Abstract
Objective: To investigate the feasibility and safety and, to a lesser extent efficacy, of inspiratory muscle training (IMT) for patients with acute complete cervical or thoracic spinal cord injury (SCI).
Design: Prospective, observational pilot study comprising a series of case reports.
Setting: Tertiary care, public hospital.
Participants: Seven adult subjects with an acute complete cervical or thoracic SCI.
Interventions: Participants received IMT as soon as their respiratory condition was stable. A high-resistance, low-repetition program of IMT using a POWERbreathe KH1 device was instituted. Training comprised 3–6 sets of 6 breaths, commenced at 50% maximum inspiratory pressure with the training load progressively increased.
Outcome measures: Feasibility (number of sessions when the criteria to participate in IMT were met/not met), safety (symptoms and physiological stability) before, during and after IMT sessions and efficacy (lung function) were measured.
Results: There were 50 sessions in total where participants met the criteria to receive IMT, with a mean (range) of 7.1 (3–11) IMT sessions per participant delivered over 10.7 (4–17) days. IMT was feasible, with all 50 planned sessions of IMT able to be delivered, and safe, with stable physiological parameters and no adverse symptoms or events recorded before, during or after IMT. Maximal inspiratory pressure increased for four participants and forced vital capacity increased for three participants over the duration of their IMT sessions.
Conclusion: A high-resistance, low-repetition program of IMT was feasible and safe in adults with an acute complete cervical or thoracic SCI whose respiratory status was stable.
Trial registration: Australian New Zealand Clinical Trials Registry (ACTRN 12614000975695).
Keywords: Spinal cord injuries, Respiratory muscle training, Breathing exercises, Safety
Introduction
The annual incidence of traumatic spinal cord injury (SCI) is approximately 15 per million in Australia, 16 per million in western Europe and 39 per million in North America.1,2 Of the injuries recorded in Australia, 53% involved cervical segments and 32% thoracic segments, with incidences of 15% and 19% for complete tetraplegia and thoracic paraplegia respectively.3 Respiratory complications are a leading cause of morbidity and mortality in the SCI population as a result of denervation of the inspiratory and expiratory respiratory muscles.4–11 The risk of respiratory complications is further increased by the autonomic dysfunction associated with the initial period of spinal shock post-SCI, resulting in mucus hypersecretion, bronchoconstriction, bradycardia and hypotension.7,8 This predisposes patients with SCI to atelectasis, sputum retention, respiratory infection and ultimately respiratory failure.6,8,10–14
Inspiratory muscle training (IMT) uses progressive resistance to load the inspiratory muscles to improve the inspiratory muscle strength and endurance of patients with a variety of chronic lung conditions.5,12,15,16 In the setting of SCI, IMT is believed to improve the strength and endurance of the accessory respiratory muscles.5 Berlowitz and Tamplin, in a Cochrane review of 11 studies, concluded that respiratory muscle training was safe (i.e. no adverse events) and effective at increasing respiratory muscle strength and perhaps lung volumes in people with a cervical SCI.5 With respect to IMT specifically (rather than respiratory muscle training), a systematic review by Sheel et al. concluded there was level 1a evidence from two randomised controlled trials that IMT significantly increased inspiratory muscle strength and level 5 evidence from one case report that IMT improved inspiratory muscle strength and decreased the number of respiratory infections.17 Similar conclusions were drawn in other systematic reviews.8,12,14 One of the randomised controlled trials referred to by Sheel et al.17 was by Van Houtte et al.9 and investigated the effectiveness of normocapnic hyperpnoea training for 14 patients with complete SCI who were at least two months post-SCI. Training resulted in significantly greater improvement in respiratory muscle strength and endurance and fewer respiratory complications compared to a control group, however the authors noted that this method of IMT was time-consuming, physically demanding and at times associated with muscle spasm/increased spasticity. The majority of studies included in these reviews included patients at least two months post-SCI, with this lag period included to control for the effect of natural recovery on outcomes. The only study identified where IMT was commenced in the acute phase post-SCI was by Derrickson et al., where the mean time post-injury was 12 days for an IMT group and 25 days for an abdominal weights group (range 2–74 days).18 Flaws in this study included the use of a very basic IMT resistor and non-standardised training (e.g. inspiratory flow rates uncontrolled, resistor selection not based on measurement of inspiratory muscle strength). Nevertheless, no adverse effects associated with IMT were reported. Thus, while there is evidence to support the use of IMT in the setting of cervical or thoracic SCI, there are a paucity of data regarding its use in the acute phase.
