Abstract
Objectives
Although weight gain on ART is common, the long-term trajectory of and factors affecting increases in fat mass in people living with HIV are not well described.
Methods
Men and women living with HIV in the Modena HIV Metabolic Clinic underwent DXA scans every 6–12 months for up to 10 years (median 4.6 years). Regression modelling in both combined and sex-stratified models determined changes in and clinical factors significantly associated with trunk and leg fat mass over the study period.
Results
A total of 839 women and 1759 men contributed two or more DXA scans. The baseline median age was 44 years and BMI 22.9 kg/m2; 76% were virologically suppressed on ART at baseline. For both sexes, trunk and leg fat consistently increased over the study period, with mean yearly trunk and leg fat gain of 3.6% and 7.5% in women and 6.3% and 10.8% in men, respectively. In multivariate analysis, factors associated with greater fat mass included female sex, per-year ART use (specifically tenofovir disoproxil fumarate and integrase strand transfer inhibitor therapy), per-unit BMI increase, no self-reported physical activity and CD4 nadir <200 cells/mm3.
Conclusions
Among people living with HIV on ART, trunk and leg fat mass increased steadily over a median of 4.6 years of follow up, particularly among women. After controlling for traditional risk factors, HIV- and ART-specific risk factors emerged.
Introduction
As ART continues to increase life expectancy for people living with HIV (PLWH), optimization of comorbid conditions, such as cardiovascular disease (CVD) and diabetes mellitus (DM), has become a primary concern. Excess body weight is a known risk factor for CVD and DM in the general population, and a growing concern among PLWH.1 Over the past 10–15 years BMI at the time of HIV seroconversion has increased, reflecting the increase in total body weight of the general population. Following initiation of ART, most individuals gain weight and many become overweight or obese.2,3 Increases in BMI following ART initiation increase CVD risk,4 and even modest weight gain is associated with greater risk of development of DM in PLWH compared with HIV-uninfected individuals.3,4 Increases in central body fat are also associated with hepatic steatosis in PLWH.5
Several studies have evaluated changes in BMI and body composition after initiating ART.6–12 However, point-prevalence and short-term longitudinal studies cannot be extrapolated to predict longer-term changes. Additionally, studies showing concomitant lean mass loss with fat mass gain on ART demonstrated that BMI alone insufficiently describes cardiometabolic risk in PLWH.7,13 The aim of this study was to understand fat mass trajectory and factors associated with fat mass quantity in a large cohort of adult men and women with HIV on ART, and to determine sex-specific risk factors for any observed differences in trunk and leg fat quantity.
Methods
Study population
This is a secondary analysis of existing longitudinal data from the multidisciplinary Modena HIV Metabolic Clinic (MHMC) at the University of Modena and Reggio Emilia, Italy. PLWH who attended the MHMC underwent DXA scans approximately every 6–12 months, beginning in 2004. We included all participants who were on ART and who had at least two DXA scans during a period of up to 10 years.
Ethics
All study procedures were in accordance with the ethical standards of the Comitato Etico Provinciale di Modena and with the Helsinki Declaration of 1975, as revised in 2000. All participants provided written, informed consent.
Definitions
Data were collected from the MHMC electronic database. The following baseline variables were collected from participants: age; smoking (number of cigarettes/day); physical activity [none, moderate (<4 h weekly), intensive (≥4 h weekly)]; hypogonadism (defined as post-menopausal in women and serum total testosterone <300 ng/dL in men);14 metabolic syndrome (using NCEP-Adult Treatment Panel III criteria);15 HCV seropositivity; duration of HIV infection; history of AIDS wasting; nadir CD4+ T lymphocyte (CD4) count; ART duration; and cumulative ART use by class and agent. Body weight was measured using a digital scale to the nearest 0.1 kg, with participants wearing light clothes without shoes. Height was measured using a wall-mounted stadiometer to the nearest 0.1 cm. BMI was defined as weight in kilograms divided by height in metres squared. Lipodystrophy was defined using the Multicenter AIDS Cohort Study definition, with anthropometric categorizations of lipoatrophy, lipohypertrophy and mixed form.16
All participants underwent venous blood sampling at 8:00 AM after an overnight fast. HCV seropositivity was determined by antibody testing (anti-HCV; Abbott HCV EIA 3.0 enzyme immunoassay, Abbott Laboratories, Chicago, IL, USA). HIV-1 RNA was measured by Abbott RealTime™ HIV-1 assay (Abbott Laboratories; lower limit of detection 50 copies/mL), CD4 count by flow cytometry, 25-hydroxy (25-OH) vitamin D by chemiluminescence immunoassay (DiaSorin, Stillwater, MN, USA) and serum total testosterone by immunochemiluminescence (ADVIA Centaur; Siemens Medical Solutions, Tarrytown, NY, USA). Vitamin D insufficiency was defined as 25-OH vitamin D concentration <30 ng/mL. All participants were scanned using the same single densitometer (Hologic Discovery W, Inc., Waltham, MA, USA). The instrument was calibrated daily with a hydroxyapatite phantom.
