Abstract
Objectives
Widespread antimicrobial resistance often limits the availability of therapeutic options to only a few last-resort drugs that are themselves challenged by emerging resistance and adverse side effects. Apramycin, an aminoglycoside antibiotic, has a unique chemical structure that evades almost all resistance mechanisms including the RNA methyltransferases frequently encountered in carbapenemase-producing clinical isolates. This study evaluates the in vitro activity of apramycin against multidrug-, carbapenem- and aminoglycoside-resistant Enterobacteriaceae and Acinetobacter baumannii, and provides a rationale for its superior antibacterial activity in the presence of aminoglycoside resistance determinants.
Methods
A thorough antibacterial assessment of apramycin with 1232 clinical isolates from Europe, Asia, Africa and South America was performed by standard CLSI broth microdilution testing. WGS and susceptibility testing with an engineered panel of aminoglycoside resistance-conferring determinants were used to provide a mechanistic rationale for the breadth of apramycin activity.
Results
MIC distributions and MIC90 values demonstrated broad antibacterial activity of apramycin against Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., Morganella morganii, Citrobacter freundii, Providencia spp., Proteus mirabilis, Serratia marcescens and A. baumannii. Genotypic analysis revealed the variety of aminoglycoside-modifying enzymes and rRNA methyltransferases that rendered a remarkable proportion of clinical isolates resistant to standard-of-care aminoglycosides, but not to apramycin. Screening a panel of engineered strains each with a single well-defined resistance mechanism further demonstrated a lack of cross-resistance to gentamicin, amikacin, tobramycin and plazomicin.
Conclusions
Its superior breadth of activity renders apramycin a promising drug candidate for the treatment of systemic Gram-negative infections that are resistant to treatment with other aminoglycoside antibiotics.
Introduction
Infectious diseases, particularly those caused by MDR pathogens, carbapenemase-producing Enterobacteriaceae (CPE) and carbapenemase-producing Acinetobacter baumannii (CPA), remain a major cause of morbidity and mortality worldwide.1–4 Widespread antimicrobial resistance often limits the availability of therapeutic options to only a very few efficacious antibiotics.5,6 Last-resort drugs such as tigecycline and colistin are themselves increasingly challenged by emerging resistance7 and compromised by significant adverse side effects.3,8 New therapeutic treatment options are therefore urgently needed.
Aminoglycoside antibiotics inhibit the essential process of protein biosynthesis9,10 by targeting the bacterial ribosome, one of the most effective targets in anti-infective history when looking back at seven decades of clinical track record.11 Aminoglycoside antibiotics are recognized for rapid bactericidal activity in serious systemic infections.12 Clinically relevant aminoglycoside antibiotics, however, are equally and increasingly challenged by emerging resistance13 due to aminoglycoside-modifying enzymes, and RNA methyltransferases frequently encountered in carbapenem-resistant isolates.14
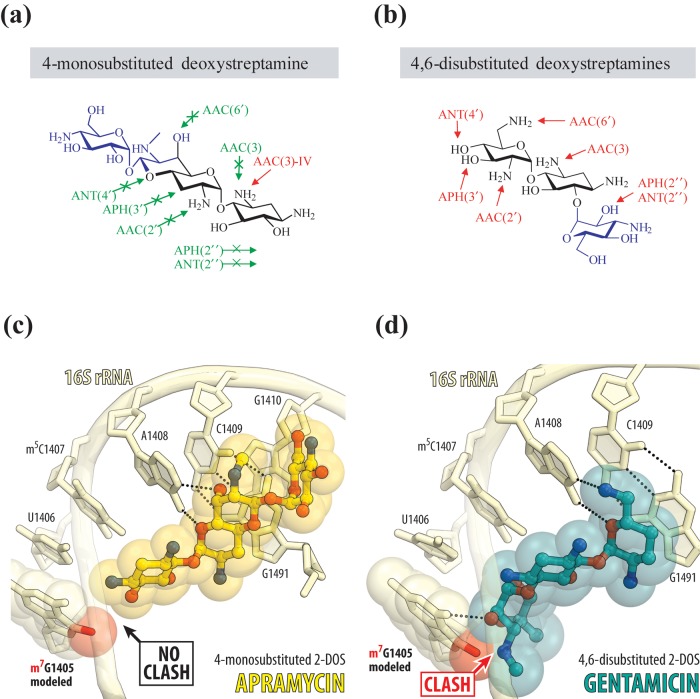
Apramycin is a monosubstituted deoxystreptamine that differs in its chemical structure from clinically relevant aminoglycoside antibiotics that are all disubstituted (Figure 1). Its unique structure renders the apramycin molecule intrinsically resilient to almost all resistance determinants typically found in MDR and XDR Gram-negative bacteria.15–17 Apramycin has also been demonstrated to be of lower toxicity than other aminoglycosides of the disubstituted deoxystreptamine drug class.18
Figure 1.
Structural rationale for the activity of apramycin in the presence of aminoglycoside resistance determinants. The chemical structures of monosubstituted (a) versus disubstituted (b) deoxystreptamine antibiotics indicating the reactive groups that are modified by acetyltransferases (AACs), phosphotransferases (APHs) and nucleotidyltransferases (ANTs). Molecular modelling of the RMTase-catalysed N7-methylation of G1405 (red sphere) onto the crystal structure of ribosome-bound apramycin (PDB entry 4AQY) reveals no clash of the methyl group with the 4-monosubstituted 2-DOS apramycin (c). Molecular modelling of the G1405 methylation onto the crystal structure of ribosome-bound gentamicin (PDB entry 4V53) reveals considerable clash with ring III of 4,6-disubstituted 2-DOS (d). Nucleotides of the 16S rRNA are shown in pale yellow, and apramycin and gentamicin are shown in yellow and teal, respectively. The E. coli nucleotide numbering is used throughout.
