Abstract
Background
The aim of this study was to evaluate the effects and safety of vasopressin receptor agonists in patients with septic shock.
Methods
PubMed, EMBASE, and Cochrane library were searched for randomized controlled trials evaluating the effects of vasopressin receptor agonists in septic shock patients. Two reviewers performed literature selection, data extraction, and quality evaluation independently. The primary outcome was mortality. And secondary outcomes included intensive care unit (ICU) length of stay, duration of mechanical ventilation, and incidence of adverse events. In addition, a trial sequential analysis (TSA) was performed.
Results
Twenty studies were eligible for meta-analysis. The results showed vasopressin receptor agonists use was associated with reduced mortality (relative risk (RR) 0.92; 95% confidence interval (CI) 0.84 to 0.99; I2 = 0%). Nevertheless, they had no significant effects on ICU length of stay (mean deviation (MD) − 0.08, 95% CI, − 0.68 to 0.52, I2 = 0%) and duration of mechanical ventilation (MD − 0.58, 95% CI − 1.47 to 0.31, I2 = 57%). Additionally, there was no significant difference in total adverse events between two groups (RR 1.28, 95% CI 0.87 to 1.90, I2 = 57%), but vasopressin receptor agonists administration could significantly increase the risk of digital ischemia (RR 4.85, 95% CI 2.81 to 8.39, I2 = 26%). Finally, there was no statistical difference of cardiovascular events (RR 0.91, 95% CI 0.53 to 1.57, I2 = 1%), arrhythmia (0.77, 95% CI 0.48 to 1.23, I2 = 23%), mesenteric ischemia (0.83, 95% CI 0.44 to 1.55, I2 = 0%), diarrhea (2.47, 95% CI 0.77 to 7.96, I2 = 49%), cerebrovascular events (1.36, 95% CI 0.18 to 10.54, I2 = 0%), and hyponatremia (1.47, 95% CI 0.84 to 2.55, I2 = 0%) between two groups. Egger’s test showed there was no significant publication bias among studies (P = 0.36).
Conclusions
The use of vasopressin might result in reduced mortality in patients with septic shock. An increased risk of digital ischemia must be taken into account.
Electronic supplementary material
The online version of this article (10.1186/s13054-019-2362-4) contains supplementary material, which is available to authorized users.
Keywords: Vasopressin, Catecholamine, Septic shock, Meta-analysis
Background
Septic shock is the leading cause of death in intensive care units. It is reported that the mortality rate of these patients can be as high as 30–60% [1–3]. Maintaining effective blood pressure is important for these patients [4]. Vasopressors are often used to reach a target mean arterial pressure (MAP), after adequate fluid resuscitation. Catecholamines, such as norepinephrine (NE), are still the first-line drugs. However, high dose of catecholamines may be associated with a higher risk of complications, including myocardial ischemia, decreased cardiac output, arrhythmias, increased tissue oxygen consumption, and pulmonary hypertension [4, 5].
Relative vasopressin deficiency often occurs in septic shock patients [6, 7]. Some pre-clinical studies showed exogenous administration of vasopressin could increase the vascular tone and improve blood pressure [8]. Several clinical studies also reported early concomitant vasopressin, and norepinephrine therapy could reduce the dose of NE, shorten the time of achieving target mean arterial pressure, and reduce catecholamine-related complications [9, 10]. Therefore, the newest Surviving Sepsis guideline suggests vasopressin could be used to raise blood pressure to target mean arterial pressure or decrease norepinephrine dosage with weak recommendations [11]. However, no consensus has been made regarding the effects of vasopressin receptor agonists on patient-centered outcomes, especially mortality. The aim of this study is to explore the effects and safety of vasopressin receptor agonists in patients with septic shock.
Methods
The present meta-analysis was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, http://www.prisma-statement.org/).
Registration and protocol
This meta-analysis was registered on PROSPERO (CRD42018104027).
Inclusion criteria
Patients: Adult septic shock patients
Intervention: Vasopressin or its analogues (e.g., terlipressin, selepressin) with or without concomitant catecholamines, irrespective of dose and duration
Comparison: Catecholamines use alone, irrespective of dose and duration
Outcomes: The primary endpoint was 28/30-day mortality, and hospital mortality and ICU mortality were equal for this analysis module. The secondary endpoints included ICU length of stay, duration of mechanical ventilation, and adverse events (total adverse events, digital ischemia, cardiovascular events, arrhythmia, mesenteric ischemia, diarrhea, cerebrovascular events, and hyponatremia).
Data source and literature search
PubMed, EMBASE, and Cochrane library were searched from inception to July 31, 2018. The detailed search strategy is showed in Additional file 1: Table S1. The bibliography of relevant articles was searched for additional articles. In addition, https://clinicaltrials.gov/ was searched for ongoing or unpublished studies.
Study selection and data extraction
Two reviewers performed literature selection independently. Firstly, we excluded duplicates through reference management tool. Then, we exclude clearly non-relevant articles by reading titles and abstracts. Finally, we decided the eligibility of each article by full-text reading.
The same two reviewers did the data extraction independently using a pre-defined datasheet. And we recorded basic information of each eligible study, characteristics of included patients, interventions, comparisons, endpoints, and other items which were essential for quality evaluation. Any discrepancy was solved by discussion or consulting with the third reviewer.
Study quality evaluation
Two reviewers evaluated the quality of each included study based on the following domains: sequence generation, allocation concealment, blinding of patients and personnel, blinding of outcome assessors, incomplete outcome data, and selective reporting. Each domain is classified as low risk, unclear risk, and high risk. Any discrepancy was solved by discussion or consulting with the third reviewer.
Statistical analysis
Relative risk (RR) was used for dichotomous data, and mean difference (MD) was used for continuous data. The heterogeneity between studies was assessed using the I2 test and chi-square test. P < 0.1 and I2 ≥ 50% indicated significant heterogeneity, and the random effects model was used. Otherwise, the fixed effects model was used. A two-sided P value < 0.05 was considered statistically significant. Publication bias was assessed by funnel plot and Egger’s test quantitatively. All statistical analyses were performed using STATA 12.0 software (SERIAL NO.40120519635) and RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
In the present study, we used the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) to evaluate the quality of evidence. And evidences were categorized as high, moderate, low, and very low, according to two group factors (factors that can reduce the quality of the evidence and factors that can increase the quality of the evidence). This process was performed on GRADEpro GDT (https://gradepro.org/).
