Abstract
Background:
Cognitive capacity may be reduced from inflammation, surgery, anesthesia, and pain. In this study, we hypothesized incision-induced nociceptive input impairs attentional performance and alters neuronal activity in the prefrontal cortex.
Methods:
Attentional performance was measured in rat using the 5 choice serial reaction time titration variant to determine the impact of surgical incision and anesthesia in a visual attention task. Neuronal activity (single spike and local field potentials) was measured in medial prefrontal cortex in animals during the task.
Results:
Incision significantly impaired attention postoperatively (area under curve (AUC) of median cue duration-time 97.2 ± 56.8 (n=9) versus anesthesia control 25.5 ± 14.5 seconds-days (n=9), P=0.002) (effect size, η2=0.456). Morphine (1 mg/kg) reduced impairment after incision (AUC of median cue duration-time 31.6±36.7 (n=11) versus saline 110 ± 64.7 seconds-days (n=10), P<0.001) (η2=0.378). Incision also decreased cell activity (n=24) (1.48+0.58 versus control 2.93+2.02 bursts/minute, P=0.002) (η2=0.098) and local field potentials (n=28) (η2=0.111) in medial prefrontal cortex.
Conclusion:
These results show that acute postoperative nociceptive input from incision reduces attention related task performance and decreases neuronal activity in medial prefrontal cortex. Decreased neuronal activity suggests nociceptive input is more than just a distraction as neuronal activity increases during audiovisual distraction with similar behavioral impairment. This suggests nociceptive input and medial prefrontal cortex may contribute to attentional impairment and mild cognitive dysfunction postoperatively. In this regard, pain may affect postoperative recovery and return to normal activities through attentional impairment by contributing to lapses in concentration for routine and complex tasks.
Summary Statement:
Nociceptive input from incision impairs the ability to perform a simple visual task; opioid analgesia improves performance after incision; and reduced neuronal activity in the medial prefrontal cortex may underlie the reduced attentional performance.
Introduction
Pain can impact functional capacity directly or from side effects of drugs to treat pain. Both are clinically relevant problems. Inadequate pain treatment may result in reduced ability to maximally perform simple tasks and impact quality of life and recovery. Moreover impaired focus or attention may be detrimental for individuals and for people affected by the individual’s performance. This has implications following surgery for return to work, school, or even routine activities of daily living which may be dependent on maximal reaction speed and focus such as slicing vegetables or driving a car.1,2
Studies of acute and chronic pain on attention have broadened our understanding of the implications and nuances of pain and subtle effects on cognitive performance.1,3–6 Distraction in clinical studies may reduce pain perception by diverting resources from painful stimuli or sensations, particularly less intense pain.7–9 However, more robust or intense pain may conversely divert resources from other tasks potentially reducing performance.1,4,6,9
Attention can readily be measured in a rodent behavioral paradigm, the 5 choice serial reaction time task (5C).10,11 This has been used to understand attention and distraction and the role of different brain regions and neurotransmitters in visual tasks.12–14 A novel variant of the 5C was developed using a titration of attentional cue duration as a measurement of attentional performance, the 5C-titration variant (5CTV).15–17 The 5CTV has been used to examine distraction and associated modulation in neuronal activity of medial prefrontal cortex (mPFC) in the rat.16 Moreover, acute nociceptive input, specifically acute abdominal nociceptive input, impairs performance in this routine and repetitive task and this can be reversed with opioid agonists and non-steroidal anti-inflammatory analgesics.17
Attention is important for optimal performance of tasks relying on cognitive and executive processes. In rat, executive function relies, in part, on input from mPFC.12 Regulation of mPFC contributes to maintaining maximal performance through balancing activity and interactions with other brain regions.12,14 Hypo- or hyper-activation in mPFC may underlie dysfunctional or disrupted attentional processing.13 Understanding the relationship of changes in neuronal activity resulting from interventions is valuable for understanding brain related changes in neuronal activation that may modulate specific behaviors. Many neurotransmitter systems play roles in attentional processing, with cholinergic and noradrenergic input to mPFC likely contributing to discrimination from interference.14,18 Neuronal activity in mPFC is predominantly increased during acute audiovisual distraction.16,18 Neuronal activity in mPFC also follows nociceptive input in an intensity and duration specific manner.19 Pain may be just another distraction, or it may disrupt attention in a distinct manner. Examining neuronal activity in mPFC in animals performing attention based tasks may permit us to determine the extent to which nociceptive input alters this region in a manner distinct from non-painful distracting stimuli. In this study we hypothesized that anesthesia and surgical incision would impair attentional performance, that impairment is related to nociceptive input and will be reduced (improved function) with morphine analgesia, and neuronal activity in mPFC in the freely behaving rat would be altered in conjunction with the reduced performance from nociceptive input using the 5CTV.
Methods
Animals.
For the experiments, a total of 46 Male, Fisher 344 rats (240–350 g, Harlan Laboratories, Indianapolis, IN) were used for this study. Twenty-two animals were used for behavior alone in the 5CTV (11 incision and morphine, 10 incision and saline); one animal did not train sufficiently in the 5CTV and never entered into the study protocol. Twenty-four animals were used for the electrophysiology experiments (9 incision electrophysiology, 9 control/anesthesia electrophysiology); 6 animals did not achieve stable performance in the 5CTV either before or after electrode placement.
Animal experiments were done in cohorts of a maximum of 8 animals with block randomization of half of the animals to treatment or control for each cohort. Animals were kept on a reversed light:dark cycle (dark 05:00–17:00) and housed in a temperature and humidity controlled room within an AAALAC accredited facility as previously described.15 Briefly, after a one week acclimation period with rats housed in pairs and given free access to standard rat chow and water, animals were singly housed and given free access to rat chow until they attained a minimum body weight of 240 g. Animals were then reduced to 90% of their free feeding weight and given sufficient rat chow thereafter to maintain normal growth and increased weight gain while maintaining 90% of average free feeding weight for Fisher 344 rats based on growth curves from the vendor. Animals were given free access to water throughout the experiment except during experimental sessions. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences (Winston-Salem, NC).
Behavior.
All behaviors were conducted in standard operant chambers controlled through a PC-compatible computer and interface using Med-PC IV software (Med Associates Inc., St. Albans, VT) as previously described.15–17 Briefly, operant chambers with standard stainless steel grid bar floors were in a sound and light attenuating cubicle (Med Associates Inc.). One wall was curved with a bank of 5 nose poke holes for the rat with LEDs located in the rear of each and an illuminated food trough with infrared head entry detection located on the opposite wall with a magazine type pellet dispenser for 45 mg food pellets. A jeweled red lens cap (Allied Electronics Inc., Fort Worth, TX) was used for the food trough lamp and a red lens was also used for the house light (Med Associates Inc.). A wide angle video camera (Genius WideCam F100, KYE Systems Inc., Doral, FL) was affixed to the operant chamber top and all sessions were observed on an external monitor in real time as well as recorded for later observation. Experiments were conducted during the dark phase of the light:dark cycle. Animals were trained in 4 phases as described.15 The final phase consisted of daily 30-min weekday sessions comprised of 100 trials each during which the animal learned to watch the bank of 5 nose poke holes until an LED (the cue) would come on randomly. Responses in the illuminated nose poke hole were reinforced by delivery of two 45 mg chocolate flavored pellets (Bio-Serv Inc., Frenchtown, PA) into the food trough signaled by illumination of the food trough light. The food trough light was turned off 2 s after head entry into the trough was detected, and the next trial began 5 s later. Incorrect or omitted responses (no response within the cue duration or 5 s, whichever is longer) resulted in no food delivery and no illumination and turning off the house light. The next trial began 5 s later as with correct trials. The cue duration was initially set to 30 s and decreased in the next trial after a correct response or increased in the next trial following incorrect or omitted responses, with cue durations being set in discreet increments according to a predetermined array ranging from 30–0.1 s.15 In this manner the animal titrated the cue duration based on the ability to maximally perform in the visual task paradigm. Each trial was signaled by illumination of the house light, followed by a 5-s intertrial interval during which the animal had to wait for illumination of an LED cue at random. Responses during this 5-s interval reset the interval timer to 5 s, such that the animal had to withhold responses until an LED cue was provided. The median cue duration (MCD) was the primary outcome measure of performance in the 5CTV task and was calculated from trials 15–100 for each session. Once the MCD was stable and titrated to <1-s duration for a minimum of 5 consecutive sessions, the animal was considered fully trained and further surgery involving implantation of electrodes was performed. The measures collected for each trial consisted of the cue duration, latency to correct or incorrect response, and the latency to retrieve food reward. Measures collected and summed across the entire session included the number of total correct, incorrect, and omitted responses, as well as the number of premature and perseverative responses and the total time required for completion of each session. Premature responses are those that occur between trials but before the LED is illuminated and perseverative responses are multiple repetitive responses in the same nose poke hole during the same trial.
