Abstract
Over 90% of neurons within the suprachiasmatic nucleus (SCN) express γ-aminobutyric acid (GABA). Although GABA is primarily an inhibitory neurotransmitter, in vitro studies suggest that the activation of GABAA receptors (GABAAR) elicits excitation in the adult SCN. The ratio of excitatory to inhibitory responses to GABA depends on the balance of chloride influx by Na+-K+-Cl− cotransporter 1 (NKCC1) and chloride efflux by K+-Cl− cotransporters (KCCs). Excitatory responses to GABA can be blocked by inhibition of the inward chloride cotransporter, NKCC1, with the loop diuretic bumetanide. Here we investigated the role of NKCC1 activity in phase shifting the circadian pacemaker in response to photic and nonphotic signals in male Syrian hamsters housed in constant darkness. In the early subjective night (CT 13.5), injection of bumetanide into the SCN reduced light-induced phase delays. However, during the late subjective night (CT 19), bumetanide administration did not alter light-induced phase advances. Injection of bumetanide during the subjective day (CT 6) did not alter the phase of free-running circadian rhythms but attenuated phase advances induced by injection of the GABAAR agonist muscimol into the SCN. These data support the hypothesis that the excitatory effects of endogenously released GABA contribute to the ability of light to induce phase delays, thereby contributing to the most important function of the circadian system, its entrainment with the day-night cycle. Further, the finding that bumetanide inhibits the phase-advancing effects of muscimol during the subjective day supports the hypothesis that the excitatory responses to GABA also contribute to the ability of nonphotic stimuli to phase shift the circadian pacemaker.
Keywords: NKCC1, phase shifting, GABAA receptors, GABA, entrainment, circadian, chloride
The suprachiasmatic nucleus (SCN) plays a key role in the circadian control of physiology and behavior in mammals, including humans (Moore and Eichler, 1972; Stephan and Zucker, 1972; Cohen and Albers, 1991). Two of the most important functions of the SCN are generating self-sustained oscillations and adjusting the period of those oscillations with the 24-h day-night cycle through the process of entrainment (Daan and Pittendrigh, 1976; Johnson et al., 2003). Light in the early subjective night phase delays the pacemaker; light in the late subjective night phase advances the pacemaker; while light in the subjective day does little to shift the clock (Decoursey, 1960; Daan and Pittendrigh, 1976). Light is transduced in specialized retinal ganglion cells and communicated to the SCN via the release of glutamate from terminals of the retinohypothalamic tract (RHT) (Hendrickson et al., 1972; Moore and Lenn, 1972; Pickard, 1982; Colwell et al., 1991; Mintz et al., 1999; Berson et al., 2002; Sollars et al., 2003).
Although the RHT appears to be the major light input pathway regulating many of the effects of the strongest zeitgeber on the SCN, other stimuli can also induce phase shifts of the pacemaker. Nonphotic phase shifting stimuli include the injection of neuropeptide Y-like peptides, discrete bouts of resident-intruder interactions, or exposure to a novel running wheel (Albers and Ferris, 1984; Mistlberger, 1991; Biello and Mrosovsky, 1995; Biello and Mrosovsky, 1996; Mistlberger et al., 2003). In nocturnal rodents, nonphotic stimuli produce a pattern of phase shifts (i.e., small phase delays during subjective night and large phase advances during subjective day) that differ greatly from those produced by light. Stimuli that induce nonphotic phase shifts are relayed to the SCN via projections from other brain regions including a direct projection from the intergeniculate leaflet and a serotonin-containing projection from the raphe (Swanson et al., 1974; Ribak and Peters, 1975; Meyer-Bernstein and Morin, 1996; Morin, 1999; Morin, 2013). Finally, the SCN provides output signals that serve to entrain physiological systems throughout the body with the day-night cycle (Kalsbeek et al., 2006; Morin, 2013).
The SCN is a network of γ-amino butyric acid (GABA)-containing cells. GABA synthesis and transporter proteins as well as GABA receptors are expressed in more than 90% of SCN neurons (van den Pol and Tsujimoto, 1985; van den Pol and Gorcs, 1986; Okamura et al., 1989; Moore and Speh, 1993; O’Hara et al., 1995; Castel and Morris, 2000; Albers et al., 2017). Two major classes of GABA receptors, GABAA and GABAB receptors (GABAARs and GABABRs, respectively), mediate the effects of GABA within the SCN. GABAARs are ligand-gated chloride ion channels whose subunit composition determines their pharmacological properties, subcellular localization, and rhythmic regulation within the SCN (Farrant and Nusser, 2005; Walton et al., 2017). GABABRs are metabotropic G-protein-coupled receptors that can be found on presynaptic, postsynaptic, and extrasynaptic membranes (Enna and McCarson, 2013). GABA can modulate the ability of the pacemaker to phase shift in response to light by acting on GABAARs and GABABRs (Ralph and Menaker, 1985; Ralph and Menaker, 1986; Ralph and Menaker, 1989; Gribkoff et al., 1998; Mintz et al., 2002; Albers et al., 2017). The GABAAR agonist muscimol and the GABABR agonist baclofen administered into the SCN inhibit phase delays in response to light in the early subjective night and decrease light-induced phase advances in the late subjective night (Gillespie et al., 1997; Gillespie et al., 1999; Novak and Albers, 2004). In addition, GABAAR and GABABR antagonists administered in the SCN enhance phase delays to light in the early subjective night (Gillespie et al., 1996). GABAARs also play a critical role in mediating the phase-delaying effects of light. The sustained activation of GABAARs (>4 h) is both necessary and sufficient to regulate the phase-delaying effects of light in the early subjective night (Hummer et al., 2015).
GABAAR signaling is also critical in SCN processing of nonphotic phase shifting stimuli (Webb et al., 2014). For example, bicuculline blocks neuropeptide Y (NPY)-induced phase advances when given in the SCN during the middle of the subjective day (Huhman et al., 1995; Huhman et al., 1997; Gribkoff et al., 1998). Interestingly, muscimol and diazepam, drugs that act on GABAARs, phase advance the pacemaker when administered during the subjective day in nocturnal rodents but induce phase delays in the diurnal grass rat (Smith et al., 1989; Mintz et al., 2002; Novak and Albers, 2004; McElroy et al., 2009). Thus, activation of GABAARs plays a key role in regulating SCN mechanisms mediating the phase shifting effects of both photic and nonphotic stimuli.
Although GABA is most commonly viewed as the primary inhibitory neurotransmitter in the adult brain, it can also have excitatory effects. The actions of cation chloride cotransporters (CCCs) are critical in determining whether GABA is hyperpolarizing or depolarizing (Xu et al., 1994; Gillen et al., 1996; Payne et al., 1996; Markadieu and Delpire, 2014). Two main types of CCCs regulate intracellular chloride. Na-K-2Cl cotransporters (NKCCs) transport chloride ions (stoichiometry 1Na+: 1K+: 2Cl−) into cells in 2 identified isoforms, NKCC1 and NKCC2. K-Cl cotransporters (KCCs) extrude chloride ions from the cell (stoichiometry 1K+: 1Cl−), with 4 identified isoforms (KCC1–4). Thus, as the GABAAR opens a chloride pore when active, the probability of depolarizing a GABAAR-expressing neuron is determined by the ratio of the activity of chloride influx (NKCC1) to chloride efflux (KCCs) (for review, see Markadieu and Delpire, 2014).
