Abstract
The flame retardant modification of epoxy (EP) is of great signification for aerospace, automotive, marine, and energy industries. In this study, a series of EP composites containing different variations of phosphorus-containing polysulfone (with a phosphorus content of approximately 1.25 wt %) were obtained. The obtained EP/polysulfone composites had a high glass transition temperature (Tg) and high flame retardancy. The influence of phosphorus-containing compounds (ArPN2, ArPO2, ArOPN2 and ArOPO2) on the thermal properties and flame retardancy of EP/polysulfone composites was investigated by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), a UL-94 vertical burning test, and cone calorimeter tests. The phosphorus-containing polysulfone enhanced the thermal stability of EP. The more stable porous char layer, less flammable gases, and a lower apparent activation energy at a high degree of conversion demonstrated the high gas inhibition effect of phosphorus-containing compounds. Moreover, the gas inhibition effect of polysulfone with a P–C bond was more efficient than the polysulfone with a P–O–C bond. The potential for optimizing flame retardancy while maintaining a high Tg is highlighted in this study. The flame-retardant EP/polysulfone composites with high thermal stability broaden the application field of epoxy.
Keywords: polysulfone, epoxy resin, flame retardant, structure-property effect, kinetics, glass transition temperature
1. Introduction
Epoxy resin (EP) and its composites, as advanced composite materials, are essential for the aerospace, automotive, marine, and energy industries. It is vital to develop the next generation of lightweight, energy-efficient EP composites, owing to their excellent specific stiffness, high strength, and good chemical resistance [1,2]. Applications in the above fields also require EP with high flame retardancy and high continuous service temperature. However, due to the plasticizing effect of flame retardants, the flame retardant modification of EP is very likely to result in a reduction in glass transition temperature (Tg) [3,4]. Hence, the main challenge in enhancing the flame retardancy of EP is to maintain a high glass transition temperature at the same time.
Present technologies for enhancing the flame retardancy of EP rely on the chemical structural modification of EP and the incorporation of flame retardants [5,6]. Unfortunately, the chemical incorporation of phosphorus into epoxide or curing agents always brings undesirable Tg decreases in the EP composites. An addition of polymeric flame retardant harbors the potential to show no or negligible negative effects on the Tg, which is highly desirable [7]. Several flame retardant elements (phosphorus, nitrogen, sulfur, silicon, etc.) and groups can be incorporated into backbones or side chains of polymeric flame retardants and play a “group synergistic effect” to improve the flame retardancy of EP, such as phosphate [8], phosphonate [9], phosphonamide [10], and phosphazene [11]. Although previous studies have shown that an addition of polysulfone containing phosphate can improve the flame retardant properties of EP, the influence of phosphorus-containing polysulfones on the glass transition temperature and flame retardancy is rarely considered [12,13].
Here, in order to determine the structure–property effect on the flame retardancy and Tg of EP composites, polysulfone with different phosphorus-containing chemical structures is used to prepare of EP composites. The amount of phosphorus content in the EP/polysulfone composites was restricted to approximately 1.25 wt %. To evaluate the influence of polysulfone with different phosphorus-containing structure, the thermal and flame-retardant properties of EP composites were carefully studied. Moreover, the flame retardant mechanisms of polysulfones in condensed and gas phases were especially investigated.
2. Experimental Section
2.1. Materials
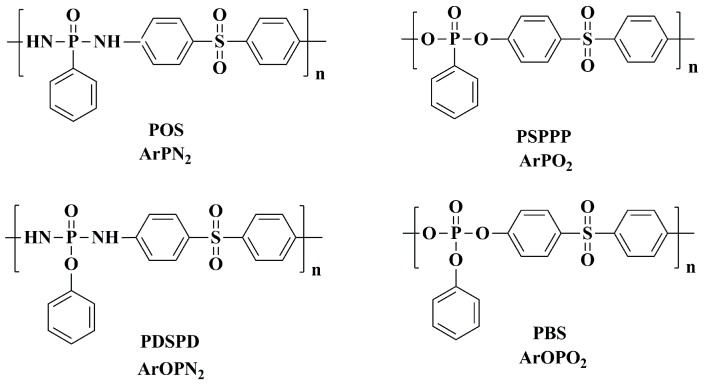
Epoxy resin (DGEBA, commercial name: E-44) was supplied by Sinopec Baling Company (Yueyang, China). Phenylphosphonic dichloride and phenylphosphate dichloride were purchased from Sigma-Aldrich (St. Louis, MO, USA) and freshly distilled before use. Acetonitrile, methanol, dimethyl sulfoxide, triethylamine, and trichloromethane were purchased from the Beijing Chemical Company, Beijing, China, and used as received. 4,4′-Diaminodiphenyl sulfone, 4,4′-diaminodiphenyl methane, 4,4′-diaminodiphenyl ether, and m-phenylenediamine (m-PDA) were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China. The poly(4,4′-diamino diphenyl sulfone phenyl phosphonamide) (ArPN2), poly(bisphenol sulfone phenyl phosphonate) (ArPO2), poly(4,4′-dia-minodiphenyl sulfone phenyl dichlorophosphate) (ArOPN2), and poly(bisphenol sulfone phenoxy phosphate) (ArOPO2) were synthesized as described in detail by Wang [9], Zhao [10], Liaw [14], and Tai [15] et al. The chemical structure of the polysulfones used is summarized in Figure 1.
Figure 1.
Chemical structures of the polysulfones used in this study.
2.2. Preparation of EP/Polysulfone Composites
Polysulfone powder and DGEBA were mixed in a one-neck flask with a magnetic stirrer to form a solution. Next, m-PDA was dropped into the DGEBA/polysulfone solution. The mixture was placed in a Teflon mold and heated at 80 °C for 2 h and post-cured at 120 °C for 2 h to obtain the EP/polysulfone composites. The amount of polysulfone is listed in Table 1. The phosphorus content of the final epoxy resin was controlled at an overall phosphorus content of approximately 1.25 wt %. The resulting neat epoxy resins are referred to as EP/ArPN2, EP/ArPO2, EP/ArOPN2, and EP/ArOPO2. The chemical compositions are summarized in Table 1.
