Abstract
Versican is a chondroitin sulfate proteoglycan found in the extracellular matrix that is important for changes in cell phenotype associated with development and disease. Versican has been shown to be involved in cardiovascular disorders, as well as lung disease and fibrosis, inflammatory bowel disease, cancer, and several other diseases that have an inflammatory component. Versican was first identified as a fibroblast proteoglycan and forms large multi-molecular complexes with hyaluronan and other components of the provisional matrix during wound healing and inflammation. The biology of versican has been well studied. Versican plays a major role in embryogenesis, particularly heart formation, where versican deletion proves lethal. The ability to purify versican to characterize and to use in experimental systems is vital to defining its role in development and disease. Protein expression systems have proven challenging to obtain milligram quantities of full length versican. Here, we describe proteoglycan biochemical purification techniques that have been developed by others, but which we have adapted to use with our source tissues and cells. We also include methods for immunohistochemical localization and quantitation of versican in tissue sections.
Keywords: Aorta, extracellular matrix, lung, vascular smooth muscle cells, versican
1.0. Introduction
Versican is a chondroitin sulfate proteoglycan found in the extracellular matrix that is important for development and disease (Wight 2016). Versican was first identified as a fibroblast proteoglycan (LeBaron and Zimmermann and Ruoslahti 1992; Zimmermann and Ruoslahti 1989) and forms large multi-molecular complexes with hyaluronan and other components of the provisional matrix during development and during wound healing and inflammation (Wight and Kang and Merrilees 2014; Wight and Kinsella and Evanko and Potter-Perigo and Merrilees 2014).
The biology of versican is complex and much studied. Versican plays a major role in embryogenesis, particularly heart formation, where versican deletion proves lethal (Hatano and Kimata and Hiraiwa and Kusakabe and Isogai and Adachi et al. 2012; Mjaatvedt and Yamamura and Capehart and Turner and Markwald 1998; Nandadasa and Foulcer and Apte 2014). Versican is involved in cardiovascular disorders such as atherosclerosis and restenosis (Wight and Merrilees 2004), lung inflammation (Andersson-Sjoland and Hallgren and Rolandsson and Weitoft and Tykesson and Larsson-Callerfelt et al. 2015; Wight and Frevert and Debley and Reeves and Parks and Ziegler 2017) and various cancers (Du and Yang and Yee 2013; Keire and Bressler and Lemire and Edris and Rubin and Rahmani et al. 2014; Theocharis and Skandalis and Tzanakakis and Karamanos 2010).
In this chapter, we will describe a relatively large scale preparative method that we use to extract and purify versican from tissues and cell cultures, as well as procedures for immunohistochemical identification of versican in tissue sections. The ability to purify versican to use in experimental systems is vital to certain kinds of studies, and it has proven difficult to obtain milligram quantities of versican using various protein expression systems. These purification and characterization techniques have been developed by others (for reviews, see (Foulcer and Day and Apte 2015; Yanagishita and Midura and Hascall 1987)), and we have adapted them to use with our source tissues and cells.
Bovine aorta provides a relatively rich and inexpensive source for versican to be used in biochemical studies. For production of human versican, we have used culture medium from human aortic smooth muscle cells (Olin and Potter-Perigo and Barrett and Wight and Chait 1999). Chaotropic agents capable of disrupting the tight association of aggregating proteoglycans with hyaluronan are used for extraction (Sajdera and Hascall 1969). The high negative charge density on the chondroitin sulfate chains is exploited using ion exchange chromatography to separate proteoglycans from more weakly charged glycoproteins (Margolis and Lalley and Kiang and Crockett and Margolis 1976). The large hydrodynamic size of versican compared to the other proteoglycans that are typically present facilitates the final purification steps by size exclusion chromatography (Olin et al. 1999).
This is a rather large scale extraction and purification procedure. The steps will be similar for smaller tissue pieces and can be scaled back accordingly. Once isolated and the purity verified, versican can be used in add-back studies (Keire et al. 2014; Merrilees and Zuo and Evanko and Day and Wight 2016), or coating of culture surfaces (Evanko and Potter-Perigo and Bollyky and Nepom and Wight 2012) to test its effect on cells, as well as biophysical (Angheloiu and Haka and Georgakoudi and Arendt and Muller and Scepanovic et al. 2011) and biochemical analyses (Olin et al. 1999; Sandy and Westling and Kenagy and Iruela-Arispe and Verscharen and Rodriguez-Mazaneque et al. 2001).
2.0. Isolation, Purification and Characterization of Versican from Bovine Aorta
2.1. Bovine Versican Extraction and Purification Protocol
-
Starting material: 300 g bovine aorta (obtained frozen from Pel Freeze)
Final yield: ~15 mg versican/300 g tissue
Thaw the aortic tissue and grind with a meat grinder to maximize extraction.
Extract with 3–5 volumes of 4 M guanidine HCl, 0.1 M Tris, pH 6.0, containing 2mM EDTA and protease inhibitors, 5 mM benzamidine, 100 mM 6-amino-hexanoic acid, 10 mM n-ethyl-maleimide, 1 mM PMSF for 24 h at 4°C with stirring. (NOTE: The tissue swells considerably and may disrupt the magnetic stir bar. Use a large 1 L or 2 L beaker)
Filter the extract through 2 layers of pre-washed cheesecloth. (NOTE: The extract is typically quite viscous and gelatinous. Alternatively, centrifugation can be used to separate the extract from the tissue residue. However, this requires a large volume bucket rotor centrifuge, and it is time consuming and less efficient.)
Re-extract the tissue residue with another 3 volumes or so of the 4 M guanidine extracting buffer overnight at 4°C.
