Abstract
Objectives
To evaluate the in vitro antimicrobial/antivirulence action of bovine lactoferrin and its ability to synergize with levofloxacin against resistant Helicobacter pylori strains and to analyse the effect of levofloxacin, amoxicillin and esomeprazole with and without bovine lactoferrin as the first-line treatment for H. pylori infection.
Methods
The bovine lactoferrin antimicrobial/antivirulence effect was analysed in vitro by MIC/MBC determination and twitching motility against six clinical H. pylori strains and a reference strain. The synergism was evaluated using the chequerboard assay. The prospective therapeutic trial was carried out on two separate patient groups, one treated with esomeprazole/amoxicillin/levofloxacin and the other with esomeprazole/amoxicillin/levofloxacin/bovine lactoferrin. Treatment outcome was determined with the [13C]urea breath test.
Results
In vitro, bovine lactoferrin inhibited the growth of 50% of strains at 10 mg/mL and expressed 50% bactericidal effect at 40 mg/mL. The combination of levofloxacin and bovine lactoferrin displayed a synergistic effect for all strains, with the best MIC reduction of 16- and 32-fold for levofloxacin and bovine lactoferrin, respectively. Bovine lactoferrin at one-fourth MIC reduced microbial motility significantly for all strains studied. In the in vivo study, 6 of 24 patients recruited had treatment failure recorded with esomeprazole/amoxicillin/levofloxacin (75% success, 95% CI 57.68%–92.32%), and in the group with esomeprazole/amoxicillin/levofloxacin/bovine lactoferrin, 2 out of 53 patients recruited had failure recorded (96.07% success, 95% CI 90.62%–101.38%).
Conclusions
Bovine lactoferrin can be considered a novel potentiator for restoring susceptibility in resistant H. pylori strains. Bovine lactoferrin added to a triple therapy in first-line treatment potentiates the therapeutic effect.
Introduction
Helicobacter pylori is involved in the development of chronic gastritis and peptic ulcer disease and has been linked to the pathogenesis of gastric lymphoma and gastric cancer; hence it is recommended that this infection should be cured whenever it is diagnosed.1–3 Clarithromycin, amoxicillin, metronidazole, tinidazole, tetracycline, rifabutin, ampicillin and fluoroquinolones have been used to treat H. pylori infection.4,5 This bacterial infection, however, has been shown to be challenging to cure. In fact, H. pylori may be resistant in various degrees to one or more of the above-mentioned antibiotics, even in subjects never treated specifically for the infection, and antibiotic resistance may be a key factor in treatment failure.6 This alarming phenomenon strongly supports the need to find novel strategies, such as the inclusion of an adjuvant aimed at enhancing the effectiveness of drugs commonly used in this specific therapy.7
Classical triple therapies with proton pump inhibitors, clarithromycin and amoxicillin or metronidazole are the mainstay of current treatment; the increasing resistance to clarithromycin, however, has reduced their effectiveness, with an eradication rate of <80% of treated cases.8,9 A recent consensus report suggests that a proton pump inhibitor/clarithromycin-containing triple therapy without prior susceptibility testing should be abandoned in those areas where the clarithromycin resistance rate is >15%.10
Resistance rates vary in different geographical areas and therefore the selection of therapeutic regimens needs to be adjusted according to the local resistance pattern, if this is known.11–15 In the region of Abruzzo, the latest data on resistance of H. pylori, isolated from already-treated (once or several times) and never-treated patients, to amoxicillin, clarithromycin, metronidazole, levofloxacin, tetracycline and rifabutin are as follows: 1.02%, 72.44%, 34.69%, 42.85%, 2.63% and 1.20%, respectively.16 Consequently, the geographical prevalence of the H. pylori resistance profile should be the basis for the selection of first-line eradication therapy aimed at avoiding primary failure.17
As an alternative to clarithromycin, many studies have examined levofloxacin and proton pump inhibitors as a first-line therapy for eradication of H. pylori infection.18 However, the eradication rates achieved with first-line levofloxacin-based treatments are not uniform and contrasting results have been reported.19–21 In regions of high clarithromycin resistance, bismuth-containing quadruple therapies are recommended for first-line therapy since bismuth may help to overcome the antibiotic resistance to clarithromycin when present.22,23 Bismuth salts, however, are not available in all countries; it is therefore useful to search for a valid substitute that may increase the effectiveness of a triple therapy regardless of the presence of H. pylori resistance to one or more antibiotics.
Recent data have called attention to the potential role of fermented milk and related whey proteins, such as bovine lactoferrin, as potential candidates for complementary therapy in settings of high antibiotic resistance or treatment failure.24
Bovine lactoferrin is a glycoprotein with multiple antimicrobial, antiviral and antifungal properties and it is widely distributed in mucosal secretions, such as saliva, tears and seminal fluid.25 As Ellison et al.26 reported, the antimicrobial activity of bovine lactoferrin is due to the sequestering of iron, which is essential for microorganism growth, and also to a direct action on the outer membrane of Gram-negative bacteria that interferes with flagellar motility. Bovine lactoferrin has been shown to have antibacterial activity against H. pylori in vitro27 and in vivo.28 Clinical studies have demonstrated that when bovine lactoferrin is used as a single drug in H. pylori-positive patients it is able to reduce the production of urea, suggesting a capacity to suppress but not eliminate H. pylori colonization.29,30
However, it has not been clarified whether bovine lactoferrin has a direct bactericidal effect on H. pylori or whether it potentiates the efficacy of a given antibiotic also in the presence of specific resistance.
