Summary
Ferulate 5‐hydroxylase (F5H) catalyses the hydroxylation of coniferyl alcohol and coniferaldehyde for the biosynthesis of syringyl (S) lignin in angiosperms. However, the coordinated effects of F5H with caffeic acid O‐methyltransferase (COMT) on the metabolic flux towards S units are largely unknown. We concomitantly regulated F5H expression in COMT‐down‐regulated transgenic switchgrass (Panicum virgatum L.) lines and studied the coordination of F5H and COMT in lignin biosynthesis. Down‐regulation of F5H in COMT‐RNAi transgenic switchgrass plants further impeded S lignin biosynthesis and, consequently, increased guaiacyl (G) units and reduced 5‐OH G units. Conversely, overexpression of F5H in COMT‐RNAi transgenic plants reduced G units and increased 5‐OH units, whereas the deficiency of S lignin biosynthesis was partially compensated or fully restored, depending on the extent of COMT down‐regulation in switchgrass. Moreover, simultaneous regulation of F5H and COMT expression had different effects on cell wall digestibility of switchgrass without biomass loss. Our results indicate that up‐regulation and down‐regulation of F5H expression, respectively, have antagonistic and synergistic effects on the reduction in S lignin resulting from COMT suppression. The coordinated effects between lignin genes should be taken into account in future studies aimed at cell wall bioengineering.
Keywords: caffeic acid O‐methyltransferase, coordinated effects, ferulate 5‐hydroxylase, lignin biosynthesis, Panicum virgatum L., switchgrass
Introduction
Lignin, which mainly deposits in secondary cell wall of vascular plants, exists as a complicated phenolic heteropolymer cross‐linking with cell wall polysaccharides to form a complex matrix. Lignin is required for structural support, water transport and plant defence in plant growth and development (Boerjan et al., 2003). p‐Coumaryl, coniferyl and sinapyl alcohols are the major precursors of lignin synthesized through the hydroxylation and methylation of derivatives from the phenylpropanoid pathway. The lignin units derived from the three monolignols are known as p‐hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units (Boerjan et al., 2003). Their proportions vary with plant species and tissue types (Chapple et al., 1992).
Over recent decades, a combinatorial approach of forward and reverse genetics has been used to investigate the lignin biosynthetic pathway widely (Bonawitz and Chapple, 2010; Fu et al., 2011a; Humphreys and Chapple, 2002; Lewis and Yamamoto, 1990; Vanholme et al., 2013). According to the currently accepted model of lignin biosynthesis, caffeyl CoA O‐methyltransferase is responsible for methylating the 3‐hydroxyl group of lignin intermediates leading to G lignin production, whereas caffeic acid O‐methyltransferase (COMT) is involved in 5‐O‐methylation leading to S lignin production. Previous studies have suggested that COMT mainly participates in the conversion of 5‐OH coniferaldehyde/5‐OH coniferyl alcohol to sinapaldehyde/sinapyl alcohol. Disruption of COMT can result in a severe reduction in S units accompanying with consequent incorporation of unusual 5‐OH G units in numerous plant species (Goujon et al., 2003; Palmer et al., 2008; Ralph et al., 2001; Rastogi and Dwivedi, 2008; Vignols et al., 1995). The accumulation of S units, however, can be affected by other lignin biosynthetic enzymes. For example ferulate 5‐hydroxylase (F5H, CYP84A1) is a cytochrome P450‐dependent monooxygenase that hydroxylates coniferaldehyde and coniferyl alcohol into 5‐OH coniferaldehyde and 5‐OH coniferyl alcohol for the subsequent formation of S lignins (Figure 1). Therefore F5H coupled with COMT can divert coniferaldehyde and coniferyl alcohol towards S lignin precursors and thereby alter both G‐ and S‐units deposition (Humphreys et al., 1999). The knockout of F5H in Arabidopsis produces a fah1 mutant comprising almost entirely of G unit and barely any S units, whereas overexpression of F5H results in low G and high S units (Chapple et al., 1992; Meyer et al., 1998). As expected, up‐regulation or down‐regulation of F5H in poplar, tobacco and alfalfa can lead to lignins consisting of significantly altered S/G ratios (Franke et al., 2000; Reddy et al., 2005). In contrast, the function of F5H has yet to be investigated in monocots.
Figure 1.
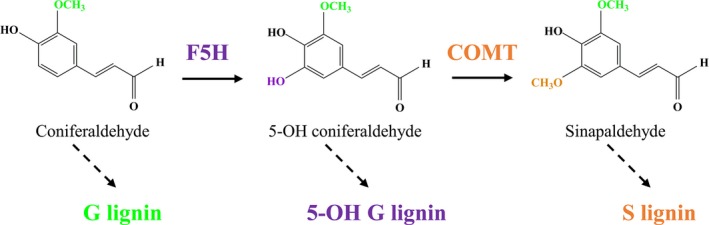
Schematic of locations of ferulate 5‐hydroxylase (F5H) and caffeic acid O‐methyltransferase (COMT) in the lignin biosynthetic pathway.
Lignin engineering is currently focusing on incorporation of atypical lignin monomers in plant cell walls for improving the digestibility of lignocellulosic biomass (Lee et al., 2017). It has been indicated that down‐regulation of F5H in Arabidopsis cinnamyl alcohol dehydrogenase (CAD) double mutant (cadc and cadd) can produce lignins derived exclusively from polymerization of coniferaldehyde (Anderson et al., 2015). The alteration of lignin composition of a fah1 cadc cadd triple mutant, however, has no negative effects on plant growth and development, whereas the lignin polymers enrich in coniferaldehyde units and the cell wall digestibility is substantially increase. In contrast, overexpression of F5H in the CAD‐deficient Arabidopsis mutant causes plant dwarfism, and the cell walls of C4H:F5H cadc cadd plants enrich sinapaldehyde units (Anderson et al., 2015). In addition, overexpression of F5H in COMT‐deficient Arabidopsis mutant, omt1, can result in lignin polymers with dramatically reduced amounts of G and S units as well as substantially increased 5‐OH G units (Vanholme et al., 2010; Weng et al., 2010). Unfortunately, overexpression of F5H in this mutant affects plant development severely, which is consistent with the previous observation in C4H:F5H cadc cadd Arabidopsis plants. Unlike the omt1 null mutant, COMT transcripts are not entirely suppressed in COMT‐antisense or ‐RNAi transgenic plants. It remains unclear if a large percentage of changes in G and S units without biomass loss can be achieved in COMT‐down‐regulated transgenic plants via concomitant regulation of F5H expression. Overall, these results suggest that simultaneous regulation of lignin biosynthetic genes can lead to lignin polymers with diverse composition and, therefore change cell wall digestibility and plant growth.
Here, we characterized the function of F5H in switchgrass (Panicum virgatum L.), a dual‐purpose forage and biofuel crop, and found that F5H was a crucial factor that affected both G and S lignin biosynthesis. Simultaneous down‐regulation of F5H and COMT synergistically reduced S lignin biosynthesis in switchgrass, whereas overexpression of F5H in the severely COMT‐suppressing background partially compensated for the loss of S lignin. Furthermore, overexpression of F5H in the moderately COMT‐suppressing background was able to fully restored S lignin biosynthesis of switchgrass. Moreover, the transgenic switchgrass lines with diverse lignin composition and elevated saccharification efficiency of cell walls may be valuable for different purposes of cell wall bioengineering in the future.
