Abstract
Background
Acinetobacter calcoaceticus–A. baumannii (Acb) complex and Stenotrophomonas maltophilia represent frequent causes of hospital-acquired infections. We evaluated the frequency and resistance rates of Acb complex and S. maltophilia isolates from medical centers enrolled in the SENTRY Program.
Methods
A total of 13 752 Acb complex and 6467 S. maltophilia isolates were forwarded to a monitoring laboratory by 259 participating sites from the Asia-Pacific region, Latin America, Europe, and North America between 1997 and 2016. Confirmation of species identification and antimicrobial susceptibility testing were performed using conventional methods and/or matrix-assisted laser desorption ionization–time of flight mass spectrometry and the broth microdilution method, respectively. Antimicrobial susceptibility results were interpreted by CLSI and EUCAST 2018 criteria.
Results
Acb complex and S. maltophilia were most frequently isolated from patients hospitalized with pneumonia (42.9% and 55.8%, respectively) and bloodstream infections (37.3% and 33.8%, respectively). Colistin and minocycline were the most active agents against Acb complex (colistin MIC50/90, ≤0.5/2 mg/L; 95.9% susceptible) and S. maltophilia (minocycline MIC50/90, ≤1/2 mg/L; 99.5% susceptible) isolates, respectively. Important temporal decreases in susceptibility rates among Acb complex isolates were observed for all antimicrobial agents in all regions. Rates of extensively drug-resistant Acb complex rates were highest in Europe (66.4%), followed by Latin America (61.5%), Asia-Pacific (56.9%), and North America (38.8%). Among S. maltophilia isolates, overall trimethoprim-sulfamethoxazole (TMP-SMX) susceptibility rates decreased from 97.2% in 2001–2004 to 95.7% in 2013–2016, but varied according to the geographic region.
Conclusions
We observed important reductions of susceptibility rates to all antimicrobial agents among Acb complex isolates obtained from all geographic regions. In contrast, resistance rates to TMP-SMX among S. maltophilia isolates remained low and relatively stable during the study period.
Keywords: gram-negative bacilli, nonfermentative, surveillance, multidrug resistance, carbapenem resistant
In the late 1970s when the first infections caused by Acinetobacter spp. were reported, this pathogen was considered a commensal opportunist of minimal clinical significance [1]. Since then, these organisms have emerged as important nosocomial pathogens [2]. It has been estimated that 45 000 US and 1 million global cases of Acinetobacter infections occur per year [3, 4]. Acinetobacter baumannii is the most clinically important species of over 60 Acinetobacter species described (http://apps.szu.cz/anemec/Classification.pdf). As some clinically relevant Acinetobacter species, such as A. baumannii, A. nosocomialis, and A. pittii, as well as the environmental species A. calcoaceticus, are difficult to distinguish from each other using conventional identification methods, these species have been collectively designated members of the so-called A. calcoaceticus–A. baumannii (Acb) complex [5]. Although Acinetobacter spp. may colonize the skin and respiratory tract of healthy individuals, community-acquired infections caused by A. baumannii are uncommon [4, 6, 7]. In contrast, in the nosocomial setting, especially intensive care units, the frequency of A. baumannii has increased over time [4]. Due to its ability to survive with minimal nutrient requirements and to resist desiccation, A. baumannii may persist in the nosocomial environment, being transmitted by hands of health care workers and/or contaminated medical equipment [2, 4, 5, 8]. Although there has been controversy regarding the mortality directly attributed to A. baumannii infections, these infections showed crude mortality rates varying from 26.0% to 61.6%, with inadequate empirical therapy importantly contributing to these elevated rates [9–11].
A. baumannii is intrinsically resistant to penicillins and has acquired genes resistant to virtually all antibiotics capable of treating gram-negative bacteria, including fluoroquinolones, aminoglycosides, and cephalosporins [2, 4, 5]. Carbapenems are usually the therapeutic agents of choice for A. baumannii infections susceptible to these antimicrobials [2, 4, 5, 9–11]. Various mechanisms of carbapenem resistance have been reported in A. baumannii, such as porin alteration, hyperexpression of efflux systems, and production of carbapenemases [2, 4, 5]. Among those, the acquisition of carbapenem-hydrolyzing class D β-lactamase (CHDL)–encoding genes, such as blaOXA-23-like, blaOXA-24/40, blaOXA-58, blaOXA-143-like, and blaOXA-235-like, is the most frequently reported [2, 5, 12]. A. baumannii also possesses the intrinsic blaOXA-51, which encodes for OXA-51, that confers resistance to carbapenems only when overexpressed [13]. In addition, A. baumannii may acquire class B β-lactamase–encoding genes, such as blaIMP, blaVIM, blaSIM, and blaNDM [4, 13]. Because of these mechanisms of resistance, polymyxins have been considered the antibiotics of choice for treating carbapenem-resistant A. baumannii infections [2, 4, 5]. However, resistance to this class of antibiotics has already been reported in many geographic regions [14–17].
Like Acinetobacter spp., Stenotrophomonas maltophilia once was also considered a pathogen of low virulence [18]. To date, S. maltophilia has been increasingly recognized as a cause of nosocomial infections, especially pneumonia in mechanically ventilated patients and bloodstream infections in neutropenic patients [19, 20]. Limited therapeutic options exist for infections due to S. maltophilia because of its intrinsic resistance to most antibiotics, including penicillin, cephalosporins, carbapenems, and aminoglycosides [18, 21]. Trimethoprim-sulfamethoxazole (TMP-SMX) has been recommended as the drug of choice for treatment of S. maltophilia infections [18, 22]; however, resistance to TMP-SMX has emerged worldwide [23–26]. The main mechanism of TMP-SMX resistance in S. maltophilia has been reported as acquisition of the sul1, sul2, and drfA genes [23–26]. Although sul genes codify a dihydropteroate synthase, dfrA genes encode for a dihydrofolate reductase resistant to action of sulfonamides and trimethoprim. Inappropriate empirical therapy for S. maltophilia infections has been associated with higher mortality rates (up to 37.5%) [27].
The SENTRY Antimicrobial Surveillance Program was designed to track antimicrobial resistance tendencies and the spectrum of activity of antimicrobials against most clinically significant pathogen isolates from North America, Europe, Asia-Pacific, and Latin America. Although previous publications have partially described the SENTRY Program results, the present study evaluated the frequency of occurrence and antimicrobial susceptibility patterns of the entire collection of Acb complex and S. maltophilia isolates from medical centers enrolled in the SENTRY Antimicrobial Surveillance Program between the years 1997 and 2016. Changes over time in susceptibility patterns were also studied.
METHODS
Bacterial Isolates
A total of 13 752 Acb complex and 6467 S. maltophilia isolates were consecutively collected from 259 medical centers located in the Asia-Pacific (49 centers, 11 countries), Latin American (17 centers, 7 countries), European (66 centers, 23 countries), and North American (127 centers, 2 countries) regions from January 1997 to December 2016 through the SENTRY Program. Data from India and China were excluded from the analysis because these countries participated in the program only for a few years and contributed highly resistant isolates, which could have introduced bias. The participating centers were guided by a common protocol to collect single isolates from patients hospitalized with pneumonia, bloodstream, skin and skin structure, intra-abdominal, or urinary tract infections. Species identification was performed at the participating medical centers and confirmed at the monitoring laboratory using conventional methods and/or matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). For the purpose of this study, isolates identified as A. baumannii, A. calcoaceticus, A. nosocomialis, A. pittii, and A. calcoaceticus–A. baumannii complex were collectively designated Acb complex isolates.
Antimicrobial Susceptibility Testing
Isolates were tested for susceptibility to various antimicrobial agents at the monitoring laboratory using the broth microdilution method [28]. Minimum inhibitory concentration (MIC) results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) [29] recommendations and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria [30] for all antimicrobials, except for ampicillin-sulbactam, ceftazidime, cefepime, and minocycline, which were interpreted only according to CLSI criteria [29]. An extensively drug-resistant (XDR) Acb complex isolate was defined as any isolate not susceptible by EUCAST criteria to ≥1 drug of ≥3 of the following classes: aminoglycosides, carbapenems, fluoroquinolones, and polymyxins; a pandrug-resistant (PDR) isolate was defined as any isolate nonsusceptible to all tested antimicrobials of the 4 classes listed for XDR. Of note, a polymyxin (colistin or polymyxin B) was not tested until 2001; thus, Acb complex isolates from 1997–2000 were excluded from the PDR analysis. Among Acb complex isolates, susceptibility rates observed in the 1997–2000 and 2013–2016 periods were compared, except for colistin and ampicillin-sulbactam, which have been tested since 2005. Among S. maltophilia isolates, TMP-SMX susceptibility rates observed in 4-year intervals were also compared over time.