Statistical analysis
Variables are expressed as median and IQR for continuous and percentage for categorical variables. To account for correlation within patients in outcome measures, mixed-effect regression models were created assuming compound symmetry variance–covariance structure for combined men and women to determine the sex-adjusted effect, and then in sex-stratified models to determine factors associated with fat mass by sex. A significant sex × year interaction in the combined model further supported the use of sex-stratified models. Mixed-effect regression models determined variables significantly (P < 0.05) associated with trunk and leg fat mass over the study period, after exclusion of variables not statistically or clinically significant in univariate analyses.
Mixed-effect models with random intercept and slope were adjusted for sex (for the combined model) and the following variables (time updated, where applicable): time in study; BMI; cumulative duration of tenofovir disoproxil fumarate use; cumulative duration of integrase strand transfer inhibitor use; age group by 5 year increments; self-reported physical activity level (none, moderate or intensive); hypogonadism; history of AIDS wasting; vitamin D insufficiency; and HCV seropositivity. Additional variables (including other ART classes/agents) were considered for inclusion, but excluded owing to either the extent of missing data or lack of significance in univariable models (P > 0.10). Stepwise forward model selection methods with backward elimination were used in building the final models. The covariates identified in the combined model were used in the sex-stratified models to examine their relationships with the outcomes in females and males separately. Annual change rates were calculated by applying simple regression to mean estimates derived from the mixed-effect models. A P value <0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 (Cary, NC, USA).
Results
Study population
A total of 839 women and 1759 men contributed at least two DXA scans [median number of scans 5 (IQR 3–7)], with a median follow-up of 4.7 years (IQR 2.1–7.7). General characteristics at the initial assessment are shown in Table 1. All participants were Caucasian, and the median age was 44 years. The median time since HIV diagnosis was 14 years, and CD4 count 528 cells/mm3; 76% had an HIV-1 RNA ≤50 copies/mL. Peripheral atrophy was diagnosed in 32% of persons, and was more frequent among men (35% versus 30%). Lipodystrophy status did not change over the follow-up period. At baseline, 7% of men were hypogonadal and 15% of women were post-menopausal, with 24% categorized as post-menopausal at any point after baseline. Median BMI was in the normal range for both sexes. Median (IQR) baseline trunk fat mass was 7.0 kg (5.2–9.2 kg) for women and 5.8 kg (4.1–8.1 kg) for men. Median (IQR) baseline leg fat mass was 3.0 kg (1.9–4.8 kg) for women and 2.0 kg (1.2–3.7 kg) for men.
Table 1.
Baseline demographic and clinical characteristics at time of first fat mass assessment
| Characteristic | Women (N = 839) | Men (N = 1759) |
|---|---|---|
| Age, years, n (%) | ||
| >55 | 37 (4) | 166 (9) |
| 51–55 | 71 (9) | 197 (11) |
| 46–50 | 183 (22) | 450 (26) |
| 41–45 | 303 (36) | 536 (31) |
| 35–40 | 182 (22) | 265 (15) |
| <35 | 63 (8) | 145 (8) |
| BMI, kg/m2 (IQR) | 21.6 (20.0–24.1) | 23.5 (21.6–25.5) |
| Smoker, n (%) | 349 (41.6) | 740 (42.1) |
| Smoking, pack years (IQR) | 10.0 (1.1–20.0) | 12.5 (0–25.0) |
| Physical activity, n (%) | ||
| none | 577 (69) | 1020 (58) |
| moderate | 184 (22) | 438 (25) |
| intensive | 44 (5) | 224 (13) |
| Hypogonadisma (%) | 124 (15) | 124 (7) |
| Metabolic syndrome, n (%) | 84 (10) | 144 (8) |
| HCV seropositivity, n (%) | 250 (30) | 468 (27) |
| Vitamin D insufficiency, n (%) | 414 (49) | 831 (47) |
| History of AIDS wasting, n (%) | 113 (13) | 81 (5) |
| CD4 nadir <200 cells/mm3, n (%) | 448 (53) | 856 (49) |
| HIV-1 viral load ≤50 copies/mL, n (%) | 646 (77) | 1319 (75) |
| Lipodystrophy, n (%) | ||
| no lipodystrophy | 118 (14) | 346 (20) |
| lipoatrophy | 209 (25) | 682 (39) |
| central fat accumulation | 97 (12) | 153 (9) |
| mixed form | 365 (44) | 514 (29) |
| ART duration, years (IQR) | 9.6 (5.6–13.1) | 8.3 (3.4–12.0) |
| TDF use, n (%) | 538 (64) | 1144 (65) |
| INSTI use, n (%) | 70 (8) | 129 (7) |
| Trunk fat mass, kg (IQR) | 7.0 (5.2–9.2) | 5.8 (4.1–8.1) |
| Leg fat mass, kg (IQR) | 3.0 (1.9–4.8) | 2.0 (1.2–3.7) |
TDF, tenofovir disoproxil fumarate; INSTI, integrase strand transfer inhibitor.
