Abstract
Background:
This study aims to evaluate the experience and challenges in managing patients with infantile hypertrophic pyloric stenosis (IHPS).
Patients and Methods:
From January 2007 to December 2015, data from patients with IHPS were retrospectively acquired and analyzed using SPSS version 15. Pearson correlation used to assess linear relationships and Student t-test to compare means. P < 0.05 was taken as statistically significant. Results were expressed as percentages, means ± standard deviation and illustrated in tables and graphs.
Results:
Twenty-six cases were managed with the mean age at diagnosis of 49.16 ± 21.4 days. Mean birth weight was 3.7 kg and mean weight at presentation was 3.3 kg. Firstborn was affected in 29%; 91% were term deliveries; 9% were post-term; none was preterm; and 36% were exclusively breastfed. Mean duration of symptoms was 25.6 ± 18.9 days. Hyponatraemia was seen in 36%, hypokalaemia 37.5%, alkalosis 35% and hypochloraemia 62%. Mean pyloric tumour length was 22.85 ± 6.56 mm and pyloric wall thickness 5.51 ± 1.36 mm. There was a significant correlation between duration of symptoms and serum potassium level (R = −0.6326, P = 0.002). Mean symptom duration in patients with hypokalaemia was 39.88 ± 23.41 days and without hypokalaemia 17.15 ± 9.78 days (P = 0.006). Mean hospital stay was 9.45 ± 3.27 days. Four patients developed four complications and three patients died (11.5%). Mean age at presentation for pre-operative mortalities was 84 ± 39 days and 46 ± 17.98 days for others (P = 0.015).
Conclusions:
IHPS presents late in our environment and occurs mainly in term males. There is a significant positive relationship between duration of symptoms and serum potassium level and the mean duration of symptoms was significantly longer in those with hypokalaemia. Pre-operative mortality was significantly associated with longer duration of symptoms.
Keywords: Challenges, developing country, experience, infantile hypertrophic pyloric stenosis
INTRODUCTION
The incidence of infantile hypertrophic pyloric stenosis (IHPS) is dropping worldwide,[1,2,3] and this has been linked to increased acceptance and practice of exclusive breastfeeding in newborns.[3,4] IHPS is an acquired cause of gastric outlet obstruction in neonates and younger infants. Demography of affected patients as well as serum electrolyte changes and pyloric dimensions on ultrasound are variable from different studies.[5,6,7,8,9,10] Some studies have also asserted variation in pyloric wall thickness and pyloric tumour length according to weight and age.[9,10] Subsequently, proposals have been made to use different diagnostic pyloric tumour wall thickness and pyloric tumour length values in preterm babies[11] to avoid diagnostic pitfalls.
We therefore aimed to evaluate the demography, serum electrolyte changes as well as ultrasonographic pyloric dimensions of our patients and compare same findings with results from studies in other parts of the world.
PATIENTS AND METHODS
A descriptive, retrospective study designed to describe our experience in managing pyloric stenosis at the Sub-department of Pediatric Surgery University of Nigeria Teaching Hospital Ituku/Ozalla Enugu, Nigeria. From January 2007 to December 2015, data from patients with the diagnosis of IHPS were retrospectively acquired using patients' folders and admission/discharge records. The retrieved data included gestational age at birth, birth weight, sex, age at presentation, weight at presentation, birth rank, initial serum electrolyte values, transabdominal ultrasound measurement of pyloric tumour length and pyloric wall thickness, method of feeding before onset of symptoms, length of hospital stay and post-operative complications. Each patient was initially adequately resuscitated with intravenous fluid and electrolytes until cardiovascular stability as well as normal serum electrolyte levels are achieved before an open pyloromyotomy through a short transverse right upper quadrant incision was done.