RESULTS
During the study period, a total of 13 752 isolates identified as Acb complex were collected from medical centers located in Asia-Pacific (16.9%), Europe (32.9%), Latin America (24.5%), and North America (25.6%). In general, Acb complex isolates were more frequently collected from patients hospitalized with pneumonia (n = 5896, 42.9% of isolates) and bloodstream infections (n = 5123, 37.3%) than other infection types, as shown in Table 1. However, Acb complex isolates were more frequently isolated from bloodstream infections (n = 1548, 46.0%) than pneumonia (n = 1297, 38.5%) in patients hospitalized at Latin American medical centers. The number of Acb complex isolates collected from bloodstream infections (n = 1805, 39.8%) was similar to that collected from pneumonia (n = 1867, 41.2%) in patients hospitalized at European medical centers.
Table 1.
Distribution of 13 752 Acinetobacter calcoacetius–A. baumannii Complex Isolates by Geographic Region and Infection Type (SENTRY Program, 1997–2016)
| Number of Organisms by Geographic Region (%) | |||||
|---|---|---|---|---|---|
| Infection Type | Asia-Pacific | Europe | Latin America | North America | Total |
| Bloodstream | 594 (25.5) | 1805 (39.8) | 1548 (46.0) | 1176 (33.3) | 5123 (37.3) |
| Intra-abdominal | 1 (0.04) | 53 (1.2) | 0 (0.0) | 32 (0.9) | 86 (0.6) |
| Pneumonia in hospitalized patient | 1271 (54.6) | 1867 (41.2) | 1297 (38.5) | 1460 (41.4) | 5895 (42.9) |
| Skin and skin structure | 422 (18.1) | 657 (14.5) | 476 (14.1) | 620 (17.6) | 2175 (15.8) |
| Urinary tract | 37 (1.6) | 131 (2.9) | 32 (1.0) | 174 (4.9) | 374 (2.7) |
| Others | 2 (0.09) | 18 (0.4) | 14 (0.4) | 65 (1.8) | 99 (0.7) |
| Total | 2327 (16.9) | 4531 (32.9) | 3367 (24.5) | 3527 (25.6) | 13 752 (100.0) |
The activity of distinct antimicrobial agents tested against 13 752 Acb complex isolates displayed by geographic region is in Table 2. Acb complex showed reduced susceptibility to most antimicrobials tested, with no antimicrobial capable of inhibiting the growth of all Acb complex isolates. Colistin was the most active agent tested (MIC50/90, ≤0.5/2 mg/L; 95.9% susceptible), followed by minocycline (MIC50/90, ≤1/>8 mg/L; 79.8% susceptible), in all geographic regions. The overall susceptibility rates to colistin varied from 93.9% in Europe to 98.1% in Latin America, whereas minocycline susceptibility rates (CLSI) varied from 70.1% in Europe to 91.1% in Latin America (Table 2). The carbapenems imipenem (MIC50/90, >8/>8 mg/L) and meropenem (MIC50/90, >8/>8 mg/L) showed limited activity against Acb complex, with imipenem susceptibility rates varying from 39.4% in Europe to 63.9% in North America. Meropenem susceptibility rates were slightly inferior to those observed for imipenem and varied from 35.6% in Europe to 59.7% in North America. Ampicillin-sulbactam (MIC50/90, >16/>16 mg/L) also showed limited activity against Acb complex, with susceptibility rates varying from 21.6% in Latin America to 54.7% in North America (Table 2). Acb complex isolates collected from bloodstream infections (n = 5123) showed higher susceptibility rates than Acb complex isolates collected from hospitalized patients with pneumonia (n = 5895) for imipenem (56.5% vs 39.2%), meropenem (53.1% vs 36.2%), minocycline (83.3% vs 76.1%), and colistin (96.6% vs 94.8%). Similarly, the percentage of Acb complex isolates exhibiting the XDR phenotype was higher among isolates collected from hospitalized patients with pneumonia (63.7%) than those with bloodstream infections (48.7%).
Table 2.
Activity of Distinct Antimicrobial Agents Tested Against 13 752 Acinetobacter calcoacetius–A. baumannii Complex Isolates by Geographic Region (SENTRY Program, 1997–2016)
| CLSIa | EUCASTa | ||||||
|---|---|---|---|---|---|---|---|
| Geographic Region (No. Tested) Antimicrobial Agent | MIC50, mg/L | MIC90, mg/L | %S | %R | %S | %R | |
| Asia-Pacific (2327)b | |||||||
| Amikacin | >32 | >32 | 42.9 | 54.8 | 41.2 | 57.1 | |
| Ampicillin-sulbactam (1799)c | >16 | >16 | 26.9 | 67.6 | d | d | |
| Cefepime | >16 | >16 | 32.9 | 60.3 | d | d | |
| Ceftazidime | >16 | >16 | 33.3 | 64.2 | d | d | |
| Colistin (1665)c | ≤0.5 | 1 | 97.5 | 2.5 | 97.5 | 2.5 | |
| Gentamicin | >8 | >8 | 34.4 | 64.0 | 34.4 | 65.6 | |
| Imipenem | >8 | >8 | 42.0 | 55.7 | 42.0 | 54.0 | |
| Levofloxacin | >4 | >4 | 34.0 | 55.2 | 32.5 | 67.1 | |
| Meropenem | >8 | >8 | 42.0 | 56.2 | 42.0 | 55.5 | |
| Minocycline (1589)c | 4 | 8 | 80.3 | 4.7 | d | d | |
| Piperacillin-tazobactam | >64 | >64 | 29.6 | 64.5 | d | d | |
| Tigecycline (1692)c | 1 | 2 | d | d | d | d | |
| Tobramycin | >8 | >8 | 42.3 | 56.4 | 42.3 | 57.7 | |
| Trimethoprim-sulfamethoxazole | >4 | >4 | 38.4 | 61.4 | 38.4 | e | |
| Europe (4531)f | |||||||
| Amikacin | >32 | >32 | 32.1 | 64.7 | 29.9 | 67.9 | |
| Ampicillin-sulbactam (3754)c | >16 | >16 | 25.2 | 65.1 | d | d | |
| Cefepime | >16 | >16 | 23.9 | 63.8 | d | d | |
| Ceftazidime | >16 | >16 | 19.8 | 74.0 | d | d | |
| Colistin (3275)c | ≤0.5 | 2 | 93.9 | 6.1 | 93.9 | 6.1 | |
| Gentamicin | >8 | >8 | 26.2 | 69.5 | 26.2 | 73.8 | |
| Imipenem | >8 | >8 | 39.4 | 58.1 | 39.4 | 55.5 | |
| Levofloxacin | >4 | >4 | 21.1 | 68.5 | 18.1 | 80.7 | |
| Meropenem | >8 | >8 | 35.6 | 60.5 | 35.6 | 53.9 | |
| Minocycline (2995)c | 2 | >8 | 70.1 | 17.7 | d | d | |
| Piperacillin-tazobactam | >64 | >64 | 18.8 | 74.9 | d | d | |
| Tigecycline (3600)c | 1 | 2 | d | d | d | d | |
| Tobramycin | 8 | >8 | 46.0 | 49.9 | 46.0 | 54.0 | |
| Trimethoprim-sulfamethoxazole | >4 | >4 | 35.2 | 64.8 | 35.2 | e | |
| Latin America (3367)g | |||||||
| Amikacin | >32 | >32 | 26.4 | 67.5 | 22.7 | 73.6 | |
| Ampicillin-sulbactam (2643)c | >16 | >16 | 21.6 | 61.9 | d | d | |
| Cefepime | >16 | >16 | 18.4 | 69.4 | d | d | |
| Ceftazidime | >16 | >16 | 14.7 | 80.2 | d | d | |
| Colistin (2246)c | ≤0.5 | 2 | 98.1 | 1.9 | 98.1 | 1.9 | |
| Gentamicin | >8 | >8 | 28.0 | 62.4 | 28.0 | 72.0 | |
| Imipenem | >8 | >8 | 44.7 | 53.1 | 44.7 | 51.6 | |
| Levofloxacin | >4 | >4 | 15.7 | 76.3 | 14.6 | 84.7 | |
| Meropenem | >8 | >8 | 41.0 | 54.6 | 41.0 | 51.3 | |
| Minocycline (2128)c | ≤1 | 4 | 91.1 | 4.9 | d | d | |
| Piperacillin-tazobactam | >64 | >64 | 12.8 | 78.0 | d | d | |
| Tigecycline (2554)c | 1 | 2 | d | d | d | d | |
| Tobramycin | >8 | >8 | 44.6 | 51.9 | 44.6 | 55.4 | |
| Trimethoprim-sulfamethoxazole | >4 | >4 | 24.7 | 75.3 | 24.7 | e | |
| North America (3527)h | |||||||
| Amikacin | ≤4 | >32 | 72.3 | 22.5 | 67.5 | 27.7 | |
| Ampicillin-sulbactam (2813)c | 8 | >16 | 54.7 | 31.9 | d | d | |
| Cefepime | 16 | >16 | 46.6 | 41.5 | d | d | |
| Ceftazidime | 16 | >16 | 48.4 | 44.9 | d | d | |
| Colistin (2461)c | ≤0.5 | 2 | 95.4 | 4.6 | 95.4 | 4.6 | |
| Gentamicin | 4 | >8 | 54.0 | 41.6 | 54.0 | 46.0 | |
| Imipenem | ≤0.5 | >8 | 63.9 | 32.0 | 63.9 | 27.0 | |
| Levofloxacin | 4 | >4 | 48.4 | 48.3 | 46.0 | 52.7 | |
| Meropenem | 1 | >8 | 59.7 | 36.5 | 59.7 | 32.6 | |
| Minocycline (2205)c | ≤1 | 8 | 81.6 | 8.2 | d | d | |
| Piperacillin-tazobactam | 32 | >64 | 44.8 | 43.5 | d | d | |
| Tigecycline (2708)c | 0.5 | 2 | d | d | d | d | |
| Tobramycin | 1 | >8 | 65.9 | 29.9 | 65.9 | 34.1 | |
| Trimethoprim-sulfamethoxazole | 1 | >4 | 53.3 | 46.7 | 53.3 | e | |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; R, resistant; S, susceptible.
