Abstract
Breast cancer (BC) remains the leading cause of death in women worldwide, despite the improvements of cancer screening and treatment methods. Recently, development of novel anticancer drugs for the improved prevention and treatment of BC is in the center of research. The anticancer effects of bromelain, as enzyme extract derived from the pineapples, contains chemicals that interfere with the growth of tumor cells. The aim of this study was to evaluate the effect of radiosensitizing of bromelain in 4T1 BC cells. This investigation utilized the 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide assay to characterize the cytotoxicity of bromelain. Colony formation method was used to establish the truth of the capability of bromelain to make sensitive to radiation therapy. Flowcytometry performed to define the contribution the apoptosis effect to bromelain mediated radiosensitization of 4T1 cells. Bromelain reduced growth and proliferation of 4T1 cell as a concentration-dependence manner significantly. The survival of 4T1 cancer cells was decreased after combined treatment in a number and size-dependent manner with regard to the control group (P < 0.05). Combination of bromelain with radiation does not influence 4T1 cell apoptosis. The results suggested that bromelain can inhibit the growth and proliferation and reduce survival of 4T1 BC cells and might be used as a candidate radiosensitizer in BC patient.
Keywords: 4T1, breast cancer, bromelain, radiosensitizing
Introduction
Breast cancer (BC) is one of the most common cancers and the original reason of cancer-related death in women worldwide. Clinically conventional treatment has been adopted for BC treatment mainly including chemotherapy, surgery, radiotherapy (RT), and some combined treatment strategies.[1,2,3,4,5] Chemotherapy is used for systemic control, whereas surgery and RT are used to the management of cancer. RT is commonly recommended as primary or adjuvant treatment modality for BC with the benefit rate as high as 80%–90%. Despite its critical role in cancer treatment, there are challenges related to RT. The maximum dose of irradiation which can be delivered to tumors and its curative effect are often limited by the similarities in the energy absorption attributes of the malignant tissues and normal ones and resulting in radiation toxicity to the nearby healthy tissues. In addition, the majority of cancer types contain a high degree of heterogeneity in microenvironment, phenotype, genotype, many hypoxic cells, and huge amount of enzymes should be protected cells to RT and variability in radiation sensitivity.[1,2,3,4] To address these challenges, tumor-selective radiosensitizers which can increase the selectivity of ionizing radiation (IR) to cancer cell without hampering the activities of healthy cells are extremely desired and have been widely investigated. The use of radiosensitizers combined with low-linear energy transfer radiations can increase normal tissue radioresistance, therapeutic efficacy by allowing to decrease the essential treatment irradiation dose, and resulting in minimizing the risk of recurrence. As is well defined there are four factors that influence radio-resistance i) repair of DNA, ii) reoxygenation, iii) redistribution of the cell cycle and iv) repopulation (the”4 R's”). Therefore, any pharmacological agents purposed at sensitizing cells to RT would require to target one or more of these factors. Whereas many agents have been discovered based on this rule, the adverse effects of the agent itself often ban its gaining application as a radiosensizer. Thus, discovery of modern radiosesitizers with low unwanted effects has become a region of interest for medical oncology researchers. Recently, the attractive in plant-taken natural materials for BC therapy and supportive care of cancer has enhanced.[1,6,7,8,9]
Bromelain is one of the cysteine protease found in the pineapple plant. Bromelain has been chemically identified since 1875 and is consumed as a phytomedical compound. It can be used to prevent diabetes and various cardiovascular diseases. A wide range of studies has been suggested that bromelain is useful for blood coagulation-related diseases, enhanced absorption of drugs, particularly of antibiotics, inflammatory, reversible inhibition of platelet aggregation, surgical traumas, and many intestinal and cancers. Since bromelain could increase the expression of p53 and Bax genes, decrease the activity of cell survival regulators such as Akt and Erk and also inhibit NF-κB, Cox-2, and PGE2 activity.[10,11] According to the best of authors’ knowledge, there is no any report document on the functions of bromelain as a radioresistant, and this work is the first to investigate its radiosensitizing effects. The purpose of the present study was to determine the ability of bromelain to sensitize mouse 4T1 BC cells to irradiation.
