Abstract
Objectives:
Cigarettes vary in rod length but are generally thought of as a constant unit. In this study, we evaluated whether the rod length of participants’ usual brand cigarettes affected their perceptions and smoking habits when switching to SPECTRUMs.
Methods:
Data were analyzed for 341 participants smoking their own brand cigarettes for one week and after switching to normal nicotine content (11.6 mg) SPECTRUMs for 2 weeks. Changes in perceptions of cigarette attributes and biomarkers of smoke exposure were evaluated using linear mixed models among 3 groups: usual length short (ULS, 72 mm); medium/king (ULM, ~84 mm); and long (ULL ≥ 100 mm).
Results:
Among the 3 cigarette length groups, only ULL smokers’ rated SPECTRUMs significantly less strong, harder to draw, lower in taste, and lower in enjoyment (p < .03) compared to usual brand. Among all groups, satisfaction was significantly lower for SPECTRUMs (p < .02). Cigarettes per day (CPD) increased significantly more for ULL (+4.75 CPD) as compared to ULM (+1.38 CPD) (p < .001). When switching to SPECTRUMs, cotinine-per-cigarette decreased among all groups, and exhaled carbon monoxide increased significantly in ULL and ULM smokers (p < .001).
Conclusion:
People who smoked long cigarettes had the largest changes in perceptions and use when switching to SPECTRUM research cigarettes.
Keywords: addiction, cigarette rod length, health behavior, healthcare policy, perceptions, reduced nicotine content, SPECTRUM cigarettes
Public health in the United States (US) continues to be threatened by the use of tobacco products, with over 480,000 Americans dying every year from tobacco-related illnesses. Approximately 44% of cigarette smokers have a high school degree or less, 25% live below the poverty level, and over 50% experience psychological distress, all of which help contribute to US health disparities.1
Nicotine addiction is considered the primary reason for continued tobacco use,2,3 and cigarette smoking provides the fastest delivery and most addictive form of nicotine self-administration.4,5 Benowitz and Henningfield2 hypothesized that if the nicotine content in cigarettes was below the threshold for addiction, the prevalence of cigarette smoking and tobacco-related morbidity and mortality would be reduced. There is evidence that reducing nicotine levels in cigarettes to a very low level can improve the overall health of the nation by reducing tobacco-related mortality.6
To reduce nicotine addiction and the harms associated with tobacco use, the US Food and Drug Administration’s (FDA) Center for Tobacco Products (CTP) has the authority to set tobacco product standards7 and funds studies to provide a scientific evidence base for regulatory activities.8 These studies use manufactured research cigarettes that have controlled levels of nicotine7–10 and allow researchers to measure the impact of differing levels of nicotine on use, perception, and dependence.9,10
To perform this research, SPECTRUM brand cigarettes (SPECTRUMs) (22nd Century Group, Inc) are provided through the National Institute of Drug Abuse (NIDA) Drug Supply Program3 and have varying nicotine contents (0.3 mg/g −16.5 mg/g) and flavors (regular and menthol), but little variance in length (~84 mm) or tobacco weight (640–700 mg per cigarette).11 However, smokers choose cigarette brands in the natural marketplace with varying rod lengths that can affect their nicotine exposure and dependence.12
Traditionally, measures of time to first cigarette (TTFC) and cigarettes smoked per day (CPD) are used by researchers as a standard measurement for nicotine addiction or dependence. For instance, the Heaviness of Smoking Index asks: “On average, how many cigarettes do you smoke each day?”13,14 In addition to TTFC, CPD is predictive of whether smokers are able to quit during cessation treatment. As CPD goes up, a smoker is less likely to be successful at quitting; therefore, this measurement is thought to represent the smoker’s degree of addiction or nicotine dependence.14
In studies that use CPD as a dependence measure, the effect of cigarette length on smoking behaviors and nicotine dependence is generally not accounted for. The standard measurement of CPD assumes that all cigarettes are similar enough to be counted as equal.13,15 It does not account for the differences in an individual’s cigarette preference, such as rod length. The US Federal Trade Commission (FTC) report of 201412 found that the percent of domestic market share of cigarettes sold by length was 3% short (68 mm - 72 mm), 57% king/medium (79 mm - 88 mm), 38% long (94 mm - 101 mm), and 1% ultralong (110 mm - 121 mm). Two years later, the 2016 report showed the percentage of long length cigarettes increased to 40%.16 With the sale of long rod length cigarettes increasing, researchers should consider the impact that cigarette length may have on smokers’ perceptions and smoking behaviors when switching to a research cigarette with a different rod length.
The length of the cigarette is not just a physical characteristic. Rather, longer cigarettes contain more tobacco by weight17 which has implications for biomarkers of tobacco exposure and consumption in addition to behavior patterns. For instance, researchers found significantly higher levels of serum cotinine and urinary NNAL in smokers of long/ultralong cigarettes compared to king sized cigarettes.18 In a study comparing king size to long length cigarettes, the authors observed that long (100 mm) cigarettes contained more tobacco filler and menthol per cigarette across several brands (Marlboro, Newport, Kool, GPC) and higher nicotine yield.19 In addition, Borland and Savvas20 found that observable aspects of the cigarette design can affect the smoker’s perception of the cigarette. The majority of participants rated the medium size (~84 mm) cigarette as most attractive, of highest quality, and most preferred. Long length cigarettes (100 mm) were ranked highest in taste.20 Preferred cigarette brand can also influence perceptions of harshness and harm among smokers.21,22
If there is a difference in cigarette perceptions, behavior patterns, and exposure among cigarette lengths (ie, a longer cigarette equals more tobacco and cotinine exposure), then this could be a factor that impacts the goals and outcomes of studies of reduced nicotine content cigarettes (RNC) including exposure reduction, adherence, and ultimately, policy regarding the manufacturing of reduced nicotine content cigarettes. The FDA announced this past year that it is taking steps to mandate reducing the nicotine content in commercially available cigarettes.23 Currently, there is only one rod length of reduced nicotine content research cigarettes available; however, this single-length design is being used to simulate the effects of reduced nicotine content cigarettes on smoking populations when switching to a research-issued design.
