Abstract
Invasive fungal infections caused by multiazole-resistant Aspergillus fumigatus are associated with increasing rates of mortality in susceptible patients. Current methods of diagnosing infections caused by multiazole-resistant A. fumigatus are, however, not well suited for use in clinical point-of-care testing or in the field. Loop-mediated isothermal amplification (LAMP) is a widely used method of nucleic acid amplification with rapid and easy-to-use features, making it suitable for use in different resource settings. Here, we developed a LAMP assay to detect a 34 bp tandem repeat, named TR34-LAMP. TR34 is a high-prevalence allele that, in conjunction with the L98H single-nucleotide polymorphism, is associated with the occurrence of multiazole resistance in A. fumigatus in the environment and in patients. This process was validated with both synthetic double-stranded DNA and genomic DNA prepared from azole-resistant isolates of A. fumigatus. Use of our assay resulted in rapid and specific identification of the TR34 allele with high sensitivity, detecting down to 10 genomic copies per reaction within 25 minutes. Fluorescent and colorimetric detections were used for the analysis of 11 clinical isolates as cross validation. These results show that the TR34-LAMP assay has the potential to accelerate the screening of clinical and environmental A. fumigatus to provide a rapid and accurate diagnosis of azole resistance, which current methods struggle to achieve.
The fungus Aspergillus fumigatus is a globally occurring saprophyte and is highly prevalent owing to its production of numerous airborne conidia.1 Clinically, A. fumigatus causes a spectrum of respiratory and invasive infections, ranging from asthma-like symptoms to invasive aspergillosis (IA) in immunocompromised hosts.2, 3 In the clinic, triazole antifungal drugs are widely used to control this fungal infection due to their high effectiveness in disrupting membrane structure and fungal cell transport.4, 5 However, fungicides are also an essential means of controlling fungal disease in agriculture with sterol demethylation inhibitor compounds, a class of chemicals that includes triazoles and imidazoles, representing the largest class of fungicides used worldwide. Environmentally, the evolution of widespread resistance to demethylation inhibitors is observed not only in plant-infecting fungal pathogens but also in the saprophytic soil-dwelling A. fumigatus. This observation suggests that the deployment of triazoles in agriculture has led to selection for antifungal resistance not only in target crop pathogens4, 6 but also those fungal species that co-inhabit in the environment and including those that can opportunistically infect humans.4
The use of long-term azole therapy to treat patients with clinical A. fumigatus infections is known to lead to the de novo evolution of resistance.7 However, naïve patients who have not been previously treated with medical azoles are increasingly being diagnosed with multiazole-resistant infections, leading to the hypothesis that these patients may have contracted infectious agents that have evolved resistance to azoles owing to their exposure to agricultural demethylation inhibitors in the environment.8, 9, 10, 11 Azole resistance in A. fumigatus is associated with a tandem repeat within the promoter and mutations in the cyp51A gene, which encodes the drug target lanosterol 14-α-demethylase. The most frequently occurring azole-resistance mechanism is characterized by a 34-bp tandem repeat (TR34) twinned with a leucine-for-histidine substitution (L98H) in cyp51A. This combination of mutations is increasingly found to be present in patients manifesting infections with azole-resistant A. fumigatus, with recent studies showing 40% to 90% of clinical triazole-resistant A. fumigatus isolates carrying the TR34/L98H allele.12, 13, 14 Importantly, A. fumigatus with this TR34/L98H combination has been also found widely in environmental samples that include soils, compost, plant bulbs, and air, suggesting that it has evolved, and amplified, in the environment as a consequence of exposure to agricultural fungicides.12, 13, 15 Epidemiologic studies using highly discriminatory genetic and genomic methods have shown the occurrence of closely related A. fumigatus genotypes containing the TR34/L98H allele both in nature and in clinical infections, strengthening the suspicion that azole-resistant isolates of this fungus in nature are causing drug-resistant infection in the clinic as a consequence of the dual use of demethylation-inhibitor antifungals.10 Consequently, there is an urgent need for rapid diagnostic methods targeted at detecting and managing multiazole-resistant A. fumigatus within both the clinic and the field. Conventional diagnostic methods, such as culture, microscopic examination, and radiology are commonly used for detecting infections caused by A. fumigatus.16 However, these methods require several days to culture the fungus, have only moderate specificity, and require further tests to phenotypically or genotypically ascertain azole resistance.17 Antibody-based tests have been developed to rapidly detect infections; however, they are not able to determine the occurrence of azole resistance.18, 19 PCR-based molecular methods have been developed due to their higher sensitivities and specificities compared to those with conventional methods and are also able to detect the presence of azole-resistance alleles.20, 21, 22, 23, 24, 25, 26, 27, 28 However, PCR-based detection requires laboratory equipment and experienced personnel. The continued increase of azole resistance, coupled with a lack of a rapid diagnostic method, raises the need for a simple, rapid, and accurate diagnostic test for antifungal mutations that is applicable in both field and clinical settings.
Loop-mediated isothermal amplification (LAMP) has been developed as a rapid, sensitive, and simple technique for the detection of pathogens. This technique provides an approach to nucleic acid detection that amplifies DNA in a highly specific, efficient, and rapid manner while under isothermal conditions.29 This technique is based on the principle of auto-cycling and strand-displacement DNA synthesis, using a DNA polymerase and a set of specially designed primers that recognize 6 distinct regions on a target DNA template. A further improvement in the speed of LAMP reaction is the introduction of two additional loop primers.30 LAMP products can be visualized in different ways, including measuring turbidity, pH-sensitive dyes, and metal indicators.31, 32, 33, 34, 35, 36, 37 Consequently, the technique has great potential for the early diagnosis of azole-resistant strains of A. fumigatus containing a tandem repeat and may facilitate the development of point-of-care devices using lab-on-a-chip technology.
