Abstract
Background
Chronic Chagas Disease (CD) diagnosis is based on serological methods employing crude, semipurified or recombinant antigens, which may result in low sensitivity or cross-reactivity. To reduce these restrictions, we developed a strategy involving use of molecules containing repetitive fragments of Trypanosoma cruzi conserved proteins. Diagnostic performance of IBMP-8.1 and IBMP-8.4 chimeric antigens (Molecular Biology Institute of Paraná - IBMP in Portuguese acronym) was assessed to diagnose T. cruzi-infected and non-infected immigrants living in Barcelona (Spain), a non-endemic setting for Chagas disease.
Methods
Reactivity of IBMP-8.1 and IBMP-8.4 was assessed using an in-house automated ELISA with 347 positive and 331 negative individuals to Chagas disease. Antigenic cross-reactivity was measured with sera samples from pregnant women with Toxoplasma gondii (n = 98) and Zika virus (n = 75) antibodies.
Results
The area under the curve values was 1 and 0.99 for the IBMP-8.1 and IBMP-8.4 proteins, respectively, demonstrating excellent diagnostic accuracy. The reactivity index was higher for IBMP-8.1 than IBMP-8.4 in positive samples and no significant difference in reactivity index was observed in negative samples. Sensitivity ranged from 99.4% for IBMP-8.1 to 99.1% for IBMP-8.4 and was not statistically different. Specificity for IBMP-8.1 reached 100 and 99.7% for IBMP-8.4, both nearly 100% accurate. No antigenic cross-reactivity was observed and reactivity index was similar to that for negative Chagas disease individuals.
Conclusions
Our results showed an outstanding performance of IBMP-8.1 and IBMP-8.4 chimeric antigens by ELISA and suggest both chimeric antigens could also be used for Chagas disease diagnosis in immigrants living in non-endemic settings.
Keywords: Chagas disease, Trypanosoma cruzi, Chimeric antigens, Immunoassay, Accuracy
Background
Chagas disease (CD) is a life-threatening infection caused by hemoflagellate protozoa Trypanosoma cruzi, generating an estimated of 14,000 deaths every year [1] and morbidity in 5.7 to 9.4 million people in the continental Western Hemisphere [2, 3]. The epidemiological pattern of CD has undergone substantial changes in last decades as a consequence of control campaigns in endemic countries, which have reduced vectorial and transfusional transmission [4]. Increasing international migration flows and more affordable traveling conditions from Latin America to non-endemic areas have contributed to epidemiology changes [5, 6]. CD is no longer limited exclusively to the impoverished rural regions of Latin America; it is transformed into a global health concern affecting people worldwide in both endemic and non-endemic countries and placing 100 million people at risk for acquiring the infection [7].
In Spain, there are more than 6 million immigrants, and more than 2 million (38%) are coming from CD endemic Latin America countries [8], posing CD as a public health challenge [9]. Indeed, Ecuador, Bolivia, and Argentina are the predominant areas of origin [7]. The diversity of the geographic areas leads to another challenge: the need for an accurate diagnostic test capable of identifying individuals infected with different T. cruzi strains. The high genetic variability of T. cruzi can be responsible for false negative results [10]. These false negative results could be avoided by using synthetic chimeric antigens with repetitive fragments of antigenic T. cruzi proteins for the detection of specific antibodies [11–14].
We performed ELISA [15, 16] and liquid microarray [17] to assess the potential diagnostic of four chimeric proteins, IBMP-8.1, IBMP-8.2, IBMP-8.3, and IBMP-8.4, to identify T. cruzi-infected individuals from several Brazilian endemic (Bahia, Goiás, Minas Gerais, and Pernambuco states; Brazil) and non-endemic settings (Paraná state; Brazil). These chimeric antigens were composed of immunodominant and conserved sequences, as described previously [15]. We obtained high-performance values and low cross-reactivity to Leishmania spp., a pathogen showing relatively high antigenic similarity to T. cruzi [15, 17]. Imprecision analyses showed that IBMP chimeric antigens are highly reproducible and IBMP-8.1 and IBMP-8.4 presented the highest performance values among the evaluated antigens. In this study, we endeavored to conduct an evaluation of the diagnostic performance of IBMP-8.1 and IBMP-8.4 chimeric antigens employing ELISA to diagnose T. cruzi-infected and non-infected immigrants living in Barcelona (Spain), a non-endemic setting for the CD.