Results
Identification and isolation of switchgrass F5H sequences
To gain insight into the F5H functions in switchgrass, we first identified F5H sequences from switchgrass. The assembled switchgrass genome (P. virgatum v4.1, Phytozome) contains a pair of F5H genes (PvF5H1a and 1b) located on chromosome 2 and share over 95% sequence identities between genes (Figure S1). The sequences of the three cytochrome P450 monooxygenases in the lignin biosynthetic pathway, F5H, cinnamate 4‐hydroxylase (C4H) and p‐coumaroyl shikimate 3′‐hydroxylase (C3H), were downloaded from eight genome‐sequenced species (switchgrass, maize, sorghum, rice, Brachypodium distachyon, Arabidopsis thaliana, Medicago truncatula and Populus trichocarpa) for the analysis of phylogenetic relationships. The phylogenetic tree showed that PvF5H1a and 1b clustered together in a group containing the typical functional F5Hs (Figure 2a). Moreover, the collinearity analysis of F5H orthologs in genome of switchgrass, maize and rice also revealed a close relationship in gene evolution and functions as well (Figure 2b). Therefore, we isolated the full‐length cDNA sequences of PvF5H1a from switchgrass for further functional investigation. Sequence alignment showed that the open reading frame of PvF5H1a shared 99% sequence identity with a previously isolated switchgrass F5H (NCBI accession No. AB608019) (Figure S1). Publicly available switchgrass gene expression atlas data revealed that PvF5H had relative high signal intensity in well‐lignified tissues and organs (Figure S2). Moreover, a high positive correlation was found between the expression pattern of PvF5H and PvCOMT (Figure 2c).
Figure 2.
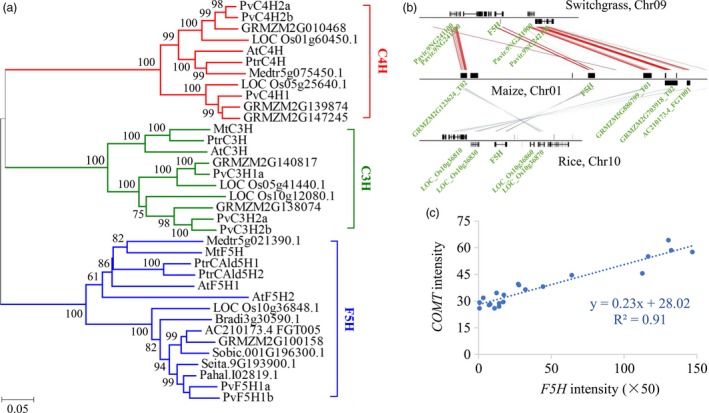
Molecular characterization of PvF5H. (a) Phylogenetic analysis of the three P450s (C4H, C3H and F5H) in the lignin biosynthetic pathway. A maximum likelihood tree was constructed in PhyML version 3.0 on the basis of multiple alignments of the deduced protein sequences from switchgrass, maize, sorghum, rice, Brachypodium distachyon, Arabidopsis thaliana, Medicago truncatula and Populus trichocarpa. Sequence data from this article can be found in Phytozome and/or Genbank under the following accession numbers: switchgrass Pavir.9NG241700.1 (PvF5H1a), Pavir.9KG138400.1 (PvF5H1b), Pavir.Fb01856.1 (PvC4H1), Pavir.5KG602000.1 (PvC4H2a), Pavir.5NG607400.1 (PvC4H2b), Pavir.3KG265800.1 (PvC3H1a), Pavir.5KG602000.1 (PvC3H2a), Pavir.5NG607400.1 (PvC3H2b); maize AC210173.4_FGT005 (F5H), GRMZM2G100158 (F5H), GRMZM2G139874 (C4H), GRMZM2G147245 (C4H), GRMZM2G010468 (C4H), GRMZM2G140817 (C3H), GRMZM2G138074 (C3H); sorghum Sobic.001G196300.1 (F5H); rice LOC_Os10g36848.1 (F5H), LOC_Os05g25640.1 (C4H), LOC_Os01g60450.1 (C4H), LOC_Os05g41440.1 (C3H), LOC_Os10g12080.1 (C3H); B. distachyon Bradi3g30590.1 (F5H); A. thaliana At4g36220 (AtF5H1), At5g04330 (AtF5H2), AT2G30490 (AtC4H), AT2G40890 (AtC3H); M. truncatula Medtr8g076290.1 (MtF5H), ABC59086.1 (MtC3H); and P. trichocarpa Potri.005G117500.1 (PtrCald5H1), Potri.007G016400.1(PtrCald5H2), Potri.013G157900.1 (PtrC4H), Potri.006G033300.1 (PtC3H). (b) Collinear relationships of F5H orthologs in genomes of switchgrass, maize and rice. A chromosomal region of PvF5H1a including 40‐kb flanking sequences were aligned with the corresponding orthologous sequences in maize (100 kb) and rice (40 kb). (c) Correlations between expression levels of F5H and COMT in different tissues and organs of switchgrass. The representative probesets of F5H (AP13ITG56842_at) and COMT (KanlowCTG00989_s_at) were retrieved from the switchgrass gene expression atlas. COMT, caffeic acid O‐methyltransferase.
Effects of F5H regulation on lignin biosynthesis
To examine the effects of F5H down‐regulation on the biosynthesis of G and S units in switchgrass, we first produced F5H‐suppressing transgenic plants with the single genotypic embryogenic callus line to exclude the potential influence of the genetic background of switchgrass on lignin biosynthesis (Figure 3a). The 485‐bp fragment of the PvF5H1a gene that can target both PvF5H1a and PvF5H1b was employed to make a hairpin structure for post‐translational gene silencing. The control plants were produced with the pANIC8D empty vector which was used as the backbone for constructing F5H‐RNAi vector. Lignin composition analysis revealed a 12.9%–35.4% reduction in S units as well as a 4.2%–22.5% increase in G units in F5HRi transgenic lines compared with those of the control plants (Figure 3b). Moreover, 5‐OH G units were also reduced in the above transgenic lines (Figure 3c).
Figure 3.
