Abstract
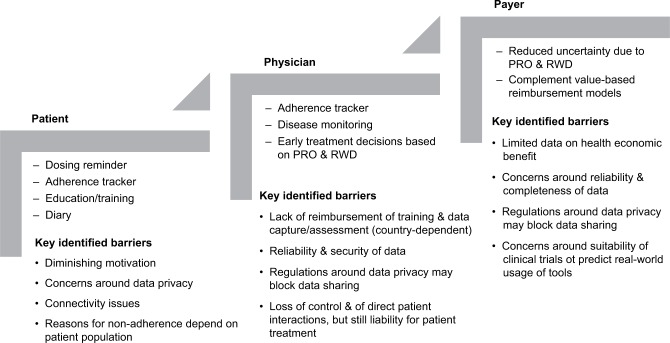
Connected drug delivery devices are increasingly being developed to support patient supervision and counseling in home setting. Features may include dosing reminders, adherence trackers, tools for patient education, and patient diaries to collect patient-reported outcomes, as well as monitoring tools with interfaces between patients and health care professionals (HCPs). Five connected devices have been selected as the basis for a review of the clinical evidence concerning the impact of electronic tools on treatment adherence and efficacy outcomes. Disease areas covered include multiple sclerosis, diabetes, hypertension, liver and renal transplant recipients, tuberculosis, hepatitis C, clinically isolated syndrome, asthma, and COPD. From studies comparing the use of electronic feedback tools to standard of care, there is an initial evidence for a higher adherence to treatment and better outcomes among patients who use the electronic tools. To substantiate the assumption that connected devices can improve adherence in an outpatient setting over a prolonged period of time, further data from controlled randomized studies are required. Key barriers to the broader adoption of connected devices include data privacy laws that may prevent data sharing with HCPs in some countries, as well as the need to demonstrate that the tools are consistently used and generate a high-quality and reproducible database. If these challenges can be addressed in a way that is agreeable to all stakeholders, it is expected that the future value of connected devices will be to 1) facilitate and improve patient involvement in disease management in a flexible care setting, 2) enable early treatment decisions, and 3) complement value-based reimbursement models.
Keywords: home-administration, self-administration, connected drug delivery device, adherence, patient-reported outcomes, real-world evidence
Introduction
Globally, health care systems are facing challenges in managing the rapidly increasing cost of medical management, while also seeking to improve patient treatment outcomes and access to care. In this context, the so-called value-based health care agreements have been introduced in some countries. Value-based care refers to a model whereby health outcomes are measured against the cost of delivering the treatment or health care service;1 this differs from a fee-for-service approach, in which providers are paid according to the amount of health care services delivered.
One way to reduce health care costs and resources might be to shift patient care from hospital to home setting.2 While the availability of oral and subcutaneous (SC) dosing regimens for small molecules and biotherapeutics makes home-administration a reality for many diseases,3 treatment adherence in a decentralized, uncontrolled setting can be negatively affected by infrequent direct interactions between patients and health care professionals (HCPs).
Thus, to ensure that patients comply with dosing regimens and to enable an ongoing dialog with HCPs, a variety of connected devices and corresponding health applications (apps) have been developed. These tools incorporate dosing reminders and educational features that help patients understand the rationale for complying with a treatment regimen. Adherence trackers capture whether patients are accurately taking the correct doses at appropriate intervals, while other apps allow patient-reported outcomes (PROs) to be recorded to enable monitoring of treatment effects as well as safety and tolerability. Information may also be accessible for payers and providers.4
Today, the potential value of electronic tools in complementing conventional treatment is generally accepted. However, there remains limited quantitative evidence on the impact of connected devices and health apps in relation to adherence to treatment, health outcomes, and overall cost effectiveness. The aims of this review article were to 1) summarize the regulatory environment within the field of connected devices and describe the regulatory status of selected devices; 2) review the impact of the selected devices on adherence to treatment and health outcomes; and 3) identify barriers that need to be overcome to unfold the full potential of these tools. The relevance of such tools in facilitating drug administration in a decentralized setting, to complement value-based health care and to facilitate early treatment decisions, is discussed. Connected devices and health apps that complement drug treatments are in-scope. The so-called “stand-alone” health apps that are not used in combination with a drug are beyond the scope of this review.
Materials and methods
The connected drug delivery devices have been selected to cover a range of different indications, administration routes, and presentations (ie, connected tools available for different molecules or in combination with a specific molecule only).
Connected health solutions that are in-scope include those that
are used as part of a drug delivery device (ie, for SC, oral, or inhaled administration) and collect device usage information with accompanying software that analyzes and reports information (ie, hardware and software). Devices can be equipped with electronic dosing reminders and/or adherence trackers
are approved by the Food and Drug Administration (FDA) or European Medicines Association (EMA) and already on the market to support administration of specific drug treatments with a number of supporting clinical trials available in the literature.
Connected health solutions that are out of scope include those that
qualify as connected drug delivery device as per above, but are not yet approved, marketed, and/or are currently in development with limited evidence available from clinical trials
have a diagnostic purpose, such as glucose monitoring tools
are not used as part of a drug delivery device, such as mobile apps and other software for patients and/or HCPs that enable monitoring and tracking, provide coaching or recommendations, or enable connectivity between HCPs and patients (ie, software only or software and hardware not involved in drug delivery).
On this basis, the connected devices selected were RebiSmart® (Merck KGaA, Darmstadt, Germany), BETACONNECT™ (Bayer AG, Leverkusen, Germany), Proteus Discover (Proteus Digital Health, Inc, Redwood City, CA, USA), SmartInhaler™ (Adherium North America, Inc, San Mateo, CA, USA; now known as Hailie™ but branded as SmartInhaler™ at the time the included studies were completed),5 and Propeller (Propeller Health, Madison, WI, USA). These technologies are either marketed in combination with one molecule (RebiSmart® with interferon β-1a, BETACONNECT™ with interferon β-1b), or marketed or developed for use with different molecules (Proteus, SmartInhaler™, and Propeller). The principal characteristics of these selected devices are summarized in Table 1. Tools that may allow an ongoing dialog between patients and HCPs, patient monitoring, or the collection of PRO data are available with some of the devices, but are not consistently applied in the different clinical studies discussed.
Table 1.
Characteristics of the selected smart devices and health apps (taken from respective product webpages)
| Device | Company | Target population/indication | Description/purpose of the device | Mechanism of action | Additional features | Associated apps | Associated software |
|---|---|---|---|---|---|---|---|
| RebiSmart® [www.RebiSmart.com] | Merck KGaA | MS | Individually adjustable electronic injection device to administer a pre-set dose of Rebif (interferon β-1a) to reduce the frequency of relapses in people with relapsing forms of MS | Administers Rebif subcutaneously, collects and stores data, and sends the information wirelessly to the MSdialog server | Allows patients to self-inject; records an accurate and objective dosing history, including the date, time, and dosage of every injection; hidden needle, multidose cartridge and adjustable comfort settings; sends injection reminders | MS Dialog App | MSdialog |
| BETACONNECT™ [www.betaseron.com] | Bayer AG | MS | Autoinjector that delivers Betaseron (interferon β-1b) to reduce the frequency of relapses in people with relapsing forms of MS | Delivers Betaseron, and pairs with myBETAapp via Bluetooth® to deliver real-time data (such as injection date, time, speed, and depth) with the BETA nurse and HCPs | Sends injection reminders; adjustable injection depth and speed; hidden needle; smooth, quiet injection; automatic needle retraction; visual and audible end-of-dose indicator; simple to unload | myBETAapp | BETACONNECT™ navigator |
| Proteus Discover [www.proteus.com] | Proteus Digital Health, Inc | Multiple indications | An ingestible pill that provides objective confirmation of adherence to oral medications. Approved with aripiprazole in schizophrenia, bipolar disorders, and depressiona | Proteus Discover is activated by taking medication with an ingestible sensor. The sensor transmits a signal from the stomach to a patch worn on the torso. A digital record is sent to the patient’s mobile device and then to the Proteus cloud where it can be accessed by HCPs | Battery-powered adhesive sensor; the patch on the torso also records patient’s heart rate, temperature, activity, and rest patterns | Discover | Discover Portal |
| SmartInhaler™ [www.smartinhaler. | Adherium North America, Inc | Asthma/COPD | Medication sensors that clip around a standard inhaler and automatically send usage data to a mobile app or PC via Bluetooth® | Contains sensors that attach to existing inhalers and record when medication is taken. These sensors automatically send usage data to a mobile app or PC via Bluetooth® | Tracks medication use; sends medication reminders | SmartInhaler™ app | SmartInhalerlive™ |
| Propeller [www.propellerhealth. | Propeller Health | Asthma/COPD | Attaches to the top of the canister of a standard inhaler. Records the location, date, and time of inhaler actuations | Records use of rescue inhaler to provide a summary of potential triggers. Connects with health care providers to share data | Reminders to take medication provided; provides useful geospatial information of asthma attacks to patients; provides personalized daily asthma forecasts | Propeller mobile app | Propeller web app |
Note:
Indicated in adults for the treatment of schizophrenia, bipolar 1 disorder, and as adjunctive treatment for major depressive disorder.
Abbreviations: HCP, health care professional; MS, multiple sclerosis; PC, personal computer.
Targeted searches were run to characterize the devices and to address the research questions for each device. Key research questions are summarized in Table S1. The searches were conducted using PubMed, Google, Google Scholar, company websites, regulatory databases, health technology assessment (HTA) databases, and trial registries (Table S2). Specific search terms used for the different devices are listed in Table S3. Potentially relevant references for each device were initially identified using title/abstract screening, and full texts were retrieved. Full texts were then screened, and all publications relevant to the research questions were included in this review.
Results
Regulatory guidance for connected devices and health apps (US and EU)
US medical device definitions and classifications As an integral part of drug treatment and patient monitoring, connected devices and related health apps can meet the definition of a medical device. To market a medical device in the United States (US), manufacturers require the submission of a Premarket Notification 510(k) prior to commercial distribution, and the FDA issues a “letter of substantial equivalence” confirming that the device is considered substantially equivalent to one legally in commercial distribution in the US.6 Similarly, in the European Union (EU), medical device commercialization requires a Conformité Européene (CE) mark that indicates compliance with specific standards of performance, quality, safety, and efficacy EU regulations. In case a medical device is associated with a specific treatment, additional regulatory requirements that also consider approval of the accompanying active substance need to be fulfilled.7
Devices are generally classified according to the risk they pose to consumers. In the US, connected devices and health apps that meet the definition of a medical device can be classified as either Class I, with the lowest risk (eg, smart devices and health apps helping users to self-manage their disease without providing specific clinical suggestions), or Class II and III, encompassing complex devices with a high risk (eg, health apps that can control another medical device or provide a patient-specific diagnosis or treatment). Most Class I devices and some Class II devices are exempt from Premarket Notification 510(k).6
In the EU, according to the 2017 Medical Device Directive 2017/745,7 health apps can be considered as non-medical devices or classified as Class I (low risk), IIa (low/medium risk), IIb (medium/high risk), or III (high risk). This classification takes into account 23 rules that consider the function, the patient’s risk, and the manufacturer’s intended use of the device.7 Under Directive 2017/745, software for diagnostic or therapeutic purposes is classified as Class IIa, and under certain conditions could even be included in Class IIb or III.
Regulatory status for selected devices
The regulatory status for the five selected devices in this section are based on publicly available information.
Approval status of selected connected devices (US)
In the US, premarket 510(k) approvals with regulatory Class II classification were obtained for devices not involving specific medications (Proteus Discover, SmartInhaler™, and Propeller).8 Furthermore, 510(k) approvals were obtained at multiple times; that is, whenever any substantial changes were made to the devices.8
For devices associated with specific treatments such as BETACONNECT™ with interferon β-1b and Proteus Discover with aripiprazole, the approval pathway involved different steps. For BETACONNECT™, supplemental Biologics License Application letters were submitted.9 A New Drug Application (NDA) was opened for Proteus Discover with aripiprazole.10 In an initial rejection letter, the FDA asked for data under real-world conditions, evaluation of use-related risks, and confirmation that customers could use the device safely and effectively.10 Proteus Discover approval was based on the NDA resubmission that contained data from “human factors validation studies.”10 The RebiSmart® is not filed in the US.
Approval status of selected smart devices (EU)
In the EU, all approved devices received a CE mark.11–14 For Proteus Discover, a submission was made to the EMA to issue a favorable opinion considering the use of Proteus technology as a “qualified method” for measuring adherence and associated relevant physiologic and behavioral parameters, such as indications of therapeutic response. The Committee for Medicinal Products for Human Use (CHMP) gave a favorable opinion for the same and noted that “if the device is intended to be marketed with a specific medicinal product, a relevant benefit/risk assessment will be carried out at time of marketing authorization application depending on the dossier.”15 BETACONNECT™ is not filed in the EU.
Impact of selected connected devices on adherence, clinical outcomes, and health care resource use
Adherence and clinical outcomes data were available for all selected connected devices. Data for the impact on health care costs and resource utilization were limited, with evidence available only for Propeller and the SmartInhaler™. The majority of clinical studies identified were uncontrolled trials (single-arm, prospective, or retrospective cohorts).
Key findings from the respective studies are presented in the following sections. For each device, randomized controlled trials (RCTs) and/or larger non-RCTs are described in detail, with statistical significance and smaller studies summarized in the respective tables. To assess the impact of patient age on adherence to treatment, the age of the study population as well as its impact on adherence rates is listed, if applicable. Where available, the focus is on trials that compare use of a connected device vs a non-connected device or standard of care.
