Abstract
Background
Cardiac remote ischemic conditioning (RIC) is a noninvasive cardioprotective method in ischemia-reperfusion injury and acute myocardial infarction (AMI). The aims of this study were to investigate the effects of RIC in a rat model of AMI.
Material/Methods
Adult male Sprague-Dawley rats included the AMI group that underwent ligation of the left anterior descending (LAD) coronary artery (n=24), the RIC group that consisted the AMI rat model treated with RIC once daily in the left hind limb until days 1, 7 and 14 (n=24), and the sham group (n=24). Myocardial infarct size was measured by routine histology with triphenyltetrazolium chloride (TTC) and Masson’s trichrome histochemical staining for myocardial necrosis and fibrosis, respectively. Serum levels of Bcl-2, Bax, caspase-3, and inducible nitric oxide synthase (iNOS) were measured by enzyme-linked immunosorbent assay (ELISA). The apoptosis index was detected using the TUNEL assay. Spectrophotometry of the myocardium was used to identify mitochondrial complexes and myocardial ATP.
Results
The RIC group showed improved cardiac hemodynamics, reduced the size of the myocardial infarction, upregulated expression of Bcl-2, and down-regulation of the levels of Bax, caspase-3, and iNOS, and reduced cardiac myocyte apoptosis and inhibited the opening of the mitochondrial permeability transition pore (MPTP).
Conclusions
In a rat model of AMI, RIC improved the hemodynamic index, reduce the levels of apoptosis and myocardial injury, and improved mitochondrial function.
MeSH Keywords: Endomyocardial Fibrosis, Mitochondria, Myocardial Infarction
Background
Clinically, several preventive methods have been used in acute myocardial infarction (AMI). Ischemic preconditioning is an intervention that does not require drug treatment and has been used in preclinical and clinical research. Ischemic preconditioning was first described in 1986 by Murry et al., and Gho et al. established the use of the non-invasive method of remote ischemic conditioning (RIC), which was applied to organs and tissue that were distant or remote from the heart, such as the kidney, brain, liver, small intestine, and distal limbs [1–3]. The method of RIC can enhance the tolerance of the myocardium to acute ischemia-reperfusion injury and reduces the degree of myocardial damage [3,4]. RIC can also have a protective role in ischemia-reperfusion injury in other organs. Therefore, RIC can improve clinical efficacy and prognosis, and it has practical clinical application.
We have previously reported the findings from the use of non-invasive limb ischemic preconditioning (LIP), used once daily for three days using RIC before ischemia-reperfusion injury of the heart in a rat model [4]. This previous study showed that RIC significantly reduced infarct size after ischemia-reperfusion injury, inhibited apoptosis, enhance cardiac tolerance to ischemia-reperfusion injury, and improved cardiac function [4]. We have also previously investigated whether long-term (4–8 weeks) non-invasive (RIC) have accelerated the recovery in a rat model of chronic myocardial infarction [5]. The findings from this previous study showed that RIC reduced myocardial injury and the levels of myocardial peroxidation in chronic myocardial infarction and significantly improved survival [5].
There have been few previously published studies on the role of RIC as an intervention during the onset of acute myocardial infarction (AMI). In 2007, Schmidt et al. first demonstrated the use of remote intermittent peripheral tissue ischemia to reduce myocardial infarction during cardiac ischemia due to coronary artery occlusion [6]. In 2010, Bøtker et al. reported the findings from a randomized clinical trial on the role of RIC before hospital admission, combined with angioplasty, in patients with AMI [7]. In 2016, Kloner reviewed the benefits and limitations of the use of non-invasive RIC from several large clinical trials in which the findings were controversial [8]. However, these previous studies reviewed single applications of RIC during AMI, the measured clinical indicators were limited, and monitoring and follow-up of the effects on AMI varied.
Based on the previous findings of its cardioprotective role in ischemia-reperfusion injury in chronic myocardial infarction using non-invasive RIC, this study aimed to investigate the effects of non-invasive RIC in a rat model of AMI.