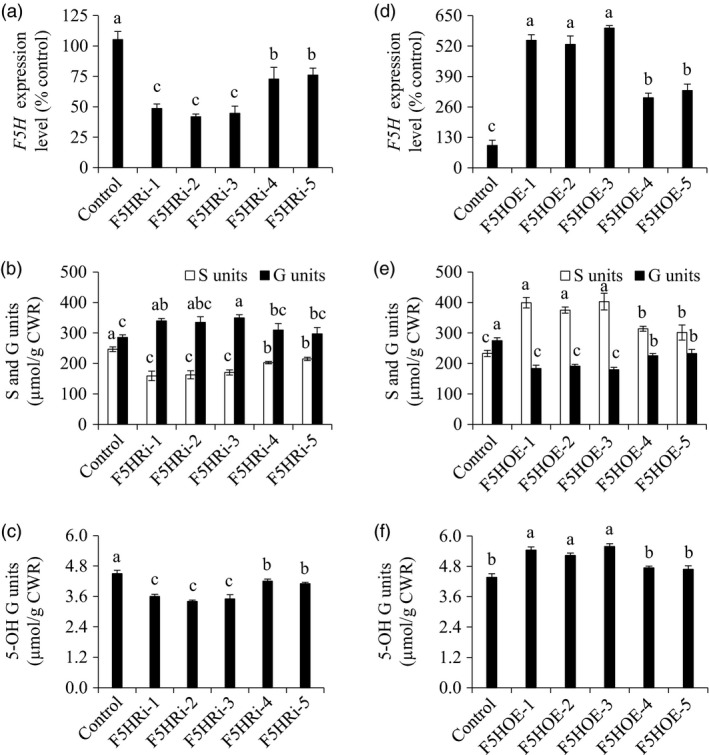
Characterization of F5HRi and F5HOE transgenic switchgrass plants. (a) Quantitative real‐time PCR analysis of F5H transcript abundances in the F5HRi transgenic lines. (b) S and G lignin monomer yield in the F5HRi transgenic lines. (c) 5‐OH G lignin monomer yield in the F5HRi transgenic lines. (d) Quantitative real‐time PCR analysis of F5H transcript abundance in the F5HOE transgenic lines. (e) S and G lignin monomer yield in the F5HOE transgenic lines. (f) 5‐OH G lignin monomer yield in the F5HOE transgenic lines. The control plants for TF5HRi and TF5HOE transgenic lines were generated with pANIC8D and pANIC6D empty vectors respectively. Stems at the R1 stage were collected. Switchgrass UBQ was used as the reference for normalization. CWR, cell wall residue. Values are mean ± SE (n = 3). Means with the different letter are significantly different (One‐way ANOVA, Duncan's test, P < 0.05).
To examine the effects of F5H up‐regulation on the biosynthesis of G and S units in switchgrass, we produced F5H‐overexpressing transgenic plants (Figure 3d). The control plants were produced with pANIC6D empty vector which was used as the backbone for constructing the F5H‐OE vector. Our results revealed a 17.4%–22.5% increase in S units as well as a 15.3%–34.7% reduction in G units in F5HOE transgenic switchgrass lines compared with those of the control plants (Figure 3e). As a consequence of F5H up‐regulation, 5‐OH G units increased in the above transgenic lines (Figure 3f).
Effects of COMT down‐regulation on F5H expression levels
The methylation of phenylpropanoid meta‐hydroxyl at the 5‐position is catalysed by COMT which is a well‐characterized key enzyme for the biosynthesis of S lignin in angiosperms. Two COMT isoforms, PvCOMT1 and 2, are found on chromosomes 2 and 6 of switchgrass respectively. Sequence alignment revealed that the PvCOMT1 protein previously isolated from switchgrass (Fu et al., 2011b) shares 84% identity with PvCOMT2. Gene Atlas analysis showed that PvCOMT1 had approximately 200‐fold higher signal intensity in different tissues than PvCOMT2 (Figure S3). Therefore, the 558‐bp fragment of the PvCOMT1 gene was employed to make a hairpin structure. To evaluate whether autonomous crosstalk occurs between COMT and F5H in the COMT‐suppressing background, we produced the COMT‐RNAi transgenic switchgrass lines to examine the expression levels of both COMT and F5H. The control plants were produced with the pANIC8B empty vector. A total of seven independent transgenic lines were subjected to analysis of F5H expression levels. Variation in F5H transcript abundances were observed among these COMT down‐regulated transgenic switchgrass lines (Figure S4).
Simultaneous down‐regulation of F5H and COMT synergistically reduced S lignin biosynthesis
Given the location of F5H in the network of the lignin biosynthetic pathway (Figure 1), we suspected that F5H‐down‐regulation may have a synergistic effect on S lignin reduction in the COMT‐suppressing background. To test our hypothesis, we re‐transformed the F5H‐RNAi cassette into a COMTRi1 transgenic line in which COMT expression was severely suppressed, and generated the double‐transformed switchgrass plants containing both COMT‐RNAi and F5H‐RNAi cassettes. Six independent double transformants were generated, and two of the six transgenic lines showed varying COMT expression levels compared with that of COMTRi1 (Table S1). Three double‐transformed switchgrass lines with comparable COMT expression level to that of COMTRi1 plants were chosen for further biochemical analysis (Figure 4a). Lignin composition analysis revealed that the transgenic lines with the double‐down‐regulation of F5H and COMT exhibited a lower proportion of S units than the COMTRi1 transgenic plants (Figure 4b). Compared with the single down‐regulation of COMT in COMTRi1 and control plants, the double‐down‐regulated transgenic lines showed a significant increase in G units (Figure 4b). Furthermore, down‐regulation of F5H in the COMT‐suppressing background reduced the accumulation of 5‐OH G units that resulted from the COMT down‐regulation; however, these double‐down‐regulated transgenic lines still showed the higher proportion of 5‐OH G units than the control plants (Figure 4c).
Figure 4.
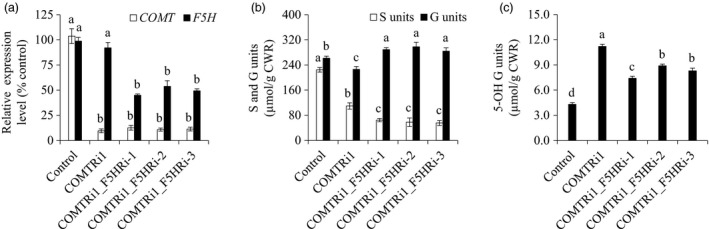
Characterization of transgenic switchgrass plants with F5H down‐regulation in the caffeic acid O‐methyltransferase (COMT)‐suppressing background. (a) Quantitative real‐time PCR analysis of COMT and F5H transcript abundances in the COMTRi1‐F5HRi transgenic lines. (b) S and G lignin monomer yield in the COMTRi1‐F5HRi transgenic lines. (c) 5‐OH G lignin monomer yield in the COMTRi1‐F5HRi transgenic lines. The control plants were generated with the pANIC8D empty vector. Stems at the R1 stage were collected. Switchgrass UBQ was used as the reference for normalization. CWR, cell wall residue. Values are mean ± SE (n = 3). Means with the different letter are significantly different (One‐way ANOVA, Duncan's test, P < 0.05).
Overexpression of F5H compensated for the loss of S lignin resulted from COMT suppression
To study whether up‐regulation of F5H in the COMT‐suppressing background can also affect S lignin accumulation, we first re‐transformed F5H‐OE cassette into the COMTRi1 transgenic line. Seven independent double transformants were generated, and two of the seven transgenic lines showed varying COMT expression levels compared with that of COMTRi1 (Table S1). As described previously, only the double‐transformed lines with comparable COMT expression level to the COMTRi1 plants were chosen for further biochemical analysis (Figure 5a). Lignin composition analysis showed that the transgenic lines with up‐regulated F5H and down‐regulated COMT expressions contained a lower proportion of G units and a higher proportion of 5‐OH G units than those of the COMTRi1 transgenic plants (Figure 5b,c). Compared with the single down‐regulated of COMT in COMTRi1 plants, the lower amount of S units resulting from COMT suppression was partially compensated in the double‐transformed lines (Figure 5b).
Figure 5.
