Abstract
Genetic diversity was assessed among 53 Indian garlic accessions using SSR markers. Initially, 24 SSR primer pairs were used for screening three selected garlic accessions. Out of 24 SSR primer pairs, 10 primer pairs which consistently showed good amplification and polymorphism were selected for DNA profiling. SSR primer pairs showed PIC values ranging from 0.30 to 0.99. Based on AMOVA we found that the greater part of the genetic diversity was expected due to intra population with 84% variation and only 16% of variation was due to among populations suggesting presence of genetic structure. The results of cluster analysis and principal component analysis largely correspond to each other. Population structure analysis revealed genetic differentiation of accessions. The results of present study revealed existence of significant variability in Indian garlic germplasm.
Keywords: Garlic, Allium sativum, SSR, UPGMA, PCA, Population structure
Introduction
Garlic, Alluim sativum L. (Alliaceae) is a diploid (2n = 2x = 16) bulb crop believed to have originated in central Asia from its wild ancestor Allium longicuspis Regel (Zohary and Hopf 2012). It is used for both culinary and medicinal purposes. It is grown worldwide, with China as a leading producer followed by India (FAO estimates; http://en.wikipedia.org/wiki/Garlic). Presence of sulfur compounds like allicin contributes to its medicinal properties (Taucher et al. 1996; Tucak et al. 2009). Garlic is mainly maintained vegetatively, due to obligate apomixes, by planting individual cloves (Pooler and Simon 1993; Bradley et al. 1996; Paredes et al. 2008). Sterile nature of garlic cultivars makes difficult to identify wild progenitors or ancestor of domesticated garlic (Zohary and Hopf 2012; Shaaf et al. 2014). Besides strict asexual life cycle, the large genome size (DNA content 32.7 pg per 2C nucleus) further complicates molecular and genetic analysis (Ranjekar et al. 1978). Mutations accumulated through cultivation history are expected to be behind variations present in domestic or cultivated garlic, which has asexual mode of reproduction unlike its wild ancestors, which harbors variations due to sexual reproduction (Shaaf et al. 2014). In contrast to general view that most garlic cultivars are sterile having flowers without fertile seed setting, few collections have been discovered from Caucuses and central Asia which are almost seed fertile or semi fertile (Kamenetsky 2007; Zohary and Hopf 2012). Diversity has been recorded in cultivated garlic for various traits (Pooler and Simon 1993; Avato et al. 1998; Figliuolo et al. 2001; Baghalian et al. 2005; Wang et al. 2014). Pooler and Simon (1993) observed high morphological diversity in garlic, for different morphological traits. In Italy, Figliuolo et al. (2001) studied a collection of cultivated garlic for 16 different morphological traits. In Iran, Baghalian et al. (2005) evaluated 24 garlic ecotypes for genetic diversity for allicin content and botanical traits like bulb mean weight, clove mean weight and clove number per bulb. Likewise, Panthee et al. (2006) studied genetic diversity of various morphological characters in garlic (Allium sativum L.) accessions from Nepal. Similarly, Wang et al. (2014) evaluated diversity of morphological traits and allicin content in garlic from China. These examples suggest that evaluation of genetic diversity in garlic is important for selection and fruitful breeding purposes. Study of genetic variation in cultivated garlic accessions is highly useful because it can lead to the identification of suitable cultivars, using clonal selection, and accessions with fertile florets so that they can be used in breeding programs (Figliuolo et al. 2001).
In India, the average productivity of garlic is 5 tons/ha which is low as compared to the other garlic growing countries (Singh et al. 2012). To increase the production of this crop for domestic as well as international market, there is an urgent need to screen the germplasm to get more divergent cultivars for qualitative traits. Since garlic is propagated by vegetative methods, the clonal selection is an important breeding method. Difficulty and absence of sexual reproduction in garlic through-out the generations, has clinched the genetic advancement of the crop through conventional breeding. However, in recent decades, scientists have aggravated advancement towards the comprehension factors involved in male sterility and fertility and recently developed a system for the production of garlic seeds (Etoh and Simon 2002; Jenderek and Hannan 2004). With that routine production of seeds, inheritance studies and the construction of linkage maps are now possible. Ipek et al. (2005) and Zewdie et al. (2005) have published first genetic maps using different marker systems.
In India, attempts have been made to collect and evaluate germplasms, but information on superior yield and yield contributing traits are very limited. It is therefore important to estimate genetic variation for germplasm selection of diverse parents which may be useful as donor for complementing various breeding programs. Although phenotypic diversity analysis has been widely used for understanding variability among genotypes for various crop plants, these phenotypic traits need intensive field trials and are also time consuming. On the contrary, use of DNA based markers like RAPD (Bradley et al. 1996; Khar et al. 2008; Paredes et al. 2008; Shaaf et al. 2014), AFLP (Ipek et al. 2003; Lampasona et al. 2003, 2012; Volk et al. 2004), SRAP (Chen et al. 2013), ISSR (Jabbes et al. 2011; Chen et al. 2014; Shaaf et al. 2014), SSR (Ma et al. 2009; Zhao et al. 2011; Cunha et al. 2012, 2014; Jo et al. 2012; Chen et al. 2014) and SNP to estimate genetic diversity is highly preferable in various crop plants including garlic and its wild relatives because of several advantages (Rafalski et al. 1996; Gupta et al. 1996). SSRs have been extensively used in genetic diversity studies because of high polymorphism detection, high abundance, co-dominant nature, high reproducibility and cost effectiveness. Moreover, application of SSRs does not require specialized bioinformatics tool (Park et al. 2009; Gous et al. 2013). Even though various diversity studies have been reported in garlic worldwide, that helped in the identification and construction of core germplasm, genetic diversity studies among Indian garlic germplasm is limited. Therefore, present study was designed to evaluate the genetic variability and diversity among Indian garlic accessions, using SSR markers.