bOrganisms include A. baumannii (1), A. calcoacetius–A. baumannii complex (2317), A. nosocomialis (4), A. pittii (5).
cLess than 90% of isolates tested against these antimicrobials; number of isolates tested is cited in parentheses.
eDilution range did not extend high enough to determine between intermediate and R, so only the S percentage is displayed.
fOrganisms include A. baumannii (4), A. calcoacetius–A. baumannii complex (4448), A. nosocomialis (5), A. pittii (74).
gOrganisms include A. baumannii (1), A. calcoacetius–A. baumannii complex (3353), A. nosocomialis (5), A. pittii (8).
hOrganisms include A. baumannii (2), A. calcoacetius–A. baumannii complex (3436), A. nosocomialis (26), A. pittii (63).
The susceptibility of selected antimicrobial agents against 13 752 isolates of Acb complex over 4-year intervals according to the geographic region is depicted in Table 3. Comparing the susceptibility rates found between the periods 1997–2000 and 2013–2016, significant decreases in the susceptibility rates were observed for nearly all tested antimicrobials in all geographic regions. Acb complex isolates collected from European medical centers in the 1997–2000 period displayed the lowest susceptibility rates to carbapenems (55.7%–64.6%) compared with Asia-Pacific (88.9%–89.2%), Latin American (81.3%–87.8), and North American (88.8%–92.6%) medical centers. A marked reduction in the carbapenem susceptibility rates was noticed for Asia Pacific and Latin America in the 2005–2008 period, whereas markedly reduced carbapenem susceptibility rates in North America occurred in the 2009–2012 period. The susceptibility rates declined continuously in the 2009–2012 and 2013–2016 periods, when the susceptibility rates to carbapenems reached their lowest values in Latin America (13.7%–14.4%) and Europe (22.2%–23.7%). In contrast, the lowest carbapenem susceptibility rates in Asia-Pacific (18.4%–18.5%) and North America (43.7%–46.8%) were observed in the 2009–2012 period. In these regions, an increase in the susceptibility rates to carbapenems, aminoglycosides, levofloxacin, ampicillin-sulbactam, and ceftazidime was observed in the 2013–2016 period. These susceptibility rate increases were more pronounced for Acb complex isolates from North American medical centers (Table 3).
Table 3.
Susceptibility of Selected Antimicrobial Agents Against 13 752 Acinetobacter calcoacetius–A. baumannii Complex Isolates Over 4-Year Intervals According to the Geographic Region (SENTRY Program, 1997–2016)
| % Susceptible by Time Perioda (No. of Isolates Tested) | ||||||
|---|---|---|---|---|---|---|
| Acb Complex | 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | Overall |
| Asia-Pacific | (314) | (346) | (535) | (674) | (458) | (2327) |
| Amikacin | 75.5 | 63.9 | 40.4 | 22.3 | 29.5 | 41.2 |
| Gentamicin | 64.0 | 51.6 | 32.5 | 18.2 | 26.9 | 34.4 |
| Tobramycin | 76.4 | 62.7 | 41.9 | 24.4 | 30.6 | 42.3 |
| Levofloxacin | 64.6 | 54.6 | 31.8 | 15.7 | 19.0 | 32.5 |
| Ampicillin-sulbactam | NT | 59.8 | 35.7 | 17.8 | 20.5 | 26.9 |
| Ceftazidime | 59.6 | 60.1 | 31.8 | 17.1 | 20.7 | 33.3 |
| Imipenem | 89.2 | 70.8 | 43.0 | 18.4 | 21.6 | 42.0 |
| Meropenem | 88.9 | 71.4 | 43.0 | 18.5 | 21.0 | 42.0 |
| Minocycline | NT | 100.0 | 84.9 | 79.8 | 74.2 | 80.3 |
| Colistin | NT | NT | 99.1 | 99.0 | 93.7 | 97.5 |
| Europe | (540) | (536) | (705) | (1125) | (1625) | (4531) |
| Amikacin | 31.7 | 40.7 | 32.9 | 32.4 | 22.7 | 29.9 |
| Gentamicin | 20.8 | 31.5 | 27.5 | 27.3 | 24.9 | 26.2 |
| Tobramycin | 48.3 | 47.4 | 50.6 | 46.4 | 42.4 | 46.0 |
| Levofloxacin | 24.4 | 26.5 | 20.9 | 15.8 | 13.7 | 18.1 |
| Ampicillin-sulbactam | NT | 40.5 | 31.5 | 25.9 | 19.2 | 25.2 |
| Ceftazidime | 25.0 | 29.5 | 23.4 | 17.3 | 15.1 | 19.8 |
| Imipenem | 64.6 | 61.6 | 50.6 | 42.4 | 23.7 | 39.4 |
| Meropenem | 55.7 | 55.7 | 47.7 | 28.2 | 22.2 | 35.6 |
| Minocycline | NT | 89.9 | 82.5 | 71.5 | 64.2 | 70.1 |
| Colistin | NT | NT | 99.2 | 97.7 | 89.6 | 93.9 |
| Latin America | (507) | (414) | (910) | (1126) | (410) | (3367) |
| Amikacin | 22.7 | 27.8 | 22.3 | 23.0 | 17.6 | 22.7 |
| Gentamicin | 27.0 | 28.0 | 30.9 | 28.2 | 22.0 | 28.0 |
| Tobramycin | 39.8 | 43.2 | 45.3 | 45.0 | 49.3 | 44.6 |
| Levofloxacin | 23.1 | 22.9 | 15.3 | 7.8 | 12.4 | 14.6 |
| Ampicillin-sulbactam | NT | 43.7 | 29.3 | 13.4 | 16.4 | 21.6 |
| Ceftazidime | 22.7 | 21.5 | 15.3 | 8.5 | 13.4 | 14.7 |
| Imipenem | 87.8 | 80.7 | 49.1 | 19.4 | 14.4 | 44.7 |
| Meropenem | 81.3 | 70.6 | 45.4 | 18.4 | 13.7 | 41.0 |
| Minocycline | NT | 100.0 | 94.2 | 90.9 | 83.9 | 91.1 |
| Colistin | NT | NT | 99.2 | 98.0 | 96.6 | 98.1 |
| North America | (472) | (479) | (548) | (823) | (1205) | (3527) |
| Amikacin | 83.7 | 74.1 | 62.2 | 54.4 | 69.9 | 67.5 |
| Gentamicin | 67.8 | 53.0 | 46.2 | 44.1 | 59.2 | 54.0 |
| Tobramycin | 80.3 | 72.0 | 60.2 | 52.4 | 69.6 | 65.9 |
| Levofloxacin | 64.4 | 45.3 | 36.7 | 37.7 | 49.1 | 46.0 |
| Ampicillin-sulbactam | NT | 65.8 | 54.6 | 45.6 | 58.9 | 54.7 |
| Ceftazidime | 67.6 | 48.4 | 38.3 | 39.6 | 51.5 | 48.4 |
| Imipenem | 92.6 | 81.8 | 62.8 | 46.8 | 57.7 | 63.9 |
| Meropenem | 88.8 | 71.8 | 58.6 | 43.7 | 54.9 | 59.7 |
| Minocycline | NT | 90.0 | 78.3 | 74.3 | 84.9 | 81.6 |
| Colistin | NT | NT | 98.4 | 96.6 | 93.6 | 95.4 |
The frequency of XDR Acb complex isolates over 4-year intervals according to the geographic region and period of time is shown in Table 4. Overall, the highest frequency of XDR Acb complex isolates was observed for Europe (66.4%), followed by Latin America (61.5%), Asia-Pacific (56.9%), and North America (38.8%). The frequency of XDR Acb complex isolates increased continuously during the study period (1997–2016) in Europe and Latin America, and from 1997–2000 to 2009–2012 in North America and the Asia-Pacific region, where the frequency of XDR Acb complex isolates decreased from 2009–2012 to 2013–2016. For this reason, the highest percentage of XDR Acb complex isolates was observed in the 2009–2012 period. In the 2013–2016 period, the highest rates of XDR Acb complex isolates were seen in isolates collected from Latin America (86.6%), followed by Europe (79.0%), and the Asia-Pacific region (72.7%). Among the 7772 XDR Acb complex isolates, colistin was the most active agent tested (MIC50/90, ≤0.5/2 mg/L; 95.1% susceptible), followed by minocycline (MIC50/90, 2/>8 mg/L; 72.7% susceptible), as expected (Table 5).
