Abstract
Background
Chloroquine, a previous highly efficacious, easy to use and affordable anti-malarial agent was withdrawn from malaria endemic regions due to high levels of resistance. This review collated evidence from published-reviewed articles to establish prevalence of Pfcrt 76T and Pfmdr-1 86Y alleles in malaria affected countries following official discontinuation of chloroquine use.
Methods
A review protocol was developed, registered in PROSPERO (#CRD42018083957) and published in a peer-reviewed journal. Article search was done in PubMed, Scopus, Lilacs/Vhl and Embase databases by two experienced librarians (AK, RS) for the period 1990-to-Febuary 2018. Mesh terms and Boolean operators (AND, OR) were used. Data extraction form was designed in Excel spread sheet 2007. Data extraction was done by three reviewers (NL, BB and MO), discrepancies were resolved by discussion. Random effects analysis was done in Open Meta Analyst software. Heterogeneity was established using I2-statistic.
Results
A total of 4721 citations were retrieved from article search (Pubmed = 361, Lilac/vhl = 28, Science Direct = 944, Scopus = 3388). Additional targeted search resulted in three (03) eligible articles. After removal of duplicates (n = 523) and screening, 38 articles were included in the final review. Average genotyping success rate was 63.6% (18,343/28,820) for Pfcrt K76T and 93.5% (16,232/17,365) for Pfmdr-1 86Y mutations. Prevalence of Pfcrt 76T was as follows; East Africa 48.9% (2528/5242), Southern Africa 18.6% (373/2163), West Africa 58.3% (3321/6608), Asia 80.2% (1951/2436). Prevalence of Pfmdr-1 86Y was; East Africa 32.4% (1447/5722), Southern Africa 36.1% (544/1640), West Africa 52.2% (1986/4200), Asia 46.4% (1276/2217). Over half, 52.6% (20/38) of included studies reported continued unofficial chloroquine use following policy change. Studies done in Madagascar and Kenya reported re-emergence of chloroquine sensitive parasites (IC50 < 30.9 nM). The average time (years) since discontinuation of chloroquine use to data collection was 8.7 ± 7.4. There was high heterogeneity (I2 > 95%).
Conclusion
The prevalence of chloroquine resistance alleles among Plasmodium falciparum parasites have steadily declined since discontinuation of chloroquine use. However, Pfcrt K76T and Pfmdr-1 N86Y mutations still persist at moderate frequencies in most malaria affected countries.
Keywords: Chloroquine, Re-emergence, Sensitivity, Plasmodium falciparum, Policy
Background
Chloroquine was once an important medicine used in malaria treatment especially due to its affordability, ease of use and high anti-malarial efficacy. However, due to high level of resistance among Plasmodium falciparum parasites, chloroquine was withdrawn from malaria treatment in most malaria endemic countries [1]. It is estimated that the loss of chloroquine to resistance was responsible for more than doubling of malaria-associated mortality in sub-Saharan Africa, a region which bears over 90% of malaria burden [2, 3].
Chloroquine resistance reached fixation levels across malaria endemic countries by late 1990s [4]. As a result artemisinin agents and their derivatives were introduced in malaria treatment and have since been the first-line anti-malarial agents [3]. The use of artemisinin-based combination therapy (ACT) in malaria treatment, however, is limited by the high cost, pill burden and currently emerging risk of decreased P. falciparum parasite sensitivity [5–7]. Due to lack of current effective alternative agents to ACT, malaria treatment faces uncertain future which could expose populations most affected by the disease to the risk of increased malaria-associated mortality as previously seen with chloroquine [2].
Recent studies have indicated emergence of P. falciparum parasites susceptible to chloroquine following cessation of its use [4, 8]. However, considerations to re-introduce chloroquine in malaria treatment is faced with the challenge of inadequacy of information on current extent of chloroquine sensitivity and the uncertainty over how this might affect future resistance. The current review was intended to collate evidence and provide current evidence on genotypic and phenotypic chloroquine resistance among P. falciparum parasites in malaria affected countries.
Methods
Protocol development
A systematic review protocol was developed following STREGA [9] and PRISMA-P [10] guidelines. The protocol was registered in International Prospective Register of Systematic Reviews, PROSPERO (#CRD42018083957, http://www.crd.york.ac.uk/prospero) and published in a peer-reviewed journal [11].
Review question
The review sought to establish the prevalence of Pfcrt K76T and Pfmdr1 N86Y alleles among Plasmodium falciparum parasites in malaria affected countries since official discontinuation of chloroquine use in malaria treatment.
Search strategy
Electronic search
Electronic search for Pubmed data base is reported in the published protocol [11]. The search terms were combined using Boolean logic ‘OR’ for synonymous terms and ‘AND’ across elements of PECOS (Population, Exposure, Comparison, Outcome and Study design).
The search terms used included, Chloroquine, ‘Antimalarial drug’, 4-aminoquinoline, ‘antimalarial agent’, Amodiaquine, piperaquine, ‘Plasmodium falciparum’, ‘malaria parasites’, ‘parasite sensitivity’, sensitivity’, ‘susceptibility’, ‘parasite susceptibility’, extent, spread, prevalence, occurrence, proportion, frequency, resistance, ‘resistance alleles’, ‘resistance mutations’, polymorphisms, ‘resistance reversal’, ‘Pfcrt K76T’, ‘Pfmdr1 N86Y’, ‘resistance reversal’, mutations, ‘1990-to-February 2018’. The search terms were restricted to title and abstract during article search in each data base. There was no language restriction in article search, articles not published in English were translated using Google translator before screening for inclusion or exclusion.
Additional searches
The reference lists of included articles were screened and for any reference that could potentially be eligible for inclusion in the review, a full text article was retrieved. In addition, authors of included articles were contacted for any relevant publications on the review topic but did obtain any response.
Data management
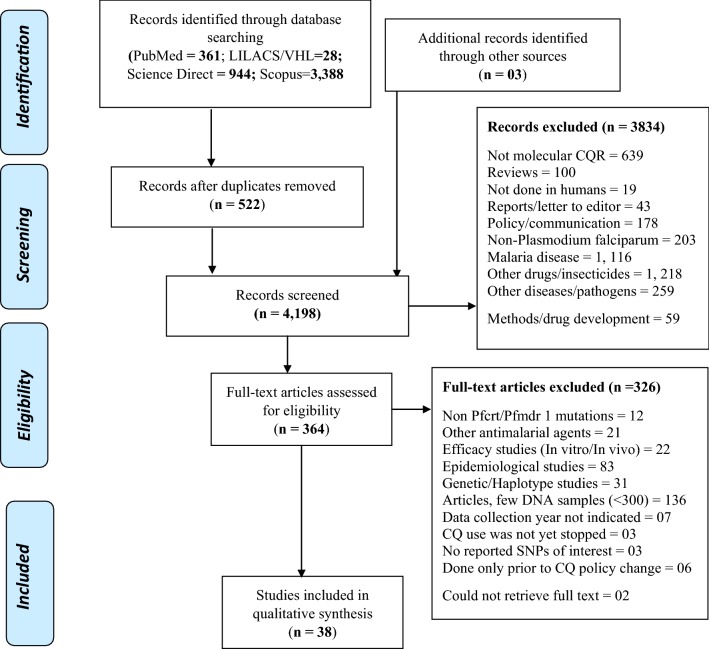
All article citations retrieved from database searches were exported into EndNote software version X7 (Thomson Reuters, 2015) and duplicates removed. The articles were grouped into relevant categories as indicated in the PRISMA flow diagram (Fig. 1).
Fig. 1.
Prisma diagram showing flow of article search and screening
Selection of studies in the review
Eligibility criteria
The titles and abstracts were screened using a priori criteria [11]. Articles that reported on at least one of the chloroquine resistance alleles, Pfcrt K76T or Pfmdr1-N86Y were considered for inclusion. Articles that reported prevalence of the above chloroquine resistance alleles and genotyped more than 300 P. falciparum DNA samples were included. Studies that reported multiple parasite resistance genotype infections among patients were included in the review. Studies that assessed prevalence of resistance alleles (Pfcrt K76T or Pfmdr1-N86Y) following cessation of chloroquine use in malaria treatment were included. The review included studies that assessed prevalence of chloroquine resistance alleles both before and after official discontinuation of chloroquine use in malaria treatment [11].
Exclusion criteria for ineligible studies
For exclusion, the following articles were considered for exclusion; focused on other drugs/insecticides, other malaria parasites, reviews, non-molecular studies, non-human studies, method development, non-Pfcrt/Pfmdr-1 genotypes, epidemiological studies, In vitro efficacy studies only without genotypic analysis, genetic studies, those that did not report any single nucleotide polymorphisms (SNPs). The articles were also considered for exclusion if they were letters to the editor/policy/communication, year of data collection is not indicated, chloroquine still being officially used after change in malaria treatment policy, data collected from countries that have not officially stopped the use of chloroquine in malaria treatment, and studies that screened few P. falciparum DNA (< 300 samples). Citations whose full text articles could not be retrieved (02) were considered for exclusion (Fig. 1).
Minimizing bias in article identification, selection and data extraction
A second librarian (RS), validated electronic search in PubMed by performing an independent and duplicate search. A second reviewer (EAO) screened all full text articles excluded by the first reviewer (MO). Any discrepancies among the reviewers were resolved by discussion and consensus. Two reviewers (NL and BB) performed duplicate and independent data extraction. Any disagreement between the reviewers was resolved by discussion and consensus. Any further disagreement between the two reviewers was referred to the third reviewer for a final decision (MO).