Data entry and analysis
Data entry and analysis were by Statistical Package for the Social Sciences (SPSS version 15, Illinois, Chicago). Pearson correlation coefficient was used to assess for linear relationships amongst various paired sets of data. Negative value suggests a negative correlation, while a positive value suggests a positive correlation. Student t-test was used to compare means. P < 0.05 was taken as statistically significant. Results were expressed as tables, graphs, percentages and means ± standard deviation, R-values and P values.
RESULTS
There were 26 cases of IHPS, 20 males (77%) and 6 females (23%) with M:F ratio of 3.3:1. Mean age at onset of symptoms was 20.00 ± 11.72 days (range 3–52 days), while the mean age at diagnosis was 49.16 ± 21.4 days (range 16–112 days) and mean duration of symptoms at presentation was 25.59 ± 18.97 days (range 5–75 days). In 14% (3/22) of the patients, onset of symptoms occurred beyond 28 days of age. In 86% (19/22) of the patients, onset of symptoms was on or before 28 days of age. Only in 16% of the cases (4/25), were the diagnoses made at or before the age of 28days. In the majority of cases (21/25, 84%) diagnoses were made beyond 28 days of age.
Average birth weight was 3.7 kg ± 0.76 kg (2.6 kg–5.10 kg) and average weight at presentation was 3.39 kg ± 0.66 kg (2.10 kg–4.6 kg).
In those with available data, the birth orders of the patients were first in 29% (6/21) of patients, second in 9.5% (2/21), third in 33% (7/21), fourth in 19% (4/21), fifth in 5% (1/21) and sixth in 5% (1/21). In all 91% (20/22) of the patients were term deliveries, 9% (2/22) post-date deliveries and there were no preterm deliveries.
Modes of delivery were by spontaneous vertex deliveries 16/22 (73%), elective caesarean section 2/22 (9%) and emergency caesarean Section 4/22 (18%).
Exclusive breastfeeding was done in 36% (8/22) before the onset of symptoms, while 64% (14/22) were not exclusively breastfed.
Average serum sodium level was 134.95 ± 7.07 (range 113–143 mmol/l); average serum potassium level 3.77 ± 0.85 (range 2.6–5.72 mmol/l); average serum bicarbonate level 27.38 ± 4.78 (range 18–36 mmol/l) and average serum chloride level was 89.76 ± 10.70 (range 70–105 mmol/l).
Hyponatraemia was seen in 8/22 (36%); hypokalaemia in 9/22 (37.5%); alkalosis in 6/17 (35%) and hypochloremia in 13/21 (62%).
Normal serum potassium levels were measured in 11/22 (50%), normal serum sodium in 14/22 (64%), normal serum chloride in 8/21 (38%) and normal serum bicarbonate in 8/17 (47%).
Hyperkalaemia was seen in 2/22 (9%) and low bicarbonate level in 3/17 (18%). Urine pH could not be assessed as this was a retrospective study and such data were not recorded in the folder.
Average pyloric tumour length was 22.85 mm ± 6.56 (15 mm–35 mm) and mean pyloric wall thickness was 5.51 mm ± 1.36 (3.2 mm–7.5 mm)
There was a strong correlation between duration of symptoms and serum potassium level with serum potassium reducing as the duration of symptoms increased (R = −0.6326, P = 0.002), [Tables 1 and 2 and Figure 1]. There was also a significant correlation between age at presentation and serum potassium level (R = −0.598, P = 0.003), [Table 1 and Figure 2].
Table 1.
Pearson correlation coefficients and P values
| Pearson correlation coefficient | P | |
|---|---|---|
| Duration of symptoms and serum potassium | −0.6326 | 0.002 |
| Duration of symptoms and serum sodium | −0.2921 | 0.1872 |
| Duration of symptoms and serum chloride level | 0.053 | 0.818 |
| Duration of symptoms and serum bicarbonate level | 0.2344 | 0.3492 |
| Duration of symptoms and pyloric tumour length | 0.0928 | 0.7071 |
| Duration of symptoms and pyloric tumour thickness | −0.3114 | 0.2244 |
| Weight and pyloric tumour length | 0.322 | 0.1546 |
| Weight and pyloric tumour thickness | −0.1244 | 0.6354 |
| Age and pyloric tumour length | 0.1597 | 0.5137 |
| Age and pyloric tumour thickness | −0.1288 | 0.6217 |
| Pyloric length and thickness | −0.1298 | 0.619 |
| Serum potassium and serum HCO3 | −0.483 | 0.042 |
| Serum chloride and HCO3 | −0.611 | 0.007 |
| Serum sodium and HCO3 | 0.068 | 0.788 |
Table 2.