Material and Methods
Animals
Specific pathogen-free (SPF) adult male Sprague-Dawley rats (mean weight 250±10 g) were obtained from the Experimental Animals Research Institute of the Military Medical Science Academy of China (SCXK-2012-0004). Rats were fed and housed in a standard animal room. All animal experiments were conducted according to the experimental protocol approved by the Laboratory Animal Care and Use Ethical Committee of Tianjin Medical University. Animal care and use were performed as required in accordance with both guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals in China and the US National Institutes of Health (NIH).
The rat model of acute myocardial infarction (AMI) and cardiac remote ischemic conditioning (RIC)
General surgical preparation of the rats was performed according to the method previously described by An et al. [5]. Rats were anesthetized with pentobarbital sodium (Sigma-Aldrich, USA) at a dose of 45 mg/kg (intraperitoneal). The trachea of each animal was intubated and ventilated with an HX-300 animal respirator (Chengdu Technology and Market Co., Ltd., China). After left thoracotomy and pericardiotomy, the left anterior descending (LAD) coronary artery was ligated at 2 mm from the lower edge of the left atrial appendage. The elevated ST-segment in the electrocardiogram (ECG) indicated that myocardial ischemia was confirmed. After surgery, all rats were placed in a warm and dry place to keep their temperatures constant and were given an intramuscular injection of penicillin sodium (80 U) to prevent infection. The model was successful after two weeks.
Non-invasive blood pressure measurements used a cuff around the thigh of the left hind limb in anesthetized rats, and a pulse sensor was placed over the dorsal artery on the surface of the foot (BL-420E Biofunction Laboratory System, Taimeng Technology Co., China) to perform cardiac remote ischemic conditioning (RIC). RIC was repeated using 5 min of inflation in the left femoral artery and 5 min of deflation for four cycles once daily on days 1, 7, and 14 of the study, as previously described [9]. Skin cyanosis and lower limb temperature indicated successful ischemia, and skin flushing and an increase in limb temperature indicated successful reperfusion.
Experimental protocol in the three rat groups
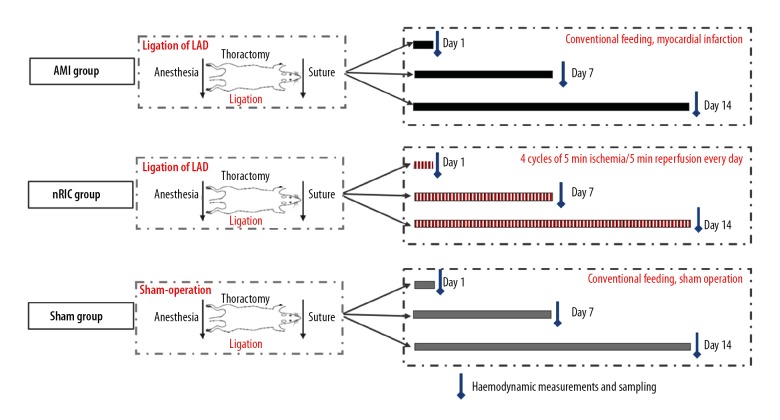
The model rats were randomly divided into three groups (Figure 1). The AMI group underwent ligation of the left anterior descending (LAD) coronary artery (n=24), the RIC that consisted of the AMI rat model treated with RIC once daily in the left hind limb until days 1, 7 and 14 (n=24), and the sham group (n=24). The AMI group were fed routinely until days 1, 7 and 14 (8 rats per session). The RIC group consisted of the rat model treated with RIC once daily in the left hind limb until days 1, 7 and 14 (8 rats per session). The sham group included rats that underwent sham surgery that did not include LAD coronary artery ligation and occlusion, fed routinely until days 1, 7 and 14 (8 rats per session).
Figure 1.
