Summary
Platelets play a critical role at the interphase of thrombosis and inflammation, key features in haemolysis-associated disorders. Exercising this role requires expression of pattern recognition receptors by platelets, including toll-like receptor 4 (TLR4) and nucleotide-binding domain leucine rich repeat containing protein 3 (NLRP3), the latter forming intraplatelet multiprotein inflammasome complexes. Platelets are a potential target of various damage-associated molecular pattern (DAMP) molecules, such as free haem, a degradation by-product of haemoglobin oxidation during haemolysis, and high-mobility group box 1 (HMGB1), a DNA-binding protein released by dying or stressed cells and activated platelets. We have recently identified platelet TLR4, NLRP3, and Bruton tyrosine kinase (BTK) as critical regulators of platelet aggregation and thrombus formation, suggesting that the BTK inhibitor ibrutinib is a potential therapeutic target. Increasing evidence suggests that these and other DAMP-driven signalling mechanisms employed by platelets might be key in mediating inflammation and thrombosis encountered in haemolytic disorders. However, the precise regulatory triggers and their clinical relevance are poorly understood. We provide new insights into these less-well characterised platelet mechanisms, which are potentially targetable in haemolytic disorders.
Keywords: platelets, inflammation, haemolysis, sickle cell disease, thrombosis
An accelerated destruction of red blood cells (haemolysis) is a critical feature of various inherited and acquired disorders that are intrinsically linked with vascular thrombosis and inflammation, including sepsis (Levi et al, 2013), valveinduced haemolysis (Edmunds et al, 1996), thrombotic thrombocytopenic purpura/haemolytic uraemic syndrome (HUS) (George & Nester, 2014), autoimmune haemolytic anaemias (Hendrick, 2003), thalassaemia (Eldor & Rachmilewitz, 2002) and sickle cell disease (SCD) (Ataga et al, 2007). Typical thrombotic complications in patients with haemolytic disorders are venous thromboembolism, pulmonary thrombosis and stroke (Hendrick, 2003; Stein et al, 2006; Ataga et al, 2007; Au et al, 2009; Levi et al, 2013). Several mechanisms may underlie the pathophysiology of the hypercoagulable and inflammatory state in haemolysis.
In SCD and thalassaemia, red blood cells express phosphatidylserine in significant amounts on the cell surface (Eldor & Rachmilewitz, 2002; Ataga et al, 2007), which promotes endothelial cell adhesion and triggers activation of thromboinflammatory pathways (Zwaal & Schroit, 1997; Setty et al, 2002). Tissue factor is a key initiator of coagulation and is upregulated on circulating mononuclear cells in SCD patients in steady state and pain crisis (Key et al, 1998). Moreover, plasma levels of procoagulant soluble CD40 ligand (sCD40L) derived from platelets are increased in SCD patients at steady state and further elevated in patients with painful crises (Lee et al, 2006).
Haemoglobin released from the ruptured red blood cells is oxidised to free haem, which regulates transcription of antioxidant enzymes, such as haem-oxygenase 1 (Ogawa et al, 2001). Free haem and other molecules, such as adenosine diphosphate (ADP) and uric acid, act as erythrocytederived damage-associated molecular pattern molecules (DAMPs) that promote thrombosis and inflammation (Gladwin & Ofori-Acquah, 2014). The DNA-binding protein high-mobility group box 1 (HMGB1) is a non-erythrocyte DAMP associated with haemolysis and abnormal coagulation, including sepsis (Wang et al, 1999), disseminated intravascular coagulation (DIC) (Hatada et al, 2005), trauma (Levy et al, 2007), HUS (Lee et al, 2013) and SCD (Xu et al, 2014). HMGB1 plasma levels are elevated in SCD patients at baseline and further increased during acute sickling events (Xu et al, 2014). HMGB1 induces thrombosis and inflammation when exported to the cell surface or released into the extracellular space by stressed or dying cells and activated platelets (Rouhiainen et al, 2000; Andersson & Tracey, 2011; Maugeri et al, 2012; Gawaz & Vogel, 2013; Vogel et al, 2014, 2015a,b, 2016). The effects of HMGB1 upregulation in the context of haemolysis are poorly understood.
