Abstract
Detecting mutations in the plasma of patients with solid tumors is becoming a valuable method of diagnosing and monitoring cancer. The TERT promoter is mutated at high frequencies in multiple cancer types, most commonly at positions -124 and -146 (designated C228T and C250T, respectively). Detection of these mutations has been challenging because of the high GC content of this region (approximately 80%). We describe development of novel probe-based droplet digital PCR assays that specifically detect and quantify these two mutations, along with the less common 242-243 CC>TT mutation, and demonstrate their application using human tumor and plasma samples from melanoma patients. Assay designs and running conditions were optimized using cancer cell line genomic DNAs with the C228T or C250T mutations. The limits of detection were 0.062% and 0.051% mutant allele fraction for the C228T and C250T assays, respectively. Concordance of 100% was observed between droplet digital PCR and sequencing-based orthogonal methods in the detection of TERT mutant DNA in 32 formalin-fixed, paraffin-embedded melanoma tumors. TERTmutant DNA was also identified in 21 of 27 plasma samples (78%) from patients with TERTmutant tumors, with plasma mutant allele fractions ranging from 0.06% to 15.3%. There were no false positives in plasma. These data demonstrate the potential of these assays to specifically detect and quantify TERTmutant DNA in tumors and plasma of cancer patients.
Liquid biopsies from plasma cell-free DNA (cfDNA) are currently being used to determine the mutational status of some solid tumors and may eventually be used to predict responses to cancer treatments and monitor disease activity1, 2, 3, 4 (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150044, last accessed June 27, 2018). The biological underpinning of this approach is that dying cells release DNA into the circulation and tumor-specific mutated DNA can be detected using PCR-based methods.5 Droplet digital PCR (ddPCR) is considered a gold standard method for the detection and absolute quantification of rare mutated alleles because of its high sensitivity, specificity, and robust quantitative performance characteristics.6, 7, 8, 9 Because ddPCR assays typically require little manipulation of the sample (no library construction or sample preamplification) and can be run quickly, the development of a few high-performing assays to detect specific mutations that are common to many cancers could simplify and accelerate the adoption of liquid biopsies into the clinic.
Recently, two mutations in the promoter region of the TERT gene [−124C>T (C228T) and −146C>T (C250T)] have been identified as widespread throughout cancer types and highly prevalent, with frequencies ranging from 40% to 70% in melanoma, bladder, liver, and central nervous system malignancies, such as glioblastoma.10, 11, 12 These promoter mutations are associated with significant increases in the transcription of telomerase, a key enzyme involved in telomere maintenance, which is critical for the survival of replicating cells.13 Therefore, developing assays to detect these TERT promoter mutations could advance the application of liquid biopsies to diagnose and monitor a wide range of cancer patients, as well as support drug research and therapeutic strategies aimed at regulating telomerase activity in cancers.14
The TERT promoter region around these mutation sites contains approximately 80% guanine and cytosine, a composition that may facilitate the formation of secondary structures that inhibit DNA polymerases from copying these regions.15 Also, the sequences surrounding each mutation are nearly identical to each other. These factors can limit read depth in next-generation sequencing platforms, potentially reducing sensitivity to detect mutant alleles. Other means of assaying these targets [eg, real-time quantitative PCR, SNaPShot (Thermo Fisher Scientific, Waltham, MA), and Sanger sequencing] do not have sufficient sensitivity or precision for quantitative measurements that may potentially be needed in clinical applications, such as monitoring cell-free DNA in plasma. We report the steps required to generate high-performance ddPCR assays for the C228T and C250T TERT promoter mutations, methods that may also be applicable to assay development for other difficult GC-rich targets of interest. Despite the high guanine and cytosine content, both assays demonstrate high specificity and sensitivity when analyzing DNA extracted from cancer cell lines, formalin-fixed, paraffin-embedded (FFPE) tissues, and human plasma samples from patients with metastatic melanoma.
Materials and Methods
Template Sources
The study was conducted using mutant and wild-type DNAs from melanoma, glioblastoma, bladder, and cutaneous squamous cell cancer cell lines (Supplemental Table S1). Negative controls included normal human peripheral blood mononuclear cell (PBMC) DNA (number G1521; Promega, Madison, WI), FFPE samples of tonsil and spleen (New York University Center for Biospecimen Research and Development, New York, NY), and frozen healthy donor plasma (BioreclamationIVT, Westbury, NY). Melanoma patient samples, both FFPE and plasma, were obtained from the New York University Interdisciplinary Melanoma Cooperative Group Biorepository. Plasma samples collected from the New York University Interdisciplinary Melanoma Cooperative Group Biorepository were stored as 1-mL aliquots at −80°C after isolation via routine centrifugation (1600 × g for 10 minutes) of whole blood collected in EDTA tubes up to 6 hours prior. Plasma samples were collected by BioreclamationIVT, according to its standard protocol, and stored at −80°C on arrival. The use of patient materials was approved by the New York University Institutional Review Board (number 10362), and all patients signed consent forms.
DNA Extractions
DNA was extracted from cell lines using the QIAmp DNA Mini Kit (Qiagen, Hilden, Germany), according to manufacturer's instructions. FFPE slides were extracted using the MoBio FFPE DNA Isolation Kit (Qiagen), as previously described.16 The plasma samples (3 to 5 mL each) were thawed at room temperature and spun at 16,000 × g for 10 minutes to remove precipitates, and DNA was extracted using the Circulating DSP NA Kit (Qiagen), according to manufacturer's instructions, and eluted into 85 μL of AVE buffer (Qiagen). Purified DNAs were quantified by two methods: FFPE and cell line DNAs were analyzed using a Nano-Drop 2000C (Thermo Fisher Scientific) spectrophotometer, and plasma samples were analyzed using Invitrogen's Qubit Fluorometer (Q32866; Thermo Fisher Scientific).
Mutation Detection Using Sanger Sequencing and/or SNaPShot
TERT promoter mutations were initially identified in tissues, cell lines, and short-term cultures using Sanger sequencing and/or SNaPShot analysis to provide orthogonal methods to validate the TERT promoter ddPCR assays. The TERT templates were amplified via PCR using primers [5′-CAGGGAGCAATGCGTCCTCGGGTTC-3′ (forward) and 5′- GCGCTGCCTGAAACTCGC-3′ (reverse)] and the HotStar Taq Plus Polymerase kit (Qiagen). The thermal cycling conditions were as follows: 95°C for 5 minutes, followed by 40 cycles of 95°C for 45 seconds, 65°C for 45 seconds, and 72°C for 1 minute, followed by 72°C for 10 minutes and a 4°C infinite hold. PCR products were purified, as previously described,16 and analyzed by Sanger sequencing (Genewiz, South Plainfield, NJ) and/or SNaPShot.17 For each Sanger sequencing sample, a quality report was given by Genewiz in the form of a quality score. For quality score values of 25 to 39, the electropherograms were manually assessed to determine peaks; for quality score values <24 (eg, too much background), a replicate DNA sample was analyzed. The SNaPShot probes were as follows: 5′-GGACCCCGCCCCGTCCCGACCCCT-3′ for C250T and 5′-GAAAGGAAGGGGAGGGGCTGGGAGGGCCCGGA-3′ for C228T.
ddPCR
Through the course of this investigation, nine pairs of primers were designed to amplify regions of the TERT promoter encompassing the most prevalent promoter mutations. However, only the final three primer pairs producing amplicon sizes, ranging from 88 to 163 bp in length, are reported herein. Mutation-specific probes were also developed to separately detect the C228T or the C250T TERT promoter single bp substitutions. Each reaction contained a mutation-specific probe (C228T or C250T) labeled with FAM and a wild-type specific probe directed to the same region (C228 or C250) labeled with HEX. Only the final optimized primer/probe combinations are described in this study. The three primer sets reported generate distinct amplicons of 88, 113, or 163 bp. Each amplicon can be tested with either pair of mutant and wild-type probes specific for either C228T or C250T. All six of these assays are available through Bio-Rad Laboratories [catalog numbers TERT C228T_113 (dHsaEXD72405942), TERT C250T_113 (dHsaEXD46675715), TERT C228T_88 (dHsaEXD20945488), TERT C250T_88 (dHsaEXD85215261), TERT C228T_163 (dHsaEXD59485034), and TERT C250T_163 (dHsaEXD33754807); Bio-Rad Laboratories, Pleasanton, CA].
The reaction mixtures for these assays consisted of the following: 10 μL ddPCR Supermix for Probes (186-3026; Bio-Rad Laboratories; 1× final concentration), 1 μL 20× primer/probe mixture, 0.25 μL of restriction enzyme (CviQI; R0639S; New England BioLabs, Ipswich, MA), 1 μL Na2EDTA at 20 mmol/L (4056-500ML; Sigma-Aldrich, St. Louis, MO), 2 μL betaine at 5 mol/L (B0300-5VL; Sigma-Aldrich), DNA template (various amounts, maximum volume of 5.75 μL per 20 μL ddPCR), and double-distilled water to a final volume of 20 μL. Reaction mixtures were processed according to the manufacturer's standard ddPCR procedures using the QX200 ddPCR system (Bio-Rad Laboratories). PCR cycling conditions were varied by changes in cycling times, amount of cycles, temperature, and titrations of additives (betaine and disodium EDTA) to establish optimal ddPCR results. The final PCR cycling conditions were as follows: 95°C for 5 minutes, followed by 50 cycles of 96°C for 30 seconds and 62°C for 1 minute, followed by 98°C for 10 minutes and a 12°C infinite hold for both C228T and C250T TERT promoter assays. Varying numbers of replicate wells (between 3 and 12) were run for each sample, depending on the amount and concentration of DNA analyzed. DNAs extracted from patient plasmas were run across 12 replicate wells because of their diluteness. Reagent-only controls (ie, no-template controls) alongside positive and negative template controls were included in every run. QuantaSoft analysis software version 1.7 (Bio-Rad Laboratories) was used to analyze all data from the ddPCR experiments.
