Abstract
Altitude is the major factor affecting both biodiversity and soil physiochemical properties of soil ecosystems. In order to understand the effect of altitude on soil physiochemical properties and bacterial diversity across the Himalayan cold desert, high altitude Gangotri soil ecosystem was studied and compared with the moderate altitude Kandakhal soil. Soil physiochemical analysis showed that altitude was positively correlated with soil pH, organic matter and total nitrogen content. However soil mineral nutrients and soil phosphorus were negatively correlated to the altitude. RT-PCR based analysis revealed the decreased bacterial and diazotrophic abundance at high altitude. Metagenomic study showed that Proteobacteria, Acidobacteria and Actinobacteria were dominant bacteria phyla at high altitude soil while Bacteroidetes and Fermicutes were found dominant at low altitude. High ratio of Gram-negative to Gram positive bacteria at Gangotri suggests the selective proliferation of Gram negative bacteria at high altitude with decrease in Gram positive bacteria. Moreover, Alphaproteobacteria was found more abundant at high altitude while the opposite was true for Betaproteobacteria. Abundance of Cytophaga, Flavobacterium and Bacteroides (CFB) were also found comparatively high at high altitude. Presence of many taxonomically unclassified sequences in Gangotri soil indicates the presence of novel bacterial diversity at high altitude. Further, isolation of bacteria through indigenously designed diffusion chamber revealed the existence of bacteria which has been documented in unculturable study of WIH (Western Indian Himalaya) but never been cultivated from WIH. Nevertheless, diverse functional free-living psychrotrophic diazotrophs were isolated only from the high altitude Gangotri soil. Molecular characterization revealed them as Arthrobacter humicola, Brevibacillus invocatus, Pseudomonas mandelii and Pseudomonas helmanticensis. Thus, this study documented the bacterial and psychrophilic diazotrophic diversity at high altitude and is an effort for exploration of low temperature bacteria in agricultural productivity with the target for sustainable hill agriculture.
Introduction
Altitude is the major factor which has confounded effects on both biodiversity and soil physiochemical properties. High altitude ecosystems are generally characterized by low temperature, variable precipitation, decreased atmospheric pressure and soil nutrient stress which have major impact on biodiversity [1]. High altitude cold environments represent the majority of the biosphere on Earth and have been successfully colonized by cold adapted microorganisms that are able to thrive, better survive and even maintain metabolic activity at subzero temperatures [2].
Western Indian Himalaya (WIH) is complex ecosystem of the planet earth which is decorated with wide range of mountains, glaciers, hot springs, cold affected soils and cold deserts. The largest proportion of Himalayan soil habitats is comprised of high altitude areas with cold and nutrient deprived soil. Heavy snowfall and repeated freezing and thawing due to seasonal variation in climate create more adverse environment [3]. Such variations in microenvironment with high altitude change the ecosystem properties which is the major factor determining the diversity of microorganims in such extreme habitats.
Extreme climatic conditions and topography of Himalaya affects the soil physiochemical properties of cold deserts. However on the other hand, increasing pressure for food and fodder on agriculture is forcing it to increase cultivation in high altitude soil ecosystem. Variable climate, precipitation, temperature, seasonal variation and snowfall alter the soil microenvironment regularly which ultimately leads to the poor soil properties. The physiochemical properties of high altitude soil were reported to vary drastically with altitudinal gradient [4]. The soil in these ecosystems is coarse textured, deserted and poor in nutrients [5]. Various soil fertility characteristics like, organic carbon, pH, total nitrogen, phosphorus and micronutrients has been found to show altitudinal variation [6]. In spite of these facts, very few studies highlighted the effect of altitudinal gradient on soil physiochemical properties of WIH, thus relation of the soil properties with altitude remain obscure. Therefore, there is immense need to study the dynamics of soil physiochemical properties across the altitudinal gradient of WIH.
Moreover, soil bacterial communities from high altitude are regarded as among the most complex and diverse assemblages of microorganisms [7]. Majority of the microorganisms in high altitude are psychrophiles and psychrotrophs which are equipped with well adaptation to thrive in high altitude associated stresses. Psychrophilic microorganisms are extensively sought for the production of cold active enzymes, cold adapted biofertilizers and metabolites which work best at low temperature [8]. WIH has been extensively studied through culturable and unculturable approaches which revealed it as a hotspot for the psychrophilic and psychrotrophic microorganims [9–11]. However, most of these studies were conducted at low to moderate altitude of WIH, leaving the high altitude mountains and glaciers soils least explored. Further, very little is known about the variation in microbial community structure of WIH in response to the change in altitude. Therefore, exploration of the microbial diversity at high altitude soil ecosystems and their comparison with microbial diversity from attitudinally different soil would provide the better insight on the effect of altitude on microbial community composition.
Gangotri, the second largest glacier of Himalaya after Siachen, is one of the extreme high altitude habitats of Himalaya. This glacier is 30 kilometers long with area (∼144 km2) and 0.5–2.5 km wide at height from 3000 to 7000 meters above sea level [12]. The permanent cold environment and scarcity of available soil nutrients at Gangotri make it the most stressed ecosystem of WIH. Previously, very few studies documented the bacterial diversity and soil chemical properties in Gangotri glacier ice and soil ecosystems [13]. Being one of the most high altitude ecosystems of WIH, Gangotri represent the most suitable soil system to study the effect of altitudinal variation on bacterial diversity. Thus, comparison of bacterial diversity of Gangotri soil to other low altitude soil system of WIH will provide the better insight of altitudinal variation on soil physiochemical properties and bacterial diversity. Moreover, Gangotri glacier is most affected by the consequences of global warming. Over the last 25 years of the 20th century it has retreated more than 850 meters [14]. Therefore, proper documentation of microbial diversity in soil ecosystem near Gangotri glacier will facilitate the studies of global warming induced changes in microbial community structure in future.
