Abstract
Background
The objective of this study was to investigate the difference in disease severity between influenza A and B among hospitalized adults using a novel ordinal scale and existing clinical outcome end points.
Methods
A prospective, observational study was conducted over the 2016–2018 influenza seasons in a central hospital. The primary outcome was the rate of clinical improvement, defined as a decline of 2 categories from admission on a 7-category ordinal scale that ranges from 1 (discharged with normal activity) to 7 (death), or hospital discharge up to day 28.
Results
In total, 574 eligible patients were enrolled, including 369 (64.3%) influenza A cases and 205 (35.7%) influenza B cases. The proportion of patients with a worse ordinal scale at admission was higher in influenza A than influenza B (P = .0005). Clinical improvement up to 28 days occurred in 82.4% of patients with influenza A and 90.7% of patients with influenza B (P = .0067). The Cox model indicated that influenza B patients had a higher clinical improvement probability than influenza A cases (adjusted hazard ratio [HR], 1.266; 95% confidence interval [CI], 1.019–1.573; P = .0335). A similar pattern was observed in weaning oxygen supplement (adjusted HR, 1.285; 95% CI, 1.030–1.603; P = .0261). In-hospital mortality for influenza A was marginally higher than influenza B (11.4% vs 6.8%; P = .0782).
Conclusions
Our findings indicated that hospitalized patients with influenza A were more ill and had delayed clinical improvement compared with those with influenza B virus infection.
Keywords: clinical outcomes, hospitalization, influenza B virus, influenza infection, mortality
Seasonal influenza is a common acute respiratory tract infection that leads to about 291 243–645 832 respiratory deaths globally each year [1]. Currently, strains from 2 subtypes of influenza A (H3N2, H1N1) and 2 lineages of influenza B viruses (Yamagata, Victoria) are the major causes of seasonal epidemics [2]. However, whether the illness severity caused by these influenza viruses is clinically similar in adults is controversial. For example, epidemiologic studies [1–3] indicate that influenza A (H3N2) subtype infections have caused higher influenza-associated hospitalizations and mortality among seasonal viruses, whereas recent hospital-based studies [4, 5] have suggested that clinical outcomes such as length of stay, mortality, pneumonia, hospitalization, intensive care unit (ICU) admission, and death did not differ by virus type. A systematic analysis pointed out that most studies have been based on population influenza surveillance or limited numbers of clinical cases and concluded that little evidence existed to show differences in the severity of illness caused by seasonal influenza viruses [6]. Therefore, more comprehensive studies are required to evaluate the comparative severity of illness caused by the 2 viruses in those hospitalized. During the 2017–2018 season, the percentage of clinical laboratory–tested specimens positive for the B/Yamagata lineage increased markedly in China [7], largely consistent with findings from the US Centers for Disease Control and Prevention [8]. Consequently, we conducted a prospective observational study to compare the clinical features and outcomes between hospitalized patients with laboratory-confirmed influenza A and B virus infection.
Recently, a novel ordinal scale end point of hospitalized patient status was introduced in a post hoc analysis of outcomes in a randomized controlled trial (RCT) of immune plasma [9]. This scale placed patients into 1 of 7 mutually exclusive clinical categories, ranging from 1 (discharge from hospital with usual function) to 7 (death). Although the RCT did not find statistically significant differences in the time to resolution of respiratory insufficiency (defined as normalization of both respiratory rate [≤20 breaths per min for adults or below the age-defined thresholds of 20–38 breaths per min for children] and SpO2 ≥93% on room air and resolution of tachypnea), the immune plasma group showed significant improvements in outcomes on the 7-day ordinal scale compared with those not receiving plasma. Subsequently, similar versions have also been used as the primary end point in several large influenza clinical trials of therapeutics for hospitalized patients (registered at Clinical.Trials.gov: NCT03376321; NCT03684044). Though the specific form and utility of the ordinal end point scale are yet to be fully validated [10], it provides a new way to assess clinical outcomes of hospitalized patients with influenza virus infection. However, the distribution of patients across the clinical status categories is obviously influenced by reasons for hospitalization, the duration and severity of illness, and treatments (antivirals, supportive care) provided. Consequently, it is possible that using a single fixed day to assess the ordinal scale could miss clinically relevant differences between patient groups of interest.
Consequently, we compared the difference in clinical severity between hospitalized patients with laboratory-confirmed influenza A and B virus infection by measuring clinical improvement based on the modified 7-category ordinal scale as the primary end point, along with other existing outcome measures.
METHODS
Study Population and Ethical Approval
A prospective observational study was conducted among hospitalized adult patients with laboratory-confirmed, seasonal influenza infection at the China-Japan Friendship Hospital. Patients admitted to the China-Japan Friendship Hospital between October 1, 2016, and June 1, 2018, were recruited. According to current guidance [11], hospitalized influenza patients were treated with neuraminidase inhibitors (NAIs) as soon as possible if they visited or were hospitalized within 48 hours from illness onset. Otherwise, the use of antiviral therapy depended on the physician. In this study, the choice of antivirals was not restricted, and dosage of oseltamivir was either the standard or double dosing regimen (75/150 mg twice daily for 5 days). As this study was an observational study, other treatments and clinical decision-making (ie, indication of IMV, ECMO, and discharge) were conducted according to guidance and local clinical practices.
