Abstract
At present, ultrasound radiation is broadly employed in medicine for both diagnostic and therapeutic purposes at various frequencies and intensities. In this review article, we focus on therapeutically-active nanoparticles (NPs) when stimulated by ultrasound. We first introduce the different ultrasound-based therapies with special attention to the techniques involved in the oncological field, then we summarize the different NPs used, ranging from soft materials, like liposomes or micro/nano-bubbles, to metal and metal oxide NPs. We therefore focus on the sonodynamic therapy and on the possible working mechanisms under debate of NPs-assisted sonodynamic treatments. We support the idea that various, complex and synergistics physical–chemical processes take place during acoustic cavitation and NP activation. Different mechanisms are therefore responsible for the final cancer cell death and strongly depends not only on the type and structure of NPs or nanocarriers, but also on the way they interact with the ultrasonic pressure waves. We conclude with a brief overview of the clinical applications of the various ultrasound therapies and the related use of NPs-assisted ultrasound in clinics, showing that this very innovative and promising approach is however still at its infancy in the clinical cancer treatment.
Keywords: Inertial cavitation, Reactive oxygen species, Sonoluminescence, Sonodynamic, Tumor Therapy, Cytotoxicity
1. Introduction
Ultrasound is defined as a type of mechanical sound wave with a periodic vibration at frequencies higher than the human hearing (20 kHz). It is generated by exciting at a proper frequency an ultrasonic transducer (usually based on a piezoelectric component or on an electromagnetic inductor) able to convert the electrical signal into a mechanical displacement [1,2]. Ultrasound devices are usually composed by a generator, a compensating amplifier and a transducer [3,4].
It is already known that ultrasonic waves cause thermal and nonthermal effects. In particular, thermal effects refer to an increase in temperature due to the absorption of the ultrasonic waves through a tissue creating mechanical compression and decompression. Part of this mechanical energy is lost due to friction effects and it is converted to heat. As a consequence, in biological systems [5] the liquidity of the phospholipid bilayer, composing the cell membranes, changes and the membrane permeability can alter [6].
The non-thermal effect of ultrasound is a complex and various set of mechanisms, comprising stable and inertial cavitation, microstreaming and radiation forces [7]. These events are able to induce both temperature increase and mechanical stresses, those in particular known as microjets and microstreams [8]. More in details, during non-inertial cavitation (also called stable cavitation) the gas pockets present in the liquid oscillate around an equilibrium radius and can persist for many acoustic compression and decompression cycles. These oscillations generate fluid streaming and the mechanical stresses create mixing of the medium [9]. On the other hand, the inertial cavitation is the process by which the gas bubbles trapped in a fluid are subjected by a rapid growth and violent collapse during exposure to ultrasound. During such collapse, high temperatures (higher than 5000 K) and pressures (more than 800 atm) are produced, releasing a high amount of energy [9]. The inertial cavitation is able to induce water thermal dissociation and thus reactive oxygen species (ROS). Furthermore, cavitation generates flashes of light, a phenomenon called sonoluminescence (SL) [9].
Ultrasound is largely employed at present in medicine for diagnostic and therapeutic purposes. The produced biological effects are related both to the intensity and frequency of the ultrasound wave used [1]. Compared to other external stimuli, it has good tissue penetration capability, it is quite safe to human health and shows low operation and instrumental costs [9,10]. Ultrasound represents an important tool for imaging and diagnosis, in a technique called sonography. In particular, the ultrasonic waves are focalized at a particular depth of diagnostic interest. Owing to the different acoustic resistances of the various tissues, the scattered signal is recovered, allowing to the imaging reconstruction of the different tissues. To enhance the echogenicity and ultrasound responsiveness of certain tissues, microbubbles were developed as contrast agents. They basically consists of various gases enhancing echogenicity stabilized within a lipid or protein shell [11,12]. It is thus possible to obtain 2D and 3D images of tissues and organs [3].
Furthermore ultrasound was used for the treatment of numerous pathologies [13], such as a remedy of soft tissue injuries, for the acceleration of wound healing, for the resolution of edema, or for the softening of scar tissues [14]. Lithotripsy procedures were applied for stones removal in urology [15]; low-intensity pulsed ultrasound found therapeutic applications for bone growth stimulation [16]. Ultrasound-assisted lipolysis and liposuction are conventional practices in cosmetic surgery for fat tissue removal [17]. However, these topics are out of the interest of the present work and the reader could refer to recent gold reviews elsewhere [18,19].
This review will focus on the use of ultrasound in the presence of both soft and solid-state nanoparticles (NPs) against tumor cells or tissues and with a special emphasis on the sonodynamic treatment (SDT). A very recent review in the field related either to NPs and nanomaterials used for SDT was reported [20]. A second review more focused on the mechanisms of SDT related to experimental medicine and biology was also recently written [21]. Here our aim is to propose an update of the most recent advances in the field focusing on the mechanisms underlying the synergistic effect of NPs and acoustic fields toward the improved sonodynamic therapeutic outcome.
2. Sonophoresis
With respect to other routes of administration, transdermal drug delivery has potential advantages since it reduces the first-pass metabolism associated with oral delivery and is less painful than parenteral administrations [22]. However, the stratum corneum limits passive diffusion to small lipophilic molecules and methods to safely render it permeable to ionic and larger molecules are needed [13]. The sonophoresis technique is based on the ability of ultrasound radiation to increase the permeability of the stratum corneum, which is considered a primary barrier to protein and drug diffusion [23]. Once a drug has traversed the stratum corneum, the next layer is easier to perfuse, and subsequently the drug can reach the capillary vessels to be absorbed [13].
While ultrasound over all the frequency ranges can enhance skin permeability, the physical mechanisms responsible for enhanced permeation are different in each regime. Initial studies focused on High Frequency ultraSound (HFS) as the first use of ultrasound to deliver therapeutics across the skin in 1954. Because the skin penetration depth of the ultrasound waves is inversely dependent to the frequency of the pressure wave, thereby its effect are limited on the stratum corneum at high frequencies [24,25]. The characteristic permeation enhancement achieved with HFS is one to ten-fold more with respect to the absence of ultrasound [26]. In contrast, the enhanced transdermal permeation of Low Frequency ultraSound (LFS) was only discovered later. Mitragotri et al. reported that the in-vitro use of 20 kHz ultrasound resulted in 1000- times greater permeability for salicylic acid and sucrose across human cadaver skin compared with that achieved with 1 MHz ultrasound [27,28].
There are several mechanisms to enhance skin permeability in sonophoresis. Among these, the acoustic cavitation [29,30], the thermal effects [27,31], the radiation forces and convection (acoustic streaming and the resulting boundary-layer reduction) [32], as well as the lipid extraction [33] were investigated.
One of the dominating mechanism for the enhancement of skin permeability is acoustic cavitation [26,34]. With respect to stable cavitation, the inertial cavitation results in higher permeability enhancement of the stratum corneum in ultrasound-assisted skin permeabilization [26]. The bubbles diameter that initially nucleate is inversely proportional on ultrasound frequency. At high frequencies, the nucleating bubbles are small (the diameter is circa 5 μm at 1 MHz) and can nucleate within the stratum corneum, giving rise to some disruption of this ordered structure. However, when using LFS, the nucleated bubbles are too large and can no longer oscillate within the stratum corneum (at 20 kHz the size is around 300 μm, that is much larger than the 20 μm thickness of the stratum corneum). Using LFS, the large bubbles form outside the skin, become unstable and implode powerfully near the solid stratum corneum surface resulting in a jet of fluid, referred to microjet. When these microjets affect the stratum corneum, they erode the dead cells and help to permeate the membrane [35–37]. Tang et al. definitively demonstrated by an experimental study that ultrasound-induced cavitation is the key mechanism via which LFS permeates the skin. They reported that cavitation, occurring outside the skin, plays the pivotal role in the skin permeation effect, while internal cavitation has no effect in the skin permeability [38].
Focusing on the NPs-assisted ultrasound as the aim of this review, a first study supporting the delivery of oligonucleotides by the application of LFS (20 kHz, 2.4 W/cm2, 50% duty cycle, 10 min) across the fullthickness pig skin was reported by Tezel et al. [39]. Similarly, Tran et al. delivered small interfering RNA loaded into cationic liposomes to melanocytic tumors present in skin to retard melanoma development. Low-frequency ultrasound supplied by a four-cymbal transducer array (20 kHz, 20% duty cycle, 15 min) enabled the penetration of nanoliposomal-siRNA complexes throughout the epidermal and dermal layers of laboratory-generated or animal skin. Nanoliposomal-mediated siRNA targeting of (V600E)B-Raf and Akt3 led to a cooperatively acting circa 65% decrease in early or invasive cutaneous melanoma compared with inhibition of each singly with negligible associated systemic toxicity [40].
3. High intensity focused ultrasound
High intensity focused ultrasound (HIFU), also called focused ultrasound surgery (FUS) [5], is a non-invasive method where high intensity ultrasonic waves are applied locally in a focal zone. The major effect is an extreme temperature rise (greater than 80 °C) due to the absorption of the ultrasound energy [7,41]. This increment results in a complete and irreversible cell death through coagulative necrosis in the focal region, minimizing the possibility of thermal damages to the tissues outside the irradiated region [1]. Non-thermal effects as acoustic cavitation, microstreaming and radiation forces also occur [8] inducing shear stress causing membrane damage and cell death [7]. With a different modulation of exposure time, number of pulses and duty cycles it is possible to obtain predominantly thermal or non-thermal effects in the focal region [41], limiting i.e. temperature increase [43].
HIFU was investigated for the treatment of various types of primary solid tumors and metastasis, including prostate, breast, kidney and liver. Moreover, HIFU was also proposed as a novel approach capable to ablate heart ectopic foci and to obtain hemostasis in acute traumatic injuries [7] and for the treatment of Alzheimer disease [43]. HIFU was also successfully used to promote the uptake of various molecules, as antineoplastic drugs, antibodies, genes and others [44], increasing temporarily the cell permeability, thanks to the capability of ultrasound to temporarily increase the cell permeability [8,45], as described more in details below. Moreover, a possibility is to enhance the drug release in a target region using HIFU by disrupting the drug trapping vesicles [46].
Despite of many promising outcomes, there are some limitations to this therapeutic approach. In particular, the achievement of elevated temperatures when the region of interest is deep or hypervascularized can be problematic. Actually, a solution could be to enhance the acoustic power or the exposition time, however it would increase the risk of side effects, as skin burns and nerve injury [47,48]. Thus, various types of micro and nano–bubbles [49], as well as other particles [50], were proposed in combination with HIFU for therapy and diagnostic imaging. These structures are indeed able to enhance HIFU-associated mechanical effects, providing cavitation nuclei [48]. Furthermore, they increase the acoustic attenuation with a consequent temperature rise [51], reducing the ultrasound intensity and the exposure time required to obtain bioeffects [47].
4. Sonoporation
This ultrasound-based permeation technique allows for the transfer of molecules between the intra- and extra-cellular medium [52–54]. Actually ultrasound can be used to temporarily render permeable the cell membrane allowing for the uptake of drugs, DNA and other therapeutic compounds from the extracellular environment [55]. Several sonoporation mechanisms were proposed and the main hypotheses of trapped microbubble interaction with cells are the push and pull mechanisms, micro-jetting, micro-streaming, and, more recently, translation of microbubbles through cells. Since the membrane alteration is transient, it leaves the drug trapped inside the treated cells after sonication.
Even if the biophysical mechanism that results in the enhancement of the cell membrane permeability under ultrasound needs further elucidation, it was reported that sonoporation is not due to inertial cavitation, but to micro-streaming and shear stresses related to stable oscillations [56,57]. In in-vitro experiments the dissolved gas in the culture medium is sufficient so that the sonication itself generates cavitation bubbles. Sonoporation is thus induced. In contrast, in in-vivo applications the lungs are very efficient at clearing out small bubbles from the circulatory system. Therefore micro and nanobubbles have to be added to induce sonoporation through ultrasound irradiation [58].
In oncological research several in vitro studies have shown ultrasound-induced membrane permeability. This mechanism has increased the uptake of anti-cancer drugs such as bleomycin, adriamycin, [59,60] and cisplatin both in-vitro and in-vivo [61].
Moreover, transcranial delivery by low-frequency ultrasound can be employed to temporarily disrupt the blood brain barrier (BBB) and thus enhance drug diffusion through microbubbles [62]. Administration of microbubbles further reduces the intensity threshold for temporarily BBB disruptions, thus allowing for much lower and safer frequencies to be applied than in their absence [63]. The targeted BBB disruption could also support the delivery of chemotherapeutic agents for brain tumors, which normally do not penetrate the BBB. More specifically, the delivery of liposome-encapsulated doxorubicin to the BBB was first investigated. The treated regions showed significantly higher concentrations of doxorubicin than the contralateral side. Moreover, the concentration of the drug in the brain tissue was observed to growth linearly with increasing the microbubble concentration [64].
Beside the use of sonoporation with chemotherapeutic molecules, this method is particularly suitable for the delivery of free nucleotides, which are otherwise prevented to cross the plasma membrane due to their negative charge and large size [57,65]. Efficient gene transfer by sonoporation was achieved when the applied ultrasound frequencies are close to those used clinically. Typically they extend from 0.5 to 4 MHz. Significant results were obtained in-vitro as well as in-vivo with focused ultrasound [66]. The level of gene expression reported after sonoporation treatments is one or two orders of magnitude higher than the level obtained with plasmid DNA alone. However, it remains lower than that obtained with chemical vectors [67]. This limitation is probably ascribable to the main difficulty in the field of ultrasound-assisted gene delivery. It consists in the lack of homogeneity both in the sonication set-up and of the acoustic conditions.