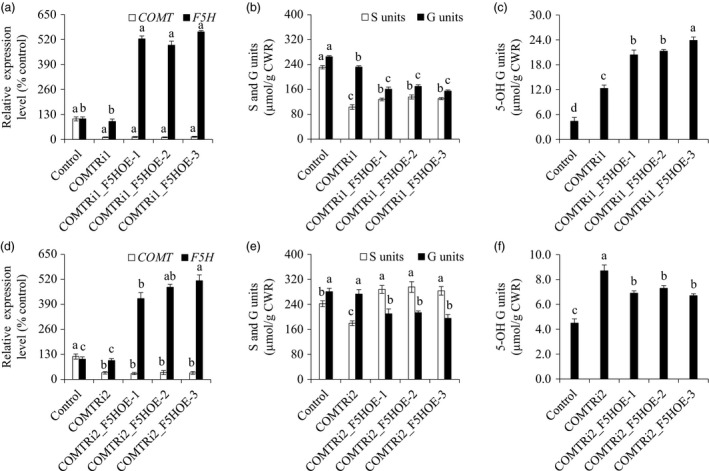
Characterization of transgenic switchgrass plants with F5H overexpression in the caffeic acid O‐methyltransferase (COMT)‐suppressing background. (a) Quantitative real‐time PCR analysis of COMT and F5H transcript abundances in the COMTRi1‐F5HOE transgenic lines. (b) S and G lignin monomer yield in the COMTRi1‐F5HOE transgenic lines. (c) 5‐OH G lignin monomer yield in the COMTRi1‐F5HOE transgenic lines. (d) Quantitative real‐time PCR analysis of COMT and F5H transcript abundance in the COMTRi2‐F5HOE transgenic lines. (e) S and G lignin monomer yield in the COMTRi2‐F5HOE transgenic lines. (f) 5‐OH G lignin monomer yield in the COMTRi2‐F5HOE transgenic lines. The control plants were generated with the pANIC6D empty vector. COMTRi1: the COMT‐RNAi line with severe down‐regulation of COMT; COMTRi2: the COMT‐RNAi line with moderate down‐regulation of COMT. Stems at the R1 stage were collected. Switchgrass UBQ was used as the reference for normalization. CWR, cell wall residue. Values are mean ± SE (n = 3). Means with the different letter are significantly different (One‐way ANOVA, Duncan's test, P < 0.05).
Given that COMTRi1 was the line with severe down‐regulation of COMT, we next employed a moderately down‐regulated COMT transgenic line, COMTRi2, to further study the antagonistic effects of F5H overexpression on S lignin biosynthesis in the COMT‐suppressing background (Figure 5d). Five independent double transformants were generated, and one of the five transgenic lines showed varying COMT expression levels compared with that of COMTRi2 (Table S1). Overexpression of F5H in the COMTRi2 transgenic switchgrass line resulted in a significant reduction in G units as expected (Figure 5e). Most strikingly, the reduction in S units resulting from COMT suppression was fully restored in the double‐transformed transgenic lines (Figure 5e). As a consequence, a relatively low proportion of 5‐OH G units were observed in the COMTRi2_F5HOE transgenic lines compared with that of the COMTRi2 plants; however, their 5‐OH G units levels were still higher than those of the control and F5HOE transgenic plants (Figures 3f and 5f). To further confirm that the negative effect of moderate down‐regulation of COMT on S lignin biosynthesis was still well sustained in the double‐transformed lines, we conducted a soluble phenolics profiling analysis by LC‐MS/MS and identified 5‐OH coniferyl alcohol glycoside, a derivative from the substrates of COMT, present in the COMTRi2_F5HOE transgenic lines (Table S2; Figure S5). Moreover, this compound specifically accumulated in the COMT‐suppressing background including COMTRi1, COMTRi2, COMTRi1_F5HOE and COMTRi2_F5HOE transgenic switchgrass plants, but not in F5HRi, F5HOE and empty vector control plants (Figure S5).
Taken together, these results imply that increasing the concentration of substrates for COMT by overexpressing F5H in the COMT‐suppressing background may compensate for the lack of COMT enzyme due to down‐regulation of COMT. To test this hypothesis, we measured COMT activity against 5‐OH coniferyl alcohol in crude plant extracts of the control, COMTRi1 and COMTRi2 plants. Our results showed that the turn‐over efficiency of crude COMT enzyme extracts prepared from COMT‐RNAi transgenic switchgrass plants was dramatically elevated by increasing the concentration of substrate (Figure S6).
Effects of diverse lignin composition on plant growth and cell wall digestibility
To evaluate whether the coordination between F5H and COMT affects switchgrass growth and development, we characterized the phenotype of the F5HRi, F5HOE, COMTRi and their double‐transformed plants. Alteration of F5H expression either in the wild type background or in the COMT‐suppressing background had no effects on plant growth and development (Figure S7). However, the double‐transformed switchgrass plants exhibited brown‐coloured stems (Figure 6). Furthermore, coloration analysis of the cross sections of internodes showed that the brown‐coloured pigment was the typical characterization of COMT suppression but not F5H‐alteration (Figure 6). No difference between the control and transgenic switchgrass plants was detected in the above ground biomass (Table 1).
Figure 6.
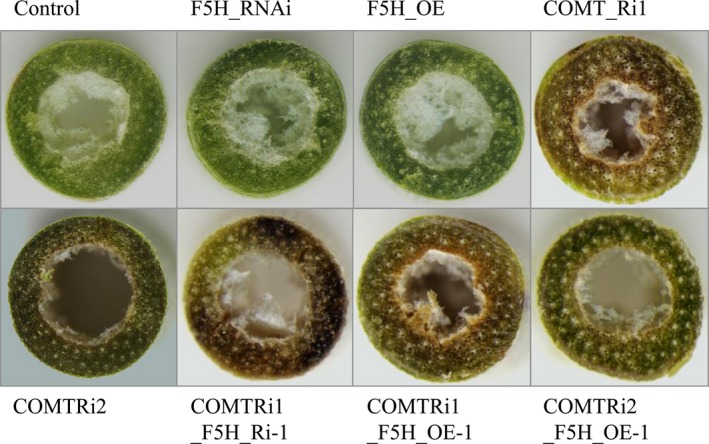
Cross sections of internodes from transgenic switchgrass plants. The control plants were generated with the pANIC empty vector. Stems at the R1 stage were collected and the different internodes were separated.
Table 1.
Effects of modification of COMT and F5H on biomass, cell wall digestibility and lignin content of transgenic switchgrass plants
| Dry matter biomass (g/plant) | Enzymatic hydrolysis efficiency (%) | Acetyl bromide lignin (mg/g CWR) | |
|---|---|---|---|
| Control | 21.7 ± 1.5a | 32.2 ± 0.9e | 234.6 ± 3.4c |
| COMTRi1 | 20.5 ± 3.9a | 40.7 ± 1.1c | 217.5 ± 1.6b |
| COMTRi2 | 23.5 ± 1.5a | 36.4 ± 0.4d | 230.1 ± 2.4c |
| F5HRi‐1 | 20.9 ± 2.2a | 33.4 ± 0.7ed | 224.8 ± 4.9c |
| F5HOE‐1 | 22.4 ± 2.1a | 32.5 ± 1.0ed | 240.5 ± 3.6c |
| COMTRi1_F5HRi‐1 | 21.3 ± 4.3a | 51.1 ± 0.9a | 195.8 ± 5.3a |
| COMTRi1_F5HRi‐2 | 20.3 ± 3.1a | 50.5 ± 0.8a | 188.8 ± 4.4a |
| COMTRi1_F5HRi‐3 | 18.8 ± 2.1a | 51.2 ± 1.4a | 187.2 ± 1.5a |
| COMTRi1_F5HOE‐1 | 23.1 ± 3.3a | 44.1 ± 1.3bc | 219.6 ± 1.9b |
| COMTRi1_F5HOE‐2 | 17.3 ± 3.6a | 45.4 ± 0.5b | 208.1 ± 0.7ab |
| COMTRi1_F5HOE‐3 | 19.5 ± 2.0a | 43.0 ± 1.9bc | 215.4 ± 1.9b |
| COMTRi2_F5HOE‐1 | 20.6 ± 2.9a | 30.8 ± 0.9e | 232.6 ± 4.3c |
| COMTRi2_F5HOE‐2 | 21.9 ± 1.6a | 31.9 ± 2.9e | 232.7 ± 1.0c |
| COMTRi2_F5HOE‐3 | 24.2 ± 3.8a | 32.1 ± 0.8e | 225.4 ± 3.7c |
The transgenic and control plants were harvested after six months of growth in the greenhouse. Values are mean ± SE (n = 3). Means with the same letter are not significantly different (One‐way ANOVA, Duncan's test, P < 0.05).