Given that research has shown that IMT is feasible, safe and effective when used in the setting of an Intensive Care Unit (ICU) for helping ventilator-dependent patients wean from mechanical ventilation, we believed it should be possible to commence IMT in the acute phase post-SCI.15,19–24 We therefore undertook a pilot study with the aim of documenting the feasibility and safety and, to a lesser extent efficacy, of IMT in a series of patients in the acute phase post-cervical or thoracic SCI.
Methods
Design
A prospective, observational pilot study comprising a series of case reports was undertaken. This design was chosen over a randomised controlled study as a slow recruitment rate was anticipated and because the main aims were feasibility and safety rather than efficacy. The study was approved by the Royal Adelaide Hospital Research Ethics Committee and registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12614000975695). As the IMT device was not on the Australian Register of Therapeutic Goods, approval was obtained through the Clinical Trials Notification scheme of the Therapeutic Goods Administration (Trial No. 2014/0587).
Setting and participants
The study was conducted at the Royal Adelaide Hospital, a 650-bed, tertiary care, urban, public hospital in Australia over a two-year period (December 2014 to December 2016). Local databases revealed that approximately 15 patients per year are admitted to the Royal Adelaide Hospital with a SCI between C4-T11.
Eligibility criteria were conservative given that IMT was being commenced at an earlier phase post-SCI than previously reported and were based on those described by Van Houtte et al.9 Inclusion criteria were adults (≥ 18 years) admitted to the Spinal Injuries Unit (SIU) or ICU of the Royal Adelaide Hospital with an acute complete (i.e. International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] assessment classification A) cervical or thoracic SCI (i.e. lesion level between C4 and T11), a forced vital capacity (FVC) less than predicted normal value and in a stable respiratory condition (defined, for the purposes of this study, as spontaneously breathing [room air or supplemental oxygen via nasal speculae] for at least 2 days). Exclusion criteria were an unwillingness to participate, inability to communicate effectively in English (e.g. insufficient understanding of English, cognitive impairment, psychiatric condition), symptomatic infection (characterised by fever [tympanic temperature ≥ 38° Celsius] and raised white cell count [ > 10 800 cells/mm3]), sub-acute phase (i.e. spontaneously breathing on room air or with nasal speculae for more than 7 days), pregnancy or other injuries/conditions that would make IMT impossible (e.g. facial injuries).
The principal investigator (TMcD) screened all new patients admitted with a SCI and approached potentially eligible patients, providing them with verbal and written information about the study. Informed consent (written or verbal for those unable to write and witnessed by family or a staff member) was obtained from those willing to participate. All participants received usual medical, nursing and allied health care with no aspect of this changing apart from the provision of IMT.
IMT protocol
The POWERbreathe KH1 device (Fig. 1) was used for IMT based on the advice of a colleague experienced in IMT (Prof Rik Gosselink, Faculty of Kinesiology and Rehabilitation Sciences, University of Leuven, Belgium), who believed it to be superior to traditional threshold-loading IMT devices. In contrast to some IMT devices where the inspiratory load can vary with inspiratory flow rate, this relatively recent hand-held electronic device provides a variable flow resistive load via an electronically controlled valve, with loading maintained at the same relative intensity throughout the breath.25 This enables practitioners to quantify the inspiratory load during IMT,26 whilst potentially being less time-consuming and physically demanding than the normocapnic hyperpnoea training described by Van Houtte et al.9
Figure 1.
POWERbreathe KH1 device.
The IMT protocol was high-resistance, low-repetition and based on that described by Bissett et al.20 for use in an ICU setting. All IMT sessions, supervised by the primary investigator (TMcD), were carried out with the participant sitting out of bed or supine in bed with the backrest elevated to 30° (or as high as possible if not medically cleared to sit to 30°), using a rigid plastic flanged mouthpiece and wearing a nose-clip if nasal speculae were not in situ. Positioning was consistent for each participant across all IMT sessions. After a 1–3 day familiarisation period where the device was used with little or no load, IMT was commenced with a training load of 50% of maximum inspiratory pressure (PImax), using the manual set-up option, with the aim of producing a rate of perceived exertion (RPE) of 6–8 (modified Borg scale). Verbal instruction and encouragement were provided during IMT. Each session comprised 3–6 sets of 6 breaths, depending on RPE and response to IMT, with rests allowed between sets as desired and a total session time less than 10 minutes. Once the participant’s RPE during IMT was < 6 and/or they were able to complete the entire IMT session, training pressure was increased by 10% per week to a maximum of 90% PImax. Training frequency was once per day for 4–5 days/week, with 2–3 rest days/week included to reduce boredom, fatigue and/or muscle injury and to coincide with our routine weekday physiotherapy service. Signs and symptoms of respiratory muscle fatigue (see Outcome measures) were monitored and if detected a rest period of up to 3 days was instituted until these subsided, at which time IMT training was recommenced at 50% PImax and progressed as tolerated. IMT sessions continued for the duration of each participant’s stay at the RAH to an arbitrary maximum of four weeks.