5. Ultrasound-triggered drug delivery system
Biological systems have demonstrated very high spatiotemporal (location and timing) sensitivity to cues and drugs. Polymer-based drug delivery systems are able to achieve a constant rate of release. Moreover they have been extensively studies for attaining localized and sustained release of bioactive molecules [68].
Recently Huebsch et al. proposed a quasi-digital ultrasound-triggered drug release, which could be accelerated and then switched back off, on demand, by applying ultrasound to disrupt ionically cross-linked hydrogels [69]. They reported that ultrasound does not permanently damage these materials. In contrast these hydrogels are able to self-repair the cross linked structure and to stop the release in the absence of the ultrasound stimulus. In-vitro studies demonstrated that a temporally short, high-dose “bursts” of drug exposure could be applied to enhance the toxicity of mitoxantrone toward breast cancer cells. Furthermore, the authors used the developed hydrogel system in-vivo to treat xenograft tumors with mitoxantrone. They found that daily ultrasound-stimulated drug release significantly reduced tumor growth with respect to the sustained drug release alone. They envisioned that the ultrasonically-assisted digital drug release will be applicable to a broad variety of polymers and bioactive molecules. This can be a potentially useful tool for studying how the timing of factor delivery controls cell fate in-vivo.
To reduce the detrimental side effects of toxic chemotherapeutic drugs, the ultimate strategy is to encapsulate the drugs in a vehicle (either an organic or inorganic micro or nano-sized carrier) showing a very low leak rate in circulation. At the same time, the carrier shows a rapid release of the drug once inside the tumor, also limiting the healthy tissue exposure [70–72].
Designing a vehicle with these two opposing properties is one of the major challenge in the field of drug delivery [73–75]. Furthermore, the design of a triggering strategy able to change the vehicle from its stable yet circulating state to its unstable thus release state can be problematic [57,76]. A unique mechanical actuation trigger is achieved by exploiting the size changes that occur when microbubbles (1–10 μm in size) interact with ultrasound allowing for rapid drug release and facilitating delivery into nearby cells. It is thus possible to focus the ultrasound to just a few cubic millimeters, allowing for precise control over the tissue location where the microbubbles are destabilized, yet able to deliver the encapsulated drug. Moreover, performing drug delivery from microbubbles by using ultrasound as trigger gives the possibility to visualize the drug-loaded microbubbles by low-pressure ultrasound [77,78]. Actually, microbubbles were born as ultrasound contrast agents and this promising technique is called “image-guided drug delivery” [79].
Different strategies were proposed to load drugs into microbubbles including drug molecules incorporated inside the hydrophobic shell, shell electrostatic binding, and drug-containing liposomes linked to the surface of microbubbles. For example, Tinkov et al. reported a 12-fold increase in local drug concentration of doxorubicin-shell-embedded microbubbles and a significant reduction in tumor growth [80]. Similarly, microbubbles loaded with 10-hydroxycamptothecin into the shell and exposed to ultrasonic excitation showed a significant drug accumulation in tumor tissues and a remarkable increase in tumor inhibition rate [81]. Docetaxel-loaded microbubble demonstrated to be both an effective ultrasound contrast agent in-vivo and enhanced the antitumor drug capability in-vitro. In fact, by exposing to ultrasound microbubbles loaded with doxorubicin-liposomes, twice more melanoma cells were killed compared to doxorubicin-liposomes alone [82]. In addition to the aforementioned small chemotherapeutic molecules, also therapeutic nucleotides (genes and siRNA) were loaded into microbubbles [83]. For example, siRNA-loaded microbubbles exposed to 1 MHz ultrasound were able to knockdown twice more the tumor suppressor gene PTEN than control siRNA alone [84].
Endo-Takahashi et al. developed polyethyleneglycol (PEG)-modified bubble liposomes containing ultrasound-contrast gas. Inside them, the authors entrapped pDNA or siRNA to be simultaneously used for ultrasound imaging and gene delivery, thus useful in the field of theranostics [44].
The main limitation to the use of microbubbles is due to their dimensions: being generally above 1 μm, it is expected that they do not extravasate into tumors, since the interstices between tumor-associated endothelial cells are in the range of 500 nm [85]. In order to avoid this drawback, “nanobubbles” small enough to extravasate through these endothelial gaps were proposed. More detail on this topic will be described in the section below.
6. Sonodynamic therapy
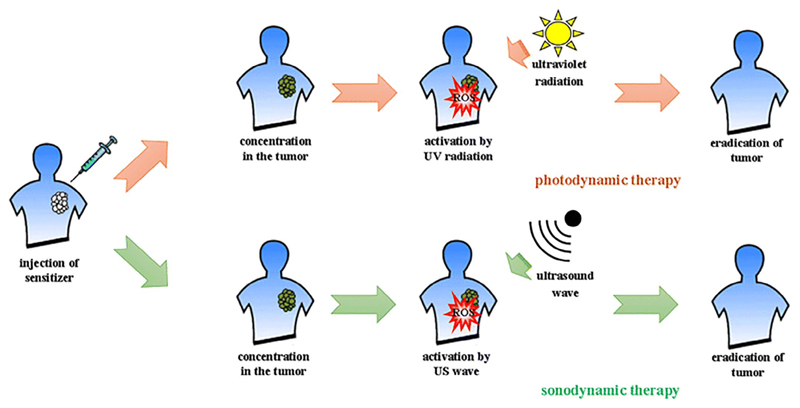
Sonodynamic therapy (SDT) emerged more recently as a novel approach for the treatment of cancer. It is based on the use of low-intensity ultrasound and a molecule, called sonosensitizer, that is sonically activated [86]. Although SDT therapeutic efficacy has been extensively demonstrated, the exact working mechanism is still under debate and will be further discussed below. Sonosensitizer could be activated by light through the sonoluminescence process [9] or with pyrolytic reactions, or upon the increase of acoustic cavitation effects [87]. SDT derives from the photodynamic therapy (PDT), where the light (typically in the ultraviolet range) is used as an external stimulus to activate a photosensitizer. However, the recognized advantage of SDT over PDT is the higher tissue penetration depth [1,88]. A scheme of both approaches is depicted in Fig. 1.
Fig. 1.
Schematic overview of both photodynamic therapy (PDT) and sonodynamic therapy (SDT) against cancer. The photo- or sonosensitizers are administered systematically or locally into the tumor tissue. They are then activated by either ultraviolet light or ultrasound radiation, respectively, leading to cancer tissue regression, or in the best-case scenario, eradication. Reprinted under a Creative Commons License. Copyright 2017 The Author(s) from Ref. [91].
Many molecules were employed in SDT, such as porphyrins, some antitumor drugs and various types of NPs [86,89]. It is also been reported in literature the use of microbubbles as adjuvant for the sonosensitizers: these can be employed both as carrier of molecules and as contrast agents for imaging purposes. Moreover, microbubbles can enhance thermal effects, perturbing the tumor vasculature [90].
6.1. NPs used in the sonodynamic therapy
As stated above, SDT implies the synergistic effect of a non-toxic and selective chemical agent, termed as sonosensitizer. The sonosensitizer is activated by low-intensity ultrasound to produce ROS.
The majority of sensitizers investigated in early SDT studies were porphyrin-based molecules or xanthene dyes, since they were originally employed in PDT. They present a comparable ROS-mediated cytotoxic effects when stimulated by ultrasound [21]. Nevertheless, most of these sonosensitizing agents are strongly hydrophobic, i.e., they easily aggregate in physiological environment, which can decrease their effectiveness and negatively affect their pharmacokinetic behavior [92]. In addition, these molecules can be toxic in some cases and show a low selectivity toward cancer tissues [1]. This wide biodistribution can seriously limit the clinical application of sonosensitizers. Actually, if the concentration gradient between the diseased tissue and adjacent normal tissues is not sufficiently high, is impossible carry out the SDT without causing undesired side-effects. In order to overcome such critical issues, the application of various type of solid and soft micro- and nano-particles in combination with SDT shows a great potential [93]. Thanks to their large surface area suitable for chemical modification and functionalization, NPs can show an improvement of biocompatibility, biodistribution and selectivity towards diseased tissues [20]. Moreover, the presence of particles in a liquid provides nucleation sites for cavitation bubbles, lowering the cavitation threshold and thus enhancing the SDT efficacy [94]. An accurate description of the mechanisms involved in the sonodynamic approach is given further below in this review.
This paragraph briefly mentions the researches developed in the last decade about NP-assisted ultrasound therapy. Depending on the function assumed in the SDT, NPs can be classified as nanosensitizers or vehicle carrying the sonosensitizer.
Liposomes-based delivery systems can be classified in this last category since they can reach the target tissue without losing their payload when circulating in the body. Liposomes are created by self-assembling lipid bilayer arrays, which separate hydrophobic and hydrophilic regions. Therefore, they can carry a huge variety of sonosensitizers, therapeutic agents, genes, proteins, peptides, as well as contrast agents. For this reason, they can be exploited for both diagnostic and imaging purposes under ultrasound irradiation [76,95].
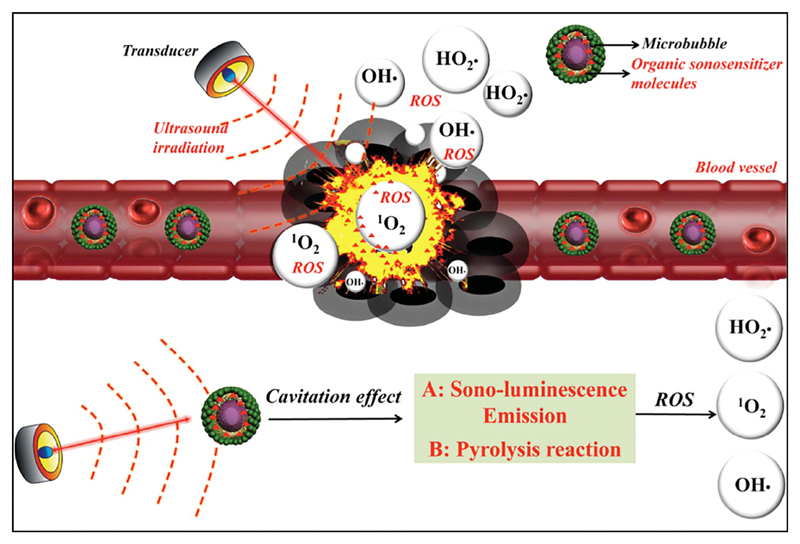
In different studies [96,97], a lipid monolayer characterized by hydrophobic tails and hydrophilic head groups, was used to stabilize the microbubbles in the physiological environment. The lipid layer also permitted the release of the hydrophobic payload in the diseased tissue by exploiting the fragmentation of the monolayer when the internal microbubble is exposed to ultrasound irradiation (Fig. 2). For example, the study of Ibsen et al. [97] was focalized on the fabrication of perfluorocarbon (PFC) gas microbubble surrounded by a lipid monolayer. The stability of these microbubbles and their ability to encapsulate the doxorubicin drug were evaluated.
Fig. 2.
Scheme of the possible mechanism of SDT assisted by microbubbles (MBs) loaded with organic sonosensitizer molecules. The MBs are injected in the blood stream and can accumulate in the tumor tissue. Upon the ultrasound stimulation, the MBs produce cavitation and thus sono-luminescence emission and pyrolysis reactions. Thanks to the organic sonosensitizer molecules included in the MBs, the formation of highly cytotoxic reactive oxygen species (ROS) and singlet oxygen (1O2) take place, leading to cancer cell death. Reprinted with permission, Copyright 2016, John Wiley & Sons Inc. [89].
Unfortunately, the attachment of sensitizer drugs or contrast agents on the lipidic surface can produce the instability of the particle. To overcome this issue, polymeric poly(L-lactide-co-glycolide) (PLGA) microbubbles were proposed [98]. Actually, PLGA microbubbles conjugated with rose bengal (RB) sensitizer resulted to be more stable than the lipid counterparts. The stability of the structures were evaluated not only in the physiological environment but also in the presence of ultrasound treatment. Indeed, while lipid-coated microbubbles were no more detectable after 24 h in the physiological environment, PLGA microbubbles resulted stable and they could be stored at 4 °C for a long period with a minimal loss number. Finally, when conjugated with RB sensitizers, PLGA microbubbles under ultrasound treatment exhibited a selective cytotoxicity in-vitro and in-vivo experiments [98].
Another study developed polymethyl methacrylate (PMMA) NPs to load the meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS) to form a new sonosensitizing agent [99]. The results of the study demonstrated that the TPPS loaded into PMMA NPs acted a major effect in the tumour suppression than the free TPPS in solution. This effect is due to the surface charge on NPs ensuring an enhanced cellular uptake. TPPS NPs not only operated as sonosensitizer vehicle, but also enhanced the cavitation activity.