Given that lignin is a crucial factor negatively affecting anthropogenic utilization of lignocellulosic biomass, we next evaluated the effect of altered lignin biosynthesis on cell wall digestibility. As anticipated, cell wall saccharification efficiencies of the COMTRi1 and COMTRi2 transgenic switchgrass plants were 26.4% and 13.0% greater than that of the control plants. Neither up‐regulation nor down‐regulation of F5H affected saccharification efficiency significantly (Table 1). Altering F5H expression levels in the COMTRi1 transgenic line, however, resulted in up to 11.6%–25.8% increases in saccharification efficiency compared with that of the COMTRi1 transgenic plants (Table 1). In contrast, the saccharification efficiencies of COMTRi2_F5HOE transgenic switchgrass lines were lower than those of COMTRi2 transgenic plants, but resembled those of the control plants (Table 1). In addition, the influence of acetyl bromide (AcBr) lignin content on digestibility of cell walls was investigated, revealing a strong negative correlation (r 2 = 0.884) between lignin content and saccharification efficiency (Figure S8). No significant correlation was observed between saccharification efficiency and 5‐OH G lignin level and S/G ratio (Figure S8).
Discussion
Genetic regulation of either F5H or COMT can affect S lignin biosynthesis severely and thereby improve forage digestibility, bioethanol production and woody pulping efficiency (Baxter et al., 2014; Chen and Dixon, 2007; Chen et al., 2004; Jung et al., 2012; Pilate et al., 2002; Shen et al., 2012). An effective method of lignin modification by RNAi‐mediated down‐regulation of COMT in switchgrass was successful in reducing S lignin biosynthesis and improving the conversion efficiency of lignocellulosic biomass into bioethanol (Fu et al., 2011a). On the other hand, the function of F5H remains largely elusive in monocot species. In this study, we characterized the function of F5H in switchgrass and revealed the coordination of F5H and COMT in the biosynthesis of S lignin.
Ferulate 5‐hydroxylase hydroxylates coniferaldehyde and coniferyl alcohol to 5‐OH coniferaldehyde and 5‐OH coniferyl alcohol, which is the master step in S lignin biosynthesis. Disruption or down‐regulation of F5H in Arabidopsis, alfalfa and rice can reduce S units and enrich G units in lignin polymers (Meyer et al., 1998; Reddy et al., 2005; Takeda et al., 2017). Conversely, overexpression of F5H in Arabidopsis, tobacco, poplar and rice can enrich S units and reduce G units (Franke et al., 2000; Meyer et al., 1998; Takeda et al., 2017). Switchgrass PvF5H1 has high amino acid sequence identity to the recently characterized rice OsCAld5H, and the collinearity analysis revealed PvF5H1 as the ortholog of OsCAld5H, implying that PvF5H1 could function in the S lignin biosynthesis as well. In line with the previous studies, both S and G lignin levels were significantly altered in transgenic switchgrass plants with either up‐regulation or down‐regulation of PvF5H1, indicating that F5H plays a crucial role in lignin biosynthesis in switchgrass. Compared with F5H, disruption of COMT results in a substantial loss of S lignin units in the Arabidopsis omt1 mutant, whereas overexpression of COMT has no effects on S lignin biosynthesis, suggesting that F5H, rather than COMT, appears to be a rate‐limiting step. Notably, a relative low level of down‐regulation for F5H in F5H‐RNAi transgenic switchgrass lines was sufficient to reduce S lignin biosynthesis significantly. In contrast, a similar loss in S units was not achieved until the transcript abundances of COMT were reduced by more than 80% in COMT‐RNAi transgenic switchgrass lines (Fu et al., 2011a; Figures 3, 4, 5). These results imply that the expression levels of F5H in switchgrass might be regulated more strictly during cell wall lignification. In addition, a recent study has shown that the phosphorylation of COMT in poplar can switch off its activity, demonstrating a novel regulation mechanism in S lignin biosynthesis (Wang et al., 2015). Unfortunately, little information on post‐translational modifications of F5H and COMT is available in switchgrass. Therefore, it would be worthwhile to investigate post‐translational modifications of F5H and COMT to refine our understanding of the complex regulatory mechanisms in S lignin biosynthesis.
Monolignols are synthesized through a complex metabolic network in plants. Therefore, it is difficult to predict the impacts of simultaneous manipulation of more than one enzyme in lignin biosynthetic pathway (Pincon et al., 2001; Zhao and Dixon, 2011). However, our results indicate that down‐regulation of F5H and COMT had a synergistic effect on S lignin biosynthesis in switchgrass. Moreover, the pattern of G lignin accumulation was similar to that of F5HRi transgenic switchgrass plants, but 5‐OH G lignin accumulation resembled that of COMTRi1 transgenics. In addition, previous studies have shown that overexpression of F5H in Arabidopsis omt1 mutant can increase 5‐OH G units incorporation in lignin polymers and reduce both G and S lignin accumulation dramatically (Vanholme et al., 2010; Weng et al., 2010). Similar results were observed in a highly down‐regulated COMT transgenic switchgrass line with concomitant overexpression of F5H. Given the fact that COMT transcripts are not entirely eliminated in the COMT‐RNAi transgenic switchgrass lines, the potential dosage effect of COMT expression has to be considered in these double‐transformed plants. Strikingly, overexpression of F5H in a background with moderate down‐regulated of COMT was able to fully restore S lignin biosynthesis. Moreover, the level of 5‐OH G units in double‐transformed switchgrass plants was still higher than that of control plants, but lower than that of the corresponding COMTRi2‐single transgenic plants. Based on the above results, we speculate that overexpression of F5H in the COMT‐down‐regulated background can significantly elevate 5‐OH coniferaldehyde/5‐OH coniferyl alcohol influx and thereby compensate for the decrease in turn‐over efficiency of COMT due to the reduction in COMT expression. The data of a COMT in vitro enzyme activity assay further supports our hypothesis. In addition, we screened numerous COMT‐RNAi transgenic switchgrass lines and found that COMT down‐regulation had the potential to trigger a significant increase in F5H expression when the biosynthesis of S lignins was disrupted in some transgenic switchgrass lines (Figure S4). Thus the antagonistic effects of up‐regulation of F5H in the COMT‐down‐regulated background on S lignin biosynthesis have to be considered carefully in the practice of lignin bioengineering.
