SUMMARY
Mucosal barriers are densely colonized by pathobiont microbes such as Candida albicans, capable of invasive disseminated infection. However, systemic infections occur infrequently in healthy individuals, suggesting that pathobiont commensalism may elicit host benefits. We show that intestinal colonization with C. albicans drives systemic expansion of fungal-specific Th17 CD4+ T cells and IL-17 responsiveness by circulating neutrophils, which synergistically protect against C. albicans invasive infection. Protection conferred by commensal C. albicans requires persistent fungal colonization and extends to other extracellular invasive pathogens such as Staphylococcus aureus. However, commensal C. albicans does not protect against intracellular influenza virus infection and exacerbates allergic airway inflammation susceptibility indicating that positively calibrating systemic Th17 responses is not uniformly beneficial. Thus, systemic Th17 inflammation driven by CD4+ T cells responsive to tonic stimulation by commensal C. albicans improves host defense against extracellular pathogens, but with potentially harmful immunological consequences.
Graphical Abstract
eTOC Blurb
Mucosal tissues are frequently colonized by microbes with pathogenic invasive potential. However, invasive systemic infection rarely occurs in healthy immune component individuals. Shao et al show commensal Candida albicans intestinal colonization uniquely activates circulating immune cells to protect against systemic infection by this, and other invasive extracellular microbial pathogens.
INTRODUCTION
Mucosal barrier tissues are densely colonized with a wide variety of pathobiont microbes capable invasive disseminated infection. This includes many important human pathogens such as Candida albicans and Staphylococcus aureus that in most individuals are associated with asymptomatic colonization, but with invasion can cause systemic infection associated with high mortality (Brown et al., 2012; Casadevall and Pirofski, 2000). Fortunately, systemic disseminated infections caused by pathobionts occur infrequently, especially considering their exceptional high rates of commensal colonization. For example, C. albicans invasive infection is estimated at only 8 cases per 100,000 individuals, or 0.008% (Pfaller and Diekema, 2007), despite intestinal colonization amongst >60% of healthy adults (Nash et al., 2017). Likewise, the annual incidence of S. aureus bloodstream infection of 26 per 100,000 individuals (0.026%) is orders of magnitude less than the ~20% and 40% rates of colonization in the intestine and anterior nares, respectively (Acton et al., 2009; Laupland, 2013; Williams, 1963). This discrepancy highlights important questions for why systemic disseminated infection, caused by pathobionts with near ubiquitous colonization, occurs so infrequently.
Addressing these questions require new models of commensal colonization by pathobiont microbes that also have the capacity for invasive infection in healthy hosts. For example, while prior intranasal Streptococcus pneumoniae administration is protective against subsequent lung infection in mice, colonization is very transient with sharply reduced pathobiont recovery within the first few days after inoculation that precludes analysis of how sustained commensal colonization impacts infection susceptibility (Wilson et al., 2015). On the other hand, while lethal infection and systemic seeding by commensal C. albicans can be induced using chemotherapeutic agents, the ensuing immune suppression and intestinal epithelial damage preclude analysis of pathobiont colonization conferred immunity in healthy hosts (Koh et al., 2008). More recently, protection against systemic C. albicans infection in mice stably colonized with attenuated isogenic mutant strains adapted for intestinal colonization has been described (Tso et al., 2018). However, the significance of these findings is uncertain since invasive infections by prototypical pathobionts such as C. albicans and S. aureus are predominantly (>80%) caused by genetically identical virulent commensal isolates (Reagan et al., 1990; Von Eiff et al., 2001; Voss et al., 1994). Thus, how pathobiont commensal colonization impacts immunity in healthy individuals remains poorly defined.
To fill these outstanding knowledge gaps, an instructive model of asymptomatic long-term intestinal colonization with virulent C. albicans was developed. In particular, recombinant C. albicans engineered to express defined model antigens was used to establish intestinal colonization to facilitate precise identification of adaptive immune components with surrogate commensal specificity. This strategy showed that C. albicans intestinal colonization primes systemic accumulation of protective Th17 activated CD4+ T cells with commensal fungi specificity and increased IL-17 responsiveness in circulating Ly6G+ neutrophils. Complementary human analysis identified positive associations between C. albicans fecal colonization density, systemic accumulation of IL-17 producing C. albicans-specific CD4+ T cells and IL-17 responsiveness by circulating neutrophils. Interestingly, commensal C. albicans conferred immunity also extends to protection against invasive infection by other extracellular pathobionts such as S. aureus, but these host defense benefits are offset by susceptibility to airway inflammation. Together, exciting facets of symbiosis between commensal C. albicans and mammalian hosts that positively calibrate systemic neutrophil-Th17 CD4+ T cell immunological responses are demonstrated.
RESULTS
C. albicans intestinal colonization protects against invasive C. albicans systemic infection.
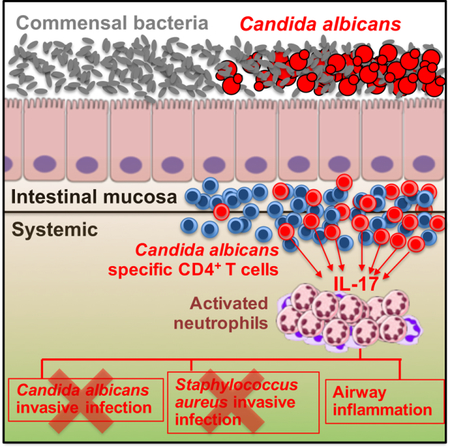
C. albicans is a common commensal of the human intestine (Nash et al., 2017). However, this fungi is rarely found in the feces of laboratory mice (Iliev et al., 2012; Skalski et al., 2018; Wheeler et al., 2016), and completely absent for mice housed in our specific pathogen free facility (Jiang et al., 2017). We reasoned the lack of commensal C. albicans could be exploited to investigate how intestinal colonization with this pathobiont shapes host immunity. Since antibiotic induced dysbiosis is a dominant risk-factor for human C. albicans colonization (Schulte et al., 2015; Spinillo et al., 1999), and in mice promotes colonization by virulent strains including SC5314 (Fan et al., 2015), how C. albicans colonization induced by bacterial dysbiosis impacts susceptibility to invasive infection was evaluated. We found a single oral C. albicans inoculation administered to mice maintained on ampicillin supplemented drinking water results in sustained (>60 days) C. albicans colonization throughout the intestinal tract (Figures 1A and 1B). C. albicans recovery in the feces was consistently achieved within 24 hours after oral inoculation, and with progressively increasing levels plateauing within the first week in ampicillin treated mice (Figure 1A). By contrast, C. albicans recovery was sporadic, and consistently at or below the limits of detection for mice without drinking water antibiotic supplementation (Figures 1A and 1B). Importantly, despite high density intestinal C. albicans colonization, no evidence of systemic fungal dissemination or other negative health consequence was observed. Tissues commonly susceptible to invasive C. albicans infection (e.g. kidney, liver, brain) remained uniformly sterile (Figure 1C), and C. albicans colonized compared with no antibiotic control mice gained weight with comparable tempo (Figure 1D). Thus, virulent isolates of the human pathobiont, C. albicans, can achieve persistent long-term asymptomatic colonization in mice.
Figure 1. Persistent C. albicans intestinal colonization facilitated by drinking water ampicillin supplementation.
(A) Recoverable C. albicans in the feces of mice with ampicillin supplementation in the drinking water compared with no antibiotic controls after oral C. albicans inoculation.
(B) Recoverable C. albicans in each intestinal segment 7 days after oral C. albicans inoculation for the mice described in (A).
(C) Recoverable C. albicans in each tissue seven days after oral C. albicans inoculation for mice with ampicillin drinking water supplementation.
(D) Weight change after oral C. albicans inoculation for the mice described in (A).
(E) Diversity and abundance of fecal bacterial species for each group of mice after drinking water ampicillin supplementation (12 days) with or without oral C. albicans inoculation (2 days after initiating ampicillin drinking water supplementation) determined by shotgun sequencing. Pie-chart area is directly proportional to the absolute abundance of fecal bacterial genomic DNA for each group of mice.
(F) Bacterial genomic equivalents for each group of mice described in (E).
(G) Diversity of fecal bacteria for each group of mice described in (E).
*p<0.05, ****p<0.0001, Bar, mean ± SEM. L.o.D., limit of detection. See also Figure S1
As expected, ampicillin drinking water supplementation caused dramatic shifts in the composition and abundance of fecal bacterial spp. as shown by shotgun sequencing (Ubeda et al., 2010) (Figures 1E and S1A). Interestingly and regardless of C. albicans colonization, the absolute abundance of fecal bacteria genomic equivalents was increased by ampicillin treatment after comparing the bacterial DNA density to a known quantity of Salinibacter ruber reference DNA added to each sample (Figure 1F). Importantly however, neither the composition, diversity nor differential abundance of commensal bacteria differed significantly between C. albicans colonized compared with non-colonized control mice maintained on ampicillin supplemented drinking water (Figures 1E-1G and S1). Thus, antibiotic induced dysbiosis is an instructive approach allowing the immune modulatory effects of C. albicans commensal colonization to be evaluated in isolation.