In order to provide a safe delivery system, different researches [100–102] focused on the manufacture of chitosan nanobubbles. Chitosan, a natural polysaccharide, was chosen for fabricating the nanobubble shell because of its high compatibility, low immunogenicity and low toxicity. the core of the nanobubble consisted of perfluoropentane. Chitosan nanobubbles had a unique polyvalent positive-charged property, so they could complex DNA. They were thus exploited as DNA-delivery systems to reach the target tissue when nanobubbles underwent to ultrasound treatment. The same nanoconstruct was studied to deliver oxygen to hypoxic tissues, exploiting the boiling point of the perfluoropentane core (32 °C). In fact, whereas this compound is liquid at room temperature, it is in vapour phase at body temperature, leading to an higher expansion of the nanobubbles and enhancing the release of loaded oxygen molecules under ultrasound irradiation [102].
In addition to liposomes and polymeric NPs, also metallic and inorganic nanoconstructs could represent efficient platforms for carrying sonosensitizer molecules. For example, gold NPs conjugated with protoporphyrin IX and ultrasound irradiation showed a significant inhibitory effect on colon carcinoma in BALB/c mice [103]. Previous studies [104] demonstrated that the presence of gold NPs prolong the non-radiative relaxation time of protoporphyrin IX, promoting the generation of singlet oxygen. Moreover, gold NPs facilitate the uptake of the sonosensitizer molecules into tumoral cells and increase the cavitation rate, acting both as cavitation nuclei and promoting the collapse activation of such cavities [103].
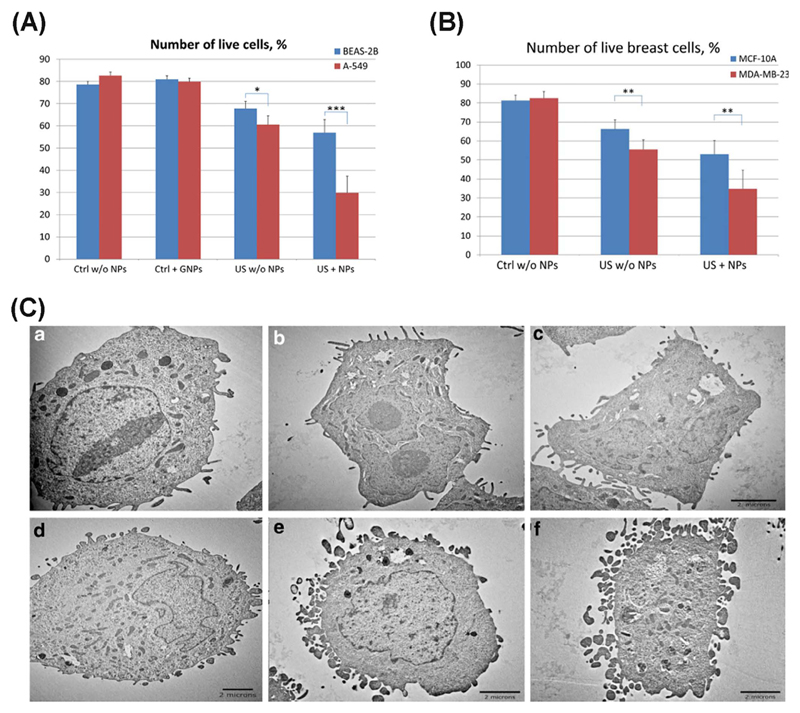
Other studies also indicated the possibility of employing gold NPs without the addition of sensitizing agents, acting themselves as nanosensitizers and carrying out the therapeutical action when activated by ultrasound (Fig. 3). In this case, the NPs acted as sources of nucleation sites, enhancing the inertial cavitation rate in biological environment, and the minor resistance of cancer cells to physical stress is exploited with respect to healthy ones [105]. A recent study [106] on gold NPs, functionalized with PEG and folic acid and activated with ultrasound, showed a promising reduction in cancer cells growth, accompanied by a significant generation of ROS species. Sazgarnia et al. [107] also found that the combination of gold NPs, ultrasound and intense pulsed light was a good strategy to further improve the therapeutic effect on tumors. In fact, the combination of these stimuli can cause a rapid heating and a subsequent vaporization of the surrounding medium, contributing to lower the cavitation pressure [108].
Fig. 3.
(A) BEAS-2B normal lung cells and A549 lung cancer cells after US treatment with gold NPs and the corresponding control cells (untreated with or without US or NPs). (B) MCF-10A normal breast cells and MDA-MB-231 breast cancer cells with/without US treatment and super-paramagnetic iron oxide NPs and the corresponding control (untreated) cells *represents P < .05, **represents P < .01, ***represents P < .001. (C) TEM images of H-184B5F5/M10 normal breast cells (a–c) and MDA-MB-231 breast cancer cells (d–f) cells, where a and d are control samples; b and e correspond to cells treated with US; c and f show the cells after combined treatment with US and magnetic NPs [105].
Similar considerations can be done about porous silicon NPs that present a good sonosensitizing efficiency, both in-vitro and in-vivo, combined with other interesting advantages, such as low toxicity, biodegradability and chance for targeting functionalization [109]. The observed decrease of cavitation threshold in the presence of porous silicon NPs was explained by the presence of nano-sized nucleation centers due to the roughness of particle surfaces [110]. The authors pointed out that this mechanism was also favoured by the presence of residual air bubbles inside the silicon nanoporous structure and by the formation of gas (hydrogen) bubbles due to silicon NPs’ dissolution [110].
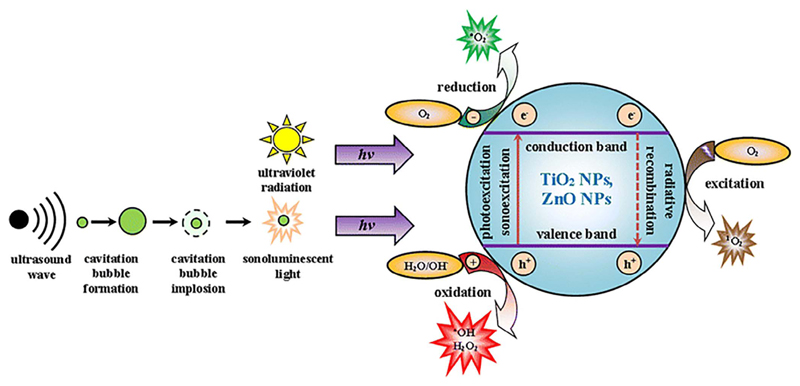
Nevertheless, concerning the class of NPs employed as nanosensitizers in SDT, the most widely studied are titanium dioxide (TiO2) NPs. TiO2, thanks to its semiconducting properties, is commonly used as photosensitizer in PDT to obtain ROS-mediated cytotoxicity. More recently, several studies [91] highlighted the possibility to activate ROS formation through ultrasound irradiation. In this case the ROS production may be triggered by several mechanisms [111], as also schematized in Fig. 4 TiO2 particles provide additional nuclei that increase the formation of ultrasound-induced cavitation bubbles. The bubble collapse induces locally high temperature increase able to generate OH radical via water pyrolysis. Moreover, thermal excitation and photo-excitation of TiO2 by sonoluminescence, resulting from bubbles implosion, also can lead to the formation of OH radicals [111]. Furthermore, TiO2 conjugation with noble metals can strongly increase the catalytic activity of the material [112]. Important therapeutical improvements are obtained with TiO2 NPs’ functionalization with targeting molecules (such as folic acid [113], avidin [114] or pre S1/S2, part of the L protein from the hepatites virus [115]). These studies reported an enhanced and preferential binding and internalization of NPs toward cancer cells, auspicious for the development of a more targeted therapy.
Fig. 4.
Scheme of the possible mechanisms in metal oxide nanoparticles (i.e. titanium dioxide, TiO2, or zinc oxide, ZnO, nanoparticles NPs) to generate reactive oxygen species (ROS) in water-based media. Here both ultraviolet (UV) and ultrasound radiations are depicted as a source to generate ROS at the surface of semiconductor metal oxide NPs in water. In particular, the ultrasound produces cavitation bubbles that, during their collapse, emit the sonoluminescent light. As a result, the semiconductor NPs are photo- or sono-excited, injecting electrons (e−) from valence to the conduction band, leaving the holes (h+) in the valence band. The separated e− and h+ react with water and gas molecules adsorbed on the semiconductor surface, generating the ROS (.O2−, .OH, H2O2). Moreover, the radiative recombination of electron-hole pair can lead to a photon emission able to generate singlet oxygen (1O2) from the oxygen molecules (O2). Reprinted under a Creative Commons License. Copyright 2017 The Author(s) from Ref. [91].
Relying on these promising results, NPs made of semiconductor metal oxides (e.g. TiO2 and ZnO) are believed to play in future a crucial role in medicine as photo- or sonosensitizers for cancer therapy [91].
Another class of metal oxide NPs that can be used in combination with ultrasound are magnetic NPs, such as magnetite (Fe3O4) or maghemite (Fe2O3) (Fig. 3). A recent study [116] indicated a synergistic effect of Fe3O4 NPs with low intensity ultrasound, causing an increase in ROS production. Indeed, it is believed that the ultrasound irradiation enhances the release of iron, necessary to trigger the Fenton reaction, responsible of the ROS generation.
A collection of the various nanomaterials used for performing SDT in-vivo on mouse models and the sorted therapeutic effects are reported in Table 1.
Table 1.
Overview of different nanoparticles and related materials used for performing SDT. Details on the particle size, the ultrasound (US) parameters used, and the obtained therapeutical effect are reported.
| Sonosensitizers | NPs size | US irradiation | Biological model | Therapeutical effect | Ref |
|---|---|---|---|---|---|
| Poly-Lactic-co-Glycolyc acid (PLGA)-microbubbles (MB)- rose Bengal (RB) conjugates (PLGA MB-RB) | 3.05 μm | 1 MHz at 3.0 W/cm2 for 3.5 min | Ectopic human tumors (BxPC-3) in mice | 4 days after cure, mice treated with PLGA MB-RB conjugates and ultrasound reveal a 34% reduction of tumor volume. In contrast, tumors treated with the PLGA MB-RB conjugates alone increased in volume by 38%. 10 days after treatment, tumors in animals treated with both the conjugates and ultrasound were still 14.6% below the original pretreatment size, while those treated with the conjugates alone increased in volume by 63.5%. |
[98] |
| Porphyrin loaded core-shell nanoparticles (TPPS-PMMANPs) | 93 nm | Shock-waves (SW): 0.88 mJ/mm2, 500 impulses, 4 impulse/sec | Breast cancer model (Rat mammary adenocarcinoma cell line-Mat B III) | Histological examinations of tumor sections of animals treated with TPPS-PMMANPs and SW highlighted a strong increase of necrotic and apoptotic features. No injury was observed on the blood vessels with blood cells extravasation with respect to untreated animals 48 h post-treatment | [117] |
| Protoporphyrin IX-gold nanoparticles conjugates | 7 nm | 1.1 MHz with a maximum intensity of 2 W/cm2 for 3 min | Colon carcinoma tumors in BALB/c mice | Protoporphyrin IX conjugated to gold nanoparticles can improve tumor response to sonodynamic therapy, reducing the relative tumor volume and increasing the cumulative survival fraction | [103] |
| Gold | 6–8 nm | Continuous mode at a frequency of 1.1 MHz with a maximum intensity of 2 W/cm2 for 3 min | Colon carcinoma tumors in BALB/c mice | Enhanced antitumor effect (evaluated by relative tumor volume and cumulative survival fraction) with ultrasound irradiation and administration of gold nanoparticles with respect to US alone | [107] |
| Porous silicon nanoparticles | 60–80 nm | 0.88 MHz at 1 W/cm2 or 2.64 MHz at 2 W/cm2 | Melanoma B16 in C57BL mice | A strong suppression of the cancer cell proliferation and tumor growth were observed after a combined treatment by the NPs and therapeutic ultrasound irradiation. The photoluminescence of porous silicon NPs was additionally used for bioimaging of cancer cells | [109] |
| Titania (TiO2) | 120 nm | 1 MHz at 1.0 W/cm2 for 60 s | HepG2 cells inoculated in BALB/c mice | As a result of the treatment repeated five times within 13 days, tumor growth was hampered up to 28 days compared with the control conditions. To enhance the anti-tumor effect of TiO2/US treatment and obtain complete regression, it might be necessary to further improve the biodistribution of the NPs to tumor region, functionalizing TiO2 NPs with targeting biomolecules | [115] |
6.2. The mechanisms of NP-assisted SDT
In the past twenty-five years, a great amount of research focused on SDT as a promising cancer therapy thank oto reduced side effects and high penetration-depth. However, the deep understanding of the mechanisms behind its cytotoxic effects is still lacking. Indeed, the exact physical/chemical mechanisms remain unclear while the SDT therapeutic efficacy, based on the combination of ROS generation and mechanical cytotoxic effects, has been extensively demonstrated [89]. This can be attributed to the complexity of the SDT process, involving together physical, chemical and biological reactions. However, since the first work on SDT by Umemura et al. in 1989, several steps towards the understanding of SDT were made.
For the purposes of this review, NP-assisted SDT will refer to the “NP-dependent sonochemical or sonophotochemical events in an acoustic field leading to cytotoxicity”, as referenced in [21]. This definition highlights the key role played by the NPs in inducing the cytotoxic effects, as initiators of the SDT process. Therefore, we will initially review the literature related to the interaction between NPs and acoustic field in aqueous environment, such as the human body. Then, we will discuss the possible processes leading to the observed cytotoxic effects. The final aim is to possibly clarify by which mechanisms can the NPs induce cytotoxic effects in an acoustic field.
