Abstract
Purpose of review:
To review the epidemiology, diagnosis, and management of cytomegalovirus retinitis (CMVR) in the post-combined antiretroviral era (cART) era.
Recent findings:
Although cART has dramatically reduced CMVR incidence and morbidity in the HIV population, CMVR continues to cause significant vision loss in both HIV and non-HIV patients, especially amongst patients without immune reconstitution. Advances in imaging including ultra-widefield fundus and autofluorescence imaging, optical coherence tomography, and adaptive optics may reflect CMVR activity; however, the diagnosis remains a clinical one. There have been minimal advances in therapy, with several agents no longer available due to market concerns.
Summary:
Despite reduced incidence and morbidity in the post-cART HIV population, CMVR continues to cause vision loss amongst HIV and non-HIV patients. Diagnosis remains primarily clinical, and therapy centers upon immune reconstitution along with systemic and/or intravitreal antivirals. Further studies are necessary to determine whether advanced imaging can influence management, and whether novel antiviral agents or adoptive immune transfer have a role in treatment of drug-resistance CMVR.
Keywords: cytomegalovirus retinitis, HAART, cART
Introduction
Cytomegalovirus (CMV) is a prevalent herpesvirus, with a seroprevalence of ~60% in the United States and Western Europe and approaching 100% in parts of South America, Asia and Africa [1, 2]. CMV is a double-stranded, enveloped virus capable of producing lifelong, latent infections [2, 3]. Primary infection in immunocompetent individuals is typically mild or asymptomatic. However, CMV can cause significant morbidity in immunocompromised hosts. In the eye, CMV is best known for causing CMV retinitis (CMVR).[3]
Epidemiology
CMVR typically affects immunocompromised hosts, including patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), recipients of bone marrow or solid organ transplants, and neonates. Among AIDS patients, CMVR remains the most common opportunistic eye infection. Before combination antiretroviral therapy (cART), the lifetime CMVR risk amongst AIDS patients was ~30% [4–6]. cART has led to an estimated 80–90% decline in CMVR [7–9], with a cumulative incidence of 1.2% and 4.2% at 4 and 10 years, respectively, in the post-cART era [10].
The single most important risk factor for CMVR development in AIDS patients is a CD4 count <50 cells/μL, with CMVR typically occurring when the CD4 count is in the single digits. [10, 11]. CD4 nadir <50 cells/μL has also been reported as a significant risk factor [9, 10]. Additional measures of AIDS disease progression, including CD8 count <400 cells/μL, HIV load >10,000 copies/mL, and presence of HIV retinopathy have also been associated with CMVR [10]. Genetic risk factors, including haplotypes of the IL-10 receptor subunit, C-C chemokine receptor type 5, and stromal cell derived factor-1 have been shown to influence CMVR risk [12, 13].
There is no current consensus regarding ophthalmologic screening in HIV disease. While some experts endorse CD-4 count-based screening (e.g., every 3–4 months for CD4 <50 cells/μL, and less often with higher counts), 2017 CDC guidelines caution that the value of this approach is unknown in the cART era [14].
CMVR also afflicts non-HIV individuals, especially those with immune compromise. In a review of 208 HIV-negative CMVR cases, Downes et al. identified a contributor to immune dysfunction in 95.5%, including underlying malignancy, most commonly leukemia or lymphoma (~29%); autoimmune disease requiring immunosuppressive therapy (~19%); bone marrow (~16%) or solid organ (~15%) transplant with systemic immunosuppression; diabetes (~6%); and Good’s syndrome (<5%) [15]. Among solid organ transplants, CMVR was noted in ~0.3%, [16–18] with the greatest risk of CMVR occurring in CMV-negative patients who received organ transplants from CMV-positive donors.[3] In this setting, CMVR occurs at a median of 9 months after solid-organ transplantation, and persists until systemic immune suppression can safely be reduced enough to allow the recipient to generate a primary T-cell response.[3]. Among hematopoietic stem cell transplant (HSCT) patients, CMVR rates are 0.12–4%, with the highest among CMV-positive recipients with CMV-negative donors [3, 19]. Other potential risk factors for CMVR include HLA mismatch, unrelated donor, delayed engraftment, longer CMV viremia duration, and higher peak CMV viral load, with only peak CMV viral load achieving statistical significance in multivariate analysis [19–22].
The incidence of CMVR may be increasing among patients receiving hematopoetic stem cell transplants, with several studies reporting higher rates of CMVR in recent years. [19, 23]
One study of HSCT patients reported an increased incidence of CMVR from 0.07% in 1985–2001 to 2.2% in 2002–2005, and another study reported an increase from 0% in 2010–2013 to 18% in 2014.[19, 23] The increased rates may be due to increased CMVR awareness among physicians, increased survival of HSCT patients, and/or increased use of aggressive immunosuppression.[19, 23, 24]
In addition to systemic immunosuppression, intra- and peri-ocular steroid administration (intravitreal triamcinolone, subtenon triamcinolone, as well as dexamethasone and fluocinolone acetonide intravitreal implants) have been implicated in non-HIV CMVR [15, 25, 26]. Finally, congenital infection with CMV may lead to CMVR in infants, even without known immune system dysfunction [27]. CMV is a significant cause of congenital viral infection, affecting ~0.6% of live births – disproportionately infants infected with or exposed to HIV and those born in developing countries [28, 29].
Clinical Signs and Symptoms
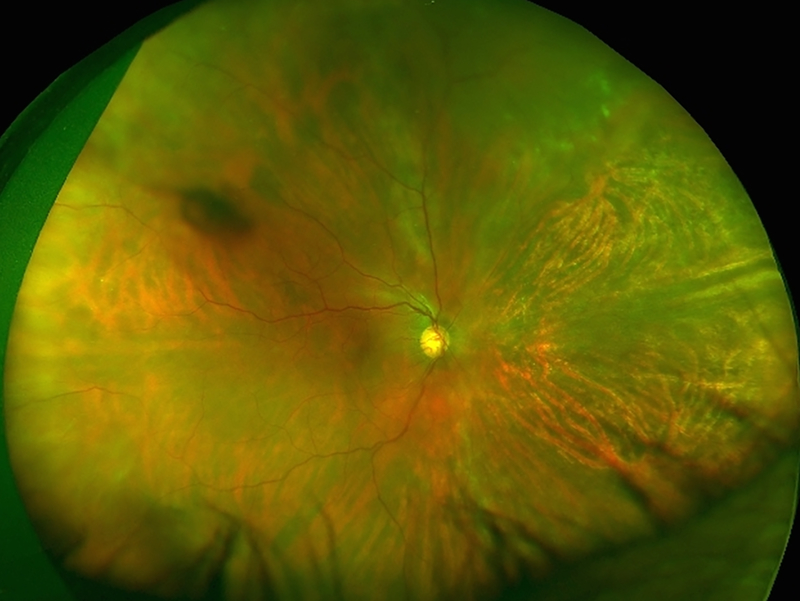
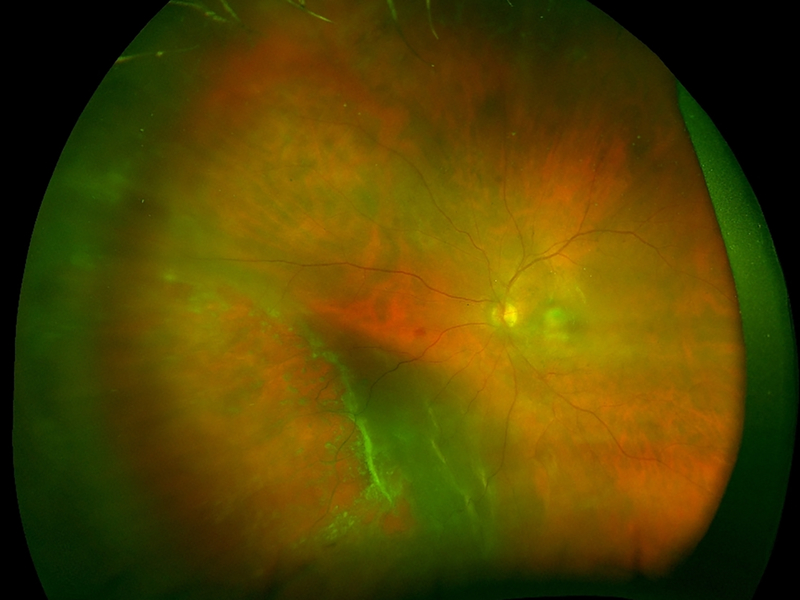
Patients with CMVR typically present with visual symptoms including blurry vision, loss of peripheral or central vision, and floaters [30]. CMVR remains a clinical diagnosis, based on the presence of characteristic fundus findings in susceptible individuals. There are several recognized patterns of CMVR including wedge-shaped areas of retinal whitening with associated hemorrhages (brush-fire, Figure 1A), small opaque white dot-like lesions (granular, Figure 1B), or rarely retinal vasculitis with perivascular sheathing (similar to frosted-branch angiitis) [31–34]. Typically, CMVR lesions are first noted peripherally and progress centripetally at a rate of 24μm per day [33, 35]. Vision loss occurs as a result of posterior pole involvement with retinal necrosis involving the macula or optic nerve, or as a result of complications including retinal detachment (RD), epiretinal membrane (ERM) or cataract [35, 36]. The clinical features of CMVR do not differ between individuals with HIV infection and those with immune compromise from other causes [37].