Table 1.
Compositions and vertical burning tests results of all epoxy composites.
| Samples | DGEBA (g) | m-PDA (g) | Polysulfone (g) | P (wt %) | UL-94 (3.2 mm) |
|---|---|---|---|---|---|
| EP | 50.00 | 6.00 | 0 | 0 | No rating |
| EP/ArPN2 | 50.00 | 6.00 | 9.88 | 1.25 | V-0 |
| EP/ArPO2 | 50.00 | 6.00 | 9.88 | 1.25 | V-1 |
| EP/ArOPN2 | 50.00 | 6.00 | 10.35 | 1.25 | No rating |
| EP/ArOPO2 | 50.00 | 6.00 | 10.35 | 1.25 | No rating |
2.3. Measurements and Characterization
Thermogravimetric analysis (TGA) was performed with a Mettler-Toledo TGA/DSC-1 thermogravimetric analyzer (Greifensee, Switzerland) under nitrogen at a heating rate of 20 °C/min. About 5.0 mg of the sample was put in an alumina crucible and heated from 50 to 700 °C. A Fourier-transform infrared spectrometer (TGA-FTIR, Nicolet iS10, Waltham, MA, USA) was coupled to the TGA analyzer by a quartz capillary at 300 °C to detect volatile pyrolysis products. Each sample was placed in an alumina crucible and heated from 50 to 600 °C at a heating rate of 20 °C/min under nitrogen. The mass of the sample was about 5 mg in each test.
Differential scanning calorimetry (DSC) was performed with a DSC Q2000 (TA Ltd., New Castle, DE, USA) at a heating rate of 20 °C/min under nitrogen. The glass transition temperatures (Tg) were read at the mid-point of the inflection curve resulting from the typical second heating in the range 40~220 °C.
A standard UL-94 vertical test was carried out on a CZF-3 instrument (Jiangning Analysis Instrument Factory, Nanjing, China) with a sample size of 125 × 12.5 × 3.2 mm3 according to ASTM D3081. The burning grade was classified as V-0, V-1, V-2, or fail according to the self-extinguishing time and dripping. Cone calorimeter (CONE) measurements were carried out in a Fire Testing Technology apparatus (East Grinstead, London, UK) at a heat flux of 50 kW/m2 according to ISO5660-1. The size of the specimens was 100 × 100 × 1.2 mm3. During the test, each specimen was mounted on aluminum foil. All the measurements were repeated three times, and the results were averaged.
X-ray photoelectron spectroscopy (XPS) was determined by a PHI Quantera II SXM (ULVAC-PHI, Inc, Chigasaki, Kanagawa, Japan) at 25 W under a vacuum lower than 10−6 Pa. The morphologies of residual char obtained from cone calorimeter tests were observed by a Hitachi S4800 (Hitachi High-Technologies Co., Tokyo, Japan) scanning electron microscope with an acceleration voltage of 15 kV. Each sample was sputtered with a gold layer to ensure surface conductivity.
3. Results and Discussion
3.1. Thermal Behaviors
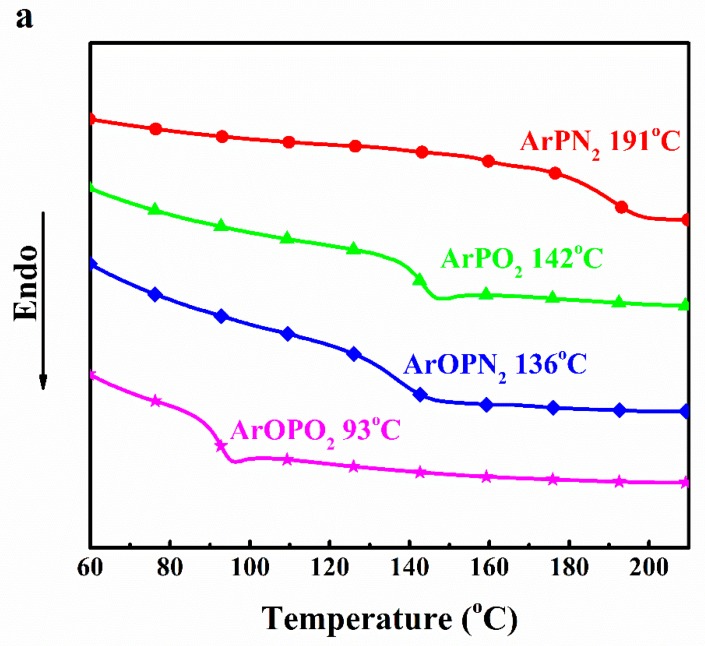
Glass transition temperature (Tg) is one of the most important physical properties of EP and significantly affects the applications of composites. Polymeric flame retardants are preferred to low-molecular flame retardants due to their better compatibility with matrix resin and their resulting in less migration [7]. The glass transition temperature of the polysulfone, EP, and EP/polysulfone composites were investigated by DSC and the main results are further summarized in Table 2. Moreover, the DSC curves of the polysulfones, EP, and EP/polysulfone composites are shown in Figure 2.
Table 2.
Thermal properties of epoxy (EP) and EP/polysulfone composites.
| Sample | Tg (°C) | Tonset (°C) | Tmax (°C) | Char a Exp./Calcd. |
|---|---|---|---|---|
| EP | 147.8 | 369 | 394 | 12.7/-- |
| EP/ArPN2 | 146.1 | 317 | 339,373 | 21.7/18.0 |
| EP/ArPO2 | 150.7 | 317 | 364 | 24.9/16.4 |
| EP/ArOPN2 | 134.0 | 313 | 365 | 25.2/17.5 |
| EP/ArOPO2 | 145.5 | 310 | 365 | 27.5/17.0 |
a Char yield at 700 °C.
