ABSTRACT
Mobility in advanced cancer patients is a major health care concern and is often lost in advanced metastatic cancers. Erosion of mobility is a major component in determining quality of life but also starts a process of loss of muscle and bone mass that further devastates patients. In addition, treatment options become limited in these advanced cancer patients. Loss of bone and muscle occurs concomitantly. Advanced cancers that are metastatic to bone often lead to bone loss (osteolytic lesions) but may also lead to abnormal deposition of new bone (osteoblastic lesions). However, in both cases there is a disruption to normal bone remodeling and radiologic evidence of bone loss. Many antitumor therapies can also lead to loss of bone in cancer survivors. Bone loss releases cytokines (TGFβ) stored in the mineralized matrix that can act on skeletal muscle and lead to weakness. Likewise, loss of skeletal muscle mass leads to reduced bone mass and quality via mechanical and endocrine signals. Collectively these interactions are termed bone‐muscle cross‐talk, which has garnered much attention recently as a prime target for musculoskeletal health. Pharmacological approaches as well as nutrition and exercise can improve muscle and bone but have fallen short in the context of advanced cancers and cachexia. This review highlights our current knowledge of these interventions and discusses the difficulties in treating severe musculoskeletal deficits with the emphasis on improving not only bone mass and muscle size but also functional outcomes. © 2019 The Authors. JBMR Plus published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
Keywords: TUMOR‐INDUCED BONE DISEASE, BONE‐MUSCLE INTERACTIONS, CANCER, CHEMOTHERAPY
Introduction
Bone loss and muscle weakness are significant sequelae of cancers metastatic to bone and of cancer therapy. Specifically, cancers of the breast, prostate, and lung have a high propensity for metastasis to bone, with 73%, 68%, and 36% of patients with advanced cancer developing a bony lesion, respectively.1 Estrogen‐receptor positive status has been identified as a potential risk for developing breast cancer bone metastases;2 however, a recent systematic analysis concluded that the primary risk factors for developing bone metastases in women with breast cancer are younger age, greater stage, and larger tumor size at diagnosis, whereas estrogen‐receptor status had no effect on bone metastasis risk.3 For patients diagnosed with prostate cancer, PSA levels ≥20 ng/mL, a Gleason score ≥8, and locally advanced disease are risk factors for developing bone metastases.4 In lung cancer, bone metastases are more commonly found in the adenocarcinoma subtype, whereas they are least common in small cell lung cancer.5 Whether the bone lesions are osteolytic (bone loss) or osteoblastic (bone formation) by X‐ray imaging, there is evidence of excess bone resorption in the majority of cancers metastatic to bone and increased risk of fractures that require surgery and spinal cord compression complications.6, 7 Cancer patients are also at increased risk of developing osteoporosis due to cancer treatment, so‐called cancer treatment‐induced bone loss (CTIBL).8, 9
Muscle weakness in patients with advanced cancer is associated with poor outcomes and exists as a spectrum that ranges from weakness in the absence of weight loss to profound muscle wasting and cachexia.10 Muscle weakness and loss of muscle mass affects between 15% and 80% of patients with cancer, depending upon tumor type and stage,11, 12 with the highest prevalence in those with advanced stages of cancer.13 Although the prevalence of combined muscle and bone loss in patients with cancer is unknown, it is logical to assume that they occur together relatively frequently given the importance of muscle‐bone cross‐talk in maintaining both tissue types.10
Bone loss and muscle weakness in cancer patients increase the risk of falls and fractures.9 In fact, a fivefold increase in fractures per year has been shown for women with newly diagnosed breast cancer receiving chemotherapy.14 These musculoskeletal events further negatively impact performance status, survival, and quality of life. Performance assessments of muscle function in cancer patients who received chemotherapy show slower chair‐rise time, reduced hand‐grip strength, and a decline in 12‐minute walk distance compared with healthy control individuals.15 Moreover, individual physician‐documented case reports show that lower‐extremity muscle weakness is a common complaint in patients receiving chemotherapy.16
The reduction in bone quality and muscle function are further exacerbated by inactivity often associated with these patients, which sets up a vicious cycle of increased immobility and reduced bone and muscle quality. In many cases, this reduces cancer treatment options, further eroding survival. Compounding the acute clinical impact of cancer metastases to bone and chemotherapy toxicities is the fact that these often cause chronic muscle weakness and exercise intolerance that can persist from months to years after remission of cancer.15, 17
Bone Loss in Cancer Patients
Long‐term sequelae of cancer therapy include an increased risk for developing osteoporosis. Several anticancer therapies (hormonal and nonhormonal) have the potential to promote bone loss through direct dysregulation of bone turnover and indirect mechanisms such as hypogonadism and nephrotoxicity.18 Such therapies include endocrine therapies for breast cancer, which mitigate the effects of estrogen; androgen deprivation therapy (ADT) for prostate cancer; and antineoplastic drugs such as platinum‐derived compounds (cisplatin), alkylating agents (ifosfamide, cyclophosphamide, doxorubicin), antimetabolites (methotrexate), glucocorticoids, and targeted therapies. Additionally, other interventions for cancer patients such as radiation therapy, gonadal ablation, bilateral orchiectomy, and oophorectomy also result in bone loss.9 Ultimately, increased bone resorption and turnover can lead to osteopenia, osteoporosis, and resultant increases in fracture risk and mortality.18
Much current work is focused on understanding cancer therapy‐induced bone loss and the best approaches to preventing or reducing bone loss. In particular, preclinical studies of breast cancer bone metastases have shed much light on this topic. Doxorubicin and carboplatin chemotherapies have been used to study musculoskeletal changes and have revealed that these agents alone cause significant reduction in bone volume.19, 20 The combination therapy Folfiri (5‐fluorouracil, leucovorin, and irinotecan) also causes reduced bone volume.21
Increased risk of fracture arises from low bone mass, low bone strength, microarchitectural disruption, and increased skeletal fragility. Further, fragility fractures (fractures that occur without trauma) are commonly found in the spine (vertebral compression fractures), hip, and wrist. The World Health Organization (WHO) has defined osteopenia and osteoporosis based on dual‐energy X‐ray absorptiometry (DXA) measurements,22 and individuals with low bone mass are at increased risk for fracture. Although age‐related bone loss and increased fracture risk is a significant issue in the general population, it is even more concerning for cancer patients and survivors. Due to cancer therapy, cancer patients and survivors suffer from accelerated bone loss. Indeed, rates of bone loss from cancer therapy can be 10 times higher than in the general population.23, 24, 25, 26
As of 2016, there were more than 7.3 million male cancer survivors and 8.1 female cancer survivors.27 Prostate cancer survivors comprise 44.8% of male cancer survivors, and breast cancer survivors comprise 43.6% of female cancer survivors. Survivors with these cancer types are at the most significant risk of developing bone metastases, and further, these cancer types are often treated with therapies that negatively impact bone mass.28, 29
Breast cancer
The majority of breast cancer patients have pathology consistent with overexpression of hormone receptors (ER+/PR+).30 Thus, the majority of breast cancer survivors are treated with endocrine therapies. Aromatase inhibitors (AIs) block estrogen production. The most common AIs are anastrozole, letrozole, and exemestane. Other drugs block estrogen's effects by binding to the estrogen receptor. Tamoxifen is a selective estrogen receptor modulator (SERM), and fulvestrant is a selective estrogen receptor degrader (SERD). Surgical interventions such as ovarian ablation will eliminate ovarian function and estrogen production. Lastly, ovarian suppression can also be induced through drugs such as goserelin or leuprolide.