Figure 1: Clinical and imaging findings in cytomegalovirus (CMV) retinitis.
(A) Ultra-widefield fundus photograph of hemorrhagic CMV retinitis with “brush-fire” pattern demonstrates areas of retinal whitening and necrosis along with overlying hemorrhage. Retinitis typically occurs along the vascular arcades and progresses centripetally towards the posterior pole. (B) Ultra-widefield fundus photograph of granular CMV retinitis demonstrates granular, hypopigmented lesions with only rare retinal hemorrhages. There are also a few faint, punctate hypopigmented lesions along the arcades. (C) Ultra-widefield fundus photograph demonstrating recurrent CMV retinitis, as noted by whitening, at the edge of the nasal chorioretinal atrophy from prior CMV retinitis and superiorly. (D) FAF imaging of the same eye at the same time showed stippled hyper- and hypo-autofluorescence in the area of healed CMV retinitis car, with a hyper-autofluorescent border in the area of reactivation as well as in the new area of retinitis superiorly. (E) Ultra-widefield fundus photograph demonstrating retinitis and whitening with numerous retinal breaks and associated rhegmatogenous retinal detachment inferonasally. (F) After repair with pars plana vitrectomy with silicone oil, the retina is attached with laser scars and CMV scars noted.
Diagnostic Testing
Standard fundus photography has proven to be a reliable means of monitoring CMVR, and has been used in all major clinical trials to determine CMVR progression. The Longitudinal Study of Ocular Complications of AIDS (LSOCA) group found that fundus photograph comparison may detect retinitis progression sooner and may provide more accurate and reproducible assessments than clinical examination [38]. Recently, ultra-widefield imaging (Optomap, Optos PLC) has emerged as a means of imaging up to 200 degrees of the peripheral retina in a single image (Figure 1). Small case series suggest that ultra-widefield imaging may be superior to standard fundus photography in the evaluation of CMVR [39, 40]. In one report, ultra-widefield imaging included 29.1–48.3% greater retinal area than standard photographs and identified 22.1% more CMVR lesions than standard nine-field photography [39, 40]. Furthermore, patients reported ultra-widefield imaging was more comfortable and less time consuming compared to standard photographs [39].
Telemedicine may provide a means of extending CMVR screening to at-risk populations in the developing world. Remote image grading has been the standard for clinical trials in the United States and elsewhere, and may have benefits over clinical examination [38]. Remote image grading has also shown promise in the developing world. A pilot study in Thailand reported 89–91% and 85–88% sensitivity and specificity, respectively, for CMVR diagnosis by remote graders, compared to a gold standard of indirect ophthalmoscopy by a trained ophthalmologist [41].
Fluorescein angiography (FA) findings in CMVR may include leakage at the optic disc and areas of active retinitis, non-perfusion in affected areas, or blocking defects in areas of hemorrhage [42, 43]. The frosted branch angiitis variant demonstrates late perivascular leakage or staining, or rarely perivascular hypofluorescence [44, 45]. Kyrieleis plaques may also be seen in CMVR and can be distinguished from frosted branch angiitis by the lack of perivascular leakage on FA [46]. FA may identify late complications of CMVR, including optic disc neovascularization, choroidal neovascularization and cystoid macular edema (CME) [47–49].
Case series suggest that fundus autofluorescence (FAF) may be useful for detecting early progression of CMVR [50]. In active retinitis, the advancing lesion border is hyper-autofluorescent, which may aid the clinician in detecting subtle areas of progression or reactivation [50]. Retinal hemorrhages and edema within areas of active retinitis appear hypo-autofluorescent with stippled hyper-autofluorescence [50]. Areas of retinal atrophy exhibit hypo-autofluorescence. In our clinical experience, FAF can facilitate early detection of lesion progression or reactivation with characteristic hyper-autofluorescence at the border of lesions (Figure 1C-D).
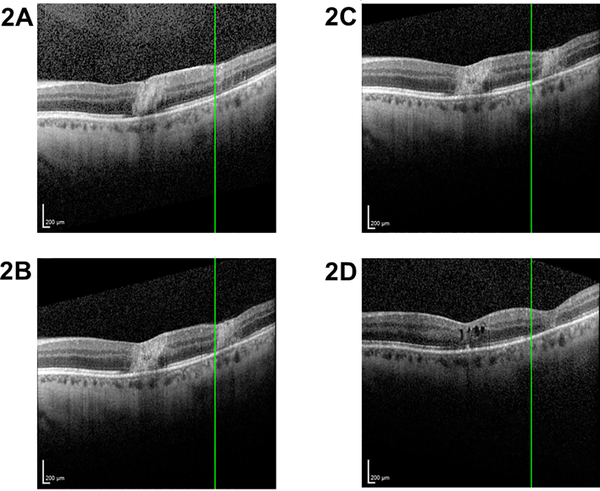
We recently reported a small retrospective series evaluating spectral domain-optical coherence tomography (SD-OCT) findings in CMVR [51]. Microstructural abnormalities in the area of active CMVR, at the leading edge, and just beyond the leading edge (as determined from fundus photographs) were evaluated by SD-OCT. Microstructural abnormalities were frequently noted beyond the leading edge of CMVR, with outer retinal disruption noted to be a potential early sign of progression, suggesting that monitoring the boundary between normal and abnormal ellipsoid zone may be useful for identifying microprogression (Figure 2) [51]. Further studies are necessary to determine the role of OCT, if any, in monitoring and managing CMVR.
Figure 2: Ellipsoid zone (EZ) disruption in cytomegalovirus (CMV) retinitis.
Spectral domain-optical coherence tomography (SD-OCT) through areas of active CMV retinitis demonstrate retinal architecture disruption with hyperreflectivity and disruption of the EZ (A). The Heidelberg viewing system marker (green line, A-B) is used to denote the border of normal and disrupted EZ at the edge of one retinitis lesion presentation. After one week (B), EZ disruption is noted to progress to the left side of the marker, in an area of previously intact EZ (A). After escalation of antiviral therapy and control of CMV retinitis, no further progression of EZ disruption is noted from this same time point (shown again in C) until 3 months later (D). The area to the right of the Heidelberg viewing system marker (green line, C-D), which had shown EZ disruption (C), shows progressive worsening of retinal architecture with retinal thinning over the same time period (D). The area to the left of the marker shows stable intact retinal architecture and EZ (C-D).
Novel imaging techniques such as OCT angiography and adaptive optics (AO) may provide ancillary information to clinicians. In one case report, AO was able to finely distinguish between areas of intact and damaged retina, with the former showing an intact cone mosaic pattern, and the latter demonstrating a honeycomb-like appearance suggesting direct visualization of the RPE due to loss of overlying photoreceptors [52]. Further studies are needed to assess the role and utility, if any, of these imaging techniques in CMVR management.
