Abstract
Physiological and biochemical changes in six-day-old hydroponically grown lentil seedlings exposed to 100 mM salinity stress with or without 5 and 10 mM Na-acetate were studied. Results showed that salt stress reduced recovery percentage, fresh weight (FW), chlorophyll (chl) content, disturbed water balance, disrupted antioxidant defense pathway by decreasing reduced ascorbate content, and caused ion toxicity resulting from increased Na+ accumulation, severe K+ loss from roots in hydroponic culture. However, exogenous application of Na-acetate improved the seedling growth by maintaining water balance and increasing chl content. Furthermore, Na-acetate application reduced oxidative damage by modulating antioxidant defense pathway, and sustained ion homeostasis by reducing Na+ uptake and K+ loss. In the second experiment in glass house, we investigated the role of Na-acetate on lentil for long-term salinity. Acetate application increased FW and dry weight, reduced oxidative and membrane damage, and lowered the accumulation of Na+ in shoot compared with salt stressed seedlings alone. From the results of both experiments, it is clear that the exogenous application of Na-acetate enhanced salt tolerance in lentil seedlings.
Electronic supplementary material
The online version of this article (10.1007/s12298-018-00640-6) contains supplementary material, which is available to authorized users.
Keywords: Ion toxicity, Lentil, Oxidative stress, Recovery, Salinity, Na-acetate
Introduction
Over 6% (800 million ha) of the total land area in the globe has already been affected by soil salinity, and the salt-affected area is increasing with time due to several natural and anthropogenic reasons (Munns and Tester 2008; Hasanuzzaman et al. 2013; Hanin et al. 2016). Soil salinity causes a significant reduction in crop growth and productivity. To secure food production for rising population, it is necessary to develop technology to grow the crop in salt-affected area.
Unfortunately, most of the crop plants, being glycophytes, are salt sensitive compared to halophytes (Ismail and Horie 2017). Soil salinity primarily affects plant growth by imposing osmotic stress followed by ionic toxicity (Munns and Tester 2008). Both the osmotic and ionic stress cause oxidative stress through over production of reactive oxygen species (ROS) (Abogadallah 2010). Reactive oxygen species are singlet oxygen (1O2), superoxide radical (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (OH.), that can cause damage to vital components of cells such as lipid, protein, and DNA (Gill and Tuteja 2010). However, production of ROS is unavoidable in plants even under the favorable growing condition, and these ROS are mainly detoxified by the antioxidant metabolic pathway (Gill and Tuteja 2010). This pathway is well equipped with non-enzymatic antioxidants such as ascorbate (AsA) and glutathione (GSH), and enzymatic antioxidants such as ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR) and glutathione reductase (GR) (Gill and Tuteja 2010). However, under salt stress condition, the rate of ROS generation is higher than the detoxification rate of ROS by antioxidant defense pathway, as a result, oxidative stress occurs (Abogadallah 2010). Evidence shows that tolerant genotypes possess better ROS detoxification ability compared to susceptible genotypes in case of rice (El-Shabrawi et al. 2010; Kibria et al.2017), barley (Seckin et al. 2010) and lentil (Singh et al. 2017). Therefore, controlling ROS production through overexpression of the genes of this pathway enhance not only salinity stress tolerance but also other abiotic stress tolerance (Ashraf 2009). In addition, salinity tolerance in glycophytes is also associated with minimal Na+ uptake, Na+ efflux to soil, reduction in NaCl-induced K+ leakage as well as maintenance of low Na+/K+ ratio (Tester and Davenport 2003; Shabala and Pottosin 2014).
Plants provide a signal by altering the metabolites and ions contents in response to an environmental condition to activate a group of genes, which in turn helps the plants to adapt in that particular environmentally challenged condition (Pastor et al. 2013; Mauch-Mani et al. 2017). For example, sensing salt stress seedlings undergoes some simultaneous changes such as increasing cytosolic Ca2+, increasing abscisic acid (ABA) and jasmonic acid (JA) accumulation to activate downstream genes to enhance tolerance (Xiong et al. 2002; Golldack et al. 2014). Thus, to enhance salt tolerance, chemicals of diverse groups (antioxidants, organic acids, phytohormones, polyamines, and osmolytes) that are induced by stress can be used. And this approach, also known as shot-gun approach, is easier to adopt, cost-effective and more promising compared to other methods such as conventional breeding and transgenic approach (Hasanuzzaman et al. 2013; Savvides et al. 2016; Rahman et al. 2017). Evidence shows that exogenous application of ascorbic acid, glutathione (GSH), nitric oxide (NO), proline (pro), salicylic acid (SA), polyamines (PAs) and organic acids are well known to enhance abiotic stress tolerance in various plants (Hasanuzzaman et al. 2013; Savvides et al. 2016; Rahman et al. 2017). Among organic acids, exogenous application of acetate improves drought stress tolerance in Arabidopsis, wheat, and maize by modulating JA signaling pathway and histone acetylation (Kim et al. 2017). Both JA signaling and histone acetylation are also required for salt tolerance (Ismail et al. 2012; Sako et al. 2015). A histone deacetylase inhibitor, Ky-2 increased histone H4 acetylation and improved salt tolerance in Arabidopsis (Sako et al. 2015). However, the role of acetate in enhancing salt tolerance remains to be elucidated.
Lentil is a legume crop, which can fix nitrogen to the soil. Furthermore, it serves as a cheap source of protein and pharmacologically important compound (Sidari et al. 2008; Afzal et al. 2014). However, this valuable crop is saline sensitive compared to other glycophytes (Misra and Saxena 2009; Hossain et al. 2017).
Therefore, in this study, we evaluated the potentiality of acetate whether it confers salt tolerance in lentil. Our results indicate that acetate can improve salt tolerance in lentil.
Materials and methods
Plant materials and treatments
Experiment I
Lentil (Lens culinaris Medik cv. BARI Lentil-7) seeds were soaked for 24 h, and then 45 seeds were placed on six-layered moistened paper towels in Petri plates for germination (Hossain et al. 2017). After incubating for 72 h in dark condition, germinated seedlings were then transferred and grown in a growth chamber flushed with nutrient solution Hyponex (Tokyo, Japan) under an irradiance of 350 μmol (photon) m−2 s−1, a temperature of 25 ± 2 °C, and a relative humidity of 65–70%. Acetate doses were fixed based on previous report (Kim et al. 2017) where they suggest 10–20 mM acetic acid effective for drought tolerance. During the preliminary trial, we used 5, 10 and 20 mM Na-acetate. Three levels of Na-acetate improved tolerance against salt stress. However, 20 mM Na-acetate caused significant growth reduction in our experimental condition. Six-day-old seedlings were exposed to these treatments. After 2-days stress treatment followed by 2-day recovery, we harvested shoots for further investigation.