Figure 2.
Differential scanning calorimetry (DSC) curves of polysulfone (a) as well as EP and EP/polysulfone composites (b).
All epoxy composites have a close glass transition temperature, suggesting that the addition of polysulfone had no obvious influence on the thermal stability of EP. Although the Tg values of the polysulfones shows great differences ranging from 93 to 191 °C, only a slight influence of the polysulfones on the Tg of epoxy resins can be found. This phenomenon agreed with a previous report that polymeric flame retardant can reduce the loss of the thermophysical properties of a resin. The main chain of polysulfone may have a complex interaction with epoxy resin, helping to ensure that EP composites maintain high thermo-properties. The order of the Tg values for all EP composites were as follows: EP/ArOPN2 (134.0 °C) < EP/ArOPO2 (145.5 °C) < EP/ArPN2 (146.1 °C) < EP (147.8 °C) < EP/ArPO2 (150.7 °C). This could be because the P-O-C bond in the side chain improves the flexibility of the polysulfone molecular chain, resulting in a slightly lower crosslinking density in the molecular structure of EP. Polysulfones show great advantages in developing EP composites with a high Tg value.
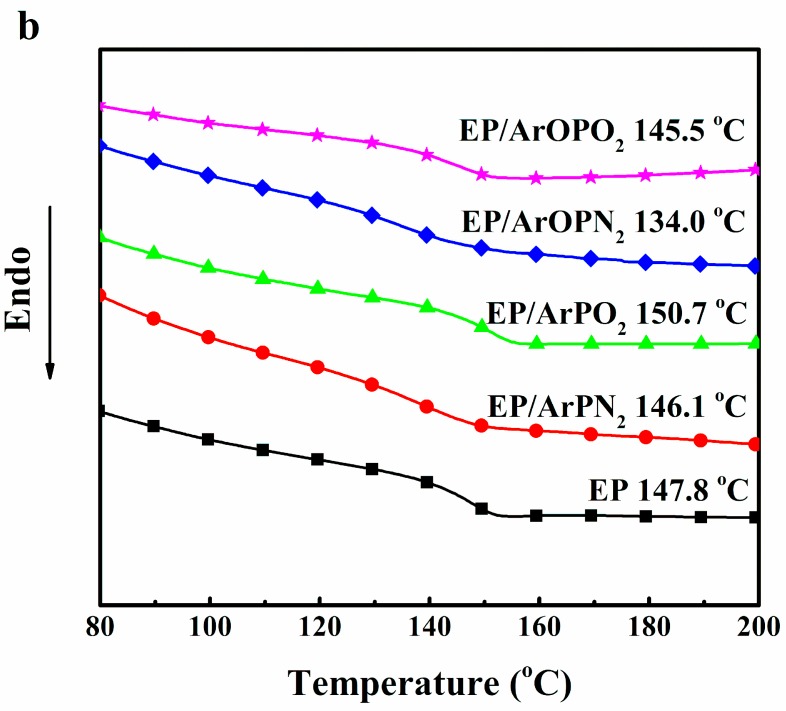
In order to investigate the relationship between the molecular structure of polysulfone and the thermal stability of EP/polysulfone, TGA was used to characterize the thermal stability of polysulfones, EP, and EP/polysulfone composites. Figure 3 shows the TGA and DTG curves of samples under nitrogen, and detailed data are shown in Table 2. TGA curves shown in Figure 3a exhibited a one-step degradation process, and the initial decomposing temperature (the temperature range of a 5% weight loss, T5%) was concentrated in a high temperature range (330–443 °C), suggesting the good thermal stability of these polysulfones. The T5% of ArPN2 and ArOPN2 was much lower than those of ArPO2 and ArOPO2, demonstrating that a higher thermal stability of phosphate than phosphamide. Moreover, the thermal degradation of polysulfones led to the formation of an intumescent char layer, which acted as a protective layer for the polymer surface. Therefore, a high residual char yield of polysulfone at 700 °C was obtained. The order was as follows: ArPO2 (37.4 wt %) < ArOPO2 (41.1 wt %) < ArOPN2 (44.5 wt %) < ArPN2 (47.9 wt %). The high thermal stability of polysulfones makes it possible to obtain high flame retardancy in EP/polysulfone composites.
Figure 3.
Thermogravimetric analysis (TGA) curves of polysulfone (a) as well as EP and EP/polysulfone composites (b).
In Figure 3b, the T5% of the EP/polysulfone composites is shifted to a lower temperature compared with those of pure EP. The residual char yield at 700 °C was much higher than that from EP as well as the calculated value. The order was as follows: EP/ArPN2 (21.7 wt %) < EP/ArPO2 (24.9 wt %) < EP/ArOPN2 (25.2 wt %) < EP/ArOPO2 (27.5 wt %). The lower decomposition temperature and higher residual char value can be explained by the catalysis effect of polysulfones on EP. The residual char yield reveals that the char formation effect of polysulfone with a P–O–C bond is more efficient than the polysulfone-containing P–C bond.
All EP/polysulfone composites decomposed at a similar temperature, ranging from 310 to 317 °C, and the maximum weight loss rate occurred at around 365 °C with a 60 wt % mass loss. Thus, phosphorus-containing structures of polysulfones with certain amounts of phosphorus have a slight influence on the thermal decomposition behavior of EP.
3.2. Flammability
The flame retardant performance of EP and EP/polysulfone composites was investigated via a UL-94 vertical burning test and CONE calorimeter analysis, and the results are given in Table 1 and Table 3. The order of UL-94 vertical burning test performances of the EP composites (with a phosphorus content of approximately 1.25 wt %) was as follows: EP/ArPN2 > EP/ArPO2 > EP/ArOPN2 ≥ EP/ArOPO2. Both EP/ArPN2 and EP/ArPO2 passed the UL-94 vertical burning test with grades of V-0 and V-1, respectively, whereas the other EP composites had not achieved any grade.
