Abstract
Background
The SENTRY Antimicrobial Surveillance Program was established in 1997 and encompasses over 750 000 bacterial isolates from ≥400 medical centers worldwide. Among the pathogens tested, Pseudomonas aeruginosa remains a common cause of multidrug-resistant (MDR) bloodstream infections and pneumonia in hospitalized patients. In the present study, we reviewed geographic and temporal trends in resistant phenotypes of P. aeruginosa over 20 years of the SENTRY Program.
Methods
From 1997 to 2016, 52 022 clinically significant consecutive isolates were submitted from ≥200 medical centers representing the Asia-Pacific region, Europe, Latin America, and North America. Only 1 isolate per patient per infection episode was submitted. Isolates were identified by standard algorithms and/or matrix-assisted laser desorption ionization-time of flight mass spectrometry. Susceptibility testing was performed by Clinical and Laboratory Standards Institute (CLSI) methods and interpreted using CLSI and European Committee on Antimicrobial Susceptibility Testing 2018 criteria at JMI Laboratories.
Results
The most common infection from which P. aeruginosa was isolated was pneumonia in hospitalized patients (44.6%) followed by bloodstream infection (27.9%), with pneumonia having a slightly higher rate of MDR (27.7%) than bloodstream infections (23.7%). The region with the highest percentage of MDR phenotypes was Latin America (41.1%), followed by Europe (28.4%). The MDR rates were highest in 2005–2008 and have decreased in the most recent period. Colistin was the most active drug tested (99.4% susceptible), followed by amikacin (90.5% susceptible).
Conclusions
Over the 20 years of SENTRY Program surveillance, the rate of MDR P. aeruginosa infections has decreased, particularly in Latin America. Whether the trend of decreasing resistance in P. aeruginosa is maintained will be documented in future SENTRY Program and other surveillance reports.
Keywords: antimicrobial, multidrug resistance, Pseudomonas aeruginosa, surveillance, susceptibility
The SENTRY Antimicrobial Surveillance Program was established in 1997 and encompasses over 750 000 bacterial isolates from more than 400 medical centers worldwide.
Among the pathogens tested in the SENTRY Program, Pseudomonas aeruginosa remains a common cause of multidrug-resistant ([MDR] nonsusceptible [NS] to at least 1 antimicrobial in 3 or more drug classes) bloodstream infections and pneumonia in hospitalized patients. Zilberberg et al [1] found that MDR P. aeruginosa was much more common in bloodstream infections (14.7%) and pneumonia (22.0%) than carbapenem-resistant Enterobacteriaceae from bloodstream infections (1.1%) and pneumonia (1.6%), which makes treatment of serious P. aeruginosa infections more challenging. Furthermore, delaying appropriate antimicrobial therapy has been associated with increased morbidity and mortality [2]. Patients with MDR P. aeruginosa have a higher 30-day mortality than patients with non-MDR P. aeruginosa [3].
Frequently, MDR P. aeruginosa isolates are resistant to carbapenems and other β-lactams, which is mediated through multiple mechanisms, including acquisition of metallo-β-lactamases, increased chromosomal AmpC production, extended spectrum β-lactamases, increased efflux, or changes in membrane permeability [4, 5]. In the present study, we reviewed geographic and temporal trends in resistant phenotypes of P. aeruginosa over the 20 years of the SENTRY Antimicrobial Surveillance Program.
METHODS
During the period from 1997 to 2016, 52 022 clinically significant P. aeruginosa isolates were submitted for testing in the SENTRY Program from ≥400 medical centers representing the Asia-Pacific (excluding China and India), European (including Turkey and Israel), Latin American, and North American regions.
Participating centers submitted bacterial clinical isolates (1 isolate per patient per infection episode) that were consecutively collected by infection type according to a common protocol. The common SENTRY Program protocol established the number of isolates for the target infection types and the time period each year during which the isolates should be collected. Each institution contributed a specified number of isolates per year with approximately 50 isolates per target infection type. Infection types included bloodstream infection (BSI), pneumonia in hospitalized patients, skin and skin structure infection (SSSI), intra-abdominal infection, and urinary tract infection. Isolates were identified by the submitting laboratory’s standard algorithms and/or matrix-assisted laser desorption ionization-time of flight mass spectrometry and confirmed at JMI Laboratories (North Liberty, IA).
