Abstract
Background
The SENTRY Antimicrobial Surveillance Program was established in 1997 and presently encompasses more than 750 000 bacterial isolates from over 400 medical centers worldwide. Among these pathogens, enterococci represents a prominent cause of bloodstream (BSIs), intra-abdominal (IAIs), skin and skin structure, and urinary tract infections (UTIs). In the present study, we reviewed geographic and temporal trends in Enterococcus species and resistant phenotypes identified throughout the SENTRY Program.
Methods
From 1997 to 2016, a total of 49 491 clinically significant enterococci isolates (15 species) were submitted from 298 medical centers representing the Asia-Pacific (APAC), European, Latin American (LATAM), and North American (NA) regions. Bacteria were identified by standard algorithms and matrix-assisted laser desorption ionization–time of flight mass spectrometry. Susceptibility (S) testing was performed by reference broth microdilution methods and interpreted using Clinical and Laboratory Standards Institute/US Food and Drug Administration and European Committee on Antimicrobial Susceptibility Testing criteria.
Results
The most common Enterococcus species in all 4 regions were Enterococcus faecalis (64.7%) and E. faecium (EFM; 29.0%). Enterococci accounted for 10.7% of BSIs in NA and was most prominent as a cause of IAIs (24.0%) in APAC and of UTIs (19.8%) in LATAM. A steady decrease in the susceptibility to ampicillin and vancomycin was observed in all regions over the 20-year interval. Vancomycin-resistant enterococci (VRE) accounted for more than 8% of enterococcal isolates in all regions and was most common in NA (21.6%). Among the 7615 VRE isolates detected, 89.1% were the VanA phenotype (91.0% EFM) and 10.9% were VanB. Several newer antimicrobial agents demonstrated promising activity against VRE, including daptomycin (99.6–100.0% S), linezolid (98.0%–99.6% S), oritavancin (92.2%–98.3% S), tedizolid (99.5%–100.0% S), and tigecycline (99.4%–100.0% S).
Conclusions
Enterococci remained a prominent gram-positive pathogen in the SENTRY Program from 1997 through 2016. The overall frequency of VRE was 15.4% and increased over time in all monitored regions. Newly released agents with novel mechanisms of action show promising activity against VRE.
Keywords: enterococci, SENTRY, surveillance, VRE
Enterococcus species currently represent the second and third most frequently observed pathogens responsible for health care–associated infections (HAIs) in the United States and Europe (EUR), respectively [1, 2]. In a recent survey (2011–2014) conducted by the National Healthcare Safety Network (NHSN) at the US Centers for Disease Control and Prevention (CDC), enterococci ranked second among antimicrobial-resistant pathogens associated with HAIs: first among pathogens associated with central line–associated bloodstream infections (CLABSIs), second among causes of surgical site infections (SSIs), and third among catheter-associated urinary tract infections [2]. Over the past 3 decades, enterococci have emerged from being considered benign commensal bacteria of low virulence to medically important multidrug-resistant (MDR; resistant to 3 or more classes of agents) HAI pathogens that are considered a serious public health threat [3–5].
Enterococci are natural colonizers of the human and animal gastrointestinal tract and are notable for their ability to survive in harsh environments [3]. Most enterococci are intrinsically resistant to aminoglycosides and many β-lactam agents (cephalosporins), and some species, such as Enterococcus faecium, have acquired a variety of genetic determinants that confer resistance to several antimicrobial classes, including chloramphenicol, tetracyclines, macrolides, lincosamides, glycopeptides, fluoroquinolones, and rarely some of the newly introduced agents such as linezolid, daptomycin, and quinupristin-dalfopristin [3, 5–12].
Trends toward an increasing prevalence of MDR enterococci as HAI pathogens have been observed [2]. Data from the 2011–2014 NHSN survey revealed that 42.5% of CLABSIs and 19.1% of SSIs due to enterococci in US medical centers were vancomycin resistant (VRE) [2]. The occurrence and spread of VRE have been documented worldwide [3, 10]. In 2011–2012, the European CDC reported VRE prevalence ranging from 3.6% to 31% in several European countries [1]. VRE has been reported in South America, Asia, and Australia, emphasizing the global occurrence of VRE in the health care environment [3, 13–20].
The majority of enterococcal infections are caused by Enterococcus faecalis and E. faecium, and until a few decades ago, E. faecalis comprised 80%–90% of the isolates [21]. More recent reports have described the emergence and dissemination of E. faecium isolates resistant to vancomycin and aminoglycosides (high-level resistance [HLAR]), which precludes using this combination as a standard therapy [3, 9, 22].
The leading factors responsible for VRE include the increased use of vancomycin for treatment of infections caused by methicillin-resistant Staphylococcus spp. coupled with intra- and interhospital dissemination of resistant clones [20, 23, 24]. VRE infections occur most commonly among at-risk patients, such as those in intensive care units, on hemodialysis, in nursing homes, those who are immunocompromised, or those being treated with selected antimicrobial agents, such as broad-spectrum cephalosporins, and are typically preceded by gastrointestinal tract colonization [3, 25–28]. VRE infections are also associated with additional morbidity, mortality, and treatment expense, especially for patients with confounding risk factors due to the reduced number of therapeutic options and potentially greater pathogenicity of these strains acquiring virulence genes [3, 5, 8, 9, 13, 20, 28–30]. VRE infections in hospitals are often clonal, with the epidemic–virulent clonal complex (CC)–17 lineage of E. faecium disseminated worldwide [3, 20, 22, 24, 31].
The epidemiology of VRE and other forms of enterococcal infection has been described in numerous single-center, sentinel, and population-based surveys conducted throughout the world [2, 5, 10, 13–19, 22, 24, 31, 32]. However, the dynamic nature of VRE trends in the United States and elsewhere suggests that this issue still merits considerable monitoring and surveillance attention [3, 5, 10, 24, 33].
The SENTRY Antimicrobial Surveillance Program is a global program that has been conducted for 20 years (1997–2016) and collects consecutive invasive and noninvasive Enterococcus spp. isolates from hospitals located in North America (NA), EUR, Latin America (LATAM), and the Asia-Pacific region (APAC) each calendar year. Enterococcus spp. isolates are evaluated for susceptibility against various antimicrobial agents used clinically to treat and prevent VRE [10, 24, 33–36]. Applying modern methods for species identification, antimicrobial susceptibility testing, and characterizing antibacterial resistance mechanisms provides a level of standardization and clarity that makes these observations very useful in the ongoing fight against antimicrobial resistance [3, 10, 24, 34, 35, 37].