The experimental protocol used to investigate the effects of non-invasive remote ischemic conditioning (RIC) on the acute myocardial infarction (AMI) in three groups of rats. In the acute myocardial infarction (AMI group) (n=24), the left anterior descending (LAD) coronary artery was ligated under spontaneous breathing conditions in the rat. The remote ischemic conditioning (RIC) group (n=24) included rats with AMI and four cycles of 5 minutes of ischemia were followed by 5 minutes of reperfusion each day on days 1, 7, and 14. In the sham group (n=24), there was no intervention.
Measurements of hemodynamic parameters
At days 1, 7 and 14 of the study, the blood pressure was measured by carotid artery intubation after anesthesia in all rats. Left ventricular (LV) catheterization was performed to record the pressure curves after the heart rate and blood pressure stabilized. All rats were anesthetized and the right carotid artery was cannulated with a heparin-filled polyethylene catheter for blood pressure monitoring. Each animal’s trachea was intubated. After the heart rate and blood pressure were stable, a catheter was continuously inserted toward the heart while observing the left ventricular pressure curve. After stabilizing the cardiac parameters for 10 min, the left ventricular systolic pressure (LVSP), the left ventricular end-diastolic pressure (LVEDP), +dp/dtmax, and −dp/dtmax were measured and an electrocardiogram (ECG) was performed using a BL-420E Biofunction Laboratory System (Taimeng Technology Co., China) which was controlled and sampled by computer.
Examination of the rat hearts at days 1, 7, and 14
After the rats were euthanized at days 1,7 and 14, the rat hearts were removed. The heart weight to body weight ratio (heart weight/body weight ratio) was measured using an electronic balance. Myocardial tissue from the anterior wall of the left ventricle was sampled at days 1, 7 and 14. Histology was performed on the rat cardiac tissue with routine hematoxylin and eosin (H&E) staining for light microscopy. Myocardial infarct size was measured using triphenyltetrazolium chloride (TTC) and Masson’s trichrome histochemical staining (Sigma-Aldrich, St. Louis MO, USA) for myocardial necrosis and fibrosis, respectively.
Measurement of B-cell lymphoma-2 (Bcl-2), Bcl-2-associated protein (Bax) and caspase-3 in the rat serum
Whole blood of the rats was collected from the abdominal aorta after they were euthanized at days 1, 7, and 14. The serum of the rats was separated to measure the amount of Bcl-2, Bax, and caspase-3. Serum was obtained by centrifuging whole blood at 3000 rpm for 15 min at 4°C. An enzyme-linked immunoassay (ELISA) method was used with primary antibodies to Bcl-2, and Bax (Cusabio, Houston, TX, USA) and to caspase-3 (LifeSpan BioScience, Seattle, WA, USA), according to the manufacturer’s instructions.
Measurement of apoptosis in the rat myocardium
Apoptosis was examined using the TUNEL with an Apoptosis Detection Kit (Roche, Basel, Switzerland), according to the manufacturer’s instructions. At days 1, 7, and 14, myocardial tissue was sampled from rats in each group. Two slices from each rat heart fixed, processed, and sectioned for histology. The histochemically stained tissue sections were examined by light microscopy, and five visual fields were selected randomly from the ischemic penumbra of each section at ×400 magnification. The ratio of positive apoptotic cells and total cells was used to express the apoptosis index.
Measurement of mitochondrial respiratory chain complexes I, II, III, and IV, adenosine triphosphate (ATP), and nitric oxide synthase (NOS) activity in the rat myocardium
At days 1, 7 and 14, myocardial tissues were taken from the anterior wall of the left ventricle of the rats. The content of the mitochondrial respiratory chain complexes I, II, III, and IV, NOS, ATP and the opening of the mitochondrial permeability transition pore (MPTP) of the extracted supernatants of the rats were measured after the tissue was homogenized and centrifuged at 3,000 rpm for 10 min at 4°C. ELISA detection kits were used and the assays were performed according to the manufacturers’ instructions (Shanghai Jiemei Gene Technology Co., Ltd., China; LifeSpan BioScience, Seattle, WA, USA; Sigma-Aldrich, St. Louis MO, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Two-way analysis of variance (ANOVA) and multiple comparison analysis was used to determine the difference between the groups. Student’s t-test was used for continuous normal variables, and a non-parametric test was used for variables with a non-normal distribution. P<0.05 and P<0.01 were considered statistically significant. All data analysis was performed using SPSS version 21.0 (IBM, Chicago, IL, USA) and GraphPad Prism version 7 (GraphPad Software, La Jolla, CA, USA).