Table 3.
Cone calorimetric data for EP and EP/polysulfone composites at 50 kW·m−2.
| Sample | TTI (s) |
PHRR (kW·m−2) |
THR (MJ·m−2) |
TML (%) |
TSR (m2) |
THR/TML (MJ·m−2·g−1) |
TSR/TML (g−1) |
TCOR/TML (g·g−1) |
|---|---|---|---|---|---|---|---|---|
| EP | 50 | 1712 | 83.7 | 93.0 | 22.4 | 3.85 | 117 | 0.11 |
| EP/ArPN2 | 29 | 847 | 61.5 | 80.2 | 18.3 | 3.27 | 110 | 0.15 |
| EP/ArPO2 | 32 | 608 | 42.7 | 82.7 | 19.2 | 2.31 | 117 | 0.18 |
| EP/ArOPN2 | 30 | 546 | 59.4 | 77.1 | 11.4 | 3.42 | 75 | 0.14 |
| EP/ArOPO2 | 30 | 726 | 55.3 | 80.8 | 14.8 | 3.09 | 94 | 0.14 |
In agreement with previous reports, it was concluded that the ArPN2 and ArPO2 with a P–C bond was more flame retardant than the ArOPN2 and ArOPO2 with a P–O–C bond [5]. It is believed that the P–C bond mainly supplied a gas phase radical effect, while P–O–C promoted the char-forming process in the condensed phase. As for EP/polysulfone composites, the gas phase radical effect seems to play a more important role than it does in the condensed phase.
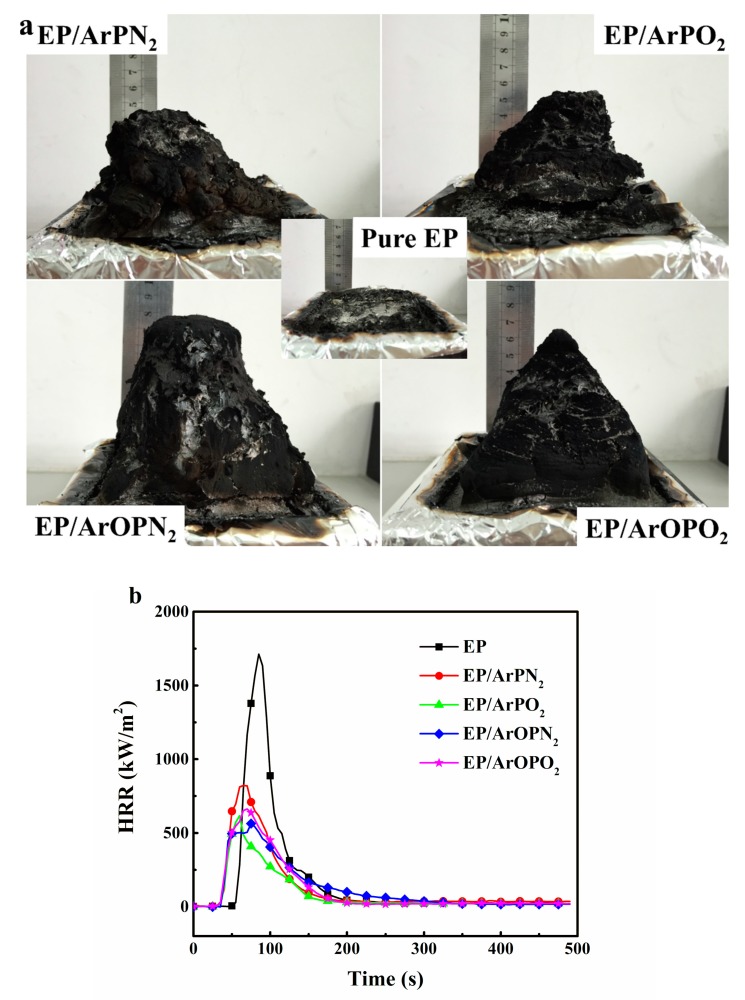
Cone calorimeters are widely used to evaluate many important parameters in real fire scenarios, such as the peak heat release rate (PHRR), total heat release (THR), peak smoke production rate (PSPR), total smoke production (TSP), and total carbon monoxide release (TCOR). Figure 4 presents macroscopic photos of residual char and the heat release rate (HRR) curves of EP composites. The characteristic results of all cone results are summarized in Table 4. Pure EP burned quickly and exhibited a limited amount of char. For EP/polysulfone composites, thick and connected three-dimensional residual char was present after cone calorimeter tests. All polysulfones showed a strong char formation effect on the epoxy resins during combustion. The improved intumescent char layer of EP/polysulfone composites acted as an insulating barrier and blocked the exchange of heat, oxygen, and flammable gases between the EP matrix and fire zone.
Figure 4.
Macroscopic photos of cone calorimeter residue (a) and heat release rate (HRR) curves of EP and EP/polysulfone composites (b).
Table 4.
X-ray photoelectron spectroscopy (XPS) results of the residual char of EP and EP/polysulfone composites.
| Sample | C (% a) | N (%) | O (%) | P (%) | S (%) |
|---|---|---|---|---|---|
| EP | 82.82 | 3.22 | 13.77 | 0.10 | 0.09 |
| EP/ArPN2 Inner | 82.49 | 1.10 | 16.10 | 0.13 | 0.18 |
| EP/ArPN2 Outer | 79.19 | 3.16 | 16.65 | 0.84 | 0.17 |
| EP/ArPO2 Inner | 81.80 | 1.11 | 16.74 | 0.14 | 0.21 |
| EP/ArPO2 Outer | 75.52 | 2.88 | 19.28 | 2.05 | 0.26 |
| EP/ArOPN2 Inner | 80.87 | 1.09 | 17.52 | 0.28 | 0.24 |
| EP/ArOPN2 Outer | 71.34 | 5.53 | 19.78 | 2.92 | 0.43 |
| EP/ArOPO2 Inner | 80.92 | 3.26 | 14.35 | 1.24 | 0.23 |
| EP/ArOPO2 Outer | 72.17 | 4.50 | 19.98 | 2.87 | 0.48 |
a Atomic concentration.