Defined as post-menopausal female or male hypogonadism.
Factors associated with trunk fat mass
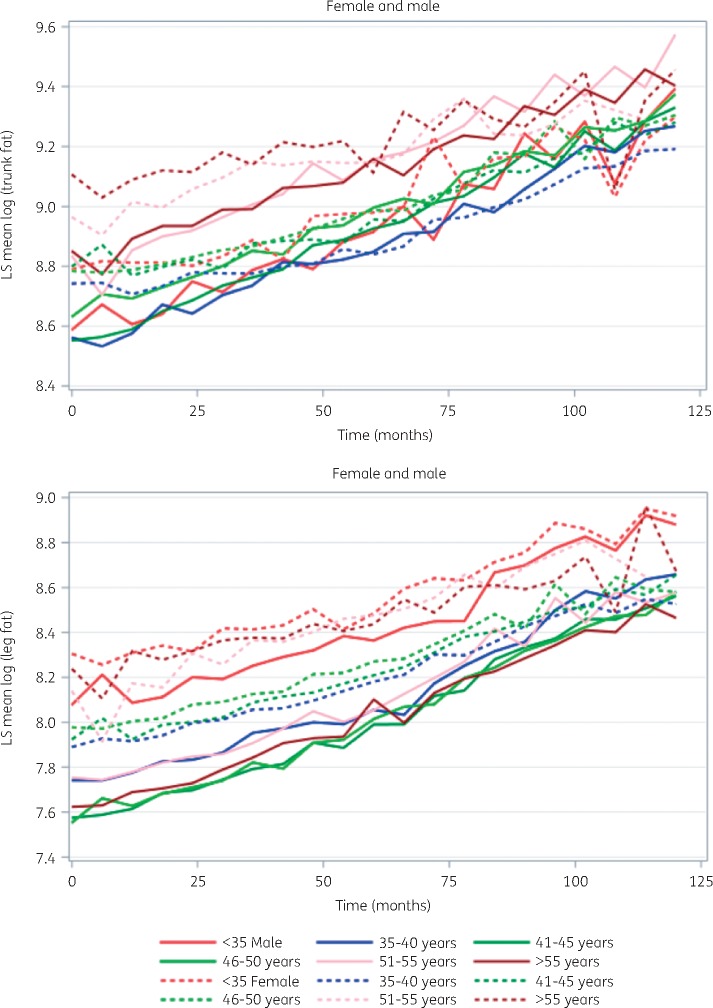
For both sexes, trunk fat mass steadily increased over the study period (Figure 1), with a mean yearly trunk fat gain of 3.6% (246 g; SD 1118 g) for women and 6.3% (438 g; SD 1026 g) for men. In combined mixed-effect models, greater trunk fat mass over the study period was associated with female sex, older age, no self-reported physical activity, CD4 nadir <200 cells/mm3, per-unit BMI increase, per-year any ART use, and per-year integrase strand transfer inhibitor and tenofovir disoproxil fumarate use (Table 2). Hypogonadism, vitamin D insufficiency, HCV seropositivity, history of AIDS wasting and HIV-1 RNA >50 copies/mL were associated with less trunk fat over the study period.
Figure 1.
Trunk and leg fat over study period. LS, log transformed.
Table 2.