The aim of this study was 2-fold: firstly to analyse the in vitro antimicrobial/antivirulence action of bovine lactoferrin alone and combined with levofloxacin against H. pylori clinical isolates previously shown to be resistant or MDR; and secondly to evaluate whether the inclusion of bovine lactoferrin in a triple therapy containing levofloxacin, amoxicillin and a proton pump inhibitor at full dosage for first-line treatment of H. pylori infection could increase the eradication rate in a geographical area where H. pylori has been shown to be resistant to levofloxacin in >40% of tested strains.
Materials and methods
Effect of bovine lactoferrin on clinical H. pylori strains in vitro
Characterization of antimicrobial susceptibility and virulence factors
Six H. pylori clinical strains were chosen for experiments. The reference H. pylori ATCC 43629 strain was included as a control. All strains studied were tested for their susceptibility profile to nine antibiotics commonly used in therapy (clarithromycin, metronidazole, ciprofloxacin, levofloxacin, moxifloxacin, tetracycline, amoxicillin, ampicillin and rifabutin) and for the main virulence factors.16
Strains were recovered from –80°C and were cultured on non-selective medium containing Columbia agar base (Oxoid) with 10% (v/v) laked horse blood plus 1% (v/v) IsoVitalex (BBL, Microbiology System, Milan, Italy) and incubated in a micro-aerobic environment at 37°C for 3–5 days (GasPak, Oxoid). The bacterial suspensions were prepared in Brucella Broth (BB) (Biolife Italiana, Milan, Italy) plus 2% FCS (Biolife), adjusted to an OD600 of 0.2, corresponding to ∼1.8 × 107 cfu/mL, and used for the experiments.
For virulence factor genotyping, the genomic DNA was extracted with the QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The DNA was stored at –20°C until use. PCRs were performed in a 2700 Thermocycler (PE Applied Biosystems) for the analysis of cagA, iceA1 and vacA s/m/i status according to previous studies by our group.31,32 The PCR was prepared in a total volume of 25 μL containing 2.5 μL of 10× PCR buffer, 1.5 mM MgCl2, 200 mM (each) deoxynucleotide triphosphates (dNTPs), 2 U of Amplitaq DNA polymerase, 20 mM each primer (Primm, Milan, Italy) and 50 ng of H. pylori DNA. For the analysis of the cagA region, primers D008 and R008, which yielded a fragment of 298 bp, were used. For the analysis of the iceA region, primers iceA1-F and iceA1-R, which yielded a fragment of 600 bp, were used. For the analysis of the vacA/s region, primers VA1-F and VA1-R, which yielded a fragment of 259 bp for the s1 variant and a fragment of 286 bp for the s2 variant, were used. For the analysis of the vacA/m region, primers VAG-F and VAG-R, which yielded a fragment of 567 bp for the m1 variant and a fragment of 642 bp for the m2 variant, were used. For the analysis of the vacA/i region, primers VAC-F1, CR1 and CR2, which yielded a fragment of 426 bp for the i1 variant and a fragment of 432 bp for the i2 variant, were used.32
The PCR products were examined by electrophoresis in 2% (w/v) agarose gel at 100 V for 30 min. Gels were stained with ethidium bromide and photographed.
Determination of MIC and MBC of bovine lactoferrin
The in vitro antimicrobial activity of bovine lactoferrin (Sigma–Aldrich) was assessed by the broth microdilution method according to CLSI guidelines.33 Bovine lactoferrin was diluted to obtain a concentration from 2.5 to 320 mg/mL. MIC values were measured by determining the lowest concentration of bovine lactoferrin needed to inhibit the visible growth of the microorganisms. The MBC was determined as the lowest concentration of bovine lactoferrin that gave complete inhibition of colony formation on plates. Each determination was performed in triplicate.
Chequerboard titration analysis
To understand the in vitro effect/synergism of bovine lactoferrin and levofloxacin, the chequerboard titration method was applied to assess the activity of the combination of these substances.34 Test tubes containing a sub-MIC concentration of bovine lactoferrin from 0.31 to 20 mg/mL and sub-MIC concentration of levofloxacin from 0.03 to 1 mg/L, respectively, in PBS were prepared and put in 96-well plates for the chequerboard configuration. The overnight bacterial inocula were prepared in BB plus 2% FCS, refreshed in the same medium and adjusted to OD600 0.12 (∼5 × 106 cfu/mL) for the experiments. The plates were incubated for 3 days at 37°C under micro-aerophilic conditions. After incubation, reduction of the OD600 value was evaluated with respect to the controls and cfu/mL values were determined. The chequerboard test was used as the basis to calculate a fractional inhibitory concentration (FIC) index35 according to the following formulas: FIC A = MIC A + B/MIC A; FIC B = MIC B + A/MIC B; and FIC index = FIC A = FIC B. The MIC A + B value is the MIC of compound A in the presence of compound B, and vice versa for MIC B + A. FIC index values were interpreted as Odds36 synergy (index ≤0.5), antagonism (>4.0) and no interaction (>0.5–4.0). Each determination was performed in triplicate. For the control, bovine lactoferrin and levofloxacin were also assayed alone.
Motility assay
Motility of H. pylori strains was assayed using semi-solid medium consisting of BB, sterile 10% FCS, sterile 10% horse blood and 0.4% bacteriological agar. Ten microlitres was inoculated on the surface of the agar with a sterile tip. Plates were incubated at 37°C under micro-aerophilic conditions. Diameters of the spreading H. pylori cells were measured after 7–10 days.37,38 The statistical significance of differences between diameters of untreated and treated samples was evaluated using Student’s t-test. Probability levels of <0.05 were considered statistically significant.