Materials and Methods
Cell culture
4T1 (mouse BC) and L929 (mouse fibroblast) cell lines were obtained from the Pasteur Institute (Tehran, Iran). Cells were maintained in Roswell Park Memorial Institute medium 1640 (Gibco Invitrogen) containing 10% fetal bovine serum (Gibco-Invitrogen), 2 g/l sodium bicarbonate, and 1% penicillin/streptomycin (Gibco-Invitrogen). Cells were grown in a humidified incubator at 37°C, at a 95% air −5% CO2 atmosphere. When the cells reached 80% confluences and subcultured, they were harvested with 0.5 g/l trypsin (Gibco Laboratories) and 0.2 g/l ethylenediaminetetraacetic acid (Gibco Laboratories) for 4 min for further passages. In all experiments, cells were seeded and then incubated 24 h before each assay.
Drug preparation
Bromelain was obtained from Merck (Darmstadt, Germany) and the stock solution was made at concentrations of 1000 μg/ml. Stock solution was filtered the final treating concentrations were obtained using diluting in a suitable culture medium.
3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide assay
Cell viability was defined by 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) assay. Almost 5000 cells/well were plated in 96-well culture dish to determine the effect of bromelain on proliferation 4T1 cells. After 24 h incubation, the cells were treated with different concentrations of bromelain, that is, 0, 5, 10, 20, 40, 50, 75, 100, 200, 300, 400, and 600 μg/ml for 24.[12] After the treatment period, 20 μl of MTT of 5 mg/ml in PBS were added to each well and left for 3–4 h at 37°C within the incubator. Following the incubation, MTT solution was eliminated and 100 μl of dimethyl sulfoxide added for 20 min at 37°C. Absorbance was measured using a multidetection microplate reader at the working wavelength of 540 nm. Experiments were repeated at least three times on different days.
Cellular-growth curve
To measure doubling time and cellular-growth curves, cells were plated in a 12-well plate with a concentration of 5–6 × 104 per well in triplicate. The culture media of each plate were changed every 3 days through the 6 days. Cell numbers were defined every 24 h by counting cells under microscope after trypsinization using trypan blue. To calculate the Doubling time (TD), growth curve was plotted on the common log scale and TD was measured by using the equation:
TD = (duration×log2)/(log(finalconcentration/initialconcentration))
Cell irradiation
Cells were irradiated by 6 MV X-ray photon using a clinical accelerator (Primus, Siemens Ltd., Germany), located in the Parsian hospital, Shahrekord, Iran, at doses of 0–6 Gy with a dose rate of 200 cGy min-1. Control groups were placed under the 6 MV photon beam for the same period of time as well. However, the controls did not receive any radiation over this procedure since the source shutter has already been closed. Irradiations were performed with a source-surface distance of 100 cm and a field size of 20 cm × 20 cm. A schematic of Plexiglas (polymethyl methacrylate [PMMA]) phantom which fabricated for this study is shown in Figure 1. Two centimeters of a Plexiglas (PMMA) sheet (water equivalent) was placed on the top of the plate to serve as a build-up material [Figure 1]. Experiments were repeated at least three times on different days.
Figure 1.
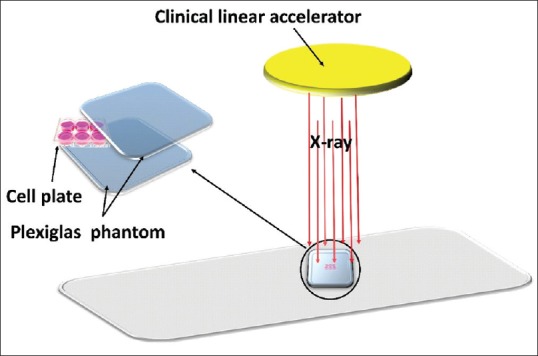
In vitro radiation experiment setup[24]
Clonogenic survival assay
Cells were seeded in 6-well microplates in triplicate different cell numbers were for different doses of irradiation (100, 200, 600, and 1000 cells were for 0, 2, 4, and 6 Gy, respectively). After overnight, cells were treated with bromelain in concentration IC50 (μg/ml) for 24 h. At the end of the treatment period, cells were irradiated by 6 MV X-ray photon at doses of 0–6 Gy. After irradiation, cells were incubated at 37 °C in a humid 5% CO2 atmosphere. Culture medium per dish was changed every 2 days. After 7 days, Colonies comprising 50 cells were stained with 0.5% crystal violet in 70% ethanol and counted by Image J software version 1.47 (National Institutes of Mental Health, Bethesda, Maryland, USA). Plating efficacy (PE) and survival fraction (SF) was calculated mathematically using the following equations:
PE = number of colonies / number of seeded cells
SF = number of colonies / (number of seeded cells × PE).
