Abstract
Background
Elevation of plasma high-density lipoprotein (HDL) cholesterol concentration reduces cardiovascular mortality and morbidity. HDLs have been shown to possess acute anti-inflammatory, antioxidant, and antithrombotic properties. We hypothesize that HDL therapy can acutely alter local and systemic manifestations of plaque instability.
Methods
Forty patients with early symptomatic carotid disease were randomized to either receive reconstituted HDL (rHDL) 40 mg/kg (n = 20) or placebo (n = 20). Carotid endarterectomies were performed 24 hr later. Plaques were obtained intraoperatively and used for measurement of thrombomodulatory genes expression. Plasma samples were collected before the infusion, 24 and 48 hr later to measure changes in systemic markers of plaque instability.
Results
No significant differences were noted in thrombomodulatory genes expression between the 2 groups. Systemic levels of tissue factor, matrix metalloproteinase 9 (MMP-9), and monocyte chemotactic factor-1 (MCP-1) were significantly reduced in the rHDL group. However, the effects on MMP-9 and MCP-1 were abolished in the immediate postoperative period. Although rHDL did not affect plasma interleukin-6 levels 24 hr following the infusion, it prevented the significant postoperative elevation seen in the placebo group.
Conclusions
A single infusion of rHDL can acutely alter plasma biomarkers associated with plaque instability and cardiovascular morbidity.
Introduction
Carotid plaque instability is at least partly responsible for the high stroke recurrence rate following an ischemic cerebrovascular event. Interestingly, the risk falls rapidly with time as the plaque heals. Therefore, early intervention is vital to prevent further neurological complications. Although good long-term results have been shown with pharmacological therapy with regard to plaque stabilization and event recurrence, the acute effects remain questionable. Therefore, surgical removal of the plaque by means of a carotid endarterectomy (CEA) remains the best option for acute protection against further embolization or vessel occlusion. Despite the significant benefit gained from early CEA in terms of prevention of event recurrence, surgical endarterectomy has been associated with relatively higher mortality and morbidity rates when compared with delayed (6–8 weeks post event) CEA and that for asymptomatic carotid disease.1 This is thought to be related to plaque instability and the acute activation of inflammatory mediators.2 It is therefore important to identify pharmacotherapeutic agents that could acutely stabilize plaques and/or modify their activity. This could result in improving surgical outcomes and reducing the rate of event recurrence while patients are awaiting surgery.
High-density lipoproteins (HDLs) and their reconstituted particles have several acute antiatherogenic properties that would make them potential therapeutic candidates. HDLs' ability to induce reverse cholesterol transport (RCT) was one of the earliest recognized properties, and many of the antiatherogenic functions were attributed to this process. More recent work showed that HDLs and their reconstituted particles have other antiatherogenic effects independent of RCT.3 These include antioxidant effects, anti-inflammatory effects, antithrombotic effects, and modification of endothelial function.4, 5, 6, 7
Many strategies have been used to raise plasma HDL concentration including intravenous administration of reconstituted HDL (rHDL).8 This treatment modality has been shown to be safe and effective in raising plasma HDL levels.9, 10 rHDL has been shown to effectively induce cholesterol reflux and inhibit proinflammatory changes and platelet aggregation.11, 12, 13 In the ERASE (Effect of Reconstituted HDL on Atherosclerosis and Efficacy) trial, it was shown that short-term rHDL infusions can induce plaque regression within 4 weeks of treatment in acute coronary syndrome patients.10 Additionally, rHDL therapy was associated with significant improvement in plaque characterization indices. More recently, Shaw et al.9 tested the effects of a single high-dose rHDL (80 mg/kg) infusion on plaques from claudicants undergoing superficial femoral artery atherectomy. They have demonstrated that rHDL infusion could lead to acute reduction in plaque lipid content and both local and systemic measures of inflammation. Theoretically, this suggests that rHDL may represent a useful agent for patients with acute ischemic cardiovascular (CV) events, but more work is needed to evaluate the acute effects on other parameters and markers of disease activity.
Our study aimed to examine the acute effects of a low-dose (40 mg/kg) infusion of rHDL, the recommended tolerable dose in humans, to modulate carotid plaque characteristics 24 hr after infusion, and systemic biomarkers of inflammation 24 and 48 hr after infusion.
Methods
Study Design and Patient Cohort
Patients were recruited from the stroke unit or the open access transient ischemic attack clinic at St George's Hospital NHS Trust, London, UK. All patients with symptomatic carotid artery stenosis that would benefit from urgent intervention (carotid stenosis >50%) were included in the trial. All patients had a carotid territory neurological event less than 1 month before CEA and trial participation. Patients' symptoms were determined by a consultant neurologist following a brain computed tomography and magnetic resonance imaging.
All patients underwent a preoperative duplex ultrasound (7.5-MHz linear transducer, SONOS 2000; Hewlett-Packard, Andover, MA) assessment by an experienced vascular technologist for plaque classification (Gray-Weale system) and quantification of degree of stenosis.
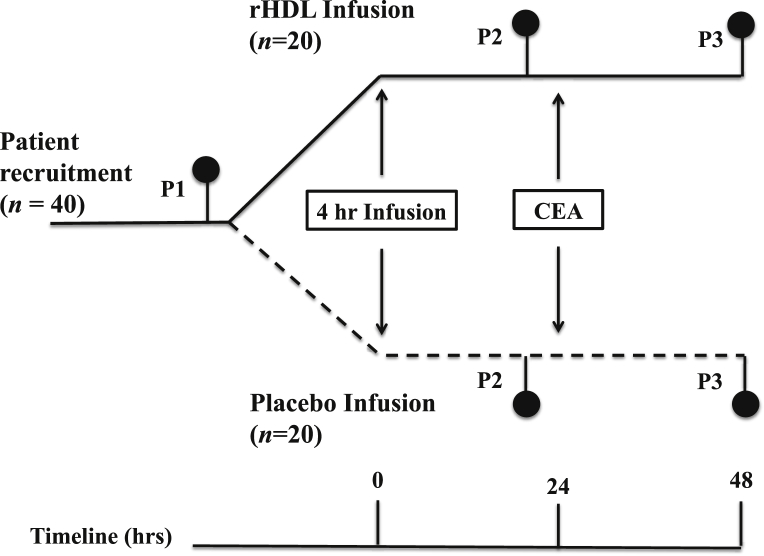
Forty patients with early symptomatic (within 1 month) carotid artery disease undergoing CEA were recruited and randomly allocated to one of the 2 groups. The first group received a preoperative infusion of rHDL at a concentration of 40 mg/kg. The second group received an equivalent volume of phosphate buffered saline (PBS). CEA was performed 24 hr later. Plaques were retrieved intraoperatively and preserved in RNAlater (Ambion, Paisley, UK). Plasma samples were collected before the infusion (P1), then 24 (P2) and 48 (P3) hours after the infusion (Fig. 1).