Here we report on a broad in vitro activity of apramycin against a variety of clinical isolates from patients in Europe, Asia, Africa and South America, including MDR, CPE and CPA. Furthermore, we elucidated the genotype of selected clinical isolates and tested engineered resistant strains to provide a mechanistic rationale for the superior spectrum of apramycin activity when compared with clinical benchmark drugs.
Materials and methods
Bacterial isolates
A total of 1132 Enterobacteriaceae and 100 A. baumannii clinical isolates were investigated in this study. The isolates were collected between 2014 and 2017 by clinical laboratories in Europe, Asia, Africa and South America, and donated without patient information.
Antimicrobial agents
Antimicrobial standards were obtained from the European Pharmacopoeia with microbiological potency values provided in each certificate of analysis. A microbiological potency value was not provided for apramycin sulphate purchased from Sigma-Aldrich. The lot-specific assay of active free base was therefore accurately determined by quantitative NMR as 554 μg of apramycin free base per mg of sulphate salt ‘as is’. Stock solutions were prepared according to CLSI guideline M07.19
Aminoglycoside susceptibility testing
CLSI broth microdilution reference methodology M0719 was used to determine MICs of antimicrobial agents. The MIC values were assessed by visual inspection with the help of an MIC 2000 Illuminated viewer (Dynatech, Kloten, Switzerland). All susceptibility testing assays were quality controlled against Escherichia coli ATCC 25922. The MIC quality control range for apramycin was determined as 2–8 mg/L (modal MIC of 4 mg/L). For all other drugs, the quality control was run against CLSI performance standards for antimicrobial susceptibility testing (M100-S25). The most recent EUCAST interpretive guidelines (www.eucast.org/clinical_breakpoints) were used to determine EUCAST breakpoints for the categorical interpretations of susceptibility and resistance for gentamicin, tobramycin, kanamycin and amikacin. For the non-clinical aminoglycoside apramycin, the MIC distributions presented in this study were used to define the following tentative ECOFFs (epidemiological cut-off values) as interpretative criteria of susceptibility and resistance: 4 mg/L for K. pneumoniae and Enterobacter spp., 8 mg/L for E. coli and 16 mg/L for A. baumannii.
Whole-genome sequencing
Clinical isolates of interest were analysed by MALDI-TOF and submitted to IIT Biotech GmbH, Bielefeld, Germany, for WGS and an ARG-ANNOT database search for antibiotic resistance genes. MALDI-TOF analysis, WGS and bioinformatics annotation of resistance genes were performed as described previously.20–22
Construction of a strain panel expressing aminoglycoside resistance determinants
The amino acid sequence of all relevant aminoglycoside resistance genes was identified by reference genes listed in Table S1 (available as Supplementary data at JAC Online) and cloned downstream of an insulated constitutive promoter of defined promoter strength.23 Each resistance cassette was then cloned into a low-copy plasmid with a pBR322 origin of replication, an ampicillin resistance cassette and a T7 terminator sequence downstream of the resistance gene. Chemically competent E. coli DH10B-derived TOP10 cells (Invitrogen) were transformed with the resulting expression plasmids, colony purified, quality-controlled by sequence analysis and antimicrobial susceptibility assessed by standard broth microdilution assays.19
Results
Broad antibacterial activity of apramycin
Apramycin demonstrated broad activity against all bacterial species tested, including MDR, CPE and CPA clinical isolates, collected between 2014 and 2017 in Europe, Asia, Africa and South America (Table 1).
Table 1.
MIC90 of apramycin in comparison with gentamicin and amikacin against clinical isolates of Enterobacteriaceae and A. baumannii isolated between 2014 and 2017
| MIC90 (mg/L) |
||||
|---|---|---|---|---|
| Species | No. | APR | GEN | AMK |
| Enterobacteriaceae (all) | 1132 | 8 | >64 | >64 |
| Escherichia coli | 250 | 8 | >64 | >64 |
| Klebsiella pneumoniae | 372 | 4 | >64 | >64 |
| Enterobacter spp. | 179 | 4 | >64 | >64 |
| Morganella morganii | 37 | 8 | >64 | 4 |
| Citrobacter freundii | 131 | 8 | >64 | >64 |
| Providencia spp. | 80 | 8 | >64 | >64 |
| Proteus mirabilis | 32 | 8 | >64 | >64 |
| Serratia marcescens | 51 | 8 | >64 | >64 |
| CPE only (all) | 406 | 4 | >128 | >128 |
| Escherichia coli | 74 | 8 | >128 | >128 |
| Klebsiella pneumoniae | 236 | 4 | >128 | >128 |
| Enterobacter spp. | 48 | 8 | >128 | >128 |
| Citrobacter freundii | 48 | 4 | >128 | >128 |
| A. baumannii | 100 | 16 | >64 | >64 |
| CPA only | 17 | 16 | >256 | >256 |
| Geographic origin | ||||
| Europe | 799 | 8 | >64 | >64 |
| Asia | 240 | 8 | >256 | >256 |
| Africa | 107 | 8 | >256 | >256 |
| South America | 86 | 4 | >256 | >256 |
APR, apramycin; AMK, amikacin; GEN, gentamicin; CPE, carbapenemase-producing Enterobacteriaceae; CPA, carbapenemase-producing A. baumannii.