In the present study, we performed the trial sequential analysis (TSA) to decrease the risks of random errors due to sparse data and repetitive testing and calculate the optimal information size for this meta-analysis. In addition to the optimal information size, an adjusted boundary line for favoring vasopressin or its analogue use and an adjusted boundary line for favoring catecholamine use alone were generated to decide whether the meta-analysis should be terminated early or the confidence interval. In this TSA model, type I error was set at 5% and type II error was set at 20%. A 10% relative risk reduction (RRR) and baseline mortality calculated from the actual meta-analyses were used to calculate the optimal information size. TSA was performed using the trial sequential analysis v.0.9.5.10 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark, available from www.ctu.dk/tsa).
Results
Literature selection process
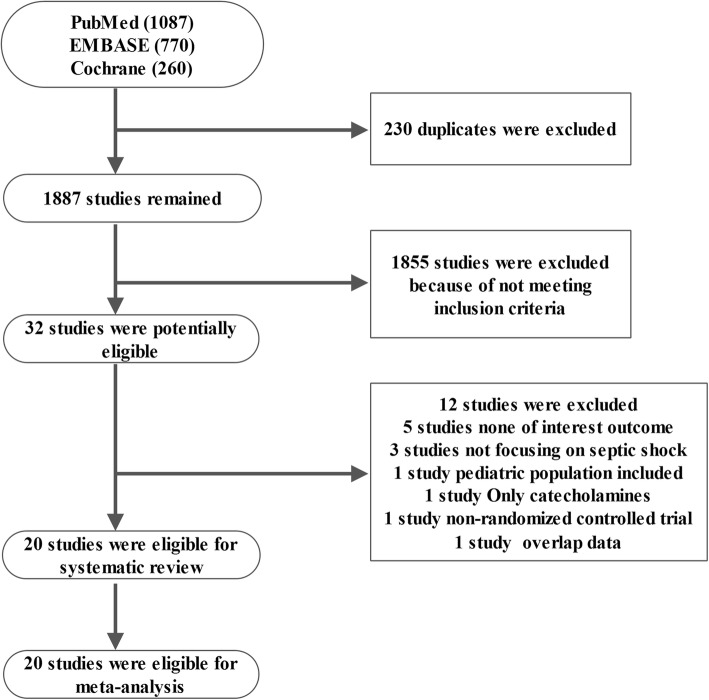
After excluding 230 duplicates, 1887 studies from 2117 hits were chosen for further evaluation. Through reading title and abstract, 1855 studies were excluded and 32 studies were potentially eligible for evaluation by reading the full text. Finally, 20 studies were included for meta-analysis [12–31]. Figure 1 shows the process of literature selection and reasons for study exclusion. Detailed information of excluded studies and ongoing studies is presented in Additional file 1: Table S2, S3.
Fig. 1.
Flow chart of literature selection
The characteristics and quality of the included studies
Twenty studies [12–31] with 2250 septic shock patients who received vasopressin receptor agonists and 2281 septic shock patients who received catecholamine alone were eligible. Patients in 9 studies and 11 studies received vasopressin [12, 17, 19–21, 28–31] and vasopressin’s analogues (pituitrin 1 [14], selepressin 1 [23], terlipressin 9 [13, 15, 16, 18, 22, 24–27]), respectively. Among them, four studies [12, 17, 18, 21] were published in abstract and relevant data were obtained from the study by McIntyre et al. [32]. Detailed information is showed in Table 1, and quality evaluation of all included studies is showed in Additional file 1: Figures S1, S2.
Table 1.
The characteristics of included studies
| Study | Year | No. of patients | Patients | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|---|
| Malay | 1999 | 10 | Septic shock | Vasopressin 0.04 U/min | NE | 24 h |
| Albanese | 2005 | 20 | Septic shock | Terlipressin: one bolus of 1 mg and a second bolus of 1 mg was given if the MAP < 65 mmHg after 20 mins | NE was started at a dose of 0.3 μg/kg/min, followed by 0.3 μg/kg/min increments at 4-min intervals to raise MAP to 65 to 75 mmHg | Hospital |
| Lauzier | 2006 | 23 | Septic shock | Arginine-vasopressin 0.04–0.20 U/min | NE 0.1–2.8 μg/kg/min | ICU |
| Russell | 2008 | 799 | Septic shock | Vasopressin 0.01–0.03 U/min or at clinician’s discretion | NE 5 to 15 mg/min or at clinician’s discretion | 90 days |
| Acevedo | 2009 | 24 | Septic shock and cirrhosis | Terlipressin 1–2 mg/4 h plus alpha-adrenergic drugs | Alpha-adrenergic drugs alone | Hospital |
| Morelli | 2009 | 45 | Septic shock | Terlipressin 1.3 μg/kg/h Vasopressin 0.03 U/min |
NE 15 μg/min | ICU |
| Han | 2012 | 139 | Septic shock | Pituitrin 1.0–2.5 U/h | Dopamine or NE 2–20 μg/kg/h | 28 days |
| Svoboda | 2012 | 30 | Septic shock | Terlipressin 4 mg/24 h for 72 h plus open-label norepinephrine | NE > 0.6 μg/kg/min for more than 24 h | 28 days |
| Fonseca Ruiz | 2013 | 30 | Septic shock | Vasopressin 0.01–0.04 U/min plus NE | NE | 28 days |
| Hua | 2013 | 32 | Septic shock patients with ARDS | Terlipressin 1.3 mg/kg/h | Dopamine < 20 mg/kg/min | 28 days |
