INTRODUCTION
Tumor ablation is broadly defined as the destruction of focal tumors by direct application of chemicals or energy.1 Tumor ablation therapies are delivered via needlelike applicators and can be broadly categorized into systems based on chemical (primarily ethanol and acetic acid) and thermal or nonthermal energy. The most widely applied thermal ablation modalities in the liver include radiofrequency (RF), microwave (MW), laser, cryoablation, and high-intensity focused ultrasonography (US). Irreversible electroporation (IRE) is generally classified as a nonthermal ablative modality, although cytotoxic temperatures can be achieved with IRE depending on the parameters used during treatment.2–4
Tumor ablation in the liver has evolved to become a well-accepted tool in the management of increasingly complex oncologic patients. Ablative therapies can be used alone, in conjunction with other ablative therapies, or in combination with other oncologic treatment strategies, such as surgery, neoadjuvant and adjuvant chemotherapy, external beam and stereotactic body radiotherapy (SBRT), and arterial liver-directed therapies, including bland embolization, chemoembolization, and/or radioembolization in the treatment of both primary and secondary hepatic malignancies.5–9
This image-rich, case-based article introduces some of the considerations that are important for physicians preparing to perform hepatic tumor ablation.
Ablation Modalities
The physical properties of each of the ablation devices are unique and these differences can affect the technical success of ablation procedures. Although an in-depth understanding of the biophysics of each device is not necessary, it is important to understand the properties of the devices available to your practice. Because operator experience has been correlated with success, it may be beneficial to concentrate experience in a few selected devices rather than dilute experience across multiple technologies.10,11
Ethanol
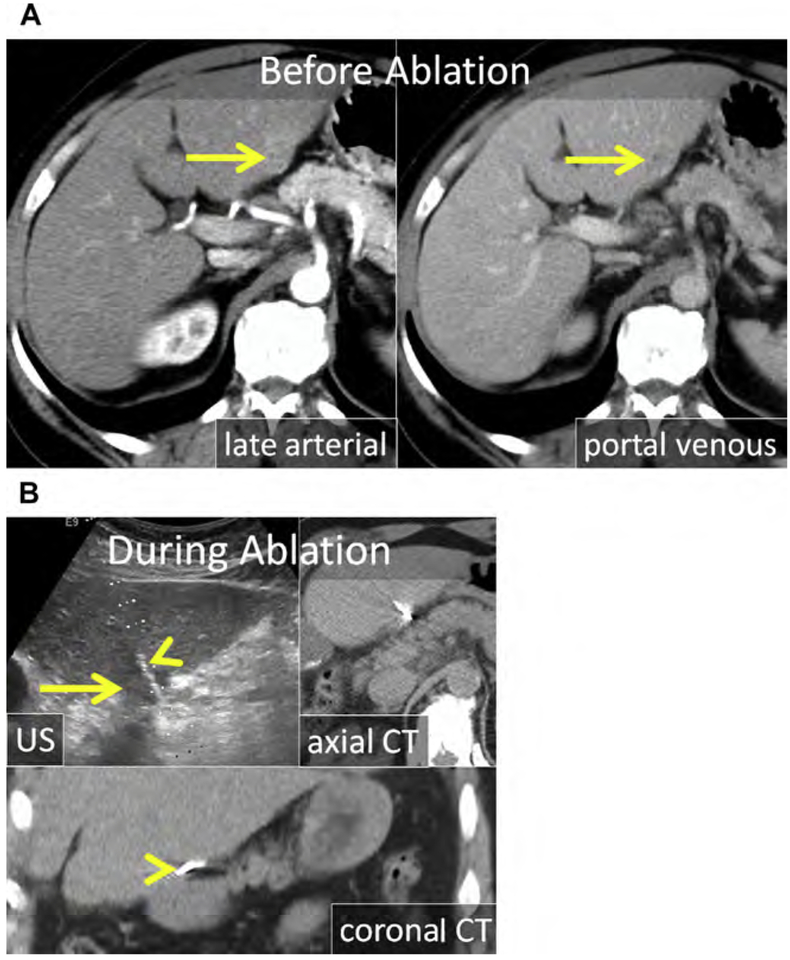
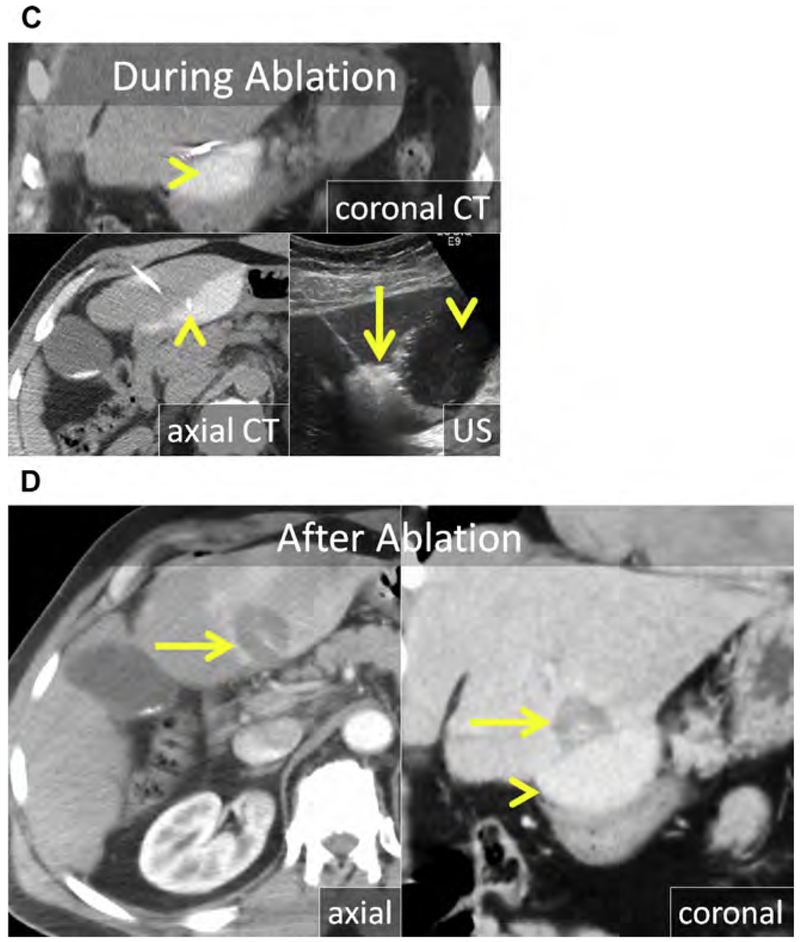
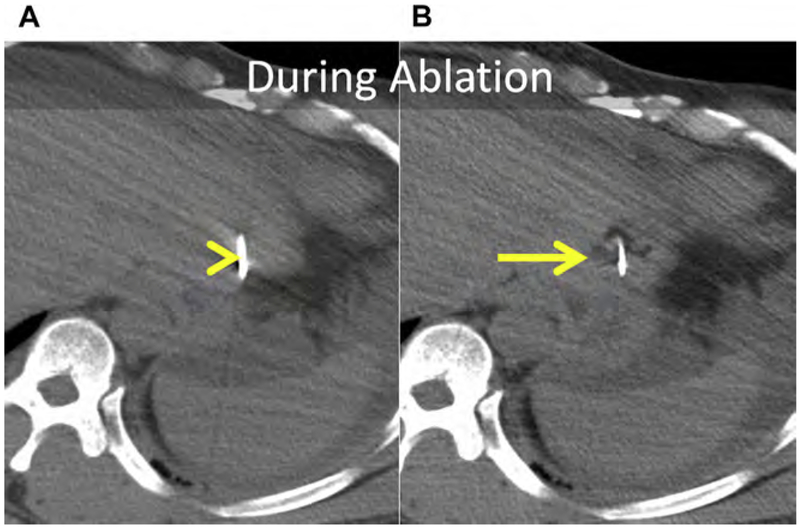
Chemical (nonenergy) ablation with ethanol was the seminal technique for percutaneous ablation. Ethanol induces coagulative necrosis via protein denaturation, cellular dehydration, and chemical occlusion of small tumor vessels. Ethanol injection is well suited for small hepatocellular carcinoma (HCC) because the firm cirrhotic liver surrounding the soft tumor limits diffusion of ethanol into the surrounding liver. Ethanol injection has little utility in the treatment of metastases in which the background liver is normal. Because of the high rate of local tumor progression and the need for repeated treatments, ethanol has largely been replaced with thermal ablation (RF and MW), for which rates of local tumor control and survival have improved.12–20 Chemical ablation has been used to augment or replace thermal ablation when treating tumors in areas of high perfusion-mediated tissue cooling or tumors in proximity to vulnerable, non-targeted structures, such as bile ducts (Fig. 1).21
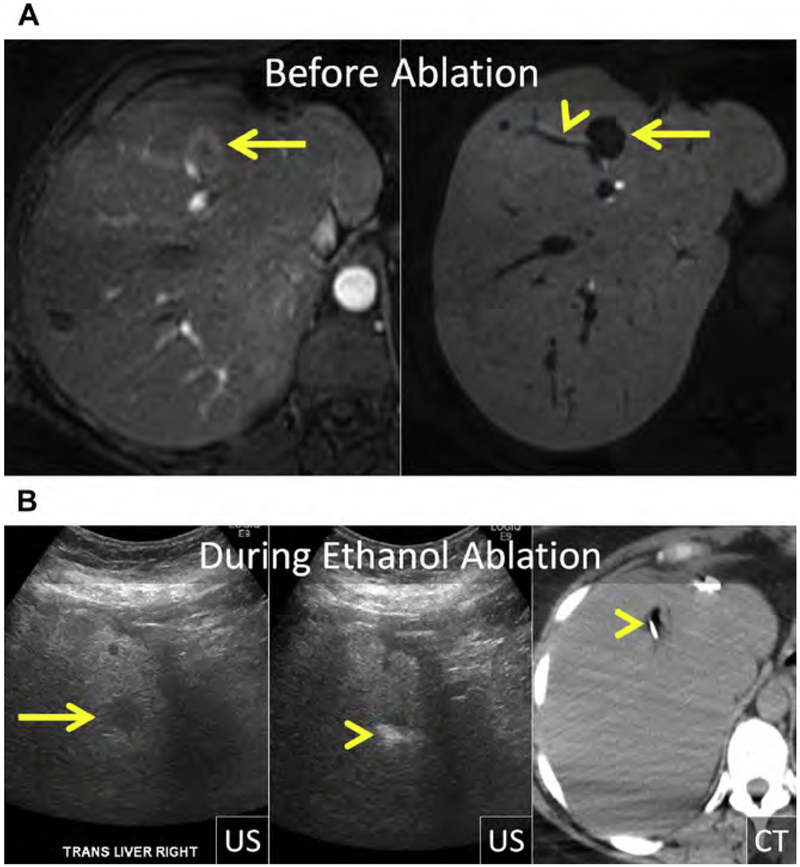
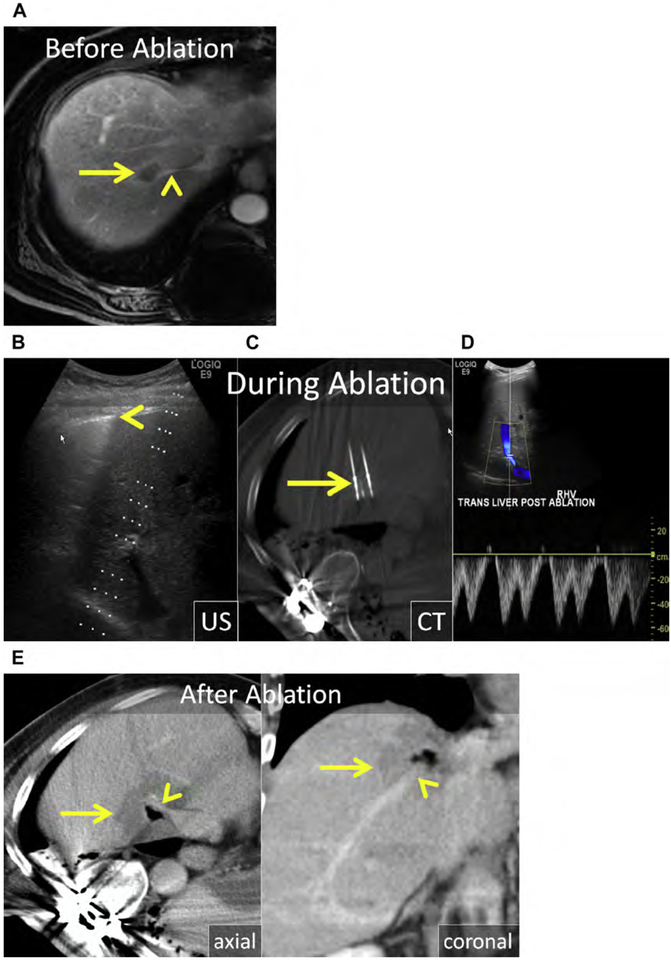
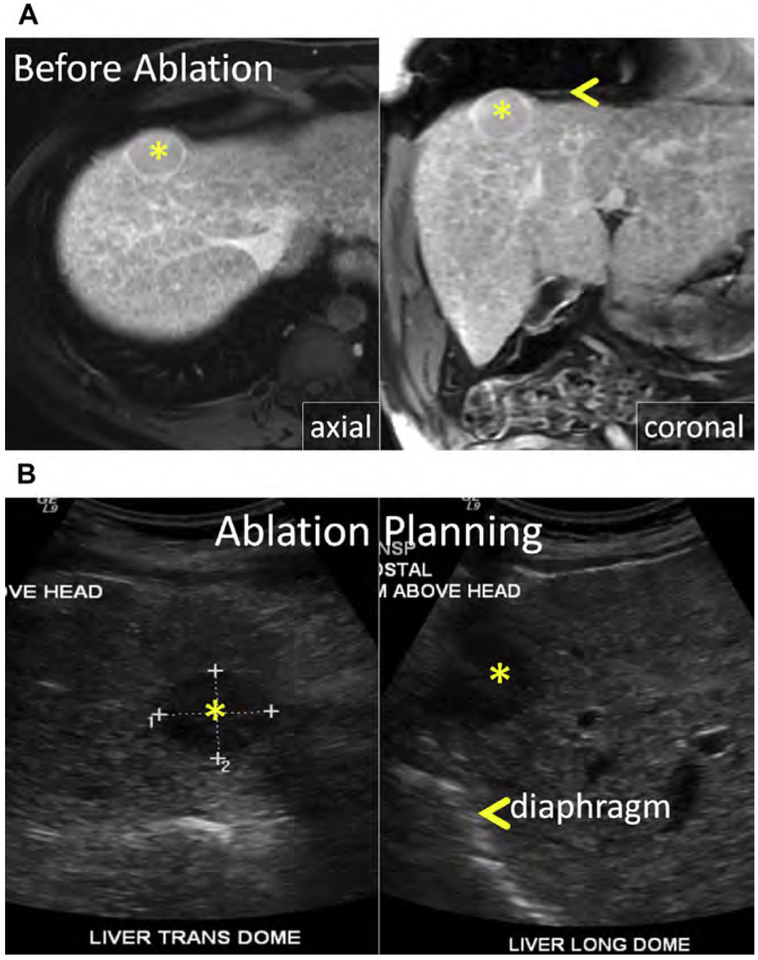
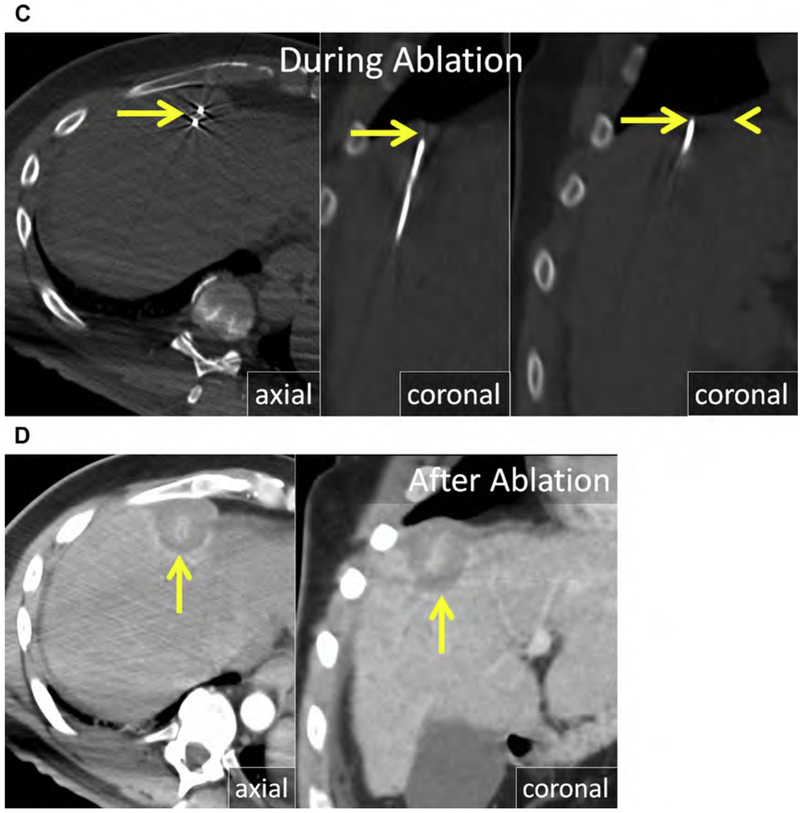
Fig. 1.