HMGB1 and haem signal through pattern recognition receptors, including toll-like receptor 4 (TLR4) (Park et al, 2004, 2006; Figueiredo et al, 2007; Belcher et al, 2014) and nucleotide-binding domain leucine rich repeat containing protein 3 (NLRP3) (Dutra et al, 2014; Li et al, 2014; Chi et al, 2015). Platelets express TLRs and nucleotide-binding oligomerization domain-like receptors (NLRs) (Shiraki et al, 2004; Hottz et al, 2013), implying that platelets are a putative target of these haemolysis-associated DAMPs. In a trauma/haemorrhagic shock model in mice with platelet-specific ablation of HMGB1, we have shown that platelets are the major source of HMGB1 within thrombi and that HMGB1 derived from platelets is a critical mediator of thrombosis and inflammation, via activation of a non-canonical TLR4/MyD88/soluble guanylate cyclase (sGC) platelet signalling pathway that was dependent on expression of platelet P-selectin (SELP, CD62P) (Vogel et al, 2015a). In another recent study, we have identified NLRP3 and Bruton tyrosine kinase (BTK) in platelets as critical co-regulators of platelet aggregation and thrombus formation (Murthy et al, 2017).
Here, we highlight the current evidence on these less-well characterised platelet danger signalling mechanisms in the context of haemolysis-associated thrombosis and inflammation, with an emphasis on SCD.
Platelets in sickle cell disease
SCD is an inherited blood disorder, caused by the presence of an abnormal haemoglobin (HbS). Polymerization of the deoxygenated HbS leads to “sickling” of red blood cells, followed by adhesion of the sickled erythrocytes to vascular endothelium, neutrophils and platelets. These cell interactions set off a cascade of downstream pathophysiological mechanisms that underlie the two hallmarks of the disease – chronic haemolysis and recurrent vaso-occlusions (Zhang et al, 2016; Thein et al, 2017). Patients with SCD experience a wide range of symptoms, including acute pain, chronic pain and multiorgan failure, due to a combination of pathological factors arising from haemolysis, inflammation and thrombosis (Thein et al, 2017). The high rate of red blood cell breakdown and release of cell-free haemoglobin and haem deplete endogenous haptoglobin and haemopexin, leading to free circulating haem, which triggers vaso-occlusions and inflammation (Belcher et al, 2014). Treatment options of SCD are still limited. Established disease management options include the myelosuppressive agent hydroxycarbamide, blood transfusions, penicillin prophylaxis, vaccinations against Streptococcus penumoniae and other pathogens, and patient education.
Even under SCD steady state conditions, circulating platelets are significantly activated in patients, and are further activated in vaso-occlusive crisis and acute clinical events, such as acute chest syndrome (Westwick et al, 1983; Wun et al, 1997; Villagra et al, 2007; Garrido et al, 2017). Beyond their role in haemostasis, platelets provide a link between thrombosis and inflammation (Semple et al, 2011; Rondina et al, 2013; Morrell et al, 2014; Vogel et al, 2015a, 2016; Murthy et al, 2017). More than two decades ago, Brittain et al (1993) proposed that activation of platelets has a contributory role in sickle vaso-occlusions. Platelets are capable of forming aggregates with monocytes and neutrophils; the aggregates were significantly elevated in SCD patients (Frelinger et al, 2014) and caused arteriolar platelet-neutrophil microemboli in the lungs of transgenic humanised SCD mice (Bennewitz et al, 2017). Platelets also facilitated the formation of circulating neutrophil-red blood cell aggregates in SCD patients in steady state, potentially contributing to the vaso-occlusion pathophysiology (Dominical et al, 2014).
The platelet activation marker P-selectin plays a critical role in the formation of platelet-neutrophil aggregates. Lung vascular permeability in sickle mice was significantly improved in the presence of a neutralising P-selectin antibody or the platelet inhibitor clopidogrel (Polanowska-Grabowska et al, 2010). Transgenic sickle mice deficient in P-selectin and E-selectin showed defective leucocyte recruitment to the vessel wall and were protected from vaso-occlusions (Turhan et al, 2002). In a double-blind, randomised phase 2 trial, SCD patients that received crizanlizumab, a P-selectin neutralising antibody, had significantly lower annual rates of sickle cell-related vaso-occlusive pain crises, suggesting that P-selectin is a therapeutic target in SCD (Ataga et al, 2017). On the other hand, the platelet inhibitor prasugrel did not significantly affect the rate of vaso-occlusive crises in children and adolescents with SCD in phase 3 trial (Heeney et al, 2016), indicating that further studies investigating the effectiveness of various platelet-specific targets in SCD are warranted.