Experiment II
To investigate the effect of acetate under long-term salt stress, we conducted another experiment in a glass house. In this experiment, pots (12 cm diameter and 9 cm in height) were filled with vermiculite and soaked with water. Seeds were sown in the pots. After germination, 15 seedlings were kept per pot. Hyponex (Tokyo, Japan) nutrient solution (diluted 1000-fold) was applied at 4-days intervals. Salt treatment was done after 15 DAS with 100 mM NaCl (gradually increased from 40 mM NaCl) with or without 10 mM Na-acetate. Seedlings were flushed with different treatment solutions every 4-days interval. Data were collected after 15 days after treatments. Therefore, the treatment combinations were Control (Con), 100 mM NaCl (S), and 100 mM NaCl+10 mM Na-acetate (S+A).
Survival percentage
To determine the survival percentage, we counted the seedlings without any leaf damages after 6 days of recovery. Survival percentage was calculated as SV (%) = Number of seedlings without chlorosis × 100/total number of seedlings (Gong et al. 2001).
Determination of chlorophyll content
To measure chlorophyll a and b, 0.1 g leaf sample were taken in a tube containing 10 mL dimethyl sulfoxide (DMSO) solution. Chlorophyll pigments are more stable in DMSO than in ethanol and acetone (Richardson et al. 2002). To extract chlorophyll, the tubes were heated at 65 °C for 1 h. After cooling the solution at room temperature, absorbance was taken at 645 and 663 nm (Hiscox and Israelstam 1979). Chlorophyll content were calculated according to Arnon (1949), and expressed as mg g−1 FW.
Estimation of proline
Proline content was quantified according to widely used method by Bates et al. (1973) and expressed as µmol g−1 FW.
Determination of lipid peroxidation
Shoot sample (0.5 g) was homogenized with 5% TCA using a mortar and pestle. Homogenized sample was centrifuged at 11,500×g for 15 min. Then, 1 mL of supernatant was added to 4 mL thiobarbituric (TBA) reagent (0.5% TBA prepared in 20% TCA). The mixture was then heated for 30 min at 95 °C. The heated solution was transferred to ice box. After cooling, the sample was again centrifuged at 11,500×g for 10 min. Then, the absorbance was taken using a spectrophotometer. Malondialdehyde (MDA) content was quantified by observing the difference in absorbance at 532 nm and 600 nm and calculated using an extinction coefficient of 155 mM−1 cm−1, and expressed as nmol g−1 FW (Heath and Packer 1968).
Determination of electrolyte leakages
Electrolyte leakage was measured according to Dionisio-Sese and Tobita (1998).
Estimation of reduced ascorbate and total glutathione content
To determine AsA and total GSH, 0.5 g shoot were homogenized in 3 mL ice-cold 5% TCA using mortar and pestle. After centrifugation at 11,500×g for 15 min at 4 °C, the supernatant was neutralized with 0.5 M potassium phosphate (K-P) buffer (pH 7.0). Reduced AsA were assayed using spectrophotometer at 265 nm in 100 mM K-P buffer (pH 6.5) with 1.0 U of ascorbate oxidase (AO) (Nahar et al. 2016).
After neutralizing supernatant with 0.5 M potassium phosphate (K-P) buffer (pH 7.0), oxidized glutathione or glutathione disulfide (GSSG) and total glutathione were measured. We followed the method of Griffiths (1980) based on enzymatic recycling.
Both reduced AsA and total GSH content was calculated from the standard curve, and expressed as µmol g−1 FW and nmol g−1 FW, respectively.
Determination of protein
Protein concentration was measured according to Bradford (1976) using bovine serum albumin (BSA) as a protein standard.
Enzyme extraction and assay
For enzyme assay, shoots (0.5 g) were homogenized in 50 mM K-P buffer (pH 7.0) containing 100 mM KCl, 1 mM ascorbate, 5 mM β-mercaptoethanol, and 10% (w/v) glycerol using a pre-chilled mortar and pestle. The homogenates were centrifuged at 11,500×g for 15 min and the supernatant was used to determine protein content and enzyme activity. During extraction, all procedures were performed at 0–4 °C.
To determine ascorbate peroxidase (APX, EC: 1.11.1.11) activity, enzyme extract was added to reaction buffer containing 50 mM K-P buffer (pH 7.0), 0.5 mM AsA, 0.1 mM H2O2, 0.1 mM EDTA. The decreased absorbance at 290 nm for 1 min was observed and APX activity was calculated using an extinction coefficient of 2800 M−1 cm−1 (Noctor et al. 2016), and expressed as µmol min−1 mg−1 protein.
According to the method of Noctor et al. (2016), monodehydroascorbate reductase (MDHAR, EC: 1.6.5.4) activity was assayed and calculated using an extinction coefficient of 6200 M−1 cm−1, and expressed as nmol min−1 mg−1 protein. Enzyme extract was added to reaction buffer containing 50 mM Tris–HCl buffer (pH 7.5), 2.5 mM AsA, 0.2 mM NADPH.
Dehydroascorbate reductase (DHAR, EC: 1.8.5.1) activity was determined according to the method of Noctor et al. (2016). Enzyme extract was added to reaction buffer containing 50 mM K-P buffer (pH 7.0), 2.5 mM GSH, 0.1 mM EDTA, 0.1 mM DHA. DHAR activity was calculated using an extinction coefficient of 14,000 M−1 cm−1, and expressed as nmol min−1 mg−1 protein.
To determine glutathione reductase (GR, EC: 1.6.4.2) activity, enzyme extract was added to reaction buffer containing 20 mM K-P buffer (pH 7.8), 1 mM EDTA, 0.1 mM GSSG, 1.35 mM NADPH. The decreased absorbance at 340 nm for 1 min was observed and GR activity was calculated using an extinction coefficient of 6200 M−1cm−1 (Noctor et al. 2016), and expressed as nmol min−1 mg−1 protein.
Catalase (CAT, EC: 1.11.1.6) activity was assayed as described by Noctor et al. (2016) and calculated using an extinction coefficient of 40 M−1 cm−1, and expressed as µmol min−1 mg−1 protein.
Determination of ion content
Sodium (Na+) and potassium (K+) ion contents were determined according to Rahman et al. (2016). Plant samples were oven dried at 80 °C for a period until weight becomes constant. Dry root and shoot (0.1 gm) were ground and digested separately with an acid mixture, nitric acid and perchloric acid (5:1) at 80 °C for 48 h. Then, sodium (Na+) and potassium (K+) were measured using an atomic absorption spectrophotometer (Hitachi Z-5000; Hitachi, Japan).
Statistical analysis
The data were subjected to analysis of variance (ANOVA) and the mean differences were compared by Fisher’s least significant difference (LSD) using XLSTAT v.2016 software. Differences at P ≤ 0.05 were considered significant.