Combined sex model for trunk fat
| Variable | Model estimate (SE) | Effect size | P value |
|---|---|---|---|
| Female sex | 0.488 (0.017) | +63% | <0.0001 |
| BMI (kg/m2)a | 0.104 (0.001) | +11% | <0.0001 |
| Age, yearsb | |||
| >55 | 0.112 (0.032) | +12% | 0.0004 |
| 51–55 | 0.162 (0.030) | +18% | <0.0001 |
| 46–50 | 0.075 (0.026) | +8% | 0.0004 |
| No regular physical activityc | 0.062 (0.009) | +6% | <0.0001 |
| Moderate physical activityc | 0.049 (0.008) | +5% | <0.0001 |
| CD4 nadir <200 cells/mm3 | 0.047 (0.012) | +5% | <0.0001 |
| Per-year ART use | 0.004 (0.001) | +0.4% | 0.0004 |
| Per-year INSTI use | 0.00005 (0.00002) | +<0.1% | 0.002 |
| Per-year TDF use | 0.004 (0.001) | +0.4% | 0.001 |
| Hypogonadism | −0.024 (0.010) | −2% | 0.02 |
| Vitamin D insufficiency | −0.016 (0.007) | −2% | 0.03 |
| HCV seropositivity | −0.046 (0.013) | −4% | 0.0005 |
| History of AIDS wasting | −0.060 (0.010) | −6% | <0.0001 |
| HIV-1 RNA >50 copies/mLd | −0.013 (0.006) | −1% | 0.02 |
SE, standard error; TDF, tenofovir disoproxil fumarate; INSTI, integrase strand transfer inhibitor.
Per-unit increase.
Reference group: <35 years of age.
Reference: intense regular physical activity.
Reference: >50 copies/mL.
Sex-stratified models (Table 3) yielded similar results with a few exceptions: among women, post-menopausal status was associated with greater trunk fat, as was per-year use of any ART, but not per-year use of tenofovir disoproxil fumarate or integrase strand transfer inhibitor. Women with AIDS wasting had less trunk fat. In men, greater trunk fat was associated with per-year use of tenofovir disoproxil fumarate and integrase strand transfer inhibitor. Less trunk fat was associated with HCV seropositivity and hypogonadism, regardless of testosterone use (data not shown). Vitamin D deficiency was not significant in the sex-stratified models.
Table 3.
Sex-stratified model for trunk fat
| Variable | Women |
Men |
||||
|---|---|---|---|---|---|---|
| model estimate (SE) | effect size | P value | model estimate (SE) | effect size | P value | |
| BMI (kg/m2)a | 0.094 (0.002) | +10% | <0.0001 | 0.110 (0.002) | +12% | <0.0001 |
| Age, yearsb | ||||||
| >55 | 0.102 (0.052) | +11% | 0.05 | 0.112 (0.038) | +12% | 0.003 |
| 51–55 | 0.158 (0.043) | +17% | 0.0003 | 0.161 (0.037) | +18% | <0.0001 |
| 46–50 | 0.086 (0.036) | +9% | 0.02 | 0.075 (0.032) | +8% | 0.02 |
| No regular physical activityc | 0.041 (0.015) | +4% | 0.006 | 0.066 (0.010) | +7% | <0.0001 |
| Moderate physical activityc | 0.030 (0.015) | +3% | 0.04 | 0.048 (0.009) | +5% | <0.0001 |
| CD4 nadir <200 cells/mm3 | 0.035 (0.017) | +4% | 0.04 | 0.052 (0.016) | +5% | 0.0009 |
| Per-year ART use | 0.007 (0.002) | +0.7% | <0.0001 | NS | NS | NS |
| Per-year INSTI use | NS | NS | NS | 0.00004 (0.00002) | +<0.1% | 0.03 |
| Per-year TDF use | NS | NS | NS | 0.006 (0.002) | +0.6% | 0.0003 |
| Hypogonadism | 0.039 (0.012) | +4% | 0.001 | −0.043 (0.015) | −4% | 0.003 |
| History of AIDS wasting | −0.095 (0.015) | −10% | <0.0001 | NS | NS | NS |
| HIV-1 RNA >50 copies/mLd | 0.019 (0.009) | +2% | 0.03 | −0.032 (0.007) | −3% | <0.0001 |
| Vitamin D insufficiency | NS | NS | NS | NS | NS | NS |
| HCV seropositivity | NS | NS | NS | −0.043 (0.017) | −4% | 0.01 |
SE, standard error; NS, not significant; TDF, tenofovir disoproxil fumarate; INSTI, integrase strand transfer inhibitor.
Per-unit increase.
Reference group: <35 years of age.
Reference: intense regular physical activity.