Culturable studies of bacterial diversity in WIH were exclusively based on the routine culturable techniques which are biased to only rapidly growing bacteria those grow only in nutrient rich medium. Thus, majority of unculturable bacteria are not trapped with such techniques [15]. Various attempts were made earlier to increase the isolation spectrum of bacteria from the environmental samples [16]. Previously Kaeberlein et al. 2002 [15] used the diffusion chamber based isolation strategy followed by in situ incubation for the isolation of novel microorganisms. However, no such attempts were ever made to cultivate bacterial diversity from WIH. Therefore, isolation of microorganisms from Gangotri in their natural soil environment through in situ incubation could increase the culturable bacterial diversity. Moreover, psychrophilic bacteria from Himalaya are known for their potential plant growth promotion [17, 18]. Nitrogen fixation in this harsh environment is of great interest because of the occurrence of high energy intensive nitrogen fixation process under cold stress in nutrients poor soil [10]. Comparative study of culturable psychrophilic diazotrophs in different altitude will emphasize the effect of altitude on culturable diazotrophic diversity. Therefore, besides revealing basic molecular mechanisms, psychrophilic diazotrophs from Gangotri could be the potential candidates for the bioinoculant development in the cold climatic agriculture.
Thus keeping above points in mind, soil chemical properties and bacterial diversity of high altitude Gangotri soil were studied and compared with previously studied Kandakhal soil to understand the effect of altitude on soil physiochemical properties and bacterial diversity [19]. Moreover, to assess the effect of altitude on psychrophilic diazotrophs, culturable diazotrophic diversity of both soils were compared. Further, indigenous diffusion chambers were constructed for the bacterial isolation from Gangotri soil as they were never used for WIH soil ecosystems.
Materials and methods
Soil sampling
Soil samples were collected from high altitude soil ecosystem of Gangotri (30.9947° N 78.9398° E). Upper 5 cm soil was taken randomly from different places with soil augur, mixed properly and kept in sterile polyethylene bags in cool packed box. Immediately after the transportation, one part of this sample was placed at 4°C for culturable study and other part was kept in -20°C for molecular studies. Some of the physiological parameters of the sampling sites were recorded on the spot and others were collected from the metrological survey. Sampling sites are depicted in S1 Fig. Sampling site did not involve endangered or protected species and no specific permissions were required to collect soil samples from this location.
Soil chemical analysis
Soil samples were analyzed for different soil physiochemical parameters, mainly soil texture, pH, total organic carbon (TOC), total Kjeldhal nitrogen (TKN), total phosphorus (P), nitrates (NO3-), ammonia (NH3), sulphates (SO4-2) and other trace elements. Soil analysis was out sourced from the Accurate Analytical Laboratory; Pune (ISO 9001–2000; Certified by TU¨ V, Germany).
Soil DNA extraction
Total soil DNA was extracted from 0.25 g soil sample using Powersoil DNA isolation kit (Mobio Lab. Inc., USA), according to the manufacturer’s instructions. DNA was quantified spectrophotometrically at 260 nm and stored in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) at −80°C till further use [18].
Real-time quantification and DGGE analysis of 16S rDNA and nifH genes
Copy number of 16S rDNA and nifH genes from collected soil samples were quantified using iCycler iQ5 Multicolor (Bio-Rad Lab, Hercules, USA) real-time polymerase chain reaction (qPCR) machine as described previously [17, 20]. DGGE was performed on a Dcode system (Bio-Rad Lab, Hercules, CA, USA). PCR for 16S rDNA and nifH DGGE analysis was performed as per earlier studies and PCR products were separated on 8% (w/v) acrylamide–bisacrylamide gel with a 40–70% denaturing gradient of urea and formamide at 60°C and 90V in 1X TAE for 16 hours [20, 21]. The gels were stained for 30 min in ethidium bromide in 1X TAE (Invitrogen, Paisley, UK) and visualized with a Gel Documentation system (Bio-Rad Lab, Hercules, CA, USA).
Metagenomic sequencing
Metagenomic sequencing was outsourced at Sandor Proteomics Pvt. Hyderabad, India. Sequencing was performed with two technical replicates. In this analysis, V3 region of the 16S rRNA gene was amplified using primer pair (341F-5′CCTACGGGAGGCAGCAG3′; 518R- 5′ATTACCGCGGCTGCTGG3′). Amplicons were purified and paired-end sequenced on an Illumina Hi-Seq platform. Singletons were removed as it might be a consequence of sequencing errors and can result in spurious OTUs. All the pre-processed reads were used to identify the OTUs using QIIME software package for constructing a representative sequence for each OTU. The representative sequence was finally aligned against Greengenes core set of sequences using PyNAST program. Representative sequence for each OTU was classified using RDP classifier and Greengenes OTU database and the sequences of those not classified were categorized as unknown. NGS data has been deposited to the NCBI Sequence Read Archive (SRA) with accession number SRR8208864.
Indigenous diffusion chamber and bacterial isolation
A diffusion chamber was made with the PVDF membrane. For its fabrication two rectangular PVDF membrane pieces were cut (14 × 7 cm) and sterilized through autoclaving. Under aseptic conditions a membrane pocket was made and sealed from the three sides leaving one side open for putting the inoculated agar slab. For making the agar slab, molten agar was poured in sterile Petri plate and cut to the appropriate size with scalpel blade so that it can fit into the pocket. A 100 μL of 10−3 dilution of sample was spread on both sides of the slab and placed under the pocket. After putting inoculated agar slab into the pocket, remaining side was sealed and diffusion chamber was kept in the moist soil bed prepared from the soil taken from the original sample site. Diffusion chamber was repeatedly turned to improved diffusion of gases. Several such diffusion chambers were made and incubated in native soil bed for different time intervals. Steps involved in the construction, inoculation and incubation of the diffusion chamber are depicted in Fig 1.
Fig 1. Schematic diagram of the construction, inoculation and incubation of diffusion chamber.
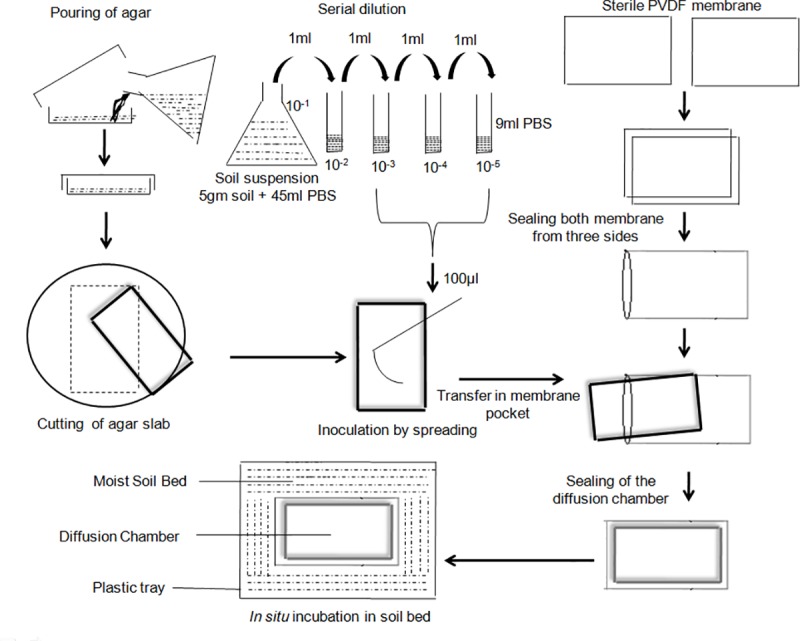
Construction of diffusion chamber and inoculation was performed in aseptic conditions under the laminar air flow.
