Abstract
Context: While there are previous systematic reviews on the effectiveness of the use of robotic-assisted gait training (RAGT) in people with spinal cord injuries (SCI), as this is a dynamic field, new studies have been produced that are now incorporated on this systematic review (SR) with meta-analysis, updating the available evidence on this area.
Objective: To synthesise the available evidence on the use of RAGT, to improve gait, strength and functioning.
Methods: SR and meta-analysis following the Cochrane Handbook for Systematic Reviews of Interventions were implemented. Cochrane Injuries Group Specialized Register, PubMed, MEDLINE, EMBASE, CINAHL, ISIWeb of Science (SCIEXPANDED) databases were reviewed for the period 1990 to December 2016.
Three researchers independently identified and categorized trials; 293 studies were identified, 273 eliminated; remaining 15 randomized clinical trials (RCT) and five SR. Six studies had available data for meta-analysis (222 participants).
Results: The pooled mean demonstrated a beneficial effect of RAGT for WISCI, FIM-L and LEMS (3.01, 2.74 and 1.95 respectively), and no effect for speed.
Conclusions: The results show a positive effect in the use of RAGT. However, this should be taken carefully due to heterogeneity of the studies, small samples and identified limitations of some of the included trials.
These results highlight the relevance of implementing a well-designed multicenter RCT powered enough to evaluate different RAGT approaches.
Keywords: Spinal cord injuries, robot-assisted gait training, locomotor training, robotics, walking
Background
Spinal Cord Injury (SCI) is a lesion that may result in sensitive, motor and autonomic impairments.1 Damage caused to the descending and ascending tracts results in postural system impairment, which affects standing, locomotion and voluntary movements.2–4 SCI affects the ability to walk and many individuals do not regain it, even though it is one of the goals of rehabilitation,5 along with upper extremity function, sexuality, bowel and bladder control.6 More than 50% of individuals with SCI present incomplete motor lesions and more than 75% of those individuals regain some form of ambulatory function.1,7 However, common consequences include slow speed walking, abnormal step length, cadence, and step symmetry that negatively impact walking efficiency.8
There has been an evolution on gait rehabilitation programs for people with spinal cord injury in the recent decades, from manually assisted over-ground training; body-weight-supported treadmill training (BWSTT) to robotic-assisted gait training (RAGT). All these interventions have the common goal of regaining or improving locomotion by providing sensory information5 and interactions with supraspinal circuits (cortical and subcortical).4,9
RAGT was introduced in the late 1990s (Lokomat, Hocoma AG, Switzerland). Today different commercial systems, such as Lokomat® (Hocoma, Volketswil, Switzerland),10 G-EO systemTM (Reha Technology AG, Switzerland), Walkbot (P&S Mechanics Co., Ltd, Korea), ReoAmbulatorTM (Motorika, USA Inc.), among others are available. The RAGT consists of a motor driven gait orthosis, controlled by a computer and secured to a patient's legs while the patient is supported by a BWS. The RAGT focuses on the correct performance of gait movements. Therapy is performed at low speed and the level of assistance by the system can be adjusted based on the patient's ability to step.
Multiple studies have demonstrated the efficacy of this orthosis to improve walking on people with incomplete motor spinal cord injury who are partially able to walk in the sub acute phase.11–13
To practice the kinematically correct stepping is thought to enhance the afferent feedback associated with normal locomotion and, therefore, to maximize plasticity within spinal and supraspinal neural circuits.11 Previous to the RAGT, BWSTT had been used, though its use is limited because of its labour-intensive requirements.11
Currently, there are 5 systematic reviews related to this theme. Swinnen et al. 2010,10 Tefertiller et al. 2011,14 Mehrholz et al. 2012,1 and Morawietz et al. 2013,15 and Karimi16et al. 2013.
Those previous studies have used gait velocity as a measure of overall motor capacity and gait recovery. Results of locomotor recovery after an intervention such as RAGT or BWSTT probably should not be assessed only by improvement on gait velocity after intervention. In fact, gait is a complex motor task produced by interaction of neurological and biomechanical systems. Additionally, the RAGT focuses on the correct performance of gait movements, and the training speed is usually slow.
According to the International Classification of Functioning, Disability and Health, functioning involves individual body function, body structures, activities and participation, and denotes the interaction between a health condition and his/her living conditions. Two constructs, ‘performance’ and ‘capacity’, are used to operationalize domains of activities and participation.17 Performance can also be measured using alternative scales that assess walking in people with spinal cord injury related to what the individual actually does in his or her current environment.17 For performance assessments the ICF, WISCI (walking index for spinal cord injury), SCIM (spinal cord independence measure), FIM (functional independence measure), among other scales may apply.
This study contributes to the available evidence on the use of RAGT in people with spinal cord injury by incorporating the latest evidence from clinical trials as well as by widening the scope with the inclusion of additional indicators of effectiveness: improve gait, strength and functioning in people with spinal cord injury in comparison to other modalities of training.
Methods
Types of studies
RCT, systematic reviews and crossover trials (only the first period) were included, although analysis were implemented independently for each study type.
Types of participants
Individuals with any level of traumatic incomplete SCI, regardless of the time since injury, sex and age, were included.
Types of interventions
The study included all trials that addressed the effectiveness of RAGT compared with other training modalities as part of a neurorehabilitation program to improve gait, strength and functioning in comparison to an alternative intervention.
RAGT interventions were required to have a main focus on gait, strength or functioning. There were no restrictions regarding to frequency and duration of the RAGT interventions.
Outcome measures
Gait parameters: Instrumented gait assessment, 10MWT, 6MWT, WISCI or any other available scale.
Strength: Isokinetic, L-Force, MRC (medical research council scale), or any other available scale.
Functioning: SCIM, FIM or any other available scale.