Efficiency Assessment
Both interassay and intra-assay amplification efficiency of the TERT assays was assessed. For interassay efficiency, the ddPCR output of the TERT assays was compared with the outputs of other ddPCR assays amplifying targets with lower GC content, specifically housekeeping genes EIF2C1 and RPP30 (57.7% GC for the 111-bp assay and 34.3% GC for the 105-bp assay, respectively), and an amplicon from the TERT coding region (TERT CR, 48.1% GC for the 106-bp assay). Four different amplicon lengths were analyzed for each gene locus to compare with the different length TERT promoter amplicons. The bp lengths were as follows: EIF2C1, 74, 111, 156, and 254 bp; RPP30, 75, 105, 155, and 247 bp; TERT CR, 74, 106, 165, and 251 bp (Table 1). The reaction mixtures for these assays consisted of the following: 10 μL ddPCR Supermix for Probes, primers (final concentration, 900 nmol/L each), probe (final concentration, 250 nmol/L), 2.5 U of restriction enzyme [HindIII for EIF2C1 and TERT CR and HaeIII for RPP30 (catalog numbers R0104 and R0108, respectively; New England BioLabs)], DNA template (PBMCs, various amounts, quantified using a Qubit 2.0 Fluorimeter), and double-distilled water to a final volume of 20 μL. For each interassay efficiency experiment, a mastermix including the DNA and all other ddPCR reagents, except for each individual assay (ie, primers and probes), was made. The mastermix was evenly divided, and the individual gene assays were added. This ensured that the input DNA amount and concentration were the same across all four assays. Three replicate wells were analyzed to generate each data point. The ddPCR cycling conditions for these assays were as follows: 95°C for 5 minutes, followed by 40 cycles of 94°C for 30 seconds and 56°C for 1 minute, followed by 98°C for 10 minutes and a 12°C infinite hold.
Table 1.
Primer and Probe Sequences for RPP30, EIF2C1, TERT CR Assays
| Gene | Amplicon length, bp | Forward sequence | Reverse sequence | FAM probe sequence | HEX probe sequence |
|---|---|---|---|---|---|
| RPP30 | 75 | 5′-AAACCCCTAAAACGCTAATATTGAT-3′ | 5′-CGTAGCCTGACTTGGTTGAA-3′ | /56-FAM/TCCTGATAGCTGTATTAAAAATAGCAAAGC/3IABkFQ/ | N/A |
| RPP30 | 105 | 5′-TGGGATCATGTTAAGTAGAAGTAGC-3′ | 5′-CTCATCTCGGTCTCCATATTGACT-3′ | /56-FAM/TATGCAAATACATGCATTTATGCAATATTAATGTAAG/3IABkFQ/ | N/A |
| RPP30 | 155 | 5′-AAGCATCGGACTGAACCAAC-3′ | 5′-CTCGGTCTCCATATTGACTTATTCT-3′ | /56-FAM/AATGGGATCATGTAAGTAGAAGTAGCTT/3IABkFQ/ | N/A |
| RPP30 | 247 | 5′-AGTTAGAAACCCCTAAAACGCT-3′ | 5′-TCGTTCTCTTTCTGTTGACAAGA-3′ | /56-FAM/AGCAAAGCATCGGACTGAACC/3IABkFQ/ | N/A |
| EIF2C1 | 74 | 5′-GGTTCGGCTTTCACCAGTC-3′ | 5′-AACTACCACTCACCCCTCTC-3′ | /56-FAM/CCTGCCATGTGGAAGATGATGC/3IABkFQ/ | N/A |
| EIF2C1 | 111 | 5′-GAGGTCTGGTTCGGCTTTC-3′ | 5′-CCGTGGGGTTCAGGTCA-3′ | /56-FAM/CAGTCTGTGCGCCCTGC/3IABkFQ/ | N/A |
| EIF2C1 | 156 | 5′-CTGGTTCGGCTTTCACCAG-3′ | 5′-AGACGTTGGTGTGAGGATCA-3′ | /56-FAM/CGCCCTGCCATGTGGAAG/3IABkFQ/ | N/A |
| EIF2C1 | 254 | 5′-AGTTCTCTGCCTGTCCCTG-3′ | 5′-AGACGTTGGTGTGAGGATCA-3′ | /56-FAM/CCTGTGGGCCGCTCCT/3IABkFQ/ | N/A |
| TERT CR | 74 | 5′-CGTGGGGATACAGTACCTGA-3′ | 5′-AGGCCATCTTTTTCTCGGACA-3′ | /56-FAM/CAATGCTTTGCAACTTGCTCCA/3IABkFQ/ | /5HEX/CAATGCTTTGCAACTTGCTCCA/3IABkFQ/ |
| TERT CR | 106 | 5′-CTGGCGTGGGGATACAGT-3′ | 5′-TTGCACCAGAGGCACTGTAT-3′ | /56-FAM/TGATTCCAATGCTTTGCAACTTGC/3IABkFQ/ | /5HEX/TGATTCCAATGCTTTGCAACTTGC/3IABkFQ/ |
| TERT CR | 165 | 5′-AGGACTTCGAGAAGCAGAGG-3′ | 5′-GAGCTGCTGCATGTGTGAGT-3′ | /56-FAM/TGGCGTGGGGATACAGTACC/3IABkFQ/ | /5HEX/TGGCGTGGGGATACAGTACC/3IABkFQ/ |
| TERT CR | 251 | 5′-TTCCAGGACTTCGAGAAGCA-3′ | 5′-GTCGGTTGGGGAATTTGCTC-3′ | /56-FAM/AGGCCTGGCGTGGGG/3IABkFQ/ | /5HEX/AGGCCTGGCGTGGGG/3IABkFQ/ |
N/A, not applicable.
Interassay efficiency was determined by comparing the ddPCR measurement of total input DNA for each assay to that of RPP30. RPP30 was chosen as the 100% standard because of the low GC content of its target amplicon and the high efficiency of the commercially available RPP30 ddPCR assay (Bio-Rad Laboratories). The efficiency of each assay was calculated as the percentage of DNA that was amplified compared with that of RPP30 in the same experiment. Each experiment was repeated three times.
Intra-assay efficiency was analyzed by comparing the ddPCR output of each assay using primer pairs that generated different amplicon lengths for each gene target. Both high-quality DNA (PBMCs) and DNA from degraded sources, specifically FFPE normal tonsil tissues (n = 10) and healthy donor plasmas (n = 6), were analyzed. These DNA samples were quantified using the Qubit 2.0 fluorimeter, and the reactions were set up as described above, adding the primers and probes last, to ensure a consistent concentration of DNA in all samples. Intra-assay efficiency was the percentage ddPCR output for each amplicon length assay compared with the shortest amplicon for that gene target.
Assessment of Analytical Specificity and Sensitivity
Specificity was assessed by analyzing DNA samples from C228T and C250T cell lines with both assays to ensure there was no cross-reactivity. Sensitivity was measured in a two-step process, as described by Armbruster and Pry.18 The limit of blank (LoB) was determined by analyzing negative controls and obtaining a background signal for the assays; the limit of detection (LoD) was calculated by determining a mutant allele concentration at which low levels of mutant DNA could be reliably distinguished from the background (LoB). ddPCR runs were analyzed using QuantaSoft version 1.7.4. Assay thresholds were set on the basis of positive and negative standards for each run. Both single- and double-positive droplets from all replicate wells (merged into a single metawell) were used to calculate the observed mutant allele fraction (MAF) because all double-positive droplets contain mutant as well as wild-type alleles. A series of cell line DNAs mixed with PBMC DNA were analyzed to achieve varying MAFs [including 0% MAF (100% PBMC DNA)] to determine each assay's LoB and LoD. More specifically, the LoB was determined using the following formula: LoB = Meanblank + 1.645*(SDblank).
The Meanblank and SDblank are calculated from three experiments using the merged data from 12 wells (metawell average) of wild-type (0% MAF) DNA to get a background signal for each assay.
Similarly, the LoD was calculated by analyzing three metawells, from three separate experiments using the following formula: LoD = LoB + 1.645*(SD0.1 sample).
The SD0.1 is the SD of the data from the three metawells using a sample with an MAF = 0.1%. The 0.1% MAF dilution was chosen because the LoD was expected to be <0.1% and have a similar SD to the 0.1% dilution. As described by Armbruster and Pry,18 the formula yields an LoD value for which 95% of samples with a mutant fractional abundance equal to the LoD will have a measurement that exceeds the defined LoB value, and only 5% will have a measurement that falls below the LoB and falsely appear as if there is no analyte.