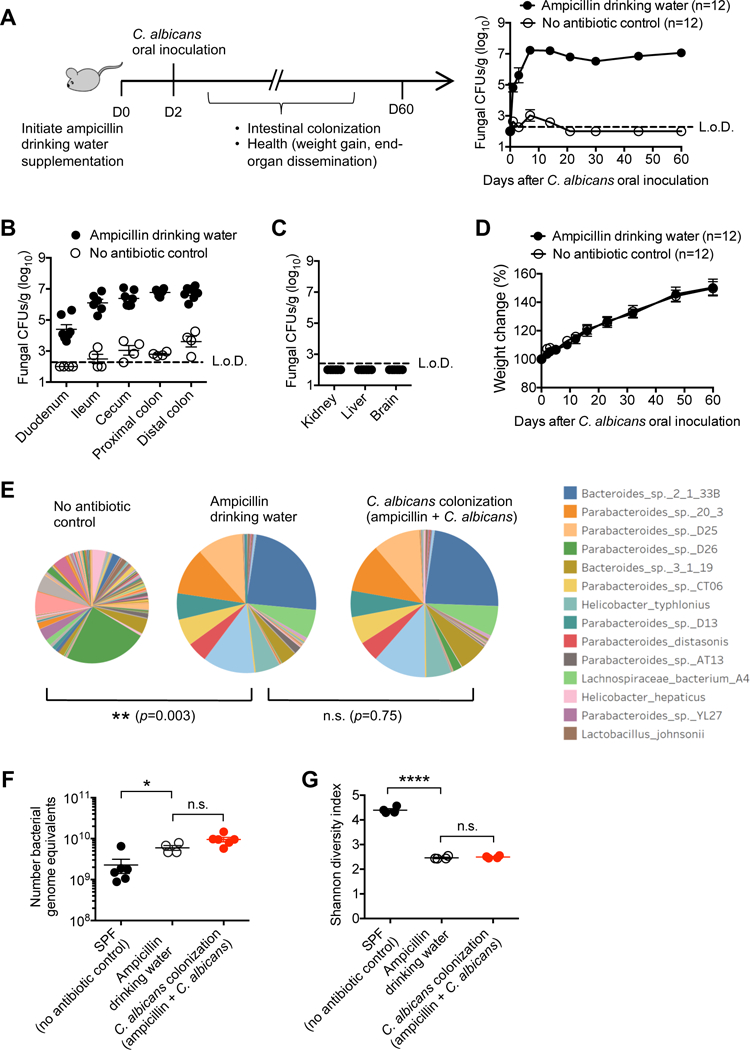
Given the lack of spontaneous disseminated infection in C. albicans colonized mice (Figures 1C, 1D), susceptibility to invasive infection was probed by intravenous inoculation with the identical or a marked isogenic virulent recombinant strain. This analysis showed sharply reduced susceptibility to systemic invasive infection conferred by intestinal colonization. Mice with commensal C. albicans had improved survival following intravenous infection with a lethal dosage of virulent strain SC5314 for immune competent mice (Jiang et al., 2015), and >100-fold reduced fungal burden in the kidneys compared with control mice maintained on ampicillin supplemented drinking water (Figure 2A). To confirm that C. albicans in the target tissue of colonized mice directly reflects reduced susceptibility to intravenous infection, as opposed to dissemination from intestinal tissue, we took advantage of GFP expression by recombinant virulent C. albicans strains (Igyártó et al., 2011) allowing oral inoculation and subsequent intravenous challenge by unique isogenic strains (Figure 2B). These experiments showed recoverable fungi in the kidney were uniformly from intravenous inoculation (GFP+), while fungi in feces were of commensal origin (GFP-) (Figure 2B). Thus, despite retaining virulence potential, pathobiont commensal C. albicans does not breech the intestinal barrier to seed systemic tissues. Moreover, protection against systemic C. albicans invasive infection is not due to enhanced immunogenicity of recombinant C. albicans, since similarly reduced susceptibility was observed in mice colonized with either recombinant or the non-recombinant SC5314 strain (Figure S2). Together, these results show commensal C. albicans intestinal colonization efficiently protects against systemic invasive infection by the same pathobiont microbe.
Figure 2. C. albicans intestinal colonization protects against systemic C. albicans invasive infection.
(A) Percent survival and recoverable C. albicans 5 days after recombinant C. albicans intravenous infection (5 × 104 CFUs) for mice with recombinant C. albicans intestinal colonization or control mice maintained on ampicillin supplemented drinking water.
(B) Fluorescence intensity of C. albicans recovered from the feces compared with kidneys 5 days after intravenous infection with GFP+ C. albicans for mice with prior GFP- C. albicans oral inoculation.
***p<0.001, ****p<0.0001, Bar, mean ± SEM. L.o.D., limit of detection. See also Figure S2
Systemic expansion of protective IL-17 producing CD4+ T cells with commensal C. albicans specificity.
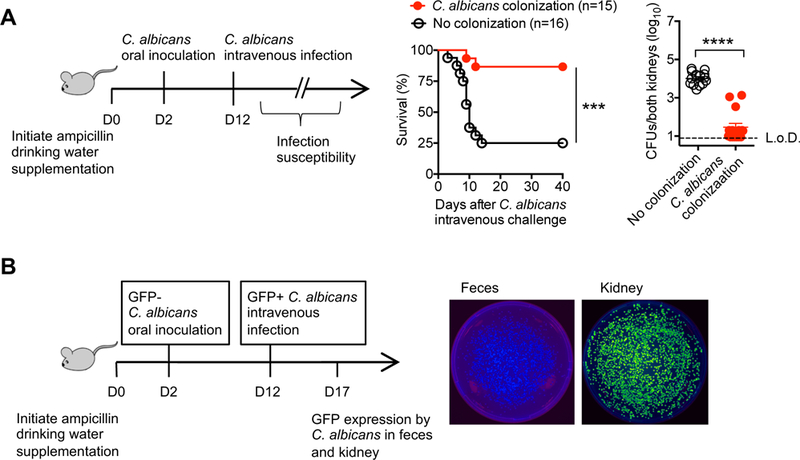
The immunological basis for protection against invasive infection conferred by intestinal colonization was addressed by evaluating systemic accumulation of adaptive immune components with commensal C. albicans specificity. We focused on CD4+ T cells since candidiasis is more prevalent amongst individuals with HIV infection or other immune comprising conditions with diminished CD4+ T cell function (Klein et al., 1984). To facilitate identification of CD4+ T cells with commensal C. albicans specificity, constitutively expression of the 2W1S55–68 variant of I-Eα by recombinant C. albicans (Igyártó et al., 2011), and high precursor frequency of endogenous CD4+ T cells with I-Ab:2W1S55–68 specificity were exploited for precise identification of cells with this surrogate commensal specificity after tetramer staining and enrichment (Moon et al., 2007). This analysis showed robust expansion of CD4+ T cells with commensal C. albicans-2W1S specificity in systemic lymphoid tissue (spleen plus peripheral lymph nodes) that were almost all CD44hi reflecting activation in response to cognate antigen stimulation (Baaten et al., 2012) (Figure 3A). Interestingly, accumulation of C. albicans-2W1S specific CD4+ T cells was not more pronounced in the mesenteric lymph node compared with splenocytes and pooled peripheral lymph node cells, highlighting the systemic response to commensal fungal colonization (Figure S3).
Figure 3. Systemic expansion of protective Th17 CD4+ T cells with commensal C. albicans specificity.
(A) Number and percent CD44hi among I-Ab:2W1S tetramer positive CD4+ T cells from spleen and peripheral lymph nodes of mice with recombinant C. albicans intestinal colonization compared with no colonization controls.
(B) Percent and number RORγt+ among I-Ab:2W1S positive (solid line) or negative (gray shaded) CD4+ T cells for mice described in (A).
(C) Percent IL-17A, IL-17F or IFN-γ production after heat-killed WT C. albicans stimulation by CD4+ splenocyte and lymph nodes cells for mice described in (A).
(D) Recoverable C. albicans five days after recombinant C. albicans intravenous infection (5 × 104 CFUs) amongst recombinant C. albicans colonized compared with control mice administered rat IgG (isotype), anti-CD4, or anti-IL-17A plus anti-IL-17F antibodies beginning one day prior to infection.
(E) Percent survival for mice described in (D).
(F) Recoverable C. albicans 5 days after recombinant C. albicans intravenous infection (5 × 104 CFUs) for Rag1-deficient mice colonized with recombinant C. albicans or no colonization controls.
** p<0.01, *** p<0.001, ****p<0.0001, Bar, mean ± SEM. L.o.D., limit of detection. See also Figures S3-S5
To investigate how C. albicans intestinal colonization stimulates the differentiation of these systemically expanded cells, their expression of canonical T helper lineage defining transcriptional regulators was evaluated. Differentiation into RORγt expressing Th17 cells accounted for the largest subset of peripheral CD4+ T cells with commensal C. albicans-2W1S specificity compared with <5% among CD4+ T cells of the same specificity in no colonization control mice (Figure 3B). Increased number and proportion of RORγt+ CD4+ T cells with C. albicans specificity persisted with antigen re-stimulation after intravenous C. albicans-2W1S challenge compared with cells in control mice undergoing primary expansion (Figure S4). Likewise, production of Th17 lineage defining cytokines IL-17A or IL-17F was selectively increased amongst CD4+ T cells from colonized compared with control mice after heat-killed C. albicans in vitro stimulation (Figure 3C). Comparatively, no differences in IFN-γ production were identified, and percent Tbet+ Th1 or FOXP3+ regulatory T cells remained similar amongst CD4+ T cells with commensal C. albicans-2W1S specificity in colonized compared with control mice (Figures 3C and S5A). Importantly, Th17 immunogenicity primed by commensal C. albicans is not restricted to antigens unique to this recombinant strain, given the increased number of IL-17A and IL-17F producing cells (~100,000 cells) after in vitro stimulation with non-recombinant heat-killed C. albicans (Figure S5B), compared with fewer than 1000 I-Ab:2W1S+ cells identified by tetramer staining (Figure 3A). Thus, C. albicans intestinal colonization in mice preferentially primes systemic accumulation of fungal-specific Th17 CD4+ T cells, in agreement with accumulation of IL-17 producing cells in the peripheral blood of healthy human volunteers (Acosta-Rodriguez et al., 2007).