(A) Metastatic neuroendocrine tumor (NET) in the ventral right liver (arrow) abutting the bile duct draining Couinaud segment VIII (arrowhead). Given prior left hepatectomy and palliative intent of ablation, avoiding a bile duct stricture and further liver volume loss was a priority. (B) US and unenhanced computed tomography (CT) images during ethanol ablation. The posterior aspect of the tumor (arrow), in proximity to the bile duct, was targeted and 4 mL of 95% ethanol was instilled (arrowheads). (C) Unenhanced CT and US images after placement of the MW antenna (arrowhead) along the anterior aspect of the tumor. When the gas clouds (arrow) became confluent the procedure was terminated. (D) MR imaging obtained 1 month after combined ethanol and MW ablation. The ablation encompasses the index lesion (arrow) and there is no bile duct stricture (arrowhead). Because a 1-cm circumferential margin could not be safely obtained, there is a substantial risk for local tumor progress (LTP).
Radiofrequency
RF is a heat-based ablation method that creates zones of coagulative necrosis through the application of heat. With RF, an alternating current is conducted through an applicator (electrode) that acts as the cathode of a closed electrical circuit with grounding pads applied to the skin acting as the anode. Ions close to the electrode vibrate rapidly as they attempt to align with the alternating current, resulting in resistive tissue heating (direct heating) that is conducted into adjacent tissues (indirect heating) as a result of the high thermal gradient.22–24 The final ablation is the result of both direct heating caused by the applied energy, and indirect heating, the result of thermal diffusion into adjacent, cooler tissues. RF is a self-limited process that has limited success in treating large tumors and tumors in regions of high tissue perfusion because of poor conductive heating and limited ability to overcome perfusion-mediated tissue cooling.23–29 As a result, RF leads to an undesirably high rate of local tumor progress (LTP) in larger tumors (Figs. 2 and 3).30–32
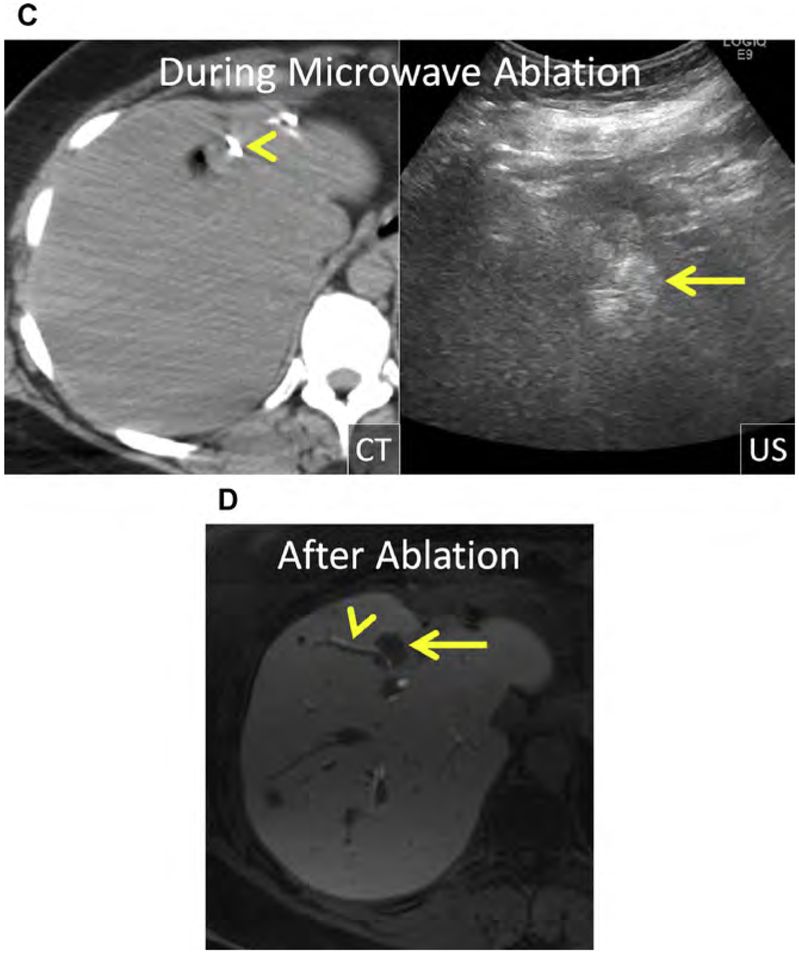
Fig. 2.
(A) Enhanced CT shows a CRLM in the medial right liver in proximity to the inferior vena cava (IVC) (arrow). (B) Enhanced CT after placement of RF electrodes (arrow). Segmental hypoenhancement of the dorsal right liver corresponds with the ablation and a hepatic infract (arrowheads). The ablation extends to the margin of the IVC and appears to encompass the index lesion. (C, D) Serial follow-up enhanced CT shows the index ablation (asterisk). (D) The new mass adjacent to the index ablation, in proximity to the IVC (arrow), represents LTP, the result of perfusion-mediated tissue cooling.
Fig. 3.
(A, B) Enhanced CT and US show a breast metastasis in the caudate lobe (arrows), in proximity to the IVC. (C, D) US and unenhanced CT after placement of the MW antennas (arrows). With this approach, the operator placed the MW antennas to mitigate the effect of perfusion-mediated tissue cooling. (E–G) Serial follow-up enhanced CT showing the index ablation (arrows). The ablation contracts over time and there is no evidence of LTP, despite proximity of the index lesion to the IVC. (E) The IVC remained patent without thrombus despite close proximity of MW antenna during ablation (arrowhead). Linear low attenuation extending from the index ablation to the liver surface corresponds with ablation of the antenna tracts.
Microwave
MWs use dielectric hysteresis to produce heat, resulting in coagulative necrosis, and are capable of penetrating tissues that are poor electrical conduits, such as lung, bone, and areas of desiccation/char. Water molecules are forced to align with an oscillating electric field emitted from an antenna, creating kinetic energy that is converted to heat. This method allows direct heating of a volume of tissue around the antenna with less reliance on conductive heating.25 MW ablation is performed at either 915 MHz or 2.45 GHz.
Compared with RF, MW has more power and can produce larger and hotter ablations. Combined with the ability to continuously power multiple antennas simultaneously, MW has improved capacity to overcome perfusion-mediated tissue cooling associated with large vessels (>2 mm) (see Fig. 3; Fig. 4).33–35 However, this increased power can result in vascular thrombosis of the portal vein, particularly in cirrhotic patients in whom portal venous flow rate is reduced (Fig. 5).36 Flow velocity within the inferior vena cava (IVC), hepatic arteries, and major hepatic veins is usually sufficient to prevent significant vascular thrombosis (see Figs. 3 and 4).37 MWs generate abundant water vapor, created by tissue out-gassing, which is well seen at both US and computed tomography (CT) (Figs. 6–8). This gas cloud seen at US correlates with the size of the ablation zone at immediate postprocedure contrast-enhanced CT and the zone of necrosis at pathology in an animal model (see Figs. 6 and 8).38
Fig. 4.
(A) CRLM in Couinaud segment VIII (arrow) abutting the right hepatic vein (arrowhead). (B, C) Three MW antennas were placed with US guidance into the mass and surrounding the right hepatic vein to overcome perfusion-mediated tissue cooling. (B) The edge of the lung (arrowhead) is easy to see and avoid during applicator placement. (C) Unenhanced CT was obtained to confirm precise antenna position (arrow). (D) Color and spectral Doppler was used intermittently during ablation to confirm patency of the right hepatic vein. (E) Axial and coronal enhanced CT immediately following MW ablation. The ablation encompasses the tumor and a margin of greater than 10 mm (arrow). The right hepatic vein remained patent (arrowhead).
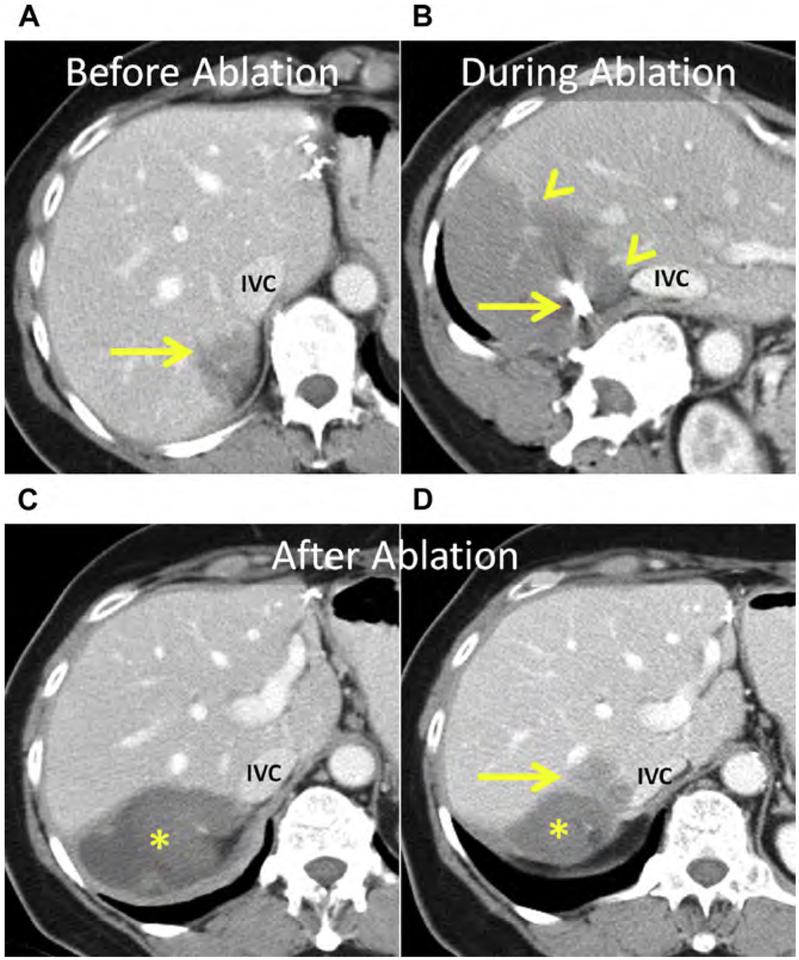
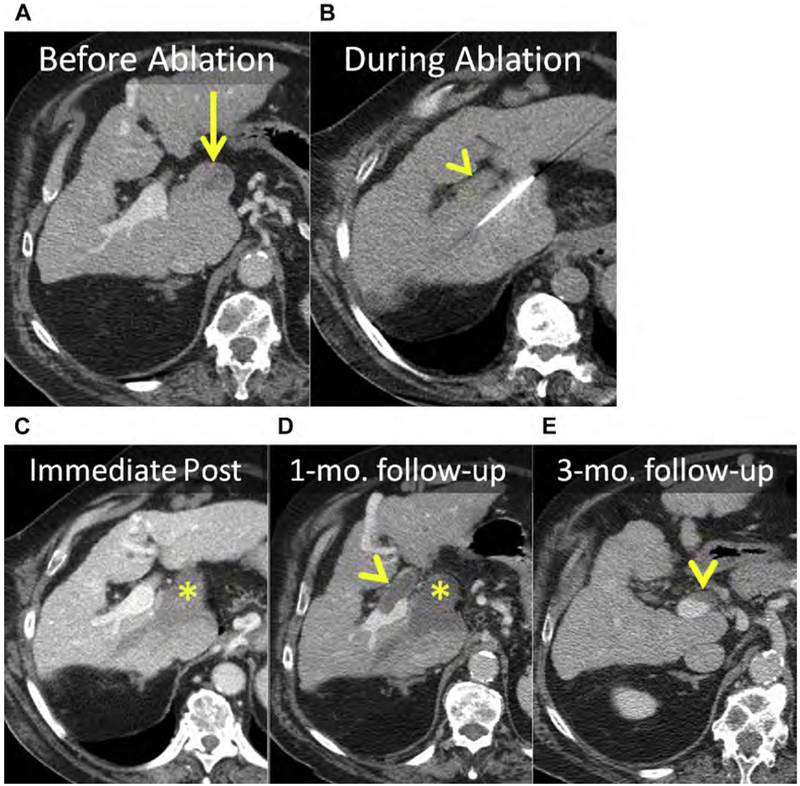
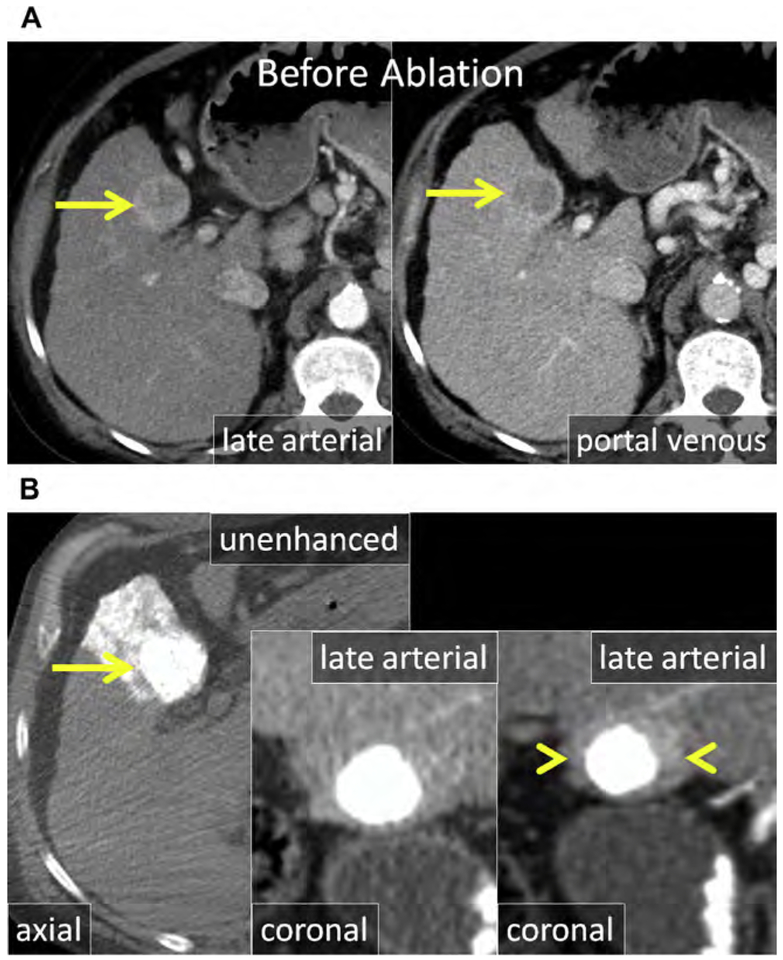
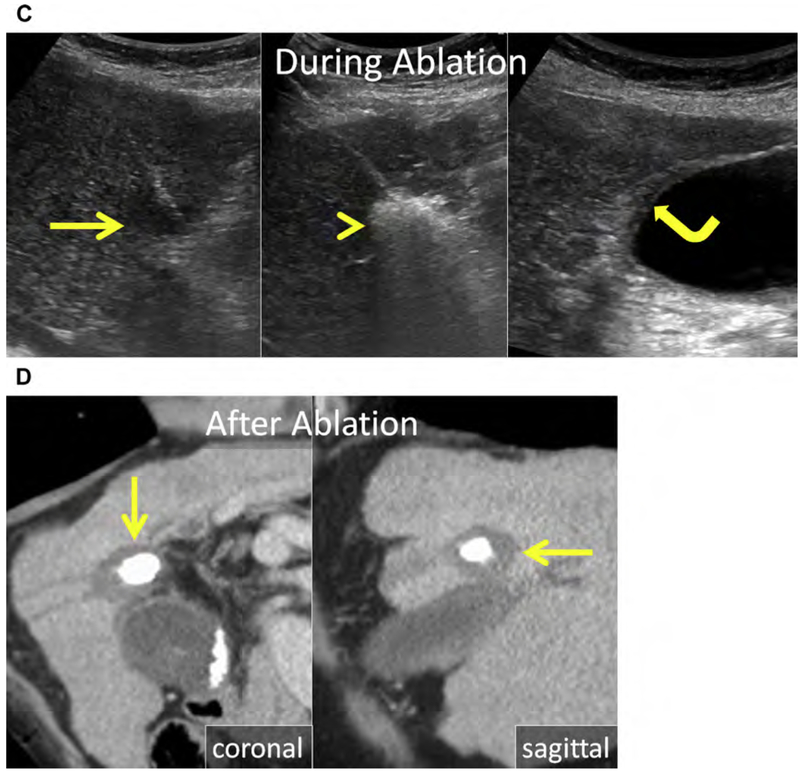
Fig. 5.
(A) Enhanced CT shows an HCC in the caudate (arrow). (B) Unenhanced CT after placement of MW antenna. Note the proximity of the main portal vein (arrowhead). (C–E) Serial follow-up enhanced CT immediately (C), 1 month (D), and 3 months after MW ablation showing the ablation (asterisks) without LTP. (C) The main portal vein was patent immediately after ablation. (D) At 1-month follow-up, there was thrombosis of the main and right portal veins (arrowhead) that (E) organized and partially resolved after anticoagulation therapy (arrowhead).
