Abstract
Background
Integrase strand transfer inhibitors (INSTIs) are highly efficacious and well tolerated antiretrovirals with fewer adverse side-effects relative to other classes of antiretrovirals. The use of INSTIs raltegravir, elvitegravir, and dolutegravir has increased dramatically over recent years. However, there is limited information about the evolution and prevalence of INSTI resistance mutations in clinical human immunodeficiency virus populations.
Methods
Human immunodeficiency virus-1-positive individuals ≥19 years were included if they received ≥1 dispensed prescription of antiretroviral therapy (ART) in British Columbia between 2009 and 2016 (N = 9358). Physician-ordered drug resistance tests were analyzed and protease inhibitor (PI), reverse-transcriptase inhibitor (RT), and INSTI resistance were defined as having ≥1 sample with a combined, cumulative score ≥30 by Stanford HIV Drug Resistance Algorithm version 7.0.1.
Results
Although most ART-treated individuals were tested for PI and RT resistance, INSTI resistance testing lagged behind the uptake of INSTIs among INSTI-treated individuals (11% in 2009; 34% in 2016). The prevalence of INSTI resistance was relatively low, but it increased from 1 to 7 per 1000 ART-treated individuals between 2009 and 2016 (P < .0001, R2 = 0.98). Integrase strand transfer inhibitor resistance mutations increased at integrase codons 66, 97, 140, 148, 155, and 263.
Conclusions
The prevalence of INSTI resistance remains low compared with PI and RT resistance in ART-treated populations but is expanding with increased INSTI use.
Keywords: dolutegravir, drug resistance, elvitegravir, HIV integrase strand transfer inhibitor, raltegravir
Integrase strand transfer inhibitors (INSTIs) are effective and well tolerated antiretrovirals (ARVs) [1, 2]. With increasing prevalence of pretreatment resistance to nonnucleoside/nonnucleotide reverse-transcriptase inhibitors (NNRTIs) [3], INSTIs are increasingly recommended in first-line and alternative first-line regimens [4–7]. Integrase strand transfer inhibitor-containing therapy also provides an alternative treatment option for individuals experiencing multidrug resistance or adverse reactions to other human immunodeficiency virus (HIV) ARVs [1, 4]. However, INSTI resistance remains a barrier to the ongoing success of HIV treatment [8]. Although ARVs effectively suppress HIV replication enabling immune restitution, selection of resistance-conferring mutations in the presence of ARVs is associated with lack of viral suppression, treatment failure, and increased likelihood of HIV transmission [8–10]. Furthermore, long-term persistence of drug resistance mutations has previously been reported and can limit regimen options [11]. Although pretreatment protease inhibitor (PI) and reverse-transcriptase (RT) inhibitor resistance testing is the current standard of care in British Columbia (BC) [12, 13], standard clinical guidelines currently do not recommend testing for INSTI resistance at initiation of therapy [14]. Instead, INSTI resistance testing is only recommended in patients who experience virologic failure while on INSTI-containing therapy [4, 13].
There have been previous reports of transmitted and treatment-emergent INSTI resistance in clinical settings [15–18]. However, to better monitor and evaluate the current modalities of INSTI resistance and assess adequacy of INSTI resistance testing, more information is required about the change in yearly prevalence of INSTI resistance and the specific integrase mutations associated with INSTI resistance selected, in large clinical HIV populations.
In a previous study, we observed low rates of treatment-emergent INSTI resistance (approximately 1 case per 100 person-years INSTI exposure) among individuals registered in BC’s Centre for Excellence in HIV/AIDS (BC-CfE) Drug Treatment Program (DTP) who initiated INSTI-containing therapies between 2012 and 2014 [19]. This longitudinal, observational study examines the prevalence of INSTI resistance and the specific INSTI resistance mutations selected in antiretroviral therapy (ART)-treated DTP individuals in each calendar year between 2009 and 2016. For context, the yearly prevalence of PI and RT (PI-RT) resistance as well as the frequency of INSTI and PI-RT resistance testing were also investigated in the same time period.
METHODS
Study Population
Participants within this study were HIV-1-positive individuals living in BC, Canada who received ART through the BC-CfE DTP. The BC-CfE monitors and maintains clinical patient profiles (ART, plasma HIV-1 ribonucleic acid [RNA] viral load, CD4 cell count, etc) and collects sociodemographic data of DTP participants. A patient information sheet describing the potential uses of data for health research is provided at DTP enrollment, and consent is not required for use of anonymized data. The DTP is an open cohort and members can enter and leave at any time, for any duration of time, or leave indefinitely. These programs have been described in detail elsewhere [20, 21]. The University of British Columbia Providence Health Care Research Ethics Board granted ethical approval for the DTP (H05-50123).
For each calendar year from January 1, 2009 to December 31, 2016, participants were counted in the denominator of ART-treated individuals if they were ≥19 years of age and were treated with at least 1 day of ART within that year. Treatment with ART was determined as ≥1 dispensed ART prescription in a calendar year through the BC-CfE’s administrative records ART prescription database.