To determine the LoB in FFPE samples, 10 normal tonsil samples were analyzed using each assay. To determine the LoB in cfDNA, 12 TERTwild-type plasma samples (six patient samples for each assay) were analyzed. LoDs were not described for either plasma or tumor samples because the LoD calculation requires a predetermined level of analyte to be present in a sample, which was not possible with patient samples. Patient samples were considered positive for the mutation if the measured MAF exceeded the FFPE or cfDNA LoB.
Results
Assay Optimization
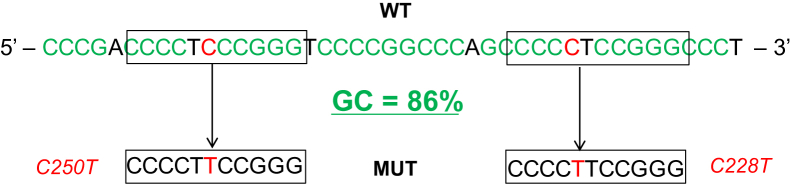
The high GC content (approximately 80%) of the TERT promoter region causes significant difficulty for the development of efficient and specific PCR assays to detect and quantify mutations in this region. The issue is further compounded by the two mutation sites being nearly identical to each other and only 22 bp apart (Figure 1). Initial testing of a primer set amplifying a 163-bp amplicon found that the addition of CviQ1 improved cluster tightness in a dye-binding detection assay (data not shown). Further development of primer/probe combinations using a 163-bp amplicon yielded poor separation of mutant and wild-type clusters, despite variations in cycling conditions and probe and primer concentrations (Figure 2A). To overcome the biochemical challenges posed by this DNA sequence, extensive optimization work was conducted with nine different primer pairs generating amplicons of varying lengths and five different probe designs. Primer and probe concentrations, amplification parameters, and reaction additives were also varied to maximize cluster separation and tightness.
Figure 1.
TERT promoter region sequence comprising the C250T and C228T point mutations (MUTs). High GC content (86%) and close proximity of the mutations make amplifying the individual mutations challenging. WT, wild type.
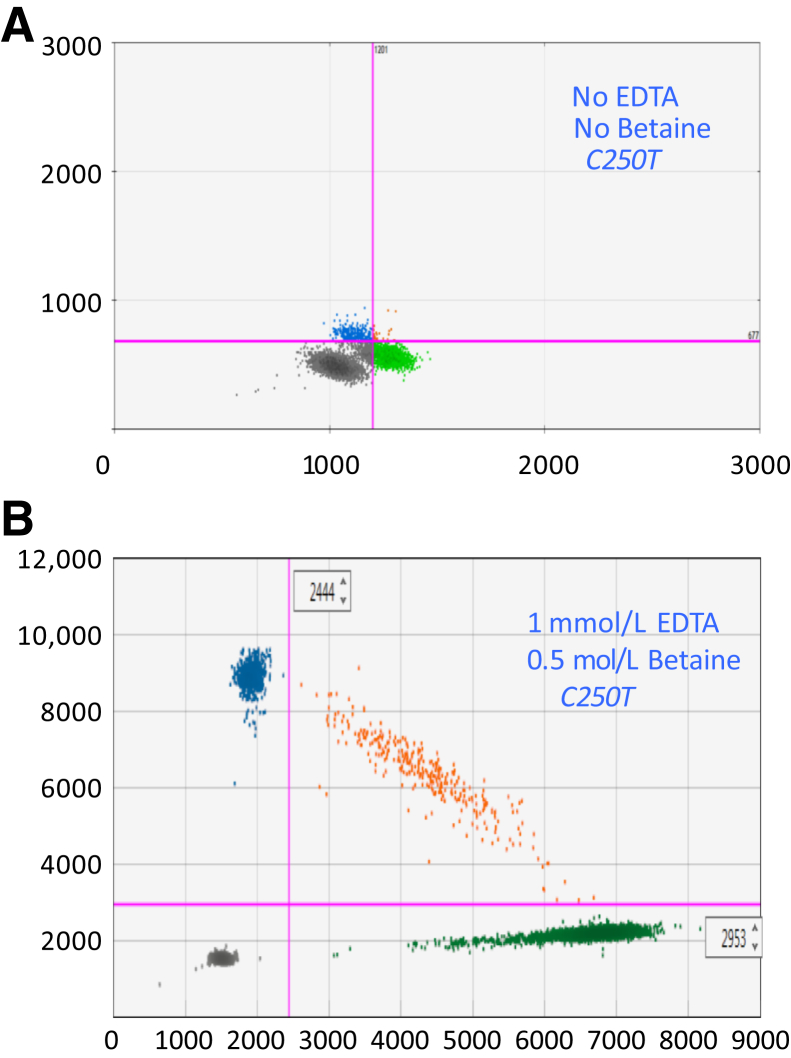
Figure 2.
Two-dimensional ddPCR plots showing the C250T TERT promoter assay with and without chemical additives. A: Initial results using standard supermix and primers and probes to amplify and detect a 163-bp fragment. B: Results of optimized assay conditions, which include betaine and disodium EDTA, and primers and probes to amplify and detect a 113-bp fragment. The annealing temperature for the assays in A and B was 62°C.
To achieve well-separated, tightly formed droplet clusters in the two-dimensional plots for both the C228T and C250T assays, temperature gradients were performed for all nine different primer designs to identify the optimal annealing/extension temperatures and times. In addition, a probe-primer dilution series experiment (1×, 2×, and 4× input for probe and primer, in varying combinations) was conducted to assess the impact of different primer and probe concentrations on cluster tightness and separation (data not shown). Although of some benefit, varying these factors did not yield satisfactory cluster tightness and separation, so the use of additives to the reaction mixtures was explored (Supplemental Figure S1). Betaine was added to the reaction mixture to inhibit possible secondary structures formed because of the high GC content of the amplicon site, and disodium EDTA was investigated to titrate the effective Mg+2 concentrations in the PCR mixture. These additives were tested in a serially titrated manner individually and in combination with a temperature gradient. The optimal reaction conditions contained a combination of 0.5 mol/L betaine and 1.0 mmol/L Na2EDTA. Another round of temperature gradient experiments (56°C to 66°C) were conducted to confirm the optimal annealing temperatures for both assays under the aforementioned modified reaction conditions (Supplemental Figure S2). The results of the second temperature gradient confirmed that the preliminary cycling conditions were still valid. The fluorescence of the mutant signal began to decrease in amplitude at temperatures >62°C, and clusters began to diffuse at temperatures <60°C. The temperature of 62°C was, therefore, chosen as the standard annealing and extension temperature for both the C228T and C250T assays, regardless of amplicon size.
In finding the betaine concentration that gave the best cluster tightness and separation, there was a modest decrease in the measured DNA concentration with betaine concentrations above approximately 0.3 mol/L (Supplemental Figure S3). At the final chosen concentration of 0.5 mol/L betaine, herein was a consistent underestimate of the DNA concentration by approximately 6.3%. This may be because of a small decrease in droplet size, which was confirmed by independent imaging analysis of replicates of the same thermocycled samples read on the QX200 Droplet Reader for DNA quantification (FlowCam Nano; Fluid Imaging Technologies, Inc., Scarborough, ME). At still higher concentrations of betaine (eg, 1 mol/L), occasional signs of droplet instability were observed as dropout wells showing high polydispersity of droplet sizes and no quantifiable data. Although this small decrease in droplet size at 0.5 mol/L betaine does not affect MAF values (nor even relative differences in absolute plasma marker concentration values during serial monitoring), QuantaSoft version 1.7 values for absolute DNA concentration will be underestimated with these TERT assays when used under the recommended running conditions. This small effect by itself can be corrected by multiplying QuantaSoft version 1.7 copies/μL values by 1.067. However, if the larger intention is to achieve accuracy in determining the absolute number of mutant target copies per mL of plasma, one would want to account for the overall TERT promoter assay efficiency, approximately 80% of the fully efficient RPP30 reference assay run under standard ddPCR conditions (which lack betaine) (discussed below). The effect of droplet size is already accounted for in this 80% efficiency value. Thus, if a more accurate value of mutant or wild-type DNA concentration is desired for high-molecular-weight DNA, the QuantaSoft version 1.7 copies/μL values should be multiplied by 1.2 when using the 113-bp C228T and C250T TERT promoter assays. Additional corrections may be necessary according to amplicon size, as with any amplicon-based quantification system, when smaller fragmented DNA samples are used, such as plasma cfDNA or FFPE DNA discussed below.
Optimized Assay Specifications and Experimental Results
Herein, we describe the results of the optimized assays, which include three primer pairs, resulting in 88-, 113-, and 163-bp amplicons, each combined with a pair of mutant (FAM) and wild-type (HEX) probes for either the C228T or the C250T mutation. The most extensive characterization described in this article was done with the 113-bp assays. Additional studies were conducted using the shorter (88-bp) assays to confirm that they showed similar analytical sensitivity, specificity, and reproducibility as the 113-bp C228T and C250T assays. The final running conditions required betaine and disodium EDTA to be added to the reaction mixtures, as described above (Figure 2B). These resulting conditions were robust across annealing temperatures ranging from 56°C to 64°C, and ddPCR quantification values were not substantially affected by changes in annealing temperatures, although cluster amplitudes, tightness, and separation were affected (Supplemental Figure S2).
