group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 16 participants by 18 months: reasons for losses to follow‐up not reported (10 in bevacizumab group, 6 in ranibizumab group) Compliance: 104/120 participants completed the 18‐month study Intention‐to‐treat analysis: no; 16 participants enrolled and randomized were not included in analysis Reported power calculation: no; "aimed to enroll a total of 120 patients...this number was arrived at by the investigators after considering the sample size of the available literature of relevant studies" Study design comment: see "Risk of bias" table regarding randomization logistics |
|
| Participants |
Country: 2 study centers in Kolkata, India Age: not reported for 120 enrolled participants (mean 64.4 years in analyzed bevacizumab group; mean 63.5 years in analyzed ranibizumab group) Gender (per cent): not reported for 120 enrolled participants (28/50 [56%] men and 22/50 [44%] women in analyzed bevacizumab group; 22/54 [41%] men and 32/54 [59%] women in analyzed ranibizumab group) Inclusion criteria: age 50 years or older; presence of subfoveal or juxtafoveal CNV of any type; active leakage pattern; baseline BCVA between 35 and 70 ETDRS letters; baseline central macular thickness ≥ 250 μm, as measured by OCT Exclusion criteria: previous treatment for CNV in either eye; macular scarring; any coexisting other ocular disease or pathology; monocular patients; history of ocular surgery within 6 months of enrollment; history of cerebrovascular accident and myocardial infarction Equivalence of baseline characteristics: gender imbalance between analyzed groups Diagnoses in participants: all with subfoveal or juxtafoveal CNV; 22/50 participants with occult CNV in bevacizumab group and 24/54 participants with occult CNV in ranibizumab group |
|
| Interventions |
Intervention 1: 1.25 mg intravitreal bevacizumab every month for first 3 months; retreatment afterward based on OCT or VA changes
Intervention 2: 0.5 mg intravitreal ranibizumab every month for first 3 months; retreatment afterward based on OCT or VA changes
Length of follow‐up Planned: 18 months Actual: 18 months |
|
| Outcomes |
Primary outcomes, as defined: "changes in BCVA and CMT from baseline (month 0) to month 18"
Secondary outcomes, as reported: blood pressure measurements; reports of unusual extremity pain
Adverse events Intervals at which outcome assessed: monthly through 18 months |
|
| Notes |
Trial registration: not reported Funding sources: reported "nil" Declarations of interest: "none declared" Study period: April 2007 to April 2009 Reported subgroup analyses: for participants with predominantly classic CNV Contacting study investigators: trial authors contacted; no additional information provided for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using random numbers tables, 60 numbers were randomly picked up from 1 to 120 and assigned to group A while the remaining sixty numbers were assigned to group B" |
| Allocation concealment (selection bias) | Unclear risk | "...randomization of the 120 numbers into two groups was done before initiation of enrolment itself. Upon initiation of enrollment, the patients were numbered sequentially based on the serial order of enrolment in the study. Depending on the enrolment number, the patients were automatically assigned to either group A or B based on the prior randomization of number 1‐120 into two equal groups using random number tables" |
| Masking of participants (performance bias) | Unclear risk | Masking of participants not reported |
| Masking of study personnel (performance bias) | Low risk | "The injections were given...by the investigators, who were blinded to the type of injection" |
| Masking of outcome assessment (detection bias) | Low risk | "All assessors were masked to the group of patients they were following up" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sixteen (13%) participants lost to follow‐up were excluded from analyses: 10 in the bevacizumab group and 6 in the ranibizumab group |
| Selective reporting (reporting bias) | Unclear risk | No protocol nor clinical trial registration was identified for this study. Outcomes were reported for stated outcomes in the Methods section of the published report; however, only P values were reported for between‐group comparisons, and no standard deviation or variance measures were reported for continuous outcomes |
| Other bias | Low risk | None observed |
BRAMD 2016.
| Methods |
Number randomized (total and per group): 332 participants randomly assigned to study treatment: 166 in bevacizumab group and 166 in ranibizumab group Exclusions after randomization: 5 participants in bevacizumab group excluded because they did not receive allocated treatment and dropped out after first injection Number analyzed (total and per group): 327 participants (161 in bevacizumab group and 166 in ranibizumab group) for all outcomes at 12 months; as participants who were lost to follow‐up, their last observation was carried forward Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 63 participants discontinued intervention owing to adverse events or refusal to continue treatment (34 in bevacizumab group and 29 in ranibizumab group) Compliance: 17 participants switched to other treatment owing to a drop in visual acuity, according to the protocol (9 in bevacizumab group and 8 in ranibizumab group) Intention‐to‐treat analysis: no; not all participants enrolled and randomized were included in the analyses Reported power calculation: yes; sample of 153 participants per group for power of 80% to conclude non‐inferiority (< 4 letters difference between drugs) at P < 0.05 Unusual study design: "the patient was labeled as a poor‐responder and treatment was changed to the other drug, if at any visit after the third injection there was a drop in BCVA of more than 10 letters compared to baseline and there was clear evidence of active CNV or leakage by qualitative SD‐OCT and/or FA assessment or at least two of the following signs of leakage on OCT; central retinal thickening >300 micron (CRT), intraretinal cysts or subretinal fluid any time after the third injection" |
|
| Participants |
Country: Netherlands Age: mean was 79 years in the bevacizumab group and 78 years in ranibizumab group Gender (per cent): 72/161 (45%) men and 89/161 (55%) women in bevacizumab group and 73/166 (44%) men and 93/166 (56%) women in ranibizumab group Inclusion criteria: "patients 60 years of age or higher; patients with primary or recurrent sub‐, juxta‐ or extrafoveal CNV secondary to AMD, including those with RAP, that may benefit from anti‐VEGF treatment in the opinion of the investigator; patients with primary or recurrent sub‐, juxta‐ or extrafoveal CNV secondary to AMD, including those with RAP, that may benefit from anti‐VEGF treatment in the opinion of the investigator; the total area of CNV (including both classic and occult components) encompassed within the lesion must be more or equal to 30% of the total lesion area; the total lesion area should be < 12 disc areas; a best corrected visual acuity (BCVA) score between 78 and 20 letters (approximately 0.63–0.05 Snellen equivalent) in the study eye" Exclusion criteria: "Ocular treatment with anti‐angiogenic drugs in the last 2 months or triamcinolone in the last 6 months; laser photocoagulation (juxtafoveal or extrafoveal) in the study eye within one month preceding baseline; patients with angioid streaks or precursors of CNV in either eye due to other causes, such as ocular histoplasmosis, trauma, or pathologic myopia; spherical equivalent of refractive error in the study eye demonstrating more than 8 diopters of myopia; cataract extraction within three months preceding baseline; IOP >25 mm Hg; active intraocular inflammation in the study eye; vitreous haemorrhage obscuring view of the posterior pole in the study eye; presence of a retinal pigment epithelial tear involving the macula in the study eye; subretinal haemorrhage in the study eye if the size of the haemorrhage is > 70% of the lesion; subfoveal fibrosis or atrophy in the study eye; history of hypersensitivity or allergy to fluorescein; inability to obtain fundus photographs, fluorescein angiograms or OCT’s of sufficient quality to be analyzed and graded by the Central Reading Centre; systemic disease with a life expectancy shorter than the duration of the study; inability to adhere to the protocol with regard to injection and follow‐up visits; legally incompetent adult; refusal to give written informed consent" Equivalence of baseline characteristics: yes Diagnoses in participants: 27% had predominantly classic CNV; 16% had minimally classic CNV; and 57% had occult CNV |
|
| Interventions |
Intervention 1: monthly injections (window, 30 ± 7 days) with 1.25 mg of bevacizumab Intervention 2: monthly injections (window, 30 ± 7 days) with 0.5 mg ranibizumab. Length of follow‐up Planned: 1 year Actual: 1 year |
|
| Outcomes |
Primary outcome, as defined: change in BCVA in the study eye from baseline to 12 months assessed with ETDRS‐like visual acuity charts at an initial distance of 4 meters
Secondary outcomes, as defined: proportion of participants with loss of BCVA < 15 letters from baseline at 12 months (responders); proportion of participants with loss or gain of BCVA < 15 letters from baseline at 12 months (stabilizers); proportion of participants with loss of 15 or more letters of BCVA from baseline at 12 months (losers); proportion of participants with gain of 15 or more letters of BCVA from baseline at 12 months (gainers); absolute and percentage changes in CRT, as measured by SD‐OCT at 4 and 12 months, as determined by the Study Reading Centre at the AMC; proportion of dropouts before final 12‐month assessments; proportion of switchers after third injection; occurrence of (serious) adverse events during 12 months of the study and costs of the 2 treatments Intervals at which outcome assessed: monthly through 12 months |
|
| Notes |
Full study name: Comparison of Bevacizumab (Avastin) and Ranibizumab (Lucentis) in Exudative Age‐related Macular Degeneration Trial registration: NTR1704 Funding sources: "this work was funded by The Netherlands Organisation for Health Research and Development (http://www.zonmw.nl/en/) (r.s.). This study was supported by Dutch health insurance companies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript" (page 1) Declarations of interest: "the authors have declared that no competing interests exist" (page 1) Study period: enrollment January 2009 to December 2011 Reported subgroup analyses: yes; treatment‐naive participants or participants with a history of treatment Contacting study investigators: trial authors not contacted as data were available in published reports Trial registration number: NTR1704 (Trialregister.nl) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation list was created in a 1:1 ratio by the TENALEA Clinical Trial Data Management System” |
| Allocation concealment (selection bias) | Low risk | “Upon randomization of a patient, an automatized email notification containing the allocation result was sent to the site's pharmacy keeping the investigator and trial personnel blinded from treatment allocation” |
| Masking of participants (performance bias) | Low risk | Triple‐masked study; “Syringes were only labelled with the patient identification number”; “Besides the identical syringes, masking was also ensured by the fact that the ophthalmologists who performed the injections did not take part in interpretation of any data or patient assessment”; “The randomization list was imported into the data management system Oracle Clinical. Upon randomization of a patient, an automatized email notification containing the allocation result was sent to the site's pharmacy, keeping the investigator and trial personnel blinded from treatment allocation” |
| Masking of study personnel (performance bias) | Low risk | Triple‐masked study; “Syringes were only labelled with the patient identification number”; “Besides the identical syringes masking was also ensured by the fact that the ophthalmologists who performed the injections did not take part in interpretation of any data or patient assessment”; “The randomization list was imported into the data management system Oracle Clinical. Upon randomization of a patient, an automatized email notification containing the allocation result was sent to the site's pharmacy keeping the investigator and trial personnel blinded from treatment allocation" |
| Masking of outcome assessment (detection bias) | Low risk | Triple‐masked study; “Syringes were only labelled with the patient identification number”; “Besides the identical syringes masking was also ensured by the fact that the ophthalmologists who performed the injections did not take part in interpretation of any data or patient assessment”; “The randomization list was imported into the data management system Oracle Clinical. Upon randomization of a patient, an automatized email notification containing the allocation result was sent to the site's pharmacy keeping the investigator and trial personnel blinded from treatment allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/332 (1.5%) participants were excluded from the study, all in the bevacizumab group. At 12 months, 63 participants did not have outcome data; last observation carried forward method was used to impute missing data for these 63 participants (34/161 in bevacizumab group and 29/166 in ranibizumab group) |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes specified a priori for 1‐year follow‐up were reported |
| Other bias | Low risk | None observed |
CATT 2011.
| Methods |
Number randomized (total and per group): 1208 participants randomly assigned to study treatment; number of participants randomized per group not reported Exclusions after randomization: 1 study center (23 participants) was excluded owing to protocol violations Number analyzed (total and per group): 1105 total participants: 265 in bevacizumab monthly group, 284 in ranibizumab monthly group, 271 in bevacizumab as needed group, and 285 in ranibizumab as needed group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 80 total participants: 21 in bevacizumab monthly group (4 died and 17 with missing data), 17 in ranibizumab monthly group (4 died and 13 with missing data), 29 in bevacizumab as needed group (11 died and 18 with missing data), 13 in ranibizumab as needed group (5 died and 8 with missing data) Compliance: limited information given: mean of 11.9 treatments given for bevacizumab monthly group, and mean of 11.7 treatments given for ranibizumab monthly group Intention‐to‐treat analysis: no; 103 participants enrolled and randomized were not included in the analyses Reported power calculation: yes; sample of 277 participants per group for power of 90% Study design comment: non‐inferiority design, 4 arms, 6 pairwise comparisons planned; at 1 year, participants in monthly dose treatment groups were re‐randomized to continue with monthly injections or switch to as‐needed injections of the same treatment drug |
|
| Participants |
Country: USA Age: mean was 80 years in bevacizumab monthly group, 79 years in ranibizumab monthly group, 79 years in bevacizumab as needed group, and 78 years in ranibizumab as needed group Gender (per cent): 732/1185 (61.8%) women and 453/1185 (38.2%) men Inclusion criteria: age 50 years or older; 1 study eye per participant with untreated active CNV due to AMD (based on presence of leakage as seen by fluorescein angiography and of fluid as seen by OCT); VA of 20/25 to 20/320 on electronic visual acuity testing Exclusion criteria: fibrosis or atrophy in center of fovea in the study eye; CNV in either eye due to other causes; retinal pigment epithelial tear involving the macula; any concurrent intraocular condition in the study eye (e.g. cataract, diabetic retinopathy) that, in the opinion of the investigator, could require medical or surgical intervention or contribute to VA loss during 3‐year follow‐up period; active or recent (within 4 weeks) intraocular inflammation; current vitreous hemorrhage in study eye; history of rhegmatogenous retinal detachment or macular hole; active infectious conjunctivitis, keratitis, scleritis, or endophthalmitis; spherical equivalent > 8 diopters; intraocular surgery (including cataract surgery) in study eye within 2 months; uncontrolled glaucoma; participants unable to be photographed to document CNV owing to known allergy to fluorescein dye, lack of venous access, or cataract obscuring the CNV; premenopausal women not using adequate contraception; pregnancy or lactation; history of other disease, metabolic dysfunction, physical examination finding, or clinical laboratory finding giving reasonable suspicion of a disease or condition that contraindicates use of an investigational drug, or that might affect interpretation of study results or render the individual at high risk for treatment complications; current treatment for active systemic infection; uncontrolled concomitant disease such as cardiovascular disease; nervous system, pulmonary, renal, hepatic, endocrine, or gastrointestinal disorders; history of recurrent significant infection or bacterial infection; inability to comply with study or follow‐up procedures Equivalence of baseline characteristics: slightly higher percentage of participants in bevacizumab monthly group had history of transient ischemic attack (8.7% vs 4% in ranibizumab monthly group, 4% in ranibizumab as‐needed group, and 6.3% in bevacizumab as‐needed group) Diagnoses in participants: 688/1185 (58%) had active neovascular AMD with CNV in foveal center; 315/1185 (27%) had fluid in foveal center; 93/1185 (8%) had hemorrhage in foveal center; 71/1185 (6%) had other foveal center involvement; and 18/1185 (1.5%) had no CNV or not possible to grade |
|
| Interventions |
Intervention 1: 1.25 mg per 0.05 mL intravitreal bevacizumab injections on a fixed schedule of every 4 weeks for 1 year; at 1 year, re‐randomization to bevacizumab every 4 weeks or as needed Intervention 2: 0.5 mg intravitreal ranibizumab injections on a fixed schedule of every 4 weeks for 1 year; at 1 year, re‐randomization to ranibizumab every 4 weeks or as needed Intervention 3: 1.25 mg intravitreal bevacizumab as needed for 2 years Intervention 4: 0.5 mg intravitreal ranibizumab as needed for 2 years Length of follow‐up Planned: 12 months for primary analysis; 24 months for secondary analyses, with modifications to 2 intervention arms, as described above Actual: 12 months for primary analysis; 24 months for secondary analyses |
|
| Outcomes | Primary outcome, as defined: change in visual acuity from baseline at 12 months with a non‐inferiority margin of 5 letters Secondary outcomes: proportion of eyes with 15‐letter change, number of injections, OCT measured change in foveal thickness, change in lesion size on OCT and also on fluorescein angiography, incidence of ocular and systemic adverse events, annual drug cost Intervals at which outcomes were assessed: weeks 4, 12, 24, 36, and 52 during first year for visual acuity; weeks 4, 8, 12, 24, and 52 for changes on OCT | |
| Notes |
Full study name: Comparison of Age‐related Macular Degeneration Treatment Trials Trial registration: NCT00593450 Funding: National Eye Institute, National Institutes of Health, USA Declarations of interest: 1 investigator reported receiving consulting fees from GlaxoSmithKline and other consulting fees from Neurotech and SurModics Study period: accrual February 2008 through December 2009; follow‐up through December 2011 Reported subgroup analyses: none, but risk factors for 2‐year VA outcomes have been reported (Ying 2015 under CATT 2011) Contacting study investigators: trial authors not contacted as data were available in published reports |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to 1 of 4 study groups. Randomization schedules were stratified according to clinical center with the use of a permuted‐block method with randomly chosen block sizes" |
| Allocation concealment (selection bias) | Low risk | Web‐based data entry system was used to allocate participants to treatment groups |
| Masking of participants (performance bias) | Unclear risk | Initially, participants were masked to which drug they received, but not to the treatment schedule. Study investigators noted that "insurance and billing documents specified ranibizumab but not study‐supplied bevacizumab. Therefore, patients may have learned or deduced their assigned drug from these financial documents" |
| Masking of study personnel (performance bias) | Low risk | Physicians were masked to drug but not to injection schedule. Physicians were uninvolved in visual acuity testing and in secondary outcome assessments |
| Masking of outcome assessment (detection bias) | Low risk | Electronic Visual Acuity system (computerized testing) was used for primary outcome. Retinal center personnel were masked. Adverse event reporting was unmasked, but medical monitor who evaluated serious adverse events was masked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 103/1208 (8.5%) participants randomized were not included in 1‐year analysis. At 2 years, outcomes were not available for all participants by their originally assigned treatment groups |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes specified a priori for 1‐year follow‐up were reported |
| Other bias | Low risk | None observed |
GEFAL 2013.