6.2.1. NP interaction with ultrasonic waves
SDT uses ultrasound at relatively low intensities (ranging from 0.5 to 4 W/cm2) that are not able to induce thermal or mechanical effects to living cells, thus they are regarded as safe. Much higher intensities are needed to cause cytotoxic effects such as temperature increase and/or inertial cavitation inception (such in the case of HIFU, as discussed above). Alternatively, high-energy shock-waves can be used to minimize the temperature effects while increasing the likelihood of cavitation [93]. Indeed, using single acoustic pulses with a wide frequency range (up to 20 MHz) and high-pressure amplitude (up to 120 MPa) combined with porphyrins-loaded polymeric NPs, Canaparo et al. were able to induce sonodynamic therapy effects on an in-vitro neuroblastoma model [99]. As stated above, the inertial cavitation refers to the rapid growth and violent collapse of bubbles after exposure to ultrasound. As ultrasound wave travels through a liquid/tissue, any gas bubbles in the liquid are forced to oscillate in the applied acoustic field. Increasing acoustic pressure, this oscillation becomes unstable and eventually the bubble implodes, generating extremely high temperatures and pressures at the center of the collapsing bubble [21]. This may be viewed as a nanometric sonochemical reactor, able to generate ROS by the homolytic cleavage of water molecules or to induce chemical changes close and/or inside the imploding bubble [118]. The minimum ultrasound intensity able to generate inertial cavitation is called cavitation threshold, and this is dependent on the characteristics of the irradiated medium, such as the viscosity, presence of impurities and temperature [119]. As reported already in some example above, it has been largely demonstrated that the presence of NPs in aqueous solutions decreases the cavitation threshold [110]. NPs are indeed able to stabilize nanobubbles on their surface and inside well-defined cavities [120–123]. These nanobubbles can initiate acoustic inertial cavitation acting as cavitation nuclei. In the context of SDT, when actuated with low-intensity ultrasound, NPs can thus initiate inertial cavitation inside and/or in close vicinity to the target cell and consequently elicit cytotoxic effects. It is indeed now generally accepted that inertialcavitation is the key mechanism behind the therapeutic effects of SDT [9]. Therefore, future research aiming at increasing the NPs-assisted STD efficacy should primarily focus on improving the ability of NPs to induce inertial cavitation. Along these lines, Yildirim et al. [124] studied the effects of the NP surface chemistry on the efficiency of inertial cavitation initiation: they showed that a rough, hydrophobic NP surface is needed to preserve the surface nanobubbles and to efficiently induce inertial cavitation. Exploiting these findings, the authors synthesized 100 nm nanoparticle ultrasound agents based on phospholipid-coated, mesoporous, hydrophobically functionalized silica nanoparticles that stabilized gas nanobubbles at their surface even once internalized by cancer cells. These ultrasound agents produced cavitating bubbles only when subjected to non-toxic levels of HIFU, leading to cancer cells death by cellular membrane destruction [48]. Using a different approach to increase the stabilization of nanobubbles on NPs surface, Kwan et al. [125] developed a novel ultrasound-responsive singlecavity polymeric NP, called nanocup, able to trap and stabilize gas nanobubbles thanks to its innovative surface morphology. Under ultrasonic irradiation, these single-cavity NPs initiatedand sustained the cavitation activity four times longer than the existing microbubble constructs, leading to the enhanced delivery of therapeutics in tissue model (Fig. 5). Mesoporous silica NPs with hydrophobic internal nanovoids were developed by Zhao and co-workers [126]. In combination with safe low-energy US (below 1 W/cm2), these mesoporous NPs lead to effective breast cancer cells killing due to acoustic cavitation initiation. In view of the above, further research isexpected toward the development of efficient cavitation-promoting NP-sensitizers- for SDT.
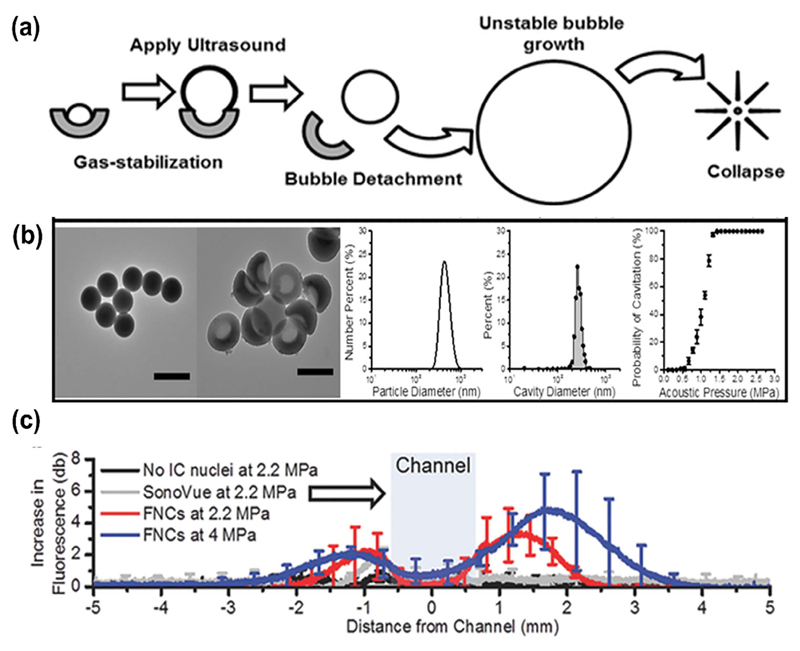
Fig. 5.
(a) Schematic of the nanocup activation upon ultrasound exposure (b) TEM images, DLS analysis and acoustic pressure needed to generate acoustic cavitation are shown. (c) Nanocups (here labeled as FNCs) penetration in a tissue model after US treatment, quantified as the average increase in fluorescence intensity of TRITCD profile taken down the center line. SonoVue®: commercial contrast agent. Adapted with permission from [125].
6.2.2. Mechanisms leading to the SDT therapeutic effect
Once the role of acoustic cavitation was defined as the first mechanism behind NP-assisted SDT, the possible processes leading to the final therapeutic effect are reviewed in the following. We distinguish between cytotoxic effects deriving directly from the collapse of the cavitating bubble and cytotoxic effects arising from the activation of the nanoparticles by cavitating bubbles.
6.2.2.1. Direct cytotoxic effects of cavitating bubbles
As discussed above, the imploding bubble can be considered as a nano-sonochemical reactor able to generate ROS in the presence of water and oxygen. These unstable molecules can exert high cytotoxic effects if generated intracellularly, such as oxidative stress, DNA damage and apoptosis, and can induce lipid peroxidation if generated close to the cell membrane [127]. Several studies showed that specific ROS scavengers such as histidine, mannitol and superoxide dismutase (SOD) protect the target cells from the SDT therapeutic effect. Their role strongly suggests an involvement of ROS in the SDT cytotoxic effect [128,129]. Therefore, ROS generated during the collapse of cavitating bubbles can be regarded as one of the possible mechanisms leading to the therapeutic effect of SDT. In addition to the chemical effects of cavitation, it was suggested that the mechanical effects arising from the implosion of cavitating bubbles can play a key role in eliciting cytotoxic effects. These mechanical effects comprises acoustic streaming, liquid microjets and shock waves. They are generated by the collapse of the cavitating bubbles and can mechanically damage cell membranes and intracellular components. Moreover, if during the expansion phase the intracellular bubble exceeds the volume of the cell, mechanical destruction of the cell can be achieved [48]. In this regard, by comparing the effects of SDT and PDT using the same photosensitizer, Hiraoka et al. concluded that the enhanced ultrasound-induced cell killing was mainly due to mechanical stress such as physical disruption of cellular membranes [130].
6.2.2.2. Cytotoxic effects arising from nanoparticle-activation by cavitating bubbles
Since NP-induced cavitation can be regarded as a mean of focusing the externally applied ultrasound energy, it should be considered the possibility that part of this energy could be transferred to the NPs. Here, we will explore how cavitating bubbles can interact with the sonosensitizer (i.e. the nanoparticle), activate it and finally lead to cytotoxic effects.
One of the effect produced by cavitation is sonoluminescence (SL). This is the emission of light from cavitating bubbles, and although the exact mechanism is still under debate, it is now generally accepted that it arises from the relaxation of excited chemical species during the bubble collapse [131]. In the earlier reports on SDT by Umemura et al., the authors suggested a role for SL based on the observation that:
light emission could be achieved using ultrasound conditions that were employed to elicit sonodynamic effects;
the used sonosensitizers, hematoporphyrin, was a photosensitizer [132].
These authors assumed that light bursts emitted by cavitating bubbles could be absorbed by the sonosensitizer and then, similarly to the photodynamic therapy mechanism, the excited sonosensitizer would generate an electron-hole (e-/h+) pair that subsequently generates ROS in aqueous environment. Moreover, Sazgarnia et al. [133] succeeded in detecting SL in gel-based phantoms using protoporphyrin IX coupled to gold NPs and thus showing that SL is effectively generated during SDT.
Since metal-oxide NPs, such as TiO2 NPs, are able to work as photosensitizers for photodynamic therapy, several authors suggested that the use of such NPs could improve the SDT efficacy exploiting sonoluminescence as mechanism to generate cytotoxic ROS [115,134]. Deepagan et al., based on this hypothesis, functionalized TiO2 NPs with gold NPs (Au-TiO2) in order to improve its quantum yield: this functionalization increased the e−/h+ recombination time by trapping the photoexcited electron while widening the absorption spectrum via surface plasmon resonance. Compared to bare TiO2 NPs, the authors showed that Au-TiO2 NPs generated a greater amount of ROS and lead to complete suppression of tumor growth, in-vivo (Fig. 6) [135]. More recently, Dai et al. [136] developed a novel nanoconstruct (NC) functionalizing two-dimensional (2D) reduced graphene oxide (GR) with TiO2 NPs (TiO2-GR NC). The high electroconductivity of graphene facilitated the separation of the sono-generated e−/h+ pairs leading to higher ROS generation in-vitro. The SDT efficacy in-vivo was compared to the bare TiO2 NPs. Being both Au-TiO2 and TiO2-GR NCs highly efficient in converting light into ROS, authors interpreted these results as a consequence of the sonoluminescent excitation of the sonosensitizers. However, this phenomenon cannot be regarded as the only mechanism behind the above-mentioned results. The size, morphology and surface chemistry of the sonosensitizers have indeed also improved the ability of TiO2 NPs to initiate acoustic cavitation by trapping more gas-nanobubbles at its surface. This would eventually lead to higher cavitation activity, higher ROS generation and thus cellular toxicity. In this regard, no report to date has definitely demonstrated the activation of sonosensitizers by sonoluminescent light. The exclusive observation that ROS are generated in SDT using photosensitizers (as metal-oxide NPs) cannot be regarded as a conclusive evidence for the sonoluminescent excitation of the sonosensitizer. Actually, ROS can be also generated just by cavitating bubbles, as discussed in the previous paragraph. In this context, it is worth mentioning that Riesz et al. [137] excluded the involvement of SL in the sonodynamic activation of the porphyrin-based sonosensitizer ATX-70 by studying the temperature effect on ROS generation. Moreover, Hachimine et al. [138] suggested that the contribution of SL to the SDT efficacy was not relevant, as this sensitizer could not absorb light, but could exert high cytotoxic effects to cancer cells under ultrasonic irradiation. Summarizing, from the available data the contribution of SL to the therapeutic efficacy of SDT has still to be fully clarified. Further research is needed to definitely unravel its role as sonosensitizer activator.
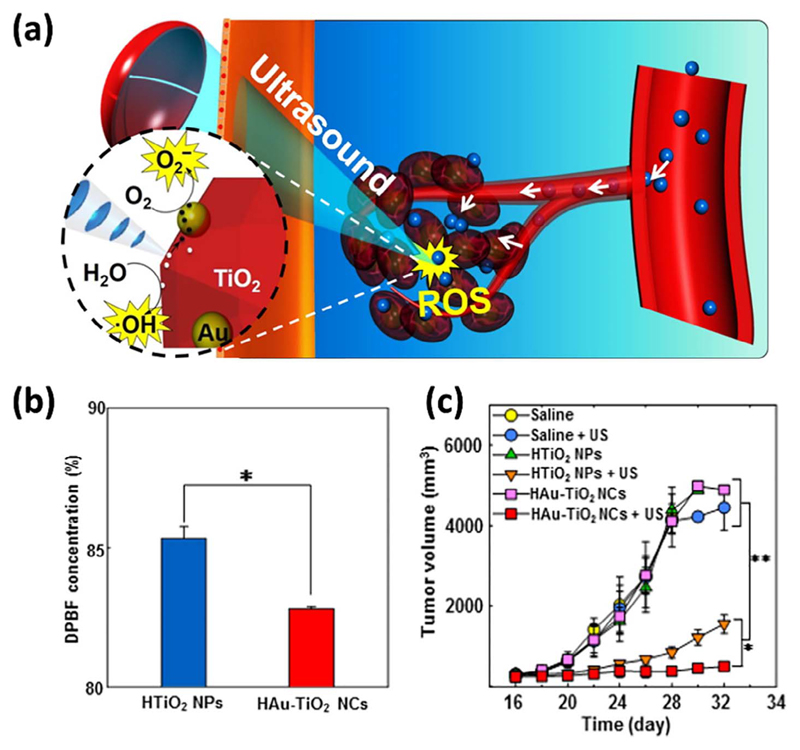
Fig. 6.