Premenopausal breast cancer patients receiving ovarian suppression in the form of goserelin have increased bone loss (10.5%) 6 years after a 2‐year treatment regimen compared with women receiving a traditional adjuvant chemotherapy regimen of cyclophosphamide, methotrexate, and 5‐fluorouracil (6.5%). The onset of bone loss from premature menopause is sudden (6 months of treatment)31 and significant (21% decreased bone density compared with age‐matched eumenorrheic women).32, 33 In postmenopausal women, several large studies have been completed examining risk of bone fracture in breast cancer patients who received tamoxifen, AIs, or no endocrine treatment. A recent meta‐analysis examined 21 independent studies in women aged 65 and younger, with stage 1 to 3 breast cancer, treated with tamoxifen or AIs, and showed: 1) fracture risk was not elevated by tamoxifen use; 2) AIs increased fracture risk by 17% compared with women who did not receive AIs; and 3) AIs increased fracture risk by 35% compared with women on tamoxifen.34 It was also observed that women on AIs have higher fracture risk during treatment than after treatment end.34 Indeed, long‐term survivors of early stage breast cancer (postmenopausal at diagnosis) are not at greater risk of osteoporotic fractures compared with age‐matched women without breast cancer.35
Prostate cancer
Significant bone loss can occur in men with prostate cancer who are treated with ADT due to hypogonadism.36 It is important to note that guidelines from the American Urological Association recommend that primary ADT alone not be included among standard options for the initial treatment of men with localized prostate cancer.37, 38 Radiation therapy is usually combined with ADT for improved outcomes in men with intermediate or high‐risk prostate cancer.39, 40, 41 Further, it is unclear if adding chemotherapy in combination with radiation therapy and ADT will improve outcomes in the adjuvant setting.42, 43 In the metastatic setting for prostate cancer, ADT is the main therapeutic approach with combination of antiandrogens and chemotherapy determined by clinical presentation. Other treatments that lower androgen levels include orchiectomy (surgical castration); luteinizing hormone‐releasing hormone agonists (leuprolide, goserelin, triptorelin, and histrelin); an inhibitor of the CYP17 enzyme, abiraterone; and antiandrogens (flutamide, bicalutamide, nilutamide, enzalutamide, and apalutamide).
Loss of bone mineral density can be detected after 6 to 9 months of ADT, and longer therapy confers a higher risk.44, 45, 46 Annual declines of bone mineral density are 2% to 8%.47, 48 Osteoporotic skeletal fractures occur in up to 20% of men within 5 years of starting ADT.49 Although several small studies have shown biclutamide, a nonsteroidal antiandrogen, to have more favorable bone health outcomes,50, 51 the prevailing consensus is that hypogonadism induced through treatment of prostate cancer is a contributor to reduced bone mineral density and that fracture risk is a concern for nonmetastatic and metastatic castration‐resistant prostate cancer.52
Although hormone‐related therapies for breast and prostate cancer survivors are the primary drivers of bone loss in these patients, there are other common cancer treatments that have been associated with bone loss, and the mechanism of action for bone loss sequela from these treatments has been well reviewed previously for breast and prostate cancer survivors.18, 53 Specifically, the use of traditional chemotherapies such as cisplatin, ifosfamide, cyclophosphamide, doxorubicin, and methotrexate all have bone‐related side effects. Newer targeted therapies are also emerging as having significant bone loss sequela.
Muscle Loss in Cancer Patients
Muscle wasting is a commonly observed phenomenon in the setting of cancer.54 As muscle is lost, patients may initially be considered sarcopenic, a term taken from Greek sárks penia, a poverty of flesh. If muscle loss continues, patients may be diagnosed with cachexia, from the Greek kákos + hexis, a bad state of the body. Thresholds to define both sarcopenia and cachexia vary enough to result in a 19‐ to 26‐fold variation in prevalence.55 The clinical definitions of sarcopenia include the presence of low skeletal muscle mass and either 1) low muscle strength, 2) low muscle function, or 3) low muscle performance. For research purposes, sarcopenia may be defined as skeletal muscle index measured via CT scan, as muscle area (cm2) standardized to height (m2), with specific cutpoints by sex and body mass index (BMI).56 Sarcopenic obesity refers to depleted muscle mass in individuals with BMI higher than 30 kg/m2.57 Sarcopenia can be found as being along the progression toward cachexia, for which there are also multiple definitions. A recent international consensus process led by experts in medical cachexia defined cachexia as weight loss of at least 5% or more in 12 months or less in the presence of underlying illness, plus three of the following criteria: decreased muscle strength, fatigue, anorexia, low fat‐free mass index, abnormal biochemistry (increased inflammatory markers [C‐reactive protein >5.0 mg/L, IL‐6 >4.0 pg/mL], anemia [<12 g/dL], and low serum albumin [<3.5 g/dL]).58
Within the setting of cancer, prevalence of sarcopenia has been reported to be 16% among long‐term breast cancer survivors59 and 56% and 60% among women and men, respectively, with stage 1 to 3 colorectal cancer.60 Further, sarcopenia is not reserved to those with a BMI <25 mg/kg2. Among patients with solid tumors of the respiratory or gastrointestinal systems, 15% of obese patients (BMI >30 kg/m2) were sarcopenic.57 To place the prevalence of sarcopenia during cancer into context, the prevalence in the general population (defined as: [(Appendicular Skeletal Muscle Mass)/BMI] <0.512 for women, or <0.789 for men) is 12% or 34% in women and men of all ages, and 48% or 27.5% among women and men older than 80 years.61 Comparison of prevalence among cancer patients and the general population suggests that sarcopenia may be more prevalent among cancer patients, though direct comparisons have yet to be made.
The prevalence of cachexia varies by stage of cancer: 0.5% in all cancer patients versus 36% to 80% among advanced cancer patients.62 The incidence rate of cachexia is often highly associated with tumor type presentation and as such, patients with pancreatic cancer, gastric cancer, and lung cancer predominantly are reported to have higher incidence of cachexia.63, 64, 65 The progression of cachexia is exacerbated in the presence of metastasis. For example, in lung cancer patients with metastasis, occurrence of cachexia is higher in metastatic patients than in nonmetastatic patients.66 As with sarcopenia, cachexia is not limited to those with a BMI <25. In one study, 35.9% of advanced cancer patients were diagnosed as cachectic. Among those, 58% and 14% were normal weight and overweight/obese, respectively.67
It is difficult to assess the impact of chemotherapy directly on skeletal muscle in patients, but one study suggests that patients treated with neoadjuvant chemotherapy experience significant muscle wasting.68 In addition to loss of muscle mass, muscle weakness is an equally important adverse effect of cancer and cancer therapy (chemotherapy, radiation, and hormone‐deprivation) in cancer patients. This aspect is gaining attention as clinicians are beginning to directly assess physical activity and functional status in their patients. For example, breast cancer patients report impaired muscle function when compared with healthy peers.69 A recent assessment of the functional capacity of breast cancer patients (stationary bicycle measure of power) showed that loss of muscle function was independent of loss of muscle mass.70 The clinical impact of muscle weakness in cancer needs to be more thoroughly investigated because outright cachexia represents one end of a very extreme spectrum with muscle weakness but not wasting on one end and muscle weakness with severe cachexia on the other end.