Ethical approval was obtained from the China-Japan Friendship Hospital Ethics Committee (Approval No. 2015–85). As an observational study, written informed consent was exempted, and only routine clinical microbiology and laboratory tests and collection of respiratory samples were permitted.
Virological Investigations
Respiratory specimens (including nasopharyngeal swab, sputum, bronchoalveolar lavage fluid, and endotracheal aspirate) were collected for detection of influenza viral RNA by real-time reverse transcription polymerase chain reaction (rRT-PCR) in our laboratory. Virus RNA was extracted from a 140-μL sample using the QIAamp Viral RNA Mini Kit (Qiagen, Hiden, Germany), from which 5 μL was used as a template for real-time amplification and detection using the SensiFAST Probe One-Step Kit (Bioline, London, UK) on the LightCycler 480 II system (Roche, Basel, Switzerland). The primers and probes targeted at FluA and FluB were provided by the Chinese National Influenza Center and the WHO Collaborating Centre for Reference and Research on Influenza. Details of laboratory confirmation of influenza virus were described previously [12]. To monitor influenza viral clearance, respiratory samples were collected daily for therapeutic monitoring and infection control. We defined the interval between symptom onset and the date of the last viral RNA-positive result of respiratory samples as the duration of viral RNA detectability.
Data Collection and Definitions
Once recruited, clinical information was recorded systematically each day from admission using a standardized electronic case record form, which included demographic characteristics, medical comorbidities, symptom/fever onset time, incident complications, requirements for oxygen therapy and ventilatory support, antiviral treatments, clinical responses, and outcomes.
The primary outcome was the integrated rate of clinical improvement up to 28 days after admission. Clinical improvement (the event) was defined as a decline of 2 categories on the modified 7-category ordinal scale of clinical status [9] from admission, or hospital discharge, whichever came first. Thus, the 7-category ordinal scale consisted of mutually exclusive categories as follows: category 7, death; 6, ICU hospitalization, requiring ECMO and/or invasive mechanical ventilation; 5, ICU hospitalization, not requiring ECMO and/or invasive mechanical ventilation; 4, non-ICU hospitalization, requiring supplemental oxygen; 3, non-ICU hospitalization, not requiring supplemental oxygen; 2, not hospitalized, but unable to resume normal activities; 1, not hospitalized with resumption of normal activities. The secondary outcomes included clinical status, assessed by the ordinal scale at fixed time points (days 7, 14, 21, and 28), time to hospital discharge or ICU discharge alive (if admitted to ICUs), time to weaning from supplemental oxygen supplement, incidence of influenza-related pneumonia, the rate of ARDS, the proportion of ICU admissions, and in-hospital mortality.
Statistical Analysis
Continuous variables were expressed as median (interquartile range [IQR]), and categorical variables were expressed as number (proportion). Two-group comparisons (influenza A vs B) were conducted by the Mann-Whitney U test or χ2 test, as appropriate. Differences between rates of continuing ICU stay, continuing mechanical ventilation, in-hospital survival, and time to undetectable viral RNA of 2 viruses were portrayed by Kaplan-Meier curves and tested by log-rank tests, respectively. Then the difference between influenza A and B of the rate of clinical improvement was compared using unadjusted and adjusted ordinal logistic regression models and Cox proportional hazard models separately between the 2 virus infections from day 1 to day 28, and so did the difference for other outcomes.
All statistical tests were 2-sided, and probabilities of less than .05 were considered to be statistically significant. Statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc.), unless otherwise indicated.
RESULTS
Admission Characteristics
Between October 1, 2016, to June 1, 2018, we enrolled 574 laboratory-confirmed influenza patients, which included 369 (64.3%) influenza A and 205 (35.7%) influenza B cases. The median age of these patients (IQR) was 63 (50–76) years. The median age (IQR) of patients with influenza A was 61 (48–74) years, significantly lower than those with influenza B (64 [55–77] years; P = .0303). There were 300 men (52.3%). The median days from illness onset to hospitalization in patients with influenza A virus infection was significantly longer than those with influenza B (median days, 5.9 vs 3.7; P = .0079). The proportions of people with diabetes, chronic obstructive pulmonary disease, heart disease, and pregnant women were similar between the 2 groups (Table 1). More patients with influenza B reported a history of malignancy, compared with those with influenza A (24.4% vs 10.3%; P < .001).
Table 1.