Flowcytometry analysis
Flowcytometry analysis was used to define the contribution the apoptosis effect to bromelain-mediated radiosensitization of 4T1 cells. In brief, 300 × 103 cells were plated onto coverslips in 6-well plates and treated with bromelain in concentration IC50 (μg/ml) for 24 h. Following the period of time, cells were irradiated at doses of 0–6 Gy by 6 MV X-ray photons and after 8 h, flowcytometry analysis was performed using a BD LSR II Flow cytometer (BD Biosciences, Heidelberg, Germany). Flowcytometry data were analyzed using Flow Jo software 7.6.1 software (Tree Star, Ashland, OR, USA). Externalized phosphatidylserine was detected by Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide staining (BD Biosciences). Annexin V-FITC-positive/propidium iodide-negative cells were assumed early apoptosis and double-positive cells were assumed late apoptotic/secondary necrotic. Experiments were repeated at least three times on different days.
Statistical analyses
All the experiments were repeated three times. All the data were analyzed by one-way ANOVA test using GraphPad in Stat (Graph Pad Prism 6, San Diego, CA, USA). Data are presented as mean ± standard deviation and P < 0.05 were deemed to be statistically significant.
Results
Bromelain-induced inhibition of proliferation
The result from the previous study was used to obtain the inhibition of 4T1 cells viability by 50% (IC50 value).[12] The values of IC50 (μg/ml) was obtained for 4T1 cells, 95 after 24 h of incubation with bromelain.
Cellular-growth curve
Cellular-growth curves were calculated for 4T1 cells as previously explained manner in materials and methods section. TD values of 19.77 h at 4T1 BC cells was determined.
Enhancement of radiation-induced clonogenic cell kill
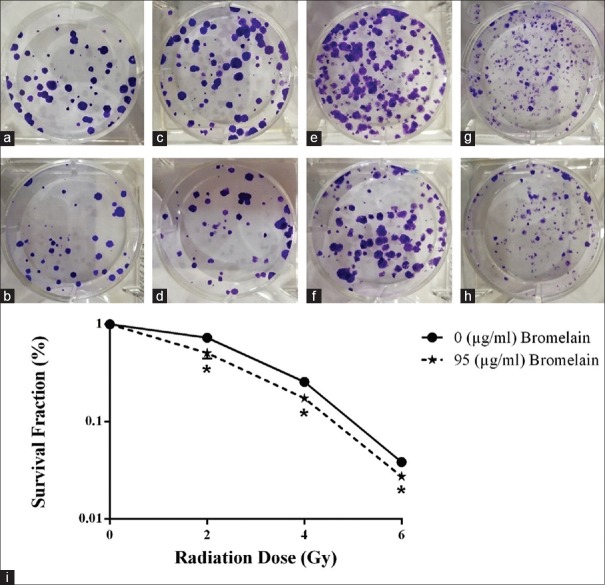
To determine the radiosensitivity effects of bromelain at IC50 (μg/ml) and various IR doses of 4T1 cells, clonogenic assays were performed. As presented in Figure 2a-h, 24 h before administration of bromelain at an IC50 of 95 (μg/ml) with X-radiation enhanced the kill of 4T1 colognes achieved by either treatment alone.
Figure 2.