All data were obtained from three independent experiments performed at least in triplicate.
Therapeutic trial of bovine lactoferrin in conjunction with levofloxacin, amoxicillin and a proton pump inhibitor
This prospective study was performed in two separate groups of patients in two pilot studies that were carried out simultaneously. One group was treated with esomeprazole, amoxicillin and levofloxacin (Group A) and the other group with esomeprazole, amoxicillin, levofloxacin and bovine lactoferrin (Group B).
For this study, 50 subjects were required for each pilot study. An effective therapy was defined as a PP cure rate of ≥90% and rates of ≤80% were prospectively deemed unacceptable. Initially the plan was to evaluate 30 patients, and the study would have been stopped at any time in each group during recruitment if ≥6 patients experienced failure [urea breath test (UBT) positive], since it became clear that a cure rate of at least 80% would be impossible to achieve. On the other hand, if at the end of recruitment of the 30 patients a cure rate >93% was achieved, the study was considered concluded and no further recruitment was necessary. Otherwise, the study would continue until 50 patients had completed the study.39
Success was assessed by the [13C]UBT 2 months after the end of treatment.
Patient selection
From January 2015 to December 2016, of the 92 consecutive UBT H. pylori-positive patients never treated for the infection, 77 were included in the study. The UBT was performed with citric acid and 75 mg of [13C]urea within 10 days prior to the start of the study.
All eligible patients agreed to participate in the study. Fifteen patients were excluded according to the following criteria: age <18 or >80 years; treatment with proton pump inhibitors (omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole), H2 blockers (ranitidine, nizatidine, cimetidine, famotidine, roxatidine) and/or antibiotics during the 4 weeks before the study; gastrointestinal malignancy; severe concomitant diseases; and previous gastric surgery. All patients enrolled in the study were affected by chronic dyspepsia without alarm symptoms. Only patients >55 years underwent an upper endoscopy before admission to the study. Patients with active gastric and/or duodenal ulcer or gastric neoplasia were excluded.39
Patients were randomly assigned to each treatment using a computer-generated list.
Ethics
Enrolled patients gave informed consent for the study, which was approved by the Scientific Committee for Human Research of the Department of Medical Sciences of ‘G. d’Annunzio’ University.
Study protocol
Patients included in Group A were treated with esomeprazole (40 mg twice daily), amoxicillin (1 g twice daily) and levofloxacin (500 mg twice daily) and patients included in Group B were treated with the same drugs and dosage as Group A with the addition of bovine lactoferrin (200 + 100 mg/day). Esomeprazole, amoxicillin and levofloxacin were administered before breakfast and dinner, and bovine lactoferrin, which expresses its major effect at pH 6.0,40 was given orally 2 h after breakfast (200 mg) and 2 h after dinner (100 mg), when esomeprazole was capable of reducing gastric acidity.40 The two groups were treated with these therapeutic agents for 10 days.
H. pylori eradication was defined as a negative result for the UBT performed at least 8 weeks after the end of treatment with a delta-over-baseline value ≤5.
Statistical analysis
Statistical evaluation was carried out using 2 analysis. A P value of ≤0.05 was considered statistically significant.
Results
In vitro test of bovine lactoferrin against clinical H. pylori strains
The antimicrobial susceptibility panel and the main virulence markers of the H. pylori strains used in the experiments are shown in Table 1. Five out of six clinical strains displayed a resistance profile with at least four drug resistances; among them, two clinical strains (H. pylori 10A/13 and H. pylori 2A/12) showed an MDR profile with resistance to three classes of antibiotics. All detected strains were cagA+, the iceA1 allelic type was found in three strains and the main vacA genotype allelic combination was s1m1i1. No correlation was detected between antibiotic resistance profiles and virulence markers.
Table 1.
Antimicrobial susceptibility panel and virulence markers of H. pylori clinical strains
| Strain | Antibiotic |
Genotype |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLR | MTZ | LVX | MXF | CIP | RFB | TET | AMP | AMX | cagA | iceA1 | vacA | |
| 9F/13 | S | S | S | S | S | S | S | S | S | + | + | s1m1i1 |
| 10A/13 | R | R | R | R | R | S | S | S | S | + | − | s1m2i1 |
| 2A/12 | R | R | R | R | R | S | S | S | S | + | + | s1m1i1 |
| 10A/11 | R | S | R | R | R | S | S | S | S | + | − | s1m1i1 |
| 11F/11 | R | S | R | R | R | S | S | S | S | + | − | s1m2i2 |
| 3A/13 | R | S | R | R | R | S | S | S | S | + | + | s1m2i2 |
| ATCC 43629 | S | S | S | S | S | S | S | S | S | + | − | s1m1i1 |
CLR, clarithromycin; MTZ, metronidazole; LVX, levofloxacin; MXF, moxifloxacin; CIP, ciprofloxacin; RFB, rifabutin; TET, tetracycline; AMP, ampicillin; AMX, amoxicillin; S, susceptible; R, resistant.