Baseline Characteristics of Study Patients on Admission
| Characteristics | Total | Flu A | Flu B | P |
|---|---|---|---|---|
| (n = 574) | (n = 369) | (n = 205) | ||
| Age, median (IQR), y | 63.0 (50.0–76.0) | 61.0 (48.0–74.0) | 64.0 (55.0–77.0) | .0303 |
| Male gender | 300 (52.3) | 195 (52.8) | 105 (51.2) | .7086 |
| Days from illness onset to hospitalization, median (IQR) | 5.4 (1.7–9.5) | 5.9 (2.4–9.9) | 3.7 (1.6–7.7) | .0079 |
| Comorbidities | ||||
| Hypertension | 237 (41.3) | 156 (42.3) | 81 (39.5) | .5192 |
| Heart disease | 146 (25.4) | 90 (24.4) | 56 (27.3) | .4404 |
| Diabetes | 164 (28.6) | 104 (28.2) | 60 (29.3) | .7830 |
| Chronic obstructive lung disease | 57 (9.9) | 39 (10.6) | 18 (8.8) | .4924 |
| Chronic kidney disease | 41 (7.2) | 28 (7.6) | 13 (6.3) | .5727 |
| Malignancies | 88 (15.3) | 38 (10.3) | 50 (24.4) | <.0001 |
| Oral (not inhaled) glucocorticoids before admission | 45 (7.8) | 28 (7.6) | 17 (8.3) | .7635 |
| Pregnancy | 4 (0.7) | 1 (0.3) | 3 (1.5) | .1099 |
| Cerebrovascular disease | 66 (11.5) | 43 (11.7) | 23 (11.3) | .8919 |
| Chronic liver disease | 163 (28.4) | 111 (30.1) | 52 (25.4) | .2300 |
| Symptoms and signs | ||||
| Axillary temperature >39°C | 119 (20.7) | 91 (24.7) | 28 (13.7) | .0018 |
| Respiratory rate >24/min | 30 (5.8) | 26 (7.9) | 4 (2.1) | .0070 |
| Pulse ≥125 beats/min | 22 (4.0) | 18 (5.1) | 4 (2.0) | .0773 |
| Systolic blood pressure <90 mmHg | 9 (1.7) | 4 (1.2) | 5 (2.7) | .2276 |
| Laboratory findings | ||||
| White blood cell count, ×109/L | ||||
| 4–10 | 369 (64.9) | 243 (66.4) | 126 (62.1) | .0556 |
| <4 | 80 (14.1) | 42 (11.5) | 38 (18.7) | |
| >10 | 120 (21.1) | 81 (22.1) | 39 (19.2) | |
| Neutrophil count, ×109/L | 4.5 (2.9, 7.2) | 4.6 (3.2, 7.5) | 4.2 (2.7, 6.2) | .0183 |
| Lymphocyte count, ×109/L | ||||
| ≥1.5 | 196 (34.5) | 130 (35.6) | 66 (32.5) | .4558 |
| <1.5 | 372 (65.5) | 235 (64.4) | 137 (67.5) | |
| Platelet count, ×109/L | ||||
| ≥100 | 521 (91.7) | 341 (93.2) | 180 (89.1) | .0927 |
| <100 | 47 (8.3) | 25 (6.8) | 22 (10.9) | |
| Creatinine, umol/L | ||||
| ≤133 | 516 (91.3) | 329 (90.9) | 187 (92.1) | .6170 |
| >133 | 49 (8.7) | 33 (9.1) | 16 (7.9) | |
| Aspartate aminotransferase, U/L | ||||
| ≤40 | 438 (77.9) | 270 (74.8) | 168 (83.6) | .016 |
| >40 | 124 (22.1) | 91 (25.2) | 33 (16.4) | |
| Lactate dehydrogenase, U/L | ||||
| ≤245 | 293 (63.3) | 184 (61.7) | 109 (66.1) | .3562 |
| >245 | 170 (36.7) | 114 (38.3) | 56 (33.9) | |
| Creatine kinase, U/L | ||||
| ≤185 | 370 (81.0) | 230 (78.0) | 140 (86.4) | .0277 |
| >185 | 87 (19.0) | 65 (22.0) | 22 (13.6) |
Data are expressed as No. (%) or median (IQR). P values were calculated by Mann-Whitney U test or chi-square test, where appropriate.
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SBP, systolic blood pressure.
Illness Measures
The proportions with abnormal physical signs (including axillary temperature over 39°C and respiratory rate over 24 beats per minute) were higher in patients with influenza A than those with influenza B (Table 1). The proportion of those admitted to general hospital wards without supplemental oxygen was lower in influenza A (72.3%) than B (86.8%) virus infections (Table 2). The proportion of patients falling into a worse category of ordinal scale at day 1 was significantly higher in patients with influenza A than those with influenza B (P = .0005).
Table 2.