Searching criteria
The search was not restricted by language or publication status. The search was limited to studies published after 1990, the year when RAGT was introduced.10
Data sources
Searches were conducted in using the following databases: Cochrane Injuries Group Specialized Register (recent issue); Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (Ovid) 1990 to december 2016; EMBASE (Ovid) 1990 to december 2016; CINAHL 1990 to december 2016; ISIWeb of Science: Science Citation Index Expanded (SCIEXPANDED) 1990 to december 2016; Pub Med (www.ncbi.nlm.nih.gov/sites/entrez/). The following terms were used for the search:
Cochrane Injuries Group Specialised Register (Search from 5 of August 2016)((spine or spinal) and (damag* or trauma* or injur* or broke* or break or fracture)) and (walk* or locomotor or rehabilitat* or robot* or orthos* or orthotic or automat* or "computer aided" or "computer assisted" or Lokomat or Locomat* or electromechanical or electro-mechanical or mechanical or mechanised or mechanized or driven) in Title, Abstract, Keywords in Cochrane Reviews
Medline (Pubmed)
exp Spinal Cord Injuries/
exp Spinal Cord Ischemia/
exp Central Cord Syndrome/
((spine or spinal) and (fracture* or wound* or trauma* or injur* or damag*)).ab,ti.
(spinal cord adj3 (contusion or laceration or transaction or trauma or ischemia)).ab,ti.
central cord injury syndrome.ab,ti.
central spinal cord syndrome.ab,ti.
exp Cervical Vertebrae/in [Injuries]
exp Spinal Cord/
SCI.ab,ti.
exp Paraplegia/
exp Quadriplegia/
(paraplegia* or quadriplegia* or tetraplegia*).ab,ti.
or/1–13
exp Gait/
exp Walking/
(locomotion and walking).ti,ab.
*Locomotion/
locomotor?training.ab,ti.
*Dependent Ambulation/
(walk* or gait* or ambulat* or mobil* or locomot* or stride*).ti,ab
or/15–21
14 and 22
exp Automation/
exp Robotics/
exp Orthotic Devices/
exp Weight-Bearing/
(weight?bearing or load?bearing).ab,ti.
(electromechanical or electro-mechanical or mechanical or mechanised or mechanized or driven or exoskeleton).ti,ab
(robot* or orthos* or orthotic* or automat* or computer?aided or computer?assisted or BWS or harness* or treadmill* or Lokomat or Locomat or G-EO).ab,ti
((gait or walk* or ambulatory) adj3 (recover* or test* or abilit* or function or speed*)).ab,ti
or/24–31
23 and 32
randomi?ed.ab,ti.
exp randomized controlled trial
controlled clinical trial.pt.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
exp review
or/ 34–41
(animals not (humans and animals)).sh.
43 not 45
(rat or rats or rodent* or mouse or mice or murine or dog or dogs or canine* or cat or cats or feline* or rabbit or rabbits or pig or pigs or porcine or swine or sheep or ovine* or guinea pig*).ti
44 not 45
33 and 46
ISI Web of Science: All databases 1990 to present
#1 Topic=(SCI OR spinal cord injuries OR central cord syndrome) AND Topic=(walk* OR gait* OR ambulat* OR mobil* OR locomot*) AND TS=(robot* OR orthos* OR electromechanical OR orthotic* OR automat* OR lokomat OR locomat OR G-EO)
#2 Topic=(randomi?ed OR randomized controlled trial OR controlled clinical trial OR placebo OR clinical trials OR randomly OR trial OR review) NOT Topic=(animal* OR rat OR rats OR rodent* OR mouse OR mice OR murine OR dog OR dogs OR canine* OR cat OR cats OR feline* OR rabbit OR rabbits OR pig OR pigs OR porcine OR swine OR sheep OR ovine OR guinea pig*)
#3 #1 and #2
Searching other resources
We contacted key authors and institutions to request details of any recently published, in press, unpublished or ongoing trials, reference lists of included studies and literature reviews, searched bibliographies of relevant studies and reviews; relevant experts in the field were also contacted.
Literature screening & study selection
Three authors independently examined titles, abstracts and keywords to identify potentially relevant studies. After the initial search, full texts of identified relevant studies were obtained; the studies were assessed by two authors and included if the inclusion criteria were met according to both researchers. Disagreements were resolved by a third reviewer.
Data extraction and management
Extracted data were filled into a pretested data collection form by at least 2 reviewers. Detailed instructions and training were provided to all authors involved in data extraction.
Assessment of risk of bias in included studies
Risk of bias was assessed to evaluate trial quality by at least two reviewers independently, using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions.18 Disagreements were resolved through consultation with a third reviewer.
Statistical analysis
The strength of the study findings was discussed by level of evidence, which was based on methodological quality. Classification of outcome measures in terms of the assessed domain (gait, strength, and functioning) was done by at least 2 reviewers independently.
All analysed outcome measures are reported as continuous variables. Mean differences (MD) and 95% confidence intervals (CIs) were calculated. A random-effects model was used for all outcomes analysed. Since it was difficult to identify the subset of participants with reported baseline and final value measurements, only final value measurements were used for the analysis. The approach suggested by Wan et al. 2014,19 was used to enter missing mean and S.D. from median and interquartile ranges. Briefly, inequalities are developed for each observation using upper and lower limits from the minimum, the three quartiles, and the maximum. These are summed to give bounds for the sum and hence the mean of the observations, the average of these bounds in the estimate.
Whenever a study presented results for several periods of follow-up for the same outcome, the last assessment was included as the final value measurement.
Studies’ authors were contacted to acquire any missing data as well as information on whether or not data could be assumed to be missing at random.
The number of participants in each meta-analysis corresponds with the number of participants included in the analysis of the published trials per group of intervention. Data were analyzed using Review Manager Software 5.3.