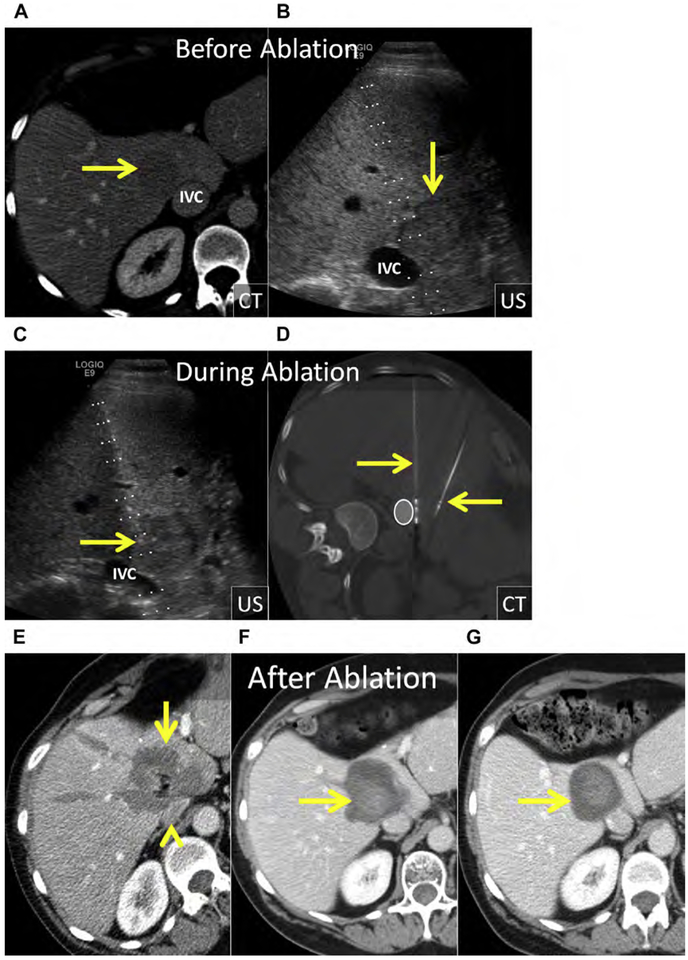
Fig. 6.
(A) HCC in the inferior right liver (arrows). Note the prior ablation in the right midliver (asterisk) without LTP. (B–D) US before and during ablation with the patient in left decubitus position. (B) At US, the HCC is hypoechoic (arrow). (C) US image after hydrodissection and placement of the MW antenna (arrowhead). (D) The gas cloud formed during MW ablation can be used to determine adequate coverage of the tumor and margin (arrows). (E) Axial and coronal enhanced CT immediately following MW ablation. The 3.3-cm ablation encompasses the index lesion, includes a margin greater than 5 mm, and corresponds with the size of the gas cloud at US (arrows).
Fig. 8.
(A) Enhanced CT in the late arterial and portal venous phase shows an exophytic HCC projecting from the inferior left liver (arrows). (B) At US, the HCC is hypoechoic (arrow). The MW antenna is well seen at both US and CT (arrowhead). (C) Artificial ascites was used to displace the stomach and pancreas; a trocar needle was placed through the left liver and a 2% iohexol-enhanced saline solution was infused (arrowheads). At CT, the stomach wall and pancreas were adequately displaced. The gas cloud at US (arrow) encompasses the HCC including a margin. (D) Enhanced CT in the late arterial (axial) and portal venous phase (coronal reformat) immediately after MW ablation. The ablation encompasses the index lesion including a 5 mm margin (arrow) without damage to the stomach or pancreas. The stomach and pancreas remained safely displaced (arrowhead) throughout the procedure and were not injured (arrowhead).
Cryoablation
Modern cryoablation equipment uses the Joule-Thomson principle of thermodynamics. High-pressure argon is forced down a narrow tube within an insulated, hollow cryoprobe; near the tip of the cryoprobe the argon escapes the tube via a small aperture into an expansion chamber, resulting in rapid cooling and formation of an ice ball. When high-pressure helium is forced down the cylinder and allowed to expand within the hollow cryoprobe, the result is rapid heating and subsequent thawing of the ice ball. This combination of rapid cooling followed by thawing brings about the cascade of events leading to cell death that direct cellular injury by formation of intracellular ice crystals and interruption of cellular metabolism and ischemia from vascular thrombosis, resulting in coagulative necrosis and apoptosis.
During cryoablation, the ice ball is readily visible at imaging, allowing assessment of adequacy of ablation and risk to nontargeted anatomy (Fig. 9). However, defining the lethal (−20°C and colder) isotherm within the visible ice,[C0]which can vary in distance from the ice edge depending on local factors, is not possible with imaging.31,39 A single cryoprobe ablation creates a very low volume of lethal ice because of perfusion-mediated tissue warming and should not be performed.40 Multiple cryoprobes can be simultaneously powered to create large ablations; however, this has been associated with an increase in both hepatic and extrahepatic complications, including fracture of the liver, hemorrhage, and a systemic inflammatory response (cryoshock) that can lead to thrombocytopenia and coagulopathy, liver failure, acute lung, and kidney injury. These risks may be exacerbated in patients with baseline coagulopathy, and are most frequently reported in patients with cirrhosis.41–49 Because cryoablation is associated with minimal pain, it is a viable treatment option for patients who are not candidates for general anesthesia or conscious sedation.
Fig. 9.
(A) CRLM in the right liver (arrow) with a dominant satellite tumor along the inferomedial margin (arrowhead). The mass is heterogeneous and echogenic at US. (B) US and enhanced CT during cryoablation. The ice ball is visible at US and CT (arrow); however, shadowing at US precludes evaluation of the deep margin. The distance between the visible ice and the lethal isotherm within the ice ball depends on local factors, including perfusion-mediated tissue warming. (C) Enhanced CT 1 year after cryoablation; there is no evidence of LTP (arrow).
Irreversible electroporation
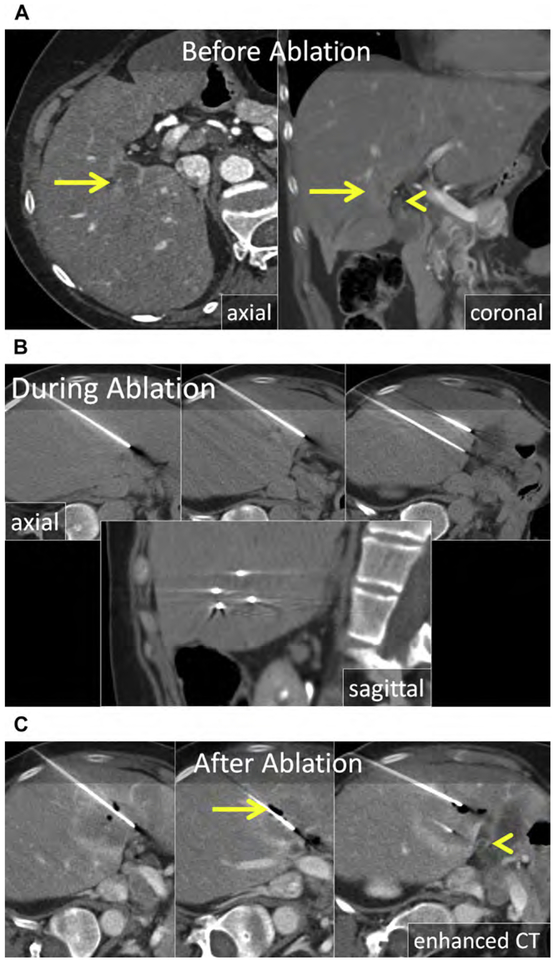
Irreversible electroporation, the only nonthermal energy-based ablative technology to date, delivers short bursts of high-voltage electrical pulses between closely approximated electrodes that create nanometer-scale pores in cell membranes, resulting in cell death.3,4 Depending on the voltage and duration of electric pulses, very high temperatures (>60 C) can be achieved, resulting in coagulative necrosis.2 Because the predominant, nonthermal mechanism of cell death allows preservation of the extracellular tissue architecture, IRE has a theoretic advantage over thermal ablation, particularly in proximity to at-risk structures such as the biliary tree (Fig. 10).4 In the liver, experience with IRE remains limited and there are no comparison studies with thermal ablation that show equivalent efficacy.
Fig. 10.
(A) Axial and coronal enhanced CT shows a CRLM in the medial right liver (arrow) in proximity to the common duct (arrowhead). (B) Axial and sagittal unenhanced CT following CT-guided placement of IRE electrodes. The electrodes are nearly parallel with near-equal spacing of 1 to 1.5 cm. Because precise electrode placement is important and a large number of electrodes are generally required for IRE, applicator placement can be time consuming. (C) Enhanced CT immediately following IRE. The ablation encompasses the tumor including a 10-mm margin and there is no apparent damage to the bile duct (arrowhead). The gas within the ablation zone (arrow) suggests that both thermal and nonthermal mechanisms of cell death were present in this case.
Irreversible electroporation is technically challenging because precise and parallel electrode placement, required to maintain the electric field, is time consuming, generally requires more applicators relative to thermal ablation and requires the use of CT guidance for applicator placement. General anesthesia with a paralytic and cardiac synchronization is required, otherwise muscle spasms and cardiac dysrhythmias can occur.
INDICATIONS AND PATIENT SELECTION
Hepatocellular Carcinoma
HCC is the most common primary liver cancer, the fourth most common type of cancer overall, and the third most common cause of cancer death worldwide.50 There are substantial variations in the epidemiology of HCC; globally, the burden of disease is related to hepatitis B virus (HBV) and is largely concentrated in developing countries. In countries where HBV is not endemic, hepatitis C and alcoholic cirrhosis remain the most common risk factors. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis are other important risk factors for cirrhosis and HCC, particularly in developed countries.51 In recent years the increased use of surveillance and improved diagnostic and therapeutic modalities have improved patient survival.52–54 Liver transplantation remains the definitive treatment of patients with both cirrhosis and HCC; however, most patients never receive transplantation because of a lack of organ availability, lack of access to a transplant center, or inability to meet transplant criteria.55
Recent advances, such as improved assessment of liver function and tumor burden, have enhanced the management of patients with HCC. Expert panels and consortia, including the European Association for the Study of the Liver and the American Association of the Study of Liver Disease, have generated evidence-based recommendations for the treatment of HCC. The Barcelona Clinic Liver Cancer (BCLC) staging system and treatment strategy incorporates tumor burden, liver function (Child-Pugh classification) and performance status to define prognosis and guide treatment decisions. Treatment options for HCC are stratified by BCLC stage. Transplantation, resection, and ablation are considered curative for very early and early stage HCC (stage 0 and A respectively). Palliative treatment options include transcatheter arterial chemoembolization (TACE) for intermediate stage HCC (stage B), and selective internal radiotherapy (SIRT) and sorafenib for advanced stage HCC (stage C). Best supportive care is reserved for terminal disease (stage D).56
Ablation
According to the BCLC criteria, image-guided tumor ablation is recommended for patients with very early and early stage HCC who are not surgical candidates.57 Very early stage includes patients with Child-Pugh A liver function, an Eastern Cooperative Oncology Group (ECOG) performance status of 0, and a single HCC less than 2 cm. Early stage includes patients with Child-Pugh A-B liver function, an ECOG performance status of 0, and a single HCC up to 5 cm or 3 HCC, each less than 3 cm.
RF ablation is considered the reference standard for the ablation of small HCC. Recently, newer generation high-powered MW (915 kHz–2.45 GHz) ablation has emerged as an alternative to RF. Compared with RF, MW systems generate higher tissue temperatures more quickly, and generally create larger ablations that are less susceptible to tissue perfusion from large (>2 mm) vessels.33–35 Available evidence to date suggests that MW is at least equivalent to RF for the treatment of very early or early stage HCC.58–67
RF ablation has been shown to be as effective as surgical resection for very early and early HCC.68,69 However, it is important to understand that patient characteristics (eg, obesity and portal hypertension) and tumor location (eg, subcapsular location; proximity to the biliary tree, colon, or diaphragm) can influence image-guided ablation results and the occurrence of complications (see Fig. 5; Fig. 11).70–73 In general, heat-based ablation, either RF or MW, should be considered first-line treatment of patients with liver dysfunction who have very early (stage 0) and early (stage A) HCC in favorable locations.
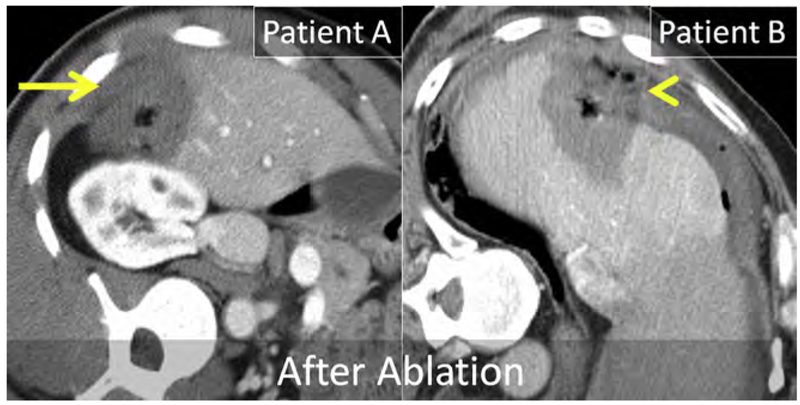
Fig. 11.
Enhanced CT immediately following MW ablation in 2 patients. Both patients are in an left posterior oblique (LPO) and received ablation procedures in the subcapsular right liver. Patient A was treated without artificial ascites, whereas patient B was treated after instillation of artificial ascites. In patient A, the ablation extended into the peritoneum and body wall (arrow) and resulted in severe postprocedure pain. In patient B, the ablation extended beyond the liver capsule into the extrahepatic fluid and fat but not into the peritoneum or body wall (arrowhead). Patient B had minimal postprocedure pain.
Other ablation devices, such as cryoablation, laser, focused US (high-intensity focused ultra-sound [HIFU]), and IRE have been successfully used in the treatment of HCC. Compared with the reference standard (RF), cryoablation is associated with a higher rate of morbidity and mortality. In some studies, the major complication rate for cryoablation exceeds 6% (compared with a major complication rate for RF that is <1%).49,67,70,74 Major complications of cryoablation include cryo-shock, hemorrhage, and liver failure. Laser and HIFU are other heat-based ablation devices that result in coagulative necrosis, similar to RF and MW. From a user standpoint, HIFU and IRE are more technically challenging than RF and MW. In addition, there are limited longitudinal data to accurately determine treatment response and equivalency of these modalities compared with RF.
Combination therapies
Exploiting synergies is the underlying principle for combination therapies. The most widely investigated combinations have been ethanol and RF, and TACE and RF.6,7,75–92 Ethanol causes coagulative necrosis and thrombosis of tumor vessels, subsequently modifying local tissue perfusion. Chemotherapy combined with embolic particles in TACE induces ischemic necrosis and modifies local tissue perfusion. Ablations with both RF and MW are larger after ethanol and TACE because of decreased perfusion-mediated tissue cooling.93–100
Hepatic artery embolization (HAE) therapies such as conventional TACE (cTACE) or TACE with drug-eluding beads (DEB-TACE) combined with ablation seem to improve technical success and decrease the incidence of LTP in select situations.84,91,101,102 For HCCs larger than 3 cm, those that are poorly encapsulated, or those that have visible satellite tumors, combination therapy should be considered. In addition, HCCs that are in poor anatomic locations, such as Couinaud segment VII and VIII or immediately adjacent to a large vessel (>2 mm), or HCC that are inconspicuous at US, may also be good candidates for combination therapy (see Fig. 1). Both RF and MW ablation have been shown to be effective in the treatment of HCC after TACE.83
There is no consensus on the optimal timing for intra-arterial therapies and ablation when used in combination. As a result, practice patterns vary widely, with ablation being performed both before and after HAE. The rationale for performing ablation before HAE is the augmented delivery of embolic particles into the hyperemic parenchyma surrounding the ablation, thereby increasing the treatment effect on the heat-damaged cells at the ablative margin; the most common location for LTP.103 The rationale for performing ablation after HAE is to reduce perfusion-mediated tissue cooling and exploit a chemotherapy-heat synergy. Another reason to perform HAE before ablation is to aid in tumor targeting when CT is used for guidance and when tumors are inconspicuous at US. If ablation is performed after TACE, it is important to treat the entire tumor in addition to at least a 5-mm circumferential margin (Figs. 12–15). This technique is well tolerated and has been shown to decrease the incidence of LTP.104
Fig. 12.
(A) At MR imaging, there is an HCC in the dome of the right liver, near the interface of Couinaud segments VII and VIII (arrow). (B, C) Because the mass was inconspicuous at US, chemoembolization with ethiodized oil was performed and followed 2 weeks later by CT-guided MW ablation. (B) Unenhanced CT with the patient in the left decubitus position. Note ethiodized oil within the index lesion (arrow) and artificial ascites interposed between the liver and body wall (arrowhead). (C) Unenhanced CT after placement of the MW antenna (arrow). (D) Enhanced CT immediately following MW ablation. The ablation encompasses the index lesion containing ethiodized oil and a 5-mm margin (arrow). Linear low attenuation extending from the index ablation corresponds with ablation of the antenna tract (arrowhead).
Fig. 15.
(A) Large (>3 cm) HCC in the right liver (arrow). (B) Unenhanced CT before and after placement of MW antennas (arrowheads). Note the excellent accumulation of ethiodized oil within the tumor (arrow) with partial clearance of ethiodized oil from the nontargeted liver in the 2-week interval between TACE and MW ablation. (C) Enhanced CT immediately following MW ablation. The ablation encompasses the tumor including a 5-mm margin (arrow). Artificial ascites was infused before and during MW ablation (arrowhead).
