Abstract
Background
Intramuscular adipose tissue (IMAT) is a feature of degenerative muscle composition and is a common feature in populations with chronic low back pain (CLBP). Avoidance behavior is a possible cause of morphological muscle composition due to disuse of the paraspinal muscles. Therefore it is of clinical interest to determine the association between fear-avoidance beliefs and IMAT of the paraspinal muscles in populations with CLBP.
Methods
In this cross-sectional study, we examined twenty-four adults, featuring a mean age of 48.63 years (SD ± 14.73), with CLBP. Axial T2-weighted Magnetic Resonance Imaging (MRI) images were selected on the same level as the intervertebral disc of segments L4-L5 and L5-S1. After determine the region of interest, the amount of IMAT was measured by an automatic-threshold method to distinguish fat from muscle tissue. Fear-avoidance beliefs were measured with the Fear-Avoidance Beliefs Questionnaire, with regard to Physical Activity (FABQ-PA). Bivariate correlation and multiple regression analysis were used to determine the association between IMAT of the paraspinal muscles and fear-avoidance beliefs.
Results
There is a significant bivariate association between the FABQ-PA and ES IMAT (r = 0.484, P = 0.017), but not for LMM (r = 0.228, P = 0.284). The association between the FABQ-PA and ES IMAT remained moderate after adjusting for covariates (β = 0.381, P = 0.028).
Conclusion
Fear-avoidance beliefs are moderately associated with ES IMAT and poorly associated with LMM IMAT in a population with CLBP. Results should be interpreted with caution due to a small and selected study population.
Keywords: Adipose tissue, Chronic low back pain, Avoidance behavior, MRI
Background
Low back pain (LBP) is a common musculoskeletal condition that affects up to 85% of the general population during their lives [1], with a high recurrence risk of 44 to 78% [2]. Chronic low back pain (CLBP) is considered to be a multifactorial condition due to a constant interaction between physical (levels of conditioning and loading exposures), psychological (stress, cognition and emotion) and social systems (culture, work, home and environment) [3].
One of the anatomical biomarkers, as part of the physical system in people with CLBP is the presence of a degenerative muscle composition in the paraspinal muscles [4–8]. Degenerative muscle composition is characterized by a decrease in the cross-sectional area (CSA) of the muscle [4, 5], and an increase of fatty infiltration, also known as “intramuscular adipose tissue” (IMAT) [6–8]. The burden of proof for the CSA as a surrogate for degenerative muscle composition is inconclusive and contradictory. In some studies, a decrease in the CSA of the paraspinal muscles is associated with LBP [9, 10], but other studies disagree [11–13]. Shahidi et al. [14] show that paraspinal muscle tissue changes are more complex than atrophy alone. IMAT appears to be a better representation of degenerative muscle composition and is a feature of decreased muscle structure and quality. In addition to IMAT, a slow-to-fast muscle fiber transformation [11], high levels of inflammation and decreased vascularity [14] are also described as features of structural remodeling of degenerative muscle tissue.
Several studies have shown an association between IMAT of the paraspinal muscles and CLBP [6]. For example, more IMAT was found in the lumbar multifidus (LMM) of patients with CLBP (23.6%), compared to people without complaints (14.5%) [15]. In addition, significantly more IMAT was found in the paraspinal muscles of patients with CLBP compared to acute LBP [16].
One of the possible explanations for the accumulation of IMAT in people with CLBP is altered individual-specific motor behaviors of paraspinal muscles [17]. It is known that IMAT is associated with decreased muscular metabolic activity [18, 19], which can lead to a reduction of intramuscular mitochondrial oxygenation of glucose, thus increasing insulin resistance and intramuscular triacylglycerol levels [20]. Intramuscular triacylglycerol levels can lead to alternative communication (adipose muscle crosstalk) between satellite cells and macrophages, where myogenic adult stem cells differentiate to fat cells instead of myocells [21], with negative consequences for the development of new sarcomeres [22, 23], thus leading to a degenerative muscle composition.
Avoidance behavior is a highly associated with pain-related factors contributing to muscle inhibition [24], and altered individual-specific motor behaviors of paraspinal muscles [17], because people with negative cognitions about pain can be afraid to move when a pain experience is considered to be dangerous [25, 26]. These pain-related factors are highly associated with a poor treatment outcome in patients with CLBP [27].
From this clinical perspective, an association between avoidance behavior and IMAT is of clinical interest because it could bear consequences for the choice of treatment and exercise intensity in people with CLBP. For example, low-intensity exercises like motor control rehabilitation presumably have a low impact on paraspinal metabolic activity and muscle quality [28, 29], when pain-related avoidance beliefs are present. It has however not yet been investigated whether avoidance behavior is associated with IMAT in a population with CLBP. Hence the research question is: What is the association between fear-avoidance beliefs and IMAT of the paraspinal muscles in patients with CLBP?
Methods
This cross-sectional study was conducted in an independent and specialized centre for Magnetic Resonance Imaging (MRI) diagnostics. MRI images were generated from participants who were referred for medical diagnostic research due to their CLBP and were blinded to MRI-findings when completing the questionnaires. The participants did not perform any additional actions for this study and consented to the use of anonymized personal information through informed consent. The Institutional Review Board (department of health studies) of HU University of Applied Sciences Utrecht approved the study protocol, reference number: 35_010_2016.
