Abstract
Background
A total of 178 825 Enterobacteriaceae isolates collected in 199 hospitals from 42 countries worldwide over 20 years (1997 to 2016) of the SENTRY Program were susceptibility tested by reference broth microdilution methods.
Methods
Trends in percentages over time were analyzed by the χ2 test. Results were reported as the percentage difference between the first (1997–2000) and the last (2013–2016) time period.
Results
Enterobacteriaceae exhibiting resistance to cephalosporins (extended-spectrum β-lactamase [ESBL] phenotype) and carbapenem resistance (CRE) significantly increased (P < 0.05; χ2 test) from 10.3% to 24.0% and 0.6% to 2.9%, respectively. Similar trends were noted for all regions and infection sources. Klebsiella pneumoniae mainly drove the CRE increase. Multidrug-resistance (MDR) rates significantly increased from 7.3% to 15.3% overall, with important trends in all regions and infection sources. Significant increases were noted for MDR K. pneumoniae and Escherichia coli, polymyxin-resistant K. pneumoniae (2.0% to 5.5% overall), and aminoglycoside-resistant E. coli (7.0% to 18.0%) and K. pneumoniae (18.1% to 26.9%) over time in North America and Latin America. Carbapenemase-encoding genes were screened after 2007, and the occurrence of these genes was compared for 2007–2009 and 2014–2016. Among 1298 CRE isolates from the 2 study periods, blaKPC was detected among 186 (49.7%) and 501 (54.2%) isolates in 2007–2009 and 2014–2016, respectively. Metallo-β-lactamase genes were detected among 4.3% of the isolates from 2007 to 2009 and 12.7% of the isolates from 2014 to 2016, mainly due to the dissemination of isolates carrying blaNDM. Genes encoding IMP and VIM enzymes were observed in 1.9% and 2.4% (7 and 9 isolates) of the isolates from 2007 to 2009 and 0.4% and 1.9% of the isolates from 2014 to 2016. OXA-48 and variants increased from 4.3% in 2007–2009 to 12.6% in 2014–2016 (mainly in Europe).
Conclusions
A change in the epidemiology of carbapenemases and important increases in ESBL, CRE, MDR, and other resistant phenotypes among virtually all geographic regions and infection sources were noted in the 20 years of surveillance, highlighting the impact of antimicrobial resistance and the importance of its continuous monitoring.
Keywords: carbapenemases, CRE, Enterobacteriaceae, ESBL, MDR
Acquired resistance among species belonging to the Enterobacteriaceae family limits the antimicrobial therapeutic options for infections caused by these organisms and is a growing cause of concern. Among the numerous resistance mechanisms observed in Enterobacteriaceae species, β-lactamases are especially worrisome because they limit the use of β-lactam agents that have broad spectrum of activity and excellent safety profiles [1]. Enterobacteriaceae isolates producing extended-spectrum β-lactamases (ESBLs), plasmid-encoded cephalosporinases, or carbapenemases are resistant to some or most β-lactams that are used as the traditional first-line options for the treatment of serious infections caused by these pathogens [2].
In addition, Enterobacteriaceae isolates producing β-lactamases often coharbor resistance mechanisms against other antimicrobial classes. Genes encoding resistance to fluoroquinolones, aminoglycosides, tetracyclines, and trimethoprim-sulfamethoxazole are often carried by mobile genetic elements that also carry β-lactamases, promoting the dissemination of resistance to multiple antimicrobial agents concomitantly [3]. Moreover, mutation-driven resistance mechanisms that reduce the affinity of the bacterial target to the antimicrobial agent or cause changes in the expression of outer membrane proteins (porins) or efflux pump systems contribute to a multidrug-resistant (MDR) phenotype among species of the Enterobacteriaceae family.
Multidrug-resistant Enterobacteriaceae isolates that were once uncommon have been reported with increasing frequency. In the European Antimicrobial Resistance Surveillance Network, resistance among Escherichia coli isolates to 3 antimicrobial classes that included the fluoroquinolones, cephalosporins, and aminoglycosides and would be considered MDR ranged from approximately 1% in 2002 [4] to 4.8% in 2016 [5]. In this European survey, MDR Klebsiella pneumoniae rates decreased from 18.9% in 2013 to 15.8% in 2016; however, in 16 European countries these rates ranged from 16.9% to 55.7% in 2016 [5].
Studies demonstrate that inappropriate antimicrobial therapy associated with β-lactamase production and MDR in Enterobacteriaceae species causes higher morbidity and mortality, significantly higher hospital costs, and prolonged hospital stays [3, 6]. Surveillance of antimicrobial resistance is recognized as an important tool at the local, national, and global levels for providing information to (1) establish better guidelines for empiric antimicrobial therapy, (2) promote awareness, and (3) prevent the dissemination of antimicrobial resistance. The SENTRY Antimicrobial Surveillance Program was initiated in 1997, and for over 20 years it has collected and published data on the global and regional resistance levels of the main organisms causing important bacterial and fungal infections.
In this study, we analyzed the trends of resistance phenotypes in the main antimicrobial classes and carbapenemase production among 178 825 Enterobacteriaceae isolates collected in 199 hospitals from 42 countries over 20 years (1997–2016) of the SENTRY Antimicrobial Surveillance Program.