A hypercoagulable state is known to contribute to the occurrence of vascular events in the microcirculation in SCD, which is at least partly mediated by upregulated activation and aggregation of circulating platelets (Ataga & Key, 2007; De Franceschi et al, 2011; Sparkenbaugh & Pawlinski, 2013). The specific mechanisms that control these activated platelet responses, and their pathophysiological significance for thromboinflammatory processes in SCD and other haemolytic conditions, however, are still poorly understood.
Pattern recognition receptor signaling in platelets
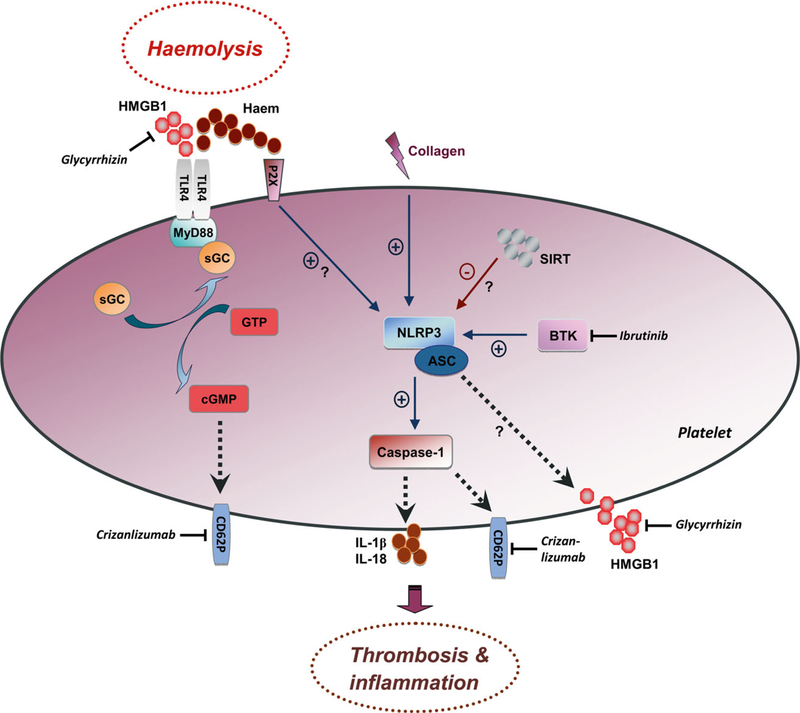
The NLRP3 inflammasome is a multiprotein complex expressed in immune cells and platelets that consists of the pattern recognition receptor NLRP3, apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (CARD) (ASC), caspase-1 and BTK (Agostini et al, 2004; Willingham et al, 2009; Hottz et al, 2013; Ito et al, 2015; Liu et al, 2017; Murthy et al, 2017). Activation of the NLRP3 inflammasome upregulates caspase-1 activity and triggers the production of bioactive interleukin-1b and other proinflammatory cytokines, such as interleukin-18 (Fig 1).
Fig 1.
Schematic overview of how platelets may orchestrate thrombosis and inflammation in haemolysis. Extracellular HMGB1 and haem are haemolysis-associated DAMPs that trigger pattern recognition receptor signalling pathways in platelets. HMGB1 signals via platelet TLR4, inducing complex formation between MyD88 and sGC leading to upregulation of enzymatic activity of sGC and upregulated platelet CD62P expression. HMGB1 sensing and secretion by platelets may also be controlled by intraplatelet NLRP3, which cooperates with ASC and caspase-1 within inflammasome complexes that activate IL-1b and IL-18 signalling. BTK and SIRT are critical regulators of the NLRP3 inflammasome. Free haem may activate both plateletspecific TLR4 and NLRP3. Activation of NLRP3 may or may not depend on purinergic P2X receptors. TLR4 and NLRP3 signalling in platelets upregulates expression of CD62P on the platelet surface. Expression of CD62P and secretion/release of IL-1b, IL-18 and HMGB1 by activated platelets are key events in regulating thrombosis and inflammation. Therapeutics that potentially target these platelet mechanisms include: Crizanlizumab, a P-selectin neutralizing antibody; glycyrrhizin, an HMGB1 inhibitor with potent inhibitory effects on platelet-specific HMGB1 signalling; and ibrutinib, an orally administered selective and covalent BTK inhibitor. (+, blue arrows) stimulatory and (-, red arrows) inhibitory signalling effects. ASC, apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain; BTK, Bruton tyrosine kinase; CD62P, P-selectin; cGMP, cyclic guanosine monophosphate; DAMP, damage-associated molecular pattern molecule; GTP, guanosine-50-triphosphate; HMGB1, high-mobility group box 1; IL-18, interleukin-18; IL-1b, interleukin-1b; MyD88, myeloid differentiation primary response gene 88; NLRP3, nucleotide-binding domain leucine rich repeat containing protein 3; sGC, soluble guanylate cyclase; SIRT, sirtuin; TLR4, toll-like receptor 4.