Results
Effects of cardiac remote ischemic conditioning (RIC) on hemodynamics in the rat model of acute myocardial infarction (AMI)
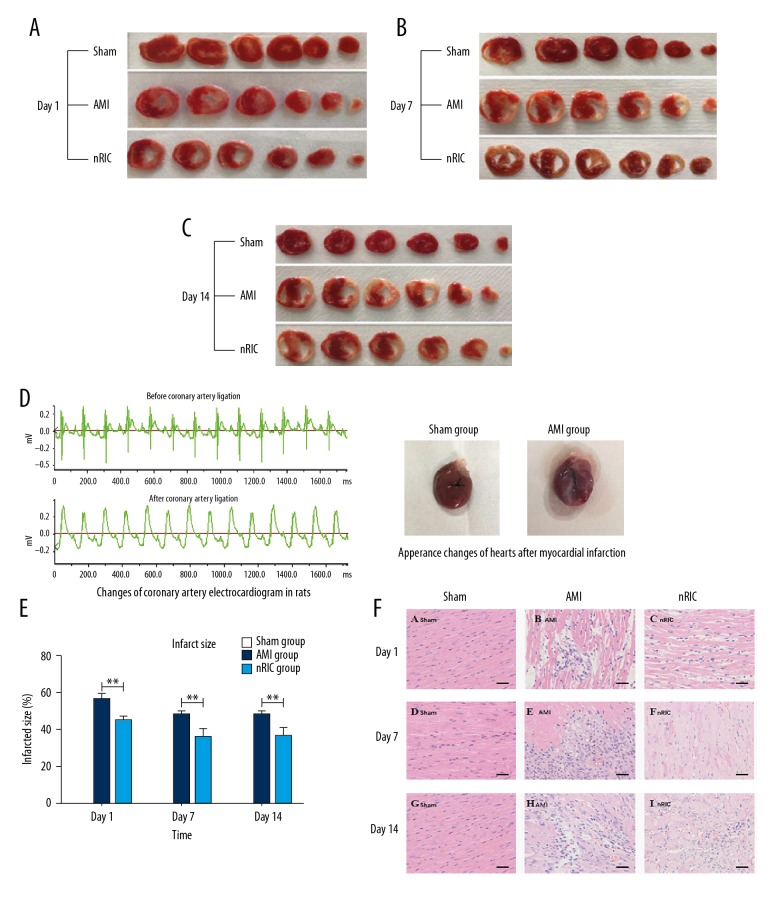
The hearts of the AMI group showed a large area of necrosis in the left ventricle, the infarct area of the anterior wall of the left ventricle became paler and thinner when compared with the sham group. Also, part of the left ventricle wall collapsed, and the amplitude of regional wall motion decreased, which was significantly different from the non-infarct area (Figure 2A–2D). Compared with the AMI rat model group, RIC significantly reduced the infarct size by 20.3%, 27.4% and 24.4% at days 1, 7 and 14, respectively (P<0.01) (Figure 2E). In the AMI group, a large number of myocardial cells were necrotic, myocardial fibers were disrupted, there was myocyte disarray, and a large number of inflammatory cells infiltrated between the cardiac myocytes when compared with the normal cardiac histology of the sham group. The histologic changes were reduced in the RIC group (Figure 2F).
Figure 2.
