Abstract
Problem:
The inflammasome is implicated in the mechanisms that lead to spontaneous preterm labor (PTL). However, whether there is inflammasome activation in the amniotic cavity of women with PTL and intra-amniotic infection (IAI) or sterile intra-amniotic inflammation (SIAI) is unknown.
Method of study:
Amniotic fluid samples were collected from women with PTL who delivered at term (n=31) or preterm without IAI or SIAI (n=35), with SIAI (n=27), or with IAI (n=17). As a readout of inflammasome activation, extracellular ASC (Apoptosis-associated Speck-like protein containing a CARD) was measured in amniotic fluid by ELISA and the expression of ASC, caspase-1, and interleukin (IL)-1β was detected in the chorioamniotic membranes by multiplex immunofluorescence. Acute inflammatory responses in amniotic fluid and the placenta were also evaluated.
Results:
1) Amniotic fluid concentrations ASC and IL-6 were higher in women with PTL and IAI or SIAI than in those who delivered preterm or at term without intra-amniotic inflammation; 2) amniotic fluid concentrations of ASC and IL-6 were lower in women with PTL and SIAI than in those with IAI; 3) there was a significant non-linear correlation between ASC and IL-6 amniotic fluid concentrations; 4) the expression of inflammasome-related proteins (ASC, caspase-1, and IL-1β) in the chorioamniotic membranes was increased in women with PTL and IAI or SIAI than in those who delivered preterm or at term without intra-amniotic inflammation; 5) inflammasome activation in the chorioamniotic membranes was weaker in women with PTL and SIAI than in those with IAI; 6) women with PTL and IAI had elevated amniotic fluid white blood cell counts compared to those without this clinical condition; and 7) severe acute placental inflammatory lesions were observed in women with PTL and IAI and in a subset of women with PTL and SIAI.
Conclusion:
Inflammasome activation occurs in the settings of intra-amniotic infection and sterile intra-amniotic inflammation during spontaneous preterm labor.
Introduction
Preterm birth is a leading cause of perinatal morbidity and mortality worldwide,1–3 which is commonly preceded by spontaneous preterm labor.4–8 Among the known etiologies, intra-amniotic infection/inflammation is the most studied causal link to spontaneous preterm labor.9–11 Intra-amniotic inflammation can be initiated as a result of microbial invasion of the amniotic cavity (i.e. intra-amniotic infection or IAI), or by damage-associated molecular patterns (DAMPs) or alarmins (i.e. sterile intra-amniotic inflammation or SIAI).12–21 Sterile intra-amniotic inflammation is an inflammatory process in which microorganisms cannot be detected using both cultivation and molecular microbiology techniques.12–21 This clinical condition is frequently observed in women: 1) with preterm labor and intact membranes,13 2) with an asymptomatic short cervix,14 3) with preterm prelabor rupture of membranes,15 and 4) with clinical chorioamnionitis at term.16 Given that sterile inflammation is induced by alarmins22–24 and that such molecules are increased in the amniotic fluid of women who deliver preterm, we have proposed and shown that alarmins can initiate the mechanisms that lead to spontaneous preterm labor.25–32
The mechanisms that lead to spontaneous preterm labor in the context of IAI or SIAI are thought to involve the inflammasome.32–39 There are several types of inflammasomes which are named based on their sensor molecule.40–43 Nucleotide-binding domain-like receptor (NLR) inflammasomes are cytoplasmic multiprotein complexes composed of 1) the sensor molecule (e.g. NLR family pyrin domain containing protein 3 or NLRP3), 2) the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) or PYD and CARD domain-containing protein (PYCARD), and (3) pro-caspase-1.44–58 Once activated, the assembled inflammasome complex induces the autocatalytic cleavage of pro-caspase-1 into its active form which, in turn, can cleave the inflammatory cytokines pro-interleukin (IL)-1β and pro-IL-18 into their mature and secreted bioactive forms,59–69 inducing a specific form of inflammatory cell death termed pyroptosis.70–72 During inflammasome activation, the ASC adaptor protein assembles into a large, microscopically visible intracellular complex (commonly referred to as a “speck”) that consists of multimers of ASC dimers.73, 74 ASC specks can serve as danger signals through release into the extracellular space, where they can amplify the inflammatory response.75, 76 Therefore, the detection of ASC specks and/or their extracellular release provides a readout of in vivo inflammasome activation.77 Recently, we provided evidence showing that in the context of IAI or SIAI there is inflammasome activation in the chorioamniotic membranes of women who deliver at term78–80 or preterm.81 In addition, inflammasome activation in the amniotic cavity was demonstrated by detecting elevated concentrations of extracellular ASC in women who underwent spontaneous labor at term.82 However, whether there is inflammasome activation in the amniotic cavity of women with spontaneous preterm labor in the context of IAI or SIAI is unknown.
Although both IAI and SIAI are associated with adverse pregnancy and neonatal outcomes,13, 83 there is evidence that the intra-amniotic inflammatory responses are different between these two clinical conditions.84 Therefore, besides determining inflammasome activation in amniotic fluid, the acute inflammatory responses in the amniotic cavity and placenta were evaluated in women with spontaneous preterm labor with IAI or SIAI.
Materials and Methods
Study design and population
This was a retrospective cross-sectional study conducted by searching our clinical database and bank of biological samples. The collection of samples was approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University, and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services. All women provided written informed consent prior to the collection of amniotic fluid.
This study included 110 amniotic fluid samples collected from women classified into the following groups (Table 1): 1) women who presented with signs of spontaneous preterm labor but delivered at term with a negative amniotic fluid culture and an IL-6 concentration <2.6ng/mL (n=31); 2) women with spontaneous preterm labor who delivered preterm without IAI or SIAI (n=35); 3) women with spontaneous preterm labor who delivered preterm with SIAI (n=27); and 4) women with spontaneous preterm labor who delivered preterm with IAI (n=17) (see diagnostic criteria below).
Table 1.
Clinical and demographic characteristics of the study population
| Preterm Delivery | |||||
|---|---|---|---|---|---|
| Preterm labor which delivered at term (n=31) | Preterm labor without sterile intra-amniotic inflammation or infection (n=35) | Preterm labor with sterile intra-amniotic inflammation (n=27) | Preterm labor with intra-amniotic infection (n=17) | p-value | |
| Maternal age (years)a | 23 (20–25.5) | 23 (19.5–25.5) | 24 (20.5–27) | 23 (20–26) | 0.7 |
| Pre-pregnancy body mass index (kg/m2)a | 21.6 (19.8–29.3)d | 23.5 (20.7–27.8)e | 28.2 (23.2–33.4)e | 24.4 (21.7–31.9)d | 0.05 |
| Raceb | 0.6 | ||||
| African American | 96.8 (30/31) | 82.9 (29/35) | 88.9 (24/27) | 94.1 (16/17) | |
| Caucasian | 0 (0/31) | 8.6 (3/35) | 7.4 (2/27) | 5.9 (1/17) | |
| Hispanic | 0 (0/31) | 5.7 (2/35) | 0 (0/27) | 0 (0/17) | |
| Other | 3.2 (1/31) | 2.9 (1/35) | 3.7 (1/27) | 0 (0/17) | |
| Gestational age at amniocentesis (weeks)a | 31.3 (30.6–32.7) | 31.4 (28.5–32.4) | 26.4 (23.8–30.2) | 26.7 (22.6–31) | 0.001 |
| Delivery routeb | 0.1 | ||||
| Vaginal | 96.8 (30/31) | 91.2 (31/34)c | 77.8 (21/27) | 88.2 (15/17) | |
| Cesarean section | 3.2 (1/31) | 8.8 (3/34)c | 22.2 (6/27) | 11.8 (2/17) | |
| Gestational age at delivery (weeks)a | 38.7 (37.4–39.4) | 34.1 (32.4–35.9) | 26.7 (24.5–31.3) | 26.7 (22.6–31) | <0.001 |
| Birthweight | 3080 (2952.5– 3362.5) | 2277.5 (1631.3–2457.5)c | 917 (592.5–1545) | 1040 (471–1370) | <0.001 |
Data are given as median (interquartile range) and percentage (n/N)
Kruskal-Wallis test with multiple comparisons.