METHODS
Bacterial Isolates
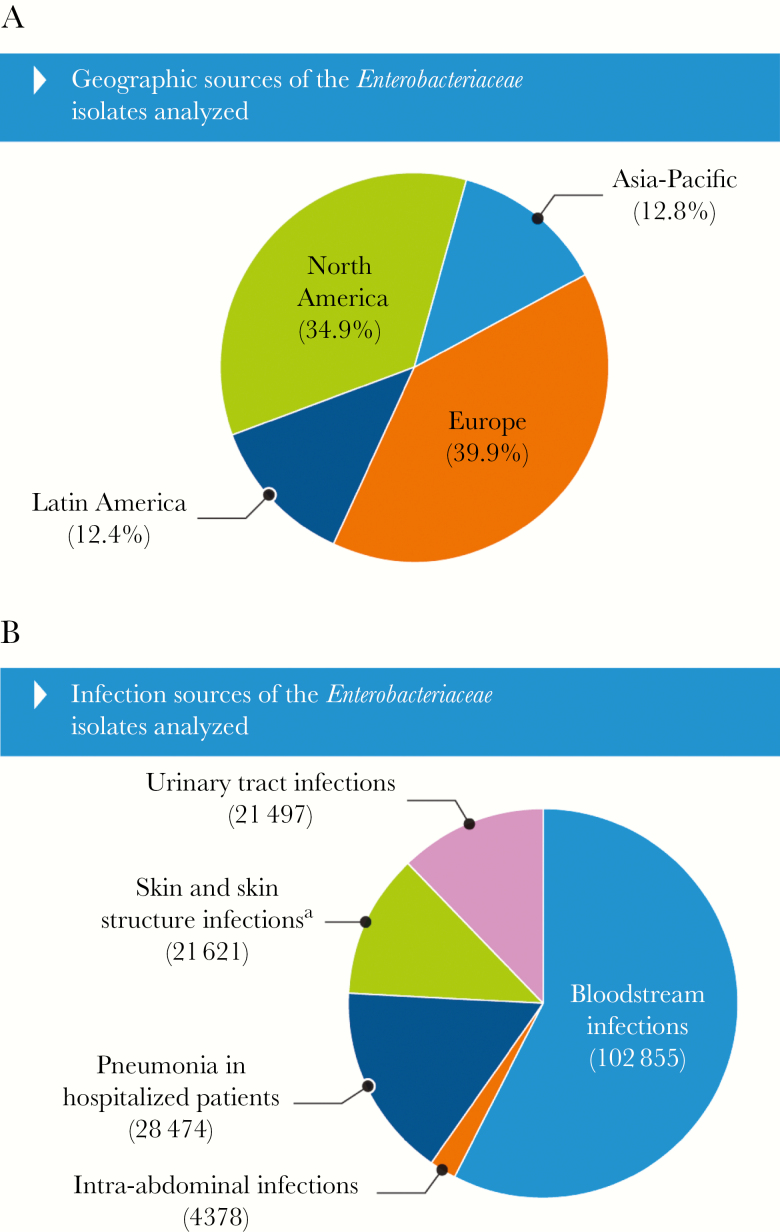
A total of 178 825 Enterobacteriaceae isolates were collected during 1997–2016 from 199 medical centers participating in the SENTRY Program that were distributed in 42 countries located in 4 main geographic regions (Figure 1A). Each participating hospital was asked to submit 1 isolate per patient episode of consecutive bacterial isolates from bloodstream infections (BSIs), skin and skin structure infections (SSSIs), pneumonia in hospitalized patients, urinary tract infections (UTIs), and intra-abdominal tract infections (Figure 1B) determined to be significant by local criteria as the reported cause of infection. Bacterial identification was primarily performed at the participating hospital and confirmed, as needed, using biochemical methods (1997–2011) and/or matrix-assisted laser desorption ionization-time of flight mass spectrometry (2012–2016).
Figure 1.
Geographic (A) and infection sources (B) of the Enterobacteriaceae isolates analyzed. aSkin and skin structure infection isolates were mainly recovered from wounds and abscesses.
Susceptibility Testing
Organisms were susceptibility tested by reference broth microdilution methods in a central laboratory according to the current Clinical and Laboratory Standards Institute (CLSI) documents [7, 8]. Validated broth microdilution panels were manufactured at JMI Laboratories (2015–2016) or by Thermo Fisher Scientific (Cleveland, OH) (1997–2014). Quality control (QC) was performed according to CLSI guidelines, and all QC minimum inhibitory concentration (MIC) results were within acceptable ranges as published in CLSI documents [7]. Categorical interpretations for antimicrobial agents were those found in the CLSI document M100 Ed28 [7], European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint tables [9], and/or the US Food and Drug Administration website (https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm410971.htm).
Phenotype Definitions
The CLSI ESBL-phenotype criteria for epidemiological detection of ESBL-producing organisms was used for E. coli, K. pneumoniae, Klebsiella oxytoca, and Proteus mirabilis and was defined as an MIC value ≥2 mg/L for ceftriaxone, ceftazidime, and/or aztreonam [7].
Isolates exhibiting doripenem, imipenem, and/or meropenem MIC values at ≥2 mg/L were categorized as carbapenem-resistant Enterobacteriaceae (CRE) with the exception of P. mirabilis and indole-positive Proteeae, which were categorized as CRE if doripenem and/or meropenem MIC values were ≥2 mg/L (due to intrinsically elevated imipenem MIC values in these isolates).
Multidrug-resistant Enterobacteriaceae was defined as any isolate nonsusceptible when applying the CLSI criteria [7] to ≥1 agent in ≥3 antimicrobial classes, including penicillins combined with a β-lactamase-inhibitor, fluoroquinolones, aminoglycosides, glycylcyclines, and the polymyxins.
Resistance to fluoroquinolones (levofloxacin, ciprofloxacin, and moxifloxacin), aminoglycosides (amikacin, gentamicin, and tobramycin), cephalosporins/monobactams (cefepime, ceftazidime, ceftriaxone, and aztreonam), carbapenems (doripenem, imipenem, and meropenem), and polymyxins (colistin and polymyxin B) was defined as resistance to any agent tested within the class when applying CLSI breakpoint criteria [7] for all agents except for colistin, which used the EUCAST breakpoint [9].