| Methods |
Number randomized (total and per group): 501 participants randomly assigned to study treatment: 255 in bevacizumab group and 246 in ranibizumab group Exclusions after randomization: 16 participants excluded because they received no injection (9 in bevacizumab group and 7 in ranibizumab group) Number analyzed (total and per group): 485 participants (246 in bevacizumab group and 239 in ranibizumab group) for safety analysis at 1 year; 404 participants (207 in bevacizumab group and 197 in ranibizumab group) for analysis on visual acuity at 1 year; most data analyzed for 374 participants (191 in bevacizumab group and 183 in ranibizumab group) with available baseline BCVA data, at least 10 months of follow‐up, and did not have major deviations from study protocol Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 81 total participants: 39 in bevacizumab group and 42 in ranibizumab group; additional 30 participants (16 in bevacizumab group and 14 in ranibizumab group) excluded from most analyses owing to protocol violations Compliance: 374/501 participants completed the study without major protocol violations Intention‐to‐treat analysis: no; not all participants enrolled and randomized were included in analyses Reported power calculation: yes; sample of 200 participants per group for power of 90% to detect 15 letter changes in BCVA Study design comment: non‐inferiority design |
|
| Participants |
Country: France (38 study centers) Age: mean age for 374 participants without major protocol violations was 79 years Gender (per cent): 248/374 (66%) women and 126/374 (34%) men Inclusion criteria: age 50 years or older; active subfoveal neovascular AMD (1 study eye eligible in bilateral cases); lesion size < 12 disk areas; recent development of lesion in cases of occult neovessels; BCVA of 20/32 to 20/320 on ETDRS scale Exclusion criteria: subretinal hemorrhage reaching foveal center and > 50% of lesion area; fibrosis or atrophy at center of fovea in study eye; CNV of other pathogenesis; retinal pigment epithelial tear reaching macula; previous or current treatment with intravitreal anti‐VEGF therapy; history of treatment 3 months before or intraocular surgery 2 months before first study injection; history of photocoagulation or intravitreal medical device in study eye; ocular or periocular infection; intraocular inflammation; diabetic retinopathy; history of autoimmune or idiopathic uveitis; IOP ≥ 25 mmHg with topical hypotensive therapy; aphakia or lack of lens capsule in study eye; known illness or condition requiring intraocular surgery within 12 months; known hypersensitivity to study drugs or allergy to agents used for ocular testing; uncontrolled arterial hypertension; history of treatment with systemic bevacizumab; premenopausal women not using adequate contraception; involvement in another clinical study; not part of French national health insurance program Equivalence of baseline characteristics: yes Diagnoses in participants: 354/374 (95%) had intraretinal and/or subretinal fluid on OCT |
|
| Interventions |
Intervention 1: 1.25 mg in 0.05 mL intravitreal bevacizumab injections every month for first 3 months; retreatment afterward based on OCT or VA changes
Intervention 2: 0.50 mg intravitreal ranibizumab injections every month for first 3 months; retreatment afterward based on OCT or VA changes Length of follow‐up Planned: 1 year Actual: 1 year |
|
| Outcomes |
Primary outcome, as defined: mean change in BCVA at 1 year (at least 10 months after inclusion), as measured on ETDRS chart
Secondary outcomes, as defined in published reports: visual acuity outcomes at 1 year: BCVA, change in BCVA, proportion with gain of ≥ 15 letters, proportion with loss of ≥ 15 letters, proportion with gain of ≥ 5 letters, proportion with loss of ≥ 5 letters; change in CNV area between baseline and final evaluations; presence of intraretinal and/or subretinal fluid; presence of pigment epithelial detachment; central subfield macular thickness; change in central subfield macular thickness; dye leakage on angiogram; number of injections; model of OCT equipment; adverse events Secondary outcomes, as defined in trial registry: efficacy of treatments at 1 year; proportions of ocular and systemic adverse events at 1 year; average number of injections and time before re‐injection during 1 year; drug profiles in blood and aqueous humor of a subset of 20 participants at 3 months; medico‐economic impact of treatments at 1 year Intervals at which outcomes were assessed: monthly through 12 months |
|
| Notes |
Full study name: Groupe d’Etude Français Avastin versus Lucentis dans la DMLA Néovasculaire Trial registration: NCT01170767 Funding sources: French Ministry of Health (Programme Hospitalier de Recherche Clinique National 2008); the French Health Insurance System co‐financed the study and funded study drugs Declarations of interest: 4 study authors declared disclosures as serving as principal investigators for trials sponsored by Novartis, Bausch & Lomb, Théa, and Alcon; serving on advisory boards for Alcon, Allergan, Bayer, Bausch & Lomb, Novartis, and Théa; receiving lecture fees from Alcon, Allergan, Bayer, Bausch & Lomb, Heidelberg Engineering, the Krys group, Novartis, Théa, and Zeiss; receiving consulting fees from Novartis, Bayer, and Allergan; or receiving honoraria from Novartis, Bayer, and Allergan; the other 4 study authors declared no conflicts of interest Study period: random enrollment June 24, 2009, to November 9, 2011 Reported subgroup analyses: none Contacting study investigators: trial authors contacted and contributed information for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was stratified by center and visual acuity (threshold: 20/100). Local hospital pharmacies were responsible for randomizing patients in each center using pre‐established lists" |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy used to conceal treatment assignments before participant enrollment and randomization (email communication with Dr. Kodjikian, dated August 7, 2014) |
| Masking of participants (performance bias) | Low risk | "Identical syringes were masked and delivered by local hospital pharmacies after aseptic preparation in authorized, centralized drug‐preparation units, using vials of Avastin 100 mg/ml and Lucentis 10 mg/ml" "The main strength of the GEFAL trial is that the study remained effectively double‐masked, unlike CATT, in which some participants received billing information, and IVAN, in which the masking differed between centers (some treating teams were aware of treatment allocation)" |
| Masking of study personnel (performance bias) | Low risk | "Identical syringes were masked and delivered by local hospital pharmacies after aseptic preparation in authorized, centralized drug‐preparation units, using vials of Avastin 100 mg/ml and Lucentis 10 mg/ml" "The main strength of the GEFAL trial is that the study remained effectively double‐masked, unlike CATT, in which some participants received billing information, and IVAN, in which the masking differed between centers (some treating teams were aware of treatment allocation)" |
| Masking of outcome assessment (detection bias) | Low risk | Only pharmacists who prepared the syringes knew about randomization assignments; ophthalmologists, study co‐ordinators, and all outcome assessors were masked like participants (email communication with Dr. Kodjikian, dated August 7, 2014) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16/501 (3%) participants randomized were not included in any analysis; most analyses reported did not include 127/501 (25%) participants |
| Selective reporting (reporting bias) | Unclear risk | Differences in outcomes between trial registration and published 1‐year results papers included the following: • Secondary visual acuity and morphology outcomes were specified clearly in the paper, but were described only as "efficacy of treatments" in trial registration • Published paper included model of OCT equipment as outcome, whereas trial registration did not • Trial registration included time before reinjection during 1 year, drug profiles in blood and aqueous humor of a subset of 20 participants at 3 months, and medico‐economic impact of treatments as outcomes, whereas published paper did not |
| Other bias | Low risk | None observed |
IVAN 2013.
| Methods |
Number randomized (total and per group) Drug randomization: 628 total participants: 305 to bevacizumab group and 323 to ranibizumab group Regimen randomization: 294/305 in bevacizumab group and 312/323 in ranibizumab group completed first 3 injections and were randomized to continue or discontinue treatment: 149 continued bevacizumab; 145 discontinued bevacizumab; 157 continued ranibizumab; and 155 discontinued ranibizumab Exclusions after randomization: 18 participants did not receive treatment and were excluded after randomization to drug treatment (9 in bevacizumab group and 9 in ranibizumab group) Number analyzed (total and per group) At 1‐year follow‐up: 561 total participants at 1 year: 136 in continued bevacizumab group; 138 in discontinued bevacizumab group; 141 in continued ranibizumab group; and 146 in discontinued ranibizumab group At 2‐year follow‐up: 525 total participants at 1 year: 127 in continued bevacizumab group; 127 in discontinued bevacizumab group; 134 in continued ranibizumab group; and 137 in discontinued ranibizumab group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up At 1‐year follow‐up: 49 total participants: 4 participants receiving treatment withdrew before completing third injection (2 in bevacizumab group and 2 in ranibizumab group); 45 participants randomized to regimen groups exited trial before 1 year (13 in continued bevacizumab group; 7 in discontinued bevacizumab group; 16 in continued ranibizumab group; and 9 in discontinued ranibizumab group) At 2‐year follow‐up: 85 total participants: 5 participants receiving treatment withdrew before completing third injection (3 in bevacizumab group and 2 in ranibizumab group); 80 participants randomized to regimen groups exited trial before 2 years (21 in continued bevacizumab group; 18 in discontinued bevacizumab group; 23 in continued ranibizumab group; and 18 in discontinued ranibizumab group) Compliance: wrong study drug was administered twice during first year At 1‐year follow‐up: adherence was 6576/6699 (98%) scheduled injections received At 2‐year follow‐up: adherence was 12761/14,640 (87%) scheduled injections received Intention‐to‐treat analysis: no; 67 participants enrolled and randomized were not included in analyses at 1 year and 103 at 2 years Reported power calculation: yes; sample of 600 participants per group for power of 90% to detect non‐inferiority Study design comment: non‐inferiority design; 2 × 2 factorial design – randomization in 2 stages: first randomized to drug treatment (bevacizumab or ranibizumab), then to treatment regimen (continue monthly injections or discontinue monthly injections and switch to as‐needed injections given in 3‐month cycles); results reported only as bevacizumab vs ranibizumab and continuous vs discontinuous |
|
| Participants |
Country: UK (23 study centers) Age: mean age for 610 participants receiving treatment was 78 years Gender (per cent): 366/610 (60%) women and 244/610 (40%) men Inclusion criteria: age 50 years or older; previously untreated neovascular AMD in study eye with any component of neovascular lesion (CNV, blood, serous pigment epithelial detachment, elevated blocked fluorescence) involving center of the fovea, confirmed by fluorescein angiography; best‐corrected VA of 25 or more letters on ETDRS chart (measured at 1 m) Exclusion criteria: neovascular lesion of 50% or more fibrosis or blood; more than 12 disc diameters; argon laser treatment in study eye within 6 months; presence of thick blood involving center of the fovea; presence of other active ocular disease causing concurrent vision loss; myopia ≥ 8 diopters; previous treatment with PDT or VEGF inhibitor in study eye; women pregnant, lactating, or of child‐bearing potential; men with spouse or partner of child‐bearing potential Equivalence of baseline characteristics: yes Diagnoses in participants: 301/610 (58%) had neovascular AMD with CNV in foveal center; 308/610 (54%) had fluid in foveal center; 90/610 (16%) had hemorrhage in foveal center; 75/610 (13%) had other foveal center involvement; and 15/610 (3%) had no CNV or not possible to grade |
|
| Interventions |
Intervention 1: 1.25 mg in 0.05 mL intravitreal bevacizumab injected monthly for 2 years Intervention 2: 0.5 mg intravitreal ranibizumab injected monthly for 2 years Intervention 3: after first 3 monthly 1.25 mg intravitreal bevacizumab injections, monthly treatment was discontinued and treatment was given as needed in cycles of 3 monthly doses Intervention 4: after first 3 monthly 0.5 mg intravitreal ranibizumab injections, monthly treatment was discontinued and treatment was given as needed in cycles of 3 monthly doses Follow‐up Planned length: 2 years Actual length: 2 years Frequency of follow‐up assessments: monthly |
|
| Outcomes |
Primary outcome, as defined: best‐corrected distance visual acuity measured as ETDRS letters at 2 years Secondary outcomes, as defined in protocol: at 1‐year and 2‐year follow‐up: frequency of adverse effects of treatment; generic and vision‐specific health‐related quality of life; treatment satisfaction; cumulative resource use/cost and cost‐effectiveness; clinical measures of vision (contrast sensitivity measured with Pelli‐Robson charts, near visual acuity measured by Bailey‐Love near reading cards, and reading speed measured with Belfast reading charts); lesion morphology (fluorescein angiography and OCT); distance visual acuity at 1 year; survival free from treatment failure Exploratory analysis: association between serum markers and cardiovascular serious adverse events Intervals at which outcomes were assessed: monthly through 24 months; various data were collected at every visit depending on assessment schedule and regimen group |
|
| Notes |
Full study name: alternative treatments to inhibit VEGF in age‐related choroidal neovascularization Trial registration: ISRCTN92166560 Funding sources: National Institute for Health Research Health Technology Assessment Program, UK Declarations of interest: various study authors reported that they were principal investigators of trials sponsored by Novartis; attending and had been remunerated for attending advisory boards for Novartis, Bayer, Neovista, Oraya, Allergan, and/or Bausch and Lomb; were employed by institution that has received payments from Novartis, Bayer, Neovista, Oraya, Alcon, and/or Pfizer; and had received honoraria from Novartis for lecture and/or teaching fees from Janssen‐Cilag Study period: random enrollment March 27, 2008, to October 15, 2010 Reported subgroup analyses: 3 genetic polymorphisms (Lotery 2013) Contacting study investigators: trial authors not contacted as data were available in published reports |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized allocations were computer generated by a third party in blocks and stratified by center" "Randomisation was stratified by centre and was blocked to ensure roughly equal numbers of participants per group within a centre" |
| Allocation concealment (selection bias) | Low risk | "Research teams at sites recruited participants and accessed a password‐protected website to randomize participants. Allocations were concealed until participants’ eligibility and identities were confirmed" "Allocations were computer generated and concealed with an internet‐based system (Sealed Envelope, London, UK). Staff in participating centres accessed the website and, on entering information to confirm a participant’s identity and eligibility, were provided with the unique study number" |
| Masking of participants (performance bias) | Low risk | From study protocol:
"Participants, clinicians and trial personnel will be masked to the VEGF inhibitor to which a participant is assigned" "We have chosen not to mask participants, clinicians and trial personnel to whether patients are allocated to continue or stop treatment at 3 months" |
| Masking of study personnel (performance bias) | Low risk | "We intended that drug allocation should be concealed by having separate masked assessment and unmasked treating teams. This system was achieved by 14 sites. At the other 9 sites, staffing levels could not support this system and an unmasked staff member prepared ranibizumab in a syringe identical to those containing bevacizumab and did not perform assessments" From study protocol: "We have chosen not to mask participants, clinicians and trial personnel to whether patients are allocated to continue or stop treatment at 3 months" |
| Masking of outcome assessment (detection bias) | Low risk | "We intended that drug allocation should be concealed by having separate masked assessment and unmasked treating teams. This system was achieved by 14 sites. At the other 9 sites, staffing levels could not support this system and an unmasked staff member prepared ranibizumab in a syringe identical to those containing bevacizumab and did not perform assessments" "Lesion morphology was assessed by independent graders masked to drug and treatment regimen" From study protocol: "We have chosen not to mask participants, clinicians and trial personnel to whether patients are allocated to continue or stop treatment at 3 months" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 67/628 (11%) participants randomized were not included in 1‐year analysis; 111/628 (18%) participants randomized were not included in 2‐year analysis |
| Selective reporting (reporting bias) | Unclear risk | Differences between protocol and published 1‐year and 2‐year results papers included the following: • 2 secondary outcomes in the protocol were not listed in paper: treatment satisfaction and survival free from treatment failure • Exploratory (serum) analysis in protocol upgraded to a secondary outcome in paper |
| Other bias | Low risk | None observed |
LUCAS 2015.
| Methods |
Number randomized (total and per group): 441 participants randomly assigned to study treatment: 220 in bevacizumab group and 221 in ranibizumab group Exclusions after randomization: 10 total participants: 7 in bevacizumab group and 3 in ranibizumab group. "All 9 patients from 1 study center were excluded because of serious protocol violations, and 1 patient was excluded after a serious retinal and vitreous hemorrhage..." Number analyzed (total and per group): 371 total participants: 184 in bevacizumab group and 187 in ranibizumab group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: none, but 60 excluded from analysis (29 in bevacizumab group and 31 in ranibizumab group), including 11 total participants who died Compliance: 371/441 participants completed study per protocol Intention‐to‐treat analysis: no; 70 participants enrolled and randomized were not included in analysis Reported power calculation: yes; 181 participants per arm to provide 80% power to detect or rule out a difference in visual acuity outcome, assuming a 10% dropout rate Study design comment: non‐inferiority design using margin of 5 letters on ETDRS chart |
|
| Participants |
Country: 10 clinical centers in Norway Age: mean 78.7 years in bevacizumab group and 78.0 in ranibizumab group Gender (per cent): 140/431 (32.5%) men and 291/431 (67.5%) women Inclusion criteria: age 50 years or older; previously untreated active neovascular AMD in study eye; BCVA in study eye between 20/25 and 20/120, measured at 4 m using an ETDRS "standardized viewer" Exclusion criteria: "pigment epithelial detachments with no associated intraretinal or subretinal edema and lesions comprising more than 50% blood or fibrosis were excluded" Equivalence of baseline characteristics: more participants in ranibizumab group had a history of myocardial infarction Diagnoses in participants: neovascular AMD; 86% had CNV under foveal center |
|
| Interventions |
Intervention 1: 1.25 mg per 0.05 mL intravitreal bevacizumab injections every 4 weeks until no signs of active AMD were found on OCT and biomicroscopic fundus examination, followed by the "treat and extend" protocol Intervention 2: 0.5 mg intravitreal ranibizumab injections every 4 weeks, followed by "treat and extend" protocol The "treat and extend" protocol for each treatment group specified that whenever a new injection was given, the "period" (interval) to the next injection was to be extended by 2 weeks up to a maximum interval of 12 weeks. Whenever recurrent neovascularization was treated, the interval was shortened by 2 weeks until the lesion was inactive. Interval extension was then restarted to a maximum of 2 weeks less than when recurrence was observed Follow‐up Planned length: 24 months Actual length: 12 months Frequency of follow‐up assessments: 4‐week intervals, modified by 2‐week increases or decreases, as described above |
|
| Outcomes |
Primary outcome, as defined: "change in BCVA at 1 year as measured on the ETDRS visual acuity chart" Secondary outcomes, as defined: "number of injections, change in CRT as measured with OCT, and change in lesion size as measured on FA" Safety outcome: occurrence of arteriothrombotic events Intervals at which outcomes were assessed: unclear, but presumably whenever participant was assessed for the need for retreatment |
|
| Notes |
Full study name: Lucentic Compared to Avastin Study Trial registration: NCT01127360 Funding sources: Oslo University Hospital, Oslo, Norway Declarations of interest: "The funding organization had no role in the design of the study but aided in the conduct of the study and data management." One study author had participated in an advisory board meeting for another anti‐VEGF agent for Bayer Study period: random enrollment March 2009 to July 2012 Reported subgroup analyses: none Contacting study investigators: pending |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated by a third party at the Norwegian University of Science and Technology, Trondheim, with the use of the block method and stratification by center" |
| Allocation concealment (selection bias) | Low risk | Drugs were allocated by unmasked study nurses, who were also responsible for aseptic filling of a syringe with assigned study drug. "The identical syringes, regardless of which drug was given, were filled by these nurses behind a screen. The syringe was then presented directly to the treating ophthalmologist" |
| Masking of participants (performance bias) | Low risk | "the patient, the treating ophthalmologist, and the assisting nurse were masked to the drug at all times" |
| Masking of study personnel (performance bias) | Low risk | "These study nurses were not involved in any other patient‐related activities in the study" |
| Masking of outcome assessment (detection bias) | Low risk | "Ophthalmic nurses, who also were masked to the drug and patient records, tested the ETDRS visual acuity" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | About 15% of participants were missing 12‐month outcome data, compared with 10% assumed in sample size calculation |
| Selective reporting (reporting bias) | Low risk | All outcomes specified were reported |
| Other bias | Low risk | No other bias was identified |
MANTA 2013.
| Methods |
Number randomized (total and per group): 321 participants randomly assigned to study treatment; number per group not reported Exclusions after randomization: 4 participants (3 owing to receiving wrong drug and 1 because participant received prior treatment and was not eligible) Number analyzed (total and per group): 317 total participants: 154 in bevacizumab group and 163 in ranibizumab group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 69 participants: reasons for losses to follow‐up not reported (33 in bevacizumab group, 36 in ranibizumab group) Compliance: 248/317 participants completed the study Intention‐to‐treat analysis: no; 4 participants enrolled and randomized were not included in analysis; data imputed using last observation carried forward method for 69 participants lost to follow‐up Reported power calculation: yes; sample of 320 participants for power of 95% Study design comment: non‐inferiority design |
|
| Participants |
Country: 10 clinical centers in Austria Age: mean 76.7 years in bevacizumab group and 77.6 years in ranibizumab group Gender (per cent): 115/317 (36.3%) men and 202/317 (63.7%) women Inclusion criteria: age 50 years or older; active primary or recurrent subfoveal lesion with CNV, measured by fluorescein angiography or OCT; BCVA in study eye between 20/40 and 20/320, measured by ETDRS charts Exclusion criteria: previous treatment for CNV or AMD; prior treatment with any intravitreal drug or verteporfin PDT in study eye; prior treatment with systemic bevacizumab; prior treatment with any intravitreal drug or verteporfin PDT in non‐study eye within 3 months; laser photocoagulation in study eye within 1 month; participation in another clinical trial within 1 month; subfoveal fibrosis or atrophy > 50% in study eye; CNV in either eye due to causes other than AMD; RPE tear involving macula of study eye; history of uncontrolled glaucoma or concurrent intraocular condition in study eye; pregnancy; allergy to fluorescein; inability to comply with study procedures Equivalence of baseline characteristics: yes Diagnoses in participants: active primary or recurrent subfoveal CNV |
|
| Interventions |
Intervention 1: 1.25 mg per 0.05 mL intravitreal bevacizumab injections every month for first 3 months; retreatment afterward based on OCT or VA changes
Intervention 2: 0.5 mg intravitreal ranibizumab injections every month for first 3 months; retreatment afterward based on OCT or VA changes
Length of follow‐up Planned: 12 months Actual: 12 months |
|
| Outcomes |
Primary outcomes, as defined: "mean change in BCVA between baseline and 1 year"
Secondary outcomes, as reported: Kaplan‐Meier proportions of the gain of 15 letters of vision, gain of 5 letters of vision, loss of 5 letters of vision, loss of 15 letters of vision; lesion size, assessed by fluorescein angiography; number of retreatments; and retinal thickness, assessed by OCT
Adverse events Intervals at which outcome assessed: monthly through 12 months |
|
| Notes |
Full study name: A Randomized Observer and Subject Masked Trial Comparing the Visual Outcome After Treatment With Ranibizumab or Bevacizumab in Patients With Neovascular Age‐related Macular Degeneration Multicenter Anti VEGF Trial in Austria Trial registration: NCT00710229 Funding sources: Austrian Ophthalmologic Society; the Ludwig Boltzmann Institute of Retinology and Biomicroscopic Lasersurgery; participating study center sites Declarations of interest: study authors reported no competing interests Study period: not reported Reported subgroup analyses: none Contacting study investigators: trial authors contacted; no additional information provided for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was stratified according to the clinical centre using a permuted block method with a fixed block size of 20" |
| Allocation concealment (selection bias) | Low risk | "Eligible patients were randomized in a 1:1 ratio to one of two groups by members of the Department of Clinical Pharmacology, Medical University of Vienna, which was otherwise not involved in the study" |
| Masking of participants (performance bias) | Low risk | "All other personnel and the patients were masked to treatment assignment" |
| Masking of study personnel (performance bias) | Low risk | "The evaluating physician was masked to treatment assignment, whereas the injecting physician was not involved in the collection of data" |
| Masking of outcome assessment (detection bias) | Low risk | "The evaluating physician was masked to treatment assignment, whereas the injecting physician was not involved in the collection of data" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/321 (1.2%) participants excluded from the study. At 12 months, 69 participants did not have outcome data; last observation carried forward method was used to impute missing data for these 69 participants |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported |
| Other bias | Low risk | None observed |
MARINA 2006.