Surgical Implantation of Electrodes.
For in vivo field-potential and spike recording rats were allowed to free feed for one week after full training in the 5CTV. Rats were initially anesthetized with pentobarbital sodium 40 mg/kg, i.p and maintained with oxygen and isoflurane. Animal breathing, reflexes, and level of anesthesia were monitored throughout surgery. A stereotaxic apparatus was used for implantation of recording electrodes in the mPFC. After sterile prep with betadine and alcohol, the skin was incised and a small burr hole was made at the location identified by the stereotactic coordinates located from the bregma + 2 mm anteriorly, 0.8 mm from midline on the right and electrodes were then placed through the burr hole −3.5 mm from the bone surface, as previously described.16 After placement, the electrodes were bent at the skull and fixed in dental cement mounted around stainless-steel mounting screws to provide further attachment and stabilization. The posterior attachment screw formed the silver wire reference/ground electrode connection to the electrodes. The wireless electrodes were manufactured with 5 Teflon coated 50 micron separate stainless steel insulated electrodes (9–10 MOhms) (NB Labs, Denison, TX) with the recording and ground electrodes attached to a 10-pin connector (A70010, Omnetics Connector Corp., Minneapolis, MN) and implanted with the connector facing upwards.20 Care was taken to provide a smooth cement surface along the base of the implant so the skin could heal around the implant. After surgery, rats were given an intramuscular injection of 300,000 units/kg penicillin G procaine to prevent infections and placed inside a heated cage to recover for at least 1 h and free fed for one week thereafter or until animals achieved presurgery weight. Animals were then food restricted as described above and began daily sessions of the 5CTV until the MCD stabilized for 5 consecutive days <1 s. Animals were then entered into the study protocol (fig. 1).
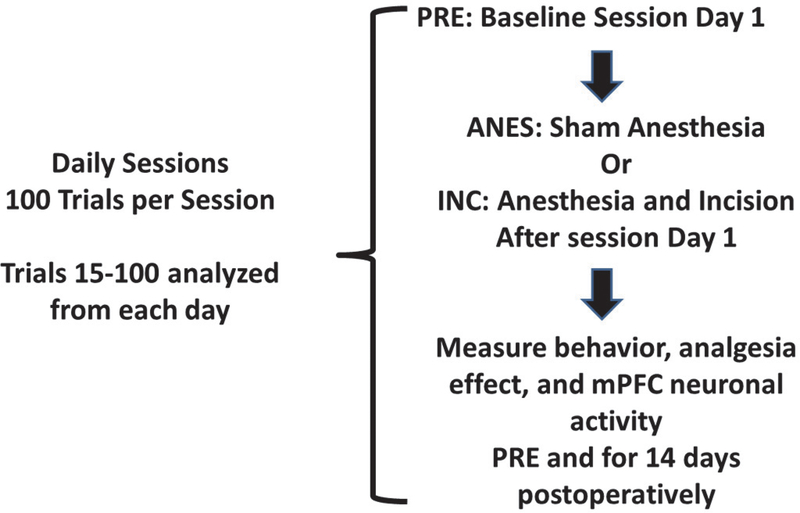
Figure 1. Study Design.
The study design for the incision protocol is presented. All animals underwent 100 trial sessions on consecutive days. After the performance was stable with a MCD <1 s for a week the protocol began: PRE: session at baseline session day 1 or time 0; Anesthesia with isoflurane (ANES) in control or incision and anesthesia (INC) after the baseline session on day PRE; Testing every day for 14 days postoperatively after INC or ANES. MCDs determined for the 15–100 trials period. Single spike data are from the same 15–100 trials period, and local field potential data are from a 900-s epoch during the same period.
Surgical Incision Protocol.
The effect of surgical incision or sham with anesthesia on performance in the cue duration titration procedure was determined in all animals. After stable recordings of MCD <1 s or after return of the MCD to <1 s following electrode placement, the animals underwent paw incision under general anesthesia with isoflurane and spontaneous ventilation as previously described using sterile technique and betadine prep.21 Sham animals were administered the same concentration of isoflurane anesthesia for the same duration, prepped, but did not undergo incision. Animals were randomized to treatment. Sutures were removed on day 10 with brief anesthesia and sham animals had the same brief anesthetic with isoflurane. The average anesthesia time for the incision and sham was 6 min, while the average anesthesia time was 3 min for suture removal. No animal developed wound dehiscence or infection during the study. For the morphine study, animals were randomly assigned to receive 1 mg/kg of morphine or saline subcutaneously daily for 14 days administered 30 min prior to the 5CTV session. Effects of on MCD were determined daily at baseline and for 14 days after the procedure. MCD, neuronal firing, and LFP were determined from the same animals. Background electrophysiological data were determined during sessions in which the animal was placed in the dark operant chamber without the operant paradigm in effect. While the technicians were blinded to treatment, blinding was not possible as the technician could see if an animal had sutures in the paw for the sham and incision groups, but remained blinded to morphine versus saline groups.
Neural Recording.
A wireless headstage was connected to the implanted electrodes of each animal prior to each session and used to record, amplify, and digitize electrical activity from each electrode using Neuroware (Triangle Biosystems Inc., Durham, NC) acquisition software for recording of extracellular local spike activity and LFP. Digital online electrophysiological data are amplified and filtered to only record spike waveforms >2.5-times baseline signal/noise using negative and positive threshold and spike template while the analog data are passed through a 1401 CED A-D converter (CED Inc., Cambridge, UK). A low pass filter was used for LFP <475 Hz, while a high pass filter was used for spikes (300 Hz–7000 Hz). One of the electrodes was selected as the reference electrode to eliminate muscle activity interference from chewing and licking. This is done with the technician blinded to treatment group. Electrode placement in the mPFC was verified in the 9 incision animals and 9 control animals with brain serial coronal cryosection at the conclusion of all experiments and compared with standard rat brain diagrams.
Neural Activity Analysis.
Offline analysis for further spike sorting was used for spike morphology to limit analysis to single cell depolarizations that were standardized and quality controlled for consistent and reproducible analytics for the primary outcomes of absolute spike count, frequency and burst count and frequency. Secondary analysis included maximum instantaneous frequency (IF), burst related IF, and spike burst and frequency in bursts. Spike probability was determined during operant sessions for 20 correct and 20 omission trials at baseline and at POD 5 after incision. This included any spike within 1 s of the cue LED going on, within 1 s of the cue LED going off, within 2 s of the house light going off, or within 2 s of head entry into the food trough as identified in real-time by observation of the video for each session. Spike probability for incorrect responses was not assessed as these are infrequent at baseline and become even less frequent after injury making probability assessment limited. NeuroExplorer version 4 (NEX Technologies, Madison, AL) was used for analysis of LFP and spike characteristics. The term ‘burst’ is a cluster of spikes from a single neuron that differs from other spikes by being more closely spaced in time than neighboring spikes thus having a higher discharge rate than the surrounding spike trains.22 Bursts were defined using the Poisson surprise method of Legéndy and Salcman.23 This method is implemented in NeuroExplorer and was used to quantify bursts between groups. The effects of incision on LFP were evaluated by using Fast Fourier Transformation and comparing the total relative spectral power (in dB or log of the power) over the 1–100 Hz range for a 900-s interval at the same time period during each of the daily sessions at baseline, and days 2, 4, 8 and 14 after surgery or control anesthesia during the 15–100 trials period. Area under the curve (AUC) was calculated using the midpoint rectangle Riemann Sum for the overall power spectral density (PSD)-frequency curve were compared between treatment groups and over time after treatment compared to baseline and then for discrete frequency bands (delta (1–3 Hz), theta (4–8 Hz), alpha (9–13 Hz), beta (14–30 Hz), and low gamma (30–50 Hz).