The NLRP3 inflammasome in platelets was initially found to be upregulated by the dengue virus (Hottz et al, 2013). We have demonstrated recently that activation of platelets by collagen or thrombin also upregulates platelet NLRP3 inflammasome activity (Murthy et al, 2017). Platelets constitutively express NLRP3, ASC and BTK. Activation of the NLRP3 inflammasome in platelets does not require an initial priming signal, such as the exposure to cytokines or a specific pattern recognition ligand as in immune cells (Hottz et al, 2015). HMGB1 (Chi et al, 2015) and haem (Dutra et al, 2014; Li et al, 2014) have been identified as regulatory triggers of NLRP3 signalling, and haem may (Li et al, 2014) or may not (Dutra et al, 2014) require purinergic P2X receptors. Cellfree haemoglobin may even synergistically increase HMGB1-mediated inflammatory responses, as shown in bone marrow-derived mouse macrophages (Lin et al, 2012).
Secretion of HMGB1 is controlled by the NLRP3 inflammasome in monocytes/macrophages (Willingham et al, 2009). Plasma derived from SCD patients at baseline and plasma from SCD mice induced TLR4 activity in a TLR4 reporter cell line in an HMGB1-dependent manner, further increased when plasma from SCD crisis patients was used (Xu et al, 2014). Other studies have shown that HMGB1 promotes inflammation in human microvascular endothelial cells (Fiuza et al, 2003) and promotes thickening of the pulmonary artery wall in a rat model of pulmonary arterial hypertension (SadamuraTakenaka et al, 2014). In a trauma/haemorrhagic shock mouse model, HMGB1-mediated activation of the TLR4/MyD88/sGC pathway in platelets induced thrombosis and the formation of neutrophil extracellular traps (NETs) (Vogel et al, 2015a). NETs formation and thrombosis are intrinsically linked with haemolysis (Ataga, 2009; Chen et al, 2014). The clinical relevance of HMGB1-mediated mechanisms in the context of haemolysis remains unknown. A schematic overview of the discussed pattern recognition receptor signalling mechanisms in platelets is presented in Fig 1.
Targeting HMGB1 and NLRP3 signalling in platelets
Hydroxycarbamide is still the main therapeutic option for SCD patients. A key component of its therapeutic effect is fetal haemoglobin induction in red blood cells and reduction of haemolysis (Bunn, 1997). Therapeutic efficacy of hydroxycarbamide also involves nitric oxide release from activation of sGC in erythroid progenitor cells (Cokic et al, 2008), reduction of neutrophil counts and activity (Saleh et al, 1999) and downregulation of proinflammatory cytokines (Lanaro et al, 2009). Targeting the adhesion molecule P-selectin, expressed by platelets and endothelial cells, with a blocking antibody has emerged as a new, promising strategy to significantly decrease vascular complications in SCD, as shown in SCD mice (Polanowska-Grabowska et al, 2010) and in a recent phase 2 trial with the humanised monoclonal P-selectin antibody crizanlizumab (Ataga et al, 2017). Crizanlizumab had an added effect in patients who were receiving concomitant hydroxycarbamide. The annual crisis rate was 32·1% lower with high-dose crizanlizumab than with placebo among patients receiving hydroxycarbamide.
P-selectin expression by platelets and platelet aggregation are critically regulated by the NLRP3 inflammasome (Murthy et al, 2017) and the HMGB1/TLR4/sGC (Vogel et al, 2015a) signalling pathway in platelets. HMGB1 and TLR4 are key players in promoting inflammation in SCD (Xu et al, 2014). Targeted ablation of HMGB1 and TLR4 in circulating platelets in transgenic mice promoted anti-thrombotic and antiinflammatory effects (Vogel et al, 2015a, 2016). The HMGB1 inhibitor Glycyrrhizin (Mollica et al, 2007), a triterpene glycoside extracted from liquorice root, exerted potent inhibitory effects on platelet HMGB1 signalling (Vogel et al, 2014, 2015a). Interfering with these pattern recognition receptor pathways in platelets might have significant effects on thrombosis and vascular inflammation in SCD via P-selectindependent and -independent mechanisms, and are currently under investigation.