Previous SENTRY Program publications from 1997 to the present have reported broad geographic trends in the isolation of various Enterococcus species from clinical specimens and the accompanying rates of antimicrobial resistance in the United States and globally [10, 24, 33, 35–42]. The present summary focuses on the geographic and temporal variations in the frequency of the Enterococcus species causing VRE and the associated antimicrobial resistance profiles using the extensive SENTRY Program database from 1997 to 2016. Specifically, this includes results for 49 491 isolates of Enterococcus species from 298 medical centers in 43 nations worldwide. This report discusses the occurrence of enterococcal infections by species and site of infection as well as the occurrence of VRE isolates and their resistance characterizations. Trends in susceptibility to a variety of established and newly introduced antimicrobials in each geographic region are also reported.
METHODS
Study Design
The SENTRY Program was initiated in early 1997 to investigate longitudinal trends in antimicrobial resistance and the frequency of pathogen occurrence. Five major objectives address the most common types of infection in a prevalence-style format: Objective A, bloodstream infections (BSIs); Objective B, community-acquired respiratory tract infections caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, and Moraxella catarrhalis; Objective C, pneumonias in hospitalized patients (PIHP); Objective D, skin and skin structure or wound infections (SSSIs); and Objective E, urinary tract infections (UTIs). In addition, intra-abdominal infections (IAIs) were monitored from 2005 through 2016. Consecutive isolates (1 per patient infection episode) were forwarded to the regional monitoring sites for reference quantitative antimicrobial susceptibility testing and confirmation of organism identification. More than 750 000 isolates, including 49 491 enterococci, have been processed from 1997 through 2016 (Table 1).
Table 1.
Variations in the Occurrence of Enterococcal Infections in Hospitals Contributing Isolates to the SENTRY Program (1997–2016)
| No. of Isolates per Infection Type (% Enterococci) | |||||
|---|---|---|---|---|---|
| Region | BSI | PIHP | IAI | SSSI | UTI |
| North America | 136 766 (10.7) | 66 012 (0.7) | 4450 (13.1) | 74 943 (5.9) | 31 832 (12.5) |
| Europe | 103 487 (8.1) | 35 780 (1.8) | 4458 (17.2) | 39 849 (7.4) | 14 874 (16.7) |
| Latin America | 37 035 (5.0) | 12 110 (1.5) | 183 (23.5) | 12 768 (9.8) | 3829 (19.8) |
| Asia-Pacific | 26 862 (5.1) | 14 602 (0.8) | 96 (24.0) | 15 484 (3.3) | 3466 (17.7) |
A total of 765 388 strains (49 491 enterococci [6.5%]) were analyzed over the 20 study years.
Abbreviations: BSI, bloodstream infection; IAI, intra-abdominal infection; PIHP, pneumonia in hospitalized patient; SSSI, skin and skin structure infection; UTI, urinary tract infection.
Participants and Monitors
Three reference laboratories acted as monitoring sites during the 1997–1999 interval: the University of Iowa College of Medicine, Iowa City, Iowa (NA and LATAM for 1997–1999 and EUR for 1999); Utrecht University, Utrecht, Netherlands (EUR for 1997–1998); and the Women’s and Children’s Hospital, Adelaide, Australia (APAC for 1998–1999). Beginning in 2000, all isolates were referred to the central monitoring laboratory, JMI Laboratories (North Liberty, IA).
Participating sites varied in number by region: 162 sites (25 206 isolates) in the NA region; 18 sites (4755 isolates) in the LATAM region; 65 sites (16 054 isolates in Europe, Israel, and Turkey) in the EUR region; and 53 sites (3476 isolates) in the APAC region.
Organisms
Participating institutions identified isolates using methods routinely employed at the submitting laboratory, which include the use of Vitek, MicroScan, API, and AuxaColor systems supplemented with classical methods for bacterial identification. Isolates were submitted to the monitoring laboratory that confirmed identication by morphological, biochemical, and molecular methods. From 2012 to 2016, isolate identity was confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker, Billerica, MA). Isolates that could not be identified by either phenotypic or proteomic methods were identified using sequence-based methods [10, 24, 33, 35, 37, 41].
Antimicrobial Agents
Representatives from all clinically important antimicrobial classes have been tested (ampicillin, penicillin, erythromycin, vancomycin, teicoplanin, chloramphenicol, doxycycline, gentamicin, streptomycin, ciprofloxacin, nitrofurantoin, and trimethoprim-sulfamethoxazole [TMP-SMZ]), as well as newer compounds such as linezolid, quinupristin-dalfopristin, daptomycin, tedizolid, telavancin, dalbavancin, oritavancin, and tigecycline. Antimicrobials were obtained from their US manufacturer or representative.
Antimicrobial Susceptibility Testing
Isolates were susceptibility tested by broth microdilution following guidelines in the Clinical and Laboratory Standards Institute (CLSI) M07 document [43] and using reference 96-well panels manufactured by JMI Laboratories or acquired from Thermo Fisher (Cleveland, OH). Quality assurance was performed by concurrently testing CLSI-recommended quality control (QC) reference strains (Stayphylococcus aureus ATCC 29213, E. faecalis ATCC 29212, and S. pneumoniae ATCC 49619). All QC results were within published acceptable ranges. Clinical breakpoints approved by the US Food and Drug Administration, CLSI [44], and/or the European Committee on Antimicrobial Susceptibility Testing [45] were applied for all tested agents. The vancomycin-resistant phenotypes (VanA and VanB) were defined as follows: VanA, resistant to vancomycin (minimum inhibitory concentration [MIC], >4 mg/L) and teicoplanin (MIC, >8 mg/L); VanB, resistant to vancomycin (MIC, >4 mg/L) but susceptible (MIC, ≤8 mg/L) to teicoplanin [44].
