SUMMARY
The flattening of leaves to form broad blades is an important adaption that maximizes photosynthesis. However, the underlying molecular mechanism of this process remains unclear. The WUSCHEL-RELATED HOMEOBOX (WOX) genes WOX1 and PRS are expressed in the leaf marginal domain to enable leaf flattening, but the nature of WOX expression establishment remains elusive. Here we report that adaxial-expressed MONOPTEROS (MP) and abaxial-enriched auxin, together, act as positional cues for patterning the WOX domain. MP directly binds to the WOX1 and PRS promoters and activates their expression. Furthermore, redundant abaxial-enriched ARF repressors suppress WOX1 and PRS expression, also through direct binding. In particular, we show ARF2 is redundantly required with ARF3 and 4 to maintain the abaxial identity. Taken together, these findings explain how adaxial-abaxial polarity patterns the mediolateral axis and subsequent lateral expansion of leaves.
Keywords: leaf, auxin, ARF, WOX, patterning
Graphical Abstract
eTOC Blurb
The flattening of leaves to form broad blades is an important adaption. Guan et al. show that the adaxial-expressed MP, abaxial-enriched auxin, and abaxial-expressed ARF repressors together position WOX expression in the middle domain. This finding describes how adaxial-abaxial polarity patterns the mediolateral axis and thus leaf flattening.
INTRODUCTION
The flattening of leaves to form broad blades is an important adaption that maximizes photosynthesis. Following initiation from the shoot apical meristem (SAM), leaf primordia develop three axes of asymmetry, a proximodistal axis, an adaxial-abaxial axis, and a mediolateral axis, to form planar leaves. Patterning the mediolateral axis (from the midrib to the margin) promotes leaf blade outgrowth, and depends on adaxial-abaxial patterning (also known as dorsoventral, or up-down polarity) [1].
Extensive molecular genetic studies have identified a transcriptional regulatory network containing leaf abaxial- and adaxial-promoting genes [2-7]. Adaxial-abaxial polarity establishment requires domain-specific expression of these transcription factors and small RNA encoding genes. It has been proposed that incipient leaf primordia may be prepatterned into adaxial and abaxial domains [6]. Regulatory genes expressed in the abaxial domain suppress those expressed in the adaxial domain and vice versa. In particular, the adaxial-expressed mobile trans-acting small interfering RNA3 (TAS3) forms a gradient [8], and restricts its targets, AUXIN RESPONSE FACTOR3 (ARF3, also known as ETTIN) and ARF4, to the abaxial domain [9-11]. Likewise, abaxial-expressed MicroRNAs 165 and 166 (miR165/166) form an opposite gradient [12-14], and restrict the expression of class III homeodomain leucine zipper (HD-ZIP III) genes PHABULOSA, PHAVOLUTA, and REVOLUTA to the adaxial domain [15, 16]. These and additional mutual repression and positive regulatory interactions confine and stabilize gene expression regions to fine-tune adaxial-abaxial polarity. In addition to gene expression, auxin transport leads to a transient adaxial low auxin domain that is required for adaxial-abaxial patterning [17].
The adaxial-abaxial polarity establishment promotes leaf blade outgrowth, and this mediolateral axis growth requires the activity of leaf meristems (also called marginal blastozones) [18-20]. Although leaves are determinate organs and do not contain anatomical features typical of meristems, transient leaf meristems, which are restricted to the marginal domains, enable leaf blade expansion [21]. Expression of WUSCHEL-RELATED HOMEOBOX1 (WOX1) and PRESSED FLOWER (PRS)/WOX3 of Arabidopsis in the marginal domain (also called the middle domain) between the adaxial and abaxial domains is critical for leaf blade outgrowth [22, 23]. WOX1 homologs in maize, petunia, Medicago, and tobacco have similar expression patterns and control leaf blade outgrowth [23-25]. WUSCHEL (WUS), which is required for the SAM [26], can substitute for WOX1 and PRS functions in the leaf [27, 28], and vice versa [29], suggesting similarities between leaf meristems and the SAM.
WOX expression in the leaf marginal domain enables leaf flattening, but little is known about how the expression domain of WOX genes is established. In fact, we also know little about the nature of activation of other stem cell-promoting WOX genes. In this study, we found that the recently identified abaxial auxin maxima work with domain-specific ARF activators and repressors to precisely activate WOX1 and PRS expression in the marginal domain, thus defining the WOX domain for leaf blade outgrowth.
RESULTS
Spatially refined auxin signaling in the middle domain of young leaf primordium
We have recently shown that transient abaxial-enriched auxin distribution contributes to leaf patterning [17]. We sought to identify downstream targets of auxin signaling that regulate leaf patterning. ARF transcription factors are important auxin signaling mediators. Therefore, we first analyzed ARF expression patterns during early leaf development to understand spatial auxin signaling.
MONOPTEROS (MP) is an ARF that regulates the expression of auxin responsive genes, and has been recently shown to regulate leaf development [17, 30]. A functional pMP::MP-GFP fluorescent marker shows that MP is expressed in young leaf primordia, and becomes obviously adaxially expressed after P2, which designates the second youngest primordium (Figure 1A, S1A, and S1C). In P3 and P4, MP expression is found in the middle domain, covering the marginal domains, and part of the adaxial domain. In addition, the expression domain of MP expands from the apex to base from P2 to P4, consistent with the basiplastic gradient of leaf expansion in Arabidopsis [31]. In addition to MP, there are four other ARF activators, NONPHOTOTROPIC HYPOCOTYL4 (NPH4)/ARF7, ARF6, 8 and 19, in Arabidopsis [32]. All these ARFs have expression in the adaxial domain to different extents in young leaf primordia (Figure S1B).
Figure 1. Spatially defined auxin signaling in the marginal and middle domains.
Optical and agarose sections through P2, P3, and P4 of vegetative shoot apices showing expression of (A) MP-GFP (green), (B) pDR5::GFPer (green) and DII-Venus (magenta), (C) pWOX1::GFP (green), (D) pPRS::GFP (green), and (E) ARF3-GFP (green). The left and middle panels, maximum intensity projections and optical sections of live imaging results with FM4-64 staining. White dotted lines indicate longitudinal sections. Pink dotted lines indicate transverse sections. The right panels, agarose sections with PI staining. Note the pWOX1::GFP reporter may have narrower expression domain than results obtained by ISH (Figures 3C and 5B). Scale bars, 20 μm. See also Figure S1.
ARFs activate auxin signal transduction in response to auxin. Consistent with a previous report [17], the auxin signaling sensor DII-Venus, whose signal indicates low auxin, is enriched in the adaxial side of the tip region, indicating reduced auxin levels in this domain from P2 to P4 (Figure 1B and S1C). Nevertheless, dispersed DII-Venus signal was also detected at a low frequency in the epidermis. By contrast, the non-responsive mDII-Venus control has uniform expression in early leaf primordia [33]. To precisely compare auxin distribution and signaling, we crossed the auxin signaling reporter pDR5::GFP-ER with p35S::DII-Venus. In plants co-expressing the two markers, we detected GFP signals in the marginal domain, initially in the tip and then expanded toward the base (Figure 1B). On the other hand, an alternative auxin signaling reporter, DR5v2 [34], had more broad expression in leaf primordia (Figure S1D). The MP expression domain partially overlaps with both DR5 and DII-Venus domains. Thus the spatial distributions of auxin and MP, as well as additional ARF activators, lead to refined auxin signaling in the marginal domain. We detected the expression of WOX1 and PRS in the marginal domain [22, 23] (Figure 1C and 1D), completely covered the DR5 regions. Thus, WOX1 and PRS expression overlaps with auxin signaling maxima in the leaf marginal domain.