To further investigate the necessity of these immune components in protection against C. albicans invasive infection, the effects of their depletion initiated one day prior to intravenous challenge was evaluated. Administration of either CD4+ T cell depleting or IL-17A plus IL-17F neutralizing antibodies each overturned the protective benefits of intestinal colonization, since fungal pathogen burden and mortality each rebounded to levels comparable to control mice (Figures 3D and 3E). Protection against invasive infection conferred by C. albicans intestinal colonization was also eliminated in Rag1-deficient mice completely devoid of all T and B cells (Figure 3F). Thus, CD4+ T cells and IL-17 cytokines are each essential for C. albicans colonization conferred protection against invasive infection.
IL-17 responsiveness in circulating neutrophils increased with C. albicans intestinal colonization.
To investigate the IL-17 responsive cell subset(s) responsible for protection, colonization induced shifts in expression of IL-17 receptor was compared amongst CD45+ leukocytes. Rather than IL-17RA, which is ubiquitously expressed in leukocyte cells (Iwakura et al., 2008), we focused on IL-17RC, an essential component of the IL-17 receptor complex whose expression is modulated by inflammation (Taylor et al., 2014). This analysis showed significantly increased frequency of IL-17RC expressing cells in the peripheral blood of C. albicans colonized compared to no colonization control mice (Figure 4A). Amongst IL-17RC+ leukocytes, the proportion of Ly6GhiLy6Cint or Gr-1+ neutrophils were each significantly increased, whereas no change (Ly6ChiLy6Glo monocytes, CD4+ or CD8+ T cells) or reciprocal reductions (B220+ B cells) were found for other leukocyte subsets (Figure 4B). Enhanced IL-17 responsiveness by circulating leukocytes was dependent on both CD4+ T cells and IL-17, since IL-17RC expression was reduced to levels comparable to no colonization control mice after administration of CD4+ T cell depleting or IL-17A plus IL-17F neutralizing antibodies (Figure 4C). Enhanced IL-17 responsiveness by neutrophils paralleled their expanded accumulation amongst circulating leukocytes (Figure 4D), and increased production of reactive oxygen species in response to C. albicans stimulation (Figure 4E). Importantly, neutrophils are essential for commensal C. albicans conferred protection, since resistance against invasive infection was efficiently overturned in mice administered anti-Ly6G or anti-Gr-1 depleting antibodies, with mortality comparable to no colonization control mice administered each depleting antibody (Figure 4F). Collectively, these results show expanded IL-17 producing CD4+ T cells primed by C. albicans intestinal colonization tonically stimulates IL-17 responsiveness by neutrophils, that in turn, protect against C. albicans invasive infection.
Figure 4. C. albicans intestinal colonization stimulates accumulation, activation and IL-17 responsiveness by circulating neutrophils.
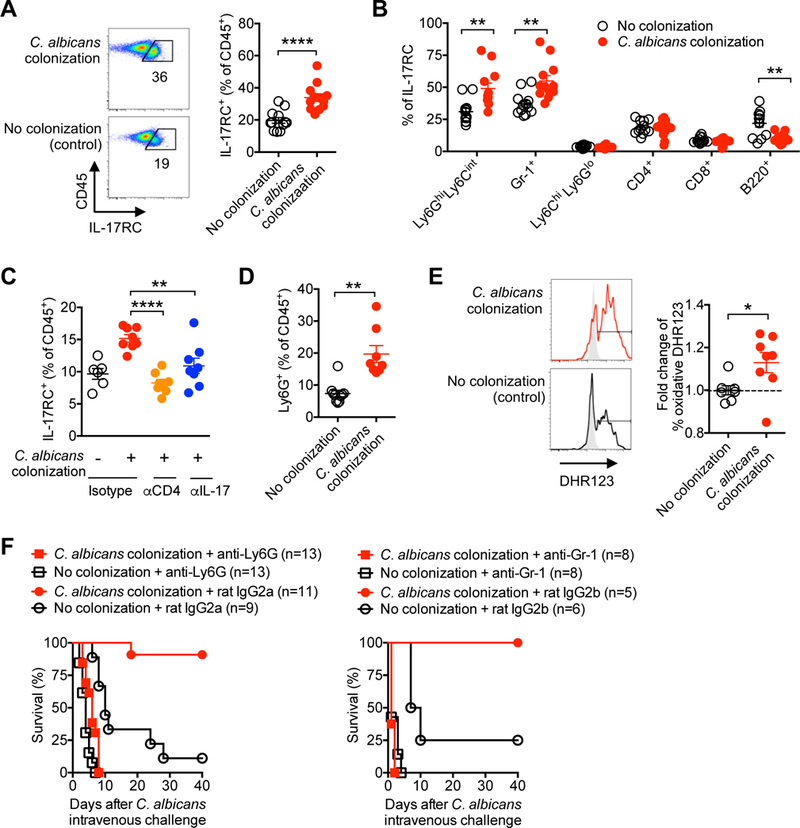
(A) Percent IL-17RC+ amongst CD45+ leukocytes in the peripheral blood of mice with recombinant C. albicans intestinal colonization compared with no colonization controls.
(B) Percent IL-17RC+ amongst each leukocyte subsets for mice described in (A).
(C) Percent IL-17RC+ amongst CD45+ leukocytes in the peripheral blood of recombinant C. albicans colonized mice 7 days after the administration of rat IgG (isotype), anti-CD4, or anti-IL-17A plus anti-IL-17F antibodies.
(D) Percent of Ly6G+Ly6Cint neutrophils amongst CD45+ leukocytes in the peripheral blood for each group of mice described in panel (A).
(E) Representative plots (solid line histogram, C. albicans extract stimulation; shaded histogram, no stimulation controls) and composite data showing the relative proportion of dihydrorhodamine (DHR)123 fluorescence amongst Ly6G+Ly6Cint neutrophils in the peripheral blood for each group of mice described in panel (A).
(F) Percent survival after recombinant C. albicans intravenous infection (5 × 104 CFUs) amongst recombinant C. albicans colonized compared with or no colonization control mice administered anti-Ly6G or anti-Gr-1 antibodies, along with each respective rat IgG isotype control antibody, beginning one day prior to infection.
*p<0.05, ** p<0.01, ****p<0.0001, Bar, mean ± SEM. L.o.D., limit of detection.
Protection against systemic infection requires persistent C. albicans intestinal colonization.
Given shifts in the composition and density of commensal microbes throughout development (Lozupone et al., 2012), we next investigated the durability of commensal C. albicans conferred protection, and, in particular, whether persistent colonization is required. These experiments utilized fluconazole, an anti-mycotic agent, that when added to ampicillin supplemented drinking water efficiently eliminates C. albicans intestinal colonization (Figure S6A) (Iliev et al., 2012). Since fluconazole would also artificially render resistance to fungal infection, we first determined the time after removing fluconazole from the drinking water when C. albicans infection susceptibility would be restored, and found similar fungal burden in mice 5 days after removing fluconazole compared with control mice (Figure S6B). Using this approach, we found protection against invasive infection and nearly all systemic immunological shifts primed by C. albicans intestinal colonization were eliminated with fungal eradication. In particular, significantly increased fungal pathogen burden was found after intravenous infection in fluconazole treated C. albicans colonized mice compared with mice with sustained C. albicans intestinal colonization (Figure 5A). Expansion of CD4+ T cells with commensal C. albicans I-Ab:2W1S specificity, and their expression of RORγt in fluconazole treated mice were each reduced to background levels found in control mice without prior C. albicans intestinal colonization (Figures 5B and 5C). Likewise, IL-17A and IL-17F production by CD4+ T cells in response to heat-killed C. albicans stimulation (Figure 5D), IL-17RC expression by circulating leukocyte and neutrophils (Figure 5E), expansion of circulating neutrophils (Figure 5F) and their production of reactive oxygen species (Figure 5G) were each overturned in fluconazole treated mice compared with mice with sustained C. albicans intestinal colonization. Thus, persistent C. albicans intestinal colonization is required for maintaining activated systemic anti-fungal Th17 immunity.
Figure 5. Protection against systemic C. albicans invasive infection requires persistent C. albicans intestinal colonization.
