Abstract
Tick-borne illnesses are increasing but are often underreported. Few cases of babesiosis have been reported from Pennsylvania. Our 4-hospital system in southeastern Pennsylvania saw a rise in cases from 7 or fewer yearly in 2008–2014 to 26 cases in 2015. There appear to be multiple potential causes of this increase in frequency.
Keywords: acorn production, Babesia, climate impact, Pennsylvania, tick-borne diseases
Vectorborne diseases transmitted by blood-feeding insects and ticks have been rising in the United States [1]. The parasite Babesia microti is transmitted by the bite of the tick Ixodes scapularis in the United States and causes a febrile illness with chills and fatigue, though clinical history and examination are often nondiagnostic [2]. Laboratory findings include normal to low leukocyte counts, thrombocytopenia, and elevated liver transaminases, but these are also nonspecific to babesiosis [2]. B. microti may be co-transmitted with other tick-borne diseases such as Borrelia burgdorferi, the agent of Lyme disease [3]. Concurrent tick infection with B. burgdorferi may in fact promote the transmission of B. microti [4].
For decades, there has been geographic spread of B. microti outward from the Massachusetts area [5], and it is also prevalent in the upper Midwestern United States. However, until recently, there was little evidence of B. microti in Pennsylvania. During 1997 to 2012, nearly 2000 Ixodes scapularis ticks found on military personnel were tested for tick-borne pathogens [6]. None of the 533 ticks from Ft. Indiantown Gap in east central Pennsylvania tested positive for B. microti, compared with 0.2%–0.6% of a comparable number at Ft. McCoy, Wisconsin, and 2.5%–3.3% at Camp Ripley, Minnesota.
In fall 2013, sampling of 1363 adult ticks from Pennsylvania showed that 47.4% were positive for B. burgdoferi and 3.5% harbored B. microti. Some 2% were coinfected with both B. burgdorferi and B. microti, 1.5% with both B. burgdorferi and Anaplasma phagocytophilum, but only 1 tick (0.07%) had both B. microti and A. phagocytophilum [7]. B. microti prevalence was highest in north central Pennsylvania at 5.5%, while the rate in southeastern Pennsylvania was near average, at 3.7% [7]. Large numbers of deer in Pennsylvania, along with the presence of Ixodes ticks, have led to numerous cases of Lyme disease in the state. Despite known tick coinfections with B. burgdorferi and B. microti, relatively few human cases of babesiosis have been reported in the state. A series of 3 Babesia cases were reported in a study from 2013 [8], which cited only 39 cases statewide from 2005 to 2013, but babesiosis is not a reportable illness in Pennsylvania.
Main Line Health System (MLHS) is located in southeastern Pennsylvania just outside Philadelphia. The 4 MLHS acute care hospitals encountered 26 cases of babesiosis in 2015, whereas no more than 7 cases had been seen in any of the preceding 7 years. This prompted us to review our babesiosis experience with a special emphasis on epidemiologic data that might explain the increase and also on effectiveness of clinical management.
METHODS
After institutional review board approval, a list of positive Babesia tests was obtained from the Clinical Microbiology Laboratory. This detailed 88 individual patients from 2008 to 2017. All cases were diagnosed via blood smear examination. Retrospective chart review located records for 84 emergency department and/or hospitalized patients; the remaining 4 were outpatients with very limited clinical information available.