The cellular responses to GABA within the SCN have been extensively studied using a variety of approaches, and yet there is little consensus on when, where, and whether GABA produces excitatory responses in SCN neurons (Wagner et al., 1997; Liu and Reppert, 2000; De Jeu and Pennartz, 2002; Itri et al., 2004; Albus et al., 2005; Aton et al., 2006; Choi et al., 2008; Irwin and Allen, 2009; Myung et al., 2012; Freeman et al., 2013; Farajnia et al., 2014; Albers et al., 2017). While there is agreement that GABA has substantial inhibitory effects in the SCN, some studies report that GABA is exclusively inhibitory, others that GABA can be excitatory during the day, and still others that GABA can be excitatory during the night. There is also controversy on whether the excitatory effects of GABA are more frequently observed in different subregions of the SCN. Importantly, NKCC1 is found in SCN neurons of the dorsal and ventral nuclei, regardless of time of day, and electrophysiologically recorded excitatory responses to GABA in the adult SCN disappear upon pharmacological inhibition of NKCC1 with bumetanide (Choi et al., 2008; Irwin and Allen, 2009; Belenky et al., 2010). Perhaps the most parsimonious view of this confusing body of evidence may be that GABA is primarily inhibitory during the day but that it has excitatory effects on a substantial number of SCN neurons at night (see Albers et al., 2017 for a review). Nevertheless, a significant number of GABA-excitable SCN neurons have been identified during the day (Liou and Albers, 1990; Wagner et al., 1997; Choi et al., 2008; Irwin and Allen, 2009; Alamilla et al., 2014).
Despite the important role of GABAAR signaling in phase shifting responses to both photic and nonphotic stimuli, the role of these CCCs in phase shifting the pacemaker is not known. Considering the prevalence of GABAAR signaling in SCN neurons, the role of GABAAR signaling in regulating the phase of the pacemaker, and the significant percentage of SCN neurons depolarized by GABA, it seems likely that excitatory responses to GABA play a functional role in entrainment within the intact SCN. The aim of these experiments was to test the hypothesis that NKCC1 activity within the SCN regulates both photic and nonphotic phase shifting in the fully intact circadian system.
MATERIALS AND METHODS
Animals, Light Treatment, and Activity Monitoring
Adult male Syrian hamsters (approximately 9–10 weeks of age; 120–140 g) were purchased from Charles River Laboratories (Wilmington, MA) and individually housed in polycarbonate cages. All hamsters were held in a 14:10 light-dark (LD) cycle for 7 to 10 days (25 ± 1 °C) and allowed to acclimate to the animal facility and the LD cycle. Food (Lab Diet #5001; Nestlé Purina Pet Care, St. Louis, MO) and water were provided ad libitum. After the acclimation period, a single unilateral cannula was implanted aimed at the SCN in each hamster, and hamsters were allowed to recover from surgery (approximately days 8–15; LD 14:10). Next, hamsters were transferred to constant darkness (DD) for 10 to 14 days allowing for the establishment of free-running activity rhythms. All hamsters were held for a total of 15 to 20 days in LD 14:10 before release into constant conditions. The light stimulus used to induce phase delays and advances (CT 13.5 and CT 19, respectively) was a 150-lux 15-min light pulse (LP). Animal facility staff used a Kodak LED Safelight (660 nm) for routine animal husbandry. Each cage was equipped with a running wheel, and locomotor activity was monitored as wheel revolutions per 10 min and collected with VitalView Software (Mini Mitter, Bend, OR). All procedures conformed to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Georgia State University.
Surgery and Drug Administration
Hamsters were deeply anesthetized (5% isoflurane induction; 2.5%–3.5% isoflurane for maintenance), placed into a stereotaxic device, and implanted with 4-mm, 26-gauge guide cannulas (PlasticsOne, Roanoke, VA) aimed at the SCN (0.8 mm anterior; 1.7 mm lateral to bregma, 10° angle toward midline). Microinjections were administered via a 33-gauge needle attached to polyethylene tubing (PE-20) and connected to a 1.0-μL Hamilton syringe. Microinjection volumes were 200 nL and were administered over 20 sec. The injection needle was left in place for 30 sec after injection to allow for diffusion of the solution. The depth of microinjection was 7.2 mm ventral to bregma. Injections and light pulses were given after approximately 10 to 14 days of exposure to DD. Bumetanide, dissolved in dimethyl sulfoxide (DMSO) as vehicle (Sigma Aldrich, St. Louis, MO), was used to inhibit NKCC1 chloride influx in order to decrease GABA-elicited excitatory responses in the SCN (Payne et al., 2003; Choi et al., 2008; Irwin and Allen, 2009). The solubility of bumetanide has been reported to range from 25 mM to more than 200 mM based on information from several different manufacturers and chemical societies. For the present study we were able to dissolve up to 1 M bumetanide in fresh (important due to the hygroscopicity of DMSO) 100% DMSO. Multiple doses, beginning with a low dose of 10 μM, were used to generate dose responses to bumetanide injected into the SCN region in vivo. Upon completion of each experiment, injection sites were verified with dye injection, histological preparation, and light microscopy. Unilateral injections of 200 nL spread slightly less than 1 mm from the injection site and, thus, diffuse to the contralateral SCN (Gillespie et al., 1999; Caldwell and Albers, 2003; Paul et al., 2005; Albers et al., 2017). Further, injections of GABA-active drugs 500 μm or more from the SCN do not induce phase shifts in activity rhythms (Hummer et al., 2015). The adult Syrian hamster SCN is around 0.6 mm × 0.3 mm × 0.6 mm (in the rostro caudal, mediolateral, and dorsoventral axes, respectively) (Lydic et al., 1982). Thus, injection sites further than 500 μm from the border of the SCN as well as sites showing SCN damage were excluded from further analysis.
Early and Late Subjective Night
To investigate the role of NKCC1 activity in light-induced phase delays at CT 13.5 and light-induced phase advances at CT 19, hamsters were microinjected with bumetanide (10 μM, 100 μM, or 1 mM) or vehicle into the SCN region immediately followed by a 15-min 150-lux LP or a sham LP (hamster removed from cage under safe light for drug injection) (Table 1). After treatment, hamsters were immediately returned to their home cages in DD, and wheel running was continually monitored for 10 to 14 more days.
Table 1.
Group sizes by time and treatment.
| Group | Vehicle | 10 μM Bum | 100 μM Bum | 1 mM Bum | 21.9 mM Mus |
|---|---|---|---|---|---|
| CT 13.5 (Bum+LP) | 9 | 6 | 5 | 7 | - |
| CT 13.5 (Bum+Sham) | 4 | 4 | 4 | 4 | - |
| CT 19 (Bum+LP) | 9 | 5 | 7 | 6 | - |
| CT 19 (Bum+Sham) | 8 | 6 | 7 | 8 | - |
| CT 6 (Bum) | 6 | 7 | 5 | 5 | - |
| CT 6 (Bum+Mus) | 6 | 6 | 8 | 5 | 7 |
Bum = NKCC1 inhibitor bumetanide; LP = 150 lux, 15-min light pulse; Mus = GABAAR agonist muscimol (21.9 mM); Sham = sham light pulse.