Isolation and characterization of psychrophilic diazotrophs
Soil sample was serially diluted and 100 μL of each 10−3 and 10−4 dilution was spread on solid nitrogen deficient Burk’s medium and incubated at 2°C for 48 hours. Individual colonies were picked and repeatedly streaked on Burk’s medium and again incubated at 2°C and finally preserved in glycerol stock at -80°C. All the selected isolates were screened for the presence of nifH gene through PCR [17]. Thereafter, growth profile with respect to temperature was studied for nifH positive isolates and only those isolates having their optimum temperature in psychrophilic range were selected. These selected putative psychrophilic diazotrophs were sequenced and identified at Microbial Culture Collection (MCC) NCCS, Pune, India.
Results
Physico-metrological parameters of sampling site
Sampling site at Gangotri had the coordinates 30.9947° N, 78.9398° E and was situated at the altitude of 3,415 meters. Comparative physico-metrological parameters of both the sampling site are given in the Table 1. Annual mean maximum and minimum temperature was 11.1 ±0.7°C and -2.3 ±0.4°C, respectively. However soil temperature at the time of sampling was 4°C and was found maximum 10°C and minimum -2°C for the sampling day. Kandakhal sampling site had the coordinates 29.8800° N and 78.5710° E with altitude 1532 meters. Annual mean maximum and minimum temperature was 25.1 ±0.7°C and 8.3 ±0.5°C, respectively. However soil temperature at the time of sampling in Kandakhal was 12°C and was found maximum 18°C and minimum 10°C for the sampling day. Mean annual snowfall in Gangotri was 257.5 ± 81.6 cm for sampling year and no snowfall was recorded in Kandakhal.
Table 1. Comparative physico-metrological parameters of Gangotri and Kandakhal sampling site.
| S. No. | Physico-metrological parameter | Gangotri | Kandakhal |
|---|---|---|---|
| 1 | Sampling time | Late November | Late November |
| 2 | Latitude | 30.9947° N | 29.8800° N |
| 3 | Longitude | 78.9398° E | 78.5710° E |
| 4 | Altitude | 3,415 | 1532 |
| 5 | Soil temperature at the time of sampling | 4 ± 05°C | 12±1°C |
| 6 | Average annual temperature | Max. 11.1 ±0.7°C Min. -2.3 ±0.4°C |
Max. 25.1 ±0.7°C Min. 8.3 ±0.5°C |
| 7 | Average precipitation | 140 mm | 200mm |
| 8 | Mean annual snowfall | 257.5 ±81.6 cm | N/A |
Soil analysis
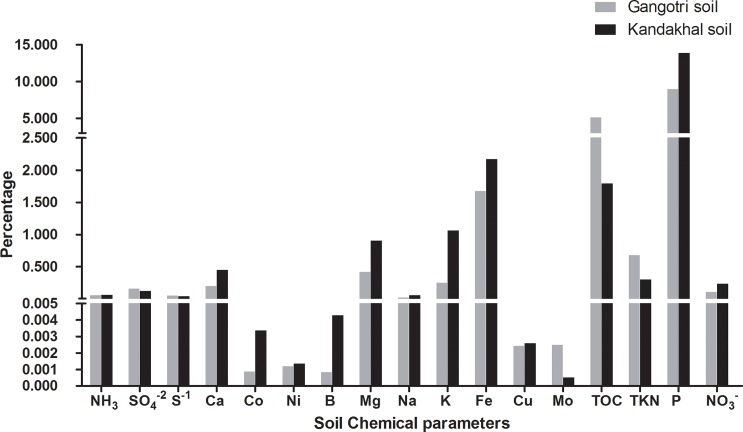
Soil analysis revealed that the chemical properties of soil vary significantly with the altitude as all the soil chemical parameters were found different in both soils (Fig 2). Soil texture was found coarse in both Gangotri and Kandakhal soil. Soil pH in Gangotri soil was 8.1 which was comparatively higher than Kandakhal where it was 7.2. Total organic carbon (TOC) for Gangotri and Kandakhal soil was 5.1006% and 1.7915%, respectively, whereas, Total Kjeldhal Nitrogen (TKN) was 0.6803% and 0.3033%, respectively. Soil nitrate in Gangotri and Kandakhal soil was 0.1107% and 0.2376%, respectively, while soil ammonium was 0.0538% and 0.0583%, respectively. Total Phosphorus in Gangotri and Kandakhal soil was 8.9387% and 13.8865%, respectively. Further, sulphates (SO4- 2) in Gangotri soil was 0.1591%, while in Kandakhal soil it was 0.1217%. Soil calcium, cobalt, nickel, boron, magnesium, sodium, potassium, iron and copper were significantly (P<0.005) higher in Kandakhal soil (S1 Table). However, molybdenum was comparatively five times higher in Gangotri soil (0.0025%) as compared to the Kandakhal soil (0.0005%). All the values of these macro-micro nutrients in both soils are given in the S1 Table.
Fig 2. Comparative soil chemical properties of Gangotri and Kandakhal soil.
Real-time quantification and DGGE analysis of 16S rDNA and nifH
Real time quantification of 16 S rDNA and nifH in Gangotri soil revealed that the copy numbers of 16S rDNA and nifH genes were 9.6×109 and 1.1 ×105, respectively. However in Kandakhal soil this was 4.63 × 1010and 8.7×105, respectively. In comparison to Kandakhal soil, Gangotri soil had significantly low copy number of 16S rDNA and nifH genes. Further, 16S rDNA and nifH DGGE profile in both soil showed the different banding pattern which indicates the difference in bacterial and diazotrophic diversity (Fig 3). Comparison of 16S rDNA and nif H DGGE profile for both soils revealed the comparatively large OTU in Gangotri, suggesting the rich bacterial and diazotrophic diversity in Gangotri soil despite the low copy number of 16S rDNA and nifH genes.