Materials and methods
Plant materials
A total of 53 accessions of garlic, including landraces, collected from different parts of India were used in the present study (Table 1).
Table 1.
List of Indian garlic (Allium sativum L.) accessions and their origin
| S. no. | Accession | Collection site | S. no. | Accession | Collection site |
|---|---|---|---|---|---|
| 1 | CL Lamba | Bareilly, Uttar Pradesh | 28 | F-13 | PAU, Ludhiana; Punjab |
| 2 | Roshnee Mota | Bareilly, Uttar Pradesh | 29 | PG-9 | PAU, Ludhiana; Punjab |
| 3 | Chachena Mota | Bareilly, Uttar Pradesh | 30 | AVTG-1 | PAU, Ludhiana; Punjab |
| 4 | Sukha 44 | Bareilly, Uttar Pradesh | 31 | BG-108 | PAU, Ludhiana; Punjab |
| 5 | Desi Lasan | Bareilly, Uttar Pradesh | 32 | AVTG-4 | PAU, Ludhiana; Punjab |
| 6 | Kadari Mota | Bareilly, Uttar Pradesh | 33 | PG-32 | PAU, Ludhiana; Punjab |
| 7 | Hari Rani | Bareilly, Uttar Pradesh | 34 | F-6-SF | PAU, Ludhiana; Punjab |
| 8 | Jawa | Bareilly, Uttar Pradesh | 35 | PG-24 | PAU, Ludhiana; Punjab |
| 9 | Kadari 4 | Bareilly, Uttar Pradesh | 36 | F-2-R | PAU, Ludhiana; Punjab |
| 10 | Cheeniaa | Bareilly, Uttar Pradesh | 37 | PG-35 | PAU, Ludhiana; Punjab |
| 11 | UP Chatta | Bareilly, Uttar Pradesh | 38 | F-2-SF | PAU, Ludhiana; Punjab |
| 12 | Kant Gola | Bareilly, Uttar Pradesh | 39 | G-282 | NHRDF, Karnal; Haryana |
| 13 | CFG-1 | Bareilly, Uttar Pradesh | 40 | G-50 | NHRDF, Karnal; Haryana |
| 14 | CFG-2 | Bareilly, Uttar Pradesh | 41 | G-323 | NHRDF, Karnal; Haryana |
| 15 | CFG-3 | Bareilly, Uttar Pradesh | 42 | Bhima Omkar | DOGR, Maharashtra |
| 16 | CFG-4 | Bareilly, Uttar Pradesh | 43 | GG-4 | DOGR, Maharashtra |
| 17 | CFG-5 | Bareilly, Uttar Pradesh | 44 | G-355 | DOGR, Maharashtra |
| 18 | CFG-6 | Bareilly, Uttar Pradesh | 45 | GG-2 | DOGR, Maharashtra |
| 19 | CFG-7 | Bareilly, Uttar Pradesh | 46 | GG-1 | DOGR, Maharashtra |
| 20 | CFG-8 | Bareilly, Uttar Pradesh | 47 | Bhima Purpule | DOGR, Maharashtra |
| 21 | F-1 | PAU, Ludhiana; Punjab | 48 | Phule Basant | DOGR, Maharashtra |
| 22 | F-2 | PAU, Ludhiana; Punjab | 49 | Godawari | DOGR, Maharashtra |
| 23 | F-3 | PAU, Ludhiana; Punjab | 50 | GHC-1 | Himachal Pradesh |
| 24 | F-4 | PAU, Ludhiana; Punjab | 51 | Single Kali | Meerut, Uttar Pradesh |
| 25 | PG-17 | PAU, Ludhiana; Punjab | 52 | Kashmiri Garlic | Kashmir, Jammu and Kashmir |
| 26 | PG-20 | PAU, Ludhiana; Punjab | 53 | Indian Garlic | Kashmir, Jammu and Kashmir |
| 27 | F-5 | PAU, Ludhiana; Punjab |
PAU Punjab Agricultural University, NHRDF National Horticulture Research Development Foundation, DOGR Directorate of Onion and Garlic Research
DNA extraction and SSR genotyping
Total genomic DNA was extracted from fresh and young leaves following CTAB method (Doyal and Doyal 1990). The quality of DNA was checked on 0.8% agarose gel and DNA concentration was determined using a Bio-Rad’s Smart Spec™ Plus spectrophotometer. PCR (polymerase chain reaction) and electrophoresis were carried out as described by Kumar et al. (2009). A total of 24 SSR primer pairs obtained from previous studies of Ma et al. (2009) and Cunha et al. (2012) were used for initial screening of three genotypes (CL Lamba, PG 20 and Godavari). Out of these 24 SSR primer pairs, 10 primer pairs showed polymorphism and they were selected for DNA profiling of 53 garlic accessions (Table 2).
Table 2.