Combine the extracts, and dialyze against distilled water. (NOTE: With such large volumes, it is more economical to dialyze against water and then add solid urea than to try to dialyze from guanidine directly into 6 M or 8 M urea. We use several tubes of large capacity dialysis tubing (Spectrum Spectra/Por, 45 mm diameter, 12–14,000 MWCO) **A white flocculent precipitate containing most of the extracted proteins and proteoglycans will appear once the extract is dialyzed into water. Important: Do not centrifuge, filter, or discard this precipitate. It will re-dissolve once urea is added in the next step.
Transfer the extract from dialysis tubing to a large beaker or flask. Add the appropriate amount of solid urea, tris, and sodium chloride to obtain 6 M urea, 0.1 M Tris, 0.2 M NaCl. Adjust the pH to 7.0. (The precipitate should dissolve, although there may be some residual cloudiness. You can add up to 8 M urea, if necessary.)
Add DEAE Sephacel (previously equilibrated into the urea buffer) directly to the extract (we use approximately 30 ml of resin per 300 g starting tissue) and mix the slurry overnight. (We avoid stir bars at this step and mix by shaking on a rotating mixer.) Let the slurry settle to the bottom of the flask before the next step.
Using a pipette, transfer the slurry of DEAE Sephacel with bound proteoglycans to an empty column and rinse with at least 5 volumes of 6 M urea, 0.1 M Tris, 0.25 M NaCl to remove non-bound and weakly bound proteins and glycoproteins.
Elute the proteoglycans with the urea buffer containing 2 M NaCl (up to 3 column volumes). For convenience and speed, we usually elute the proteoglycans batch-wise in 3 fractions, rather than using a salt gradient.
The proteoglycan fraction (containing primarily versican, biglycan, and decorin) is then concentrated using Centri-prep centrifugal concentrators (Millipore). Alternatively, the eluted total proteoglycans can be dialyzed against water and lyophilized. (NOTE: this typically yields ~100 mg or more of total proteoglycans.)
2.2. Size Exclusion Chromatography
The proteoglycans are then fractionated by size on a preparative column of Sepharose CL-4B (2 × 87 cm) in the urea buffer, collecting 2 ml fractions. (NOTE: we have also used Sepharose CL-2B.)
The fractions are monitored for sulfated glycosaminoglycan content using the DMMB assay (Farndale and Buttle and Barrett 1986).
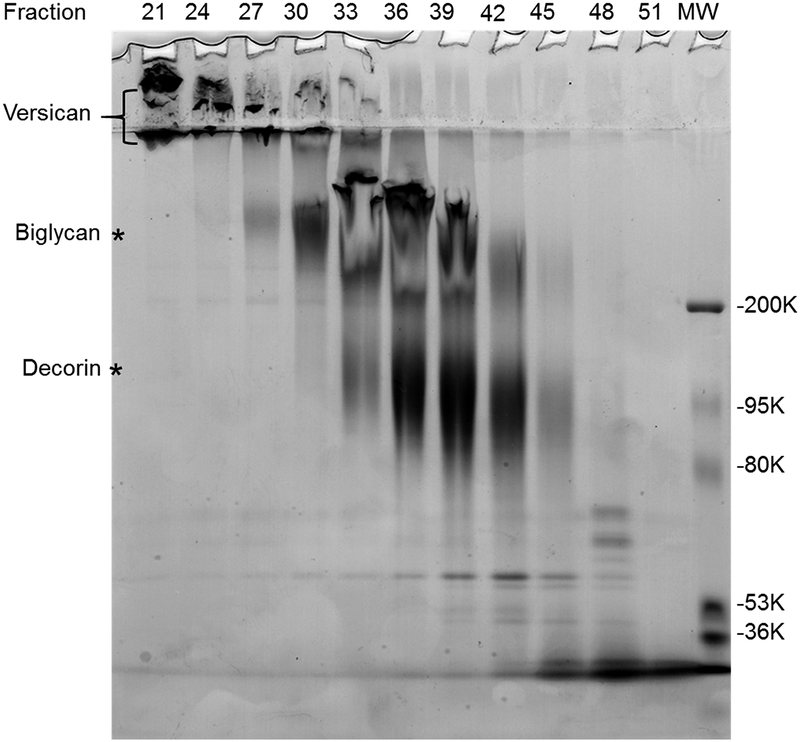
Fraction aliquots (50 μl) are then precipitated with 1 ml of 95 % ethanol containing 1.5 % potassium acetate overnight at −20°C. The fractions containing versican are identified using SDS-PAGE (Figure 3). We use 4–12% gradient gels with a 3% stacking gel. As shown, versican runs in the stacking gel and just at the top of the running gel.
Pool the appropriate fractions. (NOTE: We find that it is best to pool the fractions from the earliest part of the peak to help ensure that no small proteoglycans are present.)
Concentrate the sample by spinning in a centrifugal microconcentrator.
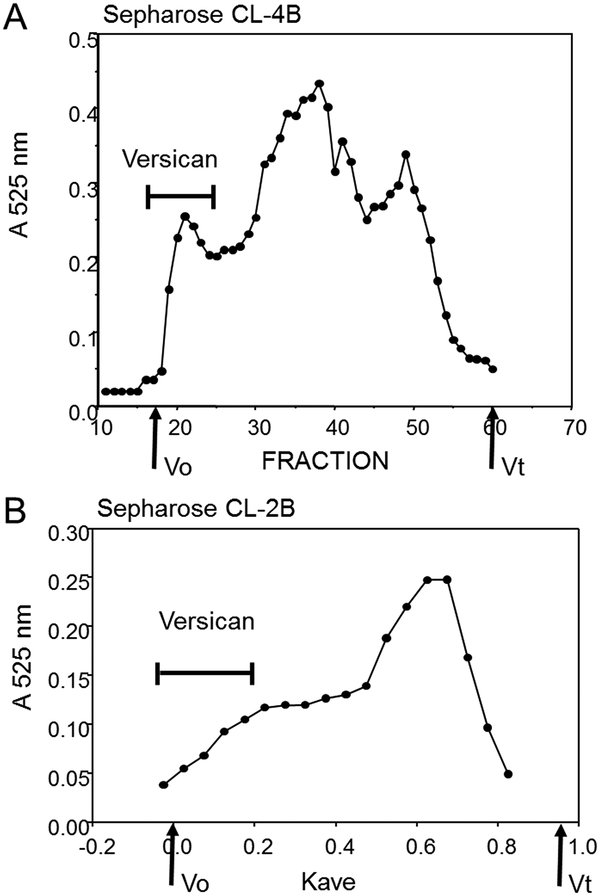
The versican-containing fractions can/should be re-chromatographed on the CL4B (or CL2B) column to remove any small proteoglycans or other small proteins that might still be in the sample (Figure 4). (NOTE: We found this step is not always necessary, especially if the pooled fractions are limited mainly to the leading fractions in the versican peak (i.e., the shoulder on CL2B).)