Comparison of the electrocardiography (ECG) and histological changes between the three rat groups, the sham group, the group with acute myocardial infarction (the AMI group), and the remote ischemic conditioning (RIC) group. (A–C) There was no myocardial infarction in the sham group. The cardiac infarct area in the AMI group was the largest among the three groups. (D) According to lead II of the electrocardiogram (ECG), the ST segment was elevated >0.2 mV after coronary artery ligation. The change in the appearance of the hearts changed after induction of acute myocardial infarction (AMI) is shown. (E) Cardiac remote ischemic conditioning (RIC) significantly reduced infarct size after AMI. (F) Photomicrograph of the histology of the myocardium shows that the myocardial fibers in the sham group are regularly arranged, the cardiac myocytes are normal, and there is no necrosis of inflammation. In the RIC group, compared with the AMI group, the degree of myocardial inflammation, cell necrosis, and injury to the cardiac myocytes was less than in the AMI group. Hematoxylin and eosin (H&E). Scale bar=200 μm. * P<0.05, ** P<0.01 vs. the AMI group. Data are presented as mean ± standard deviation (SD) (n=24) (8 rats per session).
Effects of RIC on heart rate, infarct size, and myocardial injury in the rat model of AMI
The left ventricular systolic pressure (LVSP) of the RIC group increased by 13.0% and 14.6%, −dp/dtmax increased by 34.2% and 29.8% at days 7 and 14, respectively (P<0.05, P<0.01 vs. the AMI group). The left ventricular end-diastolic pressure (LVEDP) decreased by 35.6%, 21.4% and 46.5%, +dp/dtmax raised by 27.0%, 25.1%, and 22.8% at days 1, 7 and 14, respectively (P<0.05, P<0.01 vs. the AMI group) (Figure 3A–3D). After RIC treatment, the heart rate of the rats at days 1 and 14 decreased by 6.3% and 9.1%, respectively (P<0.05, P<0.01 vs. the AMI group) (Figure 3E).
Figure 3.
The effects of remote ischemic conditioning (RIC) on the hemodynamic parameters in the rat model of acute myocardial infarction (AMI). (A–D) Following remote ischemic conditioning (RIC), the hemodynamic indices improved. The left ventricular systolic blood pressure (LVSP) and the maximum descent rate of the left ventricular end-diastolic pressure (LVEDP) (−dp/dtmax) in the RIC group were greater than those in the AMI group at days 7 and 14. (E) The heart rate of the rats increased significantly after AMI and decreased after RIC treatment. (F) The red-green fluorescence ratio for mitochondrial membrane potential changes in apoptosis. (G) The opening of the mitochondrial permeability transition pore (MPTP) in each group. (H) The heart weight/body weight ratio decreased after RIC. (I) RIC reduced the degree of myocardial fibrosis, demonstrated by Masson’s trichrome stain for collagen (blue). Scale bar=200 μm. * P<0.05, ** P<0.01 vs. the AMI group; # P<0.05, ## P<0.01 vs. the sham group. Data are presented as the mean ± standard deviation (SD) (n=24) (8 rats per session).
The red-green fluorescence ratio increased by 62.5%, 30.0% and 42.7% at days 1, 7 and 14 in RIC group, respectively (P<0.01 vs. the AMI group) (Figure 3F). The opening of the mitochondrial permeability transition pore (MPTP) was significantly reduced by 27.3%, 9.5% and 36% at days 1, 7, and 14 in the RIC group, respectively (P<0.05, P<0.01 vs. the AMI group) (Figure 3G). The heart weight/body weight ratio of the RIC group decreased by 15.1% and 7.5% respectively at days 1 and 14 (P<0.05, P<0.01 vs. the AMI group), but only by 4.7% at day 7 (P>0.05) (Figure 3H). Myocardial fibrosis in the AMI group was more severe at days 7 and 14 than day 1. Compared with the AMI group, RIC reduced the deposition of collagen fibers indicating a reduced degree of myocardial fibrosis (Figure 3I).