Subjective Day
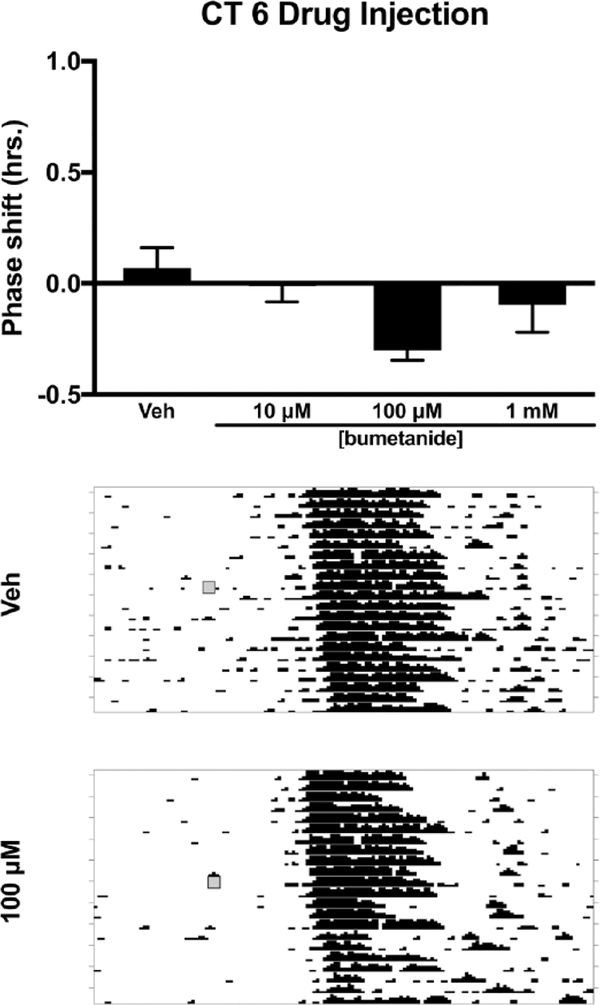
Because muscimol injection into the SCN during the subjective day (CT 6) induces phase advances in activity rhythms, we explored the role of NKCC1-mediated excitation of SCN neurons in these GABAAR-signaled phase advances in hamsters housed in DD (Smith et al., 1989; Huhman et al., 1995; Gribkoff et al., 1998; Mintz et al., 2002). This treatment mimics the phase advances induced by several nonphotic stimuli (Mrosovsky et al., 1992; Mrosovsky, 1996). At CT 6, hamsters were microinjected with bumetanide alone (10 μM, 100 μM, or 1 mM), vehicle, or the same concentrations of bumetanide in a cocktail with a phase-advancing dose of muscimol (21.9 mM) (Mintz et al., 2002) (Table 1). After injection, animals were returned to their home cages in DD, and wheel running was continually monitored for 10 to 14 more days.
Statistical Analysis
Phase shifts were quantified using the linear regression method (Daan and Pittendrigh, 1976). Regression lines were fit to activity onsets for 7 to 10 days, both for pretest baselines and postmanipulation onsets (ClockLab; Actimetrics, Wilmette, IL). Postinjection regression lines omitted 2 to 3 days of onsets following injection due to transient effects of light and handling on the free-running rhythm. Data are represented with mean (±SEM) phase shifts (hours) and were analyzed using 1-way ANOVA followed by a Tukey’s HSD post hoc test for significant ANOVAs. Significance was set at p ≤ 0.050.
RESULTS
Effect of Inhibition of NKCC1 on Light-induced Phase Delays (CT 13.5)
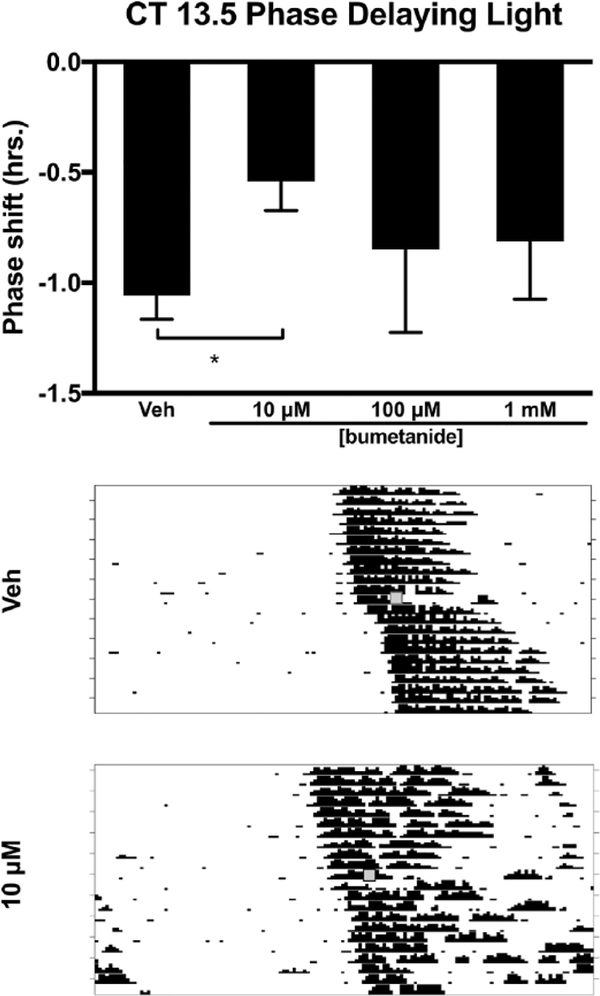
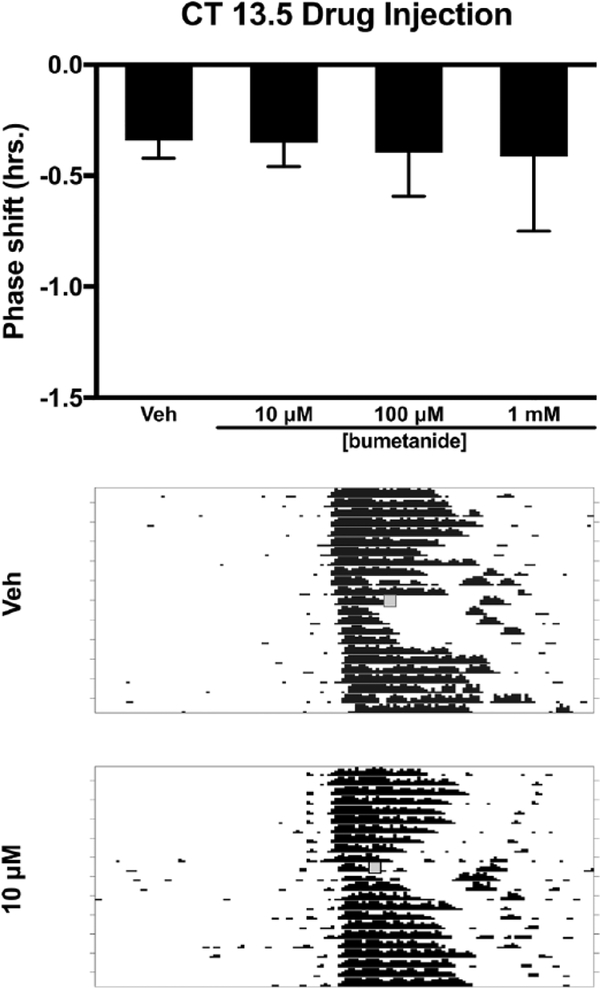
Injection of bumetanide, an inhibitor of NKCC1, into the SCN region prior to a light pulse at CT 13.5 decreased the magnitude of light-induced phase delays (F3,23 = 3.135, p = 0.045). Tukey’s HSD post hoc testing revealed that 10 μM bumetanide decreased the magnitude of the phase delay when compared with vehicle (p = 0.027). The other two concentrations (100 μM and 1 mM) of bumetanide did not differ from vehicle or 10 μM bumetanide (Fig. 1). Injection of bumetanide alone (without light) at CT 13.5 did not produce phase shifts when compared with vehicle controls (F3,12 = 0.168, p = 0.916) (Fig. 2).