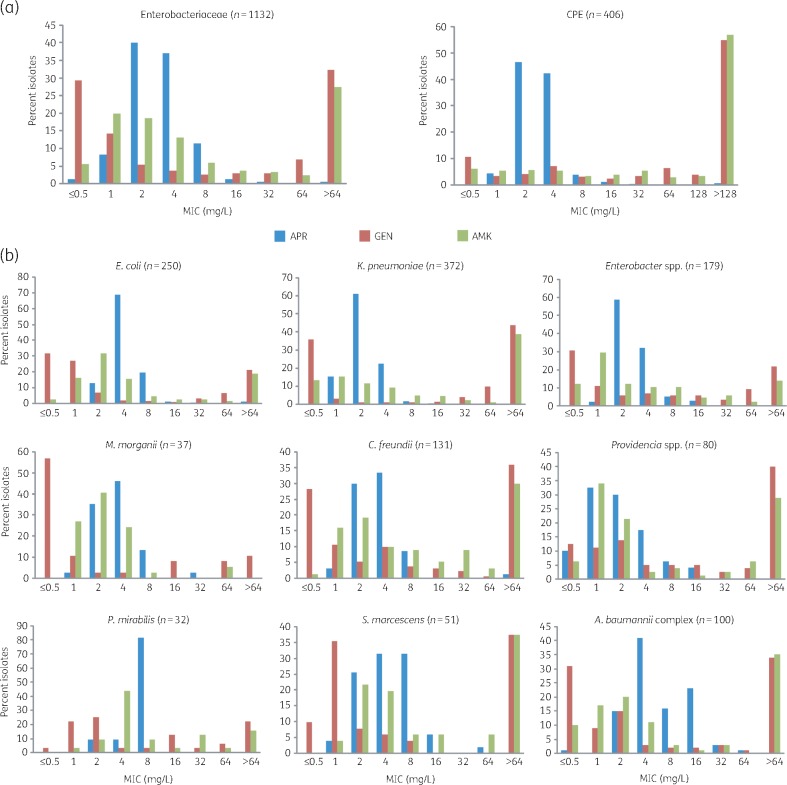
Apramycin exhibited significantly better antimicrobial activity against E. coli, Klebsiella pneumoniae, Enterobacter spp., Providencia spp., Morganella morganii, Citrobacter freundii, Proteus mirabilis, Serratia marcescens and clinical isolates of the A. baumannii complex than the clinical standard-of-care aminoglycosides gentamicin and amikacin (Table 1). An apramycin concentration of 8 mg/L inhibited 98% of all Enterobacteriaceae isolates tested. An apramycin concentration of 16 mg/L inhibited 97% of all A. baumannii isolates tested. At the level of individual Enterobacteriaceae species, an apramycin concentration of 4 mg/L inhibited 99% of K. pneumoniae and 93% of Enterobacter isolates. An apramycin concentration of 8 mg/L inhibited 99% of all E. coli, 98% of C. freundii, 96% of Providencia spp., 92% of S. marcescens, 97% of M. morganii and 100% of P. mirabilis clinical isolates (Table 1). In comparison, the MIC90 of amikacin and gentamicin was >64 mg/L for most of the Enterobacteriaceae species tested (Table 1). The epidemiological distribution of MIC values highlighted the uncompromised in vitro activity of apramycin against clinical isolates of Enterobacteriaceae and A. baumannii (Figure 2 and Figures S1 and S2).
Figure 2.
MIC distribution of apramycin in comparison with gentamicin and amikacin. (a) MIC distribution for Enterobacteriaceae clinical isolates of diverse geographic origin collected between 2014 and 2017 (left), and a subset of only carbapenemase-producing Enterobacteriaceae (CPE, right). (b) MIC distribution for Enterobacteriaceae and A. baumannii at the genus or species level. APR, apramycin; AMK, amikacin; GEN, gentamicin; CPE, carbapenemase-producing Enterobacteriaceae. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
High activity of apramycin against CPE and A. baumannii
In addition to looking at all isolates collectively, we also analysed the activity of apramycin against the carbapenemase-producing subpopulation of isolates representing the most urgent unmet medical need.4 A total of 406 carbapenemase-producing E. coli, K. pneumoniae, Enterobacter, C. freundii and Providencia spp. isolates and 17 CPA were investigated in this study. Apramycin concentrations of 4 and 8 mg/L inhibited 93% and 97% of all CPE isolates, respectively. A concentration of 16 mg/L was required to inhibit 94% of CPA isolates (Table 1). In comparison, gentamicin and amikacin showed little activity against these isolates. In plotting the MIC distribution of apramycin for only CPE and CPA, the superiority of apramycin over clinical benchmark aminoglycosides became even more apparent (Figure 2). Against individual CPE species, apramycin inhibited 97% of K. pneumoniae and 94% of C. freundii clinical isolates at ≤4 mg/L, and 94% of E. coli and 93% of Enterobacter clinical isolates at ≤8 mg/L. In comparison, gentamicin and amikacin were found to be inactive against a remarkable proportion of carbapenemase-producing E. coli, K. pneumoniae, Enterobacter, C. freundii and Providencia spp. clinical isolates (Figure 2). The higher activity of apramycin against individual CPE species became particularly apparent when plotting the MIC distribution (Figure S1).
Importantly, and in contrast to the clinically approved antibiotics gentamicin, kanamycin, tobramycin, amikacin, plazomicin and meropenem,24–27 apramycin was active against E. coli isolates that were characterized by WGS as encoding carbapenemases NDM-1, OXA-23 and IMP-1, which are the main causes of carbapenem resistance in CPE strains (Table 2).28,29 Apramycin was likewise active against K. pneumoniae strains encoding carbapenemases KPC-2, OXA-181, OXA-232 and OXA-48 (Table 2), and against E. coli strains harbouring the plasmid-mediated colistin resistance gene, mcr-1.30
Table 2.