Data extraction
Data extraction form was developed in Excel spread sheet 2007, pre-tested on 5 articles and adjusted as appropriate. The following data was extracted from included articles, author, citation, country where study was done, study design, method of sample collection, years covered by data collection, year when chloroquine use was officially stopped, duration (years) since discontinuation of chloroquine use, whether chloroquine is still being used in the study area, In vitro assay (IC50), genotyping success rate, DNA extraction method, laboratory where genotyping was done, prevalence of Pfcrt 76T before and after cessation in chloroquine use, prevalence of Pfmdr-1 86Y before and after discontinuation of chloroquine use, trends in prevalence of chloroquine resistance alleles (Pfcrt 76T, Pfmdr-1 86Y), nature of malaria transmission, prevalence of mixed genotype infections, prevalence of other mutations (184F, 1246D, 1042D), and factors associated with chloroquine malaria treatment outcomes [11].
Data synthesis of included studies
Descriptive data synthesis was conducted with findings from a single primary study being the unit of analysis. Sub-groups were created, region (West Africa, East Africa, Southern Africa, and Asia), study-design (cross sectional, cohort, RCTs and non-randomized clinical trials). Extracted data was analyzed using Open Meta Analyst software [12]. Descriptive summaries of study outcomes were generated including; year of official cessation of chloroquine use, chloroquine in vitro IC50, duration from discontinuation of chloroquine use to data collection, chloroquine resistance alleles, factors associated with chloroquine resistance, genotyping success rate, allele calling algorithms, chloroquine use, and trends in genotypic chloroquine resistance.
DerSimonian–Laird (DL) random effects analysis was performed to establish a summary estimate of prevalence of P. falciparum Pfcrt 76T and Pfmdr1 86Y resistance alleles in malaria affected regions using Open Meta Analyst software. Sub-group analysis was performed, region (East Africa, Southern Africa, West Africa, Asia) and study design (cross sectional, Non-randomized clinical trials and RCTs). Heterogeneity in included articles was inferred from the summary estimates of I2- statistic. The I2 statistic was used to indicate percentage (%) heterogeneity that could be attributed to between-study variance. Interpretation: I2 = 25% (small heterogeneity), I2 = 50% (moderate heterogeneity), I2 = 75% (large heterogeneity) [13]. Due to high level of heterogeneity in the included studies the authors were unable to perform quantitative data analysis.
Missing data and risk of bias assessment
The variables that were missing from included articles were recorded as not reported. No statistical test was applied in handling missing data. However, available information was used in recalculating some variables in addition to contacting authors. The risk of publication bias was assessed using indirect assessment of rank correlation between effect size and sample size (Kendall’s tau) method [14]. In this method correlation of the articles is interpreted from the analysis output in Open Meta (Analyst) software where 1, represent perfect correlation and 0 no correlation.
Results
Description of included studies
Article search yielded a total of 4721 citations (Pubmed = 361, Lilacs/Vhl = 28, Science Direct = 944, Scopus = 3388). Additional searches resulted in 03 articles. After removing duplicates, 4198 articles remained and their titles and abstracts were screened for inclusion using a priori criteria. A total of 3, 834 articles were excluded after title and abstract screening. Full text of the remaining 364 articles were obtained and screened using a set criteria. Two (02) citations were excluded as their full text could not be obtained. A total of 326 articles were excluded as they did not meet the a priori inclusion criteria. Review data was extracted from a total of 38 articles that met the a priori inclusion criteria (Fig. 1).
Characteristics of included studies
Eleven (11) studies were conducted in East Africa, six (6) were from Southern Africa, fourteen (14) from West Africa and seven (7) from Asia (Table 1). A total of twenty-six (26) studies were cross-sectional, eight (08) RCTs, three (03) non-randomized clinical trials and one (01) cohort study.
Table 1.
Summary of the included articles
| Author | Year | Country | Design | Study period | Year CQ PC | Years CQ not in use | CQ still used | Prev. of Pfcrt K76T after CQ policy change | Prev. of Pfmdr1 N86Y after CQ policy change |
|---|---|---|---|---|---|---|---|---|---|
| Andriantsoanirina [37] | 2010 | Madagascar | RCT | 2006–2007 | 2005 | 01 | Yes | 0.32% (2/621) | 44.5% (147/330) |
| Achieng [19] | 2015 | Kenya | CSS | 1995–2014 | BF/AF | NA | Yes | 28.5% (212/745) | 14.9% (111/745) |
| Asih [45] | 2009 | Indonesia | CSS | 2007 | 2004 | 3 | NR | 89.2% (345/387) | 42.3% (82/194) |
| Atroosh [36] | 2012 | Malaysia | CSS | 2007–2011 | NR | NA | Yes | 52.7% (39/75) | 5.3% (4/75) |
| Baraka [15] | 2015 | Burkina Faso | RCT | NR | 2005 | NA | NR | 48.7% (130/272) | 61.8% (168/267) |
| Baraka [76] | 2018 | DRC/Uganda | RCT | 2012–2014 | NR | NR | NR | NR | DRC: 37.9% (278/732) Uganda: 7.5% (55/732) |
| Bo Huang [35] | 2016 | Grande Comore Island | CSS | 2006–2014 | 2004 | 2 | Yes | 49.5% (100/202) | 66.8% (135/202) |
| Sutar [41] | 2013 | India | CSS | 2008 | 1982 | 26 | No | 77.6% (156/201) | 59.5% (119/200) |
| Das [28] | 2017 | India | CT | 2008–2013 | 1982 | 26 | Yes | Kolkata: 69.1% (540/781) Purulia: 50.97% (398/781) |
Kolkata: 44.3% (346/781) Purulia: 65.6% (512/781) |
| Frank [29] | 2011 | Gabon | CSS | 1995–2007 | 2003 | 8 | Yes | 99.2% (356/359) | NR |
| Gadalla [77] | 2015 | Tanzania | Cohort | 2003–2006 | 2001 | 2 | NR | 93.6% (219/234) | 91.6% (163/234) |
| Gupta [42] | 2018 | Mozambique | CSS | 2015 | 2002 | 13 | NR | 2.3% (8/351) | 3.1% (11/351) |
| Hemming-Schroeder [20] | 2018 | Kenya | CSS | 2003–2015 | 1999 | 4 | Yes | 54.5% (335/615) | 24.2% (142/586) |
| Akala [46] | 2014 | Kenya | CSS | 2008–2012 | 1999 | 9 | NR | 42.2% (326/772) | 20.5% (165/804) |
| Jovel [34] | 2014 | Guinea-Bissau | CT | 2001–2002 | 2008 | 7 | Yes | 36.1% (600/1662) | 36.7% (606/1650) |
| Kateera [40] | 2016 | Rwanda | CSS | 2015 | 2001 | 14 | No | 39.2% (152/388) | 3.7% (14/382) |
| Asare [26] | 2014 | Ghana | CSS | 2012 | 2004 | 8 | Yes | 72% (154/214) | NR |
| Lekana-Douki [39] | 2011 | Gabon | CSS | 2004/2009 | 2005 | 4 | No | 95.2% (219/230) | 66.5% (153/230) |
| Lucchi [22] | 2015 | Kenya | CSS | 2010–2013 | 1999 | 11 | Yes | 9.9% (20/203) | 3.5% (7/200) |
| Ly [30] | 2012 | Senegal | CSS | 2000–2009 | 2003 | BF/AF | Yes | 59.2% (316/532) | 59.2% (316/532) |
| Afsharpad [44] | 2012 | Iran | CSS | 2008–2010 | 2007 | 1 | NR | 94.7% (161/170) | 42.9% (73/170) |
| Mbogo [24] | 2014 | Uganda | CT | 2003–2012 | 2004 | 1 | Yes | 94.3% (807/856) | 34.6% (267/771) |
| Mungthin [33] | 2014 | S. Thailand | CSS | 2009 | 1995 | 14 | Yes | 100% (558/558) | 89.2% (498/558) |
| Mittra [43] | 2006 | India | CSS | 2000–2004 | 1982 | 26 | NR | 84.2% (223/265) | 30.1% (72/239) |
| Mwai [21] | 2009 | Kenya | CSS | 1993–2006 | 1999 | NA | Yes | 63% (203/322) | 74.6% (126/169) |
| Mwanza [38] | 2016 | Zambia | CSS | 2010–2013 | 2003 | 7 | No | 0 | NR |
| Ndam [32] | 2017 | Cameroon | CT | 2003–2012 | 2002 | 1 | Yes | 42.9% (111/259) | NR |
| Ndiaye [31] | 2012 | Senegal | CSS | 2009–2011 | 2003 | 6 | Yes | 18.7% (84/449) | NR |
| Ogouyemi-Hounto [23] | 2013 | Benin | CSS | 2011 | 2004 | 7 | Yes | 93.9% (200/213) | 57.1% (121/212) |
| Okombo [18] | 2014 | Kenya | CSS | 1995–2013 | 2004 | 9 | Yes | 57.9% (212/366) | 51.6% (119/231) |
| Afoakwah [27] | 2014 | Ghana | CSS | 2010–2011 | 2004 | 6 | Yes | 58.5% (144/246) | NR |
| Otienoburu [47] | 2016 | Liberia | CT | 2008–2009 | 2003 | 5 | NR | 93.5% (275/294) | 69.4% (204/294) |
| Some [78] | 2016 | Burkina Faso | CT | 2012 | 2005 | 7 | NR | 24.6% (58/236) | 19.3% (46/238) |
| Sondo [79] | 2015 | Burkina Faso | CT | 2010–2012 | 2005 | 5 | NR | 20.5% (120/584) | NR |
| Thomsen [16] | 2013 | Mozambique | CSS | 2009–2010 | 2002 | 7 | Yes | 43.1% (163/378) | 35.4% (134/378) |
| Raman [17] | 2011 | Mozambique | CSS | 2006–2010 | 2002 | 4 | Yes | 76.9% (1694/2203) | 0 |
| Duah [25] | 2013 | Ghana | CSS | 2003–2010 | 2005 | BF/AF | Yes | 52.1% (554/1063) | 47.8% (372/778) |
| Mohammed [80] | 2013 | Tanzania | CSS | 2010–2011 | 2001 | 9 | NR | 5.7% (42/741) | NR |
CSS cross-sectional, BF/AF before/after, NR not reported, CQ chloroquine, PC policy change
Genotyping errors and parasite DNA extraction methods
For Pfcrt 76T allele, genotyping was done in a total of 28,820 DNA samples with 18,343 being successfully genotyped, 63.6% (18,343/28,820). While genotyping was done for Pfmdr-1 86Y mutation in a total of 17,365 DNA samples with 16,232 being successfully genotyped, 93.5% (16,232/17,365). One study by Baraka et al. [15] done in Bukina-Faso did not report DNA extraction method used. The other 37 articles reported using either of the following DNA extraction methods, Chelex-100, Quiagen blood mini kit, Takara DNA blood mini kit, phenol–chloroform, Accu-Prep Genomic DNA extraction kit, methanol fixation method, QIAxtractor system, and Nucleospin Genomic DNA blood pure kit.