Details of SSR primers used for the genotyping of 53 garlic accessions
| S. no | Primer code | Forward and reverse primer sequence (5′–3′) | PIC | Rp | MI | Number of band | Polymorhic band | Polymorphism % |
|---|---|---|---|---|---|---|---|---|
| 1 | Asa06 | GGGGTGTTACATTCTCCCCT | 0.891 | 3.25 | 6.24 | 7 | 7 | 100 |
| ACCGCCTGATTTTGCATTAG | ||||||||
| 2 | Asa07 | CTCGGAACCAACCAGCATA | 0.509 | 4.04 | 1.14 | 4 | 3 | 75 |
| CCCAAACAAGGTAGGTCAGC | ||||||||
| 3 | Asa08 | TGATTGAAACGAATCCCACA | 0.83 | 5.47 | 6.64 | 8 | 8 | 100 |
| GGGGGTTACCTGAACCTGTTA | ||||||||
| 4 | Asa10 | TTGTTGTTCTGCCATTTT | 0.798 | 3.10 | 3.19 | 4 | 4 | 100 |
| GATCTAAGCCGAGAGAAA | ||||||||
| 5 | Asa17 | TCCACGACACACACACACAC | 0.305 | 5.77 | 0.41 | 3 | 2 | 66.66 |
| ATGCAGAGAATTTGGCATCC | ||||||||
| 6 | Asa24 | TTGTTGTGCCGAGTTCCATA | 0.525 | 2.67 | 1.05 | 2 | 2 | 100 |
| CAGCAATTTACCAAAGCCAAG | ||||||||
| 7 | Asa31 | CAGAGACTAGGGCGAATGG | 0.499 | 2.08 | 0.25 | 2 | 1 | 50 |
| ATGATGATGACGACGACGAG | ||||||||
| 8 | Asa59 | CGCTTACTATGGGTGTGTGTC | 0.999 | 0.075 | 1.99 | 2 | 2 | 100 |
| CAAGTGGGAGACTGTTGGAG | ||||||||
| 9 | EU909133 | CACAGCAACATGCACCAT | 0.499 | 2.037 | 0.25 | 2 | 1 | 50 |
| TGCCGGAACTCGATATT | ||||||||
| 10 | EU909138 | AATCTCCCTCCAAAGTCCC | 0.736 | 3.55 | 3.68 | 5 | 5 | 100 |
| CCTGTATTTTGTGTAAAGCATCA | ||||||||
| Average | 0.65 | 3.20 | 2.48 | 3.90 | 3.50 | 84.16 | ||
PIC polymorphism information content, Rp resolving power, MI marker index
SSR and molecular variance analysis
Data generated using 10 SSR primer pairs on 53 garlic accessions was scored in binary format. Polymorphism Information Content (PIC) (Botstein et al. 1980), marker index (MI) (Milbourne et al. 1997) and Resolving Power (Rp) (Prevost and Wilkinson’s 1999) were also calculated. Molecular data was processed using NTSYSpc version 2.2 software (Rohlf 1998; Exeter Software, Setauket, NY). Genetic similarity coefficients of pair-wise comparisons among the accessions analyzed were calculated based on Jaccard coefficient (Jaccard 1908) within the Similarity for Qualitative Data (SIMQUAL) module of NTSYS 2.2. The Unweighted Pair Group Method with Arithmetic Mean (UPGMA) clustering method was used to construct the dendrogram. Principal components analysis (PCA) was also performed. Analysis of molecular variance (AMOVA) (Excoffier et al. 1992; Excoffier 1993) was used to calculate variance components within and among the population with the software GenAlEX6 (Peakall and Smouse 2006).
Population structure analysis
Further, in order to estimate the number of subpopulations in the garlic germplasm, population STRUCTURE analysis was carried out using the program STRUCTURE version 2.2 (Pritchard et al. 2000). The membership of each cultivars was tested for K = 2 to K = 10 with admixture model. Three independent runs were assessed for each fixed K and each run consisted of 30,000 burn-in period and 1,00,000 number of Markov Chain Monte Carlo (MCMC) repeats. The optimal value of K was determined by examination of the ΔK statistic and L (K) (Evanno et al. 2005) using Structure Harvester program (Earl and von Holdt 2012). Exact K value could not be obtained due to log-likelihood values appeared an increasing function of K for all examined K value. Therefore, true K values may not have been represented in the model.