Fig. 1.
Flow diagram of trial design. Timing of rHDL infusion: P1 (preinfusion); P2 (24 hr postinfusion); and P3 (48 hr postinfusion).
This trial has conformed to the principles outlined in the Declaration of Helsinki. Before consenting to enroll in the trial, all patients were provided with an invitation to participate. This invitation detailed the purpose of the study and the content of the infusion they would receive, detailing that rHDL is considered a plasma product with an equivalent safety profile to other blood products. All patients were given the right to withdraw their consent at any time during or after the trial. None of the volunteer patients refused to join the trial or withdrew consent after agreeing to participate.
The study design was approved by the Local Research Ethics Committee and registered with Clinicaltrials.org (Unique Identifier: NCT00822302).
Inclusion Criteria
All symptomatic patients (symptoms <1 month) with confirmed internal carotid artery stenosis >50%, who are listed for urgent CEA.
Exclusion Criteria
-
•
Pregnant women and women with childbearing age.
-
•
Patients with impaired renal or liver function.
-
•
Patients sectioned under the Mental Health Act.
Operative Technique
CEA was standardized, where all patients were done under general anesthesia, with a shunt. All patients underwent a standard longitudinal endarterectomy with bovine pericardium patch closure.
Safety Evaluation
Patient safety was assessed by monitoring adverse events, physical examinations, and monitoring of vital signs. This was performed half hourly during the 4-hr infusion period. Following completion of the infusion, patients were assessed half an hr later, then 4 hourly until the next morning. All patients had a pre and postinfusion electrocardiogram (ECG). Patients' liver function tests, urea, and electrolytes were assessed pre and postinfusion.
Monitoring for Clinical Events and Adverse Experiences
The prespecified clinical events were all-cause mortality and the occurrence of major fatal/nonfatal vascular events or procedures. Death, MI, unstable angina, heart failure, arrhythmias, and stroke were classified by an external events classification committee blinded to treatment, assigned with the use of definitions used in other studies.14, 15 Any reaction to the study drug was noted and all events were reported to the hospital pharmacist, drug manufacturer, local research committee, and the department of Medicines and Healthcare Products Regulatory Agency.
All cardiac events were confirmed by a cardiologist, with documented evidence of myocardial ischemia (ECG changes, significantly raised cardiac enzymes, or new angiographic findings of disease). All suspected cerebrovascular events were confirmed by a stroke physician.
Power Calculation
No previous study has examined the acute effects of a single low, clinically tolerable, dose of rHDL (40 mg/kg ApoAI) on symptomatic carotid atherosclerotic plaques. However, in a previous report Shaw et al.9 demonstrated a significant reduction in plaque vascular cell adhesion molecule-1 (VCAM-1) expression 5 to 7 days following a single infusion of a high-dose infusion of rHDL (80 mg/kg ApoAI) compared with a saline infusion. We expected a more modest effect, and estimated that a sample size of 20 would provide 90% power to detect a 25% difference in the % VCAM-1 expression, using a 2-sided significance level of 0.05.
Reconstitution of HDL, Randomization, Blinding, and Infusion
Lyophilized rHDL (CSL-111), manufactured by CSL-Behring (Bern, Switzerland), is composed of human plasma ApoAI and soybean phosphatidylcholine in a molar ratio of 1:150. The rHDL is prepared using cholate dialysis according to published methods,16 and reconstituted by the addition of 50 mL of sterile water for injection, resulting in a pale yellow solution, pH 7.5 and containing 10% sucrose as a stabilizing agent and 13 mmol/L cholate. Viral safety is ensured by the use of screened blood donations, viral elimination, and inactivation steps during the preparation process.16 Therefore, rHDL has a similar safety profile to other plasma-derived products used in clinical practice.
Once reconstituted, rHDL was stored at 4°C and administered within 24 hr. Randomization was established using a random number sequence generator in St George's Hospital Pharmacy. The site pharmacist was unblinded to the individual patient treatment allocation, but had no further role in the study. All others involved, including patients, study nurses and investigators were blinded to the treatment allocation by wrapping the infusion syringes in colored cellophane, disguising the solution. Infusions were given intravenously, using constant regulated delivery via a Harvard delivery pump (Pump 44; Harvard Apparatus, Kent, UK), over 4 hr. Treatment codes were not revealed to investigators until all the analyses were completed.
Tissue Sample Collection
Twenty-four hr following infusion, carotid plaques were removed by endarterectomy, and collected in RNA stabilizing solution, RNAlater (Ambion). Tissue samples were photographed and gross pathological features recorded. To ensure random sampling from each sample, the entire biopsy was diced into 2-mm3 pieces sampled randomly for analysis. Tissue pieces were then washed for 15 min, 3 times, PBS, before embedding (10 × 2 mm3/block) in OCT™ (Tissue Tek™; ThermoFisher, Cambridge, UK) for histological (oil-red-o staining) and immunohistochemical staining.
For total RNA extraction and complementary DNA synthesis and quantitative real-time polymerase chain reaction, see supplementary material.