Susceptibility (S) testing was performed at JMI Laboratories by the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method and interpreted using CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2018 criteria [6, 7] The antimicrobials tested included amikacin, cefepime, ceftazidime, ciprofloxacin, colistin (tested 2006–2016), meropenem, piperacillin-tazobactam, and tobramycin. Gentamicin, imipenem, and levofloxacin were also tested for resistant phenotype determination.
Resistant phenotypes analyzed using EUCAST criteria were as follows: MDR (NS to at least 1 antimicrobial in ≥3 drug classes), extensively drug-resistant ([XDR] NS to at least 1 agent in all but ≤2 drug classes), and pan drug-resistant (PDR), according to Magiorakos et al [8]. Ceftazidime-NS and meropenem-NS were determined according to EUCAST interpretive criteria.
RESULTS
Infection Types
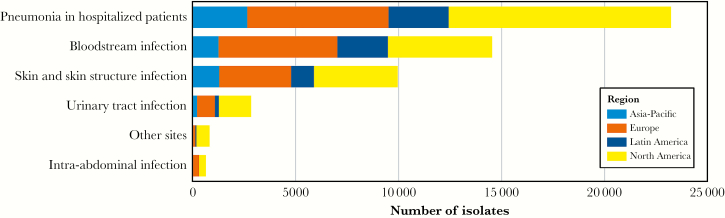
The most common infection type from which P. aeruginosa was isolated was pneumonia in hospitalized patients (44.6%, n = 23 227) followed by BSI (27.9%, n = 14 539) and SSSI (19.1%, n = 9952) as shown in Table 1. The number of isolates from each of the 4 regions by infection type is shown in Figure 1. Pseudomonas aeruginosa was most frequently isolated from pneumonia in all 4 regions.
Table 1.
Pseudomonas aeruginosa Isolates (1997–2016) Stratified by Infection Type and Percentage of Isolates With Resistant Phenotypes
| Resistant Phenotypea | Bloodstream Infection (n = 14 539) | Pneumonia in Hospitalized Patients (n = 23 227) | Skin and Skin Structure Infection (n = 9952) | Intra-abdominal Infection (n = 648) | Urinary Tract Infection (n = 2838) | Other Infection (n = 818) | Total(n = 52 022) |
|---|---|---|---|---|---|---|---|
| Multidrug resistant | 23.7% | 27.7% | 21.7% | 19.3% | 23.0% | 19.1% | 24.9% |
| Extensively drug resistant | 17.4% | 19.0% | 15.8% | 12.7% | 16.5% | 12.3% | 17.6% |
| Pan drug resistant | 0.1% | 0.1% | 0.0% | 0.5% | 0.1% | 0.0% | 0.1% |
| Ceftazidime nonsusceptible | 22.0% | 24.7% | 20.1% | 19.1% | 18.4% | 17.2% | 22.5% |
| Meropenem nonsusceptible | 22.3% | 27.1% | 20.6% | 21.9% | 19.2% | 18.1% | 23.9% |
aCriteria as published by European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2018.
Figure 1.
Distribution of Pseudomonas aeruginosa isolates by infection type and region.
Antimicrobial Susceptibility
Pneumonia had a higher rate of isolates with MDR and XDR (27.7% and 19.0%, respectively) than BSIs (23.7% and 17.4%, respectively) as shown in Table 1. Multidrug-resistant rates over time are shown in Table 2, ranging from a high of 27.5% in 2005–2008 to a low of 21.8% in 2013–2016. Extensively drug-resistant rates also peaked in 2005–2008 at 20.2%. Pan drug-resistant isolates were rare throughout the study period. First identified in 2005–2008, PDR isolates totaled 0.1% and remained at 0.1% in the 2009–2012 and 2013–2016 time frames (Table 2). The frequency of ceftazidime-NS isolates was highest (25.1%) in the 2005–2008 period and has decreased to 19.2% most recently (Table 2). The rate of meropenem-NS isolates was highest (27.3%) in 2009–2012 and decreased to 22.7% in 2013–2016 (Table 2).