(a) Schematic illustration of using Au-TiO2 NPs as sonosensitizers for efficient tumor SDT. (b) Diphenylisobenzofuran (DPBF) was used as fluorescent probe for detecting the sonogeneration of singlet oxygen. Gold NPs-functionalized TiO2 lead to higher ROS generation. (c) SDT therapeutic outcome in SCC7 tumor-bearing mice. Hydrophilized Au-TiO2 NPs (HAu-TiO2 NCs) resulted in tumor regression. Adapted with permission from [135]. Copyright 2016, American Chemical Society.
The mechanisms leading to the ultrasound-dependent enhancement of the cytotoxic action of sonosensitizers, such as porphyrins and organic dyes was reviewed by Misik and Riesz [118]. The authors suggested that SDT cytotoxic effects are due to the chemical activation of sonosensitizers inside or in the close vicinity to the collapsing bubbles. As mentioned above, the heat released by the inertial confinement of the gas in the collapsing bubble can either directly induce the pyrolysis of sonosensitizer or make it reacting with cavitation-generated ROS, eventually forming cytotoxic sensitizer-dependent free radicals. In view of what stated above in the case of NP-assisted SDT, it can be hypothesized that both cavitation-induced high temperatures and ROS chemically activate the NPs. The collapsing bubbles are thus expected to localize close to their site of origin, i.e. the NP surface. This could be the case for ROS-responsive NP-based drug delivery systems or oxidative stress responding NPs [139,140]. Moreover, acoustic cavitation can induce mechanical activation of NPs, such as formation of a mesoporous surface, structural modifications and the creation of fresh highly-reactive metal oxide surfaces. These mechanical activation modes can lead to a higher chemical reactivity of the sonosensitizing NP and thus to a possible higher cellular toxicity [141,142].
6.3. Summary of nanoparticles-assisted SDT working mechanisms
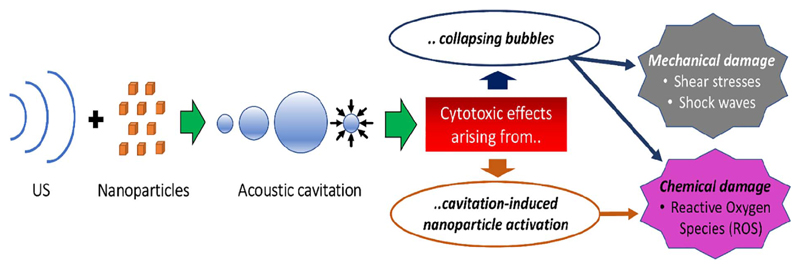
A summary of the above-suggested mechanisms is shown in Fig. 7. At first, the interaction of NPs with low-intensity ultrasonic waves can generate acoustic cavitation. Then, the collapse of cavitating bubbles can directly generate cytotoxic effects, both sono-mechanical, such as shearing stresses and shock waves, and sono-chemical, as the formation of cytotoxic ROS. Together with these mechano-chemical phenomena, acoustic cavitation also induces the activation of the NPs leading to further cellular damage. According to the most agreed theory, sonoluminescence could excite NPs in generating e−/h+ pairs and subsequently cytotoxic ROS, while shock waves and high temperatures could change their chemical and/or structural properties possibly increasing their cellular toxicity. Beside these effects, it is very complex to experimentally distinguish between the individual contribution of each mechanism to the final STD therapeutic outcome. Actually, it can depend on the type of the sonosensitizing NP, on the ultrasound parameters, and on the experimental setup. For these reasons the SDT needs to be considered as a combination of different simultaneous mechanisms.
Fig. 7.
Working hypothesis of the mechanism of SDT. The combination of US and NPs induces acoustic cavitation. This leads to cytotoxic effects via two main pathways: either collapsing bubbles directly damage cells through shock waves, shear stresses and ROS, or the cavitation-induced NPs activation leads to chemical cytotoxicity.
6.4. The cytotoxic effects of sonodynamic therapy
The cytotoxicity of SDT is a challenging topic and the comprehension of the toxicity mechanisms is not simple. Actually, such mechanisms of toxicity are strictly dependent on the nature and characteristics of both US and NPs and mostly influenced by the environmental conditions. For example, different sonosensitizers, even if belonging to the same category like metal oxide NPs, could behave differently in the same experimental condition. In the same way, different US parameters, like frequency and intensity, could generate different responses in the presence of the same sonosensitizer [87].
Usually, all studies of SDT demonstrated the toxicity of the treatment showing the effects on the cell viability. This factor is checked in different ways, through the detection of enzymatic activity (MTT, WST-1, CellTiter Glo assay), the inhibition of cell growth (clonogenic assay) and the cell lysis (Trypan Blue staining). Different works in the literature reported their individual explanations about the cell toxicity and attributed it to numerous components of SDT-cytotoxic effects, as further detailed in the following.
First, a pivotal role in SDT-induced cytotoxicity seems to be played by the oxidative stress [143]. ROS are produced over the limits tolerated by the cells, leading to oxidative stress and oxidative lesions in the cellular structures. The type of the induced cell death varies in the different contexts and either apoptosis or necrosis are reported [88,144]. The nature of the involved ROS depends on the method used for their investigation (i.e. Electron Paramagnetic Resonance Spectroscopy – ESR, Flow cytometry, Spectrofluorimetry), the molecules used for their detection (i.e. specific fluorescent and chemiluminescent sensors, ESR spin traps or ROS scavengers) and experimental conditions used (temperature, test duration). However, even if ROS are not easy to be identified, their presence is confirmed by a plethora of observed “ROS related” biological effects. These effects include the induction of apoptosis, the lipids peroxidation, the loss of the mitochondrial membrane potential, the DNA damage and the activation of different ROS signaling pathways [87].
Among all the identified ROS involved in the SDT process, the most discussed is the singlet oxygen radical. For Wang et al. it is considered the predominant mediator in sonodynamic activity [88], while others works support the idea that different types of ROS are involved in the cytotoxic effect [118]. This oxidizing compound is possibly generated by the action of sonoluminescent hotspots produced by the ultrasound energy on the sonosensitizer [9]. The singlet oxygen presence is associated with detrimental effects for cell viability like membrane and cytoskeletal damage, DNA fragmentation and loss of mitochondrial membrane potential [88,145].
The sonochemical effects associated to this highly reactive compound and, more in general to ROS generation, are often detected in association with other types of effects defined as sonomechanical ones. These effects arise either from the US action on the aqueous medium (bubble implosion and energy release) or from the direct interaction of NPs with the cell structures. The result is a mechanical damage and a consequent induction of cell death by the so called “mechanical pathway” from refs. [88,105], as also depicted in Fig. 3.
As discussed in this review, the cell death induced by the combined treatment of US and NPs is the aim of the nanoparticles-assisted SDT. However, other than illustrating the cytotoxic factors of the SDT, the following paragraph tries to give a more deep comprehension of the effects related to the single agent. Actually, the application of SDT implies the safety of each used component: neither the stimulus nor the sonosensitizer are toxic, but when they are combined together a cytotoxic event occurs. Nevertheless, either US and NPs could become toxic in specific conditions and only the comprehension of the uncombined cytotoxicity mechanisms will make possible a more precise and fine tuning of their synergistic cytotoxic effect. Here a special focus will be given on metal oxide NPs, being the most interesting in terms of cytotoxic-related effects.
6.4.1. US-related toxicity
The ability of US to induce cell toxicity was largely demonstrated in the literature and US-based therapeutic applications are currently used in the clinical setting [146–149], as further detailed in the chapter 7 of this review.
For instance, HIFU are able to generate thermal effects detrimental for cell viability and find application in tumor ablation for cancer therapy [7]. However, in the context of SDT only the cytotoxic effect of low intensity US (below 5 W/cm2) must be taken into account. The intensity of the ultrasonic waves is a pivotal parameter for cytotoxicity, and its variations, even in the range of low intensities, mediates different toxic effects in the biological systems. Umemura et al. [150], investigated the toxicity profiles of different low intensity pulsed-US (LIPUS) in a human leukemia cell line. They demonstrated that it is possible to tune the cytotoxic effect by simply modifying the US intensity. The authors thus identified an optimal condition for the generation of the apoptotic event only. Evidences of this behavior came also from Tian et al. [143]. These authors demonstrated that the proportion between cell apoptosis and necrosis could be determined by the intensity of the US stimulus employed in the experimental setting.
Focusing especially on apoptosis, the association between US and this death process is largely reported in the literature [151–155]. Nevertheless, the precise mechanisms driving this event are nor shared neither completely understood. Prausnits and coworkers demonstrated that Ca2+ plays a major role in the apoptosis and that this event is induced by an increase of cytosolic Ca2+. This correlation emerged also in other works and a partial explanation seems to lie in the mechanical stress induced by US on the cell membrane. In particular, US treatment is able to increase the permeability and pore formation in the plasma membrane and to create membrane wounds. These events lead to a Ca2+ intracellular influx and to its release from intracellular stores [156,157].
The ability of ultrasound to generate a mechanical cell damage is a challenging question. The main idea relates this event to two non-thermal effects, classified as acoustic streaming and cavitation.
As mentioned above, the cavitation induced by US relies on the formation of tiny gas bubble in the tissues as the result of US vibration [158]. In this context, the damage to the biological structures is mainly due to the inertial cavitation, where the bubble implosion results in an aggressive process able to direct injury the cell structure (Fig. 8). Different mechanisms of damage are instead associated to the acoustic streaming. This is the movement of a fluid due to an ultrasound wave, generated by the energy transfer of the ultrasound to the fluid. It can be the movement generated by the ultrasound beam propagation (bulk streaming) or the eddies of flow around a vibrating bubble (microstreaming) [159]. In both cases this is a less aggressive process than the cavitation itself. Furthermore, the sound waves are not strong enough to produce a real cell damage, but just able to displace ions and molecules. The result is a possible rearrangement of biological structures accompanied by fluid movements in the fluid around cell membranes.
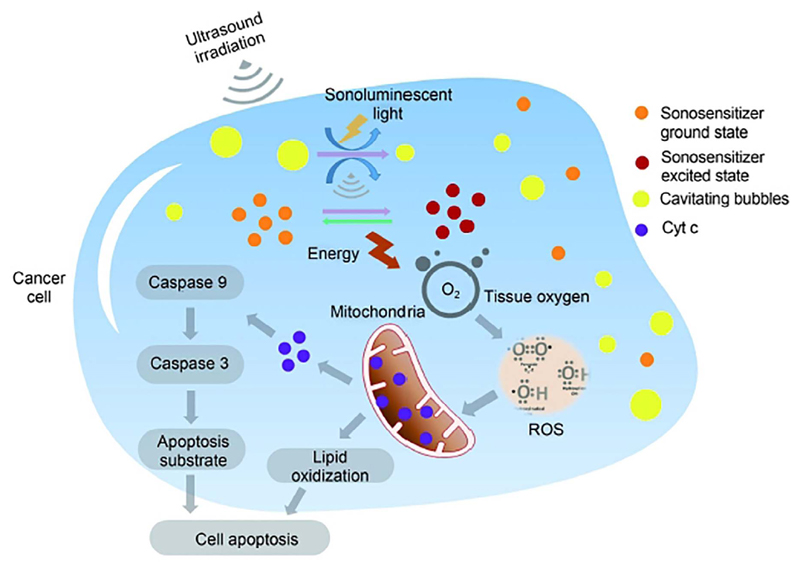
Fig. 8.
Scheme of the various cell toxicity mechanisms induced by SDT. The cavitation induced by ultrasound produces micro-sized gas bubbles in the cell. Sonoluminescent light radiation can be also produced. The sonosensitizer is excited and generate reactive oxygen species (ROS), which are also directly involved in the cellular toxicity. In particular, the damage of mitochondrial membrane and the release of Cyt c, both mediated by ROS, induce the cell apoptosis. Reprinted under a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. Copyright 2016 Cancer Biology & Medicine [88].
Moreover, other than structural effects, low-intensity US cavitation was demonstrated to produce alteration in the cellular activity, like the protein synthesis and the cytokynes production [160]. This functional effect can be explained by an activation of the mechanoreceptors able to detect the mechanical stimulations produced by US [161]. This activation could be also interpreted as a kind of “cellular recovery response”, developed by the cell to fight the damages induced by the treatment [162]. For instance, Xiong et al. recently demonstrated that LIPUS activates pathways related to the cell proliferation in mesenchymal stem cells. Similarly, Ling et al. correlated the US treatment to the proliferation signaling pathways of PI3K/AKT in human amnion-derived mesenchymal stem cells [163].
Finally, the cytotoxic effect of ultrasound is not only related to mechanical damages, but also to the production of toxic chemical species, i.e. ROS (Fig. 8). These ROS could derive from the sonochemical reactions induced by the inertial cavitation in the medium [164] or could be intracellularly produced by the cellular mitochondrial apparatus in a second moment. For this reason, in SDT one of the main role of the sonosensitizer is to enhance the production of ROS induced by the ultrasound treatment, even if the cell damage is due to the synergistic effect of these two components or just from ones, is already under debate [156].
6.4.2. Metal oxide NPs toxicity
Several metal oxide NPs were shown to exhibit intrinsic cytotoxicity both on prokaryotic and eukaryotic cells [165,166]. The mechanisms involved in the cytotoxic effect are complex and difficult to generalize. In fact, they are strictly dependent on the concentration and physicochemical properties of the specific metal oxides that also affect their behavior in the biological contest [167].