Outcome measures
Demographic and descriptive data including sex, age, level of SCI and ISNCSCI impairment classification were recorded.
Feasibility outcomes comprised the number of sessions when the criteria to participate in IMT were met or not met (and reasons why).
Safety outcomes included the response to the previous IMT session detected by reviewing the participant’s medical records/charts to ascertain the occurrence of any new respiratory complications (e.g. respiratory infection, lobar atelectasis, sputum retention) or escalation of respiratory support (e.g. higher level of supplemental oxygen, invasive or non-invasive mechanical ventilation). We also questioned participants regarding their subjective response to the previous session including symptoms that could potentially have resulted from inspiratory muscle fatigue (e.g. long-lasting complaints of general fatigue), hypercapnoea (e.g. headache, confusion) or muscle injury (e.g. delayed-onset muscle soreness [for participants with sufficient sensation]). Other safety outcomes included the measurement of physiological parameters before, during and after each IMT session until measurements had returned to near-baseline levels. These comprised respiratory rate (visual count over 30 seconds), heart rate, percutaneous oxygen saturation and blood pressure (Welch Allyn Spot Vital Signs device), RPE and symptoms of respiratory distress/depression. The occurrence of spasticity (visual observation) and any other adverse symptoms or signs were also documented. Additionally, on the first occasion of IMT, electrocardiograph monitoring (automatic external defibrillator device [AED], Medtronic) was undertaken before, during and after IMT to detect cardiac arrhythmias.
To assess the efficacy of IMT, PImax (POWERbreathe KH1 device), FVC and peak expiratory flow rate (PEFR) (EasyOne Spirometer) were measured just prior to every IMT session. Position was standardised during these measurements when possible and the best of three attempts recorded.
Sample size and data analysis
Given the observational nature of this study, a sample of 10 patients was sought. However, only seven patients were recruited over a 2-year period, in part because of an increased number of patients with incomplete SCI. In view of the small sample, data are reported for each participant and analysed descriptively. Physiological data recorded pre-IMT, immediately and 5 minutes post-IMT were used in analyses.
Results
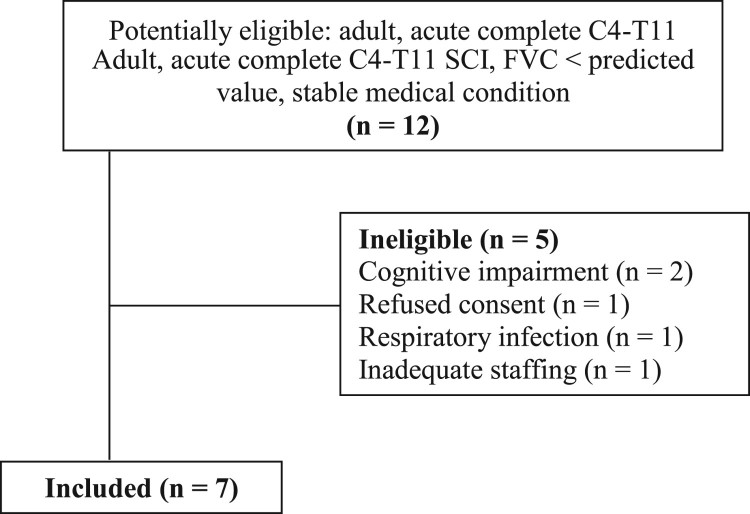
Twelve patients were screened over the 2-year study period (Fig. 2) with five patients excluded: two with acute delirium (cognitive impairment), one refused consent and one had an ongoing symptomatic respiratory infection. Staff time constraints prevented recruitment of the final excluded patient. Descriptive data for the seven participants are shown in Table 1. All seven were males, ISNCSCI impairment classification A (as per inclusion criteria), with a mean (range) age of 33.6 (22–62) years, SCI level C4 to T6 and were 10.1 (5–17) days post-SCI. The variable time post-SCI to commencement of IMT reflects the time it took for patients to meet the eligibility criteria.