Table 2.
Resistant Phenotype Percentages of Pseudomonas aeruginosa Isolates by 4-Year Period Over 20 Years of the SENTRY Program
| Resistant Phenotype a | 1997–2016 (n = 52 022) | 1997–2000(n = 9512) | 2001–2004 (n = 7928) | 2005–2008 (n = 7170) | 2009–2012(n = 10 951) | 2013–2016 (n = 16 461) |
|---|---|---|---|---|---|---|
| Multidrug resistant | 24.9% | 24.1% | 27.3% | 27.5% | 26.9% | 21.8% |
| Extensively drug resistant | 17.6% | 15.9% | 19.6% | 20.2% | 19.6% | 15.2% |
| Pandrug resistant | 0.1% | 0.0% | 0.0% | 0.1% | 0.1% | 0.1% |
| Ceftazidime nonsusceptible | 22.5% | 22.8% | 23.4% | 25.1% | 25.0% | 19.2% |
| Meropenem nonsusceptible | 23.9% | 19.3% | 24.9% | 26.6% | 27.3% | 22.6% |
aCriteria as published by European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2018.
Activities of specific antimicrobials with CLSI and EUCAST clinical breakpoints are shown in Table 3 for all isolates and MDR and XDR phenotypes. Colistin (added to testing in 2006) was the most active agent overall (99.4%/99.4%S [CLSI/EUCAST]) and against isolates with MDR (98.9%/98.9%S [CLSI/EUCAST]) and XDR (98.7%/98.7%S [CLSI/EUCAST] phenotypes (Table 3). Amikacin was the second most active agent, inhibiting 90.5%/86.0% (CLSI/EUCAST) of all isolates, 65.0%/52.7% (CLSI/EUCAST) of MDR, and 55.9%/42.6% (CLSI/EUCAST) of XDR isolates. Tobramycin was slightly less active than amikacin and inhibited 84.6%/84.6% (CLSI/EUCAST) of all isolates, 45.7%/45.7% (CLSI/EUCAST) of MDR, and 34.2%/34.2% (CLSI/EUCAST) of XDR isolates. Other commonly used antipseudomonal β-lactams (cefepime, ceftazidime, meropenem, and piperacillin-tazobactam) had susceptibilities for all isolates ranging from 73.2% to 79.3% and 13.8% to 27.5% for MDR and 5.5% to 17.6% for XDR (CLSI and EUCAST; Table 3). Ciprofloxacin had overall 73.0%S with CLSI breakpoints and 67.8%S with the lower EUCAST breakpoints, only 24.6%/17.1%S for MDR, and 13.6/7.8%S for XDR (CLSI/EUCAST). Aztreonam was the least active antimicrobial tested with overall susceptibility of 64.7%/4.6%, and only 15.6%/1.1%S for MDR and 12.0/0.5%S for XDR (CLSI/EUCAST; Table 3).
Table 3.
