Abstract
To find the effects of cross-linker phenol-formaldehyde (PF) resin on the performance of soy-based adhesives, the reaction between model compounds hydroxymethyl phenol (HPF) and glutamic acid were studied in this paper. HPF prepared in laboratory conditions showed higher content of hydroxymethyl groups than normal PF resin, which was proved by the results of Electrospray Ionization Mass Spectrometry (ESI-MS) and 13C Nuclear Magnetic Resonance (13C-NMR). The results of ESI-MS, Fourier transform infrared spectroscopy (FT-IR), and 13C-NMR based on resultant products obtained from model compounds showed better water resistance of the soy protein-based adhesive modified by PF-based resin, which indicated the reaction between PF resin and soy protein. However, it seemed that the soy-based adhesive cross-linked by HPF with the maximum content of hydroxymethyl groups did not show the best water resistance.
Keywords: phenol formaldehyde, soy protein-based adhesives, cross-link, amino acid
1. Introduction
To meet with the needs of environmentally-friendly adhesives in the wood industry, more and more attention has been given to adhesives prepared with natural materials, such as soy protein-based adhesives. It is well-known that soy-based adhesives with no modification cannot be used directly for wood panels because of their poor water resistance [1,2]. Cross-linking modification has been proved to be one of the effective modification methods to resolve this problem. The cross-linkers include amino resins [3,4], a resultant derived from epichlorohydrin and ammonium hydroxide [5], polyethyleneglycol diacrylate [6], aldehyde and its derivatives [7], and others. Phenol-formaldehyde (PF) resin is widely used as a wood adhesive for the preparation of exterior wood panels for its good water resistance. It was also reported to be used to cross-link soy-based adhesives [8]. Yang developed a soy protein-based adhesive modified by PF with a solid weight ratio of PF/soy = 30:70 for plywood [9]. Another similar soy-based adhesive for Oriented Strand Board (OSB) was introduced by Hse [10]. Kreibich used a soy adhesive and a phenol-resorcinol-formaldehyde (PRF) adhesive to prepare finger-jointed boards with a “honeymoon” process [11]. The reports mentioned above proved that PF was an effective cross-linker of soy-based adhesives. However, almost all of these reports were mainly on the performances of the resulted panels and no report has been given on the reaction between PF resin and soy protein-based adhesive.
For soy-based adhesives prepared with the most commonly-used material, defatted soy flour, the idea that the soy protein in soy flour is the foundation for the preparation of the adhesive has been widely accepted. The reactive groups in soy-based adhesives normally include –OH, –SH, –COOH, and –NH2 from the amino acid units of soy protein. The modification of soy-based adhesives, especially the improvement on its water resistance, is presumed to be based on the reaction between one or some of these reactive groups from soy protein and cross-linkers. Although the reactive group might come from –NH2 of soy protein according to the chemical structure of PF resin, there has been no direct proof or report.
The analysis on the reaction between protein and PF cross-linker seems impossible using modern instruments with the samples prepared from soy flour, which is a complicated mixture with some ingredients. Even for soy-based adhesive prepared with soy protein isolate (SPI), whose protein content is higher than 90%, the analysis on chemical structures of resulted adhesive is also very difficult because of the complicated amino acid compositions of soy protein. Although FT-IR was always used to analyze the chemical structures of soy-based adhesives [12], the information it could give was rather limited. All things considered, an amino acid, specifically glutamic acid, was chosen as a model compound to study the modification mechanism of soy adhesives by PF resin. According to the result of Tilley on the amino acid compositions in soy protein [13], glutamic acid covers 23.75 mol % in β-conglycinin, the most important composition of SPI, and stands out of the 16 measured amino acids. The most important point is that the application of pure glutamic acid makes the analysis on the reaction between protein and cross-linker possible. Unlike soy protein with some reactive groups, the reactive group in PF resin comes mainly from hydroxymethyl. Therefore, the hydroxymethyl phenol (HPF) with higher content of hydroxymethyl groups than a normal PF resin was used as a model compound of PF resin in this work.
Although PF resin is toxic, it was still used as a cross-linker of soy-based adhesives in this work for three reasons: (1) PF was proved to be useful for the cross-linking of soy-based adhesives. (2) As the first synthetic resin in industry, the chemical structure and performances of PF are relatively simple. (3) As an important aldehyde derivative, a study on the interaction between PF and soy protein will shed some light on the modification of soy-based adhesives by aldehyde and its derivatives.
The novelty of this work is mainly due to the usage of the model compound glutamic acid. Our work on the reaction between HPF and a common dipeptide has already been reported [8]. The reaction between the unit composition of the protein’s amino acid and hydroxymethyl phenol will be more helpful for the understanding of the reaction between soy protein and hydroxymethyl phenol.