| Oliveira | 2014 | 387 | Septic shock | Vasopressin 0.01–0.03 U/min with low doses of norepinephrine | NE 0.05–2.0 μg/kg/min | 28 days |
| Barzegar | 2016 | 30 | Septic shock | Vasopressin 0.03 μg/min plus NE | NE: infusion adjusted to MAP ≥ 65 mmHg | 28 days |
| Choudhury | 2016 | 84 | Cirrhotics with septic shock | Terlipressin 2–8 mg over 24 h | NE 7.5–60 μg/min | 28 days |
| Clem | 2016 | 82 | Septic shock | Vasopressin 0.04 U/min plus NE with 0.05–0.5 μg/kg/min | NE 0.05 to 0.5 μg/kg/min | 28 days |
| Gordon | 2016 | 408 | Septic shock | Vasopressin: titrated up to 0.06 U/min to maintain the MAP 65 to 75 mmHg | NE: titrated up to 12 μg/min to maintain the MAP 65 to 75 mmHg | 28 days |
| Capoletto | 2017 | 250 | Septic shock and cancer | Vasopressin | NE | 90 days |
| Chen | 2017 | 57 | Septic shock patients with ARDS | Terlipressin 0.01–0.04 U/min to maintain MAP between 65 and 75 mmHg, if necessary plus NE | NE > 1 μg/min to maintain MAP between 65 and 75 mmHg | 28 days |
| Prakash | 2017 | 184 | Cirrhosis with septic shock. | Terlipressin 2 mg/24 h and 3.75–30 μg/min of NE as needed to maintain MAP > 65 mmHg | NE 7.5–60 μg/min | 30 days |
| Russell | 2017 | 48 | Septic shock | Selepressin 1.25, 2.5, and 3.75 ng/kg/min until shock resolution or a maximum of 7 days | Placebo | 28 days |
| Liu | 2018 | 535 | Septic shock | Terlipressin 20–160 μg/h | NE 4–30 μg/min | 28 days |
No number, NE norepinephrine, ICU intensive care unit, ARDS acute respiratory distress syndrome
Meta-analysis
The primary endpoint: mortality
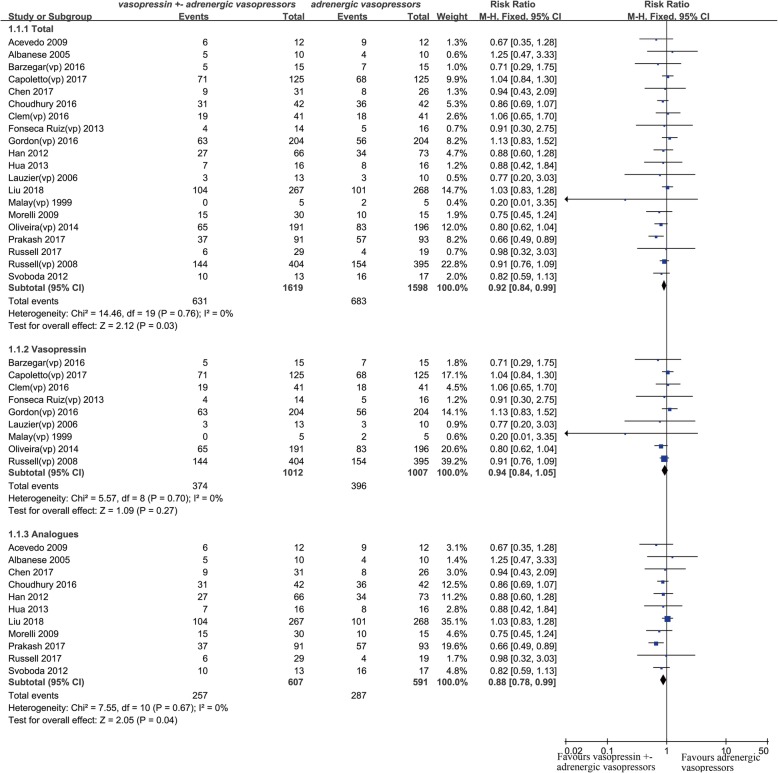
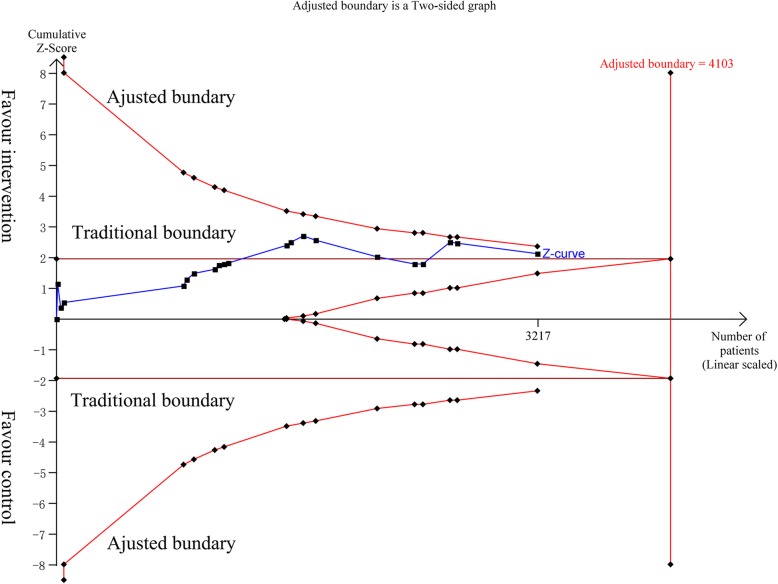
Twenty studies were included for mortality analysis [12–31], and the combined RR was 0.92 (95% confidence interval (CI) 0.84 to 0.99, P = 0.03, I2 = 0%) (Fig. 2). The quality of evidence is presented in Additional file 1: Table S4. The results of TSA indicated the optimal information size was 4103 patients for mortality and more high-quality RCTs are needed, although z curve had crossed the general boundary line, but it did not cross any adjusted boundary line favoring the intervention group or control group. And the adjusted RR was 0.92 (95% CI 0.84 to 0.99, P = 0.03, I2 = 0%), based on 10% RRR (from a baseline event rate of 43%) (Fig. 3).
Fig. 2.
Forest plot for effects of vasopressin or its analogues on 28/30-day mortality (mortality rate within 30 was equal)
Fig. 3.
Trial sequential analysis for effects of vasopressin or its analogues on 28/30-day mortality. The diversity-adjusted required information size (4103 participants) was based on a relative risk reduction of 10%, an alpha of 5%, a beta of 20%, and an event proportion of 43% in the control arm. The blue cumulative z curve was constructed using a fixed effects model
Post hoc sensitive and subgroup analysis
Firstly, the combined RR was 0.95 (95% CI 0.86–1.05, P = 0.30, I2 = 0%) [13–16, 19, 20, 22–31] for studies published in full text and 0.85 (95% CI 0.74–0.98, P = 0.02, I2 = 23%) [12, 17, 18, 21] for studies published in abstract. In addition, after removing three studies that did not report 28/30-day mortality, the combined RR was 0.92 (95% CI 0.83–1.00, P = 0.06, I2 = 0%) [12–15, 17–19, 21–29, 31]. Thirdly, the combined RR was 0.94 (95% CI 0.84–1.05, P = 0.70, I2 = 0%) [12, 17, 19–21, 28–31] for patients who received vasopressin and 0.88 (95% CI 0.78–0.99, P = 0.04, I2 = 0%) [13–16, 18, 22–27] for patients who received its analogues (Fig. 2). Finally, we performed another subgroup analysis based on different diagnoses. In patients with cirrhosis, the combined RR was 0.73 (95% CI 0.61–0.88, P = 0.001, I2 = 23%), and in other patients, the combined RR was 0.95 (95% CI 0.87–1.04, P = 0.24, I2 = 0%).
