Abstract
Background
Most antibiotic use in the United States occurs in the outpatient setting, and 10% of these prescriptions are generated by dentists. The development of comprehensive antibiotic stewardship programs (ASPs) in the dental setting is nascent, and therefore we describe the implementation of a dental ASP.
Methods
A collaborative team of dentist, pharmacist, and physician leaders conducted a baseline needs assessment and literature evaluation to identify opportunities to improve antibiotic prescribing by dentists within Illinois’ largest oral health care provider for Medicaid recipients. A multimodal intervention was implemented that included patient and provider education, clinical guideline development, and an assessment of the antibiotic prescribing rate per urgent care visit before and after the educational interventions.
Results
We identified multiple needs, including standardization of antibiotic prescribing practices for patients with acute oral infections in the urgent care clinics. A 72.9% decrease in antibiotic prescribing was observed in urgent care visits after implementation of our multimodal intervention (preintervention urgent care prescribing rate, 8.5% [24/283]; postintervention, 2.3% [8/352]; P < .001).
Conclusions
We report the successful implementation of a dental ASP that is concordant with the Centers for Disease Control and Prevention Core Elements of Antibiotic Stewardship in the Outpatient Setting. Our approach may be adapted to other dental practices to improve antibiotic prescribing.
Keywords: antibiotic stewardship, dentistry
Increasing antibiotic resistance is a global health threat that is associated with increased mortality and increased health care costs [1–4]. In fact, the United Nations General Assembly met to address this threat in September 2016; this was only the fourth time since the inception of the United Nations that a health topic was discussed in this forum [4]. In addition to resistance, antibiotics are a common cause of severe adverse effects, including Clostridioides difficile infection (CDI; formerly known as Clostridium difficile) [5, 6]. The principal means to decrease antibiotic resistance and adverse effects is to minimize unnecessary antibiotic use. To mitigate suboptimal antibiotic use and improve patient outcomes, antibiotic stewardship programs (ASPs) have been advocated for by the World Health Organization, the Centers for Disease Control and Prevention (CDC), The Joint Commission, the Centers for Medicare and Medicaid Services (CMS), the Infectious Diseases Society of America, and others [2, 3, 7–9].
Historically, the focus of ASPs has been on the inpatient setting; however, the majority of antibiotic use in the United States occurs in the outpatient setting [10]. In the community, 10% of all antibiotics are prescribed by dentists [11, 12]. Dentists frequently prescribe antibiotics for indications including prophylaxis before dental procedures, postsurgery, and for the treatment of oral infections. Available data suggest that there is a significant opportunity for improvement, given that 30%–85% of antibiotics prescribed by dentists may be suboptimal or not indicated [13, 14]. Importantly, as much as 42% of CDI occurs in the outpatient setting, and antibiotics prescribed by dentists have been associated with CDI in multiple reports [6, 15–18]. Notably, dentists are the leading prescriber of clindamycin in the United States, which is among the highest-risk agents for CDI [12].
To encourage the implementation of ASPs, CMS now requires ASPs in nursing homes, and The Joint Commission requires ASPs in acute care facilities [8, 9]. The CDC has also developed recommendations for various settings, including the Core Elements of Outpatient Antibiotic Stewardship, which includes dental providers and dental practices and can be freely accessed from the CDC [19]. The CDC Core Elements of Outpatient Antibiotic Stewardship include making a commitment to optimizing antibiotic use, implementing a policy or practice to improve prescribing, tracking prescribing and related outcomes of the intervention(s) and feeding this information back to clinicians, providing education to prescribers and patients, and ensuring access to expertise needed to improve antibiotic prescribing. Comprehensive national guidelines exist for the development of ASPs in inpatient settings, but these specifically exclude outpatient settings [20]. However, multiple clinical treatment guidelines and a systematic approach for assessing overprescribing of antibiotics are available to inform the implementation and assessment of antibiotic stewardship in outpatient medical settings [21, 22]. In dentistry, a lack of consensus guidelines in the United States for the treatment of oral infections contributes to the difficulty of standardizing practice and assessing the appropriateness of antibiotic use in dental practices. A few studies describe the outcomes of interventions to improve antibiotic use in dentistry; these have found a lack of patient education and dentist guideline adherence to be barriers to appropriate antibiotic use. These studies, primarily in the United Kingdom, are encouraging but do not describe the implementation of comprehensive ASPs [13, 23–25].