Table 4.
Frequency of Extensively Drug-Resistant Acinetobacter calcoacetius–A. baumannii Complex Isolates Over 4-Year Intervals According to the Geographic Region and Period of Time (SENTRY Program, 1997–2016)
| Frequency (%) of XDR Isolatesa by Time Period (No. Tested) | ||||||
|---|---|---|---|---|---|---|
| Geographic Region (No. of XDR Isolates) | 1997–2000 (1833) | 2001–2004 (1775) | 2005–2008 (2698) | 2009–2012 (3748) | 2013–2016 (3698) | Overall (13 752) |
| Asia-Pacific (1324) | 9.6 | 24.3 | 62.8 | 80.3 | 72.7 | 56.9 |
| Europe (3007) | 43.1 | 43.5 | 64.5 | 71.3 | 79.0 | 66.4 |
| Latin America (2072) | 17.0 | 36.2 | 67.1 | 77.3 | 86.6 | 61.5 |
| North America (1369) | 10.0 | 26.9 | 46.2 | 54.4 | 40.8 | 38.8 |
| Total (7772) | 21.6 | 33.6 | 61.3 | 71.0 | 66.6 | 56.5 |
Abbreviation: XDR, extensively drug-resistant.
aOrganisms include A. baumannii (6), A. calcoacetius-A. baumannii complex (7756), A. nosocomialis (4), A. pittii (6).
Table 5.
Activity of Distinct Antimicrobial Agents Tested Against 7772 Acinetobacter calcoacetius–A. baumannii Complexa Isolates Categorized as Extensively Drug-Resistant (SENTRY Program, 1997–2016)
| CLSIb | EUCASTb | |||||
|---|---|---|---|---|---|---|
| Antimicrobial Agent | MIC50, mg/L | MIC90, mg/L | %S | %R | %S | %R |
| Amikacin | >32 | >32 | 16.0 | 78.2 | 12.4 | 84.0 |
| Ampicillin-sulbactam | >16 | >16 | 9.2 | 76.9 | c | c |
| Cefepime | >16 | >16 | 3.6 | 85.9 | c | c |
| Ceftazidime | >16 | >16 | 2.4 | 94.1 | c | c |
| Colistin (6520)d | ≤0.5 | 2 | 95.1 | 4.9 | 95.1 | 4.9 |
| Gentamicin | >8 | >8 | 8.0 | 85.8 | 8.0 | 92.0 |
| Imipenem | >8 | >8 | 12.8 | 82.6 | 12.8 | 78.2 |
| Levofloxacin | >4 | >4 | 1.3 | 90.2 | 0.1 | 99.5 |
| Meropenem | >8 | >8 | 8.1 | 86.3 | 8.1 | 79.6 |
| Minocycline (5961)d | 2 | >8 | 72.7 | 13.6 | c | c |
| Piperacillin-tazobactam | >64 | >64 | 0.9 | 95.4 | c | c |
| Tigecycline (6981)d | 1 | 4 | c | c | c | c |
| Tobramycin | >8 | >8 | 27.0 | 68.5 | 27.0 | 73.0 |
| Trimethoprim-sulfamethoxazole | >1 | >1 | 14.7 | 85.3 | 14.7 | e |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; R, resistant; S, susceptible.
aOrganisms include A. baumannii (6), A. calcoacetius–A. baumannii complex (7756), A. nosocomialis (4), A. pittii (6).
dLess than 90% of isolates tested against these antimicrobials; number of isolates tested is cited in parentheses.
eDilution range did not extend high enough to determine between intermediate and R, so only the S percentage is displayed.
Nonsusceptibility to both carbapenems and colistin/polymyxin B was detected in 332 Acb complex isolates. Only 12.0% of these 332 isolates were susceptible to amikacin, 10.2% were susceptible to gentamicin, and 28.3% were susceptible to tobramycin based on EUCAST criteria [30]. By applying CLSI breakpoints [29], 51.0% of these isolates were susceptible to minocycline, but only 10.6% were susceptible to ampicillin-sulbactam (data not shown). An important increase in the number of colistin- and carbapenem-resistant Acb complex isolates was observed over time, especially after the year 2011, and more significantly in 2015 and 2016. From 2001 to 2005, only 10 of 2264 isolates (0.4%) exhibited this phenotype, whereas in 2016, a total of 86 of 921 (9.3%) isolates were recovered from 24 medical centers in 13 countries. Of these 86 isolates, a single Greek site and 2 Turkish sites contributed 13, 14, and 15 isolates, respectively, accounting for 48.8% of the resistant isolates collected in 2016.
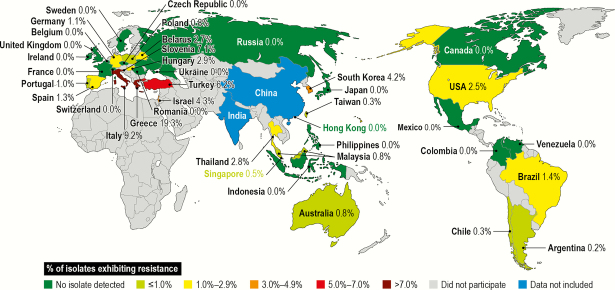
A total of 312 of 11 904 (2.6%) Acb complex isolates were categorized as PDR. These isolates were collected from 22 countries, with the highest number of isolates submitted from the United States (77 isolates, 2.5% of isolates from the United States), followed by Turkey (67 isolates, 6.2%), Greece (62 isolates, 19.3%), Italy (36 isolates, 9.2%), Brazil (18 isolates, 1.4%), and South Korea (16 isolates, 4.2%), whereas the highest prevalence of these resistant isolates was observed in the isolates from Greece (19.3%, 62/321), Italy (9.2%, 36/391), Slovenia (7.1%, 1/14), Turkey (6.2%, 67/1087), Israel (4.4%, 6/138), and South Korea (4.2%, 16/379), as shown in Figure 1. Among the 312 PDR Acb complex isolates, 277 were tested against minocycline, and approximately 48.7% of these isolates would be considered susceptible to minocycline by the CLSI criteria.
Figure 1.
Distribution of 180 Acinetobacter calcoacetius–A. baumannii complex isolates exhibiting pandrug-resistant phenotype by country: SENTRY Program (2001–2016).