The secondary endpoints
ICU length of stay
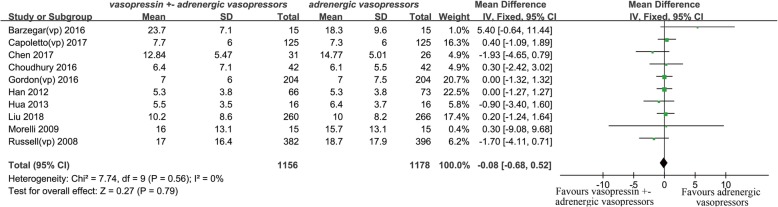
Ten studies reported ICU length of stay [12, 14–16, 19, 22, 24, 27, 28, 31]. The results showed there were no effects of vasopressin receptor agonists on ICU length of stay (MD − 0.08, 95% CI − 0.68–0.52, P = 0.79, I2 = 0%) (Fig. 4).
Fig. 4.
Forest plot for effects of vasopressin or its analogues on intensive care unit length of stay
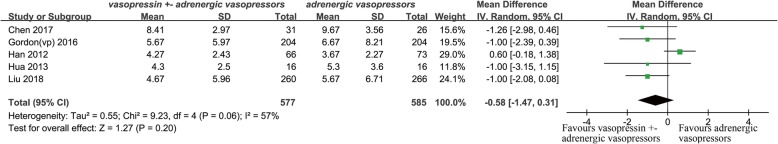
Duration of mechanical ventilation
Five studies were eligible for analysis of duration of mechanical ventilation [14, 15, 19, 24, 27]. The combined MD was − 0.58 (95% CI − 1.47–0.31, P = 0.20, I2 = 57%) (Fig. 5). The results showed vasopressin receptor agonist administration did not significantly affect the duration of mechanical ventilation. The result of the study by Han was different from the other studies [14]. A sensitive analysis was performed by removing the study by Han; the combined MD was − 1.05 (95% CI − 1.77 to − 0.32, P = 0.005, I2 = 0%).
Fig. 5.
Forest plot for effects of vasopressin or its analogues on the duration of mechanical ventilation
Adverse events
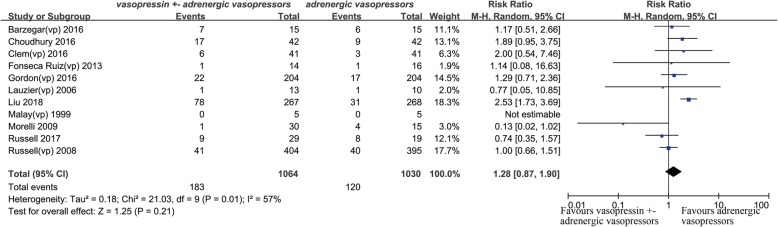
Total adverse events
Eleven studies were included in the analysis of total adverse events [16, 19–23, 27–31]. The combined RR was 1.28 (95% CI 0.87–1.90, P = 0.21, I2 = 57%) (Fig. 6). The results were driven by the study by Liu et al. [27], which carried 18.3% of the weight. A sensitive analysis was performed by removing the study by Liu et al., and the combined RR was 1.11 (95% CI 0.86–1.43, P = 0.44, I2 = 10%).
Fig. 6.
Forest plot for effects of vasopressin or its analogues on total adverse events
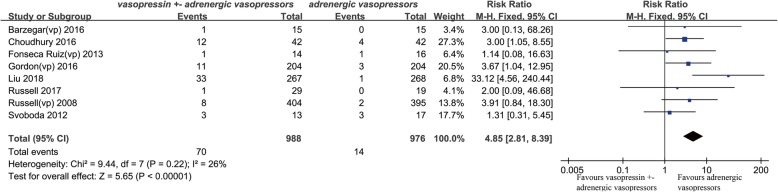
Digital ischemia
Eight studies and 1964 patients were eligible for the analysis [19, 22, 23, 25, 27–29, 31]. The combined RR was 4.85 (95% CI 2.81–8.39, P < 0.001, I2 = 26%) (Fig. 7), indicating that the use of vasopressin receptor agonists was associated with more digital ischemia events. A sensitive analysis was performed by removing the study by Liu et al. [27], due to its results that were significantly different from the other studies. And the combined RR was 2.79 (95% CI 1.54–5.05, P < 0.001, I2 = 0%), supporting the original conclusion.
Fig. 7.
Forest plot for effects of vasopressin or its analogues on digital ischemia
Other adverse events
There were no effects of vasopressin receptor agonists on cardiovascular events [19, 23, 27, 28, 30, 31], arrhythmia [16, 19, 21–23, 27, 28, 31], mesenteric ischemia [19, 23, 27, 31], diarrhea [23, 27, 31], cerebrovascular events [23, 31], and hyponatremia [27, 28, 31]. And the combined RR was 0.91 (95% CI 0.53–1.57, P = 0.73, I2 = 1%) (Additional file 1: Figure S3), 0.77 (95% CI 0.48–1.23, P = 0.28, I2 = 23%) (Additional file 1: Figure S4), 0.83 (95% CI 0.44–1.55, P = 0.55, I2 = 0%) (Additional file 1: Figure S5), 2.47 (95% CI 0.77–7.96, P = 0.13, I2 = 49%) (Additional file 1: Figure S6), 1.36 (95% CI 0.18–10.54, P = 0.77, I2 = 0%) (Additional file 1: Figure S7), and 1.47 (95% CI 0.84–2.55, P = 0.18, I2 = 0%) (Additional file 1: Figure S8), respectively. Additional subgroup analyses are showed in Table 2.
Table 2.