Antibiotic susceptibility of selected genotypes of E. coli and K. pneumoniae clinical isolates isolated between 2014 and 2017
| MIC (mg/L) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Organism | Characteristic | APR | GEN | KAN | TOB | AMK | PLZ | MEM |
| E. coli | NDM-1 | 8 | >128 | >128 | >128 | >128 | >128 | >128 |
| IMP-1 | 4 | 32 | 128 | 32 | 8 | 4 | 8 | |
| MCR-1 | 2–4 | 2 | >128 | 0.5 | 1 | 2 | 0.125 | |
| OXA-23 | 8 | 64 | >128 | 16 | 4 | 2 | 8 | |
| K. pneumoniae | KPC-2 | 2 | 128 | >128 | >128 | >128 | >128 | 128 |
| OXA-181 | 2 | 64 | 128 | 64 | 8 | 1–2 | 4 | |
| OXA-232 | 2 | >128 | >128 | >128 | >128 | >128 | 128 | |
| OXA-48 | 2 | 128 | >128 | >128 | >128 | 1 | 64 | |
APR, apramycin; GEN, gentamicin; KAN, kanamycin; TOB, tobramycin; AMK, amikacin; PLZ, plazomicin; MEM, meropenem.
Genotypic rationale for superior antibacterial activity
To assess the potency of apramycin against MDR bacteria, we compared the susceptibility and MIC distribution of MDR versus non-MDR clinical isolates of E. coli, K. pneumoniae and E. cloacae against a panel of aminoglycoside antibiotics. Clinical isolates in this analysis were classified as MDR if expressing resistance to at least three out of the following five drug classes: third-generation cephalosporins, quinolones, aminoglycosides, carbapenems and a β-lactam antibiotic with a β-lactamase inhibitor, namely piperacillin/tazobactam.31 The distribution of MICs for MDR clinical isolates was shifted to higher MIC values for the analysed benchmark aminoglycoside antibiotics, amikacin, gentamicin, kanamycin and tobramycin, when compared with non-MDR clinical isolates, but not for apramycin (Figure S3).
WGS of MDR clinical isolates of E. coli, E. cloacae and K. pneumoniae revealed the genotypes associated with the phenotypic resistance profiles of the analysed clinical isolates. The identified genotypes corresponded closely to the resistance phenotypes predicted by the aminoglycoside antibiogram (Figure 1). A broad variety of aminoglycoside resistance determinants and combinations thereof were identified in the genomes of the sequenced MDR clinical isolates including several distinct subtypes of acetyltransferases (AACs), phosphotransferases (APHs), nucleotidyltransferases (ANTs) and 16S rRNA methyltransferases (RMTases) (Table 3).
Table 3.
Phenotypes associated with the predominant aminoglycoside resistance determinants found in the analysed bacterial strains by WGS
| Resistance phenotype |
|||||
|---|---|---|---|---|---|
| Genotype | APR | GEN | AMK | KAN | TOB |
| Escherichia coli | |||||
| AAC(3)-II | S | R | S | I | I |
| AAC(6′)-I | S | S | I | R | R |
| AAC(6′)-I ANT(2′′) | S | R | R | R | R |
| AAC(6′)-I AAC(3)-II | S | R | I | R | R |
| rmtB AAC(3)-II APH(3′) | S | R | R | R | R |
| Klebsiella pneumoniae | |||||
| ANT(2′′) | S | I | I | R | I |
| AAC(3)-II APH(3′) | S | R | S | R | I |
| AAC(6′)-I APH(3′) | S | S | I | R | R |
| AAC(6′)-I AAC(3)-II | S | R | I | R | R |
| AAC(6′)-I AAC(3)-II APH(3′) | S | R | I | R | R |
| rmtB AAC(6′)-I AAC(3)-II | S | R | R | R | R |
| Enterobacter cloacae | |||||
| ANT(2′′) | S | I | I | I | I |
| AAC(6′)-I AAC(3)-II | S | R | S | R | R |
| AAC(6′)-I ANT(2′′) | S | I | I | R | R |
| AAC(6′)-I AAC(3)-V ANT(2′′) | S | R | I | R | R |
| AAC(6′)-I AAC(3)-V APH(3′) | S | R | R | R | R |
| rmtC AAC(6′)-I AAC(3)-II | S | R | R | R | R |
| Acinetobacter baumannii | |||||
| armA | S | R | R | R | R |
| AAC(6′)-I | S | I | R | R | R |
| APH(3′)-I armA | S | R | R | R | R |
| AAC(6′)-I APH(3′) | S | I | R | R | R |
| AAC(3)-I armA | S | R | R | R | R |
| APH(3′)-VI AAC(3)-I | S | R | R | R | S |
| APH(3′)-VI AAC(3)-I ANT(2′′) | S | R | R | R | R |
| AAC(6′)-I AAC(3)-I APH(3′) | S | R | R | R | R |
APR, apramycin; GEN, gentamicin; AMK, amikacin; KAN, kanamycin; TOB, tobramycin.
In the case of apramycin, ECOFFs have been used as interpretative criteria.
The predominant resistance mechanisms identified were those facilitated by the acetyltransferases AAC(6′) and AAC(3), which accounted for the majority of phenotypic non-susceptibility to gentamicin, tobramycin, amikacin and kanamycin (Table 3). The nucleotidyltransferase ANT(2′′), phosphotransferase APH(3′) and the RMTase-encoding genes armA, rmtB and rmtC were also found in clinical E. coli, K. pneumoniae, Enterobacter and A. baumannii isolates (Table 3). As predicted, the presence of these antibiotic resistance genes in the genomes of the analysed MDR clinical isolates did not negatively impact the antibacterial activity of apramycin. In conclusion, although many of the MDR clinical isolates analysed were resistant to benchmark aminoglycoside antibiotics, they retained a susceptible phenotype to apramycin when applying the ECOFF of 8 mg/L for E. coli, 4 mg/L for K. pneumoniae and Enterobacter spp., and 16 mg/L for A. baumannii (Table 3).