Figure 2.
Flow of participants through the trial. C, cervical; FVC, forced vital capacity; n, number; SCI, spinal cord injury; T, thoracic.
Table 1. Descriptive data for the seven participants.
| Subject number | Sex | Age (years) | Mechanism of SCI | Level of SCI | Relevant PMH | Days post-SCIa | Number of IMT sessions | Duration of IMT (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 30 | MBA | T6 | Nil | 5 | 3 | 5 |
| 2 | M | 62 | Fall | C4 | HT, anxiety | 17 | 7 | 11 |
| 3 | M | 28 | MVA | C7 R, T1 L | Nil | 12 | 8 | 13 |
| 4 | M | 28 | Diving | C6 | Limb fractures | 9 | 10 | 17 |
| 5 | M | 22 | MBA | T4 | Nil | 10 | 7 | 10 |
| 6 | M | 23 | Kite surfing | C6 | Scheurmann’s disease | 11 | 11 | 15 |
| 7 | M | 42 | MVA | C6 | Nil | 7 | 4 | 4 |
C, cervical; HT, hypertension; IMT, inspiratory muscle training; L, left; M, male; MBA, motor bike accident; MVA, motor vehicle accident; PMH, past medical history; R, right; SCI, spinal cord injury; T, thoracic.
aDays from SCI to first session of IMT.
Feasibility
There were 50 sessions in total when the seven participants met the criteria to participate in IMT, with a mean (range) of 7.1 (3–11) IMT sessions per participant delivered over 10.7 (4–17) days (Table 1). The variability in the number of IMT sessions and days over which it was delivered resulted from the variability in participants’ length of stay at the RAH. There were no occasions when participants failed to meet the pre-session criteria to undertake IMT and no instances when IMT had to be curtailed intra-session.
Safety
No adverse safety outcomes were identified prior to any of the 50 sessions of IMT. Cardiorespiratory responses to IMT are summarised in Table 2. Little change was seen in physiological parameters from baseline pre-IMT levels to those recorded immediately post-IMT, with return to near-baseline values by 5 minutes post-IMT training for all 50 sessions of IMT. RPE increased from a mean (range) of 1.4 (0.0–6.0) pre-IMT to 3.9 (1.7–9.5) immediately post-IMT, decreasing to 1.9 (0.0–7.8) by 5 minutes post-IMT. No cardiac arrhythmias were detected before, during or after the first sessions of IMT. There were no instances of spasticity or any other adverse symptoms or signs noted before, during or after the 50 IMT sessions.
Table 2. Cardiorespiratory response to IMT sessions for each participant.a.
| Subject number | Respiratory rate | Heart rate (beats per minute) | SpO2 (%) | Blood pressure (mmHg) | Rate of perceived exertion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-IMT | Immed post-IMT | 5′ post-IMT | Pre-IMT | Immed post-IMT | 5′ post-IMT | Pre-IMT | Immed post-IMT | 5′ post- | Pre-IMT | Immed post-IMT | 5′ post-IMT | Pre-IMT | Immed post-IMT | 5′ post-IMT | |
| 1 | 18.0 | 20.7 | 20.7 | 71.3 | 70.3 | 69.7 | 99.7 | 99.3 | 99.7 | 115.7/50.7 | 110.3/49.0 | 111.7/51.7 | 1.3 | 3.2 | 2.7 |
| 2 | 17.0 | 19.1 | 17.0 | 53.7 | 51.1 | 51.3 | 96.9 | 97.7 | 96.4 | 111.0/64.4 | 104.3/62.3 | 107.6/63.6 | 2.0 | 3.1 | 2.0 |
| 3 | 14.3 | 14.0 | 15.0 | 79.9 | 83.8 | 81.5 | 99.1 | 99.3 | 98.8 | 109.6/67.6 | 104.5/62.0 | 107.0/63.1 | 0.4 | 2.3 | 0.4 |
| 4 | 8.6 | 8.7 | 8.9 | 64.7 | 65.2 | 62.6 | 96.9 | 96.4 | 96.5 | 111.3/62.2 | 113.5/66.8 | 115.3/66.1 | 0.0 | 1.7 | 0.0 |
| 5 | 16.4 | 16.0 | 16.7 | 95.0 | 96.9 | 93.1 | 100.0 | 99.7 | 99.9 | 109.7/58.0 | 111.4/60.0 | 111.3/60.6 | 0.1 | 3.6 | 0.0 |
| 6 | 13.9 | 13.2 | 13.6 | 60.9 | 60.5 | 57.7 | 98.6 | 98.8 | 98.0 | 103.2/56.0 | 105.1/59.1 | 107.6/61.2 | 0.0 | 4.0 | 0.1 |
| 7 | 16.8 | 15.5 | 15.5 | 56.3 | 53.8 | 55.3 | 96.8 | 98.0 | 97.5 | 100.3/57.3 | 104.0/62.3 | 104.0/57.8 | 6.0 | 9.3 | 7.8 |
aData are reported as mean values for each participant across their IMT sessions.