Fisher’s exact test.
One missing data
Two missing data
Three missing data
Clinical definitions
Gestational age was determined by the date of the last menstrual period and confirmed by ultrasound examination. The gestational age derived from sonographic fetal biometry was used if the estimation was inconsistent with menstrual dating. Spontaneous preterm labor was diagnosed by the presence of regular uterine contractions (at least two contractions every 10 minutes) associated with cervical changes in patients with a gestational age between 20 and 36 (6/7) weeks. Microbial invasion of the amniotic cavity (MIAC) was defined as a positive amniotic fluid culture and/or a polymerase chain reaction with electrospray ionization mass spectrometry (PCR/ESI-MS) (Ibis® Technology–Athogen, Carlsbad, CA, USA) test result.85–88 Intra-amniotic inflammation was defined as an amniotic fluid IL-6 concentration ≥2.6 ng/mL.89–92 SIAI was defined as an amniotic fluid IL-6 concentration ≥2.6ng/mL89 without microorganisms detected by culture or PCR/ESI-MS.12–21, 93 IAI (or microbial-associated intra-amniotic inflammation) was defined as the presence of MIAC with intra-amniotic inflammation.12–21,94,95
Amniotic fluid sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis under antiseptic conditions and monitored by ultrasound. Transabdominal amniocentesis was performed for the detection of intra-amniotic inflammation and/or infection. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and centrifuged at 1300 x g for 10 min at 4°C, and the supernatant was stored at −80°C until use. A portion of this amniotic fluid was also transported to the clinical laboratory for culture of aerobic/anaerobic bacteria and genital mycoplasmas. The clinical tests also included the determination of amniotic fluid white blood cell count,96 glucose concentration,97 Gram stain,98 and IL-6 concentration.89
Determination of IL-6 in amniotic fluid
Amniotic fluid concentrations of IL-6 were determined by using a sensitive and specific enzyme immunoassay obtained from R&D Systems (Minneapolis, MN, USA). The IL-6 concentrations were determined by interpolation from the standard curve. The inter- and intra-assay coefficients of variation for IL-6 were 8.7% and 4.6%, respectively. The detection limit of the IL-6 assay was 0.09 pg/mL. The IL-6 concentrations in amniotic fluid were determined for clinical purposes.
Determination of extracellular ASC in amniotic fluid
Concentrations of extracellular ASC in the amniotic fluid were determined by using a sensitive and specific enzyme-linked immunosorbent assay (ELISA) kit obtained from LifeSpan Biosciences (Seattle, WA). This ELISA kit was initially validated in our laboratory prior to the execution of this study. Amniotic fluid concentrations of ASC were obtained by interpolation from the standard curve. The inter- and intra-assay coefficients of variation were 5.0% and 8.6%, respectively. The sensitivity of the assay was 0.131 ng/mL.
Placental histopathological examination
Sampling of the placentas was conducted according to protocols established by the Perinatology Research Branch. Five-μm-thick sections of formalin-fixed, paraffin-embedded tissue specimens were cut and mounted on SuperFrost™ Plus microscope slides (Erie Scientific LLC, Portsmouth, NH). After deparaffinization, slides were rehydrated and stained with hematoxylin-eosin. A minimum of 5 full-thickness sections of chorionic plate, 3 sections of umbilical cord, and 3 chorioamniotic membrane rolls from each case were examined by placental pathologists who were blinded to clinical history and additional testing results. Acute inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading.99–104
Multiplex immunofluorescence and phenoptics (i.e. multispectral imaging)
Tissue sections (5-μm-thick) were prepared from the chorioamniotic membranes (amnion and choriodecidua) of women who underwent spontaneous preterm labor. Multiplex immunofluorescence staining was performed using the Opal 7 kit (Cat#NEL811001KT; PerkinElmer, Waltham, MA) following the manufacturer’s instructions. Prior to multiplex immunofluorescence staining, each analyte was individually optimized with single antibody staining combined with different fluorescent TSA® reagents (PerkinElmer). After deparaffinization, slides were placed in antigen retrieval (AR) buffer and boiled using a microwave oven. Following blocking to eliminate non-specific binding, slides were incubated with antibodies against ASC (PYCARD) (Cat#AG-25B-0006-C100; AdipoGen, San Diego, CA), caspase-1 (CAT#MA5–16215; Invitrogen, Rockford, IL), or IL-1β (Cat#NBP1–19775; Novus Biologicals, Littleton, CO) at room temperature. The slides were then washed and incubated with Opal Polymer HRP Ms+Rb (Cat#ARH1001EA; PerkinElmer). Next, the slides were incubated with one of the following fluorescent TSA® reagents included in the Opal 7 kit to detect each antibody staining: Opal 520, Opal 570, or Opal 690 (dilution 1:100). After washing, the slides were counterstained with Spectral DAPI (Cat#FP1490; PerkinElmer) and mounted using ProLong Diamond Antifade Mountant (Life Technologies, Eugene, OR). Autofluorescence slides as well as slides stained with isotype (negative controls) were included. Multiplex staining was performed by consecutively staining slide-mounted tissues using the same antibody concentrations and conditions validated through single-plex staining. Each previous primary and secondary antibody was removed by boiling in AR buffer before the application of the next primary antibody. After multiplex staining, the slides were imaged using the Vectra Polaris Multi-spectral Imaging System (PerkinElmer) and images were analyzed using the InForm 2.4.1 image analysis software (PerkinElmer).
Statistical Analysis
Statistical analysis was performed using the R statistical language and environment (www.r-project.org). Data was compared between groups using unpaired Wilcoxon tests, and p-values were adjusted across comparisons and the two analytes (IL-6 and ASC) to control the false discovery rate. Adjustment for gestational age at sampling was performed using a linear regression model. An adjusted p-value (i.e. q-value) <0.05 was considered a significant result. The magnitude of differences was expressed as the difference in the means after log2 transformation of the data, to obtain log2 fold changes in the concentrations. The correlation between ASC and IL-6 levels was assessed via Spearman correlation tests and was inspected using locally weighted scatterplot smoothing (LOESS).
Results
Characteristics of the study population
The demographic and clinical characteristics of the study population are shown in Table 1. There were no differences in maternal age, body mass index, or race between the study groups (Table 1). The majority of women included in this study were African American (Table 1). Gestational age at amniocentesis and delivery was different among the study groups; therefore, statistical analysis included adjustments for gestational age at sampling (Table 1). Birthweights were also significantly different among the study groups (Table 1).