The final step in preparing versican for storage is to dialyze the pooled fractions into water, and measure the chondroitin sulfate and/or protein content. Prepare aliquots containing 100 or 200 μg of versican in individual microfuge tubes, and then freeze and lyophilize them. This is a convenient size to resuspend and work with in experiments. Our typical yield is approximately ~15 mg versican/300 g aortic tissue.
It is also important to measure endotoxin levels in the final preparation, if the versican will be used for adding to cells or to animals.
Figure 3.
SDS-PAGE analysis of selected fractions from the CL4B column shown in Fig. 2A stained with Coomassie blue and Alcian blue. Versican was enriched in fractions 19–24.
Figure 4.
Re-chromatography of the versican fractions on CL4B. Fractions 19–24 from Figure 2 were combined, concentrated, and rerun on CL4B. The earliest fractions contain nearly pure versican.
2.3. Assessment of Purity
The purity of the versican preparation is checked at each stage by SDS-PAGE using Coomassie blue and Alcian blue staining.
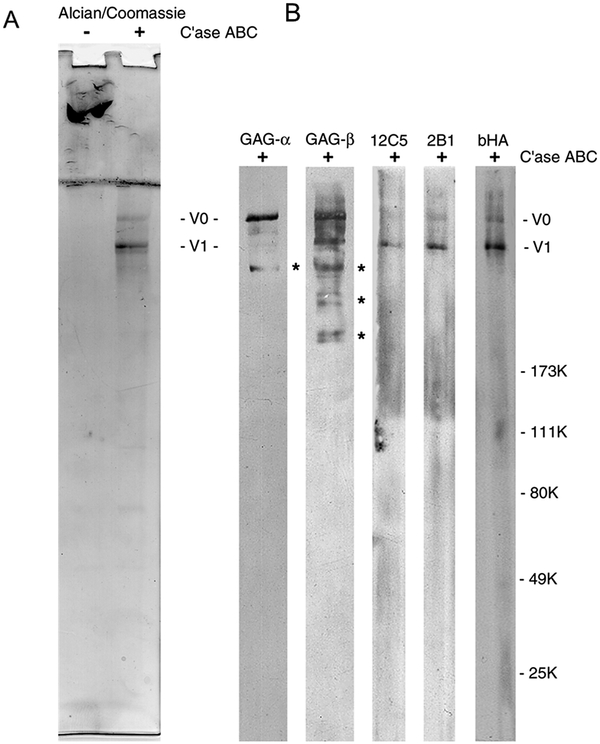
SDS-PAGE analysis of the total extract, DEAE unbound, and 1 M NaCl proteoglycan fractions intact and following digestion with chondroitin ABC lyase is shown in Figure 1. Coomassie blue and Alcian blue are used to stain proteins and glycosaminoglycan-containing proteins respectively. The predominant PGs present in aorta are the large chondroitin sulfate proteoglycan versican (arrow), which remains in the stacking gel, and biglycan and decorin, which appear as two smaller Alcian blue-positive bands. Following digestion with chondroitinase, the core proteins for versican splice variants, V0 and V1 appear at about 550 and 500 kDa, while the core proteins of decorin and biglycan run at about 45 kDa.
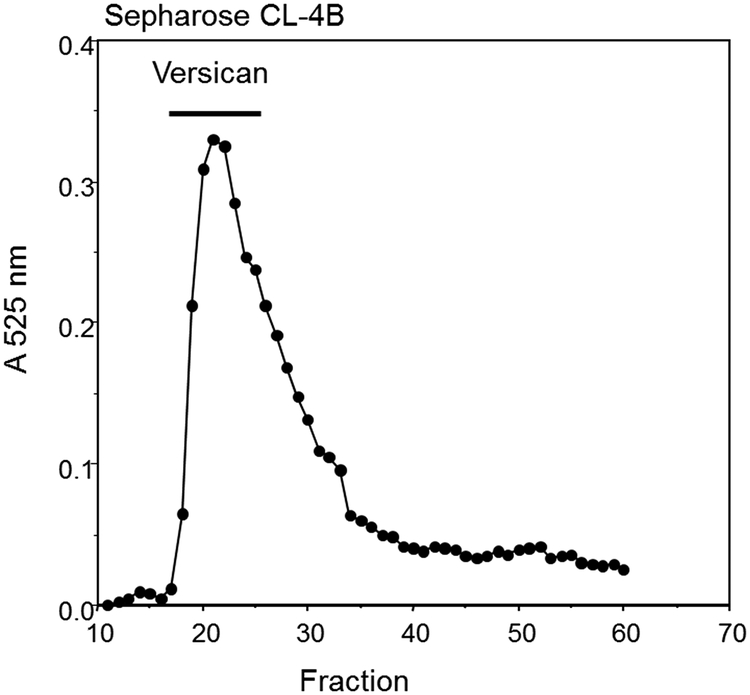
Figure 2 shows representative size profiles from each of the molecular sieve columns. With Sepharose CL-4B (Figure 2A), versican elutes early with a clear peak just at the void volume, while the small proteoglycans, mainly biglycan and decorin, elute later as a broad peak. With Sepharose CL-2B (Figure 2B), versican is found in the included volume of the column and elutes as a broad shoulder ahead of the large peak of the small proteoglycans. (**NOTE: Processing the many milligrams of total proteoglycans obtained from the DEAE purification step can require several molecular sieve column runs, (up to 12 runs, depending on the size of the preparative column).) Dividing the sample into several portions helps avoid overloading the column with too much material. The small proteoglycans massively predominate as a proportion of the total material and elute as a very large peak. Including the dialyzable detergent CHAPS (0.1%) in the urea buffer may improve recovery.