Drug Resistance Testing
Drug resistance tests were ordered by each individual’s physician and processed at the BC-CfE research laboratories. However, if an initial HIV-1 RNA viral load level was requested by an individual’s physician and the HIV-1 RNA viral load was >250 copies/mL, a drug resistance test was automatically ordered by the BC-CfE and the results were sent to the physician. Each individual in the study contributed cumulative drug resistance data from time of DTP enrollment until December 31, 2016. An individual contributed to the count of “tested for resistance” in the first year that the test was performed, and this “tested” status was automatically carried forward to each subsequent year the individual was treated with ART in the DTP. Viral nucleic acids were extracted from 500 µL plasma using the NucliSENS easyMAG from bioMérieux (Montreal, Canada). The protease, RT, and integrase genes were amplified by “nested” reverse transcription-polymerase chain reaction (PCR) using the Expand High Fidelity PCR system from Roche Diagnostics (Laval, Canada) as described elsewhere [22]. Sanger sequencing was performed bidirectionally using the BigDye Terminator version 3.1 Cycle Sequencing Kit from Applied Biosystems (ABI, Foster City, CA) on an ABI 3730 DNA Sequencer. A consensus sequence was produced, and chromatograms were analyzed by in-house custom software called RECall [23].
Prevalence of Integrase Strand Transfer Inhibitor and Protease Inhibitor and Reverse-Transcriptase Inhibitor Resistance
In each year, an individual contributed to the total count of ART-treated individuals if they were treated with ART through the DTP within that year. Because annual prevalence is calculated separately for each year, deceased and/or lost to follow-up participants are accounted for accordingly. Samples were defined as drug resistant to either INSTI or PI-RT if they contained cumulative mutations that result in intermediate- or high-level resistance to at least 1 ARV in the INSTI or PI-RT drug classes, respectively, as defined by a score ≥30 based on the Stanford University HIV Drug Resistance Database Genotypic Resistance Interpretation Algorithm version 7.0.1 [24]. To account for the potential long-term persistence of drug resistance mutations [11], each ART-treated individual tested for drug resistance was classified as having drug resistance (INSTI or PI-RT resistance) in the first year they had a sample meeting the criteria for resistance, and this resistance status was carried forward to each subsequent year the individual was treated with ART in the DTP. The annual prevalence of INSTI and PI-RT resistance per 1000 ART-treated individuals was calculated at the end of each calendar year.
Integrase Strand Transfer Inhibitor Resistance by Year of First Detection
To estimate the contribution of treatment-emergent INSTI resistance to the prevalence of INSTI resistance over time, new cases of INSTI resistance among ART-treated individuals were identified in each year and counted in the first year they were detected. It is important to note that newly identified cases of INSTI resistance do not correspond to incidence of INSTI resistance during the study period, but rather to the first year an individual who contributed to the count of prevalence had study-defined INSTI resistance during the study period. The drug associated with newly detected INSTI resistance was categorized as the last prescribed INSTI (either raltegravir, elvitegravir, or dolutegravir) before detection of resistance. If the first identified INSTI resistance occurred before the first known INSTI dispensed date in the DTP, the INSTI drug exposure was termed unclassifiable.
Prevalence of Integrase Strand Transfer Inhibitor Resistance Mutations
Each ART-treated individual with newly detected study-defined INSTI resistance was counted once per mutation position in each calender year they received ART in the DTP. Samples were defined as drug resistant to INSTIs if they contained cumulative mutations that result in intermediate- or high-level resistance to at least 1 ARV in the INSTI drug class, as defined by a score ≥30 based on the Stanford University HIV Drug Resistance Database Genotypic Resistance Interpretation Algorithm version 7.0.1 [24]. An individual’s INSTI mutation contributed to the mutation count in the first year it was detected and automatically carried forward to all subsequent years the individual received ART in the DTP.
Statistical Analysis
Generalized additive models were used to model the nonlinear trend of the prevalence of PI-RT and INSTI drug resistance [25, 26]. Statistical software R version 3.2.2 was used with the mgcv package [25], assuming a Beta distribution and a logit link. The year or percentage of patients tested for INSTI resistance were the explanatory variables being smoothed. Restricted maximum likelihood was used to estimate the parameters. A cubic regression spline was used to smooth the yearly trend in each of the outcomes.
RESULTS
Characteristics and Yearly Number of Participants
Between January 1, 2009 and December 31, 2016, 9358 unique individuals received ART through the DTP in BC. Patients were predominantly male (83%) infected with HIV-1 subtype B (81%). The median age of participants at the year of first inclusion in the study was 46 years old (25th–75th percentile [Q1–Q3] 38–53) (Table 1). In total, 3645 unique individuals (39%) received INSTI-containing therapy in the DTP during the study period. A patient may have been prescribed more than 1 INSTI during the study period. During this time period, there were 1546 individuals ever treated with raltegravir, 830 individuals ever treated with elvitegravir, and 1856 individuals ever treated with dolutegravir.
Table 1.