Reference: >50 copies/mL.
Factors associated with leg fat mass
For both sexes, leg fat also steadily increased over the study period (Figure 1), with a mean (SD) yearly leg fat gain of 7.5% (187 g) for women and 10.8% (232 g) for men. In combined-sex mixed-effect models (Table 4), leg fat mass was greater for women compared with men over the study period. Individuals with higher BMI, per-year tenofovir disoproxil fumarate and integrase strand transfer inhibitor use, and no self-reported physical activity also had greater leg fat, whereas hypogonadism/post-menopausal state was associated with less leg fat.
Table 4.
Combined-sex model for leg fat
| Variable | Model estimate (SE) | Effect size | P value |
|---|---|---|---|
| Female sex | 0.741 (0.028) | +210% | <0.0001 |
| BMI (kg/m2)a | 0.084 (0.002) | +9% | <0.0001 |
| Age, yearsb | |||
| >55 | −0.278 (0.054) | −27% | <0.0001 |
| 51–55 | −0.173 (0.051) | −17% | 0.0007 |
| 46–50 | −0.248 (0.045) | −25% | <0.0001 |
| 41–45 | −0.300 (0.043) | −30% | <0.0001 |
| 35–40 | −0.257 (0.046) | −26% | <0.0001 |
| No regular physical activityc | 0.049 (0.009) | +5% | <0.0001 |
| Moderate physical activityc | 0.043 (0.008) | +4% | <0.0001 |
| Metabolic syndrome | 0.021(0.009) | +2% | 0.03 |
| CD4 nadir <200 cells/mm3 | 0.171 (0.021) | +18% | <0.0001 |
| Per-year ART use | −0.016 (0.002) | −2% | <0.0001 |
| Per-year INSTI use | 0.00009 (0.00001) | +<0.1% | <0.0001 |
| Per-year TDF use | 0.007 (0.002) | +<0.1% | <0.0001 |
| Hypogonadism | −0.048 (0.011) | −5% | <0.0001 |
| Vitamin D insufficiency | −0.023 (0.008) | −2% | 0.003 |
| History of AIDS wasting | NS | NS | NS |
| HIV-1 RNA >50 copies/mLd | NS | NS | NS |
SE, standard error; NS, not significant; TDF, tenofovir disoproxil fumarate; INSTI, integrase strand transfer inhibitor.
Per-unit increase.
Reference group: <35 years of age.
Reference: intense regular physical activity.
Reference: >50 copies/mL.
In contrast to the combined-sex model, in sex-stratified models (Table 5), women who were postmenopausal had greater leg fat mass, whereas men with hypogonadism had less leg fat mass. Per-year tenofovir disoproxil fumarate use was associated with greater leg fat in both men and women. Among women only, less leg fat was associated with age 36–50 years compared with age <35. In men, lower physical activity and a diagnosis of metabolic syndrome were associated with greater leg fat. As with trunk fat mass, per-year integrase strand transfer inhibitor use was associated with greater leg fat mass in men.
Table 5.
Sex-stratified model for leg fat
| Variable | Women |
Men |
||||
|---|---|---|---|---|---|---|
| model estimate (SE) | effect size | P value | model estimate (SE) | effect size | P value | |
| BMI (kg/m2)a | 0.080 (0.002) | +8% | <0.0001 | 0.087 (0.002) | +9% | <0.0001 |
| Age, yearsb | ||||||
| >55 | NS | NS | NS | −0.333 (0.064) | −33% | <0.0001 |
| 51–55 | NS | NS | NS | −0.233 (0.062) | −23% | 0.0002 |
| 46–50 | −0.171 (0.078) | −17% | 0.028 | −0.292 (0.055) | −29% | <0.0001 |
| 41–45 | −0.269 (0.074) | −27% | 0.0003 | −0.319 (0.054) | −31% | <0.0001 |
| 35–40 | −0.263 (0.077) | −26% | 0.0005 | −0.239 (0.057) | −24% | <0.0001 |
| No regular physical activityc | NS | NS | NS | 0.056 (0.011) | +6% | <0.0001 |
| Moderate physical activityc | NS | NS | NS | 0.047 (0.010) | +5% | <0.0001 |
| Metabolic syndrome | NS | NS | NS | 0.28 (0.011) | +3% | 0.018 |
| CD4 nadir <200 cells/mmc | 0.035 (0.017) | +4% | 0.04 | 0.052 (0.016) | +5% | 0.0009 |
| Per-year ART use | −0.012 (0.004) | −1% | 0.006 | −0.016 (0.002) | −2% | <0.0001 |
| Per-year INSTI use | NS | NS | NS | 0.00001 (0.00002) | +<0.1% | <0.0001 |
| Per-year TDF use | 0.007 (0.002) | +0.7% | 0.005 | 0.006 (0.002) | +0.6% | 0.0003 |
| Hypogonadism | 0.031 (0.013) | +3% | 0.02 | −0.072 (0.018) | −7% | <0.0001 |
| Vitamin D insufficiency | NS | NS | NS | NS | NS | NS |
| HCV seropositivity | NS | NS | NS | NS | NS | NS |
| History of AIDS wasting | NS | NS | NS | NS | NS | NS |
| HIV-1 RNA >50 copies/mLd | NS | NS | NS | NS | NS | NS |
SE, standard error; NS, not significant; TDF, tenofovir disoproxil fumarate; INSTI, integrase strand transfer inhibitor.