In the cone calorimeter tests, the addition of polysulfones preserves more mass of the EP composites. The order of the char yields of the EP composites was as follows: EP/ArPO2 < EP/ArOPO2 < EP/ArPN2 < EP/ArOPN2. It is noted that polysulfone with a P–O–C bond played a more important role in the char-forming process of EP than polysulfone with a P–C bond. Meanwhile, higher char yields for EP/ArPN2 and EP/ArOPN2 were observed, which is illustrated by the fact that the degradation gases from the imino group promotes the formation of intumescent char layers.
The time to ignition (TTI) obtained in the cone calorimeter shows differences in the ignition behavior of the various samples. It is in the following order: EP/ArPN2 < EP/ArOPN2 ≤ EP/ArOPO2 < EP/ArPO2 < EP. The early TTI for EP/polysulfone composites was associated with the catalysis effect of polysulfones in EP. This agreed well with the TGA experiments. For EP/polysulfone composites, an intumescent char layer formed shortly after ignition. With the protective char layer, the HRR and PHRR of EP/polysulfone composites were significantly decreased. After ignition, the HRR of the EP/polysulfone composites peaked rapidly and then decreased to a low value due to the formation of an intumescent char layer. The PHRR for the EP/polysulfone composites is ordered by increasing fire risk: EP/ArOPN2 < EP/ArPO2 < EP/ArOPO2 < EP/ArPN2. The THR divided by the total mass loss (THR/TML) is a measure for the effective heat of combustion and the combustion efficiency of the volatiles [16]. The THR/TML ratio for EP/ArPO2 and EP/ArPN2 showed a lower value in comparison to EP/ArOPO2 and EP/ArOPN2, respectively. During combustion, volatiles containing phosphorus were released into the flame zone, resulting in flame inhibition. The effect of the polysulfones with a P–C bond was stronger than that of the polysulfones with a P–O–C bond. Moreover, the THR/TML ratio for EP/ArPN2 and EP/ArOPN2 was higher than that for EP/ArPO2 and EP/ArOPO2, respectively, which may be associated with the release of nitrogen-containing gases by the imino group in the main chain of polysulfones.
Smoke and carbon monoxide (CO) from combustion bring serious harm to human health. For EP/ArOPN2 and EP/AROPO2, the P–O–C structure in the side chain of polysulfones promotes the char-forming process and greatly decreases the TSR. As is well known, the enhanced flame retardant performance of EP results in incomplete combustion. The TSR/TML and TCOR/TML ratios increased in the following order: EP/ArOPN2 ≤ EP/ArOPO2 < EP/ArPN2 < EP/ArPO2, suggesting that the polysulfones with a P–C bond show a strong flame inhibition effect in gas phase flame retardancy than those with a P–O–C bond.
3.3. Gas Phase Mechanisms
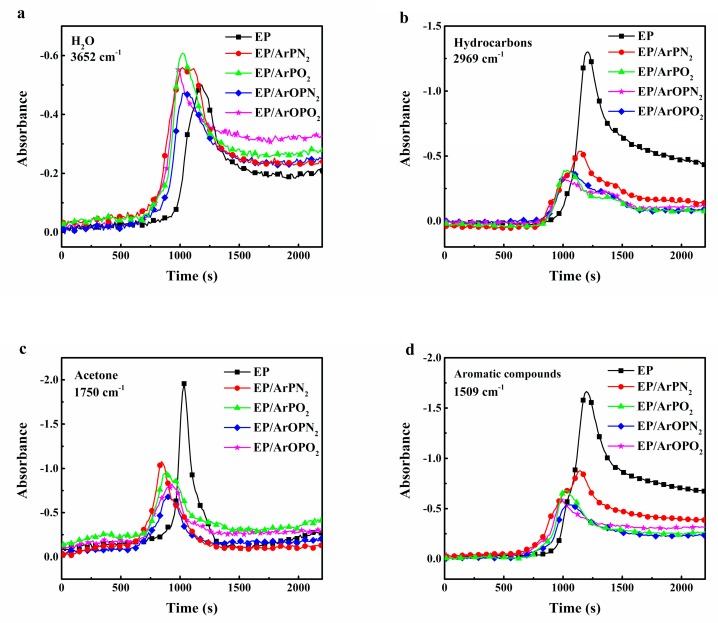
In order to study the gas phase mechanisms of polysulfones, the pyrolysis products during thermal decomposition were analyzed by TGA-FTIR, and the absorbance of the pyrolysis products vs. time is plotted in Figure 5. During the main decomposition step, EP and EP/polysulfone composites released similar products, such as water or phenol (3652 cm−1), hydrocarbons (2969 cm−1), acetone (1750 cm−1), aromatic compounds (1609 and 1509 cm−1), and ethers (1259 and 1176 cm−1) [17]. With the addition of polysulfone, the pyrolysis products from EP/polysulfone composites were formed earlier than in the case of EP. This can be explained in terms of phosphorus-containing polysulfones catalyzing the thermal decomposition of EP. In addition, the absorbance intensity of flammable gases (acetone and hydrocarbons) was lower than that for EP. Consequently, the addition of phosphorus-containing polysulfones reduced the release of flammable gases.
Figure 5.
Absorbance of gas products for EP and EP/polysulfone composites vs. time: (a) H2O; (b) hydrocarbons; (c) acetone; (d) aromatic compounds.