Susceptibilities of Pseudomonas aeruginosa for All Regions and Time Periods
| CLSIa | EUCASTa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Resistant Phenotype Antimicrobial Agent | MIC50b (mg/L) | MIC90 (mg/L) | Range (mg/L) | %S | %I | %R | %S | %I | %R |
| All isolates (52 022) | |||||||||
| Amikacin | ≤4 | 16 | ≤4 to >32 | 90.5 | 2.5 | 7 | 86.0 | 4.5 | 9.5 |
| Aztreonam | 8 | >16 | ≤0.12 to >16 | 64.7 | 13.8 | 21.5 | 4.6 | 73.9 | 21.5 |
| Cefepime | 4 | 16 | ≤0.5 to >16 | 79.3 | 10.9 | 9.8 | 79.3 | 20.7 | |
| Ceftazidime | ≤2 | >16 | ≤2 to >16 | 77.5 | 5.2 | 17.3 | 77.5 | 22.5 | |
| Ciprofloxacin | ≤0.5 | >2 | ≤0.5 to >2 | 73 | 4.5 | 22.6 | 67.8 | 32.2 | |
| Colistin | 1 | 2 | ≤0.5 to >4 | 99.4 | 0.6 | 99.4 | 0.6 | ||
| Meropenem | 0.5 | >8 | ≤0.12 to >8 | 76.1 | 6.5 | 17.4 | 76.1 | 13 | 10.9 |
| Piperacillin-tazobactam | 8 | >64 | ≤1 to >64 | 73.2 | 12.1 | 14.8 | 73.2 | 26.8 | |
| Tobramycin | 0.5 | >8 | ≤0.25 to >8 | 84.6 | 0.9 | 14.5 | 84.6 | 15.4 | |
| Multidrug resistant (12 972) | |||||||||
| Amikacin | 8 | >32 | ≤4 to >32 | 65.0 | 8.6 | 26.4 | 52.7 | 12.3 | 35 |
| Aztreonam | >16 | >16 | ≤0.12 to >16 | 15.6 | 22.1 | 62.3 | 1.1 | 36.7 | 62.3 |
| Cefepime | 16 | >16 | ≤0.12 to >16 | 26.8 | 36.5 | 36.7 | 26.8 | 73.2 | |
| Ceftazidime | >16 | >16 | ≤2 to >16 | 26.3 | 14.9 | 58.8 | 26.3 | 73.7 | |
| Ciprofloxacin | >2 | >2 | ≤0.5 to >2 | 24.6 | 7.5 | 67.9 | 17.1 | 82.9 | |
| Colistin | 1 | 2 | ≤0.5 to >4 | 98.9 | 1.1 | 98.9 | 1.1 | ||
| Meropenem | 8 | >8 | ≤0.12 to >8 | 27.5 | 13.4 | 59.1 | 27.5 | 32.1 | 40.4 |
| Piperacillin-tazobactam | >64 | >64 | ≤0.5 to >64 | 13.8 | 34.7 | 51.4 | 13.8 | 86.2 | |
| Tobramycin | >8 | >8 | ≤0.25 to >8 | 45.7 | 2.8 | 51.5 | 45.7 | 54.3 | |
| Extensively drug resistant (9161) | |||||||||
| Amikacin | 16 | >32 | ≤4 to >32 | 55.9 | 10.3 | 33.8 | 42.6 | 13.3 | 44.1 |
| Aztreonam | >16 | >16 | ≤0.12 to >16 | 12 | 21.6 | 66.4 | 0.5 | 33.1 | 66.4 |
| Cefepime | 16 | >16 | ≤0.12 to >16 | 14.2 | 39.4 | 46.3 | 14.2 | 85.8 | |
| Ceftazidime | >16 | >16 | ≤2 to >16 | 16.2 | 15.6 | 68.2 | 16.2 | 83.8 | |
| Ciprofloxacin | >2 | >2 | ≤0.5 to >2 | 13.6 | 7.3 | 79.2 | 7.8 | 92.2 | |
| Colistin | 1 | 2 | ≤0.5 to >4 | 98.7 | 1.3 | 98.7 | 1.3 | ||
| Meropenem | >8 | >8 | ≤0.12 to >8 | 17.6 | 12.4 | 70 | 17.6 | 31.7 | 50.7 |
| Piperacillin-tazobactam | >64 | >64 | ≤0.5 to >64 | 5.5 | 34.7 | 59.8 | 5.5 | 94.5 | |
| Tobramycin | >8 | >8 | ≤0.25 to >8 | 34.2 | 3.0 | 62.9 | 34.2 | 65.8 | |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing.
aCriteria as published by CLSI 2018 and EUCAST 2018.
bMIC, minimum inhibitory concentration; S, susceptible, I, intermediate, R, resistant.