Results
293 studies were identified on the electronic search. 47 of them were duplicated studies and were eliminated. Results of the search are shown in Fig. 1.
Figure 1.
Flow Diagram
The full text review was carried out for the 28 preliminary selected trials, 20 of them met the inclusion criteria for this review: five SR,1,10,14–16 and 15 RCT.20–34 Characteristics of included studies are shown in table 1.
Table 1. Characteristics of included studies.
| Study | Design | Participants | Intervention | Outcome measures |
|---|---|---|---|---|
| Alcobendas-Maestro 2012 | RCT | n= 80 Time since injury (months)= <6 AIS= C or D Level of injury= C2 to T12 |
Group 1: Patients received 40 walking reeducation sessions of equal time using a Lokomat program with overground practice. Group 2: Overground mobility therapy alone.*training in both groups consisted of 1 hour of training, the Lokomat group used the system for 30 minutes, the other 30 minutes were completed with conventional therapy. |
WISCI 6MWT FIM-L LEMS MAS VAS |
| Duffel 2014 | RCT | n= 78 patients with incomplete SCI |
Group 1: Lok group, locomotor training was provided using a robot-assisted locomotor training device Training was provided three times per week; each session lasted <1 hour, with 30–45 minutes of training. (4 w). Group 2: Tiz group,.03 mg/kg of Tizanidine was administered four times a day for four weeks. (4 w). Group 3: Control subjects received no intervention. |
Walking speed Endurance and mobility TUG 10MWT 6MWT |
| Duschau 2010 | RCT | n= 15 Participants with chronic incomplete SCI |
Group 1: POS: Position control with the stiffness of the Lokomat controller set to Khip = 1200 Nm/rad, Kknee = 900 Nm/rad2. Group 2: SOFT: Impedance control with the stiffness set to Khip = 192 Nm/rad, Kknee = 144 Nm/rad. Group 3: COOP: Path control with window set to 20% of the gait cycle and the support gain ks adjusted individually for each patient3 Group 4: COOP+: Path control with window set to 20% of the gait cycle and the support gain ks increased to 130% of the value used in the previous condition Single session |
Spatio-temporal characteristics Peek knee extension Maximal hip flexion during swing phase |
| Esclarín-Ruz 2014 | RCT | n= 88 Time since injury (months)= <6 months AIS= C or D Level of injury= C2 to T11 |
Group 1: Subgroups A1 and B1 (LKOGT) were imparted 30 minutes of conventional mobility training plus 30 minutes of robotic-assisted mobility training. Group 2: Subgroups A2 and B2 (OGT) were imparted 60 minutes of conventional mobility training. ** |
10MWT 6MWT WISCI LEMS FIM-L |
| Field-Fote 2005 | RCT *** |
n= 27 Time since injury (months)= >12 months Level of injury= at or above T10 |
Group 1: Treadmill training with manual assistance (TM). Group 2: Treadmill training with stimulation (TS). Group 3: Over- ground training with stimulation (OG). Group 4: Treadmill training with robotic assistance (LR). **** |
Walking speed Training speed Step length Step symmetry |
| Field-Fote 2011 | RCT ***** |
n= 74 AIS= C or D Level of injury= at or above T10 |
Group 1: Treadmill training with manual assistance (TM). Group 2: Treadmill training with stimulation (TS). Group 3: Over- ground training with stimulation (OG). Group 4: Treadmill training with robotic assistance (LR). **** |
Walking speed Walking distance LEMS (right/left) |
| Kressler 2013 | RCT | n= 62 AIS= C or D Level of injury= at or above T10 |
Group 1: Treadmill training with manual assistance (TM). Group 2: Treadmill training with stimulation (TS). Group 3: Over- ground training with stimulation (OG). Group 4: Treadmill training with robotic assistance (LR). **** |
VO2 Walking velocity Walking economy Substrate utilization: slow, moderate and maximal walking speeds. |
| Labruyère 2014 | Randomized cross-over | n= 9 Chronic incomplete SCI Time since injury (months)= >12 AIS= C or D |
Group 1: received 16 sessions of RAGT (45 min each) within 4 weeks followed by 16 sessions of strength training (45 min each) within 4 weeks. Group 2: received the same interventions in reversed order. |
10 MWT (preferred and max) Waking speed Balance Strength Risk of falling and pain ****** |
| Lam 2015 | RCT | n= 15 Motor incomplete SCI Time since injury (months)= >12 AIS= C or D |
Group 1: BWSTT with Lokomat-applied resistance (Loko-R). Group 2: conventional Lokomat-assisted BWSTT (Control). Training sessions were 45 min, 3 times/week for 3 months. |
Walking capacity OG (SCI-FAP) Walking speed Walking distance |
| Niu 2014 | RCT | n= 40 Incomplete SCI Spasticity at lower extremities |
Group 1:1-hour Lokomat trainings over one month. Group 2: control subjects received no interventions. |
10MWT TUG 6MWT MVC MAS |
| Nooijen 2009 | RCT | n= 51 Chronic incomplete SCI Time since injury (months)= >12 Level of injury= at or above T12 |
Group 1: BWSLT on the treadmill with manual assistance for stepping (TM). Group 2: BWSLT on the treadmill with peroneal nerve stimulation to assist stepping (TS). Group 3: BWSLT overground with peroneal nerve stimulation (WalkAide2TM, Hanger Orthopedic Group, Inc., Bethesda, MD; OG). Group 4: BWSLT on the treadmill with assistance of a locomotor robot (Lokomat, Hocoma AG, Zurich, Switzerland; LR). 12 weeks of training |
10MWT 6 mts of the walkway |
| Quinzaños 2014 | RCT | n= 31 Time since injury (months)= >6 AIS= C or D |
Group 1: 12 training sessions, 20 min., 4 times per week (3 weeks). body weight support was 50%, every week a decrease of 10% was made. Guidance was determined depending on lovet scale; 4 and 5, 20%, for a 3, the 40% was used, for a 2, 60% was used, and for 1 and 0 a guidance of 80% was assigned. Group 2: The same parameters of the Lokomat were used + an auditive feedback. |
Spatio-temporal variables Cadence Range of movement Spasticity SCI-FAP |
| Shin 2014 | RCT | n= 60 Time since injury (months)= <6 AIS= D Non progressive SCI |
Group 1: RAGT three sessions per week at duration of 40 minutes with regular physiotherapy in 4 weeks. Group 2: The conventional group underwent regular physiotherapy twice a day, 5 times a week. |
LEMS AMI SCIM WISCI |
| Tang 2014 | RCT | n= 30 Incomplete SCI AIS= D Level of injury= T8-L3 |
Group 1: The total set-up and treatment time for the Lokomat never exceeded 1 hour. The initial training speed was 1.5 km/h and it was progressively raised to 1.8 km/h. The body weight system was initiated at 35%, and 70% guidance force. Group 2: The Ergo_bike group subjects were instructed to pedal at a pedaling rate of 45 rpm with a work load of 60 W. 1 training session of 40 minutes. | P-RT 10 m máximum walking speed |
| Wu 2012 | Cross-over | n= 10 AIS= D Level of injury= C2 to T10 |
Group 1: One group received 4 weeks of assistance training followed by 4 weeks of resistance training. Group 2: The other group received 4 weeks of resistance training followed by 4 weeks of assistance training. |
Walking velocity (self-selected and fast) 6MWT balance Muscle tone Strength |
* This information was provided via email from the authors.