Apramycin evades almost all aminoglycoside resistance mechanisms
The high genotypic diversity and the presence of multiple antibiotic resistance determinants within individual isolates may in part blur a more mechanistic correlation between phenotypic resistance and individual resistance genes. Key resistance genes were therefore cloned into a DH10B-derived E. coli lab strain to allow promoter-controlled expression of individual resistance genes in an otherwise isogenic background. This enabled elucidation of the apramycin activity in the presence of individual and well-defined resistance genes including AACs, APHs, ANTs and RMTases. This analysis revealed high activity of apramycin against all known resistance mechanisms, with the exception of AAC(3)-IV (Table 4). In contrast, the clinical aminoglycosides gentamicin, amikacin, tobramycin and amikacin were each inactivated by a variety of resistance mechanisms as expected.32–34 Susceptibility to amikacin was reduced in the presence of AAC(6′) and some subtypes of APH(3′) and, similarly to plazomicin, was fully abolished in the presence of RMTases. When comparing the resistance profile of plazomicin with that of amikacin, it became apparent that susceptibility to plazomicin was retained in the presence of AAC(6′) and APH(3′), but at the cost of lower susceptibility to plazomicin than amikacin in the presence of AAC(2′) and APH(2′′). The superiority of apramycin to other aminoglycosides was particularly remarkable for RMTases. Engineered E. coli strains expressing armA, rmtB, rmtC or rmtF genes were highly resistant (MIC >64 mg/L) to all other aminoglycoside antibiotics tested, including plazomicin, while remaining susceptible to apramycin (Table 4). The only resistance determinant investigated in this study that rendered the host E. coli cells non-susceptible to apramycin was the aminoglycoside 3-N-acetyltransferase subtype IV [AAC(3)-IV] (Table 4), a resistance mechanism that was previously known to confer apramycin resistance.35
Table 4.
Apramycin activity in comparison with gentamicin, amikacin, tobramycin and plazomicin against engineered E. coli strains expressing individual aminoglycoside resistance mechanisms
| Resistance mechanism | MIC (mg/L) |
||||
|---|---|---|---|---|---|
| APR | GEN | AMK | TOB | PLZ | |
| None | 4 | 0.5 | 1–2 | 0.5 | 0.5 |
| AAC(6′)-I | 4 | 2 | 64 | 32–64 | 0.5 |
| AAC(6′)-II | 4 | 64 | 8 | 32–64 | 1 |
| AAC(3)-I | 8 | >64 | 1–2 | 1 | 0.5–1 |
| AAC(3)-II | 8 | >64 | 1 | 32 | 4 |
| AAC(3)-III | 4 | >64 | 0.5–1 | >64 | 0.5 |
| AAC(3)-IV | >64 | 2 | 1–2 | 2 | 0.5 |
| AAC(3)-VI | 4 | >64 | 1–2 | 4 | 1 |
| AAC(2′)-I | 2–4 | 4 | 1–2 | 8–16 | 8–16 |
| APH(3′)-I | 2 | 1–2 | 1–2 | 8 | 0.5 |
| APH(3′)-II | 4 | 0.5 | 8 | 0.5 | 0.5 |
| APH(3′)-III | 4 | 0.5 | 32 | 4–8 | 0.5 |
| APH(3′)-VI | 4 | 0.5 | 64 | 0.5 | 0.5–1 |
| APH(2′′)-II | 2–4 | >64 | 2–4 | 64 | 8 |
| APH(2′′)-IV | 4 | >64 | 1–2 | 32–64 | 8 |
| ANT(4′)-II | 2–4 | 0.5 | 1–2 | 0.5 | 0.5–1 |
| ANT(2′′)-I | 4 | 16–32 | 1 | 16–32 | 0.5 |
| armA | 2–4 | >64 | >64 | >64 | >64 |
| rmtB | 4 | >64 | >64 | >64 | >64 |
| rmtC | 2–4 | >64 | >64 | >64 | >64 |
| rmtF | 2–4 | >64 | >64 | >64 | >64 |
APR, apramycin; GEN, gentamicin; AMK, amikacin; TOB, tobramycin; PLZ, plazomicin.
Discussion
The results presented in this study clearly demonstrate that apramycin has a broad antibacterial activity against a variety of clinical isolates, including MDR, CPE and CPA clinical isolates of diverse geographic origin (Table 1). This is consistent with previous reports, which showed that the vast majority of the analysed CPE clinical isolates from the USA, the UK and China were more susceptible to apramycin than to other aminoglycosides.16,28,36 Our analysis provides strong evidence that the high susceptibility to apramycin is a widespread phenomenon and not geographically restricted.
Apramycin retains significant activity against a broad spectrum of MDR and XDR clinical isolates, including those with NDM-1, IMP-1, OXA-23, OXA-48, OXA-181, OXA-232 and KPC-2 genotypes (Tables 1 and 2).28,29 Previously, it was shown that in comparison with amikacin, gentamicin and tobramycin, apramycin is highly active against MDR, XDR and pan-drug-resistant (PDR) clinical isolates that include A. baumannii and P. aeruginosa.15 Furthermore, our results also demonstrate uncompromised activity of apramycin against E. coli clinical isolates harbouring the plasmid-borne resistance gene mcr-130 (Table 2), which confers resistance to the last-resort drug colistin.
Perhaps the most striking observation is that the presence of various antibiotic resistance-conferring genes, such as those encoding aminoglycoside-modifying enzymes and RMTases, did not adversely affect the distribution of apramycin MICs in the analysed clinical isolate panels. Apramycin remained active against E. coli harbouring various individual aminoglycoside resistance determinants, including those which rendered the host E. coli cells resistant against all other antibiotics tested (Tables 3 and 4). The only mechanisms found to induce significant resistance to apramycin was that of the acetyltransferase AAC(3)-IV (Table 4), which has previously been identified as a pre-determinant of resistance to apramycin and other aminoglycosides.37
Regio-specific acetylation of the 3-amino group is catalysed by a family of acetyltransferases that for the most part do not seem to recognize apramycin as a substrate. To date, AAC(3)-IV is the only subtype known to acetylate apramycin efficiently. Acetylation at C3 not only replaces an amino group with a less basic amide, but may also prevent its binding to the drug target site by means of a steric clash with the phosphate backbone of 16S rRNA (Figure S4).