Per-unit increase.
Reference group: <35 years of age.
Reference: intense regular physical activity.
Reference: >50 copies/mL.
Discussion
In this large cohort of women and men living with HIV on ART and with up to 10 years of follow-up, trunk and leg fat mass both increased steadily throughout the observation period, and HIV- and ART-related factors influenced fat mass. Importantly, many patients referred to the MHMC are treatment-experienced, and most were virologically suppressed on ART at cohort enrolment (76%). Thus, these increases in fat mass were above and beyond those associated with ART initiation, and occurred in all age groups, across ART classes and in both sexes. Not unexpectedly, women had greater trunk and leg fat mass over the study period, but the rate of fat mass increase was greater among men. Hypogonadal/post-menopausal state was associated with greater fat mass in women only, and irrespective of testosterone use (for men). Traditional factors associated with weight gain in the general population, such as reduced physical activity, were also observed in this cohort.
In this cohort of PLWH, we observed that trunk and leg fat mass increased at all age intervals for both men and women. This is in contrast to the general population, where several epidemiological studies have associated older age with stabilization of fat mass.17,18 In a sample of healthy Italian individuals, limb fat mass percentage increased in women up to 70 years of age, then remained stable.18 US epidemiological data on total and percentage body fat from the third National Health and Nutrition Examination Survey (NHANES) showed a decrease in total body fat and fat-free mass after age 55 in both men and women.19 When compared with the US population, the rates of trunk fat mass gain among PLWH in our cohort were greater than expected for age.20–23 Longitudinal data from the NHANES showed increases in trunk fat by DXA of only 0.8 kg/decade in men and 0.5 kg/decade in women.23 In a subset of participants with DXA data from the ACTG ART initiation trial A5224s, Women’s Interagency HIV Study (WIHS) and Boston Area Community Health/Bone (BACH/Bone) Survey studies, compared with HIV-uninfected individuals, PLWH had greater adjusted total, trunk and limb fat gain than expected for age both during the post-ART initiation period (weeks 0–96) and over a median of 7 years of follow-up.7 Although the mechanism of greater than expected weight gain for age in PLWH on ART has not been well defined, the steady increase in fat mass seen in our cohort during a period of up to 10 years of follow-up is consistent with previous studies looking at BMI trends,4,8,12 and worrisome in a patient population already at increased cardiometabolic risk.
Although the per-year effect sizes are small, the associations of integrase strand transfer inhibitor and tenofovir disoproxil fumarate use with greater trunk and leg fat mass in both the combined-sex model and among men in sex-stratified models are novel and clinically important. Since ART is a lifelong commitment, small per-year effects may have large cumulative effects, again creating concern for exacerbation of cardiometabolic risk. Of note, we did not explore the potential effects of all individual ART agents and/or combinations, and previously published data on weight or body composition changes with specific ART agents vary. For example, a study of ART-naive individuals starting a raltegravir- or efavirenz-based regimen showed greater weight gain in the efavirenz arm after 96 weeks.24 However, ACTG A5260s, a randomized trial comparing tenofovir disoproxil fumarate plus emtricitabine plus either raltegravir or a boosted PI, showed no difference by ART regimen in regional fat quantity by DXA scan at 96 weeks.25 A recent observational study showed a significant increase in weight in virologically suppressed individuals switched from an efavirenz-containing regimen to a dolutegravir-based regimen (versus continued efavirenz).26 Although our analysis focused on classes of ART (because of the wide variety of combinations of exposure over the study period), future analyses are needed to understand the role of individual drug exposure on weight trajectory.