Results
Na-acetate improved survival percentage under salt stress
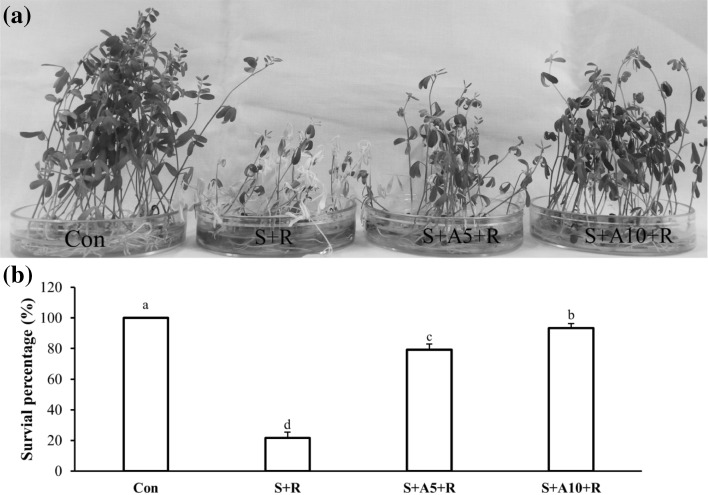
To investigate whether acetate could enhance salt tolerance, we applied 5 and 10 mM Na-acetate with 100 mM NaCl. Exogenous application of Na-acetate increased plants survival from 100 mM NaCl. Around 80% seedlings failed to recover from 100 mM NaCl stress, whereas 93% seedlings recovered from 100 mM NaCl stress when treated with 10 mM Na-acetate (Fig. 1a, b).
Fig. 1.
Phenotypic appearance of lentil seedlings under salt stress treated with or without Na-acetate in experiment I (a), survival percentage 6-day after recovery (b). The treatments were Control (Con), 100 mM NaCl (S+R), 100 mM NaCl+5 mM Na-acetate (S+A5+R), 100 mM NaCl+10 mM Na-acetate (S+A10+R). Mean (± SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying Fisher’s LSD test
Effect of Na-acetate on seedlings growth under salt stress
Seedlings’ fresh weight (FW), dry weight (DW) and chl content decreased under salt stress condition (Table 1; Supplementary Fig. 1a). At 100 mM NaCl concentration, FW decreased by 36% compared to control and the addition of Na-acetate with salt increased the FW by 30% compared with salt stressed alone in experiment I. Dry weight did not changed significantly after Na-acetate application with salt under experiment I (Table 1). Furthermore, measurement of chl revealed that chl (a + b) reduced by 25% at 100 mM NaCl stress compared with control. On the contrary, acetate treatment improved the chl (a + b) content under salt stress (Supplementary Fig. 1a) in experiment I.
Table 1.
Effect of exogenous Na-acetate on fresh weight (FW), dry weight (DW), water content (WC), malondialdehyde (MDA), other aldehyde, and electrolyte leakage (EL) in lentil seedlings
| Treatments | FW (mg shoot−1) | DW (mg shoot−1) | Water content (%) | MDA (nmol g−1 FW) | Other aldehydes (µmol g−1 FW) | Electrolyte leakage (%) | |
|---|---|---|---|---|---|---|---|
| Expt I | Con | 72 ± 3.3a | 11 ± 0.1a | 85.0 ± 0.6a | 20 ± 1.8b | 0.28 ± 0.02b | 24 ± 7.8c |
| S+R | 46 ± 6.3c | 9.8 ± 0.9b | 78.8 ± 1.2b | 47 ± 9.5a | 0.71 ± 0.07a | 85 ± 5.1a | |
| S+A5+R | 65 ± 1.7ab | 10.2 ± 0.2ab | 84.4 ± 0.6a | 19 ± 0.8b | 0.23 ± 0.04b | 49 ± 2.4b | |
| S+A10+R | 60 ± 2.2b | 9.8 ± 0.5ab | 83.6 ± 1.1a | 21 ± 0.8b | 0.21 ± 0.01b | 47 ± 2.4b | |
| Expt II | Con | 383 ± 0.02a | 44 ± 0.7a | 88.5 ± 0.5a | 21 ± 0.5b | 0.24 ± 0.02b | 7 ± 0.8c |
| S | 310 ± 0.03b | 37 ± 2.7b | 88.0 ± 0.6a | 27 ± 2.1a | 0.40 ± 0.08a | 44 ± 4.1a | |
| S+A | 366 ± 0.04ab | 45 ± 4.4a | 87.6 ± 1.1a | 20 ± 3.3b | 0.32 ± 0.04ab | 28 ± 4.8b |
Mean (± SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying Fisher’s LSD test
In experiment II, FW decreased by 19% under 100 mM NaCl stress compared to control. However, the addition of Na-acetate with salt increased the FW by 18% compared with salt stressed alone. Dry weight improved significanlty after Na-acetate application with salt under experiment II (Table 1).
Acetate improved water status under salt stress
Shoot water content (WC) decreased by 7% at 100 mM NaCl compared to control under laboratory condition. However, external application of Na-acetate along with 100 mM NaCl increased WC by 7% compared with salt treated seedlings alone. Proline content increased under salt stress condition whereas the lower amount of pro was found in Na-acetate treated plants compared to salt stressed seedlings alone (Supplementary Fig. 1b).
In experiment II, there was no significant difference regarding WC among treatments (Table 1).
Exogenous acetate lowered oxidative damage under salt stress
To understand the extent of oxidative damage in a plant cell, MDA content is determined as an indicator of lipid peroxidation. Higher MDA was observed under 100 mM NaCl stress in both experiments. Interestingly, the addition of Na-acetate along with salt significantly reduced MDA content (Table 1). Other aldehydes also showed similar trend like MDA content. Level of membrane damage is indicated by EL. Severe EL was observed in case of salt-treated plants compared to control plants. In both experimental conditions, Na-acetate reduced EL under salt stress condition (Table 1).
