Abstract
Priapism is a disorder in which prolonged penile erection persists uncontrollably, potentially leading to tissue damage. Priapism commonly afflicts patient populations with severely low nitric oxide (NO) bioavailability. As NO is a primary mediator of erection, the molecular mechanisms involved in priapism pathophysiology associated with low NO bioavailability are not well understood. The objective of this study was to identify dysregulated molecular targets and signaling pathways in penile tissue of a mouse model of low NO bioavailability that have potential relevance to priapism. Neuronal + endothelial NO synthase double knockout mice (NOS1/3−/−) were used as a model of low NO bioavailability. Priapic-like activity was demonstrated in the NOS1/3−/− mice relative to wild type (WT) mice by measurement of prolonged erections following cessation of electrical stimulation of the cavernous nerve. Penile tissue was processed and analyzed by reversed-phase liquid chromatography tandem mass spectrometry. 1279 total proteins were identified and quantified by spectral counting, 46 of which were downregulated and 110 of which were upregulated in NOS1/3−/− vs. WT (P<0.05). Ingenuity Pathway Analysis of differentially expressed proteins revealed increased protein kinase A and G-protein coupled receptor signaling in NOS1/3−/− penis which represent potential mechanisms contributing to priapism secondary to low NO bioavailability.
Keywords: Priapism, nitric oxide, erectile, mass spectrometry, PKA, GPCR, penis, proteomics
Graphical Abstract
INTRODUCTION
Priapism is an erectile disorder in which penile erection persists undesirably and uncontrollably without sexual purpose.1 Episodes of priapism can be extremely painful and result in a medical emergency. On a psychological level, priapism can result in anxiety, avoidance of social situations, embarrassment, and can adversely affect sexual relationships.2 Frequent episodes during the night can result in sleep deprivation, ultimately impacting work or scholastic performance.2 On a physiologic level, priapism can result in progressive penile fibrosis and endothelial and smooth muscle cell necrosis in the corpus cavernosum, ultimately resulting in erectile dysfunction.3 Extreme episodes of priapism can even result in penile gangrene, although this is a rare occurrence.4
The origin of priapism is often identified as idiopathic in nature, although it is known to be caused by multiple factors, including but not limited to: neurologic disorders such as spinal cord injury or autonomic neuropathy, pelvic neoplasms, toxins from scorpion stings or spider bites, recreational abuse of phosphodiesterase type 5 (PDE5) inhibitors, marijuana, or cocaine, or as undesired side effects of anticoagulants, antidepressants, antihypertensive or antianxiety agents.5 However, the disorder is commonly manifest in individuals with low nitric oxide (NO) bioavailability, such as those with glucose-6-phosphate dehydrogenase (G6PD) deficiency, β-thalassemia, and sickle cell disease (SCD), but also men with Fabry disease to a lesser extent.3,6–10 It is estimated that SCD afflicts 20–25 million individuals worldwide.3 Observational studies suggest that the probability of a man with SCD suffering from priapism in his lifetime is between 35 and 42 percent11,12; however, these numbers are thought to be a gross underrepresentation of the true prevalence.3,13 G6PD deficiency is estimated to afflict over 400 million people worldwide14,15, although the prevalence for priapism among these individuals is unknown. The pathogenesis and molecular determinants of priapism in disease states associated with low NO bioavailability is not fully understood. Furthermore, current therapies are largely reactive in the face of an acute event and hence non-preventative, resulting in frequent readmission of priapism patients to hospital emergency departments.16,17 This reflects a limited understanding of the science of priapism from which to formulate effective interventions.3
NO has been thoroughly characterized as a major mediator of penile erection.18,19 Because causally linking NOS-deficiency to priapism is counterintuitive, molecular mechanisms by which NOS-deficiency produces priapism are equally obscure. NO has a vasodilatory role through stimulation of soluble guanylate cyclase and ultimately protein kinase G.20 However, NO is known to control cellular signaling and transcriptional regulation independently of its vasodilatory role.21,22 Thus, given the versatile roles of NO, it is plausible that NO-deficiency may contribute to priapism in a number of possible ways that have not previously been explored.
The utilization of proteomics to gain systemic molecular insight into physiology and disease pathology has gained traction as the technology has advanced. In particular, recent improvements in mass spectrometry sensitivity and accuracy have yielded the ability to identify thousands of proteins in tissue samples of mixed cell type composition.23 Furthermore, improvements in bioinformatics approaches have greatly aided in the interpretation and application of these large data sets. In particular, Ingenuity Pathway Analysis (IPA) software can predict pathway activation or inhibition based on protein expression patterns, determined by causal relationships reported in the scientific literature.24 The purpose of this study was to identify dysregulated molecular targets and signaling pathways in penile tissue of a mouse model of low NO bioavailability that have potential relevance to priapism.
EXPERIMENTAL PROCEDURES
Animals
Adult (9–12 month) male double mutant neuronal NOS and endothelial NOS knockout mice (NOS1/3−/−) were used as a model of low NO bioavailability. The NOS1/3−/− mice were originally developed on a mixed sv129 and C57Bl/6 background and backcrossed for greater than 12 generations on a C57Bl/6 background.25,26 Age-matched male C57Bl/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME) to serve as the wild type control. Animals were housed in a pathogen-free, temperature controlled facility with ad libitum access to standard rodent chow and drinking water. All experimental procedures were conducted in accordance with the ethical standards of the Johns Hopkins University School of Medicine Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Measurement of Intracavernosal Pressure/Mean Arterial Pressure
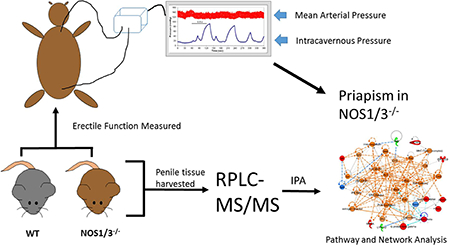
Mice were anesthetized with an intraperitoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine, and supplemented with an intramuscular injection as needed. The left carotid artery was cannulated with polyethylene (PE) tubing filled with 100 U/ml of heparinized saline connected to a pressure transducer allowing for continuous measurement of mean arterial pressure (MAP) with LabChart7 software (ADInstruments, Sydney, Australia). The shaft of the penis was freed of skin and fascia and the right crus was exposed by removing part of the ischiocavernous muscle. A 30-gauge needle connected to PE tubing and filled with 100 U/ml of heparinized saline was inserted into the crus and connected to a pressure transducer, allowing for continuous measurement of intracavernosal pressure (ICP). The bladder and prostate were exposed through a midline abdominal incision. The right major pelvic ganglion and cavernous nerve were identified posterolateral to the prostate and an electrical stimulator with a platinum bipolar hook was placed around the cavernous nerve. The cavernous nerve was stimulated with a square pulse stimulator (Grass Instruments, Quincy, MA) for 60 seconds at 0.5, 1, 2, and 4 volts, with five minutes passing between each stimulation period to allow for detumescence and measurement of the post-cavernous nerve stimulated (Post-CNS) erectile response. Erectile function was assessed by the peak ICP/MAP ratio and the area under the curve (AUC) of the ICP pressure tracing during stimulation normalized to MAP (AUC/MAP). Priapic-like activity was assessed by the peak ICP/MAP, AUC/MAP, and erection duration during the first four minutes PostCNS. Erection duration was defined as the amount of time in which ICP was greater than 2 times the baseline ICP, as described previously.27
Proteomic Analysis of Penile Tissue
Penile tissue was obtained from a separate group of mice that did not undergo nerve stimulation. Penile tissue was exposed by freeing the tissue of skin and fascia. Mice were sacrificed and penile tissue from the base to the proximal glans was immediately harvested, snap frozen in liquid nitrogen, and stored at −80°C until processing. Penile tissue was processed as described recently in cardiac tissue.28 Briefly, tissue was pulverized in liquid nitrogen and solubilized using a motor driven pestle and glass mortar in 8 M urea, 2 M thiourea, 4% CHAPS, and 1% dithiothreitol. The homogenate was centrifuged at 16,000 rpm for 20 minutes. The supernatant was separated, and protein concentrations were determined with CB-X assay kit (G-Biosciences, St. Louis, MO). 100 μg of protein was reduced, alkylated, and digested with trypsin utilizing a FASP protein digest kit (Expedeon, Cambridge, United Kingdom). Peptides were desalted with an Oasis HLB 96-well plate (Waters, Milford, MA). 2 μg of peptides were analyzed using label-free quantification by reversed-phase liquid chromatography tandem mass spectrometry (RPLC-MS/MS) online with an Orbitrap Elite mass spectrometer (Thermo Scientific, Waltham, MA) coupled to an Easy-nLC 1000 system (Thermo Scientific). Peptides were concentrated on a C18 trap column (Acclaim PepMap 100) in 0.1% aqueous formic acid, then subsequently separated on a C18 analytical column using a linear solvent gradient from 5–35% over 155 minutes, as described.28 The analysis was operated in a data-dependent mode with full scan MS spectra acquired in the Orbitrap analyzer at a 60,000 resolution, followed by MS2 acquisition of the top 10 ions in the ion trap.