Colorectal Cancer Hepatic Metastasis
Colorectal carcinoma (CRC) is the third most common cancer and the second most common cause of cancer-related mortality in the United States.105 Metastatic disease is present at diagnosis of CRC in 20% and another 30% to 50% develop liver metastasis (colorectal liver metastasis [CRLM]) during treatment. Of those patients with metastatic disease, 25% to 30% have isolated hepatic metastasis.106 Without treatment, median survival with CRC hepatic metastasis is 6.9 months, with a 5-year survival of 0%.107,108
Complete surgical resection confers the best chance for long-term survival; however, only 20% of patients with CRLM are candidates for resection. More effective neoadjuvant chemo-therapy regimens and improved surgical techniques have had a significant impact on survival. At present, 5-year survival after hepatic resection for CRLM approaches 50%.109,110 In comparison, patients with CRLM treated with chemotherapy, but who do not undergo liver resection have a 5-year overall survival (OS) of 10%.111
Although OS has improved, approximately 45% of patients experience early recurrence (within 6 months) after liver resection, whereas 75% experience recurrent disease overall. Early recurrence negatively affects prognosis, with an approximately 50% reduction in 5-year OS compared with late recurrence. With repeat resection, OS for early recurrence is similar to patients reresected for late recurrence.112 Risk factors for recurrence include T3–T4 primary tumor, synchronous liver metastasis, more than 3 liver metastases, largest liver metastasis greater than 5 cm, and a 0-mm resection margin.112,113 Response to neoadjuvant chemotherapy and administration of adjuvant chemotherapy reduce early surgical recurrence.112
Adjuvant chemotherapy has not been shown to improve OS; however, is still used because of improvement in progression-free survival (PFS).114–117 A phase II randomized controlled trial comparing systemic therapy alone with RF ablation and adjuvant chemotherapy for CRLM showed improved PFS with adjuvant chemotherapy.118
Ablation
The role of percutaneous ablation in the management of CRLM remains in debate; however, ablation is widely accepted for patients with unresectable tumors, those who are not surgical candidates, and in the setting of recurrent disease after liver resection when insufficient liver reserve precludes further resection. Although retrospective studies have concluded that resection offers better survival than ablation, no randomized controlled trials have been performed to date. Of the studies comparing surgical resection and ablation, patient selection favors surgical resection in virtually all cases.119,120 Patients in the ablation arm tend to have a greater burden of disease, a higher rate of extrahepatic tumors, and larger tumors, tend to be older, and tend to have more comorbidities. In those studies in which ablation has been used as first-line therapy for patients with resectable CRLM, the 5-year survivals are similar to those of surgical series.121,122 Other retrospective studies show comparable survival with surgical series when ablation is applied to CRLM less than 3 cm.123–126
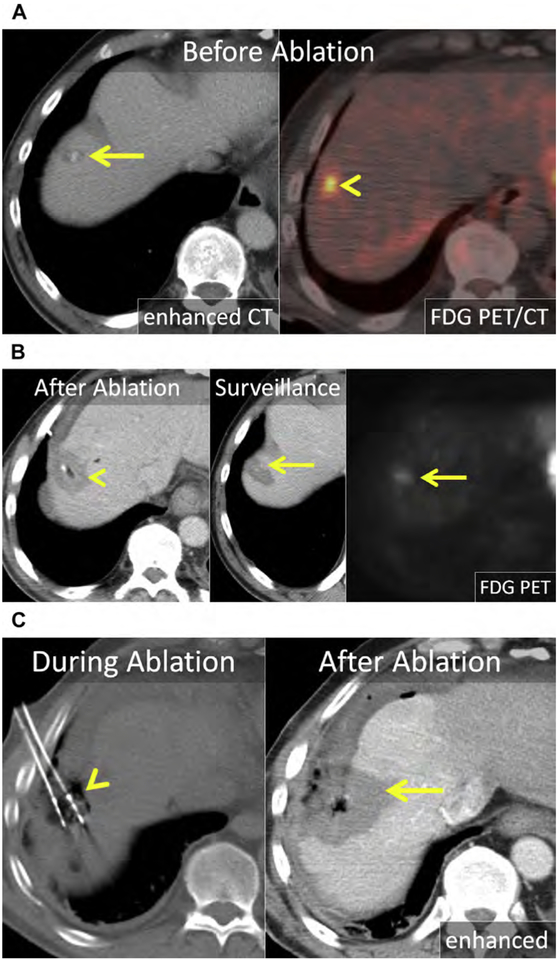
Small and medium-sized CRLM are suitable for percutaneous ablation, with the most commonly treated tumors measuring up to 3 cm (see Figs. 2, 4, 9, 10; Fig. 16). Depending on the location of the tumor and the technology, tumors of up to 5 cm can be successfully treated with an acceptable rate of LTP.121,127–130 Multiple ablations in a single session or repeat ablation can safely be performed for limited disease when there is sufficient hepatic reserve (see Fig. 16). As with surgical resection, the best results following liver ablation for CRLM are achieved in the setting of a solitary tumor less than 3 cm.123,131
Fig. 16.
(A) Enhanced CT shows a 1.2-cm CRLM in the right liver, near the dome (arrow). The mass is metabolically active on fused 18F-fluorodeoxyglucose (FDG) PET-CT (arrowhead). (B) Enhanced CT immediately following MW ablation. The 2.5-cm ablation encompasses the index lesion (arrowhead); however, it does not achieve the 1-cm margin needed to mitigate the risk of LTP. At surveillance enhanced CT and FDG PET-CT, there was LTP (arrows). (C) On unenhanced CT, 2 MW antennas are positioned within the index lesion with gas surrounding the antennas (arrowhead). Artificial ascites was infused before and during MW ablation. Enhanced CT immediately following MW ablation shows a 4.5-cm ablation (arrow) covering the prior ablation and a 10-mm margin.
Compared with resection, LTP is more common following ablation and the risk increases for larger tumors.121,127–130,132 However, these differing rates of LTP have not had an apparent effect on OS.122,124 One possible explanation for this dichotomy between LTP and OS between patients after ablation and resection may be that patients who have experienced LTP and/or distant intrahepatic tumor progression after ablation are often candidates to undergo repeat ablation (or resection) because ablation spares more healthy liver tissue relative to surgery.
Neuroendocrine Cancer Hepatic Metastasis
Neuroendocrine tumors (NETs) are a diverse group of malignancies with variable, although often indolent, biological behavior. Clinical behavior and prognosis correlate closely with histologic differentiation and World Health Organization (WHO) grade. For patients with low-grade (G1) or intermediate-grade (G2) histology and metastatic disease, survival is highly variable and can depend on factors unrelated to histology, such as primary tumor location. For example, survival in the setting of advanced carcinoid disease is much worse for patients with the primary tumor arising from the colon and lung (median OS, 7 and 17 months respectively) compared with the small bowel (median OS, 55–65 months). As a result, assessment of comparative benefit among treatment strategies is difficult, given the various natural histories of these malignancies.133
Most patients with advanced NET have hepatic metastatic disease. Management of these patients is complex and requires a multidisciplinary team approach, with consideration of age, performance status, clinical symptoms, extent, and biology of disease. Surgical resection provides clear benefit with symptom palliation and favorable long-term survival.134–138 However, progressive hepatic metastatic disease and recurrent symptoms occur in 85% to 95% of patients. In the setting of limited, isolated liver hepatic meta-static disease, orthotopic liver transplantation (OLT) has been attempted; however, the role of OLT in metastatic NET is not yet established and remains controversial.139–143
Clinical symptoms depend on a variety of factors, including the site of the primary tumor; the presence of synchronous and metachronous tumors; and the extent and location of local, regional, and distant metastasis. Clinical syndromes are the result of hormone hypersecretion from gastropancreatic NETs or release of vasoactive substances (serotonin) into the systemic circulation from hepatic metastasis. Somatostatin analogues (octreotide or lanreotide) and cytotoxic chemotherapies are useful in controlling both tumor growth and hormone-related symptoms in advanced metastatic disease.133
Most patients present with multifocal, bilobar metastatic disease and thus are not surgical candidates and are unlikely to benefit from percutaneous ablation. HAE, alone or as an adjunct to medical therapy, has been a successful palliative technique for patients with liver predominant disease with response rates, as measured by decreased hormone secretion, symptom benefit, and/or radiologic response, generally exceeding 50%. Similar tumor response rates, symptom palliation, and survival have been shown among bland embolization, chemoembolization (cTACE and DEB-TACE), and SIRT.144–152 Thus, appropriate patient selection is an important consideration to minimize treatment-related side effects among the HAE treatment options.
Ablation
The role of ablation in the management of NET liver metastasis remains undefined, although it is largely accepted as a palliative treatment option in nonsurgical candidates and as an adjunct to surgical resection in patients with oligometastatic disease (see Fig. 1). Patients who undergo resection almost always develop new sites of metastatic disease in the liver remnant. Because repeat liver resection may not be feasible because of limited hepatic reserve, percutaneous ablation, in combination with chemotherapy and somatostatin analogues, can provide relief of clinical syndromes and prolong OS.137,153 Consensus-based guidelines put forth by the National Comprehensive Cancer Network recommend cytoreductive surgery and/or ablation if near-complete eradication of hepatic tumor burden can be achieved.
Most published reports of tumor ablation are small (<40 patients) retrospective case studies. The largest series included 89 patients with hepatic metastatic NET treated with laparoscopic RF.153 In this cohort, a mean of 6 lesions (and up to 16 lesions) were treated in a single session; most patients (73%) had durable (median, 14 months) symptom relief.
Other Hepatic Tumors
For other hepatic malignancies, either primary or secondary, it is important to understand the underlying disease process and tumor biology. For example, performing tumor ablation if there is rapidly progressing metastatic disease or extensive extrahepatic metastatic disease is unlikely to be beneficial. However, patients with oligometastatic disease who have had a durable response to adjuvant therapies may benefit from liver tumor ablation.118 The size, location, and number of hepatic tumors and the extent and location of extra-hepatic metastases are important considerations.
Breast Cancer Hepatic Metastasis
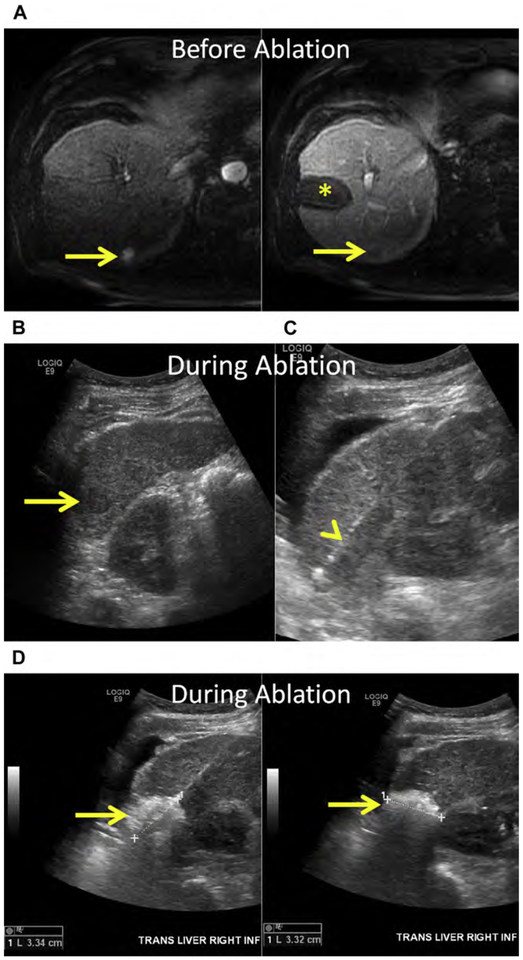
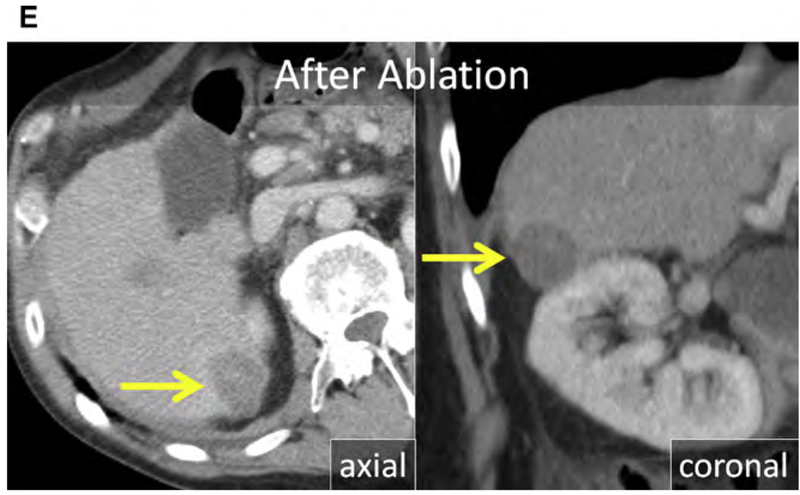
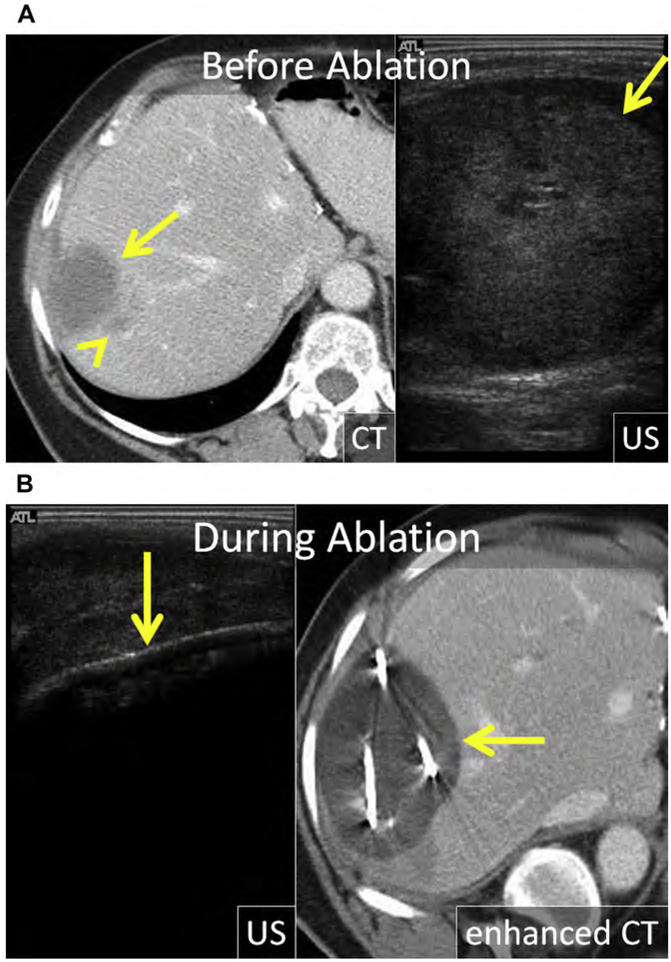
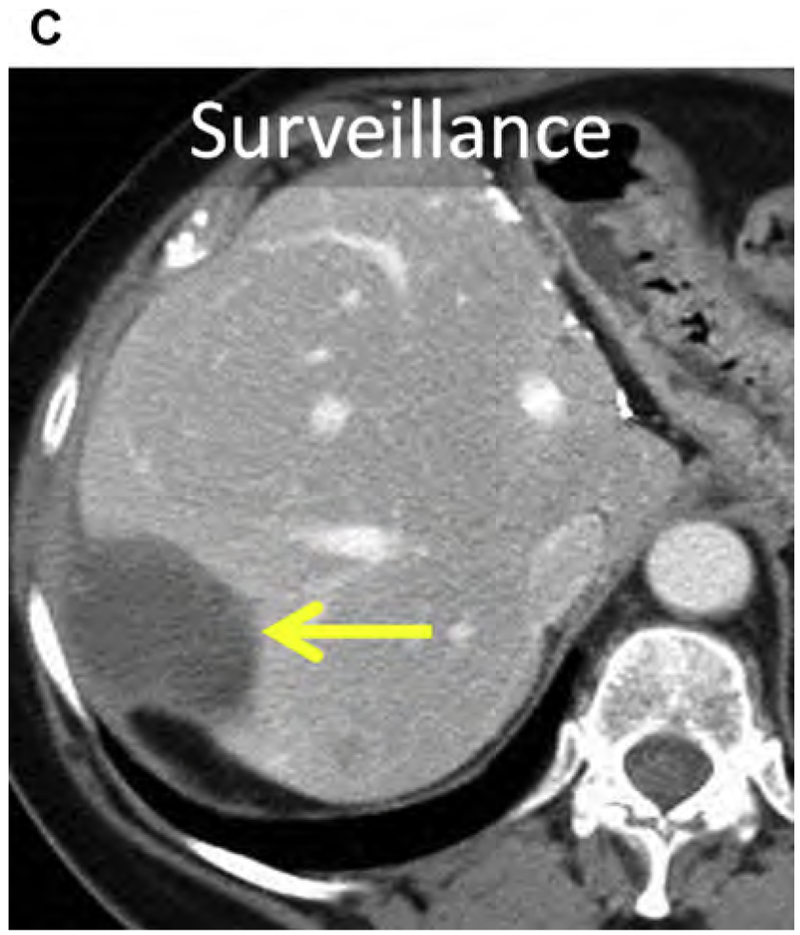
Breast cancer is the most common malignancy and the leading cause of cancer-related mortality in women.154 The prognosis remains poor for metastatic breast cancer despite the introduction of modern chemotherapeutic regimens and biologics, with a median OS of 24 to 30 months and a 5-year survival of 23%.155,156 Both synchronous and metachronous hepatic metastases occur, and usually signify metastatic disease elsewhere; most commonly the lung, bone, nodes, and brain.157 Isolated hepatic metastasis is uncommon but still portends a poor prognosis, with median OS of approximately 12 months and 5-year survival of 8.5%.155,158,159 Prognosis has been linked to human epidermal growth factor receptor 2 (HER2) expression in liver metastasis, presence of extra-hepatic metastasis, and patient age.160
Breast cancer hepatic metastases have typically been treated with chemotherapeutics alone, with surgery reserved for palliation in symptomatic patients.161 However, more recently several small retrospective series suggest a survival benefit in carefully selected patients even if there is extrahepatic disease.160,162–164 Alone or combined with surgery, thermal ablation may improve survival. In a small case series of 50 patients, RF ablation of limited hepatic metastatic disease in the setting of stable or limited extrahepatic metastatic disease improved median OS to 43 months (see Fig. 3).160,165
Benign Hepatic Tumors
Arguably, patients with benign hepatic tumors are the ideal candidates for percutaneous tumor ablation. Because the underlying disorder is benign, complete ablation may not be necessary and there is a wide window of opportunity to obtain a complete ablation, if needed. However, the treatment alternatives generally offered are surgical and are associated with significant morbidity. Thus, a minimally invasive alternative is extremely appealing for these often young and otherwise healthy patients.