Effect of acetate on enzymatic and non-enzymatic antioxidants under salt stress
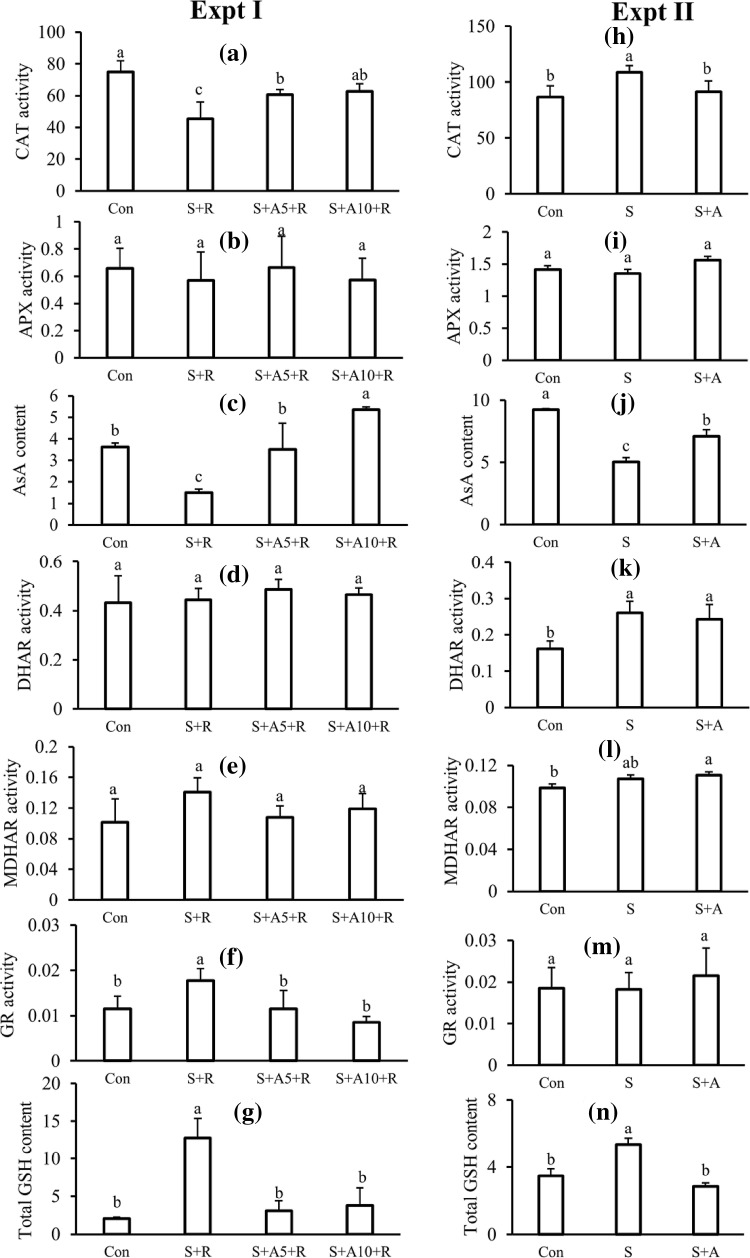
Catalase activity decreased under salt stress condition. However, acetate application increased the CAT activity under salt stress condition in experiment I (Fig. 2a). In experiment II, there was no significant change in CAT activity between salt treated and acetate with salt treated seedlings (Fig. 2h).
Fig. 2.
Response of antioxidant defense pathway with different treatments in experiment I (a–g) and experiment II (h–n). CAT activity, µmol min−1 mg−1 protein (a, h); AsA content, µmol g−1 FW (b, i); APX activity, nmol min−1 mg−1 protein (c, j); MDHAR activity, nmol min−1 mg−1 protein (d, k); DHAR activity, nmol min−1 mg−1 protein (e, l); GR activity, nmol min−1 mg−1 protein (f, m); and GSH content, µmol g−1 FW (g, n). Treatments are the same as described in Table 1. Mean (± SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying Fisher’s LSD test
In both experiments, a significant reduction in AsA content was observed in salt-treated plants compared to control. On the contrary, seedlings treated with acetate under salt stress had higher AsA content compared to salt treated seedlings alone (Fig. 2c, j).
There was a fluctuation in APX activity among treatments of two experimental conditions. However, the difference in the APX activity among treatments was not significant in experiment I (Fig. 2c, k).
Monodehydroascorbate reductase and DHAR activities between NaCl treated plants and acetate along with NaCl did not show significant difference under both experimental conditions (Fig. 2d, e, k, l).
In experiment I, glutathione reductase activity was highest at 100 mM NaCl treated seedlings. Acetate application inhibited the GR activity under 100 mM saline condition (Fig. 2f). In the case of experiment II, there was no significant change in GR activity among treatments (Fig. 2m).
Under salt stress condition, total GSH content increased (Fig. 2g) in experiment I and II. When seedlings treated with acetate under 100 mM salinity, GSH content reduced in both experiments (Fig. 2g, n).
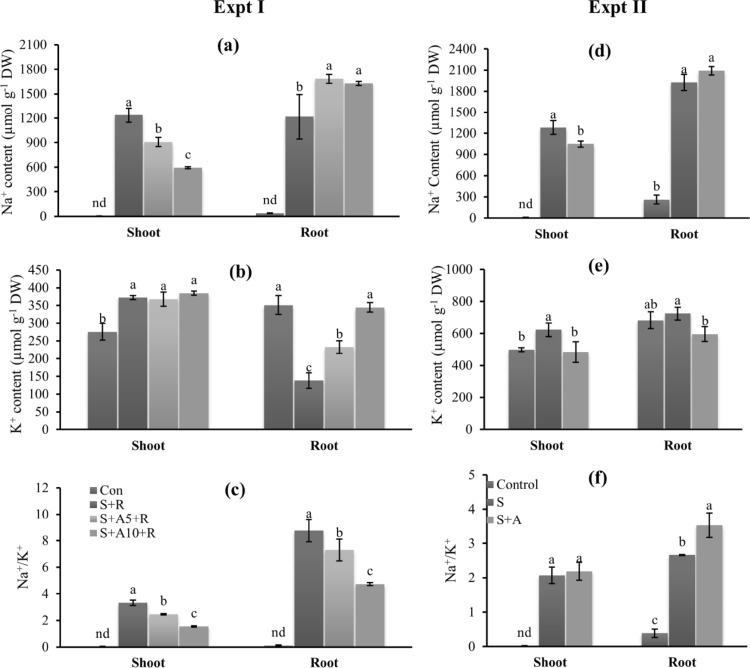
Acetate improved ion homeostasis under salt stress condition
Exogenous application of Na-acetate improved ion homeostasis under salt stress condition. Acetate treated seedlings showed lower Na+ content in the shoot but not in root under 100 mM NaCl stress compared with 100 mM salt stressed seedlings alone (Fig. 3a, d) in both experiments.
Fig. 3.
Ion homeostasis under salt stress with or without acetate in experiment I (a–c) and experiment II (d–f). Na+ content in shoot and root (a, d), K+ content in shoot and root (b, e), and Na+/K+ ratio in shoot and root (c, f). Treatments are the same as described in Table 1. Mean (± SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying Fisher’s LSD test
Root K+ significantly reduced under salt stress compared to control in experiment I. Application of Na-acetate improved the root K+ content at 100 mM NaCl (Fig. 3b). Salt stress increased the shoot K+ compared to control. Supplementation of Na-acetate along with 100 mM NaCl did not change shoot K+ content significantly compared to 100 mM NaCl treated seedlings (Fig. 3b, e).
In experiment II, shoot K+ content increased and root K+ content did not change significantly under stress condition compared to control. Acetate application reduced K+ content both in shoot and root compared to salt treated seedlings alone.
Na+/K+ ratio increased under salt stress in both root and shoot in experiment I and II (Fig. 3c, f). Acetate application reduced Na+/K+ ratio under salt stress both in root and shoot in experiment I but not in experiment II.