A significant increase of 5‐OH G units and cell wall digestibility has been achieved by overexpression of F5H in the COMT‐deficient Arabidopsis mutant (Weng et al., 2010). Our results showed that up‐regulating F5H expression in the COMT severely down‐regulated transgenic switchgrass significantly increased saccharification efficiency of cell walls compared with that of the empty vector control. Conversely, up‐regulating F5H expression in the COMT‐moderately down‐regulated transgenic switchgrass did not improve saccharification efficiency of cell walls. Furthermore, no correlation was observed between cell wall saccharification efficiency and 5‐OH G lignin level and S/G ratio; however, the saccharification efficiency was negatively correlated with AcBr lignin content. Therefore, our results imply that other factors besides lignin composition may still have important influences on cell wall digestibility. In addition, downregulating F5H expression in the severely COMT‐suppressing background of switchgrass remarkably elevated saccharification efficiency of cell walls compared with that of the single down‐regulation of COMT. These results suggest that the concomitant alteration of F5H and COMT expression levels in switchgrass plants indeed has potential to improve feedstock utilization of bioenergy crops in the future.
Experimental procedures
Plant materials and growth conditions
We used Alamo, a lowland type switchgrass cultivar, for lignin modification. According to the criteria described by Hardin et al. (2013), the development of our switchgrass plants were divided into five elongation stages (E1, E2, E3, E4 and E5) and three reproductive stages (R1, R2 and R3). Plants were grown in a greenhouse with 16 h light (390 μE/m2/S).
Identification and cloning of PvF5H
PvF5H1a and 1b were identified by blasting previously published switchgrass F5H sequences (GeneBank accession no: AB608019) against the switchgrass genome database v4.1 (http://www.phytozome.org/). MEGA 5 software suite (http://www.megasoftware.net/) was employed to conduct alignment of multiple sequences and phylogenetic tree analysis of F5H orthologs downloaded from switchgrass, maize, sorghum, rice, B. distachyon, A. thaliana, M. truncatula and P. trichocarpa. A maximum likelihood tree was constructed in PhyML version 3.0 (http://atgc.lirmm.fr/phyml/) on the basis of multiple alignments of deduced F5H protein sequences. Core‐orthologous gene pairs in switchgrass, maize and rice were employed to define orthologous blocks as described by Bai et al. (2016). The chromosomal region of PvF5H1a including 40 kb flanking sequences were aligned with the corresponding orthologous sequences in maize (100 kb) and rice (40 kb). Gene collinearity analysis of PvF5H1a was performed as described by Bai et al. (2016). The expression patterns of PvF5H and COMT were retrieved from the switchgrass Gene Expression Atlas (http://switchgrassgenomics.noble.org/). PvF5H was isolated from switchgrass stem tissues by reverse transcription polymerase chain reaction (RT‐PCR) based on the sequence of F5H1a downloaded from phytozome v12 and was subjected to sequencing and further studies.
Generation of transgenic switchgrass plants
The primers used for the cloning of fragments of COMT‐RNAi, F5H‐RNAi and F5H‐OE were designed based on the code sequence of the isolated PvCOMT and PvF5H (Table S3). The final binary vectors of pANIC8B‐COMTRi, pANIC8D‐F5HRi and pANIC6D‐F5HOE were constructed by LR recombination reactions (Invitrogen, Shanghai, China), and transferred into Agrobacterium tumefaciens strain AGL1 using the freezing/heat‐shock method.
A high‐quality, single genotype embryogenic callus line induced from an Alamo seed was employed for Agrobacterium‐mediated transformation following the procedure described by Wu et al. (2016). Transgenic switchgrass lines were grown in the greenhouse at 26 °C with 16 h light (390 μE/m2/S). In addition, the calli induced from inflorescences of the selected COMT‐RNAi transgenic lines were used for the re‐transformation of F5H‐RNAi and F5H‐OE constructs into the COMT‐suppressing background. Hygromycin and bialaphos were used as the selectable reagents to generate COMTRi‐F5HRi and COMTRi‐F5HOE transgenic switchgrass plants.
Expression levels of F5H and COMT in transgenic switchgrass plants
The positive transgenic lines were identified by genomic PCR with specific hph and bar primers. The fragments of 375 and 242 bp were the expected sizes of PCR products for hph and bar respectively (Table S3). Stems at the R1 stage were collected from each plant and ground in liquid nitrogen. Total RNA extracted by Tri‐Reagent (Invitrogen) from approximate 0.2 g stem samples were subjected to reverse transcription with Superscript III Kit (Invitrogen) after treatment with TURBO™ DNase I (Ambion, Austin, TX). The remaining samples were lyophilized for soluble phenolics, lignin and cell wall digestibility analyses. The expression levels of F5H and COMT were analysed by quantitative real‐time PCR (qRT‐PCR) as described by Wu et al. (2016). The primers used for qRT‐PCR were listed in Table S3. The cycle thresholds were determined using an ABI PRISM 7900 HT sequence detection system (Applied Biosystems, Foster City, CA), and the data were normalized using the level of switchgrass Ubq2 transcripts (GenBank accession no: HM209468).
Determination of lignin content and composition
Soluble extracts were removed from the ground lyophilized stem samples by three successive extractions with chloroform/methanol (2 : 1 v/v), methanol and water at room temperature as described by Chen and Dixon (2007), and the remaining cell wall residues (CWRs) were lyophilized. The extractive‐free CWRs were then used to quantify lignin content and composition. The AcBr method was employed to quantify lignin content (Hatfield et al., 1999). The thioacidolysis method was used to determine lignin composition (Lapierre et al., 1995).
Identification and quantification of soluble phenolics
Soluble phenolics were extracted twice from 30.0 ± 0.05 mg of the lyophilized above‐ground stem samples with 1.0 mL 50% methanol plus 1.5% acetic acid for 3 h at room temperature each time (Fu et al., 2011b). Identification and quantification of 5‐OH coniferyl alcohol glycoside was performed by LC‐PDA/ESI‐MS/MS according to a previously described method (Fu et al., 2011b). All the mass spectra were acquired using a Bruker Esquire LC equipped with an electrospray ionization (ESI) source. Mass spectra from positive‐ and negative‐ion ESI were recorded over the range of 50–2200 m/z. To confirm the aglycone structure of the deduced compound, the vacuum‐dried methanolic extracts of switchgrass internodes were resolved with 3 mL of 5 mg/mL β‐glucosidase in citric acid buffer (pH = 5.5) and the reaction was incubated at 37 °C overnight (Tian and Dixon, 2006). The β‐glucosidase hydrolysis products were vacuum‐dried, re‐dissolved in 1.0 mL 80% methanol and were identified by comparing their retention time, and UV‐visible and mass spectra with the corresponding standard compounds. The reference standard of 5‐OH coniferyl alcohol was synthesized by the Chemistry Research Solution LLC (PA, USA).
Enzyme activity assay
The stems of wild type switchgrass plants collected at the R1 stage were homogenized in liquid nitrogen. Powdered tissue (about 500 mg) was extracted for 3 h at 4 °C in extraction buffer (Fu et al., 2011a). The samples were centrifuged at 17 900 g for 20 min at 4 °C, and the extracts were desalted on PD‐10 columns (Pharmacia, Shanghai, China). Activities of COMT against 5‐OH coniferyl alcohol in crude plant extracts were determined as described by Liu et al. (2012).