| Methods |
Number randomized (total and per group): 716 participants randomly assigned to study treatment: 238 to 0.3 mg ranibizumab group, 240 to 0.5 mg ranibizumab group, and 238 to sham injection group Exclusions after randomization: none Number analyzed (total and per group): all 716 participants: 238 to 0.3 mg ranibizumab group, 240 to 0.5 mg ranibizumab group, and 238 to sham injection group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 52 participants did not complete 12 months: 12 in the 0.3 mg ranibizumab group, 14 in the 0.5 mg ranibizumab group, and 26 in the sham injection group. Reasons included death, adverse events, loss to follow‐up, participant's decision, physician's decision, participant non‐compliance, and need for other therapeutic intervention Compliance: "more than 90% of patients in each treatment group remained in the study at 12 months, and approximately 80 to 90% remained at 24 months" Intention‐to‐treat analysis: yes; using last observation carried forward for missing data Reported power calculation: yes; sample of 720 participants for power of 95% Study design comment: following primary analyses of the study at 1 year and with recommendation of the data monitoring committee, study protocol was amended to offer treatment with 0.5 mg ranibizumab to participants still being followed in the sham control group. Study protocol was amended 4 months into the study to allow photodynamic therapy for active minimally classic or occult with classic lesions that were no larger than 4 disc areas in size and were accompanied by a 20‐letter or greater loss from baseline visual acuity confirmed at consecutive study visits. When photodynamic therapy was used, scheduled study treatment was postponed until the next scheduled monthly study visit |
|
| Participants |
Country: USA Age: range 52 to 95 years; mean was 77 years in each of the 3 treatment groups Gender (per cent): 464/716 (65%) women and 252/716 (35%) men Inclusion criteria: age 50 years or older; active primary or recurrent subfoveal lesions with CNV secondary to AMD defined as (1) exhibiting at least a 10% increase in lesion size determined by comparing a fluorescein angiogram performed within 1 month before study day 0 with a fluorescein angiogram performed within 6 months preceding study day 0, (2) resulting in a visual acuity loss of greater than 1 Snellen line any time within prior 6 months, or (3) subretinal hemorrhage associated with CNV within 1 month preceding study day 0; total area of CNV encompassed within the lesion at least 50% of total lesion area; total lesion area of 12 disc areas or less in size; best‐corrected visual acuity of 20/40 to 20/320 (Snellen equivalent on ETDRS chart). Participants with lesions with an occult CNV component were included, but for participants with concomitant classic CNV, the area of classic CNV must have been less than 50% of total lesion size Exclusion criteria: prior treatment with verteporfin, external beam radiation therapy, or transpupillary thermotherapy in the study eye; previous participation in a clinical trial involving anti‐angiogenic drugs; treatment with verteporfin in non‐study eye less than 7 days before study day 0; previous intravitreal drug delivery or subfoveal focal laser photocoagulation in study eye; laser photocoagulation in study eye within 1 month preceding study day 0; history of vitrectomy surgery, submacular surgery, or other surgical intervention for AMD in study eye; participation in any studies of investigational drugs within 1 month preceding study day 0; subretinal hemorrhage in study eye involving center of the fovea if hemorrhage involves 50% or more of total lesion area or measures 1 or more disc areas in size; subfoveal fibrosis or atrophy in study eye; CNV in either eye due to other causes; retinal pigment epithelial tear involving the macula in the study eye Equivalence of baseline characteristics: yes Diagnoses in participants: 1/716 (0.1%) had predominantly classic CNV; 264/716 (37%) had minimally classic CNV; and 451/716 (63%) had occult with no classic CNV |
|
| Interventions |
Intervention 1: 0.3 mg ranibizumab intravitreal injection monthly for 2 years
Intervention 2: 0.5 mg ranibizumab intravitreal injection monthly for 2 years
Intervention 3: sham injection monthly for 2 years In all intervention groups, verteporfin photodynamic therapy for the study eye was allowed if choroidal neovascularization converted to a predominantly classic pattern Length of follow‐up Planned: 2 years Actual: 2 years |
|
| Outcomes |
Primary outcomes, as defined: proportion of participants who lost fewer than 15 letters from baseline visual acuity in study eye at 12 months
Secondary outcomes, as defined: proportion of participants who gained 15 or more letters from baseline, proportion of participants with a Snellen equivalent of 20/200 or worse, and mean change from baseline (letters over time); mean change from baseline to month 12 in size of the classic CNV component and total area of leakage from CNV Exploratory efficacy endpoints: proportion of participants with visual acuity 20/40 or better and 20/20 at 12 and 24 months (Snellen equivalent), total area of and change from baseline CNV lesion, area of leakage Adverse events: include ocular and non‐ocular adverse events and proportion of participants developing immunoreactivity to ranibizumab, intraocular inflammation, and IOP Safety assessments: IOP measurement 60 minutes after each injection, incidence and severity of ocular and non‐ocular adverse events, changes and abnormalities in clinical laboratory parameters and vital signs, and immunoreactivity to ranibizumab Intervals at which outcomes assessed: 12 and 24 months |
|
| Notes |
Full study name: Minimally Classic/Occult Trial of the Anti‐VEGF Antibody Ranibizumab in the Treatment of Neovascular Age‐Related Macular Degeneration Trial registration: NCT00056836 Funding sources: Genentech, USA, and Novartis Pharma, Switzerland Declarations of interest: various authors reported that they had received consulting fees from Genentech, Eyetech, Novartis Ophthalmics, Novartis, QLT, Alcon Laboratories, Pfizer, Regeneron, Theragenics, VisionCare, Protein Design Labs, Allergan, BioAxone, Tanox, Genaera, Jerini, Oxigene, Quark, Genzyme, iScience, ISTA, and Athenagen; lecture fees from Genentech, Eyetech, Pfizer, Jerini, Allergan, and Novartis Ophthalmics; grant support from Genentech, Novartis, Eyetech, Pfizer, Theragenics, and Genaera and Alcon Laboratories; and/or equity interest in Pfizer and/or were employees of Genentech and owned Genentech stock Study period: enrollment March 2003 to December 2003 Reported subgroup analyses: by baseline lesion (4 or fewer optic disc areas; more than 4), type of lesion (minimally classic; occult with no classic), and baseline VA (fewer than 55 letters; 55 or more letters) Contacting study investigators: trial authors contacted and contributed information for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomly assigned in a 1:1:1 ratio, using a dynamic randomization algorithm, to receive ranibizumab (LUCENTIS®, Genentech, Inc., South San Francisco, CA) 0.3 or 0.5 mg or a sham injection monthly (30±7 days) for 2 years (24 injections). Randomization was stratified by baseline visual acuity score (< 55 letters [approximately worse than 20/80] vs. ≥ 55 letters) at day 0, by choroidal neovascularization subtype (minimally classic or occult with no classic), and by study center" |
| Allocation concealment (selection bias) | Low risk | "A centralized interactive voice response system (IVRS) was used to handle the randomization" (email communication with Genentech, dated October 24, 2007) |
| Masking of participants (performance bias) | Low risk | "All other study site personnel (except those assisting with injections), patients, and central reading center personnel were masked to treatment assignment" |
| Masking of study personnel (performance bias) | Low risk | "Masking of treatment assignment required at least two investigators per study site: an evaluating physician (masked to treatment assignment) and an injecting physician (unmasked regarding ranibizumab or sham treatment but masked to ranibizumab dose)" |
| Masking of outcome assessment (detection bias) | Low risk | "All other study site personnel (except those assisting with injections), patients, and central reading center personnel were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Efficacy analyses were performed on an intent‐to‐treat basis (all randomized patients) using a last observation carried forward method to handle missing data" |
| Selective reporting (reporting bias) | Low risk | We did not have access to the protocol. We matched all outcomes reported in publications with those reported to the FDA |
| Other bias | Unclear risk | Sponsored by Genentech and Novartis Pharma. Study authors disclosed financial interests and/or were paid consultants, employees, and/or shareholders of funding companies |
PIER 2008.
| Methods |
Number randomized (total and per group): 184 participants randomly assigned to study treatment: 60 to 0.3 mg ranibizumab, 61 to 0.5 mg ranibizumab, and 63 to sham injection Exclusions after randomization: 1 participant in the 0.3 mg ranibizumab group withdrew from the study before receiving first treatment and was excluded Number analyzed (total and per group): 183 participants: 59 in the 0.3 mg ranibizumab group, 61 in the 0.5 mg ranibizumab group, and 63 in the sham injection group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 13 participants did not complete 12 months: 1 in the 0.3 mg ranibizumab group, 2 in the 0.5 mg ranibizumab group, and 8 in the sham injection group. Reasons included participant's decision, participant non‐compliance, and need for other therapeutic intervention Compliance: "...treatment compliance was good in the ranibizumab groups, with 85% or more of subjects receiving each scheduled injection. In the sham group, 27% of subjects permanently discontinued treatment before month 12, most often because the subject’s condition mandated another therapeutic intervention" Intention‐to‐treat analysis (Y/N): yes; using last observation carried forward for missing data Reported power calculation: yes; sample of 180 participants for power of 90% Study design comment: following reports of other clinical trials, the study protocol was amended (February 2006) to offer treatment with 0.5 mg ranibizumab to participants in the sham control group who had completed 12 months of follow‐up and were still being followed. The study protocol was amended again (August 2006) to switch participants in the 0.3 mg ranibizumab group to receive 0.5 mg ranibizumab, to change assessments for all participants from quarterly to monthly after month 12, and to allow treatment with ranibizumab in fellow eyes |
|
| Participants |
Country: USA (43 study centers) Age: range 54 to 94 years; mean was 79 years in each ranibizumab treatment group and 78 years in the sham injection group Gender (per cent): 110/184 (60%) women and 74/184 (40%) men Inclusion criteria: age 50 years or older; primary or recurrent subfoveal CNV secondary to AMD, with total CNV area (classic plus occult CNV) 50% or more of total lesion area and total lesion size 12 or fewer disc areas; best‐corrected visual acuity of 20/40 to 20/320 (Snellen equivalent on ETDRS chart). Participants with minimally classic or occult with no classic CNV were included if they had 10% or more increase in lesion size between 1 and 6 months before day 0, 1 or fewer Snellen lines (or equivalent) VA loss within prior 6 months, or CNV‐associated subretinal hemorrhage within 1 month before day 0 Exclusion criteria: prior treatment with verteporfin photodynamic therapy, external beam radiation therapy, transpupillary thermotherapy, or subfoveal laser photocoagulation (or juxtafoveal or extrafoveal laser photocoagulation within 1 month before day 0); subretinal hemorrhage in the study eye involving the center of the fovea, if the hemorrhage covers 50% or more of the total lesion area or measures 1 or more disc areas in size; previous inclusion in anti‐angiogenic drug trial; prior treatment with photodynamic therapy in non‐study eye within 7 days before day 0 Equivalence of baseline characteristics: yes Diagnoses in participants: 35/184 (19%) had predominantly classic CNV; 69/184 (38%) had minimally classic CNV; 79/184 (43%) had occult with no classic CNV; and 1/184 (< 1%) could not be classified |
|
| Interventions |
Intervention 1: 0.3 mg ranibizumab intravitreal injection every month for first 3 doses (day 0, months 1 and 2), followed by doses every 3 months (months 5, 8, 11, 14, 17, 20, and 23) Intervention 2: 0.5 mg ranibizumab intravitreal injection every month for first 3 doses (day 0, months 1 and 2), followed by doses every 3 months (months 5, 8, 11, 14, 17, 20, and 23) Intervention 3: sham injection every month for first 3 doses (day 0, months 1 and 2), followed by doses every 3 months (months 5, 8, 11, 14, 17, 20, and 23) Length of follow‐up Planned: 2 years Actual: 2 years |
|
| Outcomes |
Primary outcomes, as defined: mean change from baseline to 12 months in visual acuity score
Secondary outcomes, as defined: proportion of participants losing 15 or fewer letters from baseline; proportion of participants gaining 15 or more letters from baseline; proportion of participants with a Snellen equivalent of 20/200 or worse; mean change from baseline in near activities, distance activities, and vision‐specific dependency NEI VFQ‐25 subscales; and mean change from baseline in total area of CNV and total area of leakage from CNV (based on central reading center assessment) Exploratory efficacy endpoints: proportion of participants who had lost 30 or fewer letters from baseline visual acuity at 12 months; mean change in visual acuity score from baseline to 3 months; mean change in visual acuity score from 3 months to 12 months Adverse events Safety assessments: incidence and severity of ocular and non‐ocular adverse events, changes in vital signs, incidence of positive serum antibodies to ranibizumab, IOP measurement 60 minutes after each injection Intervals at which outcomes assessed: injection visits at day 0 and months 1, 2, 3, 8, 11, 14, 17, 20, and 23; clinic visits at months 3, 12, and 24 |
|
| Notes |
Full study name: A Phase IIIb, Multicenter, Randomized, Double‐Masked, Sham Injection‐Controlled Study of the Efficacy and Safety of Ranibizumab in Subjects with Subfoveal Choroidal Neovascularization With or Without Classic CNV Secondary to Age‐Related Macular Degeneration Trial registration: NCT00090623 Funding sources: Genentech, USA, and Novartis Pharma, Switzerland Declarations of interest: various authors reported that they had received consulting fees from Genentech, Novartis, OSI/Eyetech, Eyetech/Pfizer, Novartis, and Alcon; lecture fees from Genentech, Novartis, OSI/Eyetech, and Eyetech/Pfizer; and grant support from Genentech, Novartis, Alcon, Allergan, Acuity, OSI/Eyetech, and Eyetech/Pfizer; held Pfizer stock; and/or were an employee and/or a stockholder of Genentech Study period: enrollment September 7, 2004, to March 16, 2005 Reported subgroup analyses: post hoc analysis of lesion size and composition (Brown 2013) Contacting study investigators: trial authors contacted and contributed information for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a dynamic randomization algorithm, subjects were randomly assigned 1:1:1 to receive 0.3 mg ranibizumab, 0.5 mg ranibizumab, or sham injections. Randomization was stratified by VA score at day zero (≤54 letters [approximately worse than 20/80] vs ≥55 letters [approximately 20/80 or better], CNV type (minimally classic vs occult with no classic vs predominantly classic CNV), and study center" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. Study investigators were contacted but could not provide additional information (email communication with Dr. Regillo, dated May 16, 2012) |
| Masking of participants (performance bias) | Low risk | "All other study site personnel (other than those assisting with study treatment administration), central reading center personnel, and the subjects were masked to treatment assignment" "For the sham‐injected control group, an empty syringe without a needle was used, with pressure applied to the anesthetized and antiseptically prepared eye at the site of a typical intravitreal injection. Pre‐ and post‐injection procedures were identical for all groups" "No subjects were unmasked to their original treatment assignment as a result of these protocol amendments" |
| Masking of study personnel (performance bias) | Low risk | "To achieve double‐masking of treatment assignment, at least two investigators participate at each study site: an 'injecting' ophthalmologist unmasked to treatment assignment (ranibizumab vs sham) but masked to ranibizumab dose, and a masked 'evaluating' ophthalmologist for efficacy and safety assessments" |
| Masking of outcome assessment (detection bias) | Low risk | "To achieve double‐masking of treatment assignment, at least two investigators participate at each study site: an 'injecting' ophthalmologist unmasked to treatment assignment (ranibizumab vs sham) but masked to ranibizumab dose, and a masked 'evaluating' ophthalmologist for efficacy and safety assessments. All other study site personnel (other than those assisting with study treatment administration), central reading center personnel, and the subjects were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Efficacy analyses used the intent‐to‐treat approach and included all subjects as randomized. Missing values were imputed using the last‐observation‐carried‐forward method" |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes specified in the Methods section |
| Other bias | Unclear risk | Sponsored by Genentech and Novartis Pharma. Study authors disclosed financial interests and/or were paid consultants, employees, and/or shareholders of funding companies |
Sacu 2009.
| Methods |
Number randomized (total and per group): 28 participants randomly assigned to study treatment: 14 in bevacizumab group and 14 in PDT + IVTA group Exclusions after randomization: none Number analyzed (total and per group): 28 total participants: 14 in bevacizumab group and 14 in PDT + IVTA group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 1 participant in PDT + IVTA group did not complete 6‐ or 12‐month visits Compliance: not reported; no participant was excluded up to 12 months Intention‐to‐treat analysis: yes, although the paper does not state how data were imputed for the participant missing 6‐ and 12‐month follow‐up visits in the PDT + IVTA group Reported power calculation: yes; sample of 14 participants per group for power of 80% Study design comment: bevacizumab group had more follow‐up visits than PDT + IVTA group |
|
| Participants |
Country: Vienna, Austria Age: mean 78 years (range 58 to 88) Gender (per cent): 19/28 women (68%) and 9/28 men (32%) Inclusion criteria: participants with neovascular AMD of any lesion type; lesion smaller than 4 disc areas; no prior treatment for neovascular AMD; VA of 20/40 to 20/800 Exclusion criteria: participants with a history of thromboembolic events within past 3 months and predictable need for ocular surgery Equivalence of baseline characteristics: yes Diagnoses in participants: neovascular AMD |
|
| Interventions |
Intervention 1: 1 mg intravitreal bevacizumab injections; after 3 initial injections at monthly intervals, retreatment was based on OCT findings only (evidence of persistent or recurrent intra‐ or subretinal fluid); participants seen at monthly intervals
Intervention 2: standard verteporfin PDT plus same day 4 mg intravitreal triamcinolone acetonide; retreatment at 3 months if evidence of leakage by fluorescein angiography
Length of follow‐up Planned: 12 months Actual: 12 months |
|
| Outcomes |
Primary outcome, as defined: change in mean visual acuity
Secondary outcomes, as reported: change in mean 1 mm central retinal thickness; BCVA; StratusOCT; fluorescein angiography; indocyanine green angiography; microperimetry
Adverse events Intervals at which outcomes assessed: baseline, months 1, 3, 6, and 12 |
|
| Notes |
Trial registration: EudraCT no. 2005‐003288‐21 Funding sources: not reported Declarations of interest: 1 investigator reported being "an owner of the patent on the use of green porphyrins in neovasculature of the eye under the guidelines of the Wellman Laboratories of Photomedicine, Harvard Medical School, Boston, MA, USA" Study period: not reported Reported subgroup analyses: none Contacting study investigators: trial authors contacted and contributed information for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "In our study we used computer generated randomized scheme and the allocation concealment methods was used (central coordinating center)" (email communication with Dr. Stefan Sacu, dated May 19, 2012) |
| Allocation concealment (selection bias) | Low risk | "In our study we used computer generated randomized scheme and the allocation concealment methods was used (central coordinating center)" (email communication with Dr. Stefan Sacu, dated May 19, 2012) |
| Masking of participants (performance bias) | High risk | "Open label"; participants could not be masked to treatment groups |
| Masking of study personnel (performance bias) | High risk | "Open label"; physicians were not masked to treatment groups |
| Masking of outcome assessment (detection bias) | High risk | "Patients in the PDT + IVTA groups had characteristic post‐treatment hypofluorescence within the area of the PDT treatment spot..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent‐to‐treat analysis was followed |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported |
| Other bias | Low risk | None observed |
SAVE‐AMD 2017.
| Methods |
Design: RCT for neovascular AMD participants + cohort study for non‐neovascular participants Number randomized: ?? 24 (Fig. 1b); 23 (Tables 1 to 4); number per arm not reported Exclusions after randomization: Figure 1b suggests 5 (4?). Statement on page 209 states 1 excluded "due to unusable data of the FMD measurements" Number analyzed: 23 participants in Tables 1 to 4, but Table 3 footnote states that only 20 RCT participants had 12‐month follow‐up measurements Unit of analysis: unclear Losses to follow‐up: Table 3 implies that 3 (4?) participants did not have 12‐month follow‐up. On page 209, states that 20 neovascular AMD participants completed the 12‐month follow‐up examination Compliance: not reported Intention‐to‐treat analysis: unable to determine Power calculation: none reported Study design comment: RCT with small sample size was not the focus of SAVE‐AMD. Primary interest was comparison of effects of anti‐VEGF agents on neovascular vs non‐neovascular AMD eyes/patients |
|
| Participants |
Country: Switzerland Age: 76.5 ± 6.7 years (RCT overall) Women: "12 (52%)" (Table 1) Inclusion criteria: neovascular AMD documented by FA and OCT; "suitable for intravitreal anti‐VEGF therapy"; age 50 years or older; stable medications for at least 1 month Exclusion criteria: acute MI, unstable angina, stroke, or a coronary intervention/revascularization procedure 3 months or less before study entry; uncontrolled symptomatic congestive heart failure 4 weeks or less before study entry; renal failure (based on creatinine level), tachycardia, poorly controlled blood pressure despite adequate therapy (160/100 threshold), symptomatic hypotension, long‐term use of long‐acting nitrates, smoking (> 5 cigarettes/d), diabetes mellitus, dyslipidemia, cholesterol > 4.5 mmol/L, liver disease, alcohol or illicit substance abuse, hypersensitivity to study drugs or excipients, active severe intraocular inflammation, malignancy, systemic inflammatory disease, participation in another study in past month Equivalence of baseline characteristics: unknown (not reported) Diagnoses of participants: all RCT participants had neovascular AMD. Cohort participants had non‐neovascular (dry) AMD |
|
| Interventions |
Intervention 1: ranibizumab intravitreal injection (0.5 mg) at day 1 and week 4, then PRN Intervention 2: bevacizumab intravitreal injection (1.25 mg) at day 1 and week 4, then PRN Length of follow‐up: 12 months |
|
| Outcomes |
Primary: endothelium‐dependent and ‐independent vasodilatation after 8 weeks of treatment Secondary: "vascular compliance", best‐corrected visual acuity (BCVA), CNV activity based on central retinal thickness (CRT) measured using OCT, changes in systemic inflammatory markers, surrogates for oxidative stress, and systolic and diastolic 24‐hour blood pressure Adverse events: death, thromboembolic events, and changes in laboratory measurements and vital signs from baseline |
|
| Notes |
Full study name: SAVE‐AMD: Safety of VEGF Inhibitors in Age‐Related Macular Degeneration Type of study: RCT (neovascular AMD) + cohort study (neovascular vs non‐neovascular AMD) Funding sources: Swiss National Science Foundation, Swiss Heart Foundation, Werner H. Spross Foundation for Ophthalmology, Bandung Foundation, and Austrian Science Fund Declarations of interest: Department of Ophthalmology of City Hospital Triemli Zurich "has received reimbursement for research, consultancy work, and presentations of [2nd author] on behalf of Novartis and Bayer." Another author (Luscher) has received "educational and research grants from Bayer Health Care, Berliln, Germany, and Pfizer Inc., New York, NY, USA, unrelated to this project." No other potential conflicts of interest reported for other study authors Study period: not reported Reported subgroup analyses: none Registration: NCT00727753 FIrst author contact information: frank.enseleit@usz.ch |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants (performance bias) | Unclear risk | Not reported |
| Masking of study personnel (performance bias) | Unclear risk | Not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 of 23 (24?) did not complete 12 months of follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported for the comparison of primary interest to study team (neovascular vs non‐neovascular AMD cases treated with anti‐VEGF agents). However, high risk for this review because only pooled data reported for participants |
| Other bias | Unclear risk | No results reported for individual anti‐VEGF agents |
Scholler 2014.