5′ = five minutes; immed, immediately; IMT, inspiratory muscle training; mmHg = millimetres of mercury; SpO2 = percutaneous oxygen saturation.
Efficacy
Efficacy data from the first and last sessions of IMT for PImax and FVC are provided in Table 3 for each of the seven participants, with session to session variability illustrated in Figs. 3–4. Lung function parameters were variable both between and within participants. On an individual basis, four participants, who received 7–11 sessions of IMT over 10–17 days, demonstrated an increase in PImax from the first to last IMT session. Three of these four also recorded an increase in FVC and PEFR with the other having a decline in these values. Two participants, who received 3–4 sessions of IMT over 4–5 days, had a decrease in PImax and FVC over time, with one also having a decrease in PEFR and the other an increase in PEFR. One participant showed no change in PImax but an increase in FVC and PEFR after 7 IMT sessions over 11 days. As shown in Fig. 5, IMT training loads were able to be increased over the sessions for each participant as planned.
Table 3. Efficacy data from the first to last session of IMT for each participant.
| Subject number | PImax, cmH2O (% predicted)a | FVC, litres (% predicted)b | PEFR, litres per second (% predicted)b | |||
|---|---|---|---|---|---|---|
| First | Last | First | Last | First | Last | |
| 1 | 44 (34.2) | 38 (29.6) | 1.96 (42.3) | 1.43 (30.9) | 3.54 (54.5) | 3.12 (48.0) |
| 2 | 30 (32.4) | 30 (32.4) | 1.63 (34.2) | 2.10 (44.0) | 1.87 (107.5) | 3.13 (179.9) |
| 3 | 63 (49.2) | 92 (71.9) | 2.40 (58.0) | 2.66 (64.3) | 2.68 (40.9) | 3.55 (54.1) |
| 4 | 73 (57.0) | 79 (61.7) | 2.38 (53.1) | 2.75 (61.2) | 4.40 (63.7) | 4.74 (66.0) |
| 5 | 56 (43.8) | 66 (51.6) | 3.38 (65.5) | 3.59 (69.6) | 5.51 (63.8) | 6.04 (70.0) |
| 6 | 40 (31.3) | 64 (50.0) | 2.37 (53.7) | 2.19 (49.7) | 4.28 (54.7) | 3.54 (45.2) |
| 7 | 109 (93.1) | 102 (87.1) | 3.08 (55.0) | 2.73 (49.1) | 2.54 (48.0) | 3.71 (70.3) |
| Means | 59.3 (48.7) | 67.3 (54.9) | 2.46 (51.7) | 2.49 (52.7) | 3.55 (61.9) | 3.98 (76.2) |
cmH2O = centimetres of water; FVC, forced vital capacity; PEFR, peak expiratory flow rate; PImax = maximal inspiratory pressure.
aPercentage predicted values based on those reported by Sclauser Pessoa et al.29
bPercentage predicted values calculated on-line (Dynamic MT) http://dynamicmt.com/dataform3.html30 and based on those reported by Langhammer et al.31
Figure 3.
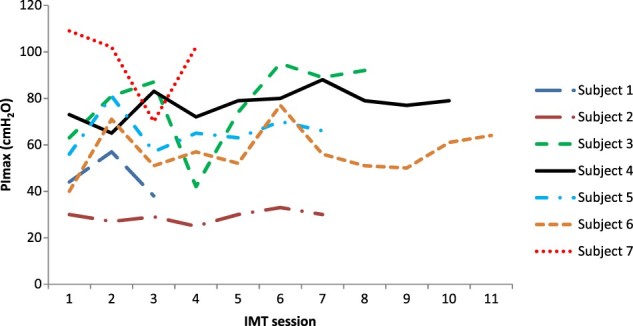
PImax variability between and within participants across IMT sessions.
