Abstract
In the ASPIRE trial, antiretroviral therapy (ART) switch to dolutegravir plus lamivudine (DTG+3TC) was comparable to 3-drug ART in maintaining viral suppression by standard viral load assays. We used an ultrasensitive assay to assess whether this switch led to increased residual viremia. At entry, levels of residual viremia did not differ significantly between arms (DTG+3TC vs 3-drug ART: mean, 5.0 vs 4.2 HIV-1 RNA copies/mL; P = .64). After randomization, no significant between-group differences were found at either week 24 or 48. These results show no evidence for increased viral replication on DTG+3TC and support its further investigation as a dual ART strategy.
Keywords: ART simplification, ART switch, dolutegravir, lamivudine, residual viremia
Two-drug antiretroviral treatment (ART) is an investigational strategy to maintain HIV suppression while reducing long-term antiretroviral drug exposure, cost, and adverse events. The Antiretroviral Strategy to Promote Improvement and Reduce Exposure (ASPIRE) study was a randomized, 48-week, controlled pilot trial that assessed the efficacy of the 2-drug regimen of dolutegravir and lamivudine (DTG+3TC) in participants who were virologically suppressed on a standard 3-drug ART regimen [1]. Virologic outcomes showed that switching to DTG+3TC was comparable to continuation of standard 3-drug maintenance therapy in maintaining viral suppression. This was supported by the Food and Drug Administration snapshot analyses, in which 93% of DTG+3TC participants maintained viral suppression at week 24 vs 91% for 3-drug ART, and 91% vs 89% at week 48, respectively. One case of virologic failure was detected in each arm, with no emergence of resistance mutations.
One potential concern about reducing treatment from 3 to 2 drugs is loss of complete viral suppression. This may be detected by use of a more sensitive viral load assay [2]. Furthermore, viral blips have been reported after switching to DTG+3TC, although this was not observed in the ASPIRE trial [1, 3]. Thus, we quantified longitudinal changes in residual viremia by the ultrasensitive integrase single-copy viral load assay to determine whether ART switch to DTG+3TC in ASPIRE participants resulted in increased low-level viral replication.
METHODS
Study Population and Samples
ASPIRE was an open-label, randomized, multicenter, 48-week clinical trial that enrolled HIV-1-infected adults who were virologically suppressed on any US Department of Health and Human Services (DHHS)–recommended/alternative or other 3-drug regimen for at least 48 weeks (NCT02263326) [1]. Inclusion criteria included age ≥18 years, ≥2 HIV-1 RNA measurements <50 copies/mL within 48 weeks of study entry, and a screening HIV-1 RNA <20 copies/mL. Exclusion criteria included a history of virologic failure (VF) after 1 year of treatment, pretreatment reverse transcriptase (RT) resistance mutation, or known integrase resistance mutations. Participants were randomized 1:1 to switch to open-label oral DTG 50 mg plus 3TC 300 mg once daily or to continue their current 3-drug ART. Postentry monitoring included plasma HIV-1 RNA using the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0 (lower detection limit, 20 copies/mL; Roche Molecular Systems), safety labs, and 4-day adherence recall. The institutional review board of each participating institution approved this study, and each participant provided informed consent.
Quantification of Residual Viremia
Low-level plasma HIV viremia were measured on plasma at study entry and at weeks 24 and 48 of treatment using the validated ultrasensitive integrase single-copy assay (iSCA; limit of detection, 0.5 HIV-1 RNA copies/mL) [4], commonly used to assess levels of residual viral load [5–12]. Plasma from the participants was spiked with an internal replication-competent ASLV long terminal repeat with a splice acceptor (RCAS) virion as a control for RNA extraction efficiency [13]. Real-time polymerase chain reactions were performed with a Roche LightCycler 480 system using primers and probes specific to a conserved region of the HIV integrase gene [4].
Statistical Methods
Differences in residual viremia between the treatment groups were analyzed by fitting a linear model accounting for possible within-patient correlation using a generalized least squares fit. We included the study time point and treatment arm in the model. Those who discontinued ART during the study or in which iSCA measurement was not available at at least 1 time point were excluded from this analysis. The participant characteristics between treatment groups were compared using the Mann-Whitney U test for continuous variables and chi-square test for categorical variables.