Duration of symptoms and mean serum electrolyte levels
| <20 days | 20-40 days | >40 days | |
|---|---|---|---|
| Mean serum sodium level (mmol/l) | 137 | 133 | 133 |
| Mean serum potassium level (mmol/l) | 4.8 | 3.6 | 3.2 |
| Mean serum bicarbonate level (mmol/l) | 26 | 30 | 28 |
| Mean serum chloride level (mmol/l) | 90 | 90 | 91 |
Figure 1.
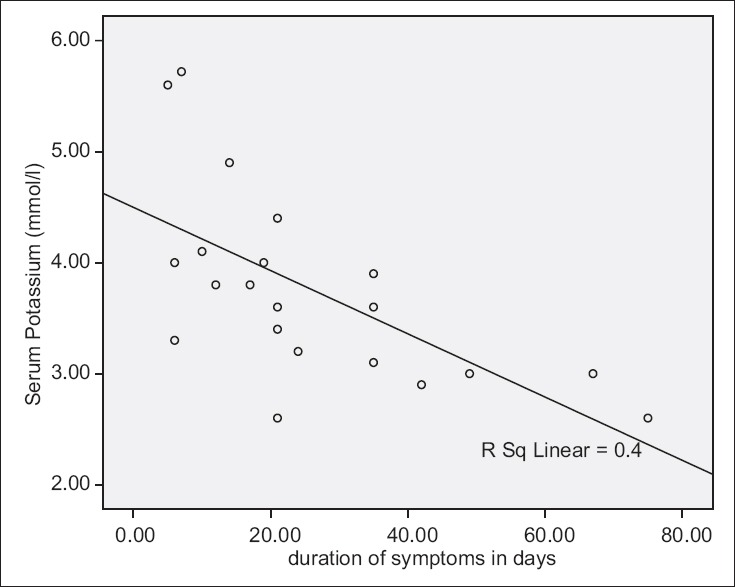
Serum potassium levels and duration of symptoms
Figure 2.
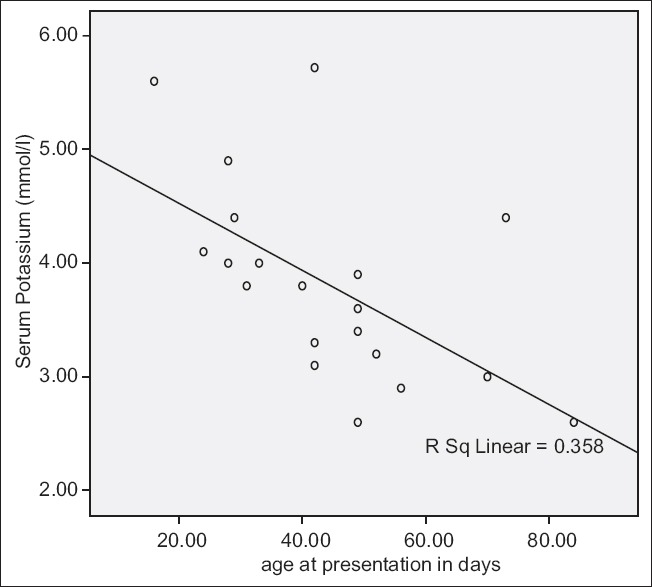
Serum potassium and age at presentation
The average duration of symptoms in patients with hypokalaemia was 39.88 ± 23.41 days and without hypokalaemia 17.15 ± 9.78 days (P = 0.006).