Data Analysis.
The primary behavioral outcome measure related to attention was the MCD that was calculated using Microsoft Excel from the cue durations for trials 15–100 for each session. A power analysis was performed a priori to detect a difference between incision and sham control for MCD based on previous data using a power of 0.9, an alpha of 0.05 and an estimated effect size of 0.4, and 14 repeated measures ANOVA between treatment difference and a correlation between measures of 0.2 and 10 subjects in each group was estimated. The experiment was set up based on this with the expectation that not all animals would train effectively or recover. As outlined in the methods, some animals were not able to participate in the study for inability to adequately train in the 5CTV initially or after electrode placement (greater after electrode placement) resulting in the sample sizes noted. Effect sizes were subsequently calculated based on the actual subjects in each experiment. Partial eta squared or eta squared (η2) (partial eta squared is when more than one variable is present) is a measure of effect size and is the proportion of the variance accounted for by each of the main effects, interactions, or error in the ANOVA. The effects of incision and morphine on MCD were analyzed using two-way mixed ANOVA with one factor repetition (time). If the interaction term was significant, within group effects were analyzed using one way repeated measures ANOVA with Holms-Sidak method used for correction and pairwise comparisons. The effects of intervention on secondary behavioral outcomes from the 5CTV were analyzed to test for treatment effects only using one way ANOVA with no pairwise comparisons. Area under the MCD-time curves was calculated using the midpoint rectangle Riemann Sum and was compared using a t-test for incision and sham/control and using a one-way ANOVA for sham/control, saline incision, and morphine incision groups. Spike, burst, and LFP analysis was performed using two-way ANOVA and within group analysis was performed if significant using one-way repeated measures ANOVA with pairwise comparisons using the Holms-Sidak method for multiple comparisons. Statistical analysis was performed using Sigmaplot (Systat Software Inc., San Jose, CA). Data are presented as means (M) and standard deviation (SD) except when not normally distributed and then median and range are presented and noted. Analysis of P-values, were determined by Sigmaplot (Systat Software Inc., San Jose, CA). Corrections for multiple comparisons were used where appropriate. Where P-values are reported, these are corrected P-values. A priori only corrected P <0.05 was considered statistically significant.
Results
Behavioral effects of Incision in the 5CTV
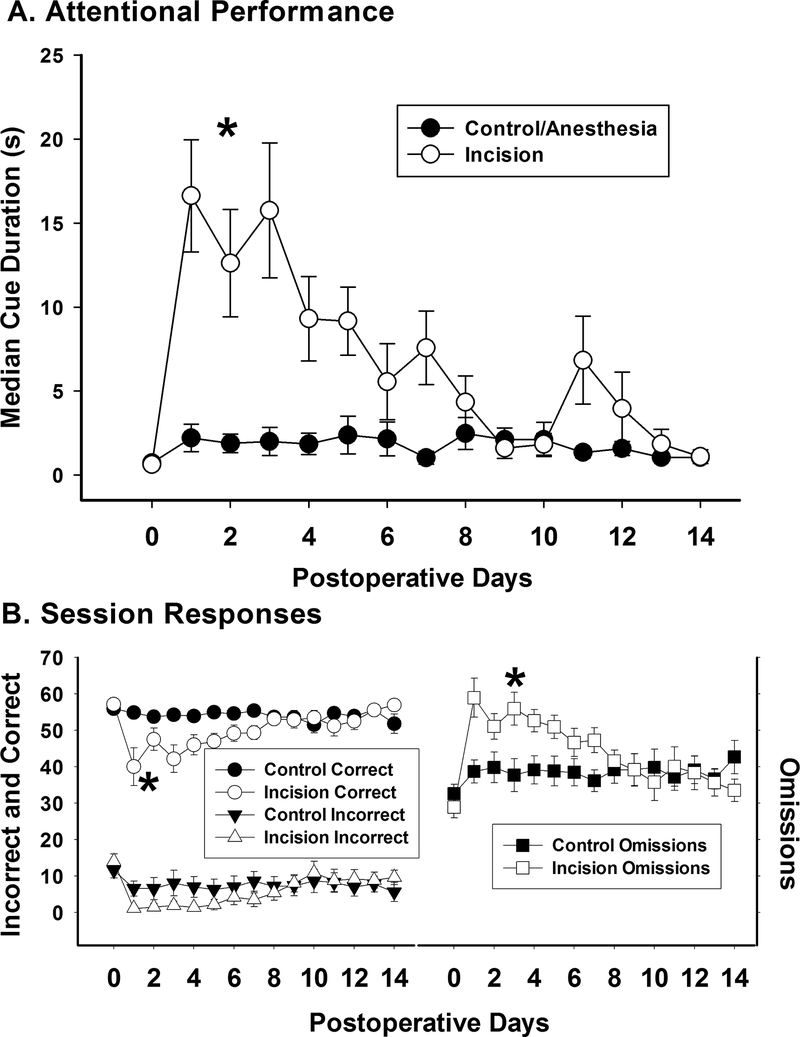
The baseline median cue duration (MCD), the measure of attentional performance in the 5CTV, was 0.6 ± 0.1 s in the sham group and 0.7 ± 0.1 s in the incision group. Paw incision increased the MCD and there was a significant effect of time (F(14,224)=7.08; P < 0.001) and treatment (F(1,224)=13.31; P = 0.002) on the MCD as well as an interaction (F(14,224)=5.92; P < 0.001) (fig. 2A). The MCD in the sham group with anesthesia alone was not different over time (F(8,14)=0.771; P = 0.70) (fig. 2A). The cumulative effect of incision (97.2 ± 56.8 seconds-days ) on performance in the 5CTV was a four-fold increase in area under the MCD-days curve compared to the anesthesia control (25.5 ± 14.5 seconds-days) representing a significant impairment in performance (t(16)=3.67; P = 0.002). Response data during the 5CTV are shown in Figure 2B. Paw incision decreased correct responses compared to anesthesia only (F(1,268)=20.36; P < 0.001) and increased the number of omission responses (F(1,168)=11.74; P < 0.001), while incorrect responses were not significantly different between groups (F(1,268)=2.85; P = 0.093) (fig. 2B). There was also a decrease in premature responses in the incision group compared to the control group (F(1,268)=15.88; P < 0.001). Representative plots of cue duration over trials are presented for a control animal and an incision animal for sessions at baseline and after incision or anesthesia only (fig. 3). The overall average latency to correct response was higher in the incision group (3.5 ± 2.6 s) compared to the control group (2.1 ± 1.3 s) (F(1,268)=35.66; P < 0.001) and the overall average latency to reward was higher in the incision group (7.4 ± 2.7 s) compared to the control group (2.4 ± 1.5 s) (F(1,268)=7.91; P=0.005). The overall average latency to incorrect responses was no different between the incision group (2.8 ± 3.3 s) compared to the control group (2.3 ± 2.0 s) (F(1,208)=1.95; P=0.164). However, while all animals had incorrect responses in all baseline sessions, there were more sessions with no incorrect responses in the incision group (36/126) compared to sessions with no incorrect responses in the control group (19/126) (X2(1, N=252)=6.72; P=0.009). The overall average rate of completion of trials within the sessions went down in the incision group (M 3.7 SD 0.7 trials/min) compared to the control group (4.4 ± 0.2 trials/min) (t(28)=−3.62; P = 0.001).
Figure 2. Effect of Incision MCD and Session Data.
A. Attentional performance was assessed using the MCD from trials 15–100 at baseline and for 14 days postoperatively from 18 animals (9 control and 9 incision animals). There was a significant increase in MCD after incision compared to control (*: one way repeated measures ANOVA between groups). B. Along with the increase in MCD, there was a significant increase in omissions and a significant decrease in correct responses (*: one way repeated measures ANOVA between groups). No difference in incorrect responses was present.