Many animal studies have shown that chemotherapy causes skeletal muscle atrophy. The common chemotherapy, cisplatin, has been shown to cause muscle atrophy that is associated with activation of NF‐κB signaling pathway and independent of the well‐characterized activation of ubiquitin proteosomal degradation.71 Another platinum coordinating therapy, carboplatin, has also been shown to lead to muscle wasting.20 Doxorubicin causes skeletal muscle weakness in part through a tumor necrosis factor receptor (TNFR1)‐dependent manner.72, 73 Finally, the combination therapy, Folfiri, has been shown to lead to skeletal muscle wasting that includes mitochondrial dysfunction.74
Multiple studies have examined the impact of muscle loss on cancer‐specific survival (CSS), disease‐free survival (DFS), and overall survival (OS), as well as time to tumor progression and chemotoxicities. For example, Prado and colleagues noted that sarcopenic women with metastatic breast cancer who were treated with capecitabine had a shorter time to tumor progression as well as elevated treatment toxicities compared with nonsarcopenic patients.75 Deluche noted that early stage breast cancer patients with sarcopenia had reduced DFS and OS.76 Two studies have assessed the impact of sarcopenia on outcomes in hepatocellular carcinoma and observed elevations in chemotoxicities77 and decreased overall survival.78 Similar findings have been noted in gastric cancer.79 In a recent systematic review that included 7843 patients from 38 studies, sarcopenia was found to be predictive of worse CCS, DFS, and OS.80 In some clinical studies, the definition of sarcopenia and cachexia have led to significant confusion, but given these highly consistent findings that muscle loss is associated with worse cancer prognosis, explorations of possible mechanisms through which muscle loss occurs is worthy of attention.
One mechanism associated with cancer cachexia is elevated inflammation, including high levels of interleukin (IL)‐6 as well as increased oxidative stress.81 These same factors are also associated with the development and progression of tumors. In turn, skeletal muscle ryanodine receptor 1 (RyR1) intracellular Ca2+ release channels, required for skeletal muscle excitation–contraction coupling, becomes oxidized in the setting of bone metastases, resulting in reduced muscle function.82 Skeletal muscle samples from human lung and breast cancer patients with bone metastases also exhibit evidence of RyR1 Ca2+ channel leak. Remaining questions in this area include whether improvements in muscle mass, function, and strength could prevent onset or progression of cachexia.
In humans, resistance exercise has been shown to prevent increases in IL‐6 during treatment for breast cancer.83 Further, low‐intensity, low‐volume resistance exercise in rodents reduced inflammatory cytokines.84 In addition, protein supplementation boosts the increases in muscle mass observed with resistance exercise training.85 An ongoing randomized controlled trial in early stage human colon cancer patients is examining the potential for resistance exercise and protein supplementation during chemotherapy to increase muscle mass, decrease chronic inflammation, and improve cancer outcomes (NCT03291951).
Exercise and Nutritional Interventions
Exercise is recommended for maintenance of bone and muscle in patients undergoing treatment for cancer.86, 87 Moderate‐intensity weight‐bearing aerobic and resistance exercise is recommended to preserve and improve bone density in adult populations with and without cancer. The American College of Sports Medicine (ACSM) specifically recommends that adults perform 30 to 60 minutes of endurance activities 3 to 5 times per week and of resistance exercise 2 to 3 times per week.87 Resistance and aerobic exercise training have been shown to preserve or improve bone density in cancer survivors and patients actively undergoing hormone therapy.88, 89, 90, 91, 92 Twenty‐six weeks of combined aerobic and resistance training has shown benefit for female cancer survivors in improved spine, hip, and whole body bone mineral density (BMD), but these results were not stratified by type of cancer or cancer therapy during primary tumor treatment.93 In another study, again not stratified by tumor type or treatment, 24 months of strength and weight training showed improved balance and muscle strength in breast cancer survivors.94 In metastatic breast cancer patients (65% of whom had bone metastases), reduced muscle strength and lower physical activity was reported compared with healthy age‐matched women.95 There are limited studies during chemotherapy to provide conclusive evidence on the effectiveness of exercise during chemotherapy to preserve bone health. Only two studies have been identified that assessed the effect of exercise on bone health during chemotherapy for women diagnosed with breast cancer, with both studies finding no significant effect.16, 96 However, neither study met the ACSM guidelines for exercise to maintain bone health.
Progressive resistance training has been shown to increase lean muscle mass and muscular strength in patients diagnosed with cancer, both during and after treatment.97, 98, 99 As such, exercise has been suggested as a therapeutic strategy to prevent or treat cancer‐related cachexia; however, there is limited clinical evidence supporting this suggestion.100 A confounding factor to most of the reported studies is that the exercise regimen (aerobic or resistance) is not consistent, nor is the duration or intensity. To get a better handle on the true effects, better controlled studies are needed in the appropriate patient populations (eg, precachexia) to determine the effect and dosage of exercise on bone health during chemotherapy treatment as well as in the prevention and treatment of cancer‐related cachexia.
Nutrition is important in the preservation of bone and muscle mass for patients diagnosed with cancer. Calcium and vitamin D supplements are potential strategies to prevent bone loss in individuals undergoing treatment for cancer. Individuals at risk for bone loss are recommended to consume 1200 to 1500 mg of calcium and 400 to 600 IU of vitamin D per day; however, there are no established guidelines for calcium and vitamin D in patients with cancer.86, 101 Studies have shown that 70% to 97.5% of patients with cancer are vitamin D deficient.102, 103, 104, 105 Calcium levels in patients with cancer vary. Up to 3% of patients with cancer experience hypercalcemia, with the majority of these patients having an advanced cancer diagnosis.106, 107 The increased calcium levels are typically due to increased parathyroid hormone–related protein (PTHrP) levels secreted by the tumor.108 PTHrP increases the bone‐resorbing activity of osteoclasts resulting in the hypercalcemia found in these patients. In contrast, hypocalcemia occurs as a result of osteoblastic lesions or the use of bone‐modifying agents such as denosumab or bisphosphonate. Few studies have looked at the total incidence of hypocalcemia in this patient population; however, a recent systematic review found that 5% of patients with cancer treated with denosumab develop hypocalcemia.109 No data have been published on the incidence and severity of either hyper‐ or hypocalcemia in patients with early stage cancer who are not receiving bone‐modifying agents. Two systematic reviews looking at the effect of calcium and vitamin D supplementation on bone health in early stage breast and prostate cancer patients receiving hormone therapy found the current recommended guidelines to be inadequate to have an effect on bone health.110, 111 Further studies are required to establish the efficacy of and guidelines for calcium and vitamin D consumption or supplementation on bone health in this population.
Cancer‐related cachexia is not reversible by conventional oral nutritional support.112 Parenteral nutrition and supplementation with branched chain amino acids and fish oil have shown promising results in preserving muscle mass loss in patients at risk for developing cachexia.113, 114, 115, 116 Interventions to increase amino acid ingestions to promote protein synthesis in patients diagnosed with cachexia have shown conflicting results.117, 118 Further clinical studies are needed to fully elucidate the effects of these interventions on muscle mass and survival in patients with cancer‐related cachexia and make meaningful progress for patients.