Subgroup analysis based on medication type
| Indicator | Vasopressin | Vasopressin’s analogues |
|---|---|---|
| ICU length of stay (MD) | − 0.17 (95% CI − 0.98–0.63, P = 0.67, I2 = 0%) | 0.03 (95% CI − 0.87–0.93, P = 0.94, I2 = 24%) |
| Duration of MV (MD) | − 1.00 (95% CI −2.39-0.39, P = 0.16)* | − 0.50 (95% CI − 1.57–0.57, P = 0.36, I2 = 63%) |
| Total adverse events (RR) | 1.13 (95% CI 0.83–1.53, P = 0.43, I2 = 0%) | 1.20 (95% CI 0.52–2.74, P = 0.67, I2 = 79%) |
| Digital ischemia (RR) | 3.33 (95% CI 1.39–7.95, P < 0.001, I2 = 0%) | 6.06 (95% CI 2.97–12.37, P < 0.001, I2 = 68%) |
| Cardiovascular events (RR) | 0.93 (95% CI 0.51–1.69, P = 0.80, I2 = 27%) | 0.84 (95% CI 0.24–2.99, P = 0.79, I2 = 0%) |
| Arrhythmia (RR) | 0.99 (95% CI 0.51–1.91, P = 0.98, I2 = 15%) | 0.57 (95% CI 0.29–1.15, P = 0.12, I2 = 35%) |
| Mesenteric ischemia (RR) | 0.77 (95% CI 0.38–1.53, P = 0.45, I2 = 0%) | 1.22 (95% CI 0.26–5.64, P = 0.80, I2 = 42%) |
| Diarrhea (RR) | 0.98 (95% CI 0.06–15.58)* | 1.64 (95% CI 0.05–54.19, P = 0.78, I2 = 71%) |
| Cerebrovascular events (RR) | 0.98 (95% CI 0.06–15.58, P = 0.99)* | 2.00 (95% CI 0.09–46.68, P = 0.67)* |
| Hyponatremia (RR) | 2.31 (95% CI 0.35–15.09, P = 0.38, I2 = 0%) | 1.39 (95% CI 0.78–2.49, P = 0.26)* |
RR relative risk, MD mean difference, CI confidence interval, ICU intensive care unit, MV mechanical ventilation
*Only one study
Publication bias
Publication bias was assessed via funnel plots and Egger’s test (Additional file 1: Figure S9). The results of Egger’s test indicated there was no significant publication bias among the included studies (P = 0.39).
Discussion
In this meta-analysis, the authors evaluated the effects and safety of vasopressin receptor agonists in patients with septic shock. The results showed vasopressin receptor agonist administration might be associated with increased survival in septic shock patients and further studies are required. However, their use could increase the risk of digital ischemia. There were no effects on ICU length of stay, duration of mechanical ventilation, cardiovascular ischemia events, arrhythmia, cerebrovascular ischemia events, mesenteric ischemia, diarrhea, and hypomania.
Generally, catecholamines, especially norepinephrine, are used as the first-line vasopressors in septic shock patients [3, 33–35]. However, with a better understanding of the pathophysiology of septic shock and growing attention to the side effects of catecholamines, alternative vasopressors are searched. Vasopressin is an endogenous hormone, and the supraoptic and paraventricular hypothalamic nuclei are the principal sources [36, 37]. Plasma vasopressin level in normal subjects does not exceed 4 pg/ml. But in patients with septic shock, the level of plasma vasopressin is reported to be abnormally low [36–39]. Moreover, exogenously administered vasopressin could increase the responsiveness to infused catecholamines and reduce the dose of catecholamines [40–42].
Vasopressin in Septic Shock Trial (VASST) failed to find a statistical difference in short-term and long-term mortality between septic shock patients who received vasopressin and norepinephrine [31]. In the present meta-analysis, we find the use of vasopressin receptor agonists is associated with increased survival when compared with those that received catecholamines alone, and this positive association may be more obvious in patients with cirrhosis who received terlipressin. Terlipressin, a synthetic analogue of vasopressin with a longer half-life, acts via V1 receptors on arteriolar smooth muscle cells. Terlipressin is generally used for hepatorenal syndrome and esophageal variceal bleeding [43, 44]. Previous small studies found a continuous infusion of terlipressin might be more effective than vasopressin in restoring hemodynamic status with less adverse events [16, 25, 45]. In the study by Choudhury et al. [22], the authors even found terlipressin is effective in improving survival of cirrhotics with septic shock, and they suggested early introduction of terlipressin rather than after failure of monotherapy. This is in agreement with the results of our study. The survival advantage of terlipressin is more obvious in cirrhotics with septic shock perhaps because it can reduce the portal pressure and result in redistribution of splanchnic blood. Additionally, terlipressin use may be useful in renal function recovery [22]. Selepressin, a more selective V1a receptor agonist, was reported to be effective in the improvement of hemodynamics in septic shock animal models and decreasing pulmonary capillary leak when used early or as first-line agent [46–48]. One small phase IIb human study reported selepressin was safe and effective in septic shock patients [49].
Several meta-analyses reached conflict conclusions [32, 50–54]. Possible reasons include different inclusion criteria. In this present study, both studies in full text and abstract were eligible. In order to reduce patient heterogeneity, only septic shock patients were included in the present study. Additionally, different endpoints and statistical methods may also account for the inconsistent outcomes.
The limitation of this study
Several limitations of the present study should be concerned. Firstly, although there was no statistical significance of Egger’s test, the possibility of publication bias cannot be completely excluded. Secondly, some endpoints were not reported in studies, which were published in the abstract. Thirdly, ICU mortality, 24 h mortality, hospital mortality, and 28/30-day mortality were regarded to be equal in the present study, and this might bias the outcome. Finally, long-term endpoints, like 90-day mortality, and some surrogate outcomes were not reported in the present study.
The implication for clinical practice and further studies
The results of this meta-analysis showed vasopressin receptor agonists improved survival with a higher risk of digital ischemia. The following reasons may account for the higher incidence of digital ischemia in the study by Liu et al. Firstly, 94% of patients with digital ischemia in their study received terlipressin and open-label noradrenaline. Furthermore, the maximum dose of terlipressin used in their study was higher than that reported in other studies [27]. However, no patient needed surgical interventions for digital ischemia. Another concern of using vasopressin in patients with septic shock is its effects on cardiac output and oxygen delivery. Vasopressin has previously been reported to be associated with a reduction of cardiac output [55], although this association is not found in other studies [16, 56]. Factors including different infusion method and dose of vasopressin, different period of fluid resuscitation, and additional medication use (inotropic infusion) may partially explain the diverse results [56]. Neto et al., in their meta-analysis, pointed that vasopressin use did not result in decreased cardiac output, except for high dose of terlipressin [52]. Additionally, Gordon et al. and Neto et al., in their studies, found vasopressin administration was associated with a significant decrease in heart rate, and this may play important role in effect on the cardiac output of vasopressin [52, 56]. In most published studies, patients in the intervention group received both vasopressin and open-label catecholamines, and this may bias the outcome. And more head-to-head comparative randomized evidence is required. The VASST study found the survival advantage of concomitant vasopressin and norepinephrine therapy was obvious in patients with less severe shock [57]. In another study, lactate concentration was reported to be associated with the hemodynamic response of vasopressin [58]. In the study by Nascente et al., they found vasopressin administration is likely to improve microcirculation in septic shock patients whose baseline noradrenaline dose was higher than 0.38 μg/kg/min [59]. Therefore, uncovering specific subgroups of septic patients who are most likely to respond to early initiation of vasopressin is important [58]. A post hoc analysis pointed that the adjunctive use of corticosteroids could increase the survival benefit of vasopressin. And in these patients, the serum vasopressin concentration significantly increased [60]. Although this association did not been observed in the following randomized controlled trial [19], adjunctive treatments with vasopressin in septic shock patients are another point requiring more studies. Moreover, the best dose, time of use [10, 61–64], infusion method (continuous or intermittent), and discontinuation strategies are also a hot topic and remain unclear [65].
