Abstract
Objective:
To determine the influence of pneumococcal penicillin non-susceptibility patterns on individual antibiotic prescription among 33 children’s hospitals using a multi-level, random intercept, logistic regression analysis.
Design:
Multi-level cross-sectional study
Setting:
33 children’s hospitals
Participants:
Children, 1–18 years of age, with CAP discharged in 2006. Hospital antibiotic susceptibility data were collected from surveys and patient data was obtained from an administrative database.
Main Exposures:
The primary exposure was the proportion of penicillin non-susceptible pneumococcal isolates reported in 2005 by each hospital. A secondary exposure included using the proportion of penicillin-resistant pneumococcal isolates to determine if a threshold of susceptibility existed.
Main Outcome Measures:
Receipt of broad spectrum empiric antibiotic therapy in 2006 (i.e., antibiotics other than penicillins or aminopenicillins).
Results:
4,888 children diagnosed with community-acquired pneumonia (CAP) were eligible. The proportion of penicillin non-susceptible isolates ranged from 9%−70% across hospitals while the proportion of penicillin resistant isolates ranged from 0%−60%. Broad spectrum antibiotics were prescribed to 93% of patients; 45% of patients received cephalosporin class antibiotics alone. There was no significant association between the proportion of pencillin nonsusceptible pneumococcal isolates at individual hospitals and narrow spectrum prescribing. However, every 10% increase in penicillin-resistant pneumococcal isolates was associated with a 39% increase in broad spectrum antibiotic prescribing (adjusted odds ratio, 1.39; 95% confidence interval: 1.08–1.69).
Conclusion:
There was substantial variability in empiric antibiotic prescribing for CAP among children’s hospitals in the U.S. High- (i.e., resistant) but not modest-levels (i.e., intermediate susceptibility) of penicillin resistance were associated with broad spectrum antibiotic prescribing.
Keywords: pneumonia, child, drug-resistance, bacterial, Streptococcus pneumoniae, hospitals, pediatric
INTRODUCTION
Antibiotic resistance is a major public health problem. Infections caused by drug-resistant bacteria lead to worse clinical outcomes than infections caused by susceptible bacteria 1. Furthermore, the rise of antibiotic resistant organisms has rapidly limited the availability of effective therapies for some infections 1. Reducing antibiotic resistance is a major focus of many national and international organizations 1–3.
Improving antibiotic prescribing practices is an important part of the global strategy to reduce antibiotic resistance. Studies aimed at improving antibiotic prescribing, including encouraging narrower spectrum antibiotic prescribing, have traditionally focused on persuasive (e.g., educational) or restrictive (e.g., formulary restriction) interventions 4. These approaches, while often successful, yield only modest improvements in prescribing practices.
Hospital “antibiograms,” bacteria-specific antimicrobial susceptibility profiles, are often used to support the need for improving antibiotic prescribing practices. However, antibiograms may also be used to influence antibiotic prescribing 5. Antibiograms are disseminated to physicians by hospitals at varying intervals, though usually at the end of each calendar year. These antibiograms are based on Clinical and Laboratory Standards Institute (CLSI ) breakpoints,6 the minimum inhibitory concentration (MIC) cut-off values that determine the level at which an organism is susceptible to specific antibiotics. The categories, susceptible, intermediate, and resistant, correspond to the likelihood of successful or unsuccessful in vitro inhibition of bacterial growth 6, 7. Physicians use antibiograms to guide empiric prescribing of broad spectrum antibiotics for infections likely to be caused by highly resistant organisms. In some cases, MIC breakpoints are altered to better align with clinical outcomes. For example, the CLSI changed the breakpoints for Streptococcus pneumoniae, the most common bacterial cause of community-acquired pneumonia (CAP), in 2008 after studies demonstrated that narrow spectrum antibiotics, such as penicillin and aminopenicillins, were effective in treating non-central nervous system (CNS) pneumococcal infections even when classified as non-susceptible in vitro 7. The proportion of pneumococcal isolates now reported as “susceptible” to penicillin has increased as a result of the change; however, the impact of hospital antibiograms on antibiotic prescribing for community-acquired infections is unknown.