Specific to smokers of commercial cigarettes in various sizes, we wanted to see if cigarette rod length affected perceptions of the cigarette, smoking habits, and measurements of exposure when switching to normal nicotine content (NNC) king size SPECTRUMs. Therefore, the purpose of this study was to compare smokers’ subjective ratings and exposure levels when using SPECTRUM cigarettes to their usual brand, and to determine if usual brand rod length had an effect on these measurements. The effects were evaluated by comparing the differences before and after switching to SPECTRUMS among the following groups: (1) all 3 usual brand rod length groups; (2) long length versus medium; and (3) long length versus short.
METHODS
This report combined data from 2 similarly designed double-blind, randomized controlled trials intended to evaluate the effects of gradually switching to RNC cigarettes. Project 1 (P1) included smokers of low socioeconomic status (less than 16 years of education) and Project 2 (P2) included smokers with mood and/or anxiety disorders. Complete details of the trial designs and methods for data collection are reported elsewhere,9,10 but the procedure is summarized here. Briefly, adult smokers (aged 18–65) of at least 5 CPD not interested in quitting in the next 6 months were recruited for an 8-month trial.
The focus of this study included the first 3 visits in the trials. During Visit 1 (V1) participants completed the baseline assessment for eligibility. Participants returned to the study for 2 more visits, one week later after smoking their usual brand cigarettes (Visit 2 [V2]) then 2 weeks later (Visit 3 [V3]) after smoking NNC SPECTRUMs. Smokers’ perceptions of SPECTRUMs were evaluated by the favorability rating scales, and their exposure levels were measured by CPD, cotinine per cigarette, and exhaled carbon monoxide (eCO). We compared these measures at baseline for 3 different rod length usual brand cigarettes groups (ULS, ULM, ULL) to the same measures after 2 weeks on one rod length SPECTRUMs.
At V1, screening for eligibility took place and consent was signed. Participants were instructed to smoke their own brand of cigarettes for one week and record what they smoked. At V2, participants were provided with a 2-week supply of the NNC SPECTRUMs at 150% of the average CPD reported on their V2 cigarette log to account for any potential increases in cigarette consumption or rescheduling of the visit. The NNC SPECTRUMs have one rod length (~84 mm), a nicotine level around 11.6 mg per cigarette, contain just under 700 mg of tobacco, and come in 2 flavors, non-menthol or menthol.24,25 The research cigarettes were provided free of charge, and the participants were asked to return all used and unused packs to the study clinic at their next visit.
The 2-week baseline period on NNC SPECTRUMs was designed to evaluate the participants’ ability to use the SPECTRUMs in place of their usual brand cigarettes. Participants were informed during the consent process at V1, and again at V2 when they received NNC SPECTRUMs, that they contain a similar amount of nicotine to their own brand cigarettes. This 2-week trial period also evaluated their ability to continue with the study before entering the randomization phase.
Measures
Demographics, cigarette characteristics and nicotine dependence.
Basic demographic information was self-reported by participants at the assessment visit (V1). Participants were asked about characteristics of their usual brand cigarettes such as cost per pack (US dollars), cigarette rod length [short (ULS, 72 mm), medium/king (ULM, 84 mm), or long (ULL, ≥100 mm)], flavor (menthol or non-menthol), and whether they bought their cigarettes by the pack, carton, or rolled their own. Participants were asked to bring their usual brand cigarette pack to V1 to allow the researcher to document the characteristics of commercial brand, flavor, and length. Nicotine dependence was assessed at each visit using the Penn State Cigarette Dependence Index (PSCDI) which is comprised of 10 items and has a total score ranging from 0–20 (0–3 not dependent, 4–8 low dependence, 9–12 medium dependence, 13+ high dependence).26
Cigarette rating scales.
Cigarettes were rated subjectively by participants on 2 scales at both V2 (own brand) and V3 (SPECTRUMs). The first scale, the modified Cigarette Evaluation Scale (mCES),27 yields 5 subscales from a 12-item questionnaire. The participant completes the questionnaire using a Likert scale (1 = not at all to 7 = extremely). The 5 subscales (and any items asked to interpret the scales) are as follows: (1) Psychological Reward (calm down, more awake, less irritable, help concentrate, reduce hunger); (2) Smoking Satisfaction (cigarette satisfying, tasting good, and enjoyment in smoking); (3) Aversion (dizziness, nauseous); (4) Enjoyment of Respiratory Tract Sensations; and (5) Craving Reduction.
The second scale is the modified Cigarette Liking Scale (mCLS),28 which was modified from a Visual Analog Scale to a 10-point Likert scale (1 = not at all to 10 = extremely). The mCLS asks participants to rate the cigarettes they smoked in the past week in regard to strength, heat, draw, harshness, taste, satisfaction, and tobacco versus just air.
Cigarette logs, CPD, other tobacco products.