2. Materials and Methods
2.1. Materials
The soy flour with 53.4% protein content was from Yuxin Soybean Protein Co., Ltd., Shandong, China. The thickness of poplar veneer for plywood was 1.5 mm and its moisture content was 8–10%. The formaldehyde solution with a concentration of 37 wt % was from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, and the formaldehyde solution of 50 wt % was from Kunming Xinfeilin Panel Board Co., Ltd., Kunming, China. l-glutamic acid was purchased from Shanghai Kangda Amino Acid Factory (Shanghai, China), with a purity of 98.5%.
2.2. Preparation of PF Resin-Based Cross-Linker
The PF resin with molar ratio F/P = 3.0 was prepared as follows: 94 parts by mass phenol, 28 parts deionized water, and 243 parts by mass formaldehyde 37% was charged into a flat bottom flask equipped with a condenser, thermometer, and a magnetic stirrer bar. The temperature was then slowly increased to 94 °C over a period of 30 min. The pH was adjusted to 8.5–9.0 with NaOH 30%. After being kept at 94 °C for 60 min, the mixture was then cooled naturally to room temperature to yield a yellow transparent resin with viscosity 750 cps and solid content 45.2%.
To retain more hydroxymethyl reactive groups, hydroxymethyl phenol (HPF) was prepared with the same molar ratio as the PF resin above to be used to study the effects of the content of hydroxymethyl on the performance of soy protein-based adhesive. HPF was prepared as the reference [8]: 94 parts by mass phenol and 180 parts by mass formaldehyde 50% was charged into a flat bottom flask equipped with a condenser, thermometer, and a magnetic stirrer bar. The pH was adjusted to 8.5–9.0 with NaOH 30%. After stirring at 30 ± 1 °C for 12, 24, and 48 h, the samples with the name of HPF-a, HPF-b, and HPF-c, respectively, with 43.0%, 44.6%, and 45.1% solid content were obtained.
2.3. Preparation of Soy-Based Adhesive
A soy-based adhesive was prepared according to [14]: 320 parts water and 80 parts by mass soy flour was put into a three-neck round-bottom flask equipped with a mechanical stirrer, thermometer, and condenser. Under continuous stirring, the mixture was heated to 45 °C. Then, 21.3 parts NaOH 30% was added to the flask. After 30 min, 20 parts urea solution 40% was added. After another 20 min, the soy protein-based adhesive with the name DS was obtained. Its solid content was 24 ± 1%.
2.4. Verification Experiment on Reaction between PF Resin and Soy-Based Adhesive
Samples of DS, DS with PF resin (PF/DS), and DS with HPF-a, HPF-b, HPF-c (HPF-a/DS, HPF-b/DS, HPF-c/DS) were dried in an oven at 180 °C to a constant weight. After being ground into a fine powder, m1 g of each sample was immersed in boiling water for 40 min. The residue was filtered, dried, and weighed as m2 g. The water resistance of the samples was characterized as ω%, which was defined as the ratio of m2/m1.
2.5. Preparation of Plywood with Soy Protein-Based Adhesive
The soy protein-based adhesive was mixed well with cross-linkers just before the preparation of three-layer plywood with a dimension of 300 mm × 220 mm × 4 mm. The solid addition amount of PF resin cross-linkers was 12% of the solid soy adhesive. During the preparation of the plywood, the veneers with double side adhesive loading of 320 g/m2 were rested at room temperature for 15 min. The assembled veneers were then sent into a XLB type single-layer hot press from Shanghai Rubber Machinery Plant (Shanghai, China) and pressed under a pressure of 1.5 MPa at 180 °C for 5 min to get a plywood sample.
2.6. Test of Dry and Wet Shear Strength of Plywood Samples
Before being cut into shear specimens with dimensions of 100mm × 25mm, the plywood panel was conditioned at (20 ± 2) °C and with relative humidity (65 ± 5)% in the laboratory for 1 day. The bonded area of each specimen was 25 mm × 25 mm. A WDS-50KN mechanical testing machine (Qingdao, China) was used to test both dry and wet shear strength of the plywood specimens. The testing method for dry and wet shear strength was performed with reference to Chinese national standard GB/T 9846.3-2004 and GB/T 17657-1999, respectively. According to the standard, the final wet shear strength was 0.9 times of the testing strength of the specimens after being immersed into boiling water for 3 h. The dry or wet shear strength was from the mean result of 8–10 specimens.
2.7. ESI-MS
PF resin-based cross-linkers, GA, and the modified soy based adhesive were respectively dissolved in chloroform to get a solution with a concentration of 10 μL/mL, that was 10 μL sample and 1 mL chloroform. The solution was then injected into the ESI source plus ion trap mass spectrometer via a syringe at a flow rate of 5 μg/s. Spectra were recorded in positive mode with ion energy of 0.3 eV and scan range of 0–1000 Da on a Waters Xevo TQ-S instrument (Milford, MA, USA).