The mitochondrial-enriched sirtuin deacetylases SIRT1 (Hwang et al, 2015) and SIRT3 (Zhao et al, 2016) have been identified as regulatory triggers that downregulate the proinflammatory effects of HMGB1 and NLRP3 signalling, respectively. Depletion or deletion of SIRT3 increased NLRP3 inflammasome activation in human macrophages and in murine kidney tissues during sepsis-induced acute kidney injury (Traba et al, 2015; Zhao et al, 2016). Platelets express functionally active SIRT1 and SIRT3 (Kumari et al, 2015), and interfering with sirtuins in platelets might affect platelet HMGB1/NLRP3 signalling.
BTK is another recently identified regulator of the NLRP3 inflammasome in immune cells (Liu et al, 2017) and platelets (Murthy et al, 2017), whose activation can be targeted with the US Food and Drug Administration-approved BTK inhibitor ibrutinib (PCI-32765) (Ito et al, 2015). Genetic ablation of BTK in platelets suppressed platelet activation, aggregation and thrombus formation, which was restored by the NLRP3 activator Nigericin, indicating a functionally relevant link between BTK and NLRP3 in platelets (Murthy et al, 2017). Thus, ibrutinib-mediated NLRP3 inhibition in platelets might be a novel therapeutic approach for targeting plateletmediated thromboinflammatory events during haemolysis. Interestingly, two SCD patients who developed chronic myeloid leukaemia (CML) reported marked reduction in the occurrence of SCD pain crises on treatment with the BCRABL1 tyrosine kinase inhibitor imatinib (Stankovic Stojanovic et al, 2011; Murphy et al, 2014). However, it is unknown if the potential effect of imatinib on vaso-occlusive crisis is due to inhibition of tyrosine kinases.
The clinical significance of platelet danger signalling mechanisms in haemolysis-driven inflammation and thrombosis is still poorly understood. Existing studies have reported promising data thus far, and further investigations in this field might identify valuable platelet-based therapeutic options for haemolytic diseases, such as SCD.
Acknowledgements
This work was supported by the NHLBI Intramural Research Program.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN & Tschopp J (2004) NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity, 20, 319–325. [DOI] [PubMed] [Google Scholar]
- Andersson U & Tracey KJ (2011) HMGB1 is a therapeutic target for sterile inflammation and infection. Annual Review of Immunology, 29, 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI (2009) Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica, 94, 1481–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI & Key NS (2007) Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology/the Education Program of the American Society of Hematology, 2007, 91–96. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Cappellini MD & Rachmilewitz EA (2007) Beta-thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. British Journal of Haematology, 139, 3–13. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, KnightMadden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW & Rother RP (2017) Crizanlizumab for the prevention of pain crises in sickle cell disease. New England Journal of Medicine, 376, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au NH, Wong AY, Vickars L, MacGillivray RT & Wadsworth LD (2009) Two new examples of Hb St. Etienne [beta 92(F8)HisGln] in association with venous thrombosis. Hemoglobin, 33, 95–100. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP & Vercellotti GM (2014) Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood, 123, 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennewitz MF, Jimenez MA, Vats R, Tutuncuoglu E, Jonassaint J, Kato GJ, Gladwin MT & Sundd P (2017) Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli. Journal of Clinical Investigation Insight, 2, e89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain HA, Eckman JR, Swerlick RA, Howard RJ & Wick TM (1993) Thrombospondin from activated platelets promotes sickle erythrocyte adherence to human microvascular endothelium under physiologic flow: a potential role for platelet activation in sickle cell vaso-occlusion. Blood, 81, 2137–2143. [PubMed] [Google Scholar]
- Bunn HF (1997) Pathogenesis and treatment of sickle cell disease. New England Journal of Medicine, 337, 762–769. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD & Frenette PS (2014) Hemeinduced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood, 123, 3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Chen H, Li F, Zhu Y, Yin W & Zhuo Y (2015) HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappaB pathway in acute glaucoma. Journal of Neuroinflammation, 12, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT & Schechter AN (2008) Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood, 111, 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L, Cappellini MD & Olivieri O (2011) Thrombosis and sickle cell disease. Seminars in Thrombosis and Hemostasis, 37, 226–236. [DOI] [PubMed] [Google Scholar]