Given the need to improve antibiotic prescribing in dental clinics and the lack of description in the literature regarding the development and implementation of systematic ASPs in dentistry, we describe our experience implementing an ASP based on the CDC Core Elements of Outpatient Antibiotic Stewardship in the dental practice setting.
METHODS/RESULTS
Setting
The University of Illinois at Chicago (UIC) College of Dentistry provides treatment to >30 000 patients each year and is Illinois’ largest oral health care provider for Medicaid recipients. UIC dental clinics provide comprehensive services, including comprehensive general dental care, urgent dental care, endodontics, oral medicine and orofacial pain, oral and maxillofacial surgery, orthodontics, pediatric dentistry, periodontics, and prosthodontics. Predoctoral students, residents, and faculty provide care at the college in its 19 general and specialty clinics. Dentists in Illinois prescribe 79.6 antibiotic prescriptions per 1000 people, higher than the national average [26].
The UIC College of Dentistry partnered with the already formalized ASP at The University of Illinois Hospital and Health Sciences System (UIH). The UIH ASP is co-led by 1 FTE infectious diseases pharmacist (A.E.G.) and 0.15 FTE infectious diseases physician (S.C.B.). The UIHASP’s goal is to empower front-line providers to use anti-infectives appropriately and thus optimize patient outcomes. These aims are facilitated through educational initiatives such as: facility-specific treatment guidelines, annual antibiograms, grand rounds presentations, and an institutional antibiotic stewardship webpage with comprehensive information. Prospective audit with intervention and feedback is conducted for patients receiving formulary-restricted anti-infectives and for patients with specific syndromes (eg, real-time rapid diagnostic-tied stewardship interventions for patients with bloodstream infections). Although historically the scope of the UIH ASP has focused mostly on inpatients, in 2016 ASP interventions were implemented in the outpatient medical setting, focusing primarily on inappropriate prescribing for acute upper respiratory tract infections in internal medicine and family medicine clinics. The current study was approved by the UIC Institutional Review Board.
Implementation of the Core Elements of Outpatient Antibiotic Stewardship at UIC Dental Practices
Commitment
In late 2016, after discussion with leadership at the UIC College of Dentistry, the UIH ASP provided a 1-hour continuing education session for the College of Dentistry clinical providers. This session introduced key concepts including the need for antibiotic stewardship in dentistry, recommendations related to the optimal use of antibiotic prophylaxis for common dental procedures, and possible mechanisms for effecting change. In the Summer of 2017, a meeting was held with representatives of the UIH ASP, College of Pharmacy Faculty, and representatives from the College of Dentistry. The aims of the meeting were to briefly review the CDC Core Elements of Outpatient Antibiotic Stewardship and discuss the feasibility of establishing an ASP at the UIC dental practices. The Associate Dean for Clinical Affairs (S.A.R., responsible for all clinical operations) at the College of Dentistry made a commitment to facilitate the development of a College of Dentistry ASP. To gain broad commitment among the dentists, the College of Dentistry ASP was discussed at a Clinical Operations Committee Meeting, and an email was sent to all College of Dentistry clinical providers highlighting the harms of antibiotic misuse and requesting that all dentists stand together in using antibiotics appropriately. Furthermore, in their annual plan, the UIH ASP included “assist with the development of the Dental ASP” as a new strategic initiative.
Action for Policy and Practice
In the Fall of 2017, a subsequent meeting between faculty from the College of Dentistry, UIH ASP pharmacist, and College of Pharmacy was held to discuss perceptions about the use of prophylactic antibiotics and antibiotics for acute oral infections, the choice of antibiotics, dose, and duration. It was suggested that there might be differences in antibiotic prescribing practices among surgical specialties. Furthermore, there may be areas for improvement in antibiotic use related to third molar extraction, bone/soft tissue grafting, and surgical implants.