A cigarette diary was given to each participant at all visits to log daily cigarette use. This diary is a paper log designed to fit on the front of a cigarette pack with a pencil the size of a cigarette to be placed in the cigarette pack. Participants also were asked to log any non-cigarette tobacco products used in the past 7 days at each visit. At V2 and V3 the researcher asked the participant if they used any other nicotine containing products including cigars, pipes, snus, snuff, dip, chew, electronic cigarettes, hookah, water pipe, dissolvable tobacco (lozenge, strips, sticks), marijuana, nicotine patch, gum, nasal spray, lozenge or inhaler.
Biomarkers, eCO, and cotinine.
Exhaled carbon monoxide (eCO, in ppm) was measured at each visit using the Bedfont Pico+ Smokerlyzer device (Bedfont Scientific Ltd, Kent, UK). Plasma cotinine levels were measured at V2 and V3 using a commercially available competitive enzyme-linked immunoassay (ELISA) kit from Calbiotech (El Cajon, CA). At the time of this report, plasma cotinine was available on a subset of 246 participants. Those who attended V2 and had plasma cotinine >15 ng/mL were included in this analysis.
Study data were collected and managed using Research Electronic Data CAPture (REDCap) electronic data capture tools.29 The following 3 sites participated in the clinical trials: (1) Penn State University (Hershey, PA; Project 1 and Project 2); (2) George Washington University (Washington, DC; Project 1); and (3) Massachusetts General Hospital (Boston, MA; Project 2).
Participant withdrawals and dropouts.
Participants who reported not smoking SPECTRUMs, not wanting to continue in the study, or who were lost to follow-up from V1 to V3 did not remain eligible to participate and were withdrawn from the study. In addition, participants were withdrawn if they smoked >10% non-research cigarettes, re duced their cigarette consumption by >50% of their average CPD (calculated from V2), or used other tobacco products at 2 or more time points during the baseline phase (first 3 weeks of the study) because they were considered to be unable to use only the SPECTRUMs. These groups were examined to investigate if the usual brand cigarette length was the cause of their dropout or withdrawal.
Data Analysis
Data analysis was done in R version 3.5.1 (https://www.r-project.org/). Demographic and cigarette characteristics overall and by usual brand rod length were used to describe the sample by calculating percentages, means, and standard deviations. Chi-square tests and one-way ANOVAs were used appropriately to determine the differences among the smokers by the 3 different usual brand rod lengths (ULS, ULM, ULL) on usual participant baseline characteristic variables.
Separate linear mixed effect regression models were used to determine the effects of cigarette length after controlling for demographic and cigarette characteristics on the outcomes of interest, which includes the subjective ratings on the mCLS items and mCES subscale scores, CPD, eCO, and cotinine per cigarette. A random intercept for each participant and random slope for visit were added to the regression models to take into account the correlated structure for each individual over different visits. No underlying structure of the correlation matrix was assumed. To account for any possible differences between the projects, a project variable (P1 vs P2) was added in the linear mixed models.
An interaction term between the visits and usual brand cigarette length was added in each model to estimate additional changes in the independent outcome variables that occurred at each visit. This particular model formulation enabled us to test for appropriate interactions (ie, if any of the effects were different among the 3 groups). Parameter estimation was done based on Restricted Maximum Likelihood (REML). From the regression models the mean scores and standard errors for each outcome variable were estimated when smoking usual brand cigarettes (V2) and after 2 weeks on NNC SPECTRUMs (V3).
To examine for statistically significant changes in outcomes from usual brand cigarettes to NNC SPECTRUMs, and to assess for group differences in these changes by usual brand rod length, Wald tests were used for the inference on fixed-effect parameters. In general, the Wald tests addressed whether there is a statistically significant difference between usual brand cigarettes and NNC SPECTRUMs for the 3 different usual brand rod length groups separately within each visit and between the visits (test of interaction). False Discovery Rate (FDR) was used to correct the p-values for multiple comparison error.
All of the results (eg, associations, estimated beta coefficients, estimated standard errors and p-values) for the longitudinal variables (mCES, mCLS, CPD, eCO, and cotinine per cigarette) were obtained from the linear mixed regression models and the corresponding Wald tests. In all of the analyses, 2-tailed p-values less than .05 were considered statistically significant.
RESULTS
Table 1 presents participant baseline demographics and cigarette characteristics, overall and by usual brand cigarette length. Overall, most participants were female (54.3%), white (68%), and had more than a high school education (56.6%). Most participants smoked usual length long (ULL) (55.1%) and menthol flavored (58.7%) cigarettes. The mean age of the participants was 44 years (SD = 12.1, range: 19–65), but there was a statistically significant difference in age among the 3 usual cigarette length groups (p < .001), with the ULL group having the oldest mean age at 46.5 years (p-value corrected by FDR). The majority of ULL smokers were black (77%) and 42.7% of ULL smokers reported a household income of less than < $20,000 per year. Although many of those who reported low income smoked ULL, the average reported cost per pack of ULL cigarettes ($6.65) was higher than that of ULM cigarettes ($5.38). Also of note, 84% of our study population reported buying cigarettes by the pack or carton versus roll-your-own.
Table 1.