Effects of RIC on mitochondrial respiratory chain complexes I, II, III, IV, ATP, and iNOS levels in the rat model of AMI
The levels of mitochondrial respiratory chain complexes (Figure 4A–4D), and the ATP level (Figure 4K) at days of 1, 7, and 14 in the RIC group were increased, but the iNOS levels (Figure 4L) decreased (P<0.05, P<0.01, vs. the AMI group). The changes of the apoptosis index using TUNEL staining in the RIC group at days 1, 7, and 14 were reduced by 12.7%, 30.9%, and 26.1%, respectively (P<0.01 vs. the AMI group) (Figure 4E, 4J).
Figure 4.
The effects of remote ischemic conditioning (RIC) on cardiac hemodynamic function and mitochondrial respiratory function in the rat model of acute myocardial infarction (AMI). (A–D) The myocardial concentrations of mitochondrial respiratory chain complexes 1, 2, 3, and 4. (E) The apoptosis index of the ischemia area for the three groups, the sham group, the AMI group, and the RIC group. (F) The serum concentrations of caspase-3 in each of the three rat groups. (G–I) The expression of Bcl-2 and Bax protein, and the ratio of Bcl-2/Bax in each group. (J) Myocardial cell apoptosis determined by TUNEL staining in rats after AMI. Scale bar=200 μm. (K–L) The levels of ATP and inducible nitric oxide synthase (iNOS) in each group. (M) Western blot assay for the protein levels of caspase-3, Bcl-2, and Bax in each group. * P<0.05, ** P<0.01 vs. the AMI group; # P<0.05, ## P<0.01 vs. the sham group. Data are presented as the mean ± standard deviation (SD) (n=24) (8 rats per session).
Effects of RIC on the expression of caspase-3, Bcl-2, and Bax in the rat model of AMI
In the RIC group, the caspase-3 levels were significantly decreased by 22.7%, 20.8%, and 30.7%, the expression of Bcl-2 protein was also significantly increased by 41.2%, 34.3%, and 27.8%, and the Bax protein level was significantly decreased by 69.8%, 81.4% and 73.4%, and the ratio of Bcl-2 and Bax (Bcl-2/Bax) significantly increased by 304.3%, 603% and 301% at days 1, 7 and 14, respectively (P<0.01 vs. the AMI group) (Figure 4F–4I). Western blot measured the expression levels of caspase-3, Bcl-2, and Bax and showed that in the RIC group, there were increased expression levels of Bcl-2 but decreased the expression levels of caspase-3 and Bax compared with the control groups (Figure 4M).
Discussion
Previous studies in our laboratory have shown that long-term noninvasive cardiac remote ischemic conditioning (RIC) could reduce infarct size, arrhythmias, cardiac myocyte apoptosis, and oxidative stress caused by ischemia-reperfusion injury in the rat heart and recovery in a rat model of chronic myocardial infarction [4,5]. Therefore, this study aimed to investigate the possible the cardioprotective effect from non-invasive RIC on myocardial morphology, biochemistry, and cardiac function in a rat model of acute myocardial infarction (AMI).
There is a difference between the two rat models of ischemia-reperfusion injury and AMI. In our previous studies, an ischemia-reperfusion injury model was used to evaluate the cardioprotective role of RIC [4,5]. The present study used a rat model of AMI to investigate the cardioprotective effect of RIC from both ischemia and infarction, ensuring that the animals in the model survived following induction of AMI. The survival rate of animal models following AMI was previously found to be approximately 70%, with pathological changes and physiological indicators being similar to those of humans [10].