Participants
Patients met the inclusion criteria if they were 18 years or older and had experienced (chronic) LBP lasting longer than 12 weeks. If there was a history of lumbar surgery, neurological disorders, spinal deformities, and recent traumatic incidents, patients were excluded from participation. MRI images with fat suppression were considered unsuitable and are excluded from this study.
Procedure imaging
Low-field Tesla 1.5 MRI images (GE Medical Systems, USA, Siemens Healthcare, Erlangen, Germany) are used with a repetition time of 4110–4400 milliseconds (ms) and an echo time of 132 ms. The slice thickness was 3 mm (mm) and the field of view 200 × 168.6 mm. The patients were placed supine with their hips and knees slightly bent (30 degrees) to maintain a neutral position of the lumbar vertebral column. The transverse recordings were selected at the intervertebral disc of L4-L5 and L5-S1 using T2-weighted sagittal images of the lumbar spine.
IMAT
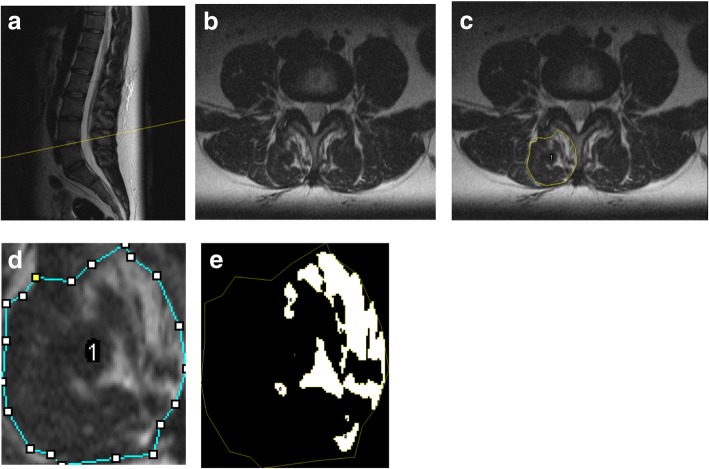
MRI data was analyzed using ImageJ 1.50i (Java-based version, public domain NIH Image Software; Research Services Branch). The T2-weighted transversal images were converted to an 8-bit gray scale. The CSA of the Erector Spinae (ES) and the LMM were bilaterally outlined at each level and were defined as the TROI. Anatomical cross-references of the LMM and ES muscles are used as proposed by Crawford et al. [30] (Fig. 1). Fatty substances between the aponeurosis of the ES and the posterior layer of the fascia thoracolumbalis, the ‘fatty tent’ between the dorsal aponeurosis of the iliocostalis and longissimus, and intrafascial triangles were not included and are considered as extramuscular fat [30]. After cropping the region of interest, the amount of IMAT was measured by an automatic-threshold method to distinguish fat from muscle tissue using a histogram (Fig. 2) [31]. The extent of IMAT was calculated by dividing the Functional Region of Interest (FROI) relative to the Total Region of Interest (TROI) by the following formula: %IMAT = FROI / TROI × 100%. This methodology is widely accepted, and features high intra-rating reliability (ICC > 0.75) [32], and concurrent validity with phantom images [33].
Fig. 1.

Anatomical cross-references used in this study. Abbreviations: IVD intervertebral disc, FT fatty tent, ES erector spinae, ST spinotransversal muscles (lumbar multifidus), QL quadratus lumborum, PM psoas major, SPC spinal canal, ZJ zygapophyseal joint, TLF fascia thoracolumbalis, IFT intrafascial triangle, ESA, aponeurosis of the ES. Orange polygonal circumference measurement = total region of interest lumbar multifidus; purple polygonal circumference measurement = total region of interest erector spinae, blue polygonal circumference measurement = quadratus lumborum, green polygonal circumference measurement = psoas major
Fig. 2.
Sagittal T2-weighted images (2a), with a selection of transverse to L4-L5 intervertebral disc, (2b), with a polygonal circumference measurement of the lumbar multifidus (2c), cropping image (2d) and automatic segmentation (2e)
Pain-related fear-avoidance beliefs
Levels of fear-avoidance beliefs were determined by the Fear-Avoidance Beliefs Questionnaire (FABQ). The FABQ consisted of 16 questions where the answers varied between 0 (disagree) to 6 (fully agree). Only the subdomain Physical Activity (FABQ-PA), the total sum ranging from 0 to 24 points, has been used in this study. The total score is the sum of items 2 to 5, with a higher score representing a greater degree of pain-related fear-avoidance beliefs [34]. A score of 14 points or higher indicates the presence of pain-related fear-avoidance beliefs. The FABQ-PA has a good inter-rater reliability of ICC = 0.90 [34], and an adequate concurrent validity with the Tampa Scale for Kinesiophobia, r = 0.62 in people with CLBP [34].
Potential confounders
A strong association has been found between IMAT and disc degeneration [35–37], age [38–40], extent of physical activity [36], degree of pain [7], and gender [41] and have been included in this research as potential confounders.