Characteristics of Individuals Within the BC Drug Treatment Program Between 2009 and 2016
| Variable | N = 9358 n (%) |
|---|---|
| ART-treated individualsa | 9358 (100) |
| INSTI-treated individualsb | 3645 (39) |
| INSTI prescribed in the DTPc | |
| Raltegravir | 1546 (17) |
| Elvitegravir | 830 (9) |
| Dolutegravir | 1856 (20) |
| Age, years, median (Q1–Q3)d | 46 (38–53) |
| Sex | |
| Male | 7768 (83) |
| Female | 1590 (17) |
| HIV subtype | |
| B subtype | 7542 (81) |
| Non-B subtype | 586 (6) |
| Unknown | 1230 (13) |
| Number of years individuals contributed resistance data, median (Q1–Q3) | 6 (2–8) |
| Number of years individuals contributed any type of data, median (Q1–Q3) | 7 (4–8) |
| Number of ART-treated individuals ever tested for PI-RT resistance | 7883 (84) |
| Number of physician-ordered resistance tests done per person, median (Q1–Q3) | 2 (1–5) |
| Number of individuals with a single resistance test only, at some point in time | 2647 (28) |
| Number of individuals with a single resistance test performed at baseline | 2109 (23) |
| Number of INSTI-treated individuals ever tested for INSTI resistance | 1244 (13) |
| Number of physician-ordered resistance tests done per person, median (Q1–Q3) | 1 (1–2) |
| Number of individuals with a single resistance test only, at some point in time | 752 (8) |
| Number of individuals with a single resistance test performed at baseline | 482 (5) |
Abbreviations: ART, antiretroviral therapy; BC, British Columbia; DTP, Drug Treatment Program; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; Q1–Q3, 25th–75th percentile range.
aAn individual contributed to the count of ART-treated if they were ever dispensed ART in the DTP during the study period.
bAn individual contributed to the count of INSTI-treated if they were ever dispensed INSTIs in the DTP during the study period.
cMedian age of individuals at first year of inclusion in the study.
dAn individual could be prescribed more than 1 INSTI during the study period.
Between 2009 and 2016, the number of individuals enrolled and receiving treatment through the DTP each year increased from 5587 to 7772 individuals (39% increase), and the proportion receiving an INSTI increased from 10% to 40% (542 to 3117 individuals) (Supplementary Table 1).
Drug Resistance Testing
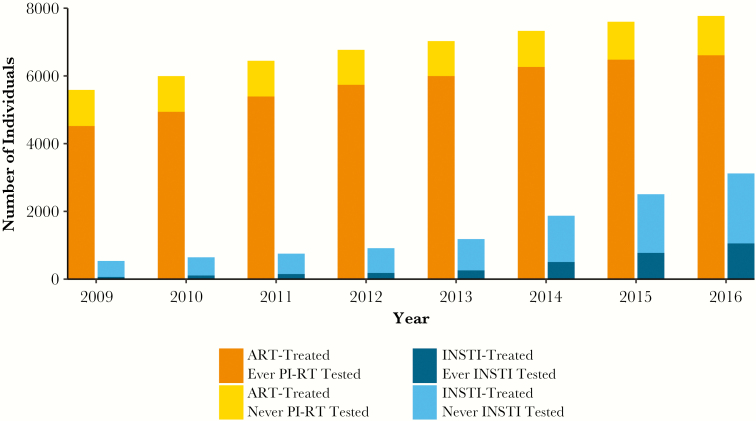
During the study period, the number of ART-treated individuals tested for PI-RT resistance increased from 4520 to 6614 individuals (81% to 85%) (Figure 1), and the number of all ART-treated individuals tested for INSTI resistance increased from 188 to 1440 individuals (3% to 19%) (P < .0001, R2 = 0.99) (Supplementary Table 1). Among those treated with INSTIs each year, the number of individuals tested for INSTI resistance increased from 60 to 1059 individuals (11% to 34%) (P < .0001, R2 = 0.97) (Figure 1).
Figure 1.
Antiretroviral therapy (ART) and drug resistance testing. All active ART-treated individuals tested and not tested for protease inhibitor and reverse-transcriptase inhibitor (PI-RT) resistance and active integrase strand transfer inhibitor (INSTI)-treated individuals tested and not tested for INSTI resistance as of December 31st of each year from 2009 to 2016 are indicated. Individuals contributed to the count of PI-RT or INSTI tested in the first year that a PI-RT or INSTI resistance test was performed, and this tested status was automatically carried forward to each subsequent year the individual was treated with ART in the Drug Treatment Program. ART-treated ever PI-RT tested, ART-treated individuals ever tested for PI-RT resistance; ART-treated never PI-RT tested, ART-treated individuals never tested for PI-RT resistance; ART-treated ever INSTI tested, ART-treated individuals ever tested for INSTI resistance; ART-treated never INSTI tested, ART-treated individuals never tested for INSTI resistance.