Results
Molecular marker analysis
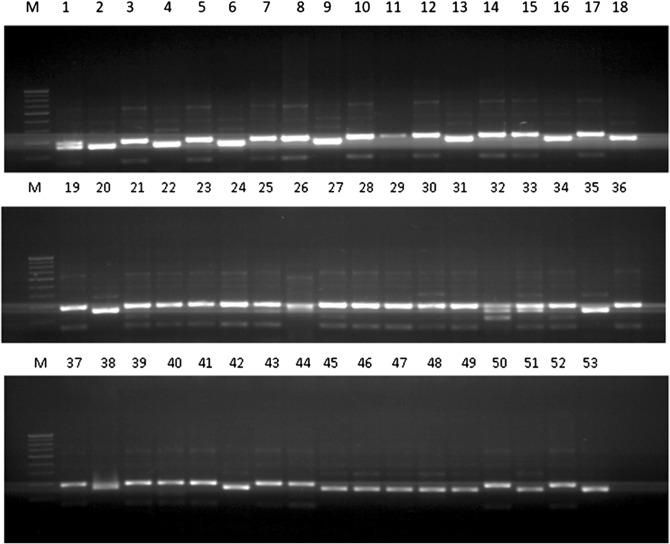
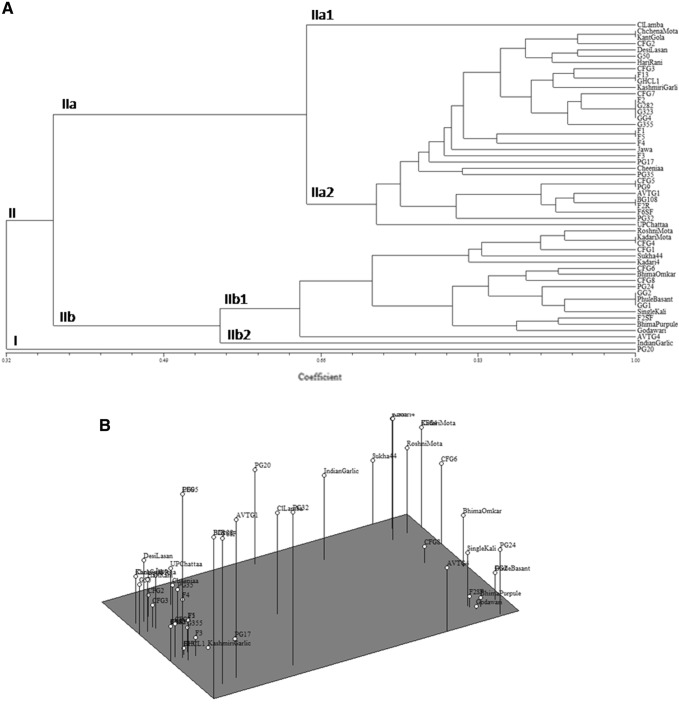
Based on the amplification by 10 SSR primer pairs, total of 39 bands were detected ranging from two to eight bands with mean value of 3.9 bands per primer pair. Out of 39 bands generated by the SSR primer pairs, 35 were polymorphic and four were monomorphic, thus generating 84.16% average polymorphism (Table 2). SSR primer Asa08 (Fig. 1), produced highest number of bands i.e. 8.0 followed by SSR primer Asa06, (7 bands). The PIC ranged from 0.30 (Asa17) to 0.99 (Asa59) with an average of 0.65. Marker index ranged from 0.25 (Asa31, EU909133) to 6.64 (Asa08) with an average 2.48. Resolving power of the primer pairs ranged from 0.07 (Asa59) to 5.77 (Asa17) with an average 3.20. The UPGMA dendrogram representing all the 53 garlic accessions clustered into two major groups (Group I to II) at the coefficient of GS = 0.32 (Fig. 2a). The group II contains maximum number of 52 accessions. Group II could be further divided into two subgroups, namely, IIa and IIb with further division into minor subgroups i.e. IIa1, IIa2, IIb1 and IIb2. Major group I contained single accession named “PG20”. Minor subgroups IIa1 and IIb2 contained solitary accession namely “CL Lamba” and Indian Garlic” respectively.
Fig. 1.
Representative gel image depicting PCR amplification using primer pairs SSR Asa08 in 53 selected accessions of garlic. M represents marker ladder
Fig. 2.
a Dendrogram of 53 garlic accessions based on UPGMA analysis and Jaccard similarity coefficient using SSR data. b Results of principal component analysis (PCA) showing distribution of 53 garlic accessions
The results of a cluster analysis based on UPGMA were mostly consistent to the results obtained by PCA (Fig. 2a, b). In both analysis, accessions namely “PG-20”, “Indian Garlic”, “AVTG-4” and “CL Lamba” are found to be distant from other accessions. PG-20, from Punjab, also showed its unique characters at morphological level (data not shown). In the PCA, the first three principal components explained 61.64% of the accumulated variation out of which first principal components contributed 46.30%, second principal components contributed 8.86% and third principal components contributed 6.48% (Fig. 2b).
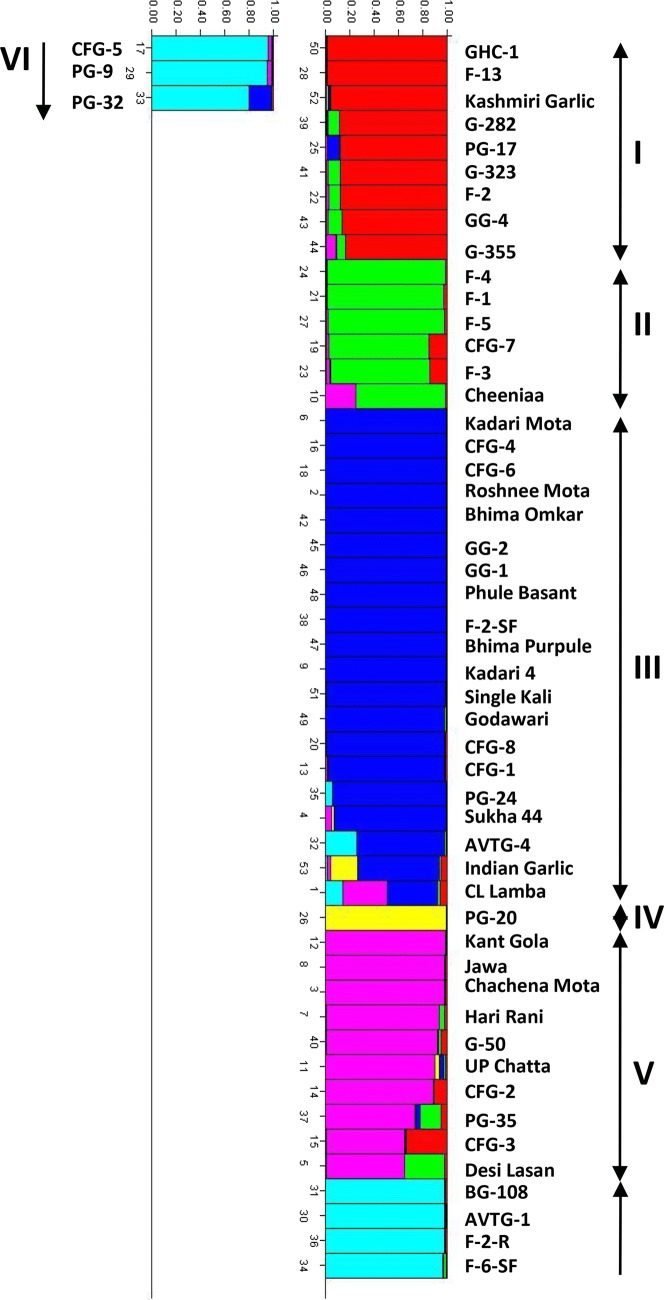
Based on AMOVA analysis, by considering location wise accessions as different representation of population, it is observed that the genetic diversity due to within population variation is about 84% and among populations is only 16% of variation (Table 3). Accession named GHC-1 from Himachal Pradesh was excluded because of the requirement of minimum two accessions from one location. Moreover, simulation based population structure model was performed to estimate the subpopulations among 53 accessions between K = 2 to K = 10. The optimum subpopulation number was determined by the maximum ΔK value obtained by the structure harvester. The most appropriate number of subpopulations was found to be K = 6 number of subpopulations. The model-based bar plot showed that 20 accessions were assigned to subpopulation III followed by subpopulation V and I with 10 and 9 accessions respectively. The sub-population IV was unique and had only accession namely PG-20.