Laboratory Testing
Aqueous or vitreous sampling with polymerase chain reaction (PCR) testing for CMV DNAis useful in identifying CMVR in unclear cases and for distinguishing from other causes of infectious retinitis [53]. CMV PCR amplification demonstrates CMV viral products in the aqueous and vitreous of infected eyes [54]. Viral titers correlate with disease activity and may be used to distinguish between active and inactive retinitis [54]. PCR testing has been used to definitively diagnose CMVR in cases of local immunosuppression from steroid injection or implant [55–57]. PCR testing may also be used to identify viral resistance to antiviral medications and may thus help guide therapy [58].
Retinitis Progression and Visual Outcomes
Without treatment, CMVR typically progresses towards the posterior pole at an average rate of 24μm per day. Treatment significantly prolongs time to progression [59–61]. In the cART era, the odds of CMVR progression have decreased by ~50% [62, 63]. The overall rate of retinitis progression in patients on cART was 0.10/person-years (PY)—a marked reduction from the rates of 3.0/PY observed in the pre-cART era [8]. For patients on cART therapy, time to CMVR progression was significantly prolonged, with a median time to progression of 1.5 years [64]. Rates of CMVR recurrence have also declined, and visual outcomes have improved due to cART therapy [4, 36, 65, 66]. In the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), vision loss to ≤20/50 and to ≤20/200 was reduced by 42% and 61%, respectively, in CMVR patients with immune recovery from cART [67].
Despite the widespread success of cART in CMVR management, CMVR still carries a poor visual prognosis, especially in patients who do not have immune reconstitution [68]. In a post-cART study, the rate of visual impairment was 0.10/eye-years and the rate of blindness was 0.06/eye-years in CMVR patients receiving cART [67].
CMVR-associated Retinal Detachment (RD)
RDs associated with CMVR (Figure 1E-F) typically occur within areas of retinal necrosis or at the border of healthy and necrotic tissue and may have multiple retinal breaks that are poorly visualized [69]. Larger areas of retinitis are associated with an increased risk of RD in pre-cART and post-cART studies [70, 71]. Other factors found to put CMVR patients at increased risk for RD include lower CD4 counts and older age [70, 71]. In the pre-cART era, RDs were a common complication of CMVR, occurring at a rate of 0.57–0.66/PY [72]. Since the introduction of cART, the rate of RD has decreased to a rate of 0.06/PY in those on cART and 0.02/PY in patients on cART with CD4 counts ≥200/μl [73]. Management should be individualized, and may include laser demarcation of peripheral RD [74], scleral buckling and/or vitrectomy with silicone oil (Figure 1F), although many authors advocate for vitrectomy with silicone oil tamponade in the majority of cases [75].
Immune Recovery Uveitis and Other Causes of Visual Morbidity
With the rise of cART, immune recovery uveitis (IRU) became an increasing source of ocular morbidity in CMVR [4]. In IRU, typically anterior uveitis or vitritis, the T-cell mediated immune reaction to residual CMV antigens is thought to be bolstered by cART-mediated immune recovery [76, 77]. The incidence of IRU has been found to range from 0.11/PY-0.83/PY [76–80]. Large areas of CMVR (greater than 25–30% retinal area), posterior pole involvement, treatment with intravitreous cidofovir, and male gender increase the risk of CMVR-related IRU [77, 79, 81].
The vitritis observed in IRU can be associated with other post-inflammatory sequelae, including CME, ERM, and cataract [76]. CME and ERM have been reported in up to 45% and 48.9%, respectively, of IRU eyes [79]. Even in the absence of IRU, ERM and cataract can develop in association with CMVR [82, 83]. [83][83] In the LSOCA cohort, IRU and posterior segment inflammatory signs were not strongly associated with cataract [76, 83]. Visual acuity (VA) of ≤20/200 is found at similar proportions in eyes with and without IRU [79]. However, when post-inflammatory complications such as CME and cataract are included in analysis, IRU accounts for up to 25% of vision loss to ≤20/200 [65].
Appropriate treatment of IRU is dependent on the location and severity of intraocular inflammation. Anterior uveitis is typically managed with topical corticosteroids, while vitritis may be treated with periocular steroids or short courses of oral steroids [84]. In eyes refractory to topical or periocular therapy, intravitreal corticosteroids have been employed [84]. Some authors recommend administering concurrent anti-CMV therapy with intravitreal steroids in order to prevent reactivation of retinitis [85]. The intravitreal flucinolone acetonide implant (retisert) has also been used for the management of refractory CME from IRU in three eyes without reactivation of CMVR [86]. However, given reports of CMVR occurring in immunocompetent individuals treated with the flucinolone implant, patients receiving this treatment for IRU should continue therapy against CMVR and should be monitored regularly by the treating ophthalmologist for the duration of the implant [84, 86, 87].
Mortality
In the pre-cART era, CMVR was associated with significant morbidity and mortality, approaching 100% mortality in many early series. One series of untreated patients described 100% mortality within 6 weeks of diagnosis of CMVR [32]. The grave mortality rates in this era are illuminated by the fact that some initial reports of AIDS-related CMVR and its treatment included post-mortem histopathologic slides [32, 31]. Mortality rates declined throughout the pre-cART era, as supportive care improved and anti-cytomegalovirus agents became available. A large study conducted in the immediate pre-cART era (1993–94) found a median survival of 262–268 days, whereas a subsequent study conducted at the time that cART became available (1994–1996) found a median survival ranging from 388–568 days, depending on the study treatment arm [61, 88]. In one large study, patients with CMVR without immune recovery had a mortality of 44.0/100 PY and 13.5 month median survival, compared to 2.7/100 PY and 27 years, respectively, for those with immune recovery [7]. In two small series of non-HIV patients with CMVR, the mortality rate ranged from 11.7–23/100 PY. [22, 89]
Treatment
The centerstone of short and long-term disease control of CMVR is immune reconstitution, whether through cART in HIV patients or modulation of systemic immunosuppressive drugs or immune reconstitution in hematopoetic stem cell transplantation [7, 89]. Among HIV-infected patients, immune recovery decreases the risk of ocular morbidity, vision loss and death [10]. The rate of bilateral blindness (VA <20/200 in both eyes) from CMVR has decreased from 14.8/100 PY in the pre-cART era to 0.4/100 PY with modern cART [7]. Antiretroviral therapy decreases the risk of RD from 2.7–8.7/100 person years to 1.0–1.3/100 person years among those with immune recovery [7, 36]. Finally, immune recovery is associated with significantly decreased mortality, from 44.0/100 PY in patients with CMVR without immune recovery to 2.7/100 PY in patients with immune recovery [7]. Systemic therapy alone may be utilized in patients with peripheral lesions that do not threaten the optic nerve or the macula. When the posterior pole is involved, retinitis progresses on systemic therapy, or the patient cannot tolerate systemic therapy, intravitreal therapy is employed. If antiviral resistance is encountered, many practitioners switch agents or use two agents in combination.
Systemic Therapy
Large, randomized controlled trials from the pre-cART era demonstrated a synergistic effect of combination systemic and local anti-CMV therapy. The ganciclovir implant alone was superior to intravenous ganciclovir alone for delaying the progression of retinitis (defined as extension of a lesion border by ≥750μm over a ≥750-μm front, the development a ≥750μm area of new retinitis, or RD within an area of retinitis) [61]. However, the addition of systemic ganciclovir to the ganciclovir implant was found to decrease the risk of mortality, visceral CMV disease and second eye disease [61]. Later studies demonstrated that combined oral and intraocular therapy delayed progression of retinitis (extension of the border by ≥750μm over a ≥750-μm front or development of a new retinitis ≥750μm in diameter) and decreased the risk of new CMV disease [88]. At present, there are several available systemic therapies that may be given orally or intravenously for induction and maintenance therapy against cytomegalovirus infections, including ganciclovir, valganciclovir, foscarnet, cidofovir and leflunomide (Table 1).
Table 1.