Figure 4.
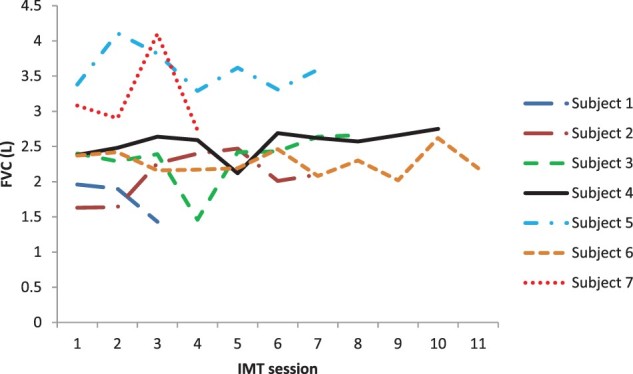
FVC variability between and within participants across IMT sessions.
Figure 5.
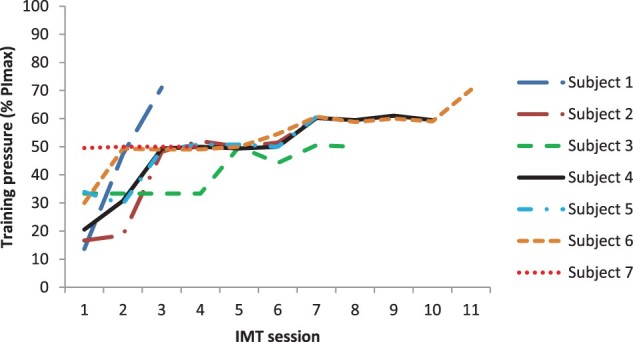
Training loads across IMT sessions.
Discussion
This pilot study investigated the feasibility and safety of commencing IMT in a series of medically stable adult patients in the acute phase post-cervical or thoracic SCI. For the seven participants, IMT was feasible with all planned sessions of IMT able to be delivered, and safe with stable physiological parameters and no adverse events associated with IMT. Improvements in lung function were seen for four of the seven participants over the duration of their IMT sessions.
Our sample, as planned, comprised patients at an earlier stage post-SCI than previous research investigating the effect of IMT for SCI patients.12 The PImax and FVC values that we recorded were similar to the means reported in the reviews by Berlowitz and Tamplin5 and Van Houtte et al.,14 but somewhat lower than those reported by Loveridge et al.27 and Silveira et al.28 which most likely reflects the acuity of our sample. Our findings of IMT being feasible and safe in acute patients were similar to Bissett et al. who investigated the use of IMT in ventilated ICU patients.20
The efficacy of IMT was not a primary aim of our study as we anticipated (and it was subsequently shown) that participants’ duration of stay in the acute hospital setting would be too short to enable a training effect. Furthermore, the variability between participants in lung function parameters and study design (i.e. lack of a control group) means the efficacy of IMT is unclear. Nevertheless, our efficacy data merit some further discussion. As noted earlier, training loads were able to be increased over the duration of the study for participants, four of whom showed improvement in most measures of lung function over time, one stayed the same and two deteriorated. Whilst those participants whose lung function improved tended to have a greater number and duration of IMT sessions (see Results), the uncontrolled nature of our study means we cannot be sure whether improvement reflects a training effect or spontaneous recovery. Whilst the deterioration in lung function seen over time for two participants (subject no. 1 and 7) could indicate an adverse effect of IMT (e.g. respiratory fatigue), we believe this is unlikely as both participants remained clinically stable. Instead, it may result from the considerable variability in lung function seen from session to session (Figs. 3 and 4) which in turn most likely reflects the effort-dependent nature of these measures, which rely on participants’ full understanding of the procedure, maximal effort and enthusiastic coaching to optimise performance.29 Whilst body position during lung function testing was standardised for each participant across the study, the time of day was not which may have affected lung function. In view of the short-term variability in volitional measures of lung function that we observed, our clinical advice is to carefully monitor the patient’s overall clinical status rather than relying solely on volitional measures of lung function to detect adverse effects from IMT in the acute phase post-SCI.