Treatments and Outcomes
| Treatments and Outcomes | Total | Flu A | Flu B | P |
|---|---|---|---|---|
| (n = 574) | (n = 369) | (n = 205) | ||
| NAI used | 461 (80.3) | 301 (81.6) | 160 (78.0) | .3091 |
| Oral oseltamivir | 454 (79.1) | 297 (80.5) | 157 (76.6) | .2706 |
| Days from illness onset to starting antiviral treatment, median (IQR) | 5.4 (1.7–9.5) | 5.9 (2.4–9.9) | 3.7 (1.6–7.7) | .0079 |
| Early NAI (≤2 days of symptom onset) | 121 (26.4) | 73 (24.5) | 48 (30.0) | .2028 |
| Late NAI (>2 days of symptom onset) | 337 (73.6) | 225 (75.5) | 112 (70.0) | .2028 |
| Time to viral RNA detection, median (IQR), d | 10.6 (9.7–14.4) | 10.7 (9.7–11.6) | 10.0 (8.6–11.6) | .9961a |
| Antibiotic | 481 (84.1) | 316 (85.9) | 165 (80.9) | .1183 |
| Oral corticosteroids | 197 (34.4) | 133 (36.1) | 64 (31.4) | .2503 |
| Influenza-related pneumonia on admission | 228 (39.8) | 171 (46.5) | 57 (27.8) | <.0001 |
| ARDS on admission | 124 (21.7) | 101 (27.5) | 23 (11.2) | <.0001 |
| ICU admission | 113 (19.7) | 91 (24.7) | 22 (10.7) | <.0001 |
| ICU length of stay, median (IQR), d | 10.8 (5.8–17.7) | 9.7 (5.8–19.4) | 12.6 (4.9–15.9) | .9624 |
| Hospital length of stay, median (IQR), d | 13.0 (8.0–17.0) | 13.0 (9.0–18.0) | 12.0 (8.0–16.0) | .2258 |
| Days from admission to discharge alive, median (IQR), d | 13.0 (8.0–17.0) | 13.0 (8.0–17.0) | 12.0 (8.0–16.0) | .4137 |
| Days from admission to death, median (IQR), d | 12.5 (9.0–22.5) | 14.0 (10.0–23.0) | 10.5 (8.0–16.0) | .3435 |
| Duration from admission to clinical improvement, median (IQR), d | 13 (12–13) | 13 (12–13) | 13 (11–13) | .0217a |
| DNR | 9 (1.6) | 8 (2.2) | 1 (0.5) | .0893 |
| Day 28 mortality | 46 (8.2) | 34 (9.5) | 12 (5.9) | .199 |
| In-hospital mortality | 56 (9.8) | 42 (11.4) | 14 (6.8) | .0782 |
| Day 28 improvement | 490 (85.4) | 304 (82.4) | 186 (90.7) | .0067 |
| 7-category scale at day 1 | ||||
| 7: Death | 0 | 0 | 0 | .0005b |
| 6: ICU, requiring IMV | 42 (7.3) | 30 (8.2) | 12 (5.9) | |
| 5: ICU, not requiring IMV | 46 (8.0) | 40 (10.9) | 6 (2.9) | |
| 4: Non-ICU, requiring oxygen | 41 (7.2) | 32 (8.7) | 9 (4.4) | |
| 3: Non-ICU, not requiring oxygen | 443 (77.3) | 266 (72.3) | 177 (86.3) | |
| 2: Discharged without resumption of normal activities | 0 | 0 | 0 | |
| 1: Discharged with resumption of normal activities | 1 (0.2) | 0 (0.0) | 1 (0.5) | |
| 7-category scale at day 7 | .0610b | |||
| 7: Death | 10 (1.7) | 7 (1.9) | 3 (1.5) | |
| 6: ICU, requiring IMV | 51 (8.9) | 39 (10.6) | 12 (5.9) | |
| 5: ICU, not requiring IMV | 24 (4.2) | 18 (4.9) | 6 (2.9) | |
| 4: Non-ICU, requiring oxygen | 45 (7.9) | 36 (9.8) | 9 (4.4) | |
| 3: Non-ICU, not requiring oxygen | 351 (61.4) | 211 (57.5) | 140 (68.3) | |
| 2: Discharged without resumption of normal activities | 0 | 0 | 0 | |
| 1: Discharged with resumption of normal activities | 91 (15.9) | 56 (15.3) | 35 (17.1) | |
| 7-category scale at day 14 | .0334b | |||
| 7: Death | 30 (5.3) | 21 (5.8) | 9 (4.4) | |
| 6: ICU, requiring IMV | 28 (4.9) | 23 (6.3) | 5 (2.4) | |
| 5: ICU, not requiring IMV | 21 (3.7) | 16 (4.4) | 5 (2.4) | |
| 4: Non-ICU, requiring oxygen | 25 (4.4) | 18 (5.0) | 7 (3.4) | |
| 3: Non-ICU, not requiring oxygen | 131 (23.1) | 81 (22.3) | 50 (24.4) | |
| 2: Discharged without resumption of normal activities | 4 (0.7) | 3 (0.8) | 1 (0.5) | |
| 1: Discharged with resumption of normal activities | 329 (57.9) | 201 (55.4) | 128 (62.4) |
Data are expressed as No. (%) or median (IQR). P values were calculated by Mann-Whitney U test or chi-square test, where appropriate.