MP acts in leaf blade outgrowth and regulates WOX1 and PRS expression
Previous studies have shown that ectopic MP activity leads to defective leaf development [17, 30]. To test if MP is involved in normal leaf development, we analyzed the leaf phenotype in arf5-1, a strong MP mutant allele. The cotyledons and rosette leaves of five-day-old arf5-1 mutant were narrower than wild-type leaves (Figure 2A and 2B). Furthermore, after growing for 17 days, about 7.5% (3/40) of arf5-1 mutants form needle-like rosette leaves that fail to flatten (Figure 2C and 2D). The frequency of needle-like lateral organs was substantially increased to ~100% in the mp-G12 nph4-1 mutant (Figure 2E and 2F), suggesting redundant roles of NPH4 with MP in leaf development. These results indicated that MP is involved in normal leaf development, especially leaf flattening.
Figure 2. MP regulates leaf patterning and expression of WOX genes.
(A) Five-day-old arf5-1 seedlings. Top, wild-type. Middle, arf5-1 with one cotyledon. Bottom, arf5-1 with two cotyledons. Scale bars, 1 mm.
(B) Quantification of length-width ratio of arf5-1 cotyledons and rosette leaves. Data are presented as mean ± SD for more than three independent experiments.
(C) A seventeen-day-old arf5-1 seedling. White arrow indicates a needle-like rosette leaf. Scale bar, 1 mm.
(D) Scanning electron micrograph (SEM) analysis of the needle-like rosette leaf indicated by white arrow in (C). Scale bar, 100 μm.
(E) A seventeen-day-old mp nph4 seedling. Dark stars indicate leaf primordium-like bulges. Scale bar, 300 μm.
(F) SEM analysis of the shoot apical meristem indicated by white dotted box in (E). Scale bar, 300 μm.
(G) FIL transcript accumulation in transverse sections of mp nph4 leaf primordium-like bulge (dark dotted lines). Scale bars, 50 μm.
(H) PRS transcript accumulation in transverse sections of mp nph4 leaf primordium-like bulge (dark dotted lines). Scale bars, 50 μm.
(I) Vegetative and rosette leaf phenotypes of two-week-old pMP::MPΔ-EAR-GR transgenic plants without or with Dex treatment. Scale bars, 1 mm.
(J) RT-qPCR analysis of WOX1 and PRS expression in pMP::MPΔ-EAR-GR vegetative meristems. Data are presented as mean ± SD for more than three independent experiments. **P < 0.01. m, meristem. r, rosette leaf, c, cotyledon. See also Figure S2.
Because WOX1 and PRS expression overlaps with auxin signaling in the marginal domain, and MP-mediated auxin signaling regulates leaf flattening, we speculated that MP and related ARF activators promote WOX expression. To this end, we first analyzed PRS expression in the mp nph4 mutant leaves. The detection of FILAMENTOUS FLOWER (FIL) confirmed that the needle-like structure had lateral organ identity [35] (Figure 2G). However, we could not detect PRS expression in the needle-like lateral organs (Figure 2H).
Because leaf development is severely compromised, we also made a presumably dominant-negative MP to understand its role in regulating WOX expression and leaf flattening. We expressed a constitutively repressive fusion protein of MP, which lacks domains III and IV and is fused to an ETHYLENE RESPONSE FACTOR-associated Amphiphilic Repression (EAR) motif and a GR domain (termed MPΔ-EAR-GR), under the endogenous MP promoter. The deletion of domains III and IV makes the fusion protein escape auxin regulation [30], and the EAR domain converts MP into a transcriptional repressor [36], so that the MPΔ-EAR-GR fusion protein constitutively represses target gene expression, and thus auxin signaling, after dexamethasone (Dex) induction. Using Dof5.8, a previously reported direct target gene of MP [37], we confirmed that the MPΔ-EAR-GR fusion protein could suppress target gene expression as designed (Figure S2A). We found that Dex induction resulted in narrower leaves suggesting a reduction of the leaf blade outgrowth (Figure 2I and S2B), reminiscent of the phenotypes observed in wox1 prs mutants [22, 23]. Furthermore, we found that a 4 hr Dex induction of MPΔ-EAR-GR resulted in a downregulation of WOX1 and PRS expression (Figure 2J). Taken together, these results indicated that MP promotes WOX1 and PRS expression and leaf flattening.
Ectopic adaxial WOX1 and PRS expression leads to defective leaf flattening
Previous studies have shown that MPΔ, which escapes auxin regulation and becomes constitutively active without auxin, leads to severe defects in leaf adaxial-abaxial polarity development [17, 30]. We went on to test if this polarity defect is caused by ectopic WOX1 and PRS expression. In transgenic pMP::MPΔ plants, leaf blade outgrowth (i.e. mediolateral axis expansion) is reduced but blade thickness is increased, suggesting ectopic adaxial-abaxial expansion (Figure 3A and H). In addition, small blade outgrowths appear on the adaxial leaf surface, a phenotype also observed in WOX1 overexpressing leaves [22]. An RT-qPCR assay indicated that WOX1 and PRS expression in pMP::MPΔ plants increased 3- and 10-fold higher, respectively, compared to the wild-type (Figure 3B). To further test if forced adaxial auxin signaling is sufficient to trigger WOX1 and PRS expression, we expressed MPΔ in the adaxial epidermis from the AS2 promoter. We observed comparable upregulation of WOX1 and PRS expression inpAS2::MPΔ plants (Figure 3B). An in situ hybridization (ISH) analysis revealed that the WOX1 and PRS expression domain expanded from the middle domain into the adaxial domain in pMP::MPΔ leaf primordia (Figure 3C and S3B). We also tested if auxin treatment can upregulate WOX expression in leaves. The structure of the Arabidopsis shoot apex prohibited access to early primordia, where auxin treatment is functional. To this end, we used tomato shoot apices with larger leaf primordia. We found the homologous SlWOX1 expression was upregulated after a 24 h IAA treatment (Figure S3A). These results indicated ectopic adaxial MP activation induced ectopic adaxial WOX gene expression, further supporting that MP activates WOX1 and PRS expression.
Figure 3. MP activates WOX1 and PRS expression in the marginal and middle domains.
(A) Rosette leaf phenotypes of Col-0 and pMP::MPΔ plants. Scale bars, 1 mm.
(B) RT-qPCR analysis of WOX1 and PRS expression in pMP::MPΔ and pAS2::MPΔ transgenic rosette leaves. Data are presented as mean ± SD for more than three independent experiments. *P < 0.05; **P < 0.01.
(C) Comparison of the WOX1 and PRS transcript accumulation in transverse sections of P4 and P5 leaf primordia of wild-type and pMP::MPΔ transgenic plants through ISH. Ladders colored in yellow indicate WOX1 and PRS expressing cells. Note expansion of expression domains and enhancement of expression levels in pMP::MPΔ plants. Scale bars, 20 μm.
(D) Vegetative phenotypes of one-month-old wild-type, wox1-2 prs, pMP::MPΔ, pMP::MPΔ wox1-2, and pMP::MPΔ prs plants. Scale bars, 10 mm.
(E) Relative expression levels of MP in plants shown in (D). Data are presented as mean ± SD for two independent transgenic progenies. **P < 0.01.
(F) The fourth rosette leaves of plants shown in (D). Scale bar, 10 mm.
(G) Quantification of length-width ratio of leaves shown in (F).
(H) SEM analysis of the fourth rosette leaves of wild-type, pMP::MPΔ, pMP::MPΔ wox1-2, and pMP::MPΔ prs plants. ad, adaxial; ab, abaxial. Scale bar, 2 mm.
See also Figure S3.