- Dominical VM, Samsel L, Nichols JS, Costa FF, McCoy JP Jr, Conran N & Kato GJ (2014) Prominent role of platelets in the formation of circulating neutrophil-red cell heterocellular aggregates in sickle cell anemia. Haematologica, 99, e214–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS & Bozza MT (2014) Hemolysisinduced lethality involves inflammasome activation by heme. Proceedings of the National Academy of Sciences of the United States of Ame ica, 111, E4110–E4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds LH Jr, Clark RE, Cohn LH, Grunkemeier GL, Miller DC & Weisel RD (1996) Guidelines for reporting morbidity and mortality after cardiac valvular operations. Ad Hoc Liaison Committee for Standardizing Definitions of Prosthetic Heart Valve Morbidity of The American Association for Thoracic Surgery and The Society of Thoracic Surgeons. Journal of Thoracic and Cardiovascular Surgery, 112, 708–711. [DOI] [PubMed] [Google Scholar]
- Eldor A & Rachmilewitz EA (2002) The hypercoagulable state in thalassemia. Blood, 99, 36–43. [DOI] [PubMed] [Google Scholar]
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV & Bozza MT (2007) Characterization of heme as activator of Toll-like receptor 4. Journal of Biological Chemistry, 282, 20221–20229. [DOI] [PubMed] [Google Scholar]
- Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH & Suffredini AF (2003) Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood, 101, 2652–2660. [DOI] [PubMed] [Google Scholar]
- Frelinger AL 3rd, Jakubowski JA, Brooks JK, Carmichael SL, Berny-Lang MA, Barnard MR, Heeney MM & Michelson AD (2014) Platelet activation and inhibition in sickle cell disease (pains) study. Platelets, 25, 27–35. [DOI] [PubMed] [Google Scholar]
- Garrido VT, Sonzogni L, Mtatiro SN, Costa FF, Conran N & Thein SL (2017) Association of plasma CD40L with acute chest syndrome in sickle cell anemia. Cytokine, 97, 104–107. [DOI] [PubMed] [Google Scholar]
- Gawaz M & Vogel S (2013) Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood, 122, 2550–2554. [DOI] [PubMed] [Google Scholar]
- George JN & Nester CM (2014) Syndromes of thrombotic microangiopathy. New England Journal of Medicine, 371, 654–666. [DOI] [PubMed] [Google Scholar]
- Gladwin MT & Ofori-Acquah SF (2014) Erythroid DAMPs drive inflammation in SCD. Blood, 123, 3689–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S & Maruyama I (2005) Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thrombosis and Haemostasis, 94, 975–979. [DOI] [PubMed] [Google Scholar]
- Heeney MM, Hoppe CC, Abboud MR, Inusa B, Kanter J, Ogutu B, Brown PB, Heath LE, Jakubowski JA, Zhou C, Zamoryakhin D, Agbenyega T, Colombatti R, Hassab HM, Nduba VN, Oyieko JN, Robitaille N, Segbefia CI & Rees DC (2016) A multinational trial of prasugrel for sickle cell vaso-occlusive events. New England Journal of Medicine, 374, 625–635. [DOI] [PubMed] [Google Scholar]
- Hendrick AM (2003) Auto-immune haemolytic anaemia–a high-risk disorder for thromboembolism? Hematology, 8, 53–56. [DOI] [PubMed] [Google Scholar]
- Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA & Bozza PT (2013) Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood, 122, 3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottz ED, Monteiro AP, Bozza FA & Bozza PT (2015) Inflammasome in platelets: allying coagulation and inflammation in infectious and sterile diseases? Mediators of Inflammation, 2015, 435783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JS, Choi HS, Ham SA, Yoo T, Lee WJ, Paek KS & Seo HG (2015) Deacetylation-mediated interaction of SIRT1-HMGB1 improves survival in a mouse model of endotoxemia. Scientific Reports, 5, 15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A & Morita R (2015) Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nature Communications, 6, 7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, Kuypers FA & Bach RR (1998) Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood, 91, 4216–4223. [PubMed] [Google Scholar]
- Kumari S, Chaurasia SN, Nayak MK, Mallick RL & Dash D (2015) Sirtuin inhibition induces apoptosis-like changes in platelets and thrombocytopenia. Journal of Biological Chemistry, 290, 12290–12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N & Costa FF (2009) Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. Journal of Leukocyte Biology, 85, 235–242. [DOI] [PubMed] [Google Scholar]
- Lee SP, Ataga KI, Orringer EP, Phillips DR & Parise LV (2006) Biologically active CD40 ligand is elevated in sickle cell anemia: potential role for platelet-mediated inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 1626–1631. [DOI] [PubMed] [Google Scholar]