Here we describe the application of LAMP chemistry for the rapid detection of long and direct tandem repeats. The described methodology, TR-LAMP, can be used as a guide for tandem repeat LAMP primer design. The methodology was validated by the design of specific LAMP primers targeting dominant triazole-resistant genotypes of A. fumigatus, and the assay was shown to have high analytical sensitivity and specificity for detecting the most prevalent cyp51A mutation, TR34. Owing to high reliability and rapid identification, the TR34-LAMP assay can be used as a novel method of diagnosing multiazole-resistant A. fumigatus, potentially coupled to point-of-care diagnostic platforms.38, 39, 40
Materials and Methods
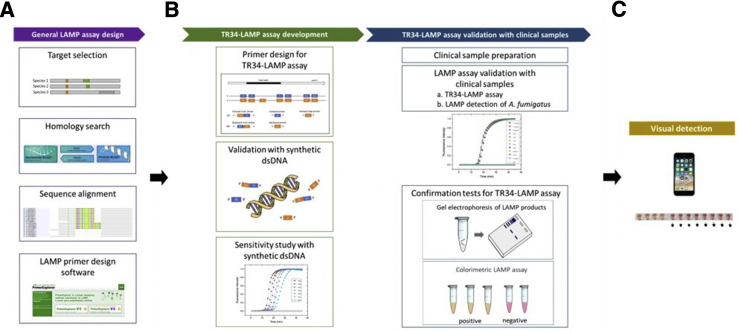
A flow diagram illustrating the overall process of TR34-LAMP assay development is shown in Figure 1. In the following sections, details of TR34-LAMP primer design, information on clinical samples, and experiments conducted in this study are presented.
Figure 1.
The workflow used in this study for 34-bp tandem repeat loop-mediated isothermal amplification (TR34-LAMP) assay. A: The azole-resistance–related gene is selected and aligned with the homologs. This alignment is used as a reference for primer design (PrimerExplorer version 5; Eiken Chemical Co. Ltd., Tokyo, Japan; https://primerexplorer.jp/e). B: TR34-LAMP assay development: forward inner primer (FIP) and/or backward inner primer (BIP) are manually designed for a specific tandem repeat detection. The developed TR34-LAMP assay is validated with synthetic double-stranded (ds)-DNA for understanding of the specificity and detection limit of the assay. TR34-LAMP assay validation with clinical samples: Novel TR34-LAMP primers are used to evaluate the feasibility of amplifying clinical samples containing the TR34 mutations. To ensure the specificity of TR34-LAMP assay, electrophoresis analysis is performed. Colorimetric LAMP assays are applied for visual detection. C: Visual detection and threshold adjustment for a binary result by using a conventional cell phone camera. A. fumigatus, Aspergillus fumigatus.
TR34-LAMP Primer Design
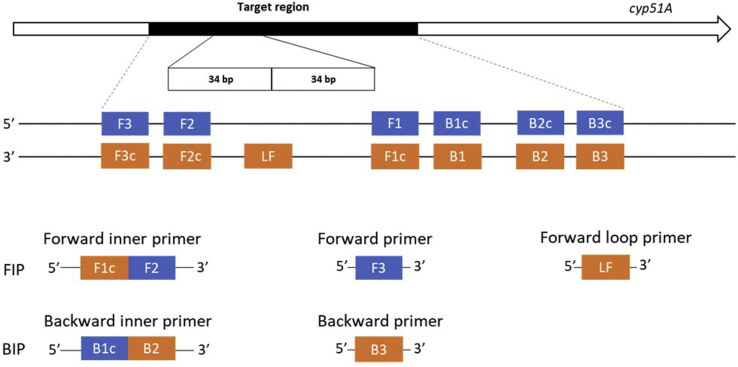
The TR34 LAMP primers were designed based on 100 cyp51A gene–related strains of A. fumigatus, which included sequences containing TR34 and TR46 mutant alleles as well as wild-type isolates (Supplemental Table S1). The sequences were downloaded from NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank) and were aligned by using a MUSCLE (multiple sequence alignment method with reduced time and space complexity) algorithm.41 The sequence alignment was verified by the identification of the tandem repeat regions. The global alignment among all strains was highly conserved, except for the 34- or 46-bp mutant regions. In total, a 250-bp nucleotide alignment was used for TR34-LAMP primer design. LAMP exhibits high specificity due to the use of four to six primers recognizing six to eight distinct regions: The forward and backward inner primers, FIP/BIP, play crucial roles in the specificity of the assay because the F2/B2 primer of FIP/BIP anneals to the F2c/B2c region in the target to initiate the LAMP reaction. The outer primers, F3/B3, are composed of fewer bases and are of a lower concentration than are FIP/BIP, initiating annealing of F3/B3 to the target in order to commence strand displacement. In addition to these four essential primers (FIP/BIP and F3/B3), the forward and backward loop primers (LF/LB) are used to accelerate the speed of the assay.30
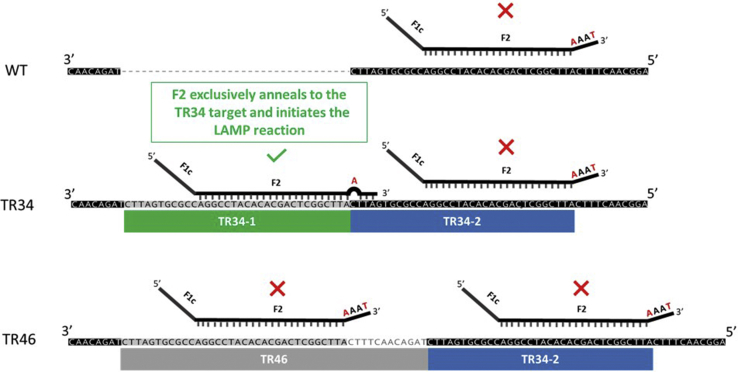
In this study, the challenge for primer design was to select six distinct regions within a long and highly repetitive location (TR34-1 and TR34-2) (Figure 2). Due to the nature of this region, the possibility of primer self-annealing and nonspecific amplification is high. To overcome these difficulties, the 3′ end of the F2 was modified (Figure 2), and the optimal region that covered as little as possible of the second repeat region for the F1c primer design while maintaining the specificity of the TR34-LAMP assay was selected (Figure 3). This modification is because FIP (F1c+F2) is crucial for initiating the reaction, and thus may affect the specificity of the LAMP assay. The details of TR34-LAMP primer design are as follows:
-
i)
Two outer primers (F3 and B3) and one inner primer (BIP) were automatically designed by using PrimerExplorer online software version 5 (Eiken Chemical Co. Ltd., Tokyo, Japan; https://primerexplorer.jp/e). Loop backward primer was not included due to design restrictions. However, due to the limitation of this software for tandem repeat LAMP primer design, the other inner primer (FIP) and one loop primer (forward) were designed manually.
-
ii)
The junction between the first tandem repeat (TR34-1) and the downstream region was targeted for designing the F2 primer, as this junction is the less-conserved region, and therefore the primer designed based on this region may be able to differentiate the TR34 mutant allele from other similar strains (Figure 2).