Calcium content reduced both in shoot and root under salt stress condition. External application of Na-acetate could not increase Ca2+ content under salt stress in experiment I (Supplementary Table 1).
A severe reduction in Mg2+ content was observed in root under salt stress. Seedling treated with 10 mM Na-acetate had higher Mg2+ content both in root and shoot under 100 mM salt stress compared to salt stress alone in experiment I (Supplementary Table 1). In experiment II, salt stress reduced the Mg2+ content in the shoot but not in root under salt stress. However, the application of Na-acetate improved the Mg2+ content in shoot under salt stress in both experiments (Supplementary Table 1).
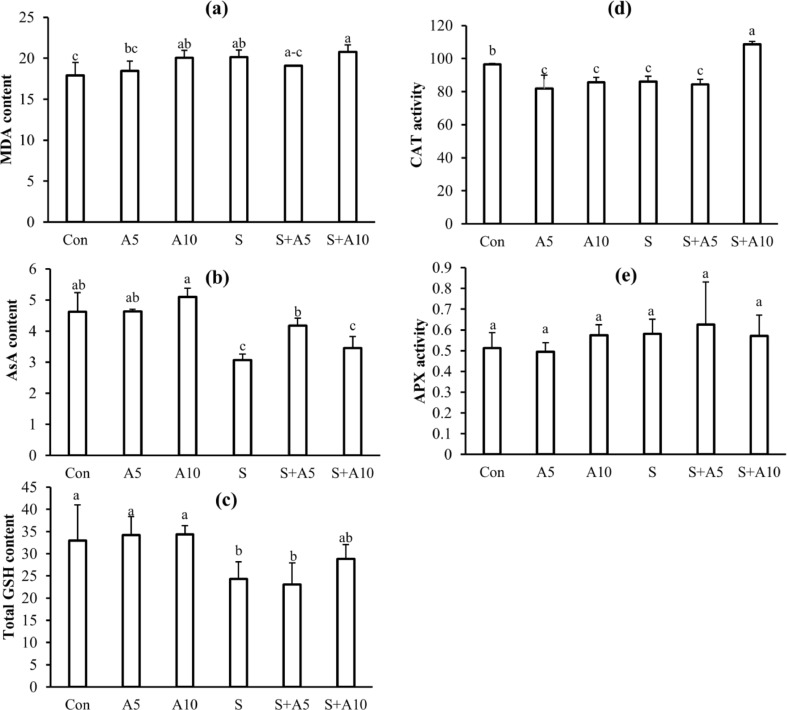
Effect of Na-acetate on MDA, AsA, and GSH content, CAT and APX activities after 2 days of salt treatments in experiment I
Malondialdehyde content was not changed significantly between salt treated, and salt plus Na-acetate treated seedlings after 2-d stress treatment (Fig. 4a).
Fig. 4.
MDA content, nmol g−1 FW (a); AsA content, µmol g−1 FW (b); GSH content, µmol g−1 FW (c); CAT activity (d); APX activity (e) after 2 days of different treatments. Treatments were Control (Con), 5 mM Na-acetate (A5), 10 mM Na-acetate (A10), 100 mM NaCl (S), 100 mM NaCl+5 mM Na-acetate (S+A5), and 100 mM NaCl+10 mM Na-acetate (S+A10). Mean (± SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying Fisher’s LSD test
Ascorbate content decreased 2 days after salt stress. However, Na-acetate increased ascorbate content under 100 mM NaCl (Fig. 4b).
After 2-day exposure to 100 mM NaCl stress, total GSH content also decreased, and acetate treatment did not change the total GSH content significantly compared to salt stressed seedlings alone (Fig. 4c).
Catalase activity decreased 2 days after salt stress. However, 10 mM Na-acetate increased CAT activity under 100 mM NaCl (Fig. 4d).
There was no significant change in APX activity among all treatments (Fig. 4e).
Correlation among the parameters
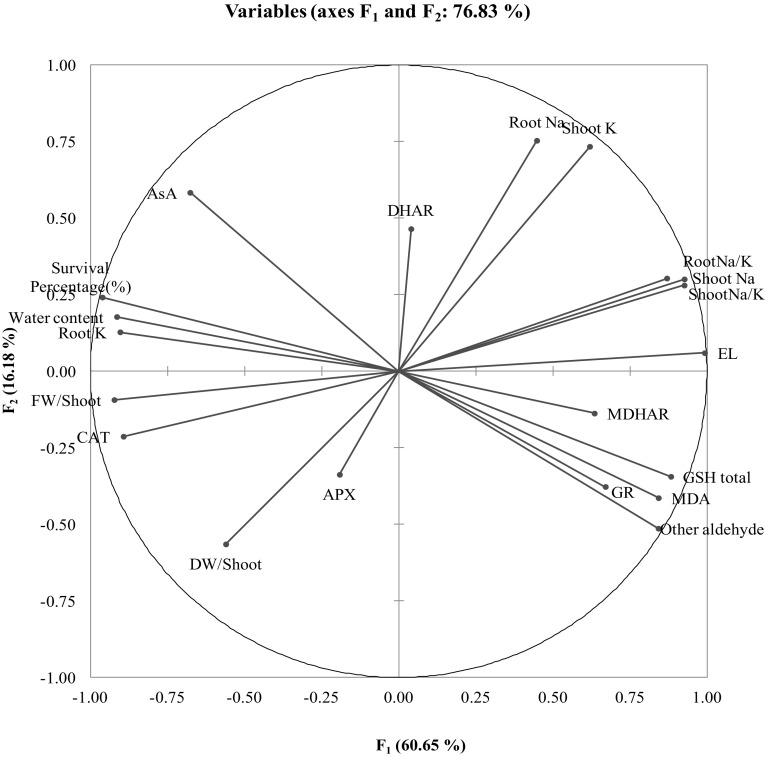
Correlation matrix shows that the antioxidant defense parameters (CAT, AsA, MDHAR, DHAR, GR, and GSH) are negatively associated with oxidative stress markers (MDA and EL), and in most cases, the correlations were significant (Table 2). The similarities among the different studied attributes are presented in Fig. 5 which shows that root and shoot ion content (K and Na) are highly affected by the treatments while APX and DHAR were not affected significantly.
Table 2.