Self-reported questionnaires
Pain intensity was measured using a numeric pain-rating scale (NPRS). The participants assessed their average pain for the week preceding the MRI on a scale of 0 (no pain) to 10 (most severe pain). The NPRS has been proven as a valid and reliable measurement tool for measuring pain intensity [42]. Physical activity was measured by asking the participants how many hours a week they participated in sporting activities on average, for the four weeks prior to the MRI.
Disc degeneration
Intervertebral degenerative changes of the disc were determined by T2-weighted sagittal images of the lumbar spine, as they provide a comprehensive impression of the disc structure [43]. The modified Pfirrmann classification of Griffith et al. [44] was used to determine the degree of disc degeneration, ranging from 1 (no degeneration) to 8 (final stage of degeneration). This research method has been proven as reliable [44].
Statistical analysis
A statistical analysis was performed using IBM SPSS Statistics 20.0. Descriptive statistics were tested together with the Shapiro-Wilk test to determine the distribution of data. The average difference in IMAT between the LMM and the ES was tested by the paired samples t-test. A bivariate correlation matrix was used to assess the independent relationship between IMAT and FABQ-PA, as well as the potential covariates. A Pearson correlation coefficient (r) was used when the bivariate association met the assumption of the parametric test [45]. A non-parametric test (Spearman’s Rho) was used when the bivariate association did not meet the assumptions of the parametric test. The bivariate association between gender and IMAT was tested by a point biserial coefficient (rpb).
A multiple regression analysis was used to determine if IMAT associated with FABQ-PA after correcting for the variance due to the covariates. At first, IMAT and FABQ-PA were entered in the regression model. Next, covariates with a significant relationship (P < 0.10) with IMAT were all entered simultaneously in the second step by a enter method. The standardized coefficients (βeta) were calculated for the association between IMAT (LMM and ES separately) and the FABQ-PA after they were corrected for covariates and are considered significant at P < 0.05. An analysis of variance (ANOVA) was used to determine whether the regression model explained a significant proportion of the statistical variance.
Results
Demographic characteristics
Demographic characteristics of the sample are shown in Table 1. All descriptive statistics showed a normal distribution of data (Shapiro-Wilk P > 0.05). Twenty-four patients met the inclusion criteria, with a mean age of 48.63 years (SD ± 14.73). The average score on the FABQ-PA was 14.92 (SD ± 5.39), of which 70.8% scored higher than 14 points. LMM IMAT (22.03%, SD ± 11.88) was significantly higher (P = 0.046) than ES IMAT (17.72%, SD ± 11.66), with an average difference of 4.28%.
Table 1.
Demographic and clinical characteristics of participants
| Variable | Value |
|---|---|
| Age (SD) | 48.63 (14.73) |
| Females (%) | 13 (52) |
| Physical Activity (weekly) | |
| 0–2 h (%) | 8 (32%) |
| 2–5 h (%) | 6 (24%) |
| 5–10 h (%) | 7 (28%) |
| > 10 h (%) | 3 (12%) |
| NPRS (SD) | 5.58 (1.91) |
| FABQ-PA (SD) | 14.92 (5.39) |
| LMM IMAT (SD) | 22.03% (11.88) |
| ES IMAT (SD) | 17.72% (11.66) |
Abbreviations: FABQ-PA Fear-Avoidance Beliefs Questionnaire subdomain Physical Activity, LMM Lumbar Multifidus, ES Erector Spinae, IMAT Intramuscular Adipose Tissue, NPRS Numeric Pain Rating Scale, SD standard deviation
NOTE. Values are presented as mean ± SD or %. N = 24
Bivariate correlation analysis
Table 2 shows the bivariate correlation matrix. Data met the full assumption for parametric testing. There was a significant association between FABQ-PA and ES IMAT (r = 0.484, P = 0.017), but not the LMM (r = 0.228, p = 0.284). LMM IMAT was found to be significantly associated with the age (r = 0.678, p < 0.001), the extent of disc degeneration on L4-L5 (r = 0.515, P = 0.010) and L5-S1 (r = 0.477, P = 0.018). LMM IMAT was not significantly associated with, gender, physical activity and pain intensity (P > 0.1). ES IMAT also showed a significant relationship with age (r = 0.472, p = 0.020), disc degeneration of L4-L5 (r = 0.480, p = 0.018) and approximate statistical significance for disc degeneration on L5-S1 (r = 0.336, p = 0.109) and physical activity (r = − 0.350, p = 0.094). No significant association has been found for ES IMAT with gender and pain intensity (P > 0.1).
Table 2.