Abbreviations: ARDS, acute respiratory distress syndrome; DNR, do not resuscitate; ICU, intensive care unit; IMV, ; IQR, interquartile range; NAI, neuraminidase inhibitor.
a P value was calculated by log-rank test.
b P values for difference in the distribution of scores on the 7-point scale at 7 and 14 days were obtained with the use of an ordinal logistic regression model, with adjustment for age, gender, heart disease, malignancies, and time from illness onset to starting antiviral treatment.
Laboratory Findings
On admission, 369 patients (64.9%) had a normal white blood cell (WBC) count. The median neutrophil count was significantly higher in patients with influenza A, compared with those with influenza B (4.6 × 109/L vs 4.2; P = .0183). In addition, higher proportions of elevated aspartate aminotransferase (>40 U/L; 25.2% vs 16.4%; P = .016) and creatinine kinase (>185 μmol/L; 22.0% vs 13.6%; P = .0277), were observed in patients infected with influenza A than those infected with influenza B. The lymphocyte count, platelet count, serum creatinine level, and serum lactate dehydrogenase showed no significant differences when comparing the 2 groups (Table 1).
Antiviral and Antibiotic Treatments
After admission, 80.3% of the patients were treated with a neuraminidase inhibitor. Most of them (79.1%) were given oral oseltamivir. The median time to starting antiviral treatments from symptom onset (IQR) was 5.4 (1.7–9.5) days. Among patients who received oseltamivir, 337 (73.6%) initiated treatment more than 2 days after illness onset. The median time to starting antiviral treatment was later in patients with influenza A than influenza B (median days, 5.9 vs 3.7 days; P = .0079). Antibiotics were administered in 481 patients (84.1%) and systemic corticosteroids in 197 (34.3%). There was no significant difference in the use of antibiotics and corticosteroids between influenza A and B virus infections (Table 2).
Outcomes
Clinical and Ordinal Scale Outcomes
In-hospital mortality was 9.8%, and by day 28 from admission, 34 (9.2%) of 369 influenza A cases and 12 (5.9%) of 205 influenza B cases died (P = .199). One hundred thirteen cases (19.7%) were admitted to the ICU. The median length of hospital stay (IQR) was 13 (8–17) days. Clinical improvement (decline of 2 categories, as assessed by 7-category scale score, or discharge) up to 28 days occurred in 82.4% of the patients with influenza A and 90.7% of the patients with influenza B (P = .0067) The median time to clinical improvement appeared to be similar between influenza A (median [IQR], 13 [12–13] days) and B (median [IQR], 13 [11–13] days) virus infections (Table 2).
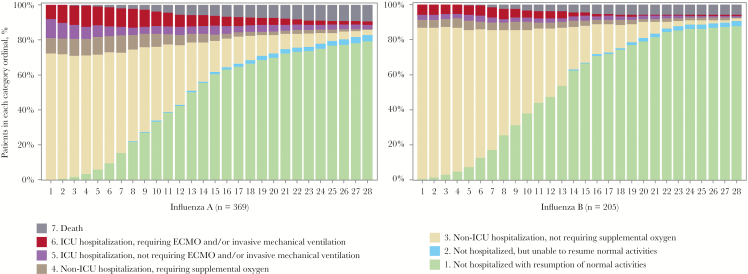
The distribution of patients falling into each category of the 7-category scale from admission to day 28 is shown in Figure 1. Patients hospitalized but not requiring supplemental oxygen (category 3) accounted for a higher proportion among those hospitalized, and a higher proportion of more severe outcomes (categories 4–7) was observed in patients with influenza A than B on admission (Figure 1 and Table 2). An ordinal logistic regression model comparing the category distributions from day 1 to day 28 showed a lower risk for worse outcomes in patients with influenza B virus infection compared with those with influenza A virus infection on most days (Figure 3; Supplementary Figure 2).
Figure 1.
Distribution of proportion falling into each category of the 7-category scale from admission to day 28. Abbreviations: ECMO, ; ICU, intensive care unit.