| Methods |
Number randomized (total and per group): 55 total: 29 in ranibizumab group and 26 in bevacizumab group Exclusions after randomization: “nine eyes were excluded” but meaning is unclear Number analyzed (total and per group): 55: 29 vs 26 at baseline. Number examined at follow‐up times not reported, but 26 in ranibizumab group and 20 in bevacizumab group finished the study. These 46 eyes could be analyzed in total Unit of analysis: unclear; appears to be eyes Losses to follow‐up: “nine eyes were excluded” appears to refer to such losses Compliance: 3 of 9 “excluded” were given ranibizumab instead of bevacizumab as assigned Intention‐to‐treat analysis: not reported; unclear as follow‐up denominators missing Reported power calculation: none Study design comment: none |
|
| Participants |
Country: Austria Age: 79.5 in ranibizumab group and 80.8 in bevacizumab group (80.15 years total) Gender (per cent): ranibizumab: 24% men, 76% women; bevacizumab: 35% men, 65% women Inclusion criteria: over 50 years of age with nAMD, which was verified in fluorescence angiography (FLA). Only patients with VA between 20/40 and 20/320 were included Exclusion criteria: previous treatment for AMD, previous systemic administration of bevacizumab, vision‐impairing cataract or other ophthalmologic disease like glaucoma, active inflammation, diabetic retinopathy, and others Equivalence of baseline characteristics: yes; similar age, BCVA, CRT, FA lesion size, gender (all P values > 0.37) Diagnoses in participants: neovascular AMD |
|
| Interventions |
Intervention 1: 3 ranibizumab injections (0.5 mg) at 30‐day intervals followed by PRN ranibizumab for another 10 months Intervention 2: 3 bevacizumab injections (1.25 mg) at 30‐day intervals followed by PRN bevacizumab for another 10 months Length of follow‐up: Planned: scheduled visits were performed monthly (30 ± 7 days) for 12 months Actual: 12 months (monthly) |
|
| Outcomes |
Primary outcome, as defined: a difference in injection frequencies of ranibizumab and bevacizumab
Secondary outcomes, as defined: effectiveness of ranibizumab and bevacizumab with respect to BCVA and CRT
Adverse events (Y/N): yes; 3 reported, all in ranibizumab arm Intervals at which outcome assessed: monthly |
|
| Notes |
Full study name: Differences of Frequency in Administration of Ranibizumab and Bevacizumab in Patients With Neovascular AMD Type of study: published Funding sources: “Birgit Weingessel and Pia Veronika Vécsei‐Marlovits received unrestricted grants from Novartis Austria during the last 5 years” Declarations of interest: study authors declare no conflicts of interest Study period: accrual: 1‐1‐2011 to 12‐31‐2011; follow‐up for 1 year yields January 2011 to December 2012 Reported subgroup analyses (Y/N): if yes, specify: no Registration: EK‐07‐192‐1007/EudraCT Nr.2007‐005157‐33 (Ethikkommission der Stadt Wien) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “For allocation of the participants, a computer‐generated list of random numbers was used on the basis of simple randomization (1:1)” |
| Allocation concealment (selection bias) | Unclear risk | No information provided on how participants could or could not possibly foresee assignments |
| Masking of participants (performance bias) | Unclear risk | No information provided on how masking of participants was done for primary and secondary outcomes |
| Masking of study personnel (performance bias) | Unclear risk | No information provided on how masking of physicians was done for primary and secondary outcomes, but outcomes are not likely to be influenced by lack of masking or blinding |
| Masking of outcome assessment (detection bias) | High risk | No information provided on how masking of outcome assessors was done for primary and secondary outcomes, and this could influence participants to respond differently |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Cannot tell; number of participants who contributed outcome data at each follow‐up time not reported, but results show that 26 participants in ranibizumab group and 20 in bevacizumab group completed the study after exclusion of 9 eyes |
| Selective reporting (reporting bias) | Unclear risk | Neither FA lesion size @ 12 months nor change in FA lesion size from baseline to 12 months reported |
| Other bias | Unclear risk | Two study authors had received unrestricted grants from Novartis Austria within 5 years (Novartis is the distributor of ranibizumab in Europe) |
Subramanian 2010.
| Methods |
Number randomized (total and per group): 28 participants randomly assigned to study treatment: 20 in bevacizumab group and 8 in ranibizumab group Exclusions after randomization: none Number analyzed (total and per group): 22 total participants: 15 in bevacizumab group and 7 in ranibizumab group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 6 participants: 3 participants voluntarily dropped out (2 in bevacizumab group, 1 in ranibizumab group); 1 participant relocated (in bevacizumab group); and 2 participants died (both in bevacizumab group) Compliance: 22/28 participants completed the study Intention‐to‐treat analysis: no; 6 participants enrolled and randomized were not included in analysis Reported power calculation: yes; 79% power for sample size of 135 participants using 2:1 randomization ratio Study design comment: although the target sample size was 135, only 28 participants were evaluated |
|
| Participants |
Country: Boston, MA, USA Age: not reported for 28 enrolled participants (mean 78 years for analyzed bevacizumab group; mean 80 years for analyzed ranibizumab group) Gender (per cent): not reported for 28 enrolled participants (all men for analyzed bevacizumab group; 6 men and 1 woman for analyzed ranibizumab group) Inclusion criteria: age 50 years or older; presence of symptomatic CNV, confirmed by intravenous fluorescein angiography and optical coherence tomography as affecting the foveal center; ability to provide informed consent; willingness to commit to regular clinic appointments and follow‐up; original protocol specified baseline VA between 20/40 and 20/200, later amended to include all baseline VAs equal to or better than 20/400 Exclusion criteria: previous treatment for wet AMD within past year; presence of subretinal hemorrhage greater than 50% of the size of the lesion on fluorescein angiography; presence of advanced glaucoma; any coexisting macular disease causing decreased vision; history of malignant or uncontrolled hypertension; intraocular inflammation; history of thromboembolic phenomena; inability to provide informed consent; participation in another concurrent ophthalmic clinical trial Equivalence of baseline characteristics: yes Diagnoses in participants: AMD |
|
| Interventions |
Intervention 1: 0.05 mL intravitreal bevacizumab injection (concentration not reported) every month for first 3 months; retreatment afterward based on OCT or VA changes
Intervention 2: 0.05 mL intravitreal ranibizumab injection (concentration not reported) every month for first 3 months; retreatment afterward based on OCT or VA changes
Length of follow‐up Planned: 12 months Actual: 12 months |
|
| Outcomes |
Primary outcomes, as defined: visual acuity
Secondary outcomes, as reported: central foveal thickness by OCT, total number of injections; blood pressure measurements
Adverse events Intervals at which outcome assessed: 1 week after injections to assess adverse events; and monthly through 12 months |
|
| Notes |
Trial registration: ISRCTN73359806 Funding sources: Veterans Affairs Boston Healthcare System, USA Declarations of interest: "The authors declare no conflict of interest" Study period: April 2007 to February 2009 Reported subgroup analyses: none Contacting study investigators: trial authors contacted and contributed information for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were enrolled by a 2:1 randomization to either the bevacizumab (2) or the ranibizumab (1) arm of the study." Study investigators were contacted, but could not provide additional information as to how the sequence was generated (email communication with Dr. Subramanian, dated May 16, 2012) |
| Allocation concealment (selection bias) | Low risk | "The Research Pharmacist at the [Veterans Affairs] Hospital Pharmacy was responsible for randomization" and "all subjects were assigned a study number" |
| Masking of participants (performance bias) | Low risk | Reported as "double‐blind"; identical syringes were used to administer agents, and all study personnel in contact with participants were masked |
| Masking of study personnel (performance bias) | Low risk | "To obtain blinding of treatment assignments, the Research Pharmacist at the [Veterans Affairs] Hospital Pharmacy was responsible for randomization, tracking and ensuring the correct study drug was administered to each patient at each visit, and dispensing the same volume of each drug in identical 1 ml syringes" |
| Masking of outcome assessment (detection bias) | Low risk | "As the only investigator with knowledge of subject assignments, the Research Pharmacist was, in turn, masked to all visual and anatomic outcomes to treatment. All other investigators, as well as other physicians, residents, and office personnel who may have inadvertently come in contact with study subjects, were masked to treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six of 28 (21%) participants enrolled were not included in the analysis: 3 voluntarily dropped out; 1 relocated; and 2 died |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were reported; however, the clinical trials register record for this trial but not published reports specified quality of life as an outcome |
| Other bias | Low risk | None observed |
VISION 2004.
| Methods |
Included trials: 2 concurrent RCTs (EOP 1003; EOP 1004) Number randomized (total and per group): 1208 participants randomly assigned to study treatment: 297 in 0.3 mg pegaptanib group, 305 in 1.0 mg pegaptanib group, 302 in 3.0 mg pegaptanib group, and 304 in sham injection group Exclusions after randomization: 22 total participants: 18 participants did not receive at least 1 injection, and 4 participants were not included in efficacy analyses because "sufficiently standardized assessment of visual acuity was not completed at baseline" Number analyzed (total and per group): 1186 participants at 1 year: 294 in 0.3 mg pegaptanib group, 300 in 1.0 mg pegaptanib group, 296 in 3.0 mg pegaptanib group, and 296 in sham injection group Unit of analysis: individuals (1 study eye per participant) Losses to follow‐up: 101 at 1 year: 23 in 0.3 mg pegaptanib group, 25 in 1.0 mg pegaptanib group, 32 in 3.0 mg pegaptanib group, and 21 in sham injection group Compliance: approximately 90% of participants completed the study Intention‐to‐treat analysis: no; 22 participants enrolled and randomized were not included in analysis Reported power calculation: yes; sample of 244 participants in each group for power of 95%; at least 270 participants were recruited for each group, assuming 10% would have missing data Study design comment: at 54 weeks, participants were re‐randomized; those in pegaptanib groups were randomized to discontinue treatment or continue with same dose; those in sham group were randomized to 1 of 5 groups: discontinue sham injections, continue with sham injections, or receive injections with 0.3, 1.0, or 3.0 mg pegaptanib |
|
| Participants |
Country: USA, Canada, Austria, Belgium, Czech Republic, Denmark, France, Germany, Hungary, Israel, Italy, the Netherlands, Poland, Portugal, Spain, Switzerland, UK, Brazil, Chile, Colombia, and Australia (117 study centers) Age: mean age in EOP 1003 was 77 years, and in EOP 1004 was 75 years Gender (per cent): 696/1190 (58%) women and 494/1190 (42%) men (based on those receiving at least 1 study treatment) Inclusion criteria: age 50 years or older; subfoveal CNV lesion secondary to AMD; BCVA of 20/40 to 20/320 in study eye and 20/800 or better in fellow eye; all angiographic subtypes of total lesion size up to and including 12 disc areas Exclusion criteria: subretinal hemorrhage in study eye ≥ 50% of lesion area; < 50% of lesion with active CNV; > 1 previous PDT treatment; PDT treatment < 8 weeks or > 13 weeks before baseline visit; IOP > 23 mmHg; without clear ocular media; inadequate pupillary dilation for stereoscopic fundus photography; atrophy > 25% of total lesion area or subfoveal scarring in study eye; history of previous subfoveal thermal laser therapy or previous or concomitant therapy with any investigational therapy to treat AMD; need for cataract surgery within 2 years; other potential causes of CNV such as myopia; ocular histoplasmosis syndrome, angioid streaks, choroidal rupture, or multifocal choroiditis; any intraocular surgery within 3 months or extrafoveal/juxtafoveal laser within 2 weeks of study entry; previous posterior vitrectomy or scleral buckling surgery; presence of retinal pigment epithelial tears or rips; participants with diabetic retinopathy, severe cardiac disease, myocardial infarction within 6 months, ventricular tachyarrhythmia requiring ongoing treatment, unstable angina, peripheral vascular disease, stroke within 12 months, acute ocular or periocular infection, previous therapeutic radiation to the eye, head, or neck; treatment with any investigational agent within 60 days; allergies to fluorescein dye or to components of the pegaptanib formulation Equivalence of baseline characteristics: yes Diagnoses in participants: 306/1190 (26%) had predominantly classic CNV; 426/1190 (36%) had minimally classic CNV; and 458/1190 (38%) had occult with no classic CNV |
|
| Interventions |
Intervention 1: 0.3 mg pegaptanib intravitreal injection every 6 weeks; at 54 weeks re‐randomization to continue or discontinue treatment
Intervention 2: 1.0 mg pegaptanib intravitreal injection every 6 weeks; at 54 weeks re‐randomization to continue or discontinue treatment
Intervention 3: 3.0 mg pegaptanib intravitreal injection every 6 weeks; at 54 weeks re‐randomization to continue or discontinue treatment
Intervention 4: sham injection every 6 weeks; at 54 weeks re‐randomization to continue sham injections, discontinue sham injections, or receive treatment with 1 of 3 pegaptanib doses (0.3, 1.0, or 3.0 mg)
Length of follow‐up Planned: 54 weeks after first randomization; 48 weeks after re‐randomization Actual: 54 weeks after first randomization; 48 weeks after re‐randomization; up to 4 years for safety outcomes |
|
| Outcomes |
Primary outcome, as defined: proportion of participants losing fewer than 15 letters of VA between baseline and 54 weeks
Secondary outcomes, as defined: proportion of participants maintaining or gaining ≤ 0, 5, 10, or 15 letters, or losing 30 or more letters; mean changes in VA at 6‐week intervals from baseline to week 54; proportion with VA 20/200 or worse at week 54; changes in size of lesion, size of CNV, and size of leakage at weeks 30 and 54 as measured by color fundus photography and fluorescein angiography
Adverse events Intervals at which outcomes assessed: 6‐week intervals from baseline to week 54; 6‐week intervals from week 54 to week 102; color fundus photography and fluorescein angiography done at baseline, and at weeks 30, 54, 78, and 102 |
|
| Notes |
Full study name: VEGF Inhibition Study in Ocular Neovascularization Trial registration: NCT00021736; NCT00321997 Funding sources: Eyetech Pharmaceuticals, Inc., New York, and Pfizer Inc., New York, USA Declarations of interest: various authors reported that they had served as a paid consultant for Eyetech Pharmaceuticals and Neovista; had received royalty income from Coherent, the manufacturer of a laser used in photodynamic therapy; and/or were employees of and shareholders in Eyetech Pharmaceuticals Study period: not reported Reported subgroup analyses: none Contacting study investigators: trial authors contacted and contributed information for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated in each trial to one of four treatment arms (sham or 0.3 mg, 1 mg, or 3 mg pegaptanib) by a dynamic procedure using a stochastic treatment allocation algorithm based on the variance method to minimize imbalances simultaneously for study center, angiographic lesion subtype and previous treatment with PDT" |
| Allocation concealment (selection bias) | Low risk | "The study coordinator randomized the patient by going on‐line to IDDI (an independent statistics/CRO) and answering eligibility and stratification questions. In response they were instructed which code on the treatment pack to use. Only when it was opened [sic] immediately prior to use would the injecting physician know whether it was active (but not which dose) or sham" (email communication with Eyetech, dated July 11, 2005) |
| Masking of participants (performance bias) | Low risk | "To maintain masking of the patients, the patients receiving sham injections and those receiving the study medication were treated identically, with the exception of scleral penetration. All patients (including those receiving sham injection) underwent an ocular antisepsis procedure and received injected subconjunctival anesthetic. The patients receiving sham injections had an identical syringe ‐ but without a needle ‐ pressed against the eye wall to mimic the active doses that were injected through the pars plana into the vitreous cavity. The injection technique precluded the patient from seeing the syringe" |
| Masking of study personnel (performance bias) | Low risk | "To maintain masking of the investigators, the study ophthalmologist responsible for patient care and for the assessments did not administer the injection" |
| Masking of outcome assessment (detection bias) | Low risk | "In all cases, a separate, certified visual‐acuity examiner masked to the treatment assignment and to previous measurements of visual acuity assessed distance visual acuity" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "For all efficacy analyses, patients were evaluated in the treatment group to which they were randomly assigned. Several analyses of the primary efficacy endpoint that accounted for missing data were also conducted." At 54 weeks, 18 participants were excluded because they had not received at least 1 study treatment; 4 participants were excluded "because a sufficiently standardized assessment of visual acuity was not completed at baseline"; and missing data for about 10% of the study population were imputed using the last observation carried forward method |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes for week 54 (first year) were reported; visual acuity outcomes defined for the first year were not reported in second year outcomes |
| Other bias | Unclear risk | Sponsored by Eyetech Pharmaceuticals and Pfizer. Study chair and some others involved in the trials were paid consultants, employees, and/or shareholders of Eyetech Pharmaceuticals |
Study acronyms: see Table 3.