** No subjects were familiar with the Lokomat robotic-assisted mobility training system before participating in the study.
***Preliminary study of the 2011 study.
**** Subjects in all groups were allotted a 60-minute intervention for each training day, with setup and take-down consuming an average of 10 to 15 minutes of this allotted time. Subjects were scheduled to train 5 days/week for 12 weeks.
***** Subjects in all groups were allotted a 60-minute intervention for each training day, with setup and take-down consuming an average of 10 to 15 minutes of this allotted time. Subjects were scheduled to train 5 days/week for 12 weeks.
******Data from the first period of this trial were provided via email by the authors.
WISCI, walking index for spinal cord injury. 6MWT, 6 minute walk test. 10MWT, 10 minute walk test. FIM-L, functional Independence measure-Locomotion. LEMS, lower estremity motor score. MAS, modified ashworth scale. VAS, visual analog scale for pain. TUG, timed up and go. OG, overground. SCI-FAP, spinal cord injury-functional ambulation profile. MVC, máximum voluntary contraction. AMI, ambulatory motor index.
Excluded studies
In the full text review eight studies were eliminated because they did not meet the inclusion criteria. From these potentially eligible trials, one was excluded because it is a protocol17 one is about a hybrid exoskeleton to restore gait,35 another uses an electromechanical gait trainer and includes only one person with SCI per group,36 and the rest of the trials12,37–40 are not RCT.
Study location
From the 15 RCT, seven trials were done in the United States20,23,26,28,30,31,34 two in Spain,25,27 two were from Switzerland,24,32 one was done in Korea,22 one in Canada,29 one in China33 and one in Mexico.21
Study participants
From the 15 RCT that met the inclusion criteria, a total of 499 participants were registered. From the five SR a total of 1,227 participants were included. The number of participants ranged between 924 to 88.25 The range of age varied from 1625 up to 70 years,24,25,27 though age is not reported in all included studies.
Only one trial did not report the proportion between men and women,34 and one study included only men.33 The total was 344 men and 132 women; the relation was 2.6:1.
60% of the trials included patients with AIS (American Spinal Injury Association Impairment Scale) C or D20,21,24–29,31 only one trial reported patients with AIS A, B, C or D.32 Three trials included only AIS D participants22,30,33 and two trials did not report the AIS.23,34 The total reported was: 140 AIS D, 61 C, two B and two A.
The aetiology is reported only in five trials.22,24,25,27,29 A total of 142 participants were reported with traumatic SCI and 93 participants were non-traumatic.
The most prevalent level of injury was cervical with 155 participants22,24,25,27,29–32; 37 for the group of T1-T624,25,29–31; 44 participants with a T7-T11 level24,25,27,29–32; and 53 below T12, 53.25,31,32 22 participants were reported including thoracic and lumbar levels without separating groups.22
Dose of intervention
The period of treatment was one day32,33; three weeks21; four weeks22,23,34; eight weeks24,25,27,30; and 12 weeks.20,26,28,29,31 The frequency was reported from three times per week during four weeks,22,34 up to five times per week for 12 weeks.20,26,28 The RAGT setup was initially prescribed for the amount of body weight supported at 60%25,27 and never less than 25%.25,27 The guidance force was set from 100%22,26,28 to 20%.21,23 The lowest initial speed was reported at 1.0 Km/h29 and in one trial23 the participants accomplished 3.4 Km/h. The length of the RAGT therapy varied from 20 minutes,21 to 45 minutes.28–30
Outcomes measures for analysis
The many diverse outcome measures recorded in included studies made it impossible for authors to analyse all of the documented data. Based on the pre stated relevant outcomes and the availability of data from specific measures in included trials, the analysis focused on speed (m/s), WISCI, strength (LEMS) and FIM-L.
Of the 15 RCT, 10 studies included outcome measures suitable for inclusion in the analysis. As measures were introduced, it was decided to use only 6 of the studies22,24,25,27,28,33 due to the different reasons listed below.