Study Participant Demographic and Smoking Characteristics: Overall and by Usual Brand Cigarette Rod Length
| Overall (N = 341) |
Usual Length Short (ULS) (4.7%, N = 16) |
Usual Length Medium/ King (ULM) (40.2%, N = 137) |
Usual Length Long (ULL) (55.1%, N = 188) |
p-value | |
|---|---|---|---|---|---|
| Male, % (N) | 45.8 (156) | 56.3 (9) | 47.5 (65) | 43.6(82) | .19a |
| Mean age (SD, range), years | 44.0 (12.1, 19–65) | 38.6 (11.3, 19–56) | 41.2 (12.0,20–65) | 46.5 (11.7, 19–65) | < .001a |
| Race, % (N) | |||||
| White | 68.0 (232) | 81.3 (13) | 81.0 (111) | 57.5 (108) | < .001a |
| Black | 24.3 (83) | 12.5 (2) | 12.4 (17) | 34.0 (64) | < .001a |
| Other | 7.6 (26) | 6.3 (1) | 6.6 (9) | 8.5 (16) | .80a |
| Education, % (N) | |||||
| ≤ High school | 43.4 (148) | 56.3 (9) | 38.0 (52) | 46.3 (87) | .03a |
| > High school | 56.6 (193) | 43.8 (7) | 62.0 (85) | 53.7 (101) | .03a |
| Income level, % (N) | |||||
| $0-$19,999 | 38.1 (102) | 38.5 (5) | 26.1 (29) | 47.2 (68) | .008a |
| $20,000-$39,999 | 35.5 (95) | 38.5 (5) | 38.7 (43) | 32.6 (47) | .60a |
| $40,000+ | 26.5 (71) | 23.1 (3) | 35.1 (39) | 20.1 (29) | .04a |
| Menthol, % (N) | 58.7 (200) | 50.0 (8) | 40.2 (55) | 72.9 (137) | < .001a |
| Cigarette type, % (N) | |||||
| Pack/carton | 84.8 (289) | 93.8 (15) | 71.5 (98) | 93.6 (176) | < .001a |
| Roll-your-own | 15.3 (52) | 6.3 (1) | 28.5 (39) | 6.4 (12) | < .001a |
| Mean cost per pack (SD, range), USD | 6.14 (2.55, 0.24–11.00) | 6.69 (2.21, 1.50–10.40) | 5.38 (2.90, 0.24–11.00) | 6.65 (2.15, 0.48–11.00) | < .001a |
| Mean PSCDI at V2 (SD, range) | 13.4 (3.4, 3.0–20.0) | 13.7 (3.1, 9.0–19.0) | 13.2 (3.4, 5.0–20.0) | 13.5 (3.4, 3.0–20.0) | .69a |
| Mean CPD at V2 (SD, range) | 19.5 (9.9, 1.3–60.0) | 22.5 (11.8, 7.3–45.6) | 21.0 (11.1, 1.3–60.0) | 18.1 (8.6, 2.2–56.6) | .88b |
| Mean eCO at V2 (SD, range), ppm | 28.8 (15.2, 4.0–100.0) | 30.7 (11.9, 10.0–58.0) | 29.6 (15.8, 4.0–100.0) | 28.0 (15.0, 4.0–85.0) | .65b |
| Mean plasma cotinine at V2 (SD, range), ng/ml | 291.0 (148.7, 18.1–918.0) | 324.2 (221.6, 131.4–918.0) | 290.0 (145.4, 23.7–812.4) | 289.2 (145.3, 18.1–708.0) | .33b |
| Mean cotinine per cigarette at V2 (SD, range), ng/ml | 16.78 (10.12, 2.28–84.05) [N=258] | 18.24 (9.35, 8.76–34.25) [N=11] | 15.72 (11.27, 2.28–84.05) [N=108] | 17.48 (9.19, 3.25–49.83) [N=139] | .37b |
| Withdrawn or lost to follow-up by V3, % (N) | 5.0 (17) | 0.0 (0) | 2.9 (4) | 6.9 (13) | .17b |
Note.
: Obtained appropriately from ANOVAs, chi-squares, and proportion test.
: Obtained from Linear Mixed models and Wald tests.
A p < .05 indicates statistically significant differences among the 3 groups.
Participants smoked an average of 19.5 (SD = 9.9) usual brand CPD, which was captured on logs from V1 to V2. Among all of the linear mixed effects models (5 models for mCES, 7 models for mCLS and 3 models for biomarkers), the project variable was marginally significant for only one model (Smoking Satisfaction, p = .04), implying considerably high consistency in data across projects.
Cigarette Rating Scales (mCES and mCLS)
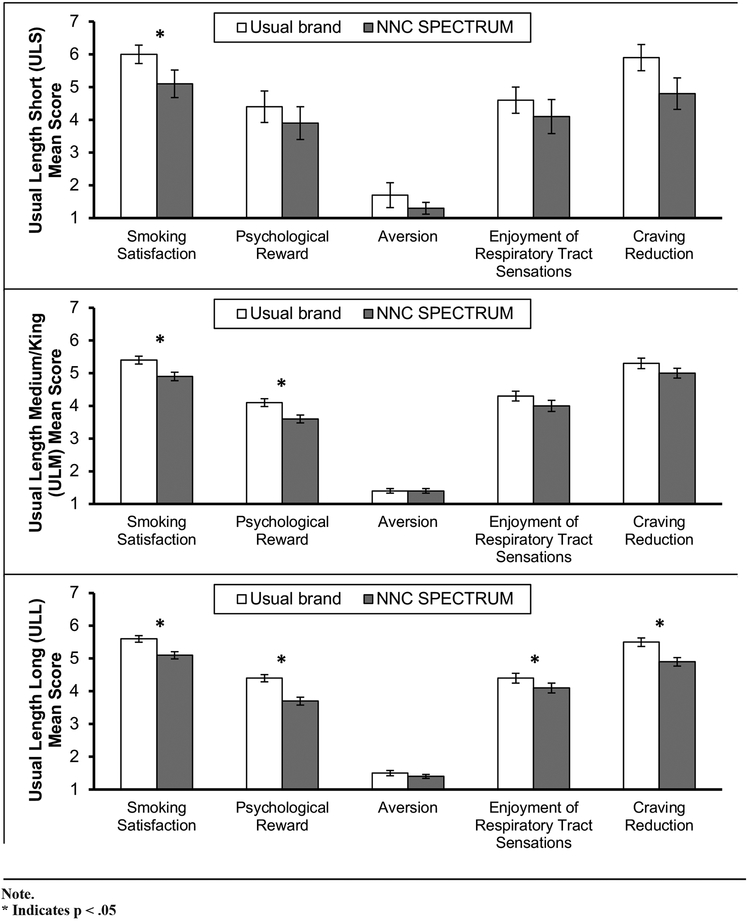
Figure 1 presents estimated mean scores and standard errors (SE) for each of the mCES sub-scales while smoking usual brand cigarettes (V2) and while smoking NNC SPECTRUMs (V3).