AMD: age‐related macular degeneration. BCVA: best‐corrected visual acuity. CMT: central macular thickness. CNV: choroidal neovascularization. CRO: clinical research organization. CRT: central retinal thickness. ETDRS: Early Treatment Diabetic Retinopathy Study. FA: fluorescein angiogram. FDA: Food and Drug Administration. FMD: XXX. IOP: intraocular pressure. IVRS: interactive voice response system. IVTA: intravitreal triamcinolone acetonide. MI: myocardial infarction. NEI VFQ‐25: National Eye Institute‐Visual Functioning Questionnaire‐25. NHS: UK National Health Service. OCT: optical coherence tomography. PDT: photodynamic therapy. PRN: pro re nata. RAP: XXX. RCT: randomized controlled trial. RPE: retinal pigment epithelium. SD‐OCT: spectral domain optical coherence tomography. VA: visual acuity. VEGF: vascular endothelial growth factor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADVANCE 2008 | Not intravitreal injection of an anti‐VEGF agent |
| ARMAST 2008 | Bevacizumab vs bevacizumab + PDT |
| Bashshur 2007 | Follow‐up less than 1 year |
| BEAT‐AMD 2009 | Follow‐up less than 1 year; also, systemic bevacizumab |
| Berger 2015 | Injection vs infusion of anti‐VEGF agent |
| Blaha 2015 | Not an RCT |
| Bolz 2008 | Dosing study: 0.3 mg or 0.5 mg intravitreal ranibizumab |
| Brown 2016 | Not an RCT |
| Cohen 2008 | Not an RCT: cost‐effectiveness assessment. States that analysis used data from a small RCT but RCT data not reported |
| Costagliola 2010 | Combination therapy: intravitreal bevacizumab alone vs intravitreal bevacizumab plus low‐fluence PDT |
| Csaky 2015 | RCT of pazopanib eye drops (6 different doses regimens) vs intravitreal ranibizumab with 1‐year follow‐up; excluded because pazopanib eye drops for treatment of AMD; not eligible for this review |
| Earnshaw 2007 | Not an RCT: cost‐effectiveness assessment |
| Eibenberger 2015 | No outcomes targeted for this review |
| EMERGE 2016 | Not an RCT to evaluate any targeted anti‐VEGF agent |
| Erdokur 2009 | Not an RCT |
| EXTEND‐I 2008 | Dosing study: 0.3 mg or 0.5 mg intravitreal ranibizumab |
| Eyetech Study 2003 | Not an RCT |
| Falkenstein 2007 | Not an RCT |
| Fletcher 2008 | Not an RCT: cost‐effectiveness assessment |
| FOCUS 2006 | Combination therapy: intravitreal ranibizumab alone vs intravitreal ranibizumab plus verteporfin PDT |
| GALATIR 2014 | Clinical trial registry record reported that the "study has been withdrawn prior to enrollment"; trial investigators had planned to compare an anti‐VEGF agent biosimilar to bevacizumab and manufactured in Russia vs 0.50 mg intravitreal ranibizumab in participants with neovascular AMD, and to evaluate the proportion losing fewer than 15 letters at 12 months as the primary outcome |
| Gallemore 2016 | Follow‐up < 1 year; not an anti‐VEGF agent targeted for this review |
| Hahn 2007 | Follow‐up less than 1 year |
| Hatta 2010 | Not an RCT |
| HAWK 2018 | Neither intervention (brolucizumab) nor control (aflibercept) targeted for this review |
| Heier 2006 | Follow‐up less than 1 year |
| Hernandez‐Pastor 2008 | Not an RCT: cost‐effectiveness assessment |
| Hernandez‐Pastor 2010 | Not an RCT: cost‐effectiveness assessment |
| Holz 2016 | Follow‐up less than 1 year: comparison of novel anti‐VEGF agent (referred to as both RTH258 and ESBA1008) vs intravitreal ranibizumab |
| Javitt 2008 | Not an RCT: cost‐effectiveness assessment |
| Kralinger 2014 | Follow‐up less than 1 year; no outcome targeted for this review |
| Lai 2009 | Dosing study: 1.25 mg (n = 24) or 2.5 mg (n = 26) intravitreal bevacizumab; follow‐up less than 1 year |
| Lazic 2007 | Follow‐up less than 1 year |
| Li 2012 | Comparison of injection schedules |
| Li 2013 | Not an RCT |
| Matthe 2011 | Not an RCT |
| MIRA‐1 2005 | Not an RCT of anti‐VEGF intravitreous injections for patients with neovascular AMD |
| Modarres 2009 | Dosing study |
| NCT00087763 | Dosing study: all participants treated with pegaptanib |
| NCT01494805 | Gene therapy intervention vs ranibizumab |
| NCT01796964 | Neither intervention (brolucizumab) nor control (aflibercept) targeted for this review |
| Neubauer 2007 | Not an RCT: statistical modeling using ANCHOR 2006 and MARINA 2006 cost data |
| Nguyen 2006 | Follow‐up less than 1 year: VEGF Trap® (aflibercept) vs placebo |
| Nowak 2012 | Not an RCT |
| Nunes 2014 | Cost analysis based on outcomes from a small RCT. No RCT data found |
| OSPREY 2014 | Follow‐up < 1 year; neither intervention nor control targeted for this review |
| Parodi 2012 | Follow‐up less than 1 year |
| PERSPECTIVES 2012 | Not an RCT |
| PrONTO Study 2009 | Not an RCT |
| Raftery 2007 | Not an RCT: cost‐effectiveness assessment |
| RATE 2011 | Clinical trial registry record reported that the "study was terminated under the political pressure of the Federal Security Service of the Russian Federation (FSB) and the Russian Society of Cardiology"; trial investigators had planned to compare 0.50 mg intravitreal ranibizumab vs 0.50 mg intravitreal ranibizumab plus PDT vs sham injection in participants with a history of coronary artery disease or cerebrovascular events, and to evaluate arterial thromboembolic events as the primary outcome |
| RIVAL 2017 | RCT of ranibizumab vs aflibercept; aflibercept excluded from this review |
| SAILOR 2009 | Dosing study: 0.3 mg (n = 1169) or 0.5 mg (n = 1209) intravitreal ranibizumab |
| Schmid‐Kubista 2011 | Follow‐up less than 1 year; RCT comparing sequential administration of intravitreal bevacizumab and pegaptanib vs intravitreal bevacizumab or pegaptanib alone |
| Sengul 2017 | Follow‐up less than 1 year; no data for any outcomes targeted for this review |
| SIGHT 2014 | Intervention (aflibercept) not targeted for this review |
| SUMMIT 2007 | 3 RCTs compared intravitreal ranibizumab alone vs intravitreal ranibizumab plus PDT |
| Suñer 2009 | Not an RCT: validation of NEI Visual Function Questionnaire using ANCHOR 2006 and MARINA 2006 data |
| Tano 2008 | Dosing study: 0.3 mg (n = 47) or 1.0 mg (n = 48) pegaptanib sodium |
| Vallance 2010 | RCT of intravitreal ranibizumab + sham PDT vs intravitreal ranibizumab + standard‐fluence verteporfin PDT |
| VERITAS 2006 | Not an RCT of anti‐VEGF intravitreous injections |
| VIEW 2014 | 2 RCTs of intravitreal aflibercept vs intravitreal ranibizumab; aflibercept not eligible for this review |
| Weigert 2008 | Follow‐up less than 1 year |
| Wolowacz 2007 | Not an RCT: cost‐effectiveness assessment |
| Zehetner 2013 | Follow‐up less than 1 year |
Study acronyms: see Table 3.
AMD: age‐related macular degeneration. NEI: National Eye Institute, National Institutes of Health, USA. PDT: photodynamic therapy. RCT: randomized controlled trial. VEGF: vascular endothelial growth factor.
Characteristics of ongoing studies [ordered by study ID]
NCT00559715.
| Trial name or title | Prevention of Vision Loss in Patients With Age‐Related Macular Degeneration (AMD) by Intravitreal Injection of Bevacizumab and Ranibizumab (VIBERA) |
| Methods |
Study design: phase 3 RCT Planned enrollment: 366 participants Length of follow‐up: 2 years |
| Participants |
Inclusion criteria: age 50 years or older; visual impairment due to active primary or recurrent CNV associated with AMD; classical or predominantly classic lesion with largest diameter of the subretinal neovascular membrane smaller than greatest distance between major temporal vascular arcades, minimally classic lesion, or occult lesion with no classic CNV; BCVA of 20/40 to 20/320 Exclusion criteria: subretinal hemorrhage involving ≥ 50% of the lesion area or ≥ 1 optic disc area; subfoveal fibrosis or atrophy; CNV of other pathogenesis; previous treatment for CNV or treatment with any anti‐angiogenic drugs; previous intravitreal drug delivery, laser photocoagulation, vitreoretinal surgery, submacular surgery, or other surgical intervention for AMD in the study eye; retinal pigment epithelial tear; active inflammation, vitreous hemorrhage, infectious conjunctivitis, keratitis, scleritis, or endophthalmitis; history of rhegmatogenous retinal detachment, macular hole, idiopathic or autoimmune‐associated uveitis, or corneal transplant; aphakia or lack of posterior capsule in the study eye; > ‐8 diopters of myopia; any intraocular condition that requires surgery or could lead to vision loss within 2 years; intraocular surgery in study eye within 2 months; uncontrolled glaucoma or history of glaucoma filtering surgery; impaired visualization of the retina precluding adequate diagnosis; premenopausal women not using adequate contraception or nursing; active systemic infection or other disease, dysfunction, or finding to contraindicate participation; hypersensitivity to study drugs or allergy to agents used for ocular testing; involvement in another clinical study within 4 weeks; unwillingness or inability to comply with study |
| Interventions | Intervention 1: 1.25 mg intravitreal bevacizumab administered monthly or on demand Intervention 2: 0.5 mg intravitreal ranibizumab administered monthly or on demand |
| Outcomes | Primary outcome, as defined: proportion of participants losing fewer than 15 letters at 1 year Secondary outcomes, as defined: proportion of participants losing fewer than 15 letters at 2 years; mean change in BCVA at 1 year and at 2 years; proportion of participants with at least 3 months treatment‐free in 2 years; number of doses of study drugs at 2 years; rate of dropout at 2 years; number of non‐responders at 2 years; retinal lesions at 2 years; adverse events at 2 years; quality of life at 2 years |
| Starting date | August 2008; primary completion date of August 2009 |
| Contact information | Bernd Muehlbauer, Professor, MD Department of Pharmacology at Klinikum Bremen Mitte Bremen, Germany, 28177 |
| Notes | "The study is designed to demonstrate the therapeutic noninferiority of the recombinant humanized monoclonal VEGF antibody bevacizumab administered by intravitreal injection in the treatment of AMD in comparison to the related fragment ranibizumab" Sponsors/Collaborators: Klinikum Bremen‐Mitte, gGmbH; Kompetenzzentrum für Klinische Studien, Bremen |
AMD: age‐related macular degeneration BCVA: best‐corrected visual acuity CNV: choroidal neovascularization RCT: randomized controlled trial
Differences between protocol and review
We modified the inclusion criteria between the 2008 publication and this update to the review. In the 2008 publication, all trials that investigated anti‐VEGF agents, alone or in conjunction with other treatments, were eligible for inclusion in the review. For this update of the review, we did not include studies in which anti‐VEGF treatment was given in combination with other AMD treatments. These combination therapies for AMD will be covered in a separate Cochrane review. Thus, we did not include in this update of the review the FOCUS 2006 trial, which was included in the 2008 publication.
The primary outcome for this update was changed from "loss of 15 or more letters of visual acuity at one year" to "gain of 15 or more letters of visual acuity at one year." We changed the primary outcome from the protocol and the 2008 publication to reflect advancements in the treatment of AMD, which now provide the potential to improve vision. We swapped the number of events with the number of non‐events for negative outcomes in our meta‐analysis to maintain the same direction of treatment effect across outcomes.
We added GRADE assessments and "Summary of findings" tables in this version of the review. Because previous versions of this review and other published systematic reviews have shown similar effects among the three anti‐VEGF agents evaluated in this review (pegaptanib, ranibizumab, and bevacizumab), we combined in our meta‐analysis data from trials that had compared any of the three agents versus a control.
Contributions of authors
Contributions to the first published version of this review (2008) Conceiving of the review: MK Designing the review: MK, SSV Co‐ordinating the review: MK, SSV Collecting data for the review ‐ Designing search strategies: CEVG Trials Search Co‐ordinator, MK, SSV ‐ Undertaking manual searches: MK, SSV ‐ Screening search results: MK, SSV ‐ Organizing retrieval of papers: MK, SSV ‐ Screening retrieved papers against inclusion criteria: MK, SSV ‐ Appraising quality of papers: MK, SSV ‐ Abstracting data from papers: MK, SSV ‐ Writing to authors of papers for additional information: MK, SSV ‐ Providing additional data about papers: MK ‐ Obtaining and screening data on unpublished studies: MK, SSV ‐ Managing data for the review: MK, SSV ‐ Entering data into RevMan: MK, SSV Analyzing data: MK, SSV Interpreting data ‐ Providing a methodological perspective: SSV, MK ‐ Providing a clinical perspective: MK, SSV ‐ Providing a policy perspective: MK Writing the review: MK, SSV Securing funding for the review: MK, CEVG US Project Performing previous work that was the foundation of the current study: MK Serving as guarantor for the review: MK
Contributions to review updates (2014 and 2018) Collecting data for the review ‐ Screening search results: KL, BSH, SSV, MK ‐ Organizing retrieval of papers: KL ‐ Screening retrieved papers against inclusion criteria: KL, BSH, SSV, MK ‐ Appraising quality of papers: KL, BSH, SSV, MK ‐ Abstracting data from papers: KL, BSH, SSV, MK ‐ Writing to authors of papers for additional information: KL, BSH ‐ Providing additional data about papers: MK, BSH ‐ Managing data for the review: KL, BSH, SSV, MK ‐ Entering data into RevMan: KL Analyzing data: KL, BSH, SSV, MK Interpreting data ‐ Providing a methodological perspective: KL, BSH, SSV, MK ‐ Providing a clinical perspective: SDS, MK, BSH, SSV ‐ Providing a policy perspective: SDS, MK, BSH Writing the review: SDS, KL, SSV , BSH, MK
Sources of support
Internal sources
Southern New England Retina Associates, Providence, RI, USA.
Brown University, Providence, RI, USA.
Johns Hopkins Medical Institutions, Johns Hopkins University, Baltimore, MD, USA.
External sources
Cochrane Eyes and Vision US Project supported by Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, Bethesda, MD, USA.
SDS and BSH received support during preparation of this review from an unrestricted grant to the Wilmer Eye Institute from Research to Prevent Blindness, New York, New York, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV), acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology
- This review update was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK Editorial base
The views expressed in this publication are those of the review authors and are not necessarily those of the NIHR, the NHS, or the Department of Health
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ABC 2010 {published and unpublished data}
- Keane PA, Heussen FM, Ouyang Y, Mokwa N, Walsh AC, Tufail A, et al. Assessment of differential pharmacodynamic effects using optical coherence tomography in neovascular age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2012;53(3):1152‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PJ, Chen FK, Cruz L, Rubin GS, Tufail A. Contrast sensitivity outcomes in the ABC Trial: a randomized trial of bevacizumab for neovascular age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2011;52(6):3089‐93. [DOI] [PubMed] [Google Scholar]
- Patel PJ, Henderson L, Sivaprasad S, Bunce C, Wormald R, Tufail A. The ABC trial ‐ a randomized double‐masked phase III study of the efficacy and safety of Avastin® (Bevacizumab) intravitreal injections compared to standard therapy in subjects with choroidal neovascularization secondary to age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 4536. [Google Scholar]
- Patel PJ, Rubin GS, Chen FK, Da‐Cruz L, Tufail A. Impact of anti‐vascular endothelial growth factor therapy on reading performance in age‐related macular degeneration: a randomized controlled trial. Investigative Ophthalmology and Visual Science 2015;56(7):4794. [Google Scholar]
- Patel PJ, Tufail A, Bunce C, Cruz L, Dowler J, Egan C, et al. A randomised, double‐masked phase III/IV study of the efficacy and safety of Avastin® (Bevacizumab) intravitreal injections compared to standard therapy in subjects with choroidal neovascularisation secondary to age‐related macular degeneration: clinical trial design. Trials 2008;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail A, Patel PJ, Egan C, Hykin P, Cruz L, Gregor Z, et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ 2010;340:c2459. [DOI] [PubMed] [Google Scholar]
ANCHOR 2006 {published and unpublished data}
- Alexander SG, Blodi BA, Webster MKW, Elledge JA, Hiner CJ, Armstrong J, et al. Ranibizumab (Lucentis®) in subjects with predominantly classic CNV: reading center evaluation of angiographic eligibility characteristics and baseline fluorescein angiographic data. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 2207. [Google Scholar]
- Barbazetto I, Saroj N, Shapiro H, Wong P, Freund KB. Dosing regimen and the frequency of macular hemorrhages in neovascular age‐related macular degeneration treated with ranibizumab. Retina 2010;30(9):1376‐85. [DOI] [PubMed] [Google Scholar]
- Barbazetto IA, Saroj N, Shapiro H, Wong P, Ho AC, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. American Journal of Ophthalmology 2010;149(6):939‐46. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012;32(9):1821‐8. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Chang TS, Fine JT, Dolan CM, Ward J, Anti‐VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age‐Related Macular Degeneration (ANCHOR) Research Group. Improved vision‐related function after ranibizumab vs photodynamic therapy: a randomized clinical trial. Archives of Ophthalmology 2009;127(1):13‐21. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision‐related function after ranibizumab treatment by better‐ or worse‐seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology 2010;117(4):747‐56. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Chang TS, Varma R, Suner I, Lee P, Dolan CM, et al. Driving ability reported by neovascular age‐related macular degeneration patients after treatment with ranibizumab. Ophthalmology 2013;120(1):160‐8. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Dolan CM, Fine J, Marceau C, Chang TS. Vision‐specific quality of life at 12 months in predominantly classic neovascular AMD in ANCHOR: a phase III trial of ranibizumab and verteporfin PDT. European VitreoRetinal Society 2006. Symposium II: Anti‐Angiogenesis (Part 2). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim R, et al. Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1432‐44. [DOI] [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age‐related macular degeneration: two‐year results of the ANCHOR study. Ophthalmology 2009;116(1):57‐65. [DOI] [PubMed] [Google Scholar]
- Brown DM, Shapiro H, Schneider S. Subgroup analysis of first‐year results of ANCHOR: a phase III, double‐masked, randomized comparison of ranibizumab and verteporfin photodynamic therapy for predominantly classic choroidal neovascularization related to age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 2963. [Google Scholar]
- Chang TS, Fine JT, Bressler N. Self‐reported vision‐specific quality of life at 1 year in patients with neovascular age‐related macular degeneration in 2 phase III randomized clinical trials of ranibizumab (Lucentis®). Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 5252. [Google Scholar]
- Ciulla TA, Shapiro H, Schneider S. Ranibizumab (Lucentis®) for neovascular age‐related macular degeneration (AMD): 1‐year visual acuity (VA) results for fellow eyes with neovascular AMD in MARINA and ANCHOR. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 4573. [Google Scholar]
- Cunningham ET, Feiner L, Chung C, Tuomi L, Ehrlich JS. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age‐related macular degeneration. Ophthalmology 2011;118(12):2447‐52. [DOI] [PubMed] [Google Scholar]
- Heier JS, Chung C, Schneider S. Time course of visual acuity changes with ranibizumab (Lucentis®) in the 2‐year ANCHOR study of patients with neovascular age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 2872. [Google Scholar]
- Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, et al. Ranibizumab for predominantly classic neovascular age‐related macular degeneration: subgroup analysis of first‐year ANCHOR results. American Journal of Ophthalmology 2007;144(6):850‐7. [DOI] [PubMed] [Google Scholar]
- Korobelnik JF. Consistency of effect of ranibizumab at 2 doses on contrast sensitivity in 3 phase III and IIIB studies of patients with CNV secondary to AMD. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Lanzetta P. Ranibizumab and patient‐reported outcomes: VFQ‐25 data from the ANCHOR and PIER trials in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 5571. [Google Scholar]
- Lanzetta P, MARINA and ANCHOR Study Groups. Combined safety of intravitreal ranibizumab in two phase III studies of CNV secondary to ARMD. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Loewenstein A, MARINA and ANCHOR Study Groups. Combined efficacy of intravitreal ranibizumab in two phase III studies of choroidal neovascularization secondary to age‐related macular degeneration. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- McDonald HR, Schneider S, Sy JP. One‐year results of ANCHOR: a phase III, multicenter, randomized, double‐masked comparison of ranibizumab and verteporfin photodynamic therapy in treatment of predominantly classic choroidal neovascularization secondary to age‐related macular degeneration. The Macula Society. 2006:114.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven‐year outcomes in ranibizumab‐treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN‐UP). Ophthalmology 2013;120(11):2292‐9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Shapiro H, Shams N, Schneider S, Depperschmidt EE, MARINA and ANCHOR Study Groups. Baseline and treatment characteristics of patients losing vision at year 1 of the MARINA and ANCHOR trials. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 4576. [Google Scholar]
- Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011;118(3):523‐30. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B. Lesion characteristics in ranibizumab (Lucentis®) treated patients with a visual acuity (VA) gain or loss in the MARINA and ANCHOR trials for wet age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 329. [Google Scholar]
- Sadda SR, Shapiro H, Schneider S. Anatomic outcomes at 2 years in the ANCHOR study comparing ranibizumab (Lucentis®) and verteporfin photodynamic therapy (PDT) in predominantly classic neovascular age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 4561. [Google Scholar]
- Sadda SR, Stoller G, Boyer DS, Blodi BA, Shapiro H, Ianchulev T. Anatomical benefit from ranibizumab treatment of predominantly classic neovascular age‐related macular degeneration in the 2‐year anchor study. Retina 2010;30(9):1390‐9. [DOI] [PubMed] [Google Scholar]
- Schwartz SG, Bressler N, Fine JT, Dolan CM, Yu EM, Ward JF, et al. Patient‐reported visual function over 24 months in predominantly classic neovascular AMD: results from ANCHOR, a phase III trial of ranibizumab and verteporfin PDT. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 1822. [Google Scholar]
- Sutter FK, Kurz‐Levin MM. Towards an optimized treatment scheme of ranibizumab in patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration: subanalysis of clinical trial data and clinical routine use. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 2884. [Google Scholar]
- Webster MK, Blodi BA, Elledge JA, Danis RP. Angiographic results of the ANCHOR study of ranibizumab in neovascular age‐related macular degeneration: reading center evaluation of angiographic data. Investigative Ophthalmology and Visual Science 2007; Vol. 48:ARVO E‐Abstract 4583.
- Weinberg DV, Shapiro H, Ehrlich JS. Ranibizumab treatment outcomes in phakic versus pseudophakic eyes: an individual patient data analysis of 2 phase 3 trials. Ophthalmology 2013;120(6):1278‐82. [DOI] [PubMed] [Google Scholar]
- Win PH, Bressler NM, Chang TS, Fine JT, Dolan CM, Ward J. Self‐reported perception of driving function after ranibizumab therapy in patients with neovascular age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 5578. [Google Scholar]
- Wolf S. Visual acuity gain with ranibizumab therapy: two‐year data from the MARINA, ANCHOR, and PIER trials in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 2883. [Google Scholar]
- Wolf S, Holz FG, Korobelnik JF, Lanzetta P, Mitchell P, Prünte C, et al. Outcomes following three‐line vision loss during treatment of neovascular age‐related macular degeneration: subgroup analyses from MARINA and ANCHOR. British Journal of Ophthalmology 2011;95(12):1713‐8. [DOI] [PubMed] [Google Scholar]
- Yang YC. Two year contrast sensitivity results from the ANCHOR study comparing intravitreal ranibizumab 0.3mg and 0.5mg with verteporfin PDT for predominantly classic CNV lesions secondary to age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 4545. [Google Scholar]
Biswas 2011 {published data only}
- Biswas P, Sengupta S, Choudhary R, Home S, Paul A, Sinha S. Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age‐related macular degeneration. Indian Journal of Ophthalmology 2011;59(3):191‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
BRAMD 2016 {published data only}
- Schauwvlieghe AME, Dijkman G, Hooymans JM, Verbraak FD, Hoyng CB, Dijkgraaf MGW, et al. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age‐related macular degeneration. The BRAMD study. PLOS ONE 2016;11(5):e0153052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwvlieghe ASME, Dijkman G, Hooymans JMM, Verbraak FD, Dijkgraaf MG, Peto T, et al. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2014;55(13):870. [Google Scholar]
CATT 2011 {published and unpublished data}
- Comparison of Age‐Related Macular Degeneration Treatment Trials. www.med.upenn.edu/cpob/studies/documents/CATTEligibilityCriteria.pdf (accessed 20 December 2011).