Correlation matrix (Pearson) of different parameters observed under laboratory condition
| Variables | Survival % | FW of shoot | DW of shoot | Water content | MDA | Other aldehyde | EL | CAT | APX | AsA |
|---|---|---|---|---|---|---|---|---|---|---|
| Survival % | 1 | 0.839** | 0.398 | 0.898** | − 0.909** | − 0.937** | − 0.932** | 0.819** | 0.132 | 0.816** |
| FW/shoot | 1 | 0.715** | 0.955** | − 0.774** | − 0.754** | − 0.938** | 0.883** | 0.332 | 0.497 | |
| DW/shoot | 0.505 | − 0.205 | − 0.201 | − 0.589* | 0.725** | 0.576 | 0.079 | |||
| Water content | 1 | − 0.880** | − 0.880** | − 0.909** | 0.810** | 0.204 | 0.655* | |||
| MDA | 1 | 0.966** | 0.811** | − 0.611* | − 0.006 | − 0.722** | ||||
| Other aldehyde | 1 | 0.807** | − 0.652* | − 0.061 | − 0.834** | |||||
| EL | 1 | − 0.913** | − 0.214 | − 0.622* | ||||||
| CAT | 1 | 0.501 | 0.538 | |||||||
| APX | 1 | − 0.144 | ||||||||
| AsA | 1 | |||||||||
| MDHAR | ||||||||||
| DHAR | ||||||||||
| GR | ||||||||||
| GSH total | ||||||||||
| Shoot Na | ||||||||||
| Root Na | ||||||||||
| Shoot K | ||||||||||
| Root K | ||||||||||
| Shoot Na/K | ||||||||||
| Root Na/K |
| Variables | MDHAR | DHAR | GR | GSH total | Shoot Na | Root Na | Shoot K | Root K | Shoot Na/K | Root Na/K |
|---|---|---|---|---|---|---|---|---|---|---|
| Survival % | − 0.590* | 0.078 | − 0.745** | − 0.914** | − 0.830** | − 0.251 | − 0.405 | 0.926** | − 0.835** | − 0.778** |
| FW/shoot | − 0.595* | − 0.061 | − 0.533 | − 0.810** | − 0.814** | − 0.379 | − 0.643* | 0.723** | − 0.805** | − 0.707* |
| DW/shoot | − 0.258 | − 0.298 | − 0.148 | − 0.268 | − 0.605* | − 0.454 | − 0.665* | 0.383 | − 0.588* | − 0.489 |
| Water content | − 0.598* | 0.046 | − 0.624* | − 0.888** | − 0.743** | − 0.189 | − 0.441 | 0.772** | − 0.739** | − 0.636* |
| MDA | 0.540 | − 0.160 | 0.690* | 0.962** | 0.629* | 0.115 | 0.272 | − 0.729** | 0.634* | 0.593* |
| Other aldehyde | 0.571 | − 0.211 | 0.765** | 0.939** | 0.602* | − 0.025 | 0.164 | − 0.779** | 0.607* | 0.544 |
| EL | 0.656* | 0.026 | 0.622* | 0.860** | 0.923** | 0.487 | 0.678* | − 0.864** | 0.921** | 0.870** |
| CAT | − 0.410 | 0.000 | − 0.490 | − 0.635* | − 0.870** | − 0.436 | − 0.681* | 0.798** | − 0.861** | − 0.772** |
| APX | 0.260 | 0.020 | − 0.228 | 0.052 | − 0.202 | − 0.004 | − 0.318 | 0.087 | − 0.162 | − 0.024 |
| AsA | − 0.429 | 0.173 | − 0.621* | − 0.707* | − 0.498 | 0.152 | 0.086 | 0.809** | − 0.523 | − 0.470 |
| MDHAR | 1 | 0.144 | 0.513 | 0.675* | 0.495 | 0.244 | 0.352 | − 0.466 | 0.501 | 0.511 |
| DHAR | 1 | 0.152 | − 0.174 | 0.177 | 0.282 | 0.312 | 0.049 | 0.166 | 0.126 | |
| GR | 1 | 0.661* | 0.516 | − 0.093 | 0.129 | − 0.657* | 0.506 | 0.414 | ||
| GSH total | 1 | 0.693* | 0.220 | 0.345 | − 0.767** | 0.700* | 0.673* | |||
| Shoot Na | 1 | 0.695* | 0.779** | − 0.881** | 0.999** | 0.968** | ||||
| Root Na | 1 | 0.894** | − 0.334 | 0.692* | 0.779** | |||||
| Shoot K | 1 | − 0.396 | 0.761** | 0.773** | ||||||
| Root K | 1 | − 0.892** | − 0.841** | |||||||
| Shoot Na/K | 1 | 0.975** | ||||||||
| Root Na/K | 1 |
**Significant at P ≤ 0.01; *Significant at P ≤ 0.05
Fig. 5.
Principal component analysis (PCA) of different studied attributes. Loading plot; APX–Ascorbate peroxidase; AsA–Ascorbate; CAT–Catalase; DHAR–Dehydroascorbate reductase; DW–Dry weight; EL–Electrolyte leakage; FW–Fresh weight; GR–Glutathione reductase; GSH–Reduced glutathione; MDA–Malondialdehyde; MDHAR–Monodehydroascorbate reductase
Discussion
At first, to know whether acetate was responsible for salt tolerance in lentil, we examined recovery percentage of seedling treated with salt with or without different concentration of acetate. Surprisingly, we observed almost all seedlings treated with 10 mM Na-acetate were able to survive after 6-days of recovery from 100 mM NaCl stress, whereas around 80% of the total seedlings without acetate treatment failed to recover from 100 mM NaCl stress (Fig. 1a, b). This result indicates that 10 mM Na-acetate enhances salt tolerance in lentil seedlings. This is the first report to show acetate-mediated salt tolerance in plants. Previously, Kim et al. (2017) reported acetate-induced drought tolerance where 75% Arabidopsis seedlings that were treated with 10 and 20 mM acetic acid survived after prolonged drought stress whereas almost all untreated seedlings failed to survive after drought stress. Salinity stress creates osmotic stress along with ionic toxicity and oxidative stress. Hossain et al. (2017) showed that damages in lentil due to salt stress is far more severe than iso-osmotic stress because of a drastic reduction in AsA content and K+ in the shoot. Shalata and Neumann (2001) suggested that successful recovery of seedlings from salt stress is related to the antioxidant activity of AsA, and not related to the osmotic component of salt stress. Therefore, we investigated whether acetate enhances salt tolerance in lentil. From the result, we assume that acetate play a role in ion homeostasis and maintaining antioxidant activity in seedlings under salt stress, which allowed the seedlings to recover from salt stress.
Generally, osmotic stress induced by salt stress immediately causes growth reduction, because the presence of salt in the growing media reduce the osmotic potential of water (Munns and Tester 2008). To know whether acetate play a role in seedlings growth by maintaining water status in the seedlings, we measured fresh weight, dry weight, chl content, water content, and pro content. Salt stress reduced the growth and chlorophyll pigments, and water content, and increased pro content in lentil shoot (Table 1; Supplementary Fig. 1a, b). Salinity-induced growth reduction, chl degradation, and osmotic stress is reported in rice (Nounjan et al. 2012); lentil (Singh et al. 2017). However, acetate application improved the FW, DW, chl content and water content, indicating the involvement of acetate in maintaining water balance and plant growth. Involvement of acetate in enhancing plant growth has been reported by Kim et al. (2017) where they found 10 mM acetic acid increases plant FW significantly compared to control. They also reported that acetate pretreated plants have higher water content under drought stress compared with drought-stressed plants alone. Other organic acids, for example, citric acid improves growth under salinity stress in Leymus chinensis (Sun and Hong 2011). These results are in line with our results, suggesting that acetate could maintain water status under salt stress condition.