Carbapenemase Screening
Carbapenem-resistant Enterobacteriaceae isolates were screened for carbapenemases by polymerase chain reaction (PCR) followed by Sanger sequencing (2007–2015) or whole-genome sequencing and analysis (2016). Multiplex PCR for carbapenemase-encoding genes followed by confirmation by singleplex PCR and sequencing of amplicons was performed as previously described [10]. Whole-genome sequencing was performed using high-quality genomic deoxyribonucleic acid on an MiSeq (Illumina, San Diego, CA) platform targeting a 30× coverage. Sequences were de novo assembled (SPAdes 3.9.0) and queried for the presence of acquired carbapenemases using a curated library and applying criteria of >94% sequencing identity and 40% minimum length coverage [11].
Statistical Analysis
Statistical analysis was performed by the χ2 test to compare the 1997–2000 period to the 2013–2016 period using SAS 9.4 (SAS Institute Inc., Cary, NC). Prevalence of carbapenemases among CRE isolates from 2007 to 2009 and 2014 to 2016 were compared. These time periods were chosen because the data were more robust and were temporally separated enough to document changes in trends.
RESULTS
Analysis of Extended-Spectrum β-Lactamases, Carbapenem-Resistant Enterobacteriaceae, and Multidrug-Resistant Phenotypes
Among 178 825 Enterobacteriaceae isolates submitted and tested during the first 20 years of the SENTRY Program, 135 059 were E. coli, K. pneumoniae, K. oxytoca, and P. mirabilis, and 24 313 (18.0% of these species) displayed an ESBL phenotype according to the CLSI criteria [7]. The ESBL-phenotype rates varied from 10.3% in the initial survey period (1997–2000) compared with 24.0% in 2013–2016. This increase was statistically significant, as was the increase observed in the ESBL phenotype for all infection sources and geographic regions (Figure 2A and B). Among the infection types, the increase in ESBL phenotype ranged from 10.6% in UTIs to 15.6% among SSSIs (Figure 2A).
Figure 2.
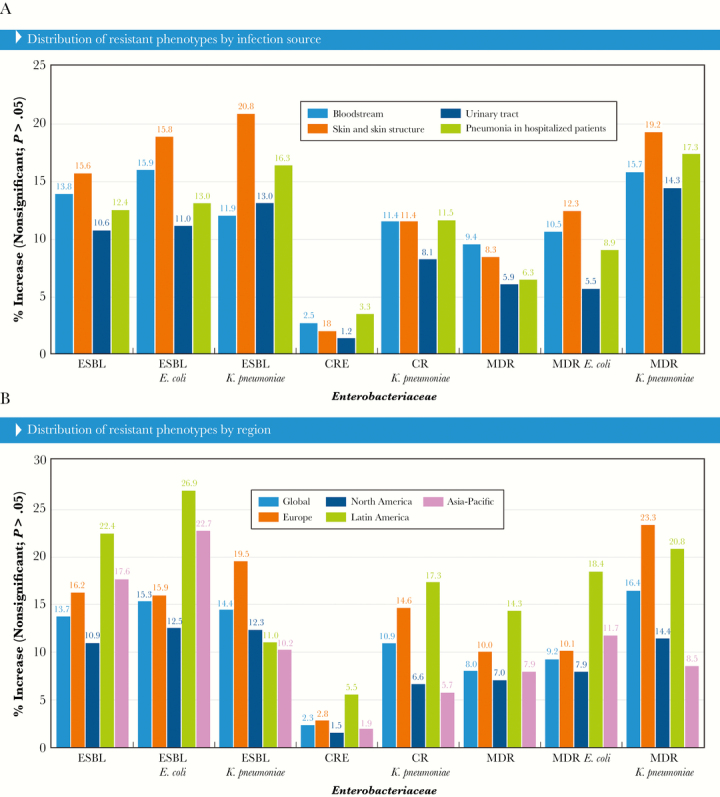
Selected antimicrobial resistance trends for all Enterobacteriaceae by (A) infection source and (B) geographic region. Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended-spectrum β-lactamase; MDR, multidrug-resistant.
The greatest increase in ESBL-phenotype rates was noted for Latin America (22.4% greater in 2013–2016 compared with 1997–2000), followed by Asia-Pacific (17.6% increase), Europe (16.2% increase), and the United States (10.9% increase) (Figure 2B). Isolates exhibiting an ESBL phenotype were mainly E. coli (47.5%) and K. pneumoniae (43.7%), and the occurrence of these isolates ranged from 3.3% to 15.8% for E. coli and 7.1% to 19.4% for K. pneumoniae when comparing the initial and later periods of the survey (Figure 2A and B).
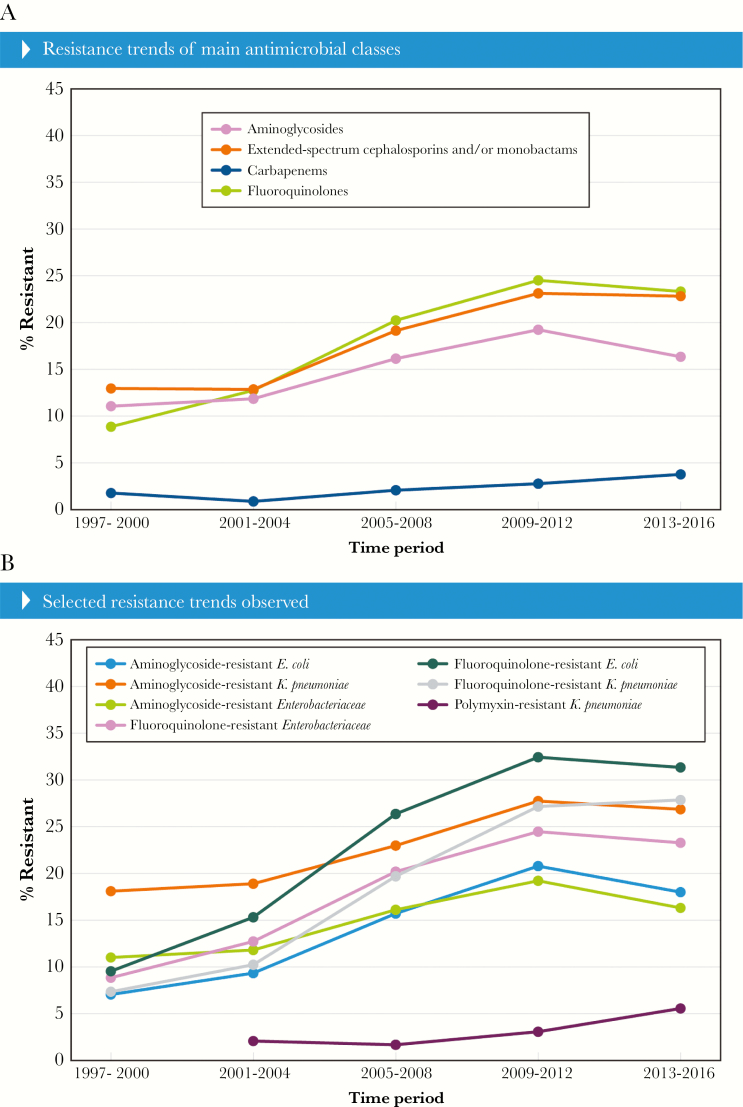
Similar to the ESBL-phenotype rates, resistance to a broad-spectrum cephalosporin (ceftazidime, cefepime, or ceftriaxone) or aztreonam among all Enterobacteriaceae isolates also increased, and 12.9% of the isolates were resistant to these agents in 1997–2000 compared with 22.4% in 2013–2016 (Figure 3A). Again, these numbers were mainly driven by E. coli and K. pneumoniae but also Enterobacter cloacae species complex (data not shown).