Figure 1.
SDS-PAGE analysis of total extract (lanes 1 and 4), DEAE unbound (lanes 2 and 5), and the 2 M NaCl fraction of total proteoglycans (lanes 3 and 6). Lanes 1–3, intact. Lanes 4–6, Chondroitinase ABC digested. The gel was stained with Coomassie blue and Alcian blue. Intact versican (indicted with bracket) remains in the stacking gel and at the top of the running gel. Putative biglycan and decorin are indicated with asterisks. Following digestion with chondroitinase, the core proteins for versican V0 and V1 appear at about 550 and 500 kDa. Biglycan and decorin core proteins and enzyme bands are indicated.
Figure 2.
Molecular sieve chromatography of total bovine aortic proteoglycans. (A) Sepharose CL-4B. (B) Sepharose CL-2B. Fractions were monitored for chondroitin sulfate using the DMMB assay. The fractions containing versican are indicated. (see Fig. 3).
3.0. Isolation of Versican from Cell Cultures
Aortic smooth muscle cells are another good source for versican (Olin et al. 1999). Culture medium can be collected over several passages to obtain microgram to milligram quantities. The use of cultured cells also allows for metabolic radiolabeling of the newly synthesized proteoglycans with either 35S-sulfate, which labels the sulfated glycosaminoglycans (primarily chondroitin sulfate) or 35S-methionine to label the core protein(s). Radiolabeling reduces the need for large amounts of material by increasing sensitivity for detecting small amounts. Nevertheless, we have obtained yields of approximately 1.4 mg versican/L conditioned medium from human smooth muscle cells.
3.1. Procedure for Isolating Versican from Cell Cultures
For isolating non-radiolabeled versican from conditioned medium, culture dishes that maximize surface area for the largest number of cells are recommended. We have used Nunc™ Cell Culture Treated TripleFlasks (Thermo-Fisher) with good results.
Collect conditioned cell culture medium (DMEM containing 10 % FBS) from cultures of aortic smooth muscle cells grown over several passages. We try to collect at least 1L of medium before isolating the proteoglycans. Protease inhibitors are added and the medium is stored at −20°C, and each medium change is added to the previously frozen supernatant.
Apply the thawed conditioned medium directly to a 20 ml column of DEAE Sephacel. (We found that DEAE Sephacel will hold approximately 10 mg of chondroitin sulfate/ml of resin.)
Rinse the column with at least 5 volumes of 8 M urea, 50 mM Tris base, 2 mM EDTA, 0.5% TX-100, pH 7.5.
Elute the proteoglycans with 8 M urea buffer containing 2 M NaCl (3 column volumes).
The eluted proteoglycans are then concentrated using Centri-prep centrifugal concentrators (Millipore). The sample is now ready for further purification (See 2.2 Size Exclusion Chromatography).
To obtain radiolabeled proteoglycans, cells can either be metabolically labeled with 80–100 μCi/ml Na235SO4 (Perkin Elmer, Waltham, MA) to label the glycosaminoglycans, or with 40 μCi/ml trans-methionine containing [35S]-(methionine and cysteine) to label the core protein(s) for 24–48 h. Shorter labeling times and pulse-chase protocols can be used for degradation studies, but are not good for preparing large amounts of versican.
Collect media with protease inhibitors and the sample is ready for DEAE Sephacel concentration and purification (1ml DEAE of Sephacel resin per 50 ml of media sample) as described above (see 3.1.3 above).
Smaller analytical sized columns of Sepharose CL-2B or CL-4B can be used with radiolabeled samples, as there is usually no danger of overloading with protein.
4.0. Characterization of the Versican Preparations by Western Blotting
Versican core proteins can be identified by western and/or ligand blot. Chondroitinase ABC digestion of the final preparation produced core proteins of approximately 550 and 500 kDa, corresponding to V0 and V1. The preparation tends to be free of contaminants as judged by Coomassie blue staining.
We have used the following antibodies and reagents: monoclonal antibody 2B1, which recognizes an epitope in the C-terminal region (Isogai and Shinomura and Yamakawa and Takeuchi and Tsuji and Heinegard et al. 1996) (Seikagaku America); monoclonal antibody 12C5, which recognizes the hyaluronan binding region in the N-terminal portion of versican (Asher and Perides and Vanderhaeghen and Bignami 1991) (Developmental Studies Hybridoma Bank; The University of Iowa); polyclonal antibodies to bovine α-GAG and β-GAG domains (provided by Maria Dours-Zimmerman, Department of Pathology, University Hospital, Zurich, Switzerland) (Dours-Zimmermann 1994) (Similar antibodies are available commercially.) The α-GAG and β-GAG antibodies allow us to identify the core proteins or fragments for V0, which contains both α and β domains; V1 which contains only the β-GAG domain; or V2, which contains only the α-GAG domain. Biotinylated hyaluronan can be used to assess the functionality of the N-terminal hyaluronan-binding region.