Therapies for CMV retinitis
| Agent | Dosing | ||
|---|---|---|---|
| Systemic | Induction | Maintenance | Notes |
| Ganciclovir (IV) | 5 mg/kg twice daily for 14–21 days | 5 mg/kg/day | |
| Valganciclovir (PO) | 900 mg twice daily | 900 mg daily | |
| Foscarnet (IV) | 90 mg/kg twice daily for 14 days | 120 mg/kg/day | |
| Cidofovir (IV) | 5 mg/kg weekly for 3 weeks | 5 mg/kg every 2 weeks | |
| Leflunomide (PO) | 100 mg daily for 5 days | 40–60 mg daily | Off-label use |
| Intravitreal | |||
| Ganciclovir | 2 mg 1–4 times as needed to halt retinitis | 2 mg weekly | |
| Foscarnet | 1.2–2.4 mg 1–2 times weekly | 1.2 mg weekly | |
| Cidofovir | 20 μg 1–8 times as needed to halt retinitis | 20 μg every 5–6 weeks | |
| Fomivirsen | 165 μg weekly for 2–3 weeks | 165 μg every other week | Approved 1998, withdrawn 2002 due to market considerations |
| Sustained delivery | |||
| Ganciclovir implant | n/a | 1 μg/h release over lifetime of implant (6–8 months) | Discontinued 2013 |
IV intravenous, PO per os, n/a not applicable
Ganciclovir is an acyclic purine nucleoside whose activation depends on the viral kinase encoded by the UL97 gene to catalyze the initial step in ganciclovir’s phosphorylation. The activated triphosphate form of ganciclovir then binds specifically to the viral DNA polymerase encoded by the UL54 gene. CMV mutations in either the UL54 or UL97 genes confers ganciclovir resistance. [90] The principle side effect of ganciclovir is myelosuppression [88, 91]. Induction therapy is given at a dose of 5mg/kg every 12 hour for 14–21 days followed by a maintenance dose of 5mg/kg/day. [88, 91]
Valganciclovir is an orally-administered prodrug of ganciclovir with excellent bioavailability. Mutations in UL54 and UL97 thus also render resistance to valganciclovir. In a randomized, controlled trial, oral valganciclovir was as effective as intravenous ganciclovir when used as initial therapy.[92] In trials, the two drugs had equivalent median time to progression, 160 days for oral valganciclovir and 125 days for intravenous ganciclovir (defined as lesion border movement ≥750μm or new lesion ≥750μm as assessed by fundus photographs) [92]. Valganciclovir may be administered as 900mg twice daily during induction therapy, and then continued at once daily dosing for maintenance [93]. A systematic review found that oral valganciclovir was preferable to intravenous ganciclovir on a cost-effectiveness basis [94]. Switching from intravenous to oral therapy resulted in a cost savings of $6866 USD per treatment period [94]. Furthermore, compared to other available therapies, the once-daily oral dosing of valganciclovir is simple and convenient, increasing patient compliance with therapy [93]. The principle therapeutic limitation of valganciclovir is myelosuppression.
Foscarnet is a pyrophosphate analogue that inhibits viral DNA replication by specifically binding to the CMV DNA polymerase encoded by the UL54 gene [95]. Thus, mutations in UL54 confer foscarnet resistance. The efficacy of foscarnet, demonstrated by randomized, controlled trials, is similar to that of ganciclovir in terms of median time to progression of retinitis [95–98]. Nephrotoxicity is the most common treatment-limiting toxicity of foscarnet [95]. Foscarnet is administered as 90mg/kg twice daily for 2 weeks for induction therapy, followed by a maintenance dose of 120mg/kg daily [99].
Cidofovir is a nucleotide analogue that is phosphorylated to its active form by cellular enzymes and then serves as a competitive inhibitor of the CMV viral DNA polymerase encoded by the UL54 gene. Mutations in UL54 confer cidofovir resistance [90]. Intravenous cidofovir was directly compared to oral and intravitreal ganciclovir in a randomized trial which suggested that both agents were similar for treatment of CMVR [100]. The long half-life of cidofovir allows for administration every 2 weeks. However, cidofovir therapy is limited by several significant side-effects including nephrotoxicity, neutropenia, uveitis and hypotony [100–102]. Cidofovir is administered as an intravenous infusion of 5mg/kg over 1 hour weekly for the first 3 weeks of induction therapy and then every 2 weeks for maintenance [90]. In order to limit nephrotoxicity, cidofovir is co-administered with intravenous hydration and probenecid (2g given 3 hours before and 1g at 2 and 8 hours after cidofovir). Despite attempts to limit nephrotoxicity, 24% of patients enrolled in one trial had to stop cidofovir because of renal toxicity [101].
Leflunomide is an immunosuppressive agent that is approved for use in rheumatoid arthritis. Leflunomide inhibits virion assembly and has been used off-label for the treatment of CMVR. Its use was initially reported in patients in India who could not afford preferred anti-CMV therapy, and has since been used in patients with drug-resistant CMVR [103–105]. Given the off-label use of leflunomide, there is no specific recommended dose from the manufacturer for CMVR. Some authors recommend using the standard dosing of 100mg once daily for 3 days followed by 20mg once daily, whereas others recommend a dose of 100–200mg daily for 5–7 days, followed by 40–60mg daily [104, 106, 107].
Intravitreal therapy
Intravitreal therapy enables delivery of a high concentration of medication directly into the eye.
Four agents, ganciclovir, foscarnet, cidofovir and fomivirsen have been used for intravitreal injections to treat CMVR. Ganciclovir was the first intravitreal therapy available for CMVR and remains a mainstay of treatment. Case series suggest efficacy of intravitreal injections for the management of CMVR [7, 108]. Intravitreal ganciclovir is typically given as a 2mg injection twice weekly as during the induction phase, followed by weekly maintenance injections (Table 1) [99].
Foscarnet may also be given intravitreally. In case series, intravitreal foscarnet was shown to reduce or halt the progression of retinitis (advance of 750μm from an existing lesion or development of new foci as assessed by clinical examination) [97, 109, 110].[110] The typical dosage of intravitreal foscarnet is a 1.2 or 2.4mg injection, given 1–2 times weekly (Table 1) [99].
Intravitreal cidofovir has also been shown to be effective at slowing the progression of CMVR, but is rarely used in clinical practive owing to higher rates of side-effects, including iritis (15–20%), CME, and irreversible hypotony that can be visually-significant [111–113].
Fomivirsen, an anti-sense oligonucleotide complementary to CMV mRNAs,[114] delayed progression (advancement of a lesion by ≥750μm along a ≥750-μm front or appearance of a new lesion ≥750μm, assessed by fundus photographs) in a randomized, controlled trial of 18 patients with CMVR [115]. Due to market concerns, fomivirsen was withdrawn in 2002.
Sustained delivery
A sustained-release formulation of ganciclovir (Vitrasert®, Bausch and Lomb) obtained FDA approval in 1996. The ganciclovir implant provided an alternative to frequent long-term injections in patients who had failed intravenous therapy. The 6mm implant was surgically implanted through the pars plana and delivered ganciclovir for 7–8 months. Complications included endophthalmitis, RD, vitreous hemorrhage, cataract, and hypotony [61, 116, 117]. In light of declining sales, the manufacturer discontinued the ganciclovir implant in 2013. Although varied means of improving intraocular ganciclovir delivery including coupling to nanoparticles of thermosensitive hydrogels have been reported [118, 119], market pressures likely limit commercial development.
Potential emerging therapies: adoptive immune transfer
Adoptive transfer of CMV-specific donor cytotoxic T-lymphocytes (CMV-CTL) is a potential emerging therapy for CMVR. Donor T-cells are obtained from healthy CMV-seropositive patients and are used to generate CMV-CTLs. The appropriate donor CMV-CTL for a given CMVR patient is selected based on partial HLA matching, with restriction in cytotoxicity to CMV epitopes presented by one of the matching HLA alleles. Intravenous infusions of CMV-CTLs are performed once weekly for three weeks. Additional cycles may be performed based on response and lack of toxicity. CMV-CTLs administered for systemic CMV infection after bone-marrow transplant have been associated with significant expansion of T-cell lines, decrease in CMV viral titers and control of systemic CMV infection [120–122]. We previously reported a single case of long-term successful treatment of multi-drug-resistant CMVR with CMV-CTLs, without concurrent systemic or intravitreal antiviral therapy [123]. Further studies are necessary to determine the long-term efficacy and safety of these CMV-specific T-cell infusions, if any, for patients with CMVR.