Methods
We used the methodology previously described by Santos et al. [18] and Brito et al. [19].
Study samples
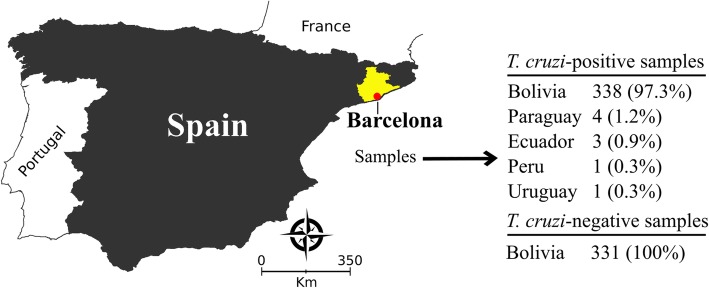
We employed anonymized human sera from individuals diagnosed at the Laboratory Clínic l’Hospitalet-Laboratori Clínic Territorial Metropolitana Sur, Catalan Institute of Health (Barcelona-Spain). The minimum sample with a 95% confidence interval, an absolute expected error of 1.1% and sensitivity of 99% was 315 sera from non-infected and 315 from T. cruzi-infected individuals. We included sera from 331 non-infected and 347 T. cruzi-infected Latin American individuals living in Barcelona (Fig. 1). The sample selection was based on non-reactivity and reactivity by two serological assays: ORTHO® T. cruzi ELISA Test System (Ortho Clinical Diagnostics Inc., Raritan, USA), which employs T. cruzi whole cell lysate antigen; and Bioelisa CHAGAS (Biokit S.A., Barcelona, Spain) or BIO-FLASH® Chagas (automated chemiluminescent assay; Biokit S.A., Barcelona, Spain), the two latter composed by recombinant T. cruzi antigens. Samples with repeatedly discrepant results between both tests or inconclusive in one of them (or in gray zone) were defined as serodiscordant. Each sample was given an identifier code in the laboratory to ensure a blinded analysis. Antigenic cross-reactivity was assessed with sera samples from Latin American pregnant women with Toxoplasma gondii (n = 98) and Zika virus (ZIKV) antibodies (n = 75). Pregnant women sera samples were employed in this study due to the availability of the biological material in Laboratory Clínic l’Hospitalet-Laboratori Clínic Territorial Metropolitana Sur serum bank. Study participants were mostly from Bolivia (97.3%), but there were individuals from other CD endemic countries (Fig. 1).
Fig. 1.
T. cruzi-positive and negative samples selected from Latin American immigrants living in Barcelona-Spain
Recombinant antigens
Proteins were expressed in Escherichia coli Bl21 star DE3 and purified from the soluble fraction of the total extract of bacterial lysate. Both IBMP-8.1 and IBMP-8.4 antigens were purified by IMAC resin first, and the best fractions dialyzed for buffer exchange and salt reduction before following a second liquid chromatography step. The second purification was conducted on ionic exchange and heparin columns, respectively. Plasmidial construct has already been described in Santos et al. [18].