Figure 1.
Effects of the NKCC1 inhibitor bumetanide injected into the SCN on light-induced phase delays of the circadian locomotor rhythm in the early subjective night (CT 13.5). Top: 10 μM bumetanide reduced phase delays in response to light at CT 13.5 compared with vehicle (*p ≤ 0.05). Middle and bottom: Representative wheel-running actograms for vehicle injection + light pulse and 10 μM bumetanide injection + light pulse, respectively. Gray boxes denote manipulation time point (i.e., drug treatment + light pulse).
Figure 2.
Effects of the NKCC1 inhibitor bumetanide injected into the SCN on the phase of the circadian locomotor rhythm in the early subjective night (CT 13.5). Top: Bumetanide did not produce phase shifts in the free-running locomotor rhythm at any concentration (p > 0.05). Middle and bottom: Representative wheel-running actograms for vehicle injection and 10 μM bumetanide injections, respectively. Gray boxes denote manipulation time point (i.e., drug treatment).
Effect of Inhibition of NKCC1 in the Late Subjective Night (CT 19)
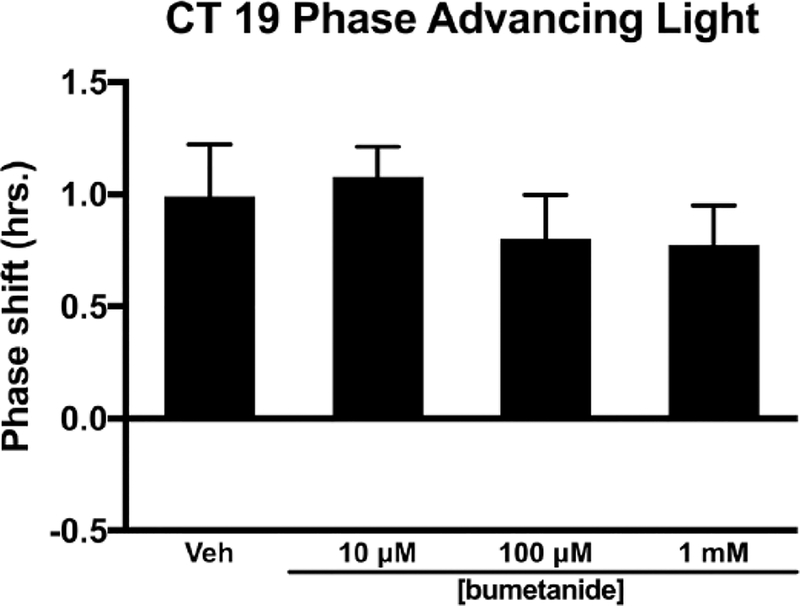
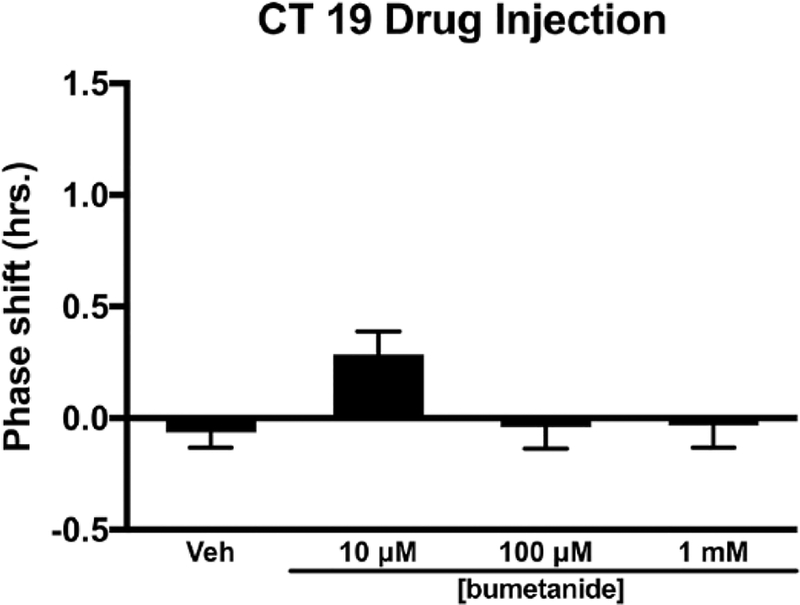
During the late subjective night (CT 19), injection of bumetanide into the SCN region did not alter the magnitude of light-induced phase advances (F3,23 = 0.445, p = 0.723) (Fig. 3). Analysis of variance of bumetanide injected into the SCN region in the late subjective night (CT 19) in the absence of light produced a significant F statistic (F3,25 = 3.092, p = 0.045). However, Tukey’s HSD post hoc analysis found no effect of treatment on circadian phase. The 10 μM concentration of bumetanide trended toward a small advance of activity onset when compared with other treatments (p = 0.054–0.091) (Fig. 4).
Figure 3.
Effects of the NKCC1 inhibitor bumetanide injected into the SCN on light-induced phase advances of the circadian locomotor rhythm in the late subjective night (CT 19). Bumetanide did not alter the magnitude of light-induced phase advances at any concentration (p > 0.05).
Figure 4.
Effects of the NKCC1 inhibitor bumetanide injected into the SCN on the phase of the circadian locomotor rhythm in the late subjective night (CT 19). Bumetanide injection into the SCN did not phase shift the circadian locomotor rhythm in the late subjective night (CT 19) (p > 0.05).