RebiSmart®
RebiSmart® is an individually adjustable electronic injection device developed to facilitate the SC self-injection of interferon β-1a for the treatment of multiple sclerosis (MS) and includes injection reminders and a web-, tablet-, and smartphone-based software app to collect and store real-time adherence, clinician-reported outcomes, and PRO data.16,17
RebiSmart® clinical studies identified in the search are detailed in Table 2 and include MS patients treated three times weekly with SC interferon β-1a. None of the trials compared RebiSmart® against standard of care without electronic tools. As no RCTs have been conducted using RebiSmart®, data from the three largest uncontrolled studies are described below.
Table 2.
Study details and results from all identified studies of RebiSmart® (adherence, clinical outcomes, and health care resources use)a
| Study name | Study objective | Study design | Indication | Sample size | Follow-up period | Key results |
|---|---|---|---|---|---|---|
| Bayas et al (2015)18 | To evaluate adherence to and effectiveness of treatment in patients with RMS using RebiSmart for self-injection of SC interferon β-1a | Multicenter, prospective, observational | RMS | 912 | 12 months or until ED | • Age in the safety population (mean ± SD) was 63.3±10.3 years • Mean cumulative adherence was 97.1±7.3% (range 30%–100%; n=791) • Adherence rates (mean ± SD) were similar in treatment-naive patients (97.3±6.5%; range 42%–100%; n=453) and patients starting interferon β-1a within 6 weeks before study entry (96.7±8.8%; range 30%–100%; n=200) • Mean adherence per patient since the last visit at 1 year or ED was 96.5±9.4% (range 20%–100%; n=759) • At month 12/ED, 72.4% (560/774) of highly adherent patients and 41.2% (7/17) of patients with a lower adherence were relapse-free • A significantly higher proportion of relapse-free compliant high-dose patients (>120 µg/week) compared with lower dose patients in clean patient subset (92.5% vs 86.7%; P=0.0146) at month 6 with nonsignificant trend remaining at week 12/ED (82.1% vs 76.6%) • AAR 0.3±0.7 in highly adherent patients (n=774) and 0.6±1.3 in less adherent patients (n=16) |
| Devonshire et al (2016)21 | To evaluate 24-week treatment adherence of RMS patients using RebiSmart® for SC injection of interferon β-1a | Multicenter, single-arm, observational | RMS | 162 | 96 weeks (24-week data were available in this publication) | • Patient age (mean ± SD) was 37.4±9.8 years • The proportion of patients with ≥80% adherence was 91.8% (95% CI 86.3, 95.2) at week 12; 82.9% (95% CI 76.2, 88.0) at week 24 • Similar treatment adherence in patients with and without anxiety at baseline (96.1% vs 95.2%, P=0.322 at week 12; 89.0% vs 89.0%, P=0.901 at week 24) |
| Fernandez et al (2016)19 | To determine long-term adherence to SC interferon β-1a treatment administered with the RebiSmart® | Multicenter, retrospective, observational | RRMS | 258 | Until device replacement (36 months maximum lifetime) treatment discontinuation | • Patient age (mean ± SD) was 40.7±9.5 years • Overall adherence was 92.6% (95% CI 90.6, 94.5) • 32% of patients (n=78) achieved an adherence rate of 100%, and 80.6% of patients (n=208) achieved an adherence rate of ≥90%; 13.2% of patients (n=34) showed an adherence of <80% • Over the study period, a slight decrease in adherence with a mean overall adherence of 94.0% (95% CI 92.0, 96.0) at 0–3 months and 90.4% (95% CI 87.4, 93.3) at the time of device replacement • The incidence of relapses decreased from 5.8% (n=258 at 0–3 months) to 4.0% (n=150 at 33–36 months) • Having experienced relapses from the beginning of treatment was significantly related to achieving an adherence of ≥80% (OR =3.06, 1.28–7.31) • Suboptimal adherence (adherence of ≥80%) was about three-times higher in subjects with relapses compared with those without relapses |
| Ghezzi et al (2017)30 | To evaluate the changes in quality of life of adolescents with RRMS receiving treatment with interferon β-1a administered subcutaneously using RebiSmart® (FUTURE study) | Multicenter, single-arm, observational, prospective study | RRMS | 50 | 52 weeks | • Adolescents (age 12–16 years) who completed the study (n=40) showed 12-, 24-, and 52-week adherence rates of 82.5%, 80.0%, and 67.5%, respectively • At end of treatment, 32 (80%) of patients were relapse-free • Self-reported quality of life: ○ PedsQL4.0 self-reported Total Scale score and all subscale scores tended to increase (nonsignificantly) from baseline to end of treatment; except for Emotional Functioning score, which showed a nonsignificant decline ○ A mean raw change over time of +0.44 points was found for PedsQL4.0 Total Scale score, with the highest improvement (+1.41) identified for the School Functioning scale • Parent-reported QoL: ○ PedsQL4.0 Total Scale score increased significantly from baseline to end of treatment (+5.27 points, P=0.041) ○ Significant increases also emerged in the parent-reported Psychosocial Health summary score (+5.90 points; P=0.015) and the School Functioning scale score (+7.84 points; P=0.029) • There was no difference in either adolescent self-reported or parent-reported PedsQL4.0 scores in relation to age (12–15 vs 16–8 years) |
| Järvinen et al (2017)76 | To investigate adherence measured by an electronic autoinjector device vs self-reported adherence and treatment convenience in subjects with RRMS | Multicenter, observational | RRMS | 31 | 24 weeks | • Patient age (mean ± SD) was 39.0±8.0 years • Mean adherence was 93.5% (95% CI 88.4, 98.2; device data); 96.6% (95% CI 94.3, 99.0; self-assessment) • Age groups 30–40 and >40 years had a mean adherence of 62% and 64%, respectively; the age group <30 years had a slightly higher mean adherence of 75% • Device found to ease patient’s treatment and perceived to be easy to use (>65% of patients) |
| Lugaresi et al (2012)28 | To assess short-term adherence to, and tolerability of, interferon β-1a administered via electronic autoinjection device in patients with RRMS (BRIDGE study) | Multicenter, single-arm, observational | RRMS | 119 | 12 weeks or early termination | • Patient age (mean ± SD) was 37.9±9.7 years • 88.2% of patients administered ≥80% of scheduled injections over 12 weeks • Significant decrease in adherence (P=0.001) from 100% through week 4 to 99.1% in weeks 5–8, to 89.0% in weeks 9–12 • Mean HADS depression (P=0.303) or anxiety (P=0.156) scores did not differ significantly from baseline • Mean (± SD) baseline PASAT score was similar in adherent and nonadherent patients (42.67±10.9 vs 42.86±13.0) |
| Lugaresi et al (2016)29 | To investigate long-term adherence to interferon β-1a administered using RebiSmart® among patients in a real-life setting (RIVER study; extension of BRIDGE study) | Multicenter, retrospective | RRMS | 57 | 20.5 months | • Patient age (mean ± SD) was 38.0±9.0 years • Overall adherence to RebiSmart® was 79.8% (median, 85.2%, range, 16%–100%) • There was no statistically significant difference in age (mean ± SD) of adherent and nonadherent patients in the entire study cohort (37.6±10.0 vs 38.1±8.6 years; P=0.845) • EDSS scores at baseline and last follow-up did not differ significantly between adherent and nonadherent patients (date not shown in publication) |
| Moccia et al (2015)22 | To investigate predictors of adherence to interferon β-1a | Retrospective analysis of prospectively collected data | RRMS | 114 | 1.5 years | • Patient age (mean ± SD) was 35.8±10.4 years • Adherence was 95.0±9.0% • Early missing (14.9%) was more likely to be associated with clinical relapse (OR =4.155; P=0.018), but not late missing (47.4%) (OR =1.454; P=0.408) vs fully adherent (37.7%) • Adherence was lower in early missing compared with late missing or fully adherent (P<0.001) • In different regression models, categories of time to first missing were not associated with age (P=0.865) |
| Paolicelli et al (2016)20 | To collect data on treatment adherence in a real-life setting, in order to identify predictors of adherence at baseline (TRACER study) | Multicenter, retrospective | RRMS | 384 | 12 months | • Patient age (mean ± SD) was 36.0±9.2 years • 89.3% of patients were adherent • Adherence decreased from 93.2% in patients aged 26–40 years (93.2%) to 87.5% in patients aged ≥41 years to 79% in patients ≤25 years (P=0.006) • 90.5% of patients with a baseline EDSS <4 showed ≥80% adherence (vs 71.4% in patients with EDSS score ≥4; P=0.016) |
| Pedersen et al (2018)26 | To evaluate patient adherence to treatment with SC interferon β-1a using RebiSmart® and assess injection-site reactions and treatment satisfaction | Multicenter, prospective, single-arm, observational | RRMS | 60b | 12 weeks | • Patient age (mean ± SD) was 43.7±7.9 years • 89% (n=48) of patients had ≥90% adherence to treatment • 94% (n=51) of patients had ≥75% adherence (95% exact CI 85, 99); only three (6%) patients had adherence <75% and could be defined as nonadherent according to the study protocol • Most patients (78%) rated convenience as the most important aspect of the device |
| Rau et al (2017)27 | To investigate adherence pattern and cognitive– behavioral variables using electronic autoinjector RebiSmart® | Multicenter, single-arm, prospective study | RRMS | 188 | 24 months | • Patient age varied from 18 to 65 years • 12-month data • Of 129 patients with 1-year adherence data, quantitative and qualitative adherence were 96.3% and 88.9%, respectively • 82% reached qualitative adherence of ≥80% • FSMC motor score increased by 9.4% (P<0.002) and cognitive score increased by 10.0%, respectively (P<0.035) • 24-month data • Of 62 patients with 2-year adherence data, quantitative and qualitative adherence were 93.4% and 84.6%, respectively • 74% reached qualitative adherence of ≥80% • Cognitive fatigue increased by 8.4% (P<0.038) |
| Solsona et al (2017)23 | To describe adherence to interferon β-1a using RebiSmart® and to explore the relationship between adherence and relapses in a Spanish cohort | Retrospective, observational | MS | 110 | 979 days (mean duration of treatment) | • Patient age (mean ± SD) was 38.8±9.3 years • Mean adherence was 96.5% (IQR 91.1–99.1) • Mean adherence was 98.7% (IQR 91.3–100) during the first 6 months and 97.6% (IQR 91.1–99.8) during the last 6 months • No statistically significant differences in adherence were detected regarding age (Spearman’s rank correlation coefficient rs =0.183, P=0.055) • Increased adherence was associated with better clinical outcomes, leading to lower relapse risk (OR =0.953; 95% CI 0.912, 0.995); each percentage unit increase in adherence decreased the likelihood of relapse by 4.7% |
| Willis et al (2014)24 | To assess adherence to SC interferon β-1a injections using data from RebiSmart® | Single-group, observational, retrospective | RMS | 225 | 24 months | • Patient age (mean ± SD) was 44.1±8.82 years • Mean adherence over 24 months was 95.0% (95% CI 93.6, 96.4) • Proportion of patients with ≥80% adherence was 91.1% (95% CI 86.6, 94.5) at 24 months • No significant differences in percentage adherence between age categories were seen at 12 months (P=0.099) or 24 months (P=0.126) |
| Zecca et al (2017)25 | To evaluate the relationship between subjectively reported and objective adherence of MS patients using RebiSmart® in Switzerland and explore variables associated with objective adherence | Multicenter, survey-based, retrospective, prospective | RRMS | 56 | 9 months | • Patient median (IQR) age was 49.0 (38.0–55.0) years • Median objective adherence was significantly higher in self-reported adherent (100%; IQR 98.8%–100%) than in self-reported nonadherent patients (93.4%; IQR 77.2%– 97.5%) (P=0.00001) • No difference between retrospective (98.8%; IQR 93%–100%) and prospective (98.8%; IQR 88.5%–100%) objective adherence (P=0.75) • Older age was significantly associated with higher objective adherence (OR=1.064; 95% CI 1.016, 1.114; P=0.008) • Median (IQR) age as per adherence rates were: 41.0 (31.5–48.0) in patients with low adherence rates; 48.5 (36.5–54.8) in patients with medium adherence and 53.5 (42.0–63.0) in patients with high adherence rates |
Notes:
Key results listed focus on the impact of the device on adherence to treatment and, if available, a correlation between adherence and efficacy outcomes;
60 patients were recruited. However, adherence data were obtained in 54 patients only due to technical problems with six devices.
Abbreviations: ED, early discontinuation; EDSS, Expanded Disability Status Scale; FSMC, Fatigue Scale for Motor and Cognitive Functions; HADS, Hospital Anxiety and Depression Scale; IFN, interferon; IQR, interquartile range; OR, odds ratio; PASAT, Paced Auditory Serial Addition Task; PedsQL, Pediatric Quality of Life Inventory; QoL, quality of life; RMS, relapsing multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SC, subcutaneous.
