Abstract
Infants who die within the first weeks to months of life may have genetic disorders, though many die without a confirmed diagnosis. Non-genetic conditions may also be responsible for unexplained infant deaths, and the diagnosis may be reliant upon studies performed in the peri-mortem period. Neonatologists, obstetricians, or pediatricians caring for these children and their families may be unsure of which investigations can and should be performed in the setting of a newborn or infant who is dying or has died. Recent advances in genomic sequencing technology may provide additional diagnostic options, though the interpretation of genetic variants discovered by this technique may be contingent upon clinical phenotype information that is obtained peri-mortem or upon autopsy. We have reviewed the current literature concerning the evaluation of an unexplained neonatal or infantile demise and synthesized a diagnostic approach, with a focus on the contribution of new and emerging genomic technologies.
Introduction
Many rare diseases present during infancy, and neonates and infants with congenital anomalies or genetic disorders contribute disproportionately to perinatal mortality (1–6). Genetic factors, ranging from common genetic variants that increase the risk of disease to rare, deleterious variants that are incompatible with life, likely contribute to unexplained neonatal and infant deaths (7). However, at present, the true contribution of genetic disorders to neonatal and infant mortality is unknown due to lack of recognition and testing. Genetic conditions potentially leading to perinatal or infant death include chromosomal anomalies (e.g. Trisomy 13, Pallister Killian syndrome [tetrasomy 12p], deletion 22q11 syndrome) as well as inborn errors of metabolism and other Mendelian disorders. With the rise of prenatal cell-free fetal DNA testing, an increasing number of chromosomal disorders are being diagnosed early in pregnancy, and congenital malformations that may or may not be genetic in origin are often found by prenatal ultrasonography. Prenatal diagnosis allows families, along with the obstetrical and neonatal team, the opportunity to prepare for an infant that may only survive for a brief period after birth. Other life-threatening conditions, however, may present unexpectedly in the newborn period and lead to death. While some of these deaths may fall into the categories of “Sudden and Unexplained Early Neonatal Death (SUEND)”, “Sudden and Unexplained Death in Infancy (SUDI)” or “Sudden Infant Death Syndrome (SIDS)” many of which are likely related to environmental or multifactorial causes (8, 9), these should be viewed as diagnoses of exclusion.
Physicians caring for infants who die in the peri-mortem period are often extremely interested in investigating the underlying disorder leading to death, but may not be familiar with all of the resources available for diagnosis. In the case of an out-of-hospital demise, the infant’s primary care pediatrician may have the opportunity to collaborate with a medical examiner, coroner, or hospital pathologist to obtain samples that may aid in post-mortem diagnosis. We therefore present a review of the literature regarding investigations for a neonatal or infant death and provide testing options for pediatric care providers to optimize their chances of identifying the condition leading to death, genetic or otherwise.
Causes of Neonatal or Infant Death
There are many broad disease categories that can lead to neonatal or infant death (see Table 1 for definitions), and some presentations may fall under multiple categories. According to the most recent National Vital Statistics System data for the United States (2005–2014), congenital malformations (which may or may not be genetic in origin) represented the most common cause of infant death, followed by prematurity and low birthweight, SIDS, maternal complications, and unintentional injuries (10). Deaths in the neonatal intensive care unit (NICU) are also commonly attributed to congenital anomalies (1–5, 11, 12) with sequelae of prematurity, infection, and neurological insults such as hypoxic-ischemic encephalopathy or intracranial and intraventricular hemorrhage also frequently observed (4). In SUEND, congenital anomalies and metabolic disorders were determined to be responsible for about half of the cases explainable by autopsy in a retrospective study, followed by pulmonary hypertension and bacterial infections (13). For SUDI, the most common cause in cases explained by autopsy was found to be infection (8), though genetic cardiac arrhythmias have also been found in these cases (14). There have been many interesting recent advances in the understanding of SUEND/SUDI/SIDS cases that involve deaths in otherwise apparently healthy neonates and infants, with no explanation found on postmortem investigation, which are outside of the scope of this article. Some neonates and infants who die of unexplained causes may have very rare or even novel genetic disorders and there may be benefit from inclusion in research studies if a comprehensive evaluation is unrevealing, as the evaluation of stillbirths and critically-ill infants has led to new disease gene discovery (15–17).
Table 1.