RESULTS
Of the 89 participants randomized to the ASPIRE study, 7 discontinued their randomized ART due to virologic rebound (n = 2), adverse events (n = 1), or noncompliance (n = 4). Additionally, 10 participants were excluded due to the lack of iSCA measurement at one or more time points. A total of 72 participants with iSCA data at study entry, week 24, and week 48 were included in the current analysis. The median CD4+ count was 663 cells/mm3, and prior ART exposure (median, 6.0 years) consisted of integrase inhibitors (40%), non-nucleoside reverse transcriptase inhibitors (29%), and protease inhibitor–based regimens (31%) at the time of study entry (Table 1).
Table 1.
Participant Characteristics
| Characteristic | DTG+3TC (n = 36) | 3-Drug ART (n = 36) | P |
|---|---|---|---|
| Age, median, y | 45.5 | 50.5 | .67 |
| Male sex, % | 92 | 86 | .71 |
| Ethnicity, % | .33 | ||
| White | 58 | 72 | |
| Black | 39 | 28 | |
| Asian | 3 | 0 | |
| CD4+ cell count, median, cells/mm3 | 677 | 637 | .09 |
| Duration of viral suppression, median, y | 5.37 | 6.04 | .82 |
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; DTG, dolutegravir.
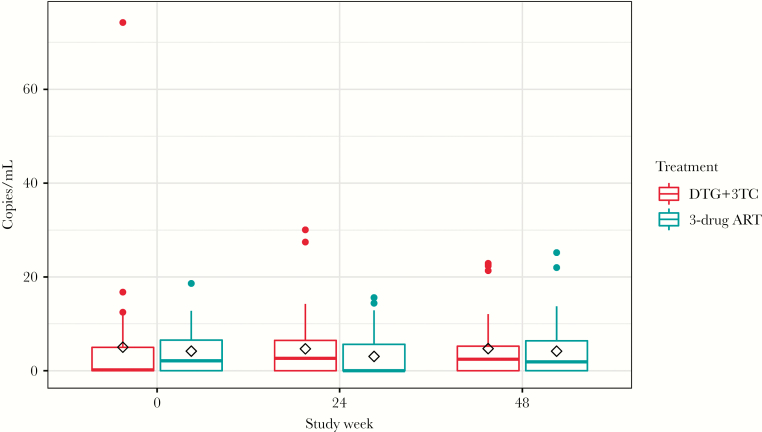
At baseline, the mean residual viremia did not differ significantly between study arms (DTG+3TC vs 3-drug ART: mean, 5.0 vs 4.2 HIV-1 RNA copies/mL; P = .64). After ART switch, no significant differences were found in the residual viremia when comparing the DTG+3TC vs 3-drug ART arms, adjusting for baseline values (mean viral load change at week 24, 1.6 copies/mL; 95% confidence interval [CI], –1.9 to 5.2; P = .37; mean viral load change at week 48, 0.5 copies/mL; 95% CI, –3.0 to 4.1; P = .76) (Figure 1). Furthermore, no significant changes in residual viremia were found after stratification by duration of prior ART treatment or CD4+ count (Supplementary Figure 1). Finally, we analyzed residual viremia as a dichotomous variable (detectable or undetectable) and found no significant differences in residual viremia detectability status between baseline and either 24 or 48 weeks in the DTG+3TC arm vs 3-drug ART arm (Supplementary Table 1).
Figure 1.
Levels of HIV viral load by the ultrasensitive integrase single-copy assay by treatment arm at study entry, 24 and 48 weeks after antiretroviral therapy switch. Tukey’s box and whisker plots; box limits: interquartile range (IQR); middle line: median; diamond: mean; vertical lines: adjacent values (1st quartile −1.5 IQR; 3rd quartile +1.5 IQR). Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; DTG, dolutegravir.
CONCLUSIONS
We have previously reported that in the ASPIRE randomized trial, switching to the 2-drug DTG+3TC regimen was comparable to continuation of a standard 3-drug maintenance therapy [1]. Using the ultrasensitive iSCA viral load assay, we found no evidence for increased low-level viral replication after a switch to DTG+3TC, as reflected by stable levels of residual viremia.