Table 3.
Summary of AMOVA results obtained by SSR genotyping of garlic accessions
| Source | df | SS | MS | Est. var. | % |
|---|---|---|---|---|---|
| Among pops | 4 | 39.853 | 9.963 | 0.7 | 16 |
| Within pops | 47 | 173.839 | 3.699 | 3.699 | 84 |
| Total | 51 | 213.692 | 4.399 | 100 |
Discussion
Garlic (Allium sativum L.) reproduces only by vegetative propagation, and yet displays considerable morphological variation within and between cultivars (Bradley et al. 1996; Abdoli et al. 2009; Singh et al. 2012). This may be due to the phenotypic plasticity and mutation presence that makes the assessment and systematic classification of garlic problematic (Jo et al. 2012). Therefore, analysis of genetic diversity and relatedness between individuals is important for garlic improvement. Jo et al. (2012) analyzed SSR based genetic diversity in 120 garlic accessions, collected from five different countries and found that genetic diversity is correlated with geographical regions. Similar to these results, molecular analysis in present study also showed that some accessions collected from same geographical location tend to be present together. Therefore, present results indicate that molecular diversity is correlated, at least in few cases, with geographical regions. As suggested by Jo et al. (2012) difference in adaptation of garlic to diverse ecogeographic conditions and local selection pressure may be involved in this correlation of molecular diversity with a particular geographical region. In the present study, group of accessions collected from the same location were considered as one population. Accessions included in the present study were obtained both from public research organization and farmers market (Table 1). Accessions from farmer market were natural landraces. Although, accessions obtained from public research organization could not be considered as true natural population, they still could possibly be categorized as populations because originally these accessions were collected from local region. Moreover, these accessions were subjected to mild selection pressure as they were not part of any improvement programme and simply maintained for distribution. Therefore, these accessions along with accessions from farmers market should represent natural genetic variation.
Low level of variation is expected in garlic because of clonal propagation that can reduce variation and consequently show low level of population structure. Results of the present study are in accordance to some previous studies in garlic (Panthee et al. 2006; Jo et al. 2012; Cunha et al. 2014; Shaaf et al. 2014; Barboza et al. 2018). Shaaf et al. (2014) studied 31 garlic accessions, collected from 31 different locations in Iran, using ISSR and RAPD molecular markers. All 31 accessions were assigned into only two clusters without admixed classes. Panthee et al. (2006) studied 179 garlic accessions from Nepal using morphological characters. All 179 accessions were grouped to only three distinct clusters suggesting presence of several duplicates in the collection. Similarly, Jo et al. (2012) studied 120 garlic accessions, from different countries, that were assigned to 4 clusters with only 3 accessions having admixed ancestry. In the present study also, 98% of garlic accessions were found to be restricted to only two clusters as revealed by UPGMA based clustering.
In the present study, AMOVA analysis revealed 84% of the variation due to differences within population variation and only 16% of variation was due to differences among populations suggesting presence of genetic structure. These values are in accordance with results of Zhao et al. (2011) who observed 84.4% variation caused by within population differences and the remaining 15.6% of variance due to divergences between groups.