Descriptions of specific terminology
| Terminology | Description |
|---|---|
| Mendelian disorder or disease | A genetic disorder that is caused by a change in a single genetic region (a variant in a gene or chromosome) and typically follows one of the patterns of inheritance defined by Gregor Mendel such as autosomal dominant, autosomal recessive, or X-linked. |
| Molecular genetic diagnosis or molecular diagnosis | The genetic variant thought to be responsible for a specific phenotype Example: The molecular diagnosis for an infant with Noonan syndrome is the pathogenic variant in PTPN11 or another Noonan-spectrum gene |
| Stillbirth | Death after 20 weeks’ gestation prior to delivery, resulting in non-liveborn birth. |
| Neonatal death | Death from birth to 28 days post-natal age |
| Infant death | Death from birth to one year of age |
| Perinatal death | Death from 20 weeks’ gestation through the early neonatal period |
| Sudden, unexpected early neonatal death (SUEND) | Seemingly healthy neonate with a postnatal collapse within the first week of age |
| Sudden, unexpected infant death (SUID) | Seemingly healthy infant with a postnatal collapse between 1 week and 1 year of age |
| Sudden infant death syndrome (SIDS) | An unexpected and unexplained death of an infant under 1 year of age |
Evaluation of Neonatal or Infant Death
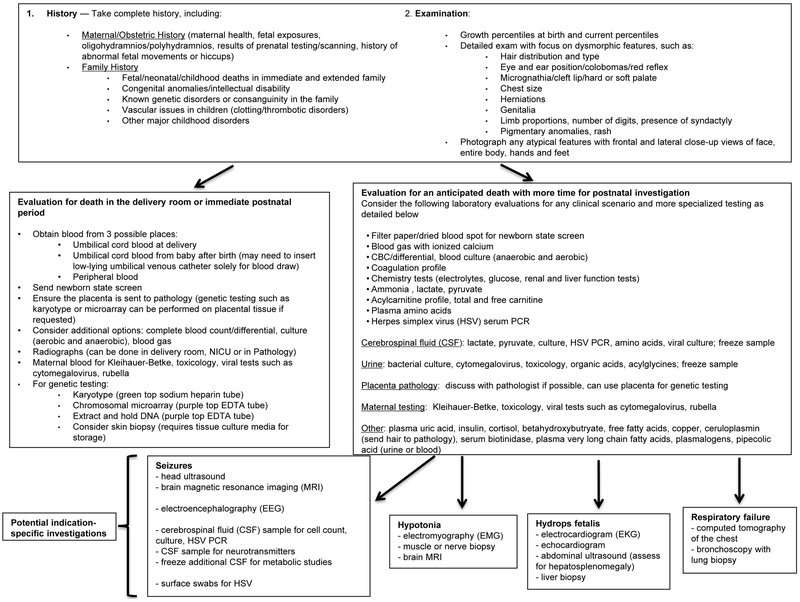
Given the diversity of etiologies known to contribute to neonatal demise, a systematic approach to the peri-mortem investigation is recommended, although the process should be tailored to the salient clinical features of a specific case. Key components of the diagnostic evaluation are reviewed below, with a particular focus on the use of whole exome sequencing (WES) and whole genome sequencing (WGS), which are becoming more easily obtained and are already changing the landscape of rare disease diagnosis. An algorithm outlining this approach is presented in Figure 1, with input from prior reviews of peri-mortem investigations (18–20). Physicians should discuss each case and approach to testing with relevant consultants, including a medical geneticist or genetic counselor where possible (consider the National Society of Genetic Counselors tool to locate a genetic counselor if necessary: https://www.nsgc.org/p/cm/ld/fid=164), pathologist, or other subspecialists as warranted.
Figure 1. Algorithm for unexpected and unexplained neonatal death.
This algorithm provides a comprehensive list of testing options; actual testing should be individualized to the neonate’s circumstance. The physician should use his or her judgment as to which investigatory approaches should be taken to ensure optimal diagnostic yield with the least amount of intervention.
History and Physical Examination
In addition to the infant’s medical history, the maternal medical history, obstetric history and family history should be reviewed carefully. The maternal history contributed to determining the cause of 26% of perinatal deaths in a retrospective study from the Wisconsin Stillbirth Service Project, a diagnostic yield approaching that of conventional autopsy in this study (30%) (21) though the yield may wane with increasing postnatal age. In the assessment for potential genetic disorders, obtaining a family history, particularly focusing on a history of birth defects, intellectual disability, recurrent (more than three) miscarriages, stillbirths, infant or early childhood deaths, or consanguinity in the family, is important. For deaths of older neonates and infants, the postnatal history becomes more relevant, and factors such as exposures and infections in addition to congenital medical conditions should be taken into account.
Clinicians should perform and document a detailed physical examination, paying close attention to possible dysmorphic features or external malformations, and to signs of external trauma (particularly for infants who have lived at home and die in the community). A recommended approach to death scene investigation in SIDS cases has been published by the Centers for Disease Control and Prevention (22). Whole-body photographs have also been shown to be helpful in the evaluation of perinatal deaths (21), and these images are easy to store and to review with specialists who may not have been immediately available at the time of death, though parental consent may be required.
Laboratory evaluation
A comprehensive approach to the laboratory evaluation is presented in Figure 1. For perinatal deaths, a maternal sample can be drawn for a Kleihauer-Betke test to evaluate for fetal-maternal hemorrhage; this has been shown to have a diagnostic yield of 8% in the Wisconsin Stillbirth Service Program (21). At a minimum, the clinician should send a newborn state screen, and it is helpful to also obtain a complete blood count, basic chemistry panel, blood gas, lactate, ammonia, and blood cultures and relevant viral studies to evaluate for infection (even if infection was not suspected clinically). For infants of any age, if an inborn error of metabolism is suspected, the basic evaluation consists of blood samples sent for amino acids, acylcarnitine profile, and total and free carnitine, as well as urine samples sent for organic acids and acylglycines. If a lumbar puncture is performed, cerebrospinal fluid (CSF) lactate, pyruvate, amino acids, and viral studies are helpful to obtain in addition to the standard CSF studies (glucose, protein, cell count, culture). Plasma amino acids, lactate, pyruvate and glucose should be obtained at the same time as CSF samples to allow for ratios to be determined which can identify disorders such as non-ketotic hyperglycinemia. If a neurotransmitter disorder is suspected as a cause of epileptic encephalopathy or other neurologic disease, this should be discussed with a neurologist or appropriate laboratory as CSF samples for these assays must be drawn into special tubes, placed on dry ice, and frozen immediately. Spare CSF should also be frozen against the possibility of further metabolic testing.