Studies included in meta-analysis comparisons
All trials involved a comparison between a RAGT and different therapeutic interventions: conventional therapy,22,25,27 no intervention,23 strength training.24 Two trials compared resisted RAGT vs. conventional RAGT,29,30 and one trial compared RAGT with acoustic feedback vs. conventional RAGT.21 Four of the studies divided the intervention in four groups; treadmill-based training with manual assistance, treadmill-based training with electrical stimulation, overground training with electrical stimulation, treadmill-based training with robotic assistance.20,26,28,31 One study compared two groups: RAGT and Ergo_bike.33 Another trial made 3 comparison groups: RAGT, tizanidine and no intervention.34 Ultimately a trial did only one training session and the participants were randomized to four different therapeutic modalities.32
Risk of bias
Risk of bias is summarized in (Figs. 2 and 3). The kappa values for inter-rater variability are >0.6 for most of the items.
Figure 2.
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies
Figure 3.

Risk of bias summary: review authors' judgments about each risk of bias for each included study
Five SR were assessed using AMSTAR. All the RCT of the five SR,1,10,14–16 were already included in our review, the studies that were not RCT or were protocols were excluded from our review.
The conclusion for the five SR is that there are not enough available studies and that the quality of existing studies is not ideal to determine the superiority of a therapeutic modality. Our review assesses 11 more trials in comparison with the latest one from Mehrholz et al. 2012, and a total of six RCT were used for the meta-analysis, of other outcomes rather than speed.
Effects of interventions
Results are described below under the comparisons carried out for each of the explored outcomes (1. Speed, m/s; 2. Strength (LEMS); 3. WISCI; 4. FIM-L).
Speed
Nine studies were included in this analysis but data for only five studies were pooled. Four studies were eliminated: two trials compared two different modalities of RAGT 21 and,29 other trial had subgroups and it was not possible to define the number of participants for each intervention group in the subgroup of maximal speed,26 and final mean was not reported in.23 The remaining five studies showed no effect compared with control groups, showing a MD of -0.00 (95% CI -0.05 to 0.04, P = 0.95) (Fig. 4).
Figure 4.
Effect on Speed
A total of 169 participants were included in the analysis of this outcome,25 and27 had the greater number of participants (54.4%), both of them with an effect size that favours RAGT therapy. The only study that reported an effect that favours the control group is.28
Strength (LEMS)
Five studies were pooled in this analysis (217 participants). The MD is 1.95 (95% CI -1.58 to 5.48, P = 0.28) in favour of the RAGT (Fig. 5). The first 2 listed studies25 and27 correspond to 40.3% of the total number of participants, both trials show a clear positive effect towards the RAGT. There is only one study that favours the control group over the use of RAGT.28
Figure 5.
Effect on LEMS
WISCI
Data from four studies,22,24,25,27 (188 participants) were pooled in this analysis. The pooled MD is 3.01 (95% CI -0.54 to 6.55, P = 0.10) in favour of the RAGT (Fig. 6).
Figure 6.
Effect on WISCI
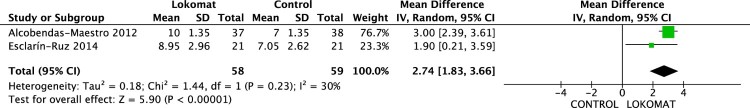
FIM-L
Data from two studies,25 and27 were pooled (117 participants) in the analysis. The MD 2.74 (95% CI -1.83 to 3.66, P = 0.00001), with a clear effect in favour of the RAGT (Fig. 7).
Figure 7.
Effect on FIM-L
Discussion
This review included 15 RCT and five SR that explored the effects of RAGT compared with different physical rehabilitation approaches. Data from six studies were available for meta-analysis.
Very good–quality evidence for three of the RCT analysed in the meta-analysis24,25,27 and moderate-quality evidence on the rest of studies analysed, showed a moderate effect of the use of RAGT for strength (LEMS), and a large effect for gait performance (WISCI) and functioning (FIM-L).
Improvement in gait was evaluated through the scores in speed and WISCI. The pooled results of 169 individuals studied for speed and 188 individuals studied for WISCI, showed no benefit in terms of speed with a MD of -0.00 (95% CI -0.05 to 0.04), but a large effect for the WISCI measurement showing a MD of 3.01 (95% CI -0.54 to 6.55). In accordance to the beneficial effect in the WISCI, the pooled results showed a large effect in terms of functioning measured by the FIM-L.
Walking speed is a very specific measure of walking capacity, while WISCI assesses physical limitation for walking secondary to impairment based on the use of assistive devices and physical assistance. Even when WISCI does not evaluate gait velocity, a medium correlation with walking speed has been reported.8 Walking speed has shown a medium correlation with lower limb’s muscle force, individual’s global independence, use of walking aids and gait performance.8 There are slight differences between both measures. Walking speed has shown higher correlation with spatial measures of gait such as step length, while WISCI-II has shown higher correlation with measures of gait symmetry such as difference between step duration of both legs.8 It is well known that step length and cadence are accommodated in response to gait velocity.41
Consequently, the different findings in terms of speed and WISCI may reflect the nature of RAGT. RAGT effect seems to be more general, addressing gait components related to use of assistive devices and gait symmetry. This needs confirmation by inclusion of specific and sensitive instrumented evaluations within clinical trials.
On the other hand, in order to increase walking speed, subjects use different muscular coordination patterns which are believed to be composed of combinations of simple neural control strategies for the co-excitation of multiple muscles, which are called motor modules.42,43 Therefore, the variations on the motor modules used by the subject will result on variations on kinetics, kinematics and spatiotemporal parameters of walking.44 In addition, biomechanical characteristics of human body determine feasibility of movements. A limitation on body biomechanics, such as an exacerbated increment on joint stiffness, could make impossible to take advantage of some motor modules, limiting their usefulness. Motor modules for walking at different speeds have been reported elsewhere.45 There are reports about reduced number and composition of motor modules used by incomplete SCI while walking.46,47 Therefore walking at faster velocities could be necessary to acquire motor modules necessary to improve walking speed.48 Moreover, results should be analysed considering biomechanical and neurological characteristics of SCI subjects.