A CD4 nadir <200 cells/mm3 was associated with greater trunk fat mass in both the combined and sex-stratified models. In a longitudinal analysis of BMI changes in the Swiss HIV Cohort Study, lower CD4 nadir had the strongest association with BMI increase during the follow-up period, which persisted into 1–4 years of ART.8 Other reports demonstrating an effect of CD4 nadir on BMI following ART initiation have also been reported.7,27 Lipodystrophy status was reassessed throughout the study period, but did not change significantly over time within individuals in spite of continuous extremity fat gain that could indicate partial reversibility of lipoatrophy. However, the lipodystrophy assessment was clinician based and not an objective measurement of fat quantity. Interestingly, in this cohort hypogonadism in men was associated with less fat mass, even after adjustment for testosterone use. Although use of testosterone in PLWH has been associated with a decrease in total body fat, it did not have an effect on visceral fat.28 Hypogonadism has also not been significantly associated with increases in BMI.29
There are several limitations to our study. First, a comparable HIV-uninfected control population was not available for comparison, and we cannot determine whether these changes are greater than in the general Italian population or consistent with normal ageing. Comparison with the US population does not fully overcome this limitation. Second, this Italian HIV cohort comprises only Caucasian participants, and fat mass quantities and changes may differ among African American and Hispanic persons.22 We also did not include detailed, person-level dietary information; however, steady increases in fat mass in the entire cohort over the study period of 10 years suggest changes at the population level. Finally, we did not have control over the type of ART used, as medications were prescribed by the participant’s primary HIV care provider. Despite these limitations, strengths of our study include fat mass measurements at several timepoints and intervals of only 6–12 months, with a median of five scans over 4.6 years, allowing us to closely trend fat mass over time. In fact, with up to 10 years’ worth of serial DXA data on participants and a high number of DXA scans per participant, our data may represent the most closely documented (greatest number of observations within the follow-up period) changes reported to date. Measurement of body composition analysis by DXA adds significantly to the understanding of observed weight changes that can be missed when only BMI is used, particularly in a population at risk of losing lean mass and with higher visceral fat-to-BMI ratios.7,30–32 Finally, our analysis included a large proportion of women living with HIV, allowing exploration of factors associated with fat mass controlling both for sex and by sex.
Conclusions
In this large, well-characterized cohort of PLWH on long-term ART, we report continued increases in trunk and leg fat mass in both men and women well beyond ART initiation. We also describe important associations between fat gain and both traditional and HIV-/ART-specific risk factors that vary by sex. Clinicians should be aware that fat gain will continue beyond ART initiation/the ‘return to health’ phase and may occur at greater rates than expected for age. Early targeted interventions are needed to prevent potential cardiometabolic complications associated with increased fat mass, particularly among those at risk of greater weight gain, including post-menopausal women and persons with prolonged ART use and/or older age. The relationship between the cumulative use of tenofovir disoproxil fumarate and integrase strand transfer inhibitors and greater fat mass requires further study.
Acknowledgements
The authors would like to thank the participants and staff of the Modena HIV Metabolic Clinic for their participation and hard work. Specifically, we would like to thank Andrea Malagoli, Stefano Zona, Marianna Menozzi, Valentina Masi, Maria Mancini, Agnese Caselgrandi and Maria Giulia Corni for their time and dedication to the Cohort’s daily operations and data management.
Funding
This study was supported by the National Institute on Ageing of the National Institute of Health (K23AG050260; R01AG054366 to K. M. E.) and the National Institute of Allergy and Infectious Diseases (K24 AI120834 to T. T. B. and K23 AI110532 to J. E. L.).
Transparency declarations
T. T. B. has served as a consultant to Gilead Sciences, Merck, BMS, Theratechnologies and EMD-Serono. J. E. L. has served as a consultant to Merck and Gilead Sciences, and receives research funding from Gilead Sciences. G. G. has served as a consultant to Gilead Sciences, Merck, and ViiV. K. M. E. has received research funding (paid to the University of Colorado) from Gilead Sciences. J. F. has served as a consultant for Theratechnologies and EMD-Serono. All other authors: none to declare.