Bromelain sensitizes 4T1 cells to irradiation. Bromelain was administered 24 h before X-rays and 7 days later clonogenic assay was carried out. Control group cells (a). Cells were treated with Bromelain (b). 2 Gy (c). Bromelain + 2 Gy (d), 4 Gy (e). Bromelain + 4 Gy (f). 6 Gy (g). Bromelain + 6 Gy (h). The survival curves derived from the clonogenic assay experiments treated with or without bromelain were significantly different (P < 0.05) (i). The experiments were performed in triplicate and data are presented as mean ± standard deviation of three separate experiments
The IC50 value obtained following the exposure of 4T1 cells to X-rays alone or in the presence of 95 (μg/ml) bromelain were 1.92 ± 0.14 and 1.25 ± 0.05 Gy, respectively. The sensitivity of 4T1 cells to radiation was increased after incubation with an IC50 of 95 (μg/ml) of bromelain after an incubation period of 24 h. Percentage of plating efficiency was found to be 52.13 after pretreatment [Figure 2a]. The SFs of the cells were 72.9% [Figure 2c and i], 25.6% [Figure 2e and i], and 3.8% [Figure 2g and i] for 2, 4, and 6 Gy alone and 50.7% [Figure 2d and i], 17.4% [Figure 2f and i], and 2.7% [Figure 2h and i] for 2, 4, and 6 Gy in the presence of bromelain, respectively. The sensitivity of 4T1 cells to radiation was increased by 95 μg/ml bromelain for all X-rays doses (P < 0.05) [Figure 2]. These results show the radiosensitizing activity of bromelain.
Flowcytometry combination of bromelain with radiation does not influence 4T1 cell apoptosis
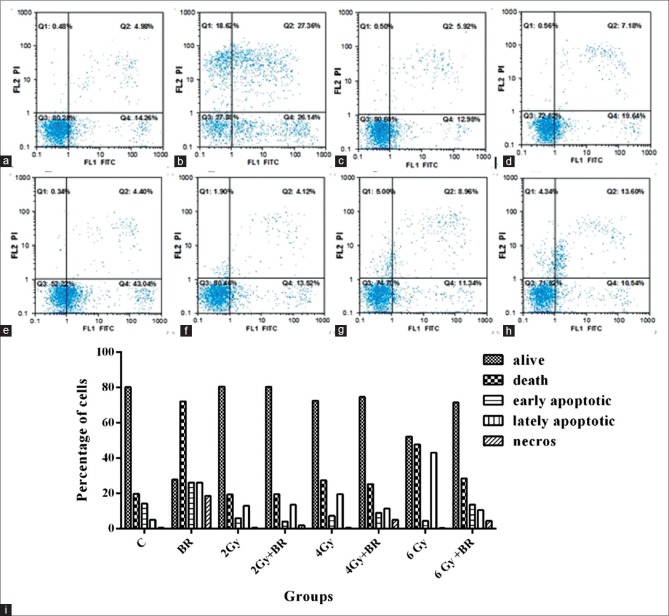
To define the contribution, the apoptosis effect to bromelain-mediated radiosensitization of 4T1 cells, Annexin V staining was determined in cells 8 h after RT. Gates were positioned according to nonstained cells and negative controls. As shown in Figure 3a, b, and i, bromelain significantly induces 26.14% apoptosis and 18.62% necrosis, 24 h treatment, compared with 14.22% and 0.5% of the control cells, respectively. However, we did not observe any enhancement in other groups – induced apoptosis treated either with RT alone or in combination with bromelain [Figure 3]. Furthermore, Figure 3 indicated an increase in late apoptotic event of individual and combination therapies compared with the control cells. We found a significant reduction in lately apoptotic event for bromelain + RT treatment; 11.34 and 10.54 compared with RT 19.64% and 43.04% in 4 Gy [Figure 3e, f, and i] and 6 Gy alone, respectively [Figure 3g, h, and i].
Figure 3.