To test if ectopic WOX expression causes leaf polarity development defects, we crossed the pMP::MPΔ transgenic plants with wox1-2 orprs mutants. Whereas both wox1-2 and prs single mutant leaves show no obvious phenotypes, wox1-2 prs double mutant leaves are narrower than wild-type leaves [22, 23]. Introduction of either wox1-2 or prs could partially, but significantly, restore leaf expansion in pMP::MPΔ (Figure 3D and 3F-3H), although the expression level of MPΔ was comparable in the wox1-2 background and even higher in the prs background (Figure 3E). We could not obtain pMP::MPΔ wox1-2prs plants. Because we observed a high frequency of seed abortion in pMP::MPΔ wox1-2 prs/+ siliques, ectopic MPΔ may lead to defective embryogenesis in the wox1-2 prs background. The results indicated that the ectopic adaxial MP activity, which is otherwise suppressed due to lack of auxin, induces ectopic WOX1 and PRS expression to alter normal leaf patterning.
MP directly upregulates WOX1 and PRS expression
To investigate whether the MP protein directly induces WOX1 and PRS expression, we constructed a Dex-inducible pMP::MPΔ-GR line. Activation of MPΔ-GR by Dex application led to an inhibition of blade outgrowth and ectopic adaxial-abaxial axis thickening (Figure 4A), which is similar topMP::MPΔ leaves (Figure 2I). An RT-qPCR analysis indicated that Dex induction of MPΔ-GR triggered WOX1 and PRS expression up-regulation within 4 hr (Figure 4B). We could also detect WOX1 and PRS induction with the presence of cycloheximide (CHX), an inhibitor of protein biosynthesis, suggesting that induction of WOX1 and PRS does not require de novo protein synthesis and that these genes are likely direct targets of MP.
Figure 4. MP directly binds to the WOX1 and PRS genomic regions.
(A) Vegetative and rosette leaf phenotypes of two-week-old pMP::MPΔ-GR transgenic plants. Scale bars, 10 mm (top) or 1 mm (bottom).
(B) RT-qPCR analysis of WOX1 and PRS expression in pMP::MPΔ-GR shoot apices. Data are presented as mean ± SD for more than three independent experiments. **P < 0.01.
(C) Schematic of the WOX1 and PRS genomic regions. Black boxes indicate AuxRE pairs (Boer et al., 2014), and stars indicate single AuxRE sites. Red stars represent TGTCGN or TGTCTG, and blue stars represent TGTCTC. The underlying lines represent the DNA fragments amplified in ChIP assays, or used for Y1H analysis. pΔ (-1347 - -1205) in white box indicates the promoter region deleted in pWOX1Δ::WOX1 construct in (J).
(D) Anti-GFP ChIP enrichment of WOX1 genomic fragments using pMP::MP-GFP inflorescences.
(E) Anti-HA ChIP enrichment of PRS genomic fragments using pMP::MP-HA inflorescences.
(F) Y1H assay of MP with WOX1 and PRS genomic fragments indicated in (C). Note fragment 3 of PRS was excluded due to strong self-activation in yeast.
(G) Transient expression assay showing MP activation of WOX1 expression. A representative image of N. benthamiana leaves 72 h after infiltration is shown. The right panel indicates the infiltrated constructs.
(H) RT-PCR analysis of MP expression in the infiltrated leaf areas shown in (G). Total RNA was extracted from leaf areas of N. benthamiana coinfiltrated with different constructs.
(I) Quantitative analysis of luminescence intensity in (G). Five independent replicates were performed. Data are presented as mean ± SD for more than three independent experiments. *P < 0.05.
(J) Rosette leaves of thirty-day-old Col-0, wox1-2 prs, pWOX1::WOX1, and pWOX1Δ::WOX1 transgenic plants in wox1-2 prs backgrounds. Scale bar, 10 mm.
(K) Quantification of length-width ratio of all rosette leaves of thirty-day-old plants. 1, 2, 3 and 4 indicate Col-0, wox1-2 prs, pWOX1::WOX1 wox1-2 prs, and pWOX1Δ::WOX1 wox1-2 prs transgenic plants, respectively.
See also Figure S4.
An analysis of the WOX1 and PRS promoter regions identified multiple auxin-response element (AuxRE) core motifs, which are sufficient for ARF recruitment [32] (Figure 4C). In particular, we found one AuxRE pair in the WOX1 promoter region, and two pairs in the PRS promoter region. Although AuxRE pairs may not be necessary for MP binding, such pairs have been identified in a few known MP target genes [38], making them good candidates as MP binding sites.
To determine whether MP associates with the promoters of WOX1 and PRS in vivo, we performed chromatin immunoprecipitation (ChIP) assays using pMP::MP-GFP and pMP::MP-HA transgenic lines [39]. Using a series of primer pairs distributed over the WOX1 and PRS promoters covering AuxRE motifs (Figure 4C), we found that MP binds 1200 base pairs (bp) upstream of the WOX1 start codon and 1500 bp upstream of the PRS start codon positions which both contain AuxRE pairs (Figure 4D and 4E).
A yeast one hybrid (Y1H) assay further confirmed that MP bound to the upstream promoter fragments containing AuxRE pairs identified by ChIP assay, but not other AuxRE motif-containing ones (Figure 4F). The additional weak binding regions detected by ChIP assays may imply that MP associates with other regions by interacting with transcription factors that binds to other elements. We performed transient expression assays using Nicotiana benthamiana leaves to further test effects of MP on pWOX1::firefly luciferase (Luc) reporter expression. We observed that MP promotes expression of pWOX1::Luc (Figure 4G-4I). Taken together, our results indicated that MP activates WOX1 and PRS expression through direct binding to their promoter regions.
To test if the AuxRE bound by ARFs in the WOX1 promoter region is critical for WOX1 activity, we made specific deletions of this region. When we fused WOX1 to its intact endogenous promoter, and transformed into wox1-2 prs mutant plants, the vast majority of the obtained transgenic lines (95%, 55/58) were rescued to wild-type-like leaf shape (Figure 4J, 4K and S4). However, deletion of the AuxREs led to a substantial portion of the transgenic lines (33%, 20/61) unable to rescue the leaf flattening defect of wox1-2 prs plants (Figure 4J, 4K and S4). This result further confirms the important role of the AuxRE region in regulating WOX1 activity.
ARF2, ARF3 and ARF4 suppress WOX1 and PRS expression in the abaxial domain
Previous studies have shown that ARF3 and ARF4 promote leaf abaxial identity [9-11]. Both ARF3 and ARF4 are targets of ta-siRNAs derived from the adaxial expressed TAS3 gene [10], and their expression is restricted to the abaxial domain [11]. A careful analysis indicated that ARF3 has relatively uniform expression in P1 and P2 stages and is restricted to the abaxial domain in the P3 stage (Figure 1E). TAS3 derived ta-siRNAs also targets ARF2 [40, 41], although the arf2 mutation alone causes no organ asymmetry phenotype [42, 43]. ARF2, ARF3, and ARF4 all encode transcriptional repressors [32], and they bind to the same AuxRE sequence as ARF activators [38, 44], leading to the hypothesis that these three ARF repressors redundantly regulate WOX expression, antagonistic to MP.
To test whether these ta-siRNA-targeted ARF genes regulate WOX1 and PRS expression, we first checked WOX gene expression in arf2-6, and arf3-1 arf4-2 mutants, as well as in ARF2 overexpression transgenic plants (ARF2ox), respectively (Figure 5A-D). The expression of WOX1 and PRS increased in both arf mutant backgrounds, and mildly decreased in the ARF2ox transgenic plants. Further ISH analyses revealed that the WOX1 and PRS transcript accumulation expanded into the abaxial domain in arf3-1 arf4-2 leaf primordia (Figure 5B and S5A). These results indicated that these ARFs negatively regulate the expression of WOX genes.
Figure 5. TAS3-targeted ARFs inhibit WOX1 and PRS expression in the abaxial domain.
(A) RT-qPCR analysis of WOX1 and PRS expression in Col-0 and arf3-1 arf4-2 rosette leaves. Data are presented as mean ± SD for more than three independent experiments. *P < 0.05, **P < 0.01.