- Altaweel MM, Daniel E, Martin DF, Mittra RA, Grunwald JE, Lai MM, et al. Outcomes of eyes with lesions composed of >50% blood in the comparison of age‐related macular degeneration treatments trials (CATT). Ophthalmology 2015;122(2):391‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATT Research Group, Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE. Five‐year outcomes with anti‐vascular endothelial growth factor treatment of neovascular age‐related macular degeneration: the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123(8):1751‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2011;364(20):1897‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciulla TA, Ying GS, Maguire MG, Martin DF, Jaffe GJ, Grunwald JE, et al. Influence of the vitreomacular interface on treatment outcomes in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2015;122(6):1203‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E, Shaffer J, Ying GS, Grunwald JE, Martin DF, Jaffe GJ, et al. Outcomes in eyes with retinal angiomatous proliferation in the Comparison of Age‐Related Macular Degeneration Treatments Trials (CATT). Ophthalmology 2016;123(3):609‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, et al. Risk of scar in the Comparison of Age‐related Macular Degeneration Treatments Trials. Ophthalmology 2014; Vol. 121, issue 3:656‐66. [DOI] [PMC free article] [PubMed]
- Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA, et al. Risk of geographic atrophy in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2014;121(1):150‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Daniel E, Ying GS, Pistilli M, Maguire MG, Alexander J, et al. Photographic assessment of baseline fundus morphologic features in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2012;119(8):1634‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Pistilli M, Daniel E, Ying GS, Fine SL, Martin DF, Maguire MG. Size and growth of geographic atrophy in the Comparison of Age‐related Macular Degeneration Treatments Trials (CATT). Investigative Ophthalmology and Visual Science 2014;55(13):1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, et al. Incidence and growth of geographic atrophy during 5 years of Comparison of Age‐related Macular Degeneration Treatments Trials. Ophthalmology 2017;124(1):97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF, et al. Growth of geographic atrophy in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2015;122(4):809‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom SA, Ying GS, Pauer GJ, Sturgill‐Short GM, Huang J, Callanan DG, et al. Pharmacogenetics for genes associated with age‐related macular degeneration in the Comparison of AMD Treatments Trials (CATT). Ophthalmology 2013; Vol. 120, issue 3:593‐9. [DOI] [PMC free article] [PubMed]
- Huang J, Ying GS, Maguire MG, Stone RA, Martin DF. Impact of change in retinal thickness on refractive error in the Comparison of Age‐Related Macular Degeneration Treatments Trials (CATT). Investigative Ophthalmology and Visual Science 2014;55(13):657. [Google Scholar]
- Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2013;120(9):1860‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire MG. Comparing treatments for age‐related macular degeneration: safety, effectiveness and cost. LDI Issue Brief 2012; Vol. 17, issue 8:1‐4. [PubMed]
- Maguire MG, Daniel E, Shah AR, Grunwald JE, Hagstrom SA, Avery RL, et al. Incidence of choroidal neovascularization in the fellow eye in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2013;120(10):2035‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age‐related macular degeneration: two‐year results. Ophthalmology 2012;119(7):1388‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith TA, McCannel CA, Barr C, Doft BH, Peskin E, Maguire MG, et al. Postinjection endophthalmitis in the Comparison of Age‐Related Macular Degeneration Treatments Trials (CATT). Ophthalmology 2015;122(4):817‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli M, Ying GS, Jaffe GJ, Toth CA, Martin DF. Resolution of retinal fluid on optical coherence tomography in the Comparison of AMD Treatments Trials (CATT). Investigative Ophthalmology and Visual Science 2015;56(7):1657. [Google Scholar]
- Shah N, Maguire MG, Martin DF, Shaffer J, Ying GS, Grunwald JE, et al. Angiographic cystoid macular edema and outcomes in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123(4):858‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Toth CA, Daniel E, Grunwald JE, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the second year of the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123(4):865‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby AS, Ying GS, Toth CA, Maguire MG, Burns RE, Grunwald JE, et al. Subretinal hyperreflective material in the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2015;122(9):1846‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying GS, Huang J, Maguire MG, Jaffe GJ, Grunwald JE, Toth C, et al. Baseline predictors for one‐year visual outcomes with ranibizumab or bevacizumab for neovascular age‐related macular degeneration. Ophthalmology 2013;120(1):122‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying GS, Maguire MG, Daniel E, Ferris FL, Jaffe GJ, Grunwald JE, et al. Association of baseline characteristics and early vision response with 2‐year vision outcomes in the Comparison of AMD Treatments Trials (CATT). Ophthalmology 2015;122(12):2523‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
GEFAL 2013 {published and unpublished data}
- Kodjikian L, Souied EH, Mimoun G, Mauget‐Faysse M, Behar‐Cohen F, Decullier E, et al. Ranibizumab versus bevacizumab for neovascular age‐related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology 2013;120(11):2300‐9. [DOI] [PubMed] [Google Scholar]
IVAN 2013 {published and unpublished data}
- A randomised controlled trial of alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation. www.ivan‐trial.co.uk/Default.aspx (accessed 4 April 2014).
- Erratum: Ranibizumab versus bevacizumab to treat neovascular age‐related macular degeneration: one‐year findings from the IVAN randomized trial (Ophthalmology (2012) 119 (1399‐1411)). Ophthalmology 2012;119(8):1508. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes S, Lotery AJ, Dakin HA, et al. A randomised controlled trial to assess the clinical effectiveness and cost‐effectiveness of alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation (IVAN). Health Technology Assessment 2015;19(78):1‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, on behalf of the IVAN study investigators. Alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation: 2‐year findings of the IVAN randomised controlled trial. Lancet 2013;382(9900):1258‐67. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age‐related macular degeneration: one‐year findings from the IVAN randomized trial. Ophthalmology 2012;119(7):1399‐411. [DOI] [PubMed] [Google Scholar]
- Foss AJ, Scott LJ, Rogers CA, Reeves BC, Ghanchi F, Gibson J, et al. Changes in intraocular pressure in study and fellow eyes in the IVAN trial. British Journal of Ophthalmology. 2016;100(12):1662‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Gibson J, Cree AJ, Downes SM, Harding SP, Rogers CA, et al. Pharmacogenetic associations with vascular endothelial growth factor inhibition in participants with neovascular age‐related macular degeneration in the IVAN study. Ophthalmology 2013;120(12):2637‐43. [DOI] [PubMed] [Google Scholar]
LUCAS 2015 {published and unpublished data}
- Berg K, Hadzalic E, Gjertsen I, Forsaa V, Berger LH, Kinge B, et al. Ranibizumab or bevacizumab for neovascular age‐related macular degeneration according to the Lucentis Compared to Avastin Study treat‐and‐extend protocol: two‐year results. Ophthalmology 2016;123(1):51‐9. [DOI] [PubMed] [Google Scholar]
- Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age‐related macular degeneration according to LUCAS treat‐and extend protocol. Ophthalmology 2015;122(1):146‐52. [DOI] [PubMed] [Google Scholar]
MANTA 2013 {published and unpublished data}
- Krebs I, Schmetterer L, Boltz A, Told R, Vécsei‐Marlovits V, Egger S, et al. A randomised double‐masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age‐related macular degeneration. British Journal of Ophthalmology 2013;97(3):266‐71. [DOI] [PubMed] [Google Scholar]
MARINA 2006 {published and unpublished data}
- Barbazetto I, Saroj N, Shapiro H, Wong P, Freund KB. Dosing regimen and the frequency of macular hemorrhages in neovascular age‐related macular degeneration treated with ranibizumab. Retina 2010;30(9):1376‐85. [DOI] [PubMed] [Google Scholar]
- Barbazetto IA, Saroj N, Shapiro H, Wong P, Ho AC, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. American Journal of Ophthalmology 2010;149(6):939‐46. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age‐related macular degeneration. Ophthalmology 2007;114(2):246‐52. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012;32(9):1821‐8. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision‐related function after ranibizumab treatment by better‐ or worse‐seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology 2010;117(4):747‐56. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Chang TS, Varma R, Suner I, Lee P, Dolan CM, et al. Driving ability reported by neovascular age‐related macular degeneration patients after treatment with ranibizumab. Ophthalmology 2013;120(1):160‐8. [DOI] [PubMed] [Google Scholar]
- Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J. Self‐reported perception of driving function following ranibizumab treatment in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 1830. [Google Scholar]
- Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR, et al. Improved vision‐related function after ranibizumab treatment of neovascular age‐related macular degeneration: results of a randomized clinical trial. Archives of Ophthalmology 2007;125(11):1460‐9. [DOI] [PubMed] [Google Scholar]
- Chang TS, Fine JT, Alexander S, Bressler N. Ranibizumab (Lucentis®) self‐reported vision specific quality of life through 12 months in age‐related macular degeneration patients with minimally classic or occult‐with‐no‐classic choroidal neovascularization in a phase III randomized clinical trial. The Macula Society. 2006:106.
- Chang TS, Fine JT, Bressler N. Self‐reported vision‐specific quality of life at 1 year in patients with neovascular age‐related macular degeneration in 2 phase III randomized clinical trials of ranibizumab (Lucentis®). Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 5252. [Google Scholar]
- Chang TS, Fine JT, Dolan CM, Marceau C, Bressler NM. Ranibizumab (Lucentis®) vision‐specific quality of life through 24 months in neovascular AMD subjects in MARINA: a phase III clinical trial. European VitreoRetinal Society 2006. Symposium II: Anti‐Angiogenesis (Part 2). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Ciulla TA, Shapiro H, Schneider S. Ranibizumab (Lucentis®) for neovascular age‐related macular degeneration (AMD): 1‐year visual acuity (VA) results for fellow eyes with neovascular AMD in MARINA and ANCHOR. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 4573. [Google Scholar]
- Cunningham ET, Feiner L, Chung C, Tuomi L, Ehrlich JS. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age‐related macular degeneration. Ophthalmology 2011;118(12):2447‐52. [DOI] [PubMed] [Google Scholar]
- Heier JS, Shapiro H, Singh AA. Randomized, controlled phase III study of ranibizumab (Lucentis®) for minimally classic or occult neovascular age‐related macular degeneration: two‐year efficacy results of the MARINA study. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 2959. [Google Scholar]
- Ho A, Shapiro H, Acharya N. Ranibizumab for neovascular age‐related macular degeneration: one‐year subanalysis of the MARINA study. The Macula Society. 2006:108.
- Holekamp NM, Acharya N, Shapiro H. Two‐year FA/OCT results of MARINA study of ranibizumab (Lucentis®) in neovascular AMD. American Academy of Ophthalmology. 2006:194.
- Hurley SF, Matthews JP, Guymer RH. Cost‐effectiveness of ranibizumab for neovascular age‐related macular degeneration. Cost Effectiveness and Resource Allocation 2008;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser PK, Blodi BA, Shapiro H, Acharya NR, MARINA Study Group. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age‐related macular degeneration. Ophthalmology 2007;114(10):1868‐75. [DOI] [PubMed] [Google Scholar]
- Kokame GT. Anatomic outcomes from the MARINA study of ranibizumab (Lucentis®) for minimally classic or occult neovascular age‐related macular degeneration. The Macula Society. 2006:110.
- Korobelnik JF. Consistency of effect of ranibizumab at 2 doses on contrast sensitivity in 3 phase III and IIIB studies of patients with CNV secondary to AMD. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Lanzetta P, MARINA and ANCHOR Study Groups. Combined safety of intravitreal ranibizumab in two phase III studies of CNV secondary to ARMD. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Loewenstein A, MARINA and ANCHOR Study Groups. Combined efficacy of intravitreal ranibizumab in two phase III studies of choroidal neovascularization secondary to age‐related macular degeneration. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Miller JW, Shapiro H, Acharya N. Randomized, controlled phase III study of ranibizumab (Lucentis®) for minimally classic or occult neovascular age‐related macular degeneration: two‐year safety results of the MARINA study. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 3539. [Google Scholar]
- Reichel E, Shapiro H, Acharya N. Subgroup analyses of two‐year results of MARINA study of ranibizumab in neovascular AMD. American Academy of Ophthalmology. 2006:194.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven‐year outcomes in ranibizumab‐treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN‐UP). Ophthalmology 2013;120(11):2292‐9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1419‐31. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Shapiro H, Shams N, Schneider S, Depperschmidt EE, MARINA and ANCHOR Study Groups. Baseline and treatment characteristics of patients losing vision at year 1 of the MARINA and ANCHOR trials. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 4576. [Google Scholar]
- Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011;118(3):523‐30. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B. Lesion characteristics in ranibizumab (Lucentis®) treated patients with a visual acuity (VA) gain or loss in the MARINA and ANCHOR trials for wet age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 329. [Google Scholar]
- Schmidt‐Erfurth U, Williams GA, MARINA Study Group. Two‐year efficacy and safety results from the MARINA study. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Suner IJ, Bressler NM, Chang TS, Fine JT, Dolan CM, Ward J. Vision‐related function after ranibizumab treatment by better‐ or worse‐seeing eye: 24 month results from MARINA. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 1808. [DOI] [PubMed] [Google Scholar]
- Sutter FK, Kurz‐Levin MM. Towards an optimized treatment scheme of ranibizumab in patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration: subanalysis of clinical trial data and clinical routine use. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 2884. [Google Scholar]
- Webster MK, Blodi BA, Glaeser Alexander S, Elledge KA, Hiner C, Hurtenbach CH, et al. Ranibizumab (Lucentis®) in subjects with minimally classic or occult CNV (MARINA): reading center evaluation of angiographic eligibility characteristics and baseline fluorescein angiographic data. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 2206. [Google Scholar]
- Weinberg DV, Shapiro H, Ehrlich JS. Ranibizumab treatment outcomes in phakic versus pseudophakic eyes: an individual patient data analysis of 2 phase 3 trials. Ophthalmology 2013;120(6):1278‐82. [DOI] [PubMed] [Google Scholar]
- Wolf S. Visual acuity gain with ranibizumab therapy: two‐year data from the MARINA, ANCHOR, and PIER trials in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐abstract 2883. [Google Scholar]
- Wolf S, Holz FG, Korobelnik JF, Lanzetta P, Mitchell P, Prünte C, et al. Outcomes following three‐line vision loss during treatment of neovascular age‐related macular degeneration: subgroup analyses from MARINA and ANCHOR. British Journal of Ophthalmology 2011;95(12):1713‐8. [DOI] [PubMed] [Google Scholar]
- Yu EB, Bressler NM, Fine JT, Ward JF, Dolan CM, Klesert T, et al. Relationship between patient‐reported visual function and visual acuity in subjects with neovascular AMD. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 1174. [Google Scholar]
PIER 2008 {published and unpublished data}
- Abraham P, Yue H, Shams N. PIER: year 1 results of a phase IIIb study of ranibizumab efficacy and safety in choroidal neovascularization due to age‐related macular degeneration. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Abraham P, Yue H, Wilson L. Randomized, double‐masked, sham‐controlled trial of ranibizumab for neovascular age‐related macular degeneration: PIER study year 2. American Journal of Ophthalmology 2010;150(3):315‐24. [DOI] [PubMed] [Google Scholar]
- Barbazetto I, Saroj N, Shapiro H, Wong P, Freund KB. Dosing regimen and the frequency of macular hemorrhages in neovascular age‐related macular degeneration treated with ranibizumab. Retina 2010;30(9):1376‐85. [DOI] [PubMed] [Google Scholar]
- Benz MS, Brown DM, Shapiro H, Tuomi L. Ranibizumab for neovascular age‐related macular degeneration (AMD): optical coherence tomography (OCT) vs. visual acuity (VA) changes in PIER study. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 347. [Google Scholar]
- Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012;32(9):1821‐8. [DOI] [PubMed] [Google Scholar]
- Brown DM, Chung CY, Tuomi L. Ranibizumab (Lucentis®) for neovascular age‐related macular degeneration (AMD): optical coherence tomography (OCT) vs. visual acuity (VA) changes in PIER study. The Macula Society. 2008:78.
- Brown DM, Tuomi L, Shapiro H, PIER Study Group. Anatomical measures as predictors of visual outcomes in ranibizumab‐treated eyes with neovascular age‐related macular degeneration. Retina 2013;33(1):23‐34. [DOI] [PubMed] [Google Scholar]
- Brown DM, Yue H, Shams N. Ranibizumab (Lucentis®) in neovascular age‐related macular degeneration (AMD): subgroup analysis of year 1 PIER efficacy data. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 4540. [Google Scholar]
- Cunningham ET, Feiner L, Chung C, Tuomi L, Ehrlich JS. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age‐related macular degeneration. Ophthalmology 2011;118(12):2447‐52. [DOI] [PubMed] [Google Scholar]
- Ho AC, Shapiro H, Wilson L. Ranibizumab (Lucentis®) for neovascular age‐related macular degeneration (AMD): two‐year angiographic results of PIER study. The Macula Society. 2008:164.
- Ho AC, Yue H, Wilson L. Ranibizumab in wet age‐related macular degeneration (AMD): crossover/rollover patient results in PIER study. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 368. [Google Scholar]
- Kaiser PK, Yue H, Shams N. Subgroup analyses of one‐year results of the PIER study of ranibizumab in neovascular AMD. American Academy of Ophthalmology. 2006:195.
- Lanzetta P. Ranibizumab and patient‐reported outcomes: VFQ‐25 data from the ANCHOR and PIER trials in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 5571. [Google Scholar]
- Michels M, Shapiro H, Wilson L. Ranibizumab (Lucentis®) for neovascular age‐related macular degeneration (AMD): 2‐year angiographic results of PIER study. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 2882. [Google Scholar]
- Mieler WF. PIER: Year 1 FA/OCT results in a study of ranibizumab (Lucentis™) for CNV due to ARMD. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
- Regillo CD, Bressler NM, Fine JT, Dolan CM, Marceau C, Chang TS. Vision‐specific quality of life at 12 months in subjects with or without classic CNV due to AMD: phase III clinical trial of ranibizumab (Lucentis™). European VitreoRetinal Society 2006. Poster Session. www.evrs.eu/2006‐evrs‐congress‐cannes/poster‐session‐amdanti‐angiogenesis (accessed 13 March 2013).
- Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double‐masked, sham‐controlled trial of ranibizumab for neovascular age‐related macular degeneration: PIER study year 1. American Journal of Ophthalmology 2008;145(2):239‐48. [DOI] [PubMed] [Google Scholar]
- Regillo CD, Chung CY, Wilson L, PIER Study Group. Ranibizumab in neovascular age‐related macular degeneration (AMD): crossover/rollover treatment effects in PIER study. The Macula Society. 2008:166.
- Wolf S. Visual acuity gain with ranibizumab therapy: two‐year data from the MARINA, ANCHOR, and PIER trials in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 2883. [Google Scholar]
Sacu 2009 {published and unpublished data}
- Michels SM, Weigert G, Geitzenauer W, Sacu S, Alina V, Schmidt‐Erfurth U. Intravitreal bevacizumab (Avastin®) therapy versus verteporfin therapy and intravitreal triamcinolone for neovascular age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 1820. [Google Scholar]
- Prager F, Michels S, Sacu S, Weigert G, Dunavölgyi R, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin®) monotherapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age‐related macular degeneration: 12 months results of a prospective, randomized, controlled clinical trial. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 1168. [DOI] [PubMed] [Google Scholar]
- Sacu S, Michels S, Prager F, Weigert G, Dunavoelgyi R, Geitzenauer W, et al. Randomised clinical trial of intravitreal Avastin® vs photodynamic therapy and intravitreal triamcinolone: long‐term results. Eye 2009;23(12):2223‐7. [DOI] [PubMed] [Google Scholar]
- Weigert G, Michels S, Sacu S, Varga A, Prager F, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin®) therapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age‐related macular degeneration: 6‐month results of a prospective, randomised, controlled clinical study. British Journal of Ophthalmology 2008;92(3):356‐60. [DOI] [PubMed] [Google Scholar]
SAVE‐AMD 2017 {published data only (unpublished sought but not used)}
- Enseleit F, Michels S, Sudano I, Stahel M, Zweifel S, Schlager O, et al. SAVE‐AMD: safety of VEGF inhibitors in age‐related macular degeneration. Ophthalmologica 2017;238:205‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scholler 2014 {published data only}
- Scholler A, Richter‐Mueksch S, Weingessel B, Vecsei‐Marlovits P‐V. Differences of frequency in administration of ranibizumab and bevacizumab in patients with neovascular AMD. Wiener Klinische Wochenschrift (Central European Journal of Medicine) 2014;126:355‐9. [DOI] [PubMed] [Google Scholar]
Subramanian 2010 {published and unpublished data}
- Donahue SP, Recchia F, Sternberg P Jr. Bevacizumab vs ranibizumab for age‐related macular degeneration: early results of a prospective double‐masked, randomized clinical trial. American Journal of Ophthalmology 2010;150(2):287. [DOI] [PubMed] [Google Scholar]
- Messori A, Fadda V, Trippoli S. Randomized study of bevacizumab vs ranibizumab for age‐related macular degeneration: inappropriate conclusions. American Journal of Ophthalmology 2010;149(5):867. [DOI] [PubMed] [Google Scholar]
- Subramanian ML, Abedi G, Ness S, Ahmed E, Fenberg M, Daly MK, et al. Bevacizumab vs ranibizumab for age‐related macular degeneration: 1‐year outcomes of a prospective, double‐masked randomised clinical trial. Eye 2010;24(11):1708‐15. [DOI] [PubMed] [Google Scholar]
- Subramanian ML, Ness S, Abedi G, Ahmed E, Daly M, Feinberg E, et al. Bevacizumab vs ranibizumab for age‐related macular degeneration: early results of a prospective double‐masked, randomized clinical trial. American Journal of Ophthalmology 2009;148(6):875‐82. [DOI] [PubMed] [Google Scholar]
- Subramanian ML, Ness S, Abedi G, Ahmed E, Daly M, Feinberg E, et al. Reply. American Journal of Ophthalmology 2010;149(5):867. [Google Scholar]
VISION 2004 {published and unpublished data}
- Chakravarthy U. Efficacy and safety of pegaptanib in age‐related macular degeneration: three‐year results of the V.I.S.I.O.N. trials. The Macula Society. 2006:116.