2.8. 13C-NMR
A 400 μL sample was dissolved in 200 μL dimethyl sulfoxide-d6 (DMSO-d6). A liquid 13C-NMR (zgig) spectrum was then obtained from a Bruker Avance spectrometer with a frequency of 600 MHz (Billerica, MA, USA). The chemical shifts were calculated relative to the control (CH3)3Si(CH2)3SO3Na dissolved in DMSO-d6. The spectra were performed at 39062.5 Hz for a number of transients of 500–800. All the tests were run with a relaxation delay of 6 s and the chemical shifts were accurate to 1 ppm. NMR spectra were integrated. The functional groups were quantified and calculated as follows [15,16,17]: With methylene glycol bond at 83 ppm as benchmark peak, integral on all absorption peak. Then, to sum all of the methylene carbon integral areas (besides methanol peak at 50 ppm and methoxyl ether peak at 56 ppm), the percentage of integral values of various types of chemical bonds to total methylene carbon were calculated.
2.9. FT-IR
Some of the liquid soy-based adhesives with or without cross-linkers were put into an oven with a temperature of 160 °C. After curing to a constant weight, the soy-based adhesive samples were ground into fine powder, and 0.001 g of each sample was mixed well with 1 g KBr to prepare a pill. Then, the dried pill was tested in a Varian 1000 infrared spectrophotometer (Palo Alto, CA, USA).
3. Results and Discussion
3.1. The Chemical Structure of Hydroxymethyl Phenol
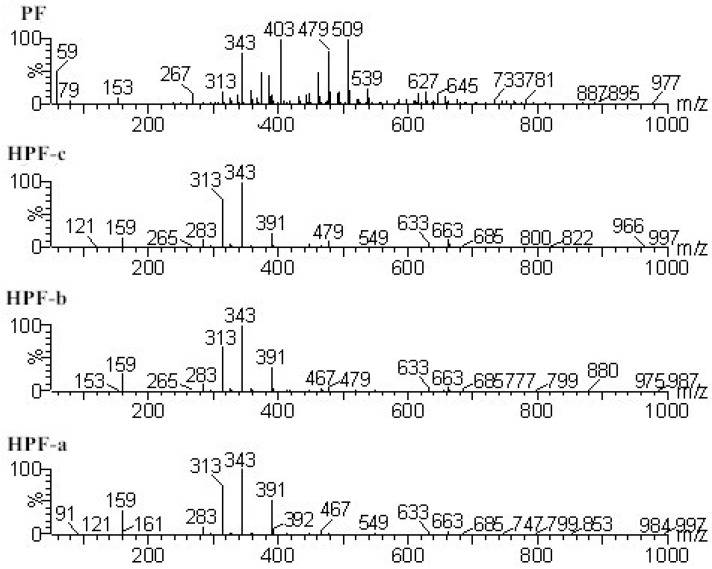
The hydroxymethyl in PF resin was thought to be the main reactive group and finally affect the properties of soy protein-based adhesives. Both HPF with higher content of hydroxymethyl groups and a normal PF resin were prepared in this work. The ESI-MS spectra of the three HPF samples and the normal PF resin were shown in Figure 1. The main ion peaks obtained from ESI-MS spectra and their assignment can be seen from Table 1.
Figure 1.
Electrospray Ionization Mass Spectrometry spectra of samples HPF-a, HPF-b, HPF-c and phenol-formaledhyde.
Table 1.
The main ion peaks of ESI-MS of PF resin-based cross-linker and their assignment.
| M+Na+ (Experimental) Da | Calculated Units |
|---|---|
| 159 |
|
| 283 |
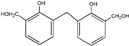
|
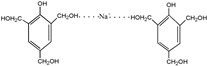
| 313 |
|
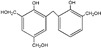
| 343 |
|
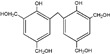
| 391 |
|
| 479 |
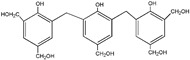
|
| 509 |
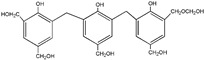
|
| 539 |
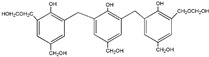
|
| 615 |
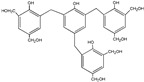
|
| 627 |
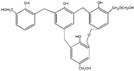
|
| 645 |
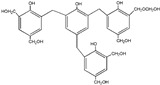
|
The ESI-MS spectra of all the three HPF samples, namely HPF-a, HPF-b, and HPF-c, looked very similar. The ion peaks mainly came from 283, 313, 343, and 391 Da, which were all assigned as dimers with two phenol molecules, while for normal PF resin, the ion peaks mainly occurred at 343, 391, 403, 479, and 509 Da, which meant that both dimmers were shown, as in HPF samples, and even some trimers or tetramers in the PF resin sample. In all, the ESI-MS results proved the lower polymerization degree of HPF samples than that of the PF resin sample, which might be good for the cross-linking of soy protein.