References
- 1. Zalesin KC, Franklin BA, Miller WM. et al. Impact of obesity on cardiovascular disease. Med Clin North Am 2011; 95: 919–37. [DOI] [PubMed] [Google Scholar]
- 2. Crum-Cianflone N, Roediger MP, Eberly L. et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5: e10106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrin M, Tate JP, Akgün KM. et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73: 228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Achhra AC, Mocroft A, Reiss P. et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med 2016; 17: 255–68. [DOI] [PubMed] [Google Scholar]
- 5. Brown TT, Mehta SH, Sutcliffe C. et al. Hepatic steatosis associated with increased central body fat by dual-energy X-ray absorptiometry and uncontrolled HIV in HIV/hepatitis C co-infected persons. AIDS 2010; 24: 811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crum-Cianflone N, Tejidor R, Medina S. et al. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS 2008; 22: 925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grant PM, Kitch D, McComsey GA. et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016; 30: 2805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasse B, Iff M, Ledergerber B. et al. Obesity trends and body mass index changes after starting antiretroviral treatment: the Swiss HIV Cohort Study. Open Forum Infect Dis 2014; 1: ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones CY, Hogan JW, Snyder B. et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis 2003; 37 Suppl 2: S69–80. [DOI] [PubMed] [Google Scholar]
- 10. Taylor BS, Liang Y, Garduño LS. et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr 2014; 65: e33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuh B, Tate J, Butt AA. et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60: 1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erlandson KM, Zhang L, Lake JE. et al. Changes in weight and weight distribution across the lifespan among HIV-infected and -uninfected men and women. Medicine 2016; 95: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yarasheski KE, Scherzer R, Kotier DP. et al. Age-related skeletal muscle decline is similar in HIV-infected and uninfected individuals. J Gerontol A Biol Med Sci 2011; 66: 332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rochira V, Guaraldi G.. Hypogonadism in the HIV-infected man. Endocrinol Metab Clin North Am 2014; 43: 709–30. [DOI] [PubMed] [Google Scholar]
- 15.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–97. [DOI] [PubMed] [Google Scholar]
- 16. Lichtenstein KA, Ward DJ, Moorman AC. et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS 2001; 15: 1389–98. [DOI] [PubMed] [Google Scholar]
- 17. Kuk JL, Saunders TJ, Davidson LE. et al. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009; 8: 339–48. [DOI] [PubMed] [Google Scholar]
- 18. Coin A, Giannini S, Minicuci N. et al. Limb fat-free mass and fat mass reference values by dual-energy X-ray absorptiometry (DEXA) in a 20-80 year-old Italian population. Clin Nutr 2012; 31: 506–11. [DOI] [PubMed] [Google Scholar]
- 19. Li C, Ford ES, Zhao G. et al. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr 2009; 90: 1457–65. [DOI] [PubMed] [Google Scholar]
- 20. Ding J, Kritchevsky SB, Newman AB. et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am J Clin Nutr 2007; 85: 405–10. [DOI] [PubMed] [Google Scholar]
- 21. Zamboni M, Zoico E, Scartezzini T. et al. Body composition changes in stable-weight elderly subjects: the effect of sex. Aging Clin Exp Res 2003; 15: 321–7. [DOI] [PubMed] [Google Scholar]
- 22. Gallagher D, Visser M, Sepúlveda D. et al. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996; 143: 228–39. [DOI] [PubMed] [Google Scholar]
- 23. Tian S, Morio B, Denis JB. et al. Age-related changes in segmental body composition by ethnicity and history of weight change across the adult lifespan. Int J Environ Res Public Health 2016; 13: 821.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lennox JL, DeJesus E, Berger DS. et al. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McComsey GA, Moser C, Currier J. et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis 2016; 62: 853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Norwood J, Turner M, Bofill C. et al. Weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 12: 527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lakey W, Yang L-Y, Yancy W. et al. From wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses 2013; 29: 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhasin S, Parker RA, Sattler F. et al. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab 2007; 92: 1049–57. [DOI] [PubMed] [Google Scholar]
- 29. Bajaj S, Pathak Y, Varma S. et al. Metabolic status and hypogonadism in human immunodeficiency virus-infected males. Indian J Endocr Metab 2017; 21: 684.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mialich MS, dos Santos AP, da Silva BR. et al. Relationship between adiposity indices, lipodystrophy, and sarcopenia in HIV-positive individuals with and without lipodystrophy. J Clin Densitom 2017; 20: 73–81. [DOI] [PubMed] [Google Scholar]
- 31. Scherzer R, Heymsfield SB, Lee D. et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS 2011; 25: 1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown TT, Xu X, John M. et al. Fat distribution and longitudinal anthropometric changes in HIV-infected men with and without clinical evidence of lipodystrophy and HIV-uninfected controls: a substudy of the Multicenter AIDS Cohort Study. AIDS Res Ther 2009; 6: 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]