The effect of bromelain on apoptosis in 4T1 cells. The cells have been treated with bromelain and/or radiation, harvested 8 h after treatment, stained with Annexin V/PI and analyzed by flow cytometric analysis. Control group cells (a). Cells were treated with bromelain (b). 2 Gy (c). Bromelain + 2 Gy (d), 4 Gy (e). Bromelain + 4 Gy (f). 6 Gy (g). Bromelain + 6 Gy (h). The bar graph shows the percentage of all apoptotic events following bromelain and/or radiation treatment (i). The data are presented as mean ± standard deviation of at least three independent experiments
Discussion
Bromelain was took out from natural pineapple which was known as an antioxidant in various in vitro study models. Bromelain has been reported to promote apoptosis, particularly in BC cells. This component also has potent anti-inflammatory effects and has been the focus of research involving inflammatory conditions. Clinical trials indicate that it is effective in reducing acute sinusitis[13] and osteoarthritis of the knee.[14] Bromelain also has the ability to control edema[15] and support fibrinolysis; in addition, it has been studied for its debriding effects on burn wounds.[16] Bromelain's mechanisms of action reduction of secretion of pro-inflammatory chemokines and cytokines.[17]
Numerous studies have shown that bromelain has immunomodulatory and antitumor properties.[18,19] In a study, Dhandayuthapani et al.[20] reported the influence of bromelain in inducing cell death by activation of the apoptosis mechanisms in GI-101A cells. They observed that with increasing the concentrations of bromelain into the mentioned cells, the apoptosis-related cell death in BC cells could be also increased.
Bromelain has varied biologic effects. To date, numerous studies have investigated the anticancer effect of bromelain. Their findings showed that bromelain made the cytotoxic effects on human prostate cancer cell (PC3), human gastric cancer cell (AGS)[21] human skin fibroblast 1184, human BC (MDA-MB-468 and MDA-MB-231)[22] and mouse BC (4T1),[12] murine lung carcinoma, melanoma, lymphoma cells[23,24] epidermoid carcinoma, melanoma,[23] and malignant peritoneal mesothelioma.[25] From the results obtained in our study, bromelain in the time of incubation 24 h reduced growth and proliferation of 4T1 cell as a concentration-dependent manner significantly. This finding in 24 h of incubation is agreeing with their results. Mechanism of action of bromelain can be related to the enhancement in the activity of caspase-3 and caspase-9 that play a vital role in apoptotic cell death induced by bromelain.[20]
Results of clonogenic formation assay showed the survival of 4T1 cells was decreased after combined treatment in a number and the size-dependent manner with respect to the control group significantly (P < 0.05).
As it is shown in Figure 3, bromelain significantly induces 26.14% apoptosis and 18.62% necrosis, 24 h treatment, compared with 14.22% and 0.5% of the control cells, respectively. However, there was no significant enhancement in induced apoptosis treated either with RT alone or in radiation combination with bromelain in other groups.
Clonogenic formation assay was used to evaluate potential radiosensitizer compounds in several studies. They have indicated a significant reduction in number, size colonies, and survival that received combination treatment with respect to the radiation therapy alone group. They find the effect of other different agents as radiosensitizer on differentiated cells, such as olaparib, rucaparib, elesclomol, and 17-DMAG on human PC3 cell lines, PC3 and LNCaP, Huaier on human BC cell lines; MCF-7 and MDA-MB-231.[1,26] Futhermore, many researchers worked on many cells, including LY2603618, MK87776, and AZD1775 on human papillomavirus(+) human head-and-neck squamous cell carcinoma (HNSCC) cells; UD-SCC-2, UM-SCC-47, and UPCI-SCC-154, EhB4 on HNSCC cells; Cal27, MSK-921, and Fadu, Human polo-like kinase 1 (PLK1) on human esophageal squamous cell carcinoma cells; KYSE 70, and KYSE 150, ganetespib on human Hepatocellular carcinoma cells; Hep3b, HepG2 and HUH, AZD6738 and ATM inhibitor KU-55933 on different cell lines; CAL27, FaDu, A549, PJ34, PJ41, T24, A2780, RT4, NCI-H1838, NCI-H1373, and DU-4475, MZ-6 and MZ-14 on human hepatocellular liver carcinoma cell line HepG2.[3,8,27,28,29,30,31] Outcomes of this study are in good agreement with their results.
On the other hand, findings of flow cytometry indicated a significant reduction in apoptotic events. It can be conducted that bromelain inhibits the effect of radiation on cancer cells. Our results of flow cytometry are not in line with the findings in the colony formation assay. In various reports have shown that radiation-induced apoptosis is enhanced by some agents such as apigenin, curcumin, genistein, sulforaphane, benzyl isothiocyanate, olaparib, rucaparib, elesclomol, 17-DMA, LY2603618, MK87776, and AZD1775, EphB4, human PLK1.[23,27,29,32,33] Our observations are opposite of their results. However, in many studies such as Rafehi et al.,[34] clonogenic formation assay was introduced as the gold standard to investigate radiosensitizers.