A total of 6467 S. maltophilia isolates were collected from North America (48.1%), Europe (31.5%), Latin America (10.9%), and Asia-Pacific (9.5%), as shown in Table 6. Overall, S. maltophilia isolates were more frequently isolated from hospitalized patients with pneumonia (3613, 55.8%) and bloodstream infections (2186, 33.8%) than other infection types. The highest number of S. maltophilia isolates was obtained from hospitalized patients with pneumonia in North America, Europe, and Asia-Pacific; however, isolates from Latin America were more frequently obtained from the bloodstream (56.8%) than pneumonia in hospitalized patients (34.8%).
Table 6.
Distribution of 6467 Stenotrophomonas maltophilia Isolates by Geographic Region and Infection Type (SENTRY Program, 1997–2016)
| No. of Organisms by Geographic Region (%) | |||||
|---|---|---|---|---|---|
| Infection Type | Asia-Pacific | Europe | Latin America | North America | Total |
| Bloodstream | 160 (26.1) | 745 (36.6) | 400 (56.8) | 881 (28.3) | 2186 (33.8) |
| Intra-abdominal | 0 (0.0) | 30 (1.5) | 0 (0.0) | 32 (1.0) | 62 (1.0) |
| Pneumonia in hospitalized patient | 384 (62.6) | 1053 (51.7) | 245 (34.8) | 1931 (62.1) | 3613 (55.8) |
| Skin and skin structure | 62 (10.1) | 174 (8.5) | 48 (6.8) | 218 (7.0) | 502 (7.8) |
| Urinary tract | 5 (0.8) | 27 (1.3) | 0 (0.0) | 44 (1.4) | 76 (1.2) |
| Others | 2 (0.3) | 9 (0.4) | 11 (1.6) | 6 (0.2) | 28 (0.4) |
| Total | 613 (9.5) | 2038 (31.5) | 704 (10.9) | 3112 (48.1) | 6467 (100.0) |
The activity of levofloxacin, minocycline, and TMP-SMX against S. maltophilia according to the geographic region of isolation is displayed in Table 7. Of note, minocycline was not included in the SENTRY Program until 2005 and was tested against only 3868 (59.8%) S. maltophilia isolates. Overall, the most active compound tested against S. maltophilia was minocycline (MIC50/90, ≤1/2 mg/L; 99.5% susceptible [CLSI]), followed by TMP-SMX (MIC5090, ≤0.5/1 mg/L; 96.2% susceptible [EUCAST]), tigecycline (MIC50/90, 0.5/2 mg/L [data not shown]), and levofloxacin (MIC50/90, 1/4 mg/L; 81.5% susceptible [CLSI]). Minocycline susceptibility rates were similar across the 4 geographic regions, ranging from 99.2% to 99.7%. TMP-SMX susceptibility rates were slightly higher among isolates collected from North America (98.2%), Europe (96.3%), and Asia-Pacific (98.3%) compared with Latin America (94.7% [EUCAST]) (Table 7). Levofloxacin MIC50/90 values were 1 and 4 mg/L, respectively, for all geographic regions, except for North America, which had an MIC90 of ≥4 mg/L. The levofloxacin susceptibility rates also varied among the geographic regions and ranged from 78.7% (North America) to 87.8% (Latin America). The TMP-SMX susceptibility rates for S. maltophilia isolates from pneumonia (95.3%) were similar to those from bloodstream infections (96.7%) and higher than those obtained from the urinary tract (89.5%) and intra-abdominal infections (91.9% [data not shown]).
Table 7.
Activity of Selected Antimicrobial Agents Tested Against 6467 Stenotrophomonas maltophilia Isolates by Geographic Region
| CLSIa | EUCASTa | |||||
|---|---|---|---|---|---|---|
| Geographic Region (No. Tested) Antimicrobial Agent | MIC50,mg/L | MIC90, mg/L | %S | %R | %S | %R |
| All geographic regions (6467) | ||||||
| Levofloxacin (6460) | 1 | 4 | 81.5 | 9.7 | b | b |
| Minocycline (3868) | ≤1 | 2 | 99.5 | 0.2 | b | b |
| Trimethoprim-sulfamethoxazole (6453) | ≤0.5 | 1 | 95.6 | 4.4 | 96.2 | 3.8 |
| Asia-Pacific (613) | ||||||
| Levofloxacin (613) | 1 | 4 | 80.9 | 9.6 | b | b |
| Minocycline (363) | ≤1 | 2 | 99.2 | 0.3 | b | b |
| Trimethoprim-sulfamethoxazole (612) | ≤0.5 | ≤0.5 | 93.8 | 6.2 | 94.1 | 5.9 |
| Europe (2038) | ||||||
| Levofloxacin (2035) | 1 | 4 | 83.6 | 8.4 | b | b |
| Minocycline (1294) | ≤1 | ≤1 | 99.2 | 0.2 | b | b |
| Trimethoprim-sulfamethoxazole (2033) | ≤0.5 | 1 | 95.8 | 4.2 | 96.3 | 3.7 |
| Latin America (704) | ||||||
| Levofloxacin (703) | 1 | 4 | 87.8 | 5.1 | b | b |
| Minocycline (342) | ≤1 | ≤1 | 99.7 | 0.0 | b | b |
| Trimethoprim-sulfamethoxazole (701) | ≤0.5 | 1 | 94.4 | 5.6 | 94.7 | 5.3 |
| North America (3112) | ||||||
| Levofloxacin (3109) | 1 | >4 | 78.7 | 11.6 | b | b |
| Minocycline (1869) | ≤1 | 2 | 99.6 | 0.2 | b | b |
| Trimethoprim-sulfamethoxazole (3107) | ≤0.5 | 1 | 96.1 | 4.4 | 96.9 | 3.1 |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; R, resistant; S, susceptible.
bBreakpoints not determined by EUCAST [30].
Overall, TMP-SMX susceptibility rates were 94.6% and 95.7% in the 1997–2001 and 2013–2016 periods, respectively. Variations in the TMP-SMX susceptibility rates were observed in the 4-year intervals, with the highest susceptibility rates observed in the 2001–2004 (97.2%) and 2009–2012 (97.1%) periods. Susceptibility rates varied in 2013–2016 according to the geographic region, with higher rates in Europe (96.5%) and North America (95.8%) and lower rates in Latin America (91.5%) and Asia-Pacific (93.5%) (Table 8). Comparing the rates obtained in the 2013–2016 period with those of 1997–2001, an increase in the TMP-SMX susceptibility rates was noticed for Asia-Pacific (91.9% vs 93.5%) and Europe (91.1% vs 96.5%); however, the opposite was observed for Latin America (96.9% vs 91.5%) and North America (96.2% vs 95.8%) (Table 8).
Table 8.
Trimethoprim-Sulfamethoxazole Susceptibility Rates of 6453 Stenotrophomonas maltophilia Isolates by Geographic Region and 4-Year Periods
| Number of Isolates (% Susceptible by Time Period)a | ||||||
|---|---|---|---|---|---|---|
| Geographic Region | 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | Overall |
| Asia-Pacific | 135 (91.9) | 100 (96.0) | 123 (91.9) | 147 (97.3) | 107 (93.5) | 612 (94.1) |
| Europe | 246 (91.1) | 355 (97.5) | 336 (98.5) | 409 (96.3) | 687 (96.5) | 2033 (96.3) |
| Latin America | 97 (96.9) | 223 (94.6) | 172 (96.5) | 115 (93.0) | 94 (91.5) | 701 (94.7) |
| North America | 632 (96.2) | 440 (98.6) | 380 (97.4) | 556 (98.4) | 1099 (95.8) | 3107 (96.9) |
| Total | 1110 (94.6) | 1118 (97.2) | 1011 (96.9) | 1227 (97.1) | 1987 (95.7) | 6453 (96.2) |
aSusceptible according to criteria published by European Committee on Antimicrobial Susceptibility Testing [30].