In a multicenter observational study, 912 RMS patients self-administered SC interferon β-1a using the RebiSmart® autoinjector three times weekly for 12 months or until early discontinuation (ED).18 The primary endpoint of mean cumulative adherence for the safety population, defined as the proportion of expected injections completed as captured by the autoinjector, was 97.1%. Mean adherence rates did not appear to differ by time since starting interferon β-1a before study entry – rates ranged from 96.7% to 97.3% across the categories. Mean adherence per patient since the last visit at month 12/ED was 96.5%. To assess the impact of full treatment adherence on disease activity, a clean patient subset (all data available, no open queries) was separately analyzed. In this analysis (n=720), a significantly higher proportion of patients treated with high-dose interferon β-1a (≥120 µg/week) were relapse-free at month 6 compared with patients with lower doses (92.5% vs 86.7%, respectively), and a nonsignificant trend was observed at month 12/ED (82.1% vs 76.6%). At month 12/ED, 72.4% of highly adherent patients (defined as cumulative adherence >75%) and 41.2% of patients with a lower adherence were relapse-free. The mean annual relapse rate (ARR) was 0.3 in highly adherent patients and 0.6 in less adherent patients.
In a multicenter, retrospective, observational study, data from 258 relapsing-remitting multiple sclerosis (RRMS) patients who received interferon β-1a treatment using RebiSmart® for 36 months or until treatment discontinuation were analyzed.19 Adherence was based on the number of SC administrations from treatment initiation to device replacement or treatment discontinuation. Overall adherence was 92.6%, while 32% achieved an adherence rate of 100%, 80.6% achieved adherence ≥90%, and 13.2% showed adherence <80%. An analysis by quarter revealed a slight decrease in adherence over time, with a mean overall adherence of 94.0% at 0–3 months and 90.4% at the time of device replacement. Over the study period, the incidence of relapses decreased from 5.8% at 0–3 months to 4.0% at 33–36 months. Suboptimal adherence (adherence of <80%) was about three times higher in subjects with relapses compared with those without relapses.
In a multicenter, retrospective, observational study, 384 RRMS patients self-administered interferon β–1a three times weekly using RebiSmart® for a period of 12 months.20 Overall, 89.3% of patients were adherent to treatment (≥80% of the scheduled injections were administered). At 12 months follow-up, adherence rates varied significantly by age groups with the highest adherence rate (93.2%) in those aged 26–40 years, followed by 87.5% in patients aged ≥41 years, and 79% in those aged ≤25 years. Moreover, 90.5% of patients with a baseline Expanded Disability Status Scale (EDSS) <4 showed ≥80% adherence (vs 71.4% in patients with EDSS score ≥4).
A number of smaller uncontrolled trials with follow-up periods between 12 weeks and 1.5 years revealed high adherence of ≥80%–90%.21–27 Lugaresi et al28,29 found a decrease in adherence (among completers) from 100% through week 4 to 99.1% in weeks 5–8, to 89.0% in weeks 9–12. After a long-term follow-up of 20.5 months, overall adherence was further decreased to 79.8%. Ghezzi et al30 reported declining adherence rates over the study period, with 12-, 24-, and 52-week adherence rates of 82.5%, 80.0%, and 67.5%, respectively.
While in the majority of trials conducted with the RebiSmart®, the impact of patient age on adherence was either not assessed or no statistically significant correlation could be detected, Zecca et al25 found a statistically significant impact of higher age on objective adherence (Table 2). Median age as per adherence rates was 41.0 years in patients with low adherence rates, 48.5 years in patients with medium adherence, and 53.5 years in patients with high adherence rates.
Predictors for adherence were assessed in a number of these smaller uncontrolled studies. In a retrospective analysis of data from RRMS patients, Moccia et al22 found that missing the first dose during the first month of observation was more likely to be associated with clinical relapse, as well as with a significantly lower adherence to treatment compared with fully adherent patients or patients who missed a dose at a later point. A correlation between high adherence and a high proportion of relapse-free patients and a very low annualized relapse rate was reported by Solsona et al.23
BETACONNECT™
The BETACONNECT™ autoinjector system, designed to simplify SC injections of interferon β-1b automatically, collects data on injection date and time, injection depth, injection speed, and injection volume. The system offers injection reminders and can transfer data to an optional mobile phone app/computer program and a navigator app to enhance communication between patients and HCPs.31
Key outcomes from clinical studies identified for BETACONNECT™ are detailed in Table 3. As no RCT has been conducted with the BETACONNECT™ device, data from the three published uncontrolled studies in MS patients treated with SC interferon β-1b are described below.
Table 3.
Study details and results from all identified studies of BETACONNECT™/myBETAapp (adherence, clinical outcomes and health care resources use)
| Study name | Study objective | Study design | Indication | Sample size | Follow-up period | Key results |
|---|---|---|---|---|---|---|
| Kleiter et al (2017)32 | To investigate adherence to therapy, satisfaction, and functional health status among patients treated with interferon β-1b using the BETACONNECT™ autoinjector | Prospective, observational | RRMS | 151 | 24 weeks | • Patient age (mean ± SD) was 41.2±11.5 years • Adherence of ≥80% declined from 72.0% (week 4) to 67.3% (week 12) and 57.9% (week 24) for patients with a least one data readout and from 81.1% at week 4 to 86.7% at week 12 to 80.5% at week 24 for patients at the respective visit • Compliance was 86.3% at week 4, 91.9% at week 12, and 92.9% at week 24 • Age tended to be a predictor of persistence at 24 weeks with patients ≥40 years being more likely to still use the BETACONNECT™ at follow-up visits (OR =1.047, 95% CI 1.003, 1.093) • Treatment-naive patients showed overall higher persistence (OR =12.246; 95% CI 2.191, 68.457) |
| Patti et al (2017)33 | To measure adherence in patients with RRMS or CIS who were treated with interferon β-1b using BETACONNECT™ | Multicenter, prospective, single-arm, observational | RRMS and CIS | 498 (474 RRMS and 26 CIS) | 24 weeks | • Patient age (median) was 44 years • Adherence (defined as completing ≥80% of prescribed injections) was assessed at baseline, week 4, week 12, and week 24. Median adherence remained stable between 93.9% and 95.4% at all visits • Higher adherence was associated with male gender, existing concomitant disease, shorter disease duration, higher SDMT score and higher satisfaction with the myBETApp |
| Rametta et al (2017)34 | To assess adherence to interferon β-1b therapy in patients who are using the BETACONNECT™ autoinjector | Multicenter, prospective, single-arm, observational | RRMS | 89 | 6 weeks | • Patient age (mean) was 52 years; majority (77.5%) of patients aged ≥45 years • Mean adherence rate was 97.6% (SD 9.0%) • 95.5% of patients reached ≥80% adherence |
Abbreviations: CIS, clinically isolated syndrome; OR, odds ratio; RRMS, relapsing-remitting multiple sclerosis; SDMT, Symbol Digit Modalities Test.
In a prospective, observational, 24-week cohort study in 151 patients with RRMS or clinically isolated syndrome (CIS) treated with interferon β-1b using the BETACONNECT™ autoinjector, adherence (defined as percentage of patients injecting ≥80% of prescribed dosages with at least one BETACONNECT™ readout) declined from 72.0% at week 4 to 67.3% at week 12 and 57.9% at week 24.32 Premature study discontinuation was the main reason for this decline in adherence (11.2% at week 4, 22.4% at week 12, and 29% at week 24). The proportion of adherent patients at each respective visit was high over the study period (81.1% at week 4, 86.7% at week 12, and 80.5% at week 24). Key predictors for persistence (defined as the number of patients still using the device at the follow-up visit) were age and “no previous treatment” – patients ≥40 years were more likely to still be using the device at follow-up than patients <40 years, and treatment-naive patients were more likely to be persistent than those who were previously treated with interferon β-1b.
In a multicenter, prospective, single-arm, observational 24-week study in 474 RRMS and 26 CIS patients treated with interferon β-1b using BETACONNECT™, median adherence (defined as completing ≥80% of prescribed injections) remained stable at between 93.9% and 95.4% at all visits.33
Higher adherence was associated with male gender, existing concomitant disease, shorter disease duration, higher Symbol Digit Modalities Test (SDMT) score, and higher satisfaction with the myBETAapp.
A smaller study in 89 RRMS patients with a 6-month observation period reported a mean adherence rate of 97.6%, with 95.5% of patients reaching ≥80% adherence.34
Proteus Discover (ingestible sensors)
The Proteus system uses ingestible sensors that communicate wirelessly to a patch worn on the body to accurately document medication adherence for oral medications. These data, as well as medication reminders, are sent to the patients’ mobile phone. Data can be shared with HCPs, allowing them to view adherence.35
For Proteus Discover, a number of RCTs and prospective trials have been identified (Table 4). Adherence was not a primary objective in most of the studies, with the focus tending to be on efficacy outcomes or cost savings using the Proteus technology. Indications included tuberculosis, hepatitis C, hypertension, type 2 diabetes mellitus (T2DM), hypercholesterolemia, and liver and renal transplant recipients. RCTs comparing Proteus technology to standard of care, as well as economic model studies, are described below.
Table 4.
Study details and results from all identified studies of Proteus Discover (ingestible sensors) (adherence, clinical outcomes, and health care resources use)
| Study name | Study objective | Study design | Indication | Sample size | Follow-up period | Key results |
|---|---|---|---|---|---|---|
| Au-Yeung and DiCarlo (2012)43 | To compare costs of direct confirmation of treatment using WOT vs SOC utilizing WHO-recommended 7-day and 3-day DOT | Prospective (economic model) | Tuberculosis | NA | NA | • Health facility cost-to-treat with WOT was 36% of 7-day DOT and 71% of 3-day DOT • Patient cost-to-be-treated with WOT was 4% of 7-day DOT and 8% of 3-day DOT • Public health worker time/subject treated using WOT was 34% of 7-day DOT and 67% of 3-day DOT • Total cost for 7-day DOT was US$3,472 (86% personnel cost; 13.5% retreatment cost) • Total cost for 3-day DOT was US$1,772 (73% personnel cost; 27% retreatment cost) • Total cost for WOT was US$1,273 (38% personnel cost; 61% retreatment cost) |
| Bonacini et al (2017)41 | To evaluate real-world adherence in chronically infected HCV patients treated with SOF/LDV and using the Digital Medicine Offering | Prospective, single-arm | HCV | 28 | 12 weeks | • Patient age (mean) was 59 years • Patients used the Digital Medicine Offering for 92% of expected days; mean ingestion adherence was 94% • Sustained virologic response at 12+ weeks was achieved in 20 of 22 patients (data for six patients were not available) |
| DiCarlo et al (2014)38 | To evaluate the utility of the Proteus® system in patients with uncontrolled hypertension | Prospective | Hypertension | 190 | 14 days | • Patient age was not reported • In patients with complete data (89%), mean medication adherence was 88% • Mean SBP decrease was −7.6 mmHg; mean DBP decrease was −3.8 mmHg • 78% patients had >70% adherence; 53% achieved BP control on prescribed therapy; 25% remained uncontrolled and required treatment modification |
| Frias et al (2017)36 | To assess the impact of digital medicine offerings on BP and glycemic and lipid control | RCT | Hypertension and T2DM | 109 | 4 and 12 weeks | • Patient age (mean ± SE) was 57.8±1.1 years in the Proteus group and 61.6±1.7 years in the usual care group • Medication adherence was ≥80% (calculated for Proteus only) • Hypertension ○ At week 4, the combined Proteus groups had a mean change in SBP from baseline of –21.8 mmHg (SE 1.5) compared with –12.7 mmHg (SE 2.8) for usual care (mean −9.1, SE 2.9, 95% CI 14.8, 3.3 mmHg) ○ A greater proportion of participants in the Proteus group achieved their BP goal (81%, 65/80) compared with usual care (33.3%, 9/27) (95% CI 18.5, 77.3) ○ At week 12, 98% (39/40) of Proteus participants achieved their BP goal compared with 51.7% of usual care participants (95% CI 7.1, 84.5) • Diabetes ○ There was a nonsignificant difference in HbA1c reduction in favor of the Proteus group, compared with usual care (4-week Proteus group mean =−0.32% SE 0.22; 12-week Proteus group mean =−0.08% SE 0.22%; usual care mean =0.28% SE 0.35%) ○ For participants with a baseline HbA1c of ≥8%, the Proteus group experienced a larger HbA1c decrease compared with an increase in HbA1c seen in the usual care group (difference from 4-week Proteus group −0.98%, 95% CI –1.72,–0.24; difference from 12-week Proteus group −0.57%, 95% CI –1.53, 0.39) ○ The differences in change in LDL-C between the Proteus groups and the usual care group were –33.2 mg/dL (95% CI –50.6,–15.8) at week 4 and –19.2 mg/dL (95% CI –36.4, –2.0) at week 12 |
| Godbehere and Wareing (2014)39 | To assess the impact of digital medicine offerings on BP | Prospective | Hypertension | 8 | 2 weeks | • Patient age varied from 49 to 62 years • Taking adherence ranged from 70% to 100% • Timing adherence ranged from 67% to 100% • BP decreased in all patients (−7/+6 mmHg to −54/–24 mmHg) |
| Kim et al (2014)44 | To assess the impact of a digital health feedback system in uncontrolled hypertensive patients | Prospective study (economic model) | Hypertension | 164 | 1 year | • Patient age was not reported • The system was estimated to result in $7.3–$18.3 million in savings ($328–$717 per BP at goal), and to lead to a 3%–9% reduction in the number of CAD and stroke events in 1 year |
| Kim et al (2015)45 | To assess the impact of digital medicines with a mobile app in patients with comorbid hypertension, diabetes, hypercholesterolemia | Prospective study (economic model) | Hypertension, T2DM, and hypercholesterolemia | NR | 1 year | • Estimated cost offsets of the Proteus service offering were $90–$185/month of use (including reimbursements) • Estimated medical cost savings (reductions in outpatient/inpatient services, monitoring, disease management, medication costs) were $850–$980 (5%–11% reduction in diabetes and CVD complications) • Revenue opportunities presented an additional value equating to $80–$95 PPPY, bringing total value of the Proteus offering to $1,020–$1,260 PPPY |
| Moorhead et al (2017)37 | To study the incremental impact of seeing vs not seeing Proteus medication dose reminders on medication taking; and to assess the safety of the Proteus medication dose reminders for the possible risk of overdosing | Cluster RCT | Hypertension and type 2 diabetes mellitus | 57 | 12 weeks | • Patient age (mean ± SD) was 58.0±10.5 years • Proteus device reminder messages were associated with a 16%±16% increase (75%±18% when seeing vs 59%±24% when not seeing mobile dose reminders) in medication taking (when not taken before dose reminder) • The average daily adherence was 85.9%±11.7% • 79% of subjects (45 of 57) achieved >80% adherence • There were no overdose events related to Proteus medication dose reminders |
| Naik et al (2017)40 | To assess the usability and acceptability of passive electronic monitoring for managing hypertension | Prospective cohort | Hypertension | 167 | 2 weeks | • Patient age (mean ± SD) was 68.0±9.0 years • Taking adherence ranged from 53% to 100%, and timing adherence ranged from 21% to 100% • SBP decreased from 154±13 to 145±18 mmHg (P<0.001) and DBP decreased from 85±11 to 80±12 mmHg (P<0.001) • 32% of registry participants were found to be capable of achieving BP control using their chronically prescribed medications |
| Sullivan et al (2017)42 | To monitor patterns of adherence using ingestible digital transmitter and to assess transplant outcomes | Prospective, single-arm, observational | Liver and renal transplant recipients | 30 | 3 months | • Patient age (mean ± SD) was 14.0±3.6 years • The average patch wear adherence was 78% and average digitized medication adherence was 89.3% • While there was no change in emergency department visit rates in the study period, there was a reduction in hospital admissions |
| Virdi et al (2016)77 | To assess the impact of a digital health feedback system in hypertensive patients | Cluster RCT | Hypertension | 103 | 4 weeks | • Patient age (mean) was 58.4 years • Proportion of patients that achieved the BP target was 83.3% in the Proteus group and 33.3% in the usual care group (95% CI 23.62, 76.38) • Average adherence measured by feedback system was 84% (80%–89%) |
Abbreviations: BP, blood pressure; CAD, coronary artery disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; DOT, directly observed therapy; HCV, hepatitis C virus; HbA1c, glycosylated hemoglobin A1C; LDL-C, low-density lipoprotein cholesterol; LDV, ledipasvir; NA, not applicable; NR, not reported; PPPY, per patient per year; RCT, randomized controlled trial; SBP, systolic blood pressure; SE, standard error; SOC, standard of care; SOF, sofosbuvir; T2DM, type 2 diabetes mellitus; WHO, World Health Organization; WOT, wirelessly observed therapy.