Effect of Inhibition of NKCC1 during the Subjective Day (CT 6)
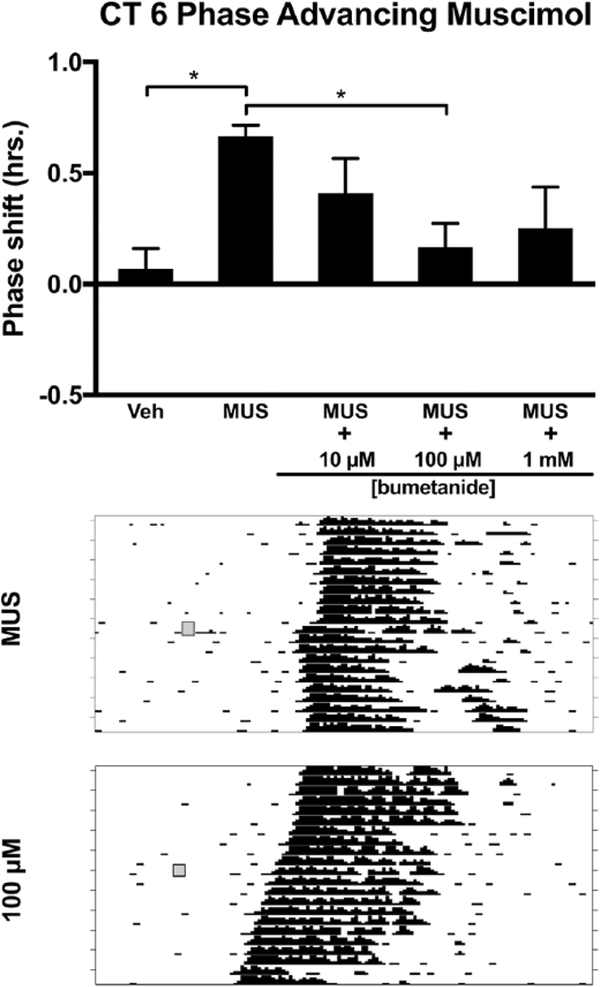
Next, we explored whether NKCC1 inhibition in the subjective day phase shifts the free-running activity rhythm. Injection of bumetanide alone into the SCN at CT 6 did not produce significant phase shifts at any concentration (F3,19 = 3.080, p = 0.052) (Fig. 5). We also examined whether NKCC1 activity modulates the phase advances produced by injection of the GABAAR agonist muscimol during the subjective day. We coadministered the same 3 concentrations of bumetanide used at CT 13.5 and CT 19 in a cocktail with a concentration of muscimol previously shown to produce phase advances during the subjective day (21.9 mM) (Mintz et al., 2002). Analysis of variance revealed a main effect of drug treatment (F4,27 = 4.152, p = 0.010). Tukey’s HSD post hoc testing revealed that muscimol (21.9 mM) administration led to a significant phase advance of the rhythm compared with controls (p = 0.010) (Fig. 6). A bumetanide concentration of 100 μM attenuated muscimol-induced phase advances (p = 0.024). Lower and higher concentrations of the NKCC1 inhibitor did not differ from muscimol administered alone (Fig. 6).
Figure 5.
Effects of the NKCC1 inhibitor bumetanide injected into the SCN on the phase of the circadian locomotor rhythm during the subjective day (CT 6). Top: Bumetanide did not produce phase shifts in the free-running locomotor rhythm at any concentration (p > 0.05). Middle and bottom: Representative wheel-running actograms for vehicle injection and 100 μM bumetanide injections, respectively. Gray boxes denote manipulation time point (i.e., drug treatment).
Figure 6.
Effects of the NKCC1 inhibitor bumetanide injected into the SCN on muscimol-induced phase advances of the circadian locomotor rhythm during the subjective day (CT 6). Top: Muscimol administration into the SCN phase advanced the circadian locomotor rhythm compared with vehicle control (*p ≤ 0.05). Muscimol-induced phase advances were reduced by injection of 100 μM bumetanide (*p ≤ 0.05). Middle and bottom: Representative wheel-running actograms for 21.9 mM muscimol injection and 100 μM bumetanide + 21.9 mM muscimol injections, respectively. Gray boxes denote manipulation time point (i.e., drug treatment).
DISCUSSION
These data indicate that injection of the NKCC1 inhibitor bumetanide within the SCN modulates the ability of the pacemaker to phase shift in response to both photic and nonphotic stimuli. In the early subjective night (i.e., CT 13.5), injection of bumetanide at a concentration (i.e., 10 μM) that inhibits SCN excitatory responses to GABA in vitro (Choi et al., 2008; Irwin and Allen, 2009; Alamilla et al., 2014; Farajnia et al., 2014; Kim et al., 2016) reduced the phase delays produced by light by approximately 50% (Fig. 1). Further support for the possibility that bumetanide reduces the ability of light to induce phase delays was the finding that bumetanide did not alter circadian phase in the absence of a light pulse at CT 13.5 (Fig. 2).
In the late subjective night (i.e., CT 19), injection of bumetanide into the SCN region did not reduce light-induced phase advances (Fig. 3). Furthermore, the injection of bumetanide into the SCN in the late subjective night in the absence of light did not lead to significant phase shifts of the rhythm at any concentration (Fig. 4). These differences suggest a different role for GABAAR-induced excitation in light-induced phase delays and phase advances or possibly no role for GABAAR excitatory responses in light-induced advances. Our laboratory has previously shown that the GABAAR agonist muscimol attenuates light-induced phase delays and advances in both night-active and day-active species (Gillespie et al., 1997; Novak and Albers, 2004). However, injection of the GABAAR antagonist bicuculline into the SCN region potentiates light-induced phase delays in the early night but not light-induced phase advances in the late night (Gillespie et al., 1997). In contrast, however, microinjection of bicuculline before phase-delaying or phase-advancing light pulses increases c-Fosimmunoreactivity within the SCN (Gillespie et al., 1999). Different GABAAR mechanisms may be involved in mediating light-induced phase delays and phase advances that may involve phase-dependent changes in the excitatory-inhibitory ratio of GABAAR containing SCN neurons and phase-dependent changes in GABAAR subunit composition (Choi et al., 2008; Irwin and Allen, 2009; Walton et al., 2017).
To determine whether the phase advances mediated by activation of GABAARs in the subjective day (i.e., CT 6) require NKCC1 activity, we examined the effects of bumetanide on muscimol-induced phase advances. As expected, injection of muscimol into the SCN region produced phase advances when compared with injections of vehicle (Fig. 6). Injection of bumetanide into the SCN in the subjective day did not induce phase shifts that differed from vehicle (Fig. 5). The injection of muscimol combined with bumetanide inhibited muscimol-induced phase advances when bumetanide was added at a concentration of 100 μM. No significant reductions in muscimol-induced phase advances were observed when bumetanide was coadministered at concentrations of 10 μM or 1 mM (Fig. 6).
The present experiments are the first to examine whether the NKCC1 inhibitor bumetanide influences the phase of the circadian pacemaker by its actions within the SCN in vivo. Prior work has focused on the effects of bumetanide on the cellular responses of SCN neurons monitored in vitro. These in vitro studies have found that micromolar concentrations of bumetanide (i.e., 10–100 μM) inhibit the excitatory effects of GABA within the SCN (Choi et al., 2008; Irwin and Allen, 2009; Alamilla et al., 2014; Farajnia et al., 2014; Kim et al., 2016). Bumetanide has a 500-fold greater affinity for NKCCs than KCCs; thus, low bumetanide concentrations (up to 100 μM) can be used in vitro to inhibit NKCCs without affecting KCCs, as observed in non-SCN brain tissue (Gamba, 2005). However, at higher concentrations, bumetanide begins to inhibit chloride efflux via actions on KCCs (Gamba, 2005; Delpire et al., 2009). These concentration-dependent, off-target actions of bumetanide on chloride efflux proteins (KCCs) may explain why we did not observe any circadian effects of 1 mM bumetanide at any time during the cycle. The effects of higher concentrations of bumetanide on SCN cellular activity in vitro, however, are not known as no dose-response work above 100 μM has been performed in the SCN, to our knowledge. Future work using microinjections of specific KCC2 inhibitors (e.g., VU024551) will be needed to address the functional role of KCC2 as well as other KCCs (1, 3, and 4) expressed in the SCN (Belenky et al., 2010; Klett and Allen, 2017).