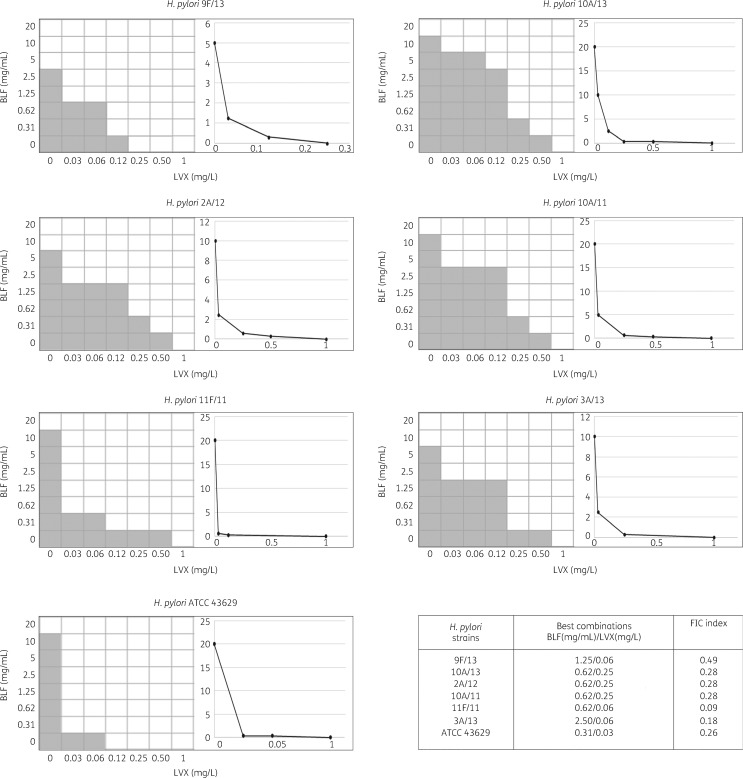
The MICs and MBCs of bovine lactoferrin for H. pylori strains are shown in Table 2. Specifically, bovine lactoferrin inhibited all strains tested, with MIC values ranging from 5 to 20 mg/mL and MBC values ranging from 40 to 160 mg/mL. When bovine lactoferrin was tested in association with levofloxacin against the H. pylori strains the MIC values of levofloxacin fell significantly to a range of 0.03–0.25 mg/L, whereas for bovine lactoferrin the range was 0.31–2.5 mg/mL. Synergy was detected in all tested strains (FIC index ≤0.5) (Figure 1).
Table 2.
Antibacterial activity of bovine lactoferrin against H. pylori strains
| Strain | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| 9F/13 | 5 | 40 |
| 10A/13 | 20 | 80 |
| 2A/12 | 10 | 60 |
| 10A/11 | 20 | 40 |
| 11F/11 | 20 | 80 |
| 3A/13 | 10 | 40 |
| ATCC 43629 | 20 | 160 |
Figure 1.
Chequerboard assays and isobolograms for evaluation of synergism between bovine lactoferrin (BLF) and levofloxacin (LVX) combinations against H. pylori strains. The calculation of the best BLF and LVX combination and the FIC index is displayed. FIC index values were interpreted as follows: synergy (FIC index ≤0.5); antagonism (>4.0); and no interaction (>0.5–4.0). Shading shows visible bacterial growth.
The chequerboard model and isobolograms for the combination of bovine lactoferrin and levofloxacin to define the synergy and to calculate the FIC index are shown in Figure 1. Synergy (FIC index ≤0.5) was detected in all strains tested, with a reduction of 4- to 16-fold in the MIC for levofloxacin. The best combinations of bovine lactoferrin and levofloxacin for each strain are indicated in Figure 1. For the H. pylori 11F/11 resistant strain, the FIC value was 0.09, with a reduction in the MICs of bovine lactoferrin and levofloxacin of 16- and 32-fold, respectively. Antagonism was not observed.
The loss of H. pylori ATCC 43629 and 2A/12 motility in the presence of bovine lactoferrin at sub-MIC values is shown in Figure 2. When treated with bovine lactoferrin at one-fourth and one-eighth of MICs, all strains studied displayed a smaller diameter of growth on soft agar in comparison with the untreated strains. Specifically, the reduction was significant at one-fourth of the MIC of bovine lactoferrin (P < 0.05).
Figure 2.
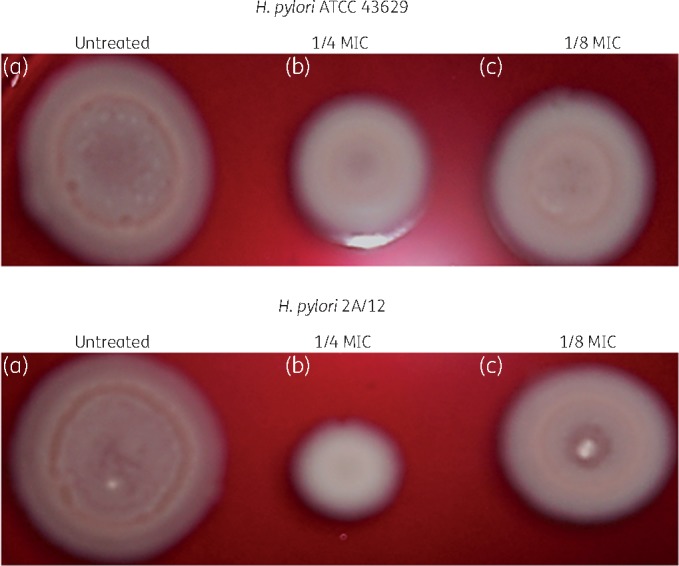
Representative images of sliding motility assay of H. pylori strains. (a) Control (ATCC 43629, diameter 19 ± 0.3 mm; 2A/12, diameter 21 ± 0.2 mm). (b) Strains cultured for 3 days and exposed to one-fourth MIC of bovine lactoferrin (BLF) (ATCC 43629, diameter 11 ± 0.1 mm; 2A/12, diameter 8 ± 0.1 mm). (c) Strains cultured for 3 days and exposed to one-eighth MIC of BLF (ATCC 43629, diameter 16 ± 0.3 mm; 2A/12, diameter 11 ± 0.1 mm).