Fig 3.
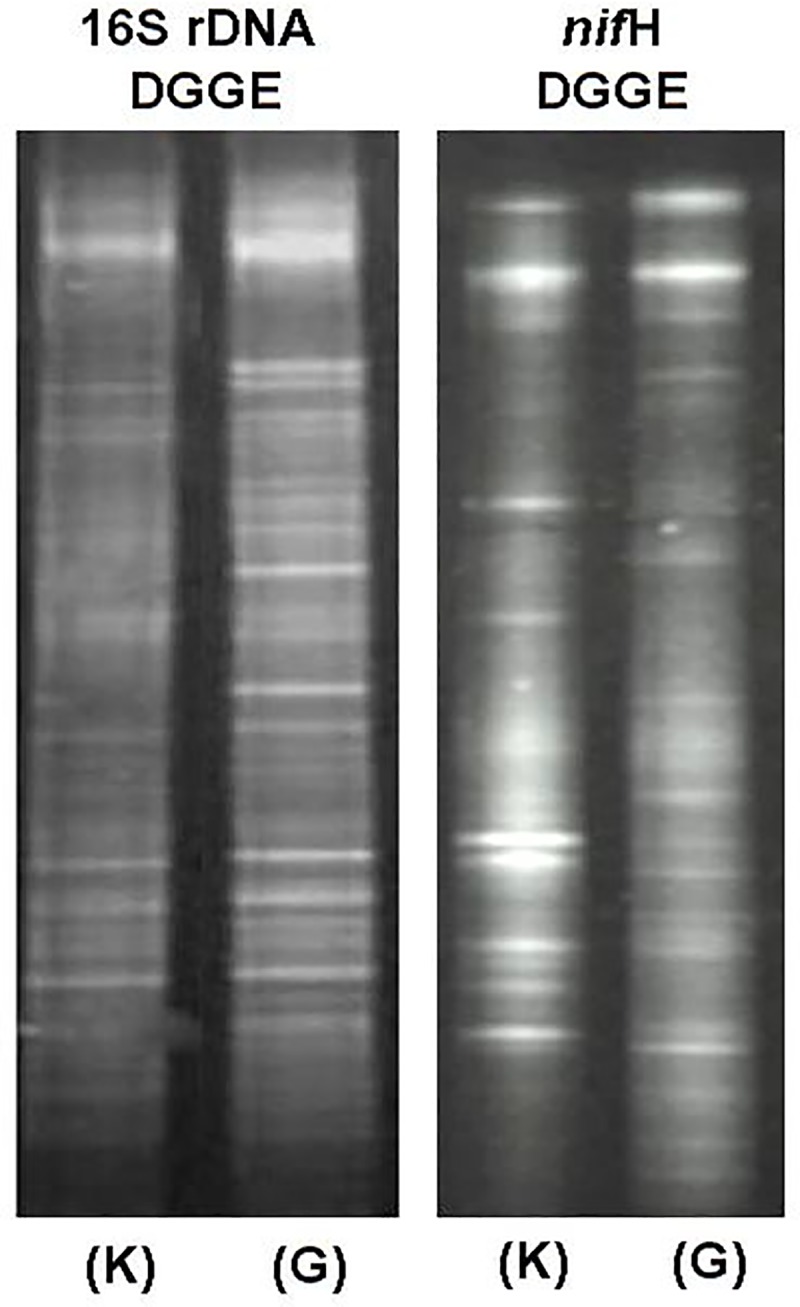
Comparative 16S rDNA (a) and nifH (b) DGGE profile in Gangotri and Kandakhal soil.
Bacterial diversity analysis of Gangotri soil
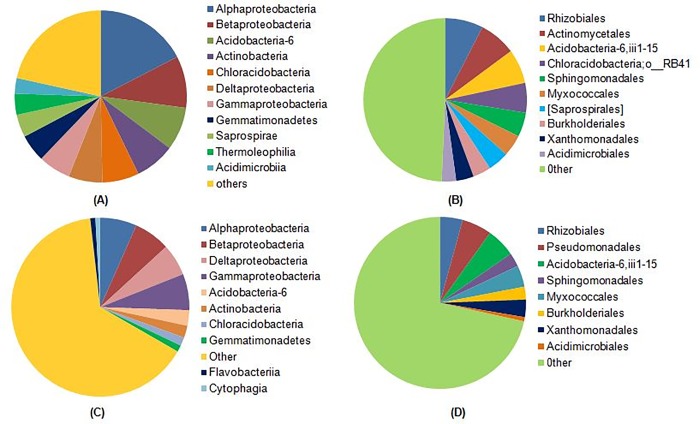
Metagenomic sequencing of two replicates of Gangotri soil DNA was performed through Illumina Hi-Seq platform. All the sequencing parameters are given in supplementary material (S2 Table). Analysis of metagenomic data revealed 115313 OTU in Gangotri soil. Shannon index (H’), Chao index, Simpson index, Equitability and PD whole tree were found 12.680, 115313, 0.998, 0.754 and 2464.99, respectively. The Shannon’s diversity index considers both richness and evenness while Chao values represent the only richness [22]. Moreover, Gangotri soil harbored diverse lineages of bacterial phyla. A total of 31 bacterial phyla were observed where, Proteobacteria (38.49%), Acidobacteria (17.88%) Actinobacteria (14.48%), Bacteroidetes (7.89%), Gemmatimonadetes (7.87%), Chloroflexi, (5.94%), Nitrospirae (1.08%),Cyanobacteria (0.93%), Firmicutes (0.9%), TM7 (0.84%), and Elusimicrobia (0.55%) were ten dominant bacterial phyla (Fig 4). Alphaproteobacteria (16.88%) was the most abundant bacterial class followed by Betaproteobacteria (9.44%), Acidobacteria-6 (7.86%) and Actinobacteria (7.33%) (Fig 5). Further analysis revealed that Rhizobiales (7.55%), Actinomycetales (7.29%), Acidobacteria-6,iii1-15 (6.81%), Chloracidobacteria;o__RB41 (5.89%) and Sphingomonadales (4.78%) were dominant orders (Fig 5) while, Sphingomonadaceae (4.52%), Hyphomicrobiaceae (3.79%) and Chitinophagaceae (3.42%) were dominant family. Taxonomic analysis showed that at phylum, class, order, family and genus level large number of reads were not identified. Presence of these large numbers of unidentified OTU suggests that the Gangotri soil had unique bacteria diversity.