Prevalence of Resistance in All Antiretroviral Therapy-Treated Individuals
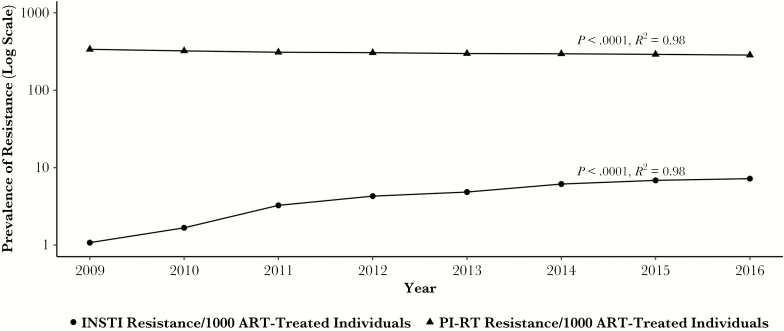
Figure 2 shows the prevalence of PI-RT and INSTI resistance in all ART-treated individuals treated with ART in BC in each calendar year from 2009 to 2016. The prevalence of study-defined PI-RT resistance in all ART-treated individuals declined significantly from 337 per 1000 ART-treated individuals in 2009 to 285 per 1000 ART-treated individuals in 2016 (P < .0001, R2 = 0.98) (Figure 2). In contrast, the prevalence of study-defined INSTI resistance was lower than that of PI-RT resistance, but it increased from 1 per 1000 ART-treated individuals in 2009 to 7 per 1000 ART-treated individuals in 2016 (P < .0001, R2 = 0.98) (Figure 2).
Figure 2.
Prevalence of protease inhibitor and reverse-transcriptase inhibitor (PI-RT) and integrase strand transfer inhibitor (INSTI) drug resistance. The annual prevalence of PI-RT and INSTI drug resistance per 1000 antiretroviral therapy (ART)-treated individuals between 2009 and 2016 within the Drug Treatment Program is shown. The trend shows a decrease in the prevalence of PI-RT resistance from 337 per 1000 ART-treated individuals in 2009 to 285 per 1000 ART-treated individuals in 2016 (P < .0001, R2 = 0.98); the trend shows an increase in the prevalence of INSTI resistance from 1 per 1000 ART-treated individuals in 2009 to 7 per 1000 ART-treated individuals in 2016 (P < .0001, R2 = 0.98).
Integrase Strand Transfer Inhibitor Resistance by Year of First Detection
From 2009 to 2016, 64 unique individuals (69% male) were newly identified as having study-defined INSTI resistance (Table 2). Median age was 47 years old ([Q1–Q3] 40–53), and the majority were infected with HIV-1 subtype B (91%). Among these individuals, 88% (56 of 64) have also had mutations conferring PI-RT resistance. The observed number of newly identified cases of INSTI resistance ranged from 4 to 15 new cases per year during the study period, and it remained relatively stable at 6–9 cases per year after peaking in 2011 (Table 2). Patient characteristics of a subset of these 64 individuals have been previously described in detail elsewhere [19]. During the study period, there was an apparent shift from raltegravir-containing regimens to elvitegravir- and dolutegravir-containing regimens (Supplementary Figure 1) and until 2014, most new cases of INSTI resistance were associated with the use of raltegravir (Table 2). In 2015 and 2016, the first 2 full years the 3 INSTIs were widely prescribed, 80% of new INSTI resistance cases (12 of 15) were attributed to elvitegravir and dolutegravir use compared with 2013 and 2014, where 88% of INSTI resistance cases (15 of 17) were associated with raltegravir use (Table 2). With decreasing use of raltegravir, most new cases of INSTI resistance have been associated with the use of elvitegravir. In 4 individuals, INSTI drug resistance mutations were documented in a sample drawn before treatment with an INSTI-containing regimen in the DTP. It is unknown whether these unclassifiable individuals had received prior INSTI treatment elsewhere (Table 2).
Table 2.
Newly Identified Cases of INSTI Resistance Within the BC Drug Treatment Program Between 2009 and 2016
| INSTI Drug Exposurea | Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Total Resistance Cases per INSTI | |
| Raltegravir | 6 | 3 | 12 | 7 | 8 | 7 | 2 | 1 | 46 |
| Elvitegravir | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 5 | 11 |
| Dolutegravir | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| Unclassifiableb | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 4 |
| Total per Year | 6 | 4 | 15 | 7 | 8 | 9 | 9 | 6 | 64 |
Abbreviations: ART, antiretroviral therapy; BC, British Columbia; DTP, Drug Treatment Program; INSTI, integrase strand transfer inhibitor.
aThe INSTI a person was last exposed to before the first detection of INSTI resistance.
bIf a person had mutations conferring INSTI resistance before the first known INSTI dispensing date in the DTP, the INSTI drug exposure was termed unclassifiable. Newly identified ART-treated individuals with mutations conferring INSTI resistance with a total score ≥30 by the Stanford University HIV Drug Resistance Database Genotypic Resistance Interpretation Algorithm version 7.0.1 to at least 1 INSTI is displayed. Note that in December 2009, as shown in Supplementary Figure 1, 475 individuals were prescribed raltegravir, 2 individuals were prescribed elvitegravir, and 0 individuals were prescribed dolutegravir, compared with December 2016, where 751 individuals were prescribed raltegravir, 579 individuals were prescribed elvitegravir, and 1467 individuals were prescribed dolutegravir.