- D'Amico DF. Macugen® in neovascular age‐related macular degeneration: exploratory subgroup analyses. American Academy of Ophthalmology. 2005:261.
- D'Amico DJ. VEGF Inhibition Study in Ocular Neovascularization (VISION): second year efficacy data. Investigative Ophthalmology and Visual Science 2005;46:ARVO E‐abstract 2309. [Google Scholar]
- D'Amico DJ, Bird AC. VEGF Inhibition Study in Ocular Neovascularization‐1 (VISION‐1): safety evaluation from the pivotal Macugen® (pegaptanib sodium) clinical trials. Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐Abstract 2363. [Google Scholar]
- Eyetech Pharmaceuticals, Inc, Pfizer. Inc. Pegaptanib sodium injection in the treatment of neovascular age‐related macular degeneration. www.fda.gov/ohrms/dockets/ac/04/briefing/2004‐4053b1.htm (accessed 20 December 2011).
- FDA. Macugen® (pegaptanib sodium injection) for the treatment of neovascular age‐related macular degeneration. www.fda.gov/ohrms/dockets/ac/04/briefing/2004‐4053b1.htm (accessed 20 December 2011).
- Gragoudas ES. VEGF Inhibition Study in Ocular Neovascularization‐1 (VISION‐1): efficacy results from phase II/III Macugen® (pegaptanib sodium) clinical trials. Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐abstract 2364. [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age‐related macular degeneration. New England Journal of Medicine 2004;351(27):2805‐16. [DOI] [PubMed] [Google Scholar]
- Jackson TL, Danis RP, Goldbaum M, Slakter JS, Shusterman EM, Oeshaughnessy DJ, et al. Retinal vascular abnormalities in neovascular age‐related macular degeneration. Retina 2014;34(3):568‐75. [DOI] [PubMed] [Google Scholar]
- Kuppermann BD. The V.I.S.I.O.N. Study: results with two years of Macugen® and outcomes of earlier treatment in early disease. American Academy of Ophthalmology. 2005:178.
- Larsen M, Sander B, Villumsen JE, Haamann PH, Cour M, Lund‐Andersen H, et al. Treatment of neovascular age‐related macular degeneration with intravitreal vascular endothelial growth factor inhibitor ‐ secondary publication. Ugeskrift for Laeger 2005;167(35):3301‐5. [PubMed] [Google Scholar]
- Leys A, Zlateva G, Shah SN, Patel M. Quality of life in patients with age‐related macular degeneration: results from the VISION study. Eye 2008;22(6):792‐8. [DOI] [PubMed] [Google Scholar]
- Macugen AMD Study Group, Apte RS, Modi M, Masonson H, Patel M, Whitfield L, et al. Pegaptanib 1‐year systemic safety results from a safety‐pharmacokinetic trial in patients with neovascular age‐related macular degeneration. Ophthalmology 2007;114(9):1702‐12. [DOI] [PubMed] [Google Scholar]
- Marcus DM. Four‐year safety of pegaptanib sodium in neovascular age‐related macular degeneration (AMD): results of the V.I.S.I.O.N. trial. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐ Abstract 5069. [Google Scholar]
- Mieler W. Safety evaluation of second year treatment of age‐related macular degeneration with pegaptanib sodium (Macugen®): VEGF Inhibition Study in Ocular Neovascularization (VISION). Investigative Ophthalmology and Visual Science 2005;46:ARVO E‐Abstract 1380. [Google Scholar]
- Mills E, Heels‐Ansdell D, Kelly S, Guyatt G. A randomized trial of pegaptanib sodium for age‐related macular degeneration used an innovative design to explore disease‐modifying effects. Journal of Clinical Epidemiology 2007;60(5):456‐60. [DOI] [PubMed] [Google Scholar]
- Patel M, Mones J, Leys A, Zlateva G, Shah SN. Quality of life in patients with age‐related macular degeneration: results from the V.I.S.I.O.N. trial. European VitreoRetinal Society 2006. Poster Session. www.evrs.eu/2006‐evrs‐congress‐cannes/poster‐session‐amdanti‐angiogenesis (accessed 13 March 2013).
- Rakic JM, Blaise P, Foidart JM, Gragoudas ES, Adamis AP, Feinsod M. Pegaptanib and age‐related macular degeneration. New England Journal of Medicine 2005;352(16):1720‐1. [DOI] [PubMed] [Google Scholar]
- Singerman LI. Two‐year systemic safety of pegaptanib in patients with neovascular age‐related macular degeneration: results of the V.I.S.I.O.N. trials. The Macula Society. 2006:120.
- Singerman LJ, Masonson H, Patel M, Adamis AP, Buggage R, Cunningham E, et al. Pegaptanib sodium for neovascular age‐related macular degeneration: third‐year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. British Journal of Ophthalmology 2008;92(12):1606‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolie AK, Mortensen KK, Hansen MH. Antiangiogenesis therapy of age‐related macular degeneration. Ugeskrift for Laeger 2005;167(35):3267. [PubMed] [Google Scholar]
- VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group, Chakravarthy U, Adamis AP, Cunningham ET Jr, Goldbaum M, Guyer DR, et al. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age‐related macular degeneration. Ophthalmology 2006; Vol. 113, issue 9:1508. [DOI] [PubMed]
- VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group, D'Amico DJ, Masonson HN, Patel M, Adamis AP, Cunningham ET, et al. Pegaptanib sodium for neovascular age‐related macular degeneration: two‐year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology 2006;113(6):1001. [DOI] [PubMed] [Google Scholar]
- Zlateva G, Patel M, Shah SN. Quality of life in patients with age‐related macular degeneration: results from the VISION study. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐Abstract 2152. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ADVANCE 2008 {unpublished data only}
- Safety and efficacy of oral PTK787 in patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration (AMD) (ADVANCE). ClinicalTrials.gov NCT00138632. [NCT00531336]
ARMAST 2008 {unpublished data only}
- Photodynamic therapy combined with bevacizumab vs bevacizumab alone for neovascular age‐related macular degeneration (ARMAST). ClinicalTrials.gov NCT0069592.
Bashshur 2007 {published data only}
- Bashshur ZF, Schakal A, Hamam RN, Haibi CP, Jaafar R, Noureddin BN. Intravitreal bevacizumab vs verteporfin photodynamic therapy for neovascular age‐related macular degeneration. Archives of Ophthalmology 2007;125(10):1357‐61. [DOI] [PubMed] [Google Scholar]
BEAT‐AMD 2009 {published data only}
- Schmid‐Kubista KE, Krebs I, Gruenberger B, Schueller J, Binder S. Systemic bevacizumab therapy for exudative neovascular age‐related macular degeneration (BEAT‐AMD‐Study). Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 304. [DOI] [PubMed] [Google Scholar]
- Schmid‐Kubista KE, Krebs I, Gruenberger B, Zeiler F, Schueller J, Binder S. Systemic bevacizumab (Avastin®) therapy for exudative neovascular age‐related macular degeneration. The BEAT‐AMD‐Study. British Journal of Ophthalmology 2009;93(7):914‐9. [DOI] [PubMed] [Google Scholar]
Berger 2015 {published data only}
- Berger BB, Yanni SE, Wenzel A, Weichselberger A, Hubschman JP. Efficacy of RTH258 (ESBA1008), an anti‐VEGF agent, applied by microvolume injection or infusion in subjects with neovascular AMD. Investigative Ophthalmology and Visual Science 2015;56 (7):821. [Google Scholar]
Blaha 2015 {published data only}
- Blaha GR, Brooks NO, Mackel CE, Pani A, Stewart AP, Price LL, et al. Changes in flare after intravitreal injection of three different anti‐vascular endothelial growth factor medications. Retina 2015;35(3):577‐81. [DOI] [PubMed] [Google Scholar]
Bolz 2008 {published data only}
- Bolz M, Pruente C, Benesch T, Ritter M, Deak G, Golbaz I, et al. The relevance of measuring central retinal thickness during intra‐vitreal therapy with ranibizumab: analyzing a multi‐center clinical trial. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 5576. [Google Scholar]
Brown 2016 {published data only}
- Brown DM, Ingerman A, Shearn SP, Slakter JS. CNV lesion characteristics as a predictor of visual outcome in wet AMD patients receiving combination therapy of intravitreal anti‐VEGF therapy and topical Squalamine lactate ophthalmic solution. Investigative Ophthalmology and Visual Science 2016;57(12):4419. [Google Scholar]
Cohen 2008 {published data only}
- Cohen SY, Bremond‐Gignac D, Quentel G, Mimoun G, Citterio T, Bisot‐Locard S, et al. Cost‐effectiveness sequential modeling of ranibizumab versus usual care in age‐related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(11):1527‐34. [DOI] [PubMed] [Google Scholar]
Costagliola 2010 {published data only}
- Costagliola C, Romano MR, Rinaldi M, dell'Omo R, Chiosi F, Menzione M, Semeraro F. Low fluence rate photodynamic therapy combined with intravitreal bevacizumab for neovascular age‐related macular degeneration. British Journal of Ophthalmology 2010;94(2):180‐4. [DOI] [PubMed] [Google Scholar]
Csaky 2015 {published data only}
- Csaky KG, Dugel PU, Pierce AJ, Fries MA, Kelly DS, Danis RP, et al. Clinical evaluation of pazopanib eye drops versus ranibizumab intravitreal injections in subjects with neovascular age‐related macular degeneration. Ophthalmology 2015;122(3):579‐88. [DOI] [PubMed] [Google Scholar]
Earnshaw 2007 {published data only}
- Earnshaw SR, Moride Y, Rochon S. Cost‐effectiveness of pegaptanib compared to photodynamic therapy with verteporfin and to standard care in the treatment of subfoveal wet age‐related macular degeneration in Canada. Clinical Therapeutics 2007;29(9):2096‐106. [DOI] [PubMed] [Google Scholar]
Eibenberger 2015 {published data only}
- Eibenberger K, Sulzbacher F, Rezar S, Roberts PK, Sacu S, Schmidt‐Erfurth U. Randomized comparison of anterior chamber inflammatory activity in eyes treated with intravitreal aflibercept or ranibizumab. Investigative Ophthalmology and Visual Science 2015;56(7):1517. [Google Scholar]
EMERGE 2016 {published data only}
- Christmas NJ. A phase 2 study (EMERGE) evaluating repeated intravitreal administration of ICON‐1 in patients with choroidal neovascularization (CNV) secondary to age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2016;57(12):4434. [Google Scholar]
- NCT02358889. Study evaluating intravitreal hl‐con1TM in patients with ehoroidal neovascularization secondary to age‐related macular degeneration (EMERGE). ClinicalTrials.gov (accessed 24 August 2018).
- Wells JA, Gonzale CR, Berger BB, Gonzalez VH, Sippy BD, Burian G. A phase I, open‐label, dose‐excalation trial to investigate safety and tolerability of single intravitreous injections of ICON‐1 targeting tissue factor in wet AMD. Ophthalmic Surgery, Lasers and Imaging Retina 2018;49:336‐45. [DOI 10.3928/23258160‐20180501‐07; PUBMED: PMID 29772044] [DOI] [PubMed] [Google Scholar]
Erdokur 2009 {published data only}
- Erdokur O, Tetikoglu M, Ozturk M, Elcioglu M. Results of comparison in use to alternative therapy methods for subfoveal choroidal neovascularization secondary to age‐related macular degeneration. Retina‐Vitreus 2009;17(4):245‐50. [Google Scholar]
EXTEND‐I 2008 {published data only}
- Tano Y. The safety and efficacy of ranibizumab in Japanese patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration: 12‐month results from the phase I/II EXTEND‐I study. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 272. [Google Scholar]
- Tano Y, Ohji M, EXTEND‐I Study Group. EXTEND‐I: safety and efficacy of ranibizumab in Japanese patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration. Acta Ophthalmologica 2010;88(3):309‐16. [DOI] [PubMed] [Google Scholar]
Eyetech Study 2003 {published data only}
- Eyetech Study Group. Anti‐vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age‐related macular degeneration: phase II study results. Ophthalmology 2003;110(5):979‐86. [DOI] [PubMed] [Google Scholar]
Falkenstein 2007 {published data only}
- Falkenstein IA, Cheng L, Morrison VL, Kozak I, Tammewar AM, Freeman WR. Standardized visual acuity results associated with primary versus secondary bevacizumab (Avastin®) treatment for choroidal neovascularization in age‐related macular degeneration. Retina 2007;27(6):701‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 2008 {published data only}
- Fletcher EC, Lade RJ, Adewoyin T, Chong NV. Computerized model of cost‐utility analysis for treatment of age‐related macular degeneration. Ophthalmology 2008;115(12):2192‐8. [DOI] [PubMed] [Google Scholar]
FOCUS 2006 {published and unpublished data}
- Antoszyk AN, Tuomi L, Chung CY, Singh A, FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age‐related macular degeneration (FOCUS): year 2 results. American Journal of Ophthalmology 2008;145(5):862‐74. [DOI] [PubMed] [Google Scholar]
- Heier JS, Boyer DS, Ciulla TA, Ferrone PJ, Jumber MJ, Gentile RC, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age‐related macular degeneration: year 1 results of the FOCUS study. Archives of Ophthalmology 2006;124(11):1532‐42. [DOI] [PubMed] [Google Scholar]
- Heier JS, FOCUS Study Group. Intravitreal ranibizumab with verteporfin photodynamic therapy for neovascular age‐related macular degeneration: year one results. Program and abstracts of the American Society of Retina Specialists 23rd Annual Meeting; 2005 July 16‐20; Montreal. 2005.
GALATIR 2014 {unpublished data only}
- NCT02036723. Multicentre double blind randomized clinical study evaluating the efficacy and safety of BCD‐021 (CJSC BIOCAD, Russia) and Lucentis® (Novartis Pharmaceuticals Canada Inc.) in patients with neovascular wet age‐related macular degeneration (GALATIR). clinicaltrials.gov/show/NCT02036723 (accessed 5 February 2017).
Gallemore 2016 {published data only}
- Gallemore RP, Elman MJ, Milton M, Zamiri P, Grosskreutz CL. A proof of concept study of intravitreal (IVT) LFG316 in patients with neovascular age related macular degeneration (NAMD). Investigative Ophthalmology and Visual Science 2016;57(12):4985. [Google Scholar]
Hahn 2007 {published data only}
- Hahn R, Sacu S, Michels S, Varga A, Weigert G, Geitzenauer W, et al. Intravitreal bevacizumab versus verteporfin and intravitreal triamcinolone acetonide in patients with neovascular age‐related macula degeneration. Der Ophthalmologe 2007;104(7):588‐93. [DOI] [PubMed] [Google Scholar]
Hatta 2010 {published data only}
- Hatta Y, Ishikawa K, Nishihara H, Ozawa S, Ito Y, Terasaki H. Effect of photodynamic therapy alone or combined with posterior subtenon triamcinolone acetonide or intravitreal bevacizumab on choroidal hypofluorescence by indocyanine green angiography. Retina 2010;30(3):495‐502. [DOI] [PubMed] [Google Scholar]
HAWK 2018 {published data only}
- Dugel PU, Warburton J, Weichselberger A, Sallstig P. A 2‐year study comparing the efficacy and safety of brolucizumab vs aflibercept in subjects with neovascular age‐related macular degeneration: testing an alternative treatment regimen. Investigative Ophthalmology and Visual Science 2016;57(12):5018. [Google Scholar]
Heier 2006 {published data only}
- Heier JS, Antoszyk AN, Pavan PR, Leff SR, Rosenfeld PJ, Ciulla TA, et al. Ranibizumab for treatment of neovascular age‐related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology 2006;113(4):642. e1‐4. [DOI] [PubMed] [Google Scholar]
Hernandez‐Pastor 2008 {published data only}
- Hernandez‐Pastor LJ, Ortega A, Garcia‐Layana A, Giraldez J. Cost‐effectiveness of ranibizumab compared with photodynamic treatment of neovascular age‐related macular degeneration. Clinical Therapeutics 2008;30(12):2436‐51. [DOI] [PubMed] [Google Scholar]
Hernandez‐Pastor 2010 {published data only}
- Hernandez‐Pastor LJ, Ortega A, Garcia‐Layana A, Giraldez J. Cost‐effectiveness of ranibizumab compared with pegaptanib in neovascular age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2010;248(4):467‐76. [DOI] [PubMed] [Google Scholar]
Holz 2016 {published data only}
- Holz FG, Dugel PU, Weissgerber G, Hamilton R, Silva R, Bandello F, et al. Single‐chain antibody fragment VEGF inhibitor RTH258 for neovascular age‐related macular degeneration: a randomized controlled study. Ophthalmology 2016;123(5):1080‐9. [DOI] [PubMed] [Google Scholar]
Javitt 2008 {published data only}
- Javitt JC, Zlateva GP, Earnshaw SR, Pleil AM, Graham CN, Brogan AJ, et al. Cost‐effectiveness model for neovascular age‐related macular degeneration: comparing early and late treatment with pegaptanib sodium based on visual acuity. Value in Health 2008;11(4):563‐74. [DOI] [PubMed] [Google Scholar]
Kralinger 2014 {published data only}
- Kralinger MT, Zehetner C, Waltl I, Kirchmayr R, Kieselbach GF. VEGF Plasma levels after intravitreal injection of aflibercept and ranibizumab. Investigative Ophthalmology and Visual Science 2014;55(13):4965. [Google Scholar]
Lai 2009 {published data only}
- Lai TY, Liu DT, Chan KP, Luk FO, Pang CP, Lam DS. Visual outcomes and growth factor changes of two dosages of intravitreal bevacizumab for neovascular age‐related macular degeneration: a randomized, controlled trial. Retina 2009;29(9):1218‐26. [DOI] [PubMed] [Google Scholar]
Lazic 2007 {published data only}
- Lazic R, Gabric N. Verteporfin therapy and intravitreal bevacizumab combined and alone in choroidal neovascularization due to age‐related macular degeneration. Ophthalmology 2007;114(6):1179‐85. [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Fang K, Tian J, Qing X, Li S, Hou J, Li J, et al. Predictors of visual response to intravitreal bevacizumab for treatment of neovascular age‐related macular degeneration. Journal of Ophthalmology 2013;2013:676049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hu Y, Sun X, Zhang J, Zhang M, Neovascular Age‐Related Macular Degeneration Treatment Trial Using Bevacizumab (NATTB). Bevacizumab for neovascular age‐related macular degeneration in China. Ophthalmology 2012;119(10):2087‐93. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li J, Zhang H, Sun P, Gu F, Liu ZL. Bevacizumab vs ranibizumab for neovascular age‐related macular degeneration in Chinese patients. International Journal of Ophthalmology 2013;6(2):169‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthe 2011 {published data only}
- Matthe E, Sandner D. Monotherapy of exudative age‐related macular degeneration with ranibizumab in patients at cardiovascular risk. Advantages of ranibizumab compared to a combination with pegaptanib. Der Ophthalmologe 2011;108(4):337‐41. [DOI] [PubMed] [Google Scholar]
MIRA‐1 2005 {published data only}
- Pulido JS, Anderson WB Jr, Flynn JT, Lichter PR, Sanders D. Multicenter prospective, randomized, double‐masked, placebo‐controlled study of rheopheresis to treat neovascular age‐related macular degeneration: interim analysis. Transactions of the American Ophthalmological Society 2002;100:85‐107. [PMC free article] [PubMed] [Google Scholar]
- Pulido JS, Sanders D, Klingel R. Rheopheresis for age‐related macular degeneration: clinical results and putative mechanism of action. Canadian Journal of Ophthalmology 2005;40(3):332‐40. [DOI] [PubMed] [Google Scholar]
Modarres 2009 {published data only}
- Modarres M, Naseripour M, Falavarjani KG, Nikeghbali A, Hashemi M, Parvaresh MM. Intravitreal injection of 2.5 mg versus 1.25 mg bevacizumab (Avastin®) for treatment of CNV associated with AMD. Retina 2009;29(3):319‐24. [DOI] [PubMed] [Google Scholar]
NCT00087763 {unpublished data only}
- NCT00087763. A phase II prospective, randomized, double‐masked, sham‐controlled, dose‐ranging, multi‐center trial to assess the effect of pegaptanib sodium on foveal thickening in patients with exudative subfoveal age‐related macular degeneration (AMD). clinicaltrials.gov/show/NCT00087763 (accessed 5 February 2017).