The aim of this multicenter study was to determine the association between pneumococcal penicillin susceptibility testing results, as reported by hospital antibiograms, and physicians’ prescribing practices for children hospitalized with CAP. We used antibiograms incorporating 2005 pneumococcal susceptibility patterns, as these antibiograms would be available to physicians when prescribing antibiotics for CAP in 2006.
METHODS
Study Design And Data Sources
This multi-level cross-sectional study used hospital data collected from surveys and patient-level data obtained from an administrative database. The Pediatric Health Information System (PHIS) was used to identify hospitals that contributed group-level data and was used to gather prescribing information for patient-level data. PHIS is a national administrative database containing resource utilization from 38 freestanding, tertiary care children’s hospitals affiliated with the Child Health Corporation of America (Shawnee Mission, KS). Participating hospitals account for 20% of all tertiary care children’s hospitals. For the purposes of external benchmarking, participating hospitals provide discharge data including patient demographics, diagnoses, and procedures. Billing data detail all of the drugs, radiologic imaging studies, laboratory tests, and supplies charged to each patient. Data quality and reliability are assured through a joint effort between the Child Health Corporation of America and participating hospitals as previously described 8, 9. The study protocol was approved by Institutional Review Boards of The Children’s Hospital of Philadelphia and the Drexel University College of Medicine.
Group-level Data.
Hospital antibiotic susceptibility patterns for S. pneumoniae were determined via written surveys sent to the microbiology laboratories of each hospital. The surveys requested information regarding antibiotic susceptibility patterns for pneumococcal isolates tested in 2005 in aggregate and, when available, by specific site (i.e., blood isolates, respiratory isolates). The cutpoints were defined using MICs for S. pneumoniae susceptibility as established by the CLSI for 2005 as follows: ≤0.06 mcg/mL, susceptible; 0.12–1.0 mcg/mL, intermediate; and ≥2.0 mcg/mL, resistant10. An isolate was considered non-susceptible if it was classified as either intermediate or resistant.
Individual-level Data.
Patient-level information for the calendar year 2006 was retrieved from the PHIS database. Children, 1–18 years of age, with CAP were eligible if they were discharged from any participating hospital between January 1 and December 31, 2006. Subjects were included if they received antibiotic therapy on the first day of hospitalization and if they satisfied one of the following International Classification of Diseases, 9th Revision (ICD-9), discharge diagnosis code criteria: 1) Primary diagnosis of pneumonia (ICD-9 codes 481–483.8, 485–486); 2) Primary diagnosis of a pneumonia-related symptom (ICD-9 codes 780.6 or 786.00–786.52 [except 786.1]) and a secondary diagnosis of pneumonia.Only patients receiving antibiotics considered conventional treatment for childhood CAP (i.e. penicillin, macrolide, cephalosporin, vancomycin, and clindamycin) on the first day of hospitalization were included.
We identified children with asthma in two ways. Asthma-related hospitalizations were defined by an ICD-9 code for asthma (493.0–493.2) in any discharge diagnosis field during any prior hospitalization in the 24 months before the current hospitalization. Chronic asthma controller medication use was defined by treatment with inhaled corticosteroids (e.g., fluticasone) or leukotriene receptor antagonists on the first day of hospitalization for CAP, which suggested that these medications were a continuation of baseline therapy.
Data from five of the thirty-eight hospitals were excluded because of incomplete patient-level information (n=3) or an incomplete antibiogram was returned (n=2). Children younger than one year of age were excluded because they experience a high rate of viral respiratory infections that are difficult to distinguish clinically from bacterial pneumonia. Patients with empyema or pleursiy were excluded as coverage for Staphylococcus aureus with a broad spectrum antibiotic is typically required. Patients with comorbid conditions that predisposed them to severe or recurrent pneumonia (e.g. cystic fibrosis, malignancy, sickle cell disease) were excluded using a previously reported classification scheme 11.
Measured Exposures
The primary exposure of interest was the proportion of penicillin non-susceptible pneumococcal isolates reported in 2005 by each hospital. An isolate was considered non-susceptible if it was classified as either intermediate or resistant. Secondary exposures included using the proportion of penicillin-resistant pneumococcal isolates to determine if a threshold of susceptibility existed, as well as restricting the exposures to blood or respiratory penicillin non-susceptible pneumococcal isolates as these isolates were more likely than aggregated isolates to represent invasive disease.