RESULTS
Nationwide surveillance programs, such as the SCOPE Program [46, 47], have provided data on nosocomial enterococcal BSIs, and the NHSN system [2, 48] also provides national data on various types of nosocomial infections due to enterococci. The SENTRY Program has documented occurrence rates for 49 491 enterococci isolates by site of infection (BSI, PIHP, SSSI, IAI, and UTI) and is the only surveillance system that analyzes data on a broad geographic scale that includes NA, EUR, LATAM, and the APAC region. During the study period (1997–2016), a total of 765 388 strains were processed by SENTRY Program participants (373 452 from NA, 236 911 from EUR, 77 314 from LATAM, and 77 711 from the APAC region), 6.5% of which were Enterococcus spp. (Table 1).
Table 1 shows the occurrence rates of enterococcal infections by site of infection within each geographic region. Enterococci accounted for 10.7% of BSI isolates in NA. The lowest rates of enterococcal infections among BSIs occurred in LATAM and APAC (5.0 and 5.1%, respectively). The highest detected rate of enterococcal UTIs was in LATAM (19.8%), followed by APAC (17.7%) and EUR (16.7%). The highest rates of IAIs due to enterococci were observed in the APAC (24.0%) and LATAM (23.5%) regions.
The frequencies of reported Enterococcus species isolates by geographic region are listed in Table 2. Of the 15 species identified in the survey, E. faecalis was the most prevalent, ranging from 62.8% of enterococci isolated in EUR to 74.1% in LATAM. E. faecium, the species in which vancomycin resistance is most prevalent, was the second most commonly identified species in all geographic regions. The proportion of E. casseliflavus and E. gallinarum isolates (species intrinsically less susceptible to vancomycin) continued to be low across all geographic regions (range, 0.4%–1.4%). Identification to the species level was not performed for 0.5%–4.6% of the isolates reported.
Table 2.
Prevalence of Enterococcus Species Isolations From All Monitored Infections Among 4 Geographic Regions in the SENTRY Program (1997–2016)
| Prevalence Among Enterococcal Isolates Tested by Region, % | |||||
|---|---|---|---|---|---|
| Enterococcus Species | North America(n = 25 206) | Europe(n = 16 054) | Latin America(n = 4755) | Asia-Pacific(n = 3476) | Total Number of Isolates |
| E. avium | 0.8 | 0.8 | 1.8 | 1.2 | 447 |
| E. casseliflavus | 0.5 | 0.5 | 0.4 | 1.0 | 256 |
| E. cecorum | 0.0 | <0.1 | 0.0 | 0.0 | 1 |
| E. devriesei | 0.0 | 0.0 | <0.1 | 0.0 | 1 |
| E. durans | 0.2 | 0.5 | 0.3 | 0.2 | 158 |
| E. faecalis | 64.2 | 62.8 | 74.1 | 64.0 | 32 015 |
| E. faecium | 28.4 | 32.6 | 18.4 | 31.3 | 14 360 |
| E. gallinarum | 0.8 | 0.9 | 1.4 | 0.8 | 446 |
| E. gilvus | <0.1 | 0.0 | 0.0 | 0.0 | 1 |
| E. hirae | 0.1 | 0.2 | 0.3 | 0.3 | 89 |
| E. italicus | 0.0 | <0.1 | 0.0 | 0.0 | 1 |
| E. malodoratus | 0.0 | 0.0 | <0.1 | 0.0 | 1 |
| E. mundtii | <0.1 | 0.0 | <0.1 | <0.1 | 3 |
| E. raffinosus | 0.3 | 0.1 | <0.1 | 0.7 | 137 |
| E. thailandicus | <0.1 | <0.1 | 0.0 | 0.0 | 2 |
| Undetermined | 4.6 | 1.6 | 3.2 | 0.5 | 1573 |
The frequency of the VanA (resistant to vancomycin and teicoplanin) [44] and VanB (resistant to vancomycin but susceptible to teicoplanin) [44] phenotypes in each region is shown in Table 3. Among 49 491 isolates of enterococci, 13.7% exhibited a VanA phenotype (range, 6.7% [APAC] to 20.0% [NA]) and 1.7% showed a VanB phenotype (range, 0.9% [LATAM] to 2.6% [APAC]). There were 6788 VanA isolates (91.0% E. faecium), 827 VanB isolates (75.3% E. faecium), and 702 VanC (E. gallinarum and E. casseliflavus) isolates (Table 2). Whereas the frequency of VanA E. faecium varied considerably among the different regions (64.7% in NA to 19.0% in EUR), the rate of VanB E. faecium was consistently low (range, 3.6% [NA] to 7.5% [APAC]) across all 4 regions (Table 3). Only 2.5% of E. faecalis isolates were resistant to vancomycin (1.9% VanA and 0.6% VanB), with little variation among the regions (Table 3). High-level resistance to streptomycin (HLR-strep; MIC, >1024 mg/L) was detected in 40.7% of E. faecium (19.4% [APAC], 19.5% [NA], 26.9% [LATAM], and 61.6% [EUR]) and 23.0% of E. faecalis (12.5% [APAC], 18.5% [NA], 27.8% [LATAM], and 28.1% [EUR]; data not shown) isolates. Resistance to ampicillin was 89.8% among E. faecium (81.6% [LATAM], 89.6% [NA], 90.8% [EUR], and 91.6% [APAC]; data not shown) and 0.4% among E. faecalis (0.1% [APAC], 0.3% [NA], 0.4% [EUR], and 0.7% [LATAM]; data not shown) isolates.
Table 3.