Prevalence of Integrase Strand Transfer Inhibitor Resistance Mutations
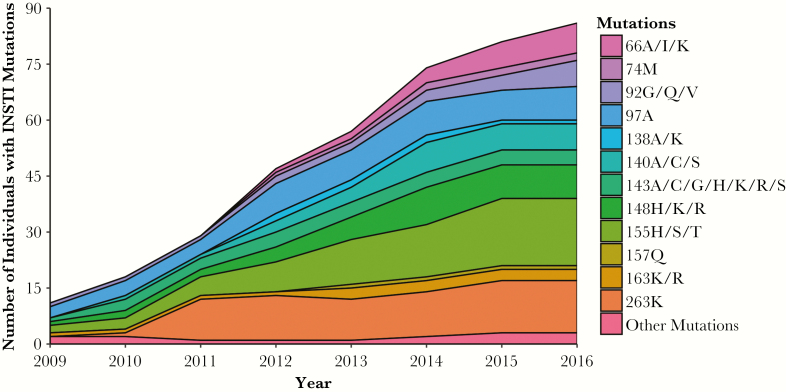
The prevalence of specific INSTI resistance mutations for individuals with newly identified study-defined INSTI resistance from 2009 to 2016 is depicted in Figure 3. Nucleotide substitutions at codons 51, 66, 74, 92, 95, 97, 118, 121, 138, 140, 143, 145, 146, 147, 148, 151, 153, 155, 157, 163, 230, and 263 of the integrase gene were identified among individuals with study-defined INSTI resistance. Overall, there was an increase in the prevalence of INSTI resistance mutations counted at codons 66, 97, 140, 148, 155, and 263 of the integrase gene (Figure 3). By the end of the study, mutation 155H/S/T was detected in 18 individuals and had the highest prevalence of all detected mutations. The second most prevalent mutation, 263K, was identified in 14 individuals by the end of 2016, after increasing from 1 to 11 individuals between 2010 and 2011.
Figure 3.
Prevalence of mutations conferring integrase strand transfer inhibitor (INSTI) resistance within newly identified INSTI-resistant individuals. The prevalence of INSTI resistance mutations in INSTI-resistant individuals within the Drug Treatment Program (DTP) between 2009 and 2016 is shown. An individual’s INSTI mutation contributed to the mutation count in the first year it was detected and automatically carried forward to all subsequent years the individual received antiretroviral therapy in the DTP. A person with mutations in 3 separate codons (eg, 92, 95, and 97) in a year will contribute 3 counts for that year, whereas a person with mutations in multiple positions within a codon only contributes a count of 1 for that codon (eg, mutations in positons 155H and 155S will only be counted once under 155H/S/T). The “Other Mutations” category represents the sum of mutation counts from 118R, 121Y, 145S, 146P, 147G, 151A/L, 153F/Y, 230R, 51Y, and 95K. NOTE: Certain specific positions associated with INSTI resistance increased in prevalence over the time period.
DISCUSSION
Drug-resistant strains of HIV have emerged for all ARVs used in clinical settings, including those of newer classes such as INSTIs [3]. In this study, we conducted a retrospective study of over 9000 HIV-1-positive, ART-treated individuals who participated in the BC DTP from 2009 to 2016. During this study period, there was a dramatic increase in the number of individuals receiving INSTIs raltegravir, elvitegravir, and dolutegravir, and a small but significant increase in the prevalence of intermediate- to high-level INSTI resistance in our ART-treated population.
Similar to findings in the United States [27], we observed an increase in the proportion of ART-treated individuals tested for INSTI resistance. This increase may be due to fewer restrictions on INSTI resistance testing in BC in recent years as well as the partially automated INSTI resistance testing implemented in 2014 for individuals treated with ART in the DTP. This automated INSTI resistance testing system involves the addition of INSTI resistance tests to the first drug resistance test ever done in BC, and, to any standard, physician-ordered PI-RT resistance test if the individual tested was currently or previously treated with INSTIs in BC. Nevertheless, although the number and proportion of INSTI-treated individuals tested for INSTI resistance increased each year during our study period, the proportion of INSTI resistance testing among INSTI-treated individuals has lagged behind the uptake of INSTI-containing therapy (11% in 2009; 34% in 2016) and lagged behind PI-RT resistance testing. This may be a result of the current physician-accessible ART guidelines, which do not support INSTI resistance testing for individuals unless there is evidence of virological failure and suspected INSTI resistance [4, 13].
Over the study period, we observed a decrease in the prevalence of PI-RT resistance in our ART-treated population. A decreasing trend in PI-RT resistance has recently been reported in other clinical HIV-positive populations [28] and may be attributed to the introduction of newer ARVs with greater genetic barriers to resistance. For example, second-generation NNRTIs rilpivirine and etravirine are not significantly impacted by single NNRTI resistance mutations [29] and have shown similar viral suppression efficacy to efavirenz [30]. In addition, increased production of novel fixed-dose combination products, which reduce pill burden and simplify regimens, may lead to better adherence and lower likelihood of ARV resistance [31, 32].