Antiviral resistance
Viral resistance occurs as a result of mutations in the viral UL97 and UL54 genes which encode viral kinase and viral DNA polymerase respectively [90]. Mutations in UL97 confer low-level resistance to ganciclovir and valganciclovir. Mutations in the viral UL54 gene confer resistance to ganciclovir, valganciclovir, foscarnet and cidofovir. Concurrent mutations in both UL97 and UL54 lead to high-level ganciclovir and valganciclovir resistance. PCR testing enables detection of antiviral resistance genes and may help tailor therapy [58]. When resistance is encountered, it is our practice to switch agents or to utilize two agents in combination for intravitreal injections. The relatively high concentrations of drug delivered intravitreally may overcome viral resistance in the management of CMVR, although systemic disease may remain difficult to treat. Systemic toxicity concerns as outlined above may also limit utility of one or more systemic antiviral agents.
Conclusions
CMVR is the most common ocular opportunistic infection in AIDS. With the success of cART for HIV, rates of CMVR have declined significantly in the past two decades. Decreasing numbers of CMVR cases have resulted in the withdrawal of two proven therapies, fomivirsen and the ganciclovir implant, owing to commercial pressures. The success of cART therapy has also caused a shift in the demographics of CMVR, with an increasingly larger portion of incident cases occurring in non-HIV-infected patients. Nevertheless, CMVR remains an important cause of ocular morbidity and vision loss in both HIV-infected patients and individuals with immune compromise from other causes. Antiviral resistance and a smaller therapeutic armamentarium provide a challenge to treating ophthalmologists, but emerging therapies such as adoptive immune transfer may provide promise for durable treatment in the near future.
Footnotes
Proprietary interests: ADP, ROA, LK, MPG: none
Human and Animal Rights: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Staras SAS, Dollard SC, Radford KW, et al. (2006) Seroprevalence of Cytomegalovirus Infection in the United States, 1988–1994. Clin Infect Dis 43:1143–1151. doi: 10.1086/508173 [DOI] [PubMed] [Google Scholar]
- 2.Cannon MJ, Schmid DS, Hyde TB (2010) Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20:202–213. doi: 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 3.Carmichael A (2012) Cytomegalovirus and the eye. Eye Lond Engl 26:237–240. doi: 10.1038/eye.2011.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holbrook JT, Jabs DA, Weinberg DV, et al. (2003) Visual loss in patients with cytomegalovirus retinitis and acquired immunodeficiency syndrome before widespread availability of highly active antiretroviral therapy. Arch Ophthalmol Chic Ill 1960 121:99–107 [DOI] [PubMed] [Google Scholar]
- 5.Hoover DR, Peng Y, Saah A, et al. (1996) Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol Chic Ill 1960 114:821–827 [DOI] [PubMed] [Google Scholar]
- 6.Jabs DA (1995) Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 93:623–683 [PMC free article] [PubMed] [Google Scholar]
- 7.Jabs DA, Ahuja A, Van Natta ML, et al. (2015) Long-term Outcomes of Cytomegalovirus Retinitis in the Era of Modern Antiretroviral Therapy: Results from a United States Cohort. Ophthalmology 122:1452–1463. doi: 10.1016/j.ophtha.2015.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabs DA, Ahuja A, Van Natta M, et al. (2010) Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology 117:2152–2161.e1–2. doi: 10.1016/j.ophtha.2010.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabs DA, Van Natta ML, Holbrook JT, et al. (2007) Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology 114:780–786. doi: 10.1016/j.ophtha.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 10.Sugar EA, Jabs DA, Ahuja A, et al. (2012) Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol 153:1016–1024.e5. doi: 10.1016/j.ajo.2011.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pertel P, Hirschtick R, Phair J, et al. (1992) Risk of developing cytomegalovirus retinitis in persons infected with the human immunodeficiency virus. J Acquir Immune Defic Syndr 5:1069–1074 [PubMed] [Google Scholar]
- 12.Sezgin E, van Natta ML, Ahuja A, et al. (2011) Association of host genetic risk factors with the course of cytomegalovirus retinitis in patients infected with human immunodeficiency virus. Am J Ophthalmol 151:999–1006.e4. doi: 10.1016/j.ajo.2010.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sezgin E, Jabs DA, Hendrickson SL, et al. (2010) Effect of host genetics on the development of cytomegalovirus retinitis in patients with AIDS. J Infect Dis 202:606–613. doi: 10.1086/654814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan JE, Benson C, Holmes KK, et al. (2009) Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep 58:1–207; quiz CE1–4 [PubMed] [Google Scholar]
- 15.Downes KM, Tarasewicz D, Weisberg LJ, Cunningham ET (2016) Good syndrome and other causes of cytomegalovirus retinitis in HIV-negative patients-case report and comprehensive review of the literature. J Ophthalmic Inflamm Infect 6:3. doi: 10.1186/s12348-016-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung H, Kim K-H, Kim J-G, et al. (2007) Retinal complications in patients with solid organ or bone marrow transplantations. Transplantation 83:694–699. doi: 10.1097/01.tp.0000259386.59375.8a [DOI] [PubMed] [Google Scholar]
- 17.Egli A, Bergamin O, Müllhaupt B, et al. (2008) Cytomegalovirus-associated chorioretinitis after liver transplantation: case report and review of the literature. Transpl Infect Dis Off J Transplant Soc 10:27–43. doi: 10.1111/j.1399-3062.2007.00285.x [DOI] [PubMed] [Google Scholar]
- 18.Fishburne BC, Mitrani AA, Davis JL (1998) Cytomegalovirus retinitis after cardiac transplantation. Am J Ophthalmol 125:104–106 [DOI] [PubMed] [Google Scholar]
- 19.Xhaard A, Robin M, Scieux C, et al. (2007) Increased incidence of cytomegalovirus retinitis after allogeneic hematopoietic stem cell transplantation. Transplantation 83:80–83. doi: 10.1097/01.tp.0000239512.94181.e9 [DOI] [PubMed] [Google Scholar]
- 20.Crippa F, Corey L, Chuang EL, et al. (2001) Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin Infect Dis Off Publ Infect Dis Soc Am 32:214–219. doi: 10.1086/318447 [DOI] [PubMed] [Google Scholar]
- 21.Hiwarkar P, Gajdosova E, Qasim W, et al. (2014) Frequent occurrence of cytomegalovirus retinitis during immune reconstitution warrants regular ophthalmic screening in high-risk pediatric allogeneic hematopoietic stem cell transplant recipients. Clin Infect Dis Off Publ Infect Dis Soc Am 58:1700–1706. doi: 10.1093/cid/ciu201 [DOI] [PubMed] [Google Scholar]
- 22.Kuo IC, Kempen JH, Dunn JP, et al. (2004) Clinical characteristics and outcomes of cytomegalovirus retinitis in persons without human immunodeficiency virus infection. Am J Ophthalmol 138:338–346. doi: 10.1016/j.ajo.2004.04.015 [DOI] [PubMed] [Google Scholar]
- 23.Larochelle MB, Phan R, Craddock J, et al. (2017) Cytomegalovirus Retinitis in Pediatric Stem Cell Transplants: Report of a Recent Cluster and the Development of a Screening Protocol. Am J Ophthalmol 175:8–15. doi: 10.1016/j.ajo.2016.09.039 [DOI] [PubMed] [Google Scholar]
- 24.Jeon S, Lee WK (2015) Cytomegalovirus Retinitis in a Human Immunodeficiency Virus-negative Cohort: Long-term Management and Complications. Ocul Immunol Inflamm 23:392–399. doi: 10.3109/09273948.2014.985385 [DOI] [PubMed] [Google Scholar]