Main Organisms and Organism Groups Stratified by Geography and Vancomycin Resistance Phenotype
| Organism/Organism Group | Asia-Pacific | Europe | Latin America | North America | Total |
|---|---|---|---|---|---|
| Enterococcus spp., No. (%) | 3476 | 16 054 | 4755 | 25 206 | 49 491 |
| Vancomycin-susceptible (≤4 mg/L) | 3135 (90.2) | 14 626 (91.1) | 4249 (89.4) | 19 544 (77.5) | 41 554 (84.0) |
| Vancomycin-resistant (VanA) | 232 (6.7) | 1095 (6.8) | 426 (9.0) | 5035 (20.0) | 6788 (13.7) |
| Vancomycin-resistant (VanB) | 89 (2.6) | 279 (1.7) | 44 (0.9) | 415 (1.6) | 827 (1.7) |
| Enterococcus faecium, No. (%) | 1089 | 5229 | 876 | 7166 | 14 360 |
| Vancomycin-susceptible (≤4 mg/L) | 780 (71.6) | 3990 (76.3) | 517 (59.1) | 2268 (31.6) | 7555 (52.6) |
| Vancomycin-resistant (VanA) | 227 (20.8) | 992 (19.0) | 323 (36.8) | 4637 (64.7) | 6179 (43.0) |
| Vancomycin-resistant (VanB) | 82 (7.5) | 246 (4.7) | 36 (4.1) | 259 (3.6) | 623 (4.3) |
| Enterococcus faecalis, No. (%) | 2225 | 10 078 | 3524 | 16 188 | 32 015 |
| Vancomycin-susceptible (≤4 mg/L) | 2213 (99.5) | 9942 (98.6) | 3413 (96.8) | 15 631 (96.6) | 31 199 (97.5) |
| Vancomycin-resistant (VanA) | 5 (0.2) | 103 (1.0) | 103 (3.0) | 398 (2.5) | 609 (1.9) |
| Vancomycin-resistant (VanB) | 7 (0.3) | 33 (0.3) | 8 (0.2) | 156 (1.0) | 204 (0.6) |
Antimicrobial susceptibility trends of the enterococcal strains tested are shown in Table 4. A decline in the susceptibility to ampicillin and vancomycin was seen over time in all geographic regions. Although neither doxycycline (range, 23.8%–55.2% susceptible) nor tetracycline (range, 24.9%–43.9% susceptible) was very active, this level of activity was maintained in all regions except NA, where susceptibility declined. Linezolid, an oxazolidinone, maintained a high level of activity (>95.0%) in all regions over the monitored 20-year period.
Table 4.
Trends in Antimicrobial Susceptibility of All Tested Enterococci in Each Monitored Region for 1997–2016: SENTRY Program
| Susceptibility, %a | |||||||
|---|---|---|---|---|---|---|---|
| Region | Time Period | No. of Isolates | AMPb | CHLc | TETd | LZD | VAN |
| NA | 1997–2000 | 4195 | 79.2 | 82.3 | 37.1 | 96.6 | 87.6 |
| NA | 2001–2004 | 3685 | 75.7 | 88.2 | 38.2 | 99.5 | 82.7 |
| NA | 2005–2008 | 6509 | 68.2 | 89.7 | 37.8 | 99.2 | 72.5 |
| NA | 2009–2012 | 6130 | 69.6 | NT | 26.4 | 99.4 | 71.8 |
| NA | 2013–2016 | 4687 | 76.9 | NT | 24.9 | 99.6 | 79.0 |
| EUR | 1997–2000 | 1593 | 83.8 | 67.9 | 33.8 | 98.8 | 96.6 |
| EUR | 2001–2004 | 2196 | 78.7 | 74.1 | 40.1 | 99.9 | 96.1 |
| EUR | 2005–2008 | 4759 | 67.4 | 74.5 | 43.9 | 99.8 | 90.6 |
| EUR | 2009–2012 | 4144 | 64.8 | NT | 38.2 | 99.7 | 87.7 |
| EUR | 2013–2016 | 3362 | 64.7 | NT | 33.1 | 99.7 | 90.1 |
| LATAM | 1997–2000 | 491 | 95.5 | 69.0 | 34.2 | 95.7 | 98.4 |
| LATAM | 2001–2004 | 560 | 86.2 | 72.3 | 31.1 | 100.0 | 94.5 |
| LATAM | 2005–2008 | 1825 | 83.9 | 73.1 | 40.1 | 99.8 | 88.7 |
| LATAM | 2009–2012 | 1326 | 80.2 | NT | 41.6 | 99.9 | 87.3 |
| LATAM | 2013–2016 | 553 | 78.1 | NT | 43.8 | 99.6 | 83.5 |
| APAC | 1997–2000 | 528 | 83.0 | 75.2 | 33.5 | 97.0 | 99.4 |
| APAC | 2001–2004 | 590 | 73.1 | 75.6 | 36.4 | 100 | 96.3 |
| APAC | 2005–2008 | 952 | 66.4 | NT | 37.2 | 99.6 | 86.2 |
| APAC | 2009–2012 | 988 | 67.9 | NT | 33.4 | 99.5 | 87.0 |
| APAC | 2013–2016 | 418 | 65.8 | NT | 33.7 | 99.5 | 86.4 |
Abbreviations: AMP, ampicillin; APAC, Asia-Pacific region; CHL, chloramphenicol; EUR, Europe; LATAM, Latin America; LZD, linezolid; NA, North America; NT, not tested; TET, tetracycline; VAN, vancomycin.
aCriteria as published by Clinical and Laboratory Standards Institute 2018 [44].
bThe results of ampicillin susceptibility tests may be used to predict susceptibility to amoxicillin-clavulanate, ampicillin-sulbactam, and piperacillin-tazobactam among non-β-lactamase- producing enterococci and imipenem for E. faecalis [44].
cChloramphenicol was tested against isolates collected during 1997–2005.
dOrganisms that are susceptible to tetracycline are also considered susceptible to doxycycline and minocycline. However, some organisms that are intermediate or resistant to tetracycline may be susceptible to doxycycline, minocycline, or both [44].
Although the frequency of infections due to VRE has been shown to increase in many areas of the world, resistance may vary within a given nation or region, supporting the need for ongoing surveillance and applying strict infection prevention practices [3, 10, 33]. Recent data from Europe found that although the rate of VRE infections has increased, the prevalence may vary as much as 10-fold across different countries [1, 49]. At the outset of the SENTRY Program in 1997–1999, considerable variation was exhibited in the frequency of VRE infections among geographic regions within the United States, with higher numbers of infections in the Northeast and North Central states compared with the Western and Southern states [33]. These differences have been muted over time; however, VRE remains most common in the Northeast (29.2% of all enterococcal infections), compared with 22% in the Midwest and South and 18.4% in the West (data not shown). Given the predominance of vancomycin-resistant E. faecium in the United States and clonal spread due to CC-17 causing inter- and intrahospital transmission of a hospital-adapted pathogen, it is not surprising that less variability is found currently in US VRE rates [3, 10, 20, 24].