RESULTS
Geographic Distribution of Babesiosis Cases
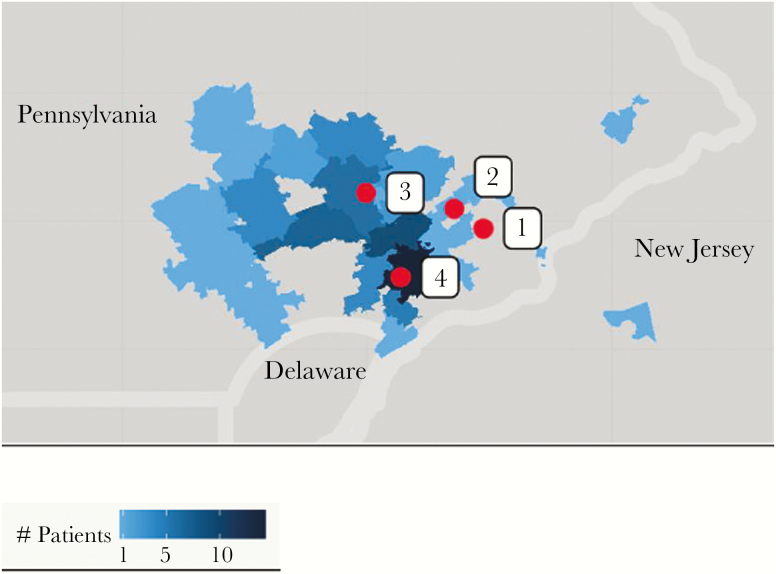
Table 1 shows the distribution of positive Babesia smears by year and submitting hospital for all patients (including 4 outpatients). Hospital 3 is the most rural of the 4 hospitals and diagnosed the most cases of babesiosis; in contrast, Hospital 1 is suburban and adjacent to the City of Philadelphia and saw the fewest. Hospitals 2 and 4 are in relatively suburban locations. The ZIP codes of the home addresses of the babesiosis patients are plotted in Figure 1, which shows the greatest density of patients around Hospitals 3 and 4.
Table 1.
Patients (n = 88) With Positive Blood Smears for Babesia by Year and Hospital
| Year | Hospital 1 | Hospital 2 | Hospital 3 | Hospital 4a | Total Cases |
|---|---|---|---|---|---|
| 2008 | 1 | 1 | |||
| 2009 | 1 | 1 | 2 | ||
| 2010 | 1 | 1 | |||
| 2011 | 2 | 4 | 6 | ||
| 2012 | 3 | 2 | 2 | 7 | |
| 2013 | 3 | 1 | 4 | 2 | 10 |
| 2014 | 1 | 2 | 1 | 4 | |
| 2015 | 2 | 7 | 11 | 6 | 26 |
| 2016 | 1 | 7 | 4 | 12 | |
| 2017 | 5 | 11 | 3 | 19 | |
| Total | 8 | 22 | 36 | 22 | 88 |
aNo data available before 2011.
Figure 1.
Density of Babesia cases by patient ZIP code of residence, 2008–2017. Hospitals in red designated 1, 2, 3, and 4 correspond to the text and Table 1. Hospital 1 is the closest to the City of Philadelphia.
Clinical Presentation
The 84 reviewed babesiosis patients had a median age (range) of 69 (22–100) years, and 71.4% were male. Risk factors for tick-borne diseases identified on admission included outdoor activities, known tick exposure, and prior Lyme disease, but information was lacking in the majority of cases. The group had a median (range) of 7 (0–42) symptomatic days, often with nondiagnostic outpatient evaluations, before hospitalization. Most patients had a high fever (75% >101oF, 56% >102oF), but physical examination was usually unremarkable. Leukocyte counts were generally low (16.7% of patients <3800/uL) or normal (76.2% of patients 3800 to 10 500/uL), and only 7.1% exceeded 10 500/uL. Low platelet counts were common (89.2% <150 000/uL, 80.6% <110 000/uL) and could be extreme (48.2% <70 000/uL, 21.7% <40 000/uL). Some 75.9% of patients had elevated transaminases, but sometimes only minimally. Concurrent Lyme disease was very common (50% of 84 patients tested).
Patients were classified as “severely ill” if they required transfusion of multiple units of blood products or met criteria published by Mareedu et al. [9], which include intensive care unit care, intubation, tracheostomy, shock, heart failure, acute respiratory distress syndrome, dialysis, or exchange transfusion. The 23 severely ill patients averaged 8.9 days in hospital after starting Babesia treatment, compared with the remaining 61 patients, who averaged 4.6 days on inpatient therapy. Severely ill patients were also older (average age, 71.8 years vs 65.7 years), though presence of concurrent Lyme disease was not significantly different (52.2% vs 49.2%). A greater percentage of the severely ill patients had underlying immunosuppression (22% = 5 of 23, consisting of chronic lymphocytic leukemia s/p splenectomy, splenectomy alone, 2 with corticosteroid therapy, and 1 with undefined immunocompromise) vs the remaining patients (6.6% = 4 of 61, consisting of 2 with lymphoma, splenectomy, and thalessemia). Degree of parasitemia >4% did correlate with worsened illness severity. Though a few patients had received blood transfusions before admission, no transfusion-related cases were identified, and there were no deaths.