-
iii)
To decrease the possibility of amplifying the second repeat region (TR34-2), a mismatch was introduced on the fourth base from the 3′ F2 primer terminus, by replacing a G with A to decrease the 3′ stability of FIP (Figure 2).
-
iv)
The location for F1c primer design was chosen to avoid covering too much of the second repeat region (TR34-2), in order to decrease the possibility of self-annealing of FIP (between F1c and F2) (Figure 3).
-
v)
Subsequently, all of these newly developed TR-LAMP primers were checked for secondary structure and primer dimer formation by using NuPack (http://www.nupack.org, last accessed May 29, 2018).42 The locations and details of LAMP primers used for the detection of TR34 mutation are shown in Figure 3 and Table 1, respectively.
Figure 2.
A schematic figure of the location of F2 for the 34-bp tandem repeat loop-mediated isothermal amplification (TR34-LAMP) assay. Partial sequences of TR34 (EU626235.1), TR46 (MF070878.1), and wild type (KJ210331.1) are shown. The green box indicates TR34 repeat unit 1 (TR34-1), the blue boxes indicates TR34 repeat unit 2 (TR34-2), and the grey box indicates TR46 repeat unit. The levels of similarity among the three sequences are highlighted and categorized: highly conserved (black), conserved between mutants (grey), and presented only in one of the sequences (white). forward inner primer (F1c+F2) used in TR34-LAMP assay is shown in black lines. The location of F2 primer plays a crucial role in the specificity of the TR34-LAMP assay. Due to the introduced mismatches (nucleotides highlighted in red) at the 3′ end of F2, a one-step yes/no reaction was developed to differentiate the TR34 mutation in a specific manner. The positive reaction (F2 attached to the target and initiate the reaction) is indicated by a green checkmark, whereas the negative reactions are indicated by red Xs. Short vertical black lines show that the primer has exact match with the template DNA. WT, wild type.
Figure 3.
A schematic diagram of cyp51A gene showing the position and composition of 34-bp tandem repeat loop-mediated isothermal amplification (TR34-LAMP) primers for amplification of the tandem repeat region. Forward outer primer (F3), backward outer primer (B3), forward inner primer FIP (F1c+F2), backward inner primer BIP (B1c+B2), loop forward primer (LF). Loop backward primer is not included in this assay due to design restrictions. F2 primer is placed within the first tandem repeat and F1c is located at the downstream of second tandem repeat to avoid self-annealing of FIP. LF primer is located in the junction of the two tandem repeats. The rest of the LAMP primers are outside of tandem repeat region.
Table 1.
TR34-LAMP Primer Sequences
| Primer | Sequence |
|---|---|
| F3 | 5′-CACCACTTCAGAGTTGTCT-3′ |
| B3 | 5′-GTATTTTATATTCACCTACCTACCA-3′ |
| FIP | 5′-TAATTAGGCAACTTTCATTCCGGATGTGTGC-TGAGCCGAATAAAT-3′ |
| BIP | 5′-ACTAAGGTGTAGTTCCAGCATACCGTAAGTA-GATCTACCTACCGTAGT-3′ |
| LF | 5′-CGGACCGCGTGATTCAT-3′ |
The introduced mismatch at 3′ of F2 primer is shown in bold.
BIP, backward inner primer; FIP, forward inner primer; LF, loop forward primer.
Samples and DNA-Extraction Method
Synthetic double-stranded DNA containing the TR34 and TR46 target were synthesized by Integrated DNA Technologies (Leuven, Belgium). For clinical samples, high-molecular-weight DNA was extracted from A. fumigatus conidia using the MasterPure yeast DNA purification kit (Lucigen, Middleton, WI) according to the manufacturer's instructions but with an additional bead-beating step. Harvested conidia were disrupted using 1.0 mm–diameter zirconia/silica beads (BioSpec Products, Bartlesville, OK) in a TissueLyser II bead mill (Qiagen, Hilden, Germany) set to a frequency of 30 (1 per second) for 45 seconds. The genomic (g)-DNA was quantified using a Qubit 3.0 fluorometer and the high-sensitivity double-stranded DNA assay kit (Life Technologies, Carlsbad, CA). Integrity of the gDNA was confirmed using a TapeStation 2200 system and gDNA ScreenTape (Agilent Technologies, Santa Clara, CA). Purified gDNA was stored at −20°C until required.
LAMP Reaction Conditions
To detect TR34 mutation in A. fumigatus using LAMP on a real-time PCR machine, the LAMP mix contained the following: 1.5 μL of 10× isothermal buffer, 0.9 μL of MgSO4 (stock at 100 mmol/L), 2.1 μL of dNTPs (stock at 10 μmol/L each), 0.375 μL of bovine serum albumin (BSA; 20 mg/mL), 2.4 μL of betaine (5 mol/L), 0.6 μL of Bst 2.0 DNA polymerase (8000 U/μL) (New England Biolabs, Hitchin, UK), 0.375 μL of Syto 9 (20 μmol/L stock) (Thermo Fisher Scientific, Waltham, MA), 1.5 μL of 10× primer mixture (20 μmol/L BIP/FIP, 10 μmol/L loop forward primer, and 2.5 μmol/L B3/F3) (Integrated DNA Technologies), 3 μL of various concentrations of gDNA (clinical samples) or synthetic double-stranded DNA (Integrated DNA Technologies) template solution ranging from 10 to 106 copies/reaction, and enough nuclease-free water to bring the volume to 15 μL. Each solution was split into 5-μL reactions (triplicates) on the 96-well PCR plate and heated at 63°C for 50 minutes. To confirm the presence of A. fumigatus in clinical samples, published primers43 and the reaction condition described in this section were used.
End-Point Colorimetric TR34-LAMP Assay
The WarmStart Colorimetric LAMP 2X master mix (New England BioLabs) contains a phenol red pH indicator that provides visual detection of the accumulation of protons during the LAMP reaction, providing a change in solution color from pink (negative) to pale orange (positive). LAMP reactions were performed in 0.2-mL PCR tubes in a 25-μL reaction volume, which contains 12.5 μL of WarmStart Colorimetric LAMP 2X master mix, 2.5 μL of TR34-LAMP primer mix (10×), 2.5 μL of gDNA extracted from clinical samples, and 7.5 μL of nuclease-free water. All reaction mixes were incubated in an end-point PCR machine (Veriti Dx 96-well Fast Thermal Cycler; Thermo Fisher Scientific) at 63°C for 30 minutes and examined by naked eyes.