3.4. Condensed Phase Mechanisms
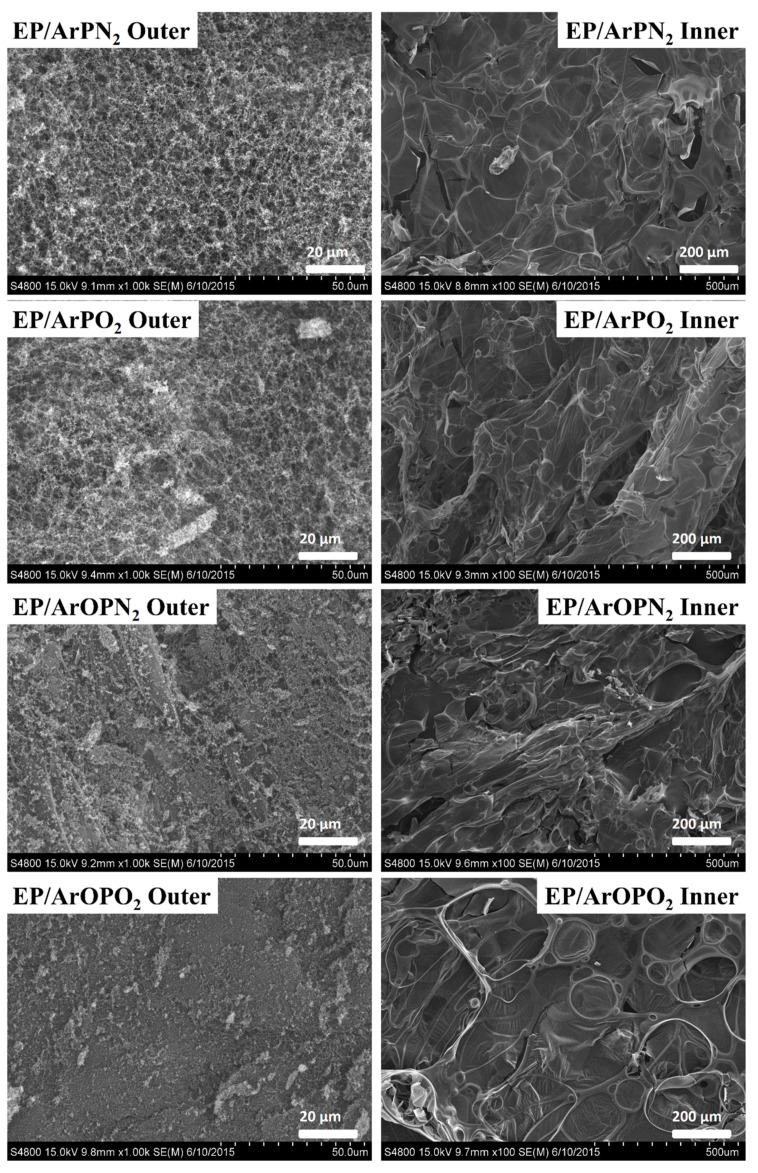
As is well known, the residual char plays a crucial role in enhancing the flame retardant performance of polymer composites. Figure 6 presents the SEM images of the outer surface and inner surface of residual chars obtained from the CONE tests. A continuous char layer can be observed on the outer surface of the residual char for all samples. As for the inner surface of residual char, it can be seen that there are many bubble-like sheets, which were caused by the pyrolysis gases in the molten polymer during combustion. As for EP/ArOPN2 and EP/ArOPO2, the outer surface of residual chars was denser than the surfaces of EP/ArPN2 and EP/ArPO2, because P–O–C mainly acted in the condensed phase and promoted the char-forming process, while P–C acted both in the condensed phase and the gas phase, and it was easy for the porous char layers to release phosphorus-containing gases.
Figure 6.
SEM images of surface for residual char of pure EP and EP/polysulfone composites.
To further understand the flame retardant mechanisms, the chemical components on the surface of the residual chars were investigated by XPS spectroscopy, and the results are summarized in Table 4. With the addition of four polysulfones, the content of oxygen and sulfur in the outer residual char of EP/polysulfone composites were much higher than that of pure EP, corresponding to a higher thermal oxidation capacity of residual char. In the case of EP/ArOPN2 and EP/ArOPO2, a high atom percent of phosphorus, nitrogen, and sulfur indicated that more P-, N-, and S-containing compounds were maintained in the outer surface of residual char. These compounds in the residual char could promote the thermal stability of the char layer and catalyze the char formation. However, it is noted that the percent of phosphorus was very low in the inner surface of EP/ArPN2 and EP/ArPO2, suggesting that P–C tended to produce phosphorus-containing pyrolysis gases and acted as a free radical in the gas phase. In summary, the P–O–C bond in the EP/polysulfone composites effectively promoted the char forming process better than the P–C bond did, resulting in a more condensed char layer. The P–C bond in the EP/polysulfone composites had a stronger flame inhibition effect in the gas phase than did the P–O–C bond.
3.5. Thermal Degradation Kinetics
To better understand the decomposition route of the EP/polysulfone composites, TGA was used to estimate the kinetic parameters of the degradation processes. Flynn–Wall–Ozawa methods (FWO) are widely used to calculate the parameters of the flame retardant composites, such as Ea (activation energies), n (apparent reaction order), and A (pre-exponential factor) [18]. The FWO method, using Dolye’s approximation for the integration, is expressed as
At a given conversion degree α, the plot of logβ against −1/Tp makes a fitted straight line with a slope of −0.457 Ea/R. The apparent activation energy Ea at a given α value can be calculated from the value of 0.457 Ea/R.