Heterogeneity of included studies
Sub-group analysis was done based on regions from where the studies were done, East Africa, Southern Africa, West Africa and Asia. A high heterogeneity, I2 > 97% was observed in all regions where the studies were conducted (East Africa: Pfcrt 76T, I2 = 99.8%; Pfmdr1 86Y, I2 = 99.6%; Southern Africa: Pfcrt 76T, 99.2%; Pfmdr1 86Y, I2 = 99.4%; West Africa: Pfcrt 76T, I2 = 99.8%; Pfmdr1 86Y, I2 = 98.1%; Asia: Pfcrt 76T, I2 = 99.2%; Pfmdr1 86Y, I2 = 99.5%). Heterogeneity was further assessed in sub-groups based on study designs (Cross-sectional, RCTs and non-randomized trials). Heterogeneity was high in articles from all study designs, I2 > 95% (RCTs: Pfcrt 76T, I2 = 99.7%; Pfmdr1 86Y, I2 = 99.3%; Non-randomized trials: Pfcrt 76T, I2 = 99.4%; Pfmdr1 86Y, I2 = 99.2%; Cross-sectional studies: Pfcrt 76T, I2 = 99.9%; Pfmdr1 86Y, I2 = 99.6%).
Aggregate prevalence of chloroquine resistance alleles in malaria affected regions
In East Africa, average prevalence of Pfcrt K76T resistance alleles is 48.9% (2528/5242) (95% CI: 22.5–75.2%) and Pfmdr-1 86Y is 32.4% (1447/5722) (95% CI 19.2–45.5%). In Southern Africa, average prevalence of Pfcrt K76T resistance allele is 18.6% (373/2163) (95% CI 14–23.1%), and Pfmdr1 86Y is 36.1% (544/1640) (95% CI 12.7–59.4%). In West Africa, average prevalence of Pfcrt K76T resistance alleles is 58.3% (3321/6608) (95% CI 40.4–76.2%) and Pfmdr1-86Y is 52.2% (1986/4200) (95% CI 41.1–63.3%). In Asia, the average prevalence of Pfcrt 76T resistance allele is 80.2% (1951/2436) (95% CI 68–92.4%) and Pfmdr1 86Y is 46.4% (1276/2217) (95% CI 22.3–70.4%).
A total of 26 studies were conducted following cross-sectional study design and reported average prevalence of chloroquine resistance alleles as Pfcrt 76T, 54.3% (5179/10,333) (95% CI 31.7–77%), Pfmdr-1 86Y, 37.7% (2765/7446) (95% CI 25–50.4%). Three studies were done following non-randomized clinical trial design and reported average prevalence of chloroquine resistance alleles as Pfcrt 76T, 82.7% (1551/1931) (95% CI 64–101.3%), Pfmdr1-86Y, 50.6% (899/2038) (95% CI 26.5–76.4%). Eight studies used RCT design and reported average prevalence of chloroquine resistance alleles was Pfcrt 76T, 33.7% (1224/3951) (95% CI 14.4–52.9%), Pfmdr1-86Y, 36.6% (1258/3851) (95% CI 20–53.3%).
Enforcement of policy on discontinuation of chloroquine use in malaria treatment
Over half, 57.9% (22/38) of included studies reported continued use of chloroquine after official discontinuation following change in malaria treatment policy. These included, Mozambique [16, 17]; Kenya [18–22]; Benin [23]; Uganda [24]; Ghana [25–27]; India [28]; Gabon [29]; Senegal [30, 31]; Cameroon [32]; Southern Thailand [33]; Guinea-Bissau [34]; Grande Comoros [35]; Malaysia [36] and Madagascar [37].
Of the 38 studies, only 4 (10.5%) done in Zambia [38], Gabon [39], Rwanda [40] and India [41] reported elimination of chloroquine use following official discontinuation (Table 1).
Prevalence of chloroquine resistance alleles after discontinuation of chloroquine use in malaria treatment
Pfcrt K76T
Nine of the 38 studies were done after more than 10 years since cessation of chloroquine use. Of these studies, 4 reported less than 50% Pfcrt K76T allele frequency, Kenya (28.4%, [19]), Mozambique (2.3%, [42]), Rwanda (39.2%, [40]), and Kenya (9.9%), [22]). Two studies reported Pfrct 76T allele frequency of more than 80%, [33], 100% (Southern Thailand) and [43], 84.2% (India) (Table 1).
Pfmdr1 N86Y
Four of the nine studies done after more than 10 years since change of chloroquine use reported less than 10% prevalence of Pfmdr1 86Y alleles, [22] (3.5%), [40] (3.7%), and [42] (2.3%). A study by Mungthin et al. [33] reported Pfmdr1 86Y allele prevalence of 89.1% 10 years after cessation of chloroquine use in malaria treatment (Table 1).
Change in malaria treatment policy from chloroquine to sulfadoxine–pyrimethamine
Majority of countries officially stopped chloroquine use in malaria treatment in 2004 (Range 1982–2008). India was the first country to stop chloroquine use in 1982. Guinea-Bissau in West Africa continued using chloroquine in malaria treatment until 2008. The average duration of time since official change in malaria treatment policy to data collection of the individual included studies is 8.7 ± 7.4 (95% CI 6.1–11.3) (Table 1).
Five studies, 13.2% (5/38) done in Zambia, Gabon, Rwanda, and India reported elimination of chloroquine use after official discontinuation. In Zambia [38], there was no detectable chloroquine resistance alleles 7 years after cessation of chloroquine use. In Gabon, there was no detectable chloroquine use 1 year after official discontinuation of chloroquine use and prevalence of Pfcrt 76T was 95.2% and Pfmdr-1 86Y, 66.5% [39]. Fourteen years after change in malaria treatment policy in Rwanda chloroquine use was stopped and prevalence of resistance alleles was, Pfcrt 76T, 39.2% and Pfmdr-1 86Y, 3.7% [40]. In India, there was no chloroquine use for over two decades after change in policy and resistance allele frequency was Pfcrt 76T, 77.6% and Pfmdr-1 86Y, 59.5% [28, 41] (Table 1).
Multiple chloroquine resistance surveys were done in eight countries (Kenya, Tanzania, Mozambique, Burkina Faso, Ghana, India, Gabon and Senegal). In all the eight countries chloroquine resistance allele prevalence decreased with increasing duration of time since discontinuation of chloroquine use in malaria treatment. None of the eight countries recorded zero prevalence of Pfcrt 76T allele after discontinuation of chloroquine use. Only Mozambique reported zero prevalence of Pfmdr1-86Y allele [17] 4 years after cessation of chloroquine use. The lowest reported Pfcrt 76T resistance allele prevalence, 2.3% was observed in Mozambique [42] 13 years after discontinuation of chloroquine use in malaria treatment. In India, a study by Mittra [43] reported the highest prevalence, 84.2% of Pfcrt 76T resistance allele after over a decade after discontinuation of chloroquine use in malaria treatment.
The average Pfcrt K76T allele frequencies varied in different countries since cessation in chloroquine use, Kenya (42.7%), Tanzania (49.7%), Burkina Faso (31.3%), Ghana (60.2%), India (70.5%), Gabon (97.2%) and Senegal (38.9%).
The average prevalence of Pfmdr-1 86Y allele varied in different countries since change in malaria treatment policy from chloroquine to sulfadoxine-pyrimethamine, Kenya (31.6%), Mozambique (19.3%), Burkina Faso (40.6%), Ghana (47.8%), India (49.9%) and Senegal (59.2%).