4.1. Sample Preparation
-
Versican should be digested with chondroitin ABC lyase prior to electrophoresis to generate the core proteins. Removing the chondroitin sulfate chains allows the core proteins to penetrate the resolving gel and may also facilitate reactivity with some antibodies. Otherwise, most of the sample will remain entirely in the stacking gel.
Preparations from DEAE-Sephacel or aliquots of column fractions are precipitated in 80% ethanol and 1.5% potassium acetate at −20°C for at least 2 hours, re-suspended in water, and re-precipitated in ethanol with 1.5% potassium acetate. Centrifuge in a microfuge for 5 minutes and discard the supernatant. Re-dissolve the pelleted samples in chondroitinase buffer, 0.02 M Tris/acetate, 0.1 mg/ml BSA, pH 8.0. Digestion of chondroitin sulfate is done with 2.0 U/mL chondroitin ABC lyase at 37°C for 3 hours. Digested and original samples are loaded in standard gel sample buffer (0.1 M Tris HCl, 3.3% SDS, 16% glycerol pH 6.8) under reducing conditions (1.7% β-mercaptoethanol in the loading buffer) to 4% to 12% gradient polyacrylamide-SDS gels with 3% polyacrylamide stacking gels.
Loading 5 μg per lane, purified versican is electrophoresed in multiple lanes of a single SDS-PAGE gel and then strips cut and incubated with various antibodies. Alternatively, the versican can be run as a single large lane and a manifold for incubating a single blotted lane with several different antibodies can be used.
The resolving portion of the gel is transferred to nitrocellulose (Schleicher and Schuell) on a semi-dry transblot apparatus (Bio-Rad).
Block the blot or strips with 2% BSA in TBS (0.1 mol/L Tris-HCl and 0.1 mol/L NaCl, pH 7.5) for 2 h at 22°C, and then expose overnight to different antibodies by using a Miniblotter manifold apparatus (Immunetics).
Incubate with an appropriate concentration of primary antibody with rocking overnight at 4°C in a large enough volume to cover the blot or blot strip. We typically use the 2B1 versican antibody at 1:4000. However, the optimal antibody concentration should be determined empirically for the Western blot system in your laboratory.
Alkaline phosphatase-conjugated goat anti-rabbit or anti-mouse IgG secondary antibody is used to visualize primary antibody binding. Sequentially incubate the blots in diluted secondary antibody at 1:20,000 in 0.1 mol/L Tris-HCl and 0.1 mol/L NaCl (pH 7.5) plus 2% BSA for 2 hours followed by the proprietary chemiluminescent substrate CSPD (Western-Light Chemiluminescent Detection Systems, Tropix Inc) in assay buffer (0.1 mol/L diethanolamine, 1 mmol/L MgCl2, 0.02% sodium azide, and 1:20 Nitro-Block [Tropix Inc.], pH 10.0) and autoradiographic exposure for 20 minutes.
Figure 5 shows the results from one preparation of bovine versican. (NOTE: the blots shown here were developed with an enhanced chemiluminescence kit.) Newer and more sensitive western blot reagents and methods are being continually developed, so your preferred reagents/methods may be used. The two major core protein bands react with all the versican antibodies that we have tested, (except for the putative V1 band, which does not react with the α-GAG antibody) and are functional in binding biotinylated hyaluronan. We see reactivity to biotinylated hyaluronan confined to the two largest bands, which is abolished when the sample is run under reducing conditions (not shown). Some smaller fragments migrating as distinct bands between ~200–400 kDa (indicated by asterisks) react with the antibodies to bovine α-GAG and/or β-GAG, but do not react with 2B1, 12C5 or biotinylated hyaluronan, suggesting they may be derived from the central region of the core proteins. These fragments are generally not detectable with Coomassie blue, however, indicating that they are present in only minor amounts.
A ligand blot is used to assess the binding of the versican core proteins or fragments to biotinylated hyaluronan (Figure 5, far right lane). Biotinylated hyaluronan should be prepared ahead of time as described (Yu and Toole 1995).
Incubate the blot with 2–4 μg/ml of biotinylated hyaluronan in TBS, 2% BSA, overnight at 4°C. (**NOTE: Hyaluronan binding is dependent on the disulfide bonds in the N-terminal globular domain of the aggregating proteoglycans (Zimmermann and Ruoslahti 1989). Therefore, versican should be run under non-reducing conditions when using labeled hyaluronan as the ligand.) As a control, the loss of binding in a sample that is run under reducing conditions (1.5% β-mercaptoethanol in the sample buffer) lends more confidence that any positive binding is real.
Figure 5.
Analysis of versican purity and characterization by western blot. (A) SDS-PAGE of intact and chondroitinase ABC-digested versican. V0 and V1 core proteins are indicated. Note the lack of Coomassie blue staining below the core proteins. (B) Reactivity of the versican preparation with anti-versican antibodies and biotinylated hyaluronan, as indicated. Minor amounts of internal fragments that react with α-GAG and β-GAG antibodies are indicated with asterisks.
5.0. Localization of Versican in Tissue using Immunohistochemistry
5.1. Background
Biochemical studies with purified versican contribute to the identification of its function and interactions with other matrix molecules. The use of immunohistochemical techniques adds contextual information by localizing versican in defined regions of tissue and cells (Chang and Tanino and Vidova and Kinsella and Chan and Johnson et al. 2014; Chang and Chan and Eriksson and Johnson and Cao and Westoo et al. 2016; Fu and Nagy and Brown and Shih and Johnson and Chan et al. 2011; Hull and Johnson and Braun and Day and Wight 2012). The immunocytochemistry of versican is routinely performed on formalin-fixed paraffin-embedded tissue using standard immunohistochemistry techniques with the addition of specific glycosaminoglycan digestion. We have also used tissue fixed with methyl Carnoy’s fixative(Evanko and Raines and Ross and Gold and Wight 1998). For some tissues, an additional heat-induced epitope retrieval (HIER) step is needed. The staining protocol should be optimized for each tissue type tested to determine the necessity of the HIER step and optimal primary concentration to use. For detection and visualization, we currently use ready-to-use polymeric horseradish peroxidase secondary antibody (HRP-poly) systems for detection (e.g., ImmPRESS™ HRP (Vector laboratories), or Mach 4™ HRP (Biocare Medical).