ASC amniotic fluid concentratio in women with spontaneous preterm labor
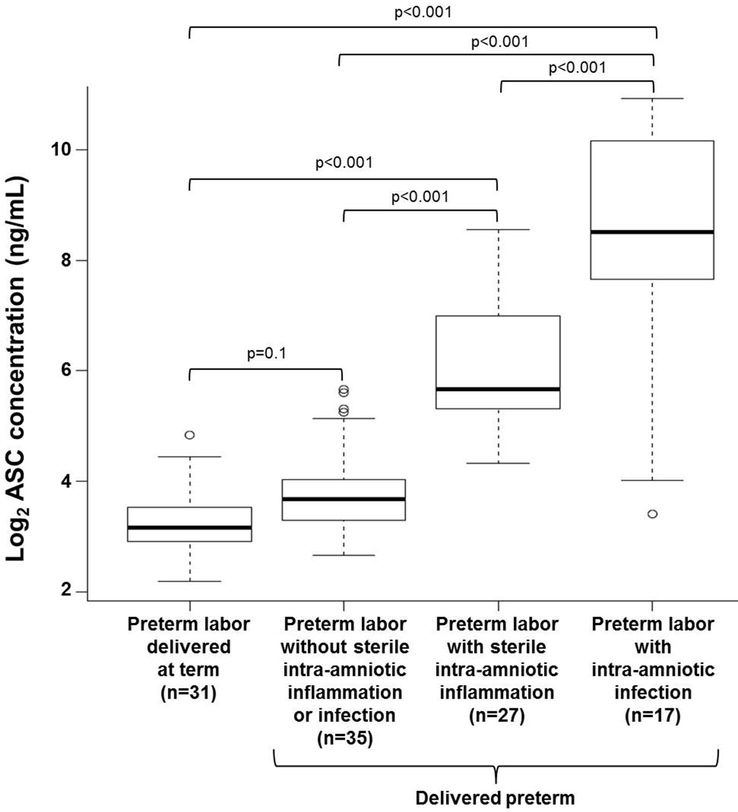
Upon inflammasome activation, the ASC protein is released into the extracellular space.75,76 As a readout of inflammasome activation, we determined whether extracellular ASC could be detected in amniotic fluid of women who underwent spontaneous preterm labor with IAI or SIAI. Amniotic fluid concentrations of ASC were significantly higher in women who underwent spontaneous preterm labor with IAI than in those who delivered preterm or at term without intra-amniotic inflammation [spontaneous preterm labor with IAI: median 365.6 ng/mL (IQR 186.7–1160 ng/mL) vs. spontaneous preterm labor without intra-amniotic inflammation: median 12.8 ng/mL (IQR 9.8–16.9 ng/mL); p<0.001 or vs. spontaneous preterm labor who delivered at term: median 8.9 ng/mL (IQR 7.5–11.8 ng/mL); (p<0.001] (Figure 1). Moreover, amniotic fluid concentrations of ASC were elevated in women who underwent spontaneous preterm labor with SIAI compared to those who delivered preterm or at term without intra-amniotic inflammation [spontaneous preterm labor with SIAI: median 50.6 ng/mL (IQR 39.7–162.7 ng/mL) vs. spontaneous preterm labor without intra-amniotic inflammation: median 12.8 ng/mL (IQR 9.8–16.9 ng/mL); p<0.001 or vs. spontaneous preterm labor who delivered at term: median 8.9 ng/mL (IQR 7.5–11.8 ng/mL); (p<0.001] (Figure 1). However, women who underwent spontaneous preterm labor with IAI had higher amniotic fluid concentrations of ASC than those with SIAI [spontaneous preterm labor with IAI: median 365.6 ng/mL (IQR 186.7–1160 ng/mL) vs. spontaneous preterm labor with SIAI: median 50.6 ng/mL (IQR 39.7–162.7 ng/mL); (p<0.001] (Figure 1). Women who underwent spontaneous preterm labor and delivered preterm without intra-amniotic inflammation tended to have greater amniotic fluid concentrations of ASC than those who delivered at term [spontaneous preterm labor without intra-amniotic inflammation: median 12.8 ng/mL (IQR 9.8–16.9 ng/mL) vs. spontaneous preterm labor who delivered at term: median 8.9 ng/mL (IQR 7.5–11.8 ng/mL); (p=0.1], but such an increase did not reach statistical significance (Figure 1).
Figure 1.
Amniotic fluid ASC concentrations in women who underwent spontaneous preterm labor. Extracellular ASC (ng/mL) was measured in amniotic fluid of women who underwent spontaneous preterm labor but delivered at term (n=31) and those who delivered preterm without sterile intra-amniotic inflammation or intra-amniotic infection (n=35), with sterile intra-amniotic inflammation (n=27), or with intra-amniotic infection (n=17). Data are shown as log2 concentration (ng/mL).
IL-6 amniotic fluid concentration in women with spontaneous preterm labor
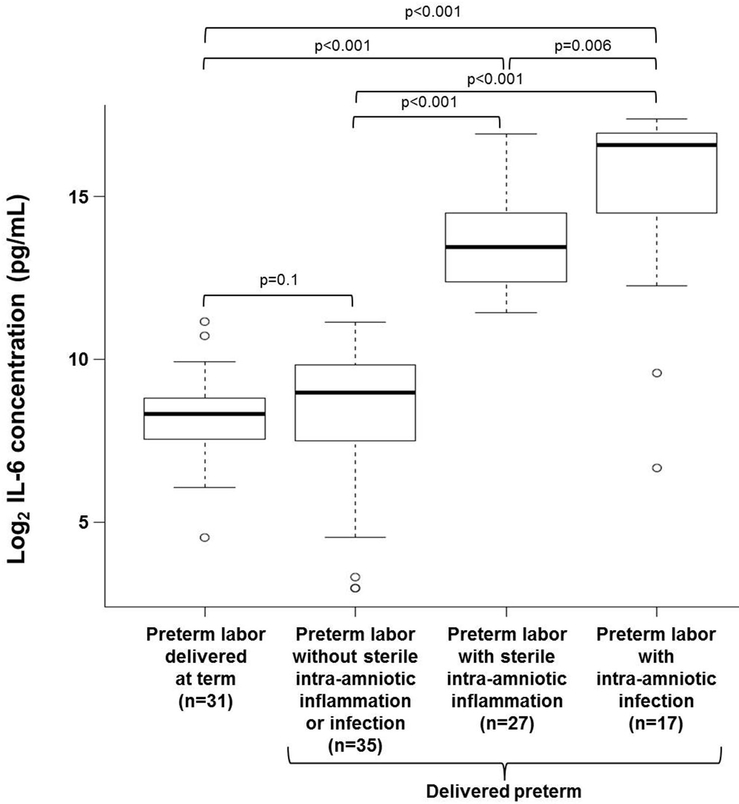
In order to correlate the ASC amniotic fluid concentrations with the degree of intra-amniotic inflammation, amniotic fluid concentrations of IL-6 were determined as previously reported.12–15,89,105 Women who underwent spontaneous preterm labor with IAI had higher amniotic fluid concentrations of IL-6 than those who delivered preterm or at term without intra-amniotic inflammation [spontaneous preterm labor with IAI: median 97,800 pg/mL (IQR 15,651–134,950 pg/mL) vs. spontaneous preterm labor without intra-amniotic inflammation: median 507 pg/mL (IQR 187.5–934 pg/mL); p<0.001 or vs. spontaneous preterm labor who delivered at term: median 322 pg/mL (IQR 185–455 pg/mL); p<0.001 (Figure 2). Women who underwent spontaneous preterm labor with SIAI also had increased amniotic fluid concentrations of IL-6 compared to those who delivered preterm or at term without intra-amniotic inflammation [spontaneous preterm labor with SIAI: median 11,247 pg/mL (IQR 5,303–23,354 pg/mL) vs. spontaneous preterm labor without intra-amniotic inflammation: median 507 pg/mL (IQR 187.5–934 pg/mL); p<0.001 or vs spontaneous preterm labor who delivered at term: median 322 pg/mL (IQR 185–455 pg/mL); p<0.001] (Figure 2). Yet, women who underwent spontaneous preterm labor with IAI had higher amniotic fluid concentrations of IL-6 than those with SIAI [spontaneous preterm labor with IAI: median 97,800 pg/mL (IQR 15,651–134,950 pg/mL) vs. spontaneous preterm labor with SIAI: median 11,247 pg/mL (IQR 5,303–23,354 pg/mL) p=0.006] (Figure 2). No differences were observed between women who underwent spontaneous preterm labor and delivered preterm without intra-amniotic inflammation and those who delivered at term (Figure 2).
Figure 2.
Amniotic fluid IL-6 concentrations in women who underwent spontaneous preterm labor. IL-6 (pg/mL) was measured in amniotic fluid of women who underwent spontaneous preterm labor but delivered at term (n=31) and those who delivered preterm without sterile intra-amniotic inflammation or intra-amniotic infection (n=35), with sterile intra-amniotic inflammation (n=27), or with intra-amniotic infection (n=17). Data are shown as log2 concentration (pg/mL).