Immunoassays
Assays were conducted according to previous reports [14, 16]. Briefly, polystyrene “Maxisorp” 96-well microplates (Nunc, Roskilde, Denmark) were coated with 25.0 ng of IBMP-8.1 and IBMP-8.4 per well diluted in coating buffer (0.05 M carbonate-bicarbonate, pH 9.6). Microplates were blocked with Well Champion reagent (Kem-En-Tec, Taastrup, Denmark) according to the manufacturer’s instructions. Serum samples were diluted in 0.05 M phosphate-buffered saline (pH 7.2)-0.5% Tween 20 (PBS-T), and 100 μl was added to each well. After 60 min of incubation at 37 °C, microplates were washed in PBS-T to remove unbound antibodies. HRP conjugated goat anti-human IgG (Bio-Manguinhos, FIOCRUZ, Rio de Janeiro, Brazil) was diluted 1:40,000 in PBS-T, and 100 μl were then added to each well, and the microplates were incubated for 30 min at 37 °C. Wells were washed five times and the immune complexes were revealed by the addition of 100 μl TBM substrate (tetramethyl-benzidine; Kem-En-Tec, Taastrup, Denmark). After a new cycle of incubation (10 min at RT in the dark), the reaction was stopped by adding 50 μl 5 N H2SO4, and the absorbance was measured at 450 nm. The protocols were automatized and the runs carried out in an automated microplate immunoanalyser (BEST 2000®, Biokit, Werfen Group Barcelona, Spain). The blank readings (buffer dilution) was subtracted from all other values.
Statistical analysis
Data were coded and entered using computer graphic software (GraphPad Software Inc., La Jolla, CA, USA). Descriptive data were presented in the form of geometric means ± standard deviation. Shapiro-Wilk test, followed by Student’s t-test, was used to test data normality. When assumed homogeneity was not confirmed, Wilcoxon’s signed rank test was adopted. Cut-off values were determined under the receiver operating characteristic curve (ROC) analyzing the whole serum panel. For data normalization, all results were expressed by plotting values in an indexed format, calculated as the ratio between a given sample’s optical density (OD) and the cut-off OD values respective to each assay. Under this index, referred to as a reactivity index (RI), all results ≥1.00 were considered positive. When a sample’s RI value was 1.0 ± 10%, the result was considered as indeterminate (i.e., in the grey zone), and these samples were deemed inconclusive. The test performances were computed using a dichotomous approach and compared regarding sensitivity, specificity, and accuracy [20]. Confidence interval was set to 95% and p < 0.05 was considered as statistically significant. A study workflow (Fig. 2) is provided according to the STARD guidelines [21]. Digital map (Fig. 1) was acquired from the Brazilian Institute of Geography and Statistics (IBGE) cartographic database in shapefile (.shp), which were formatted and analyzed using TerraView version 4.2, public-access software provided by the National Institute for Space Research from Brazil (www.dpi.inpe.br/terraview).