Figure 1.
Modified Cigarette Evaluation Subscale (mCES) Mean Scores by Usual Brand Rod Length: Usual Brand (Visit 2) versus Normal Nicotine Content (NNC) SPECTRUM (Visit 3)
There were no statistically significant differences in the mean mCES scores among the 3 usual brand cigarette length groups at V2. However, from V2 to V3, the overall ratings of NNC SPECTRUMs, combining all groups, decreased significantly for Smoking Satisfaction, Psychological Reward, Enjoyment of Respiratory Tract Sensations, and Craving Reduction (all ps < .03) after controlling for all other predictors.
Based on the regression models, there was a significantly positive relationship between nicotine dependence (PSCDI) and all the subscales of mCES except Aversion (p < .01) at V2 for usual brands. In the case of Smoking Satisfaction, the mean score significantly decreased from V2 to V3 for all the 3 usual brand cigarette length groups (p < .01). For Psychological Reward, the mean score decreased from V2 to V3 for all the 3 usual brand cigarette length groups, but the decrease was significant only for ULM and ULL (p < .001). Even though there was a decrease of Enjoyment of Respiratory Tract Sensations and Craving Reduction from V2 to V3 in all 3 groups, the only statistically significant decrease was in ULL (p < .05). There were no significant group differences in the mCES outcomes among the groups at V3. The amount of change from V2 to V3 for any of the mCES scores was not significantly different among the 3 groups after adjusting the p-values for multiple comparison error by FDR.
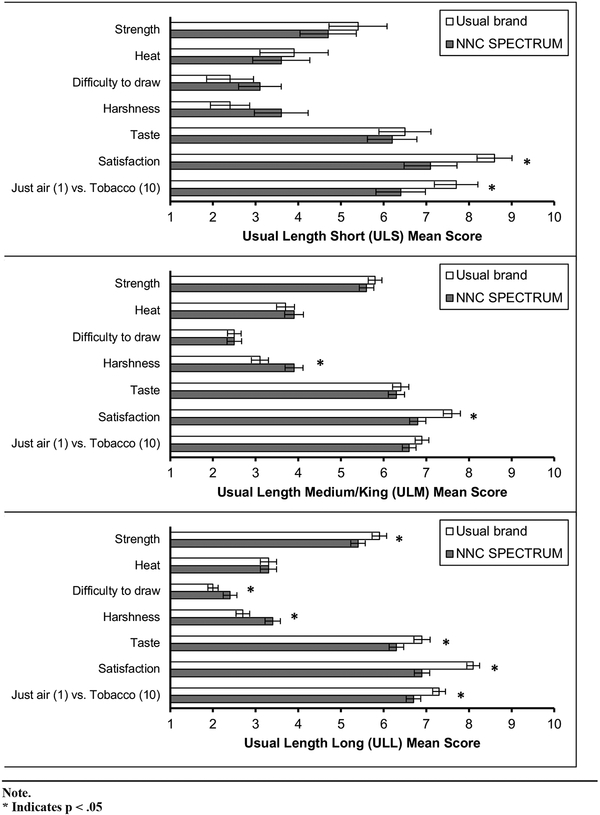
At V2, there was no statistically significant difference in the mean mCLS scores among the 3 usual brand cigarette length groups except for difficulty to draw, where there was a significant difference between ULL and ULM groups (p = .02). Figure 2 shows the estimated mean scores and SE of the mCLS questions between V2 and V3 for each usual brand cigarette length group.
Figure 2.
Modified Cigarette Liking Scale (mCLS) Mean Scores by Usual Brand Rod Length: Usual Brand (Visit 2) versus Normal Nicotine Content (NNC) SPECTRUM (Visit 3)
Note that there was a statistically significant decrease in the mean score of satisfaction between V2 and V3 for all the 3 groups (p < .05). Overall, for each usual brand cigarette length group, there was a decrease in the mean scores between V2 and V3 for strength, taste, satisfaction, and tobacco versus just air. Meanwhile, there was an increase in the mean score for difficulty to draw and harshness, even though these changes were not statistically significant in all the groups. The patterns of change from V2 to V3 were similar in direction for all 3 groups. At V3, there was no statistically significant difference among the 3 usual brand cigarette length groups on the mCLS items. Similarly, as with the mCES scores, the amount of change from V2 to V3 for any of the mCLS scores was not significantly different among the 3 groups after adjusting the p-values by FDR.