Correlation between ASC and IL-6 amniotic fluid concentrations
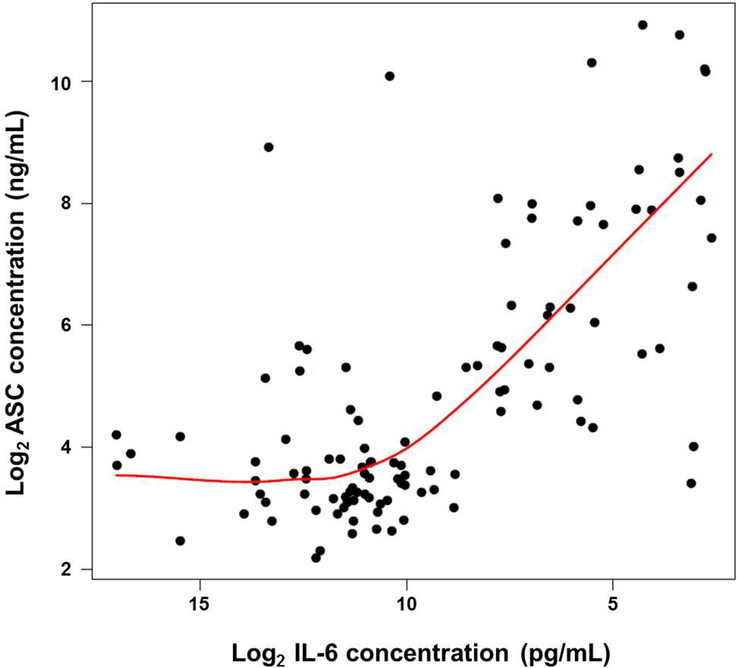
There was a significant non-linear correlation between ASC and IL-6 amniotic fluid concentrations (Figure 3) (Spearman correlation 0.61, p<0.0001). ASC amniotic fluid concentrations started to increase when IL-6 concentrations surpassed 1000 pg/mL (~10 units on the log2 scale in Figure 3). The non-linear (quadratic) relation between log2 IL-6 and ASC amniotic fluid concentrations was significantly better than a linear fit (ANOVA p<0.001).
Figure 3.
Correlation between ASC and IL-6 amniotic fluid concentrations in women who underwent spontaneous preterm labor. Data are shown as log2 concentration. The red line represents a locally weighted scatter plot smoothing (LOESS) estimating the average log2 ASC concentration as a function of log2 IL-6 concentration.
Are ASC amniotic fluid concentrations associated with inflammasome activation in the chorioamniotic membranes?
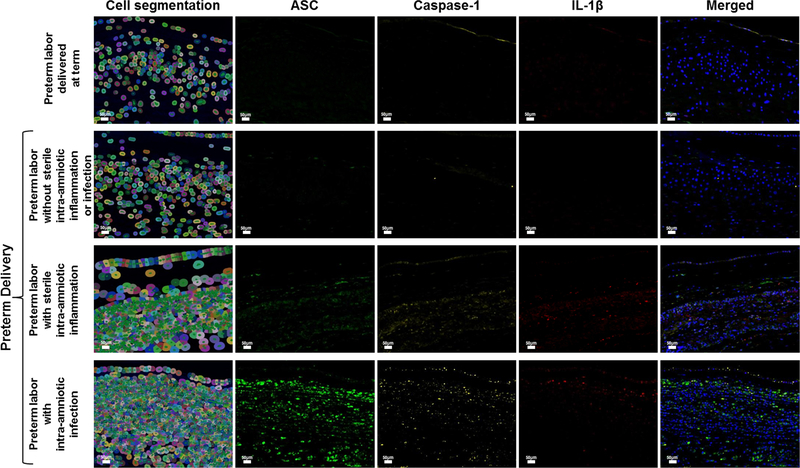
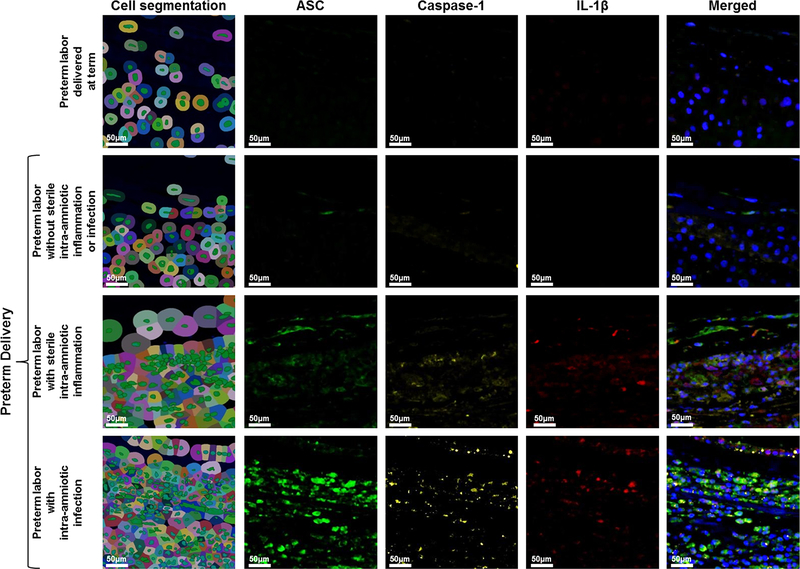
Given that ASC amniotic fluid concentrations were significantly higher in women with intra-amniotic inflammation regardless of the presence of microorganisms, we next evaluated whether both IAI and SIAI were associated with inflammasome activation in the chorioamniotic membranes. Multiplex immunofluorescence staining followed by phenoptics (i.e. multi-spectral imaging) was performed in the chorioamniotic membranes from women who underwent spontaneous preterm labor to co-localize the expression of ASC, caspase-1, and IL-1β. Figures 4 and 5 include the detection of ASC, caspase-1, and IL-1β in the chorioamniotic membranes from women in the different study groups. In order to represent cellular components and layers of the chorioamniotic membranes, we show a cell map created by using the function of cell segmentation (left column in Figures 4 and 5). In each image of Figure 4, the amnion epithelium is at the top and the decidua is at the bottom. The expression of ASC, caspase-1, and IL-1β was observed in the chorioamniotic membranes of women with IAI or SIAI, being higher in those with IAI (Figure 4). A magnification of the amnion-chorion interface is shown in Figure 5, illustrating that the three proteins are elevated in tissues from women with IAI compared to those with SIAI. The expression of ASC and caspase-1 was also minimally detected in the chorioamniotic membranes from women who underwent spontaneous preterm labor and delivered preterm without intra-amniotic inflammation but was absent in those who delivered at term (Figures 4 and 5).
Figure 4.
Expression and co-localization of inflammasome components in the chorioamniotic membranes of women who underwent spontaneous preterm labor. Multiplex immunofluorescence staining of ASC (green), caspase-1 (yellow), and IL-1β (red) was performed in the chorioamniotic membranes of women who underwent spontaneous preterm labor but delivered at term and those who delivered preterm without sterile intra-amniotic inflammation or intra-amniotic infection, with sterile intra-amniotic inflammation alone, or with intra-amniotic infection. Phenoptics was performed to generate cell segmentation images as well as separate and merged immunofluorescence images. Images are representative of 3 experiments per group. Images were taken at 400X magnification and scale bars represent 50 μm.
Figure 5.
Magnified view of inflammasome component expression at the amnion-chorion interface of women who underwent spontaneous preterm labor. Multiplex immunofluorescence staining of ASC (green), caspase-1 (yellow), and IL-1β (red) was performed in the chorioamniotic membranes of women who underwent spontaneous preterm labor but delivered at term and those who delivered preterm without sterile intra-amniotic inflammation or intra-amniotic infection, with sterile intra-amniotic inflammation alone, or with intra-amniotic infection. Phenoptics was performed to generate cell segmentation images as well as separate and merged immunofluorescence images. Images are representative of 3 experiments per group. Images were taken at 400X magnification and a close-up of the amnion-chorion interface is shown. Scale bars represent 50 μm..