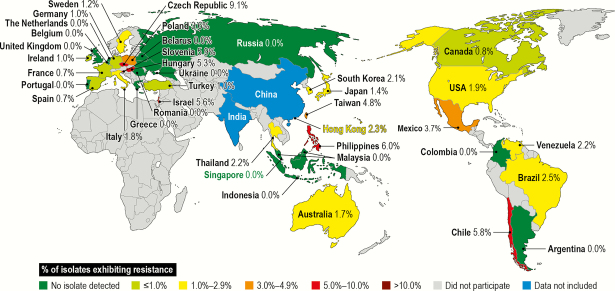
Among 6450 S. maltophilia isolates tested against TMP-SMX and levofloxacin, 112 (1.7%) exhibited resistance to both TMP-SMX (MIC, >4 mg/L [EUCAST]) and levofloxacin (MIC, ≥4 mg/L [nonsusceptible by CLSI criteria]). The highest number of isolates was collected in the United States (55 isolates, 1.9% of US isolates), followed by Brazil (7 isolates, 2.5%), Chile (6 isolates, 5.8%), Mexico (5 isolates, 3.7%), and Germany (4 isolates, 1.0%), whereas the highest prevalence of these resistant isolates was observed in the Czech Republic (9.1%, 1/11), followed by the Philippines (6.0%, 3/50), Slovenia (5.9%, 1/17), Chile (5.8%, 6/103), Israel (5.6%, 3/54), and Taiwan (4.8%, 2/42), as shown in Figure 2. This resistance phenotype has been observed since 2010 and varied according to the 4-year interval, with a higher number of isolates collected in 2013–2016 (59 of 1987 isolates, 3.0%) than in 2009–2012 (18 of 1227 isolates, 1.5%). The S. maltophilia isolates presenting this resistance phenotype were more commonly isolated from hospitalized patients with pneumonia (53 of 3613 isolates, 1.5%) and bloodstream infections (14 of 2186 isolates, 0.6%) than from other infection types. Minocycline showed good activity (MIC50/90,1/4 mg/L [data not shown]) against these isolates, inhibiting 90.0% of them at MIC ≤4 mg/L.
Figure 2.
Distribution of 112 Stenotrophomonas maltophilia isolates exhibiting resistance to trimethoprim-sulfamethoxazole (European Committee on Antimicrobial Susceptibility Testing) and with levofloxacin minimum inhibitory concentration values ≥4 mg/L by country: SENTRY Program (1997–2016).
DISCUSSION
Members of the Acb complex and S. maltophilia represent frequent causes of hospital-acquired infections worldwide, usually among intensive care unit and/or immunocompromised patients [2, 4, 5, 9–11, 18–20]. Although these pathogens can cause various types of infections, bloodstream infection and pneumonia were the most common infections according to the results of this study, which are in agreement with findings of previous studies [9–11, 19, 20].
For a major part of the SENTRY Program, routine clinical laboratories at participating medical centers used commercial identification systems like Vitek 2, Phoenix, and MicroScan WalkAway for bacterial species identification. These systems cannot accurately discriminate A. baumannii from other genetically related species that include A. calcoaceticus, A. dijkshoorniae, A. nosocomialis, A. pittii, and A. seifertii, which are commonly lumped together as the A. calcoaceticus–A. baumannii complex [5, 31, 32]. As MALDI-TOF MS was not available at the monitoring laboratory before 2014, these isolates were designated as the Acb complex in a broad sense and included in our study. Most probably, isolates included in the Acb complex are not A. calcoaceticus, an environmental species, but are mainly representatives of A. baumannii and less frequently of other closely related species because the great majority of these isolates were recovered from hospital-acquired infections [33]. Since the introduction of MALDI-TOF MS at the monitoring laboratory in 2014, no true A. calcoaceticus isolate has been added to the SENTRY Program data bank.
Treatment of infections caused by Acb complex isolates represents a significant clinical challenge because these pathogens exhibit notable inherent antibiotic resistance in addition to their ability to acquire and harbor diverse resistance determinants [2, 4, 5, 13]. In this study, no antimicrobial agent tested was active against all Acb complex isolates. Carbapenems are considered the antimicrobials of choice for treating infections caused by susceptible A. baumannii. Due to increasing carbapenem resistance rates, these agents have become a progressively more critical therapeutic option for infections caused by Acb complex isolates [4]. High carbapenem resistance rates were noted in all geographic regions, and only North American isolates showed imipenem susceptibility rates near 58.0% in the 2013–2016 period. In contrast, Acb complex isolates collected from Asia- Pacific (21.6%), Europe (23.7%), and Latin America (14.4%) showed much lower imipenem susceptibility rates during this period. Although we have not characterized the mechanisms of carbapenem resistance of the entire Acb complex collection, previous SENTRY Program studies have partially characterized such isolates. Mendes and collaborators reported that 162 of 230 (70.4%) Acinetobacter spp. isolates nonsusceptible to imipenem or meropenem, which were collected from 41 medical centers in 10 Asia-Pacific countries between 2006 and 2007, harbored blaOXA-23 more frequently than blaOXA-24/40 and blaOXA-58 [34]. At that time, only a single A. baumannii isolate from South Korea harbored blaVIM-2. In addition, Mendes et al. detected the spread of unique carbapenem-resistant Acinetobacter spp. clones among medical centers located in distinct countries [34]. Increasing carbapenem resistance caused by the dissemination of OXA-23- or OXA-58-producing clones was also detected in Turkish and Italian sites between 2000–2006 and 2007, respectively [35, 36]. In 2007, the spread of epidemic clones harboring blaOXA-24/40 or blaOXA-58 was observed in a Texas hospital [37]. blaOXA-23, blaOXA-24/40, and blaOXA-58 were also detected among 277, 77, and 29 respective Acb complex isolates from 14 European countries between 2009 and 2011. Most isolates carrying these genes belonged to international clone (IC) 2 [38]. In addition, IMP-1-producing A. baumannii isolates were detected in Brazilian and Argentinean medical centers (2001–2002), whereas IMP-2- and VIM-1-producing isolates were detected in Italy (2003) and Greece (2002–2003), respectively [39, 40].
Therapeutic options against infections caused by carbapenem-resistant Acb complex isolates are very limited. Polymyxins are often the last treatment option for infections caused by XDR Acb complex isolates [16, 17]. In this study, colistin exhibited the highest susceptibility rates in all geographic regions. Unfortunately, a slight decrease in colistin susceptibility rates was observed between 2005–2008 and 2013–2016 in all geographic regions, especially in Europe (99.2%–89.6%). Since colistin-resistant Acinetobacter spp. was first reported in the Czech Republic in 1999, the number of global reports has increased, with higher rates being reported for Asia-Pacific and Europe [14–16]. Recently, Nowak et al. reported that 31 of 65 (47.7%) A. baumannii isolates recovered from patients with ventilator-associated pneumonia enrolled in the MagicBullet trial in Europe were resistant to colistin [17]. Isolates from Greece exhibited the highest rate of colistin resistance (56.8%), followed by isolates from Italy (42.9%) and Spain (28.6%). The authors also observed that most colistin-resistant strains emerged independently, with some clones persisting over time [17].
In this SENTRY Program study, minocycline was the second most active agent against Acb complex isolates, including XDR and PDR isolates. In fact, more than 72.7% of XDR and 48.7% of PDR Acb complex isolates remained susceptible to minocycline according to the CLSI breakpoints (MIC, ≤4 mg/L). However, Wong and collaborators have warned about the need for clinical validation of minocycline breakpoints because 4 mg/L, the upper limit of the CLSI susceptible breakpoint, coincides with the mean peak blood level of minocycline when a 200-mg intravenous dose is administered. Although minocycline has shown potent in vitro activity against drug-resistant A. baumannii, the clinical experience with this agent for treatment of invasive Acb infections is still very limited [4, 41, 42].
Sulbactam is a class A β-lactamase inhibitor that has intrinsic activity against A. baumannii by possessing affinity to penicillin-binding protein (PBP)–1 and PBP-3 [43]. In most countries, sulbactam is only commercially available in combination with ampicillin. Although ampicillin-sulbactam showed similar therapeutic efficacy when compared with imipenem for treatment of Acinetobacter spp. ventilator-associated pneumonia in a small number of critically ill trauma patients, this combination should not be recommended for treatment of infections caused by Acb complex isolates based on our surveillance results (16.4%–58.9% resistant [CLSI]) [29, 44, 45]. In addition, susceptibility testing of ampicillin-sulbactam is often problematic, making it difficult to establish a good correlation between MICs and clinical response [46, 47]. Of note, EUCAST chose not to publish ampicillin-sulbactam breakpoints for Acinetobacter spp.