NCT01494805 {published data only}
- Constable IJ, Pierce CM, Lai CM, Magno AL, Degli‐Esposti MA, French MA, et al. Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal rAAV.sFLT‐1 for wet age‐related macular degeneration. EBioMedicine 2016;14:168‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CM, Magno A, Pierce C, Samulski RJ, Chalberg TW, Blumenkranz MS, et al. Results from a phase I/II clinical trial on anti‐vascular endothelial growth factor gene therapy in patients with exudative age‐related macular degeneration. Journal of Gene Medicine 2013;15(8‐9):319. [Google Scholar]
- Lai CM, Magno AL, Barone SB, Schwartz SD, Blumenkranz MS, Constable IJ, et al. Optical coherence tomography profiles, visual acuity and ranibizumab usage during 3‐year follow‐up in a rAAV.sFLT‐1 gene therapy clinical trial for wet age‐related macular degeneration. Molecular therapy. Conference: 20th Annual Meeting of the American Society of Gene and Cell Therapy, ASGCT 2017. United States 2017;25:315. [Google Scholar]
- Rakoczy EP, Lai CM, Magno A, French M, Barone SB, Schwartz SD, et al. Association of serum neutralizing antibodies with safety and efficacy outcomes in a Phase 2a trial of rAAV.sFlt‐ 1 in the treatment of wet AMD. Investigative Ophthalmology and Visual Science 2016;57(12):526. [Google Scholar]
NCT01796964 {published data only}
- Dugel PU, Jaffe GJ, Salistig P, Warburton J, Weichselberger A, Wieland M, et al. Brolucizumab versus aflibercept in participants with neovascular age‐related macular degeneration: a randomized trial. Ophthalmology 2017;124(9):1296‐304. [DOI] [PubMed] [Google Scholar]
- Dugel PU, Jaffe GJ, Sallstig P, Warburton J, Weichselberger A, Wieland M, et al. Brolucizumab versus aflibercept in participants with neovascular age‐related macular degeneration: a randomized trial. Ophthalmology 2017;124(9):1296‐304. [DOI] [PubMed] [Google Scholar]
Neubauer 2007 {published data only}
Nguyen 2006 {published data only}
- Nguyen QD, Shah SM, Hafiz G, Quinlan E, Sung J, Chu K, et al. A phase I trial of an IV‐administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age‐related macular degeneration. Ophthalmology 2006;113(9):1522. [DOI] [PubMed] [Google Scholar]
Nowak 2012 {published data only}
- Nowak MS, Jurowski P, Grzybowski A, Goś R, Pastuszka M, Kapica A, et al. A prospective study on different methods for the treatment of choroidal neovascularization. The efficacy of verteporfin photodynamic therapy, intravitreal bevacizumab and transpupillary thermotherapy in patients with neovascular age‐related macular degeneration. Medical Science Monitor 2012;18(6):CR374‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nunes 2014 {published data only}
- Nunes RP, Rodrigues E, Hirai FE, Barroso LF, Novais E, Badaro E, et al. Efficacy and cost‐effectiveness of anti‐VEGF treatments for age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2014;55(13):4939. [Google Scholar]
OSPREY 2014 {published data only}
- Singerman LJ, Weichselberger A, Sallstig P. OSPREY trial: randomized, active‐controlled, phase II study to evaluate safety and efficacy of RTH258, a humanized single chain anti‐VEGF antibody fragment, in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2015;56(7):4801. [Google Scholar]
Parodi 2012 {published data only}
- Parodi MB, Cascavilla M, Papayannis A, Kontadakis DS, Bandello F, Iacono P. Intravitreal bevacizumab in advanced‐stage neovascular age‐related macular degeneration with visual acuity lower than 20/200. Archives of Ophthalmology 2012;130(7):934‐5. [DOI] [PubMed] [Google Scholar]
PERSPECTIVES 2012 {published data only}
- Chakravarthy U, Staurenghi G, Kwok K, Tressler CS, Buggage R, PERSPECTIVES Study Group. Treating early choroidal neovascularisation with pegaptanib sodium in patients with neovascular age‐related macular degeneration: findings of the PERSPECTIVES study. British Journal of Ophthalmology 2012;96(10):1351‐4. [DOI] [PubMed] [Google Scholar]
PrONTO Study 2009 {published data only}
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable‐dosing regimen with intravitreal ranibizumab for neovascular age‐related macular degeneration: year 2 of the PrONTO study. American Journal of Ophthalmology 2009;148(1):43‐58.e1. [10.1016/j.ajo.2009.01.024.Epub 2009 Apr 18] [DOI] [PubMed] [Google Scholar]
Raftery 2007 {published data only}
- Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis®) versus bevacizumab (Avastin®): modelling cost effectiveness. British Journal of Ophthalmology 2007;91(9):1244‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
RATE 2011 {unpublished data only}
- NCT01319188. Ranibizumab for age‐related macular degeneration and the risk of arterial thromboembolic events (RATE). clinicaltrials.gov/show/NCT01319188 (accessed 5 February 2017).
RIVAL 2017 {published data only}
- Gillies MC, Hunyor AP, Arnold JJ, Pecheur F, Underhill K, Guymer RH, et al. Comparison of ranibizumab and aflibercept in patients with neovascular age‐related macular degeneration treated following a 'treat and extend' protocol: efficacy variables from the pre‐specified 12‐month interim analysis of the rival study. Clinical and Experimental Ophthalmology. Conference: 49th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists. Australia 2017;45(1):29. [Google Scholar]
SAILOR 2009 {published data only}
- Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age‐related macular degeneration. Ophthalmology 2009;116(9):1731‐9. [DOI] [PubMed] [Google Scholar]
- Reichel E, Francom S, Rubio R. Ranibizumab (Lucentis®) safety in previously treated and newly diagnosed patients with neovascular age‐related macular degeneration (AMD): the SAILOR study. The Macula Society. 2008:168.
Schmid‐Kubista 2011 {published and unpublished data}
- Schmid‐Kubista KE, Krebs I, Ansari‐Shahrezaei S, Haas P, Hagen S, Binder S. Comparing treatment of neovascular age‐related macular degeneration with sequential intravitreal Avastin and Macugen versus intravitreal mono‐therapy ‐ a pilot study. Current Eye Research 2011;36(10):958‐63. [DOI] [PubMed] [Google Scholar]
Sengul 2017 {published data only}
- Sengul A, Rasier R, Ciftci C, Artunay O, Kockar A, Bahcecioglu H, et al. Short‐term effects of intravitreal ranibizumab and bevacizumab administration on 24‐h ambulatory blood pressure monitoring recordings in normotensive patients with age‐related macular degeneration. Eye (London, England) 2017;31(5):677‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGHT 2014 {published data only}
- Li X, Chen Y, Zhang J, Xu X, Zhang F, Cheung CMG, et al. Intravitreal aflibercept versus photodynamic therapy in Chinese patients with neovascular age‐related macular degeneration: outcomes of the sight study. Journal of Ocular Pharmacology and Therapeutics 2017;33(6):435‐44. [Google Scholar]
SUMMIT 2007 {published data only}
- Slakter JS, DENALI Study Group. Combination therapy with verteporfin PDT and ranibizumab for subfoveal choroidal neovascularization due to AMD. Investigative Ophthalmology and Visual Science 2007;48:E‐Abstract 1817. [Google Scholar]
Suñer 2009 {published data only}
- Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ‐25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Investigative Ophthalmology and Visual Science 2009;50(8):3629‐35. [DOI] [PubMed] [Google Scholar]
Tano 2008 {published data only}
- Tano Y, Pegaptanib Sodium Multi‐center Study Group. Pegaptanib sodium one‐year treatment study for neovascular age‐related macular degeneration. Nippon Ganka Gakkai Zasshi 2008;112(7):590‐600. [PubMed] [Google Scholar]
Vallance 2010 {published data only}
- Vallance JH, Johnson B, Majid MA, Banerjee S, Mandal K, Bailey CC. A randomised prospective double‐masked exploratory study comparing combination photodynamic treatment and intravitreal ranibizumab vs intravitreal ranibizumab monotherapy in the treatment of neovascular age‐related macular degeneration. Eye 2010;24(10):1561‐7. [DOI] [PubMed] [Google Scholar]
VERITAS 2006 {published data only}
- Mieler WF, The VERITAS trial. VERITAS ‐ The rationale and design of a combination therapy trial for wet AMD. Investigative Ophthalmology and Visual Science 2006;47:E‐Abstract 5232. [Google Scholar]
VIEW 2014 {published data only}
- Marcus DM. Long‐term follow‐up of intravitreal aflibercept injection (IAI) for neovascular age‐related macular degeneration (nAMD) in an open‐label extension of the VIEW1 study. Investigative Ophthalmology and Visual Science. 2014; Vol. 55(13):3943.
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age‐related macular degeneration: ninety‐six‐week results of the VIEW studies. Ophthalmology 2014;121(1):193‐201. [DOI] [PubMed] [Google Scholar]
Weigert 2008 {published data only}
- Weigert G, Michels S, Sacu S, Varga A, Prager F, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin) versus photodynamic therapy plus intravitreal triamcinolone for neovascular age‐related macular degeneration: 6‐month results of a prospective, randomized controlled clinical trial. British Journal of Ophthalmology 2008;92(3):356‐8. [PUBMED: PMID: 18201136] [DOI] [PubMed] [Google Scholar]
Wolowacz 2007 {published data only}
- Wolowacz SE, Roskell N, Kelly S, Maciver FM, Brand CS. Cost effectiveness of pegaptanib for the treatment of age‐related macular degeneration in the UK. Pharmacoeconomics 2007;25(10):863‐79. [DOI] [PubMed] [Google Scholar]
Zehetner 2013 {published data only}
- Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age‐related macular degeneration, and in patients with diabetic macular oedema. British Journal of Ophthalmology 2013;97(4):454‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00559715 {unpublished data only}
- NCT00559715. Prevention of vision loss in patients with age‐related macular degeneration (AMD) by intravitreal injection of bevacizumab and ranibizumab (VIBERA). clinicaltrials.gov/show/NCT00559715 (accessed 22 August 2018).
Additional references
Aiello 1994
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New England Journal of Medicine 1994;331(22):1480‐7. [DOI] [PubMed] [Google Scholar]
AREDS 2001
- Age‐Related Eye Disease Study Research Group. A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology 2001;119(10):1414‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
AREDS2 2013
- Age‐Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega‐3 fatty acids for age‐related macular degeneration: the Age‐Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309(19):2005‐15. [DOI] [PubMed] [Google Scholar]
Boekhoorn 2007
- Boekhoorn SS, Vingerling JR, Witteman JCM, Hofman A, Jong PT. C‐reactive protein level and risk of aging macula disorder: the Rotterdam study. Archives of Ophthalmology 2007;125(10):1396‐401. [DOI] [PubMed] [Google Scholar]
Bourne 2014
- Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Prevalence and causes of vision loss in high‐income countries and in Eastern and Central Europe: 1990‐2010. British Journal of Ophthalmology 2014;98(5):629‐38. [DOI] [PubMed] [Google Scholar]
Brown 2008
- Brown MM, Brown GC, Brown HC, Peet J. A value‐based medicine analysis of ranibizumab for the treatment of subfoveal neovascular macular degeneration. Ophthalmology 2008;115(6):1039‐45. [DOI] [PubMed] [Google Scholar]
Bunce 2006
- Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health 2006;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carneiro 2012
- Carneiro AM, Mendonca LS, Falcao MS, Fonseca SL, Brandao EM, Falcao‐Reis FM. Comparative study of 1+PRN ranibizumab versus bevacizumab in the clinical setting. Clinical Ophthalmology 2012;6:1149‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
CMS 2014
- Centers for Medicare and Medicaid Services. Billing and coding information regarding uses, including off‐label uses, of bevacizumab and ranibizumab, for the treatment of ophthalmological diseases. Document ID A49034. www.cms.gov/medicare‐coverage‐database (accessed 9 April 2014).
Cohen 2014
- Cohen D. Roche and Novartis colluded over wet AMD drugs, says Italian regulator. BMJ 2014;348:g2006. [DOI] [PubMed] [Google Scholar]
Congdon 2004
- Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology 2004;122(4):477‐85. [DOI] [PubMed] [Google Scholar]
de Juan 2013
- Juan E, Laganovska G, Loewenstein A, Alster Y, Erickson S. First in‐human results of a refillable drug delivery implant providing sustained release of ranibizumab in patients with neovascular AMD. Macula Society. 2013:136‐7.
Deangelis 2007
- Deangelis MM, Ji F, Kim IK, Adams S, Capone A Jr, Ott J, et al. Cigarette smoking, CFH, APOE, ELOVL4, and risk of neovascular age‐related macular degeneration. Archives of Ophthalmology 2007;125(1):49‐54. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9. Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
EOP 1003
- Eyetech Pharmaceuticals, Inc, Pfizer, Inc. Pegaptanib sodium injection in the treatment of neovascular age‐related macular degeneration; and FDA. Macugen® (pegaptanib sodium injection) for the treatment of neovascular age‐related macular degeneration. www.fda.gov/ohrms/dockets/ac/04/briefing/2004‐4053b1.htm (accessed 20 December 2011).
EOP 1004
- Eyetech Pharmaceuticals, Inc, Pfizer, Inc. Pegaptanib sodium injection in the treatment of neovascular age‐related macular degeneration; and FDA. Macugen® (pegaptanib sodium injection) for the treatment of neovascular age‐related macular degeneration. www.fda.gov/ohrms/dockets/ac/04/briefing/2004‐4053b1.htm (accessed 20 December 2011).
Friedman 2004
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, Jong PT, et al. Prevalence of age‐related macular degeneration in the United States. Archives of Ophthalmology 2004;122(4):564‐72. [DOI] [PubMed] [Google Scholar]
Ghafour 1983
- Ghafour IM, Allan D, Foulds WS. Common causes of blindness and visual handicap in the west of Scotland. British Journal of Ophthalmology 1983;67(4):209‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillies 2014
- Gillies MC, Walton RJ, Arnold JJ, McAllister IL, Simpson JM, Hunyor AP, et al. Comparison of outcomes from a phase 3 study of age‐related macular degeneration with a matched, observational cohort. Ophthalmology 2014;121(3):676‐81. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro. Version accessed 11 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gragoudas 2004
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, the VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age‐related macular degeneration. New England Journal of Medicine 2004;351(27):2805‐16. [DOI] [PubMed] [Google Scholar]
Haddad 2006
- Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age‐related macular degeneration: a review of progress to date. Survey of Ophthalmology 2006;51(4):316‐63. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holz 2013
- Holz FG, Bandello F, Gillies M, Mitchell P, Osborne A, Sheidow T, et al. Safety of ranibizumab in routine clinical practice: 1‐year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. British Journal of Ophthalmology 2013;97(9):1161‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyman 1987
- Hyman L. Epidemiology of eye disease in the elderly. Eye 1987;1(Pt 2):330‐41. [DOI] [PubMed] [Google Scholar]
Ip 2008
- Ip MS, Scott IU, Brown GC, Brown MM, Ho AC, Huang SS, et al. Anti‐vascular endothelial growth factor pharmacotherapy for age‐related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology 2008;115(10):1837‐46. [DOI] [PubMed] [Google Scholar]
Klein 2008
- Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long‐term incidence and progression of age‐related macular degeneration. Archives of Ophthalmology 2008;126(1):115‐21. [DOI] [PubMed] [Google Scholar]
Kvanta 1996
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age‐related macular degeneration express vascular endothelial growth factor. Investigative Ophthalmology and Visual Science 1996;37(9):1929‐34. [PubMed] [Google Scholar]
Leibowitz 1980
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973‐1975. Survey of Ophthalmology 1980;24(Suppl):335‐610. [PubMed] [Google Scholar]
Li 2016
- Li E, Donati S, Virgili G, Krzystolik MG. Treatment schedules for administration of anti‐vascular endothelial growth factor agents for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 1996
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age‐related macular degeneration‐related choroidal neovascular membranes. Investigative Ophthalmology and Visual Science 1996;37(5):855‐68. [PubMed] [Google Scholar]
Mitchell 2002
- Mitchell P, Wang JJ, Smith W, Leeder SR. Smoking and the 5‐year incidence of age‐related maculopathy: the Blue Mountains Eye Study. Archives of Ophthalmology 2002;120(10):1357‐63. [DOI] [PubMed] [Google Scholar]
Mitchell 2011
- Mitchell P. A systematic review of the efficacy and safety outcomes of anti‐VEGF agents used for treating neovascular age‐related macular degeneration: comparison of ranibizumab and bevacizumab. Current Medical Research and Opinion 2011;27(7):1465‐75. [DOI] [PubMed] [Google Scholar]
Moja 2014
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011230.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MPSG 1991
- Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy: five‐year results from randomized clinical trials. Archives of Ophthalmology 1991;109(8):1109‐14. [PubMed] [Google Scholar]
Owen 2003
- Owen CG, Fletcher AE, Donoghue M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom?. British Journal of Ophthalmology 2003;87(3):312‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasmussen 2014
- Rasmussen A, Sander B. Long‐term longitudinal study of patients treated with ranibizumab for neovascular age‐related macular degeneration. Current Opinion in Ophthalmology 2014;25(3):158‐63. [DOI] [PubMed] [Google Scholar]
Review Manager 5 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rofagha 2013
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven‐year outcomes in ranibizumab‐treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN‐UP). Ophthalmology 2013;120(11):2292‐9. [DOI] [PubMed] [Google Scholar]
Sarwar 2016
- Sarwar S, Clearfield E, Soliman MK, Sadiq MA, Baldwin AJ, Hanout M, et al. Aflibercept for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD011346.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaumberg 2007
- Schaumberg DA, Hankinson SE, Guo Q, Rimm E, Hunter DJ. A prospective study of 2 major age‐related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Archives of Ophthalmology 2007;125(1):55‐62. [DOI] [PubMed] [Google Scholar]
Schmucker 2010
- Schmucker C, Ehlken C, Hansen LL, Antes G, Agostini HT, Lelgemann M. Intravitreal bevacizumab (Avastin) vs. ranibizumab (Lucentis) for the treatment of age‐related macular degeneration: a systematic review. Current Opinion in Ophthalmology 2010;21(3):218‐26. [DOI] [PubMed] [Google Scholar]
Schmucker 2012
- Schmucker C, Ehlken C, Agostini HT, Antes G, Ruecker G, Lelgemann M, et al. A safety review and meta‐analyses of bevacizumab and ranibizumab: off‐label versus gold standard. PloS One 2012;7(8):e42701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seddon 2006
- Seddon JM, George S, Rosner B, Klein ML. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age‐related macular degeneration. Human Heredity 2006;61(3):157‐65. [DOI] [PubMed] [Google Scholar]
Smith 1996
- Smith W, Mitchell P, Leeder SR. Smoking and age‐related maculopathy. The Blue Mountains Eye Study. Archives of Ophthalmology 1996;114(12):1518‐23. [DOI] [PubMed] [Google Scholar]
SST 20
- Submacular Surgery Trials Research Group, Solomon SD, Jefferys JL, Hawkins BS, Bressler NM. Incident choroidal neovascularization in fellow eyes of patients with unilateral subfoveal choroidal neovascularization secondary to age‐related macular degeneration: SST report No. 20 from the Submacular Surgery Trials Research Group. Archives of Ophthalmology 2007;125(10):1323‐30. [DOI] [PubMed] [Google Scholar]
Stein 2014
- Stein JD, Newman‐Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost‐effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology 2014;121(4):936‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tielsch 1994
- Tielsch JA. Vision problems in the US: a report on blindness and vision impairment in adults age 40 and older. Prevent Blindness. Illinois: Schaumberg, 1994. [Google Scholar]
Ting 2002
- Ting TD, Oh M, Cox TA, Meyer CH, Toth CA. Decreased visual acuity associated with cystoid macular edema in neovascular age‐related macular degeneration. Archives of Ophthalmology 2002;120(6):731‐7. [DOI] [PubMed] [Google Scholar]
Van Kerckhoven 2001
- Kerckhoven W, Lafaut B, Follens I, Laey JJ. Features of age‐related macular degeneration on optical coherence tomography. Bulletin de la Societe Belge d'Ophtalmologie 2001;281:75‐84. [PubMed] [Google Scholar]
Virgili 2007
- Virgili G, Bini A. Laser photocoagulation for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004763.pub2] [DOI] [PubMed] [Google Scholar]
Wormald 2007
- Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002030.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Krzystolik 2005
- Krzystolik MG, Woodcome HA, Reddy U. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD005139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2014
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti‐vascular endothelial growth factor for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD005139.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2016
- Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS. Intravitreal bevacizumab versus ranibizumab for treatment of neovascular age‐related macular degeneration: findings from a Cochrane systematic review. Ophthalmology 2016;123(1):70‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vedula 2008
- Vedula SS, Krzystolik M. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005139.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