Prevalence of chloroquine resistance alleles before discontinuation of chloroquine use in malaria treatment
Pfcrt K76T
In Ghana, 2 years before change (2003–2004) in policy, Pfcrt 76T allele frequency was 50-98% [25]. In Kenya, Pfcrt 76T allele frequency varied from several reports prior to cessation of chloroquine use in malaria treatment, 57% (1995), 88.9% (1999) [18]; in 1993–2003, 62.8% [19]. In Senegal, [30] (72.4%), [31] (2001: 64%; 2004: 59.5%). In Gabon Pfcrt 76T allele frequency varied, 93.8% in 2004 [39], 100% in 1995–2002 [29]. In India prior to change in chloroquine use, Pfcrt 76T prevalence varied in different studies, 91% [41], 53% [28]. In Guinea-Bissau, Pfcrt 76T allele frequency before change in policy was, 23.6%. In Iran the reported frequency was 97.7% [44]. In Uganda, there was high reported frequency prior to change in policy, 99% [24]. In Zambia, Pfcrt 76T allele frequency was 95% in 2001 prior to policy change. A study by Bo Huang et al. 2016 reported 62-98% Pfcrt 76T allele frequency prior to policy change in Grande Comore Island. In Mozambique reported allele frequency prior to policy change was 96.1% [17] (Table 2).
Table 2.
Prevalence of chloroquine resistance alleles before and after change in policy
| Author | Year of CQ PC | Prev. of Pfcrt 76T BF CQ PC | Prev. of Pfmdr-1 86Y BF CQ PC | Prev. of Pfrct 76K/T AF CQ PC | Prev. of Pfmdr-1 86N/Y AF CQ PC | Prev. of 76T/86Y AF CQ PC | Prev. of Y184F AF CQ PC | Prev. of D1246Y AF CQ PC |
|---|---|---|---|---|---|---|---|---|
| Andriantsoanirina [37] | 2005 | NR | NR | NR | 3.3% | NR | 71.7% | 33.3% |
| Achieng et al. [19] | BF/AF | 62.8% (1993–2003) | NR | 9.8% | 11.4% | NR | 40.8% | 13.8% |
| Asih et al. [45] | 2004 | NR | NR | NR | NR | 18.7% (65/348) | NR | 63.3% |
| Atroosh et al. [36] | NR | NR | NR | NR | NR | NR | NR | 4% |
| Baraka et al. [15] | 2005 | NR | NR | 21.7% | 19.5% | NR | NR | NR |
| Baraka et al. [76] | NR | NR | NR | NR | DRC: 9.3% Uganda: 1.5% |
NR | DRC: 43.5% Uganda: 32.8% |
DRC: 9.2% Uganda: 20.9% |
| Bo Huang et al. [35] | 2004 | 62–98% | 90–100% | NR | NR | NR | 42.6% | 20.8% |
| Sutar et al. [41] | 1982 | 91% (2005) | 91% (2005) | 6.97% | 9.5% | 70% (109/156) | NR | 53.19% (2013) |
| Das et al. [28] | 1982 | 53% (2005) | NR | NR | 16.13% | NR | NR | NR |
| Frank et al. [29]. | 2003 | 100% (’95–’02) | NR | NR | NR | NR | NR | NR |
| Gadalla et al. [77] | 2001 | NR | NR | 1.7% | 8.99% | NR | 2.2% | 82% |
| Gupta et al. [42] | 2002 | NR | NR | NR | NR | NR | 46.7% | NR |
| Hemming-Schroeder et al. [20] | 1999 | NR | NR | 12.7% (46/362) | 23.7% | NR | 33.3% | 25.4% |
| Akala et al. [46] | 1999 | NR | NR | 33.7% (260/772) | 17.7% | NR | NR | NR |
| Jovel et al. [34] | 2008 | 23.6% | 43.1% | NR | NR | 13% (80/615) | 42% | NR |
| Kateera et al. [40] | 2001 | NR | NR | 10.1% (39/388) | 15.4% | NR | 59.8% | 19.2% |
| Lekana-Douki et al. [39] | 2005 | 93.8% (2004) | 75% (2004) | 1.5% (2/134) | 8.2% | NR | NR | 5.2% |
| Lucchi et al. [22] | 1999 | NR | NR | 13.7% | 3% | NR | 37.3% | 6.8% |
| Afsharpad et al. [44] | 2007 | 97.7% | 41% | NR | 2.4% | NR | NR | NR |
| Mbogo et al. [24] | 2004 | 99% (2003–2004) | 19% (2003–2004) | 4% (31/776) | 58.1% | NR | 12.2% | 40.4% |
| Mungthin et al. [33] | 1995 | NR | NR | NR | NR | NR | 10.4% | NR |
| Mittra et al. [43] | 1982 | NR | NR | 4.91% | 69.1% | NR | 99.16% | NR |
| Ndam et al. [32] | 2002 | NR | NR | 19.7% (51/259) | NR | NR | NR | NR |
| Ndiaye et al. [31] | 2003 | 65% (2000) 64% (2001) 59.5% (‘04) |
NR | 10.2% (46/449) | NR | NR | NR | NR |
| Ogouyemi-Hounto [23] | 2004 | NR | NR | NR | 28.8% | 55.2% | NR | NR |
| Okombo et al. [18] | 2004 | 1995: 57% 1999: 88.9% |
1995: 57.1% 1999: 72.8% |
4.2% (8/192) | NR | NR | 30.9% | 19.1% |
| Otienoburu et al. [47]. | 2003 | NR | NR | 2.4% (7/294) | 16% | NR | 35.4% | 18.7% |
| Some et al. [78] | 2005 | NR | NR | NR | NR | NR | 70.8% | NR |
| Thomsen et al. [16] | 2002 | 90% | NR | NR | NR | 35.8% (59/165) | 28.8% | NR |
| Raman et al. [17] | 2002 | 96.1% | NR | NR | 16% | NR | NR | NR |
| Duah et al. [25]. | 2005 | 2003: 50–98% | 2003: 48–98% | NR | NR | NR | 2010: 40–80% | 2010: 35% |
| Sondo et al. [79] | 2005 | NR | NR | 20.5% (120/584) | NR | NR | NR | NR |
NR not reported, PC policy change, CQ chloroquine, BF before, AF after
Pfmdr1 N86Y
In Ghana from 2003-to-2004 prior to policy change, Pfmdr1 86Y allele frequency was 48-96%. In Kenya a study by Okombo et al. [18] reported 57.1% in 1995 and 72.8% in 1999. In 2003-2004 a study by Mbogo et al. [24] in Uganda reported Pfmdr1 86Y allele frequency of 19%. A study by Afsharp et al. [44] in Iran reported allele frequency of 41% prior to policy change. In India, a study by Sutar et al. [41] reported 91% allele frequency. A study by Bo Huang et al. [35] in Grande Comore Island reported 90–100% prevalence. In Guinea-Bissau, Pfmdr1 86Y allele frequency prior to change in policy was 43.1% (Table 2).
Majority, 63.2% (24/38) of the studies did not report the prevalence of P. falciparum chloroquine resistance alleles prior to cessation of chloroquine use in malaria treatment (Table 2). A high proportion, 65.8% (25/38) of the studies reported occurrence of mixed genotype infections. Six studies reported mixed 76T/86Y infections (Indonesia: [45]; India: [41]; Kenya: [46]; Benin: [23]; Mozambique: [16]). Thirteen studies reported presence of 76T/K and 86Y/N genotype in P. falciparum infections. Two studies [28, 41] in India reported the presence of all three genotypes 76T/K, 86Y/N and 76T/86Y in a single parasite infection (Table 2).
Phenotypic chloroquine resistance among P. falciparum after cessation of chloroquine use
Five studies reported phenotypic parasite resistance to chloroquine after official discontinuation of use in malaria treatment. In Madagascar, one (01) year after change of policy, chloroquine IC50 was 18.7 nM (95% CI 14.7–23.7 nM) [37]. India that stopped chloroquine use over two decades (26 years) prior to the study, chloroquine IC50 were, 2008 (Kolkata: 146.5 nM; Purulia: 162.25 nM), 2013 (Kolkata: 238.6 nM; Purulia: 247.42 nM) [28]. After over one decade since change of malaria treatment policy in Kenya, chloroquine IC50 values varied from year to year, 2010 (31.77 nM), 2011 (23.42 nM), 2012 (21.09 nM) and 2013 (19.85 nM) [22]. A study in Southern Thailand [33], 14 years after change in policy reported high average chloroquine IC50 value, 129.2 ± 45.2 nM. A study by Mwai et al. [21] in Kenya showed that P. falciparum parasites carrying Pfcrt 76T mutations had chloroquine IC50 of 63 ± 90 nM while those carrying Pfmdr1 86Y mutation had IC50 of 68 ± 87 nM (Table 3).
Table 3.