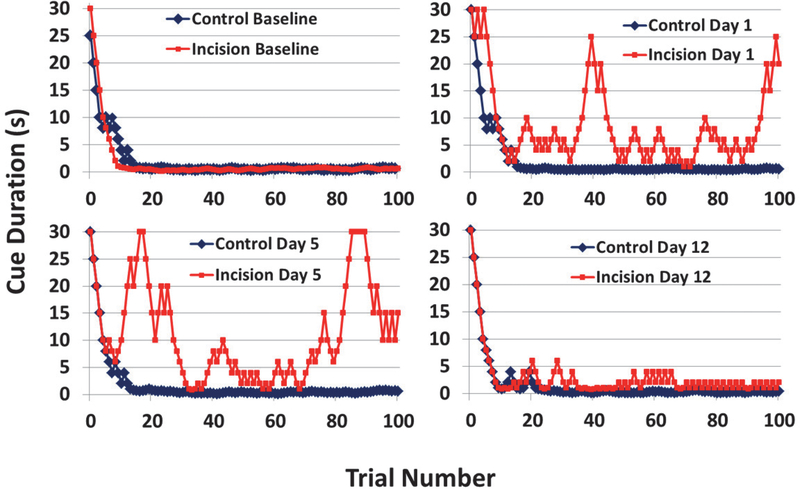
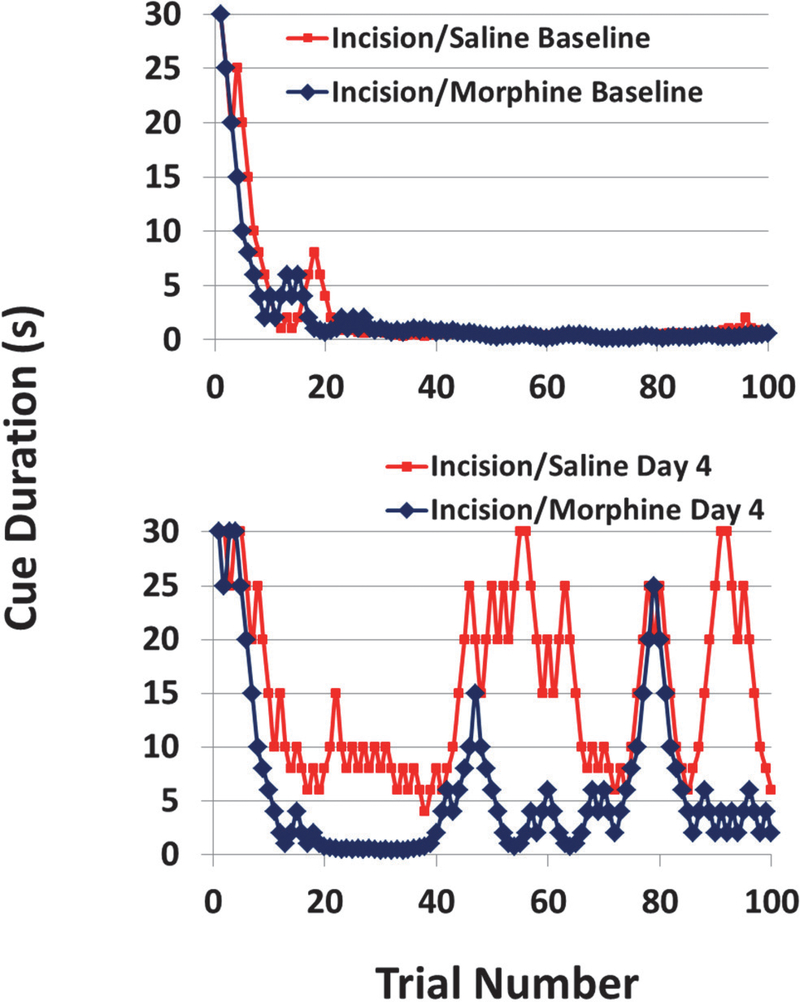
Figure 3. Representative Examples of the Effects of Incision in the 5CTV.
The blue is from a control/anesthesia only animal and the red is an animal that underwent anesthesia and incision. At baseline the behavior over the 100 trials is similar. On postoperative day 1 and 5 the inability of the animal to maintain performance after incision can readily be seen based on the persistent increase in cue duration and inability to maintain performance with a lower cue duration compared to the control animal. By day 12, there is still some reduced performance in the animal after incision, but very similar to the control and close to baseline performance.
Behavioral effects of Morphine on Performance in the 5CTV after Incision
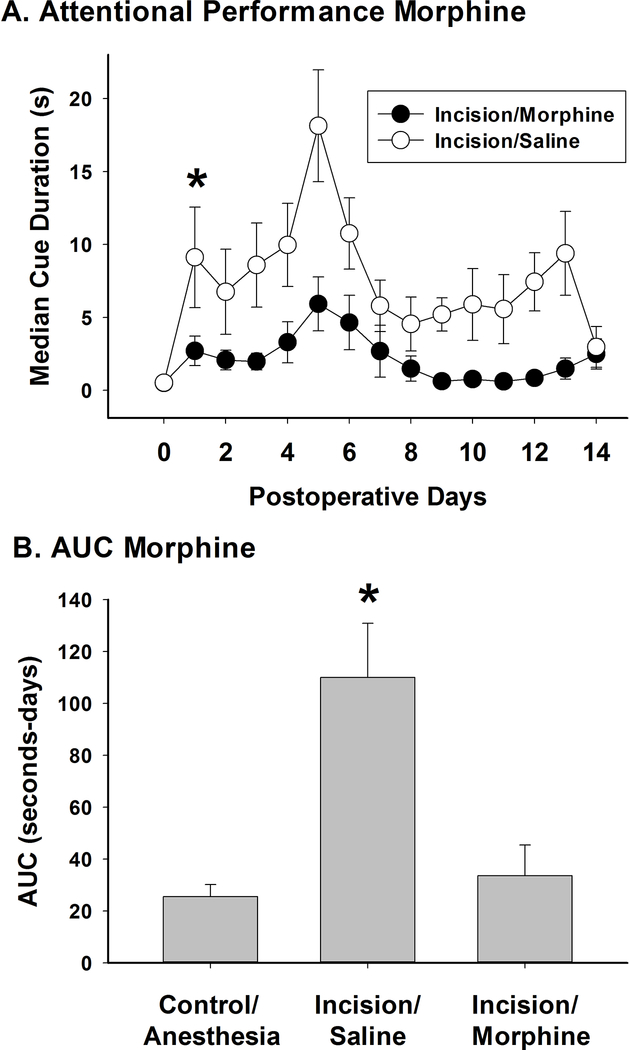
The baseline MCD was 0.5 ± 0.1 s in the incision/saline group and 0.5 ± 0.1 s in the incision/morphine group. A single dose of 1 mg/kg of morphine was used to verify the role of pain from the incision in reducing performance in the 5CTV, although a full dose response has been demonstrated using a different model of pain.17 This dose is an intermediate dose in efficacy for pain treatment in the rat and has no effect in normal animals in this task.17 MCD increased significantly over time compared to baseline in both the incision/saline group (F(14,149)=2.48; P = 0.004) and in the incision/morphine group (F(14,164)=2.27; P = 0.008). However, morphine significantly attenuated the increase in MCD after incision compared to saline (F(1,314)=10.99; P = 0.004) (fig. 4A). There was a cumulative effect of morphine on performance in the 5CTV F(2,28)=11.51; P < 0.001) and this is represented by a four-fold decrease in area under the MCD-days curve (31.6 ± 36.7 seconds-days) compared to the incision/saline control (110.0 ± 64.7 seconds-days) (t(20)=4.07; P < 0.001) (fig. 4B). This was also compared to the control anesthesia group (25.5 ± 14.5 seconds-days) and no difference in AUC was noted between the control/anesthesia group and the incision/morphine group (t(18)=0.30, P = 0.77). Representative plots of cue duration over trials are presented for sessions with an incision/saline and an incision/morphine animal at baseline and after incision (fig. 5).
Figure 4. Effect of Morphine or Saline on Performance in the 5CTV after Incision.
A. MCD was increased over time in both the incision/saline (N=10 animals) and the incision/morphine (N=11 animals) groups with a significant difference between the 2 groups (*: one way repeated measures ANOVA between groups). B. Using area under the MCD-time curve, the incision/saline group is significantly different from both the control/anesthesia (N=9 animals) group and the incision/morphine group (*: one way ANOVA between groups with pairwise comparisons using Holm-Sidak method) while no difference was seen between the control/anesthesia group and the incision/morphine groups.