5.2. Antibodies
On mouse tissue we use:
Rabbit anti-verisican antibody directed against a.a. 1360–1439 of mouse versican (EMD Millipore, #AB1033) for versican V0 and V1.
or Rabbit anti-versican antibody directed against a.a 535–598 of mouse versican (EMD Millipore, #AB1032) for versican V2.
On human tissue we use:
Mouse anti-large proteoglycan (clone 2B1) (formerly available from Seikagaku)(Isogai et al. 1996).
Rabbit anti-versican antibody (cloneEPR12277) (ABCAM # ab177480).
5.3. Procedure to Localize the Proteoglycan Versican in Tissue
The following procedure can be adapted to automated systems.
Cut 5-μm paraffin sections and mount on positively-charged slides.
Bake the slides for 10 min at 58°C to aid in the removal of paraffin wax and the adhesion of tissue.
De-wax tissue sections by incubating slides in three changes of xylene for 3 min each change.
Rehydrate the tissue sections by incubating slides in three changes of 100% ethanol for 2 min each change followed by two changes of 95% ethanol for 2 min each change, followed by 5 min in deionized water.
Incubate slides in Tris-HCL buffered saline (TBS: 50 mM Tris, 0.9% NaCl, pH 7.6) for 5 min.
OPTIONAL - When required to enhance staining, perform HIER antigen retrieval with either 10mM sodium citrate, 0.05% pH 6.0 or 10 mM Tris Base, 1 mM EDTA Solution, pH 9.0. Incubate slides in antigen retrieval solution at 110°C in a pressure cooker for 15 min. The pressure cooker may be replaced with a steamer or water bath, but incubation times in the retrieval solution may need to be increased.
Wash slides with two changes of TBS.
Remove glycosaminoglycan chains by incubating slides with 0.2 Unit/ml chondroitinase ABC in 18 mM Tris, 1 mM sodium acetate, 1 mg/ml BSA pH 8.0 in a humidified chamber for 1 h at 37°C.
Wash slides with two changes of TBS.
To quench endogenous peroxidases in the tissue, incubate slides in 3% H2O2 TBS for 10 min at room temperature.
Wash slides with two changes of TBS.
Apply a sufficient volume of blocking solution, 2% normal serum (corresponding to secondary antibody species), 1% bovine serum albumin, globulin-free (BSA) in TBS to cover the tissue section and incubate for 1 h at room temperature.
Incubate tissue sections with an appropriate concentration of primary antibody for 1 h at room temperature or overnight at 4°C.
Wash slides with three changes of TBS.
Apply either an anti-mouse IgG or anti-rabbit IgG HRP poly secondary and incubate 15–30 min.
Wash slides with three changes of TBS.
Visualize positive staining by incubating sections with diaminobenzidine or other horseradish peroxidase substrate for 2–10 min.
Wash slides with two changes of TBS and then with deionized water.
Counterstain tissue by incubating with Mayer’s hematoxylin for 5 min.
Wash slides with 2 changes of TBS.
Dehydrate stained slides by incubating slides in one change of 95% ethanol followed by three changes of 100% ethanol for 2 min each change.
Clear tissue sections by incubating slides in 3 changes of xylene for 3 min each and mount in xylene compatible mounting media.
6.0. Quantitation of Versican on Tissue Sections
6.1. Background
Advances in microscopy, digital imaging, computer hardware and analysis software have improved our ability to efficiently obtain quantitative and unbiased data from studies using immunohistochemistry to identify spatial or temporal changes of versican in tissues (Snyder and Washington and Birkland and Chang and Frevert 2015). This has led to an increased use of quantitative microscopy to understand the role of versican in regulating biological processes occurring in organs. Two techniques used for quantitative microscopy are stereology and image analysis. Morphometric analysis with these two techniques is most accurate when performed on tissue samples that have been properly fixed and processed and when immunohistochemistry is performed using validated antibodies with proper antibody and tissue controls (Frevert and Johnson and Stahl 2014; Goodwin and Johnson and Frevert 2017; Hewitt and Baskin and Frevert and Stahl and Rosa-Molinar 2014).
6.2. Stereology
Of the two quantitative techniques, stereology is considered the gold standard because it provides precise, unbiased measurements of three-dimensional (3D) structures in organs without making assumptions (Howard and Reed 1998; Muhlfeld and Ochs 2013; Ochs and Muhlfeld 2013). Rigorous sampling and counting techniques are required when using stereology and allow one to statistically extrapolate data about the cellular and structural make-up of an organ or tissue biopsy.(Gundersen and Jensen and Kieu and Nielsen 1999) The major limitation of stereology is the time required to perform these studies, which is the reason investigators often perform morphometric analysis using image analysis.
6.3. Image analysis
Image analysis is a technique often used to obtain quantitative data from tissue samples using analysis software that segments pixels in a digital image based on features such as color (i.e., RGB), density or texture. A limitation of image analysis is that it often requires assumptions to be made and only provides measurements of relative changes to the object(s) of interest in tissues. Even with its recognized limitations, image analysis is a powerful tool when used correctly to obtain quantitative data.
A recent and important addition to microscopy and digital imaging has been the development of microscopes that are used to convert stained tissue on glass slides into whole slide digital images allowing for more efficient viewing and analysis of histopathology and immunohistochemistry with computers. Most processes required to obtain unbiased data from tissue samples can be automated when integrated with whole slide digital imaging, making image analysis an efficient and effective tool to quantitate versican accumulation in tissue sections (Figure 6).