Blood Collection
Whole blood was collected in Vacutainers™ (one citrated, one K2-EDTA; Becton Dickinson, Oxford, UK) at each time point, placed immediately on ice, centrifuged (3000 rpm for 15 min) and plasma collected, aliquoted and stored at −80°C until assayed.
Histology and Immunohistochemistry
Methods were previously reported17, 18, 19 and detailed in the supplementary section.
Measurement of Serum Lipids
Direct HDL measurement was made using an ADVIA 2400 (Siemens Diagnostics Medical Solutions, Surrey, UK) and performed according to manufacturer's recommendations. Low-density lipoprotein (LDL) cholesterol was determined using the Friedewald calculation.20 ApoAI was measured by nephelometry using the IMAGE™ kit (Beckman Coulter Ltd, High Wycombe, Bucks, UK).
Measurement of Plasma Factors
Plasma concentrations of C-reactive protein (CRP), interleukin-6 (IL-6), monocyte chemotactic factor-1 (MCP-1), and matrix metalloproteinase 9 (MMP-9) were quantified using enzyme-linked immunoassays (ELIZA) (BD Biosciences, Oxford, UK; R&D systems, Oxford, UK). Inter and intra-assay variations (cv) were CRP 4.3% and 6.9%, IL-6 3.3% and 6.4%, MCP1 4.0% and 7.5%, and MMP-9 5.0% and 8.8%, respectively. Plasma concentration of tissue factor (TF) was quantified by ELIZA (Sekisui Diagnostics, Stamford, CT) according to manufacturer's recommendations. Inter and intra-assay variations (cv) were 5.1% and 8.2%, respectively.
Statistical Analysis
Results were expressed as mean ± standard deviation (SD), unless stated otherwise. The analyses were performed using GraphPad Prism for Mac (version 4.0) and SPSS for Mac (version 19).
Plasma factors analysis
Shapiro–Wilks normality test was performed for the differences (from baseline) in the 24 and 48 hr postinfusion assays in rHDL and Placebo groups. Following that, Levenes test was performed for the difference in variances between the rHDL and Placebo groups 24 and 48 hr postinfusion. Many of the normality tests had P values <0.05, but fewer of the variance tests had P < 0.05. Therefore, a nonparametric test was more appropriate.
To analyze the difference in the effect of treatment between the rHDL and Placebo groups, Wilcoxon rank-sum test was performed for the difference in the 24- and 48-hr changes from baseline levels between the 2 groups. Finally, P values were adjusted for experimentwise error using the Benjamini–Yekutieli method.
Within each group, comparisons between baseline (preinfusion) and postinfusion levels were analyzed using Mann–Whitney test.
A P value <0.05 was considered significant.
Quantitative polymerase chain reaction analysis
The distributions of messenger RNA (mRNA) levels were skewed and logarithmic transformation was used for normalization. Comparison of the gene expression between the 2 groups was made using paired Student's 2-tailed t-test. A P value <0.05 was considered significant.
Histology and immunohistochemistry analysis
Comparison between the 2 groups was analyzed using Mann–Whitney test. A P value <0.05 was considered significant.
Results
Patient Demographics
A total of 40 patients were randomized with 20 in each group. There were no significant differences in the baseline characteristics between the 2 groups (Table I). The 2 groups were matched for age: 69.0 ± 11.9 years (mean ± SD) in the rHDL group versus 72.8 ± 8.3 years in the placebo group. The groups were also matched for major risk factors and best medical therapy (aspirin and statin). There were no differences in the degree of stenosis and plaque morphology (Gray–Weale grading system) between the 2 groups (Table SI in supplementary section).
Table I.
Patient baseline demographics
| Baseline demographics | Placebo (n = 20) | rHDL (n = 20) | P value |
|---|---|---|---|
| Age | 72.8 ± 8.31 | 69.0 ± 11.99 | NS |
| Men | 17 (85) | 12 (60) | NS |
| Symptoms | |||
| Amaurosis fugax | 1 (5) | 1 (5) | NS |
| TIA | 10 (50) | 8 (40) | NS |
| Stroke | 9 (45) | 11 (55) | NS |
| Time (days) from index event to CEA | 17.8 ± 8.52 | 17.1 ± 7.10 | NS |
| Risk factors | |||
| Smokinga | 18 (90) | 13 (65) | NS |
| Dyslipidemiab | 19 (95) | 16 (80) | NS |
| Hypertensionc | 20 (100) | 20 (100) | NS |
| Diabetesd | 4 (20) | 3 (15) | NS |
| Previous CVA | 3 (15) | 2 (10) | NS |
| IHDe | 6 (30) | 5 (5) | NS |
| Antiplatelet agent | |||
| Aspirin | 20 (100) | 20 (100) | NS |
| Clopidogrel | 8 (40) | 5 (25) | NS |
| Dipyridamole | 3 (15) | 4 (20) | NS |
| Statins | |||
| Simvastatin 40 mg | 18 (90) | 19 (95) | NS |
| Atorvastatin 20 | 1 (5) | 0 (0) | NS |
| Atorvastatin 40 | 1 (5) | 1 (5) | NS |
Data are expressed as number (percentage) or mean ± SD.
CABG, coronary artery bypass graft; CVA, cardiovascular accident; HMG Co-A, 3-hydroxy-3- methylglutaryl coenzyme A; IHD, ischaemic heart disease; NS, nonsignificant; TIA, transient ischemic attack.
Past or present history of smoking.
Fasting cholesterol level >5.0 mmol/L and/or use of HMG Co-A reductase inhibitor (statin).
Systolic blood pressure >160 mm Hg and/or diastolic blood pressure >90 mm Hg and/or use of antihypertensives.
Fasting glucose level >7 mmol/L or insulin and non–insulin-dependent diabetes requiring treatment including diet controlled.
Previous history of myocardial infarction/CABG and/or angina requiring treatment.
Safety Results
Major adverse events are shown in Table SII in the supplementary section. CSL-111 was generally well tolerated, with the majority of adverse events being mild to moderate. There were no significant differences in the overall frequencies between the placebo and rHDL groups. All elevated liver function tests (alanine aminotransferase, aspartate aminotransferase, and bilirubin) spontaneously returned to normal within 4 weeks without any intervention or any significant sequelae.