Preclinical Models of Advanced Metastatic Cancer and Cachexia
Advanced cancers with metastases are the most dangerous to patients and many animal models have been developed to study tumor metastasis. Our focus in this review is on metastatic spread that includes bone. Bone metastases are common in advanced cancer such as breast, prostate, and lung cancer and associated with bone pain, fracture, hypercalcemia, and muscle weakness.119, 120 Bone‐muscle cross‐talk is a key nexus in the development of muscle weakness in bone metastases121 and may be equally important in the development and progression of cachexia. The endocrine signals between bone and muscle are of great interest and likely play a large role in the overall health of the musculoskeletal system. Bone is a uniquely capable tissue for nascent tumor cell survival and proliferation. Solid tumor metastases to bone stimulate bone destruction via osteoclast‐mediated bone resorption, releasing active transforming growth factor β (TGFβ) stored in the bone matrix to promote a feed‐forward cycle of tumor growth and bone destruction.120, 122, 123, 124, 125, 126, 127
We were the first to describe cancer cachexia in animal models of osteolytic bone metastases (human MDA‐MB‐231 cells metastatic to bone). We identified mechanism of bone‐muscle cross‐talk by which bone‐derived TGFβ contributes to muscle weakness via oxidation of skeletal muscle ryanodine receptor1 Ca2+ release channel that is critical for excitation‐contraction coupling (E‐C coupling).121 It has been previously shown that leaky skeletal muscle RyR1 Ca2+ channels cause muscle weakness.128, 129, 130 This RyR1 channel Ca2+ leak results in a reduction in the amount of Ca2+ stored in the sarcoplasmic reticulum, which directly reduces the force of skeletal muscle contraction because it is dependent on the level of tetanic Ca2+ released from the sarcoplasmic reticulum. In support of the bone microenvironment's systemic effects that promote muscle weakness in osteolytic cancer in bone, and that TGFβ is a major mediator, we have shown 1) increased serum TGFβ concentrations in mice with breast cancer bone metastases compared with mice with primary breast cancer (no bone metastases); 2) TGFβ activity increased in skeletal muscle from mice and humans with bone metastases; 3) TGFβ activity is reduced by antiresorptive therapy (bisphosphonate); 4) skeletal muscle expression of NADPH oxidase 4 (Nox4) increased and Nox4‐RyR1 binding increased in muscle from mice and humans with bone metastases; and 5) increased oxidation of skeletal muscle RyR1 and RyR1 Ca2+ leak. Nox4 is a constitutively active oxidase and TGFβ target gene that generates reactive oxygen species (ROS).131, 132 Oxidation of RyR1 results in sarcoplasmic reticulum Ca2+ leak and reduced muscle strength due to loss of the RyR1 complex stabilizing subunit, calstabin1. Calstabin1 maintains the closed state of the RyR1 channel. RyR1 oxidation and decreased calstabin1 binding is a biochemical signature associated with RyR1 sarcoplasmic reticulum Ca2+ leak.128, 129 These are direct effects of osteolytic bone metastases and not due to presence of tumor cells. In fact, a 10‐fold larger inoculum of MDA‐MB‐231 cells into the primary site (mammary fat pad) did not induce cachexia, skeletal muscle weakness, TGFβ signaling in muscle, increased oxidative stress, or RyR1 Ca2+ leak.121
A number of other murine metastatic models have been used to evaluate the biologic changes in bone induced by tumor cells. These include xenografts, either primary cells from patients or established human cell lines, and transplanted into immunocompromised murine hosts.133 Xenograph models have been useful to elucidate mechanistic events within the tumor cells and within osteocytes, and as described, above cancer cachexia in osteolytic bone metastases. However, the absence of relevant stromal‐tumor interactions and a functional immune system can be limitations to these models, depending on experimental questions. Syngeneic mouse models of metastatic breast cancer have been used with some success to evaluate stromal‐tumor interactions and the role of the immune system in controlling metastatic progression but to a lesser extent cancer cachexia. In particular, in transplantable models, tumor cells can be injected orthotopically into the mammary gland into a syngeneic host, and primary tumor growth and metastatic progression occur within weeks to months.134 These models have been established from tumor cells isolated from spontaneous mammary tumors in mice.135, 136 The 4T1 model, a collection of mammary tumor cell lines syngeneic in BALB/c mice, has been the principal transplantable mouse model used to study both tumor‐ and host‐derived factors involved in spontaneous metastasis.135, 137, 138 However, recently the E0771 metastatic model has been developed in C57/BL6 mice.136 Each of these models has specific strengths depending on the scientific question but as a group represent a power set of tools to unravel bone and muscle pathology in cancer and cancer therapy.
Potential Pharmacological and Nonpharmacological Therapeutics
As described above, mice with breast cancer bone metastases develop significant skeletal muscle weakness and cachexia. Stabilization of RyR1 with the small molecule, Rycal S107, prevents RyR1 Ca2+ leak even in the presence of oxidative stress by preventing loss of calstabin1 and did not affect tumor burden. Further, either inhibiting TGFβ directly (TGFβ receptor blockade [SD208] or TGFβ neutralizing antibody [1D11]) or indirectly (blocking TGFβ release from bone using a bisphosphonate zoledronic acid [ZA]) prevented RyR1 oxidation and restored muscle strength in mice with breast cancer bone metastases. Blockade of Nox4 (GKT137831) also restored skeletal muscle strength without affecting tumor burden. Finally, human muscle samples taken from patients with breast cancer and lung cancer with bone metastases had oxidation of RyR1 and loss of calstabin1, validating the clinical significance of these data.121 Thus, TGFβ released from the tumor‐bone microenvironment promotes oxidation of RyR1 and contributes to cancer‐associated skeletal muscle weakness.
The potential of these targets for translation in the clinic is high. Bisphosphonates, such as ZA, are already FDA‐approved. GKT137831 has recently completed phase 2 clinical trials for diabetic nephropathy (trial no. NCT02010242). Anti‐TGFβ therapies have been widely tested across multiple diseases, including recently for metastatic breast cancer in a phase 2 trial by Eli Lilly (trial no. NCT02538471). Finally, a clinical version of Rycal S107 (ARM210/S48168) has obtained FDA Orphan Drug Designation and Rare Pediatric Disease Designation for the treatment of Duchenne muscular dystrophy. Combining these novel therapeutics could lead to improvement for patients with cancer‐associated skeletal muscle weakness.
Other important regulators of cachexia (eg, TNFα, IL‐6) could lead to muscle weakness in osteolytic cancer in bone. Indeed, reduced contractile protein expression or function may also contribute to weakness in bone metastases and could be involved in the novel link we described between bone destruction and weakness via the TGFβ‐Nox4‐RyR1 axis.121 Mice with breast cancer bone metastases also exhibit severe cachexia in addition to TGFβ‐mediated Ca2+ mishandling, after initial muscle weakness is detected.121 Other factors released from the bone matrix during osteolytic bone destruction, such as activin, may contribute to muscle atrophy that is independent of RyR1 Ca2+ leak and remains to be investigated.