Geographic Resistance Trends
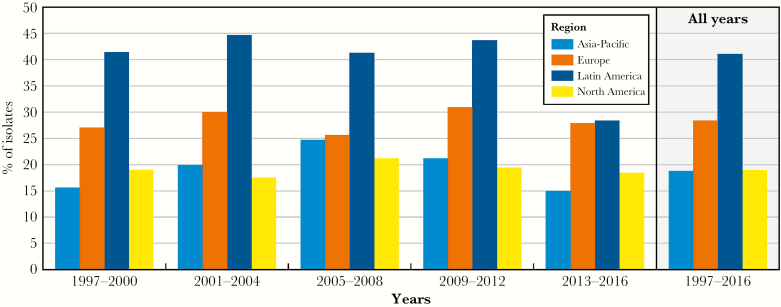
Isolates with the MDR phenotype were most frequently isolated in Latin America with 41.1%, followed by Europe with 28.4%, North America with 18.9%, and Asia-Pacific with 18.8% (Figure 2).
Figure 2.
Percentage of multidrug-resistant Pseudomonas aeruginosa in 4 regions over the 20-year SENTRY Program study period.
Table 4 shows the percentage susceptibility of the antimicrobials by 4-year period for all regions and for each individual region. Susceptibilities for North American and European isolates were relatively stable with variations within 10% between each period. The largest shift in susceptibility for North American isolates was the decrease in meropenem susceptibility from 85.2% in 1997–2000 to 79.0% in 2005–2008, with susceptibility increasing to 81.8% in 2013–2016. European isolates showed a similar shift for meropenem, from a high of 76.5% in 1997–2000 and a low of 68.9% in 2009–2012 with a return to 70.6% in 2013–2016.
Table 4.
Susceptibilities of Pseudomonas aeruginosa Isolates in Each 4-Year Period for 4 Regions
| Susceptibility % by Time Perioda | ||||||
|---|---|---|---|---|---|---|
| Region Antimicrobial Agent | 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | Overall |
| All regions (n) | (9512) | (7928) | (7170) | (10 951) | (16 461) | (52 022) |
| Amikacin | 89.3 | 88.4 | 88.9 | 90.3 | 93.1 | 90.5 |
| Cefepime | 79 | 77.0 | 77.8 | 76.5 | 83.1 | 79.3 |
| Ceftazidime | 77.2 | 76.6 | 74.9 | 75.0 | 80.8 | 77.5 |
| Ciprofloxacin | 74 | 69.9 | 70.8 | 71.7 | 75.7 | 73 |
| Colistinb | N/A | N/A | 99.4 | 99.2 | 99.5 | 99.4 |
| Meropenem | 80.7 | 75.1 | 73.4 | 72.7 | 77.4 | 76.1 |
| Piperacillin-tazobactam | 73.4 | 70.6 | 70.7 | 70.1 | 77.4 | 73.2 |
| Tobramycin | 83.4 | 80.4 | 81.6 | 84.2 | 88.8 | 84.6 |
| North America (n) | (4380) | (2763) | (2129) | (4351) | (8847) | (22 470) |
| Amikacin | 95.4 | 96.7 | 96.1 | 97.1 | 96.7 | 96.4 |
| Cefepime | 83.1 | 85.5 | 83.7 | 83.4 | 86.1 | 84.7 |
| Ceftazidime | 80.7 | 85.6 | 81.4 | 82.7 | 85.1 | 83.5 |
| Ciprofloxacin | 76.8 | 74.5 | 75.7 | 76.3 | 78.0 | 76.8 |
| Colistin | N/A | N/A | 99.9 | 99.1 | 99.6 | 99.5 |
| Meropenem | 85.2 | 83.2 | 79 | 80.3 | 81.8 | 82.1 |
| Piperacillin-tazobactam | 78.5 | 79.7 | 77.1 | 77.5 | 81.6 | 79.5 |
| Tobramycin | 91.9 | 92.7 | 90.5 | 92.4 | 93.0 | 92.4 |