Model based grouping using STRUCTURE estimated six subpopulations in garlic germplasm based on their genomic fraction (Fig. 3). These results showed the presence of genetically distinct populations. The group I (red), II (green), III (blue), IV (yellow), V (pink) and VI (light blue) had 9, 6, 20, 1, 10 and 7 accessions respectively. In the present study, an accession was allotted to a cluster if atleast 75% of its genome fraction was estimated to belong to that cluster. Accessions with membership probabilities < 0.75 were assigned to an admixed group. Out of 53 accessions, 50 were assigned to six groups, and only 3 accessions were retained in the admixed groups. Cluster III showed presence of one admixture (CL Lamba) and group V showed presence of two admixtures (Desi lasan and CFG-3). Besides these, other accessions which showed some extent of admixing are “Indian garlic”, “AVTG-4”, “PG-32”, PG-35” and “Cheeniaa”. Admixed individuals contain chromosomal segments derived from one population or another (Pritchard et al. 2000; Falush et al. 2003). Interestingly, garlic accession “PG-20” came out to be unique and therefore needs further detailed investigation for some possible wild race or species. Group III was most uniform cluster with 15 (75%) of accessions sharing at least 95% of alleles indicating presence of possible duplicates. Interestingly, out of these 15 accessions, eight (53%) belonged to Uttar Pradesh and six (40.0%) belonged to Maharashtra. This possible duplication explains the presence of garlic clones from same origin in single group. Groups I, II, V and VI also seem to contain at least three duplicate accessions based on sharing of at least 95% alleles. Cunha et al. (2014) studied 130 garlic accessions from Brazil and other countries. All garlic entries in this study could be assigned to two main groups indicating the presence of several duplicates in the collection and therefore a core set with 17 genotypes representing the entire collection was selected. In the present study also, presence of duplicates, especially in landraces, seems to be the reason of accession from same origin present together. Common practice among growers to rename accessions locally could also be the reason of this duplication (Cunha et al. 2014). Some other AFLP based diversity studies in garlic also reported presence of several duplicates (up to 64%) (Volk et al. 2004; Ipek et al. 2005; Lampasona et al. 2012). Unlike to seed grown plants, clonally propagated species like garlic requires regeneration and maintenance every year which in turn raises cost of maintenance. Therefore, molecular marker analysis is quite useful, in the identification of possible duplicates and to further reduce the cost involved in the annual maintenance. Results of cluster analysis, PCA and grouping by population structuring corresponds up to some extent, suggesting reliability of analysis.
Fig. 3.
STRUCTURE output for 53 garlic accessions with K = 6. Numbers on x-axis represents accessions. The y-axis represents genetic proportion in different clusters
Considering that garlic is almost propagated by vegetative means and thus lack genetic recombination, the level of polymorphism observed in this study may therefore be considered high. In the present study, three or more bands of uncertain origin were also observed as reported in previous molecular marker based studies (Ipek et al. 2005, 2008; Jo et al. 2012). Ipek et al. (2005, 2008) and Jo et al. (2012) reported presence of more than one allele at single locus in many garlic accessions, suggesting duplication may be common in large garlic genome. Genome duplication occasions help in multiplying the hereditary materials whereupon evolution can work and gene duplication gives extra hereditary material to mutation, drift and selection to act upon, that outcomes one of a kind or new gene functions. This functional disparity of duplication is considered important for biological evolution and increase in biological complexity. Without gene duplication, the pliancy of a genome or species in adjusting to changing situations would be seriously limited, because no more than two variants (alleles) exist at any locus within a (diploid) individual. That particular copies have added for the progression of some new functions, for instance, the production of floral structures, induction of disease resistance and stress tolerence. Moreover, whole genome duplications that have occurred in the ancestries of a several adapted crop species, including wheat (Triticum aestivum), cotton (Gossypium hirsutum), and soybean (Glycine max), have added to essential agronomic attributes, for example, organic product shape, grain quality, and blooming time. Considering this, genome duplication and presence of unique or specific alleles in a particular accession may contribute to genetic improvement in garlic.
The genotypes from highly divergent groups can be used towards their desirable/complimentary trait for the genetic improvement of garlic effectively (Gupta et al. 1992). Genetically unique accessions, avoiding duplicates, from different clusters should be preserved. As suggested by Shaaf et al. (2014), emphasis should be made on the collection and preservation of landraces, wild and weedy relatives that could help to identify novel genes suitable for local acclimatization and introgression into new cultivars . Besides this, development of fertile garlic clones as discussed by Etoh and Simon (2002) is highly advantageous. The present study shows existence of significant variability in Indian germplasm with potential of further improvement. In our study, we identified four highly diverse genotypes namely PG-20, Indian Garlic, AVTG-4 and CL-Lamba. These highly diverse genotypes can be utilized for further improvement.
Acknowledgements
The authors are grateful to Prof. Gaya Prasad, Hon’ble Vice Chancellor of the Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut, U.P. India for providing facilities and encouragement. Authors are also thankful to PAU; Ludhiana, NHRDF, Karnal and DOGR, Maharashtra for providing garlic material.
Contributor Information
Mukesh Kumar, Email: k.mukesh123@yahoo.com.
Shailendra Sharma, Email: Shgjus6@gmail.com.