The main limitations of this study were the single site, small sample size and uncontrolled design, clearly limiting the generalisability of our results. However, the slow recruitment rate justifies our choice of study design, particularly given our focus on the feasibility and safety of IMT. While the inclusion of patients with incomplete SCI would have increased our sample size, we believed that it was preferable to focus on those patients with complete SCI in order to be consistent with previous studies.9,17,18 Despite these limitations, the current study provides new evidence demonstrating that a high-resistance, low-repetition program of IMT was both feasible and safe at an earlier stage post-SCI than has previously been documented. Given that no adverse events were documented over the 50 sessions of IMT, it is possible that a less conservative IMT program may be feasible and safe in the acute phase post-SCI. Further study is needed to demonstrate the efficacy of IMT when instituted during the acute phase post-SCI.
Conclusion
A high-resistance, low-repetition program of IMT was feasible with all planned sessions able to be delivered, and safe with no adverse events recorded before, during or after IMT in adults with an acute complete cervical or thoracic SCI with stable respiratory function. Further study is required to investigate the efficacy of IMT in this setting.
Funding Statement
This work received a grant of A$700 from the Royal Adelaide Hospital Physiotherapy Centenary Fund to purchase the POWERbreathe KH1 device.
Acknowledgements
We thank Prof Rik Gosselink, Dr Anne Leditschke and Ms Bernie Bissett for their advice as experts in this field during development of the IMT protocol and the patients who participated in the study.
Disclaimer statements
Contributors Both authors contributed to all phases of this study including design, data collection and analysis and writing up. The final version of the manuscript has been approved by both authors.
Ethics approval This study was approved by the Royal Adelaide Hospital Research Ethics Committee and all participants gave their informed consent to participate.
References
- 1.Cripps RA, Lee BB, Wing P, Weerts E, Mackay J, Brown D.. A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord 2011;49(4):493–501. doi: 10.1038/sc.2010.146 [DOI] [PubMed] [Google Scholar]
- 2.ISCoS The International Spinal Cord Society. Global mapping of spinal cord injury (SCI) epidemiology: towards a living data repository (LDR). Available from http://www.iscos.org.uk/sci-global-mapping (accessed 20 March 2017).
- 3.AIHW: Norton L 2010. Spinal cord injury, Australia 2007–08. Injury research and statistics series no.52. Cat. no. INJCAT 128. Canberra: AIHW. Available from http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442458862 (accessed 20 March 2017).
- 4.Arora S, Flower O, Murray NP, Lee BB.. Respiratory care of patients with cervical spinal cord injury: a review. Crit Care Resusc 2012;14(1):64–73. [PubMed] [Google Scholar]
- 5.Berlowitz DJ, Tamplin J.. Respiratory muscle training for cervical spinal cord injury. Cochrane Database Syst Rev 2013;7:CD008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown R, DiMarco AF, Hoit JD, Garshick E.. Respiratory dysfunction and management in spinal cord injury. Respir Care 2006;51(8):853–68. [PMC free article] [PubMed] [Google Scholar]
- 7.Galeiras Vázquez R, Rascado Sedes P, Mourelo Fariña M, Montoto Marqués A, Ferreiro Velasco ME.. Respiratory management in the patient with spinal cord injury. Biomed Res Int 2013;2013:168757. doi: 10.1155/2013/168757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid WD, Brown JA, Konnyu KJ, Rurak JM, Sakakibara BM.. Physiotherapy secretion removal techniques in people with spinal cord injury: a systematic review. J Spinal Cord Med 2010;33(4):353–70. doi: 10.1080/10790268.2010.11689714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Houtte S, Vanlandewijck Y, Kiekens C, Spengler CM, Gosselink R.. Patients with acute spinal cord injury benefit from normocapnic hyperpnoea training. J Rehabil Med 2008;40(2):119–25. doi: 10.2340/16501977-0140 [DOI] [PubMed] [Google Scholar]
- 10.Winslow C, Rozovsky J.. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 2003;82(10):803–14. doi: 10.1097/01.PHM.0000078184.08835.01 [DOI] [PubMed] [Google Scholar]
- 11.Zimmer MB, Nantwi K, Goshgarian HG.. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med 2007;30(4):319–30. doi: 10.1080/10790268.2007.11753947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks D, O'Brien K, Geddes EL, Crowe J, Reid WD.. Is inspiratory muscle training effective for individuals with cervical spinal cord injury? A qualitative systematic review. Clin Rehabil 2005;19(3):237–46. doi: 10.1191/0269215505cr856oa [DOI] [PubMed] [Google Scholar]