Database Searching and Processing
All raw data from the Orbitrap Elite were converted to mzXML format using Msconvert from ProteoWizard for peaklist generation.29 All MS/MS samples were analyzed using Sorcerer 2™-SEQUEST® algorithm (Sage-N Research, Milpitas, CA, USA) searched against the concatenated target/decoy Mouse Uniprot 2016 database.30,31 The search settings included trypsin as the digestion enzyme, parent ion tolerance of 50 PPM and fragment ion tolerance of 1 Da, Carbamidomethyl of cysteine as a fixed modification and oxidation of methionine as a variable modification. The post-search analysis was performed using Scaffold 4 (Proteome Software, Inc., Portland, OR, USA) with protein and peptide probability thresholds set to 95% and 90%, respectively, and minimum two peptides required for identification.32 Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins sharing significant peptide evidence were grouped into clusters based on the following rules: 1) For two proteins to be clustered, the sum of the probabilities of their shared peptides must be at least 95%. 2) The proteins must share at least 50% of their evidence. This is determined by summing the probabilities of the shared peptides and comparing this value with the summed probabilities of all of the peptides for each individual protein. If the sum of the probabilities of the shared peptides is greater than or equal to half of the sum of the peptide probabilities for either of the individual proteins, a cluster is formed. 3) A protein may be included in an existing cluster if it meets the above criteria with a member protein of the cluster. They are by default represented by the protein that shows the highest associated probability. Relative protein quantification was obtained from MS data using spectral counting.33,34 The entire complement of proteins that were statistically significantly different (P < 0.05) between WT and NOS1/3−/− samples were uploaded into Ingenuity Pathway Analysis software (QIAGEN, Redwood City, CA) and analyzed for altered regulation of protein interaction networks and functional signaling pathways. Only proteins in which spectral counts were detected for at least half of the replicate samples in a given group were considered for statistical analysis. Statistical significance of the alterations of the networks and pathways was determined by IPA (P < 0.05). Of those networks and pathways determined to be significantly different, the molecular activity predictor (MAP) tool within IPA (Fall 2016 release) was utilized to predict activation or inhibition of proteins within networks and known biologic canonical pathways identified to be potentially relevant to smooth muscle tone regulation, blood flow regulation, or priapism pathogenesis. Proteins were analyzed by Software Tool for Rapid Annotation of Proteins (STRAP, Boston University School of Medicine, Boston, MA) for biological process and cellular compartment ontologies. The MS/MS proteomics data have been deposited to the ProteomeXchange Consortium (http://www.proteomexchange.org) via the PRIDE partner repository35 with the dataset identifier PXD007713 and 10.6019/PXD007713.
Immunoblotting Analysis
Penile tissue was obtained from a separate group of mice that did not undergo nerve stimulation. Penile tissue was exposed by freeing the tissue of skin and fascia. Mice were sacrificed and penile tissue from the base to the proximal glans was immediately harvested, snap frozen in liquid nitrogen, and stored at −80°C until processing. Tissue was homogenized in 10 μl/mg radioimmunoprecipitation assay buffer (Cell Signaling Technologies, Danvers, MA) with 1 mM PMSF on ice with a VirTishear tissue grinder (Virtis Company, Gardiner, NY). Homogenate was centrifuged at 16,000 x g for 30 min, and the soluble protein concentration was determined by the bicinchoninic acid assay (ThermoFisher Scientific). Proteins were resolved on 4–20% or 10% Tris gels (Bio-Rad), transferred to nitrocellulose membranes, and probed overnight at 4°C with anti-MLC2 (#3672), anti-MYPT1 (#2634), or anti-phospho-VASP Ser157 (#84519) primary antibodies (Cell Signaling Technologies), and then a horseradish peroxidase conjugated anti-rabbit secondary antibody (#NA934, GE Healthcare, Little Chalfont, United Kingdom). Reactive bands were detected by electrochemiluminescence and quantified by densitometry with ImageJ software. The phospho-VASP membrane was stripped and reprobed for VASP (#3132, Cell Signaling Technologies). All membranes were stripped and reprobed with an anti-β-actin primary antibody (#A5316, Sigma Aldrich) to serve as a loading control.
Data and Statistical Analysis
Genotype differences in erectile function were determined by two-way repeated measures ANOVA with Sidak’s multiple comparisons post-hoc testing. Differences in spectral counts or densitometry were determined by Student’s t-test or Mann-Whitney non-parametric testing where appropriate. An α-level of 0.05 was used to determine significance in all instances.