Importantly, the ratio of GABA-induced excitation to inhibition in SCN neurons changes over the circadian cycle, as shown in multiple studies, although the exact temporal pattern of these changes is not clear, with reports of GABA-induced excitation occurring in the day, the night, or both (Wagner et al., 1997; Choi et al., 2008; Irwin and Allen, 2009; Alamilla et al., 2014; Farajnia et al., 2014). GABA excitation of SCN neurons may also differ in the ventral and dorsal subregions, although these findings are also not consistent. Dorsal SCN neurons are more likely to display excitatory postsynaptic potentials (EPSPs) upon GABA application, and NKCC1 protein may be higher in the dorsal SCN at night, although other authors have reported no differences (Choi et al., 2008; Belenky et al., 2010). Optic chiasm stimulation of the RHT or GABA application are more likely to increase the intracellular calcium concentration ([Ca2+i]) in the dorsal SCN (Irwin and Allen, 2009). Klett and Allen (2017) used chloride sensors to show that bumetanide causes small changes in intracellular chloride concentration ([Cl−i]). Surprisingly, bumetanide increases [Cl−i] in arginine-vasopressin–positive (AVP+) neurons and decreases [Cl−i] in vasoactive intestinal polypeptide–positive (VIP+) neurons. KCC antagonism increases [Cl−i] in both AVP+ and VIP+ SCN neurons, as expected, upon inhibition of the chloride efflux protein. In addition, bumetanide blocks the effects of a specific KCC2 antagonist on [Cl−i], suggesting that NKCC1 is regulating chloride influx in SCN neurons, as shown in other brain regions (Klett and Allen, 2017). Furthermore, we cannot rule out the possibility that our microinjection of NKCC1 inhibitor acted on non-AVP+ or non-VIP+ SCN neurons, as we applied the inhibitor to the entire SCN region and these cells comprise a small percentage of total SCN network neurons: VIP ≈ 10% and AVP ≈ 20% (Welsh et al., 2010). Inhibition of NKCC1 in non-AVP/VIP cells may be responsible for the modulation of phase shifts. Of course, the data of the present study cannot discriminate among these possibilities. However, it is important to note that most, if not all, of these neuropeptidergic cells colocalize with GABA in SCN neurons (Moore and Speh, 1993). It is also possible that our injections of bumetanide may have affected glycinergic signaling within the SCN, as glycine receptors also mediate a chloride current in SCN neurons and their activation may induce phase advances during the subjective day and phase delays in the early night (Ito et al., 1991; Mordel et al., 2011). However, our findings in the subjective day suggest that bumetanide inhibits phase advances directly signaled by the GABAAR, as we inhibited subjective day advances induced by the GABAAR agonist muscimol. In addition, to our knowledge, no in vivo work has explored the role of glycine in SCN mechanisms of entrainment to light.
Importantly, the differences observed in the present study suggest that the effects of bumetanide within the SCN are both phase and concentration dependent, consistent with observations made in vitro. Concentrations of bumetanide that dramatically inhibit the excitatory effects of GABA in vitro reduce the phase-delaying effects of light at CT 13.5. These data are consistent with a theoretical model in which endogenously released GABA is proposed to increase depolarization in response to RHT signaling in neurons with a higher ratio of NKCC1:KCC2 activity (i.e., excitatory to inhibitory) but to induce hyperpolarization in response to RHT signaling in neurons with a lower ratio of NKCC1:KCC2 activity (Irwin and Allen, 2009). The findings of the present study provide support for this model and significantly extend it by demonstrating that the excitatory effects of endogenously released GABA at CT 13.5 contribute to the ability of light to induce phase delays, thereby contributing to the most important function of the circadian system—its entrainment with the day-night cycle. Indeed, reduction of the excitatory effects of endogenously released GABA reduces the phase delay produced by light at CT 13.5 (Fig. 1).
Importantly, activation of the RHT produces GABA-induced excitatory responses in some SCN neurons and GABA-induced inhibitory responses in other SCN neurons (Irwin and Allen, 2009). Likewise, the present data indicate that GABA released in response to RHT activation enhances light-induced phase delays, while previous work has found that GABAA antagonists can enhance and GABAA agonists can inhibit light-induced phase delays at CT 13.5 (Gillespie et al., 1996). How can endogenously released GABA enhance light-induced phase delays and also inhibit light-induced phase delays? Perhaps these opposite effects of GABA are the result of GABAAR activation in different subregions or on subsets of neurons within the SCN with different NKCC1:KCC ratios. For example, the enhancement of light-induced phase delays could be mediated by the excitatory effects of GABA endogenously released in the dorsal SCN, whereas the inhibitory effects of GABA on light-induced phase delays could be mediated by activation of GABAARs in the retinoreceptive ventral SCN. The inhibitory effects of GABA on light-induced phase delays could be the result of the inhibition of RHT signaling to the ventral SCN and/or the inhibition of GABA release in neurons that project from the ventral to the dorsal SCN. In support of this idea are the findings that more NKCC1 activity is seen in the dorsal SCN and that the excitatory effects of GABA may be proportionally greater in the dorsal SCN than in the ventral SCN, particularly during the night (Choi et al., 2008; Irwin and Allen, 2009).
In addition to the acute effects of activating or inhibiting SCN GABAARs on the ability of light to induce phase delays, the sustained activation of GABAARs appears to be necessary for light to induce phase delays at CT 13.5 (Hummer et al., 2015). Because phase-delaying pulses of light produce a sustained activation of SCN neurons that lasts more than 4 h, and the SCN is a largely GABAergic network, it seems likely that there is a corresponding sustained release of GABA in the SCN (Hamada et al., 2001; Kuhlman et al., 2003; LeSauter et al., 2011; DeWoskin et al., 2015). Support for the hypothesis that the sustained activation of GABAARs in the SCN is necessary for the phase-delaying effects of light comes from the findings that injection of muscimol into the SCN beginning at CT 13.5 must occur over at least 4 h to mimic phase delays to light and that the injection of bicuculline must be given for 6 consecutive hours to block the phase-delaying effects of light (Hummer et al., 2015). Whether the ability of the sustained activation of GABAARs to produce phase delays is the result of excitatory, inhibitory, or bipolar responses to GABA is not known. One hypothesis that would be consistent with the existing data is that the sustained activation of GABAARs produces sustained excitatory responses (perhaps in the dorsal SCN) and the sustained excitatory responses are necessary to phase delay the pacemaker (Choi et al., 2008; DeWoskin et al., 2015; Hummer et al., 2015). If correct, this hypothesis might also explain why bumetanide inhibits light-induced phase delays in the present study because bumetanide would reduce the duration of the excitatory responses to GABA induced by light.
During the subjective day, bumetanide inhibited the ability of muscimol to induce phase advances, supporting the hypothesis that excitatory responses to GABA contribute to the phase-advancing effects of muscimol at CT 6 (Fig. 6). A functional role for the excitatory effects of GABA during the day is consistent with electrophysiological evidence reporting that GABA has primarily excitatory effects on SCN neurons during the day (Wagner et al., 1997; Alamilla et al., 2014). Even in several of the studies that found GABA’s excitatory effects to occur primarily during the night, a significant number of excitatory responses to GABA and muscimol were also observed during the day (Choi et al., 2008; Irwin and Allen, 2009). Because activation of GABAARs appears to be necessary for the phase shifting effects of NPY as well as other nonphotic phase shifting stimuli, the excitatory effects of GABA may be partially responsible for mediating these nonphotic shifts. Thus, these data provide further support for the hypothesis that GABA’s excitatory effects in the SCN play an important functional role in circadian timekeeping.