In late 2017, the group reviewed baseline antibiotic prescribing data from September 2017 prepared by the College of Dentistry. First, a search for all prescriptions generated during September 2017 was completed, and from this, systemic antibiotic drugs were selected. These antibiotic prescriptions were then cross-referenced with the patient visit, Dental Procedures and Nomenclature (CDT) codes entered that day, the prescribing provider (student/resident/faculty), and the provider who approved (resident/faculty) the prescription. With this information, the electronic dental record was reviewed to identify presenting symptoms. Although studies related to the efficacy of utilizing pre- and postsurgical antibiotics have mixed conclusions, if the procedure has significant risk for and incidence of postoperative infection, antibiotics are generally indicated [27, 28].
With these data, we identified a few potential areas for improvement. First, we found that the prescribing rate for patients with acute dentoalveolar conditions (eg, periapical abscess) in the urgent care clinic was likely an area for improvement. We concluded this given that the prescribing rate varied widely among individual dentists, and antibiotics are often unnecessary for this indication in the absence of extraoral swelling, diffuse intraoral swelling, or systemic symptoms such as fever. We found that there was a significant number of antibiotic prescriptions generated with the CDT code “D0140 limited oral evaluation – problem focused” with student providers. This code is primarily used by student providers on rotation in the College of Dentistry’s Urgent Care Program. Furthermore, we observed variability in the choice of antibiotics for patients with acute dentoalveolar conditions. Amoxicillin was often used; however, the more narrow-spectrum agent, penicillin, was also prescribed. The second patient population identified for possible improvement per our baseline data was patients undergoing surgical placement of dental implants. Pre- and postsurgical placement prescribing patterns also varied between providers and specialty groups.
For our first practice intervention, we decided to focus on 1 area that was feasible, amenable to change primarily through education, not expected to be controversial, and likely to decrease antibiotic use for a significant portion of patients. We decided to first focus on standardizing antibiotic use for acute dentoalveolar conditions, given that this diagnosis met all of the aforementioned criteria, was identified as a need in our baseline assessment, and is a common condition in our practice.
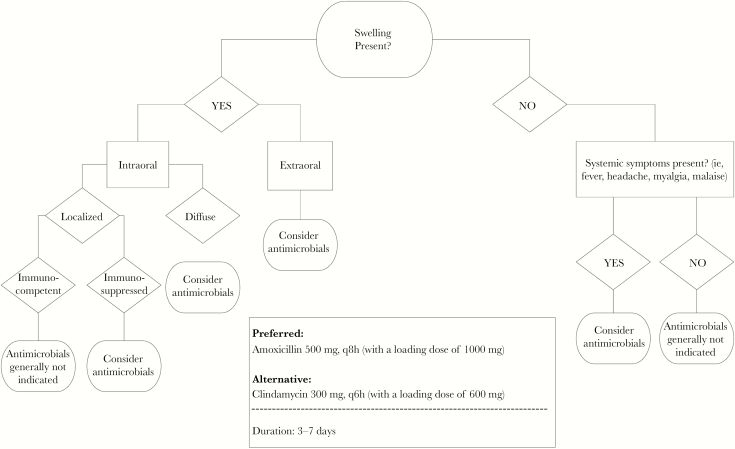
A systematic literature search was conducted to identify literature supporting best practices for antibiotic prescribing in patients with acute dentoalveolar conditions [29–31]. After a review of the available literature and international guidelines, the College of Dentistry faculty led the development of an evidence-based institutional guideline. To obtain broad input from fellow dentists and ensure buy-in, input and consensus for the guideline were gained among general dentists, endodontists, and oral and maxillofacial surgeons. The resulting guideline was designed to assist clinicians in identifying patients where antibiotics may be used based on patient symptoms (Figure 1). When antibiotics were indicated, this guideline also included preferred and alternative antibiotic therapy and a suggested duration. This guideline was reviewed and endorsed by the College’s Clinical Operations Committee and disseminated to all dentists, residents, and postgraduate and predoctoral clinical providers via email and posted in the dental clinics in March 2018.