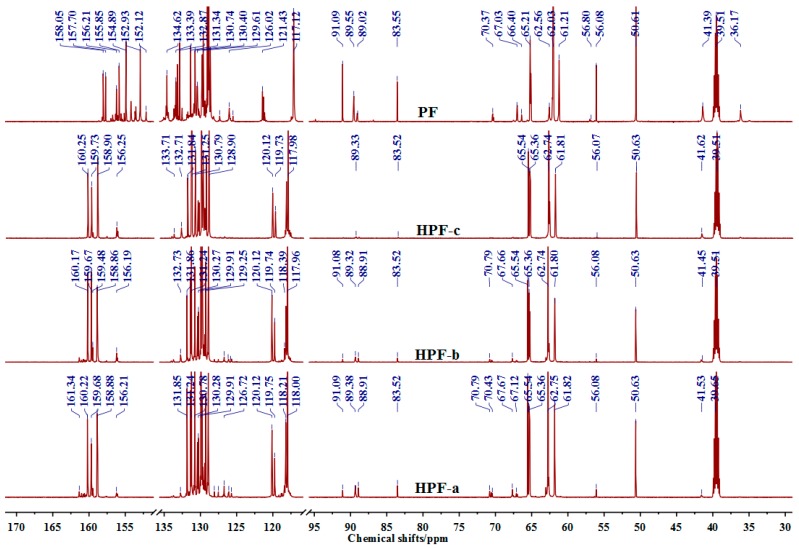
The 13C-NMR spectra of HPF and PF samples are given in Figure 2 and their quantitative results are in Table 2. The four 13C-NMR spectra looked very similar, which were easy to be understood when considering the same materials for the sample preparation and their similar chemical reaction. The main difference of these samples came from the content of hydroxymethyl groups, which were 85.1%, 89.9%, 94.9%, and 55.1% for HPF-a, HPF-b, HPF-c, and PF samples, respectively, calculated by methods mentioned in this paper. It indicated that the content of hydroxymethyl groups of HPF was all higher than that of the normal PF resin. The longer the reaction time, the more hydroxymethyl groups would be obtained. Both the 13C-NMR and the ESI-MS results showed the high content of hydroxymethyl groups in HPF samples prepared in this work.
Figure 2.
The 13C-NMR spectra of samples PF, HPF-a, HPF-b, and HPF-c.
Table 2.
Methylene carbons contents in different samples calculated from results of 13C-NMR.
| Structure | Chemical Shift (ppm) | HPF-a | HPF-b | HPF-c | PF |
|---|---|---|---|---|---|
| o–CH2–p | 34–36 | 0.0 | 0.0 | 0.8 | 18.0 |
| p–CH2–p | 39–41 | 1.2 | 1.6 | 3.2 | 10.4 |
|
o–CH2OH HOCH2–o–CH2OH Ph–(CH2OH)3–/– |
61.4–65.52 | 85.1 | 89.9 | 94.9 | 55.1 |
| Ph–(CH2O)nH(n ≥ 2) | 69.1–70.07 | 3.6 | 2.3 | 0.0 | 4.9 |
| Ph–CH2–O–CH2–Ph | 72–74 | 2.0 | 1.2 | 0.0 | 1.1 |
| HOCH2OH | 83.73 | 1.3 | 0.8 | 0.0 | 1.7 |
| formaldehyde and its derivatives | 84–92.87 | 6.8 | 4.2 | 1.1 | 8.7 |
3.2. The Interaction between HPF and Soy Protein
A verification experiment was done to prove the interaction between PF resin and soy protein-based adhesive. The results are given in Table 3. According to the design of the experiment, the ω was obtained as the total weight divided by the insoluble parts of soy adhesives. It represented the water resistance of the soy-based adhesives. As seen in Table 3, the water resistance of soy-based adhesive improved greatly after mixing with HPF and PF. It was obvious that the soy-based adhesive without cross-linker had no water resistance. The residue of soy-based adhesives with cross-linker (m2) was more than the pure HPF or PF (solid weight of cross-linker), which was a good indication of the interaction between soy-based adhesive and HPF or PF. One thing has to be pointed out—if the content of soy protein was at a normal level (50% in the soy flour used in this work), the weight of the residue from sample HPF-b/DS was even higher than the total solid amount of soy protein and cross-linker. It seemed that HPF might react with soy proteins and even non-proteins in a soy-based adhesive, such as a carbohydrate. The soy protein-based adhesive cross-linked with HPF-b containing medium content of hydroxymethyl groups showed more insoluble residue rather than HPF-c with more hydroxymethyl groups. It might be related to the complicated components of soy-based adhesive, especially after the soy flour’s hydrolysis.
Table 3.