Guidelines for collecting and storing samples for genetic testing were reviewed (18) and are presented in Table 2. Blood samples for genetic testing should ideally be obtained at least 48 hours after the last blood transfusion (though packed red blood cell transfusions for neonates are typically leukoreduced and DNA is extracted from leukocytes for genetic testing). If clinicians anticipated the need for a blood transfusion, particularly transfusion of whole blood or exchange transfusion, samples for genetic testing should be obtained prior to the transfusion. The emerging utility of molecular genetic testing for diagnosis in perinatal and neonatal death is further reviewed below (see “Genetic evaluation”).
Table 2.
Considerations for sample collection for pre- or post-mortem genetic testing
| Sample type | Use for genetic testing | Collection volume | Container | Storagea |
|---|---|---|---|---|
| Whole blood for DNA extraction | DNA extracted for: ➢ Gene sequencing (including WES, single gene, gene panels, or mitochondrial genome sequencing) ➢ Other DNA-based tests such as methylation studies or chromosomal microarray |
5 mL (minimum 1-2 mL) | EDTA (purple top) tube | For immediate (within one week) use, refrigerate at 4°C For longer-term storage, freeze at −20°C to −70°C |
| Whole blood for cell culture | Cells cultured for: ➢ Karyotype ➢ FISH |
5 mL (minimum 1-2 mL) | Sodium heparin (green top) tube | Stable at ambient temperature or refrigerated (4°C) for up to 72 hours Should not be frozen |
| Placenta for cytogenetics (see below for placental samples for gene sequencing) | Cells cultured for: ➢ Karyotype ➢ FISH |
5 mg minimum Obtain sample from fetal side of placenta (villi) |
Sterile container with tissue culture media | Room temperature or refrigerated (4°C) for up to 72 hours Should not be frozen or fixed in formalin |
| Skin biopsy | Fibroblasts grown in culture for: ➢ enzyme activity testing ➢ other functional studies DNA extracted for use as above |
3 mm punch biopsy | Sterile container with tissue culture media (normal saline can be used for very brief storage) | Can be refrigerated at 4°C for up to 72 hours prior to culturing or frozen and stored at −20°C to −70°C Should not be fixed in formalin |
| Other tissue biopsy (e.g. placenta, lung, muscle, heart) | DNA extracted for gene sequencing tests RNA sequencing |
25-30 mg of tissue | Sterile container | Freeze immediately and store at −20°C to −70°C If tissue is to be used for RNA sequencing, obtain sample within hours of death if possible and flash freeze in liquid nitrogen. Cannot be fixed in formalin or paraffin-embedded |
FISH, fluorescence in situ hybridization.
If samples are to be sent to external labs, they should be sent using overnight shipping with a reliable courier. Refrigerated samples less than 72 hours old can generally be sent at room temperature, while frozen samples should be sent on dry ice in an insulated container (e.g. Styrofoam). Please contact the receiving laboratory for specific instructions.
Tissue biopsies
Samples of affected tissue may be helpful in determining the condition leading to death. Pathologists can examine tissue biopsies prepared using standard methods to look for histologic indicators of a certain disorder. For example, muscle and nerve biopsies can reveal pathognomonic features of certain neuromuscular conditions in an infant who dies in the setting of unexplained hypotonia and liver biopsies can be useful in identifying certain inborn errors of metabolism. Tissue samples obtained prior to death may also be saved in culture media and used to grow fibroblasts for additional studies, which may be helpful in confirming the functional impact of variants found through genetic testing. Skin biopsies are often used for this purpose, and this simple procedure can be performed by neonatologists, medical geneticists, general surgeons, or dermatologists. Skin biopsies obtained for the purpose of growing fibroblasts should be collected prior to or within a few hours of death if possible (Table 2).
Tissue samples obtained prior to death or by autopsy can also be used for genetic testing, provided they are obtained and stored appropriately so that DNA can later be extracted (Table 2). Biopsy samples to be used for future genetic testing should be frozen immediately, as DNA is not easily extracted from degraded or formalin-fixed, paraffin-embedded tissue (though if necessary, methods, though not ideal, do now exist). RNA sequencing has recently emerged as a useful adjunct to gene sequencing in the diagnosis of genetic disorders (as in muscle diseases such as muscular dystrophies) as it can be helpful in determining the functional impact of genetic variants by investigating the effect on transcription, particularly in affected tissues (23). However, as RNA is more sensitive to degradation than DNA, optimal timing of sample collection (ideally within hours of death) and placement in a dedicated RNA collection tube or, if necessary, immediate freezing is critical (24).