Acute inflammatory responses in the amniotic cavity and placenta of women with spontaneous preterm labor
In order to complement our observations in amniotic fluid, other indicators of intra-amniotic inflammation (e.g. amniotic fluid white blood cell count and glucose concentration) were evaluated in our study population (Table 2). The number of white blood cells in amniotic fluid was higher in women who underwent spontaneous preterm labor with IAI compared to other groups (Table 2). Women who underwent spontaneous preterm labor with SIAI had a modest increase in the white blood cells found in amniotic fluid compared to those who delivered preterm without intra-amniotic inflammation and those who delivered at term (Table 2). As expected, women who underwent spontaneous preterm labor with IAI had a reduced amniotic fluid glucose concentration compared to the other study groups (Table 2). Women who underwent spontaneous preterm labor with SIAI had comparable amniotic fluid glucose concentrations to those who delivered preterm or at term without intra-amniotic inflammation (Table 2).
Table 2.
White blood cell count and glucose concentration in amniotic fluid and placental histopathology in the study population
| Preterm Delivery | |||||
|---|---|---|---|---|---|
| Preterm labor which delivered at term (n=31) | Preterm labor without sterile intra-amniotic inflammation or infection (n=35) | Preterm labor with sterile intra-amniotic inflammation (n=27) | Preterm labor with intra-amniotic infection (n=17) | p-value | |
| White blood cell count (cells/mm3)a | 0 (0–2.5) | 1 (0–3) | 2.5 (0.8–13.3)e | 295 (23–420) |
<0.001 |
| Amniotic fluid glucose (mg/dL)a | 30 (24–34.5) | 29 (20.5–33) | 21 (19–26)d | 10 (1–17)c | <0.001 |
| Acute maternal inflammatory response | |||||
| Stage 1 (acute subchorionitis)b | 13.8% (4/29)d | 12.9% (4/31)f | 29.2% (7/24)e | 12.5% (2/16)c | 0.3 |
| Stage 2 (acute chorioamnionitis)b | 17.2% (5/29)d | 16.1% (5/31)f | 12.5% (3/24)e | 18.8% (3/16)c | 0.9 |
| Stage 3 (acute necrotizing chorioamnionitis)b | 0% (0/29)d | 0% (0/31)f | 16.7% (4/24)e | 68.8% (11/16)c | <0.001 |
| Acute fetal inflammatory response | |||||
| Stage 1 (acute phlebitis/chorionic vasculitis)b | 13.8% (4/29)d | 9.7% (3/31)f | 20.8% (5/24)e | 31.3% (5/16)c | 0.2 |
| Stage 2 (acute arteritis)b | 3.4% (1/29)d | 3.2% (1/31)f | 4.2% (1/24)e | 50% (8/16)c | <0.001 |
Data are given as median (interquartile range) and percentage (n/N)
Kruskal-Wallis test with multiple comparisons.
Fisher’s exact test.
One missing data
Two missing data.
Three missing data.
Four missing data.
Acute maternal and fetal inflammatory responses in the placenta were also evaluated among the study groups. Mild and moderate acute maternal (stages 1 and 2) and fetal (stage 1) inflammatory responses were similarly observed among the study groups (Table 2). However, severe acute maternal (stage 3) and fetal (stage 2) inflammatory responses were more frequently observed in women who underwent spontaneous preterm labor with IAI (Table 2). A subset of women who underwent spontaneous preterm labor with SIAI presented acute necrotizing chorioamnionitis (acute maternal inflammatory response stage 3); yet, this placental lesion was not as commonly observed as in women with IAI (Table 2). Women who underwent spontaneous preterm labor with SIAI presented comparable rates of acute arteritis (acute fetal inflammatory response stage 2) to those who delivered at term or preterm without IAI or SIAI (Table 2).
Discussion
Principal findings
The principal findings of the study are as follows: 1) amniotic fluid concentrations of ASC (extracellular ASC indicative of inflammasome activation) and IL-6 were higher in women who underwent spontaneous preterm labor with IAI or SIAI than in those who delivered preterm or at term without intra-amniotic inflammation; 2) amniotic fluid concentrations of ASC and IL-6 were lower in women with PTL and SIAI than in those with IAI; 3) there was a significant non-linear correlation between ASC and IL-6 amniotic fluid concentrations; 4) the expression of inflammasome-related proteins (ASC, caspase-1, and IL-1β) in the chorioamniotic membranes was increased in women who underwent spontaneous preterm labor with IAI or SIAI than in those who delivered preterm or at term without intra-amniotic inflammation; 5) inflammasome activation in the chorioamniotic membranes was weaker in women who underwent spontaneous preterm labor with SIAI than in those with IAI; 6) women who underwent spontaneous preterm labor with IAI had elevated white blood cell counts and reduced glucose levels in amniotic fluid compared to the other 3 study groups; 7) women who underwent spontaneous preterm labor with SIAI had a modest increase in the number of white blood cells in amniotic fluid and comparable glucose levels to those who delivered preterm or at term without intra-amniotic inflammation; 8) severe acute maternal and fetal inflammatory responses in the placenta were frequently observed in women who underwent spontaneous preterm labor with IAI; and 9) a subset of women with spontaneous preterm labor and SIAI had severe acute maternal inflammatory responses in the placenta.
Inflammasome activation in spontaneous preterm labor with intra-amniotic infection
Herein, we showed that women who underwent spontaneous preterm labor with IAI had the highest amniotic fluid concentrations of extracellular ASC, which coincides with most elevated concentrations of IL-6 (i.e. intra-amniotic inflammation). These results are consistent with previous studies, which demonstrated that amniotic fluid concentrations of caspase-133 (the predominant inflammasome-activated caspase46), IL-1β,26 and IL-18106 (inflammasome-processed cytokines56) are greater in women with intra-amniotic infection/inflammation than in those without this clinical condition. More recently, it was reported that the chorioamniotic membranes from women who underwent spontaneous preterm labor with acute histologic chorioamnionitis (a placental lesion strongly associated with IAI99,100,102,107–116) displayed the following: 1) elevated mRNA and protein levels of NLRP3 (i.e. inflammasome sensor molecule), 2) increased expression and amounts of active caspase-1, 3) high concentrations of mature IL-1β and IL-18, and 4) enhanced inflammasome assembly (i.e. ASC/caspase-1 complexes), compared to those without this placental lesion.81 Furthermore, in vitro studies have found that microbial products (e.g. lipopolysacchride) induce the activation of caspase-1 and release of IL-1β in the chorioamniotic membranes35, 37, 117 and that Ureaplasma species (genital mycoplasmas are the most common microorganisms found in women with IAI12, 16, 118–123) are capable of activating the inflammasome pathway in murine macrophages.124 Together, these findings indicate that the inflammasome is involved in the mechanisms that lead to microbial-associated preterm labor and birth.
Women with IAI have numerous amniotic fluid immune cells,96,125–128 which could be of fetal and/or maternal origin,92 and commonly present severe acute inflammatory lesions in the placenta.99,129 This suggests that, besides the chorioamniotic membranes, maternal and fetal leukocytes are a source of extracellular ASC in the amniotic cavity. Further research is needed to investigate whether the different microorganisms invading the amniotic cavity can differentially activate the inflammasome in the chorioamniotic membranes and amniotic fluid immune cells.