Finally, results showed a moderate benefit in the improvement of strength, favouring the RAGT intervention. Reduction of guiding force as training progresses could be responsible for this result. As the guidance force diminishes, the subject must improve its lower limbs’ strength in order to perform the right movements. However, this improvement of strength does not transfer to gait speed because no RAGT trains ankle plantar flexors. Biomechanically, gait velocity is determined by the conversion of potential energy of the centre of mass (CoM) into kinetic energy and calf muscles determine this amount of energy. Ankle joint is also critical for propulsion, shock absorption, and balance during walking.49 Also, an improvement exclusively on body biomechanics does not result automatically on an improvement in motor performance if the neurological system cannot take advantage of it.50
RAGT devices were designed to provide appropriate sensory information to the spinal cord by imposing a normal walking pattern in order to evoke locomotor activity. This relies on the hypothesis of the existence of central pattern generators (CPG) in humans which are neural networks within the spinal cord that generate basic rhythmical motor patterns involved in walking.51–55 However it has also been shown that supraspinal pathway plays an important role in inter-limb coordination.49 Miyai and colleagues56 showed that medial sensorimotor cortices and the supplementary motor cortical areas were involved in the control of walking. Until now three types of robotic-assisted device have been developed: exoskeleton, end-effector and portable robotic exoskeletons. Up to date only RCT involving Lokomat have been performed, and no RCT of the portable robotic exoskeletons or the end-effector type. Even though we cannot generalize our conclusions to all the RAGT systems, we believe they could be applicable due to the fact that all of them have similar design, performance and therapeutic philosophy. All RAGTs control lower limb motion according to predefined joint trajectories and perform therapy at low speed (< 3.6 km/h) allowing adjustment on assistance level based on the patient's ability to step. G-EO system is an end effector robot, that can simulate other movements such as stair climbing at the expense of losing control over every lower limb joints trajectory.
Improving ankle plantar flexor recruitment is a critical component for rehabilitation. A RAGT should target plantar flexor strength and motor modules used for walking propulsion. For the design of training protocols for RAGT, it is crucial to understand how training affects patterns of muscle activity and the influence of assistance and training speed.57 Generalization of motor learning can be sensitive to speed.58 Fast walking can help improve motor function. It can be a promoter of motor plasticity48 and encourage motor exploration by requiring participants to walk at more challenging speeds allowing greater practice (more steps). It also emphasizes subcomponents of walking such as propulsion in order to allow reconstruction of motor modules.45
In addition to possible intrinsic limitations of the study methods, it is important to consider that the trials included in the review had considerable heterogeneity in terms of trial design, characteristics of the interventions and participant’s characteristics. Similarly, there are differences in treatment dosage and training parameters for each study.
There is an unclear risk of bias for allocation concealment and blinding in the majority of the studies, however, as blinding in such intervention studies is difficult, it was considered as low risk if the author mentioned that this did not influence on the obtained results.
Limitations of the review
The risk of publication bias exists in all SR. In an attempt to minimize this situation, an extensive search was made in some of the most important databases for the theme, the authors of the studies were contacted in aim to complete the missing data, and a search was made on the references of the studies, to find possible titles with our inclusion criteria. However, it remains possible that “grey literature” may have not been identified; nevertheless it is considered that this would not have a significant impact on the results.
Our study results may be limited due to the heterogeneity of the people with spinal cord injury studied, as pooling all the populations together may lead to missing the subgroup with the major benefit from this type of intervention, but there were insufficient trials and participants to conduct a subgroup analyses.
Conclusions
Results show that gait training in a robotic orthosis have positive effects in terms of improvements in performance of gait, strength and functioning, but no effect on speed, which is expected for all the reasons listed above. However, these results must be considered in light of the SR limitations.
In terms of the availability of studies that deal with the measurement of RAGT effect in people with spinal cord injury, it was found that studies are limited in number and heterogeneous in terms of treatment dosage and training parameters, with small sample sizes and lack of quality in their methodological designs. For these reasons, there is a great need to carry out larger sample multicentre randomized controlled trials that evaluate different locomotor training approaches, specify different subgroups and include specific and objective outcomes that assess functioning and performance rather than speed.
Acknowledgements
We thank the entire team at the Neurological Rehabilitation Department from the National Institute of Rehabilitation (INR) and the entire health personnel at this National Institute for their support.
Disclaimer statements
Contributors None.
Funding None.
Conflict of Interest Statement The Authors declare that there is no conflict of interest.
Ethics approval None.