Imaging
If relevant, electrographic studies such as electromyography (EMG), electrocardiography (EKG), or electroencephalography (EEG), as well as dynamic imaging studies such as echocardiogram can be performed prior to death. Static and non-electrographic imaging studies can be performed post-mortem, such as whole-body X-rays. These images can help to visualize the positions of various medical support devices that may have contributed to death, to assess for trauma, skeletal malformations, or abnormal accumulation of gas in body cavities (suggesting particular infections), and to potentially clarify the infant’s gestational age if unknown (19). Post-mortem radiography may be an important adjunct to autopsy, and may be useful if the family declines a conventional autopsy. A whole-body radiograph was found to aid in confirmation of cause of death in 16% of stillbirths and early neonatal deaths in a recent review (21). Suggested positioning for these radiographs includes anterioposterior (AP) and lateral whole-body images. The infant’s head should be mildly extended and the arms placed at the sides, extended and supinated, with legs also extended for the AP projection. For the lateral view, the infant’s arms should be placed on the chest and the hips flexed to avoid overlapping with other structures (25). If a skeletal dysplasia is strongly suspected, a dedicated skeletal survey is preferred, as pathognomonic findings of a particular disorder, such as achondroplasia, can be seen (21).
Given declining rates of autopsy (21), there has been recent interest in the utilization of post-mortem magnetic resonance imaging (MRI) for pathologic diagnosis. A prospective study from the United Kingdom assessing concordance between conventional autopsy and “minimally invasive” autopsy, consisting of whole-body MRI with blood sampling, found high rates of concordance, at 81.0% for children under one month of age and 84.9% for ages one month to one year (26). Certain congenital anomalies, such as brain malformations, may be better visualized using MRI due to autolysis after death impacting the ability to recognize findings on autopsy (26). However, brain MRI may miss cases of hypoxic-ischemic injury as it may be performed before changes are visible by this technique (27). It is important to note that certain histological findings are valuable for the diagnosis of specific infections or other rare disorders (e.g. inborn errors of metabolism) and, therefore, MRI is not the perfect substitute for a conventional autopsy (19). Therefore, MRI may be particularly helpful when complemented by molecular genetic testing or biopsies for histological examination. One major limitation to the routine use of post-mortem MRI is the lack of technological availability and clinical expertise to perform these studies at non-academic centers.
Autopsy
Although alternative approaches now exist, a full autopsy performed by a pathologist provides invaluable information. While the existing literature focuses predominantly on the evaluation following stillbirth (21, 28), there is limited information available concerning the yield of autopsy in the neonatal period. A study of NICU deaths in Portugal found that autopsy resulted in an explanatory or new diagnosis in 24.5% of cases over five years (12). In a series of SUDI cases from the United Kingdom, autopsy identified the etiology leading to death in 37%, with the cause found via macroscopic or histological examination for 76% of the explained cases (8). Another retrospective analysis revealed that over half of early neonatal deaths SUEND were etiologically explained following autopsy (13), and a small series of neonatal deaths in Turkey found that autopsy identified a new diagnosis in 27% (29).
Autopsy typically consists of an external examination followed by evaluation of the internal organs. For perinatal deaths, this would include examination of the placenta and umbilical cord. Placental pathology has been shown to help identify the cause of death in approximately 30% of cases of perinatal death, though this information is not as helpful for older infants (21). Placental samples can also be sent for cytogenetic or molecular genetic testing (see below) (19). For genetic testing of the placenta, a portion of the fetal side (i.e., the villi) should be saved and can be submitted as a fresh sample for cytogenetic or molecular genetic studies; samples can also be frozen for potential further genetic testing (of note, this provides the added benefit of being covered under the mother’s insurance as testing of a product of conception) (21). An important caveat to placental genetic testing is the possibility of fetal-placental discordance for genetic testing results, with a genetic abnormality either restricted to the placenta or absent from the placenta but present in the fetus.
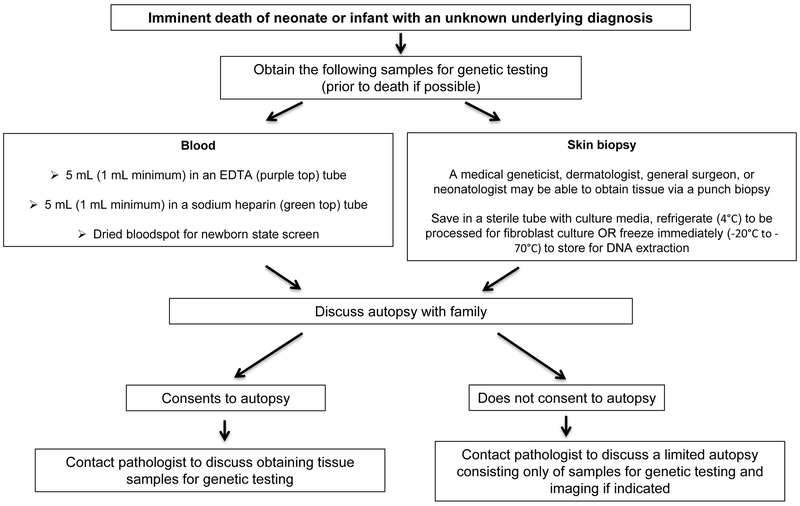
The external and internal examinations are complemented by imaging and laboratory tests (particularly microbiological or cytogenetic) (19). Special consent may be required to examine the eyes, of particular importance in suspected inborn errors of metabolism and certain other genetic disorders. Evaluation of the internal organs includes anthropomorphic measurements, a macroscopic evaluation for congenital malformations and other visible abnormalities, and a microscopic, histological evaluation with special stains if indicated, such as the use of oil-red O staining to look for abnormal deposition of fat in organs as can be seen in certain inborn errors of metabolism (19, 30). If parents are reluctant to consent to a complete autopsy, the internal examination can be limited to specific body parts. If an internal examination is declined entirely, information can still be gained by a less-invasive autopsy with or without isolated biopsy specimen options (Figure 2). For example, on external exam, dysmorphic features and skin lesions can be assessed and photographed (19).