- Lee BC, Mayer CL, Leibowitz CS, StearnsKurosawa DJ & Kurosawa S (2013) Quiescent complement in nonhuman primates during E coli Shiga toxin-induced hemolytic uremic syndrome and thrombotic microangiopathy. Blood, 122, 803–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, Schultz M & van der Poll T (2013) Sepsis and thrombosis. Seminars in Thrombosis and Hemostasis, 39, 559–566. [DOI] [PubMed] [Google Scholar]
- Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y & Billiar TR (2007) Systemic inflammation and remote organ injury following trauma require HMGB1. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 293, R1538–R1544. [DOI] [PubMed] [Google Scholar]
- Li Q, Fu W, Yao J, Ji Z, Wang Y, Zhou Z, Yan J & Li W (2014) Heme induces IL-1beta secretion through activating NLRP3 in kidney inflammation. Cell Biochemistry and Biophysics, 69, 495–502. [DOI] [PubMed] [Google Scholar]
- Lin T, Sammy F, Yang H, Thundivalappil S, Hellman J, Tracey KJ & Warren HS (2012) Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. Journal of Immunology, 189, 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Pichulik T, Wolz OO, Dang TM, Stutz A, Dillen C, Delmiro Garcia M, Kraus H, Dickhofer S, Daiber E, Munzenmayer L, Wahl S, Rieber N, Kummerle-Deschner J, Yazdi A, Franz-Wachtel M, Macek B, Radsak M, Vogel S, Schulte B, Walz JS, Hartl D, Latz E, Stilgenbauer S, Grimbacher B, Miller L, Brunner C, Wolz C & Weber AN (2017) Human NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome activity is regulated by and potentially targetable through Bruton tyrosine kinase. The Journal of Allergy and Clinical Immunology, 140, 1054–1067. [DOI] [PubMed] [Google Scholar]
- Maugeri N, Franchini S, Campana L, Baldini M, Ramirez GA, Sabbadini MG, RovereQuerini P & Manfredi AA (2012) Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity, 45, 584–587. [DOI] [PubMed] [Google Scholar]
- Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G & Bianchi ME (2007) Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chemistry & Biology, 14, 431–441. [DOI] [PubMed] [Google Scholar]
- Morrell CN, Aggrey AA, Chapman LM & Modjeski KL (2014) Emerging roles for platelets as immune and inflammatory cells. Blood, 123, 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Close J, Lottenberg R & Rajasekhar A (2014) Effectiveness of imatinib therapy for sickle cell anemia and chronic myeloid leukemia. American Journal of the Medical Sciences, 347, 254–255. [DOI] [PubMed] [Google Scholar]
- Murthy P, Durco F, Miller-Ocuin JL, Takedai T, Shankar S, Liang X, Liu X, Cui X, Sachdev U, Rath D, Lotze MT, Zeh HJ 3rd, Gawaz M, Weber AN & Vogel S (2017) The NLRP3 inflammasome and bruton’s tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation. Biochemical and Biophysical Research Communications, 483, 230–236. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H & Igarashi K (2001) Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO Journal, 20, 2835–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A & Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. Journal of Biological Chemistry, 279, 7370–7377. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A & Abraham E (2006) High mobility group box 1 protein interacts with multiple Toll-like receptors. American Journal of Physiology. Cell Physiology, 290, C917–C924. [DOI] [PubMed] [Google Scholar]
- Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, Gear AR & Linden J (2010) P-selectin-mediated plateletneutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 2392–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondina MT, Weyrich AS & Zimmerman GA (2013) Platelets as cellular effectors of inflammation in vascular diseases. Circulation Research, 112, 1506–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhiainen A, Imai S, Rauvala H & Parkkinen J (2000) Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thrombosis and Haemostasis, 84, 1087–1094. [PubMed] [Google Scholar]
- Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K, Inoue H & Maruyama I (2014) HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One, 9, e102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh AW, Hillen HF & Duits AJ (1999) Levels of endothelial, neutrophil and plateletspecific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematologica, 102, 31–37. [DOI] [PubMed] [Google Scholar]
- Semple JW, Italiano JE Jr & Freedman J. (2011) Platelets and the immune continuum. Nature Reviews Immunology, 11, 264–274. [DOI] [PubMed] [Google Scholar]
- Setty BN, Kulkarni S & Stuart MJ (2002) Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood, 99, 1564–1571. [DOI] [PubMed] [Google Scholar]
- Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, Ejiri J, Kobayashi S, Hirata K, Kawashima S & Yokoyama M (2004) Expression of Toll-like receptors on human platelets. Thrombosis Research, 113, 379–385. [DOI] [PubMed] [Google Scholar]
- Sparkenbaugh E & Pawlinski R (2013) Interplay between coagulation and vascular inflammation in sickle cell disease. British Journal of Haematology, 162, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic Stojanovic K, Thioliere B, Garandeau E, Lecomte I, Bachmeyer C & Lionnet F (2011) Chronic myeloid leukaemia and sickle cell disease: could imatinib prevent vaso-occlusive crisis? British Journal of Haematology, 155, 271–272. [DOI] [PubMed] [Google Scholar]
- Stein PD, Beemath A, Meyers FA, Skaf E & Olson RE (2006) Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. American Journal of Medicine, 119, e897–811. [DOI] [PubMed] [Google Scholar]
- Thein MS, Igbineweka NE & Thein SL (2017) Sickle cell disease in the older adult. Pathology, 49, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traba J, Kwarteng-Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, Waclawiw MA, Han K, Pelletier M, Sauve AA, Siegel RM & Sack MN (2015) Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. The Journal of Clinical Investigation, 125, 4592–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhan A, Weiss LA, Mohandas N, Coller BS & Frenette PS (2002) Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proceedings of the National Academy of Sciences of the United States of America, 99, 3047–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT & Kato GJ (2007) Platelet activation in patients with sickle disease, hemolysisassociated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood, 110, 2166–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Chatterjee M, Metzger K, Borst O, Geisler T, Seizer P, Muller I, Mack A, Schumann S, Buhring HJ, Lang F, Sorg RV, Langer H & Gawaz M (2014) Activated platelets interfere with recruitment of mesenchymal stem cells to apoptotic cardiac cells via high mobility group box 1/Toll-like receptor 4mediated down-regulation of hepatocyte growth factor receptor MET. Journal of Biological Chemistry, 289, 11068–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schaffer TE, Bohn E, Frick JS, Borst O, Munzer P, Walker B, Markel J, Csanyi G, Pagano PJ, Loughran P, Jessup ME, Watkins SC, Bullock GC, Sperry JL, Zuckerbraun BS, Billiar TR, Lotze MT, Gawaz M & Neal MD (2015a) Platelet-derived HMGB1 is a critical mediator of thrombosis. The Journal of Clinical Investigation, 125, 4638–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Borger V, Peters C, Forster M, Liebfried P, Metzger K, Meisel R, Daubener W, Trapp T, Fischer JC, Gawaz M & Sorg RV (2015b) Necrotic cell-derived high mobility group box 1 attracts antigen-presenting cells but inhibits hepatocyte growth factor-mediated tropism of mesenchymal stem cells for apoptotic cell death. Cell Death and Differentiation, 22, 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Rath D, Borst O, Mack A, Loughran P, Lotze MT, Neal MD, Billiar TR & Gawaz M (2016) Platelet-derived high-mobility group box 1 promotes recruitment and suppresses apoptosis of monocytes. Biochemical and Biophysical Research Communications, 478, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A & Tracey KJ (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science, 285, 248–251. [DOI] [PubMed] [Google Scholar]
- Westwick J, Watson-Williams EJ, Krishnamurthi S, Marks G, Ellis V, Scully MF, White JM & Kakkar VV (1983) Platelet activation during steady state sickle cell disease. Journal of Medicine, 14, 17–36. [PubMed] [Google Scholar]
- Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA & Ting JP (2009) NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. Journal of Immunology, 183, 2008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T, Paglieroni T, Tablin F, Welborn J, Nelson K & Cheung A (1997) Platelet activation and platelet-erythrocyte aggregates in patients with sickle cell anemia. Journal of Laboratory and Clinical Medicine, 129, 507–516. [DOI] [PubMed] [Google Scholar]
- Xu H, Wandersee NJ, Guo Y, Jones DW, Holzhauer SL, Hanson MS, Machogu E, Brousseau DC, Hogg N, Densmore JC, Kaul S, Hillery CA & Pritchard KA Jr (2014) Sickle cell disease increases high mobility group box 1: a novel mechanism of inflammation. Blood, 124, 3978–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Xu C, Manwani D & Frenette PS (2016) Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood, 127, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WY, Zhang L, Sui MX, Zhu YH & Zeng L (2016) Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Scientific Reports, 6, 33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RF & Schroit AJ (1997) Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood, 89, 1121–1132. [PubMed] [Google Scholar]