Fig. 2.
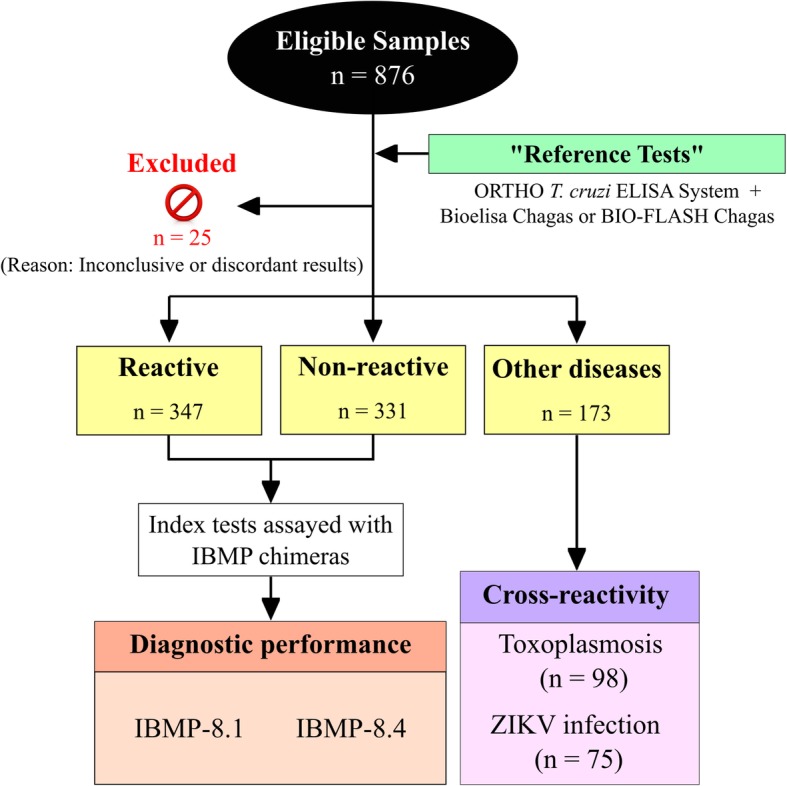
Study workflow for testing IBMP T. cruzi chimeras. RI, reactivity index; ZIKV, Zika virus
Results
Diagnostic performance
The reactivity index and assay performance parameters found for the IBMP-8.1 and IBMP-8.4 chimeric antigens are illustrated in Fig. 3. Based on 678 samples from non-infected and T. cruzi-infected individuals, the AUC (area under the curve) values were 1.000 and 0.9998 for IBMP-8.1 and IBMP-8.4 proteins, respectively, demonstrating excellent overall diagnostic accuracy. The IBMP-8.1 chimera yielded the highest diagnostic accuracy among T. cruzi-infected individuals, and no significant difference was observed between chimeric antigens in non-infected individuals. Sensitivity was 99.4% for IBMP-8.1 and 99.1% for IBMP-8.4. Only one positive sample was simultaneously false negative when assayed by IBMP-8.1 and IBMP-8.4 chimeric antigens. Specificity of IBMP-8.1 achieved 100 and 99.7% for IBMP-8.4. Both proteins showed an accuracy of nearly 100%. No statistically significant differences in sensitivity or specificity scores were found between the chimeric antigens. Using RI values of 1.0 ± 0.10 as the grey zone inconclusive interval, we observed two positive and one negative sample fell in the inconclusive space using IBMP-8.1 while two positive samples to IBMP-8.4 presented similar behavior. Overall, the number of inconclusive results was 0.44% for IBMP-8.1 and 0.29% for IBMP-8.4 (Fig. 3). No sample fell concomitantly inside the grey zone for IBMP-8.1 and IBMP8.4 chimeric antigens.
Fig. 3.
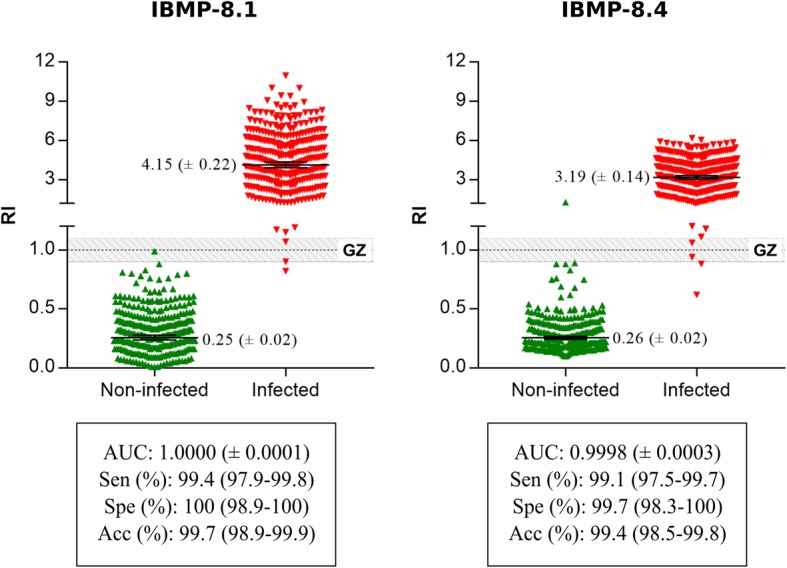
Reactivity index (RI) was obtained with serum from non-infected and T. cruzi-infected immigrants living in Barcelona-Spain. The cut-off value is RI = 1.0 and the grey zone is RI = 1.0 ± 0.10. Lines and whiskers represent geometric means (± 95% CI). AUC, area under curve; GZ, grey zone; Sen, sensitivity; Spe, specificity; Acc; accuracy
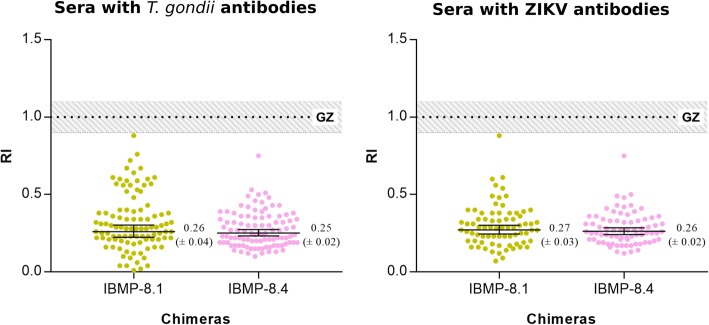
Antigenic cross-reactivity
The RI values for sampled with T. gondii and ZIKV antibodies were identical to that found for non-infected individuals, indicating extremely low reactivity. No false-positive or inconclusive samples were found with IBMP chimeric antigens (Fig. 4).