Measured Outcomes
The primary outcome was the receipt of empiric broad spectrum antibiotic therapy in 2006 (i.e., an antibiotic other than penicillin or aminopenicillins). A subanalysis was performed excluding patients who received macrolide therapy alone or in combination as it was most likely prescribed for suspicion of an atypical bacterium as the etiology of their pneumonia and not S. pneumoniae.
Data Analysis
Categorical variables were described using frequencies and percentages. Chi-square analysis was used to compare the between hospital distribution of individual level variables.
We used multi-level, random intercept, logistic regression to explain the influence of hospital penicillin non-susceptible pneumococcal patterns on individual-level antibiotic prescription for several reasons. First, the observations are not independent as patients admitted to the same hospital are similar in regards to both their exposure and outcome, precluding a simple logistic regression (rather than multi-level) modeling approach. Second, the variability within and between hospitals in the PHIS database is of interest and a generalized estimating equation (GEE) (rather than random effects) approach would treat the heterogeneous patient population in each hospital as a nuisance factor 12. Third, the inference with a random-effects model is made for a specific patient in a specific hospital while the inference with a GEE approach results in a population effect averaged over all the hospitals. The population average inference of the GEE approach does not allow for interpretation of the influence from the complex heterogeneities that exist between hospitals 13, 14.
The first model, considered the ‘empty’ model, contained the random-intercept only and no other predictor variables. This model accounted for the probability of receiving broad spectrum antibiotic prescribing as a function of which hospital the patient attended. The second model, an extension of the ‘empty’ model, added the proportion of penicillin non-susceptible pneumococcal isolates. By including the proportion of penicillin non-susceptible pneumococcal isolates as a separate measure in the model we are able to estimate it’s specific effect on antibiotic prescribing. The proportion of penicillin non-susceptible pneumococcal isolates was grand-mean centered at 52% (standard deviation [SD]: 11.4) 15. This model determined the amount of variance explained by the addition of susceptibility patterns reported from each hospital. In the additional sub-analyses, the exposures were grand-mean centered at 26% (SD: 15.1) for penicillin-resistant S. pneumoniae, 43% (SD: 15.3) for penicillin-nonsusceptible S. pneumoniae blood isolates, and 54% (SD: 13.9) for penicillin nonsusceptible S. pneumoniae respiratory isolates.
The third model tested individually the inclusion of potential effect modifiers, age and asthma status. These interaction terms, determined a priori, remained in the model if the main effect of non-susceptible pneumococcal patterns and aminopenicillin prescribing changed by 10% or more 16. The models were compared using Akaike’s information criterion (AIC) 17. The model with the smallest AIC was chosen.
The median odds ratio (OR) quantified the heterogeneity between different hospitals. The median OR, calculated using the variance of the hospitals in each model, is the median value of the ORs when comparing all possible pairs of patients with similar covariates admitted to different hospitals 19, 20. A median OR equal to 1 indicates that there is no difference between hospitals in the probability of receiving broad spectrum antibiotics and a median OR larger than 1 indicates large variation in the probability of receiving broad spectrum antibiotics between hospitals. This measure is not dependent on the prevalence of broad spectrum antibiotic prescribing and can therefore be compared with future studies. All statistical analyses were performed using SAS statistical software (version 9.1, SAS Institute Inc, Cary, N.C.).
RESULTS
Hospital Exposure
Hospitals reported the percentage of pneumococcal isolates tested in 2005 that were susceptible to penicillin, overall and, when available, by specific site (i.e., blood isolates, respiratory isolates) (Table 1).