In addition to pharmacologic targets, exercise may yield significant gains in musculoskeletal function. The mechanism(s) of exercise‐induced modulation of bone‐muscle cross‐talk in the setting of metastatic cancer patients is an evolving field. In preclinical models, we have demonstrated that 8 weeks of voluntary wheel running, a form of aerobic exercise in mice, can significantly reduce metastatic burden in the lungs and femurs using a clone in the 4T1 series (4T1.2 transfected with luciferase) that has a predilection for bone (unpublished results). The mechanism underlying the protective effect of exercise on metastases is not completely understood. Exercise may be preventing dissemination of tumor cells from the primary tumor or altering the trafficking of metastases into tissue. The entry of tumor cells into the bone microenvironment may cause a number of significant changes including an increase in growth factors (eg, parathyroid hormone–related protein), which causes an upregulation of RANKL, and downregulation of osteoprotegerin (OPG),123 which enhances osteoclast function resulting in greater bone degradation. As a consequence, TGF‐β, VEGF, IGF‐1, and other bone morphogenetic proteins are released into the bone microenvironment.139 In addition, tumor cells secrete cytokine such as IL‐6, IL‐8, and MCP‐1 that impact osteoclast differentiation.140 Exercise may be interrupting a number of the aforementioned pathways to reduce the metastatic burden in bone. In addition, aerobic and/or resistance exercise may be useful interventions to prevent or delay cancer cachexia in osteolytic bone metastases because of the reduction in inflammatory cytokines or improvement in metabolic outcomes observed with exercise. The evaluation of both aerobic and resistance exercise in the 4T1 model may allow us to evaluate exercise as a therapeutic intervention to prevent cancer cachexia and the key biological mediators involved.
Clinical trials have shown that exercise improves bone density in patients with metastatic bone lesions.141, 142 Improvements in bone density in this patient population have been associated with a significant decrease in serum markers of bone resorption such as pyridinium cross‐links pyridinoline (PYD) and C‐terminal cross‐linking telopeptide of type I collagen (CTX‐1).142 Although mechanical loading of bone and/or dietary components have well‐established mechanistic effects on osteoclast and osteoblast activity,143 it is unknown if such mechanisms translate to cachectic conditions often found in patients with bone lesions. Further, the utility of exercise as a nonpharmacological intervention for muscle mass and muscle function appears to be most beneficial in patients with metastatic cancer who are precachectic. Exercise has been shown to decrease circulating levels of IL‐6 in patients with a cancer diagnosis.83 Additionally, resistance training upregulates IGF‐1, which results in activation of the downstream pathway IRS1/PI3K/Akt, and aerobic exercise has been shown to increase production of PGC‐1α4.144 Both Akt and PGC‐1α4 inhibit FOXO3, an upregulator of two ubiquitin ligases important in sarcomere breakdown and inhibition of protein synthesis.81, 145 An important caveat to the above exercise clinical trials in patients with metastatic bone lesions is to recognize that patients were healthy enough to exercise and had limited pain and or sufficient pain management, thus impacting their choice to volunteer for an exercise study.83, 141, 142, 145 Although exercise can be tailored in many different ways, a patient's medical history, clinical presentation (comorbidities and pain), as well as previous health behaviors need to be taken into consideration for treatment choice.
In addition to the effects of exercise on musculoskeletal outcomes, exercise after a cancer diagnosis reduces the risk of cancer recurrence and improves both cancer‐specific and all‐cause mortality. Numerous meta‐analyses146, 147, 148, 149, 150, 151, 152, 153, 154, 155 and a recent systematic review156 have evaluated the strength of the evidence, and there are a considerable number of studies demonstrating a protective effect of exercise on recurrence and mortality in breast, colorectal, and prostate cancer patients. Specifically, exercise after a cancer diagnosis was associated with a 21% to 35% lower risk of cancer recurrence, a 28% to 44% lower risk of cancer‐specific mortality, and a 25% to 48% decreased risk of all‐cause mortality.156 In the aforementioned studies, exercise patterns were assessed by using a variety of self‐report and interview‐administered questionnaires that evaluated a range of exercise habits (eg, occupational, recreational). To date, it is unclear whether exercise is associated with improvements in recurrence and/or survival in patients diagnosed with other cancer types. Furthermore, more studies are need to determine the type of exercise and the dose, duration, and frequency of exercise that is the most beneficial for improving cancer outcomes. Exercise also improves quality of life in some but not all cancer survivors. Improvements in quality of life with exercise have been observed in breast cancer and hematological malignancies, but not in prostate, lung, colorectal, or gynecological cancer survivors.(156) The lack of a positive effect of exercise on quality of life in some cancer may be driven by a limited number of studies in these types of survivors and warrants further study.
Summary
Erosion of musculoskeletal function severely impacts quality of life. In cancer patients, the loss of mobility and risk for falls and fractures are a major concern and can be caused by the tumor itself but also by the therapies used to reduce tumor burden. The loss of muscle mass and function (cachexia) and the loss of bone mass are connected in a feedback loop due to the tight interconnected mechanical and endocrine functions of these tissues. The cancer continuum, from the point of diagnosis to treatment, and survivorship is a highly variable process. For certain cancers, therapeutic strategies do try to account for musculoskeletal effects. In pancreatic cancer, for example, where cachexia incidence is high, nutrition is a key focus during treatment. In bone metastases, the use of bisphosphonates or denosumab to protect against bone loss is used in conjunction with antitumor therapies. However, most treatment strategies do not fully incorporate protection of the musculoskeletal system even though this is critical to quality of life and survival (Fig. 1). A key future direction is the incorporation of musculoskeletal protection and improved function early in the cancer continuum.
Figure 1.
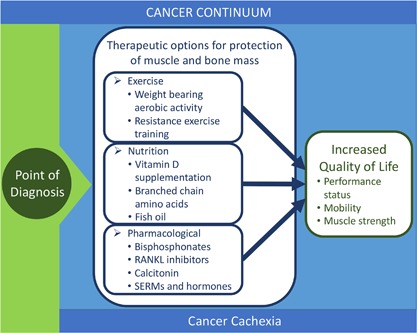
Schematic showing the therapeutic options for loss of bone and muscle throughout the cancer continuum.
Disclosures
All authors state that they do not have any conflicts of interest.
Acknowledgments
Authors’ roles: KMS, KMM, CJR, KHS, and DLW drafted the topics for this review and co‐wrote the manuscript.