Figure 2. Molecular autopsy for unexplained neonatal death.
Guidelines for obtaining samples for genetic evaluation. Tissue biopsies from affected organs can also be obtained prior to death. Pre- or post-mortem biopsied tissue to be saved for genetic testing should be frozen immediately, not formalin-fixed or paraffin-embedded. See Table 2 for further details on collection and storage.
If a sample for genetic testing has not been obtained prior to death, samples may be obtained and stored during autopsy for a molecular genetic evaluation (Figure 2, Table 2). It is important to emphasize that biopsy samples obtained by autopsy for future genetic testing (i.e. more than two days later) should ideally be frozen immediately.
Genetic evaluation: the “molecular autopsy”
Historically, the genetic evaluation of fetal and perinatal death was restricted to cytogenetic techniques, such as karyotype or fluorescence in situ hybridization (FISH), to assess for common aneuploidies such as Trisomies 21, 18, or 13. More recently, chromosomal microarray has been recommended as the first-line test in the setting of multiple congenital anomalies (31), including those found prenatally (32) (often replacing karyotype) and in the setting of stillbirths (33). While chromosomal microarray can detect aneuploidy in addition to microdeletions and duplications that may not be visible on standard karyotype, this test may miss balanced translocations (which may be clinically relevant due to the interruption of genes within the breakpoints of the translocation (34)). Chromosomal microarray may also miss triploidy (three copies of each chromosome) depending on the technology used. In stillbirths and early neonatal deaths, basic chromosomal analysis has demonstrated a 15% yield in determining an etiology of death (21). For deaths in the NICU, an older study found 23.3% were due to a genetic disorder, though molecular diagnoses were few and chromosomal in nature (3), and a recent retrospective analysis attributed 5% of deaths to a “genetic syndrome” (4). However, as many deceased infants have not undergone genetic testing or have had only a karyotype or chromosomal microarray, which will generally not detect single gene disorders, Mendelian disorders are likely under-diagnosed in this population.
In recent years, with the rise of massively-parallel (“next generation”) sequencing technologies, gene sequencing, particularly whole exome sequencing (WES) or whole genome sequencing (WGS), has become faster and cheaper and is therefore more easily available to investigate neonatal and infant deaths. WES involves sequencing of all of the protein-coding genes, which comprise 1–2% of the genome (35), while WGS includes sequencing the non-coding regions of the genome that may contain regulatory elements. Although significantly more costly, WGS may detect variants not found by WES. Rather than focusing on sequencing genes associated with a particular condition, such as an epilepsy gene panel, WES or WGS allows for a broader approach. This may be of particular value for unexplained perinatal or neonatal deaths, as these cases are less likely to have a well-defined phenotype suggesting a specific genetic test. Furthermore, sequencing a single gene or panel of genes may be less desirable in the peri-mortem setting, as there may not be an opportunity for further testing if these initial tests return negative. Additionally, WES and WGS allow an investigator to re-evaluate the data at a future point when better understanding of genetic variants, that may not have been present at the time of initial analysis, can be incorporated into the diagnostic review. This approach has been shown to increase the total diagnostic yield of WES by 11% 12 months after the initial analysis (36). Such downstream reviews not uncommonly are requested when the mother is either pregnant, or the parents are planning for another child. It is thought that “hypothesis-free” approaches, including the use of massively-parallel sequencing technologies for WES or WGS coupled with metabolomic analyses, may represent the future of the perinatal investigation (37). However, it is still important to provide as much ancillary information as possible, as interpretation of the many variants found is critically dependent upon this information. The use of WES or WGS for diagnosis of deceased patients has been termed the “molecular autopsy” and has demonstrated utility in cases of fetal death (15, 38). A study in Japan focusing on 129 genes related to inborn errors of metabolism and cardiac arrhythmias in a cohort of 71 cases of unexplained infant death found possible postmortem diagnoses in 11 infants (39). It has been suggested that WGS should be pursued in all cases of unexplained infant death (7), though a particular challenge to post-mortem testing in the United States is insurance approval, as testing that is ordered after a child has died will generally not be covered by the child’s insurance. Some companies will provide postmortem testing at a reduced cost as a compassionate service, and gene sequencing can sometimes be pursued via a research protocol that will not be billed to the family.