Figure 3.
Antimicrobial resistance trends for selected antimicrobial classes of (A) all Enterobacteriaceae or (B) bacterial species.
A statistically significant increase in CRE rates was noted over time for the overall isolates and breakdowns by all regions and infection sources (Figure 2A and B). Carbapenem-resistant Enterobacteriaceae rates increased from 0.6% in 1997–2000 to 2.9% in 2013–2016 (P < 0.05) with gradual increases of 0.8%–0.9% per period since 2005–2008. Carbapenem-resistant Enterobacteriaceae rates increased 1.5%, 1.9%, and 2.8% for the United States, Asia-Pacific, and Europe, respectively; however, a more remarkable increase was noted in Latin America, where CRE rates went from 0.8% to 6.4% (5.6% increase). The increase in CRE rates was more pronounced among isolates recovered from patients hospitalized with pneumonia and BSIs (3.3% and 2.5% increases, respectively), but rates also increased 1.8% for SSSIs and 1.2% for UTIs.
Carbapenem-resistant K. pneumoniae (CR-KPN) was the main driver of the CRE increase, and this species comprised 71.1% of the CRE isolates. The increase in CR-KPN was statistically significant in all regions and among all infection types. It is interesting to note that the increase in CR-KPN was similar among all infection types (11.4% or 11.5%) except for UTI, which had a slightly lower increase (8.1%) throughout the study. Carbapenem-resistant K. pneumoniae increases varied among geographic regions and were higher in Latin America, followed by Europe (Figure 2A and B).
The 4 most common CRE species other than K. pneumoniae were E. cloacae species complex (9.0% of the CRE), Serratia marcescens (5.4%), E. coli (4.2%), and Klebsiella (formerly Enterobacter) aerogenes (3.9%). The number of isolates among these species per year was too small for a trend analysis.
Multidrug-resistant rates significantly increased from 7.3% to 15.3%, but variability was observed among different regions and infection sources analyzed. A significant increase in MDR was noted in all regions and all infection sources (Figure 2A and B). The most common MDR species were K. pneumoniae (35.2%), E. coli (30.2%), E. cloacae (9.7%), P. mirabilis (6.3%), and S. marcescens (5.3%), comprising 86.7% of isolates. A significant increase over time in MDR rates was noted for K. pneumoniae (16.4% increase) and E. coli (9.2% increase) (Figure 2A and B).
Significant increases in resistance to specific antimicrobial classes were observed among the overall isolate population and main species (Figure 3A and B). Aminoglycoside resistance increased in E. coli (7.0% to 18.0%) and K. pneumoniae (18.1% to 26.9%) globally and increased overall in North America (4.0% to 11.3%) and Latin America (22.6% to 30.8%) (Figure 3B). Fluoroquinolone resistance increased from 8.8% to 23.3% among the Enterobacteriaceae isolates mainly because of E. coli (9.5% to 31.4%) and K. pneumoniae (7.3% to 27.9%) (Figure 3B). A significant increase (P < .0001) in polymyxins (polymyxin B or colistin) resistance rates was noted for K. pneumoniae from 2.0% in 2001–2004 when this compound started being tested to 5.5% in 2013–2016 (Figure 3A and B).
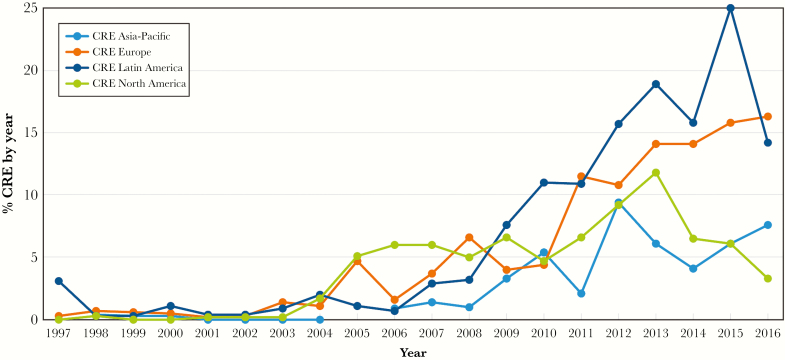
The early analysis of CRE prevalence in the 4 regions demonstrates that an increase from 1.7% to 5.1% occurred in North America from 2004 to 2005, and these rates stayed above 5% until 2016 (Figure 4). Among European countries, an increase in CRE prevalence was noted in 2005 due to the spread of isolates carrying blaVIM in Italy and Greece, but CRE rates were constantly above 5% after 2007 only. For Asia-Pacific and Latin America, CRE rates above 5% were only noted after 2010 and 2008, respectively (Figure 4).