Fig 4.
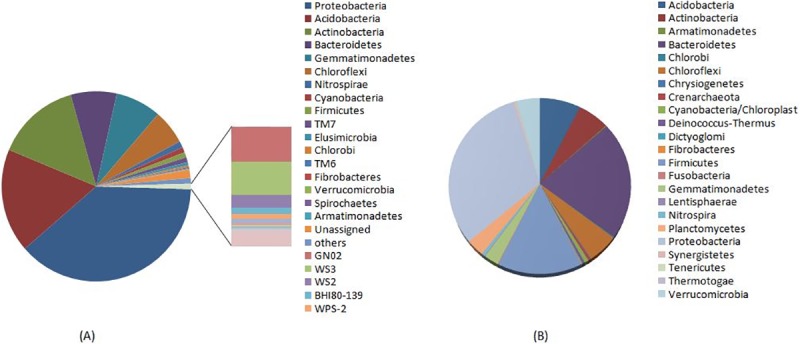
Dominant bacterial phyla in Gangotri (A) and Kandkhal soil (B).
Fig 5. Comparative representation of bacterial taxa in Gangotri and Kandakhal soil.
Where, (A) and (B) represents the relative percentage of bacterial class and order in Gangotri soil and (C) (D) represent dominant classes and order in Kandakhal soil.
Diffusion chamber and culturable diversity
Diverse bacterial species were obtained through diffusion chamber based culture-dependent strategy. Molecular identification of 14 isolates selected on the basis of different colony characteristic revealed them as Paenarthrobacter nirogunajacolicus, Dyadobacter endophyticus, Arthrobacter pascens, Paenarthrobacter siccitolerans, Pseudomonas mandelii, Acidovorax facilis, Pantoea gaviniae, Pseudomonas baetica, Pseudomonas frederiksbergensis and Arthrobacter equi (Table 2). Some of these isolates had never been documented from the WIH and are considered to be unculturable. These results suggest that this indigenous diffusion chamber could facilitate the isolation of novel/unique bacterial species.
Table 2. Identification of bacterial cultures isolated through diffusion chamber.
| S.No | Culture ID | Top-Hit taxon | Strain | Similarity (%) |
|---|---|---|---|---|
| 1 | UC-1 | Paenarthrobacter nirogunajacolicus | G2-1(T) | 99.86 |
| 2 | UC-2 | Pseudomonas mandelii | CIP 105273 (T) | 99.59 |
| 3 | UC-3 | Paenarthrobacter nirogunajacolicus | G2-1(T) | 99.86 |
| 4 | UC-6 | Arthrobacter pascens | DSM 20545 (T) | 99.37 |
| 5 | UC-8 | Pseudomonas mandelii | CIP 105273 (T) | 99.86 |
| 6 | UC-9 | Paenarthrobacter siccitolerans | G2-1(T) | 99.86 |
| 7 | UC-10 | Dyadobacter endophyticus | 65 (T) | 97.6 |
| 8 | UC-11 | Pseudomonas mandelii | CIP 105273 (T) | 99.59 |
| 9 | UC-13 | Acidovorax facilis | CCUG 2113(T) | 99.06 |
| 10 | UC-14 | Pantoea gaviniae | A18/07(T) | 98.31 |
| 11 | UC-15 | Arthrobacter nitroguajacolicus | G2-1(T) | 99.18 |
| 12 | UC-16 | Pseudomonas baetica | a390(T) | 99.59 |
| 13 | UC-17 | Pseudomonas frederiksbergensis | JAJ28(T) | 99.46 |
| 14 | UC-18 | Arthrobacter equi | IMMIB L-1606(T) | 98.76 |
Isolation of culturable psychrophilic diazotrophs
Bacterial isolation from Gangotri soil at 2°C in nitrogen deficient medium for 48 hours gave 30 bacteria isolate on the basis of differential colony characteristic. Further, only 25 isolates gave positive result in PCR based nifH amplification. Growth profiling of these 25 diazotrophs with respect to temperature suggested that only six have temperature profile in the psychrophilic temperature range (S2 Fig). All these six bacterial isolates had optimum growth temperature of 10°C and barely grow above 25°C. Molecular characterization revealed these psychrophilic diazotrophs as Pseudomonas helmanticensis, Arthrobacter humicola, Brevibacillus invocatus and Pseudomonas mandelii etc (Table 3). Further, no psychrophilic culturable diazotrophic bacteria were isolated from Kandakhal soil in nitrogen deficient medium after prolong incubation at 2°C.
Table 3. Molecular characterization of the cold adapted diazotrophs isolated from Gangotri.
| S.NO | Sample ID | Top-Hit Taxon | Top-Hit Strain | Similarity (%) |
|---|---|---|---|---|
| 1 | B-3 | Pseudomonas helmanticensis | OHA11(T) | 99.51 |
| 2 | B-8 | Arthrobacter humicola | KV-653(T) | 98.67 |
| 3 | B-25 | Brevibacillus invocatus | NCIMB 13772(T) | 99.93 |
| 4 | AB-03 | Pseudomonas mandelii | CIP 105273(T) | 99.52 |
| 5 | AB-04 | Pseudomonas helmanticensis | OHA11(T) | 99.31 |
| 6 | AB-16 | Pseudomonas mandelii | CIP 105273(T) | 99.73 |
Discussion
This study emphasized the effect of high altitude and associated factors on soil physiochemical properties and bacterial diversity. All the studied soil physiochemical parameters were comparatively different for both soils (Fig 2). Soil organic matter (SOM) is the important factor determining the microbial community structure in soil. In comparison to the Kandakhal soil, TOC was found significantly (P<0.05) higher in Gangotri soil. TOC content in Gangotri and Kandakhal soil was found 5.1006% and 1.7915%, respectively which was three times higher in Gangotri soil. Generally soil with SOM contents <0.5% is considered poor and >2.0% is desirable for agriculture [23]. Further analysis revealed that SOM was negatively correlated with temperature and positively correlated with altitude. Low temperature is reported to decrease the microbial and enzymatic activity in high altitude soil, thus rendering the SOM unaffected by microbial decomposition [24]. Therefore low temperature at high altitude is the major factor determining the high SOM at high altitude.