Table 1:
Variability in proportion of penicillin-nonsusceptibility in Streptococcus pneumoniae across all hospitalsa
| Main Exposure | Hospital Reported Data (No.) | Median (%) | Interquartile Range (%) | Range (%) |
|---|---|---|---|---|
| Penicillin-nonsusceptible S. pneumoniae | ||||
| All isolates | 33 | 52 | 46–60 | 9–70 |
| Blood isolates | 17 | 48 | 37–54 | 14–69 |
| Respiratory isolates | 14 | 56 | 48–65 | 4–72 |
| Penicillin-resistant S. pneumoniae | ||||
| All isolates | 20 | 25 | 18–30 | 0–60 |
All numbers in table are the median percentages of the hospitals
Patient Characteristics
There were 4,888 patients from 33 hospitals. Narrow spectrum antibiotic therapy was prescribed to 348 (7%) of the 4,888 children with CAP. Of these children, 295 (85%) were 1–5 years old, 37 (10%) were 6–11 years old, and 16 (5%) were 12–18 years old. Patients who received a narrow spectrum antibiotic were between 1–5 years old (85% compared to 65%; p-value <0.0001), more likely to have a prior asthma-related hospitalization (18% compared to 13%; p-value=0.01), and more likely to have received a chronic asthma medication (26% compared to 21%,; p-value=0.02) than those receiving empiric broad spectrum antibiotics.
Variability in Antibiotic Prescribing
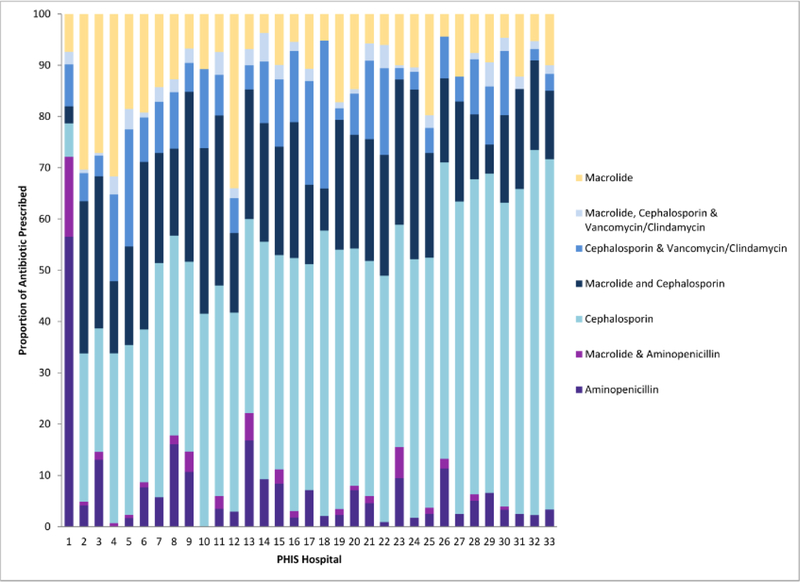
Commonly used antibiotics were classified into 7 categories based on their spectrum of antibacterial activity to describe hospital variability in antibiotic prescribing (Figure 1). Overall, 45% (n=2194) of all the patients received cephalosporins as empiric therapy for CAP; cephalosporins were also the most commonly prescribed antibiotic within each hospital. One exception was a hospital where penicillins or aminopenicillins alone were prescribed at a much higher rate, 57%, compared with other hospitals, in which penicillins or aminopenicillin alone accounted for 7% of the total proportion of antibiotics prescribed for CAP during the study period.
Figure 1.
7 categories based on their spectrum of antibacterial activity to describe hospital variability in antibiotic prescribing.
Association of Resistance and Prescribing
In the unadjusted analysis there was no correlation between the proportion of penicillin-non-susceptible pneumococcal isolates and broad spectrum antibiotic prescribing (spearman correlation= 0.070, p-value=0.703). In the adjusted analysis there was no association between the proportion of penicillin-non-susceptible pneumococcal isolates and broad spectrum antibiotic prescribing, either overall or when restricted to blood or respiratory isolates (Table 2). However, the association between the proportion of penicillin-resistant pneumococcal isolates and broad spectrum antibiotic prescribing was significant; patients were 39% more likely to receive broad spectrum antibiotics for every 10% increase in penicillin-resistant pneumococcal isolates (Table 2). There was no substantive change when patients who received macrolide therapy alone were excluded from the analysis (adjusted OR: 1.38; 95% confidence interval: 1.08, 1.67) nor when patients who received macrolide therapy in combination were excluded from the analysis (adjusted OR: 1.41; 95% confidence interval: 1.08. 1.73).