There has been a concerning report that ART switch to a 2-drug regimen may lead to increased low-level viral replication, which could eventually select for drug resistance or lead to virologic rebound [2]. Data from studies using 2-drug regimens as maintenance therapy are mixed. In a study of switching suppressed patients to raltegravir (RAL) plus maraviroc (MVC), increased virologic failure was observed despite preswitch assessment of viral tropism [2]. By contrast, switching to DTG+rilipvirine (RPV) [14] or a boosted PI+3TC [15, 16] has been a successful strategy. In addition, no significant changes in levels of systemic inflammation [17] or differences in HIV DNA decline [18] were observed. It is possible that the success of certain 2-drug regimens results from at least 1 of the agents having a high resistance barrier, a characteristic shared by DTG and boosted PIs. Our results support the virologic efficacy of selected 2-drug regimens and are consistent with multiple studies now demonstrating that DTG+3TC is an effective option either for treatment-naïve individuals [19, 20] or as a maintenance therapy [1, 21].
The use of DTG+3TC as a maintenance regimen likely has several benefits, including decreasing costs [21, 22] and avoidance of side effects associated with abacavir or tenofovir. For example, the switch to a 2-drug therapy has been reported to improve renal function and bone mineral density when tenofovir disoproxil fumarate is avoided [15], and improvements in immune function and metabolic markers have also been found with decreased nucleos(t)ide reverse transcriptase (NRTI) exposure [21, 23]. It is important to note that the ASPIRE trial excluded individuals with any history of NRTI genotypic resistance mutations, especially in light of a recent report showing that a history of M184V resistance was associated with an increased probability of viral blips in those switching to DTG+3TC [24]. This was not associated with a significantly increased risk of virologic failure, but the sample size was relatively limited, and DTG+3TC treatment should not be recommended in those with a history of resistance mutations against 3TC. Another limitation of this study is that sampling of nonperipheral blood compartments was limited to the genital tract. A combined genital tract analysis was performed for the ASPIRE participants and those in the AIDS Clinical Trials Group A5353 study of DTG+3TC for ART-naïve individuals. The frequency of genital HIV RNA shedding while virologically suppressed was similar between those on a standard 3-drug ART and those on DTG+3TC as either initial or maintenance therapy [25].
In summary, the ASPIRE trial has demonstrated that DTG+3TC is effective in maintaining viral suppression, as measured either by conventional or ultrasensitive viral load assays. These results support further investigation of DTG+3TC dual therapy for maintenance of viral suppression, as is being done in the fully powered TANGO study (NCT03446573).
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by an investigator-sponsored study grant from ViiV HealthCare to Northwestern University (NU). V.C.M., C.J.F., C.A.B., T.W., S.L.K., J.C., J.Z.L., and P.E.S. have received grant funding for this study to their institutions through NU from ViiV/GSK. V.C.M. has received funding from the Emory CFAR (P30AI050409).