References
- 1. Fang J, Xu Q. Differences of osteoblastic bone metastases and osteolytic bone metastases in clinical features and molecular characteristics. Clin Transl Oncol. 2015;17:173–9. [DOI] [PubMed] [Google Scholar]
- 2. Liede A, Jerzak KJ, Hernandez RK, Wade SW, Sun P, Narod SA. The incidence of bone metastasis after early‐stage breast cancer in Canada. Breast Cancer Res Treat. 2016;156:587–95. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H, Zhu W, Biskup E, et al. Incidence, risk factors and prognostic characteristics of bone metastases and skeletal‐related events (SREs) in breast cancer patients: a systematic review of the real world data. J Bone Oncol. 2018;11:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joniau S, Briganti A, Gontero P, et al. Stratification of high‐risk prostate cancer into prognostic categories: a European multi‐institutional study. Eur Urol. 2015;67:157–64. [DOI] [PubMed] [Google Scholar]
- 5. Oliveira MB, Mello FC, Paschoal ME. The relationship between lung cancer histology and the clinicopathological characteristics of bone metastases. Lung Cancer. 2016;96:19–24. [DOI] [PubMed] [Google Scholar]
- 6. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94. [DOI] [PubMed] [Google Scholar]
- 7. Wegener B, Schlemmer M, Stemmler J, Jansson V, Durr HR, Pietschmann MF. Analysis of orthopedic surgery of bone metastases in breast cancer patients. BMC Musculoskelet Disord. 2012;13:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg A, Leitzel K, Ali S, Lipton A. Antiresorptive therapy in the management of cancer treatment‐induced bone loss. Curr Osteoporos Rep. 2015;13:73–7. [DOI] [PubMed] [Google Scholar]
- 9. Guise TA. Bone loss and fracture risk associated with cancer therapy. Oncologist. 2006;11:1121–31. [DOI] [PubMed] [Google Scholar]
- 10. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–9. [DOI] [PubMed] [Google Scholar]
- 11. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract. 2017;32:30–9. [DOI] [PubMed] [Google Scholar]
- 12. Ryan AM, Power DG, Daly L, et al. Cancer‐associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75:199–211. [DOI] [PubMed] [Google Scholar]
- 13. Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF. Estimation of cachexia among cancer patients based on four definitions. J Oncol. 2009;2009:693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hadji P. Cancer treatment‐induced bone loss in women with breast cancer. Bonekey Rep. 2015;4:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilliam LA, St Clair DK. Chemotherapy‐induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011;15:2543–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwartz AL, Winters‐Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34:627–33. [DOI] [PubMed] [Google Scholar]
- 17. Nicolson GL, Conklin KA. Reversing mitochondrial dysfunction, fatigue and the adverse effects of chemotherapy of metastatic disease by molecular replacement therapy. Clin Exp Metastasis. 2008;25:161–9. [DOI] [PubMed] [Google Scholar]
- 18. D'Oronzo S, Stucci S, Tucci M, Silvestris F. Cancer treatment‐induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treat Rev. 2015;41:798–808. [DOI] [PubMed] [Google Scholar]
- 19. Rana T, Chakrabarti A, Freeman M, Biswas S. Doxorubicin‐mediated bone loss in breast cancer bone metastases is driven by an interplay between oxidative stress and induction of TGFbeta. PLoS One. 2013;8:e78043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hain BA, Xu H, Wilcox JR, Mutua D, Waning DL. Chemotherapy‐induced loss of bone and muscle mass in a mouse model of breast cancer bone metastases and cachexia. JCSM Rapid Commun. 2019. (in press). [PMC free article] [PubMed] [Google Scholar]
- 21. Barreto R, Kitase Y, Matsumoto T, et al. ACVR2B/Fc counteracts chemotherapy‐induced loss of muscle and bone mass. Sci Rep. 2017;7:14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–41. [DOI] [PubMed] [Google Scholar]
- 23. Higano CS. Understanding treatments for bone loss and bone metastases in patients with prostate cancer: a practical review and guide for the clinician. Urol Clin N Am. 2004;31:331–52. [DOI] [PubMed] [Google Scholar]
- 24. Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5‐year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–7. [DOI] [PubMed] [Google Scholar]
- 25. Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early‐stage breast cancer. J Clin Oncol. 2001;19:3306–11. [DOI] [PubMed] [Google Scholar]
- 26. Maillefert JF, et al. Bone mineral density in men treated with synthetic gonadotropin‐releasing hormone agonists for prostatic carcinoma. J Urol. 1999;61:1219–22. [PubMed] [Google Scholar]
- 27. Miller KD, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- 28. Jensen AO, Jacobsen JB, Norgaard M, Yong M, Fryzek JP, Sorensen HT. Incidence of bone metastases and skeletal‐related events in breast cancer patients: a population‐based cohort study in Denmark. BMC Cancer. 2011;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saad F, Clarke N, Colombel M. Natural history and treatment of bone complications in prostate cancer. Eur Urol. 2006;49:429–40. [DOI] [PubMed] [Google Scholar]
- 30. Kohler BA, et al. Annual report to the nation on the status of cancer, 1975‐2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johansen JS, Reis BJ, Hassager C, et al. The effect of a gonadotropin‐releasing hormone agonist analog (nafarelin) on bone metabolism. J Clin Endocrinol Metab. 1988;67:701–6. [DOI] [PubMed] [Google Scholar]
- 32. Cann CE, Martin MC, Genant HK, Jaffe RB. Decreased spinal mineral content in amenorrheic women. JAMA. 1984;251:626–9. [PubMed] [Google Scholar]
- 33. Miller KK, Klibanski A. Clinical review 106: amenorrheic bone loss. J Clin Endocrinol Metab. 1999;84:1775–83. [DOI] [PubMed] [Google Scholar]
- 34. Tseng OL, Spinelli JJ, Gotay CC, et al. Aromatase inhibitors are associated with a higher fracture risk than tamoxifen: a systematic review and meta‐analysis. Ther Adv Musculoskelet Dis. 2018;10:71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pawloski PA, Geiger AM, Haque R, et al. Fracture risk in older, long‐term survivors of early‐stage breast cancer. J Am Geriatr Soc. 2013;61:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–36. [DOI] [PubMed] [Google Scholar]
- 37. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2017;199:683–90. [DOI] [PubMed] [Google Scholar]
- 38. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199:990–7. [DOI] [PubMed] [Google Scholar]
- 39. Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mason MD, Parulekar WR, Sydes MR, et al. Final report of the intergroup randomized study of combined androgen‐deprivation therapy plus radiotherapy versus androgen‐deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. 2015;33:2143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fossa SD, Wiklund F, Klepp O, et al. Ten‐ and 15‐yr prostate cancer‐specific mortality in patients with nonmetastatic locally advanced or aggressive intermediate prostate cancer, randomized to lifelong endocrine treatment alone or combined with radiotherapy: final results of the Scandinavian Prostate Cancer Group‐7. Eur Urol. 2016;70:684–91. [DOI] [PubMed] [Google Scholar]
- 42. Fizazi K, Faivre L, Lesaunier F, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high‐risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015;16:787–94. [DOI] [PubMed] [Google Scholar]
- 43. James ND, Sydes MR, Clark NW, et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith MR, Lee WC, Brandman J, et al. Gonadotropin‐releasing hormone agonists and fracture risk: a claims‐based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–903. [DOI] [PubMed] [Google Scholar]
- 45. Saad F, Adachi JD, Brown JP, et al. Cancer treatment‐induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–76. [DOI] [PubMed] [Google Scholar]
- 46. Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83:1561–6. [PubMed] [Google Scholar]
- 47. Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy X‐ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–7; discussion 2367. [PubMed] [Google Scholar]
- 48. Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen‐deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100:892–9. [DOI] [PubMed] [Google Scholar]
- 49. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. [DOI] [PubMed] [Google Scholar]
- 50. Smith MR, Goode M, Zietman AL, et al. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004;22:2546–53. [DOI] [PubMed] [Google Scholar]