Fig. 4.
Analysis of the cross-reactivity of the IBMP-8.1 and IBMP-8.4 chimeras to sera with Toxoplasma gondii antibodies (n = 98) and Zika virus antibodies (n = 75). The cut-off value is 1.0 and the grey zone is RI = 1.0 ± 0.10. Lines and whiskers represent geometric means (± 95% CI). GZ, grey zone; RI, reactivity index; ZIKV, Zika virus
Discussion
Diagnosis of chronic CD is not a simple task due to the high genetic diversity of T. cruzi, which might lead to misdiagnosis [22]. In fact, T. cruzi parasite has remarkable genetic heterogeneity, it is classified into seven evolutionary genetic groups or discrete typing units (DTUs) termed TcI-TcVI and TcBat, with sub-classifications for regional strains [22–24]. Regional differences in sensitivity of serological tests had been reported, leading to negative CD diagnosis, mostly in non-endemic countries that receive immigrants from several endemic areas [25–28]. Therefore, a serological test should be able to diagnose CD regardless of the T. cruzi antigenic heterogeneity. The main benefit in the use of chimeric antigens as antigenic matrix is the increased repertoire of epitopes in comparison to non-chimeric recombinant antigens, reducing the number of false negative results. In previous studies, our group analyzed the IBMP performance to diagnose T. cruzi-infected individuals in several endemic and non-endemic geographical areas from Brazil [14, 15], a country where TcII is predominant [10]. Here, we used two chimeric antigens to capture specific anti-T. cruzi antibodies in the sera of Latin American immigrants living in Barcelona/Spain, a non-endemic setting for CD. The majority of CD-positive samples were collected from Bolivian immigrants, where TcV is the most common DTU found in Bolivia [10] and predominates in Bolivian immigrants living in Barcelona [29]. Based on the result from previous studies, we suggest that IBMP-8.1 and IBMP-8.4 antigens are able to diagnose chronic Chagas disease in areas with predominance of TcII and TcV genetic groups.
The assays exhibited high diagnostic accuracy values as demonstrated by AUC (nearly 100%), indicating a substantial discriminative power between negative and chronic CD-positive samples. Similar results were previously found in samples from several Brazilian settings both by ELISA (AUC > 99.7%) [14] and liquid microarray (AUC > 99.1%) [17]. Moreover, the reactivity index from IBMP-8.1 and IBMP-8.4 chimeric antigens, achieved from immigrants living in a non-endemic setting with CD, was higher than those previously obtained for Brazilian samples [14]. It is interesting to note that no differences were observed concerning negative samples. Further studies need to evaluate the performance of the chimeric antigens in settings where other DTUs are predominant, i.e., Argentina, Mexico and Costa Rica.
The present study showed high sensitivity and specificity for both IBMP-8.1 and IBMP-8.4, similar to those found previously using Brazilian samples [14, 15]. Also, the chimeric antigens were found to be nearly 100% accurate, suggesting that the number of misdiagnoses was negligible. In fact, only two and three CD-positive samples were misclassified when assayed with IBMP-8.1 and IBMP-8.4, respectively. In previous studies we evaluated the increase of sensitivity values and the RI signal using a multiplex methodology [17] or the equimolar mixture of the antigens [15], however no gains of were achieved. Hence, we believe that the lack of reactivity from these samples could be due to host immunological reasons or low levels of antibodies on the sera. In CD-negative samples, only one sample was classified as false-positive by IBMP-8.4 antigen. Although this sample presented low signal to IBMP-8.4 (1.28), it was classified as negative when assayed by commercial tests (RI < 0.25) and by the IBMP-8.1 chimera (0.22).