Influence of rHDL Infusion on Serum Lipids
rHDL infusion significantly elevated serum high-density lipoprotein cholesterol (HDL-C) concentration within 24 hr (P = 0.02) (Table II, Table III). Although this significant rise was not sustained beyond 24 hr, rHDL infusion prevented the postoperative (48-hr postinfusion) fall in serum HDL concentrations noted in the placebo group (8.66% vs. −19.46% in the rHDL and placebo groups consecutively, P = 0.001). As expected, these results were reciprocated by ApoAI serum concentrations.
Table II.
The effect of infusion with rHDL or placebo on plasma lipid profile
| Lipid (mmol/L) | Group | Baseline | 24 hr | 48 hr |
|---|---|---|---|---|
| HDL-C | rHDL | 1.01 ± 0.22 | 1.21 ± 0.29* | 1.10 ± 0.19 |
| Placebo | 1.15 ± 0.34 | 1.00 ± 0.37 | 0.92 ± 0.28 | |
| ApoA1 | rHDL | 1.32 ± 0.18 | 1.83 ± 0.50* | 1.40 ± 0.22* |
| Placebo | 1.43 ± 0.32 | 1.24 ± 0.32 | 1.16 ± 0.20 | |
| LDL-C | rHDL | 2.41 ± 0.80 | 2.18 ± 0.87 | 1.93 ± 0.61 |
| Placebo | 2.21 ± 1.40 | 2.00 ± 1.17 | 1.80 ± 1.08 | |
| Total cholesterol | rHDL | 4.32 ± 1.11 | 4.15 ± 1.13 | 3.67 ± 0.72 |
| Placebo | 4.18 ± 1.48 | 3.61 ± 1.35 | 3.22 ± 1.26* |
*P < 0.05 (change in plasma concentration from baseline).
Values are presented as mean ± SD.
Table III.
Comparing % change in plasma lipid profile from baseline (24 and 48 hr following infusion of rHDL or placebo)
| Lipid | % Change from baseline (mean ± SD) 24 hr following infusion |
% Change from baseline (mean ± SD) 48 hr following infusion |
||||
|---|---|---|---|---|---|---|
| rHDL group | Placebo group | P value | rHDL group | Placebo group | P value | |
| HDL-C | 22.91 ± 28.66 | −14.33 ± 11.82 | 0.001 | 8.66 ± 13.19 | −19.46 ± 13.06 | 0.001 |
| ApoA1 | 38.16 ± 31.36 | −13.07 ± 9.75 | 0.0003 | 8.50 ± 10.67 | −22.30 ± 13.7 | 0.002 |
| Cholesterol | −3.45 ± 11.71 | −13.60 ± 12.85 | 0.27 | −13.41 ± 10.93 | −23.79 ± 15.03 | 0.73 |
| LDL | −9.54 ± 16.78 | −0.18 ± 39.65 | 0.49 | −17.71 ± 17.68 | −22.90 ± 29.76 | 0.74 |
In the placebo group, there was a significant drop in total cholesterol concentration 48 hr postinfusion. This correlated well with the noted postoperative drop in HDL cholesterol plasma concentrations in this group.
No significant changes were demonstrated in serum LDL cholesterol concentrations in either group.
Effects of rHDL Infusion on Plasma Inflammatory Mediators
Infusion of rHDL did not significantly alter plasma IL-6 concentration at 24-hr postinfusion (Table IV, Table V). However, rHDL prevented the significant postoperative (48-hr postinfusion) rise in IL-6 levels seen in the placebo group (P = 0.01). This did not translate into a significant treatment effect, as the % change from baseline was not significantly different between the 2 groups at 24 and 48 hr following the infusion.
Table IV.
The effect of infusion with rHDL or placebo on plasma inflammatory mediators
| Inflammatory mediator | Group | Baseline | 24 hr | 48 hr |
|---|---|---|---|---|
| IL-6 (pg/mL) | rHDL | 54.65 ± 16.05 | 47.21 ± 10.29 | 58.57 ± 37.62 |
| Placebo | 57.95 ± 24.76 | 55.54 ± 27.68 | 89.48 ± 68.36* | |
| CRP (mg/mL) | rHDL | 8.69 ± 10.1 | 7.51 ± 6.20 | 33.60 ± 33.02† |
| Placebo | 3.31 ± 4.34 | 3.37 ± 3.74 | 45.06 ± 41.39† | |
| MCP-1 (pg/mL) | rHDL | 225.3 ± 218.9 | 157.3 ± 190.3* | 189.7 ± 183.3 |
| Placebo | 144.4 ± 116.6 | 112.2 ± 108.4 | 177.1 ± 146.7 | |
| sCD40 (pg/mL) | rHDL | 292.6 ± 191.1 | 166.5 ± 45.95* | 187.8 ± 137.9 |
| Placebo | 194.4 ± 54.01 | 186.2 ± 53.13 | 181.1 ± 54.63 |
*P < 0.05.
†P < 0.001 (change in plasma concentration from baseline).
Values are presented as mean ± SD.
Table V.