Inflammasome activation in spontaneous preterm labor with sterile intra-amniotic inflammation
We found that women who underwent spontaneous preterm labor with SIAI had higher amniotic fluid concentrations of extracellular ASC than those who delivered preterm or at term in the absence of intra-amniotic inflammation. Women with SIAI harbor a unique environment in the amniotic cavity, in which the module of IL-1α is enriched compared to those without intra-amniotic inflammation.84 IL-1α is a potent alarmin130 that is increased in the amniotic fluid of women with intra-amniotic inflammation26 and induces preterm delivery in mice,131 an effect that can be abrogated by pretreatment with the IL-1 receptor antagonist.132 Importantly, the amniotic fluid IL-1α module contained high mobility group box (HMGB)1,84 a prototypic alarmin,133,134 whose intra-amniotic concentrations are a predictor of a shorter interval to delivery13 and whose administration induces preterm labor and birth in mice.31 We proposed that the mechanisms whereby alarmins induce preterm birth involve the inflammasome since the incubation of the chorioamniotic membranes with HMGB1 induced the upregulation of inflammasome components (e.g. NLRP3), activation of caspase-1, and release of mature IL-1β.32 Taken together, these data suggest that inflammasome activation in the intra-amniotic space can occur in the setting of SIAI, a process that could be initiated by alarmins.
Amniotic fluid ASC concentrations were lower in women with SIAI than in those with IAI. It is well established that the NLRP3 inflammasome can be activated by both microbes45,135–145 and alarmins;146–154 yet, it was recently showed that sterile signals generate weaker and delayed NLRP3 inflammasome-dependent inflammatory responses compared to those triggered by microbial signals.155 In line with this concept, women with SIAI had a lower number of amniotic fluid leukocytes and presented a reduced frequency of acute placental lesions compared to those with IAI. Furthermore, a protein network analysis of women who underwent spontaneous preterm labor showed that inflammatory responses in SIAI are distinct and not as severe as in IAI.84 Collectively, these data suggest that the intra-amniotic inflammatory process initiated by alarmins is milder than that triggered by microbes. A potential source of alarmins in the context of SIAI is the choriodecidua, which undergoes cellular senescence during spontaneous preterm labor.156
Do all preterm involve inflammasome activation?
In the current study, we also showed that, in the absence of intra-amniotic inflammation, women who underwent spontaneous preterm labor and delivered preterm tended to have higher amniotic fluid concentrations of extracellular ASC and IL-6 than those who delivered at term. The chorioamniotic membranes from women who delivered preterm in the absence of intra-amniotic inflammation also displayed low expression of ASC and caspase-1. These findings are consistent with previous observations showing that the chorioamniotic membranes from women who underwent spontaneous preterm labor without acute histologic chorioamnionitis exhibit signs of inflammasome assembly (i.e. ASC/caspase-1 complexes); yet, these complexes were not as abundant as in those with this placental lesion.81 These results suggest that, in the absence of high concentrations of IL-6, preterm labor is associated with a mild intra-amniotic inflammatory response, which is only partially mediated by the inflammasome. We propose that the adaptive immune system may participate in such an inflammatory process. This concept is supported by the following observations: 1) effector T cells can activate the NLRP3 inflammasome in antigen-presenting cells, amplifying adaptive immune responses,157 2) T cells are present in the amniotic fluid128 and chorioamniotic membranes,158,159 and 3) maternal and fetal T-cell activation is associated with preterm labor and birth.160–162
Conclusion
The data presented herein showed that the process of premature labor in the context of IAI and SIAI is characterized by the activation of the inflammasome as evidenced by elevated concentrations of extracellular ASC and expression of inflammasome components in the chorioamniotic membranes. Such an inflammatory process is weaker increased in women with SIAI compared to those with IAI. Collectively, these results suggest that inflammasome activation, either driven by microbes or alarmins, is a common pathway implicated in the pathogenesis of preterm labor and birth.
Acknowledgements
We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit, as well as the research assistants from the PRB Clinical Laboratory, for their help in collecting samples. Finally, we thank Derek Miller for his critical readings of the manuscript.
Funding: This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No.HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Footnotes
Conflict of Interest Statement: The authors declared no potential conflicts of interest.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE: National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–2172. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE: Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–440. [DOI] [PubMed] [Google Scholar]
- 3.Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, Thorp JM, Caritis SN, Prasad M, Tita AT, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC, Tolosa JE, Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 2016. July;215:103.e1–103.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM: The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–429. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz GS, Blackmore-Prince C, Lapinski RH, Savitz DA: Risk factors for preterm birth subtypes. Epidemiology 1998;9:279–285. [PubMed] [Google Scholar]
- 6.Moutquin JM: Classification and heterogeneity of preterm birth. BJOG 2003;110 Suppl 20:30–33. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Culhane JF, Iams JD, Romero R: Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muglia LJ, Katz M: The enigma of spontaneous preterm birth. N Engl J Med 2010;362:529–535. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC: Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–279. [PubMed] [Google Scholar]
- 10.Gomez R, Romero R, Edwin SS, David C: Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am 1997;11:135–176. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Gotsch F, Pineles B, Kusanovic JP: Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007;65:S194–202. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L: A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L: Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM: Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2015;28:1343–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM: Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM: Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015;43:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Docheva N, Martinez-Varea A, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L: Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med 2016;44:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Chaemsaithong P, Korzeniewski SJ, Kusanovic JP, Docheva N, Martinez-Varea A, Ahmed AI, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L: Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med 2016;44:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Chaiyasit N, Ahmed AI, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L: Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med 2016;44:77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Chaiyasit N, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L, Kim YM: Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med 2016;44:53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Kusanovic JP, Yoon BH, Kim JS, Chaiyasit N, Ahmed AI, Qureshi F, Jacques SM, Kim CJ, Hassan SS, Chaiworapongsa T, Yeo L, Kim YM: Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med 2016;44:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubartelli A, Lotze MT: Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 2007;28:429–436. [DOI] [PubMed] [Google Scholar]
- 23.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T: The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev 2007;220:60–81. [DOI] [PubMed] [Google Scholar]
- 24.Oppenheim JJ, Yang D: Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 2005;17:359–365. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK: Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–1123. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA: Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–123. [DOI] [PubMed] [Google Scholar]
- 27.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS, Espinoza J: The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 2007;35:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, Edwin S, Gomez R, Hassan SS, Romero R: Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS: Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS: Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med 2012;25:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS: Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, Dong Z, Hassan SS, Gomez-Lopez N: HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol Reprod 2016;95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS: Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD: Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol 2013;191:5702–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brickle A, Tran HT, Lim R, Liong S, Lappas M: Autophagy, which is decreased in labouring fetal membranes, regulates IL-1beta production via the inflammasome. Placenta 2015;36:1393–1404. [DOI] [PubMed] [Google Scholar]
- 36.Modi BP, Teves ME, Pearson LN, Parikh HI, Haymond-Thornburg H, Tucker JL, Chaemsaithong P, Gomez-Lopez N, York TP, Romero R, Strauss JF 3rd: Mutations in fetal genes involved in innate immunity and host defense against microbes increase risk of preterm premature rupture of membranes (PPROM). Mol Genet Genomic Med 2017;5:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross SN, Potter JA, Aldo P, Kwon JY, Pitruzzello M, Tong M, Guller S, Rothlin CV, Mor G, Abrahams VM: Viral Infection Sensitizes Human Fetal Membranes to Bacterial Lipopolysaccharide by MERTK Inhibition and Inflammasome Activation. J Immunol 2017;199:2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strauss JF 3rd, Romero R, Gomez-Lopez N, Haymond-Thornburg H, Modi BP, Teves ME, Pearson LN, York TP, Schenkein HA: Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol 2018. Mar;218:294–314.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim R, Lappas M: NOD-like receptor pyrin domain-containing-3 (NLRP3) regulates inflammation-induced pro-labor mediators in human myometrial cells. Am J Reprod Immunol 2018;79:e12825. [DOI] [PubMed] [Google Scholar]