Of 1232 clinical isolates tested in this study, 4 isolates (<0.5%) were found to be resistant to all antibiotics tested including apramycin. The fact that apramycin resistance was found to be rare in a relevant panel of clinical isolates suggests a very high (>99%) probability of coverage in empirical treatment of MDR pathogens. WGS of the four isolates resistant to apramycin revealed the presence of a variety of resistance genes including aac(3)-IV (Table S2), which was in agreement with the genotypic prediction inferred from the results with engineered E. coli strains (Table 4). The fact that resistance to apramycin was exclusively associated with an aac(3)-IV-positive genotype in all cases suggests feasibility of a PCR detection kit as a more rapid diagnostic than bacterial culture or WGS. Among over a thousand clinical isolates from across the globe included herein, no other apramycin resistance determinants were found.
A literature search for additional mechanisms of apramycin resistance suggested acetylation of the 1-amino group as another possible mechanism of apramycin resistance.38,39 However, little clinical or other evidence has since corroborated the hypothesis. Putative AAC(1) enzymes were found to acetylate aminoglycoside antibiotics inconsistently.40 Structural modelling suggests that the drug-binding pocket can accommodate an acetamide at C1 without a prohibitive steric clash, unlike acetylation at C3 discussed above (Figure S4). This is in agreement with the fact that aminoglycosides such as amikacin, arbekacin or plazomicin, with the bulkier l-4-amino-2-hydroxybutyramide amide group at C1, are accepted by the drug-binding pocket without significant loss of activity when compared with the parent amines. Any loss of activity on acetamide formation at the 1-position would therefore be likely to arise owing to the loss of the basic amine (unlike amikacin, arbekacin and plazomicin in which the functionalized amide group itself carries a basic amine). The existence of such AAC(1) resistance mechanisms, however, has yet to be confirmed independently. Phylogenetic alignment of amino acid sequences revealed a very high sequence homology of putative AAC(1) sequences with AAC(3)-I proteins (Figure S5). Further studies are needed to re-analyse the functional conversion of aminoglycosides in the presence of AAC(1) proteins.
The high activity of apramycin against MDR, CPE and CPA clinical isolates is owing to the ability of apramycin both to evade aminoglycoside-modifying enzymes and to bind to a methylated drug target site.18 Drug target methylation at ribosomal site N7-G1405 by RMTases encoded by armA and rmtC, and related antibiotic resistance-conferring enzymes effectively distorts the binding pocket for clinically approved aminoglycoside antibiotics, including plazomicin (Figure 1).41,42 Consequently, although retaining antibiotic activity against many aminoglycoside-modifying enzymes, plazomicin is inactive against drug-resistant clinical isolates encoding RMTases.43 Methylation of N7-G1405 is frequently encountered in CPE clinical isolates17,36,44–47 and is therefore often associated with the resistance of CPE to all of the clinically relevant aminoglycoside antibiotics. In contrast, the binding and activity of apramycin are not prevented by methylation of N7-G1405.18 The crystal structure of apramycin bound to its target site (Figure 1) reveals sufficient space to accommodate methylation of N7-G1405, whereas otherwise it results in a steric clash with the glycosidic ring at C6 in standard-of-care aminoglycosides and plazomicin, all of which are 4,6-disubstituted deoxystreptamines.18,48 This ultimately leads to uncompromised activity of apramycin against a broad spectrum of pathogenic bacteria, including CRE and A. baumannii.
WGS of clinical A. baumannii isolates tested in this study revealed a relatively high incidence (44%) of armA-positive isolates. Because ArmA-mediated methylation inside the drug-binding pocket does not obstruct the binding of apramycin, the MIC distribution of apramycin was found to be superior to that of other aminoglycoside antibiotics for clinical isolates of the A. baumannii complex (Figure 2b).
Although some of the other clinically relevant aminoglycoside antibiotics, such as amikacin, tobramycin and gentamicin, have previously been successfully applied in the treatment of CPE infections either alone or in combination with other antimicrobial agents,49,50 increasing RMTase-mediated resistance represents a challenge for treatment of CPE and CPA bacterial infections, in particular in Southern Europe and in low- and middle-income countries in South America, Africa and Asia. The intrinsic resilience of apramycin to common mechanisms of aminoglycoside resistance including 16S rRNA-modifying enzymes translates into a superior spectrum of activity that comprises highly drug-resistant Gram-negative organisms and hence a possible therapeutic remedy for this issue. Collectively, our results are in agreement with previous reports suggesting the potential of apramycin.15,28,51
In the present study, preliminary ECOFFs were used as interpretative criteria because the pharmacokinetics (PK), probability of target attainment and, therefore, clinical breakpoints for apramycin are currently unknown. Preliminary (and as yet unpublished) data on the apramycin PK in various animal species suggest a PK profile that resembles that of other aminoglycoside antibiotics. Further preclinical evaluation is required to study the in vivo efficacy and the PK/pharmacodynamics of apramycin, which will be important in assessing the clinical potential of apramycin in the treatment of MDR Gram-negative bacterial infections in humans.
Apramycin may also represent a promising lead scaffold for further derivatization, taking advantage of the various beneficial features of apramycin including its exquisite selectivity for the bacterial over the eukaryotic cytosolic and mitochondrial ribosomes.52–54 Together with its low inherent toxicity reported earlier,18 apramycin and apramycin-like monosubstituted deoxystreptamines may represent a promising new subclass of aminoglycoside antibiotics for further optimization and development.