Assay Specificity, Sensitivity, and Limit of Detection
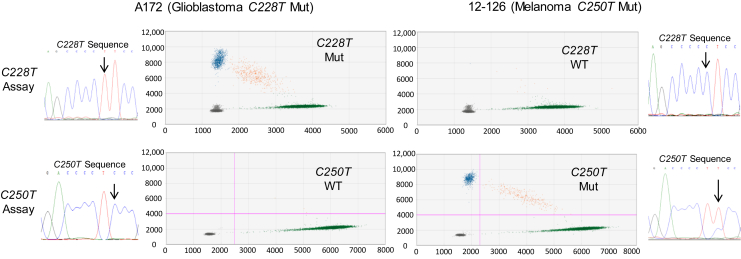
Specificity was established by testing the optimized assays on mutant templates validated using orthogonal methods (ie, Sanger sequencing and/or SNaPShot assays). The mutation status of DNAs from several cell lines using these methods is shown in Supplemental Table S1. Glioblastoma cell line A172 (TERT C228T mutant) and melanoma short-term culture NYU 12-126 (TERT C250T) were selected for the analytical validation studies because these templates had approximately 50% mutant allele frequency. Each assay yielded strong signals for its respective mutation with clear cluster separation and minimal cross-reactivity (<0.05% MAF) when analyzing a cell line with the other TERT promoter mutation (Figure 3). A roughly proportional increase in the number of false-positive droplets with higher DNA inputs and when analyzing samples from FFPE normal and tumor tissues was not observed (described below).
Figure 3.
Demonstration of specificity using cell lines with TERT promoter mutations (Muts). Cell lines A172 (C228T) and 12-126 (C250T) were analyzed with Sanger sequencing to identify their TERT promoter mutation. ddPCR using the 113-bp assays identified the specific mutations in each cell line. Representative analyses of triplicate wells using 33 ng DNA input per well are shown. Note the extremely low level of assay cross-reactivity (ie, few false-positive droplets). Black arrows indicate the single-base mutation site for the respective TERT promoter mutation. WT, wild type.
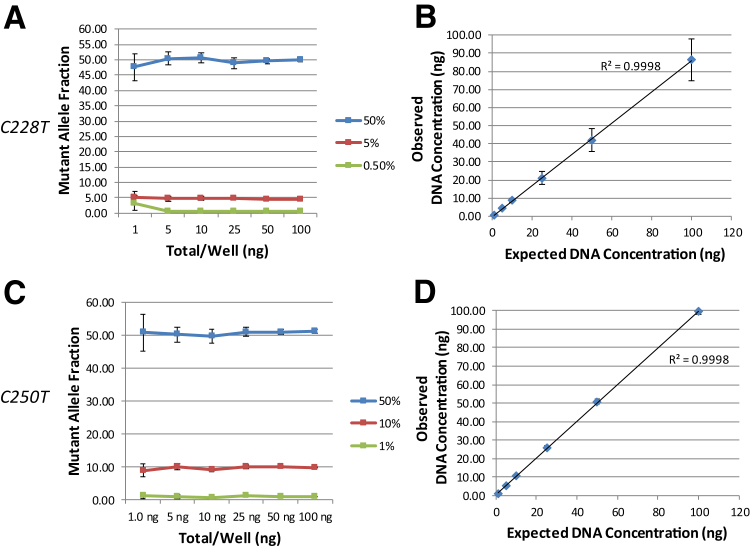
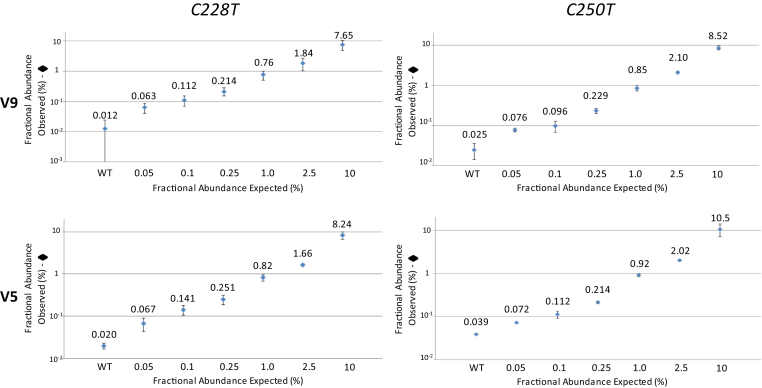
The linearity of quantification of the 113-bp assays was analyzed in dynamic range experiments. Mutant cell line DNAs at varying mutant fraction concentrations were serially diluted from 100 to 1 ng input per well. Both assays linearly quantified wild-type and mutant DNA along a large range of input DNAs with a conserved mutant fraction (Figure 4).
Figure 4.
Linearity of DNA quantification across a broad dynamic range. A: A range of input cell line DNA concentrations (1 to 100 ng per well) was analyzed in triplicate wells, at 50%, 5%, and 0.05% mutant allele fractions, using the C228T 113-bp assay (A172 cell line). C: The same range of DNA concentrations, at 50%, 10%, and 1% mutant allele fractions, was analyzed with the C250T 113-bp assay (12-126 cell line). B and D: The average absolute output for the DNA concentrations (expected versus observed) is shown for the C228T (three experiments; B) and C250T (two experiments; D) assays. Error bars are 95% CIs of the mean for the experiments.
To determine the LoDs for the 113- and 88-bp assays, serial dilutions of mutant TERT templates from cell lines were mixed with normal human PBMC DNA to generate sample series with mutant fractions ranging from 0% to 10% (0%, 0.05%, 0.1%, 0.25%, 1%, 2.5%, and 10%). Three such series of DNA samples were generated with total DNA input amounts of 6.6, 33, and 66 ng/well (0.1, 0.5, and 1.0 copies per droplet). DNA samples were run in three replicate wells using the 113-bp assays. Each sample triplicate was merged in QuantaSoft and analyzed as a metawell; thus, a total of 20, 100, or 200 ng DNA was used to calculate the MAF values. The background MAF (ie, LoB) of each assay was established using wild-type DNA from PBMCs and the LoDs using the mutant DNA mixtures described above.
Using larger amounts of total DNA in these titration experiments resulted in greater precision (both for wild-type and nonzero MAF samples) compared with experiments using lower DNA input amounts and, thus, enabled lower LoBs and LoDs to be achieved. For example, using 33 ng/well, the LoB and LoD for the C228T assay were 0.027% and 0.031%, respectively; and for the C250T assay, the values were 0.031% and 0.034% MAF, respectively. The results from assays using 6.6 ng/well (for both C228T and C250T assays) had more well-to-well variation, causing higher SDs and LoBs compared with runs using more DNA input per well. These experiments were repeated using 12 wells with 6.6 ng input DNA/well (79 ng total DNA analyzed; using both 113- and 88-bp assays). The result was lower LoBs and resulting LoDs for both assays based on the results from three independent experiments (Figure 5). For the C228T assay, the LoB was 0.029%, and the LoD was 0.062%; for the C250T assay, the LoB was 0.041%, and the LoD was 0.051% MAF. Because these experiments analyzed a similar amount of total input DNA to that of the 33 ng/well (99 ng total) experiments, and gave similar LoDs when using a metawell merge, we suggest that samples with low concentrations of DNA be run with more replicate wells to maximize the amount of DNA analyzed and to ensure the maximum sensitivity.
Figure 5.
Titration series used to determine TERT promoter assays' limits of blank (LoBs) and limits of detection (LoDs). DNAs from cell lines A172 (left panels) and 12-126 (right panels) were diluted with peripheral blood mononuclear cell (PBMC) DNA to mutant allele fractions of 10%, 2.5%, 1.0%, 0.25%, 0.1%, and 0.05% and analyzed with the C228T and C250T assays. PBMC DNA at 100% (0% mutant) was included to determine background (LoB). A total of 6.6 ng of DNA per well was analyzed in 12 replicate wells using the C228T and C250T assays. The LoB and LoD for both mutant assays with either the 113- or 88-bp amplicons were calculated, as described in Materials and Methods, using data generated from these experiments. WT, wild type.
Increasing numbers of double-positive droplets (because of cross-reactivity of the mutant probes on the wild-type template) were observed when 33 and 66 ng/well of 0% mutant DNA was tested; however, these droplets contributed negligible amounts to the mutant signal at both low and high DNA input levels, so the LoBs remained similar to that of the 6 ng/well metawell merge, approximately 0.030% MAF for both assays. Excluding these double-positive droplets from the calculations altogether seriously compromised the quantification of the mutant allele in higher DNA input and mutant fraction samples.