Conclusion
In summary, our results showed that bromelain could inhibit the growth and proliferation of mouse BC 4T1 cell line. It can reduce the survival of these cells. According to the gold standard clonogenic formation assay, bromelain can be a candidate radiosensitizer. While clinical relevant studies are needed to achieve the toxicity and the efficacy of bromelain, these findings recommend that it is necessary to further investigation as a potential radiosensitizer for BC. Further researches in vitro and in vivo of the effect of the radiosensitizing agents in combination with RT may advance progress of this treatment strategy for the clinical management of BC.
Ethics statement
All applicable international, national, and institutional guidelines for the care of human were followed.
Financial support and sponsorship
This work is a part of MSc thesis which financially supported by Isfahan University of Medical Sciences, Iran (Grant No: 395174) and Research Deputy of Shahrekord University of Medical Sciences, Iran (Grant No: 2246).
Conflicts of interest
There are no conflicts of interest.
BIOGRAPHIES

Farzaneh Raeisi obtained her BSc in Physics from Shahrekord University in 2014 and her MSc in Medical Physics from Isfahan University of Medical Sciences in 2016. Her research interests include radiationtherapy, image processing, and molecular genetics.
Email: farzaneraisi@yahoo.com

Daryoush Shahbazi-Gahrouei Professor of Medical Physics, BSc in Physics (School of Science, Isfahan University, Iran, 1987), MSc in Medical Physics (Tarbiat Modarres University, Tehran, Iran, 1991), PhD in Medical Physics (University of Western Sydney and St. George Cancer Care Centre, Sydney, Australia, 2000).
Email: shahbazi@med.mui.ac.ir

Elham Raeisi received her BSc degree from Shahid Beheshti Unuiversity, Tehran, Iran in 2002, MSc degree in Medical Physics from Tarbiat Modares University, Tehran, Iran in 2005 and PhD degree in Medical Physics/Interdisciplinary in 2011, 2013. Her research interest is Cancer Research.
Email: elham_raeisi@yahoo.com

Esfandiar Heidarian received his PhD degree in clinical biochemistry in 2005 from Isfahan University of Medical Sciences, Isfahan, Iran. He is currently a professor at Shahrekord University of Medical Sciences, Shahrekord, Iran. His research interests are focused on oxidative stress and antioxidants. He has participated in several scientific and technical committees.
Email: heidariyan46@yahoo.com
Acknowledgment
Authors would like to thank the staff in Parsian hospital, Shahrekord, Iran for their contribution to this study.
References
- 1.Ding X, Yang Q, Kong X, Haffty BG, Gao S, Moran MS, et al. Radiosensitization effect of huaier on breast cancer cells. Oncol Rep. 2016;35:2843–50. doi: 10.3892/or.2016.4630. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama N, Ogawa Y, Yasuoka M, Iwasa H, Miyatake K, Yoshimatsu R, et al. Therapeutic response to a novel enzyme-targeting radiosensitization treatment (KORTUC II) for residual lesions in patients with stage IV primary breast cancer, following induction chemotherapy with epirubicin and cyclophosphamide or taxane. Oncol Lett. 2017;13:69–76. doi: 10.3892/ol.2016.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyama N, Ogawa Y, Yasuoka M, Ohgi K, Iwasa H, Miyatake K, et al. Therapeutic results of a novel enzyme-targeting radiosensitization treatment, Kochi oxydol-radiation therapy for unresectable carcinomas II, in patients with stage I primary breast cancer. Oncol Lett. 2017;13:4741–7. doi: 10.3892/ol.2017.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui L, Her S, Borst GR, Bristow RG, Jaffray DA, Allen C, et al. Radiosensitization by gold nanoparticles: Will they ever make it to the clinic? Radiother Oncol. 2017;124:344–56. doi: 10.1016/j.radonc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Ghahremani F, Shahbazi-Gahrouei D, Kefayat A, Motaghi H, Mehrgardi MA, Javanmard SH. AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Adv. 2018;8:4249–58. [Google Scholar]