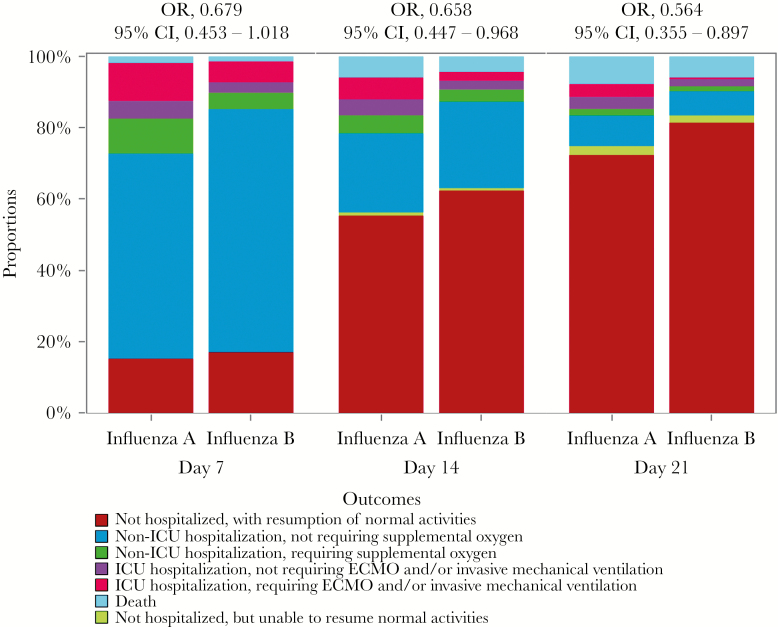
Figure 3.
Effects of influenza A virus vs influenza B virus for worse clinical outcomes among hospitalized patients at days 7, 14, and 28 according to the 7-category ordinal scale end point. Odds ratios and 95% confidence intervals were estimated by ordinal logistic models based on the 7-point ordinal outcome scale, adjusting for age, gender, heart disease, malignancies, and time from illness onset to starting antiviral treatment. Patients hospitalized with influenza A virus infection were taken as the reference when modeling. Abbreviations: CI, confidence interval; ECMO, ; ICU, intensive care unit; OR, odds ratio.
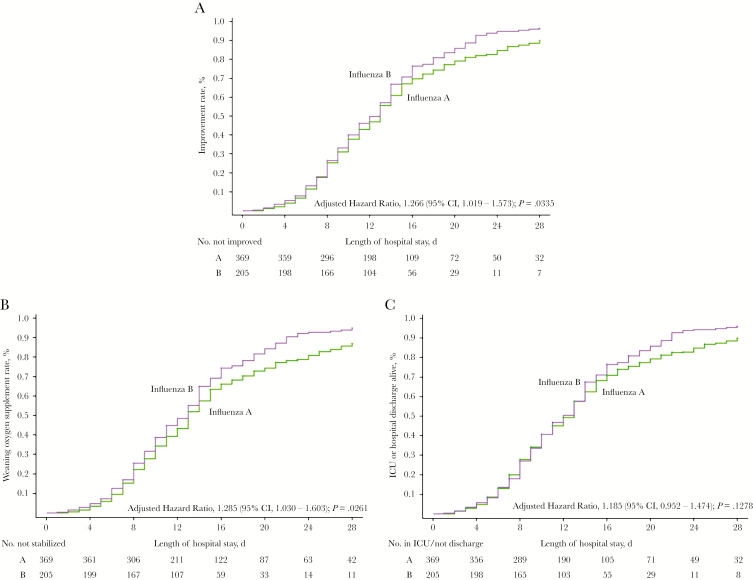
Patients with influenza B virus infection had significantly higher probability of clinical improvement compared with those with influenza A virus infection (unadjusted hazard ratio [HR], 1.225; 95% CI, 1.021–1.471; P = .0292). In an adjusted analysis that incorporated the potential confounding factors (age, gender, heart disease, malignancies, 7-category point scale at day 1, and time from illness onset to starting antiviral treatments), a similar pattern was observed (adjusted HR, 1.266; 95% CI, 1.019–1.573; P = .0335) (Figure 2A). Although pneumonia cases on admission were excluded, no significant difference was observed between infections of 2 viruses (adjusted HR, 1.25; 95% CI, 0.940–1.596; P = .1331), and further analysis on severity from day 1 to day 28 also showed a similar result (Supplementary Table 1).
Figure 2.
Overall rate of clinical improvement/weaning oxygen supplement rate/intensive care unit (ICU) or hospital discharge alive rate among hospitalized patients with influenza A or B virus infection. Shown are (A) cumulative incidences of clinical improvement, (B) weaning oxygen supplement rate, and (C) ICU or hospital discharge alive rate from admission to 28 days. Hazard ratios were adjusted for age, gender, heart disease, malignancies, and time from illness onset to starting antiviral treatments. Clinical improvement was defined as discharge or a 2-step descrese on a 7-point ordinal scale of clinical status after admission. Abbreviation: CI, confidence interval.
Other Clinical Outcomes
The proportion weaned off supplemental oxygen by day 28 was 80.8% (298/369) in patients with influenza A and 89.8% (184/205) in those with influenza B (P = .0049). The Cox model adjusted for potential confounding factors (age, gender, heart disease, malignancies, and time from illness onset to starting antiviral treatments, 7-category point scale at day 1) indicated that patients with influenza B infection had significantly higher probability of weaning oxygen supplement than those with influenza A infection (adjusted HR, 1.285; 95% CI, 1.030–1.603; P = .0261) (Figure 2B).