Figure 5. Representative Examples of the Effects of Morphine on Incision in the 5CTV.
The blue is from a morphine/incision animal and the red is an incision/saline animal. At baseline the behavior over the 100 trials is similar. On postoperative day 4 the effect of morphine on improving performance and reducing the overall cue duration after incision can readily be seen when compared to the more impaired performance and more consistently higher cue duration over the trials in the session in the incision/saline animal.
There was an overall effect of morphine on correct responses after incision with an increase in correct responses (F(1,313)=43.45; P < 0.001) in the morphine group (53.9 ± 9.7) compared to the saline group (44.8 ± 14.5), and an overall effect of morphine (34.1 ± 11.6) decreasing omissions compared to the saline group (41.0 ± 9.2) after incision (F(1,313)=34.66; P < 0.001). Morphine also increased incorrect responses (9.6 ± 8.8) compared to saline (4.2 ± 7.6) after incision (F(1,313)=34.17; P < 0.001). The overall average latency to correct responses was lower in the morphine group (1.9 ± 1.7 s) compared to the saline group after incision (4.3 ± 3.4 s) (F(1,313)=67.55; P < 0.001) and the overall average latency to reward was lower in the morphine group (1.9 ± 0.7 s) compared to the saline group after incision (3.1 ± 2.2 s) (F(1,313)=44.51; P < 0.001). The overall average latency to incorrect responses was shorter in the morphine group (1.9 ± 1.9 s) compared to the saline group (3.6 ± 4.1 s) after incision (F(1,246)=20.49; P < 0.001). There were fewer sessions with no incorrect responses in the morphine group (16/149) compared to sessions with no incorrect responses in the saline groups (50/100) after incision, reversing the effect seen from the incision (X2(1, N=325)=29.21; P < 0.001). The overall average rate of completion of trials within the sessions was not different between the morphine (5.7 ± 7.4 trials/min) and the saline groups (5.6 ± 12.5 trials/min) after incision (F(1,313)=0.003; P = 0.96).
Single Spike Activity in the mPFC from Incision during 5CTV
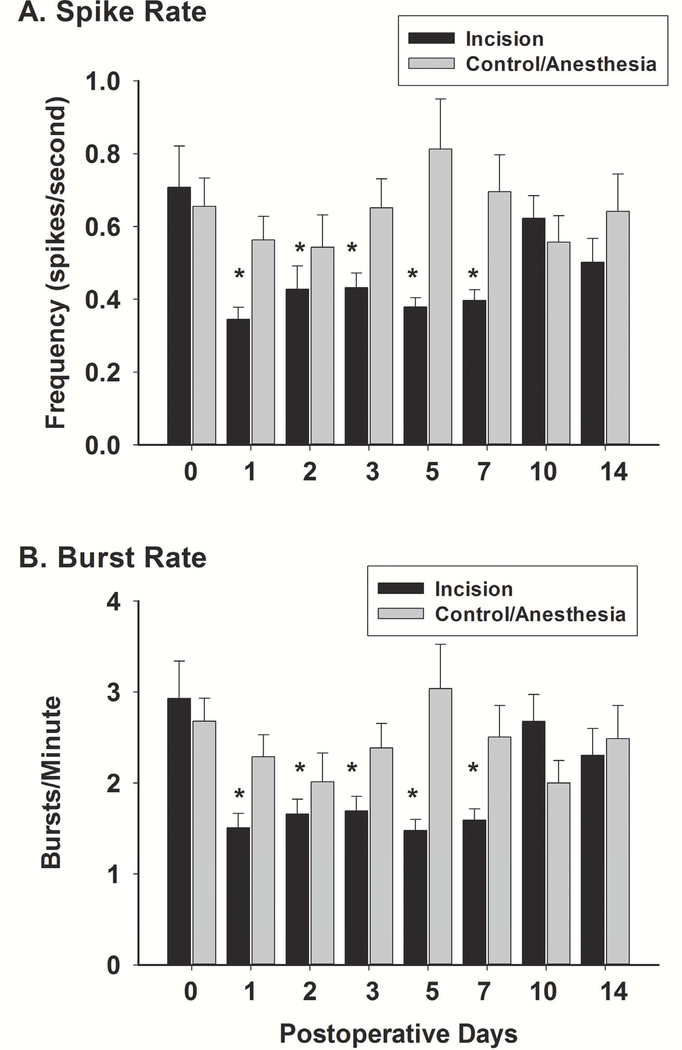
Neuronal spike analysis was performed from 24 distinct neuronal spikes isolated from 45 electrodes in 9 animals in the incision animals and 28 distinct neurons form 45 electrodes in 9 animals in the control sham group. Spike frequency during background sessions (dark chamber, no 5CTV) was found to be 0.38 ± 0.14 spikes/s and burst rate 1.43 ± 0.48 burst/min and these were increased to 0.68 ±0.48 spikes/s and 2.93 ± 2.02 burst/min during the 5CTV procedure (spike frequency F(2,77)=5.50, P = 0.006 and burst rate F(2,77)=9.22, P < 0.001). No difference was found between baseline control and incision group spike frequency and burst rate (incision 0.71 ± 0.56 spikes/s and control 0.66 ± 0.40 spikes/s and incision 2.93 ± 2.02 burst/min and control 2.68 ± 1.32 burst/min) (spike frequency (t(52)=0.391, P = 0.64 and burst rate t(52)=0.54, P = 0.594). There was an overall interaction effect of treatment and time on spike rate or spike frequency (F(7,350)=4.66; P < 0.001); with significant differences from POD1 through POD7 noted using Holms Sidak method for pairwise multiple comparisons (fig. 6A). Within group comparisons revealed that there was no overall effect of sham surgery on spike rate (F(7,216)=1.03; P = 0.412) while there was a reduction in spike frequency over time in the incision group (F(7,184)=4.214; P<0.001). Spike probability was a secondary outcome and only determined at baseline and only POD5 after incision or sham (see table 1). Spike probability was higher when the LED turned on and off for the correct responses while there was no difference between spike probability with the LED on and off during omissions. However, after incision the light off increased the spike probability for correct responses, while for incision there was a decrease in spike probability with light off with omissions. Behavior at baseline and postoperative day (POD) 5 is presented for comparison in Table 2.
Figure 6. Effects of Incision on Neuronal Action Potential Spike and Burst Rates.
Spikes and burst were determined from a total of 18 animals (N=9 control and N=9 incision animals). A total of 24 distinct neuronal spike were isolated from 45 electrodes in the incision animals and 28 distinct neurons in the control animals. A. There was no difference in spike rate between the incision and the control/anesthesia group at baseline. There was no difference in spike rate over time after sham anesthesia in the control. Spike rate was reduced significantly after incision on day one and remained so through day 7 (*: one way repeated measures ANOVA between groups with pairwise comparisons using the Holm-Sidak method). Thereafter no difference was noted. B. There was also no difference in burst rate between the incision and the control/anesthesia group at baseline. There was also no difference in burst rate over time after sham anesthesia in the control. Burst rate was reduced significantly after incision on day one and remained so through day 7 (*: one way repeated measures ANOVA between groups with pairwise comparisons using the Holm-Sidak method). Thereafter no difference was noted. Characteristics of spike in bursts are presented in Table 3 for baseline and postoperative day 5 only.
Table 1.
Behavior-Related Spike Probability
| Nose Poke Light |
||||
|---|---|---|---|---|
| Spike Probability | Spike Probability | Spike Probability | Spike Probability | |
| Pre | Post | House Light Off | Feed Reward | |
| Correct | 0.31 ± 0.11 | 0.42 ± 0.16 | NA | 0.51 ± 0.26 |
| Omission | 0.16 ± 0.09* | 0.16 ± 0.10* | 0.24 ± 0.16 | NA |
| Correct POD5 | 0.29 ± 0.17 | 0.59 ± 0.02† | NA | 0.39 ± 0.28 |
| Omission POD5 | 0.18 ± 0.11 | 0.04 ± 0.04*,‡ | 0.19 ± 0.12 | NA |
Spike probability is shown for the visual cue (Nose Poke Light) both before (Pre=any spike within 1 sec of cue light going on) and after (Post=any spike within 2 sec of cue light going off). Spike probability is shown for spikes within 1 sec after house light goes off after omission trials or for spikes within 2 sec of food trough head entry. Data were collected over 20 trials for each animal (N=11). Incorrect responses are too few to adequately evaluate. Data were analyzed using a paired t-test and P < 0.05 was considered significant. POD=postoperative day
different from correct
different from light on pre
different from baseline
Table 2.