Figure 4.
Carbapenem-resistant Enterobacteriaceae (CRE) trends over years by region.
The participation of each country in the survey was not consistent in all cases. Each country’s participation and the variations in the ESBL, CRE, and MDR phenotype rates are demonstrated in Figure 5. Among 29 countries that participated in at least 3 defined periods that include 2013–2016, the ESBL rates increased in all countries, except New Zealand, where the ESBL-phenotype rate decreased, and Greece, where the ESBL-phenotype rates were constantly high during the study. Carbapenem-resistant Enterobacteriaceae rates increased by less than 2% in 11 of the 29 countries with consistent participation. A moderate CRE increase (5.2% to 11.6%) was observed in 11 other countries, and a remarkable increase in the CRE occurrence was observed in Poland (28.8% difference from the initial and later periods). Carbapenem-resistant Enterobacteriaceae rates were unchanged or decreased in 6 countries. Finally, MDR rates increased in all countries, except Greece and Hong Kong.
Figure 5.
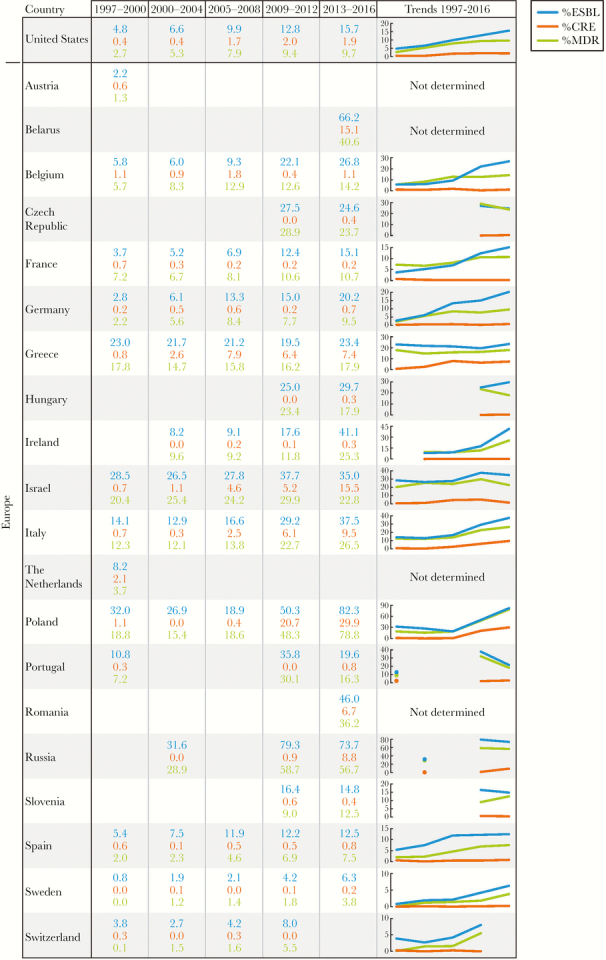
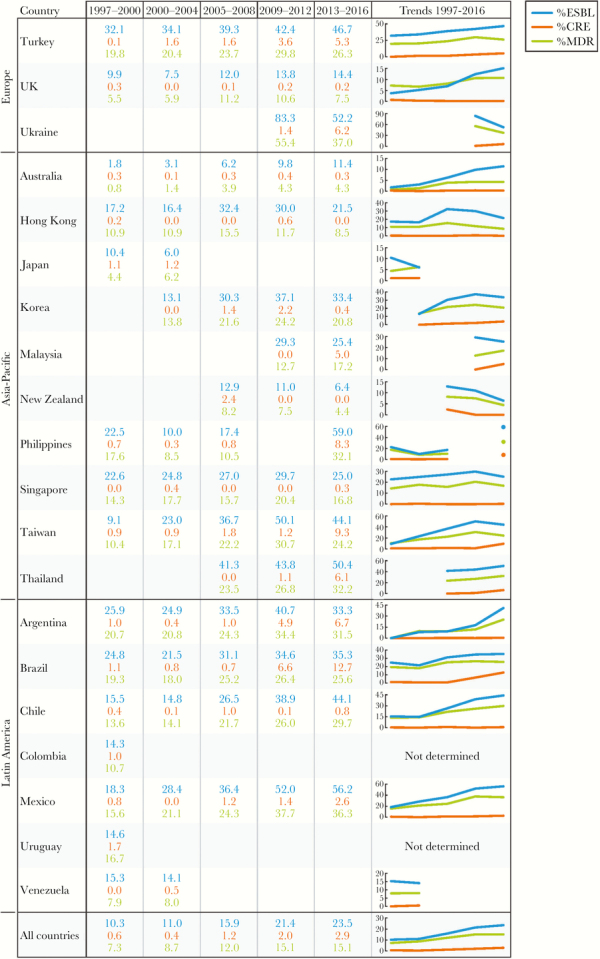
Extended-spectrum β-lactamase (ESBL), carbapenem-resistant Enterobacteriaceae (CRE), and multidrug-resistant (MDR) rates among countries participating in the SENTRY Program. (Figure continues on next page)
Analysis of Carbapenemase Screening Results
Among 2717 CRE isolates, a total of 1788 isolates collected over the years were screened for the presence of carbapenemase genes. Carbapenemase-encoding genes were consistently screened after 2007, and 2007–2009 and 2014–2016 were the periods compared in this analysis. The number of isolates and species observed for each year in these 2 periods are listed in Table 1.
Table 1.