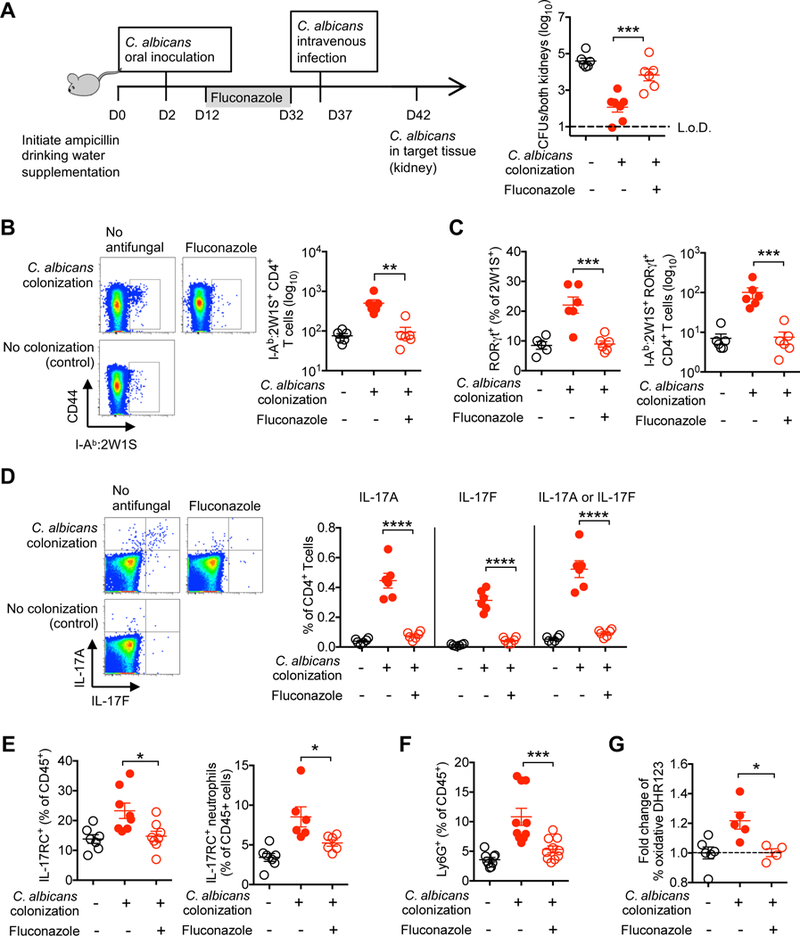
(A) Recoverable C. albicans 5 days after recombinant C. albicans intravenous infection (5 × 104 CFUs) for recombinant C. albicans colonized mice treated with fluconazole, no antifungal treatment, or no C. albicans colonization control mice.
(B) Number of I-Ab:2W1S tetramer positive CD4+ T cells from spleen and peripheral lymph nodes for recombinant C. albicans colonized mice treated with fluconazole for 20 days, no antifungal treatment, or no colonization control mice.
(C) Percent and number RORγt+ among I-Ab:2W1S positive CD4+ T cells for mice described in (B).
(D) Percent IL-17A or IL-17F production by CD4+ splenocyte and lymph nodes cells after heat-killed WT C. albicans stimulation for mice described in (B).
(E) Percent IL-17RC+ amongst CD45+ leukocytes or Ly6G+Ly6Cint neutrophils in the peripheral blood for mice described in (B).
(F) Percent of Ly6G+Ly6Cint neutrophils amongst CD45+ leukocytes in the peripheral blood for mice described in (B).
(G) Relative intensity of dihydrorhodamine (DHR)123 fluorescence amongst Ly6G+Ly6Cint neutrophils in the peripheral blood after C. albicans extract stimulation for mice described in (B).
*p<0.05, ** p<0.01, *** p<0.001, ****p<0.0001, Bar, mean ± SEM. L.o.D., limit of detection. See also Figure S6
Commensal C. albicans conferred protection extends to other extracellular pathogens.
Given neutrophil activation induced by C. albicans colonization, together with the shared importance of these cells in protection against invasive infection by other microbial pathogens (Kolaczkowska and Kubes, 2013), we next investigated the breadth of protection conferred by commensal C. albicans. Since ampicillin drinking water supplementation is required for maintaining C. albicans colonization, microbial pathogens with natural or induced ampicillin resistance were utilized for infection. Interestingly, sharply reduced susceptibility to systemic infection by the unrelated extracellular bacterial pathogen S. aureus was associated with C. albicans intestinal colonization. Mortality after intravenous infection with an inoculum of methicillin-resistant S. aureus that is lethal for ampicillin treated control mice was completely eliminated for mice with commensal C. albicans (Figure 6A). Survival paralleled reduced bacterial burden after infection with a lower S. aureus inoculum (Figure 6A). Reciprocally, S. aureus susceptibility rebounded in fluconazole treated mice in agreement with the aforementioned experiments highlighting a necessity for persistent C. albicans colonization in maintaining activated Th17 systemic immunity (Figure 6A). By contrast to definitive protection against S. aureus, no significant differences in survival, time to death, or progression of clinical symptoms were found after infection with the intracellular viral pathogen, influenza A virus amongst C. albicans colonized compared with control mice (Figure 6B). Thus, commensal C. albicans conferred protection is not restricted only to C. albicans invasive infection, but extends to other extracellular pathogens, such as S. aureus where neutrophils play a dominant role in protection (Rigby and DeLeo, 2012).
Figure 6. Commensal C. albicans protects against S. aureus infection and promotes susceptibility to Th17 airway inflammation.
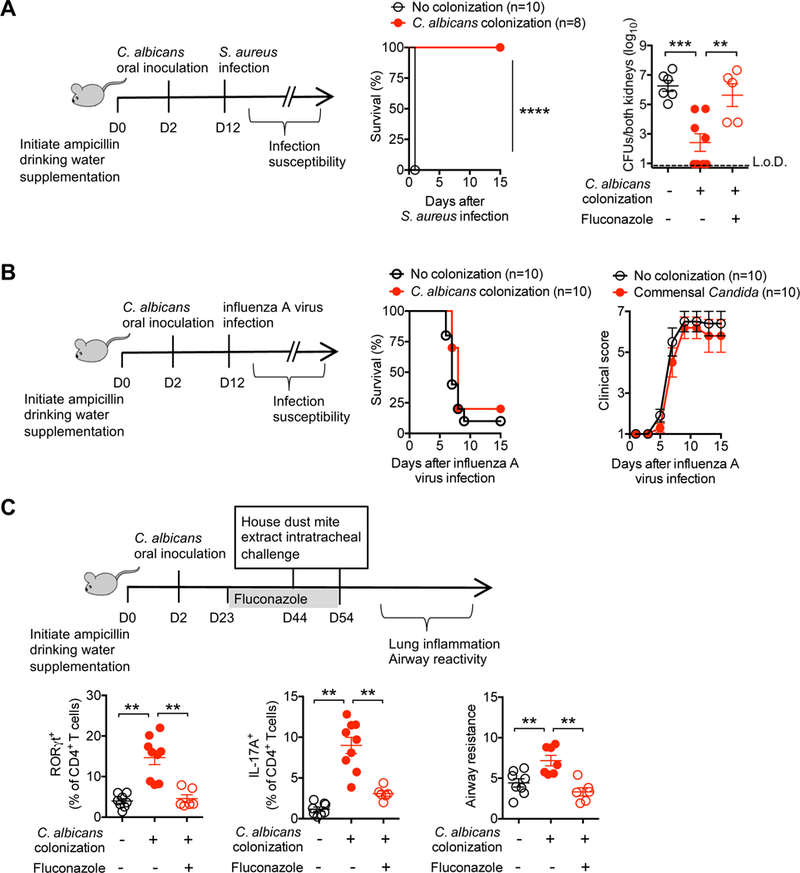
(A) Percent survival after S. aureus intravenous infection (strain USA300; 108 [left panel]), and recoverable S. aureus 5 days after infection with a reduced dosage (strain USA300; 3 × 107 CFUs [right panel]) for mice with recombinant C. albicans intestinal colonization, C. albicans colonized mice treated with fluconazole, or no colonization control mice.
(B) Percent survival and clinical score progression after influenza A virus intranasal infection (strain PR8, 2 × 105 PFU) for mice with recombinant C. albicans intestinal colonization or no colonization control mice.
(C) Airway resistance, percent RORγt+ or IL-17A production by CD4+ T cells recovered from the lungs of recombinant C. albicans colonized mice treated with fluconazole, no antifungal treatment, or no C. albicans colonization control mice. Mice were intratracheally sensitized and challenged with house dust mite extract, and airway resistance represent change over baseline after inhaled methacholine challenge.
** p<0.01, *** p<0.001, ****p<0.0001, Bar, mean ± 1 SEM. L.o.D., limit of detection.
Commensal C. albicans activation of systemic Th17 inflammation promotes susceptibility to airway inflammation.
Given these remarkable protective benefits against invasive infection, complementary studies addressed the potential for harmful consequences associated with systemic accumulation of activated neutrophils and Th17 immunity primed by C. albicans colonization. Allergic airway inflammation is increasingly recognized to be mediated by activated neutrophils and aberrant IL-17 production (Alcorn et al., 2010). Since intestinal fungi have been shown to exacerbate asthma-like symptoms in antibiotic treated mice (Li et al., 2018; Noverr et al., 2005; Skalski et al., 2018; Wheeler et al., 2016), the potential for commensal C. albicans induced shifts in airway inflammation were evaluated. We found the lungs of mice with C. albicans intestinal colonization compared with no colonization control mice contained significantly expanded levels of Th17 (RORγt+ and IL-17 producing) CD4+ T cells that parallel increased airway hyperresponsiveness after house dust mite intratracheal challenge (Figure 6C). Reciprocally, RORγt+ and IL-17 producing CD4+ T cell accumulation and airway responsiveness were each efficiently reversed with commensal C. albicans eradication by fluconazole (Figure 6C). Together, these results show commensal C. albicans tonically stimulates systemic Th17 inflammation with broad immunological host impacts, on one hand protecting against systemic invasive infection by extracellular pathogens, but also promoting susceptibility to aberrant tissue inflammation.