ORCID
Aberto Isaac Pérez-Sanpablohttp://orcid.org/0000-0003-0550-928X
References
- 1.Mehrholz J, Kugler J, Pohl M.. Locomotor training for walking after spinal cord injury (Review) Locomotor training for walking after spinal cord injury. Cochrane Collab 2012;(11):1–49. [Google Scholar]
- 2.Lyalka VF, Zelenin P V, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG.. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol 2005;94(6):3677–90. [DOI] [PubMed] [Google Scholar]
- 3.Quinzaños J, Villa A R, Flores A, Pérez R.. Proposal and validation of a clinical trunk control test in individuals with spinal cord injury. Spinal Cord 2014;52(6):449–54. [DOI] [PubMed] [Google Scholar]
- 4.Field-Fote E, Yang JF, Basso DM, Gorassini MA.. Supraspinal Control Predicts Locomotor Function and Forecasts Responsiveness To Training After Spinal Cord Injury. J Neurotrauma2016; [DOI] [PMC free article] [PubMed]
- 5.Behrman AL, Harkema SJ.. Locomotor Training After Human Spinal Cord Injury: A Series of Case Studies. J Am Phys Ther Assoc 2000;80(7):688–700. [PubMed] [Google Scholar]
- 6.Cain SA, Gohritz A, Fridén J, Zyl N Van.. Review of Upper Extremity Nerve Transfer in Cervical Spinal Cord Injury. J Brchial Plex Peripher Nerve Inj 2015;10(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: A multicenter trial. Arch Phys Med Rehabil 2005;86(4):672–80. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Sanpablo AI, Quinzaños-Fresnedo J, Loera-Cruz R, Quiñones-Uriostegui I, Reyes GR, Perez-Zavala R.. Validation of the instrumented evaluation of spatio-temporal gait parameters in patients with motor incomplete spinal cord injury. Spinal Cord2017; [DOI] [PubMed]
- 9.Donati ARC, Shokur S, Morya E, Campos DSF, Moioli RC, Gitti CM, et al. Long-Term Training with a Brain-Machine Interface-Based Gait Protocol Induces Partial Neurological Recovery in Paraplegic Patients. Sci Rep 2016;6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swinnen E, Duerinck S, Baeyens J-P, Meeusen R, Kerckhofs E.. Effectiveness of robot-assisted gait training in persons with spinal cord injury: a systematic review. J Rehabil Med 2010;42(6):520–6. [DOI] [PubMed] [Google Scholar]
- 11.Hornby TG, Zemon DH, Campbell D.. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther 2005;85(1):52–66. [PubMed] [Google Scholar]
- 12.Schwartz I, Meiner Z.. Robotic-Assisted Gait Training in Neurological Patients: Who May Benefit? Ann Biomed Eng 2015;43(5):1260–9. [DOI] [PubMed] [Google Scholar]
- 13.Wirz M, Bastiaenen C, de Bie R, Dietz V.. Effectiveness of automated locomotor training in patients with acute incomplete spinal cord injury: a randomized controlled multicenter trial. BMC Neurol 2011;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tefertiller C, Pharo B, Evans N, Winchester P.. Efficacy of rehabilitation robotics for walking training in neurological disorders: A review. J Rehabil Res Dev 2011;48(4):387–416. [DOI] [PubMed] [Google Scholar]
- 15.Morawietz C, Moffat F.. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 2013;94(11):2297–308. [DOI] [PubMed] [Google Scholar]
- 16.Karimi MT.Robotic rehabilitation of spinal cord injury individual. Ortop Traumatol Rehabil 2013;15(1):1–7. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization The ICF: An Overview 2010;1–10.
- 18.Higgins JPT, Green S.. Cochrane Handbook for systematic Reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration2011. 1–639 p.
- 19.Wan X, Wang W, Liu J, Tong T.. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field-Fote EC, Lindley SD, Sherman AL.. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther 2005;29(3):127–37. [DOI] [PubMed] [Google Scholar]
- 21.Quinzaños Fresnedo J, Sahagún Olmos RC, León Hernández SR, Pérez Zavala R, Quiñones Uriostegui I, Solano Salazar CJ, et al. Efectos a corto plazo del entrenamiento de la marcha en una órtesis robótica (Lokomat) con retroalimentación auditiva en pacientes con lesión medular incompleta crónica. Rehabilitacion 2015;49(1):30–7. [Google Scholar]
- 22.Shin JC, Kim JY, Park HK, Kim NY.. Effect of robotic-assisted gait training in patients with incomplete spinal cord injury. Ann Rehabil Med 2014;38(6):719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu X, Varoqui D, Kindig M, Mirbagheri MM.. Prediction of gait recovery in spinal cord injured individuals trained with robotic gait orthosis. J Neuroeng Rehabil 2014;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labruyere R, van Hedel HJ a, Labruyère R, A van Hedel HJ, van Hedel HJ a, Labruyere R, et al. Strength training versus robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study in patients depending on walking assistance. J Neuroeng Rehabil 2014;11(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esclarín-Ruz A, Alcobendas-Maestro M, Casado-Lopez R, Perez-Mateos G, Florido-Sanchez MA, Gonzalez-Valdizan E, et al. A comparison of robotic walking therapy and conventional walking therapy in individuals with upper versus lower motor neuron lesions: a randomized controlled trial. Arch Phys Med Rehabil 2014;95(6):1023–31. [DOI] [PubMed] [Google Scholar]
- 26.Kressler J, Nash MS, Burns PA, Field-Fote EC.. Metabolic responses to 4 different body weight-supported locomotor training approaches in persons with incomplete spinal cord injury. Arch Phys Med Rehabil 2013;94(8):1436–42. [DOI] [PubMed] [Google Scholar]
- 27.Alcobendas-Maestro M, Esclarín-Ruz A, Casado-Lopez RM, Muñoz-González A, Pérez-Mateos G, González-Valdizán E, et al. Lokomat Robotic-Assisted versus overgrpund training within 3 to 6 months of incomplete spinal cord lesion: Randomized controlled trial. Neurorehabil Neural Repair 2012;26(9):1058–63. [DOI] [PubMed] [Google Scholar]
- 28.Field-Fote EC, Roach KE.. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther 2011;91(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam T, Pauhl K, Ferguson A, Malik RN, Krassioukov A, Eng JJ.. Training with robot-applied resistance in people with motor-incomplete spinal cord injury: Pilot study. J Rehabil Res Dev 2015;52(1):113–30. [DOI] [PubMed] [Google Scholar]