CRE Isolates Recovered in 2 Periods When Isolates Were Screened for Carbapenemase-Encoding Genes
| No. of Isolates by Year | |||||||
|---|---|---|---|---|---|---|---|
| CRE Species | Total Isolates | 2007 | 2008 | 2009 | 2014 | 2015 | 2016 |
| Klebsiella pneumoniae | 997 | 75 | 79 | 95 | 224 | 273 | 251 |
| Enterobacter cloacae species complex | 69 | 11 | 19 | 16 | 19 | 24 | 23 |
| Escherichia coli | 52 | 3 | 9 | 7 | 9 | 13 | 11 |
| Serratia marcescens | 44 | 7 | 5 | 8 | 7 | 12 | 5 |
| Klebsiella oxytoca | 30 | 6 | 2 | 5 | 4 | 3 | 10 |
| Klebsiella aerogenes | 27 | 7 | 3 | 4 | 6 | 4 | 3 |
| Citrobacter freundii species complex | 9 | 1 | 5 | 5 | |||
| Proteus mirabilis | 6 | 1 | 1 | 2 | 2 | ||
| Enterobacter spp. | 4 | 1 | 2 | 1 | |||
| Raoultella ornithinolytica | 3 | 1 | 1 | 1 | |||
| Pluralibacter gergoviae | 2 | 1 | 1 | ||||
| Providencia stuartii | 2 | 1 | 1 | ||||
| Raoultella planticola | 2 | 2 | |||||
| Serratia spp. | 2 | 2 | |||||
| Hafnia alvei | 1 | 1 | |||||
| Morganella morganii | 1 | 1 | |||||
| Pantoea agglomerans | 1 | 1 | |||||
| Raoultella spp. | 1 | 1 | |||||
| Total | 1298 | 111 | 126 | 137 | 277 | 333 | 314 |
Abbreviation: CRE, carbapenem-resistant Enterobacteriaceae.
A total of 1298 CRE isolates were observed in these 2 study periods, 374 isolates from 2007 to 2009 and 924 from 2014 to 2016, and 981 isolates were positive for 1 or 2 carbapenemase-encoding genes. Among the carbapenemase-producing Enterobacteriaceae (CPE) from 2014 to 2016, K. pneumoniae was the most common species (689 of 849), followed by E. cloacae (72 of 849). Carbapenemase-producing Enterobacteriaceae isolates were detected from 68 sites in both periods, 30 hospitals in 12 countries during 2007–2009 versus 55 hospitals in 25 countries during 2014–2016. It is noteworthy that 58.6% of the CPE isolates were detected in only 10 participating hospitals.
Isolates carrying K. pneumoniae carbapenemase (KPC)-encoding genes were the most common in both periods and accounted for 186 (49.7% of the CRE) and 501 (54.2%) isolates in 2007–2009 and 2014–2016, respectively (Table 2). Differently from KPC-harboring isolates that were observed in similar rates in both study periods, a remarkable increase in isolates carrying genes encoding metallo-β-lactamases (MBLs) and oxacillinases (OXAs) was noted. Metallo-β-lactamase-carrying isolates were detected among 4.3% of the isolates from 2007 to 2009 and 12.7% of the isolates from 2014 to 2016 (Table 2). This increase was mainly due to the dissemination of isolates carrying blaNDM that were not observed in 2007–2009 and corresponded to 10.9% of the CRE isolates from 2014 to 2016. Genes encoding IMP and VIM enzymes were observed in 1.9% and 2.4% (7 and 9 isolates) of the isolates from 2007 to 2009 and 0.4% and 1.9% of the isolates from 2014 to 2016 (Table 2). Isolates carrying OXA genes, which were mainly OXA-48 and variants and 1 OXA-23, increased from 4.3% in 2007–2009 to 12.6% in 2014–2016 and were detected mainly in Turkey. The numbers described include only isolates carrying 1 carbapenemase gene; however, 26 isolates harboring 2 carbapenemase genes were noted in 2014–2016. The most common combination (19 of 26 isolates) included genes encoding an OXA-48 plus an MBL, usually blaNDM-1. Other combinations were a KPC plus MBL or OXA-48 (4 and 3 isolates, respectively). In addition, the rates of CRE isolates not carrying carbapenemases decreased remarkably from 41.5% in 2007–2009 to 17.5% in 2014–2016 (Table 2).
Table 2.
Carbapenemase Genes Detected Among CRE Isolates From 2007 to 2009 and 2014 to 2016
| 2007–2009 | 2014–2016 | ||||
|---|---|---|---|---|---|
| Carbapenemase Group/Carbapenemase | No. of Isolates From Both Periods | n | % | n | % |
| MBL | 133 | 16 | 4.3 | 117 | 12.7 |
| IMP | 11 | 7 | 1.9 | 4 | 0.4 |
| IMP-1 | 3 | 3 | 0.8 | ||
| IMP-4 | 3 | 1 | 0.3 | 2 | 0.2 |
| IMP-8 | 1 | 1 | 0.1 | ||
| IMP-18 | 1 | 1 | 0.3 | ||
| IMP-26 | 2 | 2 | 0.5 | ||
| IMP-64 | 1 | 1 | 0.1 | ||
| NDM | 95 | 95 | 10.3 | ||
| NDM-1 | 88 | 88 | 9.5 | ||
| NDM-7 | 5 | 5 | 0.5 | ||
| NDM-9 | 2 | 2 | 0.2 | ||
| VIM | 27 | 9 | 2.4 | 18 | 1.9 |
| VIM-1 | 21 | 7 | 1.9 | 14 | 1.5 |
| VIM-2 | 1 | 1 | 0.3 | ||
| VIM-4 | 3 | 3 | 0.3 | ||
| VIM-5 | 1 | 1 | 0.1 | ||
| VIM-23 | 1 | 1 | 0.3 | ||
| Serine carbapenemases | 690 | 187 | 50.0 | 503 | 54.4 |
| KPC | 687 | 186 | 49.7 | 501 | 54.2 |
| KPC-2 | 249 | 52 | 13.9 | 197 | 21.3 |
| KPC-3 | 336 | 42 | 11.2 | 294 | 31.8 |
| KPC-4 | 2 | 2 | 0.2 | ||
| KPC-6 | 1 | 1 | 0.1 | ||
| KPC-12 | 1 | 1 | 0.1 | ||
| KPC-17 | 1 | 1 | 0.1 | ||
| KPC-20 | 1 | 1 | 0.1 | ||