Characteristics of Spikes in Bursts in Incision group after surgery
| Baseline | Postoperative Day 5 | Statistics | |
|---|---|---|---|
| Burst Number (total) | 62.8 ± 45.7 | 39.2 ± 13.4 | T(23)=2.442, P = 0.023 |
| Burst Rate (number/minute) | 2.93 ± 2.02 | 1.48 ± 0.58 | T(23)=3.364, P = 0.003 |
| spikes in bursts (percent) | 57.6 ± 9.9 | 62.2 ± 8.4 | T(23)=−1.762, P = 0.091 |
| Mean frequency (Hertz) | 74 ± 36 | 70 ± 25 | T(23)=0.493, P = 0.627 |
| Mean burst duration (seconds) | 0.95 ± 0.81 | 1.46 ± 1.11 | T(23)=−2.654, P = 0.014 |
| Mean spikes in bursts (number) | 8.3 ± 1.8 | 10.1 ± 3.4 | T(23)=−2.300, P = 0.031 |
| Mean Peak frequency (Hertz) | 401 ± 88 | 406 ± 57 | T(23)=−0.384, P = 0.704 |
| Mean interburst interval (seconds) | 27.4 ± 14.9 | 46.2 ± 19.8 | T(23)=−4.648, P < 0.001 |
Baseline session and POD5 session in incision group. Significance was tested using the paired t- test and statistical data shown for the analysis. P < 0.05 is considered significant.
Burst rate was determined for each session. There was an overall interaction between treatment and time on burst rate (F(7,350)=5.99; P < 0.001) with significant differences from POD1 through POD7 noted using Holms Sidak method for pairwise multiple comparisons (fig. 6B). Within group comparisons revealed that there was no overall effect of sham on spike rate (F(7,216)=1.16; P = 0.327), while there was a reduction in spike frequency over time in the incision group (F(7,184)=5.49; P < 0.001). Characteristics of spikes in bursts were secondary outcome measures and only analyzed at baseline and on POD5 after incision or sham (see Table 3).
Table 3.
Behavior for Baseline, Spike Probability, and Burst Characteristics Postoperative Day 5
| Baseline | Postoperative Day 5 | Overall Statistics | |
|---|---|---|---|
| Median Cue Duration (seconds) | 0.6 ± 0.3 | 9.2 ± 6.1 | T(11)=4.274; P = 0.003 |
| Correct (total) | 57.1 ± 3.2 | 46.9 ± 6.5 | T(11)=5.145; P = 0.063 |
| Incorrect (total) | 9.2 ± 6.1 | 0.6 ± 0.3 | T(11)=4.274; P = 0.003 |
| Omissions (total) | 28.9 ± 8.8 | 50.9 ± 9.6 | T(11)=7.645; P < 0.001 |
| Premature (total) | 8.3 ± 5.7 | 1 ± 1.6 | T(11)=4.158; P = 0.003 |
| Perseverative (total) | 3.7 ± 3.3 | 2.9 ± 2.9 | T(11)=0.428; P = 0.68 |
LFP activity changes in the mPFC from Incision during 5CTV
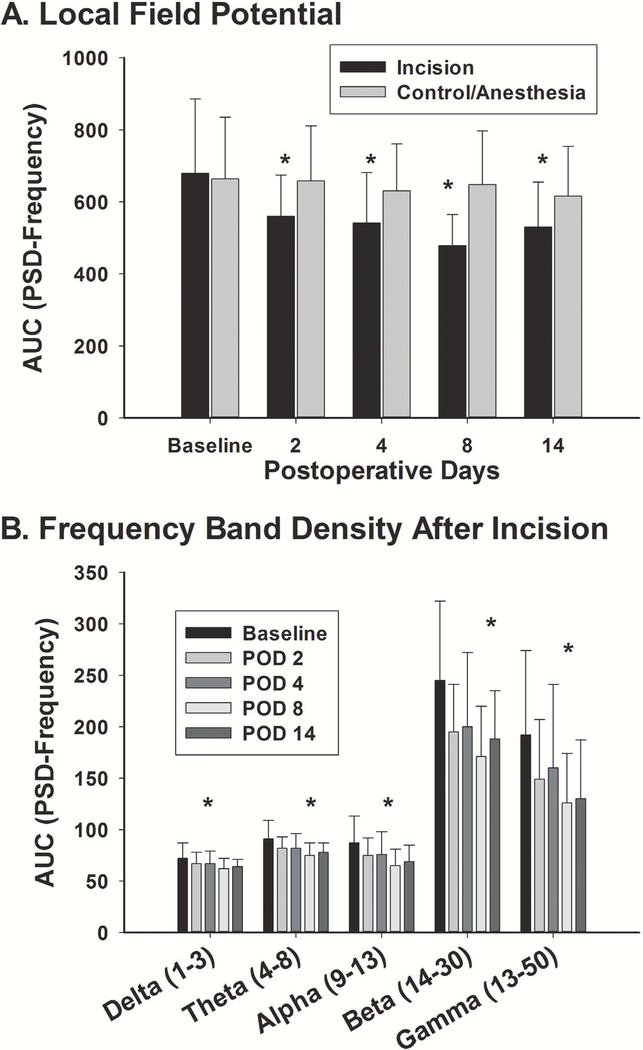
Local field potentials (LFP) were analyzed from 21 distinct neurons isolated from 45 electrodes in 9 animals in the incision animals and 28 distinct neurons form 45 electrodes in 9 animals in the control sham group. One electrode was used as a ground to reduce noise from areas outside of the location of the electrodes. The LFPs were analyzed comparing area under curve (AUC) for the power spectral density (PSD) (in dB or log of the power)-frequency curve from 0–100 Hz from baseline after incision or in sham control. There was an overall interaction effect of treatment and time on LFP (F(4,235)=2.44; P = 0.048) (fig. 7A). Within group comparisons showed the time effect was only present for the incision group (F(4,100)=5.812; P < 0.001) with a reduction in LFP AUC of the PSD-frequency curve that was apparent at POD 2 and remained through POD 14 using Holms Sidak method. This time-dependent effect was present at each of the frequencies (delta [1–3 Hz], theta [4–8 Hz], alpha [9–13 Hz], beta [14–30 Hz], and low gamma [30–50 Hz]) (fig. 7B). There was no difference in LFP over time after sham anesthesia (F(4,135)=0.487; P = 0.745).
Figure 7. Effects of Incision on Local Field Potentials.
The power spectral density is the log of the frequency domain of the local field potentials in the mPFC and was determined from 21 electrodes (N=9 animals for control) for control and 28 electrodes for incision (N= 9 incision animals) over a 900-s epoch during trials 15–100. A. There was no difference in baseline LFP between control and incision groups. There was no difference in LFP over time after sham anesthesia in the control. After incision, there was a significant decrease in LFP that persisted through day 14 (*: one way repeated measures ANOVA between groups with pairwise comparisons using the Holm-Sidak method) at which point the behavior had returned to normal. B. The LFP were further broken down into specific frequency bands to determine if any one band had a different behavior. No frequency band was different from the overall effect on LFP as a time dependent effect was present at all of the frequencies (*: one way repeated measures ANOVA over time at each frequency band revealed a similar significant effect at each frequency for the incision group for all times) (delta [1–3 Hz], theta [4–8 Hz], alpha [9–13 Hz], beta [14–30 Hz], and low gamma [30–50 Hz]).