In a cluster-randomized, prospective, open-label trial with a 12-week intervention period, 109 patients with T2DM (hemoglobin A1c [HbA1c] ≥7) and hypertension (systolic blood pressure [SBP] ≥140 mmHg) were randomized to receive medicine in combination with the Proteus ingestible sensor for 4 or 12 weeks, or usual care.36 In the Proteus groups, the medical sensor and the medication were co-encapsulated to ensure that both were taken simultaneously. The Proteus group investigators were instructed to review the adherence reports on the web portal during study visits and provide patient education/counseling or titrate medications as needed, based on these reports, in addition to other clinical data. Medication adherence, calculated only for the Proteus ingestible sensor, was >80% (not a trial objective).
In hypertension, at week 4, the combined Proteus groups had a mean change in SBP from baseline of –21.8 mmHg vs –12.7 mmHg for usual care. In addition, a greater proportion of participants in the Proteus groups achieved their BP goal (81%) compared with usual care (33.3%). At week 12, 98% of Proteus participants achieved their BP goal vs 51.7% of usual care participants.36
In diabetes, there was a nonsignificant difference in HbA1c reduction in favor of the Proteus groups (4-week Proteus group mean, −0.32%; 12-week Proteus group mean, −0.08%; usual care mean, 0.28%). For participants with a baseline HbA1c ≥8%, the Proteus groups experienced a larger HbA1c decrease compared with an HbA1c increase in the usual care group (difference from 4-week Proteus group, −0.98%; difference from 12-week Proteus group, −0.57%). Differences in change in low-density lipoprotein cholesterol (LDL-C) between the Proteus groups and the usual care group were –33.2 mg/dL at week 4 and –19.2 mg/dL at week 12.36
In a cluster RCT in 57 hypertension and T2DM patients with a 12-week follow-up, Proteus Discover-derived medication dose reminders were associated with a 16% increase in medication taking vs no reminder messages.37 Average daily adherence was 85.9, and 79% of subjects achieved >80% adherence. Moreover, there were no overdose events related to Proteus medication dose reminders.
In other uncontrolled studies with comparatively short observation periods (2–4 weeks), adherence was typically >80% in patients with hypertension,38–40 hepatitis C,41 or liver and renal transplant recipients.42 Godbehere and Wareing39 and Naik et al40 distinguished between “taking adherence” (the number of ingestible sensors detected over the number of prescribed sensors) and “timing adherence” (the number of sensors detected within ±2 hours of the average time of all detections for the dosing period). A trend for higher variability in timing adherence was observed compared with taking adherence.
No formal assessment of the impact of age on adherence rates was conducted in the studies reported for the Proteus device.
The impact of using Proteus technology on health care costs has been analyzed for treatment of tuberculosis, hypertension, T2DM, and hypercholesterolemia. Au-Yeung and DiCarlo43 compared the impact of wirelessly observed tuberculosis therapy (WOT) using Proteus technology with WHO-recommended standard of care, 7-day and 3-day directly observed therapy (DOT). Using treatment data from public sources, it was calculated that the cost of WOT would be 36% of 7-day DOT and 71% of 3-day DOT in a public health facility’s cost-to-treat analysis. In other words, the total cost for 7-day and 3-day DOT were estimated US$3,472 and US$1,772, respectively, while the total cost for WOT was estimated US$1,273.
Kim et al44 estimated the impact of the Proteus technology on outpatient services, monitoring, and cardiovascular complications in uncontrolled hypertensive patients. From a study that assessed the potential of the technology in 164 patients with a history of uncontrolled hypertension, the authors estimated that the Proteus system could result in cost savings of $7.3–18.3 million in a health plan of 1 million members and could lead to a 3%–9% reduction in the number of coronary artery disease (CAD) and stroke events in 1 year. In 2015, Kim et al calculated the value of Proteus on reducing BP, blood glucose levels, and lipids in patients with comorbid hypertension, diabetes, and hypercholester-olemia.45 The model was based on costs from the Medicare Fee schedule, Agency for Health care Research and Quality (AHRQ) databases, payer interviews, clinical and utilization assumptions from the literature, expert opinions, as well as a study using the Proteus technology in patients with uncontrolled hypertension. The authors estimated that cost offsets for Proteus would be $90–185/month of use (including reimbursements) and that medical cost savings (reductions in outpatient/inpatient services, monitoring, disease management, medication costs) would be $850–$980 per patient per year (5%–11% reduction in diabetes and CVD complications).
SmartInhaler™
The SmartInhaler™ platform includes adherence trackers for patients and HCPs, dosing reminders, and allows insights into medication usage. SmartInhaler™ medication sensors wrap around a patient’s existing dry powder or metered-dose inhaler and automatically send usage data to their smartphone using Bluetooth®. The corresponding app analyzes, stores, and monitors inhaler use.46
Both RCTs and prospective studies in asthma patients have been conducted with the SmartInhaler™ (Table 5), with RCT data comparing SmartInhaler™ to standard of care described in detail below.
Table 5.
Study details and results from all identified studies of SmartInhaler™ (adherence, clinical outcomes, and health care resources use)
| Study name | Study objective | Study design | Indication | Sample size | Follow-up period | Key results |
|---|---|---|---|---|---|---|
| Britto et al (2017)52 | To evaluate if the text messaging intervention (using Smart Inhaler™) would result in improved adherence to prescribed ICS | RCT | Asthma | 64 | 6 months | • Patient age (mean ± SD) was 17.2±1.9 years in the intervention group and 17.4±2.7 years in the control group • Receiving the text intervention resulted in an increase in adherence of 2.75% each month relative to no intervention (P<0.01); improvements were not sustained • There was modest improvement in asthma control and quality of life outcomes in the text messages group compared with control group (scores at different timepoints mentioned in figures and not reported in text in the publication) • The adolescent participants gave high ratings on the acceptability of the text messaging system |
| Burgess et al (2010)a,49 | To assess the impact of measuring adherence and providing feedback on medication usage by children with unstable asthma | RCT | Asthma | 26 | 4 months | • Patient age varied between 6 and 14 years. Mean age of patients in the intervention group was 9.1 years and in the control group was 9.3 years • Adherence was significantly higher in the intervention group than in the control group (79% vs 58%, P<0.01) • Adherence in the control group declined slightly over the study; mean adherence in the intervention group increased from 76.8% during the third month to 84.2% over the final month (P<0.01) • There was a significant improvement in asthma control in both the intervention and the control groups: at baseline, 15 of 26 subjects reported using their reliever medication three or more times/week over the previous month. During the 4-month study, two subjects reported requiring reliever medication on average three or more times/week (P=0.02) • At baseline, mean FEV1 across all subjects was 75% predicted; mean FEV1 of all participants during the study increased to 85.2% • The change in FEV1 from baseline (% predicted) was greater in those subjects receiving feedback (13.8% vs 9.8%; P<0.9) • The change in FEV1 in the final month (% predicted) was similar in both groups (87.3% vs 86.9%; P<0.4) |
| Chan et al (2015)a,48 | To investigate whether use of an inhaler with audiovisual reminders improves adherence and asthma outcomes in children presenting with asthma exacerbation | RCT | Asthma | 220 | 6 months | • Patient age (mean ± SD) was 8.9±2.5 years in the intervention group and 8.9±2.6 in the control group • Median percentage adherence was 84% in the intervention group, compared with 30% in the control group (P<0.0001) • Intervention group had an improved asthma morbidity score (P=0.008) and asthma control scores at 2, 4, and 6 months (P<0.0001), and fewer exacerbations at 2 months (6% vs 24%, P=0.015) • Mean percentage adherence at 2, 4, and 6 months was 91%, 84%, and 79%, respectively, for the intervention group, compared with 40%, 33%, and 27%, respectively, for the control group • The change in asthma morbidity score from baseline to 6 months was significantly greater in the intervention group than in the control group (P=0.008). There was a reduction of 2.0 points from a mean baseline score of 9.3 (SD 2.2) to 7.3 (2.1) in the intervention group vs 1.2 points from a baseline of 9.2 (2.5) to 8.0 (2.2) in the control group • For childhood asthma control test scores, the difference between groups was significant at all timepoints (2, 4, and 6 months; P<0.0001), with the intervention group scoring higher by an overall average of 1.57 • The median percentage number of days on which a reliever was used was 9.5% in the intervention group and 17.4% in the control group (P=0.002) • No differences in FEV1 (P=0.38), asthma-related school absences (P=0.096), emergency visits (P=0.509), or caregiver work absences (P=0.167) were reported |
| Charles et al (2007)a,50 | To determine whether an audiovisual reminder device improves adherence with ICS therapy in adult asthma patients | RCT | Asthma | 110 | 6 months | • Median (range) patient age was 39 (13–65) years in the intervention group and 35 (15–64) years in the control group • In the last 12 weeks, the proportion of medication taken was 93% in the AVRF group and 74% in the control group, with a difference of 18% (95% CI 10, 26; P<0.0001) • The proportion of subjects taking >50%, >80%, or >90% of medication was greater in the AVRF group. Corresponding ratios of proportions adherent were 1.33 (95% CI 1.10, 1.61; P=0.003), 2.27 (95% CI 1.56, 3.3; P<0.0001), and 3.25 (95% CI 1.74, 6.1%; P<0.0001), respectively |
| Foster et al (2014)a,51 | To test the effectiveness of two brief GP-delivered interventions (with and without IRF) for improving adherence and asthma control | Cluster RCT | Asthma | 143 | 6 months | • Patient age (mean ± SD) was 40.3±15.2 years • Adherence was significantly higher in the IRF group vs non-IRF groups (73%±26% vs 46±28%; P<0.0001), but not significantly different between PAD and non-PAD • The asthma control improved overall (mean change in ACT score, 4.5±4.9; P<0.0001), with no significant difference between IRF and non-IRF groups • Severe exacerbations experienced by 11% patients in IRF groups and 28% in non-IRF groups (P=0.013; after adjustment for exacerbation history; P=0.06) • There were statistically significant and clinically important improvements from T0 to T6 months in asthma-related QoL (mean change, 0.77±1.15; P<0.0001) and significant improvements in anxiety (−0.93±3.18; P=0.022) and self-reported adherence behavior (MARS-A, 0.28±0.82; P=0.008); no significant difference between IRF and non-IRF groups |
| Jochmann et al (2015)78 | To compare self-assessment of adherence in children with difficult asthma with adherence measured electronically | Prospective, single-arm | Asthma | 50 | 16 weeks | • Median (range) patient age was 12.4 (5–17) years • Median SmartInhaler adherence was 60% (range 24%–97%) • FENO improved significantly in those with SmartInhaler adherence >80% • FEV1 improved significantly following monitoring (86 at baseline to 91.5 at follow-up; P=0.01) • mPAQLQ also improved significantly following monitoring (5.1 at baseline to 5.8 at follow-up; P=0.02) |
| Kenyon et al (2016)53 | To assess feasibility and acceptability of a health worker-delivered electronic adherence monitoring intervention among the highest utilizers of acute asthma care | Prospective, single-arm | Asthma | 14 | 3 months | • Median (range) patient age was 3.5 (3–9) years • All caregivers viewed the electronic monitoring device favorably and would recommend it to friends; 56% believed the device helped to improve asthma control • Overall, ACT scores improved by a mean of 2.7 points (95% CI 0, 5.5; P=0.05) over the 3-month intervention (just below the minimally significant ACT change threshold of 3 points) |
| Morton et al (2017)a,47 | To determine whether electronic monitoring results in improved clinical outcomes in asthma patients (STAAR study) | Open-label, parallel group, RCT | Asthma | 90 | 12 months | • Patient age (mean ± SD) was 10.4±2.9 years in the intervention group and 10.2±2.9 in the control group • Adherence in the intervention group was 70% vs 49% in the control group (P ≤ 0.001) • No significant difference in change in ACQ, but intervention group required significantly fewer oral steroids courses and fewer hospital admissions compared with control group ○ Event rate (per 100 child days) for courses of oral steroids were 0.411 for the intervention arm compared with 0.676 for the control arm (P=0.008) ○ Event rate (per 100 child days) for hospital admissions was 0.0254 for the intervention arm compared with 0.129 for the control arm (P<0.001) • FEV1% improved in both treatment arms, with no significant difference between treatments at 12-months compared with baseline • Differences in GP visits in the two groups were not clinically significant |
Note:
Studies submitted to NICE.