Under abiotic stresses including salt stress, higher ROS are produced, resulting in oxidative stress (Mittler 2002, 2017). The level of damages caused by ROS can be understood by measuring MDA, other aldehyde content, and EL indicating the extent of membrane damage. Salt stressed plants had higher MDA, other aldehyde and EL, which means that higher oxidative and membrane damages happened to stressed plants (Table 1). Salinity-induced oxidative damage in lentil was also observed by Bandeoğlu et al. (2004), Singh et al. (2017) and Hossain et al. (2017). However, acetate-treated seedlings suffered much less from oxidative damage, and experienced fewer membrane damages compared with NaCl stressed seedlings alone (Table 1).
Then, we asked why acetate-treated seedlings had lower oxidative damage under salt stress. To answer this question, we investigated the antioxidant defense pathway, which controls the ROS below an extent to which ROS cannot cause oxidative damage and rather play a signaling role. In the antioxidant pathway, AsA content decreased, and total GSH content increased under salt stress in both experimental conditions. However, acetate treatment increased AsA under 100 mM NaCl condition, indicating the involvement of acetate in maintaining non-enzymatic antioxidant level under stress condition (Fig. 2). Maintaining AsA level is related to salt tolerance in lentil (Hossain et al. 2017), and exogenous application of AsA enhances salt tolerance in tomato (Shalata and Neumann 2001). However, acetate application could not upregulate the full set of antioxidant enzymes in both experiments (Fig. 2). For efficient detoxification of ROS, upregulation of the full set of the antioxidant components in the pathway may not happen all the time (Abogadallah 2010). For instance, Noreen and Ashraf (2009) found that among different enzymes, only CAT is a reliable marker for salt tolerance in pea (Pisum sativum). Hossain et al. (2017) reported that of the components antioxidant defense system, only AsA content and CAT activity is related to salt tolerance in lentil.
We then checked the MDA, AsA, and GSH content, and CAT and APX activity after two days exposure to 100 mM salt stress (Fig. 4). Although we found a slight change in MDA content among treatments, this change is very close to control. However, AsA content, GSH content and CAT activity were reduced slightly under salt stress compared to control. The results indicate that seedlings treated with salt for two days did not suffer from oxidative stress, but their antioxidant balance started to disrupt. When we allowed the salt-stressed seedlings to recover from this point and measured this parameter after 2-day recovery, we found further decrease in AsA content and CAT activity (Fig. 2a, c), and dramatic increase in MDA and GSH (Table 1; Fig. 2g). These results indicate that salt stressed seedlings had lost their antioxidant balance, and as a result, severe oxidative damage occurred. Therefore, we can assume that oxidative stress is not a sudden phenomenon. At the beginning of salt stress, plants use AsA to scavenge excess amount of ROS. With time, salt-treated seedlings lost their capability to maintain redox balance, whereas acetate treated plants maintained redox balance under salinity stress.
The detrimental effect of Na+ appears with time when Na+ build up to a toxic level in leaves (Munns and Tester 2008). The higher amount of Na+ in the cytosol leads to lowering K+ content in the cytosol. As a result, plant metabolic functions are inhibited because K+ is required for activation of many key enzymes. Thus, maintaining lower Na+/K+ ratio is important for plant metabolism under salt stress (Shabala and Pottosin 2014). We then checked the different ions content under salt stress with or without acetate application. Acetate-treated seedlings had lower Na+ in shoot, and lower Na+/K+ ratio in hydroponically grown seedling under stress condition (Table 2) But, this was not true for experiment II where Na-acetate treated seedlings accumulated higher Na+, this is simply because we applied 10 mM Na-acetate along with salt treatments which added extra 10 mM Na+ to the growth media for a long time. Our findings are in line with Hossain et al. (2017) where they found severe K+ and Mg2+ loss from the root, and higher Na+/K+ ratio in shoot and root in hydroponically grown lentil seedling under salt stress. However, Na-acetate maintained ion homeostasis in experiment I for 100 mM NaCl stress condition and lowered the shoot Na+ content under salt stress in experiment II. These results suggest that acetate can maintain ion homeostasis under salt stress condition. Previously, Sako et al. (2015) reported that increased histone H4 acetylation by using Ky-2, a histone deacetylase inhibitor, lowers the Na+ accumulation in Arabidopsis seedlings by enhancing SOS1-dependent Na+ efflux. Application of acetate also increases histone acetylation (Kim et al. 2017), therefore, we can assume that acetate-mediated histone acetylation is responsible for lower accumulation of Na+ in lentil shoot in both experiments. Lower translocation of Na+ from root to shoot is associated with salt tolerance (Maathuis 2013; Assaha et al. 2017). Therefore, it is worthy to investigate acetate-mediated root to shoot transport of Na+.
We assessed the potentiality of acetate in mitigating the damages induced by salt stress. Under different experimental conditions, the effect of acetate was evaluated under 100 mM salt stress. Results provide the evidence that exogenous application of acetate protects the seedlings under salt stress by maintaining ascorbate level and reducing Na+ uptake or reducing Na+ accumulation in the shoot. However, further investigation is required to reveal the molecular mechanism of acetate-induced tolerance of salt stress: whether acetate could enhance ascorbate synthesis, how acetate could reduce Na+ uptake and how acetate could inhibit Na+ transport from root to shoot.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Chlorophyll content (a) and Proline content (b) under salt stress with or without acetate in experiment I Treatments are the same as described in Fig. 1. Mean (± SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P≤ 0.05 applying Fisher’s LSD test (TIFF 155 kb)
Acknowledgements
This research was funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We thank Mr. Sayed Mohammad Mohsin and Khursheda Parvin, Faculty of Agriculture, Kagawa University, Japan for a critical review and editing the English of the manuscript. We also thank Dr. Md. Motiar Rohman, Bangladesh Agricultural Research Institute, Gazipur, Bangladesh for providing lentil seeds.