Therapeutic trial
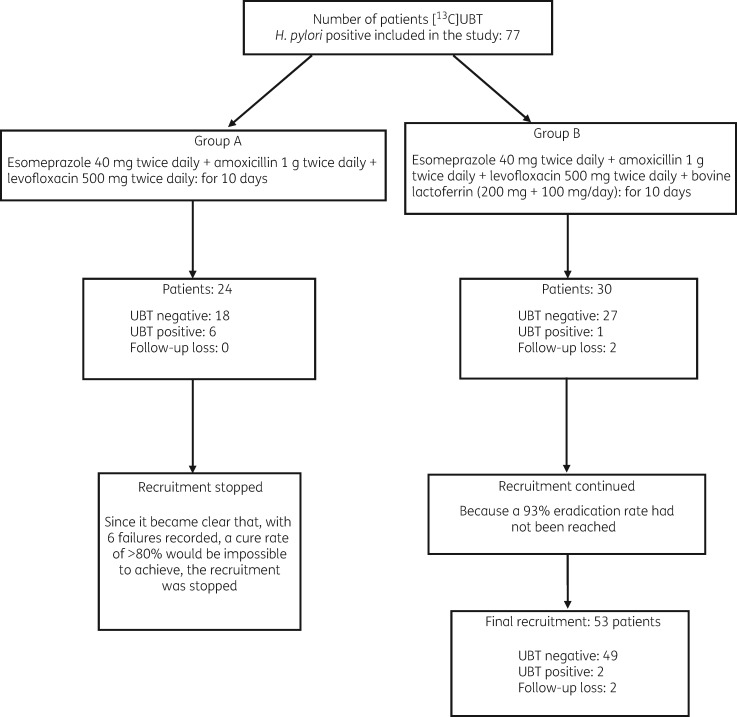
Patient characteristics are summarized in Table 3. In Group A, after recruitment of 24 patients who completed the treatment and returned for follow-up, six failures (UBT positive) were recorded. At this point recruitment was halted as it was judged to be impossible to exceed the 80% success rate even if recruitment had continued up to 30 cases. The cure rate in this group was 75% in ITT and PP analyses (95% CI 57.68%–92.32%).
Table 3.
Summary of study data
| Group A | Group B | |
|---|---|---|
| Therapeutic trial: | 40 mg ESO, 1000 mg AMX , 500 mg LVX (all twice daily) | 40 mg ESO, 1000 mg AMX, 500 mg LVX, 100 mg BLF (all twice daily) |
| Number of patients | 24 | 53 |
| Gender, n (%) | ||
| female | 16 (66.6) | 32 (60.3) |
| male | 8 (33.4) | 21 (39.7)a |
| Age, years, mean (range) | 48 (23–70) | 50 (20–75)a |
| Smoking habit, n/N (%) | ||
| female | 9/16 (56.2) | 21/32 (65.6) |
| male | 3/8 (37.5) | 10/21 (47.6)a |
| Alcohol use ≤30 g/week, n/N (%) | ||
| female | 8/16 (50) | 20/32 (62.5) |
| male | 6/8 (75) | 16/21 (76.1)a |
| Dyspepsia, n (%) | 24 (100) | 53 (100) |
| Endoscopy, n/N (%) | 7/24 (29.1) | 16/53 (30.1)a |
| Gastritis, n/N (%) | 4/7 (57.1) | 10/16 (62.5)a |
| Duodenitis, n/N (%) | 2/7 (28.5) | 5/16 (31.2)a |
| Oesophagitis (Los Angeles, grade A), n/N (%) | 1/7 (14.2) | 3/16 (18.7)a |
ESO, esomeprazole; AMX, amoxicillin; LVX, levofloxacin; BLF, bovine lactoferrin.
Not significant versus Group A (2 analysis).
In Group B, 30 patients were initially recruited and 28 completed the study, whereas 2 patients were lost to follow-up. Two failures (UBT positive) and 26 successful eradications (UBT negative) were recorded with a success rate in PP analysis of 92.8%. Since a 93% success rate was not achieved as specified in the protocol, recruitment continued up to 53 patients (Figure 3). The final analysis in this group showed that 49 of the 53 patients recruited showed successful eradication, with a cure rate of 92.45% (95% CI 86.52%–100.38%) by ITT and 49/51 of (96.07%, 95% CI 90.62%–101.38%) by PP analysis.
Figure 3.
Flow diagram for eradication of H. pylori.
The χ2 analysis of the Group A and Group B eradication rates showed a significant difference (P = 0.0058).
Adverse effects
Adverse events occurred in four patients from Group A (16.6%) and eight patients from Group B (15.09%) and were represented by diarrhoea [2 (8.3%) and 3 (5.6%), respectively], abdominal pain [1 (4.1%) and 3 (5.6%)] and nausea [1 (4.1%) and 2 (3.7%)]. These adverse effects did not induce a discontinuation of the treatment.
Discussion
This study demonstrates that bovine lactoferrin in vitro inhibits the growth and motility of H. pylori. When used in combination with levofloxacin against H. pylori strains resistant to levofloxacin and other antibiotics, bovine lactoferrin at sub-MIC values restores the effectiveness of the antibiotic through a synergistic action.
In vivo, bovine lactoferrin achieves a therapeutic gain of 21% when added to a triple therapy with a proton pump inhibitor, levofloxacin and amoxicillin in a group of patients living in a geographical area where H. pylori resistance to levofloxacin is >40% of tested strains.