There are a variety of benign liver tumors that can be treated, including simple and complex cysts, focal nodular hyperplasia, and hemangiomas and adenomas. Liver cysts and hemangiomas are generally small, follow an indolent course, and require no specific imaging or follow-up. In contrast, adenomas can rupture or infrequently undergo malignant transformation.
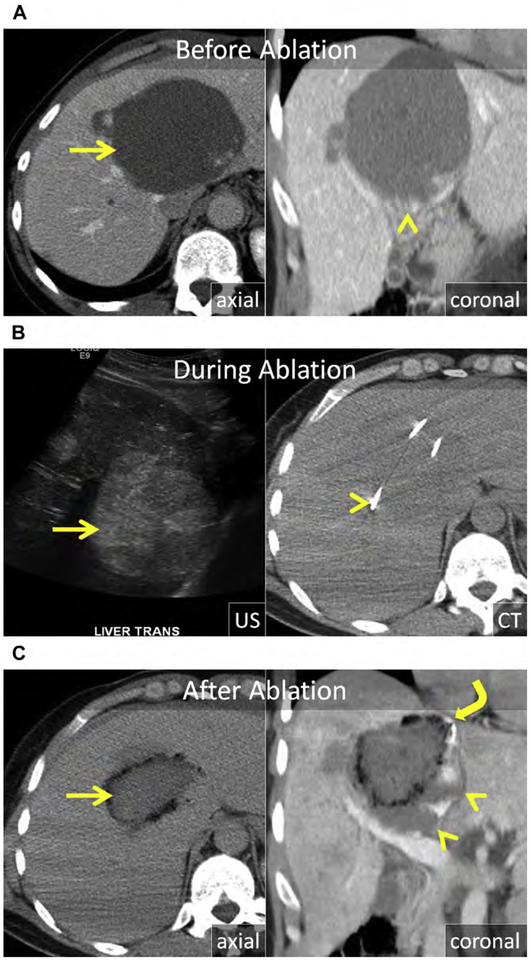
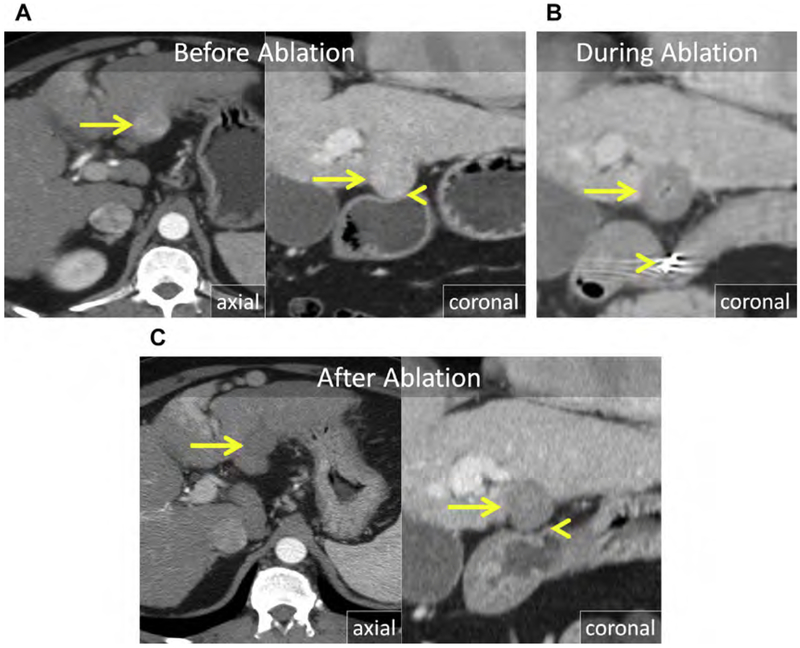
Hemangiomas can become large; cause mass effect on local structures, such as the central bile duct, stomach, or duodenum; or stretch the Glisson capsule, resulting in obstructive symptoms, anorexia, weight loss, and abdominal pain. Although surgical resection has been the historic standard of care in symptomatic patients, percutaneous ablation provides a much less invasive option with lower morbidity. Because hemangiomas are benign, complete ablation is not necessary and should be avoided when critical structures, such as central bile ducts, are at risk (Fig. 17). There are a few small case series using RF or MW to treat hemangiomas with high technical success, low morbidity, and durable symptom relief.166–171
Fig. 17.
(A) Axial and coronal enhanced CT show a giant cavernous hemangioma centered in the central liver (arrow), extending to the hilar plate (arrowhead). (B) US and unenhanced CT images during MW ablation. The hemangioma is heterogeneous and echogenic at US (arrow). Three MW antennas were placed into the mass in a triangular array (arrowhead) with approximate spacing of 1.5 cm. (C) Enhanced axial and coronal CT immediately after MW ablation. There has been substantial decrease in size of the hemangioma (arrow) with resolution of mass effect and stretch of the Glisson capsule at the dome (curved arrow). The inferior portion of the mass, near the hilar plate, was purposefully left untreated to avoid injury of the central bile ducts and vasculature (arrowheads).
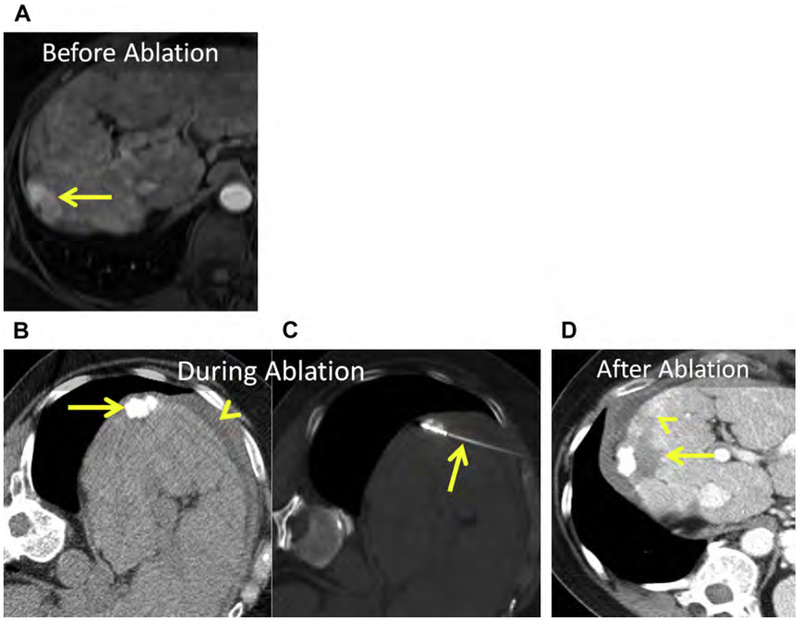
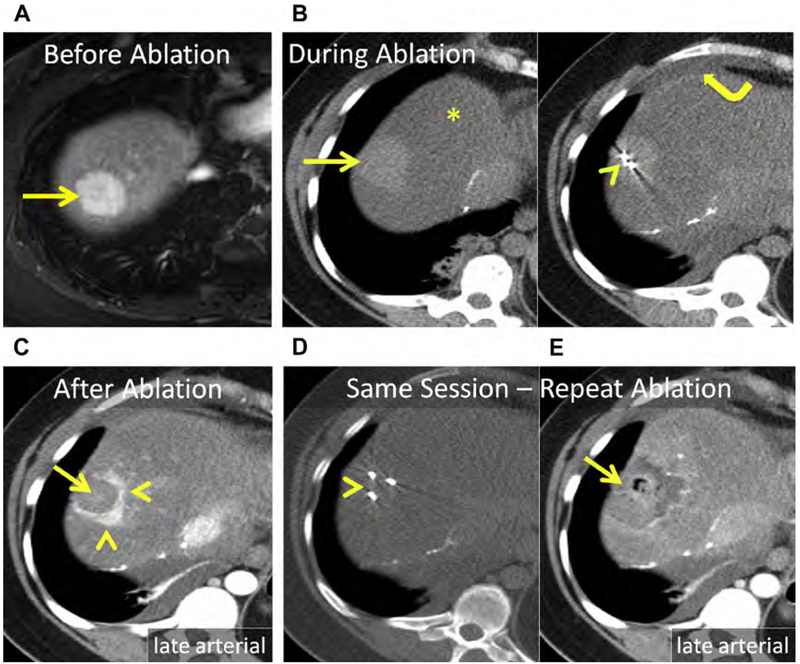
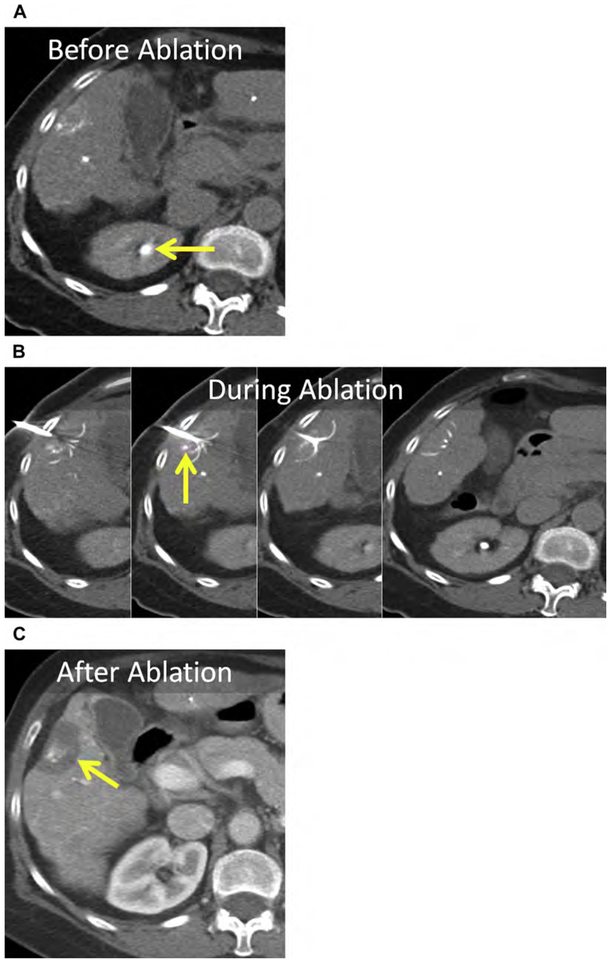
Hepatic adenomas are rare; typically arise in women using oral contraception; and infrequently occur in men, in whom they can be associated with anabolic steroid use. Glycogen storage diseases (type I and III) are associated with multiple adenomas.172 Compared with other benign tumors, management is more complex because of the risk of hemorrhage and malignant transformation. Active surveillance, surgical resection, embolization, and ablation have all been used. Adenomas larger than 5 cm, those that fail to regress or that grow despite withdrawal of hormones, those that have beta-catenin activation or dysplasia/atypia at biopsy, and adenomas in men should be surgically resected or ablated.172–174 Even though adenomas are benign, complete ablation should be achieved to avoid delayed complications, such as hemorrhage or malignant transformation (Fig. 18).
Fig. 18.
(A) Hepatic adenoma in the dome of the liver, Couinaud segment VIII (arrow). (B) Unenhanced CT after placement of 2 MW antennas (arrowhead). The mass (arrow) is conspicuous at unenhanced CT because of severe, diffuse hepatic steatosis (asterisk). Note artificial ascites used to protect the body wall and diaphragm (curved arrow). (C) Enhanced CT immediately following MW ablation. Persistent eccentric avid enhancement (arrowheads) surrounding the nonenhancing ablation (arrow) is compatible with viable tumor. (D) CT image following reinsertion of the 2 antennas and the addition of a third MW antenna (arrowhead). (E) Enhanced CT immediately following same-session repeat MW ablation. The ablation now encompasses the index lesion (arrow).
ABLATION PROCEDURE AND SURVEILLANCE
Local Tumor Progression
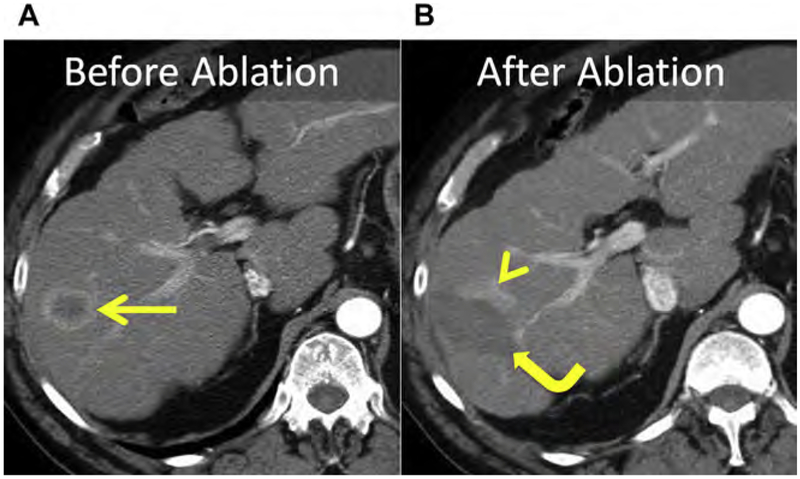
The liver surrounding a malignant liver tumor often contains satellites of carcinoma that are invisible at conventional imaging. These tumor satellites are frequently the cause for LTP in technically successful ablations (ie, ablations without imaging evidence of residual tumor on immediate or short-term follow-up; see Fig. 16; Fig. 19). The extent and distance of the satellites from the index tumor are variable, and depend on factors such as tumor type, size, degree of differentiation, and vascularity. Therefore, the goal of tumor ablation is to encompass both the index tumor and a circumferential margin appropriate for the tumor type.
Fig. 19.
(A) CT before ablation shows an HCC within the right liver (arrow). (B) At follow-up, eccentric enhancement (arrowhead) adjacent to the index ablation (curved arrow) is consistent with LTP. Satellite tumors invisible at conventional imaging are generally the cause for LTP, suggesting that an adequate margin was not achieved. For HCC, a minimum of a 5-mm circumferential margin is necessary.
Ablative margin: hepatocellular carcinoma
The size and degree of differentiation of HCC determine the presence and extent of satellite tumors. Hypervascular HCC is associated with capsular invasion and satellite tumors. As HCC progresses from well to poorly differentiated, the presence and size of satellites increases, and as the index tumor increases in size, the number of satellites increases. However, the size of the index HCC does not affect the distance of the satellite from the index HCC. For HCCs less than 2 cm and HCCs between 2 and 3 cm, the mean distance of the satellite from the index HCC was 5.3 and 4.8 mm, respectively. Nonhypervascular HCC (at CT angiogram) rarely has satellite tumors, regardless of size.175 For HCCs less than 3 cm, a minimum of a 5-mm circumferential ablative margin must be achieved in order to maximize the odds of complete ablation and reduce the rate of LTP.175–179
The overall ablation zone size can easily be underestimated for a particular tumor. For example, a 2-cm HCC should be treated with (at minimum) a 3-cm zone of ablation, provided that the tumor is centered within the ablation. If off center, the zone of ablation should be larger. If an HCC is near or abutting the liver capsule, the ablation should extend to the liver capsule. For HCCs larger than 3 cm, those that lack a capsule, or those that have gross satellites present on conventional imaging, combination therapy and/or larger circumferential margins may be necessary to achieve local tumor control.180
Ablative Margin: Colorectal liver metastasis and other hepatic metastases
The biology and perfusion of the tumor relative to the surrounding liver are important factors to consider when planning ablation of hepatic metastases. Relative to the adjacent liver, CRLMs are less well perfused than the surrounding liver. As a result, the index tumor is easier to ablate than the margin of normal liver.181 Similarly, the water content of metastatic NET relative to adjacent liver parenchyma allows easier ablation of the mass compared with the liver margin. Nevertheless, a minimum of a 1-cm circumferential ablative margin must be achieved in order to maximize the odds of complete ablation and reduce the rate of LTP in CRLM.132 For example, a 2-cm metastasis should receive (at minimum) a 4-cm zone of ablation, provided the index lesion is centered within the ablation. If off center, the zone of ablation should be larger. If a mass is within 1 cm of the liver capsule, the ablation should extend to the liver capsule. Obtaining at least a 1-cm margin in all directions is particularly important with ill-defined tumors. These tumors tend to have more satellite tumors at distances further from the index tumor.182,183
Technique-specific Considerations
Excellent preprocedure imaging, either with contrast-enhanced CT or MR imaging, is necessary for ablation planning. This imaging allows evaluation of the extent of disease within the liver and the proximity of the tumor to major vessels and nontargeted anatomy. When comparison imaging is available, interval change in burden of hepatic and extrahepatic disease can provide insight into tumor biology. For HCC, preprocedure imaging within 1 to 2 months is usually sufficient. However, the doubling rate of CRLM, among other hepatic metastases, is much shorter than that of HCC, so preprocedure imaging should be obtained within 2 weeks of ablation.