| KPC-likea | 96 | 92 | 24.6 | 4 | 0.4 |
| SME | 3 | 1 | 0.3 | 2 | 0.2 |
| SME-2 | 1 | 1 | 0.3 | ||
| SME-4 | 2 | 2 | 0.2 | ||
| OXA | 132 | 16 | 4.3 | 116 | 12.6 |
| OXA-23 | 1 | 1 | 0.1 | ||
| OXA-48 | 128 | 16 | 4.3 | 112 | 12.1 |
| OXA-163 | 1 | 1 | 0.1 | ||
| OXA-232 | 1 | 1 | 0.1 | ||
| OXA-244 | 1 | 1 | 0.1 | ||
| Double carbapenemases | 26 | 26 | 2.8 | ||
| KPC+MBL | 4 | 4 | 0.4 | ||
| KPC-2, NDM-1 | 1 | 1 | 0.1 | ||
| KPC-3, NDM-1 | 1 | 1 | 0.1 | ||
| KPC-3, VIM-1 | 1 | 1 | 0.1 | ||
| KPC-17, NDM-1 | 1 | 1 | 0.1 | ||
| MBL+OXA-48 | 19 | 19 | 2.1 | ||
| NDM-1, OXA-232 | 10 | 10 | 1.1 | ||
| NDM-1, OXA-48 | 5 | 5 | 0.5 | ||
| NDM-5, OXA-232 | 2 | 2 | 0.2 | ||
| VIM-1, OXA-48 | 2 | 2 | 0.2 | ||
| KPC+OXA-48 | 3 | 3 | 0.3 | ||
| KPC-3, OXA-48 | 3 | 3 | 0.3 | ||
| Negative | 281 | 124 | 33.2 | 157 | 17.0 |
| Not tested | 36 | 31 | 8.3 | 5 | 0.5 |
Abbreviations: CRE, carbapenemase-resistant Enterobacteriaceae; IMP, imipenemase metallo-β-lactamase; KPC, K. pneumoniae carbapenemase; MBL, metallo-β-lactamase; NDM, New Delhi metallo-β-lactamase; OXA, oxacillinase.
aAmplicon sequencing was not performed. Isolates were tested using a multiplex reaction and reamplified using singleplex.
DISCUSSION
During the 20 years of the SENTRY Program, we systematically collected and tested >178 000 Enterobacteriaceae isolates and demonstrated an increase in cephalosporin-resistance (ESBL phenotype) among E. coli and K. pneumoniae, CRE, and MDR organisms. Cephalosporin resistance in E. coli dramatically increased after the 2005–2008 period (4.6% to 10.4% in 2009–2012). This increase has been noticed by others and has been associated with the worldwide dissemination of the E. coli ST131 lineage that carries blaCTX-M-15 [12–14]. This observation is corroborated by the increase in fluoroquinolone resistance from 3.6% in 1997–2000 to 11.8% in 2001–2014 and 21.8% in 2005–2008, since it has been established that blaCTX-M-15 was acquired by an ST131 E. coli isolate that was already resistant to fluoroquinolones [15]. Moreover, some detailed studies were performed using SENTRY Program isolates from US hospitals that confirmed the presence of ST131, blaCTX-M-15, and fluoroquinolone resistance in this collection [16–18].
Among K. pneumoniae isolates, the increase in resistance to carbapenems occurred exponentially over the study, and the ESBL-phenotype rates increased almost 15% from 1997–2000 to 2013–2016 (21.7% to 36.1%). We have documented a shift from the predominance of SHV genes encoding ESBLs in K. pneumoniae to a majority of isolates carrying blaCTX-M in US hospitals after 2013 [19], similar to other literature reports [20–22]. The dissemination of carbapenemases that also encode resistance to cephalosporins contributed to the increase in the ESBL-phenotype rates in K. pneumoniae.
Another significant finding was the increase in carbapenem resistance overall and dominantly among K. pneumoniae. Carbapenem-resistant Enterobacteriaceae rates have been observed closely during the past 10 years of the SENTRY Program, and genetic analysis of these isolates has been performed to identify the occurrence of carbapenemases [10, 23]. Not only was a significant increase in the CRE rates (0.6% to 2.9%) detected worldwide and in all infection sources monitored, but a dramatic change in the epidemiology of the carbapenemase genes was observed. These changes have also been reported in the literature, and our data support the observations that KPC-encoding genes continue to prevail in several geographic areas and that the dissemination of isolates carrying genes encoding NDM and OXA-48 variants have contributed to the increasing CRE rates in the period after 2012 [24]. It is noteworthy that the CRE isolates not carrying carbapenemases decreased over time, and this trend should be monitored closely after introducing the new β-lactam/β-lactamase inhibitor combinations that display activity against serine-carbapenemase-producing isolates.
Resistance to other antimicrobial classes important for treating infections caused by Enterobacteriaceae also increased, and a remarkable increase of MDR isolates was also documented. Multidrug-resistant Enterobacteriaceae often are secondarily important because MDR nonfermentative organisms are more common and challenging for patient management [3]; however, Enterobacteriaceae isolates resistant to >3 antimicrobial classes greatly limit therapeutic options and have become more common regardless of the infection source or geographic region and are observed among the most common Enterobacteriaceae species.