RESULTS AND DISCUSSION
Erectile Response to Cavernous Nerve Stimulation
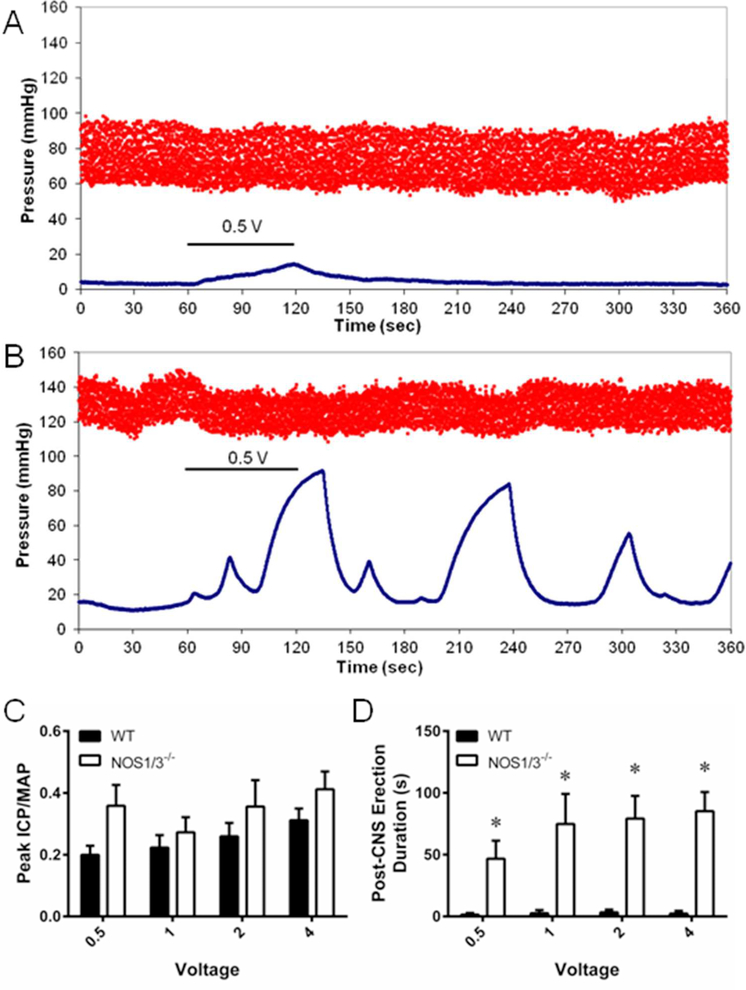
Representative pressure tracings of ICP and carotid arterial pressure at baseline, during 0.5 V of cavernous nerve stimulation, and post-stimulation are presented for WT mice (Fig. 1A) and NOS1/3−/− mice (Fig. 1B). There were no differences between groups in cavernous nerve stimulated erectile function as assessed by peak ICP/MAP during stimulation (Fig. 1C) or the area under the curve (AUC) of the ICP tracing during stimulation (Fig. S-1A). However, priapic-like activity was evident in the NOS1/3−/− mice by observing the undulation of ICP Post-CNS. Post-CNS erections were prevalent following all levels of nerve stimulation in NOS1/3−/− mice (Fig. 1D). In addition, the peak ICP/MAP value that occurred after 30 seconds of detumescence was approximately 4-fold higher in NOS1/3−/− mice compared to WT mice (Fig. S-1B). The post-CNS AUC/MAP was higher in NOS1/3−/− mice following 1 and 4 volts of CNS (Fig. S-1C). However, the undulating nature of the ICP tracings post-CNS in the NOS1/3−/− mice introduces significant variability into the AUC measurements and also indicates that the Post-CNS priapic response is not necessarily stimulation dose-dependent.
Fig. 1. Assessment of cavernous nerve stimulated erectile function and priapic activity in wild type and NOS1/3−/− mice.
Representative mean arterial pressure (MAP) and intracavernous pressure (ICP) tracings in response to 0.5 volts of cavernous nerve stimulation in (A) wild type (WT) and (B) NOS1/3−/− mice. (C) Assessment of erectile function by peak ICP/MAP following voltage dependent cavernous nerve stimulation. (D) Priapic activity assessed by erection duration following cavernous nerve stimulation. Data expressed as mean ± SEM for n = 7 animals per group. * P < 0.05 vs. WT.
Taken together, these data demonstrate uncontrolled erections following nerve stimulation, consistent with a priapism phenotype in the NOS1/3−/− mice. These results are consistent with previous studies utilizing various NOS knockout mice26 or transgenic sickle cell disease mice36,37 by our lab and others. This finding lays the groundwork for investigating the mechanisms by which NOS-deficiency specifically leads to priapism. As NOS-deficiency has been proposed as an underlying mechanism of priapism in a variety of patient populations,3,6–10 insights into molecular alterations in NOS-deficient penile tissue are of immediate clinical relevance. Furthermore, randomized controlled trials targeting the NO pathway via PDE5 inhibition are underway,38 and pharmacologic therapy involving either direct or indirect NO donation has recently been proposed to treat sickle cell disease patients suffering from priapism.39,40
Protein Quantification by Mass Spectrometry
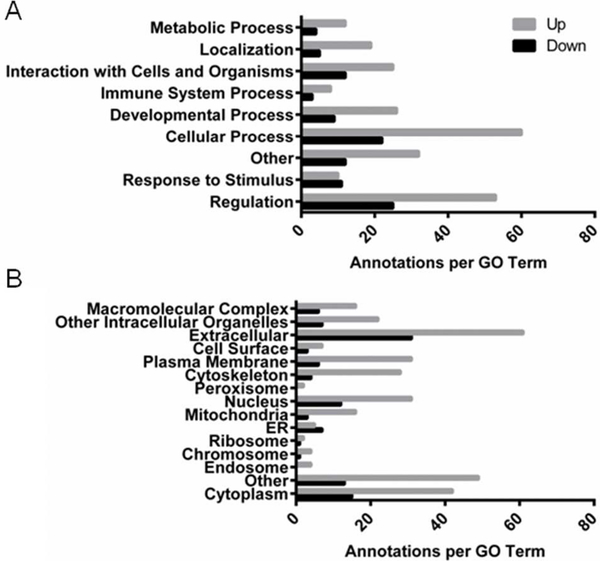
A total of 1279 proteins were identified from peptides extracted from mouse penile tissue by mass spectrometry (Table S-1). Peptides were quantified by spectral counts, with total spectral counts for each sample, as well as fold-changes and p-values for group differences presented in Supplemental Table S-1. Detailed characteristics of all peptides observed by mass spectrometry are presented in Supplemental Table S-2. There was a strong tendency for upregulation over downregulation of proteins as a result of NOS-deficiency (110 upregulated vs. 46 downregulated). Of these proteins that were differentially expressed in the NOS-deficient penis, the majority functioned in cellular regulation and processes (Fig. 2A) and were primarily localized to the extracellular space and cytoplasm (Fig. 2B), although NOS-deficiency extends to all cellular compartments to some degree.
Fig. 2. Gene ontology for proteins that were up- or down-regulated in NOS1/3−/− penis relative to wild type.
Number of annotations per gene ontology term for (A) biological process and (B) cellular component for which each up- or down-regulated protein falls, as determined by STRAP analysis.
Augmented PKA signaling in NO-Deficient Priapism
One cytoplasmic kinase identified to be upregulated in NOS1/3−/− penile tissue was protein kinase A (PKA). Adenosine is a pleiotrophic signaling molecule that targets G-protein coupled receptors, which themselves couple to adenylyl cyclase to stimulate intracellular cAMP accumulation.41 PKA is the primary receptor for cAMP, mediating its effects through phosphorylation of a variety of downstream targets primarily in the cytoplasmic and nuclear compartments.41 These targets and their IPA predicted activation/deactivation in the NOS1/3−/− penis are illustrated in figure S-2. Adenosine metabolism appears to be dysregulated in the NOS1/3−/− penis. Purine nucleoside phosphorylase was found to be downregulated by mass spectrometry, resulting in a predicted decrement in adenosine catabolism (Fig. S-3). On the contrary, adenosine kinase was found to be upregulated by mass spectrometry (Table S-1), which could potentially result in enhanced adenosine cycling to AMP.42 The erectogenic potential of adenosine has been known since the early 1990’s when intracavernous injection of adenosine was found to induce a dose-dependent increase in ICP in dogs.43 However, excessive adenosine concentrations were implicated as a potential cause of priapic-like activity when adenosine deaminase deficient mice displayed spontaneous erections.44 Adenosine can be particularly harmful for SCD patients.45 Adenosine is known to promote oxygen release from hemoglobin, which in the SCD patient promotes polymerization leading to sickled erythrocytes and ensuing hemolysis.46 Hemolysis and resultant congestion of blood within the corpora cavernosa has been proposed as a potential cause of SCD associated priapism,47 while it has recently been demonstrated that SCD bone marrow transplant to control WT mice causes priapic-like activity.48 This model of subacute hemolysis demonstrated diminished penile NOS activity, underscoring the role of diminished NO signaling in this disease state.48 However, the priapism phenotype in the NOS1/3−/− model of NOS-deficiency utilized in the present study is not the result of hemolysis or sickled erythrocytes. The results of the present study suggest PKA activation secondary to NO-deficiency, which may be of particular relevance to the SCD associated priapism; whereby there is potential for NO-deficiency to increase adenosine concentrations, adenosine to promote polymerization and hemolysis, which could drive further depletion of NO availability.