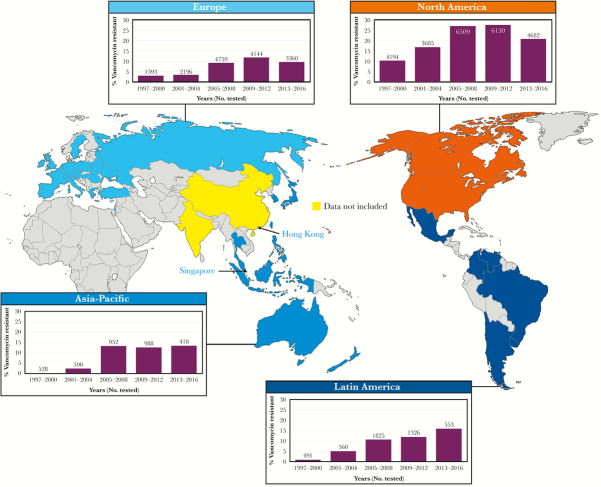
An important aspect of any antimicrobial surveillance program is longitudinality [50–52]. By conducting surveillance of specific pathogens across many years, one can assess the emergence of specific strains or species and discover changes in the antimicrobial susceptibility profiles of the organisms [10, 20, 24, 50, 51]. Furthermore, when longitudinal surveillance encompasses a broad geographic distribution, one may eventually develop a useful picture of regional, national, or even global trends or shifts in species distribution and antimicrobial resistance [10, 20, 50]. Thus, over the 20-year duration of the SENTRY Program, it is clear that the frequency of VRE (VanA and VanB only) as a cause of enterococcal infection has increased incrementally in all 4 of the monitored global regions (Figure 1). In the early years of the SENTRY Program (1997–2000), VRE was relatively uncommon (0.0%–3.0%) in all monitored regions except for NA (10.3%); however, the frequency of VRE increased in all regions through 2012. The decline in VRE in recent years (2013–2016) in EUR and NA (Figure 1) is likely due to regional and national emphasis on the prudent use of vancomycin and applying infection prevention (IP) efforts directed at controlling methicillin-resistant S. aureus and VRE [10, 53]. The global spread of the hospital-derived CC-17 VRE, coupled with less intensive IP efforts, continues to account for the progressive increases in VRE in the APAC and LATAM regions [10, 20, 24]. Clearly, VRE has become a global threat to the care of hospitalized individuals and must be addressed by enhanced antimicrobial stewardship and IP efforts. Also, the prudent application of novel and newer agents with potent activity against these MDR pathogens appears to be more necessary [3, 9, 10, 12, 20, 22, 54].
Figure 1.
Frequency of vancomycin-resistant (VanA and VanB) enterococci by geographic region: SENTRY Program, 1997–2016.
As resistance to vancomycin is usually accompanied by multiple resistance to other antimicrobial agents such as macrolides, tetracyclines, and fluoroquinolones, the activity of alternative therapeutic agents for VRE infections was evaluated. Table 5 lists, by region, the MIC50 and MIC90 values and percentage of isolates susceptible for 9 antimicrobial agents tested against VRE (VanA and VanB) in the SENTRY Program. Notably, most older agents (ampicillin, doxycycline, piperacillin-tazobactam) were largely inactive and contribute to the MDR nature of E. faecium and E. faecalis worldwide.
Table 5.
Potency and Spectrum of 9 Selected Antimicrobial Agents Tested Against 7615 Vancomycin-Resistant (VanA and VanB Phenotypes) Enterococcal Isolates in the SENTRY Program, 1997–2016
| MIC50/90 (% of Tested Isolates Susceptible), mg/La | ||||
|---|---|---|---|---|
| AntimicrobialAgent | NA(n = 5450) | EUR(n = 1374) | LATAM(n = 470) | APAC(n = 321) |
| Ampicillinb | >8/>8 (10.5) | >8/>8 (10.0) | >8/>8 (22.8) | >8/>8 (3.4) |
| Tetracyclinec | >8/>8 (35.6) | ≤4/>8 (57.5) | ≤4/>8 (64.7) | ≤4/>8 (62.3) |
| Tigecycline | ≤0.12/≤0.12 (99.2) | ≤0.12/≤0.12 (99.5) | ≤0.12/≤0.12 (99.3) | 0.12/0.25 (99.4) |
| Daptomycin | 2/2 (99.6) | 2/2 (100.0) | 1/2 (100.0) | 2/4 (99.7) |
| Oritavancind | 0.03/0.12 (92.3) | 0.015/0.06 (95.7) | 0.03/0.12 (92.2) | ≤0.008/0.06 (98.3) |
| Linezolid | 1/2 (98.0) | 1/2 (99.2) | 1/2 (99.6) | 1/2 (99.4) |
| Tedizolide | 0.12/0.25 (99.5) | 0.12/0.25 (99.5) | 0.12/0.25 (100.0) | 0.12/0.25 (100.0) |
| Quinupristin-dalfopristinf | ≤0.5/>2 (95.9) | 1/>2 (83.5) | 1/>2 (84.9) | 1/2 (92.4) |
Abbreviations: APAC, Asia-Pacific region; EUR, Europe; LATAM, Latin America; MIC, minimum inhibitory concentration; NA, North America.
aCriteria as published by Clinical and Laboratory Standards Institute 2018 [44] and European Committee on Antimicrobial Susceptibility Testing 2018 (tigecycline only) [45].
bThe results of ampicillin susceptibility tests may be used to predict susceptibility to amoxicillin-clavulanate, ampicillin-sulbactam, and piperacillin-tazobactam among non-β-lactamase-producing enterococci and imipenem for E. faecalis [44].
cOrganisms that are susceptible to tetracycline are also considered susceptible to doxycycline and minocycline. However, some organisms that are intermediate or resistant to tetracycline may be susceptible to doxycycline, minocycline, or both [44].
dSusceptible breakpoint (MIC, ≤0.12 mg/L) for vancomycin-susceptible E. faecalis was applied to all vancomycin-resistant enterococci [44]. Enterococci that are susceptible to oritavancin (VanA) may be resistant to dalbavancin and/or telavancin.
eSusceptible breakpoint (MIC, ≤0.5 mg/L) for E. faecalis was applied to all vancomycin-resistant enterococci [44].
fData for vancomycin-resistant E. faecium only.