Hospital discharge or ICU discharge alive (if admitted to ICUs) up to day 28 occurred in 308/369 (83.5%) patients with influenza A and 186/205 (90.7%) patients with influenza B (P = .0161). However, no difference was observed in the adjusted Cox model (adjusted HR, 1.185; 95% CI, 0.952–1.474; P = .1278) (Figure 2C).
During the hospital stay, viral pneumonia (39.8%) and ARDS (21.7%) were the most common complications. A higher incidence of viral pneumonia (46.5 vs 31.4%; P < .001) and ARDS (27.5 vs 11.2%; P < .001) was observed in patients infected with influenza A virus, compared with those with influenza B. Accordingly, more patients with influenza A were admitted to the ICU (24.7 vs 10.7%; P < .0001). However, the length of hospital stay did not differ between influenza A and B infections (median, 13.0 vs 12.0 days; P = .4137), and no significant difference in the length of ICU stay was found (median, 9.7 vs 12.6 days; P = .9624). A nonsignificantly higher risk of in-hospital mortality was noted in patients with influenza A compared with B (11.4 vs 6.8%; P = .2036). (Table 2). The Kaplan-Meier curves in Supplementary Figure 1 also showed no differences for rates of remaining ICU stay, rates of remaining IMV, overall survival, or rates of viral RNA positivity between the 2 virus infections.
Virology
The median (IQR) duration of viral RNA detection from illness onset was 10.6 (9.7–14.4) days. The median duration of detection was similar in the influenza A and influenza B groups (10.7 vs 10.0 days; log-rank test P = .9961) (Table 2).
DISCUSSION
In our prospective cohort study of adults hospitalized for influenza A or B virus infection, we found evidence for differences in both clinical presentation and recovery. The proportion with clinical improvement up to day 28 was significantly lower, and the proportions developing influenza-related pneumonia or ARDS and requiring ICU admission were significantly higher among patients with influenza A virus infection, compared with those with influenza B virus infection.
The clinical severity of influenza may have been affected by inherent differences in viral virulence [13, 14] and the role of cross-protective cell-medicated immunity [15]. Volunteer challenge studies have provided detailed data on frequency of symptomatic infection after infection among different influenza subtypes. A recent study also revealed that patients infected with influenza A developed more pneumonia and ICU admission than those with influenza B [6]. The participants with influenza B had a lower frequency of asymptomatic influenza infection [14]. This had previously been reported in a study analyzing the activation of the antiviral responses of human dendritic cells to influenza A or B virus infection. However, based on the present data, we found it hard to explain these differences.
The use of conventional outcomes (eg, mortality, length of stay) in previous studies does not include substantial amounts of outcome information over time and may lead to underestimation of the differences between serious influenza A and B virus illness in hospitalized adults [4, 5, 16]. For example, our study found that the length of hospital stay and the mortality rate of patients were comparable between the 2 virus infections and consistent with former studies [4, 5].
We systematically evaluated the severity of the 2 virus infections by clinical improvement based on a modified 7-category ordinal scale. Using the rate of clinical improvement, a significant difference in the rate was observed among patients with influenza A vs B virus infection. And the same pattern was found in the distribution of the proportion falling into each category of the 7-category scale at different time points. The clinical improvement assessed by the 7-category ordinal scale is able to capture a broad range of clinical states and track the status change of each patient from admission.
In this real-world study, there were some significant differences between the 2 groups, including time of initial antiviral treatment, age, and underlying diseases. To clearly identify the difference of illness between influenza A and B, these confounding factors were included in our multivariate model, and the same results were found in the multivariate analysis. Therefore, our data provided evidence that indicated that influenza A was somewhat more severe than influenza B virus infections in hospitalized adults. A similar time to viral clearance was observed in our study. In a previous observational study, early oseltamivir treatment was less beneficial for influenza B compared with influenza A virus infections in pediatric and adult outpatients [17]. Because the median time to oseltamivir initiation in our study was 5.5 days after illness onset, the opportunity for detecting clinical benefit from antiviral therapy was likely diminished [18]. Also, earlier antiviral therapy in patients with influenza B virus infection than those with influenza A may have promoted clearance of influenza B and reduced the difference in duration of viral clearance between the 2 viruses.
There are several limitations in our study. The time to clinical improvement was defined by changes in prospectively defined categories of clinical status. However, this end point does not necessarily capture full recovery. For example, a patient receiving mechanical ventilation who experiences a 2-step decrease in category to being hospitalized on supplemental oxygen would be included as clinically improved, although not recovered. Also, decisions regarding clinical care interventions like ICU admission, mechanical ventilator support, use of supplemental oxygen, and hospital discharge vary widely across institutions and practitioners. This study was conducted in a single center and needs to be validated by multicenter studies. Second, the respiratory specimens were not collected to further identify the influenza A viral subtype or influenza B lineage. Therefore, we cannot conduct further analysis among the 4 strains of influenza virus: influenza A (H3N2, H1N1) and B (Yamagata, Victoria lineage) viruses.