Bovine lactoferrin can improve the potency of traditional antimicrobial regimens by fighting antimicrobial resistance through the reduction of flagellar motility and consequently microbial colonization. Our data are in line with a recent study that emphasized an innovative strategy of using natural bioactive substances capable of synergizing with antibiotics and also expressing anti-virulence activity to combat antibiotic resistance and bacterial virulence.41
The use of natural compounds combined with antibiotics represents an important strategy to tackle the antibiotic resistance phenomenon.42 The synergistic action obtained reduces the MIC of levofloxacin, restoring the efficacy of the antimicrobial drug .
Bovine lactoferrin could also exert an antimicrobial effect against H. pylori43in vitro and in vivo by inhibition of its growth at pH 6.40 In addition, the antibacterial activity of bovine lactoferrin may be attributed to its ability to bind iron with great affinity and prevent its utilization by the bacteria.
The evidence that deferoxamine, another iron chelator, inhibits H. pylori growth may support this hypothesis.44,45 Additional properties of bovine lactoferrin that may explain its antibacterial activity include immune-modulatory activity,46 antioxidant activity47 and a significant inhibitory effect on the in vivo attachment of H. pylori to the stomach, associated with a reduction in bacterial number and inflammation.48 It has also been shown that recombinant human lactoferrin can bind and disrupt some bacterial cell membranes,49,50 and when it is co-administered with amoxicillin against Gram-negative bacteria it increases its efficacy, as found in animal models. Furthermore, in a recent study Yuan et al.51 examined the effectiveness of recombinant human lactoferrin isolated from transgenic goats as a treatment for H. pylori in vitro and in vivo. For the in vitro experiments, the results revealed that recombinant human lactoferrin not only inhibited the growth of H. pylori, but also suppressed the expression of two major virulence factors, cagA and vacA.
It has been shown that levofloxacin in combination with amoxicillin and proton pump inhibitors can be an effective regimen for first-line anti-H. pylori treatment.52,53 In a meta-analysis, it has been observed that levofloxacin-based first-line therapy and standard therapy have equivalent efficacy and safety profiles for eradication of H. pylori.54 However, H. pylori resistance to levofloxacin has clearly increased in recent years;17,55 this may explain why the triple therapy that includes this antibiotic has reduced therapeutic efficacy. Bovine lactoferrin has already been shown to possibly potentiate the efficacy of a triple therapy that included antibiotics other than levofloxacin for the treatment of H. pylori infection. In a study by de Bortoli et al.,56 the addition of bovine lactoferrin to a triple therapy with esomeprazole, amoxicillin and clarithromycin increased the success rate by 16% (ITT and PP analysis). In a similar study adding bovine lactoferrin to a therapy with rabeprazole, tinidazole and clarithromycin, Di Mario et al.57 were able to increase the eradication rate by 21%, with total eradication in 92.2% (ITT analysis) and 95.2% (PP analysis) of treated cases. In our study the addition of bovine lactoferrin to a triple therapy that included esomeprazole, amoxicillin and levofloxacin for H. pylori infection significantly increased the eradication rate compared with that of the non-supplemented regimen, with a therapeutic gain of 21%.
Since resistance of H. pylori to levofloxacin in our region is present in >40% of tested strains it may be hypothesized that bovine lactoferrin potentiates the effect of levofloxacin, reducing the level of resistance of the bacterium and thereby increasing the overall efficacy, as shown in the in vitro study.
In addition, adding bovine lactoferrin to a levofloxacin-based triple therapy may avoid a levofloxacin preliminary susceptibility test, as it is when clarithromycin-based treatment is used in geographical areas with high clarithromycin resistance.10
The limitations of our study include the absence of a preliminary culture and susceptibility testing since H. pylori resistance to levofloxacin is quite high in our region. Moreover, as reviewed by Gisbert,58 even after culture-guided rescue treatments the lowest eradication rates were obtained in patients with H. pylori strains susceptible to all antibiotics, indicating that factors other than in vitro antibiotic susceptibility, such as biofilm H. pylori production, may influence eradication rates. However, we believe that the randomized allocation of patients to the two groups would have overcome this weakness to some extent.
Therefore, it may be concluded that the 10 day quadruple therapy consisting of esomeprazole, amoxicillin, levofloxacin and bovine lactoferrin can be generally used as the first-line treatment of H. pylori infection, including those regions where H. pylori expresses high resistance to fluoroquinolones.
Acknowledgements
We thank Laurino Grossi for his useful contribution to this study and Mrs Catherine Hlywka for reviewing the English style of the manuscript.
Funding
This study was supported by internal funding and Fondo di Ataneo per la Ricerca (FAR 2017) held by Leonardo Marzio and Luigina Cellini.
Transparency declarations
None to declare.