Trends in parasite resistance after cessation of chloroquine use in malaria
| Author | Mean IC50 in parasites with Pfct K76T | Country where study was done | Year policy was changed | Trends in prev. of 76T CQR allele AF PC | Trends in prev. of 86Y CQR allele AF PC |
|---|---|---|---|---|---|
| Andriantsoanirina et al. [37] | 18.7 nM (95% CI 14.7–23.7 nM) | Madagascar | 2005 | NR | NR |
| Achieng et al. [19] | NR | Kenya | 1999 | 2008–2014: 28.5%, 2014: 2.3% | 2008–2014: 14.9% |
| Hemming-Schroeder et al. [20] | NR | Kenya | 1999 | Kakamega 2003: 80% 2005: 61% 2008: 60% 2015: 2.7% Kombewa 2003: 71% 2005: 91.9% 2008: 90% 2015: 11.8% |
Kakamega 2003: 59.2% 2005: 59.1% 2008: 40% 2015: 4.2% Kombewa 2003: 57.1% 2005: 40% 2008: 45% 2015: 5% |
| Akala et al. [46] | NR | Kenya | 1999 | 2008: 68.4% 2009: 55.4% 2010: 47.8% 2011: 12.4% 2012: 29.8% |
2008: 38.1% 2009: 24.3% 2010: 19.7% 2011: 7.8% 2012: 13.3% |
| Lucchi et al. [22] | 2010: 31.77 nM 2011: 23.42 nM 2012: 21.09 nM 2013: 19.85 nM |
Kenya | 1999 | 2010: 38.8% 2011: 28.6% 2012: 18.7% 2013: 7% |
2010: 2% 2011: 1.5% 2012: 0% 2013: 0% |
| Okombo et al. [18] | NR | Kenya | 1999 | 2006: 49.5% 2013: 17.2% |
2006: 57.5% 2013: 2.1% |
| Mwai et al. [21] | 63 ± 90 nM (5-150 nM) | Kenya | 1999 | 1993–2006: 94% to 63% | 1993–2006: 75% |
| Bo Huang et al. [35] | NR | Grande Comore Island | 2004 | 2006–2014: 72.2–19.5% | 2006–2007: 87% 2013–2014: 40.2% |
| Das et al. [28] | Kolkata 2013: 238.6 nM, (95% CI 121–321 nM) Purulia 2013: 247.42 nM, (95% CI 126–316 nM) |
India | 1982 | Kolkata 2012: 94.64% Purulia 2012: 96.15% |
Kolkata 2013: 87.23% 2009: 46.67% Purulia 2013: 87.93% 2009: 64.2% |
| Frank et al. [29] | NR | Gabon | 2003 | 1995–2002: 100%; 2005–2007: 97% | NR |
| Gadalla et al. [77] | NR | Tanzania | 2001 | 2003: 96.9% 2004: 94.9% 2005: 89.5% 2006: 100% |
2003: 67.7% 2004: 67.8% 2005: 66.2% 2006: 75% |
| Jovel et al. [34] | NR | Guinea-Bissau | 2008 | 2008: 31% 2010: 48% 2011: 57% 2012: 34% |
2008: 36% 2010: 30% 2011: 29% 2012: 34% |
| Ly [30] | NR | Senegal | 2003 | 2004–2005: 47.16%, 2006–2009: 59.46% |
|
| Afsharpad et al. [44] | NR | Iran | 2007 | 2008–2010: 94.7% | 2008–2010: 42.9% |
| Mbogo et al. [24] | NR | Uganda | 2004 | 2005: 100% 2007: 100% 2008: 98% 2009: 94% 2010: 96% 2011: 92% 2012: 67% |
2005 (53%) 2007 (49%) 2008 (16%) 2009 (44%) 2010 (19%) 2011 (16%) 2012 (8%) |
| Mwanza et al. [38] | NR | Zambia | 2003 | 2001: 95% 2006: 26% 2010–2013: 0% |
NR |
| Ndam et al. [32] | NR | Cameroon | 2002 | 2003: 53% 2012: 25.3% |
NR |
| Ndiaye et al. [31] | NR | Senegal | 2003 | 2009: 19.8% 2010: 18.2% 2011: 18% |
NR |
| Sondo et al. [79] | NR | Burkina Faso | 2005 | 2010: 27.22% 2012: 16.49% |
NR |
| Thomsen et al. [16] | NR | Mozambique | 2002 | 2009: 55.9% 2010: 33.8% |
2009: 47.2% 2010: 26.9% |
| Raman et al. [17] | NR | Mozambique | 2002 | 2006: 96.1% 2007: 91.3% 2008: 76.1% 2009: 45.5% 2010: 32.4% |
2006: 74.7% 2007: 76.4% 2008: 64.9% 2009: 51.6% 2010: 30.9% |
| Duah et al. [25] | NR | Ghana | 2005 | 2005–2006:73–95% 2007–2008:50–95%, 2010: 45–80% |
2005–2006:31–67% 2007–2008:36–67% 2010: 10–50% |
CQR chloroquine resistance, PC policy change, BF before, AF: after, NR Not reported, Prev. prevalence
Factors associated with chloroquine treatment outcomes in malaria patients
A study in Madagascar [37] showed that patients with P. falciparum parasites having a Pfmdr-1 86Y mutation (OR = 4.6, 95% CI 2.3 to 8.9), and age (OR = 1.2, 95% CI 1.1 to 1.3) were predictors of chloroquine treatment failure among malaria patients. Patients with P. falciparum parasites having Pfcrt 76T are more likely to fail on treatment Malaysia, [36]. A study in Ghana [26] showed that being infected with parasites carrying Pfcrt 76T mutation was associated with staying in a place where CQ is sold and used for malaria treatment (P < 0.0001). In India (Purulia), chloroquine treatment failure was strongly associated with P. falciparum parasites carrying Pfmdr1 86Y+ 1246Y mutation in 2008–2009. Lucchi et al. indicated that P. falciparum parasites with Pfcrt 76T and Pfmdr-1 86Y mutation had significantly higher IC50 values compared to wild-type in Kenya [22], and in Senegal [30]. A study by Afoakwah et al. [27] (Iran) showed that patients infected with P. falciparum parasites having Pfcrt 76T mutation had higher parasite density with mean density of 73,529 parasites/µl of blood compared to wild type infections.
Duration since official cessation of chloroquine use and prevalence of resistance alleles
Thirty-two (32) of the 38 included studies (84.2%) reported time duration (years) since discontinuation of chloroquine use to when data collection was done. Of which the average time (years) since cessation of chloroquine use in malaria treatment up to data collection date was 8.7 ± 7.4 (95% CI 6.1–11.3) years. India stopped use of chloroquine in malaria treatment for more than two decades (26 years) prior to data collection in all included studies.
Fifteen studies were conducted in regions/countries less than 5 years after discontinuation of chloroquine use in malaria treatment and reported average prevalence, 63.5% of Pfcrt 76T resistance allele. Twelve studies done in the same regions and time-period reported average prevalence, 52.2% of Pfmdr1 86Y. Twelve studies were conducted in countries between 6 and 10 years after cessation of chloroquine use and reported average prevalence, 49.1% of Pfcrt 76T resistance alleles. Seven studies conducted in the same countries and time-period reported average prevalence, 37.4% of Pfmdr1-86Y. Eight studies were conducted in countries after more than 10 years since cessation in use of chloroquine in malaria treatment and reported average prevalence of resistance alleles, Pfcrt 76T (56.5%) and Pfmdr1 86Y (38.8%).
A study by Mungthin et al. [33] conducted in Southern Thailand 14 years since cessation of chloroquine use reported 100% prevalence of Pfcrt 76T resistance alleles. In Gabon, Frank et al. [29] reported high prevalence, 99.2% of Pfcrt 76T resistance alleles 8 years after change in chloroquine policy in malaria treatment. In Benin, Ogouyemi-Hounto et al. [23] reported 93.9% prevalence of Pfcrt 76T 7 years after cessation of chloroquine use. In Liberia, 93.5% prevalence of Pfcrt 76T resistance alleles was reported in a study done 5 years after cessation of chloroquine use in malaria treatment [47].
The prevalence of chloroquine resistance alleles varied in different malaria endemic countries at the time of discontinuation of chloroquine use in malaria treatment. Some countries had over 90% prevalence of Pfcrt 76T resistance allele, 94.3% (Uganda: [24]), 94.7% (Iran: [44]), 95.2% (Gabon: [29]). Madagascar had the lowest prevalence, 0.32% of Pfcrt 76T 1 year after change in treatment policy [37].
Discussion
Chloroquine resistance was first detected in Thailand in Southeast Asia [48] and spread to other malaria affected regions [49, 50]. Due to high levels of resistance, chloroquine was withdrawn from malaria treatment globally except in Central America where parasites remain susceptible [1]. The review observed progressive decline in prevalence of Pfcrt 76T and Pfmdr-1 86Y resistance alleles following official discontinuation of chloroquine use across malaria endemic countries. This finding is similar to previous review which indicated a reduction in prevalence of chloroquine resistance alleles, Pfcrt 76T and Pfmdr-1 86Y since discontinuation of chloroquine use [51]. The current review further highlights presence of phenotypic P. falciparum chloroquine sensitivity. In Madagascar, a study by Andriantsoanirina et al. [37] reported chloroquine IC50, 18.7 nM (sensitive), India, [28], 146.5 nM, 162.25 nM (highly resistant), Kenya, < 25 nM (sensitive), Southern Thailand, [33], 129.2 nM (highly resistant) (highly resistant (IC50 > 101 nM), moderately resistant (IC50 30.9 nM < IC50 < 100.9 nM) and sensitive (IC50 < 30.9 nM) [52]. The observed re-emergence of chloroquine susceptibility is timely especially due to recent emergence of P. falciparum parasites with decreased artemisinin susceptibility in Southeast Asia [6, 7]. Chloroquine sensitive P. falciparum parasites that have recently emerged especially in Africa following discontinuation of chloroquine use have evolved independently from African parasites [53]. Immune individuals in high malaria transmission settings serve as reservoirs [54] and are likely to be the source of current observed chloroquine susceptible parasite strains that have emerged following cessation of chloroquine use [53].