- 30.Wu M, Landry JM, Schmit BD, Hornby TG, Yen S-C.. Robotic resistance treadmill training improves locomotor function in human spinal cord injury: a pilot study. Arch Phys Med Rehabil 2012;93(5):782–9. [DOI] [PubMed] [Google Scholar]
- 31.Nooijen CFJ, Ter Hoeve N, Field-Fote EC.. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J Neuroeng Rehabil 2009;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duschau-Wicke A, Caprez A, Riener R.. Patient-cooperative control increases active participation of individuals with SCI during robot-aided gait training. J Neuroeng Rehabil 2010;7(43):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Q, Huang Q, Hu C.. Research on Design Theory and Compliant Control for Underactuated Lower-extremity Rehabilitation Robotic Systems code: (51175368). J Phys Ther Sci 2014;26:1597–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffell L, Niu X, Brown G, Mirbagheri M.. Variability in Responsiveness to Interventions in People with Spinal Cord Injury: Do Some Respond Better Than Others? Eng Med Biol Soc 2014;978(1):5872–5. [DOI] [PubMed] [Google Scholar]
- 35.Del-Ama AJ, Koutsou AD, Moreno JC, De-los-Reyes A, Gil-Agudo Á, Pons JL.. Review of hybrid exoskeletons to restore gait following spinal cord injury. J Rehabil Res Dev 2012;49(4):497–514. [DOI] [PubMed] [Google Scholar]
- 36.Freivogel S, Schmalohr D, Mehrholz J.. Improved walking ability and reduced therapeutic stress with an electromechanical gait device. J Rehabil Med 2009;41:734–9. [DOI] [PubMed] [Google Scholar]
- 37.Hesse S, Schmidt H, Werner C, Bardeleben A, Berlin K.. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol 2003;16:705–10. [DOI] [PubMed] [Google Scholar]
- 38.Hussain S, Xie SQ, Liu G.. Robot assisted treadmill training: Mechanisms and training strategies. Med Eng Phys 2011;33:527–33. [DOI] [PubMed] [Google Scholar]
- 39.Arazpour M, Hutchins SW, Bani MA.. The efficacy of powered orthoses on walking in persons with paraplegia. Prosthet Orthot Int 2015;39(2):90–9. [DOI] [PubMed] [Google Scholar]
- 40.Wall A, Borg J, Palmcrantz S.. Clinical application of the Hybrid Assistive Limb (HAL) for gait training - a systematic review. Front Syst Neurosci 2015;9(March):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honeine J, Schieppati M, Gagey O, Do M.. By counteracting gravity, triceps surae sets both kinematics and kinetics of gait. Physiol Rep 2014;2(2):e00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen JL, Neptune RR.. Three-Dimensional Modular Control of Human Walking. J Biomech 2012;45(12):2157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen JL, Kautz SA, Neptune RR.. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin Biomech (Bristol, Avon) 2013;28(6):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ting LH, Chiel HJ, Trumbower RD, Allen JL, McKay JL, Hackney ME, et al. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 2015;86(1):38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama H, Ogawa T, Kawashima N, Shinya M, Nakazawa K.. Distinct sets of locomotor modules control the speed and modes of human locomotion. Sci Rep 2016;6:36275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox EJ, Tester NJ, Kautz SA, Howland DR, Clark DJ, Garvan C, et al. Modular control of varied locomotor tasks in children with incomplete spinal cord injuries. J Neurophysiol 2013;110(6):1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayes HB, Chvatal SA, French MA, Ting LH, Trumbower RD.. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin Neurophysiol 2014;125(10):2024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamontagne A, Fung J.. Faster is better: implications for speed-intensive gait training after stroke. Stroke 2004;35(11):2543–8. [DOI] [PubMed] [Google Scholar]
- 49.Reinkensmeyer DJ, Dietz V.. Neurorehabilitation Technology. second. Reinkensmeyer DJ, Dietz V, editors. Springer. Zurich: Springer; 2016. 413–433. [Google Scholar]
- 50.Beijersbergen CMI, Granacher U, Vandervoort AA, DeVita P, Hortobagyi T.. The biomechanical mechanism of how strength and power training improves walking speed in old adults remains unknown. Ageing Res Rev 2013;12(2):618–27. [DOI] [PubMed] [Google Scholar]
- 51.Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F.. Modular Control of Limb Movements during Human Locomotion. J Neurosci 2007;27(41):11149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scivoletto G, Ivanenko Y, Morganti B, Grasso R, Zago M, Lacquaniti F, et al. Review Article: Plasticity of Spinal Centers in Spinal Cord Injury Patients: New Concepts for Gait Evaluation and Training. Neurorehabil Neural Repair 2007;21(4):358–65. [DOI] [PubMed] [Google Scholar]
- 53.Dominici N, Ivanenko YP, Cappellini G, d’Avella A, Mondi V, Cicchese M, et al. Locomotor primitives in newborn babies and their development. Science 2011;334(6058):997–9. [DOI] [PubMed] [Google Scholar]
- 54.Dietz V.Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull 2009;78(1):I–VI. [DOI] [PubMed] [Google Scholar]
- 55.Dietz V, Grillner S, Trepp A, Hubli M, Bolliger M.. Changes in spinal reflex and locomotor activity after a complete spinal cord injury: a common mechanism? Brain 2009;132(8):2196–205. [DOI] [PubMed] [Google Scholar]
- 56.Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 2001;14(5):1186–92. [DOI] [PubMed] [Google Scholar]
- 57.Van Kammen K, Boonstra AM, Van Der Woude LH V, Reinders-Messelink HA, Den Otter R.. The combined effects of guidance force, bodyweight support and gait speed on muscle activity during able-bodied walking in the Lokomat. Clin Biomech 2016;36:65–73. [DOI] [PubMed] [Google Scholar]
- 58.Hamzey RJ, Kirk EM, Vasudevan EVL.. Gait speed influences after effect size following locomotor adaptation, but only in certain environments. Exp brain Res 2016;234(6):1479–90. [DOI] [PubMed] [Google Scholar]