Verification test of reaction between PF cross-linker and soy-based adhesive.
| Samples | Solid Weight of Soy-Based Adhesive/g | Solid Weight of Cross-Linker/g | m1/g | m2/g | Ω (m2/m1)/% |
|---|---|---|---|---|---|
| DS | 2.00 | 0.00 | 2.00 | 0.00 | 0 |
| HPF-a/DS | 1.79 | 0.21 | 2.00 | 1.14 | 57 |
| HPF-b/DS | 1.79 | 0.21 | 2.00 | 1.32 | 66 |
| HPF-c/DS | 1.79 | 0.21 | 2.00 | 1.10 | 55 |
| PF/DS | 1.79 | 0.21 | 2.00 | 1.00 | 50 |
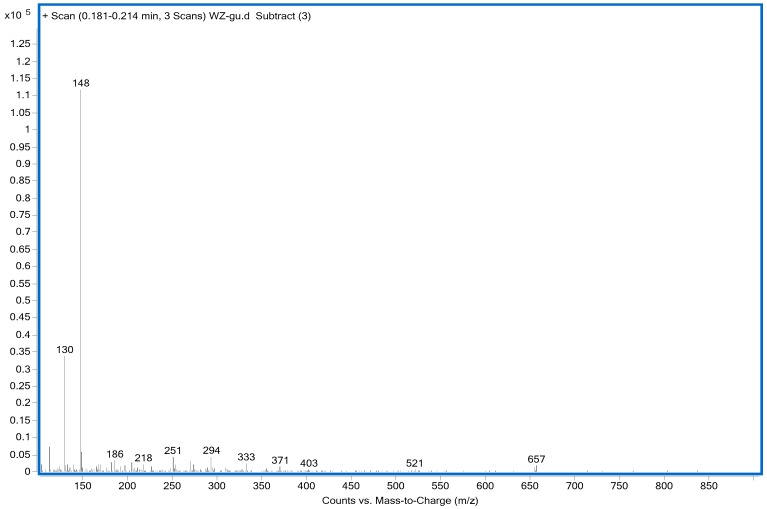
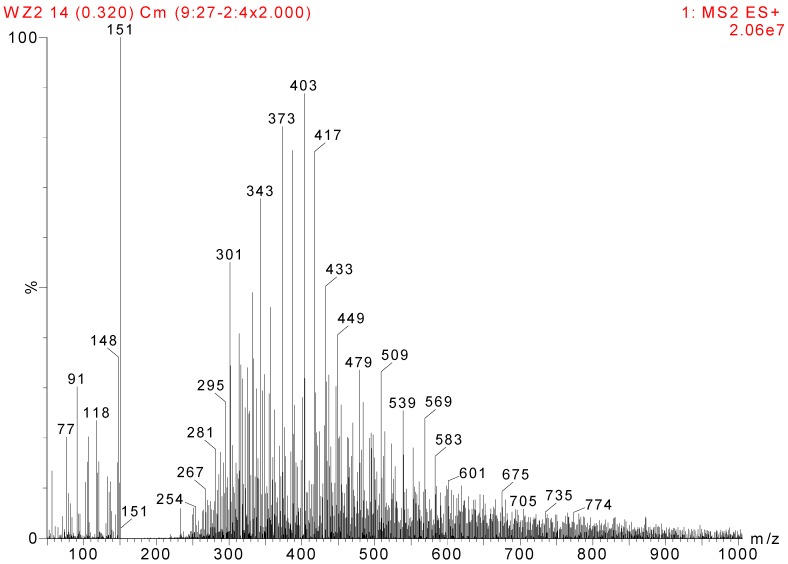
Glutamic acid (GA) was chosen as a model compound to study the interaction between HPF and soy protein. The ESI-MS spectra of GA and HPF/GA are given as Figure 3 and Figure 4, respectively. The assignments of their main ESI-MS ion peaks can be seen in Table 4. For a chemical group with a nitrogen atom, the ion peak of ESI-MS will normally be observed with a form of M + H+. By calculation, the peak at 148 Da came from the GA molecules. The peak at 295 Da was the addition product from one molecule of GA and one molecule of hydroxymethyl phenol. The peak at 417 Da was assigned as a complex with Na+ composed of one molecule of GA and two molecules of hydroxymethyl phenol. The peaks of 449, 479, 509, 539, and 569 Da with an interval of 30 Da were from the addition reaction of one molecule of GA and a dimer of HPF resin with two molecules of phenol. The difference between them was mainly in the quantities of hydroxymethyl groups. Table 4 proved the reaction between GA and HPF.
Figure 3.
ESI-MS spectra of sample l-glutamic acid.
Figure 4.
ESI-MS spectra of sample hydroxymethyl phenol/l-glutamic acid.
Table 4.