No cross-reactivity of IBMP chimeric antigens against antibodies of T. gondii and ZIKV was observed. Indeed, previous studies have shown extremely low reactivity of IBMP chimeric antigens for several infectious diseases, even for Leishmania spp. [15, 17], a pathogen phylogenetically similar to T. cruzi. T. gondii and ZIKV positive samples were used in this study due to serum bank availability and because these infectious diseases did not assay before using IBMP proteins.
Conclusion
Our results showed a remarkable performance of IBMP-8.1 and IBMP-8.4 chimeric antigens by ELISA and suggest both antigens could also be used for CD diagnosis in immigrants living in non-endemic settings. The high accuracy of IBMP-8.1 and IBMP-8.4 chimeric antigens suggests that they are useful for CD diagnosis in individuals infected with other DTUs.
Acknowledgements
The authors would like to address a special thanks to Ignacio Martínez (Departmento de Immunologia, Instituto de Investigaciones Biomedicas, Universidad Nacional Autonoma de Mexico) and Jesus Almeda (Unitat de Suport a la Recerca, Direcció d’Atenció Primària Costa de Ponent-IDIAP J Gol) for their critical review. We also thank the staff at the Laboratori Clínic Hospitalet (Laboratori Clínic Territorial Metropolitana Sud, Catalan Institute of Health, Barcelona/Spain), especially Elisabeth Castro, as well as Montse Graells and Cristina Fernández for help with sample collection and laboratory analysis.
Availability of the data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research project was funded by a Gonçalo Moniz Institute, Oswaldo Cruz Foundation (Fiocruz/BA). The funder was not involved in the design of the study, in the collection, analysis and interpretation of the data, or in writing the manuscript.
Abbreviations
- Acc
Accuracy
- AUC
Area under the curve
- CD
Chagas disease
- DTU
Discrete typing unit
- GZ
Grey zone
- HRP
Horseradish peroxidase
- IBMP
Molecular Biology Institute of Paraná (IBMP in Portuguese acronym)
- OD
Optical density
- PBS
Phosphate-buffered saline
- PBS-T
Phosphate-buffered saline-0.5% Tween 20
- RI
Reactivity index
- ROC
Receiving operator curve
- Sen
Sensitivity
- Spe
Specificity
- STARD
Standards for reporting of diagnostic accuracy studies
- T. cruzi
Trypanosoma cruzi
- T. gondii
Toxoplasma gondii
- TBM
Tetramethyl-benzidine
- ZIKV
Zika virus
Authors’ contributions
FLNS and ED were involved in the design and operational management of the study. FLNS and WVS were the lead study statistician. ED and RPDR were principal investigators in this study. All authors (ED, RPDR, BE, IU, NITZ, ES, ZM, PAFC, WVS, EDS, YMG, and FLNS) contributed to the interpretation of the data, contributed to this publication and approved the final manuscript for submission. All authors (ED, RPDR, BE, IU, NITZ, ES, ZM, PAFC, WVS, EDS, YMG, and FLNS) had access to the study data and are responsible for the veracity and completeness of the data reported.
Author information
FLNS is investigator in public health at Gonçalo Moniz Institute, Oswaldo Cruz Foundation (Fiocruz), Brazil.
Ethics approval and consent to participate
Ethical permission was sought and granted by Clinical Research Ethics Committee of Bellvitge University Hospital (Barcelona, Spain; IRB OHRP: IRB00005523), and was carried out in accordance with the guidelines of the Helsinki Declaration. With the purpose of protecting the patient’s private information, the Clinical Research Ethics Committee of Bellvitge University Hospital approved that the samples were anonymized so that the investigators do not have access to patient’s private information, therefore, avoiding the requirement of verbal or written consent. This manuscript has also been revised for its publication by the Clinical Research Ethics Committee of Bellvitge University Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eva Dopico, Email: evadopico@gmail.com.