Bivariate correlations between the lumbar IMAT infiltration and gender, age, physical activity, NPRS mean, disc degeneration and fear-avoidance beliefs
| LMM IMAT | ES IMAT | ||
|---|---|---|---|
| Gender | r pb | −0.103 | 0.088 |
| p | 0.630 | 0.683 | |
| Age | r | 0.678 | 0.472 |
| p | < 0.001 | 0.020 | |
| Physical activity | r | −0.205 | −0.350 |
| p | 0.337 | 0.094 | |
| NPRS mean | r | 0.075 | −0.188 |
| p | 0.728 | 0.379 | |
| Disc degeneration L4-L5 | r | 0.515 | 0.480 |
| p | 0.010 | 0.018 | |
| Disc degeneration L5-S1 | r | 0.477 | 0.336 |
| p | 0.018 | 0.109 | |
| FABQ-PA | r | 0.228 | 0.484 |
| p | 0.284 | 0.017 | |
Abbreviations: NPRS Numeric Pain Rating Scale, FABQ-PA Fear-Avoidance Beliefs Questionnaire subdomain Physical Activity, IMAT intramuscular adipose tissue, LMM Lumbar Multifidus, ES Erector Spinae
rpb = Point biserial coefficient
Multiple regression analysis
Tables 3 and 4 shows the multiple regression analysis. The significant explained variance of ES IMAT, physical activity, age and disc degeneration of L4-L5 (model 2) was 55.5% (ANOVA P = 0.003). After adjusting for confounders, the association between the FABQ-PA and ES IMAT was moderate (β 0.381, P = 0.028). The significant explained variance of LMM IMAT, disc degeneration and age (model 2) was 54.5% (ANOVA P = 0.003). The association between the FABQ-PA and LMM IMAT was poor (β 0.168, P = 0.307) after adjusting for confounders.
Table 3.
Multiple regression analysis IMAT ES L4-L5 & L5-S1
| Variable | Unstandardized coefficients | Standardized coefficients | P-value | |||
|---|---|---|---|---|---|---|
| B | 95% CI | βeta | ||||
| Lower | Upper | |||||
| M1 | (Constant) FABQ-PA |
3.526 0.952 |
−8.655 0.191 |
15.707 1.713 |
0.484 | 0.554 0.017 |
| M2 | (Constant) FABQ-PA DD L4 Age PA |
−9.985 0.750 0.679 0.354 −3.355 |
−26.748 0.092 −1.817 0.040 −7.126 |
6.778 1.409 3.175 0.668 0.416 |
0.381 0.110 0.447 −0.305 |
0.228 0.028 0.576 0.029 0.078 |
Abbreviations; M Model, IMAT Intramuscular Adipose Tissue, PA Physical Activity, DD Disc Degeneration, FABQ-PA Fear-avoidance beliefs questionnaire subdomain physical activity, ES Erector Spinae
Table 4.
Multiple regression analysis IMAT LMM L4-L5 & L5-S1
| Variable | Unstandardized coefficients | Standardized coefficients | P-value | |||
|---|---|---|---|---|---|---|
| B | 95% CI | βeta | ||||
| Lower | Upper | |||||
| M1 | (Constant) FABQ-PA |
15.196 0.456 |
1.394 −0.406 |
28.998 1.319 |
0.228 | 0.032 0.284 |
| M2 | (Constant) FABQ-P Age DD L4 DD L5 |
−13.341 0.336 0.430 0.766 1.003 |
−30.157 −0.334 0.104 − 1.777 − 1.333 |
3.475 1.006 0.756 3.309 3.140 |
0.168 0.533 0.122 0.174 |
0.362 0.307 0.012 0.536 0.338 |
Abbreviations; M Model, IMAT Intramuscular Adipose Tissue, DD Disc Degeneration, FABQ-PA Fear-avoidance beliefs questionnaire subdomain physical activity, LMM Lumbar Multifidus
Discussion
This study has shown a moderate relationship between pain-related fear-avoidance beliefs and ES IMAT. Avoidance behavior can be seen as a common-sense response to dealing with LBP when people have negative thoughts about their pain [24, 46], which are associated with altered motor patterns of paraspinal muscles [17, 47] and reduced physical activity [48]. One added value of our results could be its utility as additional evidence showing why avoidance behavior is not a recommended approach in CLBP.
A small association has been found between fear-avoidance beliefs and LMM IMAT, in contrast with ES IMAT. This difference may be due to different substitution patterns of the LMM as compared to other spinal muscles, which are caused by a long-loop inhibition through the medial ramus of the dorsal radix and are probably more related to pain-induced vertebral pathology [49]. Shahidi et al. [39] noted that the lumbar multifidus degenerates in people with chronic degenerative lumbar spine pathology. This corresponds with our findings, where LMM IMAT is highly associated with age (r = 0.678, P < 0.001) and disc degeneration (L4-L5 r = 0.515, P = 0.010 and L5-S1 r = 0.477, P = 0.018). In addition, rapid atrophy of the LMM has been noted within 3 days after intervertebral lesions in a porcine model [50], or pain onset in humans [9]. After pain onset, pain-induced reflex inhibitory mechanisms and disturbance in coordination are also noted in the LMM [51]. Nevertheless, we know that muscle tissue changes are more complex than atrophy alone in people with CLBP [13], and therefore a clear explanation for the underlying mechanisms of different outcomes in ES IMAT and LMM IMAT remains hypothetical.
Degenerative muscle composition with a high fat content increases with age, appears to progress faster in the LMM (0.24% per year) than the ES (0.13% per year) and corresponds to findings from this study [38]. The standardized coefficient (βeta) in the final model of the multiple regression analysis was higher for LMM IMAT (β = 0.533) and less so for ES IMAT (β = 0.447).