Abbreviations: ACQ, Asthma Controlled Questionnaire; ACT, Asthma Control Test; AVRF, audiovisual reminder function; FENO, fractional exhaled nitric oxide; GP, general practitioner; ICS, inhaled corticosteroids; IRF, inhaler reminders and feedback; MARS-A, Medication Adherence Report Scale for Asthma; NICE, National Institute of Health & Clinical Excellence; PAD, personalized adherence discussion; PAQLQ, Pediatric Asthma Quality of Life Questionnaire; QoL, quality of life; RCT, randomized controlled trial; STAAR, STudy of Asthma Adherence Reminders.
In an open-label, parallel group RCT, 90 children aged 6–16 years on regular inhaled corticosteroids (ICSs) with poorly controlled asthma were randomized to electronic adherence monitoring using SmartInhaler™ with daily reminder alarms together with feedback in the clinic (active intervention) or to adherence monitoring alone (usual care).47 The study had a 12-month follow-up, with clinic visits at 3 months intervals. Adherence rate (calculated for each 3-month period) was calculated as percentage of the number of doses actually taken vs number of doses prescribed. There was a statistically significant difference in adherence (70% for active intervention vs 49% control). There was no significant difference in change in Asthma Controlled Questionnaire (ACQ), but children in the intervention group required significantly fewer courses of oral steroids and fewer hospital admissions.
In a randomized study of 220 children aged 6–15 years with asthma exacerbation treated with ICS, audiovisual reminder functions (AVRFs) on the SmartInhaler™ device used with a preventer inhaler was either enabled (intervention group) or disabled (control group).48 The follow-up period was 6 months, and adherence was defined as the proportion of ICS taken relative to the number of doses prescribed. Median adherence was 84% and 30% in the intervention and control groups, respectively. In addition to the improved adherence, improvement in asthma morbidity score from baseline to 6 months was significantly greater in the intervention group (from 9.3 to 7.3) than in the control group (from 9.2 to 8.0). In addition, there were fewer exacerbations at 2 months in the intervention group than in the control group (6% vs 24%), but no differences in FEV1, asthma-related school absences, emergency visits, or caregiver work absences.
In a smaller study with a follow-up period of 4 months, 26 children aged 6–14 years were randomized to being informed of their adherence (intervention group) or for their adherence to remain undisclosed (control group). Adherence was collected by means of the SmartInhaler™. Similar to Chan et al,48 it was found that while adherence was significantly higher in the intervention group (79% vs 58%), this was not reflected in a statistically significant improvement in lung function.49 The change in FEV1 from baseline was 13.8% in the intervention group vs 9.8% in the control group, and the mean FEV1 in the final month was 87.3% and 86.9%, respectively, in the two groups.
In a randomized open-label parallel group study with a follow-up of 24 weeks, 110 adult or adolescent asthma patients taking ICS were randomized to receiving their medication with or without an AVRF.50 Adherence (defined as the proportion of medication taken as prescribed over the final 12 weeks of the study) was 93% in the AVRF group and 74% in the control group. In addition, the proportions of subjects taking >50%, >80%, or >90% of their medication were greater in the AVRF group.
In a cluster RCT, with general practitioner (GP) as a unit of cluster, GPs were trained to deliver the relevant intervention(s) with 143 patients from their own practice prescribed twice-daily ICS/long-acting β2-agonist for ≥1 month with a follow-up of 6 months.51 GPs were randomized to one of the following four groups: usual care (UC) (n=43 patients); UC + personalized adherence discussions (PAD, n=24 patients); usual care + inhaler reminders and feedback (IRF) (n=35 patients); UC + IRF + PAD (n=41 patients). Adherence was significantly higher in the IRF (73%) than in non-IRF groups (46%), and there was no statistically significant difference between PAD and non-PAD groups. Asthma Controlled Test (ACT) scores improved overall (mean change, 4.5), and a significant difference among IRF and non-IRF groups was observed. About 11% and 28% of patients experienced severe exacerbations in the IRF and non-IRF groups, respectively. Overall, there were no significant differences between the reminder and non-reminder groups in any other secondary outcome.
In a randomized crossover study in 64 adolescent asthma patients, Britto et al52 investigated the impact of text messaging using SmartInhaler™ on adherence to prescribed ICS. All patients underwent 3 months of receiving personalized text messages such as medication or appointment reminders or other messages of their choice (intervention arm) and 3 months without access to the text messaging tool (control arm) in a randomized order. Receiving text reminders resulted in a 2.75% increase in adherence each month, while adherence decreased in the absence of text messages. In the group that received text messages first, intervention effects were not sustained on switching to control, and adherence decreased.
No formal assessment of the impact of patient age on adherence rate was conducted in the studies reported for the SmartInhaler™.
To assess the feasibility and acceptability of health worker-delivered electronic adherence monitoring, a prospective cohort 3-month pilot study was performed in which 14 children (median age, 3.5 years) with the highest frequency of asthma-related emergency department and hospital care within a local managed care Medicaid plan were enrolled.53 The intervention included motivational interviewing, electronic monitoring of controller and rescue inhaler use, and outreach by a community health worker for predefined medication alerts. All participants initiated the use of the electronic devices, but no modem signal was transmitted after a mean of 45 days for five patients. All caregivers viewed the electronic monitoring device favorably and would recommend it to friends; 56% believed the device helped to improve asthma control.
Morton et al47 reported a trend in the reduction in GP/emergency department visits and days off school due to asthma in the SmartInhaler™ group. This difference was, however, not statistically significant. Results from multivariate analysis showed that patients receiving usual care were ~1.5 times more likely to be prescribed oral steroids (incidence rate ratio [IRR]: 1.53) and also had ~4.5 times higher rate of hospital admissions (IRR: 4.38) compared with the SmartInhaler™ group.
SmartInhalers™ were subject to a National Institute of Health & Clinical Excellence (NICE) HTA.13 The review included five RCTs in adults and children assessing asthma control using ACQ scores, medication adherence, proportion of prescribed doses taken, and days absent from school for patients using SmartInhalers™ as highlighted in Table 5.
While an improved adherence with the SmartInhaler™ technology was found across studies, NICE noted that “key uncertainties are that some of the available studies were either not designed to or were not adequately powered to show whether improved adherence is associated with significantly improved outcomes.” It concluded that the evidence for these possible resource consequences is limited and that the resource impact would be greater than standard care, because of the cost of the device and software access (£100 per unit [exclusive of VAT], plus £14.17 per month for each HCP to access cloud-based data), unless reductions in GP and hospital visits were realized.
Propeller
The Propeller system includes an electronic inhaler sensor that attaches to an existing third-party inhaler. The sensor monitors the date, time, and frequency of medication use and transmits these data back to secure servers through a smartphone app or hub-base station. Location data are collected on medication use among patients who have a smartphone. The sensors regularly transmit data back to the server or sync through the smartphone or hub.54 Similar to the SmartInhaler™ technology, Propeller is used for a variety of differed inhaler types.
Both RCTs and prospective studies in patients with asthma and COPD have been conducted with the Propeller system (Table 6). Data from RCTs and larger uncontrolled studies that compared the use of Propeller to standard of care are described in detail below. Use of rescue inhaler was monitored in most of the studies. While some studies highlight the use of a short-acting β-agonist (SABA), others did not mention the name of the rescue inhaler.
Table 6.
Study details and results from all identified studies of Propeller (adherence, clinical outcomes, and health care resources use)
| Study name | Study objective | Study design | Indication | Sample size | Follow-up period | Key results |
|---|---|---|---|---|---|---|
| Barrett et al (2018)63 | To evaluate the extent to which digital health intervention improves asthma outcomes in a real-world setting | Prospective, single-arm | Asthma | 497 | 12 months | • Patient mean age was 38 years (range 4–90 years) and 80% were adults • At 12 months of follow-up, there was a 78% reduction in SABA use, an 84% reduction in night-time SABA use, and a 48% improvement in symptom-free days from baseline to month 12 (P<0.0001) • The number of symptom-free days increased from 62% (first week) to 83% (2 months), to 88% (6 months), and to 90% (12 months) (P<0.0001) |
| Carl et al (2018)64 | To determine whether a quality improvement program employing a digital health platform could improve pediatric asthma outcomes | Prospective, single-arm | Asthma | 82 | 12 months | • Patient age varied from 4 to 18 years • Aggregate average controller adherence at 1, 2, 3, and 6 months post-enrollment timepoints demonstrated rates of 58%, 64%, 62%, and 55%, respectively • Average weekly rescue event rate at these time points demonstrated decreased events from baseline (0.597/week) to 0.21 (63%), 0.20 (69%), 0.25 (59%), and (75%), respectively |
| Chen et al (2017)62 | To assess the feasibility and clinical impact of a digital health intervention in a Medicare population with COPD or asthma | Prospective, single-arm | Asthma or COPD | 236 (198 COPD, 38 asthma) | 6 months | • Among asthma patients: • 65% of patients were 60 years or older • At 6 months, SABA use decreased from 1.15 (first week) to 0.56 uses/person/day (last week); significant decrease of 51.4% (P<0.05) • Increase in symptom-free days was 28% (46% at first week to 59% at last week) • Among COPD patients: • 78% of patients were 60 years or older • At 6 months, SABA use decreased from 1.53 (first week) to 0.74 uses/person/day (last week); significant decrease of 51.7% (P<0.01) |
| Hoch et al (2017)65 | To evaluate the feasibility of Propeller Health monitoring device, along with clinical outcomes | Prospective, single-arm | Asthma | 25 | 3 months | • Patient age varied from 6 to 17 years • Average weekly adherence ranged from 76% (week 1) to 36% (week 12). The overall average weekly adherence for the cohort was 56% (SD 12%) • Significant decrease in the rate of rescue medication; 76% (week 1) of study vs 40% (week 12) (P=0.012) • Average weekly number of rescue medications declined from 9.9±14 (week 1) to 2.4±5.2 (week 12) • There was no difference in the rate of night-time events between week 1 and week 12 of the study (P=0.727) • Average number of asthma-free days increased from 4.8 days (±2.2) to 6 days (±1.6) • FEV1 and FVC increased during the study period; however, the difference did not reach significance. FEF25–75 increased from an average of 80% predicted to 84% predicted (P=0.02) |
| Kenyon et al (2018)66 | To assess the feasibility of a mobile health, ICS adherence reminder intervention and to characterize adherence trajectories immediately following severe asthma exacerbation in high-risk urban children with persistent asthma | RCT | Asthma | 41 | 30 days | • Patient age (mean ± SD) was 5.9±2.1 years • Electronic monitoring of ICS use and adherence reminders delivered via text message were feasible for most participants, but there was no signal of effect • After adjusting for age and parental education, mean adherence rates were 36% for the intervention group and 32% for the control group (P=0.73) • Age had no significant impact on adherence rate. After adjusting for impact of intervention and education level, ICS adherence rates in <5 years old were 6% (95% CI 7, 44) compared with 43% (95% CI 30, 55) in those aged ≥5 years (P=0.14) • Mean daily medication adherence trends over the 30-day intervention interval were also similar between the two groups, with broadly overlapping SDs • Mean change in parent-reported portion of the cACT score over the 30 days of the intervention was not statistically significantly different between controls (3.1) and intervention (1.2) (P=0.16) |
| Merchant et al (2016)55 | To measure real-world effectiveness of Propeller Health Asthma Platform to reduce use of SABA and improve asthma control | RCT | Asthma | 495 | 12 months | • Patient mean age was 36.6 years in the intervention group and 36.0 years in the routine care group. Approximately 30% of patients in both the groups were between 5 and 17 years of age. • Daily mean number of SABA uses/person decreased by 0.41 for intervention group and by 0.31 for routine care between first week and remainder of the study (P<0.001) • Proportion of SABA-free days increased 21% (intervention) and 17% (control) (P<0.01) • ACT scores were not significantly different between arms; initially uncontrolled adults: • Significantly larger improvement in ACT scores in the intervention group vs routine care (+6.2 and +4.6, respectively, P<0.01) • Significantly larger improvement in the proportion with controlled asthma in intervention group vs routine care (63% controlled in the study period vs 49%, respectively; P<0.05) |
| Merchant et al (2017)68 | To assess the impact of digital health intervention, which leveraged sensors and app-based education, on ER visits, hospitalizations, inpatient days, and clinic visits | Retrospective, cohort | Asthma | 507 | Data collected between July 2011 and September 2016 | • Patient age was not reported • Significant reductions in hospitalizations (2.7 to 0.6 days; 79% reduction; P<0.0001), inpatient days (7.9 to 1.4 days; 82% reduction; P<0.001) and ER visits (19.2 to 8.3 days; 57% reduction; P<0.0001) • Non-acute asthma-related clinic visits increased (197–277 days; 41% increase; P<0.0001) |
| Su et al (2016)67 | To identify hotspots of asthma symptoms; evaluate associations between asthma symptoms and environmental covariates in real-time and space | Prospective, single-arm | Asthma | 140 | 20 months | • Patient age was not reported • By targeting environmental interventions that could have the largest impact on asthma within specific neighborhoods, 914,000 inhaler uses and $1.8 million of hospitalization costs could be avoided |
| Van Sickle et al (2010)58 | To investigate if online feedback about remotely monitored inhaled bronchodilators improves composite measures of asthma control | Prospective, single-arm | Asthma | 27 | NR | • Patient mean age was 35.5 years (range: 19–74 years) • Asthma control scores increased significantly after receiving email reports after first (P=0.01) and second month (P=0.007) • Patients reported more awareness of symptom frequency, level of control, asthma triggers, and increased adherence to preventive medication |