There was a significant correlation between Serum Potassium and bicarbonate levels with serum potassium reducing as bicarbonate level rises (R = −0.483, P = 0.042), [Table 1].
Serum chloride level also decreased as serum bicarbonate level increased (R = −0.611, P = 0.007) [Table 1 and Figure 3].
Figure 3.
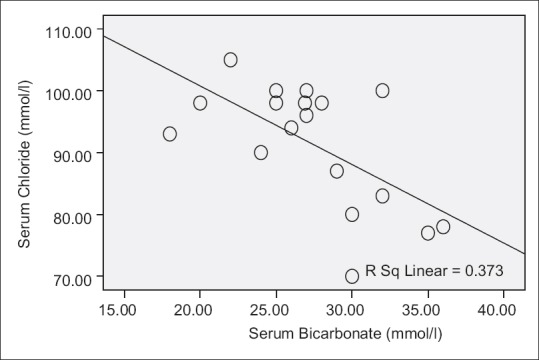
Serum chloride and serum bicarbonate levels
All had open Fredet–Ramstedt pyloromyotomy through a right upper quadrant transverse incision and under general anaesthesia.
Mean hospital stay is 9.45 ± 3.27 days (6–21 days). Mean duration from admission to surgery was 5.07 ± 3.39 days and mean post-operative day at discharge is 4.58 ± 2.03 days.
Four patients developed four post-operative complications (15% complication rate), namely, two superficial wound breakdown, one reactionary haemorrhage and one incisional hernia. There was no case of incomplete pyloromyotomy or pyloroduodenal mucosal injury.
Three patients died giving an 11.5% mortality rate: Two died pre-operatively during resuscitation probably from severe electrolyte imbalance and one died on operating table from intra-operative anaesthetic complications.
Average age at presentation for pre-operative mortalities was 84 ± 39 days and 46 ± 17.98 days for others (P = 0.015).
DISCUSSION
There has been a global trend towards reduction in the incidence of IHPS[1,2,3] though occurrence has always been more among the Caucasian population.[12] This reduction in incidence has been linked to the practice of exclusive breastfeeding in the first few months of life.[1,3,4] In this study, a record of 26 cases in 8 years is lower than other single-centre studies in the United States of America,[9] South Africa,[13] Malaysia[14] and Australia[15] but higher than another in Benin city Nigeria.[3]
Although male preponderance has been established in IHPS worldwide, the proportion of females in this study of 23% is high when compared with some other studies.[3,13,15]
Late presentation with a mean age at diagnosis of 49 days (7 weeks) in this study is higher than a mean age of 4.6 weeks, 5.4 weeks recorded by Said et al.[10] and Taylor et al.,[15] respectively, and comparable to 43.1 days recorded by Leong et al. in Taiwan.[12] Majority of our patients (86%) presented late beyond the neonatal period despite the fact that many (84%) had their onset of symptoms in the neonatal period. This is mainly due to prior visits to peripheral hospitals before presenting to a tertiary centre for care.
Since exclusive breastfeeding is thought to be protective against IHPS,[1,3,12] 36% of our patients were exclusively breastfed before onset of symptoms. The majority of the patients were however not exclusively breastfed. In another study by Osifo and Evbuomwan,[3] in a different part of the country, no IHPS was found among exclusively breastfed babies.
Some studies showed that patients are more likely to develop IHPS following caesarean deliveries,[4] but in our study, only 27% had caesarean delivery while the majority were delivered by the vaginal route.
In spite of the fact that preterm delivery is a known risk factor for IHPS,[4,16] none of our patients was born preterm, and this may be explained by high mortality of preterm babies in our environment[17,18] as they may not alive till the peak age of incidence of IHPS.
Most studies established that first birth order in a family is associated with increased risk of IHPS while the second birth and subsequent birth ranks beyond this are associated with reduced risk of IHPS.[4,19] Contrary to this established empirical evidence, the current study showed the greatest number of IHPS was found amongst the third birth order (33%), while first birth had 29%. It may be difficult to draw conclusions from this as it is not a population-based study and the sample size is small.