| Europe (n) | (2380) | (2947) | (2712) | (3762) | (5620) | (17 421) |
| Amikacin | 85.7 | 87.9 | 90.5 | 87.2 | 87.8 | 87.8 |
| Cefepime | 77.2 | 75.2 | 79.6 | 73.4 | 78.2 | 76.7 |
| Ceftazidime | 77.1 | 74.8 | 76.1 | 70.1 | 74.4 | 74.2 |
| Ciprofloxacin | 71.3 | 69.3 | 72.2 | 68.3 | 70.9 | 70.3 |
| Colistin | N/A | N/A | 99.6 | 99.4 | 99.5 | 99.5 |
| Meropenem | 76.5 | 73.3 | 74.2 | 68.9 | 70.6 | 72.1 |
| Piperacillin-tazobactam | 71.9 | 68.6 | 73.4 | 66.2 | 70.8 | 70.0 |
| Tobramycin | 75.1 | 75.9 | 81.3 | 78.5 | 82.3 | 79.3 |
| Asia-Pacific (n) | (1243) | (792) | (811) | (1327) | (1236) | (5409) |
| Amikacin | 96.5 | 94.4 | 91.6 | 94.2 | 95.0 | 94.5 |
| Cefepime | 84.8 | 82.2 | 77.4 | 79.0 | 86.8 | 82.3 |
| Ceftazidime | 81.2 | 81.4 | 75.2 | 77.5 | 81.9 | 79.6 |
| Ciprofloxacin | 85.8 | 82.1 | 77.3 | 80.8 | 83.6 | 82.2 |
| Colistin | N/A | N/A | 97.2 | 98.6 | 99.1 | 98.5 |
| Meropenem | 84.7 | 80.8 | 79.4 | 77.1 | 82.8 | 81.1 |
| Piperacillin-tazobactam | 77.7 | 74.2 | 69.4 | 71.8 | 80.2 | 75.1 |
| Tobramycin | 92.0 | 88.5 | 87.8 | 91.8 | 93.7 | 91.2 |
| Latin America (n) | (1509) | (1426) | (1518) | (1511) | (758) | (6722) |
| Amikacin | 71.4 | 70.2 | 74.8 | 74.9 | 86.8 | 74.4 |
| Cefepime | 65.1 | 61.4 | 66.5 | 62.3 | 77.2 | 65.4 |
| Ceftazidime | 64.2 | 60.1 | 63.2 | 62.8 | 74.6 | 64.0 |
| Ciprofloxacin | 60.5 | 55.7 | 57.9 | 58.8 | 71.1 | 59.7 |
| Colistin | N/A | N/A | 99.8 | 99.4 | 99.1 | 99.5 |
| Meropenem | 70.8 | 59.7 | 61.2 | 56.2 | 67.7 | 62.7 |
| Piperacillin-tazobactam | 57.3 | 54.7 | 57.7 | 57.4 | 73.1 | 58.7 |
| Tobramycin | 64.5 | 61.2 | 66.2 | 68 | 80.6 | 66.8 |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; N/A, not available.
aSusceptible based on criteria as published by CLSI 2018.
bAgent added in 2006.
The largest variations were seen in Latin America, with amikacin susceptibility increasing from 70.2% in 2001–2004 to 86.8% in 2013–2016. β-lactam susceptibilities also varied greatly with meropenem susceptibility dropping from a high of 70.8% in 1997–2000 to a low of 56.2% in 2009–2012. Meropenem susceptibility rose to 67.7% in 2013–2016. Asia-Pacific experienced a similar trend with meropenem susceptibility dropping from a high of 84.7% in 1997–2000 to a low of 77.1% in 2009–2012 and rising to 82.8% in the most recent period. Piperacillin-tazobactam susceptibility increased from 69.4% in 2005–2008 to 80.2% in 2013–2016.