| Van Sickle et al (2011)59 | To investigate if weekly feedback summarizing use of remotely monitored rescue medication improves asthma control | Prospective, single-arm | Asthma | 34 | 4 months | • Patient mean age was 41.8 years (range: 20–82 years) • Mean ACT scores increased from 17.5 at entry to 19.5 at exit (P=0.01) • Days with asthma symptoms in preceding 2 weeks declined from 6 at entry to 2.8 at exit (P=0.001) • Nights with asthma symptoms in preceding 2 weeks declined from 2.7 at entry to 1.3 at exit (P=0.08) • The ACT scores of 64% participants improved, 11% had no change and 23% worsened |
| Van Sickle et al (2013)60 | To assess whether weekly email reports on monitored use of inhaled, short-acting bronchodilators improves composite asthma control measures | Prospective, single-arm | Asthma | 30 | 4 months | • Patient mean age was 36.8 years (range: 19–74 years) • No significant difference in ACT scores between entry and first month values (P=0.66) • ACT scores increased by 1.40 points (95% CI 0.61, 2.18) for each subsequent study month after patients received feedback |
| Van Sickle et al (2014)61 | To determine the effect of sensor-enabled, mobile health asthma program on individual-level asthma outcomes, including frequency of asthma rescue medication use, asthma-free days and control status | Prospective, single-arm | Asthma | NR | 12 months | • Patient age was not reported • Improved adherence to clinical guidelines reported; 57% of participants had asthma action plan at study end vs 41% at intake • The proportion considered well-controlled increased by 33% between intake and completion • Proportion with an asthma-free day in the first month was significantly different from all subsequent months (P<0.01) |
| Van Sickle et al (2015)57 | To determine whether mobile health asthma program could improve asthma outcomes, including frequency of rescue medication use, asthma-free days, and control | Prospective, single-arm | Asthma | 299 | 12 months | • Study participants included both children and adults. Mean/median age was not reported • 57% of participants reported having an asthma action plan at study end, compared with only 41% at intake • At study exit, rescue inhaler use declined by 75%, and the proportion of participants with an asthma-free day increased significantly by 39% |
| Van Sickle et al (2016)56 | To determine whether a sensor-enabled, clinically integrated mobile health asthma quality improvement program could reduce the frequency of SABA use, and increase asthma-free days, asthma control and controller medication adherence | RCT | Asthma | 125 | 6 months | • Study participants included only adults. Mean/median age was not reported • Significant improvements with intervention vs control on all clinical outcomes, including controller medication adherence, daily SABA use, asthma-free days, and asthma control (all P<0.001) • 21-point improvement in adherence for the intervention |
Abbreviations: ACT, Asthma Control Test; cACT, Childhood Asthma Control Test; ER, emergency room; FEF25–75, forced expiratory flow at 25%–75% of the pulmonary volume; ICS, inhaled corticosteroids; NR, not reported; RCT, randomized controlled trial; SABA, short-acting β-agonist.
In an RCT enrolling 495 asthma patients with a SABA prescription at study intake, patients either received access to and feedback from the Propeller system, with physicians able to monitor patient status (intervention group) or routine care whereby patients were fitted with sensors, but did not receive feedback.55 The follow-up period was 12 months. The mean daily number of SABA uses per person decreased by 0.41 for the intervention group and by 0.31 for routine care between the first week and the end of study. In addition, the proportion of SABA-free days increased by 21% for the intervention group and 17% for routine care. ACT scores were not significantly different between arms. There was a greater improvement in the proportion of patients with controlled asthma in the intervention group vs routine care (63% vs 49%) among adults with initially uncontrolled asthma.
In a 6-month RCT, 125 adult asthma patients with a SABA prescription were randomized to an intervention (electronic inhaler sensors tracking medication use, electronic data visualizations, reminders, and personalized education) or control group (received sensors, but with no data access for the patient or care manager).56 In addition to a 21-point improvement in adherence in the intervention group, significant improvements in controller medication adherence, daily SABA use, asthma-free days, and asthma control were reported.
In 2015, Van Sickle et al57 published the results from a study in 299 asthma patients with a SABA prescription. The initial control period was 30 days and the subsequent period during which patients received the smartphone and web-based self-management tool was 12 months. At follow-up, rescue inhaler use declined by 75%, and the proportion of participants with an asthma-free day increased by 39%. The proportion with asthma-free days during the initial control period was significantly lower than all subsequent months, and the proportion considered well-controlled increased by 33% between intake and exit. In addition, while only 41% of patients were reported to have an asthma action plan at study entry, 57% had one at the end of the trial. Similar findings were reported in a number of other non-randomized trials.58–65
Suboptimal adherence trajectories following severe exacerbations independent of the use of daily text message reminders by means of the Propeller system were reported by Kenyon et al.66 In their study, children aged 2–13 years with persistent asthma were randomized to an intervention group (receiving daily text message reminders, including tips about the value of regular controller use) or control group (receiving only two reminders to sync their sensors). The impact of patient age on adherence to study treatments was assessed. After adjusting for age and parental education, mean adherence rates were 36% and 32% for the intervention and control groups, respectively. Mean daily medication adherence trends over the 30-day intervention were also similar between the two groups, with broadly overlapping standard deviations. In addition, mean change in the parent-reported portion of the childhood asthma control test (cACT) score over the 30 days was not statistically significantly different between controls (3.1) and intervention (1.2). While the study was not powered to analyze adherence, there was no signal of impact due to the text message intervention.
In a model of the impact of municipal intervention scenarios based on data from 140 asthma patients recording inhaler use with a wireless sensor that passively collected date, time, and location of inhaler use in Louisville between June 2012 and February 2014, it was estimated that $1.8 million in hospitalization costs could be avoided through targeted use of interventions such as the Propeller device.67
A retrospective study of electronic medical records compared asthma-related and non-asthma-related utilization event rates among 507 asthma patients during the intervention period (use of Propeller Sensor) to those during their pre-intervention baseline.68 All acute asthma-related utilization rates demonstrated significant reductions, including hospitalizations (2.7 to 0.6 days), inpatient days (7.9 to 1.4 days), and emergency department visits (19.2 to 8.3 days), while non-acute asthma-related clinic visits increased (197 to 277 days). Moreover, there was a non-statistically significant decrease in all non-asthma utilization events, including hospitalizations, emergency department visits, and clinic visits. The authors suggested that the increase in clinic visits may be related to providers making an attempt to address patient worsening in a non-acute setting before an acute exacerbation occurred.
Discussion and conclusion
From the five preselected connected drug delivery devices, the two that were developed by the manufacturer of combined interferons (RebiSmart® and BETACONNECT™) showed high adherence rates of >80% to 90% overall. In addition, as assessed with the RebiSmart® device, there was a correlation between higher adherence and a number of efficacy outcomes. Nevertheless, it cannot be concluded that the observed compliance with the dosing regimen was a result of the application of connected features, as the relevant trials did not include a control arm.
A different database is available for devices that were developed by specialized biotech companies aiming to apply their technologies to molecules and devices developed by potential pharma partners (Proteus Discover, SmartInhaler™, Propeller). As part of the overall value proposition, studies have been conducted comparing adherence to treatment and related outcomes with and without connected device features. Collectively, clinical studies demonstrate improved adherence to treatment with the connected device vs standard of care, with a number of trials revealing a correlation between adherence and efficacy outcomes. In addition, there is initial evidence that the use of connected devices could result in lower health care costs due to reductions in outpatient and inpatient services, patient monitoring, disease management, and medication costs.
Therefore, considering together the data available for the different connected devices, there is initial evidence that such tools could be a cost-effective modality to improve adherence to treatment and ultimately outcomes outside of a controlled stetting. Thus, digital homecare is expected to decrease uncertainty around patient adherence, while maintaining patient-centric disease management.
Through real-time collection of patient health and disease state information, connected devices can promote the early tracking of potential adverse events or complications, allowing physicians to differentiate between pharmacological resistance and improper medication use. This may prevent severe treatment-related events or treatment failure that would otherwise consume costs and resources (including hospitalization) and enable real-time decision-making concerning continuation of treatment (including whether to maintain or alter the dose) or treatment discontinuation.
Connected drug delivery devices may also play a role in complementing value-based reimbursement models. Long-term reimbursement decisions for the “companion drug” might be supported with adherence or PRO data derived from these technologies. Such data could be used to support performance and value-based payment solutions by tracking the ongoing performance post-approval to confirm the outcomes of RCTs in the real-world setting.
To ensure broad acceptance of connected devices, however, a number of barriers still need to be overcome (Figure 1). Despite initial enthusiasm, patients may lose motivation over time, which is difficult to show in a clinical trial setting. Not all patients will want to share their data with HCPs or payers, and depending on the area where people live, connectivity issues may prevent continuous application of the electronic device. In addition, as reasons for nonadherence depend on the patient and patient population, customized connected tools tailored to individual patients, patient populations, and indications need to be made available. From an HCP perspective, there will likely be concerns about lack of remuneration for patient training, data capture, and assessment. Also, aspects of reliability, completeness, and security of the data, as well as the perceived lack of control and direct patient contact while still being liable for the treatment, need to be further addressed. To unfold the full potential of connected devices from an HCP and payer perspective, more data on the health economic benefit need to become available on an individual drug and/or indication basis. The increasingly stringent laws around data privacy and patient security also have to be taken into consideration in this context. For example, the new European regulations on personal data protection that came into effect in May 2018 across its Member States69,70 will impact the European setting for connected devices and health apps. The full set of its implications on the environment for connected devices and related health apps will not be fully understood and visible for a few years.
Figure 1.
Expected value of connected devices for stakeholders and potential barriers to adoption.
Abbreviations: PRO, patient-reported outcome; RWD, real-world data.
It is expected that future connected device features enabling drug delivery in the home setting may be further improved technically by considering aspects such as patient age, general customization to individual patient needs, as well as a combination of different adherence tools. Studies on the impact of patient age on the successful use of connected devices are still limited. More work in this area is mandated, as one potential challenge with the widespread use of connected devices is the requirement to develop a certain level of computer skills in computer-naive patients. Interestingly, however, in a study with the RebiSmart, older age was associated with a higher objective adherence.25 In contrast, Barone et al71 reported a greater preference for BETACONNECT™ among younger patients (100% in patients aged 18–40 years) compared with older patients (75% in patients aged 51–70 years). Furthermore, older patients were less likely to switch away from standard injection.
In this context it becomes evident that a customization of connected device features should not only relate to a patient’s age but also to other individual needs that are specific to the person, medication, and timing of drug intake. As already seen in the field of asthma and chronic obstructive diseases, depending on individual patient needs, electronic devices are complemented with text messaging and self-managing tools (ie, web-based and mobile apps to record symptoms and monitor the disease).72 To permit a user-centered design in engaging individuals in behavior change, patients should even be involved in designing the most appropriate adherence tool.73
To further improve compliance with the treatment regimen outside the setting of a controlled hospital or physician’s office, a combination of different adherence tools has already been proposed with the aim of tracking and overcoming a number of factors leading to poor adherence. Kalantarian et al74 described a two-step system for detecting when a pill bottle is opened using commercial smart-bottle technologies, and when a pill is consumed using a custom-designed smart necklace equipped with a piezoelectric sensor. The authors suggested that combining these two mechanisms coupled with a mobile app could passively monitor adherence and inform caregivers of patient status.
The field of connected drug delivery devices is evolving rapidly. In particular, with the first FDA-approved smart pen for insulin launched in 201775 and a number of similar devices in late-stage development, the clinical database on the usefulness of electronic adherence tools linked to drug delivery devices is expected to increase significantly in the near future.