Electrolyte changes were variable showing both normal and abnormal levels. Hypochloraemia was the most common electrolytes abnormality in this study, while in some other study,[16] elevated bicarbonate was the most common. In Tutay et al.,[5] most patients had normal serum electrolytes with the most common electrolyte anomaly being hyperkalaemia.
The present report [Table 3] and some earlier studies[7,8] indicate that electrolytes may be normal in the early presentation of IHPS and gradually deranges when the duration of symptoms increase and adequate resuscitations are not promptly instituted.
Table 3.
Distribution of serum electrolyte levels amongst the patients
| Percentage elevated | Percentage normal | Percentage reduced | |
|---|---|---|---|
| Serum sodium | 0 | 64 | 36 |
| Serum potassium | 9 | 50 | 41 |
| Serum bicarbonate | 35 | 47 | 18 |
| Serum chloride | 0 | 38 | 62 |
Alkalosis drives potassium into cells hence leading to the significant negative relationship in this study between serum potassium and serum bicarbonate levels [Table 1].
There was a progressive decline of average serum potassium level as the duration of symptoms increased [Table 3]. From Nmadu report,[7] almost all had alkalosis but with normal serum potassium in early presenters and hypokalaemia in those presenting after 3 weeks of symptoms. This result corroborates findings in this study which found a significant negative relationship between duration of symptoms and hypokalaemia [Figure 1]. Furthermore, average duration of symptoms was longer in those with hypokalaemia when compared with those without hypokalaemia.
There was a significant reduction in serum chloride level as serum bicarbonate increased [Table 1]. This may be explained by the fact that the sum of serum chloride and bicarbonate remain fairly constant within a normal range so that changes in one variable cause an opposite change in the other.
The current study recorded an average pyloric tumour thickness of 5.51 mm at an average age of 49 days. Pyloric tumour wall thickness had negative correlation with weight, age and duration of symptoms. These were however not significant [Table 1]. In some other studies,[9,10] the mean pyloric tumour wall thickness had a significant positive relationship with age and weight. In consonance with our findings in patients with IHPS, another study[9] suggested that pyloric wall thickness however had a negative correlation with age and weight in those without IHPS. It is difficult to state why there is a negative correlation between pyloric wall thickness and age and weight contrary to some other studies[9,10] where the pyloric tumour wall thickness increased with age and weight.
There is a positive relationship between pyloric tumour length and age (P = 0.51, R = 0.16), duration of symptoms (P = 0.71, R = 0.09) and weight (P = 0.15, R = 0.32) though none of these was statistically significant [Table 1]. In some other studies,[9,10] however, there were significant positive relationships between age, weight and pyloric tumour length.
All had open pyloromyotomy through a transverse right upper quadrant incision. There was no use of curvilinear supraumbilical incision. Facilities for laparoscopic pyloromyotomy were absent, and hence, this method was not used. Non-operative management with the use of atropine sulphate was not employed in the patient management.
Post-operative complication rate of 15% is high but comparable to other African studies.[13] All complications were managed non-operatively. There was no incidence incomplete pyloromyotomy or pyloroduodenal mucosal perforation. In some other studies,[16,20] there were incomplete pyloromyotomies and omitted mucosal perforations requiring re-exploration.
Mortality rate in this study is higher than most other studies[12,21,22] and significantly related to late age at presentation (P = 0.015). These were two pre-operative mortalities during resuscitation and one intra-operative mortality possibly from anaesthetic complications. These may be related to the severe electrolyte imbalance related to prolonged duration of symptoms before presentation to the surgeon. Late presentation was also a strong determinant of mortality in another African publication from Tanzania.[21]
Prolonged hospital stay (mean of 9.45 days) in this study is comparable to some Asian[12] and African[21] studies but more than some other published data[23] and is mainly due to late presentation and time to adequate resuscitation. It took an average of 5.07 ± 3.39 days from admission to surgery. This duration accounted mainly for the time to full resuscitation ensuring normal serum electrolyte indices and adequate hydration before surgery is carried out. This is less than duration reported by Chalya et al. in Tanzania[21]
Limitations of study
This is a single-unit study in a low incidence area and with a limited number of cases. Despite these limitations, we believe that this study has provided basic data for our environment and from which improvements can be made in future.