Discussion
These data establish that impaired attentional performance results from incision and that nociceptive input plays a role since morphine attenuates this impairment. The functional impact of paw incision is readily assessed using the 5CTV longitudinally until resolution. This study corroborates the effect of nociceptive input on attentional performance and extends these findings by suggesting a role for mPFC neuronal activity in the effect of incisional nociceptive input as measured by the increased MCD with associated changes in individual spike activity and LFP power.17 These changes in neuronal activity after incision are distinct from changes induced by audiovisual distraction and suggest that interference from pain is not due to competing distracting stimuli.16,24
Attentional deficits from pain are reported.17,24–29 Interestingly, nerve injury did not induce deficits until 2 weeks after injury and were most marked months later. While chronic pain and acute postoperative pain may be different, one would anticipate acute postoperative nerve injury would cause disruption early. Alternatively, the standard 5C may be less sensitive at detecting impairment. Enhanced sensitivity of the 5CTV to detect impairment may be from cue titration to performance capability instead of measuring success rates in a paradigm with fixed difficulty.15–17 The titration method results in cue changes until reaching the limits of performance capability. Thus, the 5CTV seems positioned to detect small decrements in capability based on maximal performance in a given animal through enhanced sensitivity to both disruption and reversal with analgesics.24,29 The ability of the 5CTV to detect individual differences within sessions and individual differences in response to surgery are unique and powerful. This may partially explain some of the differences in responses to incision in cohorts in the study between the incision group in the sham initial experiment and the incision group in the morphine experiment.
Time and ability to respond to cue change likely reflect either a delay in processing speed or a decision to move more slowly. Based on video observation slower or less movement seems unlikely, although not specifically measured. Additionally, incision did not impair total distance travelled in open field analysis previously.30 The decreased performance seems to be the “decision” to omit or the inability to process the cue resulting in increased cue duration reflected in more omissions and less correct responses. Motivation could be a factor, and while motivation was not measured directly, it has been suggested that disruption in attentional tasks from pain is related to decreased processing speed and not decreased motivation.25
Measuring mPFC neuronal activity in behaving animals during these tasks allows further validation of its role in attentional performance and nociceptive processing. Attention-related burst firing of individual neurons occurs in PFC.31 In this study spike frequency and bursting decreased with nociception generated by incision. The character of the bursts also changed with increased burst duration, increased spikes within a burst, and increased inter-burst interval after injury. Neuronal bursts may globally increase neurotransmitter release and information processing, whereas reduced burst activity may attenuate the signal transmission efficiency.32,33 Thus, after injury signaling may be dampened as a result of ongoing nociceptive input. Interestingly, increased bursts seen with distraction and decreased bursts with nociceptive input both are associated with reduced performance in the 5CTV and other behaviors.13,15,17 This may be why nociceptive input appears to be different than distraction from the perspective of neuronal activity in the mPFC despite the similar behavior. Such findings are consistent with both hyperactivity and hypoactivity of the mPFC producing disruption in the classical 5 choice.
Nociceptive input may have a dual effect on PFC activity. The immediate response to nociceptive input is intensity-dependent increased activity.19 However, nociceptive activation also induces mPFC deactivation that may be modulated by dopamine from amygdala.34,35 This is consistent with reduced activity of total spike frequency as well as mPFC bursts in our freely moving animals during the task. The decreased activity may result from increased GABAergic tone.35 Increased synaptic activity might result in an increase in LFP from activation of inhibitory neurons and release of GABA in large quantities. This was likely not the case as LFP was also reduced. These relationships were previously studied under anesthesia so the awake or behaving state could impact these relationships. Differences in immediate and delayed nociceptive input could be from a feed forward circuit and while acute pain from surgery may activate mPFC, the longer term nociceptive input likely increases activity elsewhere to reduce mPFC output. Initial pain activation of mPFC would result in heightened attention, awareness, and focus. Persistent nociceptive input to the brain, likely through amygdala activation, produces a predominance of inhibitory input to mPFC to reduce activity and impair attentional performance.36 Neuronal activity was measured in the prelimbic (PL)-PFC and studies suggest that noxious input increases activity in PL-PFC alone, but increased activity in infra limbic (IL)-PFC may reduce or inhibit activity of pyramidal cells in the PL area.37 Thus, the reduction in bursts and spike frequency in PL-PFC seen as a result of incision could also be related to increased activity in IL modulating PL-PFC. This increased activity in IL-PFC may be a compensatory mechanism to nociceptive input persisting beyond the immediate first pain response permitting the animal to function by reducing the impact of the negative consequence of increased activity within the nociceptive circuit.38 This may be the very modulation that permits the animal to function, although with reduced maximal performance, with persistent and ongoing nociceptive input while the surgical injury is healing. Reduced cholinergic input to PFC from nociceptive input could also reduce activity in PFC since cholinergic input is considered an amplification mechanism for local glutamatergic circuits necessary for cue detection.26 It is possible that opioids may partially release this modulation to improve performance. Regardless of the etiology of reduced PFC activity, PFC is affected by pain as demonstrated by structural changes from pain as early as one week after injury.27 Further studies to understand opioid and non-opioid analgesics in improving attentional performance and altering neural responses in the face of nociceptive input will be valuable to understand the implications for when these pharmacologic interventions may improve attentional function or lead to decrements in cognitive and executive function.
Composite electrical LFP signals were measured which have contributions from multiple neuronal and non-neuronal processes.39 The mPFC LFP activity likely plays a role in memory and attentional processes and contains synaptic processing information from many sources distinct from information obtained from spiking activity.40 In our study we limited LFP activity to truly “local” electrical activity to reduce interference from distant sources, particularly motor noise.41 This was done by using a local electrode as reference instead of the wire on the skull. This reduces non-neural and non-local contamination from the reference electrode and movement. Gamma oscillations have been largely attributed to volume conducted noise and the local reference reduces gamma power immensely such that in our studies the high gamma (50–100 Hz) is so low that interpretation is not meaningful.41 Separation of LFP into distinct bands is supported by associations of band limited power signals with certain behavioral states or sensory inputs.39 Theta bands in particular are thought to be associated with learning and attention.40 In our study, all frequency bands were decreased in response to incision and remained so throughout the course of the study. Further studies involving event related changes will be valuable, particularly for understanding theta oscillations in PFC with respect to correct and incorrect responses and future response successes as a dynamic learning process altered from nociceptive input. Likely the specific signal correlations will emerge with this more granular approach and lead to greater understanding of the role of nociceptive input leading to altered processing in PFC. Further understanding will also come from defining the relationship between frequency band specific oscillations and spike probability as a function of the event outcome. This should include understanding the firing patterns and abnormalities in firing and synchrony of neural activity that underlies specific behavioral changes. Additionally, one limitation of our study was recording only on the right side (opposite the injury). Understanding the role of both sides, their interaction and the laterality may be important to understanding the impaired attention.
Pain may alter attention by increasing attentional load, disrupting information processing and altering input. The association of nociceptive input with the reduced PL-mPFC synaptic activity measured by decreased LFP power or reduced spike and burst activity may underlie the inability to maintain attention and perform maximally. These effects of incision extend beyond the day of anesthesia and surgery and may reduce attentional performance for days after a small procedure. Future improvement in understanding the effects of pain and therapeutic interventions will come from resolving changes occurring during specific actions in the 5CTV and will involve event-related spike and potential analysis. Further study is warranted as recovery time ramifications exist from the standpoint of return to optimal functioning in daily activities. This is particularly salient for the return of patients to activities with higher risks requiring maximal attention and optimum performance for the greatest safety for the patient and those affected by their performance.
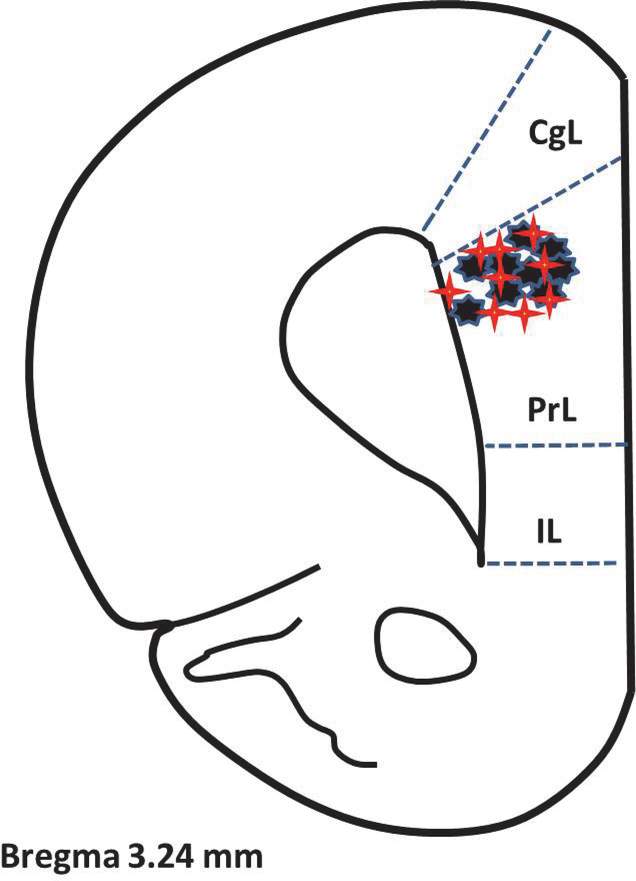
Figure 8. Location of the electrodes in the prelimbic area of the medial prefrontal cortex at the conclusion of the experiments.