Analytical Sensitivity of the TR34-LAMP Assay
Sensitivity of the assay was evaluated in serial 10-fold dilutions of synthetic TR34 DNA target, ranging from 100 to 106 copies per reaction. Each condition was run in triplicate and the experiments repeated three times. LAMP assays were performed in a LightCycler 96 System (Roche Molecular Systems, Pleasanton, CA) and data analyzed using LightCycler 96 System software version SW1.1.
Specificity of the TR34-LAMP Product
The specificity of TR34-LAMP amplification was determined by the restriction digestion of LAMP products. Based on the consensus target sequence, the restriction enzyme MluCI (New England BioLabs) was used to digest amplification products. The restriction digests were incubated at 37°C for 30 minutes, using the CutSmart buffer purchased from the manufacturer. Digested products were analyzed by gel electrophoresis on a 1.2% agarose gel, in 1× TAE (Tris base, acetic acid) buffer at 120 V for 40 minutes. The DNA bands were stained with SYBR Safe DNA Gel Stain (InvitroGen/Thermo Fisher Scientific) and visualized by illumination with a UV lamp (366 nm) using the BioSpectrum Imaging System (UVP/Thermo Fisher Scientific). Experiments were performed using gDNA from clinical isolates. All isolates were provided and characterized by Dr. Oliver Baden, University of Göttingen (Göttingen, Germany), and Dr. Darius Armstrong-James, Imperial College London (London, UK).44
Mobile Phone Imaging Protocol and Image Processing
An iPhone 6s (Apple, Cupertino, CA) was used to capture the readout under room-light conditions. The photo was taken using a phone camera with auto-focus and auto-exposure default settings. The image was subsequently processed by using open-resource ImageJ software version 1.51 (NIH, Bethesda, MD; http://imagej.nih.gov/ij).45
Results
Analytical Sensitivity of the TR34-LAMP Assay
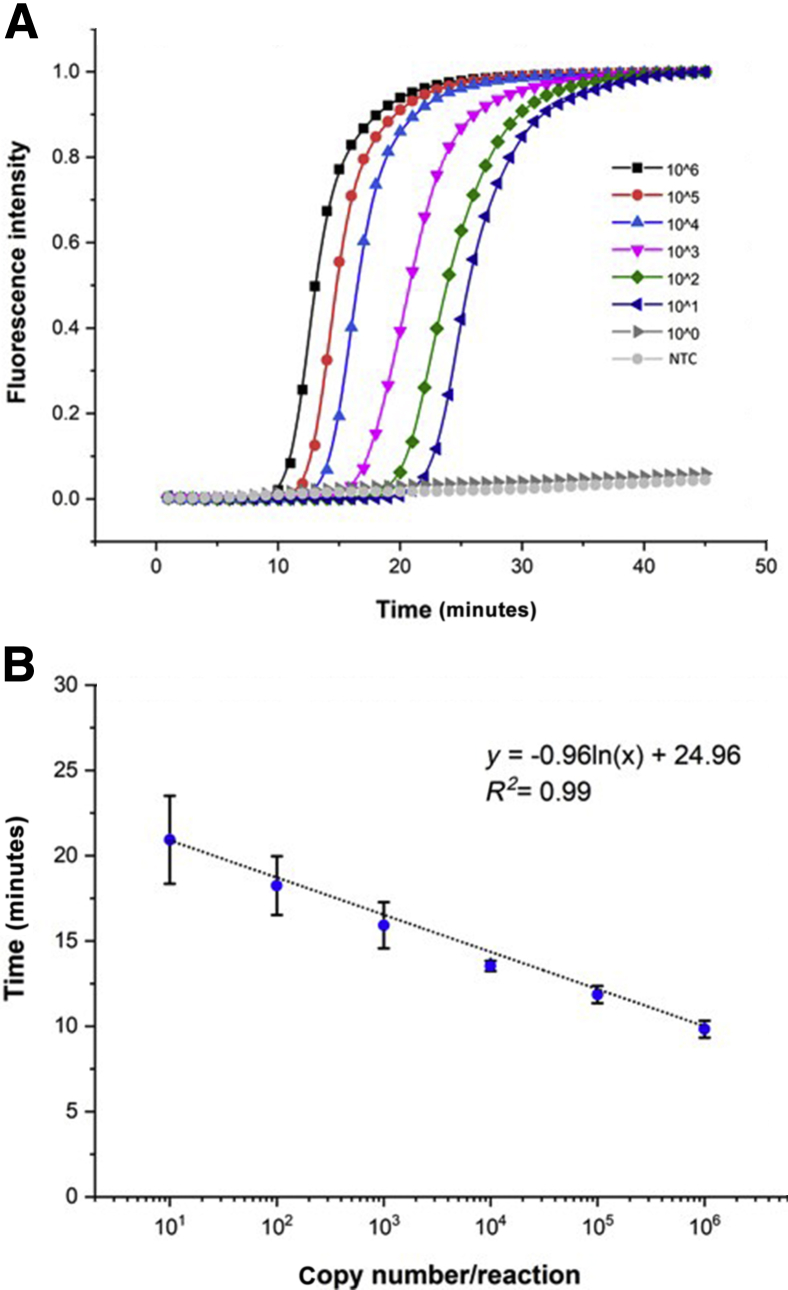
The TR34-LAMP assay showed a lower limit of detection of 10 copies/reaction. The mean times to positivity at the different DNA concentrations (ranging from 106 to 101) were 9.83 ± 0.50, 11.86 ± 0.51, 13.54 ± 0.30, 15.92 ± 1.35, 18.24 ± 1.72, and 20.94 ± 2.58 minutes, respectively (Figure 4). To evaluate the performance of the TR34-LAMP assay in the quantification of target DNA, serial dilutions of synthetic TR34 double-stranded DNA were analyzed. Time to positivity versus the logarithm of the serial dilution showed a linear regression, with a correlation coefficient of 0.99 (Figure 4).
Figure 4.
Real-time 34-bp tandem repeat loop-mediated isothermal amplification (TR34-LAMP) amplification plot and standard curve. A: Detection of different concentrations of synthetic TR34 DNA. As low as 10 copies/reaction were detected within 22 minutes. B: Standard curve for TR34-LAMP assay generated from the amplification plot between serial 10-fold dilutions of TR34 synthetic DNA. Nine replicates per concentration were used. Nontemplate controls (NTC) were also included, which remained negative for 30 minutes. Data are expressed as means ± SD.