People with CLBP demonstrate altered individual-specific motor (control) behaviors [17, 47] and show discrete loss of cortical organization of inputs to paraspinal muscles which could lead to differential central activation [52, 53]. In this case, motor rehabilitation is suggested as a possible effective treatment to restore optimal control in LBP [54]. To restore full motor control of the lower back, it is doubtful whether low-intensity exercises like motor control rehabilitation alone is effective to improve paraspinal muscle function when high levels of intramuscular fat are present. We think that ES IMAT could be an adverse secondary consequence of avoidance behavior due to altered individual specific motor patterns [17, 55], with reduced muscular metabolic activity [18, 19]. From this perspective, cognition-targeted motor control training combined with pain neuroscience education could be a more appropriate intervention to improve muscle function, also because it has been proven more effective than current best-evidence physiotherapy for improving physical function and pain cognitions in people with CLBP [56]. However, in our study it cannot be determined whether the association of fatty infiltration of the paraspinal muscles and pain-related fear-avoidance beliefs is the cause or effect of one another. To demonstrate a causal relationship between pain-related fear-avoidance beliefs and IMAT of the paraspinal muscles, studies with a longitudinal design are recommended.
This study shows that the average LMM IMAT was 22.03%, and ES IMAT 17.72%. Other studies took diverse IMAT levels for both the LMM and ES [6, 57]. Besides population-based differences like age or levels of disability, such controversial results could be explained by differences in methodology (quantitative versus qualitative measurement methods, MRI versus computed tomography scan) [30, 31]. Despite discrepancies in methodology, histological studies demonstrated that IMAT observed by MRI strongly corresponds to histologic evidence of paraspinal morphology and is therefore considered a valid method for quantifying the degree of fatty infiltration [31]. Some studies have proven reliable at distinguishing fat from muscle tissue in conventional T1-weighted MRI images based on pixel intensity and histographic methodology [58, 59]. In order to compare different studies in future investigations, it is recommended to standardize threshold and segmentation procedures.
Study limitations
A limitation in this study is the use of T2-weighted spin echoes, which can be more difficult to correct due to other changes that may occur in and around the muscle [59]. Maillard et al. [60] describes a rapid extension of the T2 relaxation time of (muscle) inflammation to as much as 100 ms/s, which closely matches the T2 relaxation time of fat (130–150 ms/s) and may affect the validity of the measurement. This difference is larger in T1-weighted spin echoes and is therefore recommended for subsequent research to quantify the degree of fatty infiltration in conventional MRI images. However, the concurrent validity and reliability are shown to be excellent in T2-weighted images [32]. Either way, MRI-based methods as DIXON/IDEAL [61] seems to be most accurate to distinguish fat from muscle tissue than normal T1- or T2-weighted spin echoes and are therefore widely recommended for subsequent testing [62, 63]. Since imaging research in this population was used for usual care, and not for the benefit of this study, it was not possible to apply these methods.
In this study, the average age was 48.63 years (SD ± 15.29) and 70.08% of the included participants were classified with pain-related fear-avoidance beliefs (FABQ-PA > 14). These factors may have led to selection bias, and for this reason it may be difficult to generalize our results to an elderly population or population with chronic lower back pain without pain-related fear-avoidance beliefs. Larger epidemiological studies of the general population can provide valuable research data. In this study, the multiple regression analysis contained one outcome variable and three covariates with 24 included participants. This model did not fully meet the recommended limit of a multiple regression analysis of at least ten observations per variable, which may have lowered the validity of the analysis [64]. An investigation using a larger population is recommended in the future.
Conclusions
This study has shown a moderate association between pain-related fear-avoidance beliefs and IMAT in ES, but a poor association with LMM IMAT. The small and selected study sample may have lowered the validity of our results, and conclusions have to be interpreted with caution. More longitudinal research with a larger population is required to demonstrate a causal relationship. MRI-based methods like DIXON/IDEAL are recommended for further research.
Acknowledgements
The authors would like to acknowledge Adri Apeldoorn, PhD, Linda Heskamp, PhD Candidate, and Marc van de Berg, MFt, for their help in analyzing MRI and statistics. The authors declare no conflict of interest by performing this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets which are used during this research are available from the corresponding author on reasonable request. This material is the result of work supported with resources and the use of facilities at the MRI Centre Amsterdam, IJsbaanpad 10-B, 1076 CV Amsterdam, the Netherlands.
Suppliers
Low-field Tesla 1.5 MRI (GE Medical Systems, USA, Siemens Healthcare, Erlangen, Germany).
ImageJ 1.50i (Java-based version, public domain NIH Image Software; Research Services Branch).
IBM SPSS Statistics 20.0.
Abbreviations
- ANOVA
Analysis of variance
- CLBP
Chronic low back pain
- CSA
Cross-sectional area
- ES
Erector spinae
- FABQ-PA
Fear-avoidance beliefs questionnaire physical activity
- FROI
Functional region of interest
- IMAT
Intramuscular adipose tissue
- LBP
Low back pain
- LMM
Lumbar multifidus
- MRI
Magnetic resonance imaging
- ms
Milliseconds
- NPRS
Numeric pain rating scale
- ROI
Region of interest
- TROI
Total region of interest
Authors’ contributions
EW was the corresponding author for this research, designed the study and was responsible for analyzing MRI data. PP was a major contributor in designing the study and helped with analyzing MRI data. NK contributed to the statistical analysis. ER and JP were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The participants did not perform any additional actions for this study and consented to the use of anonymized personal information through informed consent. The Institutional Review Board (department of health studies) of HU University of Applied Sciences Utrecht approved the study protocol, reference number: 35_010_2016.