Figure 1.
Clinical decision support tool; evidence-based recommendations for antimicrobial use for acute dentoalveolar conditions.
Education and Expertise
In addition to the institutional consensus guideline for the management of acute dentoalveolar conditions, multiple other educational interventions were implemented. In early 2018, weekly emails were sent to the College of Dentistry by the Office of Clinical Affairs that provided 1-minute updates on clinical guidelines, safety measures, and general reminders. These “Monday Minutes” focused on antibiotic stewardship in oral health for the first 12 weeks. Example educational topics each week included an overview of antibiotic stewardship, antibiotic use in dentistry including Illinois-specific prescribing data, clinical checklists to improve antibiotic prescribing in dentistry, and the 2017 updates for antibiotic prophylaxis against infective endocarditis [32, 33]. Furthermore, the recording of the previously provided 1-hour continuing education presentation was posted on the College of Dentistry’s faculty development website and was made available to all clinical providers and staff. Educational signs directed at dentists were also placed throughout the college and clinics and included the CDC tip sheets: “Seven Ways Dentists Can Act Against Antibiotic Resistance” [32, 34]. Finally, patient-facing educational posters that focused on when antibiotics may or may not be indicated at the dentist and the potential harms of antibiotics were placed in exam rooms [35].
Tracking and Reporting
The CDC Core Elements of Outpatient Antibiotic Stewardship suggests that 1 potential initial metric is tracking the percentage of all visits associated with an antibiotic [19]. In May 2018, after implementation of our educational “Monday Minutes,” signage and clinical provider and staff discussions focused on antibiotic stewardship in dentistry, antibiotic prescribing rates in faculty-supervised urgent care clinic visits were assessed and compared with baseline data from September 2017. Among all providers, the antibiotic prescribing rate per urgent care visit decreased by 72.9% before and after the multimodal intervention (preintervention urgent care prescribing rate [September 2017], 8.5% [24/283]; postintervention [May 2018], 2.3% [8/352]; P < .001). These data suggest that our initial interventions may have decreased antibiotic prescribing; these data were reported back to the College of Dentistry. During practice meetings where these data were discussed, clinical providers expressed that anecdotally they had become more conscious of appropriate antibiotic prescribing since the implementation of the educational interventions.
DISCUSSION
We have described our experience establishing a multidisciplinary ASP in an academic dental practice based on the CDC Core Elements of Outpatient Antibiotic Stewardship. To our knowledge, this is the first description of the implementation of a comprehensive antibiotic stewardship program in a dental practice. Our initial results suggest that simple educational interventions may decrease antibiotic prescribing in this setting. Although the results of our educational quality improvement initiative are limited by a lack of reporting of patient and prescriber characteristics, the metric we used is recommended by the CDC and is easily operationalized by our dental practices. Furthermore, a recent systematic review and international consensus study recommended that this metric be used in the outpatient setting for internal benchmarking purposes [36]. In addition, our dental providers have indicated an increased awareness of the appropriate use of antibiotics due to our educational efforts.
When considering which intervention to implement first with a new ASP, it is important to consider feasibility, impact, and likelihood of success [37]. Our interventions were simple, not controversial, evidence-based, and targeted one of the most common reasons for antibiotic use as identified by our baseline assessment. Our multimodal communication likely increased adoption of the educational content, given that the components were easily accessible by clinical providers, associated with continuing education credit, and included evidence-based clinical support tools. Analogous to our efforts, the aforementioned factors have been associated with facilitating physician participation in training programs related to appropriate antibiotic use for upper respiratory tract infections [38].
As part of our comprehensive stewardship program, we initially selected a high-impact target. Available data suggest that there are many potential opportunities for specific antibiotic use interventions in dentistry [14, 39–41]. In the United Kingdom, peer clinical audits of antibiotic prescribing among dentists have been used effectively for broad education and practice change purposes, including the need for better assessment of systemic signs of infection (availability and use of thermometers in dental practices) and for identifying the specific patient populations in which antibiotics are appropriate [24]. A randomized trial of audit and feedback based on local antibiotic use guidelines in Scotland has also been shown to decrease antibiotic use; however, qualitative data on common reasons for misuse were not collected [23].