C. albicans fecal colonization density positively correlates with systemic fungal-specific Th17 inflammation.
To investigate whether Th17 inflammation driven by CD4+ T cell recognition of commensal C. albicans occurs similarly in humans, the relationship between fecal C. albicans colonization density, and levels of IL-17 producing CD4+ T cells with fungal specificity and IL-17 responsiveness by circulating neutrophils was evaluated in intensive care unit patients naturally predisposed to have fungal colonization from antibiotic exposure. We reasoned comparing activation of immune cells with C. albicans fecal colonization density at a single time point for each individual would reveal a snapshot for how tonic C. albicans colonization impacts systemic immunity. Fecal abundance of C. albicans was evaluated by shotgun sequencing, followed by Kraken alignment of sequence reads against a custom comprehensive microbial genome database (Wood and Salzberg, 2014). C. albicans-specific Th17 cells were enumerated by as the percentage of IL-17A and/or IL-17F producing CD4+ peripheral blood cells after heat-killed C. albicans in vitro stimulation. Both stimulation and no stimulation control specimens were supplemented with anti-CD28 antibody to improve re-stimulation efficiency and cytokine production (Koehler et al., 2018). These experiments showed direct correlations between IL-17 production by circulating CD4+ T cells (p=0.001) and IL-17RC expression by CD15+CD16+ neutrophils (p<0.001) each compared with the relative or absolute abundance of C. albicans fecal colonization (Figures 7A, 7B, and S7). These differences in CD4+ T cell IL-17 production and shifts in neutrophil IL-17 responsiveness are restricted to C. albicans since positive associations were eliminated when compared with the fecal density of non-albicans Candida spp. (Figures 7A, 7B, and S7). Likewise, no correlations were identified between neutrophil IL-17RC expression and the density of fecal colonization by other common pathobionts including Staphylococcus aureus, Escherichia coli or Enterococcus faecalis (Figure 7C). Thus, selective activation of the Th17-neutrophil axis by commensal C. albicans is recapitulated in humans.
Figure 7. C. albicans fecal colonization density positively correlates with systemic levels of fungal-specific Th17 inflammation.
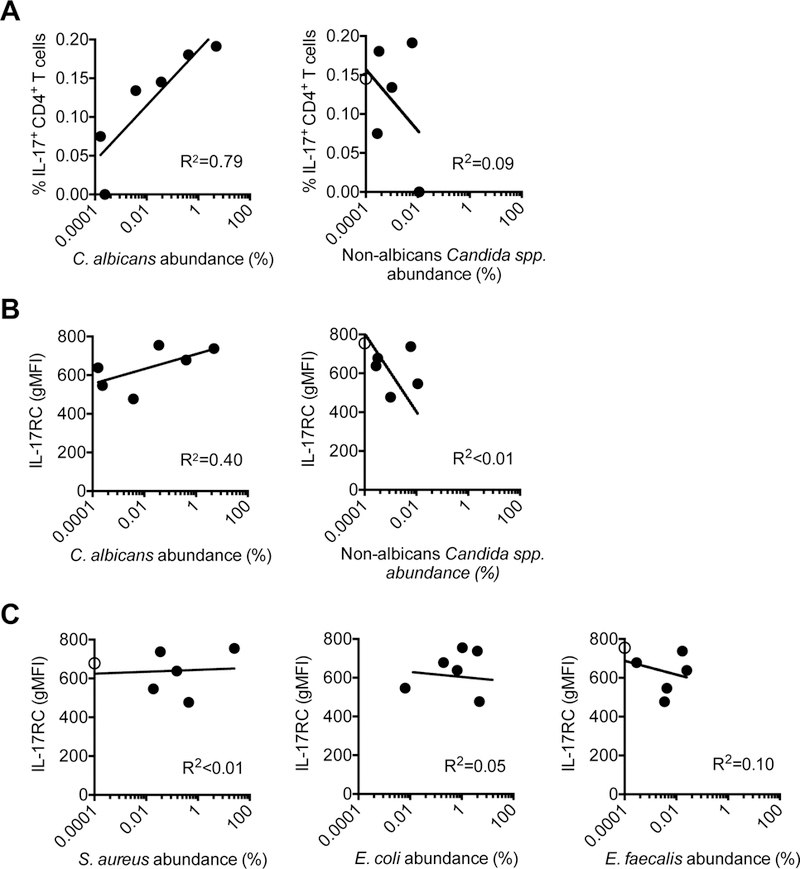
(A) Regression analysis comparing IL-17A or IL-17F production by CD4+ peripheral blood cells of after heat-killed C. albicans stimulation compared with the fecal relative abundance of C. albicans or non-albicans Candida spp. determined by shotgun sequencing for each individual.
(B) Regression analysis comparing intensity of IL-17RC staining by CD15+CD16+ neutrophils in peripheral blood cells compared with the fecal relative abundance of C. albicans or non-albicans Candida spp. for each individual.
(C) Regression analysis comparing intensity of IL-17RC staining by CD15+CD16+ neutrophils in peripheral blood cells compared with the fecal relative abundance of S. aureus, E. coli, or E. faecalis for each individual.
Levels below the limits of detection are shown as open circles on the y-axis. See also Figure S7
DISCUSSION
The intestine and other mucosal tissues harbor many virulent pathobionts capable of invasive disseminated infection (Brown et al., 2012; Casadevall and Pirofski, 2000). Despite their exceptionally high colonization prevalence, systemic infection by pathobiont microbes occurs relatively infrequently. One explanation may be that mucosal-dwelling pathobionts have limited tropism for extra-intestinal tissues, and thus rarely cause systemic infection. However, this is likely an over-simplification, since invasion and dissemination to extra-intestinal tissues are nonetheless consistent features of human clinical infection. Thus, we sought an immunological explanation by investigating systemic immunological changes primed by commensal pathobiont colonization first in healthy immune competent hosts, and later with depletion defined immune components to probe their necessity against invasive infection. We show despite high density C. albicans intestinal colonization, spontaneous disseminated infection occurs very infrequently in mice, similar to immune competent humans (Pfaller and Diekema, 2007). Interestingly, resistance to systemic infection is not passive, but actively acquired and maintained by Th17 CD4+ T cells responsive to commensal-pathobiont microbes. In turn, IL-17 stimulates accumulation, activation and enhanced IL-17 responsiveness in circulating neutrophils. This necessity for IL-17, CD4+ T cells and circulating neutrophils reveal mechanistic details for why immune suppression and, in particular, neutropenia are dominant risk factors for candidemia (Patolia et al., 2013), and extend the functional implications of Th17 dominated differentiation of C. albicans-specific memory CD4+ T cells in healthy human volunteers (Acosta-Rodriguez et al., 2007) by establishing their importance in host defense against invasive infection.
Commensal C. albicans primed expansion of protective IL-17 producing CD4+ T cells with fungal specificity also provides important clues for explaining discordance in the necessity of IL-17 in protection against C. albicans invasive infection. In particular, while IL-17A and IL-17 receptor have each been reported to be indispensable for resistance against intravenous C. albicans infection using IL-17A-or IL-17 receptor-deficient mice (Huang et al., 2004; Saijo et al., 2010; van de Veerdonk et al., 2010), others have shown completely non-essential roles for these molecules (De Luca et al., 2010). Our results suggest this discordance is explained by whether mice in each facility have commensal C. albicans colonization, since IL-17 neutralization and CD4+ T cell depletion each cause susceptibility to intravenous C. albicans infection only amongst mice with C. albicans intestinal colonization (Figure 3D). Thus, the presence of commensal fungi dictates the relative importance of these immune components in host defense against invasive infection.
Many other phenotypes attributed to fungal immunity and the immune modulatory properties of commensal fungi are likely to be influenced by the unique host mycobiome. For example, while antibiotic induced intestinal overgrowth of the commensal fungus, Wallemia mellicola enhances the severity of allergic airway disease in mice (Skalski et al., 2018), this fungal species is not present for animals in our facility (Jiang et al., 2017). Instead, we and others find similarly increased susceptibility of mice to allergic airway inflammation with C. albicans intestinal colonization (Noverr et al., 2005) (Figures 6C). Functional overlap between unique species of commensal fungi is further supported by recent independent studies showing C. tropicalis and other endogenous fungi protect against colon cancer through CARD9 signaling in intestinal myeloid cells (Malik et al., 2018; Wang et al., 2018). Together, these results suggest positive calibration of Th17 inflammation we demonstrate may not be unique to C. albicans, but shared by other fungal species that activate unique intestinal immune cell subsets, such CX3CR1+ mononuclear phagocytes, in a similar fashion (Li et al., 2018). In this regard, functional overlap for fungi would represent a sharp contrast to commensal bacteria where a majority of intestinal Th17 cells respond to the single bacterial species, segmented filamentous bacteria (Yang et al., 2014). On the other hand, the remarkable specificity whereby IL-17 responsiveness in human circulating neutrophil cells is associated with C. albicans fecal colonization density, but not non-albicans Candida spp. or other bacterial pathobionts (Figure 7), highlight potentially unique immune modulatory properties of C. albicans, distinct from other commensal fungal species. This notion is in agreement with recent human T cell analysis implicating C. albicans as the major inducer of systemic Th17 differentiation that propagates expansion of IL-17 producing CD4+ T cells cross-reactive to other fungal species (Bacher et al., in press). Thus, unique microbial features of C. albicans dominantly promote Th17 immunity shifts in humans. Whether C. albicans induced immune modulation is restricted only to humans, or present in other mammalian species, are important areas for future investigation.