Anesthesia
Tumor ablation has been performed with general anesthesia (GA), conscious sedation, and local anesthesia with or without sedation. When combined with a paralytic, GA can render the diaphragm motionless, allowing precise applicator placement. The Valsalva technique can improve tumor conspicuity and access to tumors, particularly those in the dome of the liver. Continuous hemodynamic monitoring by anesthesiology and improved pain control are other advantages of GA.
Relative to GA, deep or conscious sedation reduces procedure time and time between patients and is not associated with the same risks as GA. However, shallow tidal volumes can limit tumor conspicuity, particularly for tumors near the dome, and respiratory motion can make applicator placement more challenging. The higher rate of LTP for subcapsular tumors treated under conscious sedation may be partially explained by the fear of inducing pain decreasing treatment time and/or power.182
Expected duration of the ablation procedure, number of applicators to be placed, conspicuity of the tumor, expected ease of applicator placement, and the ability of the patient to remain in the preferred position are factors to consider when determining the need for GA.
Nontarget anatomy
A variety of techniques can be used before and/or during ablation to reduce the risk of collateral damage. Patient positioning, instillation of intraperitoneal fluid or gas, insufflation of intraperitoneal balloons, applying traction on applicators, biliary perfusion with chilled fluids, and intentional pneumothorax are techniques that have been used to protect nontargeted anatomy.
Patient positioning
A supine position is convenient, safe, and can expedite the procedure. However, nontargeted structures, such as the lung and diaphragm, frequently preclude safe access to liver masses with CT, particularly those near the dome. Even though US provides a greater variety of access points into the liver for tumor ablation, a supine position is frequently suboptimal.
Oblique and lateral decubitus positions allow access to a greater number of hepatic tumors, particularly those near the dome of the liver. These positions can allow nontargeted anatomy to move. In addition, the operator may be able to optimize placement of the applicators so as to protect non-targeted structures while ensuring adequate coverage of the index tumor (see Figs. 6, 11, 12, 16, 18; Fig. 20).
Fig. 20.
(A) At MR imaging, there is an HCC in Couinaud segment VIII (asterisks) abutting the diaphragm (arrowhead). (B) Shallow LPO improved conspicuity and access to the tumor for US-guided antenna placement (asterisks) (C) Axial and coronal unenhanced CT with 2 MW antennas (arrows) in the mass. The unenhanced CT after applicator placement allows precise localization of the applicator and is particularly useful when evaluating the proximity of nontargeted structures; the diaphragm in this case (arrowhead). (D) Axial and coronal enhanced CT immediately following MW ablation. The ablation encompasses the index lesion including a margin of greater than 5 mm (arrow).
Diaphragm
Hepatic tumors in proximity to or abutting the liver capsule, particularly those near the dome of the liver, pose unique challenges. Conspicuity of these tumors at US can be suboptimal because of rib shadow, the lung edge, or poor acoustic penetration making applicator placement difficult. As a result, primary effectiveness is reduced, the rate of LTP is higher, and patients experience more postprocedure pain.184–189 Artificial ascites, directed toward the subdiaphragmatic space, can improve tumor conspicuity at US, reduce the rate of primary failure and LTP, and reduce postprocedural pain (see Figs. 6, 11, 12, 16, 18, 20).190–196
A transthoracic CT-guided approach to tumors near the dome of the liver, without or following the creation of an artificial pneumothorax or pleural effusion, has been described and can be safe and effective. The most commonly reported complications are pneumothorax and pleural effusion.100,197–199 Although seeding the pleural space with tumor is a concern, the incidence is likely extremely low and comparable with incidence of seeding the peritoneum.
Central bile ducts
Extending a zone of ablation to central bile ducts (right and left hepatic ducts, common hepatic and common bile ducts) can result in bile duct stricture and progressive loss of subtended liver parenchyma.200 Bile leaks, cholangitis, and liver abscesses can subsequently occur, leading to significant morbidity. For patients with metastatic disease, these complications can delay initiation or reinstitution of chemotherapy, potentially resulting in decreased survival. Although high-flow bile duct cooling can be protective, ablation within 1 cm of a central bile duct should be avoided.201,202 Alternative therapies, such as IRE, ethanol ablation, HAE, and SBRT, should be considered when appropriate for a particular tumor type (see Figs. 1 and 17).
Hepatic vasculature
Complete ablation of tumors adjacent to high-flow blood vessels larger than 3 mm in diameter is challenging because of perfusion-mediated tissue cooling heat sink (see Fig. 2).23,26,27 Several strategies have been used to combat perfusion-mediated tissue cooling, including increasing power and time of ablations in proximity to major vasculature, targeted placement of applicators closer to vessels, and occlusion of hepatic vasculature with peripherally inserted balloon catheters (see Figs. 3 and 4). From a technology perspective, high-powered MW is less susceptible to perfusion-mediated tissue cooling than RF, and IRE may be unaffected by perfusion-mediated cooling (see Figs. 3 and 4).34,203
In contrast, patients with cirrhosis and slow portal venous flow are at increased risk for portal venous thrombosis (PVT) caused by heat transfer.29,37 PVT can increase portal vein pressure, leading to decreased liver function, worsening ascites, and increased risk for variceal bleeding. Because of the low incidence with RF and tendency to resolve, historically PVT has been of minimal concern.204 With the higher temperatures and larger ablations of MW, the risk for PVT may be higher.34 Anticoagulation therapy generally results in complete or near-complete resolution of PVT (see Fig. 5).
Image guidance
Many different tools have been used for imaging guidance, including US, CT, MR imaging, fluoroscopy, and more recently fused PET-CT following the administration of fluorine-18-fluorodeoxyglucose.205 Each technique has strengths and limitations; the choice of guidance technique should be tailored to the unique situation for each patient.
When technically feasible, US is an excellent guidance tool for applicator placement. The largest published series with the lowest LTP rates following percutaneous ablation almost invariably have used US guidance for applicator placement and ablation monitoring.36,128,206 Ultrasound offers high soft tissue contrast, simultaneous assessment of the tumor and applicator, fixed guides to assist applicator placement, and the ability to interrogate flow within hepatic vasculature, among other advantages (see Figs. 1, 3, 4, 6, 8, 14, 20). However, US is operator dependent and its utility may be compromised by tumor location and body habitus.
Fig. 14.
(A) Enhanced CT in the late arterial and portal venous phase shows a large (>3 cm) HCC in the medial left liver in proximity to the gallbladder (arrow). (B) The HCC was treated with TACE (arrow) because of the size and location. Ablation was initially deferred; however, at serial follow-up CT, LTP became apparent (arrowheads). (C) US during MW antenna placement and ablation. The hypoechoic HCC (arrow) becomes encompassed by the gas cloud formed during MW ablation (arrowhead). Minimal wall thickening of the gallbladder is the result of thermal injury to the serosa (curved arrow). (D) Enhanced coronal and sagittal CT immediately after MW ablation. The ablation encompasses the index ethiodized oil-laden lesion including a 5-mm margin (arrow). On follow-up images, there was no permanent damage to the gallbladder.
CT as a guidance tool allows for evaluation of the entire liver and proximity of non-target anatomy; however, CT is limited by poor soft tissue contrast. Retained ethiodized oil, from prior HAE, can provide a target for CT guidance without compromising the ability to use US (see Figs. 12–15). Radiation exposure to the patient and operator is another disadvantage of CT guidance.
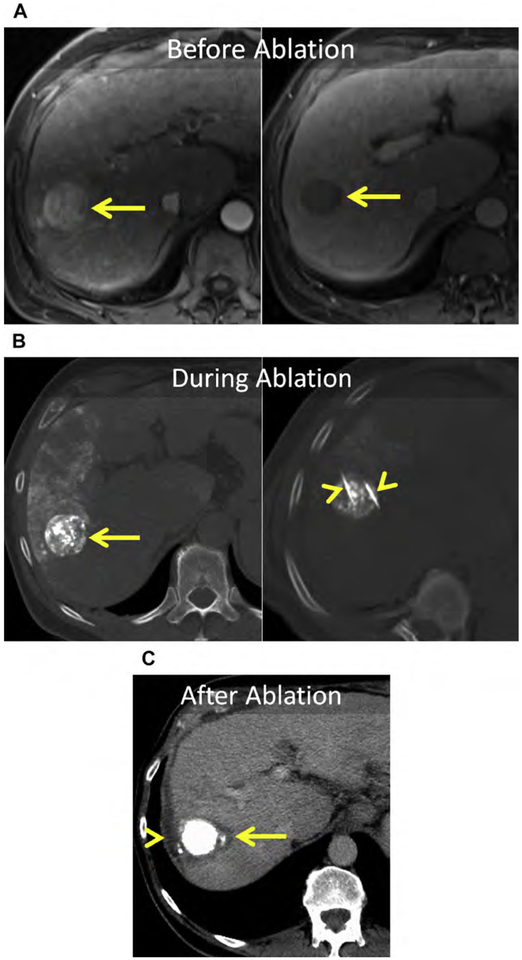
Other guidance techniques, such as MR imaging, PET-CT, and fused US/CT-MR imaging, have been used successfully as guidance tools for percutaneous ablation, but are not universally available.207–218 MR imaging offers superior lesion characterization, relative to CT, and the ability to monitor index ablation temperatures (thermometry); however, not all ablation devices are MR imaging compatible. Small bore sizes can limit applicator placement at MR imaging, particularly in obese patients.
Multiple applicator synergy
Treating large tumors with adequate margins requires the creation of large ablation zones. There are 2 methods to achieve large heat-based ablations: multiple overlapping ablations with a single applicator repositioned to different locations (sequential) and multiple applicator (simultaneous) ablation. Sequential RF and MW ablations result in smaller, less predictable ablations with clefts, whereas simultaneous ablation creates larger, confluent, and more predictable ablations (see Figs. 3, 7, 9; Fig. 21).219,220 There have been no clinical studies directly comparing sequential and simultaneous ablation techniques. However, sequential ablation has been shown to be an independent predictor of primary treatment failure with RF.221
Fig. 7.
HCC in a patient with cirrhosis during MW ablation. (A) At unenhanced CT, the MW antenna in the left liver is pointed at the stomach (arrowhead) and there is artificial ascites in the left upper quadrant. (B) The steam generated during MW ablation is also visible at CT (arrow).
Fig. 21.
(A) Enhanced CT in the late arterial (axial) and portal venous phase (coronal reformat) shows an exophytic HCC projecting from the inferior left liver (arrows), abutting the gastric wall (arrowhead). (B) Coronal enhanced CT immediately following ablation. Two trocar needles (arrowhead) were used as a mechanical lever to displace the stomach from the index ablation (arrow). (C) Enhanced CT in the late arterial (axial) and portal venous phase (coronal reformat) in follow-up after MW ablation. There is no evidence for LTP (arrow) and the gastric wall is normal (arrowhead).
Periprocedural monitoring
Monitoring ablations during treatment can facilitate complete ablation.222 During chemical and thermal ablation, US, CT, and MR imaging can be used to observe the growing ablation and confirm that the tumor and margin are sufficiently covered (see Figs. 1, 6–9, 14, 16). Evaluation of the ablation immediately following treatment with contrast-enhanced US, CT, or MR imaging is strongly recommended. If the tumor is inadequately treated, additional ablation should be performed at the same treatment session if possible (see Fig. 18).
POSTPROCEDURE SURVEILLANCE
There is no consensus on the optimal interval or frequency of postprocedure imaging. Because LTP is much more common in the first year, most investigators suggest that surveillance imaging in the first year should be more frequent.223,224 Be sides imaging, tumor markers such as alpha-fetoprotein for HCC and carcinoembryonic antigen and/or carbohydrate antigen 19–9 for CRLM, can identify disease progression and provide prognostic information.
Both CT and MR imaging are useful tools in postablation surveillance, each with unique advantages. CT is less prone to artifact compared with MR imaging and has the advantage of complete evaluation of the thorax in the same session. However, contrast-enhanced MR imaging has much better soft tissue characterization, offering improved assessment of the index ablation and progression of hepatic disease. Both CT and MR imaging are useful in the assessment of extrahepatic abdominal metastatic disease. The addition of PET-CT to surveillance in CRLM or breast cancer hepatic metastases is useful in the setting of increasing tumor marker levels if there is negative, equivocal, or indeterminate anatomic imaging (see Fig. 16). As a general rule, the postablation imaging modality can be based on the modality that best evaluated the tumor on preablation imaging.
Multiphase postcontrast imaging to include late arterial, portal venous, and delayed (3–5 minutes) phases should be considered after tumor ablation for hypervascular tumors including HCC and NET (see Figs. 8 and 21). This technique allows evaluation for both local and regional tumor progression. Growing peripheral nodular enhancement at the ablation margin indicates LTP (see Figs. 16 and 19). Dual-energy CT may improve detection of LTP because of improved definition of the ablation zone and higher lesion-to-liver contrast on the iodine map relative to standard blended images. Single-phase postcontrast CT (portal venous phase) is adequate for anatomic surveillance and restaging of CRLM and breast cancer hepatic metastases (see Figs. 2, 3, 9, 16). In addition to peripheral nodular enhancement, LTP can also present as enlargement of the index ablation. With MR imaging, hepatobiliary contrast (gadoxetic acid) is particularly useful in restaging hepatic metastatic disease (see Fig. 1).
SUMMARY
Physicians performing ablation should be familiar with the ablation devices they use and the technical aspects of the procedure that can improve primary effectiveness and reduce complications. At present, percutaneous ablation is first-line therapy for very early and early HCC and second-line therapy for CRLM, and is a treatment option for other hepatic tumors on a case-by-case basis. An understanding of the underlying tumor biology is important when weighing the potential benefits of ablation. Achieving a circumferential ablative margin, at least a 0.5 cm for HCC and at least 1.0 cm for liver metastases, decreases LTP. Surveillance imaging after ablation should be most frequent in the first year, when rates of LTP are the highest.
Fig. 13.
HCC in a patient with cirrhosis before, during, and after radiofrequency (RF) ablation. (A, B) Enhanced CTobtained for ablation planning immediately before RF electrode placement. Note that the contrast has washed out of the liver and is being excreted by the kidneys (arrow). (B) The only useful guide for placement of the RF electrode was retained ethiodized oil (arrow).(C)Enhanced CT1 month after RF ablation.The ablation encompasses the index lesion containing ethiodized oil, including a 5-mm margin (arrow).
KEY POINTS.
Percutaneous ablation is considered first-line therapy for very early and early hepatocellular carcinoma and second-line therapy for colorectal carcinoma liver metastases.
An understanding of the underlying tumor biology is important when weighing the potential benefits of ablation for other primary and secondary liver tumors.
Achieving a circumferential ablative margin, of at least a 0.5 cm for hepatocellular carcinoma and atleast 1.0 cm for liver metastases, decreases the incidence of local tumor progression.
Surveillance imaging after ablation should be more frequent in the first year, when rates of local tumor progression are the highest.
Acknowledgments
Disclosures: None (S.A. Wells, M.G. Lubner, T.J. Ziemlewicz); stockholder, member of the Board of Medical Advisors for NeuWave Medical (J.L. Hinshaw); stockholder, consultant for NeuWave Medical (C.L. Brace); patent holder and receives royalties from Covidien, stockholder and member of the Board of Directors for NeuWave Medical (F.T. Lee). This work was supported by grants R01CA142737 and R01CA149379 from the National Institutes of Health.