Supplementary Material
Acknowledgements
The authors express their gratitude to Maisra El-Bouseary, Dr Diego Andrey, Dr Ana C. Gales, Dorota Żabicka, Marek Gniadkowski, Fernando Baquero, Rafael Canton, Niels Frimodt-Møller and Reinhard Zbinden for kindly supplying clinical isolates. We acknowledge IIT Biotech GmbH Bielefeld and Dr Frank Imkamp for their expertise and services in whole-genome sequencing, and the staff at the Institute of Medical Microbiology at University of Zurich for their support in susceptibility testing contributing to Figure S3. We are grateful to Raymond Lin for critical comments on the manuscript.
Funding
Some of the research leading to these results was conducted as part of the ND4BB European Gram-Negative Antibacterial Engine (ENABLE) Consortium (www.nd4bb-enable.eu) and has received funding from the Innovative Medicines Initiative Joint Undertaking under grant agreement n°115583, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and The European Federation of Pharmaceutical Industries and Associations (EFPIA) companies in kind contribution. The ENABLE project is also financially supported by contributions from Academic and Small and medium-sized enterprise (SME) partners. The research leading to these results has been supported by the University of Zurich.
Transparency declarations
D. C., E. C. B. and S. N. H. are co-founders of Juvabis AG, a startup biotech company with an interest in aminoglycoside therapeutics. All other authors: none to declare.
References
- 1. Potron A, Poirel L, Nordmann P.. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 2015; 45: 568–85. [DOI] [PubMed] [Google Scholar]
- 2. Busani S, Serafini G, Mantovani E. et al. Mortality in patients with septic shock by multidrug resistant bacteria. J Intensive Care Med 2017; doi: 10.1177/0885066616688165. [DOI] [PubMed] [Google Scholar]
- 3. Poirel L, Kieffer N, Liassine N. et al. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect Dis 2016; 16: 281.. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-resistant Bacterial Infections. Geneva: World Health Organization, 2017. (WHO/EMP/IAU/2017.12). [Google Scholar]
- 5. Blair JM, Webber MA, Baylay AJ. et al. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 2015; 13: 42–51. [DOI] [PubMed] [Google Scholar]
- 6. Fischbach M, Walsh C.. Antibiotics for emerging pathogens. Science 2009; 325: 1089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu YY, Wang Y, Walsh TR. et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016; 16: 161–8. [DOI] [PubMed] [Google Scholar]
- 8. Osei Sekyere J, Govinden U, Bester LA. et al. Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: emerging resistance mechanisms and detection methods. J Appl Microbiol 2016; 121: 601–17. [DOI] [PubMed] [Google Scholar]
- 9. Juhas M. Pseudomonas aeruginosa essentials: an update on investigation of essential genes. Microbiology (Reading, Engl) 2015; 161: 2053–60. [DOI] [PubMed] [Google Scholar]
- 10. Lee SA, Gallagher LA, Thongdee M. et al. General and condition-specific essential functions of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 2015; 112: 5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCoy LS, Xie Y, Tor Y.. Antibiotics that target protein synthesis. Wiley Interdiscip Rev RNA 2011; 2: 209–32. [DOI] [PubMed] [Google Scholar]
- 12. Krause KM, Serio AW, Kane TR. et al. Aminoglycosides: an overview. Cold Spring Harb Perspect Med 2016; 6: a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michiels JE, Van den Bergh B, Verstraeten N. et al. In vitro emergence of high persistence upon periodic aminoglycoside challenge in the ESKAPE pathogens. Antimicrob Agents Chemother 2016; 60: 4630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karah N, Dwibedi CK, Sjöström K. et al. Novel aminoglycoside resistance transposons and transposon-derived circular forms detected in carbapenem-resistant Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 2016; 60: 1801–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang AD, Smith KP, Eliopoulos GM. et al. In vitro apramycin activity against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 2017; 88: 188–91. [DOI] [PubMed] [Google Scholar]
- 16. Smith KP, Kirby JE.. Evaluation of apramycin activity against carbapenem-resistant and -susceptible strains of Enterobacteriaceae. Diagn Microbiol Infect Dis 2016; 86: 439–41. [DOI] [PubMed] [Google Scholar]
- 17. Wachino J, Arakawa Y.. Exogenously acquired 16S rRNA methyltransferases found in aminoglycoside-resistant pathogenic Gram-negative bacteria: an update. Drug Resist Updat 2012; 15: 133–48. [DOI] [PubMed] [Google Scholar]
- 18. Matt T, Ng CL, Lang K. et al. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc Natl Acad Sci USA 2012; 109: 10984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Tenth Edition: Approved Standard M07-A10. CLSI, Wayne, PA, USA, 2015. [Google Scholar]
- 20. Schulthess B, Bloemberg GV, Zbinden A. et al. Evaluation of the Bruker MALDI biotyper for identification of fastidious Gram-negative rods. J Clin Microbiol 2016; 54: 543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stucki D, Ballif M, Egger M. et al. Standard genotyping overestimates transmission of Mycobacterium tuberculosis among immigrants in a low-incidence country. J Clin Microbiol 2016; 54: 1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta SK, Padmanabhan BR, Diene SM. et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 2014; 58: 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davis JH, Rubin AJ, Sauer RT.. Design, construction and characterization of a set of insulated bacterial promoters. Nucleic Acids Res 2011; 39: 1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. López-Diaz MD, Culebras E, Rodríguez-Avial I. et al. Plazomicin activity against 346 extended-spectrum-β-lactamase/AmpC-producing Escherichia coli urinary isolates in relation to aminoglycoside-modifying enzymes. Antimicrob Agents Chemother 2017; 61: pii: e02454-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodríguez-Avial I, Pena I, Picazo JJ. et al. In vitro activity of the next-generation aminoglycoside plazomicin alone and in combination with colistin, meropenem, fosfomycin or tigecycline against carbapenemase-producing Enterobacteriaceae strains. Int J Antimicrob Agents 2015; 46: 616–21. [DOI] [PubMed] [Google Scholar]