Biomass measurement and internode coloration observation
The positive transgenic switchgrass plants were subjected to morphological analysis. The controls were generated from a population including the plants derived from the transgenic plants with pANIC8B, pANIC8D and pANIC6D empty vectors. Transgenic and control plants were harvested after 6‐months of growth in a greenhouse and dried in an oven at 40 °C for 1 month to evaluate the above‐ground dry matter biomass yield.
The second internode collected from the stem at the R1 stage were free‐hand sectioned with a razor blade and pictures of unstained samples were immediately taken under an Olympus SZX12‐Fluorescent Stereo Microscope system (Olympus, Tokyo, Japan) for internode coloration characterization.
Determination of cell wall digestibility
The switchgrass stems (R1 stage) collected in a paper bag were oven‐dried for 7 days at 40 °C. The dried stem samples were ground through a Thomas model 4 Wiley® mill with a 1‐mm sieve and used for CWR preparation as described by Chen and Dixon (2007). The protocol (LAP‐009, Enzymatic Saccharification of Lignocellulosic Biomass; http://www.nrel.gov/biomass/analytical_procedures.html) described by National Renewable Energy Laboratory were then used to determine saccharification efficiency of the extractive‐free CWRs. The phenol‐sulphuric acid assay was employed to measure the amount of fermentable sugars (Dubois et al., 1956). The ratio of sugars released by enzymatic hydrolysis to the amount of total sugars present in cell wall materials before the enzymatic hydrolysis treatment was determined as saccharification efficiency of cell walls.
Statistical analysis
Primary transgenic switchgrass plants were propagated by transferring the same number of tillers into each pot. Three copies of each line were grown in 1‐gallon pots. Stems at the R1 stage were collected from the three copies of each transgenic line. Two technical replicates were conducted for lignin and cell wall digestibility analyses of each sample. The mean values were used for statistical analysis. Data from each trait were subjected to one‐way analysis of variance (ANOVA). The significance of treatments was tested at the P = 0.05 and 0.01 level. Standard error of the mean is provided in all figures and tables as appropriate. Means with the different letter are significantly different (One‐way ANOVA, Duncan's test, P < 0.05).
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Figure S1 Alignment of switchgrass F5H nucleic acid sequences.
Figure S2 Gene expression atlas analysis of PvF5H.
Figure S3 Gene expression atlas analysis of PvCOMT1 and PvCOMT2.
Figure S4 Quantitative RT‐PCR analysis of PvF5H transcript abundances in COMT‐RNAi transgenic switchgrass plants.
Figure S5 5‐OH coniferyl alcohol glucoside yield in methanolic extracts of stems of control and transgenic switchgrass plants.
Figure S6 Extractable COMT enzyme activity in stems of control and COMT‐RNAi transgenic switchgrass plants.
Figure S7 Morphological characterization of transgenic switchgrass plants.
Figure S8 Relationships between saccharification efficiency and lignin content and composition.
Table S1 Varying COMT and F5H expression levels in the double transgenic switchgrass lines.
Table S2 Identification of 5‐OH coniferyl alcohol glycoside by LC‐MS/MS in COMT‐RNAi transgenic switchgrass plants.
Table S3 Primers used in this study.
Acknowledgements
The work was supported by the National Key Technologies Research & Development Program‐Seven Major Crops Breeding Project (No. 2016YFD0101803), the National Natural Science Foundation of China (No. 31470390 and 31500241) and Major Program of Shandong Province Natural Science Foundation (No. ZR2018ZB0213).
References
- Anderson, N.A. , Tobimatsu, Y. , Ciesielski, P.N. , Ximenes, E. , Ralph, J. , Donohoe, B.S. , Ladisch, M. et al (2015) Manipulation of guaiacyl and syringyl monomer biosynthesis in an Arabidopsis cinnamyl alcohol dehydrogenase mutant results in atypical lignin biosynthesis and modified cell wall structure. Plant Cell, 27, 2195–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Z. , Chen, J. , Liao, Y. , Wang, M. , Liu, R. , Ge, S. , Wing, R.A. et al (2016) The impact and origin of copy number variations in the Oryza species. BMC Genom. 17, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, H.L. , Mazarei, M. , Labbe, N. , Kline, L.M. , Cheng, Q. , Windham, M.T. , Mann, D.G. et al (2014) Two‐year field analysis of reduced recalcitrance transgenic switchgrass. Plant Biotechnol. J. 12, 914–924. [DOI] [PubMed] [Google Scholar]
- Boerjan, W. , Ralph, J. and Baucher, M. (2003) Lignin biosynthesis. Annu. Rev. Plant Biol. 54, 519–546. [DOI] [PubMed] [Google Scholar]
- Bonawitz, N.D. and Chapple, C. (2010) The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu. Rev. Genet. 44, 337–363. [DOI] [PubMed] [Google Scholar]
- Chapple, C.C. , Vogt, T. , Ellis, B.E. and Somerville, C.R. (1992) An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell, 4, 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. and Dixon, R.A. (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 25, 759–761. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Auh, C.K. , Dowling, P. , Bell, J. , Lehmann, D. and Wang, Z.Y. (2004) Transgenic down‐regulation of caffeic acid O‐methyltransferase (COMT) led to improved digestibility in tall fescue (Festuca arundinacea). Funct. Plant Biol. 31, 235–245. [DOI] [PubMed] [Google Scholar]
- Dubois, M. , Gilles, K.A. , Hamilton, J.K. , Rebers, P.A. and Smith, F. (1956) Colorimetric method for dertermination of sugars and related substances. Anal. Chem. 28, 350–356. [Google Scholar]
- Franke, R. , McMichael, C.M. , Meyer, K. , Shirley, A.M. , Cusumano, J.C. and Chapple, C. (2000) Modified lignin in tobacco and poplar plants over‐expressing the Arabidopsis gene encoding ferulate 5‐hydroxylase. Plant J. 22, 223–234. [DOI] [PubMed] [Google Scholar]
- Fu, C. , Mielenz, J.R. , Xiao, X. , Ge, Y. , Hamilton, C.Y. , Rodriguez, M. , Chen, F. et al (2011a) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl Acad. Sci. USA, 108, 3803–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, C. , Xiao, X. , Xi, Y. , Ge, Y. , Chen, F. , Bouton, J. , Dixon, R. et al (2011b) Downregulation of cinnamyl alcohol dehydrogenase (CAD) leads to improved saccharification efficiency in switchgrass. Bioenerg. Res. 4, 153–164. [Google Scholar]
- Goujon, T. , Sibout, R. , Pollet, B. , Maba, B. , Nussaume, L. , Bechtold, N. , Lu, F. et al (2003) A new Arabidopsis thaliana mutant deficient in the expression of O‐methyltransferase impacts lignins and sinapoyl esters. Plant Mol. Biol. 51, 973–989. [DOI] [PubMed] [Google Scholar]
- Hardin, C.F. , Fu, C.X. , Hisano, H. , Xiao, X.R. , Shen, H. , Stewart, C.N. , Parrott, W. et al (2013) Standardization of switchgrass sample collection for cell wall and biomass trait analysis. Bioenerg. Res. 6, 755–762. [Google Scholar]
- Hatfield, R.D. , Grabber, J. , Ralph, J. and Brei, K. (1999) Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J. Agric. Food Chem. 47, 628–632. [DOI] [PubMed] [Google Scholar]