The emergence of VRE has prompted the clinical development of several novel and modified antimicrobial compounds with potent activity against most VRE strains, including the oxazolidinones (linezolid and tedizolid), the lipopeptides or lipoglycopeptides (oritavancin, dalbavancin, and telavancin), and a glycylcycline (tigecycline) [6, 9, 10, 22]. In contrast to the older agents (ampicillin and tetracycline) shown in Table 5, linezolid, tedizolid, daptomycin, oritavancin, and tigecycline were all highly active (92.2%–100.0% susceptible) against the VRE from the SENTRY Program and, in most cases, were more active than quinupristin-dalfopristin, especially those from EUR and LATAM (Table 5). Among these agents, daptomycin, linezolid, oritavancin, quinupristin-dalfopristin, and tedizolid are indicated for treatment of infections due to VRE, whereas telavancin and tigecycline are not approved for the treatment of VRE [6]. In contrast to oritavancin, neither dalbavancin (4.2% susceptible at the CLSI E. faecalis vancomycin-susceptible breakpoint of ≤0.25 mg/L) nor telavancin (1.8% susceptible at the CLSI E. faecalis vancomycin-susceptible breakpoint of ≤0.25 mg/L) were active against VanA-VRE (data not shown). Dalbavancin (81.8% susceptible at the CLSI E. faecalis vancomycin-susceptible breakpoint of ≤0.25 mg/L) and telavancin (79.4% susceptible at the CLSI E. faecalis vancomycin-susceptible breakpoint of ≤0.25 mg/L) showed moderate activity against VanB-VRE (data not shown).
Enterococcal resistance has been described for quinupristin-dalfopristin and linezolid and more recently for daptomycin and tigecycline [6, 9, 22, 55–57]. In the context of the SENTRY Program, despite observing a low rate of linezolid resistance (1.6% of VRE), characterization of linezolid-resistant E. faecalis and E. faecium isolates revealed that alterations in 23S rRNA (G2576T mutations) were the dominant oxazolidinone resistance mechanism in E. faecium, whereas the plasmid-borne resistance gene optrA became more prevalent in E. faecalis [58]. Thus, data from the SENTRY Program continue to document the global dissemination of optrA-carrying E. faecalis isolates recovered from patients in countries beyond the APAC region, including China, Ireland, Sweden, and the United States [58]. As such, monitoring the emergence and spread of this resistance determinant at local and regional levels is important, especially due to the potential for E. faecalis bacteria to serve as a reservoir for spreading optrA to MDR pathogens (ie, E. faecium).
DISCUSSION
The SENTRY Antimicrobial Surveillance Program was designed to track antimicrobial resistance trends and the spectrum of microbial pathogens causing human infection on a global scale. The SENTRY Program has unique features that distinguish it from other excellent surveillance projects, such as the SCOPE Program [46, 47], the NHSN [2, 48], the European Antimicrobial Resistance Surveillance Network (EARS-Net) [49], and population-based surveillance programs conducted in the United States [5, 29], Australia [14], Canada [13, 16, 17], China [18], India [15], South Korea [59], Norway [60], and Taiwan [19]. Whereas these cited programs are usually based in a single country, may track only nosocomial infections, and/or rely primarily on a wide variety of susceptibility testing results/methods from participating centers, the SENTRY Program monitors nosocomial and community-onset infections on a global scale using validated reference identification and antimicrobial susceptibility testing methods in a central monitoring laboratory design, including central quality assurance [10, 24, 33, 35–42].
When the SENTRY Program began in 1997, VRE was an uncommon cause of HAI in most world regions except NA [33, 46]. Subsequently, VRE rates increased steadily in all geographic regions, as did resistance to commonly used anti-enterococal antimicrobial agents such as penicillins (ampicillin and piperacillin-tazobactam) and tetracyclines and teicoplanin in EUR. The introduction of new agents with potent activity against VRE E. faecium, such as linezolid, tedizolid, daptomycin, and tigecycline, offers great promise in the treatment of VRE infections. However, the clinical data supporting their wide monotherapy use in severe, complicated infections are relatively limited, and the role of these new agents in the current armamentarium remains to be established [6, 9, 22, 54]. Each of these agents demonstrates excellent, often bactericidal, activity, and each agent inhibits more than 98.0% of VanA and VanB enterococci across all 4 monitored regions (Table 5). Moreover, the breadth and duration of the SENTRY Program allows for the detection of emerging resistance to these agents, as they are employed throughout the world [10, 24, 37, 58]. As an example, despite the low frequency of resistance to oxazolidinones among E. faecalis and E. faecium isolates, the SENTRY Program confirmed the identification of phenotypically nonsusceptible strains and documented the presence of ribosomal mutations in both species as well as the plasmid-borne (eg, optrA) resistance genes in E. faecalis from multiple geographic locations and numerous other oxazolidinone resistance mechanisms [58]. Implementing proteomic and molecular characterization of HAI pathogens, such as the enterococci in the context of a global surveillance program, allows detailed and accurate characterization of infecting strains that will be useful in defining resistance and evaluating new candidate agents for the prevention and/or treatment of these serious infections.
In comparing these data, it is important to realize that the results of most surveillance studies have potential biases that reflect the population surveyed, the method for data collection, and the underlying purposes for data collection [50, 51, 61–64]. Significant differences may exist regarding patterns of antimicrobial resistance and usage, and these differences are likely to affect the ability to compare data among different studies [50, 51, 61–64]. Thus, longitudinal surveillance (SENTRY Program) by the same reference methods and study sites is important in providing accurate estimates of trends in antibacterial and antifungal resistance [10, 24, 34, 35, 37, 65].
Acknowledgments
The authors thank all participants of the SENTRY Program for their work in providing isolates.
Financial support. Funding for the manuscript was provided by JMI Laboratories.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. ECDC. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011–2012.2013. http://ecdc.europa.eu/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf. Accessed June 25, 2018.