In summary, connected drug delivery devices offer the potential to ensure a high quality of care while supporting drug administration in a decentralized setting. There is already a comparatively large database on the impact of connected features on adherence to treatment and efficacy outcomes. To further apply data generated by connected devices for enabling early treatment decisions and to even complement value-based reimbursement models, aspects such as HCP reimbursement, data reliability, as well as data privacy need to be assessed in greater detail.
Supplementary materials
Table S1.
Predefined research questions
| Research questions |
|---|
| • Is there any regulatory/HTA guidance for connected devices in the US and EU, including apps or software in particular? |
| • What was the approval pathway for any connected devices that have been reviewed by regulatory authorities? |
| • Which clinical studies or other studies were required by regulatory and HTA bodies in order to grant approval of the selected connected devices? |
| • What is the impact of selected connected devices on adherence (clinical studies or RWE)? |
| • What is the impact of selected connected devices on clinical outcomes? |
| • What is the impact of selected connected devices on health care costs and resources, including doctors’ visits (scheduled and unscheduled visits)? |
| • What are the potential barriers that need to be overcome to unfold the full potential of these tools? |
Abbreviations: EU, European Union; HTA, Health technology assessment; RWE, real-world evidence; US, United States.
Table S2.
Data sources searched
| Data sources searched |
|---|
| PubMed |
| Google Scholar |
| Company websites |
| HTA databases (AETSA; AHRQ; CADTH; CTAF-ICER; HAS; INAHTA; IQWiG; NHS; NICE; PBAC; SMC) |
| USFDA website |
| EMA website |
| Clinicaltrials.gov |
| International Clinical Trials Registry Platform |
Abbreviations: AETSA, Agencia de Evaluación de Tecnologías Sanitarias de Andalucía; AHRQ, The Agency for Health care Research and Quality; CADTH, Canadian Agency for Drugs and Technologies in Health; CTAF-ICER, The California Technology Assessment Forum – Institute for Clinical and Economic Review; EMA, European Medicines Agency; HAS, Haute Autorité de Santé; HTA, Health technology assessment; INAHTA, The International Network of Agencies for Health Technology Assessment; IQWiG, The Institute for Quality and Efficiency in Health care; NHS, National Health Service; NICE, National Institute for Health and Clinical Excellence; PBAC, Pharmaceutical Benefits Advisory Committee; SMC, Scottish Medicines Consortium; USFDA, United States Food and Drug Administration.
Table S3.
Search terms used
| Device | Synonym/local language translation |
|---|---|
| Proteus Discover | Proteus pill, Proteus patch, and other translated terms of the device in different languages (eg, Proteus-Pille [German], Proteus pillola [Italian], pilule Proteus [French], píldora de Proteus [Spanish]) |
| RebiSmart® | Msdialog and other translated terms of the device in different languages (same as that of English) |
| BETACONNECT™ | BETACONNECT™ system, BETACONNECT™, and other translated search terms of the device in different languages (eg, Beta verbinden [German], Beta connettersi [Italian]; while French and Spanish terms remained same as that of English language) |
| SmartInhaler™ | Smart inhaler, SmartInhaler medication sensors, SmartInhaler sensors, Smartturbo, Smarttouch and other translated terms of the device in different languages (eg, Intelligenter Inhalator [German], Inalatore intelligente [Italian], Inhalateur intelligent [French], Inhalador inteligente [Spanish]) |
| Propeller | Propeller Sensor, specific device terms including Ellipta, Respimat, Diskus, MDI, metered-dose inhaler, and other translated terms of the device in different languages (eg, Elica [Italian], Hélice [French & Spanish]); while the German term remained same as that of English language |
Note: General searches were also undertaken to identify guidance documents for connected devices overall.
Abbreviation: MDI, metered-dose inhaler.
Acknowledgments
The authors acknowledge Magdalena Wanner for support in literature search and documentation. The authors would also like to thank Rachel Danks for assistance with the preparation of this manuscript. The literature review and editorial assistance for the manuscript was funded by F. Hoffmann-La Roche.
Footnotes
Disclosure
BB, CSC, and JS are employed by F. Hoffman-La Roche Ltd. The authors report no other conflicts of interest in this work.
References
- 1.Chee TT, Ryan AM, Wasfy JH, Borden WB. Current state of value-based purchasing programs. Circulation. 2016;133(22):2197–2205. doi: 10.1161/CIRCULATIONAHA.115.010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Masson G, Solé G, Desnuelle C, et al. Home versus hospital immunoglobulin treatment for autoimmune neuropathies: a cost minimization analysis. Brain Behav. 2018;8(2):e00923. doi: 10.1002/brb3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–440. doi: 10.1007/s40259-018-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan S. Advanced Delivery Devices - How data hubs & smart devices are enabling the rise of therapeutic ecosystems. Drug Development & Delivery. Jan-Feb. 2016. [Accessed November 2, 2018]. Available from: https://drug-dev.com/advanced-delivery-devices-how-data-hubs-smart-devices-are-enabling-the-rise-of-therapeutic-ecosystems/
- 5.Adherium Adherium Launches the Hailie™ Global Brand to Connect People with Asthma and COPD to Better Care [press release] [Accessed November 2018]. Available from: https://www.adherium.com/news/adherium-launches-the-hailie-global-brand-to-connect-people-with-asthma-and-copd-to-better-care/
- 6.United States Food and Drug Administration Medical Device Overview. Sep 9, 2018. [Accessed November 2018]. Available from: https://www.fda.gov/forindustry/importprogram/importbasics/regulatedproducts/ucm510630.htm.
- 7.European Union Regulation EU 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation EC 178/2002 and Regulation EC1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Apr 5, 2017. [Accessed November 3, 2018]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745.
- 8.United States Food and Drug Administration 510(k) Premarket Notification. Sep 9, 2018. [Accessed November 2018]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm.
- 9.United States Food and Drink Administration Betaconnect – supplemental BLA letters. Sep 25, 2015. [Accessed November 4, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/103471Orig1s5186ltr.pdf.
- 10.United States Food and Drink Administration Drug Approval Package: Abilify MyCite (aripiprazole) Sep 13, 2017. [Accessed November 4, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/207202Orig1s000TOC.cfm.
- 11.Proteus Digital Health Aug 13, 2010. [Accessed November 4, 2018]. Available from: www.proteus.com/press-releases/proteus-biomedical-announces-european-ce-mark-approval-of-ingestible-sensor-and-monitor-system/
- 12.Excell S, Thristan M, Dangond F, Marhardt K, St Charles-Krohe M, Turner-Bowker DM. A novel electronic application of patient-reported outcomes in multiple sclerosis – Meeting the necessary challenge of assessing quality of life and outcomes in daily clinical practice. Eur Neurolog Rev. 2014;9(1):49–55. [Google Scholar]
- 13.National Institute for Health and Care Excellence Smartinhaler for asthma. Jan 11, 2017. [Accessed November 4, 2018]. Available from: https://www.nice.org.uk/guidance/mib90/resources/smartinhaler-for-asthma-pdf-63499461673669.
- 14.Propeller Propeller Health receives FDA clearance for the Propeller platform in association with GSK’s Elipta® inhaler. Jul 11, 2016. [Accessed November 4, 2018]. Available from: https://www.propellerhealth.com/2016/11/07/propeller-health-receives-fda-clearance-for-the-propeller-platform-in-association-with-gsks-ellipta-inhaler/
- 15.European Medicines Agency Qualification opinion on ingestible sensor system for medication adherence as biomarker for measuring patient adherence to medication in clinical trials. Feb 15, 2016. [Accessed November 4, 2018]. Available from: https://www.ema.europa.eu/documents/regulatory-procedural-guideline/qualification-opinion-ingestible-sensor-system-medication-adherence-biomarker-measuring-patient_en.pdf.
- 16.Lugaresi A. RebiSmart™ (version 1.5) device for multiple sclerosis treatment delivery and adherence. Expert Opin Drug Deliv. 2013;10(2):273–283. doi: 10.1517/17425247.2013.746311. [DOI] [PubMed] [Google Scholar]
- 17.Bayas A. Improving adherence to injectable disease-modifying drugs in multiple sclerosis. Expert Opin Drug Deliv. 2013;10(3):285–287. doi: 10.1517/17425247.2013.763793. [DOI] [PubMed] [Google Scholar]
- 18.Bayas A, Ouallet JC, Kallmann B, et al. Adherence to, and effectiveness of, subcutaneous interferon β-1a administered by RebiSmart® in patients with relapsing multiple sclerosis: results of the 1-year, observational smart study. Expert Opin Drug Deliv. 2015;12(8):1239–1250. doi: 10.1517/17425247.2015.1057567. [DOI] [PubMed] [Google Scholar]
- 19.Fernández O, Arroyo R, Martínez-Yélamos S, et al. Long-term adherence to IFN beta-1a treatment when using RebiSmart® device in patients with relapsing-remitting multiple sclerosis. PLoS One. 2016;11(8):e0160313. doi: 10.1371/journal.pone.0160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paolicelli D, Cocco E, Di Lecce V, et al. Exploratory analysis of predictors of patient adherence to subcutaneous interferon beta-1a in multiple sclerosis: tracer study. Expert Opin Drug Deliv. 2016;13(6):1–7. doi: 10.1517/17425247.2016.1158161. [DOI] [PubMed] [Google Scholar]
- 21.Devonshire VA, Feinstein A, Moriarty P. Adherence to interferon β-1a therapy using an electronic self-injector in multiple sclerosis: a multicentre, single-arm, observational, phase IV study. BMC Res Notes. 2016;9:148. doi: 10.1186/s13104-016-1948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moccia M, Palladino R, Russo C, et al. How many injections did you miss last month? A simple question to predict interferon beta-1a adherence in multiple sclerosis. Expert Opin Drug Deliv. 2015;12(12):1829–1835. doi: 10.1517/17425247.2015.1078789. [DOI] [PubMed] [Google Scholar]
- 23.Solsona MDE, Boquet EM, Estruch BC, et al. Impact of adherence on subcutaneous interferon beta-1a effectiveness administered by Rebismart® in patients with multiple sclerosis. Patient Prefer Adherence. 2017;11:415–421. doi: 10.2147/PPA.S127508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis H, Webster J, Larkin AM, Parkes L. An observational, retrospective, UK and Ireland audit of patient adherence to subcutaneous interferon beta-1a injections using the RebiSmart(®) injection device. Patient Prefer Adherence. 2014;8:843–851. doi: 10.2147/PPA.S54986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zecca C, Disanto G, Mühl S, Gobbi C. Subjective patient-reported versus objective adherence to subcutaneous interferon β-1a in multiple sclerosis using RebiSmart®: the CORE study. BMC Neurol. 2017;17(1):171. doi: 10.1186/s12883-017-0952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen ED, Stenager E, Vadgaard JL, et al. Adherence to subcutaneous interferon beta-1a treatment using an electronic injection device: a prospective open-label Scandinavian noninterventional study (the ScanSmart study) Patient Prefer Adherence. 2018;12:569–575. doi: 10.2147/PPA.S154417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rau D, Roßnagel F, Gössling J, et al. Adherence, cognition and behavioral performance in relapsing-remitting MS RRMS patients using the electronic autoinjector RebiSmart: 1 and 2-year follow-up from the German multicenter RebiSmart study. Mult Scler J. 2017;23(3 Suppl):889–890. [Google Scholar]
- 28.Lugaresi A, Florio C, Brescia-Morra V, et al. Patient adherence to and tolerability of self-administered interferon β-1a using an electronic autoinjection device: a multicentre, open-label, phase IV study. BMC Neurol. 2012;12:7. doi: 10.1186/1471-2377-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lugaresi A, De Robertis F, Clerico M, et al. Long-term adherence of patients with relapsing-remitting multiple sclerosis to subcutaneous self-injections of interferon β-1a using an electronic device: the RIVER study. Expert Opin Drug Deliv. 2016;13(7):931–935. doi: 10.1517/17425247.2016.1148029. [DOI] [PubMed] [Google Scholar]
- 30.Ghezzi A, Bianchi A, Baroncini D, et al. A multicenter, observational, prospective study of self- and parent-reported quality of life in adolescent multiple sclerosis patients self-administering interferon-β1a using RebiSmart™-the FUTURE study. Neurol Sci. 2017;38(11):1999–2005. doi: 10.1007/s10072-017-3091-6. [DOI] [PubMed] [Google Scholar]
- 31.Ziemssen T, Sylvester L, Rametta M, Ross AP. Patient satisfaction with the new interferon beta-1b autoinjector (BETACONNECT™) Neurol Ther. 2015;4(2):125–136. doi: 10.1007/s40120-015-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleiter I, Lang M, Jeske J. Adherence, satisfaction and functional health status among patients with multiple sclerosis using the BETACONNECT™ autoinjector: a prospective observational cohort study. BMC Neurol. 2017;17(1):174. doi: 10.1186/s12883-017-0953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti F, Martínez Ginés ML, Norenberg C, Duarte Caron F. BetaEval Global: Prospective, non-interventional, multinational, observational cohort study of patients using BETACONNECT. Mult Scler J. 2017;23(3 Suppl):954. [Google Scholar]
- 34.Rametta M, Germino R, Mcleod K, et al. Impact of Betaconnect auto-injector on patient adherence of betaseron therapy. Annual meeting of the Consortium of Multiple Sclerosis Centers; 2017 May 24-27; New Orleans, LA, USA. [Google Scholar]