Total phosphorus (P) was found low, in Gangotri soil than Kandakhal. Further, less acidic pH of Gangotri soil would also reduce the bioavailability of P to the living system. Soil ammonium content was observed almost same in both the soils but soil nitrate and TKN were found two fold higher in Gangotri soil. Levels of ammonium nitrogen in soil vary from 2–10 ppm, but under cold conditions it was reported above 10 ppm [25]. TKN in Gangotri and Kandakhal soil was found 0.6803% and 0.3033%, respectively. Total nitrogen in agriculture soil is generally reported to vary from 0.10% to 0.15%, thus both the soils were rich in total nitrogen. Increased soil temperature is reported as a primary environmental factor that decrease the N mineralization processes thus influencing the bioavailability of soil nitrogen [26]. Nitrogen associated with SOM is not readily mineralized, thus comparatively high total N content of the soil at high altitude could be the result of high SOM. Though, the soil TOC, N and S content was found high in Gangotri soil, their bioavailability to the living system is major factor. Most of the N, P and S remain bound to the SOM, which is not degraded sufficiently under low temperature. Previously, nitrogen mineralization rate, SOM degradation rate and soil P content were found to decrease with temperature [27, 28]. Therefore, low nutrient status of high altitude soil is the result of low temperature induced decrease in mineralization and decomposition.
Further, mineral nutrients analysis revealed that calcium, cobalt, nickel, boron, magnesium, sodium, potassium, iron and copper were found decreased at high altitude soil. Similar trend in micronutrient dynamics with respect to altitudinal variation was found, previously [5]. However, Mo was found comparatively very high in Gangotri soil. Mo is usually present in soil in a concentration of 0.25–5 ppm [29]. High pH could be the possible reason for this increased Mo in Gangotri soil as pH is positively correlated with Mo concentration in soil. Thus Gangotri soil is alkaline, rich in SOM and TKN and poor in mineral nutrients. Therefore, this study showed the negative impact of increasing altitude on soil nutrient status which in turn influences microbial diversity.
Quantitative study of total bacteria and diazotrophs through q-PCR showed that bacterial and diazotrophic counts were significantly (P < 0.05) influenced by altitudinal differences. At Gangotri soil, high altitude and associated stresses decrease the total bacterial and diazotrophic count. Low available carbon and other soil nutrients at high altitude could negatively affect the growth of heterotrophic bacteria thus reducing the total bacterial count (TBC). On the other hand, low available nitrogen could provide selective advantages to the diazotrophs thus enhancing the diazotrophic count to the level which could sustain with other available nutrient resources [30]. Further, high soil nitrate under decreased N mineralization conditions indicates that the high nitrate concentration at high altitude soil could be the functional attribute of the diazotrophs which is further supported by the rich diazotrophic count in Gangotri soil.
Moreover, unculturable study of Gangotri soil concludes that this soil is rich in bacterial diversity as evident by the appearance of large number of different bacterial taxa. Further, presence of many taxonomically unclassified sequences indicates the presence of novel bacterial diversity. Moreover, Chao1 index, Shannon’s diversity index, Simpson index, Equitability and PD whole tree was significantly higher for Gangotri soil than Kandkhal soil. Comparison of metagenomic data of these two soils revealed the dominance of Proteobacteria in both soils with comparatively high relative abundance in Gangotri soil than Kandakhal. Besides, Proteobacteria, Acidobacteria and Actinobacteria had high abundance in Gangotri soil, while Bacteroidetes and Fermicutes were found higher in Kandakhal. Firmicutes was the third most abundant phylum in Kandakhal, but in Gangotri their abundance was very low indicating the negative impact of high altitude on the diversity of gram positive bacteria. High ratio of Gram-negative to Gram positive bacteria in Gangotri soil is due to the selective increase in proteobacterial diversity and decrease in the diversity of Firmicutes. Thus altitude has profound effect on the ratio of Gram-negative/Gram positive bacteria in the high altitude ecosystems. Many other bacterial phyla, Bacteroidetes, Gemmatimonadetes, Nitrospirae, Verrucomicrobia, Armatimonadetes, Cyanobacteria, Planctomycetes, and Chloroflexi were present in both soils. The abundance of Cytophaga, Flavobacterium and Bacteroides (CFB) were found positively correlated with high altitude. Presence of these phyla in cold deserts have also been reported in many studies [31–33].
Community composition of bacteria within above mentioned phyla was significantly different in both soils. In Gangotri, Alphaproteobacteria (16.88%) was the most abundant bacterial class of Proteobacteria followed by Betaproteobacteria (9.44%), Deltaproteobacteria (6.17%) and Gammaproteobacteria (5.9%). However, in Kandakhal soil all the above mention classes of Proteobacteria were uniformly distributed each having 8% abundance. Distribution of proteobacterial class in cold deserts is sensitive to seasonal variation and show dominance of Betaproteobacteria in summer, while Alphaproteobacteria shows equal abundance throughout the all seasons. Though, Betaproteobacteria is reported the most abundant proteobacterial class in high altitude, but little is known about the distribution of Proteobacteria in glacier ecosystem [34]. Previous studies of the permafrost glaciers revealed the Alphaproteobacteria as a dominant Proteobacteria in glacier soil. Thus glacier conditions at Gangotri could be the possible factor determining the dominance of Alphaproteobacteria in Gangotri soil.
Incubation in indigenous diffusion chamber resulted in the isolation of bacteria which were least characterized and supposed to be putative novel. This was the first time when culturable bacteria from high altitude cold desert soil were isolated through diffusion chamber based strategy. All the bacteria isolated through this technique were identified as Paenarthrobacter nirogunajacolicus, Dyadobacter endophyticus, Arthrobacter pascens, Paenarthrobacter siccitolerans, Pseudomonas mandelii, Acidovorax facilis, Pantoea gaviniae, Pseudomonas baetica, Pseudomonas frederiksbergensis and Arthrobacter equi. Comparison of these culturable isolates to the unculturable study revealed that these isolates belong to the phylum Proteobacteria, Actinobacteria and Bacteroidetes which were the most dominant bacterial phylum in Gangotri soil. Most of these isolates had never been isolated from the Gangotri and other regions of WIH. Therefore, these results indicate the effectiveness of “Indigenous Diffusion Chamber” for the isolation of rare bacterial species. Moreover, repeated full length sequencing and phylogenetic analysis of one isolate showed that this belongs to the genus Dyadobacter, showing highest similarity of 97.6% with Dyadobacter endophyticus 65 (T). Previous literature have shown that the Dyadobacter endophyticus 65 (T) is the entophyte of the maize plant, however no such cropping was done at high altitude Gangotri [35]. Thus this strain could be the putative novel and is being characterized further.