Comparing % change in plasma inflammatory mediators from baseline (24 and 48 hr following infusion of rHDL or placebo)
| Inflammatory mediator | % Change from baseline (mean ± SD) 24 hr following infusion |
% Change from baseline (mean ± SD) 48 hr following infusion |
||||
|---|---|---|---|---|---|---|
| rHDL group | Placebo group | P value | rHDL group | Placebo group | P value | |
| IL-6 | −10.56 ± 17.72 | −2.56 ± 24.61 | 0.26 | 15.41 ± 91.11 | 62.99 ± 139.20 | 0.08 |
| CRP | 5.78 ± 55.86 | 55.88 ± 265.05 | 0.89 | 765.92 ± 772.53 | 3924.36 ± 6988.29 | 0.06 |
| MCP-1 | −5.04 ± 155.04 | −9.26 ± 63.57 | 0.40 | 35.70 ± 211.35 | 55.16 ± 105.04 | 0.14 |
| sCD40 | −27.29 ± 30.25 | −1.80 ± 22.84 | 0.15 | −16.79 ± 81.17 | −2.13 ± 29.88 | 0.19 |
CRP levels were unaffected by rHDL infusion in the 24-hr postinfusion period. Postoperatively, CRP levels were significantly elevated in both groups.
rHDL infusion significantly reduced plasma MCP-1 levels 24 hr postinfusion (P = 0.03). These effects were not sustainable beyond 24 hr. rHDL infusion also significantly reduced sCD40 plasma concentrations at 24 hr but not at 48 hr postinfusion (P = 0.01 and 0.08, respectively). Again, when comparing the % change, this did not translate into significant treatment effects between the 2 groups for both MCP-1 and sCD40, at 24 and 48 hr postinfusion.
Effects of rHDL on Plasma MMP-9, TF Antigen, d-Dimer, and Fibrinogen
While no alterations in MMP-9 levels were noted in the placebo group, rHDL therapy induced a significant reduction in plasma MMP-9 within 24 hr of the infusion (P = 0.003) (Table VI, Table VII). As with MCP-1 and sCD40, the effects were transient and levels were not significantly different from the baseline postoperatively (48 hr postinfusion). There was also a significant treatment effect between the 2 groups at 24 hr postinfusion (P = 0.002) and this effect was lost 48 hr postinfusion.
Table VI.
The effect of infusion with rHDL or placebo on plasma MMP-9, TF antigen, d-dimer, and fibrinogen
| Plasma markers of plaque activity | Group | Baseline | 24 hr | 48 hr |
|---|---|---|---|---|
| MMP-9 (ng/mL) | rHDL | 398.4 ± 293.1 | 102.3 ± 160.2* | 345.7 ± 286.0 |
| Placebo | 362.0 ± 270.6 | 280.9 ± 214.5 | 399.6 ± 255.7 | |
| TF (pg/mL) | rHDL | 320.4 ± 215.5 | 254.0 ± 199.6 | 251.3 ± 192.9 |
| Placebo | 320.4 ± 247.2 | 474.9 ± 282.8 | 453.8 ± 294.4 | |
| d-Dimer (mg/mL) | rHDL | 0.81 ± 1.43 | 0.61 ± 1.28 | 0.87 ± 1.32 |
| Placebo | 0.54 ± 0.49 | 0.55 ± 0.54 | 0.70 ± 0.70 | |
| Fibrinogen (g/L) | rHDL | 4.14 ± 0.87 | 3.78 ± 1.01 | 3.95 ± 0.93 |
| Placebo | 3.87 ± 1.00 | 3.61 ± 0.71 | 3.71 ± 0.58 |
*P < 0.05 (change in plasma concentration from baseline).
Values are presented as mean ± SD.
Table VII.
Comparing % change in plasma MMP-9, TF antigen, d-dimer, and fibrinogen from baseline (24 and 48 hr following infusion of rHDL or placebo)
| Plasma markers of plaque activity | % Change from baseline (mean ± SD) 24 hr following infusion |
% Change from baseline (mean ± SD) 48 hr following infusion |
||||
|---|---|---|---|---|---|---|
| rHDL group | Placebo group | P value | rHDL group | Placebo group | P value | |
| MMP-9 | −69.40 ± 27.94 | −12.76 ± 27.24 | 0.002 | 24.78 ± 108.26 | 34.24 ± 91.98 | 0.27 |
| TF | −30.05 ± 22.53 | 22.77 ± 54.10 | 0.001 | −25.83 ± 38.26 | 62.96 ± 53.57 | 0.0003 |
| d-Dimer | −10.20 ± 27.30 | 4.60 ± 81.62 | 0.51 | 50.39 ± 67.17 | 40.44 ± 52.99 | 0.60 |
| Fibrinogen | −7.66 ± 20.30 | −4.08 ± 15.66 | 0.97 | −3.56 ± 17.40 | 1.01 ± 27.43 | 0.89 |
Infusion of rHDL did not result in significant changes in plasma TF concentrations in the 24- and 48-hr postinfusion levels. However, although not statistically significant, there was a steady reduction in TF plasma concentrations in the rHDL group at 24 and 48 hr postinfusion. Conversely, in the placebo group, there was a steady rise (not statistically significant) in plasma TF concentrations 24 and 48 hr postinfusion. This has translated into a significant treatment effect of rHDL at 24 and 48 hr (P = 0.001 and P = 0.0003, respectively).
No significant effects on plasma d-dimer and fibrinogen levels were noted in either group.
Effects of rHDL Infusion on Carotid Plaque Biology
There were no significant differences in gene expression of TM, TF, tissue factor pathway inhibitor, urokinase-type plasminogen activator, tissue plasminogen activator, plasminogen activator inhibitor-1, or CD68 mRNA comparing carotid plaques from rHDL and placebo groups (Table VIII).
Table VIII.
Summary of gene expression (arbitrary unit normalized to 18S rRNA) of candidate thrombomodulatory factors
| mRNA (per 18s RNA) | rHDL group (n = 20) | Placebo group (n = 20) | P value |
|---|---|---|---|
| TM | 0.60 (0.46–0.74) | 0.57 (0.34–0.75) | 0.33 |
| TF | 1.28 (1.03–1.42) | 1.21 (0.98–1.45) | 0.30 |
| TFPI | 1.44 (1.16–1.58) | 1.33 (1.11–1.59) | 0.34 |
| tPA | 1.49 (1.33–1.86) | 1.57 (1.25–1.79) | 0.33 |
| uPA | 1.58 (1.44–1.84) | 1.55 (1.33–1.80) | 0.28 |
| PAI-I | 1.32 (1.06–1.48) | 1.16 (1.01–1.53) | 0.32 |
| CD-68 | 1.79 (1.52–1.89) | 1.79 (1.40–1.99) | 0.38 |
Values represent median and interquartile range.