- 35.Belknap R, Weis S, Brookens A, et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS One. 2013;8(1):e53373. doi: 10.1371/journal.pone.0053373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res. 2017;19(7):e246. doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moorhead P, Zavala A, Kim Y, Virdi NS. Efficacy and safety of a medication dose reminder feature in a digital health offering with the use of sensor-enabled medicines. J Am Pharm Assoc. 2017;57(2):155–161. doi: 10.1016/j.japh.2016.12.067. [DOI] [PubMed] [Google Scholar]
- 38.Dicarlo L, Kim YA, Young J, Bezhadi Y. Real-time assessment of medication taking and activities of daily living in patients with uncontrolled hypertension. Value Health. 2014;17(7):A475. doi: 10.1016/j.jval.2014.08.1360. [DOI] [PubMed] [Google Scholar]
- 39.Godbehere P, Wareing P. Hypertension assessment and management: role for digital medicine. J Clin Hypertens. 2014;16(3):235. doi: 10.1111/jch.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naik R, Macey N, West RJ, et al. First use of an ingestible sensor to manage uncontrolled blood pressure in primary practice: the UK hypertension registry. J Community Med Health Educ. 2017;7(1):1–5. [Google Scholar]
- 41.Bonacini M, Ya K, Pitney C, et al. A novel digital medicine offering to assess and optimize adherence for oral hepatitis C treatment. Hepatol. 2017;66(Suppl 1):394A. [Google Scholar]
- 42.Sullivan S, Cutright L, McConnell L, et al. Use of digitized medications to enhance adherence and transplant outcomes. Pediatr Transplant. 2017;21(Supplement 1):33. [Google Scholar]
- 43.Au-Yeung KY, DiCarlo L. Cost comparison of wirelessly vs directly observed therapy for adherence confirmation in anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2012;16(11):1498–1504. doi: 10.5588/ijtld.11.0868. [DOI] [PubMed] [Google Scholar]
- 44.Kim YA, Virdi N, Raja P, Dicarlo L. Modeling the impact of a digital health feedback system in uncontrolled hypertensive patients. Value Health. 2014;17(7):A479. doi: 10.1016/j.jval.2014.08.1382. [DOI] [PubMed] [Google Scholar]
- 45.Kim YA, Raja P, Dicarlo L, Virdi N, Park H. An economic model of the impact of digital medicines with a mobile application in patients with comorbid hypertension, diabetes, and hypercholesterolemia. Value Health. 2015;18(3):A40. [Google Scholar]
- 46.Chen AH, Harrison J, Black PN, Mitchell EA, Foster JM. Using electronic monitoring devices to measure inhaler adherence: a practical guide for clinicians. J Allergy Clin Immunol Pract. 2015;3(3):335–349. doi: 10.1016/j.jaip.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 47.Morton RW, Elphick HE, Rigby AS, et al. STAAR: A randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax. 2017;72(4):347–354. doi: 10.1136/thoraxjnl-2015-208171. [DOI] [PubMed] [Google Scholar]
- 48.Chan AHY, Stewart AW, Harrison J, Camargo CA, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med. 2015;3(3):210–219. doi: 10.1016/S2213-2600(15)00008-9. [DOI] [PubMed] [Google Scholar]
- 49.Burgess SW, Sly PD, Devadason SG. Providing feedback on adherence increases use of preventive medication by asthmatic children. J Asthma. 2010;47(2):198–201. doi: 10.3109/02770900903483840. [DOI] [PubMed] [Google Scholar]
- 50.Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. J Allergy Clin Immunol. 2007;119(4):811–816. doi: 10.1016/j.jaci.2006.11.700. [DOI] [PubMed] [Google Scholar]
- 51.Foster JM, Usherwood T, Smith L, et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol. 2014;134(6):1260–1268. doi: 10.1016/j.jaci.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 52.Britto MT, Rohan JM, Dodds CM, Byczkowski TL. A randomized trial of user-controlled text messaging to improve asthma outcomes: a pilot study. Clin Pediatr. 2017;56(14):1336–1344. doi: 10.1177/0009922816684857. [DOI] [PubMed] [Google Scholar]
- 53.Kenyon CC, Chang J, Wynter SA, Fowler JC, Long J, Bryant-Stephens TC. Electronic adherence monitoring in a high-utilizing pediatric asthma cohort: a feasibility study. JMIR Res Protoc. 2016;5(2):e132. doi: 10.2196/resprot.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merchant R, Inamdar R, Henderson K, et al. Digital health intervention for asthma: patient-reported value and usability. JMIR Mhealth Uhealth. 2018;6(6):e133. doi: 10.2196/mhealth.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract. 2016;4(3):455–463. doi: 10.1016/j.jaip.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Van Sickle D, Barrett M, Humblet O, et al. Randomized, controlled study of the impact of a mobile health tool on asthma SABA use, control and adherence. Eur Respir J. 2016;48:PA1018. [Google Scholar]
- 57.Van Sickle D, Su J, Barrett M, et al. Impact of a mobile health pilot study on asthma rescue inhaler use, control and self-management. Eur Respir J. 2015;46:PA2528. [Google Scholar]
- 58.Van Sickle D, Magzamen S, Truelove S. Online feedback about remotely monitored inhaled bronchodilators improves composite measures of asthma control. Am J Respir Crit Care Med. 2010;181:A3127. [Google Scholar]
- 59.Van Sickle D, Morrison TA. Weekly feedback summarizing use of remotely monitored rescue medication improves asthma control. Am J Respir Crit Care Med. 2011;183:A4755. [Google Scholar]
- 60.Van Sickle D, Magzamen S, Truelove S, et al. Remote monitoring of inhaled bronchodilator use and weekly feedback about asthma management: an open-group, short-term pilot study of the impact on asthma control. PLoS One. 2013;82:e55335. doi: 10.1371/journal.pone.0055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Sickle D, Barrett M, Marcus J, Humblet O, Smith T. Impact of a technology-driven asthma program on symptoms, control and self-management: the clinical perspective. 142nd APHA Annual Meeting & Exposition 2014 Annual Meeting & Exposition; New Orleans, LA, USA. 2014; p. 303269. [Google Scholar]
- 62.Chen JS, Jones-Ford GS, Tuffli M, Barrett M, van Sickle D. Feasibility and clinical impact of deploying a digital health intervention in a Medicare population with COPD. Am J Respir Crit Care Med. 2017;195:A1722. [Google Scholar]
- 63.Barrett M, Combs V, Su JG, et al. AIR Louisville: Addressing asthma with technology, crowdsourcing, cross-sector collaboration, and policy. Health Aff. 2018;37(4):525–534. doi: 10.1377/hlthaff.2017.1315. [DOI] [PubMed] [Google Scholar]
- 64.Carl JC, Isakov D, Winners S, et al. Use of remote electronic monitoring improves asthma outcomes through improved adherence. Am J Resp Crit Care Med. 2018;197:A1164. [Google Scholar]
- 65.Hoch H, Meier MR, Brinton JT, Szefler SJ. A feasibility study of daily monitoring of controller and rescue medication use in a pediatric patient population at high risk of an asthma exacerbation. Am J Resp Crit Care Med. 2017;195:A198. [Google Scholar]
- 66.Kenyon CC, Gruschow SM, Quarshie WO, et al. Controller adherence following hospital discharge in high risk children: a pilot randomized trial of text message reminders. J Asthma. 2018;13:1–9. doi: 10.1080/02770903.2018.1424195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su J, Barrett M, Henderson K, et al. Application of wireless inhaler sensors to enhance asthma surveillance and inform municipal interventions. APHA 2016 Annual Meeting & Exposition; Denver, CO, USA. 2016; p. 4382. [Google Scholar]
- 68.Merchant R, Tuffli M, Barrett M, et al. Impact of a digital health intervention on asthma healthcare utilization. Eur Respir J. 2017;50:PA4692. [Google Scholar]
- 69.European Commission What does the general data protection regulation (GDPR) govern? 2018. [Accessed November 2018]. Available from. Available from: https://ec.europa.eu/info/law/law-topic/data-protection/reform/what-does-general-data-protection-regulation-gdpr-govern_en.
- 70.European Union Regulation (EU) 2016/679 of the European Parliament and of the council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) [April 27, 2016]. [Accessed November 2018]. Available from https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679&from=EN.
- 71.Barone DA, Singer BA, Merkov L, Rametta M, Suarez G. Survey of US patients with multiple sclerosis: comparison of the new electronic interferon beta-1b autoinjector (BETACONNECT™) with mechanical autoinjectors. Neurol Ther. 2016;5(2):155–167. doi: 10.1007/s40120-016-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blakey JD, Bender BG, Dima AL, et al. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Respir J. 2018;52(5) doi: 10.1183/13993003.01147-2018. 180114722 11 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blixen C, Sajatovic M, Moore DJ, et al. Patient participation in the development of a customized m-Health intervention to improve medication adherence in poorly adherent individuals with bipolar disorder (BD) and hypertension (HTN) Int J Healthc. 2018;4(1):25–35. doi: 10.5430/ijh.v4n1p25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalantarian H, Motamed B, Alshurafa N, Sarrafzadeh M. A wearable sensor system for medication adherence prediction. Artif Intell Med. 2016;69:43–52. doi: 10.1016/j.artmed.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Klonoff DC, Kerr D. Smart pens will improve insulin therapy. J Diabetes Sci Technol. 2018;12(3):551–553. doi: 10.1177/1932296818759845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Järvinen E, Multanen J, Atula S. Subcutaneous interferon β-1a administration by electronic auto-injector is associated with high adherence in patients with relapsing remitting multiple sclerosis in a real-life study. Neurol Int. 2017;9(1):6957. doi: 10.4081/ni.2017.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Virdi NS, Kim Y, Raja P, Savage G, Osterberg L. Optimizing treatment in patients with uncontrolled hypertension and type 2 diabetes by using a digital health offering. J Am Soc Hypertens. 2016;10(4):e72–e73. [Google Scholar]
- 78.Jochmann A, Nagakumar P, Hall P, et al. Improvement in asthma control and airway inflammation during a period of electronic monitoring. Eur Respir Soc. 2015;46:OA4775. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Predefined research questions
| Research questions |
|---|
| • Is there any regulatory/HTA guidance for connected devices in the US and EU, including apps or software in particular? |
| • What was the approval pathway for any connected devices that have been reviewed by regulatory authorities? |
| • Which clinical studies or other studies were required by regulatory and HTA bodies in order to grant approval of the selected connected devices? |
| • What is the impact of selected connected devices on adherence (clinical studies or RWE)? |
| • What is the impact of selected connected devices on clinical outcomes? |
| • What is the impact of selected connected devices on health care costs and resources, including doctors’ visits (scheduled and unscheduled visits)? |
| • What are the potential barriers that need to be overcome to unfold the full potential of these tools? |
Abbreviations: EU, European Union; HTA, Health technology assessment; RWE, real-world evidence; US, United States.
Table S2.
Data sources searched
| Data sources searched |
|---|
| PubMed |
| Google Scholar |
| Company websites |
| HTA databases (AETSA; AHRQ; CADTH; CTAF-ICER; HAS; INAHTA; IQWiG; NHS; NICE; PBAC; SMC) |
| USFDA website |
| EMA website |
| Clinicaltrials.gov |
| International Clinical Trials Registry Platform |
Abbreviations: AETSA, Agencia de Evaluación de Tecnologías Sanitarias de Andalucía; AHRQ, The Agency for Health care Research and Quality; CADTH, Canadian Agency for Drugs and Technologies in Health; CTAF-ICER, The California Technology Assessment Forum – Institute for Clinical and Economic Review; EMA, European Medicines Agency; HAS, Haute Autorité de Santé; HTA, Health technology assessment; INAHTA, The International Network of Agencies for Health Technology Assessment; IQWiG, The Institute for Quality and Efficiency in Health care; NHS, National Health Service; NICE, National Institute for Health and Clinical Excellence; PBAC, Pharmaceutical Benefits Advisory Committee; SMC, Scottish Medicines Consortium; USFDA, United States Food and Drug Administration.
Table S3.
Search terms used
| Device | Synonym/local language translation |
|---|---|
| Proteus Discover | Proteus pill, Proteus patch, and other translated terms of the device in different languages (eg, Proteus-Pille [German], Proteus pillola [Italian], pilule Proteus [French], píldora de Proteus [Spanish]) |
| RebiSmart® | Msdialog and other translated terms of the device in different languages (same as that of English) |
| BETACONNECT™ | BETACONNECT™ system, BETACONNECT™, and other translated search terms of the device in different languages (eg, Beta verbinden [German], Beta connettersi [Italian]; while French and Spanish terms remained same as that of English language) |
| SmartInhaler™ | Smart inhaler, SmartInhaler medication sensors, SmartInhaler sensors, Smartturbo, Smarttouch and other translated terms of the device in different languages (eg, Intelligenter Inhalator [German], Inalatore intelligente [Italian], Inhalateur intelligent [French], Inhalador inteligente [Spanish]) |
| Propeller | Propeller Sensor, specific device terms including Ellipta, Respimat, Diskus, MDI, metered-dose inhaler, and other translated terms of the device in different languages (eg, Elica [Italian], Hélice [French & Spanish]); while the German term remained same as that of English language |
Note: General searches were also undertaken to identify guidance documents for connected devices overall.
Abbreviation: MDI, metered-dose inhaler.