Table 2:
Random-intercept multilevel model predicting the probability of being prescribed a broad spectrum antibiotic c
| Exposure | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratioa (95% CI) |
|---|---|---|
| Penicillin-nonsusceptible S. pneumoniae | ||
| All isolates | 1.13 (0.76, 1.49) | 1.12 (0.76, 1.48) |
| Blood isolates | 0.99 (0.59, 1.38) | 0.97 (0.58, 1.37) |
| Respiratory isolates | 0.97 (0.58, 1.37) | 1.10 (0.62, 1.58) |
| Penicillin-resistant S. pneumoniae | ||
| All isolatesb | 1.40 (1.08, 1.72) | 1.39 (1.08, 1.69) |
Abbreviations: CI, confidence interval
Adjusted for age, prior hospitalization for asthma, and chronic asthma medication
The outlier hospital did not report overall resistance and therefore is not included in this model
Odds ratios are given for every 10% change in penicillin-nonsusceptible or resistant pneumococcal isolate
One hospital was unique in its prescribing practices (Figure 1) and its inclusion increased the variance component in the models. There was no association between proportion of penicillin non-susceptible S. pneumoniae isolates and broad spectrum prescribing when this hospital was excluded (adjusted OR: 1.07; 95 % confidence interval: 0.79, 1.35) but the overall fit of the model improved (AIC with the outlier was 2144.3 versus 1984.8 without the outlier). This finding indicates that some, but not all, of the variability between hospitals is due to this hospital which had a disproportionate amount of narrow spectrum antibiotic prescribing. In addition we found no statistical association between proportion of penicillin non-susceptible pneumococcal isolates and broad spectrum antibiotic prescribing when limiting the dataset to the twenty hospitals that reported information in each of the three breakpoints (adjusted OR: 1.31; 95% confidence interval: 0.97, 1.66).
The median OR for penicillin non-susceptible isolates, overall and site-specific, indicated large variability among hospitals in broad spectrum prescribing (Table 3). In contrast, there was less variability between hospitals in the probability of prescribing broad spectrum antibiotics when adjusting for penicillin-resistant pneumococcal isolates; on average a patient had 1.62 higher odds of receiving a broad spectrum antibiotic solely based on which hospital they were admitted. The least amount of variability exists between hospitals in prescribing broad spectrum antibiotics when only accounting for the proportion of penicillin-resistant S. pneumoniae isolates.
Table 3:
Median odds ratio for unadjusted and adjusted models to quantify the heterogeneity in broad spectrum antibiotic prescribing across hospitals.
| Exposure | Unadjusted Median Odds Ratio (95% CI) | Adjusted Median Odds Ratioa (95% CI) |
|---|---|---|
| Penicillin-nonsusceptible S. pneumoniae | ||
| All isolates | 2.65 (1.42, 4.96) | 2.68 (1.40, 5.12) |
| Blood isolates | 2.98 (1.03, 8.59) | 3.04 (1.01, 9.11) |
| Respiratory isolates | 2.98 (0.99, 8.93) | 3.04 (0.98, 9.48) |
| Penicillin-Resistant S. pneumoniae | ||
| All isolatesb | 1.68 (1.23, 2.30) | 1.62 (1.21, 2.17) |
Abbreviations: CI, confidence interval
Adjusted for age, prior hospitalization for asthma, and chronic asthma medication
The outlier hospital did not report overall resistance and therefore is not included in this model
DISCUSSION
This multicenter study found substantial variability in empiric antibiotic prescribing for CAP among children’s hospitals in the U.S. High- (i.e., resistant) but not modest-levels (i.e., intermediate susceptibility) of penicillin resistance were associated with broad spectrum antibiotic prescribing. As narrow spectrum antibiotics effectively treat most non-CNS pneumococcal infections, our findings suggest that physician prescribing for CAP is responsive to hospital pneumococcal antibiotic susceptibility patterns. The broader implication of this finding is that strategies to optimally align antibiotic susceptibility patterns and clinical outcomes can lead to meaningful decreases in broad spectrum antibiotic prescribing.