Detection and Quantification of DNA from Clinical Sample by TR34-LAMP Assay
The applicability of the TR34-LAMP assay in detecting TR34 mutant allele in clinical samples was validated by real-time and end-point amplifications. Data obtained with the real-time instrument showed positive reactions in only TR34 samples. This result suggests that there was no cross reaction with TR46 and wild-type strains, confirming the high specificity of the TR34-LAMP assay. The real-time assay yielded results within 17 minutes in the detection of the four clinical samples containing the TR34 mutant allele. The detection times were 16.45 ± 0.31 minutes for CL-Asp164, 15.85 ± 0.17 minutes for CL-Asp168, 16.03 ± 0.13 minutes for CL-Asp251, and 15.92 ± 0.20 minutes for E076 (Table 2). Obtained times to positive results were used to estimate the concentration of each clinical sample by using the regression formula from Figure 4: [y = −0.96ln(x) + 24.96].
Table 2.
Validation of the TR34-LAMP Assay with Clinical Isolates
| Type | Clinical sample |
A. fumigatus detection |
TR34 mutation detection |
|||
|---|---|---|---|---|---|---|
| LAMP A. fumigatus∗ |
TTP, means ± SD, minutes | TR34-LAMP† | TTP, means ± SD, minutes | Estimated concentration‡ | ||
| TR34 | CL-Asp164 | + | 23.19 ± 0.25 | + | 16.45 ± 0.31 | 7.38 × 103 |
| CL-Asp168 | + | 23.29 ± 0.50 | + | 15.85 ± 0.17 | 1.38 × 104 | |
| CL-Asp251 | + | 24.6 ± 1.14 | + | 16.03 ± 0.13 | 1.14 × 104 | |
| E076 | + | 22.23 ± 0.28 | + | 15.92 ± 0.20 | 1.28 × 104 | |
| TR46 | E224 | + | 22.82 ± 0.27 | − | − | − |
| E357 | + | 21.32 ± 0.29 | − | − | − | |
| E276 | + | 22.55 ± 0.23 | − | − | − | |
| WT | E409 | + | 25.82 ± 0.42 | − | − | − |
| ARAF016 | + | 22.72 ± 0.21 | − | − | − | |
| AF293 | + | 24.98 ± 0.81 | − | − | − | |
| CL-ASP237 | + | 22.39 ± 0.14 | − | − | − | |
SDs were calculated from replicates.
+, positive reaction; −, negative reaction; A. fumigatus, Aspergillus fumigatus; LAMP, loop-mediated isothermal amplification; TTP, time to positivity (detection within 30-minute timeframe); WT, wild type.
LAMP A. fumigatus, result of LAMP assay by using published LAMP primers43 for A. fumigatus detection.
TR34-LAMP, result of TR34-LAMP assay by using clinical samples.
Estimation of initial concentration (Copy number/Reaction) by linear regression formula [y = −0.96ln(x) + 24.96].
The obtained values were 7.38 × 103, 1.38 × 104, 1.14 × 104, 1.28 × 104 copies/reaction for CL-Asp164, -168, -251, and E076, respectively (Table 2).
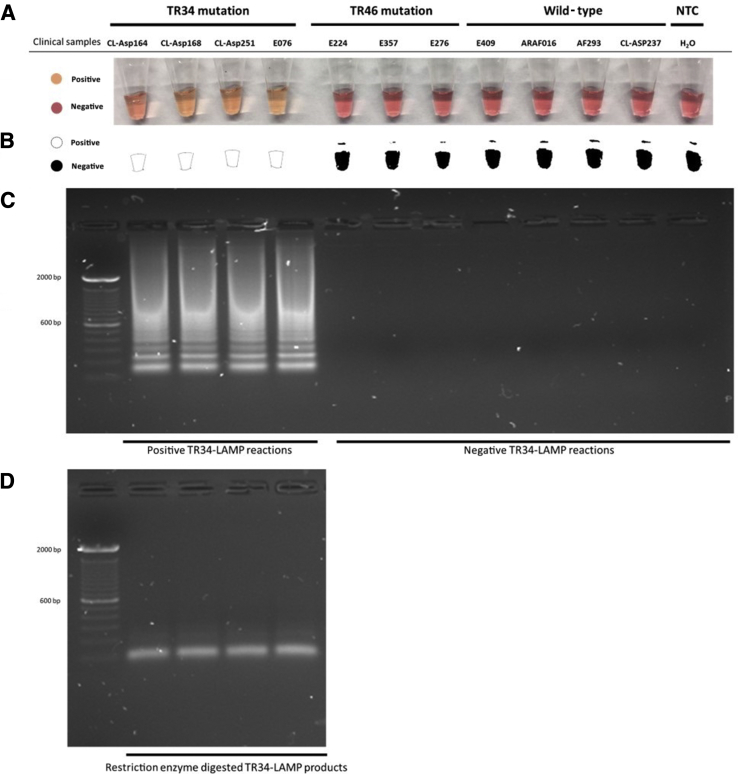
Colorimetric end-point experiments showed that the TR34-LAMP assay could successfully be visualized by the naked eye and/or using a cell phone camera after a 20-minute reaction. Positive reactions, TR34 clinical samples, were observed as pale orange, whereas negative reactions, TR46 and wild-type samples, were observed as pink (Figure 5). Analysis via gel electrophoresis confirmed that the presence of TR34-LAMP products in the tubes had caused the color to change from pink to pale orange, indicating that the TR34 primer set is specific for the TR34 mutation (Figure 5).
Figure 5.
Specificity of the 34-bp tandem repeat loop-mediated isothermal amplification (TR34-LAMP) assay. A: Visualization of the TR34-LAMP products by colorimetric LAMP method. B: Binary results obtained by adjusting the threshold of the photo taken by an iPhone 6 camera. C: Assessment is based on gel electrophoresis analysis of TR34-LAMP products. D: Restriction enzyme digestion of positive TR34-LAMP products. NTC, nontemplate controls.
Specificity of the TR34-LAMP Assay
The specificity of the amplification was confirmed by restriction digestion of the amplified LAMP products. As expected, after MluCI restriction enzyme digestion, two bands were observed (103 and 206 bp) (Figure 5). These results indicate that the LAMP products were specifically amplified from the TR34 region within A. fumigatus.