Diagram from Watson and Paxinos. There were five electrodes placed in each animal, but the location of each was in close proximity to the other for each animal (N=18) and the five are represented by a single red star (control) or black star (incision).
Acknowledgments
Research Support
Funding provided by the National Institutes of Health through grants NS074357 (TJM), GM113852 (TJM, MDB, DGR, JCE), and GM104249 (DGR, TJM).
Footnotes
Competing Interests
No conflicts of interest to report.
References
- 1.Veldhuijzen DS, van Wijck AJ, Wille F, Verster JC, Kenemans JL, Kalkman CJ, Olivier B, Volkerts ER: Effect of chronic nonmalignant pain on highway driving performance. Pain 2006; 122:28–35 [DOI] [PubMed] [Google Scholar]
- 2.Dale MT, Naik R, Williams JP, Lloyd AJ, Thompson JP: Impairment of sustained attention after major gynaecological surgery. Eur J Anaesthesiol 2005; 22:843–7 [DOI] [PubMed] [Google Scholar]
- 3.Keogh E, Moore DJ, Duggan GB, Payne SJ, Eccleston C: The disruptive effects of pain on complex cognitive performance and executive control. PLoS One 2013; 8:e83272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dick BD, Rashiq S: Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg 2007; 104:1223–9 [DOI] [PubMed] [Google Scholar]
- 5.Grisart JM, Plaghki LH: Impaired selective attention in chronic pain patients. Eur J Pain 1999; 3:325–33 [DOI] [PubMed] [Google Scholar]
- 6.Eccleston C, Crombez G: Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 1999; 125:356–66 [DOI] [PubMed] [Google Scholar]
- 7.Veldhuijzen DS, Kenemans JL, de Bruin CM, Olivier B, Volkerts ER: Pain and attention: attentional disruption or distraction? J Pain 2006; 7:11–20 [DOI] [PubMed] [Google Scholar]
- 8.Villemure C, Schweinhardt P: Supraspinal pain processing: distinct roles of emotion and attention. Neuroscientist 2010; 16:276–84 [DOI] [PubMed] [Google Scholar]
- 9.Eccleston C: Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther 1995; 33:391–405 [DOI] [PubMed] [Google Scholar]
- 10.Bari A, Dalley JW, Robbins TW: The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 2008; 3:759–67 [DOI] [PubMed] [Google Scholar]
- 11.Amitai N, Markou A: Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behav Neurosci 2011; 125:764–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalley JW, Cardinal RN, Robbins TW: Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 2004; 28:771–84 [DOI] [PubMed] [Google Scholar]
- 13.Pezze M, McGarrity S, Mason R, Fone KC, Bast T: Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J Neurosci 2014; 34:7931–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins TW: The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002; 163:362–80 [DOI] [PubMed] [Google Scholar]
- 15.Martin TJ, Grigg A, Kim SA, Ririe DG, Eisenach JC: Assessment of attention threshold in rats by titration of visual cue duration during the five choice serial reaction time task. J Neurosci Methods 2015; 241:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ririe DG, Boada MD, Schmidt BS, Martin SJ, Kim SA, Martin TJ: Audiovisual distraction increases prefrontal cortical neuronal activity and impairs attentional performance in the rat. J Exp Neurosci 2017; 11:1179069517703080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin TJ, Strassburg TJ, Grigg AL, Kim SA, Ririe DG, Eisenach JC: Assessment of behavioral disruption in rats with abdominal inflammation using visual cue titration and the Five-choice Serial-reaction Time Task. Anesthesiology 2017; 127:372–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill TM, Sarter M, Givens B: Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci 2000; 20:4745–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Tomida M, Katayama Y, Kawakami Y: Response durations encode nociceptive stimulus intensity in the rat medial prefrontal cortex. Neuroscience 2004; 125:777–85 [DOI] [PubMed] [Google Scholar]
- 20.Nicolelis MA, Lin RC, Woodward DJ, Chapin JK: Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of awake rats. Proc Natl Acad Sci U S A 1993; 90:2212–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ririe DG, Vernon TL, Tobin JR, Eisenach JC: Age-dependent responses to thermal hyperalgesia and mechanical allodynia in a rat model of acute postoperative pain. Anesthesiology 2003; 99:443–8 [DOI] [PubMed] [Google Scholar]
- 22.Lobb C: Abnormal bursting as a pathophysiological mechanism in Parkinson’s disease. Basal Ganglia 2014; 3:187–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legéndy CR, Salcman M: Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 1985; 53:926–39 [DOI] [PubMed] [Google Scholar]
- 24.Pais-Vieira M, Lima D, Galhardo V: Sustained attention deficits in rats with chronic inflammatory pain. Neurosci Lett 2009; 463:98–102 [DOI] [PubMed] [Google Scholar]
- 25.Higgins GA, Silenieks LB, Van Niekerk A, Desnoyer J, Patrick A, Lau W, Thevarkunnel S: Enduring attentional deficits in rats treated with a peripheral nerve injury. Behav Brain Res 2015; 286:347–55 [DOI] [PubMed] [Google Scholar]
- 26.Sarter M, Paolone G: Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci 2011; 125:825–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M: Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A 2009; 106:2423–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freitas KC, Hillhouse TM, Leitl MD, Negus SS: Effects of acute and sustained pain manipulations on performance in a visual-signal detection task of attention in rats. Drug Dev Res 2015; 76:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyette-Davis JA, Thompson CD, Fuchs PN: Alterations in attentional mechanisms in response to acute inflammatory pain and morphine administration. Neuroscience 2008; 151:558–63 [DOI] [PubMed] [Google Scholar]
- 30.Ewan EE, Martin TJ: Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg 2014; 118:854–62 [DOI] [PubMed] [Google Scholar]
- 31.Womelsdorf T, Ardid S, Everling S, Valiante TA:Burst firing synchronizes prefrontal and anterior cingulate cortex during attentional control. Curr Biol. 2014. November 17;24(22):2613–21. [DOI] [PubMed] [Google Scholar]
- 32.Cooper DC: The significance of action potential bursting in the brain reward circuit. Neurochem Int 2002; 41:333–40 [DOI] [PubMed] [Google Scholar]
- 33.Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC: Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci 2003; 26:161–7 [DOI] [PubMed] [Google Scholar]
- 34.Onozawa K, Yagasaki Y, Izawa Y, Abe H, Kawakami Y. Amygdala-prefrontal pathways and the dopamine system affect nociceptive responses in the prefrontal cortex. BMC Neurosci 2011; 12:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji G, Neugebauer V: Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J Neurophysiol 2011; 106:2642–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V: Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 2010; 36:5451–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji G, Neugebauer V: Modulation of medial prefrontal cortical activity using in vivo recordings and optogenetics. Mol Brain 2012; 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A: Neuronal circuits of fear extinction. Eur J Neurosci 2010; 31:599–612 [DOI] [PubMed] [Google Scholar]
- 39.Einevoll GT, Kayser C, Logothetis NK, Panzeri S: Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci 2013; 14:770–85 [DOI] [PubMed] [Google Scholar]
- 40.Benchenane K, Tiesinga PH, Battaglia FP: Oscillations in the prefrontal cortex: A gateway to memory and attention. Curr Opin Neurobiol 2011; 21:475–85 [DOI] [PubMed] [Google Scholar]
- 41.Whitmore NW, Lin SC: Unmasking local activity within local field potentials (LFPs) by removing distal electrical signals using independent component analysis. Neuroimage 2016; 132:79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]