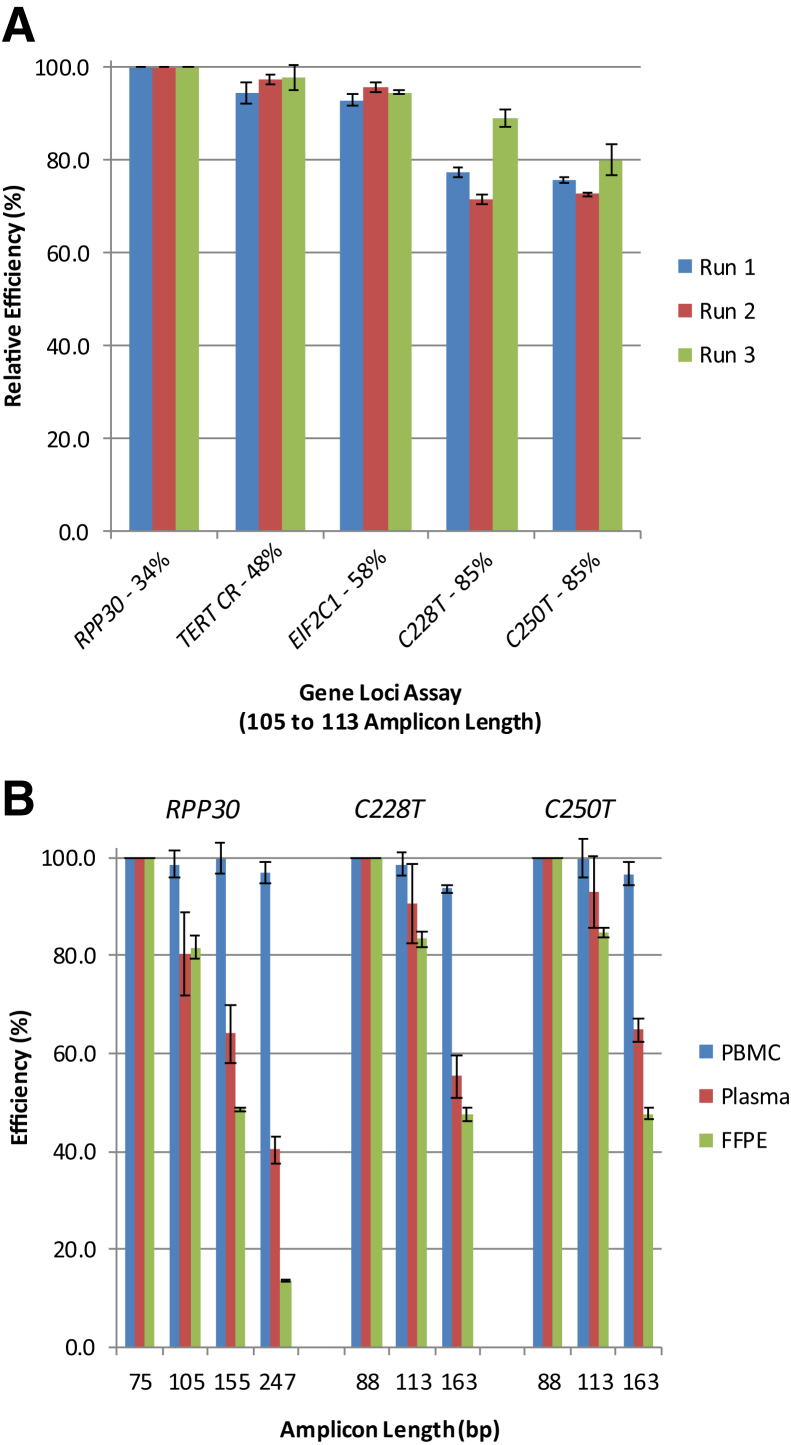
Assay Efficiency
Because of the high GC content of the TERT promoter assay target sequences and the concern that it might not allow for efficient quantification, the assay efficiencies of the TERT promoter assays were compared with assays for other targets with much lower GC content running under standard ddPCR conditions, including RPP30, EIF2C1, and a target within the coding region of the TERT gene (TERT CR). Data were compiled from three separate runs using PBMC DNA as the template. Target amplicons were RPP30 (105 bp), TERT CR (106 bp), EIF2C1 (111 bp), and TERT promoter (113 bp). ddPCR results were normalized against RPP30 to standardize the differences between genes. Compared with RPP30, the overall efficiencies of the EIF2C1 and TERT CR assays ranged between 95% and 100%, whereas the TERT promoter assays were approximately 80% efficient (Figure 6A).
Figure 6.
TERT promoter assay efficiency. A: Interassay efficiency comparing with housekeeping genes. Peripheral blood mononuclear cell (PBMC) DNA (5 to 25 ng) was amplified in each run using primers generating amplicon lengths between 105 and 113 bp for RPP30, the TERT coding region (CR), EIF2C1, C228T, and C250T (113-bp assay). Percentage of GC content is shown for each amplicon to demonstrate the effect of GC content on amplification efficiency. The blue, red, and green bars for each assay represent three different runs. The efficiencies of the assays were normalized against RPP30 by dividing the copies/μL of each assay by that of RPP30 and are plotted on the y axis. B: Intra-assay efficiency with respect to amplicon length and DNA template quality. Extracted DNAs from PBMCs, normal tissues, and plasma samples were analyzed using the RPP30, C250T, and C228T assays. Three or four primer pairs were used to generate different sized amplicons for each gene (specified on the x axis); detection probes were the same among all assays for a given gene. Intra-assay efficiencies were calculated by dividing the copies/μL of a given amplicon length by the copies/μL of the shortest amplicon length for that gene (not normalized against RPP30). DNAs analyzed: PBMC (blue), healthy donor plasma (red), and normal tonsil formalin-fixed, paraffin-embedded (FFPE) tissue (green). Data for the graph can be found in Supplemental Table S2. The data shown are from three replicate wells using the same DNA sample across all three assays and are representative of the overall results from multiple patient samples (Materials and Methods).
Amplicon Size Effect
Because the intended use of these assays is to detect and quantify mutated TERT alleles present in fragmented DNA from human tumor and plasma samples, differences in amplification efficiencies of the different amplicon length assays were also examined using DNA templates from different sample types. The amplification efficiencies of the 88-, 113-, and 163-bp assays were compared using DNAs from PBMCs, healthy donor plasma, and FFPE normal tissues. For comparison, RPP30 assays amplifying 75-, 105-, 155-, and 247-bp amplicons were examined. In general, as amplicon length increased, detection efficiency decreased for all assays (Figure 6B and Supplemental Table S2). The decrease was minimal when analyzing high-molecular-weight DNA from PBMCs; it was substantial when analyzing fragmented DNAs extracted from FFPE and plasma samples. For example, using amplicons ranging in length from 155 to 163 bp, there was an approximately 50% decrease in quantification efficiency for the FFPE DNA and an approximately 40% decrease in efficiency for plasma cfDNA when compared with the smallest amplicon for that particular assay (ie, RPP30 or TERT promoter) using the same DNA template source. Similar results were found for EIF2C1 and TERT coding region assays, in which amplicon lengths also ranged from approximately 75 to approximately 250 bp (data not shown).
Mutation Detection in Formalin-Fixed, Paraffin-Embedded Samples
Before using the assays to analyze tumor material, formalin-fixed normal tonsil tissues (n = 10) were tested to determine the background mutant fraction level (LoB) for both assays. A slightly increased level of mutant-positive droplets was found in both assays when analyzing DNA input from these formalin-fixed normal tissues, including some single-positive mutant droplets rarely seen with unfixed DNA samples (ie, from cell lines, PBMCs, or plasma cfDNA). The LoBs did not exceed 0.2% MAF for both assays (Supplemental Figure S4).
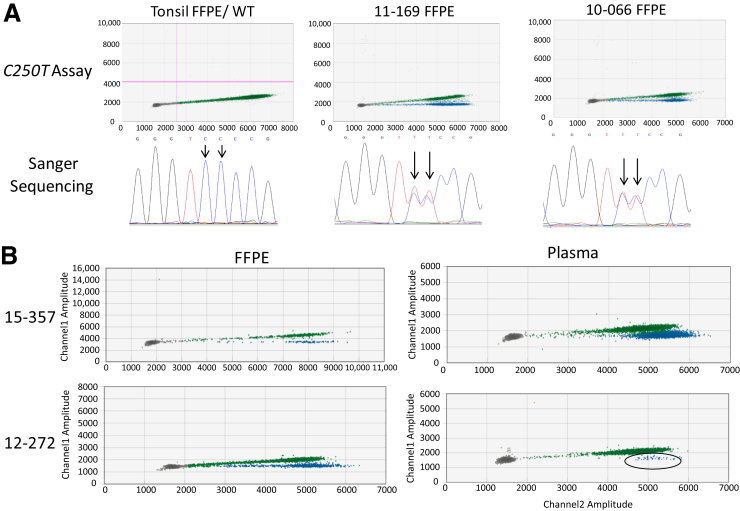
To determine the accuracy of the assays to detect mutations in human tumor samples, the mutational status of FFPE metastatic melanoma tumors was identified from 34 patients using orthogonal methods (ie, SNaPShot and Sanger sequencing) and then the samples were analyzed with both 113-bp ddPCR assays (C228T and C250T). There were 10 tumors mutant for C228T and five tumors mutant for C250T, each of which was concordant between ddPCR and Sanger sequencing (Supplemental Figure S5). Interestingly, four tumor samples from four independent patients with an abnormal cluster of droplets below the normal HEX signal were also identified for the C250T assay. The new cluster was completely flat along the x axis, whereas the normal HEX signal has a slight upward tilt. These samples were analyzed by Sanger sequencing, and a rare 2-bp mutation, 242-243 CC>TT, was detected in all of the samples that displayed the forked cluster (Figure 7A). Three of the samples were part of separate, larger studies of TERT promoter mutations in melanoma and were included because of the novelty of this finding. There were also 15 tumors that lacked any of these TERT mutations. Overall, the results of the ddPCR assays were 100% concordant with the orthogonal methods (Supplemental Figure S6). The two-dimensional plots in Figure 8 are representative of FFPE samples positive for the C228T and C250T mutations.
Figure 7.