The prevalence of INSTI resistance reported in our study is similar to a recent study observing the prevalence of transmitted INSTI resistance in a Swiss HIV cohort [33] but is lower than those reported in other North American and European cohorts [15, 16, 27, 28]. This difference may in part be due to our method of defining drug resistance, which was restricted to individuals who had intermediate- to high-level INSTI resistance as defined by the Stanford University HIV Drug Resistance Database Genotypic Resistance Interpretation Algorithm version 7.0.1. Other studies have defined INSTI resistance as individual [15, 16, 27, 33] and/or cumulative [16, 27] presence of mutations. Nevertheless, there is an increase in the prevalence of intermediate- to high-level INSTI resistance within our population from 2009 to 2016 (1 to 7 per 1000 ART-treated individuals), likely due to the increase in the number of individuals receiving INSTI-containing therapies. In previous studies, we have identified <80% ARV adherence and CD4 cell count <200 cells/μL as strong risk factors of emergent INSTI resistance in DTP participants treated with INSTI-containing regimens [19], and these factors may contribute to the increase in the prevalence of INSTI resistance over time.
Since 2009, usage of elvitegravir and dolutegravir have risen considerably in the DTP since their introduction in BC in 2013 and 2014, respectively, whereas there has been a decline in the use of raltegravir, the first INSTI [34]. The observed increase in usage of dolutegravir may be due to its association with fewer adverse drug effects and its higher selective barrier for multidrug resistance mutations compared with elvitegravir and raltegravir [35–37]. This shift in prescribed drug therapies coincides with the shift in INSTIs associated with newly detected cases of INSTI resistance. Over time in our ART-treated population, the observed number of new raltegravir-associated resistance cases decreased as use of raltegravir declined (6 cases in 2009; 1 case in 2016). Elvitegravir-associated resistance accounted for the majority of new cases in 2015 and 2016, whereas only 3 new cases were associated with dolutegravir use, despite a marked increase in dolutegravir prescribing. Overall, the number of newly identified cases of individuals with study-defined INSTI resistance increased and then subsequently decreased during the study period.
Individuals with newly identified study-defined INSTI resistance appear to have mutations selected at codons 66, 97, 140, 148, 155, and 263. Mutation 66I is expected with increased elvitegravir usage [38]. Mutation 97A, in combination with other INSTI mutations, confers reduced susceptibility to raltegravir and dolutegravir [39, 40]. Mutations 140A/S are associated with dolutegravir and raltegravir resistance, and, in combination with mutation 148H, it is associated with reduced virological suppression [41]. Mutations 148H/R and 155H are mutations associated with 2 distinct mutational pathways that reduce susceptibility to all current INSTIs [42, 43]. Mutation 263K is associated with decreased HIV-deoxyribonucleic acid integration, viral replication capability, and integrase enzyme capacity but may also confer low- to intermediate-level susceptibility to dolutegravir and elvitegravir [44, 45]. The 263K mutation has previously been noted as an emergent INSTI mutation in treatment-experienced, INSTI-naive DTP individuals initiating raltegravir, elvitegravir, or dolutegravir for the first time [19] as well as in other treatment-experienced HIV-1-positive populations [35].
There are several limitations within our study. First, the incomplete coverage of INSTI resistance testing limits our ability to adequately characterize INSTI resistance mutations and may also underestimate the prevalence of INSTI resistance. We speculate that this poor coverage could be due to the relatively new nature of INSTIs and their significance might be overlooked. This study also observed cumulative mutations that confer intermediate- to high-level resistance to INSTIs, and this threshold does not include individual mutations that may confer low-level resistance and may be polymorphic or treatment-emergent. Most INSTI resistance tests are requested in response to virological failure rather than before initiation of ART therapy, and, therefore, individuals with intermediate- to high-level resistance to INSTIs were included in our study because they were more likely to be identified during routine clinical care. Without comprehensive baseline INSTI resistance testing, the prevalence of individual low-level resistance mutations cannot be accurately estimated. As a result, we cannot determine the true prevalence of INSTI mutations present in our ART-treated population and our INSTI prevalence may be underestimated. Our prevalence of INSTI resistance may also be underestimated do to Sanger sequencing’s inability to detect rare but clinically significant drug resistance mutations present at low viral populations [46]. In addition, our assessment of nucleotide substitutions within the integrase gene can limit key identification of mutations outside of the integrase gene, which may confer high-level resistance to raltegravir, elvitegravir, and dolutegravir [47].
CONCLUSIONS
In summary, our results indicate that the prevalence of INSTI resistance in the DTP is low, but it is gradually increasing over time as INSTI prescribing increases. The ability to precisely characterize and determine the frequency of INSTI resistance is hampered by the limited usage of INSTI resistance testing. Although previous research suggests dolutegravir’s genetic barrier to resistance is high, the genetic barrier of other currently prescribed INSTIs, raltegravir and elvitegravir, is comparable to other ARVs [37]. Integrase strand transfer inhibitor-treated individuals with suboptimal adherence may require more extensive monitoring to permit early detection of emergent INSTI resistance mutations. Further drug resistance monitoring is required to detect any changes in the prevalence of INSTI resistance with the introduction of newer INSTIs, such as bictegravir.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the technical assistance provided by the BC Centre for Excellence in HIV/AIDS. We also thank all of the participants of the Drug Treatment Program.