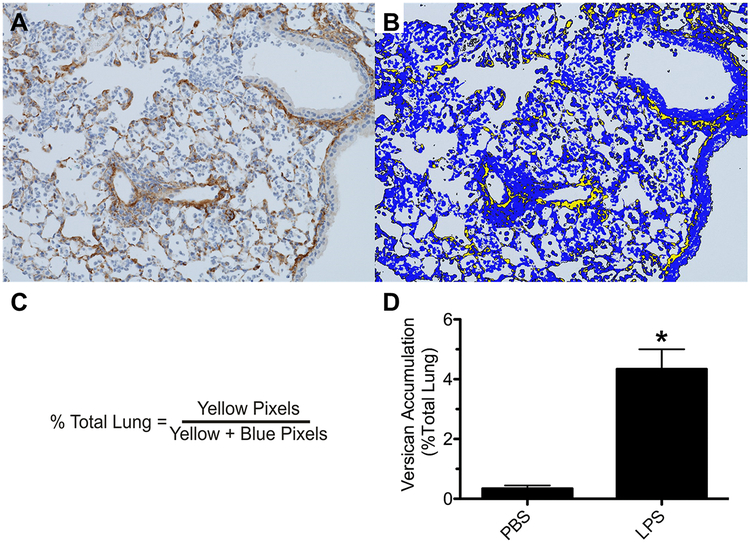
Figure 6.
Image analysis provides unbiased quantitative data on the relative amount of versican accumulation in lung tissue (% Total tissue) of mice treated with phosphate buffered saline (PBS) or lipopolysaccharide (LPS) for 48 hours. (A) Positive immunostaining for versican (brown) in lung tissue obtained from a mouse 48 h after oropharyngeal instillation of LPS. Hematoxylin was the counterstain (blue). (B) Segmentation of the digital image shown in (A) using Visopharm® software where yellow pixels designate lung tissue that stains positive for versican and blue pixels define lung tissue stained only with hematoxylin. (C) Formula used to determine the relative area of lung tissue stained positive for versican. (D) Accumulation of versican is significantly increased in the lungs of mice treated with LPS. Values are the mean ± SEM with n = 4 for PBS- and n = 8 for LPS-treated mice. (*) significantly different (p = 0.0084) than mice treated with PBS using Mann Whitney U-test.
6.4. Quantitation of Versican using Whole Slide Digital Images
Following treatment of mice with phosphate buffered saline (PBS) or lipopolysaccharide (LPS) for 48 h, mice were euthanized and the lungs collected and processed to perform immunohistochemistry as described in Section 5.0. The following image analysis protocol was used to quantitate versican accumulation in lung tissue.
Glass slides of versican immunohistochemistry are scanned with a Hamamatsu Nanozoomer®-HT whole slide imaging system (Bridgewater, NJ) using a 20× objective (NA 0.75) to create whole slide digital images.
The whole slide digital image is imported into Visopharm® Image Analysis Software (Hoersholm, Denmark).
The first step in image processing is an automated tissue detection feature that outlines lung tissue as a region of interest (ROI).
The second step in processing the digital images is the development of a protocol that segments the image into different components based on pixel values. This is accomplished by converting the digital images into gray scale values using two features, RGB-Red and RGB-Blue, with a mean filter of 5 pixels by 5 pixels.
This training session results in a protocol that discriminates pixels with positive immunostaining for versican (brown) from pixels stained with the counter stain, which is hematoxylin (Figure 6A).
Using the project-specific configuration and automated processing, each whole slide image is segmented into discrete regions based on a threshold of pixel values to generate the desired outputs, which are seen as yellow pixels for versican accumulation and blue pixels for the hematoxylin counterstain (Figure 6B).
The relative area of versican accumulation is calculated as a ratio of the area of versican stained pixels to the area of total lung tissue (versican + hematoxylin stained pixels) for each whole slide digital image that was created using the Hamamatsu whole slide scanner (Figure 6C).
Image analysis of the versican immunohistochemistry shows that very little versican is present in lungs of mice treated with the vehicle control, which is PBS. In contrast, positive immunostaining for versican is increased in lungs of mice treated with LPS (Figure 6D), which has been previously reported (Chang et al. 2014; Snyder et al. 2015).
Acknowledgements
We thank Megan Larmore and Brian Johnson from the University of Washington Histology and Imaging Core for their work on whole slide digital imaging and image analysis and Dr. Virginia M. Green from the Matrix Biology Program at BRI for her help in editing and preparing the manuscript.
This work was supported by National Institutes of Health grants P01 HL098067 (CWF and TNW), U19 AI125378 (TNW), and P30DK089507 (CWF).