- 51. Wadhwa VK, Weston R, Parr NJ. Bicalutamide monotherapy preserves bone mineral density, muscle strength and has significant health‐related quality of life benefits for osteoporotic men with prostate cancer. BJU Int. 2011;107:1923–9. [DOI] [PubMed] [Google Scholar]
- 52. Lowrance WT, et al. Castration‐resistant prostate cancer: AUA guideline amendment 2018. J Urol. 2018;200:1264–72. [DOI] [PubMed] [Google Scholar]
- 53. Handforth C, D'Oronzo S, Coleman R, Brown J. Cancer treatment and bone health. Calcif Tissue Int. 2018;102:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Batsis JA, Mackenzie TA, Lopez‐Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999‐2004. Nutr Res. 2015;35:1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS Study. JAMA Oncol. 2017;3:e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prado CM, Lieffers JR, McCarger LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 58. Argiles JM, Lopez‐Soriano FJ, Toledo M, et al. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle. 2011;2:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Villasenor A, Ballard‐Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Broughman JR, Williams GR, Deal AM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. J Geriatr Oncol. 2015;6:442–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers‐update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fearon KC, Baracos VE. Cachexia in pancreatic cancer: new treatment options and measures of success. HPB (Oxford). 2010;12:323–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan BH, Fladvad T, Braun TP, et al. P‐selectin genotype is associated with the development of cancer cachexia. EMBO Mol Med. 2012;4:462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 66. Shiono M, Huang K, Downey RJ, et al. An analysis of the relationship between metastases and cachexia in lung cancer patients. Cancer Med. 2016;5:2641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun L, Quan XQ, Yu S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer. 2015;67:1056–62. [DOI] [PubMed] [Google Scholar]
- 68. Daly LE, Ni Bhuachalla EB, Power DG, et al. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. 2018;9:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Christensen JF, Jones LW, Andersen JL, et al. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25:947–58. [DOI] [PubMed] [Google Scholar]
- 70. Ballinger TJ, Reddy A, Althouse SK, et al. Impact of primary breast cancer therapy on energetic capacity and body composition. Breast Cancer Res Treat. 2018;172:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Damrauer JS, Stadler ME, Acharyya S, et al. Chemotherapy‐induced muscle wasting: association with NF‐kappaB and cancer cachexia. Eur J Transl Myol. 2018;28:7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gilliam LA, Ferreira LF, Bruton JD, et al. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol (1985). 2009;107:1935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gilliam LA, Moylan JS, Callahan LA, Sumandea MP, Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve. 2011;43:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barreto R, Mandili G, Witzmann FA, et al. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol. 2016;7:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prado CM, Baracos VE, McCarger LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–6. [DOI] [PubMed] [Google Scholar]
- 76. Deluche E, Leobon S, Desport JC, et al. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer. 2018;26:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mir O, et al. Sarcopenia predicts early dose‐limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. 2012;7:e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–30. [DOI] [PubMed] [Google Scholar]
- 79. Kawamura T, Mukuuchi R, Tokunaga M, et al. Long‐term outcomes of gastric cancer patients with preoperative sarcopenia. Ann Surg Oncol. 2018;25:1625–32. [DOI] [PubMed] [Google Scholar]
- 80. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 81. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. [DOI] [PubMed] [Google Scholar]
- 82. Waning DL, Guise TA. Cancer‐associated muscle weakness: what's bone got to do with it? Bonekey Rep. 2015;4:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schmidt ME, Meynkohn A, Habermann N, et al. Resistance exercise and inflammation in breast cancer patients undergoing adjuvant radiation therapy: mediation analysis from a randomized, controlled intervention trial. Int J Radiat Oncol Biol Phys. 2016;94:329–37. [DOI] [PubMed] [Google Scholar]
- 84. Zanchi NE, Lira FS, de Siqueira Filho MA, et al. Chronic low frequency/low volume resistance training reduces pro‐inflammatory cytokine protein levels and TLR4 mRNA in rat skeletal muscle. Eur J Appl Physiol. 2010;109:1095–102. [DOI] [PubMed] [Google Scholar]
- 85. Xia Z, Cholewa J, Zhao Y, et al. Hypertrophy‐promoting effects of leucine supplementation and moderate intensity aerobic exercise in pre‐senescent mice. Nutrients. 2016;8 pii: E246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Swenson KK, Henly SJ, Shapiro AC, Schroeder LM. Interventions to prevent loss of bone mineral density in women receiving chemotherapy for breast cancer. Clin J Oncol Nurs. 2005;9:177–84. [DOI] [PubMed] [Google Scholar]
- 87. Kohrt WM, Bloomfield SA, Little KD, et al. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–96. [DOI] [PubMed] [Google Scholar]
- 88. Hojan K, Milecki P, Leszczynski P. The impact of aerobic exercises on bone mineral density in breast cancer women during endocrine therapy. Pol Orthop Traumatol. 2013;78:47–51. [PubMed] [Google Scholar]
- 89. Hojan K, Milecki P, Molinska‐Glura M, Roszak A, Leszczynski P. Effect of physical activity on bone strength and body composition in breast cancer premenopausal women during endocrine therapy. Eur J Phys Rehabil Med. 2013;49:331–9. [PubMed] [Google Scholar]
- 90. Winters‐Stone KM, Dobek J, Nail LM, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Winters‐Stone KM, Dobek J, Bennett JA, et al. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc. 2014;46:1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Saarto T, Sievanen H, Kellokumpu‐Lehtinen P, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. a 12‐month randomised controlled trial. Osteoporos Int. 2012;23:1601–12. [DOI] [PubMed] [Google Scholar]
- 93. Almstedt HC, Grote S, Korte JR, et al. Combined aerobic and resistance training improves bone health of female cancer survivors. Bone Rep. 2016;5:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Twiss JJ, Waltman NL, Berg, K , et al. An exercise intervention for breast cancer survivors with bone loss. J Nurs Scholarsh. 2009;41:20–7. [DOI] [PubMed] [Google Scholar]
- 95. Yee J, Davis GM, Beith JM, et al. Physical activity and fitness in women with metastatic breast cancer. J Cancer Surviv. 2014;8:647–56. [DOI] [PubMed] [Google Scholar]
- 96. Swenson KK, Nissen MJ, Anderson E, et al. Effects of exercise vs bisphosphonates on bone mineral density in breast cancer patients receiving chemotherapy. J Support Oncol. 2009;7:101–7. [PubMed] [Google Scholar]
- 97. Brown JC, Schmitz KH. Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. J Clin Oncol. 2015;33:2184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nilsen TS, Raastad T, Skovlund E, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54:1805–13. [DOI] [PubMed] [Google Scholar]
- 99. Winters‐Stone KM, Dobek J, Nail L, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011;127:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Michaud LB, Goodin S. Cancer‐treatment‐induced bone loss, part 2. Am J Health Syst Pharm. 2006;63:534–46. [DOI] [PubMed] [Google Scholar]
- 102. Abulkhair O, et al. Vitamin D levels and breast cancer characteristics: findings in patients from Saudi Arabia. J Steroid Biochem Mol Biol. 2016;164:106–9. [DOI] [PubMed] [Google Scholar]
- 103. Acevedo F, Perez V, Perez‐Sepulveda A, et al. High prevalence of vitamin D deficiency in women with breast cancer: the first Chilean study. Breast. 2016;29:39–43. [DOI] [PubMed] [Google Scholar]
- 104. Aguirre M, Manzano N, Salas Y, et al. Vitamin D deficiency in patients admitted to the general ward with breast, lung, and colorectal cancer in Buenos Aires, Argentina. Arch Osteoporos. 2016;11:4. [DOI] [PubMed] [Google Scholar]