The diagnostic yield of WES has been previously demonstrated to be 25% in a predominantly pediatric cohort (40), and the use of WES or WGS for the diagnosis of critically-ill neonates has been increasing in popularity, with a diagnostic yield of about 50% (16, 17, 41–44). It may therefore be anticipated that the known genetic contribution to neonatal death will increase over time as more diagnoses are realized, although these studies typically do not include samples taken postmortem. In addition, the molecular autopsy in perinatal death holds great potential to increase the understanding of known disease genes and for novel disease gene discovery, as extreme phenotypes, such as in utero lethality, are likely attributable to highly-damaging variants in genes associated with prenatal human development (15, 38). As an example, a recent study using WES for diagnosis of perinatal deaths found that 32% of diagnoses represented disorders that had not previously been described as perinatal lethal and 32% of diagnoses resulted in the description of novel disease genes (15).
Ideally, WES or WGS should be performed as a trio, with samples from the mother, father, and child. This aids in diagnostic yield, as it improves the ability to detect de novo disease-causing variants (those that arose anew in the child), and to confirm the phase of variants that are inherited in a suspected autosomal recessive pattern (one variant inherited from each parent). Regardless, the yield using proband- (affected child) only exome has also been established in infancy (44). Additionally, in cases of fetal death with no available fetal sample, sequencing of only the parents can suggest a genetic diagnosis, particularly in consanguineous families (15). WES or WGS can be performed on a blood sample or from tissue samples that have been properly collected and stored (Table 2). WES from a clinical laboratory has a turnaround time of approximately 3–4 months, although protocols have been established on a research basis for rapid WGS, with a turnaround time of 26 hours (17, 41–43). Clinical genetic testing laboratories now offer a “rapid exome” product with a 1–2 week turnaround time that may be useful in the event of an impending neonatal death as results may return in time to impact management or aid in decisions regarding redirection of care, as has been demonstrated in recent studies (16, 41, 42, 44, 45).
There are multiple possible results from WES or WGS (as well as from other genetic tests). A “positive” result indicates that a variant was found in a known disease gene that either is known to cause disease or is very likely to cause disease on the basis of established criteria (46). It is important however, to correlate the clinical findings with the genetic results, especially in cases reported as “likely pathogenic” as this is not, of itself, confirmatory. A “negative” result means that no such variant was found, though does not necessarily mean that the disorder is not genetic. There are also results that are unclear, termed “variants of uncertain significance”, or VUS. In order to establish causality from a VUS, supporting data from other investigations, such as other laboratory tests or tissue biopsies, may be critical. For example, if a VUS is found in a gene associated with an inborn error of metabolism, the results of the basic metabolic evaluation (amino acids, acylcarnitines, acylglycines, organic acids) may help to support or refute the diagnosis. If a skin biopsy was obtained and fibroblasts cultured, enzyme activity can be measured in the fibroblasts that can also support or refute the diagnosis. Histological or imaging studies may also provide information important to the interpretation of the genomic data.
When pursuing genetic testing, particularly WES or WGS, the involvement of a medical geneticist or genetic counselor is important, as pre- and post-test counseling and informed consent are essential aspects of the testing process. Many centers do not have direct access to a clinical geneticist, or to a cytogenetics or molecular genetics laboratory. If the appropriate samples are saved, particularly frozen blood in an EDTA (purple top) tube, genetic testing can still be coordinated post-mortem with the aid of professional societies, such as the National Society of Genetic Counselors (NSGC). The NSGC has developed a website detailing resources available for post-mortem genetic evaluations, including appropriate post-mortem samples to be saved for genetic testing; an information sheet for family members; an authorization form to release autopsy results and samples for testing; sample letters of medical necessity for insurance companies; and contact information for reaching the NSGC Post-mortem Group of genetic counselors (47).
Importance of Finding a Diagnosis
While a diagnosis obtained prior to death can provide information to aid in the decision to limit therapies that are considered futile, or to aid in the decision to withdraw life-sustaining medical technologies (or even suggest interventions to prevent neonatal demise), a confirmed diagnosis that is obtained post-mortem can still be valuable to the family. In the setting of a genetic disorder, there may be implications for reproductive counseling to the parents regarding recurrence risk (the chance of having another affected child). If a molecular genetic diagnosis is found, clinicians can offer families the opportunity for reproductive planning using in vitro fertilization with pre-implantation genetic diagnosis to avoid having another child with the same condition. There may also be implications for testing of other family members who may be hidden carriers of a disease-causing variant that has not yet manifested clinically (e.g. inherited cardiac arrhythmias). Regardless of the etiology, understanding the reasons for a child’s death can be a crucial part of the grieving process and is important to the medical community in preventing future deaths.
Conclusion
In the event of a perinatal death or death in early infancy, many options exist for investigation of the underlying cause. Traditional investigations, including a history and physical examination and a complete autopsy, provide a large amount of useful information. Newer genomic sequencing technologies hold expanding potential to provide answers to families and care providers and to provide valuable information to the medical and scientific community.
Table 3.