(B) Comparison of the WOX1 and PRS transcript accumulation in transverse sections of P4 and P5 leaf primordia of Col-0 and arf3-1 arf4-2 mutants through ISH. Ladders colored in yellow indicate WOX1 and PRS expressed cells. Note expansion of expression domains and enhancement of expression levels in pMP::MPΔ plants. Scale bar, 20 μm.
(C) RT-qPCR analysis of WOX1 and PRS expression in Col-0 and arf2-6 rosette leaves. Data are presented as mean ± SD for more than three independent experiments. *P < 0.05.
(D) RT-qPCR analysis of WOX1 and PRS expression in Col-0 and ARF2ox transgenic rosette leaves. Data are presented as mean ± SD for more than three independent experiments. *P < 0.05.
(E) Cross section analysis of wild-type, arf2-6, and ARF2ox rosette leaves. Ad, adaxial. Scale bar, 100 μm.
(F) Rosette leaf phenotypes of arf3-1 arf4-2 double and arf3-1 arf4-2 arf2-6 triple mutants. Scale bar, 10 mm.
(G) Phenotypes of wox1-2 prs p35S::amiR-ARF rosette leaves. White arrows indicate needle-like leaves in wox1-2 prs p35S::amiR-ARF plants. Scale bars, 10 mm.
(H) SEM analysis of the needle-like rosette leaf indicated by white arrow in (G). Scale bar, 500 μm.
(I) Cross section of Col-0 and wox1-2 prs p35S::amiR-ARF leaf petioles. Scale bars, 100 μm (top) or 10 μm (bottom). ad, adaxial; xy, xylem; ph, phloem.
See also Figure S5 and S6.
To determine whether ARF2 is involved in leaf development, we compared the cross sections of arf2-6 and ARF2ox rosette leaf blades with that of wild-type plants (Figure 5E). In wild-type leaves, there is a steep contrast of adaxial and abaxial mesophyll morphology: Cells in the adaxial two layers are larger, round, and densely packed, whereas cells in the abaxial two layers are more branched and are separated by spacious air spaces. In arf2-6, abaxial mesophyll cells are less branched, and all mesophyll cells in ARF2ox are round-shaped. Furthermore, we constructed arf2-6 arf3-1 arf4-2 triple mutant plants (Figure 5F, S5B and S5C). Whereas arf3-1 arf4-2 leaves have mild leaf polarity defects [11], arf2-6 arf3-1 arf4-2 leaves have more severe leaf patterning defects when plants are mature (~20 d after germination). We observed frequent deep lobes and occasional trumpet-like leaves in the triple mutant but not the double mutant. In addition, there are more adventitious small blade protrusions on the abaxial side in the triple mutant. We also designed an artificial miRNA (amiRNA) targeting ARF2, ARF3 and ARF4 [45], amiR-ARF, and constitutively expressed it under the control of the Cauliflower Mosaic Virus 35S promoter (p35S). Leaves of p35S::amiR-ARF lines resemble the observed arf3-1 arf4-2 leaf phenotypes (Figure S6A and S6B), possibly due to residual expression of any of the three ta-siRNA-targeted ARFs (Figure S6C). In summary, ARF2 is redundantly involved in leaf polarity development along with ARF3 and ARF4.
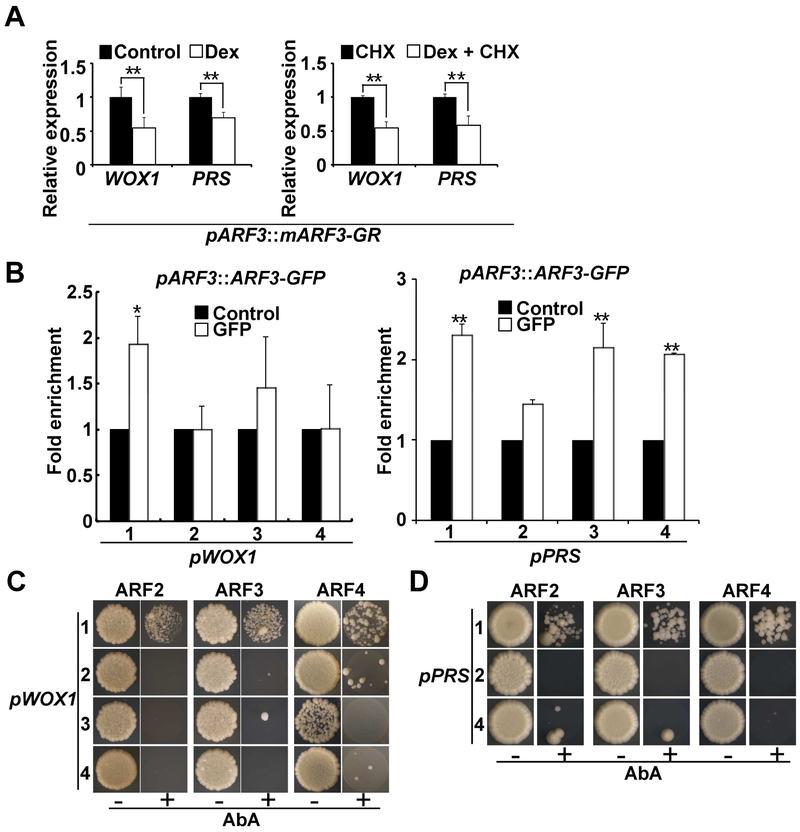
To reveal whether the three ta-siRNA-targeted ARFs directly suppress WOX1 and PRS expression, we used a Dex inducible pARF3::mARF3-GR line, which has TAS3-independent ARF3-GR expression [46]. We measured the effect of ARF3 activation in pARF3::mARF3-GR plants on WOX1 and PRS expression by RT-qPCR. ARF3 activation resulted in rapid reduction of both WOX1 and PRS transcripts levels within 4 hr, in the absence or presence of the protein synthesis inhibitor CHX (Figure 6A), suggesting direct transcriptional suppression. We further performed ChIP and Y1H assays to confirm direct binding of ARF3 to WOX1 and PRS promoter regions and to test if ARF2 and ARF4 also bind to the same regions. ChIP results indicated that ARF3 bound to the WOX1 and PRS promoters (Figure 6B). The Y1H experiments suggested that all three ARFs bound to the MP-bound promoter regions of WOX1 and PRS (Figure 6C and 6D). Both regions harbor one or two AuxRE pairs. Taken together, these results support that the three ARF repressors redundantly suppress WOX1 and PRS expression by directly binding to their promoter regions that are also targeted by MP.
Figure 6. TAS3-targeted ARFs directly bind to the WOX1 and PRS genomic regions.
(A) RT-qPCR analysis of WOX1 and PRS expression in pARF3::mARF3-GR vegetative apices. Data are presented as mean ± SD for more than three independent experiments. **P < 0.01.
(B) Anti-GFP ChIP enrichment of WOX1 and PRS genomic fragments using pARF3::ARF3-GFP inflorescences. *P < 0.05, **P < 0.01.
(C and D) Y1H assay of ARF2, ARF3 and ARF4 with WOX1 (B) and PRS (C) genomic fragments indicated in Figure 4C. Note fragment 3 of PRS was excluded due to strong self-activation in yeast.
MP and ARF3 play antagonistic roles in regulating WOX1 promoter activity
Since MP and ta-siRNA-targeted ARF repressors have opposite effects on WOX1 and PRS expression (Figures 2-5), and all these ARFs can bind to the same promoter regions (Figures 4 and 6), we speculated that ARF activators and ARF repressors compete for access to WOX1 and PRS promoters and thereby refine spatial expression of WOX1 and PRS in the leaf marginal domain. To test this hypothesis, we performed transient expression assays using Nicotiana benthamiana leaves to test effects of MP and ARF3 on pWOX1::Luc reporter expression. We observed that MP promotes pWOX1::Luc reporter expression, and ARF3 interfered with MP activation of pWOX1::Luc expression (Figure 7A-C). Thus, ARF3 as a repressor antagonizes MP activation of WOX1 expression, presumably through competing for access to the same promoter regions.