Conclusions
The use of vasopressin might result in reduced mortality in patients with septic shock. An increased risk of digital ischemia must be taken into account, and more studies are required.
Additional file
Table S1. Study search strategy. Table S2. Information on excluded studies. Table S3. List of Ongoing studies. Table S4. GRADE. Figure S1. Risk of bias summary. Figure S2. Risk of bias graph. Figure S3. Forest plot for vasopressin or its analogues on cardiovascular events. Figure S4. Forest plot for vasopressin or its analogues on arrhythmia. Figure S5. Forest plot for vasopressin or its analogues on mesenteric ischemia events. Figure S6. Forest plot for vasopressin or its analogues on diarrhea. Figure S7. Forest plot for vasopressin or its analogues on cerebrovascular events. Figure S8. Forest plot for vasopressin or its analogues on hyponatremia. Figure S7. Funnel plot for publication bias. (DOCX 554 kb)
Acknowledgements
The authors thank Yongan Xu, Emergency department, the second affiliated hospital, Zhejiang University School of Medicine.
Funding
Zhejiang medical and health science and technology project (2017ky372) and Hangzhou yuhang district medical and health research key subject construction (2016009) supported this work.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article and its supplementary information files.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- ICU
Intensive care unit
- MD
Mean difference
- NE
Norepinephrine
- RR
Relative risk
- TSA
Trial sequential analysis
Authors’ contributions
YS and LBJ conceived of and designed the study. XF and JW did the literature search, selection, and data extraction. LBJ, XF, and YS did quality evaluation. LBJ and JW did analyze and interpreted the data. YS and LBJ drafted or revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Libing Jiang, Phone: +086057187783921, Email: 2515168@zju.edu.cn.
Yi Sheng, Email: sshengyi@126.com.
Xia Feng, Email: f405776789@163.com.
Jing Wu, Email: 21618157@zju.edu.cn.
References
- 1.Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, Caixeta N, Salomao R, Angus DC, Pontes Azevedo LC. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis. 2017;17(11):1180–1189. doi: 10.1016/S1473-3099(17)30322-5. [DOI] [PubMed] [Google Scholar]
- 2.SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42(12):1980-9. [DOI] [PubMed]
- 3.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 4.Scheeren TWL, Bakker J, De Backer D, Annane D, Asfar P, Boerma EC, Cecconi M, Dubin A, Dunser MW, Duranteau J, et al. Current use of vasopressors in septic shock. Ann Intensive Care. 2019;9(1):20. [DOI] [PMC free article] [PubMed]
- 5.Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41(1):143–150. doi: 10.1097/CCM.0b013e318265ea64. [DOI] [PubMed] [Google Scholar]
- 6.Russell JA. Vasopressin in vasodilatory and septic shock. Curr Opin Crit Care. 2007;13(4):383-91. [DOI] [PubMed]
- 7.Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31(6):1752–1758. doi: 10.1097/01.CCM.0000063046.82359.4A. [DOI] [PubMed] [Google Scholar]
- 8.Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, DAlessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122–1125. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 9.De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 10.Hammond DA, Ficek OA, Painter JT, McCain K, Cullen J, Brotherton AL, Kakkera K, Chopra D, Meena N. Prospective open-label trial of early concomitant vasopressin and norepinephrine therapy versus initial norepinephrine monotherapy in septic shock. Pharmacotherapy. 2018;38(5):531–538. doi: 10.1002/phar.2105. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 12.Capoletto C, Almeida J, Ferrari G, Fukushima J, Nakamura R, Risk S, Osawa E, Park C, Oliveira G, Galas F, et al. 37th International Symposium on Intensive Care and Emergency Medicine (part 2 of 3) abstracts. Critical Care. 2017;21(Suppl 1):P168
- 13.Acevedo JG, Fernandez J, Escorsell A, Mas A, Gines P, Arroyo V. Clinical efficacy and safety of terlipressin administration in cirrhotic patients with septic shock. J Hepatol. 2009;50:S73. [Google Scholar]
- 14.Han XD, Sun H, Huang XY, Zhang SY, Wang YD, Ren K, Li F. A clinical study of pituitrin versus norepinephrine in the treatment of patients with septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012;24(1):33–37. [PubMed] [Google Scholar]
- 15.Chen Z, Zhou P, Lu Y, Yang C. Comparison of effect of norepinephrine and terlipressin on patients with ARDS combined with septic shock: a prospective single-blind randomized controlled trial. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29(2):111–116. doi: 10.3760/cma.j.issn.2095-4352.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Morelli A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Cecchini V, Bachetoni A, D'Alessandro M, Van Aken H, Pietropaoli P, et al. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Crit Care. 2009;13(4):R130. doi: 10.1186/cc7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira S, Dessa F, Rocha C, Oliveira F. Early vasopressin application in shock study. Crit Care. 2014;18(1):P158. [Google Scholar]
- 18.Prakash V, Choudhury AK, Sarin SK. Early introduction of a combination of low dose terlipressin and noradrenaline as vasopressors is superior to hig dose noradrenaline alone in patients of cirrhosis with septic shock ( NCT02468063). The 68th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting 2017. Hepatology. 2017;66(S1):1–1185. doi: 10.1002/hep.29500. [DOI] [PubMed] [Google Scholar]
- 19.Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, Pogson DG, Aya HD, Anjum A, Frazier GJ, et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock the VANISH randomized clinical trial. J Am Med Assoc. 2016;316(5):509–518. doi: 10.1001/jama.2016.10485. [DOI] [PubMed] [Google Scholar]
- 20.Malay MB, Ashton RC, Jr, Landry DW, Townsend RN. Low-dose vasopressin in the treatment of vasodilatory septic shock. J Trauma. 1999;47(4):699–703. doi: 10.1097/00005373-199910000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Clem O, Painter J, Cullen J, McCain K, Kakkera K, Meena N, Hammond D. Norepinephrine and vasopressin vs norepinephrine alone for septic shock randomized controlled trial. Crit Care Med. 2016;44(12):413.