Recommendations
We recommend large-scale prospective studies since incidence dropping worldwide. Furthermore the effects of macrolide antibiotics, maternal smoking during early pregnancy, maternal overweight in pre-pregnancy period and young maternal age (<25 years) on development of IHPS should be studied in our environment.
CONCLUSIONS
PS occurs mainly in term neonates. Presentation is generally late with a significant negative relationship existing between duration of symptoms and serum potassium level. Average duration of symptoms was significantly longer in those with hypokalaemia than those with normal serum potassium level. There are also significant inverse relationships between serum bicarbonate levels and serum chloride and potassium levels. The most common electrolyte anomaly is hypochloraemia. No significant relationships between age, weight, duration of symptoms and pyloric tumour length and wall thickness were noted. Pre-operative mortality was significantly associated with longer duration of symptoms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Krogh C, Biggar R, Fischer TK, Lindholm M, Wohlfahrt J, Melbye M. Bottle-feeding and risk of pyloric stenosis. Pediatrics. 2012;130:1–7. doi: 10.1542/peds.2011-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommerfield T, Chalmers J, Youngson G, Heeley C, Fleming M, Thomson G, et al. The changing epidemiology of infantile hypertrophic pyloric stenosis in Scotland. Arch Dis Child. 2008;93:1007–11. doi: 10.1136/adc.2007.128090. [DOI] [PubMed] [Google Scholar]
- 3.Osifo DO, Evbuomwan I. Does exclusive breastfeeding confer protection against infantile hypertrophic pyloric stenosis? A 30-year experience in Benin City, Nigeria. J Trop Pediatr. 2009;55:132–4. doi: 10.1093/tropej/fmn094. [DOI] [PubMed] [Google Scholar]
- 4.Svenningsson A, Svensson T, Akre O, Nordenskjöld A. Maternal and pregnancy characteristics and risk of infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2014;49:1226–31. doi: 10.1016/j.jpedsurg.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 5.Tutay GJ, Capraro G, Spirko B, Garb J, Smithline H. Electrolyte profile of pediatric patients with hypertrophic pyloric stenosis. Pediatr Emerg Care. 2013;29:465–8. doi: 10.1097/PEC.0b013e31828a3006. [DOI] [PubMed] [Google Scholar]
- 6.Breaux CW, Jr, Hood JS, Georgeson KE. The significance of alkalosis and hypochloremia in hypertrophic pyloric stenosis. J Pediatr Surg. 1989;24:1250–2. doi: 10.1016/s0022-3468(89)80561-5. [DOI] [PubMed] [Google Scholar]
- 7.Nmadu PT. Alterations in serum electrolytes in congenital hypertrophic pyloric stenosis: A study in Nigerian children. Ann Trop Paediatr. 1992;12:169–72. doi: 10.1080/02724936.1992.11747564. [DOI] [PubMed] [Google Scholar]
- 8.Touloukian RJ, Higgins E. The spectrum of serum electrolytes in hypertrophic pyloric stenosis. J Pediatr Surg. 1983;18:394–7. doi: 10.1016/s0022-3468(83)80188-2. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal CW, Rivard DC, Mortellaro VE, Sharp SW, St. Peter SD. Evaluation of ultrasonographic parameters in the diagnosis of pyloric stenosis relative to patient age and size. J Pediatr Surg. 2012;47:1542–7. doi: 10.1016/j.jpedsurg.2012.03.068. [DOI] [PubMed] [Google Scholar]