However, from all the proposed cytotoxicity mechanisms by the literature, three of them merit consideration in the application of SDT: (i) the generation of ROS; (ii) the mechanical destruction of cell membranes; (iii) the metal-ion release.
For the first case, some works evidence the ability of metal oxides NPs to produce ROS even in absence of any stimulus. In particular, for elements or compounds belonging to the group of semiconductors, this ability lies in their chemical structure. Electrons can easily migrate to the particle surface where they react with the adsorbed species, leaving extremely reactive holes in the conduction band. This mechanism seems to be enhanced by the numerous defects in the NPs crystal structure [168] and could be efficiently induced by the US stimulus in the context of SDT [88]. Furthermore, ROS could derive from a more indirect mechanism, involving the interaction between NPs and the electron transport chain placed in the cell mitochondrial apparatus [169].
The second interesting mechanism associated to metal oxide NPs, or even more in general to the presence of NPs, is the mechanical destruction of cell membranes [170]. Damages in the cell membrane due to NP treatment are well reported in the literature [171]. Nevertheless the precise toxicity mechanism is still under debate. Vidic and coworkers explained that the point defects, such as atoms at edges, give rise to an abrasive NPs surface able to the injury the cell membrane [172]. Despite the exact identification of the causes, this intriguingly effect can be favorably exploited by SDT. In fact, the dissociation of NPs induced by the ultrasound action could enhance the physical interactions with the cell membrane leading to an increase of the cytotoxic effect [173].
Finally the metal-ion release due to the dissolution of metal oxide NPs in aqueous media is frequently considered as the major or even the only cause of metal oxide NPs toxicity [20]. The mechanistic causes of this metal-associated toxicity are poorly explored and seems to be very specific for each metal. For instance, the dissolution of ZnO NPs into Zn2+ induces a mitochondrial-driven apoptosis and a protein disequilibrium toxicity, due to the activation of specific different cellular responses [174]. Also in this case, this characteristic metal oxide NP effect could be exploited by SDT, being the metal ions release facilitated by ultrasound stimulation [175].
7. Clinical applications of NPs assisted ultrasound in cancer treatments
Ultrasound is the most commonly used imaging technique in clinics [146]. Several contrast-enhanced ultrasound are clinically approved in more than 50 countries, mainly in Europe, Asia and Canada with a broad spectrum of applications [176]. In the last decade, microbubbles (MBs) developed as ultrasound contrast agent (UCA) were also proposed for drug delivery purposes via sonoporation [177]. MBs coupled with ultrasound lead to the creation of pores in the cell membrane. They can also open the endothelial junctions, thus enhancing vessel permeability and improving the extravasation of co-administered drugs. This technique is currently employed in different preclinical and also few clinical trials [146]. The studies from Postema et al. and Dimcevski et al. reported that phospholipidic MB (SonoVue®) in combination with ultrasound can be successfully used to increase the response of cancer patients to gemcitabine [147,178].
Ultrasound-based approaches were also adopted to facilitate drug delivery across the blood–brain barrier (BBB) and a first clinical trial (NCT02343991) has been started to evaluate the safety of BBB disruption in combination with lipidic MBs and magnetic resonance imaging-guided ultrasound to enhance the accumulation of doxorubicin in brain tumors [179].
Recently, nanoscale UCAs like nanobubbles, echogenic liposomes, micelles and nanodroplets were proposed to overcome the size limit of the MBs [146]. Indeed, in contrast to MBs, the above-mentioned nanostructures are able to extravasate from the blood vessels to the tissues and transport the drugs deeper into the malignant cells, enabling new theranostic capabilities. Although a lot of refinements concerning nanoscale UCAs are currently ongoing and it is expectable a substantial impact on future drug delivery field, their clinical translation has not been initiated so far [177]. Doxorubicin-containing liposomes were widely used in ultrasound-based drug delivery studies [146]. The reason for their popularity in the research studies resides in fact that several forms, known as Doxil, Caelyx, DaunoXome and Myocet, are already clinically approved by FDA [180]. The engineering of these liposomes for ultrasound-based applications requires an efficient drug loading, stability during circulation, and efficient release of the cargo upon insonation. For example, stable long-circulating liposomes containing doxorubicin reduce drug-related toxicity, and liposome formulations, where doxorubicin can be locally released by heat, are now in clinical trials [78,181].
There are still few obstacles to translate the ultrasound-mediated drug delivery from nanostructures to a clinical setting. For instance, the comprehension of the cytotoxic mechanisms taking place in-vivo has still to be clarified. Similarly, the biological effects will need to be closely monitored and it will also be important to understand the biodistribution and pharmacokinetics of the NPs coupled with ultrasound when delivered in-vivo [180]. However, advances in the field are in progress as more in-vitro and in-vivo studies are being performed.
SDT has exhibited profound physical and chemical changes on cellular structure. It has also shown notable efficacy against a variety of neoplastic cell lines in both in-vitro and in-vivo studies (see a prominent review elsewhere [149] on this topic). The optimization of sonosensitizers with great sonodynamic efficiency and biocompatibility represents a key issue for SDT clinical applications [21].
SDT has shown efficacy in both in-vitro and in-vivo against multiple adherent neoplastic cell lines, with a particular promise against leukemia cells. Nevertheless, at the clinical level the assessment of this technique has been limited to solid tumors only [149].
X. J. Wang et al. employed a new sonosensitizing agent (referred Sonoflora 1™ (SF1)) developed by SonneMed, LLC able to produce singlet oxygen upon interaction with the proper ultrasound wave and induce cellular necrosis. The authors reported preliminary in-vitro and animal studies [182] and successively they published initial clinical data using SDT with SF1 for the treatment of an advanced breast cancer [183]. Three patients with metastasized breast carcinoma were studied. Their carcinoma failed to respond to the conventional therapy and spread to the whole body. The SDT agent was administered through lingual absorption and a combination of sonodynamic and photodynamic therapies was applied irradiating the tumor for 20 min daily for 4 days. The treatment was repeated every two weeks. After 2 or 3 cycles of SDT all the patients showed a positive partial result. These primary clinical data showed that SDT with SF1 was well tolerated. The authors thus concluded that SDT has a significant therapeutic effect for patients with advanced breast cancer [183,184].
Another research group employed SDT with two new chlorophyll-derived sono-photo-sensitizing agents developed by EEC Biotech and approved for clinical use [185]. By previous in-vitro experiments with human breast and lung cancer cell-lines, they showed that SDT was strongly synergetic with chemotherapy. By means of animal studies, the authors demonstrated that the sensitizers were specifically absorbed into tumor cells and that SDT inhibited the growth of mouse S-180 sarcoma. They started the clinical study with seven patients who were pathologically subjected to advanced esophagocadiac and gastric adenocarcinomas. The sono-photo-dynamic therapy was concurrent with chemotherapy within the range from moderate to half of conventional dosages. On the light of the preliminary data, the authors suggested that sono-photo-dynamic therapy had almost no toxicity, but might dramatically enhance the conventional therapeutic efficacy in advanced refractory esophagocadiac and gastric adenocarcinomas [185].
Other clinical case studies were conducted in patients with locally advanced and inoperable pancreatic cancers. They were treated using a customized configuration of commercial clinical ultrasound scanners in the presence of MBs. The combination of ultrasound, microbubbles, and the chemotherapy in these clinical settings increased the number of treatment cycles, prolonging the quality of life in patients with pancreatic adenocarcinomas compared to chemotherapy treatment alone [147,178,186–190].
The use of hard-matter NPs (yet metal or metal oxide NPs) in clinics assisting ultrasound can offer the advantage of having an engineered type of nano-sensitizers acting themselves as therapeutics with ultrasound stimulation (US) [191]. Actually, NPs can sufficiently disperse in aqueous solution and, thanks to their dimensions ranging from 1 to 200 nm, have more changes to avoid the activation of the complement cascade and their eventual clearance by immune cells and macrophages [20]. The fast nanotechnological evolution in the production of different kinds of NPs supports early disease diagnosis and staging, thus enabling also image-guided therapy and personalized therapy [192–196]. NPs application in theranostics allows to integrate diagnostic and therapeutic capabilities into a single nanostructure. This all-in-one approach will enable the NPs in-vitro and in-vivo use for monitoring the sites of bioaccumulation and for evaluating their therapeutic value [197]. The design and the production of engineered hard-matter NPs, as reported above, demonstrate a wide range of possibilities to solve the limitation of sonosensitizer molecules and MBs-based SDT. NPs can be able to invade tissues with an effective and localized accumulation, release the carried drugs, improving the treatment’s efficiency, and minimizing the side effects [20]. By tuning the NPs design and, more in details, their surface functionalization, it is possible to improve active tumor targeting and avoid the hematologic toxicities. In Harada et al. [198] the effect of US combined with the use of titanium dioxide NP (TiO2) on C32 melanoma cells was studied in-vitro and in-vivo. The results showed that when the tumors were treated with TiO2 alone or only irradiated by ultrasound, the cancer progression was unaffected as compared to control mice. Strikingly, th tumors treated by a combination of TiO2 NPs and ultrasound irradiation resulted in a significant inhibition of tumor growth compared with the untreated mice. Recently, long-circulating hydrophilized TiO2NPs (HTiO2 NPs) were designed by You et al. the authors, The authors demonstrated that, when systemically administered to mice, HTiO2 NPs can suppress the progression of a deep side located tumor [199]. Moosavi Nejda et al. [200] reported that, the increased intensity of US (20, 32, 55, 73 W cm−2) in in-vitro experiments using US-excited TiO2 NPs (100 μg mL−1), improved the damage in oral squamous cell carcinoma until reaching cells necrosis at 73 W cm−2 and 3 s. Yamaguchi et al. proposed water-dispersed TiO2 NPs and SDT as a novel cure for malignant gliomas. In particular, water-dispersed TiO2 NPs were functionalized through polyethylene glycol (PEG) on their surface (TiO2/PEG) and the survival rate of U251 human glioblastoma cells remarkably decreased depending on the US intensity used for each treatment [191].
8. Expert opinion and future perspective
NPs are successfully assisting ultrasound applications, in particular sonodynamic, competing with others more traditional techniques for cancer diagnosis and treatment. At present, the wide collection of results coming out from in-vitro and in-vivo experiments confirms the efficacy of NP-assisted ultrasound against different types of malignant cells and tissues. However, as highlighted in the previous sections, more studies and clinical trials will be necessary. Further researches and sustained technological improvements are requested to develop new and more biocompatible and effective NPs-based sensitizers. In particular, the increase the NPs-assisted STD efficacy should primarily focus on improving the ability of NPs to induce inertial cavitation, i.e. exploiting the optimization of both the chemistry and the morphology of the NP surface.
Furthermore, the role of NP sonosensitizer should be further investigated and understood, especially for those particles having a multifuncitonal and synergistic effect with the ultrasound action.
Together with the NPs properties, ultrasound irradiation can be optimized to improve the generation of cavitating bubbles and consequently the therapeutic outcome of NPs-assisted STD. Indeed, inertial cavitation phenomenon strongly depends on ultrasonic frequency, pressure amplitude, and, if pulsed ultrasound is used, on pulse repetition frequency and the number of cycles in each pulse [201]. In this regard, dual frequency ultrasound irradiation has also been recently proposed [111].
Further research toward the optimization of the ultrasound irradiation is expected. Moreover, it is fundamental to deeply understand the mechanism of action of ultrasound on biological tissues. The difficulty of discriminating the thermal from the mechanical effects poses serious challenges in this regard, as well as their synergistic effect. However, this understanding will be indispensabile to proceed toward the clinical trials.
Concerning the SDT mechanisms, from the available data, the contribution of sonoluminescence to the therapeutic efficacy of SDT has still to be fully clarified. Further research is needed to definitely unravel its role as sonosensitizer activator.
When studying and writing this review, we also noted a lack in uniformity of the published data describing the ultrasound set up used by different authors. In particular, data describing the power densities, pressures, presence or absence of cavitation, and type of cavitation obtained are all necessary to compare the different literature works among each other.
Altogether, the optimization of both nano-sonosensitizers, with great sonodynamic efficiency and biocompatibility, and ultrasound protocols represents a key issue for leading SDT to successful clinical applications.
9. Conclusions
Ultrasound-based therapies and diagnostics play a fundamental role in the scientific research panorama and in the clinical context. Several kinds of both soft and hard-material NPs were proposed for assisting US-based therapies. As it clearly appears from the literature, SDT is an innovative treatment against cancer offering a huge potential. The cellular damage arises only when both non-toxic stimulus and sensitizers are combined, thus drastically lowering side-effects compared to traditional treatments. However, the exact physical/chemical mechanisms behind the observed therapeutic efficacy still need to be unraveled: this would open up the possibility to rationally optimize SDT with the ultimate goal to bring this technology to the clinics.
More in general, we reported on the efficacy of NP-assisted US against tumor cells and tissues where the working mechanism, even in association with other therapeutic approaches in clinics, has still to be fully understood. Therefore, further interdisciplinary scientific research on NP-assisted US mechanisms is expected and the engagement of biologists and physicians together with physicists, chemists and engineers is highly encouraged for the future.
Highlights.
Ultrasound-based therapies are opening new horizons in the oncological field.
Innovative and promising solutions derive from different nanoparticles-assisted ultrasound treatments.
Sonodynamic therapy (SDT) emerged recently as a novel approach for cancer treatment.
Different and complex cell death mechanisms are involved in nanoparticles-assisted SDT.
Nanoparticles-assisted ultrasound is still at its infancy in clinics.