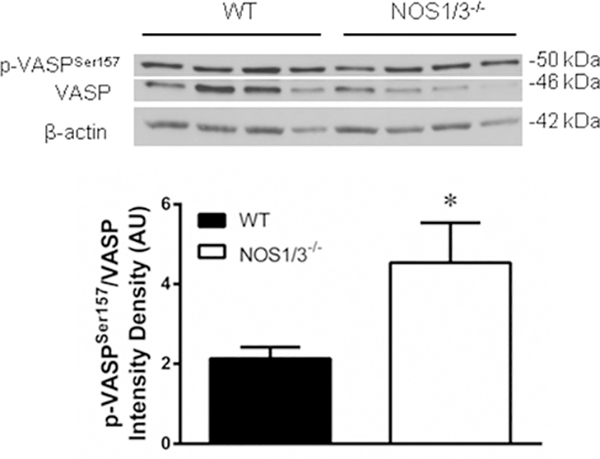
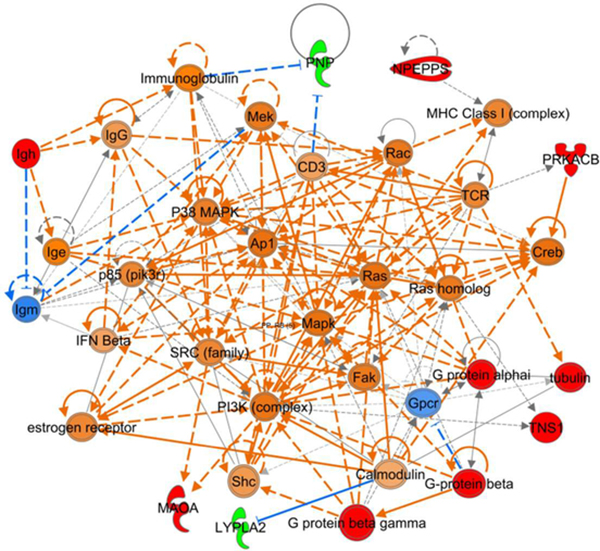
In addition to the increased spectral counts of the catalytic subunit of PKA (Table S-1), NOS1/3−/− penile tissue demonstrated an increase in VASP phosphorylation at Ser157 relative to unphosphorylated VASP (Fig. 3; p = 0.038). Phosphorylation at Ser157 induces an electrophoretic mobility shift of VASP from 46 to 50 kDa, allowing unphosphorylated VASP to be detected with this antibody at the 46 kDa band.49 Ser157 of VASP is a primary target for PKA, thus the p-VASPSer157-to-unphosphorylated-VASP ratio is a well recognized index of PKA activity. IPA further implicates PKA involvement in three interactive protein networks dysregulated in NOS1/3−/− penile tissue (Figs. 4, S-4, S-5). The network depicted in Fig. 4 is suggestive of aberrant cell signaling, with projected increased activities of several major cell signaling kinases and increased G protein contents. Increased content of PKA catalytic subunit beta (PRKACB) was projected to activate cyclic AMP-responsive element-binding protein (Creb), which influences several cell signaling pathways. This network also consisted of projections for other major signaling kinases, predicting increased activities of phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK), P38 MAPK, and MAPK kinase (Mek). To our knowledge, none of these kinases have previously been implicated in priapism pathophysiology, but are potential targets for future investigation. Increased spectral counts for monoamine oxidase A (MAOA) were observed in NOS1/3−/− penile tissue and implicated in the network depicted in Fig 4, which may stem from the increased PKA and PI3K activity. MAOA is a flavoprotein localized to the outer mitochondrial membrane that is an enzymatic source of reactive oxygen species in the mitochondrial matrix and cytosol.50 Increased oxidative stress has been associated with priapism by several studies.8,48,51–53 The role of MAOA in the penis has been previously unexplored; thus, MAOA may represent a previously unidentified contributor to oxidative stress in priapism. PKA activation is implicated by IPA in a network of cell regulatory factors (Fig. S-4), suggesting an alteration of protein homeostasis, removal of damaged proteins, DNA damage repair, apoptosis, and cell cycle progression in NOS-deficient cavernous tissue. Further, this network predicts an activation of vascular endothelial growth factor (Vegf), which is a known regulator of erection physiology.54 Vegf has not previously been implicated in priapism pathophysiology, which remains a potential area of future investigation.
Fig. 3. Augmented protein kinase A (Pka)-mediated cell signaling in the NOS1/3−/− penis.
Western blot for the Pka downstream target VASP. Phosphorylation of VASP at Serine 157, the primary Pka directed site, is detected at the 50 kDa band. Unphosphorylated VASP is detected at the 46 kDa band. Beta-actin served as the loading control. Densitometry of VASP phosphorylated at Ser157 normalized to unphosphorylated VASP is quantified for n = 8 per group. * P < 0.05 vs. WT.
Fig. 4. Dysregulated cell signaling in the NOS1/3−/− penis.
IPA for up- and down-regulated proteins in NOS1/3−/− penis yielded network interactions involving cell signaling kinases and G proteins. Protein expression determined by spectral counting is denoted by red (increased) and green (decreased) filled symbols. IPA determined predictions are denoted by orange (increased) and blue (decreased) filled symbols and connecting arrows. Solid arrows indicate direct action, hatched arrows indicate indirect action.
One of the current leading hypotheses behind the pathogenesis of priapism in SCD is the activation of opiorphins.3 Opiorphins are a family of peptides with potent vasodilatory effects in corporal smooth muscle.55 The vasodilatory effects of opiorphins may be in part mediated through their activation of adenosine signaling.56 Opiorphins have been shown to induce a G-protein coupled receptor-mediated activation of the A2B adenosine receptor in corporal smooth muscle cells exposed to hypoxic conditions.56 Further, genes encoding opiorphin homologues were upregulated in SCD mouse corporal smooth muscle. Neprilysin (MME), the mouse equivalent of opiorphin, was found to be drastically increased in NOS1/3−/− penile tissue in the present study (Table S-1, Fig. S-4). Of interest, transfection of cultured cavernous smooth muscle cells with neprilysin siRNA has been shown to knock down gene expression of multiple GPCRs.57 These findings implicate NOS-deficiency as a factor in opiorphin- and adenosine-mediated priapism in SCD. A separate hypothesis is that shear stress induces ATP release from endothelial cells, which is converted to adenosine.44,58 The adenosine then signals through the A2B adenosine receptor to activate PI3K and Akt to phosphorylate eNOS to produce NO that drives sustained erections.44,58 Given that eNOS is knocked out in our mouse model, this mechanism of adenosine action cannot be a factor in the priapic phenotype, implicating a more prominent role for PKA activation under NOS-deficient conditions.