- 13.Roth EJ, Stenson KW, Powley S, Oken J, Primack S, Nussbaum SB, et al Expiratory muscle training in spinal cord injury: a randomized controlled trial. Arch Phys Med Rehabil 2010;91(6):857–61. doi: 10.1016/j.apmr.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 14.Van Houtte S, Vanlandewijck Y, Gosselink R.. Respiratory muscle training in persons with spinal cord injury: a systematic review. Resp Med 2006; 100(11):1886–95. doi: 10.1016/j.rmed.2006.02.029 [DOI] [PubMed] [Google Scholar]
- 15.Bissett B, Leditschke IA, Paratz JD, Boots RJ.. Respiratory dysfunction in ventilated patients: can inspiratory muscle training help? Anaesth Intensive Care 2012: 40(2):236–46. [DOI] [PubMed] [Google Scholar]
- 16.Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G.. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011;37(2):416–25. doi: 10.1183/09031936.00031810 [DOI] [PubMed] [Google Scholar]
- 17.Sheel AW, Reid WD, Townson AF, Ayas N.. Respiratory management following spinal cord injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, et al., editors. Spinal Cord Injury Rehabilitation Evidence. Version 5.0, Vancouver; 2014. p. 1–55. https://scireproject.com/evidence/rehabilitation-evidence/respiratory-management/ (accessed 20 March 2017). [Google Scholar]
- 18.Derrickson J, Ciesla N, Simpson N, Imle PC.. A comparison of two breathing exercise programs for patients with quadriplegia. Phys Therapy 1992;72(11):763–9. doi: 10.1093/ptj/72.11.763 [DOI] [PubMed] [Google Scholar]
- 19.Bissett B, Leditschke IA.. Inspiratory muscle training to enhance weaning from mechanical ventilation. Anaesth Intensive Care 2007;35(5):776–9. [DOI] [PubMed] [Google Scholar]
- 20.Bissett B, Leditschke IA, Green M.. Specific inspiratory muscle training is safe in selected patients who are ventilator-dependent: a case series. Intensive Crit Care Nurs 2012;28(2):98–104. doi: 10.1016/j.iccn.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Cader SA, Vale RG, Castro JC, Bacelar SC, Biehl C, Gomes MC, et al. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. J Physiother 2010;56(3):171–7. [DOI] [PubMed] [Google Scholar]
- 22.Caruso P, Denari SDC, Ruiz SA, Bernal KG, Manfrin GM, Friedrich C, et al. Inspiratory muscle training is ineffective in mechanically ventilated critically ill patients. Clinics (Sao Paulo) 2005;60(6):479–84. doi: 10.1590/S1807-59322005000600009 [DOI] [PubMed] [Google Scholar]
- 23.Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, et al Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care 2011;15(2):R84. doi: 10.1186/cc10081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprague SS, Hopkins PD.. Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys Ther 2003;83(2):171–81. [PubMed] [Google Scholar]
- 25.Charususin N, Gosselink R, deCramer McConnell, Saey D, Maltais F, et al. Inspiratory muscle training protocol for patients with chronic obstructive pulmonary disease (IMTCO study): a multicentre randomised controlled trial. BMJ Open 2013:3:3003101. doi: 10.1136/bmjopen-2013-003101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer D, Jacome C, Charususin N, Scheers H, McConnell A, Decramer M, et al. Measurement validity of an electronic inspiratory loading device during a loaded breathing task in patients with COPD. Respir Med 2013;107(4):633–5. doi: 10.1016/j.rmed.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 27.Loveridge B, Badour M, Dubo H.. Ventilatory muscle endurance training in quadriplegia: effects on breathing pattern. Paraplegia 1989;27(5):329–39. [DOI] [PubMed] [Google Scholar]
- 28.Silveira JM, Gastaldi AC, de Matos Boaventura C, Souza HC.. Inspiratory muscle training in quadriplegic patients. J Bras Pneumol 2010;36(3):313–9. doi: 10.1590/S1806-37132010000300008 [DOI] [PubMed] [Google Scholar]
- 29.Sclauser Pessoa IMB, Franco Parreira V, Fregonezi GA, Sheel AW, Chung F, Reid WD.. Reference values for maximal inspiratory pressure: a systematic review. Can Respir J 2014;21(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dynamic MT.Pulmonary function – reference normal predicted values calculator. http://dynamicmt.com/dataform3.html (accessed 20 March 2017).
- 31.Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L.. Forced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord-Trøndelag Study. Eur Respir J 2001;18(5):770–9. doi: 10.1183/09031936.01.00255301 [DOI] [PubMed] [Google Scholar]