A previous review by Venkatesan et al. [51] showed continued prevalence of Pfcrt 76T alleles in malaria endemic regions, East Africa (67.6%), and West Africa (73.3%) following discontinuation of chloroquine use. The current review observed a further decline in Pfcrt 76T prevalence rates from those reported by Venkatesan et al. [51], East Africa (48.9%, 2528/5242), Southern Africa (18.6%, 373/2163), West Africa (58.3%, 3321/6608) and Asia (80.2%, 1951/2436). A similar trend in Pfmdr1 86Y mutation was seen by Ventaketsan et al. [51] in different regions of Africa, East Africa (61.1%), and West Africa (48.7%). A recent review by Okell et al. [55] also indicated a reduction in prevalence of Pfmdr1 86Y gene following change in malaria treatment policy. The current review observed lower average prevalence rate of Pfmdr-1 86Y mutation, East Africa (32.4%, 1447/5722), Southern Africa (12.7%, 544/1640), West Africa (52.2%, 1986/4200), and Asia (22.3%, 1276/2217). Despite an overall decline in frequency, chloroquine resistance alleles have persisted in malaria endemic countries following official change in policy more than a decade ago. Persistence of chloroquine resistance alleles in the population could be due to development of compensatory mutations in P. falciparum parasites that restore parasite fitness in the absence of drug pressure [56]. This incomplete reversal of resistance is a drawback to efforts considering potential re-emerging role of chloroquine in malaria control and elimination efforts. Chloroquine resistance alleles Pfcrt 76T and Pfmdr-1 86Y are selected for by the current artemisinin-based combinations used in malaria treatment [57, 58]. The wide spread use of artemisinin agents in malaria treatment across malaria endemic regions could be contributing to the observed persistence of chloroquine resistance alleles in the population. Duraisingh et al. [59] indicated that increased prevalence of Pfmdr-1 86 N alleles was associated with decreased P. falciparum parasite sensitivity to lumefantrine and mefloquine. The persistence of P. falciparum parasites carrying Pfcrt 76T and Pfmdr-1 86Y mutations could indicate the high efficacy of artemisinin and partner drug (lumefantrine) especially among African parasites [51, 60].
The review observed varying extents of decline in prevalence of Pfcrt 76T and Pfmdr-1 86Y chloroquine resistance alleles across malaria affected countries following discontinuation of chloroquine use. In addition, chloroquine resistance allele frequencies prior to policy change varied across malaria endemic countries. The drivers for observed variations in rates of re-emergence of chloroquine sensitive P. falciparum genotype are not clear. However, continued chloroquine use after official discontinuation, parasite biology, fixation of chloroquine resistance alleles prior to change in malaria policy could be having a role. A study by Laufer et al. [53] indicated that the re-emerging susceptible P. falciparum parasite strains are an expansion of susceptible parasite population that survived during the period when chloroquine was still being used for malaria treatment. Therefore, the observed variation in prevalence of chloroquine resistance alleles could also be due to differences in baseline levels of Pfcrt 76T and Pfmdr-1 86Y alleles in the population at the time of discontinuation of chloroquine use across malaria endemic regions. Drug-susceptible organisms may regain predominance as long as there is a population that survives due to exposure to non-lethal drug concentration despite prolonged drug pressure. The review showed that the rate of decline of prevalence of Pfcrt 76T and Pfmdr-1 86Y resistance alleles do not correspond to the duration of time since cessation in chloroquine use in most malaria endemic countries. Previous studies have shown that the extent of resistance corresponds to the prevailing drug pressure [61].
Continued use of chloroquine was observed in majority of malaria affected regions. This may be indicative of the challenges in implementation of malaria treatment policies across malaria affected countries [5]. This is especially the case in sub-Saharan Africa where chloroquine remained accessible to the general population through unofficial channels. In some countries, chloroquine continued to be imported for use in other indications such as P. vivax infections, and inflammatory conditions such as arthritis [5]. The ease of access of medicines over the counter in most low and middle income countries provides avenue for continued access and use of chloroquine in malaria treatment despite official discontinuation of its use [62, 63]. The low price coupled with ease of taking chloroquine compared to current artemisinin-based combinations, further influences continued non-prescribed use of chloroquine in malaria treatment [63]. Resistance selection in an area is an inevitable consequence of antimicrobial use especially when resistant organisms to the same medicine have already emerged in other areas [51, 61, 64]. It follows that reduction in extent of antimicrobial use would result in emergence of more susceptible micro-organisms [61]. This is exemplified by chloroquine in which withdrawal from malaria treatment resulted in the return of susceptible parasites [4, 65]. This pattern of re-emergence of chloroquine susceptible P. falciparum parasites across malaria affected regions has been observed in the current review. However, complete reversal to chloroquine sensitive parasites was not observed. This is likely due to continued drug pressure as chloroquine and related drugs are still accessed and used in the population [53]. The continued use of amodiaquine, a partner drug in artemisinin-based combinations for example has been shown to select for resistance to chloroquine [66].
Ursing et al. showed that even in presence of resistance, a change in dosing regimen restores chloroquine efficacy among P. falciparum parasites [67]. The study indicated that in vivo chloroquine failure rate can be decreased by giving the drug twice per day instead of once daily. Furthermore, doubling of chloroquine dose achieved 95% cure despite pre-existing parasite resistance with no observed increase in adverse events. In Guinea-Bissau, Ursing et al. [68] showed that doubling chloroquine dose helped stabilize spread of chloroquine resistance allele Pfcrt 76T. Pharmacodynamics modelling revealed that higher doses of chloroquine can eliminate resistant P. falciparum parasites [69]. Ginsburg [70] suggested that there is a finite concentration of chloroquine above which resistance will not develop. The hypothesized explanation for this suggestion is that the energy consumption required by the parasite to survive in presence of high chloroquine concentrations causes decrease in general fitness such that the parasites will not prevail [71]. A study by Nosten et al. [72] in Thailand also showed that if parasites develop resistance to a drug, combining that drug with an appropriate partner agent may in some cases effectively reduce drug pressure and allow for re-emergence of susceptible forms of the parasite. This helps inform current efforts considering re-introduction of chloroquine in malaria treatment. From recent evidence chloroquine can only be considered for re-introduction in malaria treatment in combination with other agents [73]. While a partner drug is sought to be combined with chloroquine, considerations should be made on the half- lives of potential agents with aim of having agents with similar half-lives to chloroquine [74, 75]. These observations are important in guiding efforts re-evaluating the potential emerging role of chloroquine in malaria treatment.
The review had some limitations, some of the articles did not report on all the review variables. Some variables were re-calculated from the data provided in the articles in addition to contacting authors to obtain more information and what was not established was reported as missing. Majority of the articles were from sub-Saharan African region however, since a wide article search criteria that was set a priori was applied, this is unlikely to affect the outcomes of the review.
Conclusion
There is a general uneven distribution of decline without complete disappearance of chloroquine resistance alleles Pfcrt 76T and Pfmdr-1 86Y across malaria endemic regions following official discontinuation of chloroquine use. Implementation of complete withdrawal of chloroquine following policy change was not achieved in most countries thus its continued access and use could have contributed to persistence of resistance alleles in the population.
Evidence from the current review of prevalence of chloroquine resistance coupled with presence of highly efficacious artemisinin agents does not support re-introduction of chloroquine in malaria treatment in areas where chloroquine was previously used.
Authors’ contributions
MO drafted the initial concept of the study. RS and AAK conducted database article search. MO, EAO, DA screened articles for inclusion in the review. MO, SN and KM developed data analysis plan and MO performed analysis of data regarding K13-mutations reported in different malaria affected regions. All authors read and approved final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The review has received funding from a Grant Number D43TW010132, supported by Office of the Director, National Institutes of Health (OD), National Institute of Dental & Craniofacial Research (NIDCR), National Institute of Neurological Disorders and Stroke (NINDS), National Heart, Lung, and Blood Institute (NHLBI), Fogarty International Center (FIC), and National Institute on Minority Health and Health Disparities (NIMHD). Additional funding was obtained from Malaria training Grant Number D43TW010526. The funders have had no role in the design of the study and in writing of the review protocol.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analysis
- STROBE
Strengthening Reporting of Observational studies in Epidemiology
- PROSPERO
International Prospective Register of Systematic Reviews
- LMIC
low and middle income countries
- STREGA
Strengthening the Reporting of Genetic Association Studies
References
- 1.World Health Organization . World Malaria Report 2008. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64:12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 3.WHO . World Malaria Report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 4.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell KA, Gatakaa H, Poyer S, Njogu J, Evance I, Munroe E, et al. Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries. Malar J. 2011;10:326. doi: 10.1186/1475-2875-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211:670–679. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frosch AEP, Venkatesan M, Laufer MK. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J. 2011;10:116. doi: 10.1186/1475-2875-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, von Elm E, et al. Strengthening the Reporting of Genetic Association Studies (STREGA)—an extension of the STROBE statement. PLoS Med. 2009;6:e1000022. doi: 10.1371/journal.pmed.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Stewart L, Shekelle P. Implementing PRISMA-P: recommendations for prospective authors. Syst Rev. 2016;5:15. doi: 10.1186/s13643-016-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ocan M, Akena D, Nsobya S, Kamya MR, Senono R, Kinengyere AA, et al. Prevalence of chloroquine resistance alleles among Plasmodium falciparum parasites in countries affected by malaria disease since change of treatment policy: a systematic review protocol. Syst Rev. 2018;7:108. doi: 10.1186/s13643-018-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS ONE. 2013;8:e69930. doi: 10.1371/journal.pone.0069930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendall MG. A new measure of Rank correlation. Biometrika. 1938;0:81–3.