Rodrigo Pimenta Del-Rei, Email: rdelrei@gmail.com.
Bertha Espinoza, Email: besgu@iibiomedicas.unam.mx.
Itziar Ubillos, Email: itziar.ubillos@gmail.com.
Nilson Ivo Tonin Zanchin, Email: nizanchin@gmail.com.
Elena Sulleiro, Email: esuillig@gmail.com.
Zaira Moure, Email: zaira_moure@hotmail.com.
Paola Alejandra Fiorani Celedon, Email: paolaorbi@gmail.com.
Wayner Vieira Souza, Email: wayner@cpqam.fiocruz.br.
Edimilson Domingos da Silva, Email: Edmilson@bio.fiocruz.br.
Yara Miranda Gomes, Email: yara@cpqam.fiocruz.br.
Fred Luciano Neves Santos, Email: fred.santos@bahia.fiocruz.br.
References
- 1.Hotez PJ, Dumonteil E, Woc-Colburn L, Serpa JA, Bezek S, Edwards MS, et al. Chagas disease: “the new HIV/AIDS of the Americas”. PLoS Negl Trop Dis. 2012;6(5):e1498. doi: 10.1371/journal.pntd.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90(6):33–43. [PubMed] [Google Scholar]
- 3.Global Burden of Disease Study 2013 Collaborators, Collaborators GB of DS 2013 Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano Arandes A, Muñoz Gutierrez J, Vergés Navarro M, Castells Doménech C, Portús Vinyeta M, Gascón Brustenga J. Prevalence of Chagas disease in the Latin American immigrant population in a primary health Centre in Barcelona (Spain) Acta Trop. 2009;112(2):228–230. doi: 10.1016/j.actatropica.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Angheben A, Boix L, Buonfrate D, Gobbi F, Bisoffi Z, Pupella S, et al. Chagas disease and transfusion medicine: a perspective from non-endemic countries. Blood Transfus. 2015;13(4$):540–550. doi: 10.2450/2015.0040-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pane S, Giancola ML, Piselli P, Corpolongo A, Repetto E, Bellagamba R, et al. Serological evaluation for Chagas disease in migrants from Latin American countries resident in Rome, Italy. BMC Infect Dis. 2018;18(1):212. doi: 10.1186/s12879-018-3118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strasen J, Williams T, Ertl G, Zoller T, Stich A, Ritter O. Epidemiology of Chagas disease in Europe: many calculations, little knowledge. Clin Res Cardiol. 2014;103(1):1–10. doi: 10.1007/s00392-013-0613-y. [DOI] [PubMed] [Google Scholar]
- 8.Eurostat. Total number of long-term immigrants arriving into the reporting country during the reference year. Available from: http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&plugin=1&pcode=tps00176&language=fr. Accessed 16 Oct 2017.
- 9.Instituto Nacional de Estadística. Población residente por fecha, sexo, edad, nacionalidad (agrupación de países) y lugar de nacimiento (agrupación de países). Available from: http://www.ine.es/jaxiPx/tabla.do?type=pcaxis&path=/t20/p321/serie/2001/l0/&file=01005.px&L=1. Accessed 18 Oct 2017.