Detection of a rare 242-243CC>TT TERT promoter mutation. A: Normal tonsil and metastatic melanoma tumor formalin-fixed, paraffin-embedded (FFPE) samples analyzed with both the 113-bp C250T TERT assay and Sanger sequencing of the promoter region. The diagonal HEX cluster is the normal wild-type (WT) cluster observed with the C250T 113-bp assay. In both tumor samples (but not the tonsil sample), there is a second, flatter line that is forked below the diagonal cluster (blue). This corresponds to droplets that contain copies of the 242-243CC>TT mutated DNA. Sanger sequencing on the wild-type tonsil DNA shows two cytosine peaks at 242 to 243, whereas both tumor samples have two thymine peaks at 242 to 243. Black arrows indicate the two bases that are affected by the 242-243CC>TT mutation. B: Metastatic melanoma tumor FFPE DNA and corresponding plasma DNAs from two patients are analyzed with the C250T 113-bp assay. Left panels: The same forked cluster (blue) can be observed in both tumor samples. Right panels: The plasma samples for each patient have a secondary cluster below the diagonal cluster, demonstrating the ability to detect this mutation in plasma samples (cluster is circled for sample 12-272). Because of large amounts of DNA in the plasma sample for 15-357, only one well was shown in the two-dimensional plot (metawell is shown for all others).
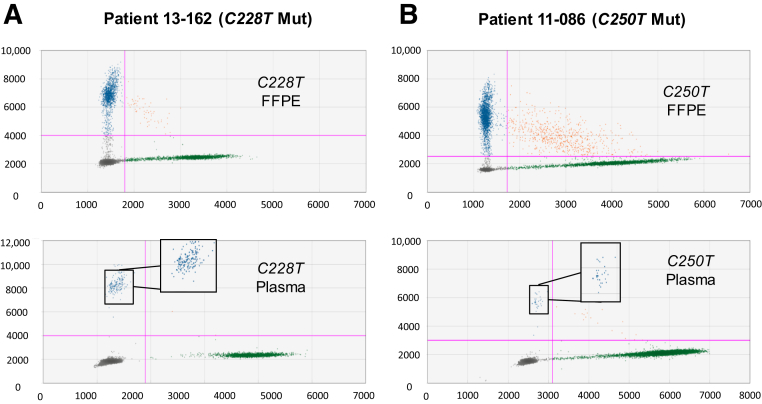
Figure 8.
TERT promoter mutations (Muts) detected in metastatic melanoma patient samples. Tumor and plasma samples from two metastatic melanoma patients were positive for either C228T (A) or C250T (B). Top panels: Formalin-fixed, paraffin-embedded (FFPE) tumor DNA. Bottom panels: Plasma sample from the corresponding patient. The 113-bp assays were used to analyze these samples. The expanded boxes are highlighting the single mutant droplets detected by each assay for plasma samples.
Mutation Detection in Plasma Samples
To assess the sensitivity of the ddPCR assays to detect cell-free circulating tumor DNA, 39 plasma samples were analyzed from 39 patients with metastatic melanoma using either the 113- or 88-bp assays. Twenty-seven patients had tumors with TERT mutations detected using the ddPCR assays; 12 patients had tumors lacking mutations. The LoB was established by analyzing 12 plasma samples from the patients whose tumors lacked both TERT mutations. The LoBs for the C228T and C250T assays were 0.063% and 0.031% MAF, respectively (Supplemental Figure S7). Six plasma samples were analyzed with the C228T assay, and six were analyzed with the C250T assay (each sample was analyzed for one mutation using 12 replicate wells). Overall, 21 of 27 plasma samples (78%) from patients with TERTmutant tumors had detectable TERTmutant DNA that matched the TERT promoter mutation in the patient's tumor (Supplemental Figure S8). These positive samples ranged from 0.06% MAF (low positive) to 15.3% MAF (high positive). Figure 8 shows representative two-dimensional plots of plasma samples that are positive for the C228T and C250T mutations. The two-dimensional plots depict the results of 12 replicate wells merged into a metawell using QuantaSoft software. Included in this data set were three plasma samples from patients with tumors that were typed as 242-243 CC>TT using the C250T assay (Figure 7B). These three plasma samples were all positive for the 242-243 CC>TT with the C250T assay.
Discussion
The development of robust droplet digital PCR assays independently detecting either of the major mutations in the TERT promoter region proved to be a substantial technical challenge for several reasons. First, the region is characterized by a high GC content, approximately 80%, making efficient and specific PCR amplification of targets generally difficult.15 Second, the two recurrent promoter mutations are separated by only 22 bp, so the amplified product included both mutation target sites. Finally, the mutated sequences surrounding the two hotspots are identical over a span of 11 bp and only differ by one dinucleotide in their wild-type sequences falling in the middle of each 11-bp span (Figure 1). We ultimately succeeded in developing highly sensitive and specific assays detecting each hotspot independently through iterative optimization of primer and probe designs operating at elevated annealing-extension temperatures, extra amplification cycles, and development of amplification conditions that leveraged betaine and EDTA as reaction mix modifiers.
The impact of betaine on the experimental ddPCR assays was investigated because its zwitterion properties have been shown to increase the binding of oligonucleotides to GC-rich regions.19 Betaine at a concentration of 0.5 mol/L improved cluster tightness and separation; however, it modestly reduced droplet size and, therefore, resulted in a slight but consistent underestimate of absolute target concentration when using the TERT promoter assays under the recommended conditions. Furthermore, it was found that excessively high betaine concentrations >0.75 mol/L (eg, 1 mol/L) droplet stability were adversely affected and should be avoided. Disodium EDTA further improved cluster separation. Disodium EDTA titrated the level of free Mg+2 in the PCR mixture, which presumably affected the stability of primer-template structures.20 A similar approach may be applied to other GC-rich genomic targets to develop successful ddPCR assays to detect and reliably quantify them. After optimization of the reaction mixture, the number of amplification cycles was lowered to the standard amount (50 to 40) and there was a clear decrease in amplitude of both wild-type and mutant droplet clusters, but a serial dilution series was never performed to measure whether the assay was able to detect at the same sensitivity despite the decrease in amplitude (data not shown).
Overall, the optimized set of assays yields amplification products of 88, 113, or 163 bp. Each assay is characterized by well-separated, tightly formed clusters, allowing for unambiguous thresholding. Detection efficiency of the final assay designs was only moderately lower (approximately 80% efficiency) in high-molecular-weight DNA than that of assays targeting less GC-rich regions of housekeeping genes, such as RPP30, EIF2C1, and the TERT gene coding region. Fragmented DNA templates from FFPE tumor and plasma sources were most efficiently amplified using primers generating the shortest amplicons (Figure 7). Taken together, the assays demonstrated excellent sensitivity and specificity in detecting the C228T and C250T mutations in various DNA templates, including cell lines, FFPE tumor samples, and cell-free, circulating tumor DNA. None of the RPP30 assays used herein was the standard commercially available assay that has been shown to be 100% efficient in amplifying DNA.21 However, given the close similarities in high A-T content between the standard RPP30 assay and the assays used herein, the performance of the shorter amplicon length RPP30 assay would be equivalent to the standard assay.
One of the barriers to the liquid biopsy field is the potentially low concentration of mutant templates in plasma cell-free DNA in certain clinical situations. This potential limitation makes assay amplification efficiency and high specificity especially important for samples with low DNA concentrations and MAFs. When assay efficiency is low, assay sensitivity suffers. In comparison to the benchmark RPP30 assay, it was found that the optimized TERT assays detected approximately 80% of the DNA copies when amplifying products of approximately 110 bp. In addition, amplicon length was critical when designing assays to be used on fragmented DNA sources, such as plasma cell-free DNA and DNA from FFPE tissues. Cell-free DNA is believed to originate from various forms of cell death, such as apoptosis and necrosis. Cellular DNA from these dying cells is exposed to nucleases that cut the DNA between nucleosomes, generating approximately 160-bp fragments.22 By different means, tumor tissue processed by formalin fixation results in a range of mostly short DNA fragment lengths because of chemical reactions between formaldehyde and nucleic acids.23 As expected, fragmented DNA was found to be more efficiently amplified by assays targeting shorter amplicons because there is a greater chance of both primers binding to the shorter (versus longer) target sequence. With FFPE DNA, there was an increase in low-amplitude droplets, likely because of the high fragmentation of the DNA, which may lead to incomplete and late starting PCR cycling for some templates.24 Nevertheless, a consistent 80% relative efficiency of the TERT assays was observed compared with the RPP30 assay, across all amplicon sizes and template sources, suggesting that the decrease in efficiency is because of the intrinsic difficulties in amplifying the region.
The optimized TERT promoter mutation assays appear to have clinical potential in the liquid biopsy field. TERTmutant circulating tumor DNA was identified in 74% of the plasma samples from patients with TERTmutant metastatic melanomas. This level of sensitivity is similar to that of the ddPCR assay for BRAFV600E, which detected mutations in approximately 80% of the metastatic plasma samples that had corresponding V600E/K mutant tumors.16, 25 To achieve this level of sensitivity, the ability of ddPCR assays to analyze low MAF samples across multiple replicate wells was used. Because larger elution volumes from column-based DNA extraction methods can potentially yield higher amounts of purified DNA,26 the DNA extraction procedure was adjusted to use a maximal elution volume that would permit nearly the entire sample to be analyzed in 12 replicate wells. This design maximizes the sensitivity to detect rare events from a 5-mL plasma sample. In addition, it made for a simple workflow to analyze each sample in a uniform manner.