REFERENCES
- 1.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology 2014;273(1):241–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology 2013;266(2):462–70. [DOI] [PubMed] [Google Scholar]
- 3.Golberg A, Yarmush ML. Nonthermal irreversible electroporation: fundamentals, applications, and challenges. IEEE Trans Biomed Eng 2013;60(3): 707–14. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol 2012; 23(12):1613–21. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg SN, Kamel IR, Kruskal JB, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol 2002;179(1): 93–101. [DOI] [PubMed] [Google Scholar]
- 6.Takaki H, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas 5 cm or smaller: risk factors for local tumor progression. J Vasc Interv Radiol 2007; 18(7):856–61. [DOI] [PubMed] [Google Scholar]
- 7.Yamakado K, Nakatsuka A, Takaki H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 2008;247(1):260–6. [DOI] [PubMed] [Google Scholar]
- 8.Lewis MA, Hobday TJ. Treatment of neuroendocrine tumor liver metastases. Int J Hepatol 2012; 2012:973946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang G, Lin M, Xie X, et al. Combined radiofrequency ablation and ethanol injection with a multi-pronged needle for the treatment of medium and large hepatocellular carcinoma. Eur Radiol 2014; 24(7):1565–71. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrand P, Leibecke T, Kleemann M, et al. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol 2006;32(4):430–4. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 2004;239(4):441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228(1):235–40. [DOI] [PubMed] [Google Scholar]
- 13.Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or 54 cm. Gastroenterology 2004;127(6):1714–23. [DOI] [PubMed] [Google Scholar]
- 14.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129(1):122–30. [DOI] [PubMed] [Google Scholar]
- 15.Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54(8):1151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepato-cellular carcinoma: a randomized controlled trial. Scand J Gastroenterol 2008;43(6):727–35. [DOI] [PubMed] [Google Scholar]
- 17.Orlando A, Leandro G, Olivo M, et al. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol 2009;104(2):514–24. [DOI] [PubMed] [Google Scholar]
- 18.Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009;49(2):453–9. [DOI] [PubMed] [Google Scholar]
- 19.Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocellular carcinoma: a meta-analysis. J Hepatol 2010;52(3):380–8. [DOI] [PubMed] [Google Scholar]
- 20.Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004; 40(6):1352–60. [DOI] [PubMed] [Google Scholar]
- 21.Lencioni R, Cioni D, Della Pina C, et al. Hepatocellular carcinoma: new options for image-guided ablation. J Hepatobiliary Pancreat Sci 2010;17(4): 399–403. [DOI] [PubMed] [Google Scholar]
- 22.Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21(8 Suppl):S179–86. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J VascInterv Radiol 1998;9(1 Pt 1):101–11. [DOI] [PubMed] [Google Scholar]
- 24.Patterson EJ, Scudamore CH, Owen DA, et al. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg 1998;227(4):559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol 2013;36(2):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radio-frequency ablation of liver tumors. J Vasc Interv Radiol 2003;14(10):1267–74. [DOI] [PubMed] [Google Scholar]
- 27.Lu DS, Raman SS, Vodopich DJ, et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol 2002;178(1):47–51. [DOI] [PubMed] [Google Scholar]
- 28.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009; 38(3):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang J, Hynes K, Brace CL. Flow-dependent vascular heat transfer during microwave thermal ablation. Conf Proc IEEE Eng Med Biol Soc 2012; 2012:5582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YS, Rhim H, Cho OK, et al. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol 2006;59(3):432–41. [DOI] [PubMed] [Google Scholar]
- 31.Bhardwaj N, Strickland AD, Ahmad F, et al. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology 2009;41(2):168–72. [DOI] [PubMed] [Google Scholar]
- 32.Schramm W, Yang D, Wood BJ, et al. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Open Biomed Eng J 2007;1:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lubner MG, Brace CL, Ziemlewicz TJ, et al. Microwave ablation of hepatic malignancy. Semin Intervent Radiol 2013;30(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol 2008;19(7):1087–92. [DOI] [PubMed] [Google Scholar]
- 35.Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol 2014;83(3):552–8. [DOI] [PubMed] [Google Scholar]
- 36.Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 2012;35(4):868–74. [DOI] [PubMed] [Google Scholar]
- 37.Chiang J, Willey BJ, Del Rio AM, et al. Predictors of thrombosis in hepatic vasculature during microwave tumor ablation of an in vivo porcine model. J Vasc Interv Radiol 2014;25(12):1965–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang J, Brace C, Andreano A, et al. Ultrasound-based relative elastic modulus imaging for visualizing thermal ablation zones in a porcine model. Phys Med Biol 2010;55(8):2281–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Littrup PJ, Ahmed A, Aoun HD, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol 2007;18(3):383–92. [DOI] [PubMed] [Google Scholar]
- 40.Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol 2009;20(10):1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bageacu S, Kaczmarek D, Lacroix M, et al. Cryosurgery for resectable and unresectable hepatic metastases from colorectal cancer. Eur J Surg Oncol 2007;33(5):590–6. [DOI] [PubMed] [Google Scholar]
- 42.Bagia JS, Perera DS, Morris DL. Renal impairment in hepatic cryotherapy. Cryobiology 1998;36(4): 263–7. [DOI] [PubMed] [Google Scholar]
- 43.Chapman WC, Debelak JP, Wright Pinson C, et al. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann Surg 2000; 231(5):752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunne RM, Shyn PB, Sung JC, et al. Percutaneous treatment of hepatocellular carcinoma in patients with cirrhosis: a comparison of the safety of cryoablation and radiofrequency ablation. Eur J Radiol 2014;83(4):632–8. [DOI] [PubMed] [Google Scholar]
- 45.Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Br J Surg 2002;89(11): 1396–401. [DOI] [PubMed] [Google Scholar]
- 46.Sohn RL, Carlin AM, Steffes C, et al. The extent of cryosurgery increases the complication rate after hepatic cryoablation. Am Surg 2003;69(4):317–22 [discussion: 322–3]. [PubMed] [Google Scholar]
- 47.Washington K, Debelak JP, Gobbell C, et al. Hepatic cryoablation-induced acute lung injury: histopathologic findings. J Surg Res 2001;95(1):1–7. [DOI] [PubMed] [Google Scholar]
- 48.Xu KC, Niu LZ, He WB, et al. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol 2008;14(9):1430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Wang C, Lu Y, et al. Outcomes of ultrasound-guided percutaneous argon-helium cryoablation of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2012;19(6):674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127(12):2893–917. [DOI] [PubMed] [Google Scholar]
- 51.Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15(Suppl 4):5–13. [DOI] [PubMed] [Google Scholar]
- 52.Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and non-patient factors. Hepatology 2013;57(5):1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004;126(4):1005–14. [DOI] [PubMed] [Google Scholar]
- 54.Han KH, Kim do Y, Park JY, et al. Survival of hepatocellular carcinoma patients may be improved in surveillance interval not more than 6 months compared with more than 6 months: a 15-year prospective study. J Clin Gastroenterol 2013;47(6):538–44. [DOI] [PubMed] [Google Scholar]
- 55.Kim WR, Gores GJ, Benson JT, et al. Mortality and hospital utilization for hepatocellular carcinoma in the United States. Gastroenterology 2005;129(2): 486–93. [DOI] [PubMed] [Google Scholar]
- 56.Reig M, Darnell A, Forner A, et al. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin Liver Dis 2014;34(4):444–55. [DOI] [PubMed] [Google Scholar]
- 57.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42(5):1208–36. [DOI] [PubMed] [Google Scholar]
- 58.Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 2014;28(12):3429–34. [DOI] [PubMed] [Google Scholar]
- 59.Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82(9): 1379–84. [DOI] [PubMed] [Google Scholar]
- 60.Groeschl RT, Pilgrim CH, Hanna EM, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 2014;259(6):1195–200. [DOI] [PubMed] [Google Scholar]
- 61.Iida H, Aihara T, Ikuta S, et al. A comparative study of therapeutic effect between laparoscopic microwave coagulation and laparoscopic radiofrequency ablation. Hepatogastroenterology 2013; 60(124):662–5. [DOI] [PubMed] [Google Scholar]
- 62.Jiao DC, Zhou Q, Han XW, et al. Microwave ablation treatment of liver cancer with a 2,450-MHz cooled-shaft antenna: pilot study on safety and efficacy. Asian Pac J Cancer Prev 2012;13(2): 737–42. [DOI] [PubMed] [Google Scholar]
- 63.Poggi G, Montagna B, DI Cesare P, et al. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res 2013;33(3):1221–7. [PubMed] [Google Scholar]
- 64.Simo KA, Sereika SE, Newton KN, et al. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol 2011; 104(7):822–9. [DOI] [PubMed] [Google Scholar]
- 65.Takami Y, Ryu T, Wada Y, et al. Evaluation of intraoperative microwave coagulo-necrotic therapy (MCN) for hepatocellular carcinoma: a single center experience of 719 consecutive cases. J Hepatobiliary Pancreat Sci 2013; 20(3):332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 67.Ziemlewicz TJ, Hinshaw JL, Lubner MG, et al. Percutaneous microwave ablation of hepatocellular carcinoma with a gas-cooled system: initial clinical results with 107 tumors. J Vasc Interv Radiol 2015; 26(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho YK, Kim JK, Kim WT, et al. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology 2010;51(4):1284–90. [DOI] [PubMed] [Google Scholar]
- 69.Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: our experience and published work review. Hepatol Res 2015;45(1):59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226(2): 441–51. [DOI] [PubMed] [Google Scholar]
- 71.Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 2006; 43(5):1101–8. [DOI] [PubMed] [Google Scholar]
- 72.Kim SW, Rhim H, Park M, et al. Percutaneous radio-frequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: assessment of safety and therapeutic efficacy. Korean J Radiol 2009;10(4):366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer 2003;97(5):1253–62. [DOI] [PubMed] [Google Scholar]
- 74.Song KD, Lim HK, Rhim H, et al. Repeated hepatic resection versus radiofrequency ablation for recurrent hepatocellular carcinoma after hepatic resection: a propensity score matching study. Radiology 2015;275:599–608. [DOI] [PubMed] [Google Scholar]
- 75.Kurokohchi K, Watanabe S, Masaki T, et al. Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol 2005;11(10):1426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee JM, Lee YH, Kim YK, et al. Combined therapy of radiofrequency ablation and ethanol injection of rabbit liver: an in vivo feasibility study. Cardiovasc Intervent Radiol 2004;27(2):151–7. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed M, Weinstein J, Liu Z, et al. Image-guided percutaneous chemical and radiofrequency tumor ablation in an animal model. J Vasc Interv Radiol 2003;14(8):1045–52. [DOI] [PubMed] [Google Scholar]
- 78.Sakr AA, Saleh AA, Moeaty AA, et al. The combined effect of radiofrequency and ethanol ablation in the management of large hepatocellular carcinoma. Eur J Radiol 2005;54(3):418–25. [DOI] [PubMed] [Google Scholar]
- 79.Gasparini D, Sponza M, Marzio A, et al. Combined treatment, TACE and RF ablation, in HCC: preliminary results. Radiol Med 2002;104(5–6):412–20. [PubMed] [Google Scholar]
- 80.Lee MW, Kim YJ, Park SW, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol 2009;82(983):908–15. [DOI] [PubMed] [Google Scholar]
- 81.Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology 2012;262(2):689–700. [DOI] [PubMed] [Google Scholar]
- 82.Hoffmann R, Rempp H, Syha R, et al. Transarterial chemoembolization using drug eluting beads and subsequent percutaneous MR-guided radiofrequency ablation in the therapy of intermediate sized hepatocellular carcinoma. Eur J Radiol 2014;83(10):1793–8. [DOI] [PubMed] [Google Scholar]
- 83.Ginsburg M, Zivin SP, Wroblewski K, et al. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol 2015; 26(3):330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng ZW, Chen MS, Liang HH, et al. A case-control study comparing percutaneous radiofrequency ablation alone or combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Eur J Surg Oncol 2010;36(3):257–63. [DOI] [PubMed] [Google Scholar]
- 85.Zhang YJ, Liang HH, Chen MS, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology 2007;244(2):599–607. [DOI] [PubMed] [Google Scholar]
- 86.Fujimori M, Takaki H, Nakatsuka A, et al. Survival with up to 10-year follow-up after combination therapy of chemoembolization and radiofrequency ablation for the treatment of hepatocellular carcinoma: single-center experience. J Vasc Interv Radiol 2013;24(5):655–66. [DOI] [PubMed] [Google Scholar]
- 87.Fujimori M, Takaki H, Nakatsuka A, et al. Combination therapy of chemoembolization and radiofrequency ablation for the treatment of hepatocellular carcinoma in the caudate lobe. J Vasc Interv Radiol 2012;23(12):1622–8. [DOI] [PubMed] [Google Scholar]
- 88.Kang SG, Yoon CJ, Jeong SH, et al. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol 2009;20(12):1570–7. [DOI] [PubMed] [Google Scholar]
- 89.Ke S, Ding X, Gao J, et al. Solitary huge hepatocellular carcinomas 10 cm or larger may be completely ablated by repeated radiofrequency ablation combined with chemoembolization: initial experience with 9 patients. Mol Med Rep 2012; 5(3):832–6. [DOI] [PubMed] [Google Scholar]
- 90.Shiraishi R, Yamasaki T, Saeki I, et al. Pilot study of combination therapy with transcatheter arterial infusion chemotherapy using iodized oil and percutaneous radiofrequency ablation during occlusion of hepatic blood flow for hepatocellular carcinoma. Am J Clin Oncol 2008;31(4):311–6. [DOI] [PubMed] [Google Scholar]
- 91.Takaki H, Yamakado K, Uraki J, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol 2009;20(2):217–24. [DOI] [PubMed] [Google Scholar]
- 92.Kurokohchi K, Watanabe S, Masaki T, et al. Combination therapy of percutaneous ethanol injection and radiofrequency ablation against hepatocellular carcinomas difficult to treat. Int J Oncol 2002;21(3): 611–5. [PubMed] [Google Scholar]
- 93.Chung H, Kudo M, Minami Y, et al. Radiofrequency ablation combined with reduction of hepatic blood flow: effect of Lipiodol on coagulation diameter and ablation time in normal pig liver. Hepatogastroenterology 2007;54(75):701–4. [PubMed] [Google Scholar]
- 94.Guang C, Kawai N, Sato M, et al. Effect of interval between transcatheter hepatic arterial embolization and radiofrequency ablation on ablated lesion size in a swine model. Jpn J Radiol 2011;29(9): 649–55. [DOI] [PubMed] [Google Scholar]
- 95.Iwamoto T, Kawai N, Sato M, et al. Effectiveness of hepatic arterial embolization on radiofrequency ablation volume in a swine model: relationship to portal venous flow and liver parenchymal pressure. J Vasc Interv Radiol 2008;19(11):1646–51. [DOI] [PubMed] [Google Scholar]
- 96.Morimoto M, Numata K, Nozawa A, et al. Radiofrequency ablation of the liver: extended effect of transcatheter arterial embolization with iodized oil and gelatin sponge on histopathologic changes during follow-up in a pig model. J Vasc Interv Radiol 2010;21(11):1716–24. [DOI] [PubMed] [Google Scholar]
- 97.Mostafa EM, Ganguli S, Faintuch S, et al. Optimal strategies for combining transcatheter arterial chemoembolization and radiofrequency ablation in rabbit VX2 hepatic tumors. J Vasc Interv Radiol 2008;19(12):1740–8. [DOI] [PubMed] [Google Scholar]
- 98.Nakai M, Sato M, Sahara S, et al. Radiofrequency ablation in a porcine liver model: effects of trans-catheter arterial embolization with iodized oil on ablation time, maximum output, and coagulation diameter as well as angiographic characteristics. World J Gastroenterol 2007;13(20):2841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamanaka T, Yamakado K, Takaki H, et al. Ablative zone size created by radiofrequency ablation with and without chemoembolization in small hepatocellular carcinomas. Jpn J Radiol 2012; 30(7):553–9. [DOI] [PubMed] [Google Scholar]
- 100.Toyoda M, Kakizaki S, Horiuchi K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol 2006;12(4):608–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamakado K, Nakatsuka A, Ohmori S, et al. Radio-frequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol 2002;13(12):1225–32. [DOI] [PubMed] [Google Scholar]
- 102.Liu HC, Shan EB, Zhou L, et al. Combination of percutaneous radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: observation of clinical effects. Chin J Cancer Res 2014;26(4):471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lencioni R, Crocetti L, Petruzzi P, et al. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: a pilot clinical study. J Hepatol 2008;49(2):217–22. [DOI] [PubMed] [Google Scholar]
- 104.Kim JH, Kim PN, Won HJ, et al. Viable hepatocellular carcinoma around retained iodized oil after transarterial chemoembolization: radiofrequency ablation of viable tumor plus retained iodized oil versus viable tumor alone. AJR Am J Roentgenol 2014;203(5):1127–31. [DOI] [PubMed] [Google Scholar]
- 105.Kelly ME, Spolverato G, Lê GN, et al. Synchronous colorectal liver metastasis: a network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J Surg Oncol 2015; 111(3):341–51. [DOI] [PubMed] [Google Scholar]
- 106.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27(22): 3677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma S, Camci C, Jabbour N. Management of hepatic metastasis from colorectal cancers: an update. J Hepatobiliary Pancreat Surg 2008;15(6): 570–80. [DOI] [PubMed] [Google Scholar]
- 108.Lewis AM, Martin RC. The treatment of hepatic metastases in colorectal carcinoma. Am Surg 2006; 72(6):466–73. [PubMed] [Google Scholar]
- 109.Mavros MN, de Jong M, Dogeas E, et al. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg 2013; 100(5):711–8. [DOI] [PubMed] [Google Scholar]
- 110.Tanaka K, Adam R, Shimada H, et al. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg 2003; 90(8):963–9. [DOI] [PubMed] [Google Scholar]
- 111.Sanoff HK, McLeod HL. Predictive factors for response and toxicity in chemotherapy: pharmacogenomics. Semin Colon Rectal Surg 2008;19(4): 226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vigano L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 2014;21(4):1276–86. [DOI] [PubMed] [Google Scholar]