Antibiotic induced bacterial dysbiosis is a pervasive risk factor for human fungal colonization (Schulte et al., 2015; Spinillo et al., 1999), and susceptibility to invasive fungal infections (Berdal et al., 2014; Yu et al., 2013). These clinical aspects of fungal colonization and invasive infection susceptibility were investigated by supplementing the drinking water of mice with ampicillin. Despite the relatively narrow spectrum of this single β-lactam antibiotic, dramatic shifts in the composition of fecal commensal bacteria were identified (Fan et al., 2015; Ubeda et al., 2010) (Figures 1E-1G and S1), that grossly recapitulate loss of diversity and bacterial composition shifts by antibiotics in humans (Zaura et al., 2015). Thus, while the impacts of ampicillin cannot be definitively excluded, bacterial dysbiosis was experimentally controlled given the similar composition, abundance and diversity of intestinal bacteria in C. albicans colonized compared with ampicillin treated control mice (Figures 1E-1G and S1).
Protection against C. albicans invasive infection has been used to show innate immune cells without antigen-specificity (e.g. monocyte, macrophage) can be trained to remember prior infectious encounters. Classical experiments show recent prior intravenous infection with a sublethal C. albicans inoculum improves survival of both wildtype and Rag1-deficient mice after C. albicans intravenous challenge (Quintin et al., 2012). More recently, Rag1-deficient mice were used to show non-essential roles for adaptive immune components in protection against C. albicans invasive infection conferred by colonization with attenuated mutant strains adapted for intestinal commensalism (Tso et al., 2018). By contrast, we show a necessity for adaptive immune cells, and in particular CD4+ T cells, in systemic host defense primed by virulent C. albicans intestinal colonization, since susceptibility to invasive infection rebounds to levels found in non-colonized control mice with CD4+ T cell depletion or in Rag1-deficient mice (Figures 3D-3F). The reason for this discordance remains uncertain, but likely reflect intrinsic differences in immunogenicity of virulent pathobionts compared with attenuated commensal microbes, the spectrum of antibiotics used to facilitate C. albicans intestinal colonization or intrinsic differences between commensal microbiota for mice housed in each respective facility.
Systemic expansion of IL-17 producing CD4+ T cells with commensal C. albicans specificity (Figures 3C and S5), together with enhanced IL-17 responsiveness by circulating neutrophils which require exposure to IL-17 and CD4+ T cells in mice with C. albicans intestinal colonization (Figure 4C), suggests that even innately trained immune cells can be further educated by adaptive immune components with commensal specificity. Durability is one important distinction between non-antigen-specific immune cells trained by transient exposure to defined microbial compounds or acute infection conditions which have relatively short functional longevity (Quintin et al., 2012; Tso et al., 2018), compared with the more sustained activation of neutrophils in mice with C. albicans intestinal colonization that we show relies on CD4+ T cell recognition of commensal C. albicans. However, durability in this context is also not absolute, since tonic stimulation by commensal C. albicans is needed for sustained activation and expansion of protective neutrophils and Th17 CD4+ T cells (Figure 5). Further supporting the need for tonic commensal stimulation is the positive correlation in humans between C. albicans fecal density and levels of systemic C. albicans-specific Th17 CD4+ T cells plus IL-17 responsiveness by circulating neutrophils (Figures 7A, 7B and S7). Thus, despite inherent differences in the composition of commensal microbes across species, this complementary analysis of immunity in humans and mice highlights conserved shifts in systemic immunity primed by C. albicans. Existence of this dynamic interplay between commensal C. albicans and systemic immune cells, that not only sense the presence of C. albicans colonization, but also transient shifts in colonization levels, open up exciting new opportunities to target fungi with mycotic probiotics and/or antifungal agents for therapeutically fine tuning systemic immunity.
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information, and requests for resources or reagents should be directed to and will be fulfilled the Lead Contact, Dr. Sing Sing Way (singsing.way@cchmc.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6 and Rag1-deficient mice were purchased from Charles River laboratories and Jackson laboratories respectively, housed under specific pathogen-free conditions, and used between 6–8 weeks of age. All experiments were performed using sex- and age-matched controls under Cincinnati Children’s Hospital Research Foundation IACUC approved protocols.
Microbes
Wildtype C. albicans (strain SC5314) and the isogenic recombinant virulent strain expressing GFP plus 2W1S55–68 peptide was provided by Dr. Daniel Kaplan (University of Pittsburgh) (Igyártó et al., 2011). Methicillin resistant S. aureus (strain USA300) was provided by Dr. Matthew Flick (Cincinnati Children’s Hospital). Mouse adapted H1N1 influenza A virus (strain PR8) was provided by Monica Malone McNeal (Cincinnati Children’s Hospital). Each C. albicans strain (wildtype or recombinant) was cultured in YPAD media at 30ºC with shaking (200 rpm). S. aureus was cultured in BHI media at 37ºC with shaking (200 rpm). For infection, C. albicans and S. aureus were each back-diluted to early log phase growth (OD600 ~0.1), then washed and diluted in sterile saline. PR8 virus was grown and tittered in Madin-Darby Canine kidney epithelial cells, stored as individual aliquots −70 ºC, and thawed immediately prior to infection.
Human subjects
Informed consent to use discard anticoagulated blood collected for routine clinical care was obtained under Cincinnati Children’s Hospital Institutional Review Board (IRB) approved protocols. Inclusion criteria were patients scheduled for routine early morning blood collection so that analysis could be performed on excess sample after clinical processing, and those willing and able to provide a stool specimen. Exclusion criteria were patients with lymphopenia or neutropenia (absolute lymphocyte and neutrophil count each > 500 × 103/µl), recent (past 30 days) exposure to antifungal compounds, or being critically ill that precluded being able to obtain oral and written consent. The final analysis included patients fitting these criteria (age range, 15 months to 5 years) and of both genders.
METHOD DETAILS
Antibiotic and antimycotic agents
To establish C. albicans intestinal colonization, the drinking water of mice was supplemented with ampicillin (1 mg/ml) two days prior to oral C. albicans inoculation. Thereafter mice were maintained on ampicillin supplemented drinking water throughout the experiment. To eradicate commensal fungi, ampicillin treated drinking water was further supplemented with fluconazole (0.5 mg/ml).
Microbial propagation and infection
For oral inoculation, 30 µl of the overnight culture C. albicans was administered dropwise into the mouths of mice. For intravenous infection, each C. albicans strain (wildtype or recombinant) (5 × 104 CFUs in 200µl) or S. aureus (108 CFUs or 3 × 107 CFUs each in 200µl) was injected into mice via the lateral tail vein. For influenza A virus infection, frozen aliquots of PR8 virus were individually thawed, diluted in saline to 2 × 105 PFU/30 µl, and intranasally administered to anesthetized (ketamine/xylazine) mice (Jiang et al., 2017). Mice were checked daily and assigned the following clinical disease score (1 healthy; 2 limited ruffled fur; 3 ruffled fur throughout; 4 mild lethargy; 5 limited movement; 6 moribund or uncontrolled spastic movements; 7 deceased) as described (Turner et al., 2017). For enumerating the number of recoverable C. albicans and S. aureus colony forming units, individual fetal pellets or each tissue from mice was sterilely dissected, weighed, and homogenized in sterile saline. Serial dilutions on the organ homogenate were spread onto BHI media plates (S. aureus) or BHI media plates supplemented with antibiotics (C. albicans) (ampicillin [2.5 µg/mL), gentamicin (2.5 µg/mL), metronidazole (2.5 µg/mL), neomycin (2.5 µg/mL), and vancomycin (1.25 µg/mL), and the number of individual colonies enumerated after incubation at 37ºC for 24 hours.