- 6.Arab-Bafrani Z, Shahbazi-Gahrouei D, Abbasian M, Fesharaki M. Multiple MTS assay as the alternative method to determine survival fraction of the irradiated HT-29 colon cancer cells. J Med Signals Sens. 2016;6:112–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Bando SI, Hatano O, Takemori H, Kubota N, Ohnishi K. Potentiality of syringetin for preferential radiosensitization to cancer cells. Int J Radiat Biol. 2017;93:286–94. doi: 10.1080/09553002.2017.1242815. [DOI] [PubMed] [Google Scholar]
- 8.Dillon MT, Barker HE, Pedersen M, Hafsi H, Bhide SA, Newbold KL, et al. Radiosensitization by the ATR inhibitor AZD6738 through generation of acentric micronuclei. Mol Cancer Ther. 2017;16:25–34. doi: 10.1158/1535-7163.MCT-16-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saberi A, Shahbazi-Gahrouei D, Abbasian M, Fesharaki M, Baharlouei A, Arab-Bafrani Z, et al. Gold nanoparticles in combination with megavoltage radiation energy increased radiosensitization and apoptosis in colon cancer HT-29 cells. Int J Radiat Biol. 2017;93:315–23. doi: 10.1080/09553002.2017.1242816. [DOI] [PubMed] [Google Scholar]
- 10.Pavan R, Jain S, Singh S, Kumar A. Properties and therapeutic application of bromelain: A review. Biotechnol Res Int 2012. 2012:976203. doi: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lencastre Novaes LC, Jozala AF, Lopes AM, de Carvalho Santos-Ebinuma V, Mazzola PG, Pessoa A., Jr Stability, purification, and applications of bromelain: A review. Biotechnol Prog. 2016;32:5–13. doi: 10.1002/btpr.2190. [DOI] [PubMed] [Google Scholar]
- 12.Raeisi F, Raeisi E, Shahbazi-Gahrouei D, Heidarian E, Amiri M, Gholami M. Cytotoxicity effect of pineapple extract on breast cancer cells (4T1) J Isfahan Med Sch. 2016;34:946–51. [Google Scholar]
- 13.Akhtar NM, Naseer R, Farooqi AZ, Aziz W, Nazir M. Oral enzyme combination versus diclofenac in the treatment of osteoarthritis of the knee – A double-blind prospective randomized study. Clin Rheumatol. 2004;23:410–5. doi: 10.1007/s10067-004-0902-y. [DOI] [PubMed] [Google Scholar]
- 14.Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Picciariello V, Inchingolo AD, et al. Clinical trial with bromelain in third molar exodontia. Eur Rev Med Pharmacol Sci. 2010;14:771–4. [PubMed] [Google Scholar]
- 15.Rosenberg L, Lapid O, Bogdanov-Berezovsky A, Glesinger R, Krieger Y, Silberstein E, et al. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: A preliminary report. Burns. 2004;30:843–50. doi: 10.1016/j.burns.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Secor ER, Carson WF, Singh A, Pensa M, Guernsey LA, Schramm CM, et al. Oral bromelain attenuates inflammation in an ovalbumin-induced murine model of asthma. Evid Based Complement Alternat Med. 2008;5:61–9. doi: 10.1093/ecam/nel110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalra N, Bhui K, Roy P, Srivastava S, George J, Prasad S, et al. Regulation of p53, nuclear factor kappaB and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skin. Toxicol Appl Pharmacol. 2008;226:30–7. doi: 10.1016/j.taap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Bhui K, Tyagi S, Srivastava AK, Singh M, Roy P, Singh R, et al. Bromelain inhibits nuclear factor kappa-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G (2)/M arrest to apoptosis. Mol Carcinog. 2012;51:231–43. doi: 10.1002/mc.20769. [DOI] [PubMed] [Google Scholar]