- 22.Choudhury A, Kedarisetty CK, Vashishtha C, Saini D, Kumar S, Maiwall R, Sharma MK, Bhadoria AS, Kumar G, Joshi YK, et al. A randomized trial comparing terlipressin and noradrenaline in patients with cirrhosis and septic shock. Liver Int. 2017;37(4):552–561. doi: 10.1111/liv.13252. [DOI] [PubMed] [Google Scholar]
- 23.Russell JA, Vincent JL, Kjolbye AL, Olsson H, Blemings A, Spapen H, Carl P, Laterre PF, Grundemar L. Selepressin, a novel selective vasopressin V1A agonist, is an effective substitute for norepinephrine in a phase IIa randomized, placebo-controlled trial in septic shock patients. Critical care (London, England) 2017;21(1):213. doi: 10.1186/s13054-017-1798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua F, Wang X, Zhu L. Terlipressin decreases vascular endothelial growth factor expression and improves oxygenation in patients with acute respiratory distress syndrome and shock. The Journal of emergency medicine. 2013;44(2):434–439. doi: 10.1016/j.jemermed.2012.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svoboda P, Scheer P, Kantorova I, Doubek J, Dudra J, Radvan M, Radvanova J. Terlipressin in the treatment of late phase catecholamine-resistant septic shock. Hepato-gastroenterology. 2012;59(116):1043–1047. doi: 10.5754/hge10550. [DOI] [PubMed] [Google Scholar]
- 26.Albanese J, Leone M, Delmas A, Martin C. Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit Care Med. 2005;33(9):1897–1902. doi: 10.1097/01.ccm.0000178182.37639.d6. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZM, Chen J, Kou Q, Lin Q, Huang X, Tang Z, Kang Y, Li K, Zhou L, Song Q, et al. Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med. 2018;44(11):1816-25. [DOI] [PubMed]
- 28.Barzegar E, Ahmadi A, Mousavi S, Nouri M, Mojtahedzadeh M. The therapeutic role of vasopressin on improving lactate clearance during and after vasogenic shock: microcirculation, is it the black box? Acta medica Iranica. 2016;54(1):15–23. [PubMed] [Google Scholar]
- 29.Fonseca-Ruiz N, Lemos Cano A, Ortiz Carmona PD, Correa Aguirre M, Gómez Hernández PM, Osorio García C, Cuesta D, Molina Saldarriaga JF. Uso de vasopresina en pacientes con choque séptico refractario a catecolaminas. Acta Colombiana de Cuidado Intensivo. 2013;13(2):114-23.
- 30.Lauzier F, Levy B, Lamarre P, Lesur O. Vasopressin or norepinephrine in early hyperdynamic septic shock: a randomized clinical trial. Intensive Care Med. 2006;32(11):1782–1789. doi: 10.1007/s00134-006-0378-0. [DOI] [PubMed] [Google Scholar]
- 31.Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre WF, Um KJ, Alhazzani W, Lengyel AP, Hajjar L, Gordon AC, Lamontagne F, Healey JS, Whitlock RP, Belley-Cote EP. Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: a systematic review and meta-analysis. JAMA. 2018;319(18):1889–1900. doi: 10.1001/jama.2018.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Crit Care Med. 2000;28(8):2758–2765. doi: 10.1097/00003246-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, Perez P, Meziani F. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med. 2010;36(12):2019–2029. doi: 10.1007/s00134-010-2045-8. [DOI] [PubMed] [Google Scholar]
- 35.Schmittinger CA, Torgersen C, Luckner G, Schroder DCH, Lorenz I, Dunser MW. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 2012;38(6):950–958. doi: 10.1007/s00134-012-2531-2. [DOI] [PubMed] [Google Scholar]
- 36.Ba ZF, Chaudry IH. Role of estrogen receptor subtypes in estrogen-induced organ-specific vasorelaxation after trauma-hemorrhage. Am J Phys Heart Circ Phys. 2008;295(5):H2061–H2067. doi: 10.1152/ajpheart.00707.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell JA. Bench-to-bedside review: vasopressin in the management of septic shock. Critical Care (London, England) 2011;15(4):226. doi: 10.1186/cc8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang G, Li T, Xu J, Liu L. PKC plays an important mediated effect in arginine vasopressin induced restoration of vascular responsiveness and calcium sensitization following hemorrhagic shock in rats. Eur J Pharmacol. 2010;628(1–3):148–154. doi: 10.1016/j.ejphar.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien A, Clapp L, Singer M. Terlipressin for norepinephrine-resistant septic shock. Lancet (London, England) 2002;359(9313):1209–1210. doi: 10.1016/S0140-6736(02)08225-9. [DOI] [PubMed] [Google Scholar]
- 40.Kopel T, Losser MR, Faivre V, Payen D. Systemic and hepatosplanchnic macro- and microcirculatory dose response to arginine vasopressin in endotoxic rabbits. Intensive Care Med. 2008;34(7):1313–1320. doi: 10.1007/s00134-008-1058-z. [DOI] [PubMed] [Google Scholar]
- 41.Yang G, Liu L, Xu J, Li T. Effect of arginine vasopressin on vascular reactivity and calcium sensitivity after hemorrhagic shock in rats and its relationship to Rho-kinase. J Trauma. 2006;61(6):1336–1342. doi: 10.1097/01.ta.0000197928.99745.22. [DOI] [PubMed] [Google Scholar]
- 42.Holmes CL, Walley KR. Vasopressin in the ICU. Curr Opin Crit Care. 2004;10(6):442–448. doi: 10.1097/01.ccx.0000144769.19213.0c. [DOI] [PubMed] [Google Scholar]
- 43.Morelli A, Ertmer C, Pietropaoli P, Westphal M. Terlipressin: a promising vasoactive agent in hemodynamic support of septic shock. Expert Opin Pharmacother. 2009;10(15):2569–2575. doi: 10.1517/14656560903257808. [DOI] [PubMed] [Google Scholar]
- 44.Lange M, Ertmer C, Westphal M. Vasopressin vs. terlipressin in the treatment of cardiovascular failure in sepsis. Intensive Care Med. 2008;34(5):821–832. doi: 10.1007/s00134-007-0946-y. [DOI] [PubMed] [Google Scholar]
- 45.Asfar P, Hauser B, Ivanyi Z, Ehrmann U, Kick J, Albicini M, Vogt J, Wachter U, Bruckner UB, Radermacher P, et al. Low-dose terlipressin during long-term hyperdynamic porcine endotoxemia: effects on hepatosplanchnic perfusion, oxygen exchange, and metabolism. Crit Care Med. 2005;33(2):373–380. doi: 10.1097/01.ccm.0000152253.45901.fb. [DOI] [PubMed] [Google Scholar]