Author contributions
MSH conceived, designed, and performed the experiment and prepared the manuscript. MMHS, and MHMBB actively participated in executing the experiment. MH designed the experiment, analyzed the data and edited the manuscript. MF conceived, designed, and monitored the experiment. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Abogadallah GM. Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav. 2010;5:369–374. doi: 10.4161/psb.5.4.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal F, Khan T, Khan A, Khan S, Raza H, Ihsan A, Ahanger MA, Kazi AG. Nutrient deficiencies under stress in legumes. In: Azooz MM, Ahmad P, editors. Legumes under environmental stress: yield, improvement and adaptations. 1. West Sussex: Wiley; 2014. pp. 53–65. [Google Scholar]
- Arnon DT. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Assaha DV, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol. 2017;8:509. doi: 10.3389/fphys.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeoğlu E, Eyidoğan F, Yücel M, Öktem HA. Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul. 2004;42:69–77. doi: 10.1023/B:GROW.0000014891.35427.7b. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teari D. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245:85–96. doi: 10.1007/s00709-010-0144-6. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Golldack D, Li C, Mohan H, Probst N. Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci. 2014;5:151. doi: 10.3389/fpls.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, Chen BO, Li ZG, Guo LH. Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J Plant Physiol. 2001;158:1125–1130. doi: 10.1078/0176-1617-00327. [DOI] [Google Scholar]
- Griffiths OW. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci. 2016;7:1787. doi: 10.3389/fpls.2016.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Fujita M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmed P, Azooz MM, Prasad MNV, editors. Ecophysiology and responses of plants under salt stress. New York: Springer; 2013. pp. 25–87. [Google Scholar]
- Heath RL, Packer L. Photo peroxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hiscox JT, Israelstam G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Hossain MS, Alam MU, Rahman A, Hasanuzzaman M, Nahar K, Al Mahmud J, Fujita M. Use of iso-osmotic solution to understand salt stress responses in lentil (Lens culinaris Medik.) South Afr J Bot. 2017;113:346–354. doi: 10.1016/j.sajb.2017.09.007. [DOI] [Google Scholar]
- Ismail A, Riemann M, Nick P. The jasmonate pathway mediates salt tolerance in grapevines. J Exp Bot. 2012;63(5):2127–2139. doi: 10.1093/jxb/err426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AM, Horie T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu Rev Plant Biol. 2017;68:405–434. doi: 10.1146/annurev-arplant-042916-040936. [DOI] [PubMed] [Google Scholar]
- Kibria MG, Hossain M, Murata Y, Hoque MA. Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci. 2017;24:155–162. doi: 10.1016/j.rsci.2017.05.001. [DOI] [Google Scholar]
- Kim JM, To TK, Matsui A, Tanoi K, Kobayashi NI, Matsuda F, Bashir K. Acetate-mediated novel survival strategy against drought in plants. Nat Plants. 2017;3:17097. doi: 10.1038/nplants.2017.97. [DOI] [PubMed] [Google Scholar]
- Maathuis FJ. Sodium in plants: perception, signalling, and regulation of sodium fluxes. J Expt Bot. 2013;65:849–858. doi: 10.1093/jxb/ert326. [DOI] [PubMed] [Google Scholar]
- Mauch-Mani B, Baccelli I, Luna E, Flors V. Defense priming: an adaptive part of induced resistance. Annual Rev Plant Biol. 2017;68:485–512. doi: 10.1146/annurev-arplant-042916-041132. [DOI] [PubMed] [Google Scholar]
- Misra N, Saxena P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009;177:181–189. doi: 10.1016/j.plantsci.2009.05.007. [DOI] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R. ROS are good. Trends in Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanism of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Rahman A, Alam MM, Mahmud JA, Suzuki T, Fujita M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci. 2016;7:1104. doi: 10.3389/fpls.2016.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH. Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ. 2016;39:1140–1160. doi: 10.1111/pce.12726. [DOI] [PubMed] [Google Scholar]
- Noreen Z, Ashraf M. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol. 2009;166:1764–1774. doi: 10.1016/j.jplph.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Nounjan N, Nghia PT, Theerakulpisut P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J Plant Physiol. 2012;169:596–604. doi: 10.1016/j.jplph.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V. Primed plants do not forget. Environ Exp Bot. 2013;94:46–56. doi: 10.1016/j.envexpbot.2012.02.013. [DOI] [Google Scholar]
- Rahman A, Hossain MS, Mahmud JA, Nahar K, Hasanuzzaman M, Fujita M. Manganese-induced salt stress tolerance in rice seedlings: regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol Mol Biol Plants. 2016;22:291–306. doi: 10.1007/s12298-016-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Nahar K, Al Mahmud J, Hasanuzzaman M, Hossain MS, Fujita M. Salt stress tolerance in rice: emerging role of exogenous phytoprotectants. In: Li JQ, editor. Advances in international rice research. Rijeka: InTech; 2017. pp. 139–174. [Google Scholar]
- Richardson AD, Duigan SP, Berlyn GP. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002;153:185–194. doi: 10.1046/j.0028-646X.2001.00289.x. [DOI] [Google Scholar]
- Sako K, Kim JM, Matsui A, Nakamura K, Tanaka M, Kobayashi M, Yoshida M. Ky-2, a histone deacetylase inhibitor, enhances high-salinity stress tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2015;57:776–783. doi: 10.1093/pcp/pcv199. [DOI] [PubMed] [Google Scholar]
- Savvides A, Ali S, Tester M, Fotopoulos V. Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci. 2016;21:329–340. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Seckin B, Turkan I, Sekmen AH, Ozfidan C. The role of antioxidant defense systems at differential salt tolerance of Hordeum marinum Huds. (sea barleygrass) and Hordeum vulgare L. (cultivated barley) Environ Exp Bot. 2010;69:76–85. doi: 10.1016/j.envexpbot.2010.02.013. [DOI] [Google Scholar]
- Shabala S, Pottosin I. Regulation of potassium transport in plants under hostile conditions: implication for abiotic and biotic stress tolerance. Physiol Plant. 2014;151:257–279. doi: 10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- Shalata A, Neumann PM. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot. 2001;52:2207–2211. doi: 10.1093/jexbot/52.364.2207. [DOI] [PubMed] [Google Scholar]
- Sidari M, Santonoceto C, Anastasi U, Preiti G, Muscolo A. Variations in four genotypes of lentil under NaCl-salinity stress. Am J Agric Biol Sci. 2008;3(1):410–416. doi: 10.3844/ajabssp.2008.410.416. [DOI] [Google Scholar]
- Singh D, Singh CK, Kumari S, Tomar RSS, Karwa S, Singh R, Pal M. Discerning morpho-anatomical, physiological and molecular multiformity in cultivated and wild genotypes of lentil with reconciliation to salinity stress. PLoS ONE. 2017;12:e0177465. doi: 10.1371/journal.pone.0177465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YL, Hong SK. Effects of citric acid as an important component of the responses to saline and alkaline stress in the halophyte Leymus chinensis (Trin.) Plant Growth Regul. 2011;64:129–139. doi: 10.1007/s10725-010-9547-9. [DOI] [Google Scholar]
- Tester M, Davenport RJ. Na+ transport and Na+ tolerance in higher plants. Ann Bot. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:165–183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chlorophyll content (a) and Proline content (b) under salt stress with or without acetate in experiment I Treatments are the same as described in Fig. 1. Mean (± SD) were calculated from three replicates for each treatment. Values with different letters are significantly different at P≤ 0.05 applying Fisher’s LSD test (TIFF 155 kb)