- 113.Dexiang Z, Li R, Ye W, et al. Outcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive cases. Ann Surg Oncol 2012;19(9): 2860–8. [DOI] [PubMed] [Google Scholar]
- 114.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008; 371(9617):1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013; 14(12):1208–15. [DOI] [PubMed] [Google Scholar]
- 116.Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 2008;26(30):4906–11. [DOI] [PubMed] [Google Scholar]
- 117.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006; 24(31):4976–82. [DOI] [PubMed] [Google Scholar]
- 118.Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23(10):2619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gleisner AL, Choti MA, Assumpcao L, et al. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 2008;143(12):1204–12. [DOI] [PubMed] [Google Scholar]
- 120.Agcaoglu O, Aliyev S, Karabulut K, et al. Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: an analysis of 395 patients. World J Surg 2013; 37(6):1333–9. [DOI] [PubMed] [Google Scholar]
- 121.Hammill CW, Billingsley KG, Cassera MA, et al. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol 2011;18(7):1947–54. [DOI] [PubMed] [Google Scholar]
- 122.Otto G, Düber C, Hoppe-Lotichius M, et al. Radio-frequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 2010;251(5):796–803. [DOI] [PubMed] [Google Scholar]
- 123.Kim KH, Yoon YS, Yu CS, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc 2011;81(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reuter NP, Woodall CE, Scoggins CR, et al. Radio-frequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg 2009;13(3):486–91. [DOI] [PubMed] [Google Scholar]
- 125.Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197(6):728–36. [DOI] [PubMed] [Google Scholar]
- 126.Oshowo A, Gillams A, Harrison E, et al. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 2003;90(10):1240–3. [DOI] [PubMed] [Google Scholar]
- 127.Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol 2012;30(7):567–74. [DOI] [PubMed] [Google Scholar]
- 128.Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265(3):958–68. [DOI] [PubMed] [Google Scholar]
- 129.Veltri A, Sacchetto P, Tosetti I, et al. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol 2008;31(5):948–56. [DOI] [PubMed] [Google Scholar]
- 130.Nielsen K, van Tilborg AA, Meijerink MR, et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg 2013;37(6):1340–7. [DOI] [PubMed] [Google Scholar]
- 131.Gillams AR, Lees WR. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol 2008; 19(5):712–7. [DOI] [PubMed] [Google Scholar]
- 132.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36(1): 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rind G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman TF, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon (France): International Agency for Research on Cancer (IARC); 2010. p. 13. [Google Scholar]
- 134.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190(4):432–45. [DOI] [PubMed] [Google Scholar]
- 135.Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 1998; 187(1):88–92 [discussion: 92–3]. [DOI] [PubMed] [Google Scholar]
- 136.Givi B, Pommier SJ, Thompson AK, et al. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery 2006;140(6):891–7 [discussion: 897–8]. [DOI] [PubMed] [Google Scholar]
- 137.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010; 17(12):3129–36. [DOI] [PubMed] [Google Scholar]
- 138.Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010; 12(6):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gedaly R, Daily MF, Davenport D, et al. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg 2011;146(8):953–8. [DOI] [PubMed] [Google Scholar]
- 140.Lang H, Oldhafer KJ, Weimann A, et al. Liver transplantation for metastatic neuroendocrine tumors. Ann Surg 1997;225(4):347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Le Treut YP, Delpero JR, Dousset B, et al. Results of liver transplantation in the treatment of metastatic neuroendocrine tumors. A 31-case French multi-centric report. Ann Surg 1997;225(4):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marin C, Robles R, Fernández JA, et al. Role of liver transplantation in the management of unresectable neuroendocrine liver metastases. Transplant Proc 2007;39(7):2302–3. [DOI] [PubMed] [Google Scholar]
- 143.van Vilsteren FG, Baskin-Bey ES, Nagorney DM, et al. Liver transplantation for gastroenteropancreatic neuroendocrine cancers: defining selection criteria to improve survival. Liver Transpl 2006; 12(3):448–56. [DOI] [PubMed] [Google Scholar]
- 144.Sward C, Johanson V, Nieveen van Dijkum E, et al. Prolonged survival after hepatic artery embolization in patients with midgut carcinoid syndrome. Br J Surg 2009;96(5):517–21. [DOI] [PubMed] [Google Scholar]
- 145.Christante D, Pommier S, Givi B, et al. Hepatic artery chemoinfusion with chemoembolization for neuroendocrine cancer with progressive hepatic metastases despite octreotide therapy. Surgery 2008;144(6):885–93 [discussion: 893–4]. [DOI] [PubMed] [Google Scholar]
- 146.Loewe C, Schindl M, Cejna M, et al. Permanent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. AJR Am J Roentgenol 2003;180(5):1379–84. [DOI] [PubMed] [Google Scholar]
- 147.de Baere T, Deschamps F, Teriitheau C, et al. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol 2008;19(6):855–61. [DOI] [PubMed] [Google Scholar]
- 148.Kennedy AS, Dezarn WA, McNeillie P, et al. Radio-embolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31(3):271–9. [DOI] [PubMed] [Google Scholar]
- 149.Kennedy A, Bester L, Salem R, et al. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-liver-metastases consensus conference. HPB (Oxford) 2015;17(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247(6):1029–35. [DOI] [PubMed] [Google Scholar]
- 151.Gaur SK, Friese JL, Sadow CA, et al. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc Intervent Radiol 2011;34(3): 566–72. [DOI] [PubMed] [Google Scholar]
- 152.Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer 2005;104(8):1590–602. [DOI] [PubMed] [Google Scholar]
- 153.Akyildiz HY, Mitchell J, Milas M, et al. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery 2010;148(6):1288–93 [discussion: 1293]. [DOI] [PubMed] [Google Scholar]
- 154.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 155.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol 2008;19(12):2012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chua TC, Saxena A, Liauw W, et al. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer 2011;47(15):2282–90. [DOI] [PubMed] [Google Scholar]
- 157.Singletary SE, Walsh G, Vauthey JN, et al. A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist 2003;8(3):241–51. [DOI] [PubMed] [Google Scholar]
- 158.Ruiterkamp J, Ernst MF. The role of surgery in metastatic breast cancer. Eur J Cancer 2011;47(Suppl 3):S6–22. [DOI] [PubMed] [Google Scholar]
- 159.Pentheroudakis G, Fountzilas G, Bafaloukos D, et al. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat 2006;97(3): 237–44. [DOI] [PubMed] [Google Scholar]
- 160.Dittmar Y, Altendorf-Hofmann A, Schüle S, et al. Liver resection in selected patients with metastatic breast cancer: a single-centre analysis and review of literature. J Cancer Res Clin Oncol 2013;139(8): 1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.O’Rourke TR, Tekkis P, Yeung S, et al. Long-term results of liver resection for non-colorectal, nonneuroendocrine metastases. Ann Surg Oncol 2008;15(1):207–18. [DOI] [PubMed] [Google Scholar]
- 162.Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg 2008;247(5): 732–8. [DOI] [PubMed] [Google Scholar]
- 163.Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol 2007;14(12):3345–51. [DOI] [PubMed] [Google Scholar]
- 164.Ruiterkamp J, Ernst MF, van de Poll-Franse LV, et al. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol 2009;35(11):1146–51. [DOI] [PubMed] [Google Scholar]
- 165.Livraghi T, Goldberg SN, Solbiati L, et al. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology 2001;220(1):145–9. [DOI] [PubMed] [Google Scholar]
- 166.Cui Y, Zhou LY, Dong MK, et al. Ultrasonography guided percutaneous radiofrequency ablation for hepatic cavernous hemangioma. World J Gastroenterol 2003;9(9):2132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gao J, Ding X, Ke S, et al. Radiofrequency ablation in the treatment of large hepatic hemangiomas: a comparison of multitined and internally cooled electrodes. J Clin Gastroenterol 2014; 48(6):540–7. [DOI] [PubMed] [Google Scholar]
- 168.Gao J, Ke S, Ding XM, et al. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery 2013;153(1):78–85. [DOI] [PubMed] [Google Scholar]
- 169.Park SY, Tak WY, Jung MK, et al. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol 2011;54(3): 559–65. [DOI] [PubMed] [Google Scholar]
- 170.Tang XY, Wang Z, Wang T, et al. Efficacy, safety and feasibility of ultrasound-guided percutaneous microwave ablation for large hepatic hemangioma. J Dig Dis 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 171.Ziemlewicz TJ, Wells SA, Lubner MA, et al. Microwave ablation of giant hepatic cavernous hemangiomas. Cardiovasc Intervent Radiol 2014;37(5): 1299–305. [DOI] [PubMed] [Google Scholar]
- 172.Liau SS, Qureshi MS, Praseedom R, et al. Molecular pathogenesis of hepatic adenomas and its implications for surgical management. J Gastrointest Surg 2013;17(10):1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rhim H, Lim HK, Kim YS, et al. Percutaneous radio-frequency ablation of hepatocellular adenoma: initial experience in 10 patients. J Gastroenterol Hepatol 2008;23(8 Pt 2):e422–7. [DOI] [PubMed] [Google Scholar]
- 174.van Vledder MG, van Aalten SM, Terkivatan T, et al. Safety and efficacy of radiofrequency ablation for hepatocellular adenoma. J Vasc Interv Radiol 2011;22(6):787–93. [DOI] [PubMed] [Google Scholar]
- 175.Ikeda K, Seki T, Umehara H, et al. Clinicopathologic study of small hepatocellular carcinoma with microscopic satellite nodules to determine the extent of tumor ablation by local therapy. Int J Oncol 2007;31(3):485–91. [PubMed] [Google Scholar]
- 176.Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95(9):1931–7. [DOI] [PubMed] [Google Scholar]
- 177.Maeda T, Takenaka K, Taguchi K, et al. Small hepatocellular carcinoma with minute satellite nodules. Hepatogastroenterology 2000;47(34):1063–6. [PubMed] [Google Scholar]
- 178.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 2007;188(2):480–8. [DOI] [PubMed] [Google Scholar]
- 179.Liu CH, Arellano RS, Uppot RN, et al. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol 2010;20(4):877–85. [DOI] [PubMed] [Google Scholar]
- 180.Ke S, Ding XM, Qian XJ, et al. Radiofrequency ablation of hepatocellular carcinoma sized >3 and </= 5 cm: is ablative margin of more than 1 cm justified? World J Gastroenterol 2013;19(42): 7389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Z, Ahmed M, Sabir A, et al. Computer modeling of the effect of perfusion on heating patterns in radiofrequency tumor ablation. Int J Hyperthermia 2007;23(1):49–58. [DOI] [PubMed] [Google Scholar]
- 182.Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005;242(2):158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shirabe K, Takenaka K, Gion T, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg 1997; 84(8):1077–80. [PubMed] [Google Scholar]
- 184.Smolock AR, Lubner MG, Ziemlewicz TJ, et al. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. AJR Am J Roentgenol 2015;204(1):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li M, Yu XL, Liang P, et al. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 2012;28(3):218–26. [DOI] [PubMed] [Google Scholar]
- 186.Kim PN, Choi D, Rhim H, et al. Planning ultrasound for percutaneous radiofrequency ablation to treat small (</= 3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: a multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol 2012;23(5):627–34. [DOI] [PubMed] [Google Scholar]
- 187.Tang Z, Fang H, Kang M, et al. Percutaneous radio-frequency ablation for liver tumors: is it safer and more effective in low-risk areas than in high-risk areas? Hepatol Res 2011;41(7):635–40. [DOI] [PubMed] [Google Scholar]
- 188.Kang TW, Rhim H, Kim EY, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol 2009;10(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Head HW, Dodd GD 3rd, Dalrymple NC, et al. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology 2007;243(3):877–84. [DOI] [PubMed] [Google Scholar]
- 190.Hinshaw JL, Laeseke PF, Winter TC 3rd, et al. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol 2006; 186(5 Suppl):S306–10. [DOI] [PubMed] [Google Scholar]
- 191.Kitchin D, Lubner M, Ziemlewicz T, et al. Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia 2014;30(5):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kang TW, Rhim H, Lee MW, et al. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol 2011;196(4):907–13. [DOI] [PubMed] [Google Scholar]
- 193.Song I, Rhim H, Lim HK, et al. Percutaneous radio-frequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol 2009; 19(11):2630–40. [DOI] [PubMed] [Google Scholar]
- 194.Rhim H, Lim HK, Kim YS, et al. Percutaneous radio-frequency ablation with artificial ascites for hepato-cellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol 2008;190(1): 91–8. [DOI] [PubMed] [Google Scholar]
- 195.Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging 2008;33(4):428–36. [DOI] [PubMed] [Google Scholar]
- 196.Uehara T, Hirooka M, Ishida K, et al. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol 2007;42(4):306–11. [DOI] [PubMed] [Google Scholar]
- 197.Takaki H, Yamakado K, Nakatsuka A, et al. Frequency of and risk factors for complications after liver radiofrequency ablation under CT fluoroscopic guidance in 1500 sessions: single-center experience. AJR Am J Roentgenol 2013;200(3):658–64. [DOI] [PubMed] [Google Scholar]
- 198.Shibata T, Shibata T, Maetani Y, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol 2004;15(11):1323–7. [DOI] [PubMed] [Google Scholar]
- 199.de Baere T, Dromain C, Lapeyre M, et al. Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology 2005; 236(2):666–70. [DOI] [PubMed] [Google Scholar]
- 200.Marchal F, Elias D, Rauch P, et al. Biliary lesions during radiofrequency ablation in liver. Study on the pig. Eur Surg Res 2004;36(2):88–94. [DOI] [PubMed] [Google Scholar]
- 201.Marchal F, Elias D, Rauch P, et al. Prevention of biliary lesions that may occur during radiofrequency ablation of the liver: study on the pig. Ann Surg 2006;243(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ogawa T, Kawamoto H, Kobayashi Y, et al. Prevention of biliary complication in radiofrequency ablation for hepatocellular carcinoma-Cooling effect by endoscopic nasobiliary drainage tube. Eur J Radiol 2010;73(2):385–90. [DOI] [PubMed] [Google Scholar]
- 203.Kingham TP, Karkar AM, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg 2012; 215(3):379–87. [DOI] [PubMed] [Google Scholar]
- 204.Akahane M, Koga H, Kato N, et al. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics 2005;25(Suppl 1): S57–68. [DOI] [PubMed] [Google Scholar]
- 205.Ryan ER, Sofocleous CT, Schöder H, et al. Split-dose technique for FDG PET/CT-guided percutaneous ablation: a method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology 2013;268(1): 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu FY, Yu XL, Liang P, et al. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia 2010;26(5): 448–55. [DOI] [PubMed] [Google Scholar]
- 207.Krucker J, Xu S, Venkatesan A, et al. Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol 2011;22(4):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Venkatesan AM, Kadoury S, Abi-Jaoudeh N, et al. Real-time FDG PET guidance during biopsies and radiofrequency ablation using multimodality fusion with electromagnetic navigation. Radiology 2011; 260(3):848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yu X, Liu F, Liang P, et al. Microwave ablation assisted by a computerised tomography-ultrasonography fusion imaging system for liver lesions: an ex vivo experimental study. Int J Hyperthermia 2011;27(2): 172–9. [DOI] [PubMed] [Google Scholar]
- 210.Minami Y, Kudo M. Ultrasound fusion imaging of hepatocellular carcinoma: a review of current evidence. Dig Dis 2014;32(6):690–5. [DOI] [PubMed] [Google Scholar]
- 211.Clasen S, Rempp H, Boss A, et al. MR-guided radiofrequency ablation of hepatocellular carcinoma: long-term effectiveness. J Vasc Interv Radiol 2011; 22(6):762–70. [DOI] [PubMed] [Google Scholar]
- 212.Clasen S, Rempp H, Hoffmann R, et al. Image-guided radiofrequency ablation of hepatocellular carcinoma (HCC): is MR guidance more effective than CT guidance? Eur J Radiol 2014;83(1):111–6. [DOI] [PubMed] [Google Scholar]
- 213.Lee MW, Rhim H, Cha DI, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol 2012;198(6): 1438–44. [DOI] [PubMed] [Google Scholar]
- 214.Mauri G, Cova L, De Beni S, et al. Real-Time US CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with us: results in 295 cases. Cardiovasc Intervent Radiol 2015; 38(1):143–51. [DOI] [PubMed] [Google Scholar]
- 215.Rempp H, Clasen S, Pereira PL. Image-based monitoring of magnetic resonance-guided thermoablative therapies for liver tumors. Cardiovasc Intervent Radiol 2012;35(6):1281–94. [DOI] [PubMed] [Google Scholar]
- 216.Song KD, Lee MW, Rhim H, et al. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol 2013;201(5):1141–7. [DOI] [PubMed] [Google Scholar]
- 217.Terraz S, Cernicanu A, Lepetit-Coiffé M, et al. Radiofrequency ablation of small liver malignancies under magnetic resonance guidance: progress in targeting and preliminary observations with temperature monitoring. Eur Radiol 2010;20(4): 886–97. [DOI] [PubMed] [Google Scholar]
- 218.Wu B, Xiao YY, Zhang X, et al. Magnetic resonance imaging-guided percutaneous cryoablation of hepatocellular carcinoma in special regions. Hepatobiliary Pancreat Dis Int 2010;9(4):384–92. [PubMed] [Google Scholar]
- 219.Brace CL, Sampson LA, Hinshaw JL, et al. Radio-frequency ablation: simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol 2009;20(1):118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010; 21(8 Suppl):S192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fernandes ML, Lin CC, Lin CJ, et al. Risk of tumour progression in early-stage hepatocellular carcinoma after radiofrequency ablation. Br J Surg 2009;96(7):756–62. [DOI] [PubMed] [Google Scholar]
- 222.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013; 34(1):11–29. [DOI] [PubMed] [Google Scholar]
- 223.Boas FE, Do B, Louie JD, et al. Optimal imaging surveillance schedules after liver-directed therapy for hepatocellular carcinoma. J Vasc Interv Radiol 2015;26(1):69–73. [DOI] [PubMed] [Google Scholar]
- 224.Liu D, Fong DY, Chan AC, et al. Hepatocellular carcinoma: surveillance CT schedule after hepatectomy based on risk stratification. Radiology 2015; 274(1):133–40. [DOI] [PubMed] [Google Scholar]