Psychrophilic bacterial population has been reported to be increased with altitude [36]. Considering the high altitude and permanent cold stress, psychrophilic nitrogen fixing bacteria from Gangotri soil were isolated. Molecular characterization of selected psychrophilic diazotrophs identified them as Pseudomonas helmanticensis, Arthrobacter humicola, Brevibacillus invocatus and Pseudomonas mandelii. To the best of our knowledge these isolates has been reported as cold adapted but not with nitrogen fixing attribute. Further, nitrogen fixation is an enzymatic process and negatively affected by temperature other than the optimal temperature for nitrogenase [37]. However, growth in nitrogen deficient medium at 2°C revealed that the nitrogen fixation machinery in these bacteria was least affected by very low temperature. Thus, molecular study of these isolates could enhance the present knowledge of the molecular mechanism of cold stressed nitrogen fixation at low temperature.
Conclusion
This study comprehended that the altitude has confounded effects on the both biotic and abiotic factors of the ecosystem. Comparison of high altitude Gangotri soil with Kandakhal soil revealed that soil chemical properties and microbial community structure both varies significantly with altitudinal gradient. This study concludes that high altitudes soil is alkaline, rich in SOM and TKN and poor in mineral nutrients. Moreover, at high altitude, soil microbial count decreases with the increase in the Cytophaga, Flavobacterium and Bacteroides (CFB), while ratio of Gram-negative/Gram positive bacteria, abundance of proteobacteria and culturable psychrophilic bacteria increases. Unculturable and diffusion chamber based study revealed the presence of unique taxonomically unclassified bacteria in this soil. Further, exploration for psychrophilic diazotrophs revealed the presence of functional psychrophilic diazotrophs in Gangotri soil which have good potential to fix nitrogen even at 2°C. These psychrophilic diazotrophs are potential candidates for low temperature bioinoculants. Therefore, WIH ecosystems are unique for their microbial community structure where altitudinal gradient is major factor determining the soil physiochemical properties and microbial community composition.
Supporting information
(TIF)
(JPG)
(DOCX)
(DOCX)
Acknowledgments
Author SK acknowledges the Council of Scientific and Industrial Research (CSIR) for granting the SRF fellowship and DCS acknowledges the Science and Engineering Research Board (SERB) Young Scientist scheme during course of this study.
Data Availability
All relevant data are within the paper and its Supporting Information files. Next generation sequencing data has been deposited in the NCBI Sequence Read Archive with accession number SRR8208864.
Funding Statement
Author SK acknowledges the Council of Scientific and Industrial Research (CSIR) for granting the SRF fellowship and DCS acknowledges the Science and Engineering Research Board (SERB) Young Scientist scheme during course of this study. We acknowledge the G.B. Pant University of Agriculture and Technology for providing the financial grant to conduct this research work (Project Code - 211). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Morán-Tejeda E. L, Moreno JI, Beniston M. The changing roles of temperature and precipitation on snowpack variability in Switzerland as a function of altitude. Geophysical Research Letters. 2013;40(10):2131–6. [Google Scholar]
- 2.D'Amico S, Collins T, Marx JC, Feller G, Gerday C. Psychrophilic microorganisms: challenges for life. EMBO Rep. 2006;7(4):385–9. 10.1038/sj.embor.7400662 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi D, Kumar S, Suyal DC, Goel R. The Microbiome of the Himalayan Ecosystem Mining of Microbial Wealth and MetaGenomics: Springer, Singapore; 2017. p. 101–16. [Google Scholar]
- 4.Shedayi AA, Xu M, Naseer I, Khan B. Altitudinal gradients of soil and vegetation carbon and nitrogen in a high altitude nature reserve of Karakoram ranges. Springerplus. 2016;5:320 10.1186/s40064-016-1935-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charan G, Bharti VK, Jadhav SE, Kumar S, Acharya S, Kumar P, et al. Altitudinal variations in soil physico-chemical properties at cold desert high altitude. Journal of soil science and plant nutrition. 2013;13(2):267–77. [Google Scholar]
- 6.He X, Hou E, Liu Y, Wen D. Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China. Sci Rep. 2016;6:24261 10.1038/srep24261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen C, Ni Y, Liang W, Wang J, Chu H. Distinct soil bacterial communities along a small-scale elevational gradient in alpine tundra. Front Microbiol. 2015;6:582 10.3389/fmicb.2015.00582 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav AN, Sachan SG, Verma P, Kaushik R, Saxena AK. Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. J Basic Microbiol. 2016;56(3):294–307. 10.1002/jobm.201500230 . [DOI] [PubMed] [Google Scholar]
- 9.Kasana RC. Bacterial Diversity in Cold Environments of Indian Himalayas Mining of Microbial Wealth and MetaGenomics: Springer, Singapore; 2017. p. 83–99. [Google Scholar]
- 10.Suyal DC, Kumar S, Yadav A, Shouche Y, Goel R. Cold Stress and Nitrogen Deficiency Affected Protein Expression of Psychrotrophic Dyadobacter psychrophilus B2 and Pseudomonas jessenii MP1. Front Microbiol. 2017;8:430 10.3389/fmicb.2017.00430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahay H, Babu BK, Singh S, Kaushik R, Saxena AK, Arora DK. Cold-active hydrolases producing bacteria from two different sub-glacial Himalayan lakes. J Basic Microbiol. 2013;53(8):703–14. 10.1002/jobm.201200126 . [DOI] [PubMed] [Google Scholar]
- 12.Singh P, Haritashya UK, Kumar N, Singh Y. Hydrological characteristics of the Gangotri glacier, central Himalayas, India. Journal of Hydrology. 2006;327(1–2) 55–67. [Google Scholar]
- 13.Baghel VS, Tripathi RD, Ramteke PW, Gopal K, Dwivedi S, Jain RK, et al. Psychrotrophic proteolytic bacteria from cold environment of Gangotri glacier, Western Himalaya, India. Enzyme and Microbial Technology,. 2005;36(5–6):654–9. [Google Scholar]
- 14.Bhattacharya A, Bolch T, Mukherjee K, Pieczonka T, KROPÁČEK J, Buchroithner MF. Overall recession and mass budget of Gangotri Glacier, Garhwal Himalayas, from 1965 to 2015 using remote sensing data. Journal of Glaciology. 2016;62(236):1115–33. [Google Scholar]
- 15.Kaeberlein T, Lewis K, Epstein SS. Isolating "uncultivable" microorganisms in pure culture in a simulated natural environment. Science. 2002;296(5570):1127–9. 10.1126/science.1070633 . [DOI] [PubMed] [Google Scholar]
- 16.Stewart EJ. Growing unculturable bacteria. J Bacteriol. 2012;194(16):4151–60. 10.1128/JB.00345-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Suyal DC, Dhauni N, Bhoriyal M, Goel R,. Relative plant growth promoting potential of Himalayan Psychrotolerant Pseudomonas jesenii strain MP1 against native Cicer arietinum L., Vigna mungo (L.) Hepper; Vigna radiata (L.) Wilczek., Cajanus cajan (L.) Millsp. and Eleusine coracana (L.)Gaertn. Afri J Microbiol Res. 2014; 8(50):3931–43. [Google Scholar]
- 18.Suyal DC, Yadav A, Shouche Y, Goel R. Diversified diazotrophs associated with the rhizosphere of Western Indian Himalayan native red kidney beans (Phaseolus vulgaris L.). 3 Biotech. 2015;5(4):433–41. 10.1007/s13205-014-0238-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S. 'Documentation of the bacterial and diazotrophic diversity from Garhwal Himalaya through culturable and unculturable approaches'. PhD Thesis, Govind Ballabh Pant University of Agriculture &Technology, Pantnagar, India, 2018.