Another study from the UK assessed the appropriateness of antibiotic use in dentistry based on adherence to Scottish and UK guidelines [25]. This cross-sectional study included patients with a pulpal, apical, or periodontal pathology, and data were collected including clinical presentation, signs and symptoms, diagnosis, antibiotic choice, dose, and duration. The authors found that 57% of the 568 enrolled patients received an antibiotic and only 19% of antibiotics were prescribed according to local guidelines. Furthermore, factors associated with an antibiotic prescription in the absence of an infection included previous operative treatment failure, presence of an acute periodontal condition, patient refusal of definitive operative treatment, dentist report of insufficient time to conduct definitive operative treatment, and patient request for antibiotics. These data suggest time resource limitations in these practices as a cause for inappropriate antibiotic use, but also factors related to patient education. Educating patients verbally and/or through educational posters in exam rooms on the harms of antibiotics and their appropriate use may decrease inappropriate use in this setting. Antibiotic prophylaxis is another potential target given that the implementation of national guidelines into practice is often delayed; recent US data have found regional variability in the rates of antibiotic prescribing and antibiotic prophylaxis, and this suggests another opportunity for improvement and standardization [41, 42].
In the future, we plan to expand the Dental ASP to other areas. We plan on mandating indications when prescribing antibiotics and integrating clinical decision tools into computerized medication order entry to further facilitate appropriate antibiotic use [38]. We will target other potential indications for antibiotics including acute periodontal conditions, infective endocarditis and orthopedic implant prophylaxis, and antibiotic prescribing associated with surgical treatment. The sustainability of our simple educational intervention will also be assessed in the future; other data suggest that simple educational interventions in the outpatient setting including easily accessible treatment guidelines for antibiotic prescribing can have a sustained decrease in antibiotic use even 3 years after dissemination [43]. We also plan on implementing peer comparison of prescribing rates in the urgent care setting. Finally, we are implementing antibiotic stewardship topics in the core College of Dentistry curriculum and believe this is likely an area that can be improved at other Colleges of Dentistry.
Although our Dental ASP is in an academic setting and was able to partner with a preexisting ASP, the resources required for ASP implementation were minimal and are likely to be similarly feasible and effective in community dental practices. Resources from the CDC and the Illinois Department of Public Health were helpful and were used in our ASP [32, 44]. Other state health departments may also be interested in partnering with dental practices to facilitate local ASP development.
Our report has some limitations. Reviewing and improving prescribing of dental providers is an emerging area in dentistry, and therefore queries by prescriptions cross-referenced with procedure codes are not a standard report in our electronic health record. We collaborated with our information services staff to generate a custom report to compile our data, and this also required manual review and classification of medication prescribed that may be subject to misclassification. Furthermore, the search to identify urgent care visits was based on CDT Codes, and visits may have been missed based on coding errors. The number of patient visits evaluated in the 2 time periods was significant, but the number of patient visits per provider was varied, and some providers had very few visits during the months queried. A longer time frame would be required to make conclusions about individual provider- level data and confirm the continued impact of the intervention. Furthermore, we have so far only assessed 1 quantitative outcome. However, about 13% of our 30 000 annual patient encounters occur in the urgent care clinic; thus this represents a significant portion of our patient encounters. This initial outcome was based on our practice’s identified needs, and future interventions and metrics tied to those will provide further evidence of the success of the ASP.
CONCLUSIONS
In conclusion, dentists prescribe a significant amount of antibiotics, and the implementation of systematic dental practice ASPs can facilitate appropriate antibiotic use to mitigate unintended consequences, such as antibiotic resistance and CDI. We have presented an approach to implementing a formalized ASP consistent with the CDC Core Elements of Outpatient Antibiotic Stewardship. We have discussed potential opportunities to improve antibiotic use and barriers and facilitators to effecting change so that others may potentially adapt lessons learned to implement antibiotic stewardship in their dental practices.