- 26. Denervaud-Tendon V, Poirel L, Connolly LE. et al. Plazomicin activity against polymyxin-resistant Enterobacteriaceae, including MCR-1-producing isolates. J Antimicrob Chemother 2017; 72: 2787–91. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Kashikar A, Bush K.. In vitro activity of plazomicin against β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE). J Antimicrob Chemother 2017; 72: 2792–5. [DOI] [PubMed] [Google Scholar]
- 28. Hu Y, Liu L, Zhang X. et al. In vitro activity of neomycin, streptomycin, paromomycin and apramycin against carbapenem-resistant Enterobacteriaceae clinical strains. Front Microbiol 2017; 8: 2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Logan LK, Weinstein RA.. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 2017; 215: S28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MacNair CR, Stokes JM, Carfrae LA. et al. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat Commun 2018; 9: 458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hombach M, Wolfensberger A, Kuster SP. et al. Influence of clinical breakpoint changes from CLSI 2009 to EUCAST 2011 antimicrobial susceptibility testing guidelines on multidrug resistance rates of Gram-negative rods. J Clin Microbiol 2013; 51: 2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jakobsen L, Sandvang D, Jensen VF. et al. Gentamicin susceptibility in Escherichia coli related to the genetic background: problems with breakpoints. Clin Microbiol Infect 2007; 13: 830–2. [DOI] [PubMed] [Google Scholar]
- 33. Rosvoll TC, Lindstad BL, Lunde TM. et al. Increased high-level gentamicin resistance in invasive Enterococcus faecium is associated with aac(6')Ie-aph(2″)Ia-encoding transferable megaplasmids hosted by major hospital-adapted lineages. FEMS Immunol Med Microbiol 2012; 66: 166–76. [DOI] [PubMed] [Google Scholar]
- 34. Jaimee G, Halami PM.. Conjugal transfer of aac(6′)Ie-aph(2″)Ia gene from native species and mechanism of regulation and cross resistance in Enterococcus faecalis MCC3063 by real time-PCR. Microb Pathog 2017; 110: 546–53. [DOI] [PubMed] [Google Scholar]
- 35. Davies J, O’Connor S.. Enzymatic modification of aminoglycoside antibiotics: 3-N-acetyltransferase with broad specificity that determines resistance to the novel aminoglycoside apramycin. Antimicrob Agents Chemother 1978; 14: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Livermore DM, Mushtaq S, Warner M. et al. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 2011; 66: 48–53. [DOI] [PubMed] [Google Scholar]
- 37. Magalhaes ML, Blanchard JS.. The kinetic mechanism of AAC3-IV aminoglycoside acetyltransferase from Escherichia coli. Biochemistry 2005; 44: 16275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lovering AM, White LO, Reeves DS.. AAC(1): a new aminoglycoside-acetylating enzyme modifying the Cl aminogroup of apramycin. J Antimicrob Chemother 1987; 20: 803–13. [DOI] [PubMed] [Google Scholar]
- 39. Ramirez MS, Tolmasky ME.. Aminoglycoside modifying enzymes. Drug Resist Updat 2010; 13: 151–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sunada A, Nakajima M, Ikeda Y. et al. Enzymatic 1-N-acetylation of paromomycin by an actinomycete strain #8 with multiple aminoglycoside resistance and paromomycin sensitivity. J Antibiot (Tokyo) 1999; 52: 809–14. [DOI] [PubMed] [Google Scholar]
- 41. Schmitt E, Galimand M, Panvert M. et al. Structural bases for 16 S rRNA methylation catalyzed by ArmA and RmtB methyltransferases. J Mol Biol 2009; 388: 570–82. [DOI] [PubMed] [Google Scholar]
- 42. Doi Y, Wachino JI, Arakawa Y.. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am 2016; 30: 523–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cox G, Ejim L, Stogios PJ. et al. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis 2018; 4: 980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poirel L, Bonnin RA, Nordmann P.. Analysis of the resistome of a multidrug-resistant NDM-1-producing Escherichia coli strain by high-throughput genome sequencing. Antimicrob Agents Chemother 2011; 55: 4224.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ho PL, Lo WU, Yeung MK. et al. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS One 2011; 6: e17989.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rahman M, Prasad KN, Pathak A. et al. RmtC and RmtF 16S rRNA methyltransferase in NDM-1-producing Pseudomonas aeruginosa. Emerg Infect Dis 2015; 21: 2059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poirel L, Goutines J, Aires-de-Sousa M. et al. High rate of association of 16S rRNA methylases and carbapenemases in Enterobacteriaceae recovered from hospitalized children in Angola. Antimicrob Agents Chemother 2018; 62: pii: e00021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polikanov YS, Melnikov SV, Söll D. et al. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat Struct Mol Biol 2015; 22: 342–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rafailidis PI, Falagas ME.. Options for treating carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis 2014; 27: 479–83. [DOI] [PubMed] [Google Scholar]
- 50. Shields RK, Clancy CJ, Press EG. et al. Aminoglycosides for treatment of bacteremia due to carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 2016; 60: 3187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Truelson KA, Brennan-Krohn T, Smith KP. et al. Evaluation of apramycin activity against methicillin-resistant, methicillin-sensitive, and vancomycin-intermediate Staphylococcus aureus clinical isolates. Diagn Microbiol Infect Dis 2018; 92: 168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mandhapati AR, Yang G, Kato T. et al. Structure-based design and synthesis of apramycin-paromomycin analogues: importance of the configuration at the 6′-position and differences between the 6′-amino and hydroxy series. J Am Chem Soc 2017; 139: 14611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perez-Fernandez D, Shcherbakov D, Matt T. et al. 4′-O-substitutions determine selectivity of aminoglycoside antibiotics. Nat Commun 2014; 5: 3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hobbie SN, Kalapala SK, Akshay S. et al. Engineering the rRNA decoding site of eukaryotic cytosolic ribosomes in bacteria. Nucleic Acids Res 2007; 35: 6086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.