Authors contributions. K. J. L. and P. R. H. conceived of the presented study idea. P. R. H. and R. B. gained funding for the study. R. B. provided access to the Drug Treatment Program. W. W. Z. carried out the experimental procedures. C. W., W. C., M. A. R., and B. Y. were involved in the acquisition of the data. C. W., B. Y., and V. D. L. contributed to the analyses of the data. K. K. also contributed to the analyses of the data and took lead in writing the manuscript. P. R. H., K. J. L., B. Y., A. O., and J. B. J. provided critical feedback on the manuscript. P. R. H. and J. B. J. supervised the project. All authors have read and approved the final manuscript.
Financial support. This study was funded in part by a Large-Scale Applied Research Project in Genomics and Personalized Health contract (Grant Number HIV142) from Genome Canada (to P. R. H.), which was paid to P. R. H.’s institution.
Potential conflicts of interest. P. R. H. has previously received grant support from Merck. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections, February 2016, Boston, MA.
References
- 1. Lennox JL, DeJesus E, Lazzarin A, et al. . Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 2. Sax PE, DeJesus E, Mills A, et al. . Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. HIV drug resistance report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 4. Panel on Antiretroviral Guidelines for Adults and Adolescents DHHS. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 4 June 2018. [Google Scholar]
- 5. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 4 June 2018. [Google Scholar]
- 6.European AIDS Clinical Society. European AIDS Clinical Society Guidelines v9.0. 2017; p. 12–13. Available at: http://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf. Accessed 31 January 2019.0. [Google Scholar]
- 7. Saag MS, Benson CA, Gandhi RT, et al. . Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA 2018; 320:379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quashie PK, Mesplède T, Wainberg MA. Evolution of HIV integrase resistance mutations. Curr Opin Infect Dis 2013; 26:43–9. [DOI] [PubMed] [Google Scholar]
- 9. Anstett K, Brenner B, Mesplede T, Wainberg MA. HIV drug resistance against strand transfer integrase inhibitors. Retrovirology 2017; 14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen MS, Chen YQ, McCauley M, et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbour JD, Hecht FM, Wrin T, et al. . Persistence of primary drug resistance among recently HIV-1 infected adults. AIDS 2004; 18:1683–9. [DOI] [PubMed] [Google Scholar]
- 12. Primary Care Guidelines Panel of the BC Centre for Excellence in HIV/AIDS. Primary Care Guidelines for the Management of HIV/AIDS in British Columbia On behalf of the Primary Care Guidelines Panel BC Centre for Excellence in HIV/AIDS. Available at: http://www.cfenet.ubc.ca/sites/default/files/uploads/primary-care-guidelines/primary-care-guidelines_015-09-15.pdf. Accessed 4 June 2018. [Google Scholar]
- 13. Committee for Drug Evaluation and Therapy of the British Columbia Centre for Excellence in HIV/AIDS. Therapeutic Guidelines for Antiretroviral (ARV) Treatment of Adult HIV Infection. Available at: http://www.cfenet.ubc.ca/sites/default/files/uploads/Guidelines/bccfe-art-guidelines-Oct_14_2015.pdf. Accessed 8 August 2018. [Google Scholar]
- 14. Günthard HF, Calvez V, Paredes R, et al. . Human immunodeficiency virus drug resistance: 2018 recommendations of the International Antiviral Society-USA Panel. Clin Infect Dis 2019; 68:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji H, Patterson A, Taylor T, et al. . Prevalence of primary drug resistance against HIV-1 integrase inhibitors in Canada. J Acquir Immune Defic Syndr 2018; 78:e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zoufaly A, Kraft C, Schmidbauer C, Puchhammer-Stoeckl E. Prevalence of integrase inhibitor resistance mutations in Austrian patients recently diagnosed with HIV from 2008 to 2013. Infection 2017; 45:165–70. [DOI] [PubMed] [Google Scholar]
- 17. Fulcher JA, Du Y, Zhang TH, et al. . Emergence of integrase resistance mutations during initial therapy containing dolutegravir. Clin Infect Dis 2018; 67:791–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menza TW, Billock R, Samoff E, et al. . Pretreatment integrase strand transfer inhibitor resistance in North Carolina from 2010–2016. AIDS 2017; 31:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lepik KJ, Harrigan PR, Yip B, et al. . Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS 2017; 31:1425–34. [DOI] [PubMed] [Google Scholar]
- 20. BC Centre for Excellence in HIV/AIDS. Drug Treatment Program. Available at: http://www.cfenet.ubc.ca/drug-treatment-program. Accessed 12 May 2018. [Google Scholar]
- 21. Patterson S, Cescon A, Samji H, et al. . Cohort profile: HAART observational medical evaluation and research (HOMER) cohort. Int J Epidemiol 2015; 44:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez-Serna A, Min JE, Woods C, et al. . Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis 2014; 58:1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woods CK, Brumme CJ, Liu TF, et al. . Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol 2012; 50:1936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanford University HIV Drug Resistance Database. HIVdb Program: Mutation List Analysis - HIV Drug Resistance Database. Available at: https://hivdb.stanford.edu/hivdb/by-mutations/. Accessed 17 May 2018. [Google Scholar]