ACKNOWLEDGMENTS
We thank Dr. Daniel Hummer and Alisa Norvelle for assistance with these experiments. We also thank Ancilla Titus-Scotland, Robert Bynes, and Christopher Barrow for animal husbandry. This work was supported by the National Institutes of Health grants R01NS078220 to H.E.A. and F32NS092545 to J.C.W.
Footnotes
CONFLICTS OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Alamilla J, Perez-Burgos A, Quinto D, and Aguilar-Roblero R (2014) Circadian modulation of the Cl(−) equilibrium potential in the rat suprachiasmatic nuclei. BioMed Res Int 2014:424982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE and Ferris CF (1984) Neuropeptide Y: role in light-dark cycle entrainment of hamster circadian rhythms. Neurosci Lett 50:163–168. [DOI] [PubMed] [Google Scholar]
- Albers HE, Walton JC, Gamble KL, McNeill JK, and Hummer DL (2017) The dynamics of GABA signaling: revelations from the circadian pacemaker in the suprachiasmatic nucleus. Front Neuroendocrinol 44:35–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus H, Vansteensel M, Michel S, Block G, and Meijer J (2005) A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol 15:886–893. [DOI] [PubMed] [Google Scholar]
- Aton S, Huettner J, Straume M, and Herzog E (2006) GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci U S A 103:19188–19193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky M, Sollars P, Mount D, Alper S, Yarom Y, and Pickard G (2010) Cell-type specific distribution of chloride transporters in the rat suprachiasmatic nucleus. Neuroscience 165:1519–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, and Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073. [DOI] [PubMed] [Google Scholar]
- Biello SM and Mrosovsky N (1995) Blocking the phase-shifting effect of neuropeptide Y with light. Proc Biol Sci 259:179–187. [DOI] [PubMed] [Google Scholar]
- Biello SM and Mrosovsky N (1996) Phase response curves to neuropeptide Y in wildtype and tau mutant hamsters. J Biol Rhythms 11:27–34. [DOI] [PubMed] [Google Scholar]
- Caldwell HK and Albers HE (2003) Short-photoperiod exposure reduces vasopressin (V1a) receptor binding but not arginine-vasopressin-induced flank marking in male Syrian hamsters. J Neuroendocrinol 15:971–977. [DOI] [PubMed] [Google Scholar]
- Castel M and Morris J (2000) Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain’s circadian pacemaker. J Anat 196(pt 1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Lee C, Schroeder A, Kim Y, Jung S, Kim J, Kim DY, Son E, Han H, Hong S, Colwell C, and Kim Y (2008) Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci 28:5450–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA and Albers HE (1991) Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: a case study. Neurology 41:726–729. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Foster RG, and Menaker M (1991) NMDA receptor antagonists block the effects of light on circadian behavior in the mouse. Brain Res 554:105–110. [DOI] [PubMed] [Google Scholar]
- Daan S and Pittendrigh C (1976) A functional analysis of circadian pacemakers in nocturnal rodents, II: the variability of phase response curves. J Comp Physiol A Neuroethol Sense Neural Behav Physiol 106:253–266. [Google Scholar]
- De Jeu M and Pennartz C (2002) Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J Neurophysiol 87:834–844. [DOI] [PubMed] [Google Scholar]
- Decoursey PJ (1960) Phase control of activity in a rodent. Cold Spring Harb Symp Quant Biol 25:49–55. [DOI] [PubMed] [Google Scholar]
- Delpire E, Days E, Lewis LM, Mi D, Kim K, Lindsley CW, and Weaver CD (2009) Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A 106:5383–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWoskin D, Myung J, Belle MD, Piggins HD, Takumi T, and Forger DB (2015) Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping. Proc Natl Acad Sci U S A 112:E3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enna SJ and McCarson KE (2013) Characterization of GABA receptors. Curr Protoc Pharmacol 63:Unit 1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, van Westering TL, Meijer JH, and Michel S (2014) Seasonal induction of GABAergic excitation in the central mammalian clock. Proc Natl Acad Sci U S A 111:9627–9632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M and Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. [DOI] [PubMed] [Google Scholar]
- Freeman G, Krock R, Aton S, Thaben P, and Herzog E (2013) GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron 78:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba G (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85:423–493. [DOI] [PubMed] [Google Scholar]
- Gillen CM, Brill S, Payne JA, and Forbush B III (1996) Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human: a new member of the cation-chloride cotransporter family. J Biol Chem 271:16237–16244. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Huhman KL, Babagbemi TO, and Albers HE (1996) Bicuculline increases and muscimol reduces the phase-delaying effects of light and VIP/PHI/GRP in the suprachiasmatic region. J Biol Rhythms 11:137–144. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Mintz EM, Marvel CL, Huhman KL, and Albers HE (1997) GABA(A) and GABA(B) agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res 759:181–189. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Van Der Beek EM, Mintz EM, Mickley NC, Jasnow AM, Huhman KL, and Albers HE (1999) GABAergic regulation of light-induced c-Fos immunoreactivity within the suprachiasmatic nucleus. J Comp Neurol 411:683–692. [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, and Yocca FD (1998) Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J Neurosci 18:3014–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti J, and Silver R (2001) Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci 21:7742–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson AE, Wagoner N, and Cowan WM (1972) An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z Zellforsch Mikrosk Anat 135:1–26. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Babagbemi TO, and Albers HE (1995) Bicuculline blocks neuropeptide Y-induced phase advances when microinjected in the suprachiasmatic nucleus of Syrian hamsters. Brain Res 675:333–336. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Marvel CL, Gillespie CF, Mintz EM, and Albers HE (1997) Tetrodotoxin blocks NPY-induced but not muscimol-induced phase advances of wheel-running activity in Syrian hamsters. Brain Res 772:176–180. [DOI] [PubMed] [Google Scholar]
- Hummer D, Ehlen JC, Larkin T, McNeill J, Pamplin J, Walker C, Walker P, Dhanraj D, and Albers HE (2015) Sustained activation of GABAA receptors in the suprachiasmatic nucleus mediates light-induced phase delays of the circadian clock: a novel function of ionotropic receptors. Eur J Neurosci 42:1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R and Allen C (2009) GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci 30:1462–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito C, Wakamori M, and Akaike N (1991) Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol 260:C213–218. [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek J, and Colwell C (2004) Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol 92:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Elliott JA, and Foster R (2003) Entrainment of circadian programs. Chronobiol Int 20:741–774. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FAJL, Perreau Lenz S, Ruiter M, Kreier F, Cailotto C, and Buijs RM (2006) SCN outputs and the hypothalamic balance of life. J Biol Rhythms 21:458–469. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim YB, Kim WB, Lee SW, Oh SB, Han HC, Lee CJ, Colwell CS, and Kim YI (2016) Histamine 1 receptor-Gbetagamma-cAMP/PKA-CFTR pathway mediates the histamine-induced resetting of the suprachiasmatic circadian clock. Mol Brain 9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett NJ and Allen CN (2017) Intracellular chloride regulation in AVP+ and VIP+ neurons of the suprachiasmatic nucleus. Sci Rep 7:10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, and McMahon DG (2003) Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci 23:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R, Cloues R, and Witkovsky P (2011) Light exposure induces short- and long-term changes in the excitability of retinorecipient neurons in suprachiasmatic nucleus. J Neurophysiol 106:576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou SY and Albers HE (1990) Single unit response of neurons within the hamster suprachiasmatic nucleus to GABA and low chloride perfusate during the day and night. Brain Res Bull 25:93–98. [DOI] [PubMed] [Google Scholar]