DISCUSSION
Over the 20 years of SENTRY Program surveillance, the rates of MDR and other resistant phenotypes for P. aeruginosa were highest in 2005–2008 and decreased in the most recent period. Latin America showed the sharpest decrease in MDR rate, which was associated with a rise in susceptibility to aminoglycosides and β-lactams. The metallo-β-lactamase SPM-1 that has been reported in multiple Brazilian institutions may be contributing to the meropenem resistance reported there [9, 10]. Among the 6722 P. aeruginosa isolates collected from the Latin American region, 3057 (45.5%) were collected from Brazilian medical centers. The results could have been directly influenced by any changes in the epidemiology within Brazilian medical centers. The high carbapenem resistance rates found in Brazilian hospitals have been mainly caused by the spread of the XDR P. aeruginosa ST277 clone, which chromosomally encodes for SPM-1 and RmtD, a 16S ribosomal ribonucleic acid (rRNA) methylase [10, 11]. This clone also possesses mutations on the quinolone-resistant determining regions of gyrA and parC, and it harbors sul1 and aminoglycoside-modifying enzyme-encoding genes such as aac(6′)-Ib-cr and aadA7 [11]. In general, SPM-1-producing P. aeruginosa ST277 isolates are susceptible only to polymyxins. Although no studies that include isolates from all Brazilian regions have been carried out, studies evaluating isolates from specific regions or single institutions have shown a decrease in the frequency of SPM-1-producing P. aeruginosa isolates [12, 13]. These studies may support the increase in the antimicrobial susceptibility rates in Latin America, especially for aminoglycosides and carbapenems, observed by the SENTRY Program study in the 2013–2016 period. Cacci et al [13] also found that as the frequency of the SPM-1 clone decreased, carbapenem-resistant isolates displayed the more commonly observed resistance mechanisms overall, including porin loss and efflux overproduction [14].
The Asia-Pacific region had an overall lower frequency of MDR P. aeruginosa than Latin America and Europe (Figure 2). The region saw an increase in MDR P. aeruginosa, from 15.6% in 1997–2000 to 24.7% in 2005–2008 and decreased to 15.0% in 2013–2016. A study by Pfaller et al [15] found that frequency of meropenem-resistant P. aeruginosa varied by country in the period 2013–2015, with South Korea having the highest rate (46.3%). Studies in the Asia-Pacific region have shown an increasing prevalence of metallo-β-lactamases and carbapenemases in P. aeruginosa, particularly the ST235 clone, which may explain the increase in MDR seen in 2005–2008 [16–18]. Because strain typing was not performed in this study, it is unknown whether the decrease in resistance is due to a decrease in the prevalence of ST235 or other causes. The European and North American medical centers had a stable frequency of MDR P. aeruginosa, with the North American and European rates ranging from 17.5% to 21.2% and 25.6% to 30.9%, respectively, over the 20-year period. Isolates from both regions showed a decrease in meropenem susceptibility in 2009–2012, although susceptibility improved in the most recent time period for both regions. The ST235 clone and others that have been globally disseminated may have contributed to the increase in meropenem resistance and, perhaps, to the variations observed [19].
CONCLUSIONS
This study has shown variation in the resistance rates over time and over geography; however, MDR P. aeruginosa remains a cause of serious infections. The improved activities of newer agents, such as ceftolozane-tazobactam and ceftazidime-avibactam, against P. aeruginosa including MDR isolates have been published elsewhere, and those agents may be effective treatment options, especially for patients with infections caused by meropenem-resistant isolates [20, 21]. Whether the trend of decreasing resistance in P. aeruginosa is maintained will be documented in future SENTRY Program and other international surveillance studies.
Acknowledgments
We thank all participants of the SENTRY Program for their work in providing isolates.