- 10.Said M, Shaul DB, Fujimoto M, Radner G, Sydorak RM, Applebaum H, et al. Ultrasound measurements in hypertrophic pyloric stenosis: Don't let the numbers fool you. Perm J. 2012;16:25–7. doi: 10.7812/tpp/12.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leaphart CL, Borland K, Kane TD, Hackam DJ. Hypertrophic pyloric stenosis in newborns younger than 21 days: Remodeling the path of surgical intervention. J Pediatr Surg. 2008;43:998–1001. doi: 10.1016/j.jpedsurg.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Leong MM, Chen SC, Hsieh CS, Chin YY, Tok TS, Wu SF, et al. Epidemiological features of infantile hypertrophic pyloric stenosis in Taiwanese children: A Nation-Wide Analysis of Cases during 1997-2007. PLoS One. 2011;6:e19404. doi: 10.1371/journal.pone.0019404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saula PW, Hadley GP. Hypertrophic pyloric stenosis in the third world. Trop Doct. 2011;41:204–10. doi: 10.1258/td.2011.110145. [DOI] [PubMed] [Google Scholar]
- 14.Tan HL, Blythe A, Kirby CP, Gent R. Gastric foveolar cell hyperplasia and its role in postoperative vomiting in patients with infantile hypertrophic pyloric stenosis. Eur J Pediatr Surg. 2009;19:76–8. doi: 10.1055/s-2008-1039199. [DOI] [PubMed] [Google Scholar]
- 15.Taylor ND, Cass DT, Holland AJ. Infantile hypertrophic pyloric stenosis: Has anything changed? J Paediatr Child Health. 2013;49:33–7. doi: 10.1111/jpc.12027. [DOI] [PubMed] [Google Scholar]
- 16.Gotley LM, Blanch A, Kimble R, Frawley K, Acworth JP. Pyloric stenosis: A retrospective study of an Australian population. Emerg Med Australas. 2009;21:407–13. doi: 10.1111/j.1742-6723.2009.01218.x. [DOI] [PubMed] [Google Scholar]
- 17.Eke CB, Ezomike UO, Chukwu BF, Chinawa JM, Korie FC, Chukwudi N, et al. Pattern of neonatal mortality in a tertiary health facility in Umuahia, South East Nigeria. Int J Trop Dis Health. 2014;4:136–46. [Google Scholar]
- 18.Lawoyin TO, Onadeko MO, Asekun-Olarimoye EO. Neonatal mortality and perinatal risk factors in rural Southwestern Nigeria: A community-based prospective study. West Afr J Med. 2010;29:19–23. doi: 10.4314/wajm.v29i1.56183. [DOI] [PubMed] [Google Scholar]
- 19.Shaoul R, Enav B, Steiner Z, Mogilner J, Jaffe M. Clinical presentation of pyloric stenosis: The change is in our hands. Isr Med Assoc J. 2004;6:134–7. [PubMed] [Google Scholar]
- 20.Hulka F, Harrison MW, Campbell TJ, Campbell JR. Complications of pyloromyotomy for infantile hypertrophic pyloric stenosis. Am J Surg. 1997;173:450–2. doi: 10.1016/S0002-9610(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 21.Chalya PL, Manyama M, Kayange NM, Mabula JB, Massenga A. Infantile hypertrophic pyloric stenosis at a tertiary care hospital in Tanzania: A surgical experience with 102 patients over a 5-year period. BMC Res Notes. 2015;8:690. doi: 10.1186/s13104-015-1660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carneiro PM. Infantile hypertrophic pyloric stenosis in Dar Es Salaam. Cent Afr J Med. 1991;37:93–6. [PubMed] [Google Scholar]
- 23.Jlidi S, Ben Youssef D, Ghorbel S, Mattoussi N, Khemakhem R, Nouira F, et al. Infantile hypertrophic pyloric stenosis. Report of 142 cases. Tunis Med. 2008;86:63–7. [PubMed] [Google Scholar]