The main ion peaks of ESI-MS of GA and HPF/GA and their assignment.
| Samples | M+H+ (exp.) | Calculated Units |
|---|---|---|
| GA | 148 |
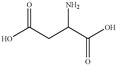
|
| HPF/GA | 295 |
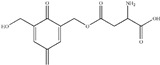
|
| 417 |
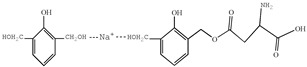
|
|
| 449 |
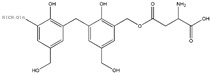 n = 1,2,3,4,5 |
|
| 479 | ||
| 509 | ||
| 539 | ||
| 569 |
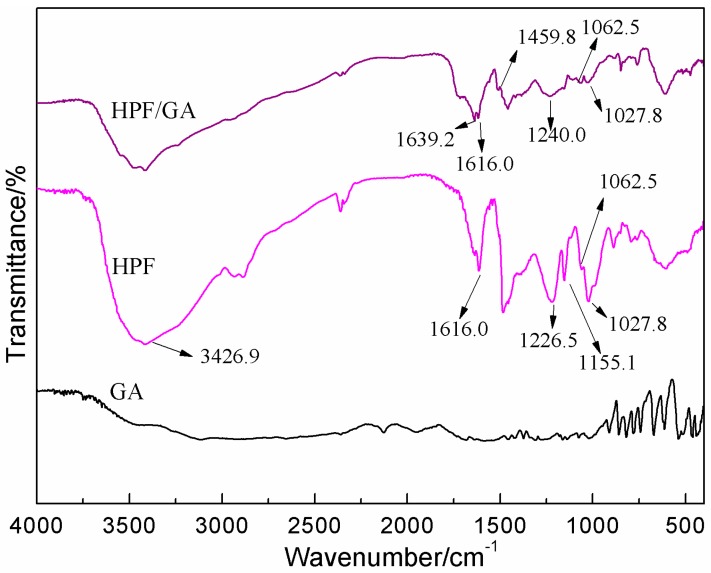
The FT-IR spectra of samples GA, HPF-b, and their mixture (HPF-b/GA) are given as Figure 5. For HPF, the broad band observed in 3462.9 cm−1 was assigned to the bound O–H groups. The absorption band of C=C from the phenyl ring was observed at 1616.0 cm−1. The absorption of C–C stretching from the bonding of the phenolic ring and hydroxymethyl group was seen at 1155.1 cm−1. The absorption of C–O of phenolic hydroxyl groups, ph–C–O groups, and hydroxymethyl groups was seen at 1226.5, 1062.5, and 1027.8 cm−1, respectively [18,19].
Figure 5.
FT-IR spectra of GA, HPF-b, and HPF-b/GA.
In the FT-IR spectrum of HPF-b/GA, the absorption of C–O of phenolic hydroxyl groups moved from 1226.5 to 1240.0 cm−1. The intensified cloud density on the phenolic ring caused by the reaction between hydroxymethyl and soy protein might be responsible for the shift [20].
A new absorption at 1459.8 cm−1 appeared in the FT-IR result of the sample HPF-b/GA, which could be assigned to the C–N stretching vibration obtained from the reaction between hydroxymethyl groups from HPF and amino groups from soy protein [21].
After mixing with GA, the absorption at 1027.8 cm−1, which was assigned to the C–O from hydroxymethyl groups, decreased. It meant that large quantities of the hydroxymethyl groups were consumed after mixing with GA. At the same time, the absorption from ph–C–O groups at 1062.5 cm−1 increased. Since the reaction among hydroxymethyl phenol was small or even invisible for its high activation energy [22,23,24], the observed increase of the absorption of ph–C–O should be caused by the new C–O groups from the reaction between hydroxymethyl phenol and GA. For GA, the reactive groups might come from both –COOH and –OH groups.
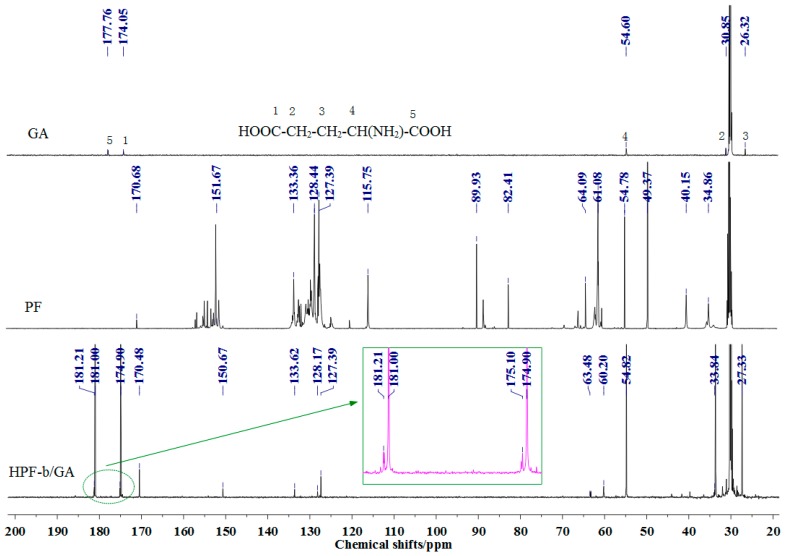
The 13C-NMR spectra of samples GA, HPF-b (PF), and HPF-b/GA are showed in Figure 6. A magnified figure in the range of 172–182 ppm of sample HPF-b/GA was also given. The labelled five absorptions in the 13C-NMR spectra of the GA sample were assigned as five carbons of GA with different chemical environments, respectively, seen from the GA’s chemical formula given in Figure 6. The absorptions from two carboxyl groups were seen exactly at 174.05 and 177.76 ppm. However, for the sample HPF-b/GA, several more peaks were seen around 174 and 177 ppm, which indicated the change of the charge density of carbonyl groups because of the reaction on carboxyl groups.