G-Protein Mediated Smooth Muscle Tone Regulation
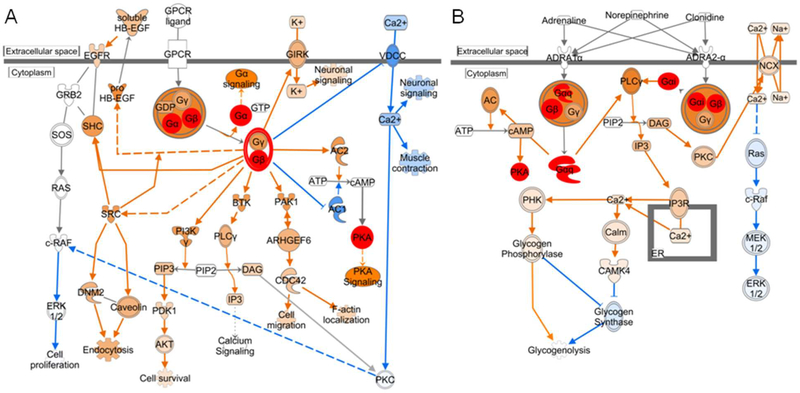
Enhanced G-protein coupled receptor (GPCR) signaling in NOS1/3−/− tissue may play an important role in vascular tone regulation. IPA predicted an augmentation in Gβγ-signaling, which may provide an upstream signal for PKA activation (Fig 5A). Increases in intracellular [Ca2+] are important stimuli for contraction of vascular smooth muscle. The predicted decrement in cellular Ca2+ entry through the voltage dependent calcium channel (VDCC) mediated by Gβγ-signaling could spur vasodilation in NOS-deficient cavernous tissue. Acute NOS-deficiency has been found to augment the blood pressure lowering effects of VDCC inhibition59 and Rho-kinase inhibition,60 suggesting a heightened sensitivity to changes in Ca2+ levels in the NOS-deficient state, potentially leading to exacerbated vasodilation in NOS-deficient tissue. However, α-adrenergic mediated G-protein activation of PLCγ and IP3 may augment Ca2+ release from the endoplasmic and sarcoplasmic reticulum (Fig. 5B), potentially providing a stimulus for vasoconstriction. This constrictor activity may act to counter the decreased Ca2+ influx from VDCC in an attempt to restore vascular homeostasis, and this push-pull mechanism may be in part responsible for the undulating nature of the post-CNS ICP in NOS1/3−/− mice (Fig 1B).
Fig. 5. Augmented G-protein signaling in the NOS1/3−/− penis.
Canonical pathways for (A) Gβγ signaling and (B) α-adrenergic signaling with activation predicted by the molecular activity predictor tool within IPA. Increased protein expression determined by spectral counting is denoted by red filled symbols. IPA determined predictions are denoted by orange (increased) and blue (decreased) filled symbols and connecting arrows. Solid arrows indicate direct action, hatched arrows indicate indirect action.
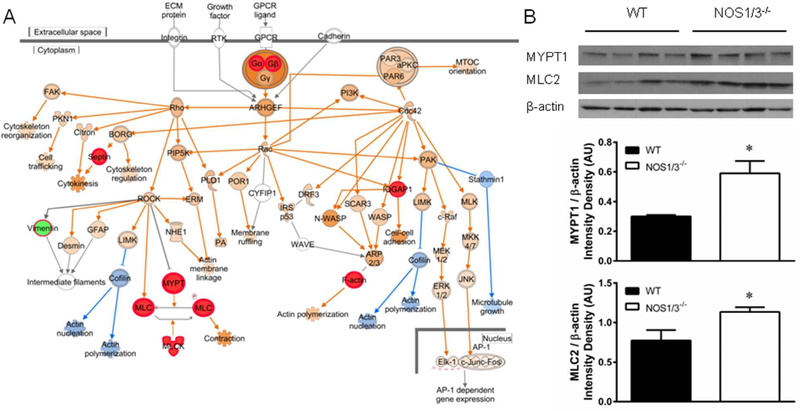
These counter regulatory forces could further be mediated by G-protein mediated induction of the Rho-family GTPases (Fig. 6A). Increased spectral counts were observed for MYPT1, MLC-3, and MLCK, and indeed we demonstrated increased MYPT1 (p < 0.001) and the smooth muscle isoform of MLC2 (p = 0.025) in NOS1/3−/− penile tissue by Western blot (Fig. 6B). These increases in the contractile machinery may implicate an increased regulatory requirement in NO-deficient tissue. IPA also predicts a decrement in actin polymerization in NO-deficient penile tissue. As actin polymerization increases vascular tone through vasoconstriction independent of MLC phosphorylation,61 decreased actin polymerization in NOS1/3−/− represents a possible contributor to NOS-deficient priapism. Furthermore, PKA-mediated VASP phosphorylation has been shown to inhibit actin polymerization,62 providing another mechanism by which actin polymerization may be inhibited in addition to the suppression of cofilin through the Rho-family GTPases as predicted by IPA.
Fig. 6. G-protein mediated effects on smooth muscle tone regulation.
(A) Canonical pathway for signaling by Rho family GTPases with activation predicted by the molecular activity predictor tool within IPA. Protein expression determined by spectral counting is denoted by red (increased) and green (decreased) filled symbols. IPA determined predictions are denoted by orange (increased) and blue (decreased) filled symbols and connecting arrows. (B) Western blot for the smooth muscle tone regulatory proteins MYPT1 and the smooth muscle isoform of MLC2. Beta-actin served as the loading control. Band densitometry was quantified and normalized to β-actin for n = 8 per group. * P < 0.05 vs. WT.
Smooth Muscle Structural Remodeling
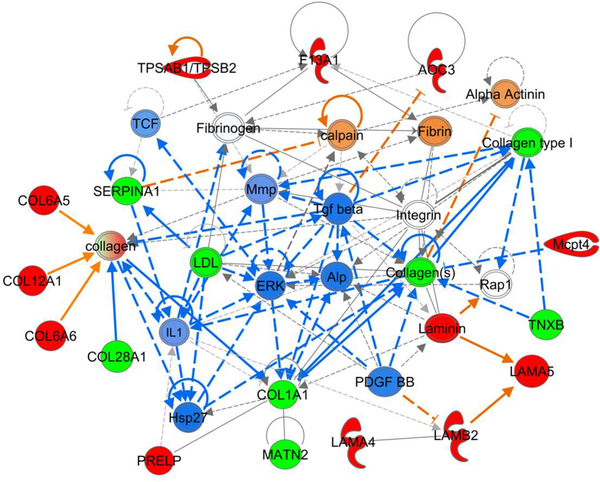
Corporal fibrosis is a prominent feature of erectile dysfunction;63 however, increased collagen deposition has been observed in penile tissue of priapic mice as well.36,64 Structural remodeling of erectile tissue is a complex process that is regulated by several pathways. For example, the atherosclerotic process drives collagen deposition in the intima of arteries and arterioles.65 Atherosclerosis is regulated largely by lipoproteins, of which apolipoprotein A-II (APOA2), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) are all decreased in penile tissue of NOS1/3−/− mice (Table S-1). Furthermore, transforming growth factor-β (TGF-β) is predicted to be decreased in NOS1/3−/− erectile tissue (Fig. 7), which is a fibrotic factor that induces collagen Iα1 (COL1A1) and collagen Vα1 (COL5A1),66 both of which were determined to be decreased in NOS1/3−/− penile tissue by mass spectrometry. COL1A1 and COL5A1 are both fibril forming collagens that intercalate together to provide tensile strength and stiffness to tissue.67 Transforming growth factor beta-1-induced transcript 1 protein (TGFB1I1) was increased 2-fold in NOS1/3−/− tissue (Table S-1, Fig S-5). TGFB1I1, also known as hydrogen peroxide-inducible clone 5, is a focal adhesion protein that is induced under oxidative stress, while the activity and subcellular localization of TGFB1I1 is regulated by focal adhesion kinase (FAK; Fig 4),68 which itself has been shown to mediate pro-fibrotic signaling.69 Membrane primary amine oxidase (AOC3) is a cell adhesion protein with monoamine oxidase activity that is increased in NOS1/3−/− tissue and is involved in this protein network (Fig. 7), which may be a source of hydrogen peroxide production and ensuing oxidative stress.70 P38 MAPK is also predicted to be increased (Fig. 4), which has previously been shown to be activated by the hypertensive condition of mechanical stretch, which promotes fibrosis in a redox- and FAK-independent manner.71 Similarly, tensile strain is known to induce collagen XIIα1 (COL12A1),72 which was increased in NOS1/3−/− tissue. COL12A1 binds decorin, fibromodulin, and cartilage oligomeric matrix protein to form flexible bridges between neighboring collagen fibrils which may absorb stress upon pressure loading.73 Collagen VIα6 (COL6A6) was the most upregulated protein in NOS1/3−/− vs. WT in the mass spectrometry dataset (Table S-1). COL6A6 and COL6A5 have been found to be drastically upregulated in fibrotic skeletal muscle of Duchenne’s Muscular Dystrophy patients, implicating both in extracellular matrix fibrotic remodeling under tensile stress.74 Collagen XXVIIIα1 (COL28A1) was found to be decreased in NOS1/3−/− penile tissue, which is known to be heavily expressed in the basal lamina of Schwann cells in the peripheral nervous system.75 COL5A1 is also required for Schwann cell myelination,76 which combined with the decrement in COL28A1 implicates a decrement in innervation and/or myelination of the NO-deficient penis. Taken together, this protein network (Fig. 7) implicates a shift in structure from collagen fibers that provide tensile strength to those that absorb high amounts of stress/pressure along with a potential nervous system decompensation in the NOS-deficient penis.