Glossary of acronyms
| Acronym | Meaning |
|---|---|
| CSF | Cerebrospinal fluid |
| EEG | Electroencephalography |
| EMG | Electromyography |
| EKG | Electrocardiography |
| FISH | Fluorescence In Situ Hybridization |
| HSV | Herpes Simplex Virus |
| MRI | Magnetic Resonance Imaging |
| NICU | Neonatal Intensive Care Unit |
| NSGC | National Society of Genetic Counselors |
| PCR | Polymerase Chain Reaction |
| SIDS | Sudden Infant Death Syndrome |
| SUDI | Sudden Unexpected Death in Infancy |
| SUEND | Sudden Unexpected Early Neonatal Death |
| VUS | Variant of Uncertain Significance |
| WES | Whole Exome Sequencing |
| WGS | Whole Genome Sequencing |
Acknowledgments
We thank Drs. Christine Bryke and Jonathan Hecht for their assistance in the development of an earlier version of our clinical algorithm for use at Beth Israel Deaconess Medical Center. MH Wojcik is supported by NIH T32GM007748.
Funding Source: NIH T32GM007748 [MHW]
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a post-peer-review, pre-copyedit version of an article published in the Journal of Perinatology. The final authenticated version is available online at: http://dx.doi.org/10.1038/s41372-018-0187-7
References
- 1.Sankaran K, Chien LY, Walker R, Seshia M, Ohlsson A, Canadian Neonatal N. Variations in mortality rates among Canadian neonatal intensive care units. CMAJ. 2002;166(2):173–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson CD, Ye XY, Hellmann J, Tomlinson C. Trends in cause-specific mortality at a Canadian outborn NICU. Pediatrics. 2010;126(6):1538. [DOI] [PubMed] [Google Scholar]
- 3.Hudome SM, Kirby RS, Senner JW, Cunniff C. Contribution of genetic disorders to neonatal mortality in a regional intensive care setting. Am J Perinatol 1994;11(2):100–3. [DOI] [PubMed] [Google Scholar]
- 4.Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135(1):59. [DOI] [PubMed] [Google Scholar]
- 5.Weiner J, Sharma J, Lantos J, Kilbride H. How infants die in the neonatal intensive care unit: trends from 1999 through 2008. Arch Pediatr Adolesc Med 2011;165(7):630–4. [DOI] [PubMed] [Google Scholar]
- 6.Wojcik MH, Schwartz T, Yamin I, Edward H, Genetti C, Towne M, et al. Genetic Disorders and Mortality in Infancy and Early Childhood: Delayed Diagnoses and Missed Opportunities. Genet Med Epub ahead of print 12 April 2018; doi: 10.1038/gim.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JA. The genomic load of deleterious mutations: relevance to death in infancy and childhood. Front Immunol 2015;6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber MA, Ashworth MT, Risdon RA, Hartley JC, Malone M, Sebire NJ. The role of post-mortem investigations in determining the cause of sudden unexpected death in infancy. Arch Dis Child. 2008;93(12):1048–53. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein RD, Kinney HC, Willinger M. Sudden Unexpected Death in Fetal Life Through Early Childhood. Pediatrics. 2016;137(6):4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathews TJ, Driscoll AK. Trends in Infant Mortality in the United States, 2005–2014 NCHS data brief, no 279. Hyattsville, MD:National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 11.Michel MC, Colaizy TT, Klein JM, Segar JL, Bell EF. Causes and circumstances of death in a neonatal unit over 20 years. Pediatr Res 2018;83(4):829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa S, Rodrigues M, Centeno MJ, Martins A, Vilan A, Brandao O, et al. Diagnosis and cause of death in a neonatal intensive care unit--how important is autopsy? J Matern Fetal Neonatal Med 2011;24(5):760–3. [DOI] [PubMed] [Google Scholar]
- 13.Weber MA, Ashworth MT, Risdon RA, Brooke I, Malone M, Sebire NJ. Sudden unexpected neonatal death in the first week of life: autopsy findings from a specialist centre. J Matern Fetal Neonatal Med 2009;22(5):398–404. [DOI] [PubMed] [Google Scholar]
- 14.Baruteau AE, Tester DJ, Kapplinger JD, Ackerman MJ, Behr ER. Sudden infant death syndrome and inherited cardiac conditions. Nat Rev Cardiol 2017;14(12):715–726. [DOI] [PubMed] [Google Scholar]
- 15.Shamseldin HE, Kurdi W, Almusafri F, Alnemer M, Alkaff A, Babay Z, et al. Molecular autopsy in maternal-fetal medicine. Genet Med 2018;20(4):420–427. [DOI] [PubMed] [Google Scholar]
- 16.Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of Exome Sequencing for Infants in Intensive Care Units: Ascertainment of Severe Single-Gene Disorders and Effect on Medical Management. JAMA Pediatr 2017;171(12):e173438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med 2012;4(154):154ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middleton O, Baxter S, Demo E, Honeywell C, Jentzen J, Miller F, et al. National Association of Medical Examiners Position Paper: Retaining Postmortem Samples for Genetic Testing. Acad Forensic Pathol 2013;3(2):191–4. [Google Scholar]
- 19.Wright C, Lee RE. Investigating perinatal death: a review of the options when autopsy consent is refused. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.British Association for Perinatal Medicine. Guidelines for the investigation of newborn infants who suffer a sudden and unexpected postnatal collapse in the first week of life: Recommendations from a professional group on sudden unexpected postnatal collapse. London; Wellchild: 2011. [Google Scholar]