- 25. Wood S. Generalized Additive Models: An Introduction with R. Chapman; and Hall/CRC, Boca Raton, Florida; 2006. [Google Scholar]
- 26. Clark M. Generalized Additive Models: Getting Started with Additive Models in R. Center for Social Research, University of Notre Dame. 2013. Available at: https://m-clark.github.io/docs/GAMS.pdf. Accessed 4 December 2015. [Google Scholar]
- 27. Hurt CB, Sebastian J, Hicks CB, Eron JJ. Resistance to HIV integrase strand transfer inhibitors among clinical specimens in the United States, 2009–2012. Clin Infect Dis 2014; 58:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davy-Mendez T, Eron JJ, Brunet L, et al. . New antiretroviral agent use affects prevalence of HIV drug resistance in clinical care populations. AIDS 2018; 32:2593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Teeranaipong P, Sirivichayakul S, Mekprasan S, et al. . Role of rilpivirine and etravirine in efavirenz and nevirapine-based regimens failure in a resource-limited country: a cross-sectional study. PLoS One 2016; 11:e0154221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen CJ, Molina JM, Cassetti I, et al. . Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS 2013; 27:939–50. [DOI] [PubMed] [Google Scholar]
- 31. Legorreta A, Yu A, Chernicoff H, et al. . Adherence to combined Lamivudine + Zidovudine versus individual components: a community-based retrospective medicaid claims analysis. AIDS Care 2005; 17:938–48. [DOI] [PubMed] [Google Scholar]
- 32. Kauf TL, Davis KL, Earnshaw SR, Davis EA. Spillover adherence effects of fixed-dose combination HIV therapy. Patient Prefer Adherence 2012; 6:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scherrer AU, Yang WL, Kouyos RD, et al. . Successful prevention of transmission of integrase resistance in the Swiss HIV cohort study. J Infect Dis 2016; 214:399–402. [DOI] [PubMed] [Google Scholar]
- 34. Government of Canada. Drug Product Database Online Query. Available at: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp. Accessed 19 August 2018. [Google Scholar]
- 35. Cahn P, Pozniak AL, Mingrone H, et al. . Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 36. Lepik KJ, Yip B, Ulloa AC, et al. . Adverse drug reactions to integrase strand transfer inhibitors. AIDS 2018; 32:903–12. [DOI] [PubMed] [Google Scholar]
- 37. Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res 2017; 239:1–9. [DOI] [PubMed] [Google Scholar]
- 38. Mesplède T, Wainberg MA. Resistance against integrase strand transfer inhibitors and relevance to HIV persistence. Viruses 2015; 7:3703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fransen S, Gupta S, Danovich R, et al. . Loss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathways. J Virol 2009; 83:11440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardy I, Brenner B, Quashie P, et al. . Evolution of a novel pathway leading to dolutegravir resistance in a patient harbouring N155H and multiclass drug resistance. J Antimicrob Chemother 2015; 70:405–11. [DOI] [PubMed] [Google Scholar]
- 41. Wensing AM, Calvez V, Günthard HF, et al. . 2017 update of the drug resistance mutations in HIV-1. Top Antivir Med 2017; 24:132–3. [PMC free article] [PubMed] [Google Scholar]
- 42. Hazuda D, Miller M, Nguyen B, Zhao J. Resistance to the HIV-integrase inhibitor raltegravir: analysis of protocol 005 a phase II study in patients with triple-class resistant HIV-1 infection. In: Program and abstracts of the 16th International HIV Drug Resistance Workshop; June 12–16, 2007; Barbados, West Indies. 2007. Abstract 8. [Google Scholar]
- 43. Gatell JM, Katlama C, Grinsztejn B, et al. . Long-term efficacy and safety of the HIV integrase inhibitor raltegravir in patients with limited treatment options in a Phase II study. J Acquir Immune Defic Syndr 2010; 53:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mesplède T, Quashie PK, Osman N, et al. . Viral fitness cost prevents HIV-1 from evading dolutegravir drug pressure. Retrovirology 2013; 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blanco JL, Varghese V, Rhee SY, et al. . HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis 2011; 203:1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cozzi-Lepri A, Noguera-Julian M, Di Giallonardo F, et al. . Low-frequency drug-resistant HIV-1 and risk of virological failure to first-line NNRTI-based ART: a multicohort European case-control study using centralized ultrasensitive 454 pyrosequencing. J Antimicrob Chemother 2015; 70:930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malet I, Subra F, Charpentier C, et al. . Mutations located outside the integrase gene can confer resistance to HIV-1 integrase strand transfer inhibitors. MBio 2017; 8:e00922-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.