- 25.Shapira Y, Mimouni M, Vishnevskia-Dai V (2017) Cytomegalovirus retinitis in HIV-negative patients - associated conditions, clinical presentation, diagnostic methods and treatment strategy. Acta Ophthalmol (Copenh). doi: 10.1111/aos.13553 [DOI] [PubMed] [Google Scholar]
- 26.Takakura A, Tessler HH, Goldstein DA, et al. (2014) Viral retinitis following intraocular or periocular corticosteroid administration: a case series and comprehensive review of the literature. Ocul Immunol Inflamm 22:175–182. doi: 10.3109/09273948.2013.866256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tagami M, Honda S, Morioka I, et al. (2016) An unusual case of congenital cytomegalovirus infection-related retinopathy. BMC Ophthalmol 16:81. doi: 10.1186/s12886-016-0246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghekiere S, Allegaert K, Cossey V, et al. (2012) Ophthalmological findings in congenital cytomegalovirus infection: when to screen, when to treat? J Pediatr Ophthalmol Strabismus 49:274–282. doi: 10.3928/01913913-20120710-03 [DOI] [PubMed] [Google Scholar]
- 29.Lanzieri TM, Dollard SC, Bialek SR, Grosse SD (2014) Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis IJID Off Publ Int Soc Infect Dis 22:44–48. doi: 10.1016/j.ijid.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedberg DN (1997) Cytomegalovirus retinitis: diagnosis and status of systemic therapy. J Acquir Immune Defic Syndr Hum Retrovirology Off Publ Int Retrovirology Assoc 14 Suppl 1:S1–6 [DOI] [PubMed] [Google Scholar]
- 31.D’Amico DJ, Talamo JH, Felsenstein D, et al. (1986) Ophthalmoscopic and histologic findings in cytomegalovirus retinitis treated with BW-B759U. Arch Ophthalmol Chic Ill 1960 104:1788–1793 [DOI] [PubMed] [Google Scholar]
- 32.Holland GN, Pepose JS, Pettit TH, et al. (1983) Acquired immune deficiency syndrome. Ocular manifestations. Ophthalmology 90:859–873 [DOI] [PubMed] [Google Scholar]
- 33.Holland GN, Shuler JD (1992) PRogression rates of cytomegalovirus retinopathy in ganciclovir-treated and untreated patients. Arch Ophthalmol 110:1435–1442. doi: 10.1001/archopht.1992.01080220097029 [DOI] [PubMed] [Google Scholar]
- 34.Spaide RF, Vitale AT, Toth IR, Oliver JM (1992) Frosted branch angiitis associated with cytomegalovirus retinitis. Am J Ophthalmol 113:522–528 [DOI] [PubMed] [Google Scholar]
- 35.Holland GN, Vaudaux JD, Jeng SM, et al. (2008) Characteristics of Untreated AIDS-related Cytomegalovirus Retinitis. I. Findings before the Era of Highly Active Antiretroviral Therapy (1988 to 1994). Am J Ophthalmol 145:5–11.e10. doi: 10.1016/j.ajo.2007.09.023 [DOI] [PubMed] [Google Scholar]
- 36.Thorne JE, Holbrook JT, Jabs DA, et al. (2007) Effect of cytomegalovirus retinitis on the risk of visual acuity loss among patients with AIDS. Ophthalmology 114:591–598. doi: 10.1016/j.ophtha.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 37.Kim DY, Jo J, Joe SG, et al. (2017) COMPARISON OF VISUAL PROGNOSIS AND CLINICAL FEATURES OF CYTOMEGALOVIRUS RETINITIS IN HIV AND NON-HIV PATIENTS. Retina Phila Pa 37:376–381. doi: 10.1097/IAE.0000000000001144 [DOI] [PubMed] [Google Scholar]
- 38.Studies of Ocular Complications of AIDS Research Group (1996) Assessment of cytomegalovirus retinitis. Clinical evaluation vs centralized grading of fundus photographs. Studies of Ocular Complications of AIDS Research Group, AIDS Clinical Trials Group. Arch Ophthalmol Chic Ill 1960 114:791–805 [PubMed] [Google Scholar]
- 39.Mudvari SS, Virasch VV, Singa RM, Maccumber MW (2010) Ultra-Wide–Field Imaging for Cytomegalovirus Retinitis. Ophthalmic Surg Lasers Imaging 41:311–315. doi: 10.3928/15428877-20100430-03 [DOI] [PubMed] [Google Scholar]
- 40.Virasch V, MacCumber M, Mudvari S (2008) Ultra-Widefield Imaging in CMV Retinitis. Invest Ophthalmol Vis Sci 49:4275 [Google Scholar]
- 41.Ausayakhun S, Skalet AH, Jirawison C, et al. (2011) Accuracy and reliability of telemedicine for diagnosis of cytomegalovirus retinitis. Am J Ophthalmol 152:1053–1058.e1. doi: 10.1016/j.ajo.2011.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babu K, Murthy KR, Sudarshan S, Biswas J (2010) BILATERAL ARTERITIS WITH CYTOMEGALOVIRUS RETINITIS IN A PATIENT INFECTED WITH HUMAN IMMUNODEFICIENCY VIRUS: Retin Cases Brief Rep 4:31–33. doi: 10.1097/ICB.0b013e318199d9dc [DOI] [PubMed] [Google Scholar]
- 43.Kozak I, McCutchan JA, Freeman WR HIV-Associated Infections. In: Retina, Fifth Edition. pp 1441–1472 [Google Scholar]
- 44.Churgin D, Relhan N, Davis JL, Albini TA (2015) Perivascular hypofluorescence in frosted branch angiitis. Ophthalmic Surg Lasers Imaging Retina 46:396–397. doi: 10.3928/23258160-20150323-30 [DOI] [PubMed] [Google Scholar]
- 45.Walker S, Iguchi A, Jones NP (2004) Frosted branch angiitis: a review. Eye 18:527–533. doi: 10.1038/sj.eye.6700712 [DOI] [PubMed] [Google Scholar]
- 46.Patel A, Pomykala M, Mukkamala K, Gentile RC (2011) Kyrieleis plaques in cytomegalovirus retinitis. J Ophthalmic Inflamm Infect 1:189–191. doi: 10.1007/s12348-011-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogie GJ, Nanda SK (2001) Neovascularization associated with cytomegalovirus retinitis. Retina Phila Pa 21:85–87 [DOI] [PubMed] [Google Scholar]
- 48.Chan CK, Lin SG (2008) Subfoveal choroidal neovascularization associated with cytomegalovirus retinitis and AIDS. Can J Ophthalmol J Can Ophtalmol 43:488–489. doi: 10.3129/i08-072 [DOI] [PubMed] [Google Scholar]
- 49.Golen JR, Eichenbaum D (2013) Neovascularization of the optic disk and vitreous hemorrhage after immune recovery and treatment of cytomegalovirus retinitis in an HIV-positive patient. Retin Cases Brief Rep 7:395–398. doi: 10.1097/ICB.0b013e318298bdb5 [DOI] [PubMed] [Google Scholar]
- 50.Yeh S, Forooghian F, Faia LJ, et al. (2010) Fundus autofluorescence changes in cytomegalovirus retinitis. Retina Phila Pa 30:42–50. doi: 10.1097/IAE.0b013e3181bfbdb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta MP, Patel S, Orlin A, et al. (2017) SPECTRAL DOMAIN OPTICAL COHERENCE TOMOGRAPHY FINDINGS IN MACULA-INVOLVING CYTOMEGALOVIRUS RETINITIS. Retina Phila Pa. doi: 10.1097/IAE.0000000000001644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arichika S, Uji A, Yoshimura N (2016) Retinal structural features of cytomegalovirus retinitis with acquired immunodeficiency syndrome: an adaptive optics imaging and optical coherence tomography study. Clin Experiment Ophthalmol 44:62–64. doi: 10.1111/ceo.12577 [DOI] [PubMed] [Google Scholar]
- 53.Harper TW, Miller D, Schiffman JC, Davis JL (2009) Polymerase Chain Reaction Analysis of Aqueous and Vitreous Specimens in the Diagnosis of Posterior Segment Infectious Uveitis. Am J Ophthalmol 147:140–147.e2. doi: 10.1016/j.ajo.2008.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith IL, Macdonald JC, Freeman WR, et al. (1999) Cytomegalovirus (CMV) retinitis activity is accurately reflected by the presence and level of CMV DNA in aqueous humor and vitreous. J Infect Dis 179:1249–1253. doi: 10.1086/314710 [DOI] [PubMed] [Google Scholar]
- 55.Gupta S, Vemulakonda GA, Suhler EB, et al. (2013) Cytomegalovirus retinitis in the absence of AIDS. Can J Ophthalmol J Can Ophtalmol 48:126–129. doi: 10.1016/j.jcjo.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 56.Saidel MA, Berreen J, Margolis TP (2005) Cytomegalovirus retinitis after intravitreous triamcinolone in an immunocompetent patient. Am J Ophthalmol 140:1141–1143. doi: 10.1016/j.ajo.2005.06.058 [DOI] [PubMed] [Google Scholar]