- 20.Soni R, Goel R. Triphasic approach for assessment of Bacterial Population in Different Soil systems. Ekologija. 2010;56(3–4):94–8. [Google Scholar]
- 21.Kumar S., Suyal D.C., Bhoriyal M., Goel R. Plant growth promoting potential of psychrotolerant Dyadobacter sp. for pulses and finger millet and impact of inoculation on soil chemical properties and diazotrophic abundance. J plant Nutrition. 2018;41(8):1035–46. [Google Scholar]
- 22.MacDonald ZG, Nielsen SE, Acorn JH. Negative relationships between species richness and evenness render common diversity indices inadequate for assessing long-term trends in butterfly diversity. Biodiversity and Conservation. 2017;26(3): 617–29. [Google Scholar]
- 23.Hardy DH, Tucker MR, Stokes CE. Understanding the soil test report. NC Department of Agriculture & Consumer Services, Agronomic Division; 2013. [Google Scholar]
- 24.Wang G, Jia Y, Li W. Effects of environmental and biotic factors on carbon isotopic fractionation during decomposition of soil organic matter. Sci Rep. 2015;5:11043 10.1038/srep11043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horneck DA, Sullivan DM, Owen JS, Hart JM. Soil test interpretation guide. [Corvallis, Or: ]: Oregon State University, Extension Service; 2011. [Google Scholar]
- 26.Bu R, Lu J, Ren T, Liu B, Li X, Cong R. Particulate Organic Matter Affects Soil Nitrogen Mineralization under Two Crop Rotation Systems. PLoS One. 2015;10(12):e0143835 10.1371/journal.pone.0143835 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao H, Bai J, He X, Zhao Q, Lu Q, Wang J. High temperature and salinity enhance soil nitrogen mineralization in a tidal freshwater marsh. PLoS One. 2014;9(4):e95011 10.1371/journal.pone.0095011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walz J, Knoblauch C, Böhme L, Pfeiffer EM. Regulation of soil organic matter decomposition in permafrost-affected Siberian tundra soils-Impact of oxygen availability, freezing and thawing, temperature, and labile organic matter. Soil Biology and Biochemistry. 2017;110:34–43. [Google Scholar]
- 29.Tallkvist J, & Oskarsson, A. Molybdenum. Handbook on the Toxicology of Metals. (Fourth Edition) ed2015. p. 1077–89.
- 30.Chan YK, Barraquio WL, Knowles R. N2‐fixing pseudomonads and related soil bacteria. FEMS microbiology reviews. 1994;13(1): 95–117. [Google Scholar]
- 31.Demergasso C, Casamayor EO, Chong G, Galleguillos P, Escudero L, Pedros-Alio C. Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama Desert, Northern Chile. FEMS Microbiol Ecol. 2004;48(1):57–69. 10.1016/j.femsec.2003.12.013 . [DOI] [PubMed] [Google Scholar]
- 32.Yang GL, Hou SG, Le Baoge R, Li ZG, Xu H, Liu YP, et al. Differences in Bacterial Diversity and Communities Between Glacial Snow and Glacial Soil on the Chongce Ice Cap, West Kunlun Mountains. Sci Rep. 2016;6:36548 10.1038/srep36548 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Cong J, Lu H, Li G, Xue Y, Deng Y, et al. Soil bacterial diversity patterns and drivers along an elevational gradient on Shennongjia Mountain, China. Microb Biotechnol. 2015;8(4):739–46. 10.1111/1751-7915.12288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skidmore M, Anderson SP, Sharp M, Foght J, Lanoil BD. Comparison of microbial community compositions of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Appl Environ Microbiol. 2005;71(11):6986–97. 10.1128/AEM.71.11.6986-6997.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao JL, Sun P, Wang XM, Qiu TL, Lv FY, Yuan M, et al. Dyadobacter endophyticus sp. nov., an endophytic bacterium isolated from maize root. Int J Syst Evol Microbiol. 2016;66(10):4022–6. 10.1099/ijsem.0.001304 . [DOI] [PubMed] [Google Scholar]
- 36.Margesin R, Jud M, Tscherko D, Schinner F. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol Ecol. 2009;67(2):208–18. 10.1111/j.1574-6941.2008.00620.x . [DOI] [PubMed] [Google Scholar]
- 37.Stal LJ. The effect of oxygen concentration and temperature on nitrogenase activity in the heterocystous cyanobacterium Fischerella sp. Sci Rep. 2017;7(1):5402 10.1038/s41598-017-05715-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(JPG)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Next generation sequencing data has been deposited in the NCBI Sequence Read Archive with accession number SRR8208864.