- 21.McPherson E, Nestoridi E, Heinke D, Roberts DJ, Fretts R, Yazdy MM, et al. Alternatives to Autopsy for Fetal and Early Neonatal (Perinatal) Deaths: Insights from the Wisconsin Stillbirth Service Program. Birth Defects Res 2017;109(18):1430–1441. [DOI] [PubMed] [Google Scholar]
- 22.Iyasu S, Rowley DL, Hanzlick RL. Guidelines for Death Scene Investigation of Sudden, Unexplained Infant Deaths: Recommendations of the Interagency Panel on Sudden Infant Death Syndrome. Morbidity and Mortality Weekly Report: Recommendations and Reports. 1996;45(RR-10):22. [Google Scholar]
- 23.Cummings BB, Marshall JL, Tukiainen T, Lek M, Donkervoort S, Foley AR, et al. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med 2017;9(386): 10.1126/scitranslmed.aal5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project. Biopreserv Biobank. 2015;13(5):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griscom NT, Driscoll SG. Radiography of stillborn fetuses and infants dying at birth. Am J Roentgenol 1980;134(3):485–9. [DOI] [PubMed] [Google Scholar]
- 26.Thayyil S, Sebire NJ, Chitty LS, Wade A, Chong W, Olsen O, et al. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet. 2013;382(9888):223–33. [DOI] [PubMed] [Google Scholar]
- 27.Leadbetter KZ, Vesoulis ZA, White FV, Schmidt RE, Khanna G, Shimony JS, et al. The role of post-mortem MRI in the neonatal intensive care unit. J Perinatol 2017;37(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nijkamp JW, Sebire NJ, Bouman K, Korteweg FJ, Erwich JJHM, Gordijn SJ. Perinatal death investigations: What is current practice? Semin Fetal Neonatal Med 2017;22(3):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nese N, Bulbul Y. Diagnostic value of perinatal autopsies: analysis of 486 cases. J Perinat Med 2018;46(2):175–181. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Mishima H, Mizukami H, Fukahori Y, Umehara T, Murase T, et al. Metabolic autopsy with next generation sequencing in sudden unexpected death in infancy: Postmortem diagnosis of fatty acid oxidation disorders. Mol Genet Metab Rep 2015;5:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet 2010;86(5):749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wapner RJ, Martin CL, Levy B, Ballif BC, Eng CM, Zachary JM, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med 2012;367(23):2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy UM, Page GP, Saade GR, Silver RM, Thorsten VR, Parker CB, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med 2012;367(23):2185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talkowski ME, Ordulu Z, Pillalamarri V, Benson CB, Blumenthal I, Connolly S, et al. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N Engl J Med 2012;367(23):2226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grody WW, Thompson BH, Hudgins L. Whole-exome/genome sequencing and genomics. Pediatrics. 2013;132(Suppl 3):211. [DOI] [PubMed] [Google Scholar]
- 36.Ewans LJ, Schofield D, Shrestha R, Zhu Y, Gayevskiy V, Ying K, et al. Whole-exome sequencing reanalysis at 12 months boosts diagnosis and is cost-effective when applied early in Mendelian disorders. Genet Med. Epub ahead of print 29 March 2018. doi: 10.1038/gim.2018.39. [DOI] [PubMed] [Google Scholar]
- 37.Hutchinson JC, Arthurs OJ, Sebire NJ. Postmortem research: innovations and future directions for the perinatal and paediatric autopsy. Arch Dis Child Educ Pract Ed. 2016;101(1):54–6. [DOI] [PubMed] [Google Scholar]
- 38.Filges I, Friedman JM. Exome sequencing for gene discovery in lethal fetal disorders--harnessing the value of extreme phenotypes. Prenat Diagn 2015;35(10):1005–9. [DOI] [PubMed] [Google Scholar]
- 39.Oshima Y, Yamamoto T, Ishikawa T, Mishima H, Matsusue A, Umehara T, et al. Postmortem genetic analysis of sudden unexpected death in infancy: neonatal genetic screening may enable the prevention of sudden infant death. J Hum Genet 2017;62(11):989–95. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med 2013;369(16):1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soden SE, Saunders CJ, Willig LK, Farrow EG, Smith LD, Petrikin JE, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med 2014;6(265):265ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med 2015;3(5):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith LD, Willig LK, Kingsmore SF. Whole-Exome Sequencing and Whole-Genome Sequencing in Critically Ill Neonates Suspected to Have Single-Gene Disorders. Cold Spring Harb Perspect Med 2015;6(2):a023168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med 2016;18(11):1090–6. [DOI] [PubMed] [Google Scholar]
- 45.Tan TY, Dillon OJ, Stark Z, Schofield D, Alam K, Shrestha R, et al. Diagnostic Impact and Cost-effectiveness of Whole-Exome Sequencing for Ambulant Children With Suspected Monogenic Conditions. JAMA Pediatr 2017;171(9):855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Society of Genetic Counselors. Postmortem Genetic Testing FAQs. https://www.nsgc.org/postmortem. Updated 2017. Accessed 11/14/2017. [Google Scholar]