In contrast to what was observed in Europe and Latin America, a decrease in the antimicrobial resistance rates for all antimicrobials, including carbapenems, was observed in Asia Pacific and North America in the 2013–2016 period. These results are in accordance with those reported by the US National Healthcare Safety Network (NHSN), probably because more than 98.0% of North American medical centers are located in the United States [48]. Although the time periods studied were slightly different, the last NHSN report about antimicrobial susceptibility data for pathogens causing hospital-acquired infections between 2011 and 2014 reported a decrease in the magnitude of the resistance percentages among Acinetobacter spp. across all hospital-acquired infection types. The authors specified that the cause of this decrease and whether it represented a true decrease in the prevalence of resistant pathogens were unknown [47, 48].
In this SENTRY Program study, the reduction of susceptibility rates to antimicrobials, including carbapenems, in most geographic regions showed a direct relationship with the increase of the XDR Acb complex phenotype. The reduced rates might be caused by the worldwide expansion of international clones that exhibit resistance to carbapenems due to production of CHDLs [17, 49]. Eight clonal lineages (ICs) that have spread worldwide have been described that encompass the previously described European clones I, II, and III [2, 5, 12]. In general, the international clone IC2 is the predominant clonal lineage globally, but the frequency of these clones may vary within a medical center and/or country [12, 17, 38, 49]. Also, local carbapenem-resistant A. baumannii clones have emerged in some medical centers [12, 36, 38, 50].
Although the results presented here are comprehensive, the limitations of the study should be noted. Because proper identification of the Acb complex was not carried out in the earlier years of the program, and A. nosocomialis and A. pittii are usually more susceptible than A. baumannii isolates, variations in the Acb complex species distributions among medical centers and geographic regions may have affected the susceptibility rates. Thus, the decrease of resistance rates to carbapenems in North America and Asia-Pacific must be confirmed by continued monitoring with proper species identification.
Infections due to S. maltophilia occur in 7.4 to 37.7 patients in 10 000 at risk for opportunistic infections [51]. TMP-SMX remains the therapy of choice for treatment of such infections [18, 22]. In this study, the activity of TMP-SMX was similar among S. maltophilia isolated from distinct geographic regions with a slight variation in susceptibility rates that ranged from 94.1% (Asia-Pacific) to 96.9% (North America). Overall, the TMP-SMX susceptibility rates showed little variation over time in the studied regions. These variations were more significant (>5%) in Europe and Latin America. Although TMP-SMX susceptibility rates increased in Europe from 91.1% to 96.5% between 1997–2000 and 2013–2016, these rates decreased in Latin America (96.9% vs 91.5%). Our results corroborate findings of previous studies that showed that TMP-SMX resistance rates varied geographically but were generally less than 10% [18, 52].
Fluoroquinolones have been prescribed as alternative agents for treating S. maltophilia infections. Clinical studies have shown similar outcomes for patients treated with TMP-SMX and fluoroquinolones for S. maltophilia infections, with few reports of emerging resistance during fluoroquinolone treatment [53, 54]. In contrast to other gram-negative bacilli, mutations on quinolone resistance–determining regions of topoisomerases-encoding genes are not the main resistance mechanism to quinolones in S. maltophilia isolates [55]. In this species, resistance to fluoroquinolones is conferred by hyperexpression of efflux systems (smeDEF, smeIJK, smeABC, smeVWX) and chromosomally encoded qnr [56]. In addition, it was recently demonstrated that overexpression of SmeDEF or SmeVWX also contributes to resistance to co-trimoxazole in S. maltophilia [57] isolates. In this SENTRY Program study, levofloxacin in vitro activity was lower than that observed for TMP-SMX, with the highest resistance rates observed in North America (11.6% [CLSI]). Overusing fluoroquinolones may have contributed to the increased levofloxacin resistance rates observed in this study [18]. Minocycline was the most active agent against S. maltophilia isolates, with susceptibility rates exceeding 99.0% across all geographic regions. Esposito and collaborators also noted a higher activity of minocycline than TMP-SMX against S. maltophilia isolated from cystic fibrosis patients [58]. Additionally, minocycline showed excellent activity (MIC50/90, 1/4 mg/L; 90% susceptible) against TMP-SMX-resistant S. maltophilia isolates exhibiting levofloxacin MIC values ≥4 mg/L. These findings are similar to those previously reported [23]. The clinical efficacy of minocycline was compared with that of TMP-SMX for treating S. maltophilia infections in a small retrospective study. Clinical success rates were similar between both groups [22]. In another retrospective analysis of 93 patients who had received minocycline 100 mg intravenously q12h for treatment of S. maltophilia infections, clinical success was documented for 58% of the patients. However, 22 (24%) and 17 (18%) patients experienced partial clinical success and failure, respectively. Patients with higher APACHE II scores and a minocycline MIC value of 4 mg/L were more likely to fail therapy [59]. Although minocycline and levofloxacin seem to be effective treatments for S. maltophilia infections, randomized clinical trials are necessary to definitively establish the optimal treatment.
In conclusion, these results from the SENTRY Program confirm previous reports regarding the increasing resistance rates to carbapenems and, more recently, to colistin in Acb complex isolates worldwide, emphasizing the need for new antimicrobial agents and/or vaccines against these pathogens. Implementing successful infection control measures to restrain XDR Acb complex clones from disseminating within distinct hospitals and/or geographic areas is critically important. Fortunately, in general, TMP-SMX resistance among S. maltophilia isolates remained low over the study period.
Acknowledgments
The authors thank all participants of the SENTRY Program for their work in providing isolates.
Financial support. Funding for the manuscript was provided by JMI Laboratories.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Glew RH, Moellering RC Jr, Kunz LJ. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 1977; 56:79–97. [DOI] [PubMed] [Google Scholar]
- 2. Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 2007; 5:939–51. [DOI] [PubMed] [Google Scholar]
- 3. Spellberg B, Rex JH. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov 2013; 12:963–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong D, Nielsen TB, Bonomo RA, et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30:409–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008; 21:538–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seifert H, Dijkshoorn L, Gerner-Smidt P, et al. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol 1997; 35:2819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dexter C, Murray GL, Paulsen IT, Peleg AY. Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert Rev Anti Infect Ther 2015; 13:567–73. [DOI] [PubMed] [Google Scholar]
- 8. Wendt C, Dietze B, Dietz E, Rüden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol 1997; 35:1394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robenshtok E, Paul M, Leibovici L, et al. The significance of Acinetobacter baumannii bacteraemia compared with Klebsiella pneumoniae bacteraemia: risk factors and outcomes. J Hosp Infect 2006; 64:282–7. [DOI] [PubMed] [Google Scholar]
- 10. Sunenshine RH, Wright MO, Maragakis LL, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007; 13:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwon KT, Oh WS, Song JH, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother 2007; 59:525–30. [DOI] [PubMed] [Google Scholar]
- 12. Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 2010; 65:233–8. [DOI] [PubMed] [Google Scholar]
- 13. Da Silva GJ, Domingues S. Insights on the horizontal gene transfer of carbapenemase determinants in the opportunistic pathogen Acinetobacter baumannii. Microorganisms 2016; 4:E29–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hejnar P, Kolár M, Hájek V. Characteristics of Acinetobacter strains (phenotype classification, antibiotic susceptibility and production of beta-lactamases) isolated from haemocultures from patients at the teaching hospital in Olomouc. Acta Univ Palacki Olomuc Fac Med 1999; 142:73–7. [PubMed] [Google Scholar]
- 15. David MD, Gill MJ. Potential for underdosing and emergence of resistance in Acinetobacter baumannii during treatment with colistin. J Antimicrob Chemother 2008; 61:962–4. [DOI] [PubMed] [Google Scholar]
- 16. Cai Y, Chai D, Wang R, et al. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 2012; 67:1607–15. [DOI] [PubMed] [Google Scholar]
- 17. Nowak J, Zander E, Stefanik D, et al. ; MagicBullet Working Group WP4 High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother 2017; 72:3277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 2015; 6:893–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Velázquez-Acosta C, Zarco-Márquez S, Jiménez-Andrade MC, et al. Stenotrophomonas maltophilia bacteremia and pneumonia at a tertiary-care oncology center: a review of 16 years. Support Care Cancer 2018; 26:1953–60. [DOI] [PubMed] [Google Scholar]
- 20. Tseng CC, Fang WF, Huang KT, et al. Risk factors for mortality in patients with nosocomial Stenotrophomonas maltophilia pneumonia. Infect Control Hosp Epidemiol 2009; 30:1193–202. [DOI] [PubMed] [Google Scholar]
- 21. Nicodemo AC, Araujo MR, Ruiz AS, Gales AC. In vitro susceptibility of Stenotrophomonas maltophilia isolates: comparison of disc diffusion, etest and agar dilution methods. J Antimicrob Chemother 2004; 53:604–8. [DOI] [PubMed] [Google Scholar]
- 22. Hand E, Davis H, Kim T, Duhon B. Monotherapy with minocycline or trimethoprim/sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. J Antimicrob Chemother 2016; 71:1071–5. [DOI] [PubMed] [Google Scholar]
- 23. Rizek C, Ferraz JR, van der Heijden IM, et al. In vitro activity of potential old and new drugs against multidrug-resistant gram-negatives. J Infect Chemother 2015; 21:114–7. [DOI] [PubMed] [Google Scholar]
- 24. Kaur P, Gautam V, Tewari R. Distribution of class 1 integrons, sul1 and sul2 genes among clinical isolates of Stenotrophomonas maltophilia from a tertiary care hospital in north India. Microb Drug Resist 2015; 21:380–5. [DOI] [PubMed] [Google Scholar]
- 25. Barbolla R, Catalano M, Orman BE, et al. Class 1 integrons increase trimethoprim-sulfamethoxazole MICs against epidemiologically unrelated Stenotrophomonas maltophilia isolates. Antimicrob Agents Chemother 2004; 48:666–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toleman MA, Bennett PM, Bennett DM, et al. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis 2007; 13:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falagas ME, Kastoris AC, Vouloumanou EK, et al. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol 2009; 4:1103–9. [DOI] [PubMed] [Google Scholar]
- 28. CLSI. M100Ed28E. Performance Standards for Antimicrobial Susceptibility Testing: 28th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 29. CLSI. M07Ed11E. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard: 11th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 30. European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MIC’s and zone diameters. Version 8.0.2018. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdfAccessed January 2018.