Potential conflicts of interest. V.C.M. has received research funding from Gilead. B.O.T. has served as a paid consultant to ViiV, Gilead, and Janssen and on the Clinical Care Options speakers bureau and has received grant funding to his institution from ViiV/ GlaxoSmithKline (GSK). C.B.M. has received a consulting fee from NU. C.J.F. has received payment for service on the Clinical Care Options speakers bureau and grant funding to his institution from ViiV, Gilead, Merck, Janssen, and Pfizer. C.A.B. has received payment for GSK board membership and Infectious Diseases Society of America board membership, speakers bureau, and travel and is an associate editor for Clinical Infectious Diseases; she has received grant funding to her institution from Gilead. T.W. has served as a paid consultant for ViiV/GSK and has received grant funding to his institution from ViiV/GSK, Gilead, and Bristol-Myers Squibb; his spouse holds stock options at Johnson & Johnson. S.L.K. has received grant funding to her institution from Gilead. E.P.A. reported nonfinancial support from GSK/ViiV. J.Z.L. has served as a paid consultant and received grant funding from Gilead and Merck. P.E.S. has served as a paid consultant to Gilead, Abbvie, Merck, Janssen, ViiV/GSK, and Bristol-Myers Squibb and has received grant funding to his institution from Gilead and ViiV/GSK. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir plus lamivudine maintains human immunodeficiency virus-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis 2018; 66:1794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campillo-Gimenez L, Assoumou L, Valantin MA, et al. ROCnRAL ANRS 157 Study Group Switch to maraviroc/raltegravir dual therapy leads to an unfavorable immune profile with low-level HIV viremia. AIDS 2015; 29:853–6. [DOI] [PubMed] [Google Scholar]
- 3. Blanco JL, Rojas J, Paredes R, et al. DOLAM Study Team Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73:1965–71. [DOI] [PubMed] [Google Scholar]
- 4. Cillo AR, Vagratian D, Bedison MA, et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol 2014; 52:3944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li JZ, Gallien S, Ribaudo H, et al. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014; 28:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henrich TJ, Hu Z, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis 2013; 207:1694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 2014; 161:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li JZ, Heisey A, Ahmed H, et al. ACTG A5197 Study Team Relationship of HIV reservoir characteristics with immune status and viral rebound kinetics in an HIV therapeutic vaccine study. AIDS 2014; 28:2649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li JZ, Arnold KB, Lo J, et al. Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis 2015; 2(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salantes DB, Zheng Y, Mampe F, et al. HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J Clin Invest 2018; 128:3102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scully EP, Rutishauser RL, Simoneau CR, et al. Inconsistent HIV reservoir dynamics and immune responses following anti-PD-1 therapy in cancer patients with HIV infection. Ann Oncol 2018; 29:2141–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 2003; 41:4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839–49. [DOI] [PubMed] [Google Scholar]
- 15. Fabbiani M, Gagliardini R, Ciccarelli N, et al. Atazanavir/ritonavir with lamivudine as maintenance therapy in virologically suppressed HIV-infected patients: 96 week outcomes of a randomized trial. J Antimicrob Chemother 2018; 73:1955–64. [DOI] [PubMed] [Google Scholar]
- 16. Pulido F, Ribera E, Lagarde M, et al. DUAL-GESIDA-8014-RIS-EST45 Study Group Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression: randomized, open-label, noninferiority DUAL-GESIDA 8014-RIS-EST45 trial. Clin Infect Dis 2017; 65:2112–8. [DOI] [PubMed] [Google Scholar]
- 17. Belmonti S, Lombardi F, Quiros-Roldan E, et al. Systemic inflammation markers after simplification to atazanavir/ritonavir plus lamivudine in virologically suppressed HIV-1-infected patients: ATLAS-M substudy. J Antimicrob Chemother 2018; 73:1949–54. [DOI] [PubMed] [Google Scholar]
- 18. Lombardi F, Belmonti S, Quiros-Roldan E, et al. AtLaS-M Study Group Evolution of blood-associated HIV-1 DNA levels after 48 weeks of switching to atazanavir/ritonavir+lamivudine dual therapy versus continuing triple therapy in the randomized AtLaS-M trial. J Antimicrob Chemother 2017; 72:2055–9. [DOI] [PubMed] [Google Scholar]
- 19. Taiwo BO, Zheng L, Stefanescu A, et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA <500000 copies/mL. Clin Infect Dis 2018; 66:1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cahn P, Madero JS, Arribas JR, et al. GEMINI Study Team Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. [DOI] [PubMed] [Google Scholar]
- 21. Maggiolo F, Gulminetti R, Pagnucco L, et al. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis 2017; 17:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis 2016; 62:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quiros-Roldan E, Magro P, Raffetti E, et al. Biochemical and inflammatory modifications after switching to dual antiretroviral therapy in HIV-infected patients in Italy: a multicenter retrospective cohort study from 2007 to 2015. BMC Infect Dis 2018; 18:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borghetti A, Baldin G, Lombardi F, et al. Efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multicentre cohort of patients with suppressed HIV-1 replication. HIV Med 2018; XXX(X):XXX–XX. [DOI] [PubMed] [Google Scholar]
- 25. Gianella S, Marconi VC, Berzins B, et al. Genital HIV-1 shedding with dolutegravir (DTG) plus lamivudine (3TC) dual therapy. J Acquir Immune Defic Syndr 2018; 79:e112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.