- Humphreys, J.M. and Chapple, C. (2002) Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 5, 224–229. [DOI] [PubMed] [Google Scholar]
- Humphreys, J.M. , Hemm, M.R. and Chapple, C. (1999) New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5‐hydroxylase, a multifunctional cytochrome P450‐dependent monooxygenase. Proc. Natl Acad. Sci. USA, 96, 10045–10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J.H. , Fouad, W.M. , Vermerris, W. , Gallo, M. and Altpeter, F. (2012) RNAi suppression of lignin biosynthesis in sugarcane reduces recalcitrance for biofuel production from lignocellulosic biomass. Plant Biotechnol. J. 10, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Lapierre, C. , Pollet, B. and Rolando, C. (1995) New insights into the molecular architecture of hardwood lignins by chemical degradative methods. Res. Chem. Intermediat. 21, 397–412. [Google Scholar]
- Lee, S. , Mo, H. , Kim, J.I. and Chapple, C. (2017) Genetic engineering of Arabidopsis to overproduce disinapoyl esters, potential lignin modification molecules. Biotechnol. Biofuels, 10, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, N.G. and Yamamoto, E. (1990) Lignin: occurrence, biogenesis and biodegradation. Annu. Rev. Plant Phys. 41, 455–496. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Shi, R. , Li, Q. , Sederoff, R.R. and Chiang, V.L. (2012) A standard reaction condition and a single HPLC separation system are sufficient for estimation of monolignol biosynthetic pathway enzyme activities. Planta, 236, 879–885. [DOI] [PubMed] [Google Scholar]
- Meyer, K. , Shirley, A.M. , Cusumano, J.C. , Bell‐Lelong, D.A. and Chapple, C. (1998) Lignin monomer composition is determined by the expression of a cytochrome P450‐dependent monooxygenase in Arabidopsis. Proc. Natl Acad. Sci. USA, 95, 6619–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, N.A. , Sattler, S.E. , Saathoff, A.J. , Funnell, D. , Pedersen, J.F. and Sarath, G. (2008) Genetic background impacts soluble and cell wall‐bound aromatics in brown midrib mutants of sorghum. Planta, 229, 115–127. [DOI] [PubMed] [Google Scholar]
- Pilate, G. , Guiney, E. , Holt, K. , Petit‐Conil, M. , Lapierre, C. , Leplé, J.C. , Pollet, B. et al (2002) Field and pulping performances of transgenic trees with altered lignification. Nat. Biotechnol. 20, 607–612. [DOI] [PubMed] [Google Scholar]
- Pincon, G. , Chabannes, M. , Lapierre, C. , Pollet, B. , Ruel, K. , Joseleau, J.P. , Boudet, A.M. et al (2001) Simultaneous down‐regulation of caffeic/5‐hydroxy ferulic acid‐O‐methyltransferase I and cinnamoyl‐coenzyme A reductase in the progeny from a cross between tobacco lines homozygous for each transgene. Consequences for plant development and lignin synthesis. Plant Physiol. 126, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph, J. , Lapierre, C. , Lu, F. , Marita, J.M. , Pilate, G. , Van Doorsselaere, J. , Boerjan, W. et al (2001) NMR evidence for benzodioxane structures resulting from incorporation of 5‐hydroxyconiferyl alcohol into lignins of O‐methyltransferase‐deficient poplars. J. Agric. Food Chem. 49, 86–91. [DOI] [PubMed] [Google Scholar]
- Rastogi, S. and Dwivedi, U.N. (2008) Manipulation of lignin in plants with special reference to O‐methyltransferase. Plant Sci. 174, 264–277. [Google Scholar]
- Reddy, M.S. , Chen, F. , Shadle, G. , Jackson, L. , Aljoe, H. and Dixon, R.A. (2005) Targeted down‐regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proc. Natl Acad. Sci. USA, 102, 16573–16578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, H. , He, X. , Poovaiah, C.R. , Wuddineh, W.A. , Ma, J. , Mann, D.G. , Wang, H. et al (2012) Functional characterization of the switchgrass (Panicum virgatum) R2R3‐MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol. 193, 121–136. [DOI] [PubMed] [Google Scholar]
- Takeda, Y. , Koshiba, T. , Tobimatsu, Y. , Suzuki, S. , Murakami, S. , Yamamura, M. , Rahman, M.M. et al (2017) Regulation of CONIFERALDEHYDE 5‐HYDROXYLASE expression to modulate cell wall lignin structure in rice. Planta, 246, 337–349. [DOI] [PubMed] [Google Scholar]
- Tian, L. and Dixon, R.A. (2006) Engineering isoflavone metabolism with an artificial bifunctional enzyme. Planta, 224, 496–507. [DOI] [PubMed] [Google Scholar]
- Vanholme, R. , Ralph, J. , Akiyama, T. , Lu, F. , Pazo, J.R. , Kim, H. , Christensen, J.H. et al (2010) Engineering traditional monolignols out of lignin by concomitant up‐regulation of F5H1 and down‐regulation of COMT in Arabidopsis. Plant J. 64, 885–897. [DOI] [PubMed] [Google Scholar]
- Vanholme, R. , Cesarino, I. , Rataj, K. , Xiao, Y. , Sundin, L. , Goeminne, G. , Kim, H. et al (2013) Caffeoyl shikimate esterase (CSE) is an enzyme in the lignin biosynthetic pathway in Arabidopsis. Science, 341, 1103–1106. [DOI] [PubMed] [Google Scholar]
- Vignols, F. , Rigau, J. , Torres, M.A. , Capellades, M. and Puigdomenech, P. (1995) The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O‐methyltransferase. Plant Cell, 7, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.P. , Chuang, L. , Loziuk, P.L. , Chen, H. , Lin, Y.C. , Shi, R. , Qu, G.Z. et al (2015) Phosphorylation is an on/off switch for 5‐hydroxyconiferaldehyde O‐methyltransferase activity in poplar monolignol biosynthesis. Proc. Natl Acad. Sci. USA, 112, 8481–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, J.K. , Mo, H. and Chapple, C. (2010) Over‐expression of F5H in COMT‐deficient Arabidopsis leads to enrichment of an unusual lignin and disruption of pollen wall formation. Plant J. 64, 898–911. [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Cao, Y. , Yang, R. , Qi, T. , Hang, Y. , Lin, H. , Zhou, G. et al (2016) Switchgrass SBP‐box transcription factors PvSPL1 and 2 function redundantly to initiate side tillers and affect biomass yield of energy crop. Biotechnol. Biofuels, 9, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. and Dixon, R.A. (2011) Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci. 16, 227–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Alignment of switchgrass F5H nucleic acid sequences.
Figure S2 Gene expression atlas analysis of PvF5H.
Figure S3 Gene expression atlas analysis of PvCOMT1 and PvCOMT2.
Figure S4 Quantitative RT‐PCR analysis of PvF5H transcript abundances in COMT‐RNAi transgenic switchgrass plants.
Figure S5 5‐OH coniferyl alcohol glucoside yield in methanolic extracts of stems of control and transgenic switchgrass plants.
Figure S6 Extractable COMT enzyme activity in stems of control and COMT‐RNAi transgenic switchgrass plants.
Figure S7 Morphological characterization of transgenic switchgrass plants.
Figure S8 Relationships between saccharification efficiency and lignin content and composition.
Table S1 Varying COMT and F5H expression levels in the double transgenic switchgrass lines.
Table S2 Identification of 5‐OH coniferyl alcohol glycoside by LC‐MS/MS in COMT‐RNAi transgenic switchgrass plants.
Table S3 Primers used in this study.