Cell staining, stimulation and flow cytometry
Fluorophore- or biotin-conjugated antibodies used mouse cell analysis are as follows: anti-CD4 (clone GK1.5), anti-CD8a (clone 53–6.7), anti-CD11b (clone M1/70), anti-CD11c (clone N418), anti-F4/80 (clone BM8), anti-B220 (clone RA3–6B2), anti-CD44 (clone IM7), anti-CD45 (clone 30F11), anti-Ly6G (clone 1A8), anti-Ly6C (clone HK1.4), anti-Gr-1 (clone RB6–8C5), anti-IL-17RC (polyclonal IgG), anti-FOXP3 (clone FJK-16S), anti-RORγt (clone Q31–378), anti-T-bet (clone 4B10), anti-IFN-γ (clone XMG1.2), anti-IL17A (clone TC 11–18H10.1), anti-IL17F (clone 9D3.1C8). Fluorophore-conjugated antibodies were used for human cell analysis are as follows: anti-CD4 (clone OKT4), anti-CD15 (clone H198), anti-CD16 (clone 3G8), anti-CD45(clone HI30), anti-IL-17A (clone eBio64DEC17), anti-IL17F (clone O33–782), anti-IL17RC (clone 309822) with staining in the presence of Human Fc block (BD Bioscience). For detecting cytokine production by individual cells, 106 cells from the spleen and peripheral lymph nodes were stimulated with heat-killed (65°C for 90 minutes) WT C. albicans (106 CFU equivalents) in 200 µl complete DMEM medium (supplemented with 10% fetal bovine serum, 1% L-glutamine, 10 mM HEPES, 1% penicillin-streptomycin) at 37°C and 5% CO2 for 24 hours, and with GolgiPlug supplementation to the media for the last 6 hours, and intracellular staining was performed after cell permeabilization (BD PharMingen) according to the manufacturers’ instructions. Single cell suspensions from spleen and peripheral lymph nodes were stained with APC or PE conjugated I-Ab:2W1S55–68 tetramer at room temperature for 60 minutes, and enriched using anti-APC or anti-PE antibody conjugated magnetic beads (Militenyi Biotec) as described (Moon et al., 2007). Samples were acquired using an FACSCanto (BD) cytometer and analyzed using FlowJo software (Tree Star).
Isolation of kidney leukocytes
Kidneys were minced and digested with collagenase D (1 mg/mL) and DNAse (0.1 mg/mL) in complete DMEM medium at 37℃ 200 rpm for 30 minutes. The digest was then passed through a 70 µm filter to obtain single cell suspensions. Leukocytes were isolated by centrifugation at 2000 rpm for 20 minutes on a 40% and 80% Percoll gradient. Residual red blood cells were lysed by hypertonic solution (10 mM HKCO3, 16 mM NH4Cl, pH 7.3) before further manipulation. Single cell suspensions were stained with APC conjugated I-Ab:2W1S55–68 tetramer at room temperature for 60 minutes before surface and intranuclear staining.
In vivo cell depletion or cytokine neutralization
The following antibodies were administered to mice for in vivo cell depletion or cytokine neutralization: anti-CD4 (clone GK1.5); anti-IL-17A (clone 17F3), anti-IL17F (clone MM17F8F5), anti-Gr-1 (clone RB6–8C5), anti-Ly6G (clone 1A8), or each respective isotype antibody control (mouse IgG1, rat IgG2b, and rat IgG2a) by intraperitoneal injection (0.6 µg each antibody per mouse) beginning 1 day prior to C. albicans intravenous infection or 7 days prior to blood collection. Thereafter, 0.3 µg of the same antibody was administered every two days until the end of the experiment.
Imaging
BHI agar plates containing fungal colonies were imaged by the In Vivo Imaging System (Perkin Elmer) using the GFP and Cy5.5 filter sets. Fluorescence intensity was quantified by region-of-interest calibration using the Cy5.5-filter set image as background and showing the ratio properties as pseudo-color images (rainbow pseudo-color shows ratio high to low as red to blue), and analyzed using Nikon Elements software.
Reactive oxygen production
Peripheral blood was collected via retro-orbital bleeding from mice. To detect production of reactive oxygen species, RBC lysed peripheral blood cells were seeded into 96-well round-bottom plates (2 × 105/well) in the presence of 0.45 µM dihydrorhodamine 123 for 15 minutes, then stimulated with 100 µg/mL C. albicans extract (Greer Laboratories) for 60 minutes.
Airway reactivity and inflammation
Mice were anesthetized with ketamine/xylazine and hung by their front incisors on an angled stand. 100 µg house dust mite extract (Greer Laboratories) in 40 µL sterile saline was administered intratracheally. 10 days later, mice were challenged with the same amount of house dust mite extract. 48 hours after the second intratracheal challenge, airway resistance in response to inhaled methacholine (100 mg/mL) was measured using FlexiVent (SCIREQ). Saline perfused lungs were minced and digested with collagenase D (1 mg/mL) and DNAse (0.1mg/mL) in complete DMEM media for 30 minutes at 37ºC with gentle shaking (200 rpm). The tissue digest was then passed through a 70 µm filter to obtain single cell suspensions. For intracellular cytokine staining, single cell preparations from the lung were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin (eBioscience Cell Stimulation Cocktail) for 4.5 hours at 37°C and 5% CO2 in the presence of BD GolgiPlug.
Human peripheral blood cells
Anticoagulated peripheral blood was RBC lysed, and cells seeded into 96-well round-bottom plates (2 × 105/well). Thereafter, triplicate wells were stimulated with heat-killed C. albicans (106 CFU equivalents) and anti-human CD28 (5 µg/mL, BD Bioscience) or anti-human CD28 for 24 hours at 37ºC, and with supplementation of the media with GolgiPlug (BD Biosciences) for the last 6 hours.
Fecal DNA extraction and shotgun metagenome sequencing
DNA extraction was performed by mixing 0.1 gram stool with 0.35 mL Epicentre Masterpure Yeast DNA Purification lysis buffer to which was added 3500U of Epicenter Ready Lyse solution. Two 5-mm stainless steel beads were added and the samples were vortexed for 1 hour at room temperature. The samples were frozen at −80ºC briefly then thawed and the supernatant was transferred to a new tube, to which 0.15 mL MPC Protein Precipitation Reagent was as added. After centrifugation (13,000 x g) for 10 minutes the supernatant was added to new tube to which 0.2 mL 100% ethanol was added. The samples were incubated at - 20ºC for 30 min. DNA was then purified on Invitrogen PureLink DNA columns following the manufacturer’s instructions. DNA concentration was determined using Qubit analyzer, and diluted to 200 ng/mL. Salinibacter ruber genomic DNA (which is not a component of the human microbiota) was then added to each sample at a fixed concentration (1.4 pg/mL) as a reference standard. Nextera XT adapters following manufacturer’s instructions, and sequencing was performed on an Illumina NextSeq500 machine using 150-bp DNA paired end reads to a depth of approximately 2 million base pairs per sample. Raw sequence data was demultiplexed and converted to fasta format and subjected to downstream analysis.
Taxonomic assignment of DNA reads
Paired-end sequencing reads from each sample were aligned with Kraken (Wood and Salzberg, 2014) against a custom genome database consisting of the human genome and approximately 40,054 bacterial, fungal, viral and parasitic genomes. The database was derived initially from all bacteria, fungi, and viruses in the RefSeq genome database (ftp://ftp.ncbi.nlm.nih.gov/genomes/refseq/, accessed 11/27/2017) as well as the human genome database (GR38Ch;ftp://ftp.ncbi.nlm.nih.gov/genomes/refseq/vertebrate_mammalian/Homo_sapiens/latest_assembly_versions/). Manual curation was used to add additional Bacteroides, Parabacteroides, and Clostridia genomes including draft genomes from NCBI Assemblies (https://www.ncbi.nlm.nih.gov/assembly) and PATRIC (https://www.patricbrc.org/view/Taxonomy/2#view_tab=genomes). Additional fungal and viral genome sequences were recovered from the above two resources and dedicated viral (https://www.viprbrc.org/brc/home.spg?decorator=vipr, http://www.virusite.org/) and fungal (http://fungidb.org/fungidb/) databases. Reads were assigned initially using Kraken and then using Bracken, which uses a Baysean approach to rebalance reads that might have had several possible assignments. Taxonomic count data was normalized by rarefaction. Species that contributed less than 0.01% of overall mapped reads or were present in less than 10% of the samples were removed. To calculate genome equivalents of total bacterial species in each fecal sample, total read counts were first normalized across samples. Read counts per sample were then multiplied by 150 (the length of sequence read) then divided by the genomic size of each bacterial species, which yielded normalized genome equivalents per sample. Genomic equivalents were then then normalized to the known quantity of added Salinibacter ruber genome equivalents. Finally, genome abundance of all bacterial species were summed to yield the total bacterial abundance (genome equivalents) per sample.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical tests were performed using Prism (Graphpad) software. The unpaired two-tailed Student’s t test was used to compared differences between two groups. One-way ANOVA with Tukey’s test for multiple comparisons was used to evaluate experiments containing more than two groups. Repeated measures two-way ANOVA was used to evaluate time course experiments. To identify species that significantly differed between patient groups, Wilcoxon pairwise rank sum test was used to identify taxa that were significantly different between patient groups and the resulting p-values were subjected to Bonferroni correction. Shrinkage Linear Discriminant Analysis (SLDA; a form of Linear Discriminant Analysis) was utilized to calculate effect size. Corrected p-values with FDR < 0.1 and SLDA effect size > 0.2 are considered significantly different between groups. As the number of species differing among groups was very large, the much more stringent cutoffs of effect size ≥10, and FDR<0.05 (Wilcoxon rank sum test with Bonferroni correction) were applied to the data for the purposes of graphical representation. Survival curves were analyzed by Log-rank (Mantel-Cox) test. Non-linear regression was used to evaluate the association between human CD4+ T cell IL-17 production or neutrophil IL-17RC expression each compared with fecal colonization density.
Supplementary Material
HIGHLIGHTS.