Consent for publication
Written informed consent was obtained from the participants for publication of their individual details and accompanying images in this manuscript. The consent form is held by the authors and is available for review by the Editor-in-Chief.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eddo Wesselink, Phone: +3138-3856999, Email: eddo_wesselink@msn.com.
Edwin de Raaij, Email: Edwin.deraaij@hu.nl.
Philip Pevenage, Email: Philip.pevenage@mricentrum.nl.
Nick van der Kaay, Email: Nick.vanderkaay@hu.nl.
Jan Pool, Email: Jan.pool@hu.nl.
References
- 1.Becker A, Held H, Redaelli M, Strauch K, Chenot JF, Leonhardt C, Keller S, Baum E, Pfingsten M, Hildebrandt J, Basler HD, Kochen MM, Donner-Banzhoff N. Low back pain in primary care: costs of care and prediction of future health care utilization. Spine (Phila Pa 1976) 2010;35:1714–1720. doi: 10.1097/BRS.0b013e3181cd656f. [DOI] [PubMed] [Google Scholar]
- 2.Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? A review of studies of general patient populations. Eur. Spine J. 2003;12:149–165. doi: 10.1007/s00586-002-0508-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’ Sullivan P, Caneiro JP, O’Keeffe M, O’Sullivan K. Unraveling the complexity of low back pain. J Orthop Sports Phys Ther. 2016;46(11):932–937. doi: 10.2519/jospt.2016.0609. [DOI] [PubMed] [Google Scholar]
- 4.Fortin M, Lazary A, Varga PP, McCall I, Battié MC. Paraspinal muscle asymmetry and fat infiltration in patients with symptomatic disc herniation. Eur Spine J. 2016;25(5):1452–1459. doi: 10.1007/s00586-016-4503-7. [DOI] [PubMed] [Google Scholar]
- 5.Ploumis A, Michailidis N, Christodoulou P, Kalatizoglou I, Gouvas G, Beris A. Ipsilateral atrophy of paraspinal and psoas muscle in unilateral back pain patients with monosegmental degenerative disc disease. Br J Radiol. 2011;84(1004):709–713. doi: 10.1259/bjr/58136533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician. 2016;19(7):985–1000. [PubMed] [Google Scholar]
- 7.Sions JM, Coyle PC, Velasco TO. Elliot JM, Hicks GE. Multifidi muscle characteristics and physical function among older adults with and without chronic low back pain. Arch Phys Med Rehabil. 2017;98(1):51–57. doi: 10.1016/j.apmr.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sions JM, Elliot JM, Pohlig RT, Hicks GE. Trunk muscle characteristics of the multifidi, erector spinae, psoas and Quadratus Lumborum in older adults with and without chronic low back pain. J Orthop Sports Phys Ther. 2017;47(3):173–179. doi: 10.2519/jospt.2017.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19(2):165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic low back pain and healthy asymptomatic subjects. Man Ther. 2008;13(1):43–49. doi: 10.1016/j.math.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Hodges PW, James G, Blomster L, Hall L, Schmid A, Shu C, Little C, Melrose J. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine (Phila Pa 1976) 2015;40(14):1057–1071. doi: 10.1097/BRS.0000000000000972.. [DOI] [PubMed] [Google Scholar]
- 12.D’hooge R, Cagnie B, Crombez G, Vanderstraeten G, Dolphens M, Danneels L. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man Ther. 2012;17(6):584–588. doi: 10.1016/j.math.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 13.D’hooge R, Cagnie B, Crombez G, Vanderstraeten G, Achten E, Danneels L. Lumbar muscle dysfunction during remission of unilateral recurrent nonspecific low-back pain: evaluation with muscle function MRI. Clin J Pain. 2013;29(3):187–194. doi: 10.1097/AJP.0b013e31824ed170. [DOI] [PubMed] [Google Scholar]
- 14.Shahidi B, Hubbard JC, Gibbons MC, Ruoss S, Zlomislic V, Allen RT, Garfin SR, et al. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res. 2017;35(12):2700–2706. doi: 10.1002/jor.23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mengiardi B, Schmid MR, Boos N, Pfirrmann CWA, Brunner F, Elfering A, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology. 2006;240(3):786–792. doi: 10.1148/radiol.2403050820. [DOI] [PubMed] [Google Scholar]
- 16.Wan Q, Lin C, Li X, Zeng W, Ma C. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol. 2015;88(1053). 10.1259/bjr.20140546. [DOI] [PMC free article] [PubMed]
- 17.Ross GB, Sheahan PJ, Mahoney B, Gurd BJ, Hodges PW, Graham RB. Pain catastrophizing moderates changes in spinal control in response to noxiously induced low back pain. Biomechanics. 2017;14(58):64–70. doi: 10.1016/j.jbiomech.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Goubert D, de Pauw R, Meeus M, Willems T, Cagnie B, Schouppe S, van Oosterwijck J, Dhondt E, Danneels L. Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross sectional study. Spine J. 2017;17(9):1285–1296. doi: 10.1016/j.spinee.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9(4):213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 20.Rivas DA, Mc Donald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging, but not obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):561–569. doi: 10.1152/ajpregu.00198.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agley CC, Rowlerson AM, Velloso CP, Lazarus NR, Harridge SD. Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. J Cell Sci. 2013;126:5610–5625. doi: 10.1242/jcs.132563. [DOI] [PubMed] [Google Scholar]