References
- Andersson-Sjoland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, et al. (2015). Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology 25:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angheloiu GO, Haka AS, Georgakoudi I, Arendt J, Muller MG, Scepanovic OR, et al. (2011). Detection of coronary atherosclerotic plaques with superficial proteoglycans and foam cells using real-time intrinsic fluorescence spectroscopy. Atherosclerosis 215:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher R, Perides G, Vanderhaeghen JJ, Bignami A (1991). Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res 28:410–421. [DOI] [PubMed] [Google Scholar]
- Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, et al. (2014). A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol 34:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YT, Chan CK, Eriksson I, Johnson PY, Cao X, Westoo C, et al. (2016). Versican accumulates in vascular lesions in pulmonary arterial hypertension. Pulm Circ 6:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dours-Zimmermann MT, Zimmerman DR (1994). A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem 269:32992–32998. [PubMed] [Google Scholar]
- Du WW, Yang W, Yee AJ (2013). Roles of versican in cancer biology--tumorigenesis, progression and metastasis. Histol Histopathol 28:701–713. [DOI] [PubMed] [Google Scholar]
- Evanko S, Raines EW, Ross R, Gold LI, Wight TN (1998). Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics and the proximity of platelet-derived growth factor and transforming growth factor-β. Am J Pathol 152:533–546. [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN (2012). Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol 31:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ (1986). Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883:173–177. [DOI] [PubMed] [Google Scholar]
- Foulcer SJ, Day AJ, Apte SS (2015) Isolation and purification of versican and analysis of versican proteolysis In Balagurunathan Kuberan, Nakato Hiroshi, Desai RU, eds. Methods in molecular biology. Springer, 587–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert CW, Johnson B, Stahl WL (2014). Immunohistochemistry: Antibody specificity In Mitchell RN, McManus LM, eds. Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms. Kidlngton, Oxford UK, Elsevier Ltd [Google Scholar]
- Fu Y, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, et al. (2011). Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J Histochem Cytochem 59:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin P, Johnson B, Frevert C (2017) Microscopy, immunohistochemistry, digital imaging, and quantitative microscopy In Treuting PM, Dintzis SM, Montine KS, eds. Comparative anatomy and histology: a mouse and human atlas. Amsterdam; Boston, Elsevier/Academic Press, 55–66 [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J (1999). The efficiency of systematic sampling in stereology--reconsidered. J Microsc 193:199–211. [DOI] [PubMed] [Google Scholar]
- Hatano S, Kimata K, Hiraiwa N, Kusakabe M, Isogai Z, Adachi E, et al. (2012). Versican/PG-M is essential for ventricular septal formation subsequent to cardiac atrioventricular cushion development. Glycobiology 22:1268–1277. [DOI] [PubMed] [Google Scholar]
- Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E (2014). Controls for immunohistochemistry: the Histochemical Society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem 62:693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard V, Reed MG (1998) Unbiased stereology : three-dimensional measurement in microscopy. New York, Springer [Google Scholar]
- Hull RL, Johnson PY, Braun KR, Day AJ, Wight TN (2012). Hyaluronan and hyaluronan binding proteins are normal components of mouse pancreatic islets and are differentially expressed by islet endocrine cell types. J Histochem Cytochem 60:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai Z, Shinomura T, Yamakawa N, Takeuchi J, Tsuji T, Heinegard D, et al. (1996). 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG-M/versican. Cancer Res 56:3902–3908. [PubMed] [Google Scholar]
- Keire PA, Bressler SL, Lemire JM, Edris B, Rubin BP, Rahmani M, et al. (2014). A role for versican in the development of leiomyosarcoma. J Biol Chem 289:34089–34103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron RG, Zimmermann DR, Ruoslahti E (1992). Hyaluronate binding properties of versican. J Biol Chem 267:10003–10010. [PubMed] [Google Scholar]
- Margolis RU, Lalley K, Kiang WL, Crockett C, Margolis RK (1976). Isolation and properties of a soluble chondroitin sulfate proteoglycan from brain. Biochem Biophys Res Commun 73:1018–1024. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Zuo N, Evanko SP, Day AJ, Wight TN (2016). G1 domain of versican regulates hyaluronan organization and the phenotype of cultured human dermal fibroblasts. J Histochem Cytochem 64:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR (1998). The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol 202:56–66. [DOI] [PubMed] [Google Scholar]
- Muhlfeld C, Ochs M (2013). Quantitative microscopy of the lung: a problem-based approach. Part 2: stereological parameters and study designs in various diseases of the respiratory tract. Am J Physiol Lung Cell Mol Physiol 305:L205–221. [DOI] [PubMed] [Google Scholar]
- Nandadasa S, Foulcer S, Apte SS (2014). The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol 35:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs M, Muhlfeld C (2013). Quantitative microscopy of the lung: a problem-based approach. Part 1: basic principles of lung stereology. Am J Physiol Lung Cell Mol Physiol 305:L15–22. [DOI] [PubMed] [Google Scholar]
- Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A (1999). Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J Biol Chem 274:34629–34636. [DOI] [PubMed] [Google Scholar]
- Sajdera SW, Hascall VC (1969). Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem 244:77–87. [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, et al. (2001). Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem 276:13372–13378. [DOI] [PubMed] [Google Scholar]
- Snyder JM, Washington IM, Birkland T, Chang MY, Frevert CW (2015). Correlation of versican expression, accumulation, and degradation during embryonic development by quantitative immunohistochemistry. J Histochem Cytochem 63:952–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK (2010). Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J 277:3904–3923. [DOI] [PubMed] [Google Scholar]
- Wight TN (2016). Provisional matrix: A role for versican and hyaluronan. Matrix Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN, Frevert CW, Debley JS, Reeves SR, Parks WC, Ziegler SF (2017). Interplay of extracellular matrix and leukocytes in lung inflammation. Cell Immunol 312:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN, Kang I, Merrilees MJ (2014). Versican and the control of inflammation. Matrix Biol 35:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN, Kinsella MG, Evanko SP, Potter-Perigo S, Merrilees MJ (2014). Versican and the regulation of cell phenotype in disease. Biochim Biophys Acta 1840:2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN, Merrilees MJ (2004). Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res 94:1158–1167. [DOI] [PubMed] [Google Scholar]
- Yanagishita M, Midura RJ, Hascall VC (1987). Proteoglycans: isolation and purification from tissue cultures. Methods Enzymol 138:279–289. [DOI] [PubMed] [Google Scholar]
- Yu Q, Toole BP (1995). Biotinylated hyaluronan as a probe for detection of binding proteins in cells and tissues. Biotechniques 19:122–124, 126–129. [PubMed] [Google Scholar]
- Zimmermann DR, Ruoslahti E (1989). Multiple domains of the large fibroblast proteoglycan, versican. EMBO J 8:2975–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]