Figure 7. MP and ARF3 play antagonistic roles in regulating WOX1 expression.
(A) Transient expression assay showing MP activates WOX1 while ARF3 antagonizes MP effect on the expression of WOX1. Representative image of a N. benthamiana leaf 72 h after infiltration was shown. The right panel indicates the infiltrated constructs.
(B) Quantitative analysis of luminescence intensity in (A). Five independent replicates were performed. Data are presented as mean ± SD for more than three independent experiments. **P < 0.01.
(C) RT-PCR analysis of MP and ARF3 expression in the infiltrated leaf areas indicated in (A).
(D) Conceptual model of how spatial auxin signaling controls leaf patterning in leaf primordium.
ARF2, ARF3 and ARF4 contribute to leaf blade outgrowth
To further understand the genetic interactions between the ta-siRNA-targeted ARF genes and WOX genes, we introduced p35S::amiR-ARF into wox1-2 prs. Suppression of ARF2, ARF3 and ARF4 expression further enhanced the leaf blade outgrowth defect of wox1-2 prs (Figure 5G-5I). We observed a ‘bladeless’ phenotype at high frequency (Figure 5G and 5H), that resembles the classical lam1 mutant of Nicotiana sylvestris [47]. Thus, these ta-siRNA-targeted ARF genes also contribute to leaf blade outgrowth, especially when WOX activity is missing. The wox1-2 prs p35S::amiR-ARF leaves are radially symmetric (Figure 5I), which is also observed in lam1 and related Medicago stf mutant [24, 47]. In contrast to tobacco, Medicago and maize, Arabidopsis wox1 prs mutants only have mild leaf blade outgrowth phenotype. These ta-siRNA-targeted ARF genes may provide the remaining leaf blade outgrowth activity.
DISCUSSION
Leaf flattening promotes efficient photosynthesis, and is an excellent model to study organ patterning. Although leaves exhibit determinate growth with a finite period of development, it has long been proposed that leaves also require meristem activity that ensures cell proliferation [18-21]. Similar to the SAM and the root apical meristem (RAM), leaf meristems have active proliferative activity to produce cells that further differentiate to enable leaf blade outgrowth. Notably, leaf meristems share highly conserved genes with the SAM and RAM. The WOX genes, which are key for maintaining stem cells both in SAM and RAM, are also expressed in the leaf meristems [22-25]. WOX1 and PRS for the leaf meristems and WUS for the SAM are partially interchangeable [27-29], further supporting the similarity between leaf meristems and the SAM. Unlike the apical meristems, leaf meristems do not maintain indeterminate self-renewal and cell proliferation. Cell division ceases in leaves after a certain time period, similar to the floral meristem. Thus, leaf patterning, especially flattening, depends on activities of leaf meristems.
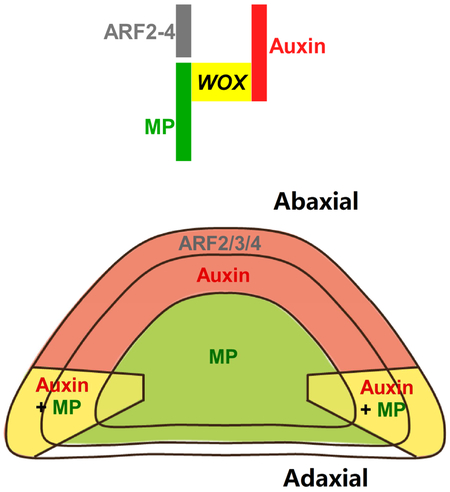
It has long been proposed that the establishment of adaxial-abaxial polarity conditions establishment of the mediolateral axis and subsequent leaf flattening [1]. By showing how WOX expression is defined, our study establishes a molecular link between the two axes (Figure 7D). Auxin is depleted from the adaxial domain during early leaf development [17]. On the other hand, MP (and possibly other ARF activators) is expressed in the adaxial and marginal domains right after leaf initiation (P2 in Figure 1A and S1C). The combination of auxin and ARF activator distribution results in restricted auxin signaling in the marginal domain (Figure 1B). In the root, HD-ZIP III transcription factors activate MP expression [48]. The same HD-ZIP IIIs are expressed in the leaf adaxial domain, and may set up the initial adaxial-enriched MP expression. Because auxin signaling promotes MP expression [49], this positive feedback loop may further refine MP expression to the marginal domain (Figure 1A). MP promotes the expression and polarization of the auxin efflux carrier PIN-FORMED1 [49, 50], further reinforcing the positive feedback loop and the marginal auxin signaling maxima. Nevertheless, weak auxin signaling may also exist in other regions, as suggested by the uniform DR5v2 expression (Figure S2D). DR5v2 is expected to be more sensitive to low level auxin signaling than the classical DR5 reporter [34].
Our genetic and molecular analysis indicated that the marginal auxin signaling activates WOX1 and PRS expression (Figures 3 and 4), mediated by direct binding of MP to WOX promoter regions (Figure 4). Furthermore, the three abaxially expressed ta-siRNA-targeted ARFs, which are transcriptional repressors [32], suppress WOX1 and PRS expression in the abaxial domain by binding to the same AuxRE-containing promoter elements targeted by MP (Figures 4-6). Recent structural analysis showed that both MP and ARF1, another ARF repressor, each form homodimers and bind to related AuxRE pairs [38]. It is conceivable that ARF repressors compete with ARF activators to restrict WOX expression. Albeit both under auxin signaling regulation, the expression domains of PRS and WOX1 do not fully overlap (Figure 1C and 1D) [22], suggesting additional regulators further specify their expression.
Our results also indicated that the three abaxially expressed ta-siRNA-targeted ARF repressors may regulate leaf patterning at two levels. The three ARF repressors promote the abaxial fate and restrict WOX expression (Figure 5B). Loss of these ARF repressors lead to adventitious small blade protursions on the abaxial side, which is associated with ectopic abaxial WOX activities (Figure 5F, S5 and S6) [11]. In addition, the abaxial-expressing ARF repressors may also contribute to leaf blade outgrowth. Introducing amiR-ARF into the wox1 prs double mutant lead to synergistic effect (Figure 5G-5I). The wox1 prs amiR-ARF plants showed a ‘bladeless’ phenotype, suggesting strongly enhanced mediolateral growth defects. Thus, these ARF repressor genes also contribute to leaf blade outgrowth. In contrast, loss of WOX activity alone is sufficient to induce a ‘bladeless’ phenotype in tobacco and Medicago [24, 47], suggesting different contributions of ARF repressors to leaf blade outgrowth in different plant species.
Overall, our analyses show that the adaxial-abaxial distribution of auxin, ARF activators, and ARF repressors collectively defines WOX1 and PRS expression, and the leaf meristem to the marginal domain. The leaf marginal meristem enables leaf expansion, and establishes the mediolateral axis [22, 24]. Finally, the leaf marginal meristem is gradually suppressed by multiple NGATHA (NGA) and CINCINNATA-class-TCP (CIN-TCP) transcription factors, resulting in determinate leaf growth [21].
STAR★Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yuling Jiao (yljiao@genetics.ac.cn).
Experimental Model and Subject Details
Growth conditions
Arabidopsis thaliana plants were grown in soil under constant light at 22°C. For imaging and ISH, seeds were stratificated for 2 d on 1/2 Murashige and Skoog (MS) medium (Duchefa), 1% (w/v) sucrose, and 0.8% (w/v) agar in the dark at 4°C and then under short-day conditions (8 hr light/16 hr dark) for 15 d.