- 22.Farup J, Madaro L, Puri PL, Mikkelsen UR. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 2015;6:1830. doi: 10.1038/cddis.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vettor R, Milan G, Franzin C, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:987–998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 24.Verbunt JA, Seelen HA, Vlaeyen JW, Bousema EJ, van der Heijden GJ, Heuts PH, Knottnerus JA. Pain-related factors contributing to muscle inhibition in patients with chronic low back pain: an experimental investigation based on superimposed electrical stimulation. Clin J Pain. 2005;21(3):232–240. doi: 10.1097/00002508-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Bunzli S, Smith A, Schütze R, Lin L, O’Sullivan P. Making sense of low Back pain and pain-related fear. J Orthop Sports Phys Ther. 2017;47(9):628–636. doi: 10.2519/jospt.2017.7434. [DOI] [PubMed] [Google Scholar]
- 26.Hodges PW, Smeets RJ. Interaction between pain, movement, and physical activity: short-term benefits, long-term consequences, and targets for treatment. Clin J Pain. 2015;31(2):97–107. doi: 10.1097/AJP.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 27.Wertli MM, Rasmussen- Barr E, Held U, Weiser S, Bachmann LM, Brunner F. Fear-avoidance beliefs - a moderator of treatment efficacy in patients with low back pain: a systematic review. Spine J. 2014;14(11):2658–2678. doi: 10.1016/j.spinee.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Colado JC, Pablos C, Chulvi-Medrano I, Garcia-Masso X, Flandez J, Behm DG. The progression of paraspinal muscle recruitment intensity in localized an global strength training exercises is not based on instability alone. Arch Phys Med Rehabil. 2011;92(11):1875–1883. doi: 10.1016/j.apmr.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol (1985) 2006;100(2):433–441. doi: 10.1152/japplphysiol.01203.2005.. [DOI] [PubMed] [Google Scholar]
- 30.Crawford RJ, Cornwall J, Abbott R, Elliott JM. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskeletal Disord. 2017;18(1):25. doi: 10.1186/s12891-016-1378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranson CA, Burnett AF, Kerslake R, Batt ME. O’ Sullivan PB. An investigation into the use of MR imaging to determine the functional cross sectional area of lumbar paraspinal muscles. Eur Spine J. 2006;15(6):764–773. doi: 10.1007/s00586-005-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry DB, Padwal J, Johnson S, Parra CL, Ward SR, Shahidi B. Methodologic considerations in region of interest definitions for paraspinal muscles in axial MRIs of the lumbar spine. BMC Musculoskeletal Disord. 2018;19(1):135. doi: 10.1186/s12891-018-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0T: relaxation times and image contrast. AJR Am J Roentgenol. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343. [DOI] [PubMed] [Google Scholar]
- 34.George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;(4):197–205. 10.2519/jospt.2010.3298. [DOI] [PubMed]
- 35.Teichtahl AJ, Urquhart DM, Wang Y, Wluka Y, O’Sullivan R, Jones G, Cicuttini FM. Lumbar disc degeneration is associated with modic change and high paraspinal fat content – a 3. 0T magnetic resonance imaging study BMC Musculoskeletal Disorders. 2016;17(1):439. doi: 10.1186/s12891-016-1297-z.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, O’Sullivan R, Jones G, Cicuttini FM. Physical inactivity is associated with narrower lumbar intervertebral discs, high fat content of paraspinal muscles and low back pain and disability. Arthritis Res Ther. 2015;17:114. doi: 10.1186/s13075-015-0629-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, Wijethilake P, O’Sullivan R, Cicuttini FM. Fat infiltration of paraspinal muscles is associated with low back pain, disability and structural abnormalities in community-based adults. Spine J. 2015;15(7):1593–1601. doi: 10.1016/j.spinee.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 38.Crawford R, Filli L, Elliot J, Nanz D, Fischer M, Marcon M, Ulbrich E. Age and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. Am J Neuroradiol. 2016;37(4):742–746. doi: 10.3174/ajnr.A4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahidi B, Parra CL, Berry DB, Hubbard JC, Gombatto S, Zlomisclic V, Allen RT, Hughes-Austin J, Garfin S, Ward SR. Contribution of lumbar spine pathology and age to paraspinal muscle size and fatty infiltration. Spine (Phila Pa 1976) 2016;42(8):616–623. doi: 10.1097/BRS.0000000000001848.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkkola R, Kormano M. Lumbar disc and back muscle degeneration on MRI: correlation to age and body mass. J Spinal Disord. 1992;5(1):86–92. doi: 10.1097/00002517-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Le Cara EC, Marcus RL, Dempsey AR, Hoffman MD, Hebert JJ. Morphology versus function: the relationship between lumbar multifidus intramuscular adipose tissue and muscle function among patients with low back pain. Arch Phys Med Rehabil. 2014;95(10):1846–1852. doi: 10.1016/j.apmr.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Jensen MP, Turner Ja. Romano JM, fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain 1999;83(2):157–162. [DOI] [PubMed]