Fig. 7. Regulation of structural remodeling factors.
IPA for up- and down-regulated proteins in NOS1/3−/− penis yielded network interactions for several regulatory proteins of fibrosis and structural remodeling. Protein expression determined by spectral counting is denoted by red (increased) and green (decreased) filled symbols. IPA determined predictions are denoted by orange (increased) and blue (decreased) filled symbols and connecting arrows. Solid arrows indicate direct action, hatched arrows indicate indirect action.
Experimental Considerations
The results of the present study provide ample clues into the response of the penile proteome to conditions of chronic low NO availability; however, these alterations may be the result of tissue damage resultant from acute priapic episodes or changes associated with chronic priapism. Furthermore, we assessed relative changes in protein abundance, which does not account for potential changes in post-translational modifications which may influence priapic activity. Accordingly, predictions made by IPA for altered protein networks and canonical signaling pathways were based on statistically significant differences in spectral counts between the two groups. It should be noted that the cumulative knowledge base of IPA is continuously expanding, and thus predictions made by the MAP tool within IPA are likely to change over time. The inferences herein should thus be viewed with caution.
CONCLUSIONS
Our results expand the pool of candidate proteins that may play a role in NO-deficient priapism. Because NO is a major physiologic mediator of penile erection, the mechanisms by which pathologic NO deficiency result in priapism have been perplexing. These proteins that are differentially expressed may play a direct role in the pathogenesis of priapism, or may be altered merely as a result of NOS-deficiency, low NO availability, the high-pressure conditions of priapism, other physiologic changes associated with priapism, or a combination of these factors. These analyses of the molecular changes provide understanding of the compensatory mechanisms through which the penis responds to NO deficiency to alter molecular signaling, physiology, and organ architecture. Future studies involving pharmacological or genetic manipulation of these proteins or pathways will be required to definitively elucidate their roles in NOS-deficient priapism. Nevertheless, several targets are identified for such future investigations. Furthermore, these data should be considered in future studies that attempt to alleviate priapism symptoms through restoration of NO bioavailability.
Supplementary Material
Supplementary Figure S-1: Additional measures of erectile function and priapic activity
Supplementary Figure S-2: Protein Kinase A signaling pathway
Supplementary Figure S-3: Adenosine nucleotide degradation pathway
Supplementary Figure S-4: Network of cell regulatory factors
Supplementary Figure S-5: Network of smooth muscle regulatory factors
Supplementary Table S-1: Spectral counts for wild type and NOS1/3−/− penile tissue
ACKKNOWLEDGEMENTS
This research was supported in part by a grant from the National Kidney Foundation of Maryland (J.D.L), and National Institutes of Health grants R01DK067223 (A.L.B.) and R01DK093917 (A.L.B.). J.D.L. is supported by Ruth L. Kirschstein National Research Service Award F32DK100082 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Koen Raedschelders for providing helpful comments on the manuscript draft.
REFERENCES
- (1).Montague DK; Jarow J; Broderick GA; Dmochowski RR; Heaton JPW; Lue TF; Nehra A; Sharlip ID; Members of the Erectile Dysfunction Guideline Update Panel; Americal Urological Association. American Urological Association guideline on the management of priapism. J. Urol 2003, 170 (4 Pt 1), 1318–1324. [DOI] [PubMed] [Google Scholar]
- (2).Addis G; Spector R; Shaw E; Musumadi L; Dhanda C The physical, social and psychological impact of priapism on adult males with sickle cell disorder. Chronic Illn. 2007, 3 (2), 145–154. [DOI] [PubMed] [Google Scholar]
- (3).Bivalacqua TJ; Musicki B; Kutlu O; Burnett AL New insights into the pathophysiology of sickle cell disease-associated priapism. J. Sex. Med 2012, 9 (1), 79–87. [DOI] [PubMed] [Google Scholar]
- (4).Khoriaty N; Schick E Penile gangrene: an unusual complication of priapism. How to avoid it? Urology 1980, 16 (3), 280–283. [DOI] [PubMed] [Google Scholar]
- (5).Salonia A; Eardley I; Giuliano F; Hatzichristou D; Moncada I; Vardi Y; Wespes E; Hatzimouratidis K; European Association of Urology. European Association of Urology guidelines on priapism. Eur. Urol 2014, 65 (2), 480–489. [DOI] [PubMed] [Google Scholar]
- (6).Backenroth R; Landau EH; Goren M; Raas-Rothschild A Fabry disease and G6PD in three family members with priapism: is the nitric oxide pathway to blame? J. Sex. Med 2010, 7 (4 Pt 1), 1588–1591. [DOI] [PubMed] [Google Scholar]
- (7).Burnett AL; Bivalacqua TJ Glucose-6-phosphate dehydrogenase deficiency: an etiology for idiopathic priapism? J. Sex. Med 2008, 5 (1), 237–240. [DOI] [PubMed] [Google Scholar]
- (8).Lagoda G; Sezen SF; Cabrini MR; Musicki B; Burnett AL Molecular analysis of erection regulatory factors in sickle cell disease associated priapism in the human penis. J. Urol 2013, 189 (2), 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Mallat NS; Wehbe D; Haddad A; Cappellini MD; Marcon A; Koussa S; Abboud MR; Radwan A; Taher AT Priapism, an emerging complication in β-thalassemia intermedia patients. Hemoglobin 2014, 38 (5), 351–354. [DOI] [PubMed] [Google Scholar]
- (10).Tzortzis V; Mitrakas L; Gravas S; Mamoulakis C; Meissner A; Kyriakou D; Melekos MD Oral phosphodiesterase type 5 inhibitors alleviate recurrent priapism complicating thalassemia intermedia: a case report. J. Sex. Med 2009, 6 (7), 2068–2071. [DOI] [PubMed] [Google Scholar]
- (11).Adeyoju AB; Olujohungbe ABK; Morris J; Yardumian A; Bareford D; Akenova A; Akinyanju O; Cinkotai K; O’Reilly PH Priapism in sickle-cell disease; incidence, risk factors and complications - an international multicentre study. BJU Int. 2002, 90 (9), 898–902. [DOI] [PubMed] [Google Scholar]
- (12).Emond AM; Holman R; Hayes RJ; Serjeant GR Priapism and impotence in homozygous sickle cell disease. Arch. Intern. Med 1980, 140 (11), 1434–1437. [PubMed] [Google Scholar]
- (13).Broderick GA; Kadioglu A; Bivalacqua TJ; Ghanem H; Nehra A; Shamloul R Priapism: pathogenesis, epidemiology, and management. J. Sex. Med 2010, 7 (1 Pt 2), 476–500. [DOI] [PubMed] [Google Scholar]
- (14).Beutler E G6PD deficiency. Blood 1994, 84 (11), 3613–3636. [PubMed] [Google Scholar]
- (15).Hundsdoerfer P; Vetter B; Kulozik AE Chronic haemolytic anaemia and glucose-6 phosphate dehydrogenase deficiency. Case report and review of the literature. Acta Haematol. 2002, 108 (2), 102–105. [DOI] [PubMed] [Google Scholar]