Genetic material
The Arabidopsis thaliana ecotypes Columbia (Col-0) and Landsberg erecta (Ler) were used as the wild-types. The arf5-1, mp-G12 nph4-1 [50], arf2-6 (CS24600) [42], arf3-1 (CS24603) [43], arf4-2 (SALK_070506C) [11], wox1-2 (8AAJ85), and prs (SALK_127850) [23] mutants used in this study are in the Col-0 background. The transgenic lines pARF6::n3GFP, pARF8::n3GFP, pARF19::n3GFP, and pNPH4::n3GFP [51], p35S::DII-Venus [52], pDR5::GFP-ER [53], pDR5v2::ntdTomato pDR5::n3GFP [34], pMP::MP-GFP, pMP::MP-HA [39], pMP::MPΔ [17], pWOX1::SV40-3XGFP, pPRS::SV40-3XGFP [54], and ARF2ox [55] are in Col-0, and pARF3::ARF3-GFP and pARF3::mARF3-GR are in Ler [46].
Method Details
Construction of transgenic plants
To construct pMP::MPΔ-GR, a 6,695-bp MP genomic DNA fragment (containing 3,231 bp upstream and 3,461 bp downstream of the start condon, respectively) was amplified using the forward primer MP-F and the reverse primer MP-R and fused in-frame to GR through cloning between the XhoI and XbaI sites of the binary vector pG0229. To construct pMP::MPΔ-EAR-GR, a 120-bp fragment coding for 183-222 amino acids of AtERF4 was amplified using the primer AtERF4-F and AtERF4-R and fused to the C-terminus of pMP::MPΔ through the SacI site in the pEASY-Blunt vector (TransGen). The resulting fusion gene was cloned between the XhoI and XbaI sites of the binary vector pG0229. These constructs were transformed into Col-0 plants, and more than 10 independent stable transgenic lines were characterized for each construct.
To construct pWOX1::WOX1-mCherry, a 6,482-bp WOX1 genomic fragment (containing 4,456 bp upstream and 2,023 bp downstream of the start condon, respectively) was amplified using the forward primer WOX1-F and the reverse primer WOX1-R and fused in-frame to mCherry through cloning between the BamHI and XhoI sites of the binary vector pYBA1128. To obtain the pWOX1Δ::WOX1-mCherry construct, a 142-bp fragment (1,347-bp to 1,205-bp upstream of the start codon) was deleted using PCR and the deleted version of WOX1 genomic fragment was cloned into pYBA1128 between the BamHI and XhoI sites. The two constructs were transformed into wox1-2 prs double mutants, and more than 50 independent stable transgenic lines were characterized for each construct.
For transformation of Arabidopsis plants, Agrobacterium tumefaciens strain GV3101 and floral dip method were used in all the transformation experiments [56].
RNA extraction and RT-PCR
Total RNA was extracted from inflorescences or leaves using the RNeasy Micro kit (Qiagen) according to the manufacturer’s instructions. For Dex/CHX treatment experiments, transgenic shoot apexes were treated by 10 μM Dex alone or with 10 μM CHX for 4 hr. The first strand of cDNA was synthesized using TransScript One-Step gDNA Removal and cDNA synthesis SuperMix (TransGen), and then used as templates for reverse transcription quantitative PCR (RT-qPCR). RT-qPCR was performed through the Bio-Rad CFX96 real-time PCR detection system using KAPA SYBR FAST qPCR kit (KAPA Biosystems). The relative expression levels of target genes were normalized to ACTIN2 (At3g18780) levels. All primers used in RT-qPCR were listed in Table S1.
ChIP-PCR analysis
Inflorescences of four-week-old pMP::MP-GFP, pMP::MP-HA, and pARF3::ARF3-GFP transgenic plants were harvested and frozen in liquid nitrogen. One gram of inflorescences was used in ChIP experiments. The inflorescences were grinded to fine powder and then fixed with 1% formaldehyde (10 mM phosphate buffer, 0.1 M NaCl, 10 mM mercapto-ethanol, 1M hexylene glycol) for 10 min. Then 0.125 M glycine was added to stop the crosslink through incubate for 5 min. The solution was filtered through 4 layers of miracloth. Nuclei were isolated through centrifugation at 12,000 rpm for 10 min. The nuclei precipitate was rinsed for 3 times with buffer containing 10 mM phosphate buffer, 0.1 M NaCl, 10 mM mercapto-ethanol, 1 M hexylene glycol, 10 mM MgCl2 and 0.5% Triton X-100. Nuclei were lysed with nuclei lysis buffer (50 mM Tris-HCl, pH8.0, 10 mM EDTA, 1% SDS). Protease inhititors were added in all the above liquids. The chromatin was sheared to an average size of 500 bp by sonication. The supernatant after centrifugation was used as input. Immunoprecipitations were performed using anti-GFP or anti-HA antibodies. No-antibody controls were used to calculate enrichment. PCR was performed using the precipitated DNA as templates. The enrichment of DNA fragments was determined by quantitative PCR analysis. Three independent biological repeats were performed for each ChIP analysis. All primers used in ChIP-PCR were listed in Table S1.
Yeast one-hybrid assay
To make the yeast one-hybrid assay bait constructs, promoter fragments of WOX1 and PRS were amplified from genomic DNA using specific primers (see Supplementary Table 3). The PCR products were ligated into pAbAi vector (Clontech). Then the bait plasmids were linearized by BstBI and integrated into yeast strain Y1HGOLD using PEG-mediated transformation according to the manufacturer’s instructions (Yeast Hand Book; Clontech, PT3024-1). Transformants were selected on media lacking uracil, verified by PCR using a promoter-specific primer and a yeast chromosome primer, and tested for auto-activation according to the manufacturer’s instructions. All AD-TF prey clones were derived from pDEST22 (Life Technologies).
Yeast one-hybrid assay was performed according to the user manual of Matchmaker™ Gold Yeast One-Hybrid Library Screenig System (Clontech, 630491). Briefly, AD-TF plasmids were directly transformed into Y1HGOLD bait strains harboring genomic promoter-reporters through PEG-mediated transformation, and transformants were selected on media lacking uracil and tryptophan but containing 800 ng/ml aureobasidin A (AbA). An equal amount of transformed yeast culture was plated on medium lacking uracil and tryptophan without addition of AbA to control for transformation efficiency. Positive interactions were identified based on growth ability after transformation, on AbA-containing medium for 3 days. All interactions were validated by retesting using the same procedure.
In situ hybridizations (ISH)
ISH was performed on 8 μm paraffin sections. Details of methods used for fixation of plants, embedding in paraffin, and in situ hybridization can be found at http://www.its.caltech.edu/~plantlab/protocols/insitu.html. Sections were cut with a Leica RM2255 ortary microtome. WOX1 and PRS probes were generated through amplifying of nucleotides 235 to 1030 of WOX1 and 192 to 828 of PRS coding sequences by PCR with the forward primers 5’-CCGACACCAGATCAGTTAAG-3’, 5’- GAATGCGGTGCAGATACAACA -3’ and reverse primers 5’-CATAGAAACAGTGAATGCCA-3’, 5’- GGTACTGTCTTGTTTGGAGT-3’, respectively. The fragments were subsequently cloned into the pEASY-Blunt vector.
Tissue preparation for confocal analysis
Shoot apices with leaves removed were collected and immediately placed in 2.5% paraformaldehyde (PFA; Sigma-Aldrich) at pH 7.0 at 4°C, vacuum infiltrated for 30 min, and then were stored overnight at 4°C. Fixed tissue samples were washed with 10% (w/v) sucrose and 1% PFA at pH 7.0 for 20 min, 20% sucrose and 1% PFA at pH 7.0 for 20 min, and 30% sucrose and 1% PFA at pH 7.0 for 30 min, successively. Samples were then embedded in 5 to 7% (w/v) low melting point agarose (Promega) liquid gel at 30°C and placed at 4°C for 15 min to solidify. Sections of 50 μm were made using a Leica VT1000S vibratome. For high-resolution images, samples were stained with 50 μg/mL propidium iodide (PI, Sigma-Aldrich).