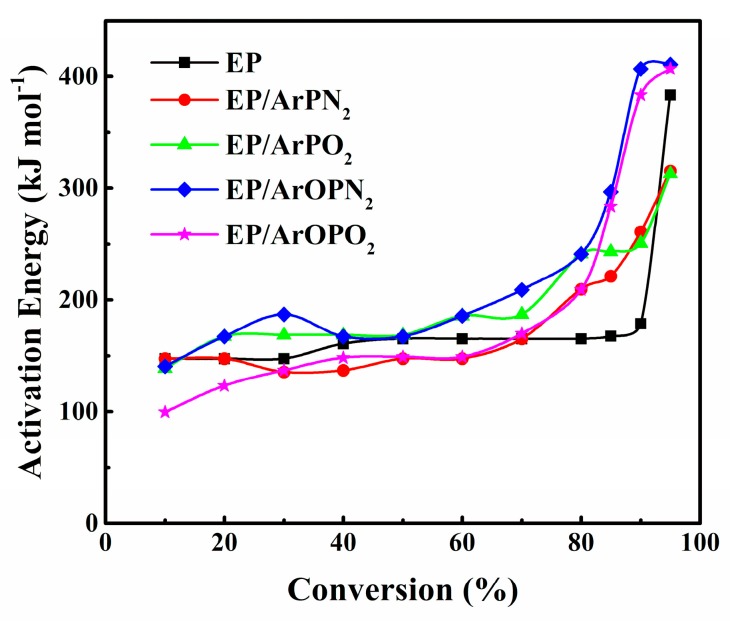
The calculated Ea values are given in Table 5. The results fitted with the FWO method are shown in Figure 7. As for EP/ArOPO2, the calculated Ea with a value of 99.57 kJ/mol is much lower than that of pure EP (147.37 kJ/mol) and other EP composites, and this was caused by the strong catalyzing effect of P–O–C. With the reaction degree increased from 20% to 40%, EP/ArOPN2 had the highest Ea value, which illustrated that the addition of ArOPN2 could promote the formation of an intumescent char layer and enhance the thermal stability of the char layer. This is also shown by the highest char layer of EP/ArOPN2 in the CONE tests. When α rises to 70~100%, the Ea value of EP/ArOPN2 and EP/ArOPO2 are the highest among all EP composites, suggesting that a P–O–C bond in the side chain improves the thermal stability of EP at high temperatures.
Table 5.
Calculated Ea at various conversions using the FWO method for EP/polysulfone composites.
| α | EP | EP/ArPN2 | EP/ArPO2 | EP/ArOPN2 | EP/ArOPO2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | r | Ea (kJ/mol) | r | Ea (kJ/mol) | r | Ea (kJ/mol) | r | Ea (kJ/mol) | r | |
| 0.10 | 147.37 | 0.9967 | 147.37 | 0.9967 | 138.74 | 0.9716 | 140.61 | 0.9816 | 99.57 | 0.9145 |
| 0.20 | 147.37 | 0.9967 | 147.37 | 0.9967 | 167.26 | 0.9593 | 167.26 | 0.9593 | 123.07 | 0.9210 |
| 0.30 | 147.37 | 0.9967 | 135.22 | 0.9907 | 168.51 | 0.9884 | 187.00 | 0.9665 | 136.91 | 0.9220 |
| 0.40 | 160.52 | 0.9974 | 136.91 | 1.0000 | 168.51 | 0.9884 | 167.26 | 0.9593 | 148.01 | 0.9586 |
| 0.50 | 165.05 | 0.9959 | 147.37 | 0.9967 | 168.51 | 0.9884 | 167.26 | 0.9593 | 148.74 | 0.9189 |
| 0.60 | 165.05 | 0.9959 | 147.37 | 0.9967 | 185.75 | 0.9876 | 185.75 | 0.9876 | 148.74 | 0.9189 |
| 0.70 | 165.05 | 0.9959 | 165.05 | 0.9959 | 187.00 | 0.9665 | 209.03 | 0.9650 | 169.86 | 0.9215 |
| 0.80 | 165.05 | 0.9959 | 209.40 | 0.9956 | 240.97 | 0.9757 | 240.97 | 0.9757 | 209.03 | 0.9650 |
| 0.85 | 167.34 | 0.9949 | 221.17 | 0.9962 | 243.09 | 0.9370 | 296.63 | 0.9657 | 283.60 | 0.9604 |
| 0.90 | 178.58 | 0.9836 | 260.79 | 0.9636 | 250.73 | 0.8971 | 406.83 | 0.9740 | 383.36 | 0.9849 |
| 0.95 | 383.36 | 0.9849 | 315.60 | 0.9846 | 312.94 | 0.9335 | 410.74 | 0.9220 | 406.83 | 0.9740 |
Figure 7.
Activation energy curves of pure EP and EP/polysulfone composites.
As for EP/ArPN2 and EP/ArPO2, the Ea values were similar to pure EP when α was lower than 70%. When the conversion rate increased to 70~85%, their Ea values were higher than those of pure EP, which means much more energy was needed to promote the decomposition process. However, when α reached 90%, the Ea values for the two composites were even lower than pure EP, which indicated a lower thermal stability of residual char. The residual char of EP/ArPN2 and EP/ArPO2 was beneficial for the release of phosphorus-containing pyrolysis gases during the fire. This is in agreement with the porous carbon layer observed from the CONE tests.
4. Conclusions
The thermal properties and flame retardancy of four polysulfones (ArPN2, ArPO2, ArOPN2, and ArOPO2) and their EP composites (phosphorus content was maintained at 1.25 wt %) were studied. The addition of all polysulfones showed a slight influence on the Tg of the EP, and the EP composites maintained good stability. All the polysulfones enhanced the thermal stability of the EP and showed a strong intumescent char formation effect on the EP. The improved intumescent char layer during the combustion of EP/polysulfone composites acted as an insulating barrier to block the exchange of heat, oxygen, and flammable gases between the EP matrix and the fire zone, and the PHRR and THR were both greatly reduced. UL-94 vertical burning tests and cone calorimeter tests indicated that the polysulfones with a P–C bond showed a higher flame retardancy than those with a P–O–C bond. TGA and cone calorimeter tests illustrated that the polysulfones containing a P–O–C bond showed a stronger char forming effect on the EP composites than those with a P–C bond, which was mainly responsible for the flame retardant effect in the condensed phase. This work provides a novel method for the design of valuable flame retardant EP used in high temperature environments.