Our data demonstrated increasing resistance trends that the medical and scientific communities have been monitoring over the years. Since 1995, when the American Society for Microbiology assembled a task force to address the issue of the increase in antimicrobial resistance, surveillance initiatives have been highlighted as an important tool for assisting in this endeavor [25]. Different surveillance programs fulfill the interests of prescribing physicians, microbiologists, infection control specialists, public health and regulatory authorities, and the pharmaceutical industry, and all have well recognized biases or inaccuracies [26, 27]. Nevertheless, these surveys provide important information that allows the identification of trends in pathogen incidence and antimicrobial resistance. The importance of surveillance has been continuously reinforced by several organizations and expert panels recognize that surveillance in local, regional, and global levels is essential to address antimicrobial resistance issues. Furthermore, global surveillance is recognized as providing (1) early warnings of emerging threats and (2) data to identify and act on long-term trends. Knowing local and regional resistance levels provides important information for empiric antimicrobial therapy decision options (https://amr-review.org).
CONCLUSIONS
The SENTRY Program is the largest and longest-operating worldwide surveillance initiative that comprises the most important human infections, provides consistently high-quality data, and evaluates timely trends in antimicrobial resistance. This program does not address important issues such as antibiotic use, how antibiotic therapy benefits patients directly and indirectly, or clinical outcomes; however, it addresses issues that have been a great concern to the infectious diseases and clinical microbiology communities for many years.
Acknowledgments
We thank all participants of the SENTRY Program for their work in providing isolates. The authors would like to thank C. Smith for performing the statistical analysis.
Financial support. Funding for the manuscript was provided by JMI Laboratories.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Rodríguez-Baño J, Pascual A. Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther 2008; 6:671–83. [DOI] [PubMed] [Google Scholar]
- 2. Rodriguez-Bano J, Gutierrez-Gutierrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev 2018; 31:e00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delgado-Valverde M, Sojo-Dorado J, Pascual A, Rodríguez-Baño J. Clinical management of infections caused by multidrug-resistant Enterobacteriaceae. Ther Adv Infect Dis 2013; 1:49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ECDC. Antimicrobial Resistance Surveillance in Europe: Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net), 2009. European Centre for Disease Prevetion and Control; 2010. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf. Accessed 30, August 2018. [Google Scholar]
- 5. ECDC. Surveillance of Antimicrobial Resistance in Europe: Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2016. European Centre for Disease Prevention and Control; 2017. Available at: https://ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-Europe-2016.pdf. Accessed 30, August 2018. [Google Scholar]
- 6. Pitout JD. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs 2010; 70:313–33. [DOI] [PubMed] [Google Scholar]
- 7. CLSI. M100Ed28E. Performance Standards for Antimicrobial Susceptibility Testing: 28th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 8. CLSI. M07Ed11E. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. 11th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 9. EUCAST. Breakpoint Tables for Interpretation of MIC’s and Zone Diameters. Version 8.0, January 2018. European Committee on Antimicrobial Susceptibility Testing; 2018. Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf. Accessed 31, January 2018. [Google Scholar]
- 10. Castanheira M, Mendes RE, Woosley LN, Jones RN. Trends in carbapenemase-producing Escherichia coli and Klebsiella spp. from Europe and the Americas: report from the SENTRY antimicrobial surveillance programme (2007-09). J Antimicrob Chemother 2011; 66:1409–11. [DOI] [PubMed] [Google Scholar]
- 11. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rossolini GM, D’Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect 2008; 14(Suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- 13. Cantón R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol 2006; 9:466–75. [DOI] [PubMed] [Google Scholar]
- 14. Livermore DM, Canton R, Gniadkowski M, et al. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 2007; 59:165–74. [DOI] [PubMed] [Google Scholar]
- 15. Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev 2014; 27:543–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson JR, Porter S, Thuras P, Castanheira M. The pandemic H30 subclone of sequence type 131 (ST131) as the leading cause of multidrug-resistant Escherichia coli infections in the United States (2011–2012). Open Forum Infect Dis 2017; 4:ofx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson JR, Porter S, Thuras P, Castanheira M. Epidemic emergence in the United States of Escherichia coli sequence type 131-H30 (ST131-H30), 2000 to 2009. Antimicrob Agents Chemother 2017; 61:e00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson JR, Johnston B, Clabots C, et al. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 2010; 51:286–94. [DOI] [PubMed] [Google Scholar]
- 19. Castanheira M, Mendes RE, Jones RN, Sader HS. Changes in the frequencies of β-lactamase genes among Enterobacteriaceae isolates in U.S. Hospitals, 2012 to 2014: activity of ceftazidime-avibactam tested against β-lactamase-producing isolates. Antimicrob Agents Chemother 2016; 60:4770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rocha FR, Pinto VP, Barbosa FC. The spread of CTX-M-type extended-spectrum β-lactamases in Brazil: a systematic review. Microb Drug Resist 2016; 22:301–11. [DOI] [PubMed] [Google Scholar]
- 21. Calbo E, Garau J. The changing epidemiology of hospital outbreaks due to ESBL-producing Klebsiella pneumoniae: the CTX-M-15 type consolidation. Future Microbiol 2015; 10:1063–75. [DOI] [PubMed] [Google Scholar]
- 22. D’Andrea MM, Arena F, Pallecchi L, Rossolini GM. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 2013; 303:305–17. [DOI] [PubMed] [Google Scholar]
- 23. Kaiser RM, Castanheira M, Jones RN, et al. Trends in Klebsiella pneumoniae carbapenemase-positive K. pneumoniae in US hospitals: report from the 2007-2009 SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis 2013; 76:356–60. [DOI] [PubMed] [Google Scholar]
- 24. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 2017; 215:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones RN. The emergent needs for basic research, education, and surveillance of antimicrobial resistance. Problems facing the report from the American Society for Microbiology Task Force on Antibiotic Resistance. Diagn Microbiol Infect Dis 1996; 25:153–61. [DOI] [PubMed] [Google Scholar]
- 26. Dalhoff A. Resistance surveillance studies: a multifaceted problem–the fluoroquinolone example. Infection 2012; 40:239–62. [DOI] [PubMed] [Google Scholar]
- 27. Tacconelli E, Sifakis F, Harbarth S, et al. Surveillance for control of antimicrobial resistance. Lancet Infect Dis 2018; 18:e99–106. [DOI] [PubMed] [Google Scholar]