- 31. Nemec A, Krizova L, Maixnerova M, et al. Acinetobacter seifertii sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex isolated from human clinical specimens. Int J Syst Evol Microbiol 2015; 65:934–42. [DOI] [PubMed] [Google Scholar]
- 32. Cosgaya C, Marí-Almirall M, Van Assche A, et al. Acinetobacter dijkshoorniae sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex mainly recovered from clinical samples in different countries. Int J Syst Evol Microbiol 2016; 66:4105–11. [DOI] [PubMed] [Google Scholar]
- 33. Gerner-Smidt P. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol 1992; 30:2680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mendes RE, Bell JM, Turnidge JD, et al. Emergence and widespread dissemination of OXA-23, -24/40 and -58 carbapenemases among Acinetobacter spp. in Asia Pacific nations: report from the SENTRY Surveillance Program. J Antimicrob Chemother 2009; 63:55–9. [DOI] [PubMed] [Google Scholar]
- 35. Gur D, Korten V, Unal S, et al. Increasing carbapenem resistance due to the clonal dissemination of oxacillinase (OXA-23 and OXA-58)-producing Acinetobacter baumannii: report from the Turkish SENTRY Program sites. J Med Microbiol 2008; 57:1529–32. [DOI] [PubMed] [Google Scholar]
- 36. Mendes RE, Spanu T, Deshpande L, et al. Clonal dissemination of two clusters of Acinetobacter baumannii producing OXA-23 or OXA-58 in Rome, Italy. Clin Microbiol Infect 2009; 15:588–92. [DOI] [PubMed] [Google Scholar]
- 37. Castanheira M, Wanger A, Kruzel M, et al. Emergence and clonal dissemination of OXA-24- and OXA-58-producing Acinetobacter baumannii strains in Houston, Texas: report from the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol 2008; 46:3179–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castanheira M, Costello SE, Woosley LN, et al. Evaluation of clonality and carbapenem resistance mechanisms among Acinetobacter baumannii-Acinetobacter calcoaceticus complex and Enterobacteriaceae isolates collected in European and Mediterranean countries and detection of two novel β-lactamases, GES-22 and VIM-35. Antimicrob Agents Chemother 2014; 58:7358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sader HS, Castanheira M, Mendes RE, et al. Dissemination and diversity of metallo-beta-lactamases in Latin America: report from the SENTRY antimicrobial surveillance program. Int J Antimicrob Agents 2005; 25:57–61. [DOI] [PubMed] [Google Scholar]
- 40. Fritsche TR, Sader HS, Toleman MA, et al. Emerging metallo-beta-lactamase-mediated resistances: a summary report from the worldwide SENTRY antimicrobial surveillance program. Clin Infect Dis 2005; 41(Suppl 4):S276–8. [DOI] [PubMed] [Google Scholar]
- 41. Ritchie DJ, Garavaglia-Wilson A. A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin Infect Dis 2014; 59(Suppl 6):S374–80. [DOI] [PubMed] [Google Scholar]
- 42. Lashinsky JN, Henig O, Pogue JM, Kaye KS. Minocycline for the treatment of multidrug and extensively drug-resistant A. baumannii: a review. Infect Dis Ther 2017; 6:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Penwell WF, Shapiro AB, Giacobbe RA, et al. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother 2015; 59:1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wood GC, Hanes SD, Croce MA, et al. Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of Acinetobacter ventilator-associated pneumonia. Clin Infect Dis 2002; 34:1425–30. [DOI] [PubMed] [Google Scholar]
- 45. Garnacho-Montero J, Dimopoulos G, Poulakou G, et al. ; European Society of Intensive Care Medicine Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med 2015; 41:2057–75. [DOI] [PubMed] [Google Scholar]
- 46. Oliveira MS, Costa SF, Pedri Ed, et al. The minimal inhibitory concentration for sulbactam was not associated with the outcome of infections caused by carbapenem-resistant Acinetobacter sp. treated with ampicillin/sulbactam. Clinics (Sao Paulo) 2013; 68:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Higgins PG, Wisplinghoff H, Stefanik D, Seifert H. In vitro activities of the beta-lactamase inhibitors clavulanic acid, sulbactam, and tazobactam alone or in combination with beta-lactams against epidemiologically characterized multidrug-resistant Acinetobacter baumannii strains. Antimicrob Agents Chemother 2004; 48:1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zarrilli R, Pournaras S, Giannouli M, Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 2013; 41:11–9. [DOI] [PubMed] [Google Scholar]
- 50. Vasconcelos AT, Barth AL, Zavascki AP, et al. The changing epidemiology of Acinetobacter spp. producing OXA carbapenemases causing bloodstream infections in Brazil: a BrasNet report. Diagn Microbiol Infect Dis 2015; 83:382–5. [DOI] [PubMed] [Google Scholar]
- 51. Garazi M, Singer C, Tai J, Ginocchio CC. Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-year review. J Hosp Infect 2012; 81:114–8. [DOI] [PubMed] [Google Scholar]
- 52. Chung HS, Hong SG, Kim YR, et al. Antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from Korea, and the activity of antimicrobial combinations against the isolates. J Korean Med Sci 2013; 28:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang YL, Scipione MR, Dubrovskaya Y, Papadopoulos J. Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 2014; 58:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cho SY, Kang CI, Kim J, et al. Can levofloxacin be a useful alternative to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob Agents Chemother 2014; 58:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Valdezate S, Vindel A, Saéz-Nieto JA, et al. Preservation of topoisomerase genetic sequences during in vivo and in vitro development of high-level resistance to ciprofloxacin in isogenic Stenotrophomonas maltophilia strains. J Antimicrob Chemother 2005; 56:220–3. [DOI] [PubMed] [Google Scholar]
- 56. Sanchez MB, Hernandez A, Martinez JL. Stenotrophomonas maltophilia drug resistance. Future Microbiol 2009; 4:655–60. [DOI] [PubMed] [Google Scholar]
- 57. Sanchez MB, Martinez JL. Overexpression of the efflux pumps SmeVWX and SmeDEF is a major cause of resistance to co-trimoxazole in Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2018; 62:301–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Esposito A, Pompilio A, Bettua C, et al. Evolution of Stenotrophomonas maltophilia in cystic fibrosis lung over chronic infection: a genomic and phenotypic population study. Front Microbiol 2017; 8:1590–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jacobson S, Junco Noa L, Wallace MR, Bowman MC. Clinical outcomes using minocycline for Stenotrophomonas maltophilia infections. J Antimicrob Chemother 2016; 71:3620. [DOI] [PubMed] [Google Scholar]