References
- 1. Ford AC, Gurusamy KS, Delaney B. et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst Rev 2016; issue 4: CD003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuo SH, Cheng AL.. Helicobacter pylori and mucosa-associated lymphoid tissue: what’s new. Hematol Soc Hematol Educ Program 2013; 2013: 109–17. [DOI] [PubMed] [Google Scholar]
- 3. Bornschein J, Malfertheiner P.. Helicobacter pylori and gastric cancer. Dig Dis 2014; 32: 249–64. [DOI] [PubMed] [Google Scholar]
- 4. Diaconu S, Predescu A, Moldoveanu A. et al. Helicobacter pylori infection: old and new. J Med Life 2017; 10: 112–7. [PMC free article] [PubMed] [Google Scholar]
- 5. De Francesco V, Bellesia A, Ridola L. et al. First-line therapies for Helicobacter pylori eradication: a critical reappraisal of updated guidelines. Ann Gastroenterol 2017; 30: 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vakil N. H. pylori treatment: new wine in old bottles? Am J Gastroenterol 2009; 104: 26–30. [DOI] [PubMed] [Google Scholar]
- 7. Kaur I. Novel strategies to combat antimicrobial resistance. J Infect Dis Ther 2016; 4: 2–6. [Google Scholar]
- 8. Kim JS, Park SM, Kim BW.. Sequential or concomitant therapy for eradication of Helicobacter pylori infection: a systematic review and meta-analysis. J Gastroenterol Hepatol 2015; 30: 1338–45. [DOI] [PubMed] [Google Scholar]
- 9. Calvet X, Lopez-Lorente M, Cubells M. et al. Two-week dual vs. one-week triple therapy for cure for Helicobacter pylori infection in primary care: a multicentre, randomized trial. Aliment Pharmacol Ther 1999; 13: 781–6. [DOI] [PubMed] [Google Scholar]
- 10. Malfertheiner P, Megraud F, O’Morain CA. et al. ; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 11. Perez Aldana L, Kato M, Nakagawa S. et al. The relationship between consumption of antimicrobial agents and the prevalence of primary Helicobacter pylori resistance. Helicobacter 2002; 7: 306–9. [DOI] [PubMed] [Google Scholar]
- 12. Megraud F, Coenen S, Versporten A. et al. ; Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62: 34–42. [DOI] [PubMed] [Google Scholar]
- 13. De Francesco V, Giorgio F, Hassan C. et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis 2010; 19: 409–14. [PubMed] [Google Scholar]
- 14. Megraud F. H. pylori antibiotic resistance: prevalence, importance and advances in testing. Gut 2004; 53: 1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyanova L, Mitov I.. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther 2010; 8: 59–70. [DOI] [PubMed] [Google Scholar]
- 16. Di Giulio M, Di Campli E, Di Bartolomeo S. et al. In vitro antimicrobial susceptibility of Helicobacter pylori to nine antibiotics currently used in Central Italy. Scand J Gastroenterol 2016; 3: 263–9. [DOI] [PubMed] [Google Scholar]
- 17. Huang JQ, Hunt RH.. Treatment after failure. The problem of ‘non-responders’. Gut 1999; 45: 140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuadrado-Lavín A, Salcines-Caviedes JR, Carrascosa MF. et al. Levofloxacin versus clarithromycin in a 10 day triple therapy regimen for first-line Helicobacter pylori eradication: a single-blind randomized clinical trial. J Antimicrob Chemother 2012; 67: 2254–9. [DOI] [PubMed] [Google Scholar]
- 19. Hung IF, Chan P, Leung S. et al. Clarithromycin amoxycillin-containing triple therapy: a valid empirical first-line treatment for Helicobacter pylori eradication in Hong Kong? Helicobacter 2009; 14: 505–11. [DOI] [PubMed] [Google Scholar]
- 20. Molina-Infante J, Perez-Gallardo B, Fernandez-Bermejo M. et al. Clinical trial: clarithromycin vs. LEV in first-line triple and sequential regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther 2010; 31: 1077–84. [DOI] [PubMed] [Google Scholar]
- 21. Iacopini F, Crispino P, Paoluzi OA. et al. One-week once-daily triple therapy with esomeprazole, LEV and azithromycin compared to a standard therapy for Helicobacter pylori eradication. Dig Liver Dis 2005; 37: 571–6. [DOI] [PubMed] [Google Scholar]
- 22. Malfertheiner P, Bazzoli F, Delchier JC. et al. ; Pylera Study Group. Helicobacter pylori eradication with a capsule containing bismuth citrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomized, open-label, non inferiority phase 3 trial. Lancet 2011; 377: 905–13. [DOI] [PubMed] [Google Scholar]
- 23. Ciccaglione AF, Cellini L, Grossi L. et al. A triple and quadruple therapy with doxycycline and bismuth for first-line treatment of Helicobacter pylori infection: a pilot study. Helicobacter 2015; 20: 390–6. [DOI] [PubMed] [Google Scholar]
- 24. Sachdeva A, Rawat S, Nagpal J.. Efficacy of fermented milk and whey proteins in Helicobacter pylori eradication: a review. World J Gastroenterol 2014; 20: 724–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jenssen H, Hancock RE.. Antimicrobial properties of lactoferrin. Biochimie 2009; 91: 19–29. [DOI] [PubMed] [Google Scholar]
- 26. Ellison RT, Giehl TJ, LaForce FM.. Damage of the outer membrane of enteric Gram-negative bacteria by lactoferrin and transferrin. Infect Immun 1988; 56: 2774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamazaki N, Yamuchi K, Kawase K. et al. Antibacterial effects of lactoferrin and a pepsin-generated lactoferrin peptide against Helicobacter pylori in vitro. J Infect Chemother 1997; 3: 85–9. [Google Scholar]
- 28. Wada T, Aiba Y, Shimizu K. et al. The therapeutic effect of bovine lactoferrin in the host infected with Helicobacter pylori. Scand J Gastroenterol 1999; 34: 238–43. [DOI] [PubMed] [Google Scholar]