CPD, Cotinine per Cigarette, and eCO
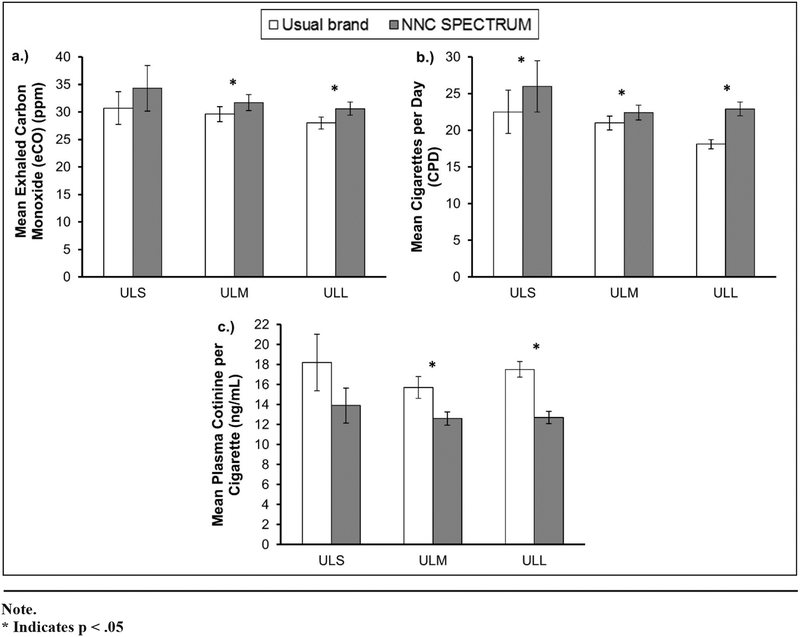
Table 2 is separated by cigarette length categories and compares mean CPD, plasma cotinine per cigarette, and eCO at V2 on usual brand cigarettes versus at V3 on NNC SPECTRUMs. Figure 3 illustrates the means and SE.
Table 2.
Mean Cigarettes per Day (CPD), Plasma Cotinine per Cigarette, and Exhaled Carbon Monoxide (eCO) by Usual Brand Rod Length (Visit 2) versus Normal Nicotine Content (NNC) SPECTRUM (Visit 3)
| Usual Brand | NNC SPECTRUM | p-value | |
|---|---|---|---|
| Usual Length Short (ULS) | |||
| Mean CPD (SD) | 22.5 (11.8) | 26.0 (14.0) | .01* |
| Mean plasma cotinine per cigarette (SD) | 18.2 (9.4) | 13.9 (5.5) | .05 |
| Mean exhaled CO, in ppm (SD) | 30.7 (11.9) | 34.3 (16.5) | .50 |
| Usual Length Medium/King (ULM) | |||
| Mean CPD (SD) | 21.0 (11.1) | 22.4 (11.5) | .001* |
| Mean plasma cotinine per cigarette (SD) | 15.7 (11.3) | 12.6 (6.7) | <.001* |
| Mean exhaled CO, in ppm (SD) | 29.6 (15.8) | 31.7 (16.9) | <.001* |
| Usual Length Long (ULL) | |||
| Mean CPD (SD) | 18.1 (8.6) | 22.9 (12.5) | <.001* |
| Mean plasma cotinine per cigarette (SD) | 17.5 (9.2) | 12.7 (7.1) | <.001* |
| Mean exhaled CO, in ppm (SD) | 28.0 (15.0) | 30.6 (16.1) | <.001* |
Note.
Indicates p < .05 when comparing usual brand to NNC SPECTRUMs using Wald test from corresponding linear mixed models.
Figure 3.
Mean Exhaled Carbon Monoxide (eCO), Cigarettes per Day (CPD), and Cotinine per Cigarette by Usual Brand Rod Length: Usual Brand (Visit 2) versus Normal Nicotine Content (NNC) SPECTRUM (Visit 3)
At V2, there was no statistically significant difference between the usual brand cigarette length groups in terms of mean CPD; however, the mean CPD significantly increased for each of the 3 groups from V2 to V3 (p < .04). CPD increased significantly more for ULL as compared to ULM (+4.75 CPD vs +1.38 CPD, p < .001), but the rates of change in CPD were not significantly different between ULL and ULS or between ULM and ULS after controlling the p-values by FDR. At V3, there was a statistically significant difference in mean CPD among the 3 groups (p < .05).
There was no significant difference in mean cotinine per cigarette among the 3 groups at V2 or at V3 separately. For all the 3 groups, cotinine per cigarette decreased significantly from V2 to V3 (p < .03). But unlike CPD, the between-group comparison for decrease in cotinine per cigarette was not significantly different after controlling the p-values for multiple comparison error using FDR.
For eCO, there was no significant difference in the means among the 3 groups at V2 or at V3 separately; however, the mean eCO increased from V2 to V3 for all the 3 groups but was significant in only the ULM and ULL groups (p < .02). Similar to cotinine per cigarette, the amount of change in eCO from V2 to V3 was not significantly different among the 3 groups. The direction of change from V2 to V3 for the mean CPD, mean eCO, and mean cotinine per cigarette was similar for all the 3 usual brand cigarette length groups.
Participant Withdrawals and Dropouts
Participant dropout data were examined to investigate if usual brand cigarette length may have affected dropout or withdrawal rates. In total, 5% (17/341) of participants left the study by V3. Of these 17 participants, 13 were in ULL, 4 were in ULM, and none were in ULS. Although most of the participants who dropped out of or withdrew from the study prior to V3 smoked usual brand long rod length, a multinomial proportion test found no evidence of significant difference between ULS, ULM, and ULL proportions of dropouts due to lack of power.