Acknowledgments
Disclaimer. The opinions expressed are those of the authors and do not represent those of the Department of Veterans Affairs, the US Government, or the Agency for Healthcare Research or Quality.
Financial support. We received no specific funding for this work. Research reported in this publication was partially supported by the Agency for Healthcare Research and Quality under award number R01 HS25177 (PI: Suda).
Potential conflicts of interest. A.E.G. has received consulting fees from Paratek outside of the submitted work. All other authors have no potential conflicts to disclose. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hauck C, Cober E, Richter SS, et al. Antibacterial Resistance Leadership Group Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect 2016; 22:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Antibiotic resistance: global report on surveillance, 2014 http://www.who.int/drugresistance/documents/surveillancereport/en/. Accessed 1 September 2018.
- 3. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013 http://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed 9 February 2019.
- 4. United Nations. Draft political declaration of the high-level meeting on the General Assembly on antibiotic resistance https://www.un.org/pga/71/wp-content/uploads/sites/40/2016/09/DGACM_GAEAD_ESCAB-AMR-Draft-Political-Declaration-1616108E.pdf. Accessed 1 September 2018.
- 5. Dantes R, Mu Y, Hicks LA, et al. Association between outpatient antibiotic prescribing practices and community-associated Clostridium difficile infection. Open Forum Infect Dis 2015; 2(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 2013; 173:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed 3 September 2018. [DOI] [PMC free article] [PubMed]
- 8. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) changes to promote innovation, flexibility, and improvement in patient care https://www.gpo.gov/fdsys/pkg/FR-2016-06-16/pdf/2016–13925.pdf. Accessed 9 February 2019.
- 9. The Joint Commission. Antibiotic Stewardship Standard, MM.09.01.01 https://www.jointcommission.org/assets/1/6/New_Antimicrobial_Stewardship_Standard.pdf. Accessed 26 October 2018.
- 10. Suda KJ, Hicks LA, Roberts RM, et al. Antibiotic expenditures by medication, class, and healthcare setting in the United States, 2010–2015. Clin Infect Dis 2018; 66:185– 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60:1308–16. [DOI] [PubMed] [Google Scholar]
- 12. Suda KJ, Roberts RM, Hunkler RJ, Taylor TH. Antibiotic prescriptions in the community by type of provider in the United States, 2005–2010. J Am Pharm Assoc (2003) 2016; 56:621–6 e1. [DOI] [PubMed] [Google Scholar]
- 13. Löffler C, Böhmer F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care—a systematic review. PLoS One 2017; 12:e0188061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suda KJ, Henschel H, Patel U, et al. Use of antibiotic prophylaxis for tooth extractions, dental implants, and periodontal surgical procedures. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blossom DB, Lewis FM, McDonald LC. The changing spectrum of Clostridium difficile associated disease: implications for dentistry. J Am Dent Assoc 2008; 139:42–7. [DOI] [PubMed] [Google Scholar]
- 16. Hansen D, Pollan LD, Fernando H. Fulminant Clostridium difficile colitis: a complication of perioperative antibiotic prophylaxis. J Oral Maxillofac Surg 2013; 71:1880–5. [DOI] [PubMed] [Google Scholar]
- 17. Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol 2012; 107:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mora Pinzon MC, Buie R, Liou J, et al. Outcomes of community and healthcare onset Clostridium difficile infection. Clin Infect Dis. 2018. Epub ahead of print. doi:10.1093/cid/ciy715 [DOI] [PubMed] [Google Scholar]
- 19. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65:1–12. [DOI] [PubMed] [Google Scholar]
- 20. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. Antibiotic prescribing and use in Doctor’s Offices https://www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/index.html. Accessed 26 October 2018.