Live imaging
To dissect vegetative SAMs, seedlings grown under short-day conditions for 15 days were transferred to the dissecting medium (3% agarose) and fixed into a hole using forceps. Leaf primordia older than P4 were carefully removed using a fine needle tip under a stereomicroscope (Nikon, SMZ18). FM4-64 (Thermo Fisher, 10 μg/mL) was applied to the SAM for 10 min. The dissected meristem with P2 or P3 was transferred to the imaging medium (1/2 MS medium with 1% agarose on the top) before live imaging. All live imaging was performed using 60× water dipping lens.
Confocal microscopy, optical microscopy, and scanning electron microscopy
Confocal images were taken with a Nikon A1 confocal microscope. Excitation and detection windows setups for GFP, Venus, and tdTomato were as described [17]. For imaging GFP and autofluorescence together, both were excited with a 488 nm laser and the emission was splited with a 500-550 nm band-pass filter for GFP and a 660-700 nm filter for autofluorescence. To detect the signal of GFP together with FM4-64 or PI, 488 nm for GFP and 561 nm for FM4-64 or PI lasers were used for excitation, 500-530 nm band-pass filter for GFP and 570-620 nm band-pass filter for FM4-64 or PI were used for detection. For the combination of GFP, Venus and FM4-64, GFP and FM4-64 were detected together as described above and Venus was imaged alone using a 514 nm laser excitation and a 524-550 nm band-pass filter emission. Then the two scan results stacked together automatically. To image tdTomato, a 561 nm laser was used for excitation and a 570-620 nm band-pass filter was used for detection. All images were scanned with 1024 × 1024 pixels. All optical photographs were taken with a Nikon SMZ1000 stereoscopic microscope or an Olympus BX60 microscope equipped with a Nikon DS-Ri1 camera head. Scanning electron microscopy was performed using the Hitachi S-3000N variable pressure scanning electron microscopy.
Transient expression assays
The transient expression assay was performed in N. benthamiana leaves essentially as previously described [57]. The WOX1 promoter was isolated using the forward primer 5’-GCGGTACCATGCTTGAAAATATCTCTGT-3’ and the reverse primer 5’-CACTCGAGCTCTGGTTGCGTGTCGCATC-3’ and cloned into pGL3-basic vector (Promega) at the KpnI (5’-end) and XhoI (3’-end) sites upstream of LUC. The WOX1 promoter with the mutated MP binding sites (pWOX1Δ) was amplified using the forward primer 5’-TCTCATATTCTTTTTAAAATTTATATTAATCT-3’ and the reverse primer 5’-GATTAATATAAATTTTAAAAAGAATATGAGATCCC-3’ from pGL3-pWOX1-LUC plasmid. Then the fused pWOX1::LUC and pWOX1Δ::LUC genes were subcloned into the KpnI and BamHI sites of pCAMBIA1300 vector. To generate MP effector construct, the MP cDNA was PCR amplified with the forward primer 5’- CACCCGGGATGATGGCTTCATTGTCTTG-3’ and reverse primer 5’- CGACTAGTTTATGAAACAGAAGTCTTAA-3’, and then was subcloned into the SmaI and SpeI sites downstream of the p35S promoter of the HA-pBA vector. For the 35S::mARF3 construct, the mutated form of ARF3 cDNA was obtained as previously described [58] and subcloned into the SmaI and SacI sites downstream of the p35S promoter of the HA-pBA vector.
The above constructs were transformed into GV3101 and the obtained Agrobacterium strains were used to infiltrate N. benthamiana leaves. Infiltrated plants were incubated at 22°C for 72 h before CCD imaging. The LUC images were captured using a low-light cooled CCD imaging apparatus (NightOWL II LB983 with indigo software). The transformed leaves were sprayed with 100 mM luciferin and placed in darkness for 5 min before luminescence detection. Error bars represent SD. Experiments were repeated at least five times.
Quantification and Statistical Analysis
For phenotypic quantification (as shown in Figure 2B, 3G, and 4K), leaf length and width were measured using the Nikon NIS-Elements software and the data were shown in mean ± SEM, and n indicates the number of leaves used for quantification. The details are as follows: Figure 2B: Col-0 cotyledon (n=12), arf5-1 cotyledon (n=12), Col-0 rosette leaf (n=10), arf5-1 rosette leaf (n=20). Figure 3G: WT (n=6), wox1-2 prs (n=6), MPΔ (n=6), MPΔ wox1-2 (n=3), MPΔ prs (n=4). Figure 4K: Col-0 (n=24), wox1-2 prs (n=18), pWOX1::WOX1 (n=24), pWOX1Δ::WOX1 (n=18). Statistical analysis was performed through Student’s t test in Excel and P values less than 0.05 was considered significant for any set of data.
Supplementary Material
Highlights.
Adaxial-expressed MP and abaxial-enriched auxin define middle domain WOX expression.
Redundant abaxial-enriched ARF repressors suppress WOX expression.
Spatial auxin signaling transforms adaxial-abaxial polarity into leaf flattening.
ACKNOWLEDGMENTS
We thank Zhizhong Gong, Chun-Ming Liu, Michiel Vandenbussche, Dolf Weijers, and the ABRC for seeds. We thank Jiyan Qi and Ying Wang for assistance with imaging, and Ying Wang for comments on the manuscript. This research was supported by National Natural Science Foundation of China (NSFC) grant 31430010, National Basic Research Program of China grant 2014CB943500, and National Program for Support of Top-Notch Young Professionals to Y.J., NSFC grant 31401232 to C.G., National Institute of General Medical Sciences of the National Institutes of Health award R15GM114733 to N.T.K., and the State Key Laboratory of Plant Genomics.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and one table and can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Waites R, and Hudson A. (1995). phantastica: a gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121, 2143–2154. [Google Scholar]
- 2.Barton MK (2010). Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Dev Biol 341, 95–113. [DOI] [PubMed] [Google Scholar]
- 3.Bowman JL, and Floyd SK (2008). Patterning and polarity in seed plant shoots. Annu Rev Plant Biol 59, 67–88. [DOI] [PubMed] [Google Scholar]
- 4.Braybrook SA, and Kuhlemeier C. (2010). How a plant builds leaves. Plant Cell 22, 1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efroni I, Eshed Y, and Lifschitz E. (2010). Morphogenesis of simple and compound leaves: a critical review. Plant Cell 22, 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husbands AY, Chitwood DH, Plavskin Y, and Timmermans MC (2009). Signals and prepatterns: new insights into organ polarity in plants. Genes Dev 23, 1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L, Yang L, and Huang H. (2007). Transcriptional, post-transcriptional and post-translational regulations of gene expression during leaf polarity formation. Cell Res 17, 512–519. [DOI] [PubMed] [Google Scholar]
- 8.Chitwood DH, Nogueira FT, Howell MD, Montgomery TA, Carrington JC, and Timmermans MC (2009). Pattern formation via small RNA mobility. Genes Dev 23, 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, and Carrington JC (2006). Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr Biol 16, 939–944. [DOI] [PubMed] [Google Scholar]
- 10.Garcia D, Collier SA, Byrne ME, and Martienssen RA (2006). Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol 16, 933–938. [DOI] [PubMed] [Google Scholar]
- 11.Pekker I, Alvarez JP, and Eshed Y. (2005). Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17, 2899–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nogueira FT, Madi S, Chitwood DH, Juarez MT, and Timmermans MC (2007). Two small regulatory RNAs establish opposing fates of a developmental axis. Genes Dev 21, 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao X, Wang H, Li H, Yuan Z, Li F, Yang L, and Huang H. (2009). Two types of cis-acting elements control the abaxial epidermis-specific transcription of the MIR165a and MIR166a genes. FEBS Lett 583, 3711–3717. [DOI] [PubMed] [Google Scholar]
- 14.Tatematsu K, Toyokura K, Miyashima S, Nakajima K, and Okada K. (2015). A molecular mechanism that confines the activity pattern of miR165 in Arabidopsis leaf primordia. Plant J 82, 596–608. [DOI] [PubMed] [Google Scholar]
- 15.Juarez MT, Kui JS, Thomas J, Heller BA, and Timmermans MC (2004). microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428, 84–88. [DOI] [PubMed] [Google Scholar]