Diagnosis and Management
Time to diagnosis from first contact with a health care provider improved from an average of 8.1 days before 2013 to 4.5 days in 2013–2014, and then 2.1 days in 2015–2017. However, the average number of outpatient visits before diagnosis only declined from 1.93 to 1.62 over the study period. The possibility of babesiosis causing low platelet counts was often not recognized on presentation, and this laboratory abnormality was most commonly attributed to “viral syndrome” or intrinsic marrow suppression. Lyme disease testing was commonly done, but testing for Ehrlichia and Anaplasma infections was inconsistent; even in 2017, only 57% of patients were tested.
DISCUSSION
Updated information from the Pennsylvania Health Department shows an increase in yearly Babesia cases from 35 in 2011 to 80 in 2017 (Pennsylvania Department of Health, unpublished data); however, due to lack of required reporting, this is likely an underestimate. The Pennsylvania Department of Health analysis of 244 confirmed and probable cases of babesiosis from 2011 to 2017 revealed 73% from southeastern counties, 20% from the northeast, and 8% from central and western counties. The Department of Public Health of Philadelphia, where babesiosis is reportable, noted only 1–3 Babesia infections yearly from 2013 to 2016, but 5 cases in 2017 [10].
Time to babesiosis diagnosis improved in the Main Line Health System as more Babesia cases were encountered, but the possibility of concurrent illnesses, especially anaplasmosis, was not always considered. Additional laboratory testing for Babesia m., including polymerase chain reaction (PCR) and antibody-based studies, has become available in recent years but has not been widely used, as this is done by special request with a delay in results. Only 1 of the 84 patients with smear-proven babesiosis had a Babesia m. PCR sent, and none were tested for Babesia antibody; however, the use of these tests in patients in whom babesiosis was eventually ruled out is not known.
Babesiosis has increased in southeastern Pennsylvania, with a rise in 2015 likely in part reflecting ongoing geographic spread. Goethert et al. reported that a specific parasitic lineage was responsible for expansion in the northeastern United States but did not speculate on causes [11]. Wisconsin reported a rise in Babesia cases starting in 2011, partly due to better reporting, and increased frequency there has persisted [12]. Forest fragmentation due to suburbanization has also increased the contact of humans with ticks, leading to more babesiosis cases [13].
Climate change, including warmer, wetter weather, has been proposed as a cause of increased infection frequency [14]. Increased acorn production (“mast”) has also been suggested as a driver of increased Lyme disease cases in the eastern United States [15] and southeastern New York [16]. The rise in these cases lags the large “mast,” as the increased number of mammals supported by the acorns is associated with more questing-stage nymphal ticks to transmit infection the following year.
A review of rainfall and temperature in Pennsylvania showed much higher than average values for some periods during 2010–2012 and predicted very large acorn crops in 2013–2015 [17]. In addition, another review stated that the winters of 2015–2016, 2016–2017, and 2017–2018 were warmer than usual and predicted greater survival of tick hosts and longer tick seasons [18]. Although circumstantial, these reports may help explain the persistent increase in babesiosis cases seen by our health system starting in 2015.
Health care providers and public health authorities in Pennsylvania should be aware of the rising number of Babesia cases, frequent concurrent Lyme disease, and the possible predictive value of monitoring acorn production and mouse populations. Babesiosis should be made a reportable disease in Pennsylvania to improve management strategies, as has been advocated by clinicians [19] and the Council of State and Territorial Epidemiologists [20].
Supplementary Material
Acknowledgments
Authors’ contributions. H.H.L.: case review, data analysis, manuscript writing; L.C.: manuscript writing and review; O.G.: microbiologic data, manuscript review; G.D.: pathology information, manuscript review; P.M.: patient epidemiology, manuscript review; E.A.N.: data from PA State Department of Health, manuscript review; K.V.: forest ecology data, manuscript review; L.K.: case review, data analysis, manuscript writing and review.
Financial support. This work was supported by the Sharpe-Strumia Research Foundation of the Bryn Mawr Hospital, Bryn Mawr, Pennsylvania (grant numbers SSRF2017-05, SSRF2018-03 to L.K., H.H.L.).