- 23. Elouafkaoui P, Young L, Newlands R, et al. Translation Research in a Dental Setting (TRiaDS) Research Methodology Group An audit and feedback intervention for reducing antibiotic prescribing in general dental practice: the RAPiD cluster randomised controlled trial. PLoS Med 2016; 13:e1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer NA, Dailey YM, Martin MV. Can audit improve antibiotic prescribing in general dental practice? Br Dent J 2001; 191:253–5. [DOI] [PubMed] [Google Scholar]
- 25. Cope AL, Francis NA, Wood F, Chestnutt IG. Antibiotic prescribing in UK general dental practice: a cross-sectional study. Community Dent Oral Epidemiol 2016; 44:145–53. [DOI] [PubMed] [Google Scholar]
- 26. Roberts RM, Bartoces M, Thompson SE, Hicks LA. Antibiotic prescribing by general dentists in the United States, 2013. J Am Dent Assoc 2017; 148:172–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Surapaneni H, Yalamanchili PS, Basha MH, et al. Antibiotics in dental implants: a review of literature. J Pharm Bioallied Sci 2016; 8:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. Cochrane Database Syst Rev. 2013; (7):CD004152. doi:. 10.1002/14651858.CD004152.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Association of Endontists position statement: AAE guidance on the use of systemic antibiotics in endodontics. J Endod 2017; 43:1409–13. [DOI] [PubMed] [Google Scholar]
- 30. Segura-Egea JJ, Gould K, Şen BH, et al. European Society of Endodontology position statement: the use of antibiotics in endodontics. Int Endod J 2018; 51:20–5. [DOI] [PubMed] [Google Scholar]
- 31. Morrow SG. Use and abuse of antibiotics. Endontics: colleagues for excellence: American Association of Endontists 2012. https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/ecfewinter12final.pdf. Accessed 4 January 2019.
- 32. Centers for Disease Control and Prevention. Checklist for antibiotic prescribing in dentistry www.cdc.gov/antibiotic-use/community/downloads/dental-fact-sheet-FINAL.pdf. Accessed 4 January 2019.
- 33. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135:e1159–95. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Seven ways dentists can act against antibiotic resistance https://www.cdc.gov/antibiotic-use/community/pdfs/17_275237-B_Reagan_Dental_Infographic-01.pdf. Accessed 26 October 2018.
- 35. Centers for Disease Control and Prevention. Antibiotic safety: do’s and don’ts at the dentist https://www.cdc.gov/antibiotic-use/community/downloads/Dos-and-Donts-FINAL.pdf. Accessed 26 October 2018.
- 36. Versporten A, Gyssens IC, Pulcini C, et al. Metrics to assess the quantity of antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73(suppl_6):vi59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilde AM, Gross AE. A new practitioner’s guide to antimicrobial stewardship. Am J Health Syst Pharm 2013; 70:2180, 2–3. [DOI] [PubMed] [Google Scholar]
- 38. Allaire AS, Labrecque M, Giguere A, et al. What motivates family physicians to participate in training programs in shared decision making? J Contin Educ Health Prof 2012; 32:98–107. [DOI] [PubMed] [Google Scholar]
- 39. Dana R, Azarpazhooh A, Laghapour N, et al. Role of dentists in prescribing opioid analgesics and antibiotics: an overview. Dent Clin North Am 2018; 62:279–94. [DOI] [PubMed] [Google Scholar]
- 40. Fluent MT, Jacobsen PL, Hicks LA; OSAP, the Safest Dental Visit Considerations for responsible antibiotic use in dentistry. J Am Dent Assoc 2016; 147:683–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koppen L, Suda KJ, Rowan S, et al. Dentists’ prescribing of antibiotics and opioids to Medicare Part D beneficiaries: medications of high impact to public health. J Am Dent Assoc 2018; 149:721–30. [DOI] [PubMed] [Google Scholar]
- 42. Durkin MJ, Hsueh K, Sallah YH, et al. An evaluation of dental antibiotic prescribing practices in the United States. J Am Dent Assoc 2017; 148:878–86 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weiss K, Blais R, Fortin A, et al. Impact of a multipronged education strategy on antibiotic prescribing in Quebec, Canada. Clin Infect Dis 2011; 53:433–9. [DOI] [PubMed] [Google Scholar]
- 44. Illinois Department of Public Health. Antibiotic Stewardship Toolkit for Dental Providors http://dph.illinois.gov/sites/default/files/publications/opps-antibiotic-stewardship-toolkit-dentists-final-112017.pdf. Accessed 9 February 2019.