- 29. Okuda M, Nakazawa T, Yamauchi K. et al. Bovine lactoferrin is effective to suppress Helicobacter pylori colonization in the human stomach: a randomized, double-blind, placebo-controlled study. J Infect Chemother 2005; 11: 265–9. [DOI] [PubMed] [Google Scholar]
- 30. Guttner Y, Windsor HM, Viiala CH. et al. Human recombinant lactoferrin is ineffective in the treatment of human Helicobacter pylori infection. Aliment Pharmacol Ther 2003; 17: 125–9. [DOI] [PubMed] [Google Scholar]
- 31. Cellini L, Grande R, Di Campli E. et al. Analysis of genetic variability, antimicrobial susceptibility and virulence markers in Helicobacter pylori identified in Central Italy. Scand J Gastroenterol 2006; 41: 280–7. [DOI] [PubMed] [Google Scholar]
- 32. Grande R, Di Giulio M, Di Campli E. et al. A model of Helicobacter pylori persistence in a case of gastric cancer. New Microbiol 2010; 33: 343–9. [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria—Third Edition: M45. CLSI, Wayne, PA, USA, 2016. [Google Scholar]
- 34. Magi G, Marini E, Facinelli B.. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A streptococci. Front Microbiol 2015; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cellini L, Di Campli E, Masulli M. et al. Inhibition of Helicobacter pylori by garlic extract (Allium sativum). FEMS Immunol Med Microbiol 1996; 13: 273–7. [DOI] [PubMed] [Google Scholar]
- 36. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 2003; 52: 1.. [DOI] [PubMed] [Google Scholar]
- 37. Roncarati D, Danielli A, Spohn G. et al. Transcriptional regulation of stress response and motility functions in Helicobacter pylori is mediated by HspR and HrcA. J Bacteriol 2007; 189: 7234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsang J, Hoover TR.. Requirement of the flagellar protein export apparatus component FliO for optimal expression of Flagellar Genes in Helicobacter pylori. J Bacteriol 2014; 15: 2709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham DY. Efficient identification and evaluation of effective Helicobacter pylori therapies. Clin Gastroenterol Hepatol 2009; 7: 145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dial EJ, Hall LR, Serna H. et al. Antibiotic properties of bovine lactoferrin on Helicobacter pylori. Dig Dis Sci 1998; 43: 2750–6. [DOI] [PubMed] [Google Scholar]
- 41. Marini E, Di Giulio M, Magi G. et al. Curcumin, an antibiotic resistance breaker against a multiresistant clinical isolate of Mycobacterium abscessus. Phytother Res 2018; 32: 488–95. [DOI] [PubMed] [Google Scholar]
- 42. Brown D. Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void? Nat Rev Drug Discov 2015; 14: 821–32. [DOI] [PubMed] [Google Scholar]
- 43. Sachdeva A, Nagpal J.. Meta-analysis: efficacy of bLF in Helicobacter pylori eradication. Aliment Pharmacol Ther 2009; 29: 720–30. [DOI] [PubMed] [Google Scholar]
- 44. Sanchez L, Calvo M, Brock J.. Biological role of lactoferrin. Arch Dis Child 1992; 67: 657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Illingworth D, Walter K, Griffiths P. et al. Siderophore production and iron-regulated envelope proteins of Helicobacter pylori. Zentralbl Bakteriol 1993; 280: 113–9. [PubMed] [Google Scholar]
- 46. Singh PK, Parsek MR, Greenberg EP. et al. A component of innate immunity prevents bacterial biofilm development. Nature 2002; 417: 552–5. [DOI] [PubMed] [Google Scholar]
- 47. Baldwin DA, Jenny ER, Aisen P.. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem 1984; 259: 13391–4. [PubMed] [Google Scholar]
- 48. Borody TJ, Ashman O.. Lactoferrin: milking ulcers? Dig Liver Dis 2003; 35: 691–3. [DOI] [PubMed] [Google Scholar]
- 49. Mielke S, Reddy R, Osato MS. et al. Direct activity of recombinant, human lactoferrin against Helicobacter pylori. J Clin Microbiol 1996; 34: 2593–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dial EJ, Romero JJ, Headon DR. et al. Recombinant human lactoferrin is effective in the treatment of Helicobacter felis-infected mice. J Pharm Pharmacol 2000; 52: 1541–6. [DOI] [PubMed] [Google Scholar]
- 51. Yuan Y, Wu Q, Cheng G. et al. Recombinant human lactoferrin enhances the efficacy of triple therapy in mice infected with Helicobacter pylori. Int J Mol Med 2015; 36: 363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nista EC, Candelli M, Zocco MA. et al. LEV-based triple therapy in first-line treatment for Helicobacter pylori eradication. Am J Gastroenterol 2006; 101: 1985–90. [DOI] [PubMed] [Google Scholar]
- 53. Telaku S, Manxhuka-Kerliu S, Kraja B. et al. The efficacy of LEV-based triple therapy first-line Helicobacter pylori eradication. Med Arch 2013; 67: 348–50. [DOI] [PubMed] [Google Scholar]
- 54. Peedikayil MC, AlSohaibani FI, Alkhenizan AH.. LEV-based first-line therapy versus standard first-line therapy for Helicobacter pylori eradication: meta-analysis of randomized controlled trials. PLoS One 2014; 9: e85620.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marzio L, Cellini L, Amitrano M. et al. Helicobacter pylori isolates from proximal and distal stomach of patients never treated and already treated show genetic variability and discordant antibiotic resistance. Eur J Gastroenterol Hepatol 2011; 23: 467–72. [DOI] [PubMed] [Google Scholar]
- 56. de Bortoli N, Leonardi G, Ciancia E. et al. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastroenterol 2007; 102: 951–6. [DOI] [PubMed] [Google Scholar]
- 57. Di Mario F, Aragona G, Dal Bó N. et al. Use of lactoferrin for Helicobacter pylori eradication. Dig Liv Dis 2003; 35: 706–10. [DOI] [PubMed] [Google Scholar]
- 58. Gisbert JP. “Rescue” regimens after Helicobacter pylori treatment failure. World J Gastroenterol 2008; 14: 5385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]