- 40.Martinon F, Burns K, Tschopp J: The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–426. [DOI] [PubMed] [Google Scholar]
- 41.Petrilli V, Papin S, Tschopp J: The inflammasome. Curr Biol 2005;15:R581. [DOI] [PubMed] [Google Scholar]
- 42.Ogura Y, Sutterwala FS, Flavell RA: The inflammasome: first line of the immune response to cell stress. Cell 2006;126:659–662. [DOI] [PubMed] [Google Scholar]
- 43.Sharma D, Kanneganti TD: The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol 2016;213:617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutterwala FS, Ogura Y, Flavell RA: The inflammasome in pathogen recognition and inflammation. J Leukoc Biol 2007;82:259–264. [DOI] [PubMed] [Google Scholar]
- 45.Mariathasan S, Monack DM: Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 2007;7:31–40. [DOI] [PubMed] [Google Scholar]
- 46.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G: The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009;10:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jha S, Ting JP: Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol 2009;183:7623–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latz E: The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 2010;22:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroder K, Tschopp J: The inflammasomes. Cell 2010;140:821–832. [DOI] [PubMed] [Google Scholar]
- 50.Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G: Inflammasomes as microbial sensors. Eur J Immunol 2010;40:611–615. [DOI] [PubMed] [Google Scholar]
- 51.Lamkanfi M, Dixit VM: Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol 2011;187:597–602. [DOI] [PubMed] [Google Scholar]
- 52.Horvath GL, Schrum JE, De Nardo CM, Latz E: Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol Rev 2011;243:119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franchi L, Munoz-Planillo R, Nunez G: Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012;13:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rathinam VA, Vanaja SK, Fitzgerald KA: Regulation of inflammasome signaling. Nat Immunol 2012;13:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franchi L, Nunez G: Immunology. Orchestrating inflammasomes. Science 2012;337:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Latz E, Xiao TS, Stutz A: Activation and regulation of the inflammasomes. Nat Rev Immunol 2013;13:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanaja SK, Rathinam VA, Fitzgerald KA: Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol 2015;25:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo H, Callaway JB, Ting JP: Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015;21:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP: A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem 1989;264:5323–5326. [PubMed] [Google Scholar]
- 60.Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA: Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A 1989;86:5227–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. : A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992;356:768–774. [DOI] [PubMed] [Google Scholar]
- 62.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. : Molecular cloning of the interleukin-1 beta converting enzyme. Science 1992;256:97–100. [DOI] [PubMed] [Google Scholar]
- 63.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS: Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 1997;275:206–209. [DOI] [PubMed] [Google Scholar]
- 64.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H: Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997;386:619–623. [DOI] [PubMed] [Google Scholar]
- 65.Dinarello CA: Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci 1998;856:1–11. [DOI] [PubMed] [Google Scholar]
- 66.Fantuzzi G, Dinarello CA: Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol 1999;19:1–11. [DOI] [PubMed] [Google Scholar]
- 67.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A: Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 2000;12:581–590. [DOI] [PubMed] [Google Scholar]
- 68.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR: Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol 2005;175:7611–7622. [DOI] [PubMed] [Google Scholar]
- 69.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA: Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol 2015;33:49–77. [DOI] [PubMed] [Google Scholar]
- 70.Cookson BT, Brennan MA: Pro-inflammatory programmed cell death. Trends Microbiol 2001;9:113–114. [DOI] [PubMed] [Google Scholar]
- 71.Miao EA, Rajan JV, Aderem A: Caspase-1-induced pyroptotic cell death. Immunol Rev 2011;243:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shalini S, Dorstyn L, Dawar S, Kumar S: Old, new and emerging functions of caspases. Cell Death Differ 2015;22:526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES: The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 2007;14:1590–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vajjhala PR, Mirams RE, Hill JM: Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J Biol Chem 2012;287:41732–41743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, Bujan S, Couillin I, Brough D, Arostegui JI, Pelegrin P: The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 2014;15:738–748. [DOI] [PubMed] [Google Scholar]
- 76.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmuller W, Latz E: The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 2014;15:727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stutz A, Horvath GL, Monks BG, Latz E: ASC speck formation as a readout for inflammasome activation. Methods Mol Biol 2013;1040:91–101. [DOI] [PubMed] [Google Scholar]
- 78.Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM, Hassan SS, Yeo L, Gomez-Lopez N: A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol 2018;79:e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, Miller D, Abrahams VM, Hassan SS: Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol 2017;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, Chaemsaithong P, Chaiworapongsa T, Dong Z, Tarca AL, Abrahams VM, Yeo L, Hassan SS: A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci 2017;24:934–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM, Yeo L, Hassan SS: A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci 2017;24:1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, Pacora P, Erez O, Yeo L, Hassan SS, Hsu CD: In vivo evidence of inflammasome activation during spontaneous labor at term. J Matern Fetal Neonatal Med 2018;17:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, Miller H, Luthy D, Pereira L, Nageotte M, Robilio PA, Fortunato S, Simhan H, Baxter JK, Amon E, Franco A, Trofatter K, Heyborne K, ProteoGenix/Obstetrix Collaborative Research N: Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014. February;210:125.e1–125.e15. [DOI] [PubMed] [Google Scholar]
- 84.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L: Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 2015. December;213:836.e1–836.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA: Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA: Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DiGiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Seok KS, Gomez R, Mittal P, Gotsch F, Chaiworapongsa T, Oyarzun E, Kim CJ, Relman DA: Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med 2010;38:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiGiulio DB, Gervasi MT, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Seok KS, Gomez R, Mittal P, Gotsch F, Chaiworapongsa T, Oyarzun E, Kim CJ, Relman DA: Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med 2010;38:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK: Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136. [DOI] [PubMed] [Google Scholar]
- 90.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L: A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med 2016;29:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L: A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med 2016;29:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, Maymon E: Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017. December;217:693.e1–693.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pacora P, Romero R, Erez O, Maymon E, Panaitescu B, Kusanovic JP, Tarca AL, Hsu CD, Hassan SS: The diagnostic performance of the beta-glucan assay in the detection of intra-amniotic infection with Candida species. J Matern Fetal Neonatal Med 2017. December 27:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Musilova I, Bestvina T, Hudeckova M, Michalec I, Cobo T, Jacobsson B, Kacerovsky M: Vaginal fluid IL-6 concentrations as a point-of-care test is of value in women with PPROM. Am J Obstet Gynecol. 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 95.Musilova I, Bestvina T, Hudeckova M, Michalec I, Cobo T, Jacobsson B, Kacerovsky M: Vaginal fluid interleukin-6 concentrations as a point-of-care test is of value in women with preterm prelabor rupture of membranes. Am J Obstet Gynecol 2016. November;215:619.e1–619.e12. [DOI] [PubMed] [Google Scholar]
- 96.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC: Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991;165:821–830. [DOI] [PubMed] [Google Scholar]
- 97.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP: Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 1990;163:968–974. [DOI] [PubMed] [Google Scholar]
- 98.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S: The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 1988;159:114–119. [DOI] [PubMed] [Google Scholar]
- 99.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM: Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213:S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, Society for Pediatric Pathology PSAFINC: Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003;6:435–448. [DOI] [PubMed] [Google Scholar]
- 101.Redline RW: Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301. [DOI] [PubMed] [Google Scholar]
- 102.Redline RW: Classification of placental lesions. Am J Obstet Gynecol 2015;213:S21–28. [DOI] [PubMed] [Google Scholar]
- 103.Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AE, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PG, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der Voorn JP, van Lijnschoten I, Gordijn SJ: Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med 2016;140:698–713. [DOI] [PubMed] [Google Scholar]