These assays compare favorably with a recently published ddPCR assay to detect TERTmutant DNA.27 That assay used locked nucleic acid chemistry for the primers and a single probe to detect both mutations, so the assay cannot specify which mutation is present in a sample. On the basis of the published figure, there was less separation of mutant and wild-type clusters compared with the assays we report, and the cluster separation for the C250T assay was limited. Such results can pose challenges to thresholding and reproducibility. Finally, they identified TERTmutant DNA in 8 of 15 patients (53%) with TERTmutant metastatic melanomas compared with the 21 of 27 patients (78%) identified herein. Although it is not clear if the differences in the patient sample sets contributed to the potentially higher clinical sensitivity of this assay, one potential reason for the differences in sensitivity may be amplicon length. The 113- and 88-bp assays were used for analyzing plasma samples; the published assay amplifies a 163-bp target that was found to be only approximately 60% as efficient in our own designs as the 88-bp assays.
In conclusion, we describe the development and optimization of ddPCR assays specifically detecting the C228T, C250T, and CC242-243TT promoter mutations in the TERT gene. The assays performed with high sensitivity and specificity in the analysis of tumor and plasma samples from patients with metastatic melanoma. Given the similarities in performance between the 88- and 113-bp assays, as well as the somewhat higher efficiency of the 88-bp assays when analyzing plasma DNA, we recommend and are using this assay when analyzing clinical samples from patients with melanoma and other tumors. These results suggest these assays have the potential to become valuable assets in diagnosing and monitoring patients with cancers possessing TERT promoter mutations, as well as in drug development research targeting telomerase expression.
Footnotes
Supported by NIH grants R21CA198495 (D.P.), UH2CA206124 (D.P.), and CA016087 (I.O. and Y.S.).
Disclosures: G.K.-N. owns stock in Bio-Rad Laboratories and is the Director of Scientific Affairs at Bio-Rad's Digital Biology Center. S.C. owns stock in Bio-Rad and was a bioinformatics manager at Bio-Rad when this work was performed. Bio-Rad prepared probes and primers for this work.
Supplemental material for this article can be found at https://doi.org/10.1016/j.jmoldx.2018.09.003.
Supplemental Data
Titrations of chemical additives used to optimize reaction conditions. Two-dimensional plots demonstrating the effect of different levels of additives when introduced into the standard mastermix preparation. Addition of EDTA helped separate clusters, whereas introduction of betaine helped form tighter clusters. Cluster separation diminishes at a higher betaine concentration. From these investigations, 1 mmol/L EDTA and 0.5 mol/L betaine were chosen as optimal. (See also Supplemental Figure S3 regarding minor effects of betaine on droplet size at higher concentrations.)
Assessment of assay robustness through a temperature gradient ranging from 56°C to 66°C. A: Analysis of cell line A172 with the 113-bp assay containing probe C228T. B: Analysis of cell line 12-126 with the 113-bp assay containing probe C250T. All two-dimensional plots are shown on the same scale; thresholding parameters were adjusted on the basis of cluster separation and amplitude for each temperature condition. Quantification results and mutant allele fraction (MAF) are shown for each annealing temperature.
Betaine concentration (Conc) effect on ddPCR quantification of sample DNA input. A series of experiments were run to investigate the potential effect of betaine concentration on quantification of known input DNA and droplet size. Percentage quantification of DNA using different concentrations of betaine was normalized to a 0 mol/L betaine and a 1 mmol/L Na2EDTA reaction mixture (y axis). Betaine concentration (mol/L) is varied across the x axis (shown are 0, 0.2, 0.3, 0.4, 0.5, 0.75, and 1 mol/L). Also shown is 0 mol/L betaine and 0 mmol/L EDTA mixture (0/0). Two replicate experiments were run to analyze the effect of betaine on droplet size and DNA quantification. The results of experiment 1 (blue) and experiment 2 (red) were averaged (green). On the basis of DNA concentration measurements at betaine concentrations varying from 0 to 1 mol/L and a constant 1 mmol/L Na2EDTA, a small decrease in the calculated DNA concentration was observed at >0.2 to 0.3 mol/L betaine. At the optimal concentration for TERT promoter assay performance, 0.5 mol/L, there is a 6.3% decrease in the calculated input DNA concentration. Red dotted boxed areas are highlighting percentage amplification with no betaine and 0.5 mol/L betaine (optimized condition). Ctrl, control; WT, wild type.
False-positive mutant background signals of TERT promoter assays using normal tonsil formalin-fixed, paraffin-embedded (FFPE) DNA. Ten samples with DNA inputs ranging from 6 to 30 ng per well were analyzed with the 113-bp TERT assays to determine the limit of blank (LoB; ie, background level) for mutant-positive FFPE samples. The averages of the 10 samples for both assays are depicted, and error bars are derived from LoB calculations (see Materials and Methods).
Concordance between ddPCR and Sanger sequencing for two TERT-mutant (MUT) melanoma formalin-fixed, paraffin-embedded (FFPE) tumor samples. A: Left panel: Peripheral blood mononuclear cell (PBMC) DNA [wild type (WT)]. Middle panel: Metastatic melanoma tumor with a thymine peak at the 228 site. Right panel: The 113-bp ddPCR assay detection of the C228T mutation in concordance with Sanger results. B: Left panel: PBMC DNA (wild type). Middle panel: Metastatic melanoma tumor with a thymine peak at the 250 site. Right panel: The 113-bp ddPCR assay detection of the C250T mutation in concordance with Sanger results. Black arrows indicate the mutation site for the respective TERT promoter mutation.
Analysis of metastatic melanoma tumor and plasma samples. A: Thirty-four metastatic melanoma patient's tumors were analyzed with both Sanger sequencing and both of the 113-bp TERT promoter assays. Nineteen of the samples were positive for a TERT promoter mutation in both Sanger sequencing and the respective ddPCR assays. Fifteen of the samples were negative for a mutation in both Sanger sequencing and the ddPCR assays. There were no false-positive samples in which the ddPCR assays detected a TERT promoter mutation without Sanger sequencing detecting it as well. There were also no cases in which ddPCR could not detect a TERT promoter mutation that Sanger sequencing detected. B: Thirty-nine plasma samples from 39 metastatic melanoma patients were analyzed using the 113- or 88-bp C228T or C250T TERT promoter assay (based on the tumor mutation detected). Of 27 plasma samples from patients with TERT mutant tumor samples, 21 (78%) were positive. No false positives were detected. FFPE, formalin-fixed, paraffin-embedded; WT, wild type.
Background mutant allele fractions (MAFs) of metastatic melanoma plasma samples lacking TERT promoter mutations. Twelve plasma samples from individual metastatic melanoma patients with TERT wild-type (WT) tumors were analyzed with either the C228T or the C250T assay (six patient samples for each assay). The MAFs for each assay were averaged, and limits of blank were calculated as described in Materials and Methods.
Range of circulating tumor DNA levels in metastatic melanoma patient plasma samples. Representative results of plasma samples with varying levels of mutant DNA analyzed using the 88-bp C228T and C250T assays. Positive plasma samples can be low positive [0.06% mutant allele fraction (MAF)] and range to high levels (15.3% MAF). Each sample is from a different patient. Assay choice (C228T or C250T) matches the specific TERT mutation in the patient's tumor sample.