- 57.Vannozzi L, Bacherini D, Sodi A, et al. (2016) Cytomegalovirus retinitis following intravitreal dexamethasone implant in a patient with central retinal vein occlusion. Acta Ophthalmol (Copenh) 94:e158–160. doi: 10.1111/aos.12783 [DOI] [PubMed] [Google Scholar]
- 58.Jabs DA, Martin BK, Ricks MO, et al. (2006) Detection of ganciclovir resistance in patients with AIDS and cytomegalovirus retinitis: correlation of genotypic methods with viral phenotype and clinical outcome. J Infect Dis 193:1728–1737. doi: 10.1086/504270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gross JG, Bozzette SA, Mathews WC, et al. (1990) Longitudinal study of cytomegalovirus retinitis in acquired immune deficiency syndrome. Ophthalmology 97:681–686 [DOI] [PubMed] [Google Scholar]
- 60.Studies of Ocular Complications of AIDS Research Group (1996) Combination foscarnet and ganciclovir therapy vs monotherapy for the treatment of relapsed cytomegalovirus retinitis in patients with AIDS. The Cytomegalovirus Retreatment Trial. The Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group. Arch Ophthalmol Chic Ill 1960 114:23–33 [DOI] [PubMed] [Google Scholar]
- 61.Musch DC, Martin DF, Gordon JF, et al. (1997) Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med 337:83–90. doi: 10.1056/NEJM199707103370203 [DOI] [PubMed] [Google Scholar]
- 62.Jabs DA, Martin BK, Forman MS, et al. (2003) Cytomegalovirus resistance to ganciclovir and clinical outcomes of patients with cytomegalovirus retinitis. Am J Ophthalmol 135:26–34 [DOI] [PubMed] [Google Scholar]
- 63.Palella FJ, Delaney KM, Moorman AC, et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860. doi: 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- 64.Macdonald JC, Karavellas MP, Torriani FJ, et al. (2000) Highly active antiretroviral therapy-related immune recovery in AIDS patients with cytomegalovirus retinitis. Ophthalmology 107:877–881; discussion 881–883 [DOI] [PubMed] [Google Scholar]
- 65.Thorne JE, Jabs DA, Kempen JH, et al. (2006) Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology 113:1441–1445. doi: 10.1016/j.ophtha.2006.03.022 [DOI] [PubMed] [Google Scholar]
- 66.Doan S, Cochereau I, Guvenisik N, et al. (1999) Cytomegalovirus retinitis in HIV-infected patients with and without highly active antiretroviral therapy. Am J Ophthalmol 128:250–251 [DOI] [PubMed] [Google Scholar]
- 67.Thorne JE, Jabs DA, Kempen JH, et al. (2006) Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology 113:1432–1440. doi: 10.1016/j.ophtha.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 68.Jacobson MA, Stanley H, Holtzer C, et al. (2000) Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am 30:231–233. doi: 10.1086/313612 [DOI] [PubMed] [Google Scholar]
- 69.Goldberg DE, Smithen LM, Angelilli A, Freeman WR (2005) HIV-associated retinopathy in the HAART era. Retina Phila Pa 25:633–649; quiz 682–683 [DOI] [PubMed] [Google Scholar]
- 70.Freeman WR, Friedberg DN, Berry C, et al. (1993) Risk factors for development of rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis. Am J Ophthalmol 116:713–720 [DOI] [PubMed] [Google Scholar]
- 71.Jabs DA, Enger C, Haller J, de Bustros S (1991) Retinal detachments in patients with cytomegalovirus retinitis. Arch Ophthalmol Chic Ill 1960 109:794–799 [DOI] [PubMed] [Google Scholar]
- 72.Studies of Ocular Complications of AIDS Research Group (1997) Rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis: the Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial. The Studies of Ocular Complications of AIDS (SOCA) Research Group in Collaboration with the AIDS Clinical Trials Group (ACTG). Am J Ophthalmol 124:61–70 [PubMed] [Google Scholar]
- 73.Jabs DA, Van Natta ML, Thorne JE, et al. (2004) Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2. Second eye involvement and retinal detachment. Ophthalmology 111:2232–2239. doi: 10.1016/j.ophtha.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 74.Althaus C, Loeffler KU, Schimkat M, et al. (1998) Prophylactic argon laser coagulation for rhegmatogenous retinal detachment in AIDS patients with cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Für Klin Exp Ophthalmol 236:359–364 [DOI] [PubMed] [Google Scholar]
- 75.Shah GK, Vander J (1998) Rhegmatogenous retinal detachments with cytomegalovirus retinitis. Curr Opin Ophthalmol 9:6–10 [DOI] [PubMed] [Google Scholar]
- 76.Goldberg DE, Wang H, Azen SP, Freeman WR (2003) Long term visual outcome of patients with cytomegalovirus retinitis treated with highly active antiretroviral therapy. Br J Ophthalmol 87:853–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karavellas MP, Azen SP, MacDonald JC, et al. (2001) Immune recovery vitritis and uveitis in AIDS: clinical predictors, sequelae, and treatment outcomes. Retina Phila Pa 21:1–9 [DOI] [PubMed] [Google Scholar]
- 78.Karavellas MP, Plummer DJ, Macdonald JC, et al. (1999) Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis 179:697–700. doi: 10.1086/314639 [DOI] [PubMed] [Google Scholar]
- 79.Kempen JH, Min Y-I, Freeman WR, et al. (2006) Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology 113:684–694. doi: 10.1016/j.ophtha.2005.10.067 [DOI] [PubMed] [Google Scholar]
- 80.Nguyen QD, Kempen JH, Bolton SG, et al. (2000) Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am J Ophthalmol 129:634–639 [DOI] [PubMed] [Google Scholar]
- 81.Song M-K, Azen SP, Buley A, et al. (2003) Effect of anti-cytomegalovirus therapy on the incidence of immune recovery uveitis in AIDS patients with healed cytomegalovirus retinitis. Am J Ophthalmol 136:696–702 [DOI] [PubMed] [Google Scholar]
- 82.Kozak I, Vaidya V, Van Natta ML, et al. (2014) The prevalence and incidence of epiretinal membranes in eyes with inactive extramacular CMV retinitis. Invest Ophthalmol Vis Sci 55:4304–4312. doi: 10.1167/iovs.14-14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kempen JH, Sugar EA, Lyon AT, et al. (2012) Risk of cataract in persons with cytomegalovirus retinitis and the acquired immune deficiency syndrome. Ophthalmology 119:2343–2350. doi: 10.1016/j.ophtha.2012.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Urban B, Bakunowicz-Łazarczyk A, Michalczuk M (2014) Immune recovery uveitis: pathogenesis, clinical symptoms, and treatment. Mediators Inflamm 2014:971417. doi: 10.1155/2014/971417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holland GN (2008) AIDS and ophthalmology: the first quarter century. Am J Ophthalmol 145:397–408. doi: 10.1016/j.ajo.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 86.Hu J, Coassin M, Stewart JM (2011) Fluocinolone acetonide implant (Retisert) for chronic cystoid macular edema in two patients with AIDS and a history of cytomegalovirus retinitis. Ocul Immunol Inflamm 19:206–209. doi: 10.3109/09273948.2010.538120 [DOI] [PubMed] [Google Scholar]
- 87.Ufret-Vincenty RL, Singh RP, Lowder CY, Kaiser PK (2007) Cytomegalovirus retinitis after fluocinolone acetonide (Retisert) implant. Am J Ophthalmol 143:334–335. doi: 10.1016/j.ajo.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 88.Martin DF, Kuppermann BD, Wolitz RA, et al. (1999) Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med 340:1063–1070. doi: 10.1056/NEJM199904083401402 [DOI] [PubMed] [Google Scholar]
- 89.Iu LP, Fan MC, Lau JK, et al. (2016) Long-term Follow-up of Cytomegalovirus Retinitis in Non-HIV Immunocompromised Patients: Clinical Features and Visual Prognosis. Am J Ophthalmol 165:145–153. doi: 10.1016/j.ajo.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 90.Biron KK (2006) Antiviral drugs for cytomegalovirus diseases. Antiviral Res 71:154–163. doi: 10.1016/j.antiviral.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 91.Drew WL, Ives D, Lalezari JP, et al. (1995) Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. Syntex Cooperative Oral Ganciclovir Study Group. N Engl J Med 333:615–620. doi: 10.1056/NEJM199509073331002 [DOI] [PubMed] [Google Scholar]