Discussion
IA is a serious threat in immunocompromised individuals, and many studies have shown that azole resistance in A. fumigatus isolates is primarily due to the presence of the TR34/L98H polymorphism, first reported in 2007 in isolates from Spain and the Netherlands46 and now detected worldwide.3 Azole-resistance genotypes are increasingly found to occur in environmental isolates of the fungus. For example, van der Linden et al47 found that 26 of 140 environmental resistant isolates contained the TR34/L98H mutation, whereas 14 strains possessed a novel polymorphism that causes multiazole resistance, TR46/Y121F/T289A. Recently, an analysis of 455 environmental isolates in Germany found that 45 had the TR34/L98H mutation and six had a TR46/Y121F/T289A mutation.48 The increasing worldwide prevalence of azole resistance and its rapid evolutionary diversification raise the need for antifungal stewardship alongside the development of novel chemotherapeutics to treat IA infections.
On-site diagnosis of the TR34 resistance allele is important, since it will allow the clinician to make more rapid decisions as to how to manage these patients' infections. The current diagnosis of IA includes direct examination of respiratory specimens, culture of the organism, histopathologic examination, galactomannan antigen detection, β-d-glucan assay, and molecular-based detection (eg, PCR) techniques.21, 22, 23, 24, 25, 26, 27, 28, 49, 50 However, the sensitivity of current diagnostic methods for IA is generally low (except for PCR) and may even return a false-negative result in some individuals at high risk.51 Therefore, to obtain an accurate clinical diagnosis, most studies suggest that the best approach is to combine different assays.52, 53 Apart from their low sensitivity and specificity, other disadvantages of these methods are that they are time consuming and rely on laboratory-based instruments. To overcome these limitations, a recent study developed a potential point-of-care device, a lateral-flow device that detected the mannoprotein produced by growing Aspergillus spp. and was able to diagnose an IA-infected patient within 15 minutes.18, 19 However, subsequent studies indicate that lateral-flow devices have only moderate sensitivity in IA detection and that they should be used only in combination with other diagnostic procedures.53 Furthermore, they do not currently allow the detection of azole-resistance alleles. Consequently, these methods, including those considered to be the gold standards, are far from adequate with respect to addressing current diagnostic needs.
Compared to the current methods, the LAMP assay has the advantage of a short amplification time with high sensitivity and specificity.29, 30 Moreover, PCR and other molecular methods can be conducted only in well-equipped laboratories and thus are impracticable in resource-limited settings, whereas LAMP does not involve thermal cycling steps and is able to amplify target DNAs in a short period of time, to detectable levels. Another advantage of the LAMP assay is that the results can qualitatively be detected by the naked eye,31, 32, 33, 34, 35, 36, 37 which makes it a suitable technique in a resource-limited setting. All of these advantages of the LAMP assay potentially provide time-saving and cost-effectiveness in comparison to conventional, laboratory- and fluorescence-based methods.
In this study, a rapid and highly specific LAMP reaction for TR34 detection was established. This TR34-LAMP assay shows exceptionally high sensitivity (10 copies/reaction) within 30 minutes of reaction time. The high sensitivity and rapid detection of the assay are achieved by applying four primers that target six regions of a DNA template, and one loop primer to accelerate the reaction. Full complementarity between the DNA template and primer sequence is generally considered essential for a specific amplification; therefore, introducing primer–template mismatches at the 3′ end of a primer has the potential to increase the amplification discrimination significantly between similar targets. In this study, the challenge in designing the primers was to place them in a long direct tandem repeat section that is flanked by highly conserved regions. To increase the amplification discrimination, primer F2 was constructed based on the sequence of the junction between the tandem repeat mutation and the conserved region, and a primer–template G–A mismatch was introduced at 3′ end of F2. The results showed that by replacing this single mismatch at the 3′ end of F2, only the TR34 strain was amplified, instead of showing a delay in time to positivity between TR34 and TR46 amplifications. Several PCR-based studies on the effects of 3′ primer mismatches on PCR detection of single-nucleotide polymorphisms have been published54, 55; however, this report is the first to examine the effects of primer–template mismatch in a tandem repeat LAMP assay. The procedure presented in this study, TR-LAMP, can be applied to the detection of other tandem repeat mutations, such as TR46 and TR53 cyp51A mutations. A further potential use of TR-LAMP is for on-site forensic detection, which usually detects hypervariable tandem repeats, minisatellite (about 30 bp per repeat unit) variations, among individuals. The novel methodology described here, combined with a lab-on-chip platform, has the potential to be developed into a fingerprint detection device in digital forensics.
In conclusion, the one-step TR34-LAMP assay developed in this study is useful for rapid detection of the TR34 mutation in the cyp51A gene of A. fumigatus. Due to its high reliability, fast identification, and ease of use, this method has the potential to determine the prevalence of the TR34 mutation in both the environmental and clinical settings. Future development will be focused on applying the TR-LAMP detection system to a lab-on-chip platform, which will allow all of the analytical steps to be conducted in a rapid, effective, and automated manner.
Acknowledgments
L.-S.Y. and J.R.-M. conceived the original idea of TR-LAMP design and planned and performed experiments; L.-S.Y. wrote the manuscript; K.M.-C. performed experiments and analyzed data; T.S., O.B., and D.A.-J. provided as well as prepared the clinical samples; M.C.F. and P.G. supervised the project; all authors wrote the manuscript.
Footnotes
Supported by Ministry of Science and Technology of Taiwan grant 105-2911-I-037-504 (L.-S.Y.), a Natural Environment Research Council (NERC) grant (M.C.F. and T.S.), and Engineering and Physical Sciences Research Council Centre for Doctoral Training in High Performance Embedded and Distributed Systems studentship grant EP/L016796/1 (K.M.C.).
L.-S.Y. and J.R.-M. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.jmoldx.2018.10.004.
Contributor Information
Ling-Shan Yu, Email: ling-shan.yu10@imperial.ac.uk.
Jesus Rodriguez-Manzano, Email: j.rodriguez-manzano@imperial.ac.uk.