DISCUSSION
The purpose of this study was to determine if the rod length of participants’ usual brand cigarettes affected their evaluation and use of the single rod length NNC SPECTRUMs during a 3-week baseline period in 2 randomized controlled clinical trials.9,10 Like Veldheer et al30 who found that menthol and non-menthol SPECTRUMs were acceptable to smokers in randomized trials, we found that smokers of varying usual brand cigarette rod lengths will use the single rod length NNC SPECTRUMs in a research trial, based on subjective ratings and use of the SPECTRUMs.
It is interesting to consider that cigarettes are typically thought of as constant units (eg, the pack-year concept) and that the extra layer of variability that accompanies differing cigarettes lengths is not typically addressed. However, cigarette length is inherently tied to cigarette design.31 For example, Richter et al11 defined the tobacco mass of the SPECTRUM 600 cigarette (non-menthol) to be 693 mg and that of the SPECTRUM 601 cigarette (menthol) to be 657 mg. Comparing medium/king length SPECTRUMs to a popular brand’s long length cigarette, such as Marlboro Red, the long length Marlboro cigarette contains much more tobacco by weight at 780 mg per cigarette.32 Cigarette design has implications when measuring nicotine dependence, smoking behaviors such as CPD, smoking exposures when measuring biomarkers, and cigarette perceptions because not all cigarettes are alike.
We found that there were changes in participants’ biomarkers and subjective ratings for all 3 groups when switching from their usual brand to SPECTRUMs. In addition, most of the statistically significant changes from baseline occurred for the ULL group. The increase in CPD and changes in perception as ULL smokers switched to SPECTRUMs could likely be explained by the difference in rod length and tobacco weight between usual brand long cigarettes and the one rod length SPECTRUMs. This suggests that because SPECTRUMs contain less tobacco, when smokers of ULL cigarettes switched to SPECTRUMs, they required more CPD to maintain the same cotinine level they achieved while smoking their usual brand. This cigarette characteristic is important to consider in all research trials and we believe that researchers should think carefully about changes in measurements of dependence (CPD) and perceptions when cigarette rod length varies from usual brand to research cigarette.
Across all rod length groups, our study showed reduced favorability of SPECTRUMs compared to their usual brand. These changes in cigarette favorability most likely reflect a brand switching effect and may be due to the psychological response to the SPECTRUMs’ plain packaging, unfamiliarity, and universal rod length.20,21,33 Although this may not be a surprising finding, it is interesting to note that the ULL group had the most significant negative changes in strength, difficulty to draw, and taste. In addition, ULL also had the most significant negative changes in the likeability of the SPECTRUMs, rating them as significantly less favorable in 4 of the 5 categories compared to their usual brand cigarette. This suggests that rod length is an important factor in brand switching.
Hukkanen et al34 found that smokers will self-titrate their nicotine intake from cigarettes through puff frequency, intensity, and duration.34 We found that all groups had lower CPD at V2 compared to V3. These differences are likely related to the fact that the SPECTRUMs were provided at no cost,35 but it is noteworthy that the majority of significant changes were found in the ULL group. Although there was no difference between the 2 projects when choosing length of cigarette, we found that most people who chose to smoke ULL are of black race with lower household incomes. These characteristics show that there is some subject variability among the groups. Therefore, those conducting future research should document and take into account the rod length and tobacco weight in usual brand cigarettes as well as participant characteristics related to choice of usual brand cigarette length when switching smokers from ULL to medium length SPECTRUMs.
Limitations of this study include self-report of cigarettes per day, imprecise measurement of exactly how much of each cigarette was smoked (usual brand or research), and limited data on the specifications of nicotine content and tobacco weight of usual brand cigarettes. In addition, the 2 study groups were of low SES and with mental health disorders; although these populations make up a large proportion of current smokers, the results may not be generalized to smokers without these conditions. No cause-effect relationship can be inferred due to the cross-sectional design of the study. Self-reported cigarette consumption is subject to potential recall bias.
Conclusion
Smoking is not only driven by nicotine exposure but also smokers’ perceptions and patterns of use. We found that these effects changed when switching to a different rod length cigarette in low socioeconomic groups and smokers with psychological stress. Researchers should consider length of usual brand cigarettes when having smokers switch to single rod length SPECTRUMs. If very low nicotine cigarettes become available in the US, manufacturing them in varying rod lengths may help to reduce changes in favorability to the cigarettes and lessen compensatory behaviors. Although we may want to reduce favorability of cigarettes, considering the smoker’s perceptions when using a potential reduced nicotine content design may enhance their use of this type of cigarette.
Human Subjects Statement
The studies were approved by the Penn State Hershey, George Washington University, and Partners Human Research Committee (Massachusetts General Hospital) Institutional Review Boards. Written informed consent was obtained from all persons prior to their participation in the research.
Acknowledgements
Research reported in this publication is supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036107 and the Center for Tobacco Products of the US Food and Drug Administration (FDA). The REDCap tools used in this project were supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) through grant UL1TR000127 and TR00214. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
Conflict of Interest Statement
SMH, VM, CL, SV, JMY, SIA, NMK, JL, LR, JM, JPR, JEM, and KH have no disclosures to report related to this publication. AEE has received research grant support from Pfizer, Forum Pharmaceuticals, and GSK and has provided consultation to Pfizer and Reckitt Benckiser. JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience.
References
- 1.US Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults in the United States. Available at: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed December 13, 2017.
- 2.Benowitz N, Henningfield J. Establishing a nicotine threshold for addiction – the implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Drug Abuse. Nicotine Research Cigarettes Drug Supply Program. Available at: https://www.drugabuse.gov/nicotine-research-cigarette-drug-supply-program. Accessed December 12, 2017.