- 43.Pfirrmann CW, Metzdorf A, Zanetti M. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 44.Griffith JF, Wang JYX, Antonio GE, Choi KC, Yu A, Ahuja AT, Leung PC. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine. 2007;(24):708–12. 10.1097/BRS.0b013e31815a59a0. [DOI] [PubMed]
- 45.Vetter TR. Fundamentals of research data and variables: the devil is in the details. Anasth Analg. 2017;125(4):1375–1380. doi: 10.1213/ANE.0000000000002370. [DOI] [PubMed] [Google Scholar]
- 46.Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaption to pain. Pain. 2011;152(3):90–98. doi: 10.1016/j.pain.2010.10.020.. [DOI] [PubMed] [Google Scholar]
- 47.van Dieen JH, Flor H, Hodges PW. Low-back pain patients learn to adapt motor behavior with adverse secondary consequences. Exerc Sports Sci Rev. 2017;45(4):223–229. doi: 10.1249/JES.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 48.Elfving B, Andersson T, Grooten WJ. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother Res Int. 2007;12(1):14–24. doi: 10.1002/pri.355. [DOI] [PubMed] [Google Scholar]
- 49.Bierry G, Kremer S, Kellner F, Abu Eid M, Bogorin A, Dietemann JL. Disorders of paravertebral lumbar muscles: from pathology to cross-sectional imaging. Skelet Radiol. 2008;37(11):967–977. doi: 10.1007/s00256-008-0494-8. [DOI] [PubMed] [Google Scholar]
- 50.Hodges P, Holm AK, Hansson T, Holms S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976) 2006;31(25):2926–2933. doi: 10.1097/01.brs.0000248453.51165.0b.. [DOI] [PubMed] [Google Scholar]
- 51.Freeman MD, Woodham M, Woodham AW. The role of the lumbar multifidus in chronic low back pain: a review. PM&R Journal. 2010;2:142–146. doi: 10.1016/j.pmrj.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Tsao H, Danneels LA, Hodges PW. ISSL prize winner: smudging the motor brain in young adults with recurrent low back pain. Spine (Phila Pa 1976) 2011;36(11):1721–1727. doi: 10.1097/BRS.0b013e31821c4267.. [DOI] [PubMed] [Google Scholar]
- 53.Schabrun SM, Elgueta-Cancino EL, Hodges PW. Smudging of the motor cortex is related to the severity of low back pain. Spine (Phila Pa 1976) 2017;42(15):1172–1178. doi: 10.1097/BRS.0000000000000938.. [DOI] [PubMed] [Google Scholar]
- 54.Tsao H, Druitt TR, Schollum TM, Hodges PW. Motor training of the lumbar paraspinal muscles induces immediate changes in motor coordination in patients with recurrent low back pain. J Pain. 2010;11(11):1120–1128. doi: 10.1016/j.jpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–22. [DOI] [PubMed]
- 56.Malfliet A, Kregel J, Coppetiers I, de Pauw R, Meeus M, Roussel N, Cagnie B, Danneels L, Nijs J. Effect of pain neuroscience education combined with cognition-targeted motor control training on chronic spinal pain. Jama Neurol. 2018:E1–E11. 10.1001/jamaneurol.2018.0492 [DOI] [PMC free article] [PubMed]
- 57.Hebert JJ, Kjaer P, Fritz JM, Walker BF. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine. 2014;39(17):1417–1425. doi: 10.1097/BRS.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 58.Rossi A, Zoico E, Goodpaster BH, Sepe A, Di Francesco V, Fantin F. Quantification of intermuscular adipose tissue in the erector spinae muscle by MRI: agreement with histological evaluation. Obesity. 2010;18(12):2379–2384. doi: 10.1038/oby.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kan HE, Scheenen TW, Wohlgemuth M, Klomp DW, Van Loosbroek-Wagenmans I. Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 2009;19:357–362. doi: 10.1371/journal.pone.0132717.. [DOI] [PubMed] [Google Scholar]
- 60.Maillard SM, Jones R, Owens C, Pilkington C, Woo P, Wedderburn LR, Murray KJ. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology. 2004;43(5):603–608. doi: 10.1093/rheumatology/keh130. [DOI] [PubMed] [Google Scholar]
- 61.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 62.Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28(3):543–558. doi: 10.1002/jmri.21492.. [DOI] [PubMed] [Google Scholar]
- 63.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5):1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why and how. BMJ. 2009;338:375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets which are used during this research are available from the corresponding author on reasonable request. This material is the result of work supported with resources and the use of facilities at the MRI Centre Amsterdam, IJsbaanpad 10-B, 1076 CV Amsterdam, the Netherlands.