Acknowledgements
The funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 678151 – Project Acronym “TROJANANOHORSE” – ERC starting Grant) and the Regional program entitled “Attrarre Docenti di Qualità con Starting Grant” from the Compagnia di Sanpaolo, D.R. 349-2016 are gratefully acknowledged.
References
- [1].Shibaguchi H, Tsuru H, Kuroki M, Kuroki M. Sonodynamic cancer therapy: a non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011;31:2425–2429. [PubMed] [Google Scholar]
- [2].Lewin PA, Ziskin MC. Ultrasound Exposimetry. CRC Press; 1992. [Google Scholar]
- [3].Carovac A, Smajlovic F, Junuzovic D. Application of ultrasound in medicine. Acta Inf Med. 2011;19:168–171. doi: 10.5455/aim.2011.19.168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Santos HM, Lodeiro C, Capelo-Martínez JL. The Power of Ultrasound, Ultrasound in Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2009. pp. 1–16. [Google Scholar]
- [5].Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015;31:302–309. doi: 10.3109/02656736.2014.969789. [DOI] [PubMed] [Google Scholar]
- [6].Zhou QL, Chen ZY, Wang YX, Yang F, Lin Y, Liao YY. Ultrasound-mediated local drug and gene delivery using nanocarriers. BioMed Res Int. 2014;2014:13. doi: 10.1155/2014/963891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: current potential and oncologic applications, AJR. Am J Roentgenol. 2008;190:191–199. doi: 10.2214/AJR.07.2671. [DOI] [PubMed] [Google Scholar]
- [8].Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2:8–27. doi: 10.5306/wjco.v2.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy–a review of the synergistic effects of drugs and ultrasound, Ultrason. Sonochem. 2004;11:349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [10].Urban C, Urban AS, Charron H, Joshi A. Externally modulated theranostic nanoparticles. Trans Cancer Res. 2013;2:292–308. doi: 10.3978/j.issn.2218-676X.2013.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hyvelin JM, Gaud E, Costa M, Helbert A, Bussat P, Bettinger T, Frinking P. Characteristics and echogenicity of clinical ultrasound contrast agents: an in vitro and in vivo comparison study. J Ultrasound Med. 2017;36:941–953. doi: 10.7863/ultra.16.04059. [DOI] [PubMed] [Google Scholar]
- [12].Ignee A, Atkinson NSS, Schuessler G, Dietrich GF. Ultrasound contrast agents. Endosc Ultrasound. 2016;5:355–362. doi: 10.4103/2303-9027.193594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31:623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93:111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [15].Lingeman JE, McAteer JA, Gnessin E, Evan AP. Shock wave lithotripsy: advances in technology and technique. Nat Rev Urol. 2009;6:660–670. doi: 10.1038/nrurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Griffin XL, Smith N, Parsons N, Costa ML. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database of Syst Rev. 2012;15 doi: 10.1002/14651858.CD008579.pub2. CD008579. [DOI] [PubMed] [Google Scholar]
- [17].Kennedy J, Verne S, Griffith R, Falto-Aizpurua L, Nouri K. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29:1679–1688. doi: 10.1111/jdv.12994. [DOI] [PubMed] [Google Scholar]
- [18].Miller D, Smith N, Bailey M, Czarnota G, Hynynen K, Makin I. C American Institute of Ultrasound in Medicine Bioeffects, Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31:623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Elmansy HE, Lingeman JE. Recent advances in lithotripsy technology and treatment strategies: a systematic review update. Int J Surg. 2016;36:676–680. doi: 10.1016/j.ijsu.2016.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu H, Zhang X, Han R, Yang P, Ma H, Song Y, Lu Z, Yin W, Wu X, Wang H. Nanoparticles in sonodynamic therapy: state of the art review. RSC Adv. 2016;6:50697–50705. [Google Scholar]
- [21].McHale AP, Callan JF, Nomikou N, Fowley C, Callan B. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. In: Escoffre J-M, Bouakaz A, editors. Therapeutic Ultrasound. Springer International Publishing; Cham: 2016. pp. 429–450. [DOI] [PubMed] [Google Scholar]
- [22].Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Exp Opin Drug Delivery. 2014;11:393–407. doi: 10.1517/17425247.2014.875528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery – A general review. Exp Opin Drug Delivery. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. Int J Pharm. 2002;243:1–15. doi: 10.1016/s0378-5173(02)00299-5. [DOI] [PubMed] [Google Scholar]
- [25].Oberli MA, Schoellhammer CM, Langer R, Blankschtein D. Ultrasound-enhanced transdermal delivery: recent advances and future challenges. Ther Delivery. 2014;5:843–857. doi: 10.4155/tde.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Controlled Release. 2011;152:330–348. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mitragotri S. Transdermal Drug Delivery using Low-Frequency Sonophoresis. In: Ferrari M, Desai T, Bhatia S, editors. BioMEMS and Biomedical Nanotechnology: Volume III Therapeutic Micro/Nanotechnology. Springer; US, Boston, MA: 2007. pp. 223–236. [Google Scholar]
- [28].Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- [29].Bhatnagar S, Schiffter H, Coussios C-C. Exploitation of acoustic cavitation-induced microstreaming to enhance molecular transport. J Pharm Sci. 2014;103:1903–1912. doi: 10.1002/jps.23971. [DOI] [PubMed] [Google Scholar]
- [30].Bhatnagar S, Kwan JJ, Shah AR, Coussios C-C, Carlisle RC. Exploitation of sub-micron cavitation nuclei to enhance ultrasound-mediated transdermal transport and penetration of vaccines. J Controlled Release. 2016;238:22–30. doi: 10.1016/j.jconrel.2016.07.016. [DOI] [PubMed] [Google Scholar]
- [31].Boucaud A, Montharu J, Machet L, Arbeille B, Machet MC, Patat F, Vaillant L. Clinical, histologic, and electron microscopy study of skin exposed to low-frequency ultrasound. Anat Rec. 2001;264:114–119. doi: 10.1002/ar.1122. [DOI] [PubMed] [Google Scholar]
- [32].Simonin J-P. On the mechanisms of in vitro and in vivo phonophoresis. J Controlled Release. 1995;33:125–141. [Google Scholar]
- [33].Alvarez-Román R, Merino G, Kalia YN, Naik A, Guy RH. Skin permeability enhancement by low frequency sonophoresis: lipid extraction and transport pathways. J Pharm Sci. 2003;92:1138–1146. doi: 10.1002/jps.10370. [DOI] [PubMed] [Google Scholar]
- [34].Seto JE, Polat BE, Lopez RFV, Blankschtein D, Langer R. Effects of ultrasound and sodium lauryl sulfate on the transdermal delivery of hydrophilic permeants: comparative in vitro studies with full-thickness and split-thickness pig and human skin. J Controlled Release. 2010;145:26–32. doi: 10.1016/j.jconrel.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Scarponi C, Nasorri F, Pavani F, Madonna S, Sestito R, Simonacci M, De Pità O, Cavani A, Albanesi C. Low-Frequency Low-intensity ultrasounds do not influence the survival and immune functions of cultured keratinocytes and dendritic cells. J Biomed Biotechnol. 2009;2009:193260. doi: 10.1155/2009/193260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Polat BE, Blankschtein D, Langer R. Low-frequency sonophoresis: application to the transdermal delivery of macromolecules and hydrophilic drugs. Exp Opin Drug Delivery. 2010;7:1415–1432. doi: 10.1517/17425247.2010.538679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ashokkumar M, Lee J, Iida Y, Yasui K, Kozuka T, Tuziuti T, Towata A. Spatial distribution of acoustic cavitation bubbles at different ultrasound frequencies. ChemPhysChem. 2010;11:1680–1684. doi: 10.1002/cphc.200901037. [DOI] [PubMed] [Google Scholar]
- [38].Tang H, Wang CCJ, Blankschtein D, Langer R. An investigation of the role of cavitation in low-frequency ultrasound-mediated transdermal drug transport. Pharm Res. 2002;19:1160–1169. doi: 10.1023/a:1019898109793. [DOI] [PubMed] [Google Scholar]
- [39].Tezel A, Dokka S, Kelly S, Hardee GE, Mitragotri S. Topical delivery of antisense oligonucleotides using low-frequency sonophoresis. Pharm Res. 2004;21:2219–2225. doi: 10.1007/s11095-004-7674-6. [DOI] [PubMed] [Google Scholar]
- [40].Tran MA, Gowda R, Sharma A, Park EJ, Adair J, Kester M, Smith NB, Robertson GP. Targeting (V600E) B-Raf and Akt3 using Nanoliposomal-siRNA Inhibits Cutaneous Melanocytic Lesion Development. Cancer Res. 2008;68:7638–7649. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hoogenboom M, Eikelenboom D, den Brok MH, Heerschap A, Fütterer JJ, Adema GJ. Mechanical High-Intensity Focused Ultrasound Destruction of Soft Tissue: Working Mechanisms and Physiologic Effects. Ultrasound in Medicine and Biology. 41:1500–1517. doi: 10.1016/j.ultrasmedbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- [42].van den Bijgaart RJE, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, Adema GJ. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66:247–258. doi: 10.1007/s00262-016-1891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Leinenga G, Götz J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci Transl Med. 2015;7:278ra233. doi: 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- [44].Endo-Takahashi Y, Negishi Y, Nakamura A, Suzuki D, Ukai S, Sugimoto K, Moriyasu F, Takagi N, Suzuki R, Maruyama K, Aramaki Y. pDNA-loaded Bubble liposomes as potential ultrasound imaging and gene delivery agents. Biomaterials. 2013;34:2807–2813. doi: 10.1016/j.biomaterials.2012.12.018. [DOI] [PubMed] [Google Scholar]
- [45].Han H, Lee H, Kim K, Kim H. Effect of high intensity focused ultrasound (HIFU) in conjunction with a nanomedicines-microbubble complex for enhanced drug delivery. J Controlled Release. 2017;266:75–86. doi: 10.1016/j.jconrel.2017.09.022. [DOI] [PubMed] [Google Scholar]
- [46].Luo Z, Jin K, Pang Q, Shen S, Yan Z, Jiang T, Zhu X, Yu L, Pang Z, Jiang X. On-demand drug release from dual-targeting small nanoparticles triggered by high-intensity focused ultrasound enhanced glioblastoma-targeting therapy. ACS Appl Mater Interfaces. 2017;9:31612–31625. doi: 10.1021/acsami.7b10866. [DOI] [PubMed] [Google Scholar]
- [47].Ahmad Reza Dibaji S, Al-Rjoub MF, Myers MR, Banerjee RK. Enhanced heat transfer and thermal dose using magnetic nanoparticles during hifu thermal ablation—An in-vitro study. J Nanotechnol Eng Med. 2014;4:040902. [Google Scholar]
- [48].Yildirim A, Chattaraj R, Blum NT, Shi D, Kumar K, Goodwin AP. Phospholipid capped mesoporous nanoparticles for targeted high intensity focused ultrasound ablation. Adv Healthcare Mater. 2017;6:1700514. doi: 10.1002/adhm.201700514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Blum N, Yildirim A, Chattaraj R, Goodwin A. Nanoparticles formed by acoustic destruction of microbubbles and their utilization for imaging and effects on therapy by high intensity focused ultrasound. Theranostics. 2017;7:694–702. doi: 10.7150/thno.17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].You Y, Wang Z, Ran H, Zheng Y, Wang D, Xu J, Wang Z, Chen Y, Li P. Nanoparticle-enhanced synergistic HIFU ablation and transarterial chemoembolization for efficient cancer therapy. Nanoscale. 2016;8:4324–4339. doi: 10.1039/c5nr08292g. [DOI] [PubMed] [Google Scholar]
- [51].Bera C, Devarakonda SB, Kumar V, Ganguli AK, Banerjee RK. The mechanism of nanoparticle-mediated enhanced energy transfer during high-intensity focused ultrasound sonication. Phys Chem Chem Phys. 2017;19:19075–19082. doi: 10.1039/c7cp03542j. [DOI] [PubMed] [Google Scholar]
- [52].Delalande A, Kotopoulis S, Postema M, Midoux P, Pichon C. Sonoporation: mechanistic insights and ongoing challenges for gene transfer. Gene. 2013;525:191–199. doi: 10.1016/j.gene.2013.03.095. [DOI] [PubMed] [Google Scholar]
- [53].Mullick Chowdhury S, Lee T, Willmann JK. Ultrasound-guided drug delivery in cancer. Ultrasonography. 2017;36:171–184. doi: 10.14366/usg.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Michiel P, Odd Helge G. Ultrasound-directed drug delivery. Curr Pharm Biotechnol. 2007;8:355–361. doi: 10.2174/138920107783018453. [DOI] [PubMed] [Google Scholar]
- [55].Bouakaz A, Zeghimi A, Doinikov AA. Sonoporation: Concept and Mechanisms. In: Escoffre J-M, Bouakaz A, editors. Therapeutic Ultrasound. Springer International Publishing; Cham: 2016. pp. 175–189. [Google Scholar]