- 15.Baraka V, Tinto H, Valea I, Fitzhenry R, Delgado-Ratto C, Mbonye MK, et al. In vivo selection of Plasmodium falciparum Pfcrt and pfmdr1 variants by artemether-lumefantrine and dihydroartemisinin-piperaquine in Burkina Faso. Antimicrob Agents Chemother. 2015;59:734–737. doi: 10.1128/AAC.03647-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen TT, Madsen LB, Hansson HH, Tomas ELE, Charlwood D, Bygbjerg IBC, et al. Rapid selection of Plasmodium falciparum chloroquine resistance transporter gene and multidrug resistance gene-1 haplotypes associated with past chloroquine and present artemether–lumefantrine use in Inhambane District, Southern Mozambique. Am J Trop Med Hyg. 2013;88:536–541. doi: 10.4269/ajtmh.12-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raman J, Mauff K, Muianga P, Mussa A, Maharaj R, Barnes KI. Five years of antimalarial resistance marker surveillance in Gaza Province, Mozambique, following artemisinin-based combination therapy roll out. PLoS ONE. 2011;6:e25992. doi: 10.1371/journal.pone.0025992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okombo J, Kamau AW, Marsh K, Sutherland CJ, Ochola-Oyier LI. Temporal trends in prevalence of Plasmodium falciparum drug resistance alleles over two decades of changing antimalarial policy in coastal Kenya. Int J Parasitol Drugs Drug Resistance. 2014;4:152–163. doi: 10.1016/j.ijpddr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achieng AO, Muiruri P, Ingasia LA, Opot BH, Juma DW, Yeda R, et al. Temporal trends in prevalence of Plasmodium falciparum molecular markers selected for by artemether–lumefantrine treatment in preACT and post-ACT parasites in western Kenya. Int J Parasitol Drugs Drug Resistance. 2015;5:92–99. doi: 10.1016/j.ijpddr.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemming-Schroeder E, Umukoro E, Lo E, Fung B, Toma´s-Domingo P, Zhou G, et al. Impacts of antimalarial drugs on Plasmodium falciparum drug resistance markers, Western Kenya, 2003–2015. Am J Trop Med Hyg. 2018;98:692–699. doi: 10.4269/ajtmh.17-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mwai L, Ochong E, Abdirahman A, Kiara SM, Ward S, Kokwaro G, et al. Chloroquine resistance before and after its withdrawal in Kenya. Malar J. 2009;8:106. doi: 10.1186/1475-2875-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucchi NW, Komino F, Okoth SA, Goldman I, Onyona P, Wiegand RE, et al. In vitro and molecular surveillance for antimalarial drug resistance in Plasmodium falciparum parasites in Western Kenya reveals sustained artemisinin sensitivity and increased chloroquine sensitivity. Antimicrob Agents Chemother. 2015;9:7540–7547. doi: 10.1128/AAC.01894-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogouyèmi-Hounto A, Ndam NT, Gazard DK, d’Almeida S, Koussihoude L, Ollo E, et al. Prevalence of the molecular marker of Plasmodium falciparum resistance to chloroquine and sulphadoxine/pyrimethamine in Benin seven years after the change of malaria treatment policy. Malar J. 2013;12:147. doi: 10.1186/1475-2875-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbogo GW, Nankoberanyi S, Tukwasibwe S, Baliraine FN, Nsobya SL, Conrad MD, et al. Temporal changes in prevalence of molecular markers mediating antimalarial drug resistance in a high malaria transmission setting in Uganda. Am J Trop Med Hyg. 2014;91:54–61. doi: 10.4269/ajtmh.13-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duah NO, Wilson MD, Chansah A, Abuaku B, Edoh D, Quashie NB, et al. Mutations in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance genes, and treatment outcomes in Ghanian children with uncomplicated malaria. J Trop Pediatr. 2013;53:27–31. doi: 10.1093/tropej/fml076. [DOI] [PubMed] [Google Scholar]
- 26.Asare KK, Boampong JN, Afoakwah R, Ameyaw EO, Sehgal R, Quashie NB. Use of proscribed chloroquine is associated with an increased risk of pfcrt T76 mutation in some parts of Ghana. Malar J. 2014;13:246. doi: 10.1186/1475-2875-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afoakwah R, Boampong JN, Egyir-Yawson A, Nwaefuna EK, Verner ON, Asare KK. High prevalence of PfCRT K76T mutation in Plasmodium falciparum isolates in Ghana. Acta Trop. 2014;136:32–36. doi: 10.1016/j.actatropica.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Tripathy S, Chattopadhayay S, Das B, Mahapatra SK, Hati AK, et al. Progressive increase in point mutations associates chloroquine resistance: even after withdrawal of chloroquine use in India. Int J Parasitol Drugs Drug Resistance. 2017;7:251–261. doi: 10.1016/j.ijpddr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank M, Lehners N, Mayengue PI, Gabor J, Dal-Bianco M, Kombila DU, et al. A thirteen-year analysis of Plasmodium falciparum populations reveals high conservation of the mutant pfcrt haplotype despite the withdrawal of chloroquine from national treatment guidelines in Gabon. Malar J. 2011;10:304. doi: 10.1186/1475-2875-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ly O, Gueye PEO, Deme AB, Dieng T, Badiane AS, Ahouidi AD, et al. Evolution of the pfcrt T76 and pfmdr1 Y86 markers and chloroquine susceptibility 8 years after cessation of chloroquine use in Pikine, Senegal. Parasitol Res. 2012;111:1541–1546. doi: 10.1007/s00436-012-2994-7. [DOI] [PubMed] [Google Scholar]
- 31.Ndiaye M, Faye B, Tine R, Ndiaye JL, Lo A, Abiola A, et al. Assessment of the molecular marker of Plasmodium falciparum chloroquine resistance (Pfcrt) in senegal after several years of chloroquine withdrawal. Am J Trop Med Hyg. 2012;87:640–645. doi: 10.4269/ajtmh.2012.11-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndam NT, Basco LK, Ngane VF, Ayouba A, Ngolle EM, Deloron P, et al. Reemergence of chloroquine-sensitive pfcrt K76 Plasmodium falciparum genotype in southeastern Cameroon. Malar J. 2017;16:130. doi: 10.1186/s12936-017-1783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mungthin M, Intanakom S, Suwandittakul N, Suida P, Amsakul S, Sitthichot N, et al. Distribution of pfmdr1 polymorphisms in Plasmodium falciparum isolated from Southern Thailand. Malar J. 2014;13:117. doi: 10.1186/1475-2875-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jovel IT, Kofoed P-E, Rombo L, Rodrigues A, Ursinga J. Temporal and seasonal changes of genetic polymorphisms associated with altered drug susceptibility to chloroquine, lumefantrine, and quinine in Guinea-Bissau between 2003 and 2012. Antimicrob Agents Chemother. 2014;59:872–879. doi: 10.1128/AAC.03554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang B, Wang Q, Deng C, Wang J, Yang T, Huang S, et al. Prevalence of crt and mdr-1 mutations in Plasmodium falciparum isolates from Grande Comore island after withdrawal of chloroquine. Malar J. 2016;15:414. doi: 10.1186/s12936-016-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atroosh WM, Al-Mekhlafi HM, Mahdy MAK, Surin J. The detection of pfcrt and pfmdr1 point mutations as molecular markers of chloroquine drug resistance, Pahang, Malaysia. Malar J. 2012;11:251. doi: 10.1186/1475-2875-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andriantsoanirina V, Ratsimbasoa A, Bouchier C, Tichit M, Jahevitra M, Rabearimanana S, et al. Chloroquine clinical failures in P. falciparum malaria are associated with mutant Pfmdr-1, Not Pfcrt in Madagascar. PLoS ONE. 2010;5:13281. doi: 10.1371/journal.pone.0013281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mwanza S, Joshi S, Nambozi M, Chileshe J, Malunga P, Kabuya J-BB, et al. The return of chloroquine-susceptible Plasmodium falciparum malaria in Zambia. Malar J. 2016;15:584. doi: 10.1186/s12936-016-1637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lekana-Douki JB, Boutamba SDD, Zatra R, Edou SEZ, Ekomy H, Bisvigou U, et al. Increased prevalence of the Plasmodium falciparum Pfmdr1 86N genotype among field isolates from Franceville, Gabon after replacement of chloroquine by artemether–lumefantrine and artesunate–mefloquine. Infection, Genetics and Evolution. 2011;11:512–517. doi: 10.1016/j.meegid.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Kateera RF, Nsobya SL, Tukwasibwe S, Hakizimanaa E, Mutesaf L, Mens PF, et al. Molecular surveillance of Plasmodium falciparum drug resistance markers reveals partial recovery of chloroquine susceptibility but sustained sulfadoxine-pyrimethamine resistance at two sites of different malaria transmission intensities in Rwanda. Acta Trop. 2016;164:329–336. doi: 10.1016/j.actatropica.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutar SKD, Dhangadamajhia G, Kar SK, Ranjit M. Molecular monitoring of antimalarial drug resistance among Plasmodium falciparum field isolates from Odisha, India. Acta Trop. 2013;126:84–87. doi: 10.1016/j.actatropica.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Gupta H, Macete E, Bulo H, Salvador C, Warsame M, Carvalho E, et al. Drug-resistant polymorphisms and copy numbers in Plasmodium falciparum, Mozambique, 2015. Emerg Infect Dis. 2018;24:40–48. doi: 10.3201/eid2401.170864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittra P, Vinayak S, Chandawat H, Das MK, Singh N, Biswas S, et al. Progressive increase in point mutations associated with chloroquine resistance in Plasmodium falciparum isolates from India. J Infect Dis. 2006;193:1304–1312. doi: 10.1086/502979. [DOI] [PubMed] [Google Scholar]