- (16).Ridyard DG; Phillips EA; Vincent W; Munarriz R Use of High-Dose Phenylephrine in the Treatment of Ischemic Priapism: Five-Year Experience at a Single Institution. J. Sex. Med 2016, 13 (11), 1704–1707. [DOI] [PubMed] [Google Scholar]
- (17).Sui W; Onyeji IC; James MB; Stahl PJ; RoyChoudhury A; Anderson CB Risk Factors for Priapism Readmission. J. Sex. Med 2016, 13 (10), 1555–1561. [DOI] [PubMed] [Google Scholar]
- (18).Burnett AL; Lowenstein CJ; Bredt DS; Chang TS; Snyder SH Nitric oxide: a physiologic mediator of penile erection. Science 1992, 257 (5068), 401–403. [DOI] [PubMed] [Google Scholar]
- (19).Burnett AL; Musicki B The nitric oxide signaling pathway in the penis. Curr. Pharm. Des 2005, 11 (31), 3987–3994. [DOI] [PubMed] [Google Scholar]
- (20).Ignarro LJ; Buga GM; Wood KS; Byrns RE; Chaudhuri G Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U. S. A 1987, 84 (24), 9265–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hess DT; Stamler JS Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem 2012, 287 (7), 4411–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bogdan C Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001, 11 (2), 66–75. [DOI] [PubMed] [Google Scholar]
- (23).Michalski A; Damoc E; Lange O; Denisov E; Nolting D; Müller M; Viner R; Schwartz J; Remes P; Belford M; et al. Ultra high resolution linear ion trap Orbitrap mass spectrometer (Orbitrap Elite) facilitates top down LC MS/MS and versatile peptide fragmentation modes. Mol. Cell. Proteomics 2012, 11 (3), O111.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Krämer A; Green J; Pollard J; Tugendreich S Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30 (4), 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Barouch LA; Harrison RW; Skaf MW; Rosas GO; Cappola TP; Kobeissi ZA; Hobai IA; Lemmon CA; Burnett AL; O’Rourke B; et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 2002, 416 (6878), 337–339. [DOI] [PubMed] [Google Scholar]
- (26).Champion HC; Bivalacqua TJ; Takimoto E; Kass DA; Burnett AL Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc. Natl. Acad. Sci. U. S. A 2005, 102 (5), 1661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Bivalacqua TJ; Ross AE; Strong TD; Gebska MA; Musicki B; Champion HC; Burnett AL Attenuated RhoA/Rho-kinase Signaling in Penis of Transgenic Sickle Cell Mice. Urology 2010, 76 (2), 510.e7–510.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kaushik G; Spenlehauer A; Sessions AO; Trujillo AS; Fuhrmann A; Fu Z; Venkatraman V; Pohl D; Tuler J; Wang M; et al. Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci. Transl. Med 2015, 7 (292), 292ra99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Chambers MC; Maclean B; Burke R; Amodei D; Ruderman DL; Neumann S; Gatto L; Fischer B; Pratt B; Egertson J; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol 2012, 30 (10), 918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Apweiler R; Bairoch A; Wu CH; Barker WC; Boeckmann B; Ferro S; Gasteiger E; Huang H; Lopez R; Magrane M; et al. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004, 32 (Database issue), D115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Elias JE; Gygi SP Target-decoy search strategy for increased confidence in largescale protein identifications by mass spectrometry. Nat. Methods 2007, 4 (3), 207–214. [DOI] [PubMed] [Google Scholar]
- (32).Searle BC Scaffold: a bioinformatic tool for validating MS/MS-based proteomic studies. Proteomics 2010, 10 (6), 1265–1269. [DOI] [PubMed] [Google Scholar]
- (33).Old WM; Meyer-Arendt K; Aveline-Wolf L; Pierce KG; Mendoza A; Sevinsky JR; Resing KA; Ahn NG Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol. Cell. Proteomics 2005, 4 (10), 1487–1502. [DOI] [PubMed] [Google Scholar]
- (34).Zybailov B; Coleman MK; Florens L; Washburn MP Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal. Chem 2005, 77 (19), 6218–6224. [DOI] [PubMed] [Google Scholar]
- (35).Vizcaíno JA; Côté RG; Csordas A; Dianes JA; Fabregat A; Foster JM; Griss J; Alpi E; Birim M; Contell J; et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013, 41 (Database issue), D1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bivalacqua TJ; Musicki B; Hsu LL; Gladwin MT; Burnett AL; Champion HC Establishment of a transgenic sickle-cell mouse model to study the pathophysiology of priapism. J. Sex. Med 2009, 6 (9), 2494–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ning C; Wen J; Zhang Y; Dai Y; Wang W; Zhang W; Qi L; Grenz A; Eltzschig HK; Blackburn MR; et al. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1α mediated reduction of PDE5 gene expression. FASEB J. 2014, 28 (6), 2725–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Burnett AL; Anele UA; Trueheart IN; Strouse JJ; Casella JF Randomized controlled trial of sildenafil for preventing recurrent ischemic priapism in sickle cell disease. Am. J. Med 2014, 127 (7), 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Anele UA; Burnett AL Nitrergic Mechanisms for Management of Recurrent Priapism. Sex. Med. Rev 2015, 3 (3), 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Anele UA; Le BV; Resar LMS; Burnett AL How I treat priapism. Blood 2015, 125 (23), 3551–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Johnson DA; Akamine P; Radzio-Andzelm E; Madhusudan; Taylor SS Dynamics of cAMP-Dependent Protein Kinase. Chem. Rev 2001, 101 (8), 2243–2270. [DOI] [PubMed] [Google Scholar]
- (42).Decking UK; Schlieper G; Kroll K; Schrader J Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ. Res 1997, 81 (2), 154–164. [DOI] [PubMed] [Google Scholar]
- (43).Takahashi Y; Ishii N; Lue TF; Tanagho EA Effects of adenosine on canine penile erection. J. Urol 1992, 148 (4), 1323–1325. [DOI] [PubMed] [Google Scholar]
- (44).Mi T; Abbasi S; Zhang H; Uray K; Chunn JL; Xia LW; Molina JG; Weisbrodt NW; Kellems RE; Blackburn MR; et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J. Clin. Invest 2008, 118 (4), 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Zhang Y; Dai Y; Wen J; Zhang W; Grenz A; Sun H; Tao L; Lu G; Alexander DC; Milburn M V; et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat. Med. 2011, 17 (1), 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Zhang Y; Xia Y Adenosine signaling in normal and sickle erythrocytes and beyond. Microbes Infect. 2012, 14 (10), 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Burnett AL Priapism pathophysiology: clues to prevention. Int. J. Impot. Res 2003, 15 Suppl 5 (S5), S80–5. [DOI] [PubMed] [Google Scholar]