Figure 6.
The 13C-NMR spectra of sample GA, PF, and HPF-b/GA.
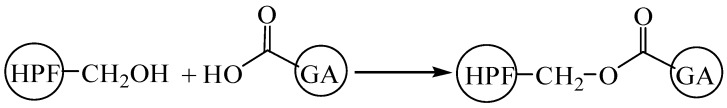
According to the analysis on the results of ESI-MS, FT-IR, and NMR, the possible reaction between PF or HPF and GA was shown in Scheme 1.
Scheme 1.
The possible reaction between phenol-formaldehyde or hydroxymethyl phenol and l-glutamic acid.
It also represents the possible reaction when using PF or HPF to cross-link soy protein-based adhesives.
3.3. Performance of Soy Protein-Based Adhesives Cross-Linked by Hydroxymethyl Phenol
The performance of plywood prepared with soy-based adhesive cross-linked by HPF and PF is shown in Table 5. The dry shear strength for all of the plywood specimens with soy-based adhesives was good enough to meet with the requirement of the relative Chinese national standard (GB/T 9846.3-2004, ≥0.70MPa). In fact, even for those without a cross-linker, there was no problem for the dry shear strength to satisfy the requirement. The cross-linkers were mainly added to improve the water resistance of soy protein-based adhesives. As seen in Table 5, the wet shear strength of the adhesives indeed increased with the addition of PF or HPF. The immeasurable wet shear strength indicated the poor water resistance of the soy-based adhesive without a cross-linker. However, all of the wet shear strengths of soy protein-based adhesive with PF or HPF satisfied the relative standard (GB/T 9846.3-2004, ≥0.70MPa). As seen in Table 5, the specimens with HPF-b showed the highest wet shear strength, which was consistent with the result of Table 3.
Table 5.
The bonding performance of soy protein-based adhesives.
| Adhesive | Viscosity/mPa·s | Dry Shear Strength/MPa | Wet Shear Strength/MPa |
|---|---|---|---|
| DS | 280 | 0.98(0.06) | - |
| HPF-a/DS | 440 | 1.82(0.16) | 0.98(0.06) |
| HPF-b/DS | 396 | 1.81(0.12) | 1.18(0.08) |
| HPF-c/DS | 310 | 1.68(0.13) | 0.82(0.06) |
| PF/DS | 1020 | 2.04(0.15) | 0.84(0.03) |
4. Conclusions
To find the effects of cross-linker phenol-formaldehyde (PF) resin on the performance of soy-based adhesives, the reaction between model compounds hydroxymethyl phenol (HPF) and glutamic acid were studied in this paper. HPF prepared in the laboratory condition showed higher content of hydroxymethyl groups than a normal PF resin, which was proved by the results of ESI-MS and 13C-NMR. The results of ESI-MS, FT-IR, and 13C-NMR based on resultant products obtained from model compounds showed better water resistance of the soy protein-based adhesive modified by PF-based resin, which indicated the reaction between PF resin and soy protein. However, it seemed that the soy-based adhesive cross-linked by HPF with the maximum content of hydroxymethyl groups did not show the best water resistance.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 31660176 and 31800481), Science-technology Support Foundation of Guizhou Province of China (No. [2019]2325 and [2019]2308).