Supplemental Data
References
- 1.Mullins J., Harvey R., Seaton A. Sources and incidence of airborne Aspergillus fumigatus (Fres) Clin Exp Allergy. 1976;6:209–217. doi: 10.1111/j.1365-2222.1976.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais T.R., Keller N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meis J.F., Chowdhary A., Rhodes J.L., Fisher M.C., Verweij P.E. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond B Biol Sci. 2016;371 doi: 10.1098/rstb.2015.0460. 20150460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ECDC . European Centre for Disease Prevention and Control; Stockholm, Sweden: 2013. Risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species. [Google Scholar]
- 5.Price C.L., Parker J.E., Warrilow A.G., Kelly D.E., Kelly S.L. Azole fungicides--understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci. 2015;71:1054–1058. doi: 10.1002/ps.4029. [DOI] [PubMed] [Google Scholar]
- 6.Cools H.J., Fraaije B.A. Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag Sci. 2013;69:150–155. doi: 10.1002/ps.3348. [DOI] [PubMed] [Google Scholar]
- 7.Snelders E., Melchers W.J., Verweij P.E. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol. 2011;6:335–347. doi: 10.2217/fmb.11.4. [DOI] [PubMed] [Google Scholar]
- 8.Hagiwara D., Watanabe A., Kamei K., Goldman G.H. Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front Microbiol. 2016;7:1382. doi: 10.3389/fmicb.2016.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lelièvre L., Groh M., Angebault C., Maherault A.C., Didier E., Bougnoux M.E. Azole resistant Aspergillus fumigatus: an emerging problem. Med Mal Infect. 2013;43:139–145. doi: 10.1016/j.medmal.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Snelders E., Huis in 't Veld R.A., Rijs A.J., Kema G.H., Melchers W.J., Verweij P.E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol. 2009;75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snelders E., van Der Lee H.A., Kuijpers J., Rijs A.J., Varga J., Samson R.A., Mellado E., Donders A.R., Melchers W.J., Verweij P.E. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5:1629–1637. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivero-Menendez O., Alastruey-Izquierdo A., Mellado E., Cuenca-Estrella M. Triazole resistance in Aspergillus spp.: a worldwide problem? J Fungi (Basel) 2016;2 doi: 10.3390/jof2030021. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verweij P.E., Chowdhary A., Melchers W.J., Meis J.F. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdolrasouli A., Scourfield A., Rhodes J., Shah A., Elborn S., Fisher M.C., Schelenz S., Armstrong-James D. High prevalence of triazole resistance in clinical Aspergillus fumigatus isolates in a specialist cardio-thoracic centre. Int J Antimicrob Agents. 2018;52:637–642. doi: 10.1016/j.ijantimicag.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhary A., Kathuria S., Randhawa H.S., Gaur S.N., Klaassen C.H., Meis J.F. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J Antimicrob Chemother. 2012;67:362–366. doi: 10.1093/jac/dkr443. [DOI] [PubMed] [Google Scholar]
- 16.De Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T., Pappas P.G., Maertens J., Lortholary O., Kauffman C.A., Denning D.W., Patterson T.F., Maschmeyer G., Bille J., Dismukes W.E., Herbrecht R., Hope W.W., Kibbler C.C., Kullberg B.J., Marr K.A., Muñoz P., Odds F.C., Perfect J.R., Restrepo A., Ruhnke M., Segal B.H., Sobel J.D., Sorrell T.C., Viscoli C., Wingard J.R., Zaoutis T., Bennett J.E. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuenca-Estrella M., Bassetti M., Lass-Flörl C., Ráčil Z., Richardson M., Rogers T.R. Detection and investigation of invasive mould disease. J Antimicrob Chemother. 2011;66(Suppl):i15–i24. doi: 10.1093/jac/dkq438. [DOI] [PubMed] [Google Scholar]
- 18.Miceli M.H., Maertens J. Role of non-culture-based tests, with an emphasis on galactomannan testing for the diagnosis of invasive aspergillosis. Semin Respir Crit Care Med. 2015;36:650–661. doi: 10.1055/s-0035-1562892. [DOI] [PubMed] [Google Scholar]
- 19.White P.L., Parr C., Thornton C., Barnes R.A. Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol. 2013;51:1510–1516. doi: 10.1128/JCM.03189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong G.L., van De Sande W.W., Dingemans G.J., Gaajetaan G.R., Vonk A.G., Hayette M.P., van Tegelen D.W., Simons G.F., Rijnders B.J. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol. 2015;53:868–874. doi: 10.1128/JCM.03216-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa C., Costa J.M., Desterke C., Botterel F., Cordonnier C., Bretagne S. Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis. J Clin Microbiol. 2002;40:2224–2227. doi: 10.1128/JCM.40.6.2224-2227.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnelly J.P. Polymerase chain reaction for diagnosing invasive aspergillosis: getting closer but still a ways to go. Clin Infect Dis. 2006;42:487–489. doi: 10.1086/499818. [DOI] [PubMed] [Google Scholar]
- 23.Kawazu M., Kanda Y., Nannya Y., Aoki K., Kurokawa M., Chiba S., Motokura T., Hirai H., Ogawa S. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1->3)-β-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J Clin Microbiol. 2004;42:2733–2741. doi: 10.1128/JCM.42.6.2733-2741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeffler J., Kloepfer K., Hebart H., Najvar L., Graybill J.R., Kirkpatrick W.R., Patterson T.F., Dietz K., Bialek R., Einsele H. Polymerase chain reaction detection of aspergillus DNA in experimental models of invasive aspergillosis. J Infect Dis. 2002;185:1203–1206. doi: 10.1086/339824. [DOI] [PubMed] [Google Scholar]
- 25.Marr K.A., Leisenring W. Design issues in studies evaluating diagnostic tests for aspergillosis. Clin Infect Dis. 2005;41(Suppl):S381–S386. doi: 10.1086/430920. [DOI] [PubMed] [Google Scholar]
- 26.Pickering J.W., Sant H.W., Bowles C.A., Roberts W.L., Woods G.L. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raad I., Hanna H., Sumoza D., Albitar M. Polymerase chain reaction on blood for the diagnosis of invasive pulmonary aspergillosis in cancer patients. Cancer. 2002;94:1032–1036. [PubMed] [Google Scholar]