- 92.Martin DF, Sierra-Madero J, Walmsley S, et al. (2002) A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med 346:1119–1126. doi: 10.1056/NEJMoa011759 [DOI] [PubMed] [Google Scholar]
- 93.Cvetković RS, Wellington K (2005) Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs 65:859–878 [DOI] [PubMed] [Google Scholar]
- 94.Mahadevia PJ, Gebo KA, Pettit K, et al. (2004) The epidemiology, treatment patterns, and costs of cytomegalovirus retinitis in the post-haart era among a national managed-care population. J Acquir Immune Defic Syndr 1999 36:972–977 [DOI] [PubMed] [Google Scholar]
- 95.Jacobson MA, O’Donnell JJ (1991) Approaches to the treatment of cytomegalovirus retinitis: ganciclovir and foscarnet. J Acquir Immune Defic Syndr 4 Suppl 1:S11–15 [PubMed] [Google Scholar]
- 96.Palestine AG, Polis MA, De Smet MD, et al. (1991) A randomized, controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Ann Intern Med 115:665–673 [DOI] [PubMed] [Google Scholar]
- 97.Tognon MS, Turrini B, Masiero G, et al. (1996) Intravitreal and systemic foscarnet in the treatment of AIDS-related CMV retinitis. Eur J Ophthalmol 6:179–182 [DOI] [PubMed] [Google Scholar]
- 98.(1992) Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. Studies of Ocular Complications of AIDS Research Group, in collaboration with the AIDS Clinical Trials Group. N Engl J Med 326:213–220. doi: 10.1056/NEJM199201233260401 [DOI] [PubMed] [Google Scholar]
- 99.Port AD, Orlin A, Kiss S, et al. (2017) Cytomegalovirus Retinitis: A Review. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. doi: 10.1089/jop.2016.0140 [DOI] [PubMed] [Google Scholar]
- 100.Studies of Ocular Complications of AIDS Research Group. The AIDS Clinical Trials Group. (2001) The ganciclovir implant plus oral ganciclovir versus parenteral cidofovir for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: The Ganciclovir Cidofovir Cytomegalovirus Retinitis Trial. Am J Ophthalmol 131:457–467 [DOI] [PubMed] [Google Scholar]
- 101.Lalezari JP, Holland GN, Kramer F, et al. (1998) Randomized, controlled study of the safety and efficacy of intravenous cidofovir for the treatment of relapsing cytomegalovirus retinitis in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirology Off Publ Int Retrovirology Assoc 17:339–344 [DOI] [PubMed] [Google Scholar]
- 102.Lalezari JP, Stagg RJ, Kuppermann BD, et al. (1997) Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann Intern Med 126:257–263 [DOI] [PubMed] [Google Scholar]
- 103.Dunn JH, Weinberg A, Chan LK, et al. (2013) Long-term suppression of multidrug-resistant cytomegalovirus retinitis with systemically administered leflunomide. JAMA Ophthalmol 131:958–960. doi: 10.1001/jamaophthalmol.2013.1589 [DOI] [PubMed] [Google Scholar]
- 104.John GT, Manivannan J, Chandy S, et al. (2004) Leflunomide therapy for cytomegalovirus disease in renal allograft recepients. Transplantation 77:1460–1461 [DOI] [PubMed] [Google Scholar]
- 105.Levi ME, Mandava N, Chan LK, et al. (2006) Treatment of multidrug-resistant cytomegalovirus retinitis with systemically administered leflunomide. Transpl Infect Dis Off J Transplant Soc 8:38–43. doi: 10.1111/j.1399-3062.2006.00128.x [DOI] [PubMed] [Google Scholar]
- 106.Avery RK (2008) Update in management of ganciclovir-resistant cytomegalovirus infection. Curr Opin Infect Dis 21:433–437. doi: 10.1097/QCO.0b013e328307c7b4 [DOI] [PubMed] [Google Scholar]
- 107.Avery RK, Mossad SB, Poggio E, et al. (2010) Utility of leflunomide in the treatment of complex cytomegalovirus syndromes. Transplantation 90:419–426. doi: 10.1097/TP.0b013e3181e94106 [DOI] [PubMed] [Google Scholar]
- 108.Jabs DA, Ahuja A, Van Natta M, et al. (2013) Comparison of treatment regimens for cytomegalovirus retinitis in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 120:1262–1270. doi: 10.1016/j.ophtha.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diaz-Llopis M, España E, Muñoz G, et al. (1994) High dose intravitreal foscarnet in the treatment of cytomegalovirus retinitis in AIDS. Br J Ophthalmol 78:120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ausayakhun S, Watananikorn S, Ngamtiphakorn S, Prasitsilp J (2005) Intravitreal foscarnet for cytomegalovirus retinitis in patients with AIDS. J Med Assoc Thail Chotmaihet Thangphaet 88:103–107 [PubMed] [Google Scholar]
- 111.Kersten AJ, Althaus C, Best J, Sundmacher R (1999) Cystoid macular edema following immune recovery and treatment with cidofovir for cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Für Klin Exp Ophthalmol 237:893–896 [DOI] [PubMed] [Google Scholar]
- 112.Kirsch LS, Arevalo JF, Chavez de la Paz E, et al. (1995) Intravitreal cidofovir (HPMPC) treatment of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology 102:533–542; discussion 542–543 [DOI] [PubMed] [Google Scholar]
- 113.Rahhal FM, Arevalo JF, Munguia D, et al. (1996) Intravitreal cidofovir for the maintenance treatment of cytomegalovirus retinitis. Ophthalmology 103:1078–1083 [DOI] [PubMed] [Google Scholar]
- 114.Orr RM (2001) Technology evaluation: fomivirsen, Isis Pharmaceuticals Inc/CIBA vision. Curr Opin Mol Ther 3:288–294 [PubMed] [Google Scholar]
- 115.Vitravene Study Group (2002) A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol 133:467–474 [DOI] [PubMed] [Google Scholar]
- 116.Chang M, Dunn JP (2005) Ganciclovir implant in the treatment of cytomegalovirus retinitis. Expert Rev Med Devices 2:421–427. doi: 10.1586/17434440.2.4.421 [DOI] [PubMed] [Google Scholar]
- 117.Martin DF (1994) Treatment of Cytomegalovirus Retinitis With an Intraocular Sustained-Release Ganciclovir Implant: A Randomized Controlled Clinical Trial. Arch Ophthalmol 112:1531. doi: 10.1001/archopht.1994.01090240037023 [DOI] [PubMed] [Google Scholar]
- 118.Duvvuri S, Janoria KG, Pal D, Mitra AK (2007) Controlled delivery of ganciclovir to the retina with drug-loaded Poly(d,L-lactide-co-glycolide) (PLGA) microspheres dispersed in PLGA-PEG-PLGA Gel: a novel intravitreal delivery system for the treatment of cytomegalovirus retinitis. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther 23:264–274. doi: 10.1089/jop.2006.132 [DOI] [PubMed] [Google Scholar]
- 119.Wang Q, Sun C, Xu B, et al. (2018) Synthesis, physicochemical properties and ocular pharmacokinetics of thermosensitive in situ hydrogels for ganciclovir in cytomegalovirus retinitis treatment. Drug Deliv 25:59–69. doi: 10.1080/10717544.2017.1413448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Einsele H, Roosnek E, Rufer N, et al. (2002) Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99:3916–3922 [DOI] [PubMed] [Google Scholar]
- 121.Feuchtinger T, Opherk K, Bethge WA, et al. (2010) Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 116:4360–4367. doi: 10.1182/blood-2010-01-262089 [DOI] [PubMed] [Google Scholar]
- 122.Mackinnon S, Thomson K, Verfuerth S, et al. (2008) Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis 40:63–67. doi: 10.1016/j.bcmd.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 123.Patel M, Coombs P, Prockop SE, et al. (2015) Treatment of Cytomegalovirus (CMV) Retinitis with CMV-Specific T-Lymphocyte Infusion. Ophthalmic Surg Lasers Imaging Retina 46:80–82. doi: 10.3928/23258160-20150101-14 [DOI] [PMC free article] [PubMed] [Google Scholar]