The degree of variability in empiric therapy prescribing for CAP in this study is similar to prior studies investigating general antibiotic prescribing 21. In contrast to recommended first line therapy for children with CAP,22 broad spectrum antibiotics were more commonly prescribed as empiric therapy for CAP than narrow spectrum antibiotics in our study. Studies in adults23 and children24 demonstrated that in vitro resistance did not correlate with in vivo resistance for non-CNS pneumococcal infections, thereby decreasing the necessity of using an antibiotic other than penicillin. Findings such as these informed the CLSI decision to change the breakpoints in 2008 to better mirror the clinical effectiveness of penicillin for non-CNS pneumococcal infections 25. Our study supports the rationale behind the decision of the CLSI, as we found an association between penicillin resistance and penicillin prescribing.
The Centers for Disease Control and Prevention reported that the number of non-meningitis pneumococcal isolates categorized as resistant decreased from 10.3% to 1.2% using the 2008 CLSI breakpoints 7. Given that only high levels of resistance seemed to influence prescribing practices, this relatively low level of resistance under the new breakpoints should influence physicians to prescribe narrow spectrum antibiotics to treat S. pneumoniae. In previous studies, however, clinicians typically used antibiograms to prescribe broader spectrum empiric therapy and continued broad spectrum antibiotic therapy even when the bacteria were identified as susceptible to narrower spectrum antibiotics 26. This limited use of a potentially powerful tool contributes to the public health problem of antibiotic resistance.
CLSI determines breakpoints by reviewing the MICs, the pharmacokinetic and pharmacodynamic information for each antimicrobial/pathogen combination, and the data from clinical trials or well documented case series 27. The site from which the isolate originates (e.g. blood, respiratory secretions, CNS) is not always taken into account when developing the breakpoints. Therefore the breakpoints do not always accurately reflect the potency of the antimicrobial in inhibiting the growth of the infecting pathogen at specific sites of infection.
CLSI breakpoints that define the interpretative categories in antibiograms must align with clinical outcomes as they influence the choice of empiric therapy. Hospital antibiograms are known to overestimate community-level drug resistance and may, in the community setting, prompt broad spectrum prescribing. Urinary tract infections, predominantly caused by Eschericha coli, are example of how breakpoints determined in vitro may contribute to broad spectrum antibiotic prescribing 28. This “false” equating of drug resistance with clinical treatment failure promotes a culture of broad spectrum antimicrobial prescribing for pathogens that are otherwise susceptible to narrower spectrum drugs in clinical settings. Urinary tract infections, however, also offer an opportunity for intervention whereby aligning in vitro susceptibility results with clinical outcomes could encourage narrower spectrum antibiotic use.
This study had several limitations. First, there is no information on the patients from whom these isolates were obtained and reported in the antibiograms. Variability in reported susceptibility patterns across hospitals may be due in part to the differences in the underlying patient populations and in part to the number of isolates used to determine susceptibility patterns at each hospital 5. Antibiograms may overestimate community-level resistance because isolates are obtained from patients with specific indications for invasive testing and from patients with chronic medical conditions and consequently greater antibiotic exposure. Better measures of community-level resistance and better diagnostic tests to identify the cause of CAP in the emergency department or hospital settings are needed.
Second, we assumed that the proportion of resistant or non-susceptible pneumococcal isolates reported by each hospital was the only measure disseminated to physicians and, consequently, to influence their prescribing practices. The outlier hospital in our study reported 46% of pneumococcal isolates to be non-susceptible to penicillin. However, this hospital had the highest proportion of narrow-spectrum antibiotic prescribing (69%) across all hospitals. Therefore, there may be other determinants that affect antimicrobial prescribing such as hospital policies to direct prescribing (e.g. formulary restriction or prior authorization required), the preference of antibiotic in each subspecialty, and the dynamic and expertise of the team of health professionals providing care 29.