- 44.Afsharpad M, Zakeri S, Pirahmadi S, Djadid ND. Molecular monitoring of Plasmodium falciparum resistance to antimalarial drugs after adoption of sulfadoxine–pyrimethamine plus artesunate as the first line treatment in Iran. Acta Trop. 2011;121:13–18. doi: 10.1016/j.actatropica.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Asih PBS, Rogers WO, Susanti AI, Rahmat A, Rozi IE, Kusumaningtyas MA, et al. Seasonal distribution of anti-malarial drug resistance alleles on the island of Sumba, Indonesia. Malar J. 2009;8:222. doi: 10.1186/1475-2875-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akala HM, Achieng AO, Eyase FL, Juma DW, Ingasia L, Cheruiyot AC, et al. Five-year tracking of Plasmodium falciparum allele frequencies in a holoendemic area with indistinct seasonal transitions. J Multidiscip Healthc. 2014;7:515–523. doi: 10.2147/JMDH.S67252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otienoburu SD, Maïga-Ascofaré O, Schramm B, Jullien V, Jones JJ, Zolia YM, et al. Selection of Plasmodium falciparum pfcrt and pfmdr1 polymorphisms after treatment with artesunate–amodiaquine fixed dose combination or artemether–lumefantrine in Liberia. Malar J. 2016;15:452. doi: 10.1186/s12936-016-1503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talisuna AO, Bloland P, D’Alessandro U. History, dynamics, and public health importance of malaria parasite resistance. Clin Microbiol Rev. 2004;17:235–254. doi: 10.1128/CMR.17.1.235-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein EY, Smith DL, Boni MF, Laxminarayan R. Clinically immune hosts as a refuge for drug-sensitive malaria parasites. Malar J. 2008;7:67. doi: 10.1186/1475-2875-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkatesan M, Gadalla NB, Stepniewska K, Dahal P, Nsanzabana C, Moriera C, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether–lumefantrine and artesunate–amodiaquine. Am J Trop Med Hyg. 2014;91:833–843. doi: 10.4269/ajtmh.14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerutti C, Jr, Marques C, de Alencar FEC, Durlacher RR, Alween A, Segurado AAC, et al. Antimalarial drug susceptibility testing of plasmodium falciparum in brazil using a Radioisotope Method. Mem Inst Oswaldo Cruz, Rio de Janeiro. 1999;94:803–809. doi: 10.1590/S0074-02761999000600017. [DOI] [PubMed] [Google Scholar]
- 53.Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine OC, Taylor TE, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J Infect Dis. 2010;202:801–808. doi: 10.1086/655659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May JF, Mockenhaupt P, Ademowo OG, Falusi AG, Olumese PE, Bienzle U, et al. High rate of mixed and subpatent malarial infections in southwest Nigeria. Am J Trop Med Hyg. 1999;61:339–343. doi: 10.4269/ajtmh.1999.61.339. [DOI] [PubMed] [Google Scholar]
- 55.Okell LC, Reiter LM, Ebbe LS, Baraka V, Bisanzio D, Watson OJ, et al. Emerging implications of policies on malaria treatment: genetic changes in the Pfmdr-1 gene affecting susceptibility to artemether–lumefantrine and artesunate–amodiaquine in Africa. BMJ Glob Health. 2018;3:e000999. doi: 10.1136/bmjgh-2018-000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laufer MK, Plowe CV. Withdrawing antimalarial drugs: impact on parasite resistance and implications for malaria treatment policies. Drug Resist Updat. 2004;7:279–288. doi: 10.1016/j.drup.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Dokomajilar C, Nsobya SL, Greenhouse B, Rosenthal PJ, Dorsey G. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether–lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob Agents Chemother. 2006;50:1893–1895. doi: 10.1128/AAC.50.5.1893-1895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphreys GS, Merinopoulos I, Ahmed J, Whitty CJ, Mutabingwa TK, Sutherland CJ, et al. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob Agents and Chemother. 2007;51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duraisingh MT, Cowman AF. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005;93:181–190. doi: 10.1016/j.actatropica.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Conrad MD, Bigira V, Kapisi J, Muhindo M, Kamya MR, Havlir DV, et al. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan Children. PLoS ONE. 2014;9:e105690. doi: 10.1371/journal.pone.0105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levin BR, Perrot V, Walker N. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics. 2000;154:985–997. doi: 10.1093/genetics/154.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11:692–701. doi: 10.1016/S1473-3099(11)70054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ocan M, Bwanga F, Bbosa GS, Bagenda D, Waako P, Ogwal-Okeng J, et al. Patterns and practices of self-medication in Northern Uganda. PLoS ONE. 2014;9:e92323. doi: 10.1371/journal.pone.0092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin BR, Lipsitch M, Perrot V, Schrag S, Antia R, Simonsen L, et al. The population genetics of antibiotic resistance. Clin Infect Dis. 1997;24:9–16. doi: 10.1093/clinids/24.Supplement_1.S9. [DOI] [PubMed] [Google Scholar]
- 65.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 66.Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourté Y, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 67.Ursing J, Schmidt BA, Lebbad M, Kofoed P-E, Dias F, Gil JP, et al. Chloroquine resistant P. falciparum prevalence is low and unchanged between 1990 and 2005 in Guinea-Bissau: an effect of high chloroquine dosage? Infect Genet Evol. 2007;7:555–561. doi: 10.1016/j.meegid.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Ursing J, Rombo L, Kofoed PE, Gil JP. Carriers, channels and chloroquine efficacy in Guinea-Bissau. Trends Parasitology. 2008;24:49–51. doi: 10.1016/j.pt.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Hoshen MB, Stein WD, Ginsburg H. Modelling the chloroquine chemotherapy of falciparum malaria: the value of a split dose. Parasitology. 1998;116:407–416. doi: 10.1017/S0031182098002480. [DOI] [PubMed] [Google Scholar]
- 70.Ginsburg H. Should chloroquine be laid to rest? Acta Trop. 2005;96:16–23. doi: 10.1016/j.actatropica.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 71.Kofoed PE, Ursing J, Poulsen A, Rodrigues A, Bergquist Y, Aaby P, et al. Different doses of amodiaquine and chloroquine for treatment of uncomplicated malaria in children in Guinea-Bissau. Implications for future treatment recommendations. Trans R Soc Trop Med Hyg. 2007;101:231–238. doi: 10.1016/j.trstmh.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 72.Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, et al. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/S0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 73.Laufer MK, Thesing PC, Dzinjalamala FK, Nyirenda OM, Masonga R, Laurens MB, et al. A longitudinal trial comparing chloroquine as monotherapy or in combination with artesunate, azithromycin or atovaquone-proguanil to treat malaria. PLoS ONE. 2012;7:e42284. doi: 10.1371/journal.pone.0042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hastings IM, Watkins WM, White NJ. The evolution of drug-resistant malaria: the role of drug elimination half-life. Philos Trans R Soc Lond B Biol Sci. 2002;357:505–519. doi: 10.1098/rstb.2001.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hastings IM, Watkins WM. Tolerance is the key to understanding antimalarial drug resistance. Trends Parasitol. 2006;2:71–77. doi: 10.1016/j.pt.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 76.Baraka V, Mavoko HM, Nabasumba C, Francis F, Lutumba P, Alifrangis M, et al. Impact of treatment and re-treatment with artemether–lumefantrine and artesunate–amodiaquine on selection of Plasmodium falciparum multidrug resistance gene-1 polymorphisms in the Democratic Republic of Congo and Uganda. PLoS ONE. 2018;13:e0191922. doi: 10.1371/journal.pone.0191922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gadalla NB, Tavera G, Mu J, Kabyemela ER, Fried M, Duffy PE, et al. Prevalence of Plasmodium falciparum anti-malarial resistance-associated polymorphisms in pfcrt, pfmdr1 and pfnhe1 in Muheza, Tanzania, prior to introduction of artemisinin combination therapy. Malar J. 2015;14:129. doi: 10.1186/s12936-015-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Somé AF, Sorgho H, Zongo I, Bazié T, Nikiéma F, Sawadogo A, et al. Polymorphisms in K13, pfcrt, pfmdr1, pfdhfr, and pfdhps in parasites isolated from symptomatic malaria patients in Burkina Faso. Parasite. 2016;23:60. doi: 10.1051/parasite/2016069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sondo P, Derra K, Tarnagda Z, Nakanabo SD, Zampa O, Kazienga A, et al. Dynamic of Plasmodium falciparum chloroquine resistance transporter gene Pfcrt K76T mutation five years after withdrawal of chloroquine in Burkina Faso. Pan Afr Med J. 2015;21:101. doi: 10.11604/pamj.2015.21.101.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohammed A, Ndaro A, Manjurano A, Mosha JF, Mosha DF, van Zwetselaar M, et al. Trends in chloroquine resistance marker, Pfcrt-K76T mutation ten years after chloroquine withdrawal in Tanzania. Malar J. 2013;12:415. doi: 10.1186/1475-2875-12-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.