- Liu C and Reppert S (2000) GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25:123–128. [DOI] [PubMed] [Google Scholar]
- Lydic R, Albers HE, Tepper B, and Moore-Ede MC (1982) Three-dimensional structure of the mammalian suprachiasmatic nuclei: a comparative study of five species. J Comp Neurol 204:225–237. [DOI] [PubMed] [Google Scholar]
- Markadieu N and Delpire E (2014) Physiology and pathophysiology of SLC12A1/2 transporters. Pflügers Archiv Eur J Physiol 466:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass J, and Prosser R (2009) Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience 164:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL and Morin LP (1996) Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci 16:2097–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, Jasnow AM, Gillespie CF, Huhman KL, and Albers HE (2002) GABA interacts with photic signaling in the suprachiasmatic nucleus to regulate circadian phase shifts. Neuroscience 109:773–778. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, and Albers HE (1999) Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci 19:5124–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger R (1991) Effects of daily schedules of forced activity on free-running rhythms in the rat. J Biol Rhythms 6:71–80. [DOI] [PubMed] [Google Scholar]
- Mistlberger R, Antle M, Webb I, Jones M, Weinberg J, and Pollock M (2003) Circadian clock resetting by arousal in Syrian hamsters: the role of stress and activity. Am J Physiol Regul Integr Comp Physiol 285:25. [DOI] [PubMed] [Google Scholar]
- Moore RY and Eichler V (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206. [DOI] [PubMed] [Google Scholar]
- Moore R and Speh J (1993) GABA is the principal neurotransmitter of the circadian system. Neurosci Lett 150:112–116. [DOI] [PubMed] [Google Scholar]
- Moore RY and Lenn NJ (1972) A retinohypothalamic projection in the rat. J Comp Neurol 146:1–14. [DOI] [PubMed] [Google Scholar]
- Mordel J, Karnas D, Inyushkin A, Challet E, Pevet P, and Meissl H (2011) Activation of glycine receptor phase-shifts the circadian rhythm in neuronal activity in the mouse suprachiasmatic nucleus. J Physiol 589:2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP (1999) Serotonin and the regulation of mammalian circadian rhythmicity. Ann Med 31:12–33. [DOI] [PubMed] [Google Scholar]
- Morin LP (2013) Neuroanatomy of the extended circadian rhythm system. Exp Neurol 243:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N (1996) Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc 71:343–372. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Salmon PA, Menaker M, and Ralph MR (1992) Nonphotic phase shifting in hamster clock mutants. J Biol Rhythms 7:41–49. [DOI] [PubMed] [Google Scholar]
- Myung J, Hong S, Hatanaka F, Nakajima Y, De Schutter E, and Takumi T (2012) Period coding of Bmal1 oscillators in the suprachiasmatic nucleus. J Neurosci 32:8900–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM and Albers HE (2004) Novel phase-shifting effects of GABAA receptor activation in the suprachiasmatic nucleus of a diurnal rodent. Am J Physiol Regul Integr Comp Physiol 286:5. [DOI] [PubMed] [Google Scholar]
- O’Hara B, Andretic R, Heller H, Carter D, and Kilduff T (1995) GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Brain Res Mol Brain Res 28:239–250. [DOI] [PubMed] [Google Scholar]
- Okamura H, Berod A, Julien JF, Geffard M, Kitahama K, Mallet J, and Bobillier P (1989) Demonstration of GABAergic cell bodies in the suprachiasmatic nucleus: in situ hybridization of glutamic acid decarboxylase (GAD) mRNA and immunocytochemistry of GAD and GABA. Neurosci Lett 102:131–136. [DOI] [PubMed] [Google Scholar]
- Paul KN, Fukuhara C, Karom M, Tosini G, and Albers HE (2005) AMPA/kainate receptor antagonist DNQX blocks the acute increase of Per2 mRNA levels in most but not all areas of the SCN. Brain Res Mol Brain Res 139:129–136. [DOI] [PubMed] [Google Scholar]
- Payne J, Rivera C, Voipio J, and Kaila K (2003) Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci 26:199–206. [DOI] [PubMed] [Google Scholar]
- Payne J, Stevenson T, and Donaldson L (1996) Molecular characterization of a putative K-Cl cotransporter in rat brain: a neuronal-specific isoform. J Biol Chem 271:16245–16252. [DOI] [PubMed] [Google Scholar]
- Pickard GE (1982) The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J Comp Neurol 211:65–83. [DOI] [PubMed] [Google Scholar]
- Ralph MR and Menaker M (1986) Effects of diazepam on circadian phase advances and delays. Brain Res 372:405–408. [DOI] [PubMed] [Google Scholar]
- Ralph MR and Menaker M (1989) GABA regulation of circadian responses to light, I: involvement of GABAA-benzodiazepine and GABAB receptors. J Neurosci 9:2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR and Menaker M (1985) Bicuculline blocks circadian phase delays but not advances. Brain Res 325:362–365. [DOI] [PubMed] [Google Scholar]
- Ribak CE and Peters A (1975) An autoradiographic study of the projections from the lateral geniculate body of the rat. Brain Res 92:341–368. [DOI] [PubMed] [Google Scholar]
- Smith R, Inouye S, and Turek F (1989) Central administration of muscimol phase-shifts the mammalian circadian clock. J Comp Physiol A 164:805–814. [DOI] [PubMed] [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, and Pickard GE (2003) Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci 20:601–610. [DOI] [PubMed] [Google Scholar]
- Stephan FK and Zucker I (1972) Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A 69:1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM, and Jones EG (1974) An autoradiographic study of the efferent connections of the ventral lateral geniculate nucleus in the albino rat and the cat. J Comp Neurol 156:143–163. [DOI] [PubMed] [Google Scholar]
- van den Pol AN and Gorcs T (1986) Synaptic relationships between neurons containing vasopressin, gastrin-releasing peptide, vasoactive intestinal polypeptide, and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus: dual ultrastructural immunocytochemistry with gold-substituted silver peroxidase. J Comp Neurol 252:507–521. [DOI] [PubMed] [Google Scholar]
- van den Pol AN and Tsujimoto KL (1985) Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience 15:1049–1086. [DOI] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, and Yarom Y (1997) GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387:598–603. [DOI] [PubMed] [Google Scholar]
- Walton JC, McNeill JK, Oliver KA, and Albers HE (2017) Temporal regulation of GABAA receptor subunit expression: role in synaptic and extrasynaptic communication in the suprachiasmatic nucleus. eNeuro 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Antle MC, and Mistlberger RE (2014) Regulation of circadian rhythms in mammals by behavioral arousal. Behav Neurosci 128:304–325. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, and Kay SA (2010) Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72:551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JC, Lytle C, Zhu TT, Payne JA, Benz E Jr, and Forbush B III (1994) Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc Natl Acad Sci U S A 91:2201–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]