Author Contributions
Conceptualization, W.Z.; investigation, W.Z., Y.L. and Q.L.; writing—original draft preparation, W.Z.; writing—review and editing, W.Z. and Y.W.; supervision, W.Z. and G.W.
Funding
Financial support from the Beijing Natural Science Foundation (2184136, 3194064, and 3194063), the Key Research Program of Frontier Sciences, CAS, Grant NO. QYZDB-SSW-JSC050-01), and the Prospective Project of Technology and Engineering Center for Space Utilization, CAS, Grant NO. CSU-QZKT-201708) is greatly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pomázi Á., Toldy A. Particle distribution of solid flame retardants in infusion moulded composites. Polymers. 2017;9:250. doi: 10.3390/polym9070250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao X.M., Zhang L., Alonso J.P., Delgado S., Martínez-Miranda M.R., Wang D.Y. Influence of phenylphosphonic amide on rheological, mechanical and flammable properties of carbon fiber/RTM6 composites. Compos. Part B. 2017;149:74–81. doi: 10.1016/j.compositesb.2018.05.018. [DOI] [Google Scholar]
- 3.Schartel B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials. 2010;3:4710–4745. doi: 10.3390/ma3104710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomázi Á., Szolnoki B., Toldy A. Flame retardancy of low-viscosity epoxy resins and their carbon fibre reinforced composites via a combined solid and gas phase mechanism. Polymers. 2018;10:1081. doi: 10.3390/polym10101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun U., Balabanovich A.I., Schartel B., Knoll U., Artner J., Ciesielski M., Döring M., Perez R., Sandler J.K., Altstädt V., et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer. 2006;47:8495–8508. doi: 10.1016/j.polymer.2006.10.022. [DOI] [Google Scholar]
- 6.Salmeia K.A., Fage J., Liang S., Gaan S. An overview of mode of action and analytical methods for evaluation of gas phase activities of flame retardants. Polymers. 2015;7:504–526. doi: 10.3390/polym7030504. [DOI] [Google Scholar]
- 7.Täuber K., Marsico F., Wurm F.R., Schartel B. Hyperbranched poly(phosphoester)s as flame retardants for technical and high performance polymers. Polym. Chem. 2014;5:7042–7053. doi: 10.1039/C4PY00830H. [DOI] [Google Scholar]
- 8.Zhao W., Liu J.P., Zhang Y., Ban D.M. Simple green synthesis of solid polymeric bisphenol A bis(diphenyl phosphate) and its flame retardancy in epoxy resins. RSC Adv. 2015;5:80415–80423. doi: 10.1039/C5RA06762F. [DOI] [Google Scholar]
- 9.Wang Y.Z., Zheng C.Y., Yang K.K. Synthesis and characterization of polysulfonyl diphenylene phenyl phosphonate. Polym. Mat. Sci. Eng. 1999;15:53–56. [Google Scholar]
- 10.Zhao W., Wang G., Liu J.P., Ban D.M., Cheng T.J., Liu X.D., Li Y.X., Huang Y.Q. Synthesis, structure–property and flame retardancy relationships of polyphosphonamide and its application on epoxy resins. RSC Adv. 2017;7:49863–49874. doi: 10.1039/C7RA08318A. [DOI] [Google Scholar]
- 11.Yang G., Wu W.H., Wang Y.H., Jiao Y.H., Lu L.Y., Qu H.Q., Qin X.Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019;366:78–87. doi: 10.1016/j.jhazmat.2018.11.093. [DOI] [PubMed] [Google Scholar]
- 12.Perez R.M., Sandler J.K.W., Altstädt V., Hoffmann T., Pospiech D., Ciesielski M., Döring M., Braun U., Balabanovich A.I., Schartel B. Novel phosphorus-modified polysulfone as a combined flame retardant and toughness modifier for epoxy resins. Polymer. 2007;48:778–790. doi: 10.1016/j.polymer.2006.12.011. [DOI] [Google Scholar]
- 13.Lin C.H., Chen J.C., Huang C.M., Jehng J.M., Chang H.C., Juang T.Y., Su W.C. Side-chain phenol-functionalized poly(ether sulfone) and its contribution to high-performance and flexible epoxy thermosets. Polymer. 2013;54:6936–6941. doi: 10.1016/j.polymer.2013.10.054. [DOI] [Google Scholar]
- 14.Liaw D.J., Shen W.C. Synthesis of aromatic polyphosphonate: low temperature solution polycondensation of 4,4′-sulphonyldiphenol with phenoxy dichlorophosphate. Polymer. 1993;34:1336–1338. doi: 10.1016/0032-3861(93)90798-F. [DOI] [Google Scholar]
- 15.Tai Q.L., Hu Y., Yuen R.K.K., Song L., Lu H.D. Synthesis, structure-property relationships of polyphosphoramides with high char residues. J. Mater. Chem. A. 2011;21:6621–6627. doi: 10.1039/c0jm03959d. [DOI] [Google Scholar]
- 16.Zhao W., Liu J.P., Peng H., Liao J.Y., Wang X.J. Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym. Degrad. Stab. 2015;118:120–129. doi: 10.1016/j.polymdegradstab.2015.04.023. [DOI] [Google Scholar]
- 17.Zhou Y., Feng J., Peng H., Qu H.Q., Hao J.W. Catalytic pyrolysis and flame retardancy of epoxy resins with solid acid boron phosphate. Polym. Degrad. Stab. 2014;110:395–404. doi: 10.1016/j.polymdegradstab.2014.10.009. [DOI] [Google Scholar]
- 18.Tian N.N., Gong J., Wen X., Yao K., Tang T. Synergistic effect between a novel char forming agent and ammonium polyphosphate on flame retardancy and thermal properties of polypropylene. RSC Adv. 2013;52:10905–10915. doi: 10.1021/ie401058u. [DOI] [Google Scholar]