- 105. Churilla TM, Brereton HD, Klem M, Peters CA. Vitamin D deficiency is widespread in cancer patients and correlates with advanced stage disease: a community oncology experience. Nutr Cancer. 2012;64:521–5. [DOI] [PubMed] [Google Scholar]
- 106. Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5:2091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jick S, Li L, Gastanaga VM, Liede A. Prevalence of hypercalcemia of malignancy among cancer patients in the UK: analysis of the Clinical Practice Research Datalink database. Cancer Epidemiol. 2015;39:901–7. [DOI] [PubMed] [Google Scholar]
- 108. Goldner W. Cancer‐related hypercalcemia. J Oncol Pract. 2016;12:426–32. [DOI] [PubMed] [Google Scholar]
- 109. Qi WX, Lin F, He AN, et al. Incidence and risk of denosumab‐related hypocalcemia in cancer patients: a systematic review and pooled analysis of randomized controlled studies. Curr Med Res Opin. 2013;29:1067–73. [DOI] [PubMed] [Google Scholar]
- 110. Datta M, Schwartz GG. Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: a critical review. Oncologist. 2012;17:1171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Datta M, Schwartz GG. Calcium and vitamin D supplementation and loss of bone mineral density in women undergoing breast cancer therapy. Crit Rev Oncol Hematol. 2013;88:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta‐analysis. J Natl Cancer Inst. 2012;104:371–85. [DOI] [PubMed] [Google Scholar]
- 113. Murphy RA, Mourtzakis M, Chu QS, et al. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer. 2011;117:1775–82. [DOI] [PubMed] [Google Scholar]
- 114. Obling SR, Wilson BV, Pfeiffer P, Kjeldsen J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin Nutr. 2019;38:182–90. [DOI] [PubMed] [Google Scholar]
- 115. Eley HL, Russell ST, Tisdale MJ. Effect of branched‐chain amino acids on muscle atrophy in cancer cachexia. Biochem J. 2007;407:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Laviano A, Muscaritoli M, Cascino A, et al. Branched‐chain amino acids: the best compromise to achieve anabolism? Curr Opin Clin Nutr Metab Care. 2005;8:408–14. [DOI] [PubMed] [Google Scholar]
- 117. Williams JP, Phillips BE, Smith K, et al. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr. 2012;96:1064–70. [DOI] [PubMed] [Google Scholar]
- 118. Winter A, MacAdams J, Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin‐resistant patients with lung cancer cachexia. Clin Nutr. 2012;31:765–73. [DOI] [PubMed] [Google Scholar]
- 119. Hofbauer LC, Rachner T, Singh SK. Fatal attraction: why breast cancer cells home to bone. Breast Cancer Res. 2008;10:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Waning DL, Mohammad KS, Reiken S, et al. Excess TGF‐beta mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21:1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor‐beta (TGF‐beta)‐binding protein‐1 by osteoclasts. A cellular mechanism for release of TGF‐beta from bone matrix. J Biol Chem. 2002;277:21352–60. [DOI] [PubMed] [Google Scholar]
- 123. Guise TA, Yin JJ, Taylor SD, et al. Evidence for a causal role of parathyroid hormone‐related protein in the pathogenesis of human breast cancer‐mediated osteolysis. J Clin Invest. 1996;98:1544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kang Y, He W, Tulley S, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. [DOI] [PubMed] [Google Scholar]
- 126. Korpal M, Yan J, Lu X, et al. Imaging transforming growth factor‐beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med. 2009;15:960–6. [DOI] [PubMed] [Google Scholar]
- 127. Yin JJ, Selander K, Chirgwin JM, et al. TGF‐beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Andersson DC, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bellinger AM, Reiken S, Carlson C, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Brillantes AB, Ondrias K, Scott A, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506‐binding protein. Cell. 1994;77:513–23. [DOI] [PubMed] [Google Scholar]
- 131. Ago T, Kitazono T, Ooboshi H, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–33. [DOI] [PubMed] [Google Scholar]
- 132. Carmona‐Cuenca I, Roncero C, Sancho P, et al. Upregulation of the NADPH oxidase NOX4 by TGF‐beta in hepatocytes is required for its pro‐apoptotic activity. J Hepatol. 2008;49:965–76. [DOI] [PubMed] [Google Scholar]
- 133. DeRose YS, Wang G, Lin YC, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Varticovski L, Hollingshead MG, Robles AI, et al. Accelerated preclinical testing using transplanted tumors from genetically engineered mouse breast cancer models. Clin Cancer Res. 2007;13:2168–77. [DOI] [PubMed] [Google Scholar]
- 135. Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 136. Johnstone CN, Smith YE, Cao Y, et al. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6‐mouse‐derived model of spontaneously metastatic mammary cancer. Dis Model Mech. 2015;8:237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Eckhardt BL, Parker BS, van Laar RK, et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res. 2005;3:1–13. [PubMed] [Google Scholar]
- 138. Kusuma N, Denoyer D, Eble JA, et al. Integrin‐dependent response to laminin‐511 regulates breast tumor cell invasion and metastasis. Int J Cancer. 2012;130:555–66. [DOI] [PubMed] [Google Scholar]
- 139. Chen YC, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 2010;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kinder M, Chislock E, Bussard KM, Shuman L, Mastro AM. Metastatic breast cancer induces an osteoblast inflammatory response. Exp Cell Res. 2008;314:173–83. [DOI] [PubMed] [Google Scholar]
- 141. Cormie P, Galvao DA, Spry N, et al. Functional benefits are sustained after a program of supervised resistance exercise in cancer patients with bone metastases: longitudinal results of a pilot study. Support Care Cancer. 2014;22:1537–48. [DOI] [PubMed] [Google Scholar]
- 142. Rief H, Omlor G, Akbar M, et al. Biochemical markers of bone turnover in patients with spinal metastases after resistance training under radiotherapy—a randomized trial. BMC Cancer. 2016;16:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Willems HME, van den Heuvel E, Schoemaker RJW, Klein‐Nulend J, Bakker AD. Diet and exercise: a match made in bone. Curr Osteoporos Rep. 2017;15:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rommel C, et al. Mediation of IGF‐1‐induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. [DOI] [PubMed] [Google Scholar]
- 145. Antoun S, Raynard B. Muscle protein anabolism in advanced cancer patients: response to protein and amino acids support, and to physical activity. Ann Oncol. 2018;29:ii10–ii17. [DOI] [PubMed] [Google Scholar]
- 146. Ibrahim EM, Al‐Homaidh A. Physical activity and survival after breast cancer diagnosis: meta‐analysis of published studies. Med Oncol. 2011;28:753–65. [DOI] [PubMed] [Google Scholar]
- 147. Des Guetz G, Uzzan B, Bouillet T, et al. Impact of physical activity on cancer‐specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract. 2013;2013:340851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta‐analysis of epidemiological studies. Acta Oncol. 2015;54:635–54. [DOI] [PubMed] [Google Scholar]
- 149. Otto SJ, Korfage IJ, Polinder S, et al. Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta‐analysis. Support Care Cancer. 2015;23:1237–50. [DOI] [PubMed] [Google Scholar]
- 150. Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22:4766–75. [DOI] [PubMed] [Google Scholar]
- 151. Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta‐analysis of prospective cohort studies. Int J Cancer. 2013;33:1905–13. [DOI] [PubMed] [Google Scholar]
- 152. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta‐analysis. Ann Oncol. 2014;25:1293–311. [DOI] [PubMed] [Google Scholar]
- 153. Zhong S, Jiang T, Ma T, et al. Association between physical activity and mortality in breast cancer: a meta‐analysis of cohort studies. Eur J Epidemiol. 2014;29:391–404. [DOI] [PubMed] [Google Scholar]
- 154. Li T, Wei S, Shi Y, et al. The dose‐response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50:339–45. [DOI] [PubMed] [Google Scholar]
- 155. Wu W, Guo F, Ye J, et al. Pre‐ and post‐diagnosis physical activity is associated with survival benefits of colorectal cancer patients: a systematic review and meta‐analysis. Oncotarget. 2016;7:52095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment‐related adverse effects. Epidemiol Rev. 2017;39:71–92. [DOI] [PubMed] [Google Scholar]