C. albicans intestinal colonization protects against C. albicans invasive infection
Systemic fungal specific Th17 CD4+ T cell accumulation with intestinal colonization
Tonic neutrophil stimulation augments host defense against extracellular pathogens
Antimicrobial immunity balanced by susceptibility to allergic airway inflammation
ACKNOWLEDGEMENTS
We are indebted to Dr. Paul E. Steele for help obtaining human blood samples, and Dr. Matthew Kofron for imaging assistance. We thank members of Drs. Alenghat, Deshmukh, Haslam, Qualls and Way laboratories for helpful suggestions. This work was supported by NIH through grants F30-DK107199 and T32-GM63483 (T.T.J.), R21-AI123089, R21-AI128932, DP1-AI131080 (S.S.W.) and P30-DK078392 (Pathology Core of the Cincinnati Digestive Diseases Research Center). S.S.W. is supported by the HHMI Faculty Scholar’s program and Burroughs Wellcome Fund Investigator in the Pathogenesis Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, and Napolitani G (2007). Surface phenotype and antigenic specificity of human interleukin 17–producing T helper memory cells. Nature immunology 8, 639–646. [DOI] [PubMed] [Google Scholar]
- Acton D, Plat-Sinnige MT, van Wamel W, de Groot N, and van Belkum A (2009). Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? European journal of clinical microbiology & infectious diseases 28, 115. [DOI] [PubMed] [Google Scholar]
- Alcorn JF, Crowe CR, and Kolls JK (2010). TH17 cells in asthma and COPD. Annual review of physiology 72, 495–516. [DOI] [PubMed] [Google Scholar]
- Baaten BJG, Tinoco R, Chen AT, and Bradley LM (2012). Regulation of antigen-experienced T cells: lessons from the quintessential memory marker CD44. Frontiers in immunology 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher P, Hohnstein T, Beerbaum E, Rocker M, Blango MG, Kaufmann S, Rohmel J, Eschenhagen P, Seidel K, Rickerts V, et al. (2019). Instruction of human anti-fungal Th17 immunity and immune pathology by cross-reactivity against a single member of the microbiota. Cell (in press). [DOI] [PubMed]
- Berdal J-E, Haagensen R, Ranheim T, and Bjørnholt JV (2014). Nosocomial candidemia; risk factors and prognosis revisited; 11 years experience from a Norwegian secondary hospital. PloS one 9, e103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, and White TC (2012). Hidden killers: human fungal infections. Science translational medicine 4, 165rv113. [DOI] [PubMed] [Google Scholar]
- Casadevall A, and Pirofski L.-a. (2000). Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infection and immunity 68, 6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Zelante T, D’angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti R, Bonifazi P, Renauld J-C, Bistoni F, et al. (2010). IL-22 defines a novel immune pathway of antifungal resistance. Mucosal immunology 3, 361–373. [DOI] [PubMed] [Google Scholar]
- Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, and Koh AY (2015). Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nature medicine 21, 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, and Schwarzenberger P (2004). Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. The Journal of infectious diseases 190, 624–631. [DOI] [PubMed] [Google Scholar]
- Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al. (2011). Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. (2012). Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science, 1221789. [DOI] [PMC free article] [PubMed]
- Iwakura Y, Nakae S, Saijo S, and Ishigame H (2008). The roles of IL‐17A in inflammatory immune responses and host defense against pathogens. Immunological reviews 226, 57–79. [DOI] [PubMed] [Google Scholar]
- Jiang TT, Chaturvedi V, Ertelt JM, Xin L, Clark DR, Kinder JM, and Way SS (2015). Commensal enteric bacteria lipopolysaccharide impairs host defense against disseminated Candida albicans fungal infection. Mucosal immunology 8, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TT, Shao T-Y, Ang WG, Kinder JM, Turner LH, Pham G, Whitt J, Alenghat T, and Way SS (2017). Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell host & microbe 22, 809–816. e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Harris CA, Small CB, Moll B, Lesser M, and Friedland GH (1984). Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. New England Journal of Medicine 311, 354–358. [DOI] [PubMed] [Google Scholar]
- Koehler FC, Cornely OA, Wisplinghoff H, Schauss AC, Salmanton-Garcia J, Ostermann H, Ziegler M, Bacher P, Scheffold A, Alex R, et al. (2018). Candida-reactive T cells for the diagnosis of invasive Candida infection–a prospective pilot study. Frontiers in microbiology 9, 1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, and Pier GB (2008). Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS pathogens 4, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, and Kubes P (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology 13, 159–175. [DOI] [PubMed] [Google Scholar]
- Laupland KB (2013). Incidence of bloodstream infection: a review of population-based studies. Clinical microbiology and infection 19, 492–500. [DOI] [PubMed] [Google Scholar]
- Li X, Leonardi I, Semon A, Doron I, Gao IH, Putzel GG, Kim Y, Kabata H, Artis D, Fiers WD, et al. (2018). Response to Fungal Dysbiosis by Gut-Resident CX3CR1+ Mononuclear Phagocytes Aggravates Allergic Airway Disease. Cell host & microbe 24, 847–856. e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, and Knight R (2012). Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A, Sharma D, Malireddi RS, Guy CS, Chang T-C, Olsen SR, Neale G, Vogel P, and Kanneganti T-D (2018). SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity 49, 515–530. e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, and Jenkins MK (2007). Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, et al. (2017). The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, and Huffnagle GB (2005). Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infection and immunity 73, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patolia S, Kennedy E, Zahir M, Patolia S, Gulati N, Narendra D, Vadde R, Pokharel S, Schmidt FM, Enriquez D, et al. (2013). Risk factors for candida blood stream infection in medical ICU and role of colonization-A retrospective study. British Journal of Medical Practitioners 6, a618. [Google Scholar]
- Pfaller M, and Diekema D (2007). Epidemiology of invasive candidiasis: a persistent public health problem. Clinical microbiology reviews 20, 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J, Saeed S, Martens JH, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg B-J, Wijmenga C, et al. (2012). Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell host & microbe 12, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan D, Pfaller M, Hollis R, and Wenzel R (1990). Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. Journal of clinical microbiology 28, 2733–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby KM, and DeLeo FR (2012). Neutrophils in innate host defense against Staphylococcus aureus infections. Seminars in immunopathology 34, 237–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung S.-h., et al. (2010). Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691. [DOI] [PubMed] [Google Scholar]
- Schulte DM, Sethi A, Gangnon R, Duster M, Maki DG, and Safdar N (2015). Risk factors for Candida colonization and Co-colonization with multi-drug resistant organisms at admission. Antimicrobial resistance and infection control 4, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalski JH, Limon JJ, Sharma P, Gargus MD, Nguyen C, Tang J, Coelho AL, Hogaboam CM, Crother TR, and Underhill DM (2018). Expansion of commensal fungus Wallemia mellicola in the gastrointestinal mycobiota enhances the severity of allergic airway disease in mice. PLoS pathogens 14, e1007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinillo A, Capuzzo E, Acciano S, De Santolo A, and Zara F (1999). Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. American journal of obstetrics and gynecology 180, 14–17. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Roy S, Leal SM Jr, Sun Y, Howell SJ, Cobb BA, Li X, and Pearlman E (2014). Activation of neutrophils by autocrine IL-17A–IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nature immunology 15, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, Lai GC, Srinivasan K, Yurieva M, Liao W, et al. (2018). Experimental evolution of a fungal pathogen into a gut symbiont. Science 362, 589–595. [DOI] [PubMed] [Google Scholar]
- Turner LH, Kinder JM, Wilburn A, D’Mello RJ, Braunlin MR, Jiang TT, Pham G, and Way SS (2017). Preconceptual Zika virus asymptomatic infection protects against secondary prenatal infection. PLoS pathogens 13, e1006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, et al. (2010). Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. The Journal of clinical investigation 120, 4332–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Kullberg BJ, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, and Netea MG (2010). Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock 34, 407–411. [DOI] [PubMed] [Google Scholar]
- Von Eiff C, Becker K, Machka K, Stammer H, and Peters G (2001). Nasal carriage as a source of Staphylococcus aureus bacteremia. New England Journal of Medicine 344, 11–16. [DOI] [PubMed] [Google Scholar]
- Voss A, Hollis RJ, Pfaller MA, Wenzel RP, and Doebbeling BN (1994). Investigation of the sequence of colonization and candidemia in nonneutropenic patients. Journal of clinical microbiology 32, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Fan C, Yao A, Xu X, Zheng G, You Y, Jiang C, Zhao X, Hou Y, Hung M-C, et al. (2018). The Adaptor Protein CARD9 Protects against Colon Cancer by Restricting Mycobiota-Mediated Expansion of Myeloid-Derived Suppressor Cells. Immunity 49, 504–514. e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, et al. (2016). Immunological consequences of intestinal fungal dysbiosis. Cell host & microbe 19, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R (1963). Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriological reviews 27, 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, and Brown JS (2015). Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal immunology 8, 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, and Salzberg SL (2014). Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome biology 15, R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, et al. (2014). Focused specificity of intestinal T H 17 cells towards commensal bacterial antigens. Nature 510, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Du L, Yuan T, Zheng J, Chen A, Chen L, and Shi L (2013). Risk factors and clinical analysis for invasive fungal infection in neonatal intensive care unit patients. American journal of perinatology 30, 589–594. [DOI] [PubMed] [Google Scholar]
- Zaura E, Brandt BW, de Mattos MJT, Buijs MJ, Caspers MP, Rashid M-U, Weintraub A, Nord CE, Savell A, Hu Y, et al. (2015). Same exposure but two radically different responses to antibiotics: resilience of the salivary microbiome versus long-term microbial shifts in feces. MBio 6, e01693–01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