- 19.Dhandayuthapani S, Perez HD, Paroulek A, Chinnakkannu P, Kandalam U, Jaffe M, et al. Bromelain-induced apoptosis in GI-101A breast cancer cells. J Med Food. 2012;15:344–9. doi: 10.1089/jmf.2011.0145. [DOI] [PubMed] [Google Scholar]
- 20.Raeisi F, Raeisi E, Shahbazi-Gahrouei D, Amini Chermahini F. A comparison of Thiazolyl blue (MTT) versus Sulforhodamine B (SRB) assay in assessment of antiproliferation effect of bromelain on 4T1, AGS and PC3 cancer cell lines. J Isfahan Med Sch. 2017;35:1056–61. [Google Scholar]
- 21.Nasiri R, Almaki JH, Idris A, Nasiri M, Irfan M, Majid FA, et al. Targeted delivery of bromelain using dual mode nanoparticles: Synthesis, physicochemical characterization, in vitro and in vivo evaluation. RSC Adv. 2017;7:40074–94. [Google Scholar]
- 22.Braun JM, Schneider B, Beuth HJ. Therapeutic use, efficiency and safety of the proteolytic pineapple enzyme bromelain-POS in children with acute sinusitis in germany. In Vivo. 2005;19:417–21. [PubMed] [Google Scholar]
- 23.Beuth J, Braun JM. Modulation of murine tumor growth and colonization by bromelaine, an extract of the pineapple plant (Ananas comosum L.) In Vivo. 2005;19:483–5. [PubMed] [Google Scholar]
- 24.Raeisi F, Raeisi E, Shahbazi-Gahrouei D, Heidarian E, Amiri M. Comparison of the radiosensitivity of cancer and normal cells to x-ray irradiation using MTT assay: An in vitro study. J Isfahan Med Sch. 2018;36:246–50. [Google Scholar]
- 25.Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150–60. doi: 10.1097/CAD.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 26.Rae C, Mairs RJ. Evaluation of the radiosensitizing potency of chemotherapeutic agents in prostate cancer cells. Int J Radiat Biol. 2017;93:194–203. doi: 10.1080/09553002.2017.1231946. [DOI] [PubMed] [Google Scholar]
- 27.Busch CJ, Kröger MS, Jensen J, Kriegs M, Gatzemeier F, Petersen C, et al. G2-checkpoint targeting and radiosensitization of HPV/p16-positive HNSCC cells through the inhibition of chk1 and wee1. Radiother Oncol. 2017;122:260–6. doi: 10.1016/j.radonc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S, Hirsch K, Sharma J, Oweida A, Griego A, Keysar S, et al. Enhancing radiosensitization in EphB4 receptor-expressing head and neck squamous cell carcinomas. Sci Rep. 2016;6:38792. doi: 10.1038/srep38792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JL, Chen JP, Huang YS, Tsai YC, Tsai MH, Jaw FS, et al. Radiosensitization in esophageal squamous cell carcinoma: Effect of polo-like kinase 1 inhibition. Strahlenther Onkol. 2016;192:260–8. doi: 10.1007/s00066-016-0951-6. [DOI] [PubMed] [Google Scholar]
- 30.Chettiar ST, Malek R, Annadanam A, Nugent KM, Kato Y, Wang H, et al. Ganetespib radiosensitization for liver cancer therapy. Cancer Biol Ther. 2016;17:457–66. doi: 10.1080/15384047.2016.1156258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gach K, Grądzka I, Wasyk I, Męczyńska-Wielgosz S, Iwaneńko T, Szymański J, et al. Anticancer activity and radiosensitization effect of methyleneisoxazolidin-5-ones in hepatocellular carcinoma HepG2 cells. Chem Biol Interact. 2016;248:68–73. doi: 10.1016/j.cbi.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohara M, Kimura S, Tanaka A, Ohnishi K, Okayasu R, Kubota N, et al. Benzyl isothiocyanate sensitizes human pancreatic cancer cells to radiation by inducing apoptosis. Int J Mol Med. 2011;28:1043–7. doi: 10.3892/ijmm.2011.770. [DOI] [PubMed] [Google Scholar]
- 34.Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A, Karagiannis TC, et al. Clonogenic assay: Adherent cells. J Vis Exp. 2011 doi: 10.3791/2573. pii: 2573. [DOI] [PMC free article] [PubMed] [Google Scholar]