CD-68, cytoplasmic domain-68; PAI-I, plasminogen activator inhibitor-I; rRNA, ribosomal ribonucleic acid; TFPI, tissue factor pathway inhibitor; TM, thrombomodulin; tPA, tissue plasminogen activator.
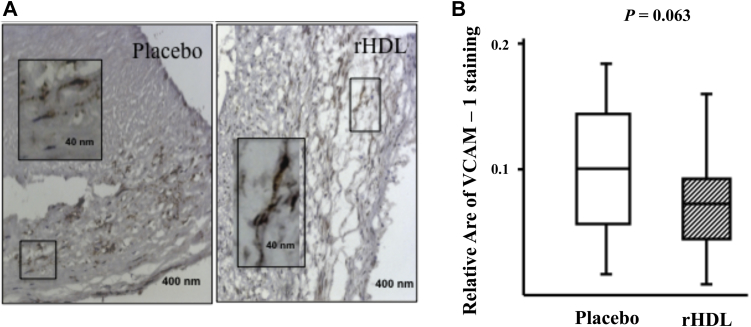
Specific VCAM-1 expression was detected in smooth muscle cells and on endothelial cells within CEA sections. No significant difference in plaque expression of VCAM-1 [median and interquartile range 0.1 (0.018–0.189) placebo vs. 0.067 (0.01–0.165) rHDL, P = 0.0638] was determined through semiquantitative morphometric image analysis using hue/saturation intensity agreed with that obtained by scoring intensity by eye. However, the median values suggest a trend toward rHDL infusion reducing VCAM-1 expression (Fig. 2). Similar analyses examining the expression of P-selectin, ICAM-1, and CD68 showed no significant difference between the 2 arms of the study.
Fig. 2.
Effect of rHDL infusion on carotid plaque expression of VCAM-1. Representative sections (A) stained with VCAM-1. Morphometric analysis (B) using semiquantitative analysis shows median and interquartile range of VCAM-1 expression.
Discussion
This study demonstrated that a single infusion of rHDL, at 40 mg/kg, is able to (i) reduce basal and surgically induced plasma concentrations of TF; (ii) reduce surgically induced plasma IL-6 elevation, and (iii) reduce basal activity of plasma MMP-9. We have demonstrated that rHDL infusion reduced plasma concentrations of TF antigen, supporting the idea that HDLs can influence coagulation. Interestingly, this single low-dose rHDL infusion did not influence the neutral fat content, adhesion molecule abundance, or gene expression in the carotid atherosclerotic plaque, which may be because of the time from infusion to analysis (24 hr) being too short to observe such changes, or because of the lower concentration of infusion or degree of heterogeneity within the carotid plaque specimens.
There are a number of pharmacological strategies for raising plasma HDL-C concentration; infusion of rHDL is one such approach. The clinical findings of the majority of such studies to date are difficult to compare, because of differing experimental designs and study cohorts. The earliest studies examined the pharmacokinetics of rHDL in normal young healthy male and reported an increase in pre-β HDL particles, which are efficient at cholesterol acceptance.21 Subsequent studies, using the higher dose of rHDL (80 mg/kg) in hypercholestrolemic male subjects, examined the effect of rHDL on vascular reactivity, found that rHDL restored vascular function through influencing nitric oxide bioavailability.22, 23 Improvements to vascular function were also demonstrated in high-dose infusion in type 2 diabetic patient cohorts.24, 25 The largest study to date, the ERASE study, compared high- and low-dose multiple infusions of rHDL in acute coronary syndrome (ACS) patients to a saline control group. The low-dose arm showed an early effect in improving plaque characteristics following multiple infusions of rHDL in ACS patients and a small but not significant difference in plaque volume.10 Infusion of rHDL at 80 mg/kg resulted in greater than 50% of the group having 5 times the upper limit of normal (ULN) of ALT and 3 times the ULN of AST. Consequently, this arm of ERASE was discontinued and 40 mg/kg is now the recommended highest tolerated dose in human subjects.
In agreement with other studies, we have demonstrated a significant elevation in plasma HDL concentration after intravenous infusion of rHDL.9, 22 Although previous studies showed that the effects persisted longer than 72 hr,21 our study showed that the significant elevation was unsustainable postoperatively (i.e., 48 hr postinfusion). However, in the rHDL group, postoperative HDL and ApoA1 levels remained higher than the baseline, but decreased in the placebo group. The reduction in plasma HDL-C following the placebo infusion is puzzling and is unlikely to be explained by a dilution effect, because both arms of the study received similar volumes of fluid. However, if this does represent a dilution effect, it may be that the modest increase in plasma HDL-C found in the rHDL infused group is underestimated. Studies in different patient populations found that low perioperative HDL-C and ApoA1 levels were associated with higher mortality and morbidity.26 Therefore, perioperative elevation of plasma HDL-C and ApoA1 levels could translate into improved clinical and operative outcomes.
CRP and IL-6 are recognized markers of acute inflammation. Both have important proatherogenic properties and elevated plasma levels can be predictive of the risk of CV complications.27, 28 It has been suggested that CRP and IL-6 are triggers of perioperative CV mortality.29 In this study, although rHDL did not affect IL-6 or CRP levels 24 hr postinfusion, it prevented the significant postoperative surge in plasma IL-6 seen in the placebo group. Similar but nonsignificant trends were demonstrated with plasma CRP. These are potentially important effects demonstrating that pretreatment with rHDL could protect against triggers of undesirable perioperative CV events.
MCP-1 is an important chemokine associated with atherogenesis. It regulates plaque infiltration by inflammatory cells, induces migration and proliferation of smooth muscle cells, MMP expression, migration of ECs, neovascularization, oxidative stress, and thrombosis.30 HDL has been shown to be effective in inhibiting MCP-1 production in activated vascular smooth muscle cells.31 Furthermore, the expression of ApoA1 in ApoE knockout mice resulted in a significant reduction in plaque expression of MCP-1.32 In our study, we have shown that rHDL significantly reduced plasma levels of MCP-1 24 hr following the infusion. These effects were transient and the postoperative levels of MCP-1 were similar in the 2 groups. Therefore, the ability of rHDL to acutely influence plasma MCP-1 levels may represent a mechanism by which rHDL therapy could affect plaque instability and CV-related mortality.