References
- 1.Oxnard G.R., Thress K.S., Alden R.S., Lawrance R., Paweletz C.P., Cantarini M., Yang J.C.-H., Barrett J.C., Jänne P.A. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non–small-cell lung cancer. J Clin Oncol. 2016;34:3375–3382. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran H., Zhang J., Vasquez M., Fossella F., Simon G., Tsao A., Gibbons D.L., Elamin Y., Banks K., Lanman R. P2. 03b-030 Retrospective review clinical use of a cfDNA blood test for identification of targetable molecular alterations in patients with lung cancer. J Thorac Oncol. 2017;12 S952. [Google Scholar]
- 3.Wan J.C., Massie C., Garcia-Corbacho J., Mouliere F., Brenton J.D., Caldas C., Pacey S., Baird R., Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 4.Cobas EGFR MUTATION TEST v2: Somatic Gene Mutation Detection System: Premarket Approval. US Food & Drug Administration; Silver Spring, MD: 2016. [Google Scholar]
- 5.Gormally E., Caboux E., Vineis P., Hainaut P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: practical aspects and biological significance. Mutat Res. 2007;635:105–117. doi: 10.1016/j.mrrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., Bright I.J., Lucero M.Y., Hiddessen A.L., Legler T.C. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacher A.G., Paweletz C., Dahlberg S.E., Alden R.S., O'Connell A., Feeney N., Mach S.L., Jänne P.A., Oxnard G.R. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016;2:1014–1022. doi: 10.1001/jamaoncol.2016.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanmamed M.F., Fernández-Landázuri S., Rodríguez C., Zárate R., Lozano M.D., Zubiri L., Perez-Gracia J.L., Martín-Algarra S., González A. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015;61:297–304. doi: 10.1373/clinchem.2014.230235. [DOI] [PubMed] [Google Scholar]
- 9.Olmedillas-López S., García-Arranz M., García-Olmo D. Current and emerging applications of droplet digital PCR in oncology. Mol Diagn Ther. 2017;21:493–510. doi: 10.1007/s40291-017-0278-8. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich B., Rachakonda P.S., Hemminki K., Kumar R. TERT promoter mutations in cancer development. Curr Opin Genet Dev. 2014;24:30–37. doi: 10.1016/j.gde.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Huang F.W., Hodis E., Xu M.J., Kryukov G.V., Chin L., Garraway L.A. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn S., Figl A., Rachakonda P.S., Fischer C., Sucker A., Gast A., Kadel S., Moll I., Nagore E., Hemminki K. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 13.Vaziri H., Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 14.Bell R.J., Rube H.T., Xavier-Magalhães A., Costa B.M., Mancini A., Song J.S., Costello J.F. Understanding TERT promoter mutations: a common path to immortality. Mol Cancer Res. 2016;14:315–323. doi: 10.1158/1541-7786.MCR-16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey U.H., Bachmann H.S., Peters J., Siffert W. PCR-amplification of GC-rich regions: “slowdown PCR. Nat Protoc. 2008;3:1312–1317. doi: 10.1038/nprot.2008.112. [DOI] [PubMed] [Google Scholar]
- 16.Chang G.A., Tadepalli J.S., Shao Y., Zhang Y., Weiss S., Robinson E., Spittle C., Furtado M., Shelton D.N., Karlin-Neumann G., Pavlick A., Osman I., Polsky D. Sensitivity of plasma BRAFmutant and NRASmutant cell-free DNA assays to detect metastatic melanoma in patients with low RECIST scores and non-RECIST disease progression. Mol Oncol. 2016;10:157–165. doi: 10.1016/j.molonc.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yancovitz M., Litterman A., Yoon J., Ng E., Shapiro R.L., Berman R.S., Pavlick A.C., Darvishian F., Christos P., Mazumdar M. Intra-and inter-tumor heterogeneity of BRAFV600Emutations in primary and metastatic melanoma. PLoS One. 2012;7:e29336. doi: 10.1371/journal.pone.0029336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armbruster D.A., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29:S49. [PMC free article] [PubMed] [Google Scholar]
- 19.Henke W., Herdel K., Jung K., Schnorr D., Loening S.A. Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res. 1997;25:3957–3958. doi: 10.1093/nar/25.19.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saiki R.K. Palgrave MacMillan; London, UK: 1989. The Design and Optimization of the PCR: PCR Technology; pp. 7–16. [Google Scholar]
- 21.Berman J., Heredia N., Regan J., Cooper S., Tzonev S., Hefner E., Karlin-Neumann G. Droplet Digital™ PCR: high-resolution copy number variation analysis. Bio-Rad Bulletin. 2012;6475:1–2. [Google Scholar]
- 22.Thierry A., El Messaoudi S., Gahan P., Anker P., Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35:347–376. doi: 10.1007/s10555-016-9629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan M., Sedmak D., Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F., Wang L., Briggs C., Sicinska E., Gaston S.M., Mamon H., Kulke M.H., Zamponi R., Loda M., Maher E. DNA degradation test predicts success in whole-genome amplification from diverse clinical samples. J Mol Diagn. 2007;9:441–451. doi: 10.2353/jmoldx.2007.070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santiago-Walker A., Gagnon R., Mazumdar J., Casey M., Long G.V., Schadendorf D., Flaherty K., Kefford R., Hauschild A., Hwu P. Correlation of BRAF mutation status in circulating-free DNA and tumor and association with clinical outcome across four BRAFi and MEKi clinical trials. Clin Cancer Res. 2016;22:567–574. doi: 10.1158/1078-0432.CCR-15-0321. [DOI] [PubMed] [Google Scholar]
- 26.Qiagen: QIAamp Circulating Nucleic Acid Handbook. Qiagen; Hilden, Germany: 2013. [Google Scholar]
- 27.McEvoy A.C., Calapre L., Pereira M.R., Giardina T., Robinson C., Khattak M.A., Meniawy T.M., Pritchard A.L., Hayward N.K., Amanuel B. Sensitive droplet digital PCR method for detection of TERT promoter mutations in cell free DNA from patients with metastatic melanoma. Oncotarget. 2017;8:78890–78900. doi: 10.18632/oncotarget.20354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Titrations of chemical additives used to optimize reaction conditions. Two-dimensional plots demonstrating the effect of different levels of additives when introduced into the standard mastermix preparation. Addition of EDTA helped separate clusters, whereas introduction of betaine helped form tighter clusters. Cluster separation diminishes at a higher betaine concentration. From these investigations, 1 mmol/L EDTA and 0.5 mol/L betaine were chosen as optimal. (See also Supplemental Figure S3 regarding minor effects of betaine on droplet size at higher concentrations.)
Assessment of assay robustness through a temperature gradient ranging from 56°C to 66°C. A: Analysis of cell line A172 with the 113-bp assay containing probe C228T. B: Analysis of cell line 12-126 with the 113-bp assay containing probe C250T. All two-dimensional plots are shown on the same scale; thresholding parameters were adjusted on the basis of cluster separation and amplitude for each temperature condition. Quantification results and mutant allele fraction (MAF) are shown for each annealing temperature.
Betaine concentration (Conc) effect on ddPCR quantification of sample DNA input. A series of experiments were run to investigate the potential effect of betaine concentration on quantification of known input DNA and droplet size. Percentage quantification of DNA using different concentrations of betaine was normalized to a 0 mol/L betaine and a 1 mmol/L Na2EDTA reaction mixture (y axis). Betaine concentration (mol/L) is varied across the x axis (shown are 0, 0.2, 0.3, 0.4, 0.5, 0.75, and 1 mol/L). Also shown is 0 mol/L betaine and 0 mmol/L EDTA mixture (0/0). Two replicate experiments were run to analyze the effect of betaine on droplet size and DNA quantification. The results of experiment 1 (blue) and experiment 2 (red) were averaged (green). On the basis of DNA concentration measurements at betaine concentrations varying from 0 to 1 mol/L and a constant 1 mmol/L Na2EDTA, a small decrease in the calculated DNA concentration was observed at >0.2 to 0.3 mol/L betaine. At the optimal concentration for TERT promoter assay performance, 0.5 mol/L, there is a 6.3% decrease in the calculated input DNA concentration. Red dotted boxed areas are highlighting percentage amplification with no betaine and 0.5 mol/L betaine (optimized condition). Ctrl, control; WT, wild type.
False-positive mutant background signals of TERT promoter assays using normal tonsil formalin-fixed, paraffin-embedded (FFPE) DNA. Ten samples with DNA inputs ranging from 6 to 30 ng per well were analyzed with the 113-bp TERT assays to determine the limit of blank (LoB; ie, background level) for mutant-positive FFPE samples. The averages of the 10 samples for both assays are depicted, and error bars are derived from LoB calculations (see Materials and Methods).
Concordance between ddPCR and Sanger sequencing for two TERT-mutant (MUT) melanoma formalin-fixed, paraffin-embedded (FFPE) tumor samples. A: Left panel: Peripheral blood mononuclear cell (PBMC) DNA [wild type (WT)]. Middle panel: Metastatic melanoma tumor with a thymine peak at the 228 site. Right panel: The 113-bp ddPCR assay detection of the C228T mutation in concordance with Sanger results. B: Left panel: PBMC DNA (wild type). Middle panel: Metastatic melanoma tumor with a thymine peak at the 250 site. Right panel: The 113-bp ddPCR assay detection of the C250T mutation in concordance with Sanger results. Black arrows indicate the mutation site for the respective TERT promoter mutation.
Analysis of metastatic melanoma tumor and plasma samples. A: Thirty-four metastatic melanoma patient's tumors were analyzed with both Sanger sequencing and both of the 113-bp TERT promoter assays. Nineteen of the samples were positive for a TERT promoter mutation in both Sanger sequencing and the respective ddPCR assays. Fifteen of the samples were negative for a mutation in both Sanger sequencing and the ddPCR assays. There were no false-positive samples in which the ddPCR assays detected a TERT promoter mutation without Sanger sequencing detecting it as well. There were also no cases in which ddPCR could not detect a TERT promoter mutation that Sanger sequencing detected. B: Thirty-nine plasma samples from 39 metastatic melanoma patients were analyzed using the 113- or 88-bp C228T or C250T TERT promoter assay (based on the tumor mutation detected). Of 27 plasma samples from patients with TERT mutant tumor samples, 21 (78%) were positive. No false positives were detected. FFPE, formalin-fixed, paraffin-embedded; WT, wild type.
Background mutant allele fractions (MAFs) of metastatic melanoma plasma samples lacking TERT promoter mutations. Twelve plasma samples from individual metastatic melanoma patients with TERT wild-type (WT) tumors were analyzed with either the C228T or the C250T assay (six patient samples for each assay). The MAFs for each assay were averaged, and limits of blank were calculated as described in Materials and Methods.
Range of circulating tumor DNA levels in metastatic melanoma patient plasma samples. Representative results of plasma samples with varying levels of mutant DNA analyzed using the 88-bp C228T and C250T assays. Positive plasma samples can be low positive [0.06% mutant allele fraction (MAF)] and range to high levels (15.3% MAF). Each sample is from a different patient. Assay choice (C228T or C250T) matches the specific TERT mutation in the patient's tumor sample.