- 46.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 47.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 48.Boucheix O, Blakytny R, Haroutunian G, Henriksson M, Laporte R, Milano S, Reinheimer TM. Selepressin and arginine vasopressin do not display cardiovascular risk in atherosclerotic rabbit. PLoS One. 2016;11(10):e0165422. doi: 10.1371/journal.pone.0165422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saad AF, Maybauer MO. The role of vasopressin and the vasopressin type V1a receptor agonist selepressin in septic shock. J Crit Care. 2017;40:41–45. doi: 10.1016/j.jcrc.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Belletti A, Musu M, Silvetti S, Saleh O, Pasin L, Monaco F, Hajjar LA, Fominskiy E, Finco G, Zangrillo A, et al. Non-adrenergic vasopressors in patients with or at risk for vasodilatory shock. A systematic review and meta-analysis of randomized trials. PloS one. 2015;10(11):e0142605. doi: 10.1371/journal.pone.0142605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polito A, Parisini E, Ricci Z, Picardo S, Annane D. Vasopressin for treatment of vasodilatory shock: an ESICM systematic review and meta-analysis. Intensive Care Med. 2012;38(1):9–19. doi: 10.1007/s00134-011-2407-x. [DOI] [PubMed] [Google Scholar]
- 52.Serpa Neto A, Nassar AP, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Damasceno MC, Russell JA. Vasopressin and terlipressin in adult vasodilatory shock: a systematic review and meta-analysis of nine randomized controlled trials. Crit Care. 2012;16(4):R154. doi: 10.1186/cc11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagendran M, Maruthappu M, Gordon AC, Gurusamy KS. Comparative safety and efficacy of vasopressors for mortality in septic shock: a network meta-analysis. J Intensive Care Soc. 2016;17(2):136–145. doi: 10.1177/1751143715620203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan JX, Chen H, Chen XY, Zhang D, He FM. Vasopressin and its analog terlipressin versus norepinephrine in the treatment of septic shock: a meta-analysis. Int J Clin Exp Med. 2016;9(7):14183–14190. [Google Scholar]
- 55.Klinzing S, Simon M, Reinhart K, Bredle DL, Meier-Hellmann A. High-dose vasopressin is not superior to norepinephrine in septic shock. Crit Care Med. 2003;31(11):2646–2650. doi: 10.1097/01.CCM.0000094260.05266.F4. [DOI] [PubMed] [Google Scholar]
- 56.Gordon AC, Wang N, Walley KR, Ashby D, Russell JA. The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest. 2012;142(3):593–605. doi: 10.1378/chest.11-2604. [DOI] [PubMed] [Google Scholar]
- 57.Gordon AC, Russell JA, Walley KR, Singer J, Ayers D, Storms MM, Holmes CL, Hebert PC, Cooper DJ, Mehta S, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36(1):83–91. doi: 10.1007/s00134-009-1687-x. [DOI] [PubMed] [Google Scholar]
- 58.Sacha GL, Lam SW, Duggal A, Torbic H, Bass SN, Welch SC, Butler RS, Bauer SR. Predictors of response to fixed-dose vasopressin in adult patients with septic shock. Ann Intensive Care. 2018;8(1):35. doi: 10.1186/s13613-018-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nascente AP, Freitas FG, Bakker J, Bafi AT, Ladeira RT, Azevedo LC, Lima A, Machado FR. Microcirculation improvement after short-term infusion of vasopressin in septic shock is dependent on noradrenaline. Clinics. 2017;72(12):750–757. doi: 10.6061/clinics/2017(12)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell JA, Walley KR, Gordon AC, Cooper DJ, Hebert PC, Singer J, Holmes CL, Mehta S, Granton JT, Storms MM, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med. 2009;37(3):811–818. doi: 10.1097/CCM.0b013e3181961ace. [DOI] [PubMed] [Google Scholar]
- 61.Hammond DA, Cullen J, Painter JT, McCain K, Clem OA, Brotherton AL, Chopra D, Meena N: Efficacy and safety of the early addition of vasopressin to norepinephrine in septic shock. J Intensive Care Med 2017:885066617725255. [DOI] [PubMed]
- 62.Reardon DP, DeGrado JR, Anger KE, Szumita PM. Early vasopressin reduces incidence of new onset arrhythmias. J Crit Care. 2014;29(4):482–485. doi: 10.1016/j.jcrc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Bihari D, Prakash S, Bersten A. Low-dose vasopressin in addition to noradrenaline may lead to faster resolution of organ failure in patients with severe sepsis/septic shock. Anaesth Intensive Care. 2014;42(5):671–674. [PubMed] [Google Scholar]
- 64.Wu JY, Stollings JL, Wheeler AP, Semler MW, Rice TW. Efficacy and outcomes after vasopressin guideline implementation in septic shock. Ann Pharmacother. 2017;51(1):13–20. doi: 10.1177/1060028016669163. [DOI] [PubMed] [Google Scholar]
- 65.Musallam N, Altshuler D, Merchan C, Zakhary B, Aberle C, Papadopoulos J. Evaluating vasopressor discontinuation strategies in patients with septic shock on concomitant norepinephrine and vasopressin infusions. Ann Pharmacother. 2018;52(8):733–739. doi: 10.1177/1060028018765187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study search strategy. Table S2. Information on excluded studies. Table S3. List of Ongoing studies. Table S4. GRADE. Figure S1. Risk of bias summary. Figure S2. Risk of bias graph. Figure S3. Forest plot for vasopressin or its analogues on cardiovascular events. Figure S4. Forest plot for vasopressin or its analogues on arrhythmia. Figure S5. Forest plot for vasopressin or its analogues on mesenteric ischemia events. Figure S6. Forest plot for vasopressin or its analogues on diarrhea. Figure S7. Forest plot for vasopressin or its analogues on cerebrovascular events. Figure S8. Forest plot for vasopressin or its analogues on hyponatremia. Figure S7. Funnel plot for publication bias. (DOCX 554 kb)
Data Availability Statement
All data generated or analyzed during the present study are included in this published article and its supplementary information files.