Third, the use of ICD-9 codes to identify patients with CAP may result in misclassification of disease. However, the ICD-9 CM codes used in this study are similar to previous studies that have shown a relatively high sensitivity and specificity for identifying CAP compared with medical record review 30, 31. Additional criteria that likely increased the specificity of these algorithms included restriction of the cohort to those receiving antibiotics conventionally used to treat CAP in children on the first day of hospitalization and exclusion of children with comorbid conditions. While this approach may exclude some previously healthy children with CAP, such as those with delayed recognition of CAP, these exclusions likely have a negligible influence on the overall estimates produced from this analysis. Children who received non-conventional antibiotics accounted for less than 7% (n=369) of the original cohort.
Lastly, limitations exist in the use of multi-level analysis. The fixed variables available for this analysis may not have accounted for all the different factors that drive a physician’s prescribing practice (i.e. the patient’s medical history). However, these unmeasured variables are by default incorporated into the random intercept in the model and could also explain some of the variability that was seen in the model 14.
In conclusion, high levels of resistance reported in an antibiogram were associated with broad spectrum empiric antibiotic therapy. The decision by the Clinical Laboratory Standards Institute to modify penicillin breakpoints for S. pneumoniae, the most common bacterial cause of childhood pneumonia, was based on considerable supporting clinical and microbiologic data. However, few empiric data were available to suggest that physicians are responsive to the antibiotic susceptibility patterns and that the breakpoint changes would actually change prescribing behavior. Our study suggests that physician’s response to the penicillin-resistant pneumococcal antibiotic susceptibility patterns may result in improved antibiotic prescribing in light of the recently modified MIC breakpoints for S. pneumoniae.
ACKNOWLEDGEMENTS
We thank the following investigators for contributing pneumococcal susceptibility data used in this study: Fariba Asghari, Rady Children’s Hospital, San Diego, CA; Elaine B. Dowell, Children’s Hospital, Aurora, CO; Rangaraj Selvarangan, Children’s Mercy Hospital, Kansas City, MO; Anami Patel, Le Bonheur Children’s Medical Center, Memphis, TN; Joseph Campos, Children’s National Medical Center, Washington, D.C.; Robert Wadowsky, Children’s Hospital of Pittsburgh, Pittsburgh, PA; Cindy Maurer, Akron Children’s Hospital, Akron, OH; Keith Zucker, Children’s Hospital Central California, Madera, CA; Caroline Tyndall, Children’s Hospital of The King’s Daughter, Norfolk, VA; Kathleen Eisenach, Arkansas Children’s Hospital, Little Rock, AR; Dennis Kmetz, Children’s Hospital of Orange County, Orange, CA; Sylvia Cuate, Driscoll Children’s Hospital, Corpus Christi, TX; Maria Staeheli, Miami Children’s Hospital, Miami, FL; Mario Marcon, Nationwide Children’s Hospital, Columbus, OH; Linda Snow, Cook Children’s Medical Center, Fort Worth, TX; Kathy Smith, Children’s Hospital of New Orleans, New Orleans, LA; Jane Schilleci, The Children’s Hospital of Alabama, Birmingham, AL; Jeanette Manley, Children’s Hospital and Medical Center, Omaha, NE; Karen Sue Kehl, Children’s Hospital of Wisconsin, Milwaukee, WI; Vera Concho, Texas Children’s Hospital, Houston, TX; Joel E. Mortensen, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Gregory A. Storch, Washington University School of Medicine/St. Louis Children’s Hospital, St. Louis, MO; Robert C. Jerris, Children’s Healthcare of Atlanta, Atlanta, GA; Xuan Qin, Seattle Children’s Hospital, Seattle, WA; Karin L. McGowan, Children’s Hospital of Philadelphia, Philadelphia, PA; Patricia Ackerman, Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN; Hossein Salimnia, Children’s Hospital of Michigan, Detroit, MI; Charles Stratton, Vanderbilt Children’s Hospital, Nashville, TN; Phyllis Della-Latta, Children’s Hospital of New York-Presbyterian, New York, NY; Shari Young, Children’s Medical Center of Dallas, Dallas, TX; Kristie Vetterli, Children’s Hospital and Research Center Oakland, Oakland, CA; Pravin H. Patel, Women and Children’s Hospital of Buffalo, Buffalo, NY; Eileen Gorss, Children’s Hospital Boston, Boston, MA
Financial disclosure: This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [grant number K01 A173729] and the Robert Wood Johnson Foundation under its Physician Faculty Scholar program for Dr. Shah.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
No conflict of interest exists for any of the authors.