- 4.Benowitz N Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83(4):531–541. [DOI] [PubMed] [Google Scholar]
- 5.Foulds J, Ramstrom L, Burke M, Fagerstrom K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob Control. 2003;12(4):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apelberg BJ, Feirman SP, Salazar E, et al. Potential public health effects of reducing nicotine levels in cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. [DOI] [PubMed] [Google Scholar]
- 7.Family Smoking Prevention and Tobacco Control Act, 21 USC, 2009. Available at: https://www.fda.gov/tobaccoproducts/labeling/rulesregulationsguidance/ucm237092.htm. Accessed December 12, 2017.
- 8.Ashley D, Backinger C, van Bemmel D, Neveleff D. Tobacco regulatory science: research to inform regulatory action at the Food and Drug Administration’s Center for Tobacco Products. Nicotine Tob Res. 2014;16(8):1045–1049. [DOI] [PubMed] [Google Scholar]
- 9.Allen S, Foulds J, Pachas G, et al. A two-site, two-arm, 34-week, double-blind, parallel-group, randomized controlled trial of reduced nicotine cigarettes in smokers with mood and/or anxiety disorders: trial design and protocol. BMC Public Health. 2017;17(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krebs N, Allen S, Veldheer S, et al. Reduced nicotine content cigarettes in smokers of low socioeconomic status: study protocol for a randomized control trial. Trials. 2017;18(1):300 [Correction:] Krebs N, Allen S, Veldheer S, et al. Trials. 2017;18(1):598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter P, Steven P, Bravo R, et al. Characteristics for SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federal Trade Commission. Federal Trade Commission Cigarette Report for 2014. Available at: https://www.ftc.gov/system/files/documents/reports/federal-trade-commission-cigarette-report-2014-federal-trade-commission-smokeless-tobacco-report/ftc_cigarette_report_2014.pdf. Accessed December 12, 2017.
- 13.Heatherton T, Kozlowski L, Frecker R, Fagerström K. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 14.Borland R, Yong H, O’Connor R, et al. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country Study. Nicotine Tob Res. 2010;12(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire to assess dependence on electronic cigarettes in a large sample of ex-smoking e-cig users. Nicotine Tob Res. 2015;17(2):186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federal Trade Commission. Federal Trade Commission Cigarette Report for 2016. Available at: https://www.ftc.gov/system/files/documents/reports/federal-trade-commission-cigarette-report-2016-federal-trade-commission-smokeless-tobacco-report/ftc_cigarette_report_for_2016_0.pdf. Accessed December 12, 2017.
- 17.Celebucki C, Wayne G, Connolly G, et al. Characterization of measured menthol in 48 U.S. cigarette sub-brands. Nicotine Tob Res. 2005;7(4):523–531. [DOI] [PubMed] [Google Scholar]
- 18.Agaku I, Vardavas C, Connolly G. Cigarette rod length and its impact on serum cotinine and urinary total NNAL levels, NHANES 2007–2010. Nicotine Tob Res. 2013;16(1):100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly G, Alpert H, Wayne G, Koh H. Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005. Tob Control. 2007;16(5):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borland R, Savvas S. Effects of stick design features on perceptions of characteristics of cigarettes. Tob Control. 2013;22(5):331–337. [DOI] [PubMed] [Google Scholar]
- 21.Skaczkowski G, Durkin S, Kashima Y, Wakefield M. Influence of premium versus value brand names on smoking experience in a plain packaging environment: an experimental study. BMJ Open. 2017;7:e014099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutti S, Hammond D, Borland R, et al. Beyond light and mild: cigarette brand descriptors and perceptions of risk in the International Tobacco Control (ITC) Four Country Survey. Addiction. 2011;106(6):1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. FDA announces comprehensive regulatory plan to shift trajectory of tobacco-related disease, death. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm568923.htm. Accessed December 17, 2017.
- 24.Richter P, Steven P, Bravo R, et al. Characteristics for SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding YS, Richter P, Hearn B. Chemical characterization of mainstream smoke from SPECTRUM variable nico tine research cigarettes. Tob Regul Sci. 2017;3(1):18–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res. 2015;17(2):186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappelleri JC, Bushmakin AG, Baker CL, et al. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 28.Gross J, Lee J, Stitzer ML. Nicotine-containing versus denicotinized cigarettes: effects on craving and withdrawal. Pharmacol Biochem Behav. 1997;57(1–2):159–165. [DOI] [PubMed] [Google Scholar]
- 29.Harris P, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. (Research Support, N.I.H., Extra-mural). J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldheer S, Midya V, Lester C, et al. Acceptability of switching from usual brand to SPECTRUM research cigarettes with normal nicotine content. Tob Regul Sci. 2018;4(1):573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Counts M, Morton M, Laffoon S, et al. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41(3):185–227. [DOI] [PubMed] [Google Scholar]
- 32.Agnew-Heard K, Lancaster V, Bravo R, et al. Multivariate statistical analysis of cigarette design feature influence on ISO TNCO yields. Chem Res Toxicol. 2016;29(6):1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercincavage M, Smyth JM, Strasser AA, Branstetter SA. Reduced nicotine content expectancies affect initial responses to smoking. Tob Regul Sci. 2016;2(4):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hukkanen J, Pleyton J, Benowitz N. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 35.Shiffman S, Scholl S. Increases in cigarette consumption and decreases in smoking intensity when non-daily smokers are provided with free cigarettes. Nicotine Tob Res. 2018;20(10):1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]