- (48).Sopko NA; Matsui H; Hannan JL; Berkowitz D; Champion HC; Hsu LL; Musicki B; Burnett AL; Bivalacqua TJ Subacute Hemolysis in Sickle Cell Mice Causes Priapism Secondary to NO Imbalance and PDE5 Dysregulation. J. Sex. Med 2015, 12 (9), 1878–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Smolenski A; Bachmann C; Reinhard K; Hönig-Liedl P; Jarchau T; Hoschuetzky H; Walter U Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J. Biol. Chem 1998, 273 (32), 20029–20035. [DOI] [PubMed] [Google Scholar]
- (50).Cadenas E; Davies KJ Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med 2000, 29 (3–4), 222–230. [DOI] [PubMed] [Google Scholar]
- (51).Kanika ND; Melman A; Davies KP Experimental priapism is associated with increased oxidative stress and activation of protein degradation pathways in corporal tissue. Int. J. Impot. Res 2010, 22 (6), 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lagoda G; Sezen SF; Hurt KJ; Cabrini MR; Mohanty DK; Burnett AL Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J. 2014, 28 (1), 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Musicki B; Bivalacqua TJ; Champion HC; Burnett AL Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J. Sex. Med 2014, 11 (2), 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Lee Y-C; Huang S-P; Tsai C-C; Cheng K-H; Juan Y-S; Wu W-J; Bao B-Y; Huang C-N; Wang C-J; Liu C-C Associations of VEGF Gene Polymorphisms With Erectile Dysfunction and Related Risk Factors. J. Sex. Med 2017, 14 (4), 510–517. [DOI] [PubMed] [Google Scholar]
- (55).Davies KP The role of opiorphins (endogenous neutral endopeptidase inhibitors) in urogenital smooth muscle biology. J. Sex. Med 2009, 6 Suppl 3, 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Fu S; Tar MT; Melman A; Davies KP Opiorphin is a master regulator of the hypoxic response in corporal smooth muscle cells. FASEB J. 2014, 28 (8), 3633–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Tong Y; Tiplitsky SI; Tar M; Melman A; Davies KP Transcription of G-Protein Coupled Receptors in Corporeal Smooth Muscle is Regulated by the Endogenous Neutral Endopeptidase Inhibitor Sialorphin. J. Urol 2008, 180 (2), 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Dai Y; Zhang Y; Phatarpekar P; Mi T; Zhang H; Blackburn MR; Xia Y Adenosine signaling, priapism and novel therapies. J. Sex. Med 2009, 6 Suppl 3, 292–301. [DOI] [PubMed] [Google Scholar]
- (59).Zicha J; Behuliak M; Pintérová M; Bencze M; Kuneš J; Vaněčková I The interaction of calcium entry and calcium sensitization in the control of vascular tone and blood pressure of normotensive and hypertensive rats. Physiol. Res 2014, 63 Suppl 1, S19–27. [DOI] [PubMed] [Google Scholar]
- (60).Brunová A; Bencze M; Behuliak M; Zicha J Acute and chronic role of nitric oxide, renin-angiotensin system and sympathetic nervous system in the modulation of calcium sensitization in Wistar rats. Physiol. Res 2015, 64 (4), 447–457. [DOI] [PubMed] [Google Scholar]
- (61).Gunst SJ; Zhang W Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol 2008, 295 (3), C576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Harbeck B; Hüttelmaier S; Schlüter K; Jockusch BM; Illenberger S Phosphorylation of the Vasodilator-stimulated Phosphoprotein Regulates Its Interaction with Actin. J. Biol. Chem 2000, 275 (40), 30817–30825. [DOI] [PubMed] [Google Scholar]
- (63).Song SH; Park K; Kim SW; Paick J-S; Cho MC Involvement of Rho-Kinase/LIM Kinase/Cofilin Signaling Pathway in Corporal Fibrosis after Cavernous Nerve Injury in Male Rats. J. Sex. Med 2015, 12 (7), 1522–1532. [DOI] [PubMed] [Google Scholar]
- (64).Wen J; Jiang X; Dai Y; Zhang Y; Tang Y; Sun H; Mi T; Phatarpekar PV; Kellems RE; Blackburn MR; et al. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J. 2010, 24 (3), 740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Katsuda S; Okada Y; Minamoto T; Oda Y; Matsui Y; Nakanishi I Collagens in human atherosclerosis. Immunohistochemical analysis using collagen type-specific antibodies. Arterioscler. Thromb. a J. Vasc. Biol 1992, 12 (4), 494–502. [DOI] [PubMed] [Google Scholar]
- (66).Lan HY Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci 2011, 7 (7), 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Gelse K; Pöschl E; Aigner T Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev 2003, 55 (12), 1531–1546. [DOI] [PubMed] [Google Scholar]
- (68).Shibanuma M; Mori K; Kim-Kaneyama J-R; Nose K Involvement of FAK and PTP-PEST in the regulation of redox-sensitive nuclear-cytoplasmic shuttling of a LIM protein, Hic-5. Antioxid. Redox Signal 2005, 7 (3–4), 335–347. [DOI] [PubMed] [Google Scholar]
- (69).Zhao X-K; Cheng Y; Liang Cheng M; Yu L; Mu M; Li H; Liu Y; Zhang B; Yao Y; Guo H; et al. Focal Adhesion Kinase Regulates Fibroblast Migration via Integrin beta-1 and Plays a Central Role in Fibrosis. Sci. Rep 2016, 6 (1), 19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Mathys KC; Ponnampalam SN; Padival S; Nagaraj RH Semicarbazide-sensitive amine oxidase in aortic smooth muscle cells mediates synthesis of a methylglyoxal-AGE: implications for vascular complications in diabetes. Biochem. Biophys. Res. Commun 2002, 297 (4), 863–869. [DOI] [PubMed] [Google Scholar]
- (71).Paravicini TM; Montezano AC; Yusuf H; Touyz RM Activation of vascular p38MAPK by mechanical stretch is independent of c-Src and NADPH oxidase: influence of hypertension and angiotensin II. J. Am. Soc. Hypertens 2012, 6 (3), 169–178. [DOI] [PubMed] [Google Scholar]
- (72).Trächslin J; Koch M; Chiquet M Rapid and reversible regulation of collagen XII expression by changes in tensile stress. Exp. Cell Res 1999, 247 (2), 320–328. [DOI] [PubMed] [Google Scholar]
- (73).Chiquet M; Birk DE; Bönnemann CG; Koch M Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils. Int. J. Biochem. Cell Biol 2014, 53, 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Sabatelli P; Gualandi F; Gara SK; Grumati P; Zamparelli A; Martoni E; Pellegrini C; Merlini L; Ferlini A; Bonaldo P; et al. Expression of collagen VI α5 and α6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol. 2012, 31 (3), 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Veit G; Kobbe B; Keene DR; Paulsson M; Koch M; Wagener R Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J. Biol. Chem 2006, 281 (6), 3494–3504. [DOI] [PubMed] [Google Scholar]
- (76).Hubert T; Grimal S; Carroll P; Fichard-Carroll A Collagens in the developing and diseased nervous system. Cell. Mol. Life Sci 2009, 66 (7), 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S-1: Additional measures of erectile function and priapic activity
Supplementary Figure S-2: Protein Kinase A signaling pathway
Supplementary Figure S-3: Adenosine nucleotide degradation pathway
Supplementary Figure S-4: Network of cell regulatory factors
Supplementary Figure S-5: Network of smooth muscle regulatory factors
Supplementary Table S-1: Spectral counts for wild type and NOS1/3−/− penile tissue