- 104.Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, Bhatti G, Kim JS, Qureshi F, Jacques SM, Jung EJ, Yeo L, Panaitescu B, Maymon E, Hassan SS, Hsu CD, Erez O: The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med 2018;46:613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero R, Chaemsaithong P, Chaiyasit N, Docheva N, Dong Z, Kim CJ, Kim YM, Kim JS, Qureshi F, Jacques SM, Yoon BH, Chaiworapongsa T, Yeo L, Hassan SS, Erez O, Korzeniewski SJ: CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. Am J Reprod Immunol 2017;78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH: Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol 2000;183:1138–1143. [DOI] [PubMed] [Google Scholar]
- 107.Blanc WA: Amniotic infection syndrome; pathogenesis, morphology, and significance in circumnatal mortality. Clin Obstet Gynecol 1959;2:705–734. [PubMed] [Google Scholar]
- 108.Russell P: Inflammatory lesions of the human placenta: Clinical significance of acute chorioamnionitis. Am J Diagn Gynecol Obstet 1979;2:127–137. [Google Scholar]
- 109.Blanc WA: Pathology of the placenta and cord in ascending and in haematogenous infection. Ciba Found Symp 1979:17–38. [DOI] [PubMed] [Google Scholar]
- 110.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA: A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988;319:972–978. [DOI] [PubMed] [Google Scholar]
- 111.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA: Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol 1991;165:955–961. [DOI] [PubMed] [Google Scholar]
- 112.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, Bracken MB: The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol 1992;166:1382–1388. [DOI] [PubMed] [Google Scholar]
- 113.Redline RW: Placental inflammation. Semin Neonatol 2004;9:265–274. [DOI] [PubMed] [Google Scholar]
- 114.Fox H, Sebire NJ: Infections and inflammatory lesions of the placenta In Pathology of the placenta, 3rd edn Edinburgh, Elsevier Saunders, 2007, pp 303–354. [Google Scholar]
- 115.Benirschke K, Burton G, Baergen R: Infectious Diseases In Pathology of the Human Placenta, Springer; Berlin Heidelberg, 2012, pp 557–655. [Google Scholar]
- 116.Anders AP, Gaddy JA, Doster RS, Aronoff DM: Current concepts in maternal-fetal immunology: Recognition and response to microbial pathogens by decidual stromal cells. Am J Reprod Immunol 2017;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lappas M: Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1beta secretion. Am J Reprod Immunol 2014;71:189–201. [DOI] [PubMed] [Google Scholar]
- 118.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS: Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 1982;145:1–8. [DOI] [PubMed] [Google Scholar]
- 119.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC: Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–824. [DOI] [PubMed] [Google Scholar]
- 120.Yoneda N, Yoneda S, Niimi H, Ueno T, Hayashi S, Ito M, Shiozaki A, Urushiyama D, Hata K, Suda W, Hattori M, Kigawa M, Kitajima I, Saito S: Polymicrobial Amniotic Fluid Infection with Mycoplasma/Ureaplasma and Other Bacteria Induces Severe Intra-Amniotic Inflammation Associated with Poor Perinatal Prognosis in Preterm Labor. Am J Reprod Immunol 2016;75:112–125. [DOI] [PubMed] [Google Scholar]
- 121.Cox C, Saxena N, Watt AP, Gannon C, McKenna JP, Fairley DJ, Sweet D, Shields MD, S LC, Coyle PV: The common vaginal commensal bacterium Ureaplasma parvum is associated with chorioamnionitis in extreme preterm labor. J Matern Fetal Neonatal Med 2016;29:3646–3651. [DOI] [PubMed] [Google Scholar]
- 122.Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, Gomez-Lopez N, Romero R, Yoon BH: Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol 2017. June;216:604.e1–604.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh KJ, Hong JS, Romero R, Yoon BH: The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2019. February;32:527–541. [Epub ahead of print: 2017 Oct 11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marques LM, Rezende IS, Barbosa MS, Guimaraes AM, Martins HB, Campos GB, do Nascimento NC, Dos Santos AP, Amorim AT, Santos VM, Farias ST, Barrence FA, de Souza LM, Buzinhani M, Arana-Chavez VE, Zenteno ME, Amarante-Mendes GP, Messick JB, Timenetsky J: Ureaplasma diversum genome provides new insights about the interaction of the surface molecules of this bacterium with the host. PLoS One 2016;11:e0161926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, Chaiyasit N, Yeo L, Shaman M, Lannaman K, Cher B, Hassan SS, Gomez-Lopez N: Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med 2017;45:523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS, Panaitescu B: Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol 2017;78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, Jacques SM, Panaitescu B, Garcia-Flores V, Hassan SS: Neutrophil extracellular traps in the amniotic cavity of women with intra-amniotic infection: a new mechanism of host defense. Reprod Sci 2017;24:1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, Hassan SS, Hsu CD: The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol 2018;79:e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gomez-Lopez N, Romero R, Leng Y, Garcia-Flores V, Xu Y, Miller D, Hassan SS: Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol 2017;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Paolo NC, Shayakhmetov DM: Interleukin 1alpha and the inflammatory process. Nat Immunol 2016;17:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romero R, Mazor M, Tartakovsky B: Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991;165:969–971. [DOI] [PubMed] [Google Scholar]
- 132.Romero R, Tartakovsky B: The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992;167:1041–1045. [DOI] [PubMed] [Google Scholar]
- 133.Harris HE, Raucci A: Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep 2006;7:774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ: HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248–251. [DOI] [PubMed] [Google Scholar]
- 135.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G: Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 2006;281:36560–36568. [DOI] [PubMed] [Google Scholar]
- 136.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ: ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol 2008;10:1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J: The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 2008;452:103–107. [DOI] [PubMed] [Google Scholar]
- 138.Thomas PG, Dash P, Aldridge JR Jr., Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD: The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009;30:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP: The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009;30:556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP: Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol 2009;182:6460–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS: Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 2009;183:3578–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A: Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 2009;206:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Menu P, Vince JE: The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol 2011;166:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA: TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 2012;150:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clay GM, Sutterwala FS, Wilson ME: NLR proteins and parasitic disease. Immunol Res 2014;59:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–241. [DOI] [PubMed] [Google Scholar]
- 147.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM: Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006;440:228–232. [DOI] [PubMed] [Google Scholar]
- 148.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E: Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008;9:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J: Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS: The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A 2008;105:9035–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, Grant EP, Coyle AJ, Misaghi A, Hoffman HM, Gallo RL: NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem 2009;284:12762–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cassel SL, Joly S, Sutterwala FS: The NLRP3 inflammasome: a sensor of immune danger signals. Semin Immunol 2009;21:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cassel SL, Sutterwala FS: Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol 2010;40:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leemans JC, Cassel SL, Sutterwala FS: Sensing damage by the NLRP3 inflammasome. Immunol Rev 2011;243:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bezbradica JS, Coll RC, Schroder K: Sterile signals generate weaker and delayed macrophage NLRP3 inflammasome responses relative to microbial signals. Cell Mol Immunol 2017;14:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gomez-Lopez N, Romero R, Plazyo O, Schwenkel G, Garcia-Flores V, Unkel R, Xu Y, Leng Y, Hassan SS, Panaitescu B, Cha J, Dey SK: Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol 2017. November;217:592.e1–592.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yao Y, Chen S, Cao M, Fan X, Yang T, Huang Y, Song X, Li Y, Ye L, Shen N, Shi Y, Li X, Wang F, Qian Y: Antigen-specific CD8(+) T cell feedback activates NLRP3 inflammasome in antigen-presenting cells through perforin. Nat Commun 2017;8:15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F: Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol 2013;69:212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim CJ, Romero R, Chaemsaithong P, Kim JS: Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015;213:S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Ahn H, Panaitescu B, Vadillo-Ortega F, Sanchez-Torres C, Salisbury KS, Hassan SS: In vivo T-cell activation by a monoclonal alphaCD3epsilon antibody induces preterm labor and birth. Am J Reprod Immunol 2016;76:386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Schwenkel G, St Louis D, Hassan SS, Mial TN: In vivo activation of invariant natural killer T cells induces systemic and local alterations in T-cell subsets prior to preterm birth. Clin Exp Immunol 2017;189:211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee TH, Keating S, Busch MP, Norris PJ, Tang Q, Cruz G, Barcellos LF, Gomez-Lopez N, Romero R, MacKenzie TC: Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med 2018. April 25;10(438). [DOI] [PMC free article] [PubMed] [Google Scholar]