The mitochondrial apoptotic pathway is the major apoptosis pathway in vertebrates. Intervention pathways of apoptosis can be used as a strategy for the treatment of AMI, ischemia-reperfusion injury, and other cardiac diseases [11]. The anti-apoptotic protein Bcl-2 and the Bax pro-apoptotic protein are members of the Bcl-2 family [12], which plays an important role in mitochondrial-mediated apoptosis [13,14]. The Bcl-2/Bax ratio determines cell apoptosis, as increasing Bax/Bcl-2 activates the mitochondrial apoptosis pathway, while increasing the expression of the anti-apoptotic protein Bcl-2 or decreasing the expression of pro-apoptotic protein Bax or Bad inhibits the mitochondrial apoptosis pathway [15]. Both the external and internal apoptotic pathways require caspase-3 [16]. Binding to apoptotic protease activator 1, caspase-9, and cytochrome-C activates caspase-3 downstream and leads to apoptosis [17].
The findings of the present study showed that after RIC intervention, the expression of Bcl-2 increased, the expression of Bax and caspase-3 decreased, and Bcl-2/Bax ratio increased, which indicated that RIC inhibited cell apoptosis by increasing Bcl-2/Bax, enhancing the stability of mitochondrial membrane and reducing the release of pro-apoptotic factors. Inhibition of the expression of pro-apoptotic proteins and reduction of the apoptotic index may be the mechanism by which RIC protects the myocardium from ischemic injury. Therefore, improving mitochondrial function is another strategy for the treatment of ischemic heart disease. In this study, the activity of mitochondrial complexes was the lowest in the AMI group, and ATP production was decreased, indicating that myocardial cells were severely damaged after AMI. RIC inhibited the opening of the mitochondrial permeability transition pore (MPTP), reducing calcium overload, and maintains the structure and function of mitochondria to promote the recovery of oxidative energy release from the oxidative respiratory chain.
In the AMI rat group in this study, there was a significant increase in the heart weight/body weight ratio, indicating that myocardial contractility and cardiac output was reduced following induction of AMI. Cardiac compensatory mechanisms increased the thickness of non-infarcted ventricular muscle, possibly mediated by inducible NOS (iNOS) [18]. Overexpression of iNOS in the myocardium of mice has been shown to cause peroxynitrite production, atrioventricular block, and even sudden death, which support the involvement of iNOS in promoting the deterioration of heart disease [19]. Saito et al. showed that iNOS can cause abnormal cardiac function and structural changes after AMI [20]. Also, iNOS has been shown to catalyze increased concentrations of NO, which combine with O2 to produce more harmful peroxynitrite and peroxide [21]. These strong oxidants can inhibit electron transfer in the mitochondrial respiratory chain and damage mitochondria resulting in dysfunction of endothelial cells in the microvasculature [21]. However, in this study, the RIC group had a significantly lower heart weight/body weight ratio, indicating that RIC can reduce ventricular remodeling after AMI. The results showed that RIC could down-regulate the expression of iNOS, reduce the production of NO and reduce oxidative stress, which might indicate that RIC has the ability to reduce ischemic myocardial fibrosis.
This study had several limitations. This was a preliminary study in a rat model of AMI and the study was conducted for a short duration of up to 14 days. Further studies in animal models are needed, but it is hoped that non-invasive methods of RIC will be investigated in large scale controlled clinical trials in patients following AMI. Following the findings of this preliminary study, the mechanism involved in the effects of RIC will be studied, with the focus on the mitochondrial transduction pathways in rat cardiomyocytes. Further studies are also required to investigate whether the combination of RIC and drug treatment further improve the therapeutic effects following AMI in the rat model.