References
- Abdoli M, Habibi-Khaniani B, Baghalian K, Shahnazi S, Rassouli H. Classification of Iranian garlic (A. sativum L.) ecotypes using RAPD marker. J Med Plants. 2009;8:45–51. [Google Scholar]
- Avato P, Miccolis V, Tursi F. Agronomic evaluation and essential oil content of garlic (Allium sativum L.) ecotypes grown in southern Italy. Adv Hort Sci. 1998;4:201–204. [Google Scholar]
- Baghalian K, Ziai SA, Naghavi MR, Badi HN, Khalighi A. Evaluation of allicin content and botanical traits in Iranian garlic (Allium sativum L.) ecotypes. Sci Hortic. 2005;103:155–166. doi: 10.1016/j.scienta.2004.07.001. [DOI] [Google Scholar]
- Barboza K, Beretta V, Kozub PC, et al. Microsatellite analysis and marker development in garlic: distributionin EST sequence, genetic diversity analysis, and marker transferability across Alliaceae. Mol Gen Genom. 2018 doi: 10.1007/s00438-018-1442-5. [DOI] [PubMed] [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Bradley KF, Rieger MA, Collins GG. Classification of Australian garlic cultivars by DNA fingerprinting. Aust J Exp Agric. 1996;36:613–618. doi: 10.1071/EA9960613. [DOI] [Google Scholar]
- Chen S, Zhou J, Chen Q, Chang Y, Du J, Meng H. Analysis of the genetic diversity of garlic (Allium sativum L.) germplasm by SRAP. Biochem Syst Ecol. 2013;50:139–146. doi: 10.1016/j.bse.2013.03.004. [DOI] [Google Scholar]
- Chen S, Chen W, Shen X, Yang Y, Qi F, Liu Y. Analysis of the genetic diversity of garlic (Allium sativum L.) by simple sequence repeat and inter simple sequence repeat analysis and agro-morphological traits. Biochem Syst Ecol. 2014;55:260–267. doi: 10.1016/j.bse.2014.03.021. [DOI] [Google Scholar]
- Cunha CP, Hoogerheide ES, Zucchi MI, Monteiro M, Pinheiro JB. New microsatellite markers for garlic, Allium sativum (Alliaceae) Am J Bot. 2012;99:e17–e19. doi: 10.3732/ajb.1100278. [DOI] [PubMed] [Google Scholar]
- Cunha CP, Resende FV, Zucchi MI, Pinheiro JB. SSR-based genetic diversity and structure of garlic accessions from Brazil. Genetica. 2014;142:419–431. doi: 10.1007/s10709-014-9786-1. [DOI] [PubMed] [Google Scholar]
- Doyal JJ, Doyal JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Earl DA, von Holdt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res. 2012;4:359–361. doi: 10.1007/s12686-011-9548-7. [DOI] [Google Scholar]
- Etoh T, Simon PW. Diversity, fertility and seed production of garlic. In: Rabinowitch HD, Currah L, editors. Allium crop science: recent advances. New York: CAB International; 2002. pp. 101–117. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L (1993) Analysis of molecular variance (AMOVA) version 1.55. In: Genetics and Biometry Laboratory, University of Geneva, Switzerland
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction sites. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figliuolo G, Candido V, Logozzo G, Miccolis V, Spagnoletti PL, Zeuli PL. Genetic evaluation of cultivated garlic germplasm (Allium sativum L. and A. ampeloprasum L.) Euphytica. 2001;121:325–334. doi: 10.1023/A:1012069532157. [DOI] [Google Scholar]
- Gous M, Rafii MY, Ismail MR, Puteh AB, Rahim HA, Islam KN, Latif MA. A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. Int J Mol Sci. 2013;14:22499–22528. doi: 10.3390/ijms141122499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VP, Rathore PK, Plaha P. Divergence analysis of some powdery mildew resistance lines of pea. Crop Improv. 1992;19:18–22. [Google Scholar]
- Gupta PK, Baliyan HS, Sharma PC, Ramesh B. Microsatellite in plants: a new class molecular markers. Curr Sci. 1996;70:45–54. [Google Scholar]
- Ipek M, Ipek A, Simon PW. Comparison of AFLPs, RAPD markers, and isozymes for diversity assessment of garlic and detection of putative duplicates in germplasm collection. J Am Soc Hortic Sci. 2003;128:46–252. [Google Scholar]
- Ipek M, Ipek AS, Almquist G, Simon PW. Demonstration of linkage and development of the first low-density genetic map of garlic, based on AFLP markers. Theor Appl Genet. 2005;110:228–236. doi: 10.1007/s00122-004-1815-5. [DOI] [PubMed] [Google Scholar]
- Ipek M, Ipek A, Simon PW. Rapid characterization of garlic clones with locus specific DNA markers. Turk J Agric Sci. 2008;32:357–362. [Google Scholar]
- Jabbes N, Geoffriau E, Le Clerc V, Dridi B, Hannechi C. Inter simple sequence repeat fingerprints for assess genetic diversity of Tunisian garlic populations. J Agric Sci. 2011;3:77–85. [Google Scholar]
- Jaccard P. Nouvelles recherché sur la distribution florale. Bull Soc Vaud Sci Nat. 1908;44:223–270. [Google Scholar]
- Jenderek MM, Hannan RM. Variation in reproductive characteristics and seed production in the USDA garlic germplasm collections. HortScience. 2004;39:48–488. doi: 10.21273/HORTSCI.39.3.485. [DOI] [Google Scholar]
- Jo MH, Ham I, Moe KT, Kwon SW, Lu FH, Park YJ, Kim WS, Won MK, Kim T, Lee EM. Classification of genetic variation in garlic (Allium sativum L.) using SSR markers. Aust J Crop Sci. 2012;6:25–631. [Google Scholar]
- Kamenetsky R. Garlic: botany and horticulture. Hortic Rev. 2007;33:123–172. [Google Scholar]
- Khar A, Devi AA, Lawande KE. Analysis of genetic diversity among Indian garlic (Allium sativum L.) cultivars and breeding lines using RAPD markers. Indian J Genet Plant Breed. 2008;68:52–57. [Google Scholar]