- 2. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012; 10:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC. Antibiotic resistance threats in the United States, 2013.Centers for Disease Control and Prevention; 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed May 15, 2018. [Google Scholar]
- 5. Chiang HY, Perencevich EN, Nair R, et al. Incidence and outcomes associated with infections caused by vancomycin-resistant Enterococci in the United States: systematic literature review and meta-analysis. Infect Control Hosp Epidemiol 2017; 38:203–15. [DOI] [PubMed] [Google Scholar]
- 6. Abbas M, Paul M, Huttner A. New and improved? A review of novel antibiotics for gram-positive bacteria. Clin Microbiol Infect 2017; 23:697–703. [DOI] [PubMed] [Google Scholar]
- 7. Erlandson KM, Sun J, Iwen PC, Rupp ME. Impact of the more-potent antibiotics quinupristin-dalfopristin and linezolid on outcome measure of patients with vancomycin-resistant Enterococcus bacteremia. Clin Infect Dis 2008; 46:30–6. [DOI] [PubMed] [Google Scholar]
- 8. Hayakawa K, Martin ET, Gudur UM, et al. Impact of different antimicrobial therapies on clinical and fiscal outcomes of patients with bacteremia due to vancomycin-resistant enterococci. Antimicrob Agents Chemother 2014; 58:3968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linden PK. Optimizing therapy for vancomycin-resistant enterococci (VRE). Semin Respir Crit Care Med 2007; 28:632–45. [DOI] [PubMed] [Google Scholar]
- 10. Mendes RE, Castanheira M, Farrell DJ, Flamm RK, Sader HS, Jones RN. Longitudinal (2001–14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010–13) analysis of oritavancin in vitro potency. J Antimicrob Chemother 2016; 71:3453–8. [DOI] [PubMed] [Google Scholar]
- 11. Munita JM, Bayer AS, Arias CA. Evolving resistance among gram-positive pathogens. Clin Infect Dis 2015; 61(Suppl 2):S48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfaller MA, Mendes RE, Streit JM, Hogan PA, Flamm RK. Five-year summary of in vitro activity and resistance mechanisms of linezolid against clinically important gram-positive cocci in the United States rom the LEADER Surveillance Program (2011 to 2015). Antimicrob Agents Chemother 2017; 61:e00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Billington EO, Phang SH, Gregson DB, et al. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis 2014; 26:76–82. [DOI] [PubMed] [Google Scholar]
- 14. Coombs GW, Daley DA, Thin Lee Y, et al. Australian Group on Antimicrobial Resistance Australian Enterococcal Sepsis Outcome Programme annual report, 2014. Commun Dis Intell Q Rep 2016; 40:E236–43. [DOI] [PubMed] [Google Scholar]
- 15. Ghoshal U, Garg A, Tiwari DP, Ayyagari A. Emerging vancomycin resistance in enterococci in India. Indian J Pathol Microbiol 2006; 49:620–2. [PubMed] [Google Scholar]
- 16. McCracken M, Wong A, Mitchell R, et al. Molecular epidemiology of vancomycin-resistant enterococcal bacteraemia: results from the Canadian Nosocomial Infection Surveillance Program, 1999–2009. J Antimicrob Chemother 2013; 68:1505–9. [DOI] [PubMed] [Google Scholar]
- 17. Simor AE, Williams V, McGeer A, et al. Prevalence of colonization and infection with methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus and of Clostridium difficile infection in Canadian hospitals. Infect Control Hosp Epidemiol 2013; 34:687–93. [DOI] [PubMed] [Google Scholar]
- 18. Sun H, Wang H, Xu Y, et al. Molecular characterization of vancomycin-resistant Enterococcus spp. clinical isolates recovered from hospitalized patients among several medical institutions in China. Diagn Microbiol Infect Dis 2012; 74:399–403. [DOI] [PubMed] [Google Scholar]
- 19. Wang JT, Chang SC, Wang HY, et al. High rates of multidrug resistance in Enterococcus faecalis and E. faecium isolated from inpatients and outpatients in Taiwan. Diagn Microbiol Infect Dis 2013; 75:406–11. [DOI] [PubMed] [Google Scholar]
- 20. Willems RJ, Top J, van Santen M, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis 2005; 11:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev 1990; 3:46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Harten RM, Willems RJL, Martin NI, Hendrickx APA. Multidrug-resistant enterococcal infections: new compounds, novel antimicrobial therapies? Trends Microbiol 2017; 25:467–79. [DOI] [PubMed] [Google Scholar]
- 23. Chavers LS, Moser SA, Benjamin WH, et al. Vancomycin-resistant enterococci: 15 years and counting. J Hosp Infect 2003; 53:159–71. [DOI] [PubMed] [Google Scholar]
- 24. Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis 2007; 58:163–70. [DOI] [PubMed] [Google Scholar]
- 25. Fridkin SK, Edwards JR, Courval JM, et al. The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 U.S. adult intensive care units. Ann Intern Med 2001; 135:175–83. [DOI] [PubMed] [Google Scholar]
- 26. Kaye KS, Engemann JJ, Mozaffari E, Carmeli Y. Reference group choice and antibiotic resistance outcomes. Emerg Infect Dis 2004; 10:1125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oprea SF, Zaidi N, Donabedian SM, et al. Molecular and clinical epidemiology of vancomycin-resistant Enterococcus faecalis. J Antimicrob Chemother 2004; 53:626–30. [DOI] [PubMed] [Google Scholar]
- 28. Rice LB, Hutton-Thomas R, Lakticova V, et al. Beta-lactam antibiotics and gastrointestinal colonization with vancomycin-resistant enterococci. J Infect Dis 2004; 189:1113–8. [DOI] [PubMed] [Google Scholar]
- 29. DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis 2005; 41:327–33. [DOI] [PubMed] [Google Scholar]
- 30. Leavis HL, Willems RJ, Top J, et al. Epidemic and nonepidemic multidrug-resistant Enterococcus faecium. Emerg Infect Dis 2003; 9:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klare I, Konstabel C, Mueller-Bertling S, et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur J Clin Microbiol Infect Dis 2005; 24:815–25. [DOI] [PubMed] [Google Scholar]
- 32. Han SH, Chin BS, Lee HS, et al. Vancomycin-resistant enterococci bacteremia: risk factors for mortality and influence of antimicrobial therapy on clinical outcome. J Infect 2009; 58:182–90. [DOI] [PubMed] [Google Scholar]
- 33. Low DE, Keller N, Barth A, Jones RN. Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: results from the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis 2001; 32(Suppl 2):S133–45. [DOI] [PubMed] [Google Scholar]