As with MCP-1, the effects of rHDL on MMP-9 were short lived, not lasting beyond the postoperative period. MMP-9 is a proteolytic enzyme that degrades vascular extracellular matrix. It has been implicated in the initiation and complication of atherosclerosis.33 Loftus et al.34 also observed that plasma MMP-9 levels were elevated in patients with embolizing carotid plaques. Our results suggest that rHDL therapy could acutely modify the unstable plaque's activity, reducing the risk of embolization and event recurrence. This could also have a potential implication on the perioperative risk of CV morbidity and mortality.
TF is a protein, which is highly expressed in unstable atherosclerotic plaques. It has major influences on thrombogenesis and coagulation.35, 36, 37 TF activity can be rapidly upregulated by thrombin, tumor necrosis factor, and IL-1. Thrombin-induced TF upregulation has been shown to be inhibited by rHDL.38 In this study, rHDL therapy had a significant effect on the surgically induced elevation of plasma TF seen in the placebo group.
Although normal range of antigenic TF in plasma is yet to be established, and the precise nature of circulating TF antigen awaits clarification, the relative differences between the 2 groups tested in our study remain worthy of consideration. In future studies, it will be essential to investigate our observation of the effects of rHDL infusion on TF-dependent procoagulant activity and to evaluate TF positivity of microparticles, in addition to blood-borne TF antigen. The effect we report following a single low-dose rHDL infusion on blood-borne TF antigens may provide another important protective mechanism against ACS, and may warrant further investigation.
Plaque thrombogenicity can be assessed through the measurement of its lipid content and inflammatory activity. It has been previously shown that a single infusion of rHDL before femoral atherectomy in patients with symptomatic peripheral vascular disease resulted in a significant reduction in plaque fat content and inflammation (measured by the presence of VCAM-1-positive cells).9 Contrary to these findings, our study showed no difference in plaque neutral lipid content, and a small, but nonsignificant trend to reduce VCAM-1 expression in carotid plaques from patients infused with rHDL in comparison with the placebo group. The inability to reproduce these data may relate to the fact that we infused rHDL at half the concentration used by Shaw et al., and examined carotid plaques 24 hr following infusion of rHDL while the femoral artery plaques were not recovered for examination until 7 days following a single infusion of high dose (80 mg/kg). Although cholesterol removal from cell membranes is known to be a rapid process, the removal of the extracellular fats associated with complex plaques may require extensive time and a higher initial dose of rHDLs. The increased calcification and fibrosis seen in femoral plaques in comparison with carotid plaques may also contribute to the different responses observed between the 2 studies.
This study has a number of limitations. First, the inherent heterogeneity of the carotid plaque tissue confounded the capacity to evaluate the effect of rHDL on active inflammation using the experimental strategies we adopted to ensure random sampling of tissue. The decision to randomly select tissue for analysis may have resulted in sampling bias. 3 may have been underestimates as the test group, that received rHDLs, had significantly lower plasma concentration of HDL-C and higher concentrations of MCP-1 and CRP at baseline.
Conclusion
The results of this study demonstrate acute effects of rHDL infusion on reducing certain activation markers, which are elevated by chronic pathology and following surgery. The potential intraoperative protective effects of this reagent may be worthy of further study to explore any potential clinical benefit of elevating HDLs before endarterectomy.
In summary, reduction of basal and stress-induced TF and reduction of basal plasma MMP-9 could potentially present a more stable biological environment for surgical removal of symptomatic plaques. This is the first report of rHDL being able to reduce human plasma concentration of TF.
Future Work
This study aimed to look at the acute effects of rHDL infusion on markers of plaque instability. It was not designed to assess clinical outcomes. So far, we have shown conflicting effects on plaque thrombogenicity with different rHDL doses. Additionally, this study demonstrated that the anti-inflammatory, antiproteolytic effects of rHDL were transient. These results indicate the need for further studies aimed at dose response assessment and the comparison of repeated doses with a single infusion. Given the current findings, it would also appear prudent to suggest further studies to evaluate the clinical effects of rHDL infusion on neurological recovery and operative mortality and morbidity, which need to be validated by large multicenter trials.
Although rHDL at 40 mg/kg was well tolerated in CV patients without any serious adverse events, rHDL was potentially hepatotoxic when given at higher doses, which raised a lot of concerns among investigators. Consequently, the drug manufacturers (CSL-Behring) are currently designing a new variant of CSL-111 (product used in this trial), which is potentially more antiatherogenic with greater cholesterol efflux capacity, without the hepatotoxic properties of CSL-111. More clinical trials are still awaited to assess the clinical utility of this product.
Acknowledgments
The authors wish to thank Mr Vindoh Kumar for randomization, reconstitution of rHDL, and dispensing, and the vascular surgeons Mr Loosemore, Derodra, Ray, and Jones for providing patients for this study and Dr Jan Poloniecki for statistical advice.
Footnotes
Sources of Funding: G.W.C. was the recipient of a Pfizer International HDL award. H.N. was supported by the Dunhill Medical Trust, in association with The Royal College of Surgeons of England.
Disclosures: Dr Basser is an employee and has ownership and stock options in Commonwealth Serum Laboratories Ltd. No other authors have financial disclosures to report.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.avsg.2015.04.084.