- 16.Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, Barton MK, and Bartel DP (2004). MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5' region. EMBO J 23, 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi J, Wang Y, Yu T, Cunha A, Wu B, Vernoux T, Meyerowitz E, and Jiao Y. (2014). Auxin depletion from leaf primordia contributes to organ patterning. Proc Natl Acad Sci U S A 111, 18769–18774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagemann W, and Gieissberg S. (1996). Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst Evol 199, 121–152. [Google Scholar]
- 19.Nardmann J, and Werr W. (2013). Symplesiomorphies in the WUSCHEL clade suggest that the last common ancestor of seed plants contained at least four independent stem cell niches. New Phytol 199, 1081–1092. [DOI] [PubMed] [Google Scholar]
- 20.Ichihashi Y, and Tsukaya H. (2015). Behavior of leaf meristems and their modification. Front Plant Sci 6, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez JP, Furumizu C, Efroni I, Eshed Y, and Bowman JL (2016). Active suppression of a leaf meristem orchestrates determinate leaf growth. eLife 5, e15023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakata M, Matsumoto N, Tsugeki R, Rikirsch E, Laux T, and Okada K (2012). Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell 24, 519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenbussche M, Horstman A, Zethof J, Koes R, Rijpkema AS, and Gerats T (2009). Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. Plant Cell 21, 2269–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tadege M, Lin H, Bedair M, Berbel A, Wen J, Rojas CM, Niu L, Tang Y, Sumner L, Ratet P, et al. (2011). STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris. Plant Cell 23, 2125–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nardmann J, Ji J, Werr W, and Scanlon MJ (2004). The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development 131, 2827–2839. [DOI] [PubMed] [Google Scholar]
- 26.Laux T, Mayer KF, Berger J, and Jurgens G (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122, 87–96. [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Niu L, McHale NA, Ohme-Takagi M, Mysore KS, and Tadege M (2013). Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc Natl Acad Sci U S A 110, 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu R, Ji J, Kelsey E, Ohtsu K, Schnable PS, and Scanlon MJ (2009). Tissue specificity and evolution of meristematic WOX3 function. Plant Physiol 149, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolzblasz A, Nardmann J, Clerici E, Causier B, van der Graaff E, Chen J, Davies B, Werr W, and Laux T (2016). Stem cell regulation by Arabidopsis WOX genes. Mol Plant 9, 1028–1039. [DOI] [PubMed] [Google Scholar]
- 30.Krogan NT, and Berleth T (2012). A dominant mutation reveals asymmetry in MP/ARF5 function along the adaxial-abaxial axis of shoot lateral organs. Plant Signal Behav 7, 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, and Dengler NG (1999). Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215, 407–419. [DOI] [PubMed] [Google Scholar]
- 32.Guilfoyle TJ, and Hagen G (2007). Auxin response factors. Curr Opin Plant Biol 10, 453–460. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Wang J, Shi B, Yu T, Qi J, Meyerowitz EM, and Jiao Y (2014). The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell 26, 2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao CY, Smet W, Brunoud G, Yoshida S, Vernoux T, and Weijers D. (2015). Reporters for sensitive and quantitative measurement of auxin response. Nat Methods 12, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarojam R, Sappl PG, Goldshmidt A, Efroni I, Floyd SK, Eshed Y, and Bowman JL (2010). Differentiating Arabidopsis shoots from leaves by combined YABBY activities. Plant Cell 22, 2113–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta M, Matsui K, Hiratsu K, Shinshi H, and Ohme-Takagi M. (2001). Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13, 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi M, Donner TJ, Scarpella E, and Yanagisawa S (2015). MONOPTEROS directly activates the auxin-inducible promoter of the Dof5.8 transcription factor gene in Arabidopsis thaliana leaf provascular cells. J. Exp. Bot 66, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boer DR, Freire-Rios A, van den Berg WA, Saaki T, Manfield IW, Kepinski S, Lopez-Vidrieo I, Franco-Zorrilla JM, de Vries SC, Solano R, et al. (2014). Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156, 577–589. [DOI] [PubMed] [Google Scholar]
- 39.Schlereth A, Moller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jurgens G, and Weijers D. (2010). MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464, 913–916. [DOI] [PubMed] [Google Scholar]
- 40.Allen E, Xie Z, Gustafson AM, and Carrington JC (2005). MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121, 207–221. [DOI] [PubMed] [Google Scholar]
- 41.Williams L, Carles CC, Osmont KS, and Fletcher JC (2005). A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proc Natl Acad Sci U S A 102, 9703–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okushima Y, Mitina I, Quach HL, and Theologis A (2005). AUXIN RESPONSE FACTOR 2 (ARF2): a pleiotropic developmental regulator. Plant J. 43, 29–46. [DOI] [PubMed] [Google Scholar]
- 43.Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. (2005). Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulmasov T, Liu ZB, Hagen G, and Guilfoyle TJ (1995). Composite structure of auxin response elements. Plant Cell 7, 1611–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez JP, Pekker I, Goldshmidt A, Blum E, Amsellem Z, and Eshed Y (2006). Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell 18, 1134–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Dinh TT, Li D, Shi B, Li Y, Cao X, Guo L, Pan Y, Jiao Y, and Chen X. (2014). AUXIN RESPONSE FACTOR 3 integrates the functions of AGAMOUS and APETALA2 in floral meristem determinacy. Plant J. 80, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McHale NA, and Marcotrigiano M (1998). LAM1 is required for dorsoventrality and lateral growth of the leaf blade in Nicotiana. Development 125, 4235–4243. [DOI] [PubMed] [Google Scholar]
- 48.Müller CJ, Valdés AE, Wang G, Ramachandran P, Beste L, Uddenberg D, and Carlsbecker A. (2016). PHABULOSA mediates an auxin signaling loop to regulate vascular patterning in Arabidopsis. Plant Physiol 170, 956–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatia N, Bozorg B, Larsson A, Ohno C, Jönsson H, and Heisler MG (2016). Auxin acts through MONOPTEROS to regulate plant cell polarity and pattern phyllotaxis. Curr Biol 26, 3202–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krogan NT, Marcos D, Weiner AI, and Berleth T. (2016). The auxin response factor MONOPTEROS controls meristem function and organogenesis in both the shoot and root through the direct regulation of PIN genes. New Phytol 212, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rademacher EH, Moller B, Lokerse AS, Llavata-Peris CI, van den Berg W, and Weijers D. (2011). A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J. 68, 597–606. [DOI] [PubMed] [Google Scholar]
- 52.Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, et al. (2011). The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 7, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, and Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. [DOI] [PubMed] [Google Scholar]
- 54.Xu TT, Ren SC, Song XF, and Liu CM (2015). CLE19 expressed in the embryo regulates both cotyledon establishment and endosperm development in Arabidopsis. J Exp Bot 66, 5217–5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Hua D, He J, Duan Y, Chen Z, Hong X, and Gong Z. (2011). Auxin Response Factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet. 7, e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clough SJ, and Bent AF (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16, 735–743. [DOI] [PubMed] [Google Scholar]
- 57.Sun J, Qi L, Li Y, Chu J, and Li C. (2012). PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet 8, e1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yifhar T, Pekker I, Peled D, Friedlander G, Pistunov A, Sabban M, Wachsman G, Alvarez JP, Amsellem Z, and Eshed Y. (2012). Failure of the tomato trans-acting short interfering RNA program to regulate AUXIN RESPONSE FACTOR3 and ARF4 underlies the wiry leaf syndrome. Plant Cell 24, 3575–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.