- 28.White P.L., Wingard J.R., Bretagne S., Löffler J., Patterson T.F., Slavin M.A., Barnes R.A., Pappas P.G., Donnelly J.P. Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin Infect Dis. 2015;61:1293–1303. doi: 10.1093/cid/civ507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagamine K., Hase T., Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16:223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- 31.Goto M., Honda E., Ogura A., Nomoto A., Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 32.Mori Y., Hirano T., Notomi T. Sequence specific visual detection of LAMP reactions by addition of cationic polymers. BMC Biotechnol. 2006;6:3. doi: 10.1186/1472-6750-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori Y., Nagamine K., Tomita N., Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289:150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Manzano J., Karymov M.A., Begolo S., Selck D.A., Zhukov D.V., Jue E., Ismagilov R.F. Reading out single-molecule digital RNA and DNA isothermal amplification in nanoliter volumes with unmodified camera phones. ACS Nano. 2016;10:3102–3113. doi: 10.1021/acsnano.5b07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomita N., Mori Y., Kanda H., Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Li R., Quan S., Shen P., Zhang D., Shi J., Yang L. GMO detection in food and feed through screening by visual loop-mediated isothermal amplification assays. Anal Bioanal Chem. 2015;407:4829–4834. doi: 10.1007/s00216-015-8652-z. [DOI] [PubMed] [Google Scholar]
- 37.Zhang F., Wang R., Wang L., Wu J., Ying Y. Tracing phosphate ions generated during DNA amplification and its simple use for visual detection of isothermal amplified products. Chem Commun. 2014;50:14382–14385. doi: 10.1039/c4cc06973k. [DOI] [PubMed] [Google Scholar]
- 38.Moser N., Rodriguez-Manzano J., Lande T.S., Georgiou P. A scalable ISFET sensing and memory array with sensor auto-calibration for on-chip real-time DNA detection. IEEE Trans Biomed Circuits Syst. 2018;12:390–401. doi: 10.1109/TBCAS.2017.2789161. [DOI] [PubMed] [Google Scholar]
- 39.Moser N., Rodriguez-Manzano J., Yu L.S., Kalofonou M., De Mateo S., Li X., Lande T.S., Toumazou C., Georgiou P. 2017. Live demonstration: A CMOS-based ISFET array for rapid diagnosis of the Zika virus.10.1109/ISCAS.2017.8050721 IEEE International Symposium on Circuits and Systems (ISCAS) doi: [Google Scholar]
- 40.Miscourides N., Yu L.S., Rodriguez-Manzano J., Georgiou P. A 12.8k current-mode velocity-saturation ISFET array for on-chip real-time DNA detection. IEEE Trans Biomed Circuits Syst. 2018;12:1202–1214. doi: 10.1109/TBCAS.2018.2851448. [DOI] [PubMed] [Google Scholar]
- 41.Edgar R.C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zadeh J.N., Steenberg C.D., Bois J.S., Wolfe B.R., Pierce M.B., Khan A.R., Dirks R.M., Pierce N.A. NUPACK: analysis and design of nucleic acid systems. J Comput Chem. 2011;32:170–173. doi: 10.1002/jcc.21596. [DOI] [PubMed] [Google Scholar]
- 43.Tang Q., Tian S., Yu N., Zhang X., Ji X., Zhai H., Sun Q., Han L. Development and evaluation of a loop-mediated isothermal amplification method for rapid detection of Aspergillus fumigatus. J Clin Microbiol. 2016;54:950–955. doi: 10.1128/JCM.01751-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bader O., Weig M., Reichard U., Lugert R., Kuhns M., Christner M., Held J., Peter S., Schumacher U., Buchheidt D., Tintelnot K., Groß U., MykoLabNet-D Partners Cyp51A-based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob Agents Chemother. 2013;57:3513–3517. doi: 10.1128/AAC.00167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellado E., Garcia-Effron G., Alcázar-Fuoli L., Melchers W.J.G., Verweij P.E., Cuenca-Estrella M., Rodríguez-Tudela J.L. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51:1897–1904. doi: 10.1128/AAC.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Linden J.W., Snelders E., Kampinga G.A., Rijnders B.J., Mattsson E., Debets-Ossenkopp Y.J., Kuijper E.J., Van Tiel F.H., Melchers W.J., Verweij P.E. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg Infect Dis. 2011;17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bader O., Tünnermann J., Dudakova A., Tangwattanachuleeporn M., Weig M., Groß U. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob Agents Chemother. 2015;59:4356–4359. doi: 10.1128/AAC.00100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koo S., Bryar J.M., Baden L.R., Marty F.M. Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J Clin Microbiol. 2010;48:1255–1260. doi: 10.1128/JCM.02281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koo S., Bryar J.M., Page J.H., Baden L.R., Marty F.M. Diagnostic performance of the (1->3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis. 2009;49:1650–1659. doi: 10.1086/647942. [DOI] [PubMed] [Google Scholar]
- 51.Adam O., Aupérin A., Wilquin F., Bourhis J.H., Gachot B., Chachaty E. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin Infect Dis. 2004;38:917–920. doi: 10.1086/383148. [DOI] [PubMed] [Google Scholar]
- 52.Aguado J.M., Vazquez L., Fernandez-Ruiz M., Villaescusa T., Ruiz-Camps I., Barba P., Silva J.Y., Batlle M., Solano C., Gallardo D., Heras I., Polo M., Varela R., Vallejo C., Olave T., Lopez-Jimenez J., Rovira M., Parody R., Cuenca-Estrella M., PCRAGA Study Group. Spanish Stem Cell Transplantation Group. Study Group of Medical Mycology of the Spanish Society of Clinical Microbiology and Infectious Diseases. Spanish Network for Research in Infectious Diseases Serum galactomannan versus a combination of galactomannan and polymerase chain reaction-based aspergillus DNA detection for early therapy of invasive aspergillosis in high-risk hematological patients: a randomized controlled trial. Clin Infect Dis. 2015;60:405–414. doi: 10.1093/cid/ciu833. [DOI] [PubMed] [Google Scholar]
- 53.Hoenigl M., Prattes J., Spiess B., Wagner J., Prueller F., Raggam R.B., Posch V., Duettmann W., Hoenigl K., Wölfler A., Koidl C., Buzina W., Reinwald M., Thornton C.R., Krause R., Buchheidt D. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol. 2014;52:2039–2045. doi: 10.1128/JCM.00467-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J., Huang S., Sun M., Liu S., Liu Y., Wang W., Zhang X., Wang H., Hua W. An improved allele-specific PCR primer design method for SNP marker analysis and its application. Plant Methods. 2012;8:34. doi: 10.1186/1746-4811-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyle B., Dallaire N., MacKay J. Evaluation of the impact of single nucleotide polymorphisms and primer mismatches on quantitative PCR. BMC Biotechnol. 2009;9:75. doi: 10.1186/1472-6750-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.