CONCLUSION
Our findings indicate that influenza A infection may result in a worse clinical improvement than influenza B among hospitalized patients with influenza virus infection. The rate of clinical improvement assessed by ordinal scale might be a reasonable end point for patients hospitalized with influenza infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Jiefeng Xia and Liang Rong for data management.
Financial support. This work was supported by The National Science Fund for Distinguished Young Scholars (81425001/H0104); Emergency Special Project of the Ministry of Science and Technology (10600100000015001206); National Science and Technology Major Project (2017ZX10204401004).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Iuliano AD, Roguski KM, Chang HH, et al. . Global Seasonal Influenza-Associated Mortality Collaborator Network Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van de Sandt CE, Bodewes R, Rimmelzwaan GF, de Vries RD. Influenza B viruses: not to be discounted. Future Microbiol 2015; 10:1447–65. [DOI] [PubMed] [Google Scholar]
- 3. Wie SH, So BH, Song JY, et al. . A comparison of the clinical and epidemiological characteristics of adult patients with laboratory-confirmed influenza A or B during the 2011–2012 influenza season in Korea: a multi-center study. PLoS One 2013; 8:e62685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su S, Chaves SS, Perez A, et al. . Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin Infect Dis 2014; 59:252–5. [DOI] [PubMed] [Google Scholar]
- 5. Chagvardieff A, Persico N, Marmillot C, et al. . Prospective comparative study of characteristics associated with influenza A and B in adults. Med Mal Infect 2018; 48:180–7. [DOI] [PubMed] [Google Scholar]
- 6. Caini S, Kroneman M, Wiegers T, et al. . Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: a systematic literature review. Influenza Other Respir Viruses 2018; 12:780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chinese National Influenza Center. 2017–2018 influenza season report http://wwwchinaivdccn/cnic/zyzx/lgzb/201801/t20180108_157998htm.
- 8. Garten R, Blanton L, Elal AIA, et al. . Update: influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beigel JH, Tebas P, Elie-Turenne MC, et al. . IRC002 Study Team Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med 2017; 5:500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peterson RL, Vock DM, Powers JH, et al. . INSIGHT FLU-IVIG Study Group Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials 2017; 14:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Health Commission of the People’s Republic of China. Clinical guidance for patients with influenza infection http://wwwnhcgovcn/xxgk/pages/viewdocumentjsp? dispatchDate=&staticUrl=%2Fyzygj%2Fpqt%2F201811%2Fdd748b43df0640e0bf 33c526ca8c9ddfshtml&wenhao=%E5%9B%BD%E5%8D%AB%E5%8A%9E %E5%8C%BB%E5%87%BD%E3%80%942018%E3%80%951020%E5%8F%B7 &utitle=%E5%85%B3%E4%BA%8E%E8%BF%9B%E4%B8%80%E6%AD% A5%E5%8A%A0%E5%BC%BA%E6%B5%81%E8%A1%8C%E6%80%A7%E6% 84%9F%E5%86%92%E5%8C%BB%E7%96%97%E5%B7%A5%E4%BD%9C%E7 %9A%84%E9%80%9A%E7%9F%A5&topictype=&topic=&publishedOrg=%E5% 8C%BB%E6%94%BF%E5%8C%BB%E7%AE%A1%E5%B1%80&indexNum= 000013610%2F2018-00308&manuscriptId=dd748b43df0640e0bf33c526ca 8c9ddf.
- 12. Wang Y, Guo Q, Yan Z, et al. . CAP-China Network Factors associated with prolonged viral shedding in patients with avian influenza A(H7N9) virus infection. J Infect Dis 2018; 217:1708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim YH, Kim HS, Cho SH, Seo SH. Influenza B virus causes milder pathogenesis and weaker inflammatory responses in ferrets than influenza A virus. Viral Immunol 2009; 22:423–30. [DOI] [PubMed] [Google Scholar]
- 14. Carrat F, Vergu E, Ferguson NM, et al. . Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008; 167:775–85. [DOI] [PubMed] [Google Scholar]
- 15. Österlund P, Strengell M, Sarin LP, et al. . Incoming influenza A virus evades early host recognition, while influenza B virus induces interferon expression directly upon entry. J Virol 2012; 86:11183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutiérrez-Pizarraya A, Pérez-Romero P, Alvarez R, et al. . Unexpected severity of cases of influenza B infection in patients that required hospitalization during the first postpandemic wave. J Infect 2012; 65:423–30. [DOI] [PubMed] [Google Scholar]
- 17. Kawai N, Ikematsu H, Iwaki N, et al. . A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis 2006; 43:439–44. [DOI] [PubMed] [Google Scholar]
- 18. Ramirez J, Peyrani P, Wiemken T, et al. . A randomized study evaluating the effectiveness of oseltamivir initiated at the time of hospital admission in adults hospitalized with influenza-associated lower respiratory tract infections. Clin Infect Dis 2018; 67:736–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.