Potential conflicts of interest. H.H.L.: advisory boards for Melinta, Tetraphase, Shionogi Pharmaceuticals; L.K.: advisory board for Merck; L.C.: none; O.G.: none; G.D.: none; P.M.: none; E.A.N.: none; K.V.: none. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. Preliminary study results were presented at the 18th International Congress on Infectious Diseases; March 2018; Buenos Aires, Argentina.
References
- 1. Rosenberg R, Lindsey NP, Fischer M, et al. Vital signs: trends in reported vectorborne diseases cases – United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 2018; 67:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vannier E, Krause PJ. Human babesiosis. New Eng J Med 2012; 366:397–407. [DOI] [PubMed] [Google Scholar]
- 3. Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol 2016; 32:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn JM, Krause PJ, Davis S, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the Northeastern United States. PLoS One 2014; 9:e115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diuk-Wasser MA, Liu Y, Steeves TK, et al. Monitoring human babesiosis emergence through vector surveillance, New England, USA. Emerg Infect Dis 2014; 20:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stromdahl E, Harner S, Jenkins S, et al. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the Midwestern and the Northeastern United States over a 15 year period (1997–2012). J Parasit Vectors 2014; 7:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hutchinson ML, Strohecker MD, Simmons TW, et al. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J Med Entomol 2015; 52:693–8. [DOI] [PubMed] [Google Scholar]
- 8. Acosta ME, Ender PT, Smith EM, Jahre JA. Babesia microti infection, Eastern Pennsylvania, USA. Emerg Infect Dis 2013; 19:1105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mareedu N, Schotthoefer AM, Tompkins J, et al. Risk factors for severe infection, hospitalization, and prolonged antimicrobial therapy in patients with babesiosis. Am J Trop Med Hyg 2017; 97:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.City of Philadelphia, Department of Public Health, Philadelphia, PA. https://phila.gov/DataReports/TickborneDiseases. Accessed 23 December 2018.
- 11. Goethert HK, Molloy P, Berardi V, et al. Zoonotic Babesia microti in the Northeastern U.S.: evidence for the expansion of a specific parasite lineage. PLoS One 2018; 13:e0193837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein E, Elbadawi LI, Kazmierczak JK, Davis JP. Babesiosis surveillance – Wisconsin, 2001–2015. MMWR Morb Mortal Wkly Rep 2017; 66:687–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh MG. The relevance of forest fragmentation on the incidence of human babesiosis: investigating the landscape epidemiology of an emerging tick-borne disease. Vector Borne Zoonotic Dis 2013; 13:250-55. [DOI] [PubMed] [Google Scholar]
- 14. Fisman DN, Tuite AR, Brown KA. Impact of El Niño Southern Oscillation on infectious disease hospitalization risk in the United States. Proc Natl Acad Sci U S A 2016; 113:14589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones CG, Ostfeld RS, Richard MP, et al. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 1998; 279:1023–6. [DOI] [PubMed] [Google Scholar]
- 16. Ostfeld RS, Levi T, Keesing F, et al. Tick-borne disease risk in a forest food web. Ecology 2018; 99:1562–73. [DOI] [PubMed] [Google Scholar]
- 17.Frantz V. State College, PA: AccuWeather. https://accuweather.com/en/weather-news/acorn-production-hindcast-not/80849. October 10, 2012. Accessed 23 December 2018.
- 18.IGeneX, Inc., Milpitas, CA. https://igenex.com/ticktalk/2018/01/12/current-trends-leading-to-an-increase-in-tick-borne-diseases/. January 12, 2018. Accessed 23 December 2018.
- 19. Sherr VT. Human babesiosis–an unrecorded reality. Absence of formal registry undermines its detection, diagnosis and treatment, suggesting need for immediate mandatory reporting. Med Hypotheses 2004; 63:609–15. [DOI] [PubMed] [Google Scholar]
- 20.Kemperman MM, DeMaria Jr A, Kazmierczak J, Feldman K, Slavinski S, Danila R, Brown C. Atlanta, GA: Council of State and Territorial Epidemiologists. https:// c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/10-ID-27.pdf. Accessed 22 December 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.