Conclusions
The aims of this study were to investigate the effects of the non-invasive method of cardiac remote ischemic conditioning (RIC) and its cardioprotective roles in a rat model of acute myocardial infarction (AMI). The findings showed that RIC improved the hemodynamic index, reduce the levels of apoptosis and myocardial injury, and improved mitochondrial function in the rat model. When combined with the findings from our previous studies on the role of RIC in chronic myocardial infarction in the rat model, there is support for further studies on the long-term cardioprotective benefits RIC in acute and chronic cardiac ischemia.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Natural Science Foundation of China (Grant No.81072631), the Natural Science Foundation of Tianjin, China (Grant No. 09JCZDJC21100), the Youth Project of Natural Science Foundation of China (Grant No. 81102446), and the Youth Project of Tianjin Natural Foundation, China (Grant No.14JCQNJC13700)
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Gho BCG, Shoemaker RG, van den Doel MA, et al. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 3.Candilio L, Malik A, Hausenloy DJ. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med. 2013;14:193–205. doi: 10.2459/JCM.0b013e328359dd7b. [DOI] [PubMed] [Google Scholar]
- 4.Li SJ, Wu YN, Kang Y, et al. Noninvasive limb ischemic preconditioning protects against myocardial I/R injury in rats. J Surg Res. 2010;164:162–68. doi: 10.1016/j.jss.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 5.An MY, Li Y, Chen WH, et al. Effects of non-invasive remote ischemic conditioning on rehabilitation after myocardial infarction. Biochem Biophys Res Commun. 2017;488:278–84. doi: 10.1016/j.bbrc.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt MR, Smerup M, Konstantinov IE, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–90. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- 7.Bøtker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 8.Kloner RA. Remote ischemic conditioning: its benefits and limitations. J Cardiovasc Pharmacol Ther. 2016;21:219–21. doi: 10.1177/1074248415618816. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Kang Y, Lou J. The optimal strategy of noninvasive limb ischemic preconditioning for protecting heart against ischemia-reperfusion injury in rats. J Surg Res. 2012;174:e47–54. doi: 10.1016/j.jss.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Janahmadi Z, Nekooeian AA, Moaref AR, Emamghoreishi M. Oleuropein offers cardioprotection in rats with acute myocardial infarction. Cardiovasc Toxicol. 2015;15:61–68. doi: 10.1007/s12012-014-9271-1. [DOI] [PubMed] [Google Scholar]
- 11.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–29. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill KL, Huang K, Zhang J, et al. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 2016;30:973–88. doi: 10.1101/gad.276725.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korytowski W, Basova LV, Pilat A, et al. Permeabilization of the mitochondrial outer membrane by Bax/Truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation mechanistic implications for the intrinsic pathway of oxidative apoptosis. J Biol Chem. 2011;286:26334–43. doi: 10.1074/jbc.M110.188516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Saez AJ. The secrets of the Bcl 2 family. Cell Death Differ. 2012;19:1733–40. doi: 10.1038/cdd.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XP, Li SY, Zeng ZB, et al. Notch1 signaling inhibits apoptosis of human dental follicle stem cells via both the cytoplasmic mitochondrial pathway and nuclear transcription regulation. Int J Biochem Cell Biol. 2017;82:18–27. doi: 10.1016/j.biocel.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q. Lentivirus mediated interference of caspase-3 expression ameliorates the heart function on rats with acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2014;18:1852–58. [PubMed] [Google Scholar]
- 17.Chen S, Peng H, Rowat A, et al. The effect of concentration and duration of normobaric oxygen in reducing caspase-3 and -9 expression in a rat-model of focal cerebral ischaemia. Brain Res. 2015;1618:205–11. doi: 10.1016/j.brainres.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Khanna S, Singh GB, Khullar M. Nitric oxide synthases and diabetic cardiomyopathy. Nitric Oxide. 2014;43:29–34. doi: 10.1016/j.niox.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Mungrue IN, Gros R, You X, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–43. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito T, Hu F, Tayara L, et al. Inhibition of NOS II prevents cardiac dysfunction in myocardial infarction and congestive heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H339–45. doi: 10.1152/ajpheart.00596.2001. [DOI] [PubMed] [Google Scholar]
- 21.Dayal S, Blokhin IO, Erger RA, et al. Protective vascular and cardiac effects of inducible nitric oxide synthase in mice with hyperhomocysteinemia. PLoS One. 2014;9:e107734. doi: 10.1371/journal.pone.0107734. [DOI] [PMC free article] [PubMed] [Google Scholar]