Author Contributions
Z.W. and X.X. contributed the testing and the analysis of the ESI-MS and FT-IR. H.L. contributed the design of the experiment and the analysis of the 13C-NMR results. J.L. (Jiankun Liang) and J.L. (Jingjing Liao) contributed the preparation of the samples and the testing of the plywood samples. G.D. contributed the analysis of the results and the edit of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lei H., Wu Z., Du G. Cross-linked soy-based wood adhesives for plywood. Int. J. Adhes. Adhes. 2014;50:199–203. doi: 10.1016/j.ijadhadh.2014.01.026. [DOI] [Google Scholar]
- 2.Guo M., Wang G. Milk Protein Polymer and Its Application in Environmentally Safe Adhesives. Polymers. 2016;8:324. doi: 10.3390/polym8090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Q., Shi Q., Zhang S. Soybean meal-based adhesive enhanced by MUF resin. J. Appl. Polym. Sci. 2012;125:3676–3681. doi: 10.1002/app.36700. [DOI] [Google Scholar]
- 4.Fan D., Qin T., Chu F. A soy flour-based adhesive reinforced by low addition of MUF resin. J. Adhes. Sci. Technol. 2011;25:323–333. doi: 10.1163/016942410X524147. [DOI] [Google Scholar]
- 5.Jang Y., Huang J., Li K. A new formaldehyde-free wood adhesive from renewable materials. Int. J. Adhes. Adhes. 2011;31:754–759. doi: 10.1016/j.ijadhadh.2011.07.003. [DOI] [Google Scholar]
- 6.Gao Q., Qin Z., Li C., Zhang S., Li J. Preparation of wood adhesives based on soybean meal modified with PEGDA as a crosslinker and viscosity reducer. Bioresources. 2013;8:5380–5391. doi: 10.15376/biores.8.4.5380-5391. [DOI] [Google Scholar]
- 7.Lei H., Du G., Pizzi A. Effects of glyoxal on chemical structure and properties of protein-based adhesives. J. Southwest For. Univ. China. 2011;31:70–73. [Google Scholar]
- 8.Lei H., Wu Z., Cao M., Du G. Study on the soy protein-based wood adhesive modified by hydroxymethyl phenol. Polymers. 2016;8:256. doi: 10.3390/polym8070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang I., Kuo M., Myers D.J. Bond quality of soy-based phenolic adhesives in southern pine plywood. J. Am. Oil Chem. Soc. 2006;83:231–237. doi: 10.1007/s11746-006-1198-7. [DOI] [Google Scholar]
- 10.Hse C.Y., Fu F., Bryant B.S. Green Chemistry for Adhesives, Proceedings of the Wood Adhesives 2000, South Lake Tahoe, CA, USA, 22–23 June 2000. Forest products Society; Madison, WI, USA: 2001. Development of formaldehyde-based wood adhesives with co-reacted phenol/soybean flour; pp. 13–19. [Google Scholar]
- 11.Kreibich R.E., Steynberg P.J., Hemingway R.W. End jointing green lumber with SoyBond; Proceedings of the Wood Residues into Revenue, Residual Wood Conference; Richmond, BC, Canada. 4–5 November 1998. [Google Scholar]
- 12.Wu Z., Lei H., Du G. Disruption of soy-based adhesive treated by Ca(OH)2 and NaOH. J. Adhes. Sci. Technol. 2013;3:1–7. doi: 10.1080/01694243.2013.768140. [DOI] [Google Scholar]
- 13.Riblett A., Herald T., Schmidt K., Tilley K.A. Tyrosine Cross-Links: Molecular Basis of Gluten Structure and Function. J. Agric. Food Chem. 2001;49:4983–4989. doi: 10.1021/jf0105081. [DOI] [PubMed] [Google Scholar]
- 14.Lei H., Wu Z., Du G. Application of soy protein-based adhesives modified by cross-linker. China Wood Ind. 2013;27:8–11. [Google Scholar]
- 15.Lei H., Du G., Pizzi A. Structural Progressing of Phenol-urea-formaldehyde Resin under Alkaline Condition. China Chem. Ind. For. Prod. 2009;29:63–68. [Google Scholar]
- 16.Wu Z., Du G., Lei H., Xi X., Cao M., Guo X. Preparation of eco-friendly urea-formaldehyde resin by urea-formaldehyde precondensate and soy protein. Chin. J. For. Eng. 2016;1:31–36. [Google Scholar]
- 17.Li T., Guo X., Liang J., Wang H., Xie X., Du G. Competitive Formation of the Methylene and Methylene ether Bridges in the Urea-formaldehyde Reaction in Alkaline Solution: A Combined Experimental and Theoretical Study. Wood Sci. Technol. 2015;49:475–493. doi: 10.1007/s00226-015-0711-2. [DOI] [Google Scholar]
- 18.Li J., Luo J., Li X., Zhang Y., Gao Q., Li J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015;74:613–618. doi: 10.1016/j.indcrop.2015.05.066. [DOI] [Google Scholar]
- 19.Wu Y., Li X., Zuo Y. Research status on the utilization of forest and agricultural biomass in inorganic wood-based panel. Chin. J. For. Eng. 2016;1:8–15. [Google Scholar]
- 20.Zuo R. Master’s Thesis. Chongqing University of China; Chongqing, China: 2007. Preparation and Characterization of L-Alanyl-L-Glutamine-Zn2+ Chelate. [Google Scholar]
- 21.Zhang X. Master’s Thesis. Beijing University of Chemical Technology; Beijing, China: 2013. Multi-aldehydes Modified Environmentally Friendly Phenolic Resin Adhesive. [Google Scholar]
- 22.Genier-Loustalot M.F., Larroque S., Grenier P. Phenolic resins: 4 Self-condensation of methylolpheols in formaldehyde-free media. Polymer. 1996;37:955–964. doi: 10.1016/0032-3861(96)87277-6. [DOI] [Google Scholar]
- 23.Nair C.P. Advances in addition-cure phenolic resins. Prog. Polym. Sci. 2004;29:401–498. doi: 10.1016/j.progpolymsci.2004.01.004. [DOI] [Google Scholar]
- 24.Hatfield G., Maciel G. Solid-state NMR study of the hexamethylenetetramine curing of phenolic resins. Macromolecules. 1987;20:608–615. doi: 10.1021/ma00169a024. [DOI] [Google Scholar]