Financial support. Funding for the manuscript was provided by JMI Laboratories.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Zilberberg MD, Shorr AF, Micek ST, et al. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care 2014; 18:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morata L, Cobos-Trigueros N, Martínez JA, et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 2012; 56:4833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castanheira M, et al. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009–2011 in 14 European and Mediterranean countries. J Antimicrob Chemother 2014; 69:1804–14. [DOI] [PubMed] [Google Scholar]
- 6. CLSI. M100 Performance Standards for Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 7. EUCAST. Breakpoint tables for interpretation of MIC’s and zone diameters. Version 8.0, January 2018. European Committee on Antimicrobial Susceptibility Testing; 2018 Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf. Accessed January 2018. [Google Scholar]
- 8. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 9. Picão RC, Poirel L, Gales AC, Nordmann P. Diversity of beta-lactamases produced by ceftazidime-resistant Pseudomonas aeruginosa isolates causing bloodstream infections in Brazil. Antimicrob Agents Chemother 2009; 53:3908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gales AC, Menezes LC, Silbert S, Sader HS. Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-beta-lactamase. J Antimicrob Chemother 2003; 52:699–702. [DOI] [PubMed] [Google Scholar]
- 11. Nascimento APB, Ortiz MF, Martins WMBS, et al. Intraclonal genome stability of the metallo-beta-lactamase SPM-1-producing Pseudomonas aeruginosa ST277, an endemic clone disseminated in Brazilian hospitals. Front Microbiol 2016; 7:1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campana EH, Xavier DE, Petrolini FV, et al. Carbapenem-resistant and cephalosporin-susceptible: a worrisome phenotype among Pseudomonas aeruginosa clinical isolates in Brazil. Braz J Infect Dis 2017; 21:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cacci LC, Chuster SG, Martins N, et al. Mechanisms of carbapenem resistance in endemic Pseudomonas aeruginosa isolates after an SPM-1 metallo-β-lactamase producing strain subsided in an intensive care unit of a teaching hospital in Brazil. Mem Inst Oswaldo Cruz 2016; 111:551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castanheira M, Mills JC, Farrell DJ, Jones RN. Mutation-driven β-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother 2014; 58:6844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfaller MA, Shortridge D, Sader HS, et al. Ceftolozane-tazobactam activity against drug-resistant enterobacteriaceae and Pseudomonas aeruginosa causing health care-associated infections in the Asia-Pacific region (APAC; minus China, Australia and New Zealand): report from an antimicrobial surveillance program (2013–2015). Int J Antimicrob Agents 2018; 51:181–9. [DOI] [PubMed] [Google Scholar]
- 16. Lee S, Park YJ, Kim M, et al. Prevalence of Ambler class A and D beta-lactamases among clinical isolates of Pseudomonas aeruginosa in Korea. J Antimicrob Chemother 2005; 56:122–7. [DOI] [PubMed] [Google Scholar]
- 17. Hong JS, Yoon EJ, Lee H, et al. Clonal dissemination of Pseudomonas aeruginosa sequence type 235 isolates carrying blaIMP-6 and emergence of blaGES-24 and blaIMP-10 on novel genomic islands PAGI-15 and -16 in South Korea. Antimicrob Agents Chemother 2016; 60:7216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim MJ, Bae IK, Jeong SH, et al. Dissemination of metallo-β-lactamase-producing Pseudomonas aeruginosa of sequence type 235 in Asian countries. J Antimicrob Chemother 2013; 68:2820–4. [DOI] [PubMed] [Google Scholar]
- 19. Oliver A, Mulet X, López-Causapé C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 2015; 21–22:41–59. [DOI] [PubMed] [Google Scholar]
- 20. Shortridge D, Castanheira M, Pfaller MA, Flamm RK. Ceftolozane-tazobactam activity against Pseudomonas aeruginosa clinical isolates from U.S. hospitals: report from the PACTS antimicrobial surveillance program, 2012 to 2015. Antimicrob Agents Chemother 2017; 61:e00465–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sader HS, Castanheira M, Shortridge D, et al. Antimicrobial activity of ceftazidime-avibactam tested against multidrug-resistant enterobacteriaceae and Pseudomonas aeruginosa isolates from U.S. Medical Centers, 2013 to 2016. Antimicrob Agents Chemother 2017; 61:e01045–17. [DOI] [PMC free article] [PubMed] [Google Scholar]