REFERENCES
- 1.Nugent R, Back E, Beith A. The Race Against Drug Resistance. Washington D.C.: Center for Global, Development; 2010. [Google Scholar]
- 2.Peters NK, Dixon DM, Holland SM, Fauci AS. The research agenda of the National Institute of Allergy and Infectious Diseases for antimicrobial resistance. J Infect Dis 2008;197:1087–93. [DOI] [PubMed] [Google Scholar]
- 3.(IDSA) IDSoA. Bad bugs, No drugs. Alexandria, VA; 2004. [Google Scholar]
- 4.Davey P, Brown E, Fenelon L, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients (Review). The Cochrane Library 2009. [DOI] [PubMed] [Google Scholar]
- 5.Lacy MK, Klutman NE, Horvat RT, Zapantis A. Antibiograms: New NCCLS Guidelines, Development, and Clinical Application. Hospital Pharmacy 2004;39:542–53. [Google Scholar]
- 6.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement.. Wayne, PA: Clinical Laboratory Standards institute; 2011. [Google Scholar]
- 7.Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae--United States, 2006–2007. MMWR Morb Mortal Wkly Rep 2008;57:1353–5. [PubMed] [Google Scholar]
- 8.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA 2008;299:2048–55. [DOI] [PubMed] [Google Scholar]
- 9.Shah SS, Hall M, Srivastava R, Subramony A, Levin JE. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis 2009;49:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feigin R, Cherry J, Demmler-Harrison G, Kaplan S, eds. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Philadelphia: Saunder S. Elsevier; 2009. [Google Scholar]
- 11.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics 2001;107:E99. [DOI] [PubMed] [Google Scholar]
- 12.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- 13.Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology;21:467–74. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian SV, O’Malley AJ. Modeling neighborhood effects: the futility of comparing mixed and marginal approaches. Epidemiology;21:475–8; discussion 9–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raudenbush SW, Bryk AS. Hierarchical linear models : applications and data analysis methods. 2nd ed. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- 16.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- 17.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- 18. Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. New York: Churchill Livingstone; 2008. [Google Scholar]
- 19.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006;60:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen K, Petersen JH, Budtz-Jorgensen E, Endahl L. Interpreting parameters in the logistic regression model with random effects. Biometrics 2000;56:909–14. [DOI] [PubMed] [Google Scholar]
- 21.Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children’s hospitals. Pediatrics;126:1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu VL, Chiou CC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis 2003;37:230–7. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso MR, Nascimento-Carvalho CM, Ferrero F, et al. Penicillin-resistant pneumococcus and risk of treatment failure in pneumonia. Arch Dis Child 2008;93:221–5. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein MP, Klugman KP, Jones RN. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin Infect Dis 2009;48:1596–600. [DOI] [PubMed] [Google Scholar]
- 26.Saha SK, Darmstadt GL, Baqui AH, et al. Rapid identification and antibiotic susceptibility testing of Salmonella enterica serovar Typhi isolated from blood: implications for therapy. J Clin Microbiol 2001;39:3583–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen JH. Who defines resistance? The clinical and economic impact of antimicrobial susceptibility testing breakpoints. Semin Pediatr Infect Dis 2004;15:105–8. [DOI] [PubMed] [Google Scholar]
- 28.Khawcharoenporn T, Vasoo S, Ward E, Singh K. High rates of quinolone resistance among urinary tract infections in the ED. Am J Emerg Med. [DOI] [PubMed] [Google Scholar]
- 29.Hulscher ME, Grol RP, van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. In: Lancet Infect Dis. 2010/02/27 ed:167–75. [DOI] [PubMed] [Google Scholar]
- 30.Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM, Breiman RF. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol 1999;149:282–9. [DOI] [PubMed] [Google Scholar]
- 31.Whittle J, Fine MJ, Joyce DZ, et al. Community-acquired pneumonia: can it be defined with claims data? Am J Med Qual 1997;12:187–93. [DOI] [PubMed] [Google Scholar]