- Kumar V, Sharma S, Sharma AK, Sharma S, Bhat KV. Comparative analysis of diversity based on morpho-agronomic traits and microsatellite markers in common bean. Euphytica. 2009;170:249–262. doi: 10.1007/s10681-009-9965-9. [DOI] [Google Scholar]
- Lampasona GS, Martınez L, Burba JL. Genetic diversity among selected Argentinean garlic clones (Allium sativum L.) using AFLP (Amplified Fragment Length Polymorphism) Source. Euphytica. 2003;132:115–119. doi: 10.1023/A:1024606004596. [DOI] [Google Scholar]
- Lampasona GS, Asprelli P, Burba JL. Genetic analysis of a garlic (Allium sativum L.) germplasm collection from Argentina. Sci Hortic. 2012;138:183–189. doi: 10.1016/j.scienta.2012.01.014. [DOI] [Google Scholar]
- Ma KH, Kwag JG, Zhao W, Dixit A, Lee GA, Kim HH, Chung IM, Kim NS, Lee JS, Ji JJ, Kim TS, Park YJ. Isolation and characteristics of eight novel polymorphic microsatellite loci from the genome of garlic (Allium sativum L.) Sci Hortic. 2009;122:355–361. doi: 10.1016/j.scienta.2009.06.010. [DOI] [Google Scholar]
- Milbourne D, Meyer R, Bradshaw JE, Baird E, Bonar N, Provan J, Powell W, Waugh R. Comparison of PCR-based marker systems for the analysis of genetic relationships in cultivated potato. Mol Breed. 1997;3:127–136. doi: 10.1023/A:1009633005390. [DOI] [Google Scholar]
- Panthee DR, Kc RB, Regmi HN, Subedi PP, Bhattarai S, Dhakal J. Diversity analysis of garlic (Allium sativum L.) germplasms available in Nepal based on morphological characters. Genet Resour Crop Evol. 2006;53:205–212. doi: 10.1007/s10722-004-6690-z. [DOI] [Google Scholar]
- Paredes CM, Becerra VV, Gonzalez AMI. Low genetic diversity among garlic (Allium sativum L.) accessions detected using random amplified polymorphic DNA (RAPD) Chil J Agric Res. 2008;68:3–12. doi: 10.4067/S0718-58392008000100001. [DOI] [Google Scholar]
- Park YJ, Lee JK, Kim NS. Simple sequence repeat polymorphisms (SSRPs) for evaluation of molecular diversity and germplasm classification of minor crops. Molecules. 2009;14:4546–4569. doi: 10.3390/molecules14114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Smouse PE. GENALEX6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes. 2006;6:288–295. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler MR, Simon PW. Characterization and classification of isozyme and morphological variation in a diverse collection of garlic clones. Euphytica. 1993;68:121–130. doi: 10.1007/BF00024161. [DOI] [Google Scholar]
- Prevost A, Wilkinson M. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet. 1999;98:107–112. doi: 10.1007/s001220051046. [DOI] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski JA, Vogel JM, Morgante M, Powel W, Andre C, Tingey SV. Generating and using DNA markers in plants. In: Birren B, Lai E, editors. Non mammalian genomics analysis. A practical guide. San Diego: Academic Press; 1996. pp. 75–134. [Google Scholar]
- Ranjekar PK, Pallotta D, Lafontaine JG. Analysis of plant genomes V. Comparative study of molecular properties of DNAs of seven Allium species. Biochem Genet. 1978;16:957–970. doi: 10.1007/BF00483747. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ (1998) NTSYSpc. Numerical taxonomy and multivariate analysis system. Version 2.20e, Exeter Software, New York
- Shaaf S, Sharma R, Kilian B, Walthe A, Ozkan H, Karami E, Mohammadi B. Genetic structure and eco-geographical adaptation of garlic landraces (Allium sativum L.) in Iran. Genet Res Crop Evol. 2014;61:1565–1580. doi: 10.1007/s10722-014-0131-4. [DOI] [Google Scholar]
- Singh RK, Dubey BK, Gupta RP. Studies on variability and genetic divergence in elite lines of garlic (Allim sativum L.) J Spices Arom Crops. 2012;21:136–144. [Google Scholar]
- Taucher J, Hansel A, Jordan A, Lindinger W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J Agric Food Chem. 1996;44:3778–3782. doi: 10.1021/jf960640e. [DOI] [Google Scholar]
- Tucak M, Cupic T, Popovic S, Stjepanovic M, Gantner R, Meglic V. Agronomic evaluation and utilization of red clover (Trifolium pratense L.) germplasm. Not Bot Horti Agrobot Cluj Napoca. 2009;37:206–210. [Google Scholar]
- Volk GM, Henk AD, Richards CM. Genetic diversity among U.S. garlic clones as detected using AFLP methods. J Am Soc Hortic Sci. 2004;129:559–569. doi: 10.21273/JASHS.129.4.0559. [DOI] [Google Scholar]
- Wang H, Li X, Shen D, Oiu Y, Song J. Diversity evaluation of morphological traits and allicin content in garlic (Allium sativum L.) from China. Euphytica. 2014;198:243–254. doi: 10.1007/s10681-014-1097-1. [DOI] [Google Scholar]
- Zewdie Y, Havey MJ, Prince JP, Jenderek MM. The first genetic linkages among expressed regions of the garlic genome. J Am Soc Hortic Sci. 2005;130(4):569–574. doi: 10.21273/JASHS.130.4.569. [DOI] [Google Scholar]
- Zhao WG, Chung JW, Lee GA, Ma KH, Kim HH, Kim KT, Chung IM, Lee JK, Kim NS, Kim SM, Park YJ. Molecular genetic diversity and population structure of a selected core set in garlic and its relatives using novel SSR markers. Plant Breed. 2011;130:46–54. doi: 10.1111/j.1439-0523.2010.01805.x. [DOI] [Google Scholar]
- Zohary D, Hopf M. Domestication of plants in the Old World. 4. New York: Oxford University Press; 2012. [Google Scholar]