- 34. Mendes RE, Woosley LN, Farrell DJ, et al. Oritavancin activity against vancomycin-susceptible and vancomycin-resistant enterococci with molecularly characterized glycopeptide resistance genes recovered from bacteremic patients, 2009–2010. Antimicrob Agents Chemother 2012; 56:1639–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mutnick AH, Biedenbach DJ, Jones RN. Geographic variations and trends in antimicrobial resistance among Enterococcus faecalis and Enterococcus faecium in the SENTRY Antimicrobial Surveillance Program (1997-2000). Diagn Microbiol Infect Dis 2003; 46:63–8. [DOI] [PubMed] [Google Scholar]
- 36. Pfaller MA, Jones RN, Doern GV, Kugler K. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). Antimicrob Agents Chemother 1998; 42:1762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deshpande LM, Ashcraft DS, Kahn HP, et al. Detection of a new CFR-like gene, cfr(B), in Enterococcus faecium recovered from human specimens in the United States: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 2015; 59:6256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997-2002). Diagn Microbiol Infect Dis 2004; 50:59–69. [DOI] [PubMed] [Google Scholar]
- 39. Diekema DJ, Pfaller MA, Jones RN, Group SP. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY Antimicrobial Surveillance Program, 1997–2000. Int J Antimicrob Agents 2002; 20:412–8. [DOI] [PubMed] [Google Scholar]
- 40. Jones RN, Della-Latta P, Lee LV, Biedenbach DJ. Linezolid-resistant Enterococcus faecium isolated from a patient without prior exposure to an oxazolidinone: report from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis 2002; 42:137–9. [DOI] [PubMed] [Google Scholar]
- 41. Jones RN, Deshpande LM. Distribution of fsr among Enterococcus faecalis isolates from the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol 2003; 41:4004–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pfaller MA, Jones RN. Global view of antimicrobial resistance. Findings of the SENTRY Antimicrobial Surveillance Program, 1997–1999. Postgrad Med 2001; 109(2 Suppl):10–21. [DOI] [PubMed] [Google Scholar]
- 43. CLSI. M07Ed11E. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 11th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 44. CLSI. M100Ed28E. Performance Standards for Antimicrobial Susceptibility Testing: 28th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 45. EUCAST. Breakpoint Tables for Interpretation of MIC’s and Zone Diameters. Version 8.0. European Committee on Antimicrobial Susceptibility Testing; 2018. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf. Accessed January 4, 2018.
- 46. Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. SCOPE Hospital Study Group. Diagn Microbiol Infect Dis 1997; 29:95–102. [DOI] [PubMed] [Google Scholar]
- 47. Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24 179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–17. [DOI] [PubMed] [Google Scholar]
- 48. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. ECDC. Antimicrobial resistance surveillance report Europe 2013 2014. http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2013.pdf. Accessed June 2016.
- 50. Jones RN. Detection of emerging resistance patterns within longitudinal surveillance systems: data sensitivity and microbial susceptibility. MYSTIC Advisory Board. Meropenem Yearly Susceptibility Test Information Collection. J Antimicrob Chemother 2000; 46(Suppl T2):1–8. [PubMed] [Google Scholar]
- 51. Jones RN, Masterton R. Determining the value of antimicrobial surveillance programs. Diagn Microbiol Infect Dis 2001; 41:171–5. [DOI] [PubMed] [Google Scholar]
- 52. Pfaller MA, Diekema DJ. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J Clin Microbiol 2002; 40:3551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber SG, Huang SS, Oriola S, et al. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: position statement from the Joint SHEA and APIC Task Force. Am J Infect Control 2007; 35:73–85. [DOI] [PubMed] [Google Scholar]
- 54. Beganovic M, Luther MK, Rice LB, et al. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin Infect Dis 2018; 67:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gonzales RD, Schreckenberger PC, Graham MB, et al. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179. [DOI] [PubMed] [Google Scholar]
- 56. Lewis JS, Owens A, Cadena J, et al. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother 2005; 49:1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sader HS, Jones RN, Stilwell MG, et al. Tigecycline activity tested against 26 474 bloodstream infection isolates: a collection from 6 continents. Diagn Microbiol Infect Dis 2005; 52:181–6. [DOI] [PubMed] [Google Scholar]
- 58. Deshpande LM, Castanheira M, Flamm RK, Mendes RE. Evolving oxazolidinone resistance mechanisms in a worldwide collection of enterococcal clinical isolates: results from the SENTRY Antimicrobial Surveillance Program. J Antimicrob Chemother 2018; 73:2314–22. [DOI] [PubMed] [Google Scholar]
- 59. Kim MC, Woo GJ. Characterization of antimicrobial resistance and quinolone resistance factors in high-level ciprofloxacin-resistant Enterococcus faecalis and Enterococcus faecium isolates obtained from fresh produce and fecal samples of patients. J Sci Food Agric 2017; 97:2858–64. [DOI] [PubMed] [Google Scholar]
- 60. Simonsen GS, Småbrekke L, Monnet DL, et al. Prevalence of resistance to ampicillin, gentamicin and vancomycin in Enterococcus faecalis and Enterococcus faecium isolates from clinical specimens and use of antimicrobials in five Nordic hospitals. J Antimicrob Chemother 2003; 51:323–31. [DOI] [PubMed] [Google Scholar]
- 61. Hunter PA, Reeves DS. The current status of surveillance of resistance to antimicrobial agents: report on a meeting. J Antimicrob Chemother 2002; 49:17–23. [DOI] [PubMed] [Google Scholar]
- 62. Jones RN. The emergent needs for basic research, education, and surveillance of antimicrobial resistance. Problems facing the report from the American Society for Microbiology Task Force on Antibiotic Resistance. Diagn Microbiol Infect Dis 1996; 25:153–61. [DOI] [PubMed] [Google Scholar]
- 63. Masterton RG. Surveillance studies: how can they help the management of infection? J Antimicrob Chemother 2000; 46(Suppl T2):53–8. [PubMed] [Google Scholar]
- 64. Perez F, Villegas MV. The role of surveillance systems in confronting the global crisis of antibiotic-resistant bacteria. Curr Opin Infect Dis 2015; 28:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Castanheira M, Deshpande LM, Davis AP, et al. Monitoring antifungal resistance in a global collection of invasive yeasts and moulds: application of CLSI epidemiological cutoff values and whole genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob Agents Chemother 2017; 61:e00906. [DOI] [PMC free article] [PubMed] [Google Scholar]