Supplementary Data
References
- 1.Rothwell P.M., Eliasziw M., Gutnikov S.A. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 2.Karkos C.D., McMahon G., McCarthy M.J. The value of urgent carotid surgery for crescendo transient ischemic attacks. J Vasc Surg. 2007;45:1148–1154. doi: 10.1016/j.jvs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Assmann G., Gotto A.M., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109(23 Suppl. 1):III8–III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 4.Navab M., Hama S.Y., Cooke C.J. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41:1481–1494. [PubMed] [Google Scholar]
- 5.Cockerill G.W., Rye K.A., Gamble J.R. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 6.Carson S.D. Plasma high density lipoproteins inhibit the activation of coagulation factor X by factor VIIa and tissue factor. FEBS Lett. 1981;132:37–40. doi: 10.1016/0014-5793(81)80422-x. [DOI] [PubMed] [Google Scholar]
- 7.Nofer J.R., van der Giet M., Tölle M. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson M.M., Reed S.C., Cockerill G.W. Therapeutic approaches to raising plasma HDL-cholesterol levels. Nat Clin Pract Cardiovasc Med. 2004;1:84–89. doi: 10.1038/ncpcardio0044. [DOI] [PubMed] [Google Scholar]
- 9.Shaw J.A., Bobik A., Murphy A. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 10.Tardif J.C., Grégoire J., L'Allier P.L. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 11.Newton R.S., Krause B.R. HDL therapy for the acute treatment of atherosclerosis. Atheroscler Suppl. 2002;3:31–38. doi: 10.1016/s1567-5688(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls S.J., Dusting G.J., Cutri B. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 13.Lerch P.G., Spycher M.O., Doran J.E. Reconstituted high density lipoprotein (rHDL) modulates platelet activity in vitro and ex vivo. Thromb Haemost. 1998;80:316–320. [PubMed] [Google Scholar]
- 14.Furberg C.D., Byington R.P., Crouse J.R. Pravastatin, lipids, and major coronary events. Am J Cardiol. 1994;73:1133–1134. doi: 10.1016/0002-9149(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 15.Furberg C.D., Adams H.P., Jr., Applegate W.B. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90:1679–1687. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 16.Lerch P.G., Förtsch V., Hodler G. Production and characterization of a reconstituted high density lipoprotein for therapeutic applications. Vox Sang. 1996;71:155–164. doi: 10.1046/j.1423-0410.1996.7130155.x. [DOI] [PubMed] [Google Scholar]
- 17.Sayed S., Cockerill G.W., Torsney E. Elevated tissue expression of thrombomodulatory factors correlates with acute symptomatic carotid plaque phenotype. Eur J Vasc Endovasc Surg. 2009;38:20–25. doi: 10.1016/j.ejvs.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Jones A., Deb R., Torsney E. Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation. 2009;119:3125–3132. doi: 10.1161/CIRCULATIONAHA.109.852467. [DOI] [PubMed] [Google Scholar]
- 19.Sousa A.R., Lane S.J., Nakhosteen J.A. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol. 1994;10:142–147. doi: 10.1165/ajrcmb.10.2.8110469. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Nanjee M.N., Doran J.E., Lerch P.G. Acute effects of intravenous infusion of ApoA1/phosphatidylcholine discs on plasma lipoproteins in humans. Arterioscler Thromb Vasc Biol. 1999;19:979–989. doi: 10.1161/01.atv.19.4.979. [DOI] [PubMed] [Google Scholar]
- 22.Spieker L.E., Sudano I., Hurlimann D. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- 23.Bisoendial R.J., Hovingh G.K., Levels J.H. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwdorp M., Vergeer M., Bisoendial R.J. Reconstituted HDL infusion restores endothelial function in patients with type 2 diabetes mellitus. Diabetologia. 2008;51:1081–1084. doi: 10.1007/s00125-008-0975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel S., Drew B.G., Nakhla S. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53:962–971. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Bennett-Guerrero E., Feierman D.E., Barclay G.R. Preoperative and intraoperative predictors of postoperative morbidity, poor graft function, and early rejection in 190 patients undergoing liver transplantation. Arch Surg. 2001;136:1177–1183. doi: 10.1001/archsurg.136.10.1177. [DOI] [PubMed] [Google Scholar]
- 27.Ridker P.M., Rifai N., Rose L. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 28.Wannamethee S.G., Whincup P.H., Rumley A. Inter-relationships of interleukin-6, cardiovascular risk factors and the metabolic syndrome among older men. J Thromb Haemost. 2007;5:1637–1643. doi: 10.1111/j.1538-7836.2007.02643.x. [DOI] [PubMed] [Google Scholar]
- 29.Devereaux P.J., Goldman L., Cook D.J. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173:627–634. doi: 10.1503/cmaj.050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egashira K. Molecular mechanisms mediating inflammation in vascular disease: special reference to monocyte chemoattractant protein-1. Hypertension. 2003;41(3 Pt 2):834–841. doi: 10.1161/01.HYP.0000051642.65283.36. [DOI] [PubMed] [Google Scholar]
- 31.Tölle M., Pawlak A., Schuchardt M. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol. 2008;28:1542–1548. doi: 10.1161/ATVBAHA.107.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rong J.X., Li J., Reis E.D. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 33.Newby A.C. Do metalloproteinases destabilize vulnerable atherosclerotic plaques? Curr Opin Lipidol. 2006;17:556–561. doi: 10.1097/01.mol.0000245262.48258.b4. [DOI] [PubMed] [Google Scholar]
- 34.Loftus I.M., Naylor A.R., Goodall S. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31:40–47. doi: 10.1161/01.str.31.1.40. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox J.N., Smith K.M., Schwartz S.M. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonderman D., Teml A., Jakowitsch J. Coronary no-reflow is caused by shedding of active tissue factor from dissected atherosclerotic plaque. Blood. 2002;99:2794–2800. doi: 10.1182/blood.v99.8.2794. [DOI] [PubMed] [Google Scholar]
- 37.Seljeflot I., Hurlen M., Hole T. Soluble tissue factor as predictor of future events in patients with acute myocardial infarction. Thromb Res. 2003;111:369–372. doi: 10.1016/j.thromres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Viswambharan H., Ming X.F., Zhu S. Reconstituted high-density lipoprotein inhibits thrombin-induced endothelial tissue factor expression through inhibition of RhoA and stimulation of phosphatidylinositol 3-kinase but not Akt/endothelial nitric oxide synthase. Circ Res. 2004;94:918–925. doi: 10.1161/01.RES.0000124302.20396.B7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.