- 10.Zingales B. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Camussone C, Gonzalez V, Belluzo MS, Pujato N, Ribone ME, Lagier CM, et al. Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clin Vaccine Immunol. 2009;16(6):899–905. doi: 10.1128/CVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández P, Heimann M, Riera C, Solano M, Santalla J, Luquetti AO, et al. Highly effective serodiagnosis for Chagas’ disease. Clin Vaccine Immunol. 2010;17(10):1598–1604. doi: 10.1128/CVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houghton RL, Benson DR, Reynolds LD, McNeill PD, Sleath PR, Lodes MJ, et al. A multi-epitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in radioimmunoprecipitation-confirmed and consensus-positive sera. J Infect Dis. 1999;179(5):1226–1234. doi: 10.1086/314723. [DOI] [PubMed] [Google Scholar]
- 14.Mucci J, Carmona SJ, Volcovich R, Altcheh J, Bracamonte E, Marco JD, et al. Next-generation ELISA diagnostic assay for Chagas disease based on the combination of short peptidic epitopes. PLoS Negl Trop Dis. 2017;11(10):e0005972. doi: 10.1371/journal.pntd.0005972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos FL, Celedon PA, Zanchin NI, Souza WV, Silva ED, Foti L, et al. Accuracy of chimeric proteins in the serological diagnosis of chronic Chagas disease - a phase II study. PLoS Negl Trop Dis. 2017;11(3):e0005433. doi: 10.1371/journal.pntd.0005433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos FL, Campos AC, Amorim LD, Silva ED, Zanchin NI, Celedon PA, et al. Highly accurate chimeric proteins for the serological diagnosis of chronic Chagas disease: a latent class analysis. Am J Trop Med Hyg. 2018;99(5):1174–1179. doi: 10.4269/ajtmh.17-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos FL, Celedon PA, Zanchin NI, Leitolis A, Crestani S, Foti L, et al. Performance assessment of a Trypanosoma cruzi chimeric antigen in multiplex liquid microarray assays. J Clin Microbiol. 2017;55(10):2934–2945. doi: 10.1128/JCM.00851-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos FL, Celedon PA, Zanchin NI, Brasil TA, Foti L, Souza WV, et al. Performance assessment of four chimeric Trypanosoma cruzi antigens based on antigen-antibody detection for diagnosis of chronic Chagas disease. PLoS One. 2016;11(8):e0161100. doi: 10.1371/journal.pone.0161100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brito VS, Santos FL, Gonçalves NL, Araujo TH, Nascimento DS, Pereira FM, et al. Performance of commercially available serological screening tests for human T-cell lymphotropic virus infection in Brazil. J Clin Microbiol. 2018;56(12):e00961–e00918. doi: 10.1128/JCM.00961-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leeflang MM, Allerberger F. How to: evaluate a diagnostic test. Clin Microbiol Infect. 2019;25(1):54–59. doi: 10.1016/j.cmi.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799. doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tibayrenc M, Ayala FJ. Towards a population genetics of microorganisms: the clonal theory of parasitic protozoa. Parasitol Today. 1991;7(9):228–232. doi: 10.1016/0169-4758(91)90234-F. [DOI] [PubMed] [Google Scholar]
- 23.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7):1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 24.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, et al. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Umezawa ES, Bastos SF, Camargo ME, Yamauchi LM, Santos MR, Gonzalez A, et al. Evaluation of recombinant antigens for serodiagnosis of Chagas’ disease in south and Central America. J Clin Microbiol. 1999;37(5):1554–1560. doi: 10.1128/jcm.37.5.1554-1560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umezawa ES, Bastos SF, Coura JR, Levin MJ, Gonzalez A, Rangel-Aldao R, et al. An improved serodiagnostic test for Chagas’ disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion. 2003;43(1):91–97. doi: 10.1046/j.1537-2995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 27.Verani JR, Seitz A, Gilman RH, LaFuente C, Galdos-Cardenas G, Kawai V, et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80(3):410–415. doi: 10.4269/ajtmh.2009.80.410. [DOI] [PubMed] [Google Scholar]
- 28.Martin DL, Marks M, Galdos-Cardenas G, Gilman RH, Goodhew B, Ferrufino L, et al. Regional variation in the correlation of antibody and T-cell responses to Trypanosoma cruzi. Am J Trop Med Hyg. 2014;90(6):1074–1081. doi: 10.4269/ajtmh.13-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abras A, Gállego M, Muñoz C, Juiz NA, Ramírez JC, Cura CI, et al. Identification of Trypanosoma cruzi discrete typing units (DTUs) in Latin-American migrants in Barcelona (Spain) Parasitol Int. 2017;66(2):83–88. doi: 10.1016/j.parint.2016.12.003. [DOI] [PubMed] [Google Scholar]