- [56].Forbes MM, Steinberg RL, O'Brien WD. Frequency-dependent evaluation of the role of definity in producing sonoporation of chinese hamster ovary cells. J Ultrasound Med. 2011;30:61–69. doi: 10.7863/jum.2011.30.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Udroiu I. Ultrasonic drug delivery in oncology. JBUON. 2015;20:381–390. [PubMed] [Google Scholar]
- [58].Barnett SB, Ter Haar GR, Ziskin MC, Nyborg WL, Maeda K, Bang J. Current status of research on biophysical effects of ultrasound. Ultrasound Med Biol. 1994;20:205–218. doi: 10.1016/0301-5629(94)90060-4. [DOI] [PubMed] [Google Scholar]
- [59].Escoffre JM, Piron J, Novell A, Bouakaz A. Doxorubicin delivery into tumor cells with ultrasound and microbubbles. Mol Pharmaceutics. 2011;8:799–806. doi: 10.1021/mp100397p. [DOI] [PubMed] [Google Scholar]
- [60].Wu J, Pepe J, Rincón M. Sonoporation, anti-cancer drug and antibody delivery using ultrasound. Ultrasonics. 2006;44:e21–e25. doi: 10.1016/j.ultras.2006.06.033. [DOI] [PubMed] [Google Scholar]
- [61].Watanabe Y, Aoi A, Horie S, Tomita N, Mori S, Morikawa H, Matsumura Y, Vassaux G, Kodama T. Low-intensity ultrasound and microbubbles enhance the antitumor effect of cisplatin. Cancer Sci. 2008;99:2525–2531. doi: 10.1111/j.1349-7006.2008.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- [63].McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology. 2006;241:95–106. doi: 10.1148/radiol.2411051170. [DOI] [PubMed] [Google Scholar]
- [64].Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- [65].Delalande A, Bouakaz A, Renault G, Tabareau F, Kotopoulis S, Midoux P, Arbeille B, Uzbekov R, Chakravarti S, Postema M, Pichon C. Ultrasound and microbubble-assisted gene delivery in Achilles tendons: long lasting gene expression and restoration of fibromodulin KO phenotype. J Controlled Release. 2011;156:223–230. doi: 10.1016/j.jconrel.2011.08.020. [DOI] [PubMed] [Google Scholar]
- [66].Huber PE, Pfisterer P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Teraphy. 2000;7:1516–1525. doi: 10.1038/sj.gt.3301242. [DOI] [PubMed] [Google Scholar]
- [67].Pichon C, Kaddur K, Midoux P, Tranquart F, Bouakaz A. Recent advances in gene delivery with ultrasound and microbubbles. J Exp Nanosci. 2008;3:17–40. [Google Scholar]
- [68].Cao Y, Langer R. Optimizing the delivery of cancer drugs that block angiogenesis. Sci Transl Med. 2010;2:15ps13. doi: 10.1126/scitranslmed.3000399. [DOI] [PubMed] [Google Scholar]
- [69].Huebsch N, Kearney CJ, Zhao X, Kim J, Cezar CA, Suo Z, Mooney DJ. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc Natl Acad Sci USA. 2014;111:9762–9767. doi: 10.1073/pnas.1405469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yoon YI, Yoon T-J, Lee HJ. Optimization of ultrasound parameters for microbubble-nanoliposome complex-mediated delivery. Ultrasonography. 2015;34:297–303. doi: 10.14366/usg.15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hesham GM, Ana MM, Ghaleb AH. Review on Triggered Liposomal Drug Delivery with a Focus on Ultrasound. Current Cancer Drug Targets. 2015;15:282–313. doi: 10.2174/1568009615666150311100610. [DOI] [PubMed] [Google Scholar]
- [72].Ahmed SE, Martins AM, Husseini GA. The use of ultrasound to release chemotherapeutic drugs from micelles and liposomes. J Drug Targeting. 2015;23:16–42. doi: 10.3109/1061186X.2014.954119. [DOI] [PubMed] [Google Scholar]
- [73].Ting C-Y, Fan C-H, Liu H-L, Huang C-Y, Hsieh H-Y, Yen T-C, Wei K-C, Yeh C-K. Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33:704–712. doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- [74].Fan C-H, Ting C-Y, Liu H-L, Huang C-Y, Hsieh H-Y, Yen T-C, Wei K-C, Yeh C-K. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34:2142–2155. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- [75].Kumar P, Gulbake A, Jain SK. Liposomes a vesicular nanocarrier: potential advancements in cancer. Chemotherapy. 2012;29:355–419. doi: 10.1615/critrevtherdrugcarriersyst.v29.i5.10. [DOI] [PubMed] [Google Scholar]
- [76].Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Dev Therapy. 2013;7:375–388. doi: 10.2147/DDDT.S31564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liu H-L, Fan C-H, Ting C-Y, Yeh C-K. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4:432–444. doi: 10.7150/thno.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ferrara KW, Borden MA, Zhang H. Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res. 2009;42:881–892. doi: 10.1021/ar8002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fokong S, Theek B, Wu Z, Koczera P, Appold L, Jorge S, Resch-Genger U, van Zandvoort M, Storm G, Kiessling F, Lammers T. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J Controlled Release. 2012;163:75–81. doi: 10.1016/j.jconrel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- [80].Tinkov S, Coester C, Serba S, Geis NA, Katus HA, Winter G, Bekeredjian R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Controlled Release. 2010;148:368–372. doi: 10.1016/j.jconrel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- [81].Li P, Zheng Y, Ran H, Tan J, Lin Y, Zhang Q, Ren J, Wang Z. Ultrasound triggered drug release from 10-hydroxycamptothecin-loaded phospholipid microbubbles for targeted tumor therapy in mice. J Controlled Release. 2012;162:349–354. doi: 10.1016/j.jconrel.2012.07.009. [DOI] [PubMed] [Google Scholar]
- [82].Lentacker I, Geers B, Demeester J, De Smedt SC, Sanders NN. Tumor cell killing efficiency of doxorubicin loaded microbubbles after ultrasound exposure. J Controlled Release. 2010;148:e113–e114. doi: 10.1016/j.jconrel.2010.07.085. [DOI] [PubMed] [Google Scholar]
- [83].Rychak JJ, Klibanov AL. Nucleic acid delivery with microbubbles and ultrasound. Adv Drug Delivery Rev. 2014;72:82–93. doi: 10.1016/j.addr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Otani K, Yamahara K, Ohnishi S, Obata H, Kitamura S, Nagaya N. Nonviral delivery of siRNA into mesenchymal stem cells by a combination of ultrasound and microbubbles. J Controlled Release. 2009;133:146–153. doi: 10.1016/j.jconrel.2008.09.088. [DOI] [PubMed] [Google Scholar]
- [85].Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Controlled Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chen H, Zhou X, Gao Y, Zheng B, Tang F, Huang J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discovery Today. 2014;19:502–509. doi: 10.1016/j.drudis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- [87].Costley D, Mc Ewan C, Fowley C, McHale AP, Atchison J, Nomikou N, Callan JF. Treating cancer with sonodynamic therapy: a review. Int J Hyperthermia. 2015;31:107–117. doi: 10.3109/02656736.2014.992484. [DOI] [PubMed] [Google Scholar]
- [88].Wan G-Y, Liu Y, Chen B-W, Liu Y-Y, Wang Y-S, Zhang N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol Med. 2016;13:325–338. doi: 10.20892/j.issn.2095-3941.2016.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Qian X, Zheng Y, Chen Y. Micro/nanoparticle-augmented sonodynamic therapy (sdt): breaking the depth shallow of photoactivation. Adv Mater (Deerfield Beach, Fla) 2016;28:8097–8129. doi: 10.1002/adma.201602012. [DOI] [PubMed] [Google Scholar]
- [90].Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41:905–928. doi: 10.1016/j.ultrasmedbio.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bogdan J, Plawinska-Czarnak J, Zarzynska J. Nanoparticles of titanium and zinc oxides as novel agents in tumor treatment: a review. Nanoscale Res letters. 2017;12:225. doi: 10.1186/s11671-017-2007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Vargas A, Pegaz B, Debefve E, Konan-Kouakou Y, Lange N, Ballini JP, van den Bergh H, Gurny R, Delie F. Improved photodynamic activity of porphyrin loaded into nanoparticles: an in vivo evaluation using chick embryos. Int J Pharm. 2004;286:131–145. doi: 10.1016/j.ijpharm.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [93].Serpe L, Foglietta F, Canaparo R. Nanosonotechnology: the next challenge in cancer sonodynamic therapy. Nanotechnol Rev. 2012;1:173–182. [Google Scholar]
- [94].Tuziuti T, Yasui K, Sivakumar M, Iida Y, Miyoshi N. Correlation between acoustic cavitation noise and yield enhancement of sonochemical reaction by particle addition. J Phys Chem A. 2005;109:4869–4872. doi: 10.1021/jp0503516. [DOI] [PubMed] [Google Scholar]
- [95].Huang SL. Liposomes in ultrasonic drug and gene delivery. Adv Drug Delivery Rev. 2008;60:1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [96].McEwan C, Owen J, Stride E, Fowley C, Nesbitt H, Cochrane D, Coussios CC, Borden M, Nomikou N, McHale AP, Callan JF. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J Controlled Release. 2015;203:51–56. doi: 10.1016/j.jconrel.2015.02.004. [DOI] [PubMed] [Google Scholar]
- [97].Ibsen S, Benchimol M, Simberg D, Schutt C, Steiner J, Esener S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J Controlled Release. 2011;155:358–366. doi: 10.1016/j.jconrel.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].McEwan C, Fowley C, Nomikou N, McCaughan B, McHale AP, Callan JF. Polymeric microbubbles as delivery vehicles for sensitizers in sonodynamic therapy. Langmuir. 2014;30:14926–14930. doi: 10.1021/la503929c. [DOI] [PubMed] [Google Scholar]
- [99].Canaparo R, Varchi G, Ballestri M, Foglietta F, Sotgiu G, Guerrini A, Francovich A, Civera P, Frairia R, Serpe L. Polymeric nanoparticles enhance the sonodynamic activity of meso-tetrakis (4-sulfonatophenyl) porphyrin in an in vitro neuroblastoma model. Int J Nanomed. 2013;8:4247–4263. doi: 10.2147/IJN.S51070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cavalli R, Bisazza A, Trotta M, Argenziano M, Civra A, Donalisio M, Lembo D. New chitosan nanobubbles for ultrasound-mediated gene delivery: preparation and in vitro characterization. Int J Nanomed. 2012;7:3309–3318. doi: 10.2147/IJN.S30912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cavalli R, Bisazza A, Rolfo A, Balbis S, Madonnaripa D, Caniggia I, Guiot C. Ultrasound-mediated oxygen delivery from chitosan nanobubbles. Int J Pharm. 2009;378:215–217. doi: 10.1016/j.ijpharm.2009.05.058. [DOI] [PubMed] [Google Scholar]
- [102].Magnetto C, Prato M, Khadjavi A, Giribaldi G, Fenoglio I, Jose J, Gulino GR, Cavallo F, Quaglino E, Benintende E, Varetto G, et al. Ultrasound-activated decafluoropentane-cored and chitosan-shelled nanodroplets for oxygen delivery to hypoxic cutaneous tissues. RSC Adv. 2014;4:38433–38441. [Google Scholar]
- [103].Sazgarnia A, Shanei A, Meibodi NT, Eshghi H, Nassirli H. A novel nanosonosensitizer for sonodynamic therapy: in vivo study on a colon tumor model. J Ultrasound Med. 2011;30:1321–1329. doi: 10.7863/jum.2011.30.10.1321. [DOI] [PubMed] [Google Scholar]
- [104].Jiménez Pérez JL, Cruz-Orea A, Ramón-Gallegos E, Gutierrez Fuentes R, Sanchez Ramirez JF. Photoacoustic spectroscopy to determine in vitro the non radiative relaxation time of protoporphyrin IX solution containing gold metallic nanoparticles. Eur Phys J Spec Top. 2008;153:353–356. [Google Scholar]
- [105].Kosheleva OK, Lai TC, Chen NG, Hsiao M, Chen CH. Selective killing of cancer cells by nanoparticle-assisted ultrasound. J Nanobiotechnol. 2016;14:46. doi: 10.1186/s12951-016-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Brazzale C, Canaparo R, Racca L, Foglietta F, Durando G, Fantozzi R, Caliceti P, Salmaso S, Serpe L. Enhanced selective sonosensitizing efficacy of ultrasound-based anticancer treatment by targeted gold nanoparticles. Nanomedicine. 2016;11:3053–3070. doi: 10.2217/nnm-2016-0293. [DOI] [PubMed] [Google Scholar]
- [107].Sazgarnia A, Shanei A, Taheri AR, Meibodi NT, Eshghi H, Attaran N, Shanei MM. Therapeutic effects of acoustic cavitation in the presence of gold nanoparticles on a colon tumor model. J Ultrasound Med. 2013;32:475–483. doi: 10.7863/jum.2013.32.3.475. [DOI] [PubMed] [Google Scholar]
- [108].Farny CH, Wu T, Holt RG, Murray TW, Roy RA. Nucleating cavitation from laser-illuminated nano-particles. Acoust Res Lett. 2005;6:138–143. Online. [Google Scholar]
- [109].Osminkina LA, Nikolaev AL, Sviridov AP, Andronova NV, Tamarov KP, Gongalsky MB, Kudryavtsev AA, Treshalina HM, Timoshenko VY. Porous silicon nanoparticles as efficient sensitizers for sonodynamic therapy of cancer. Microporous Mesoporous Mater. 2015;210:169–175. [Google Scholar]
- [110].Sviridov AP, Osminkina LA, Nikolaev AL, Kudryavtsev AA, Vasiliev AN, Timoshenko VY. Lowering of the cavitation threshold in aqueous suspensions of porous silicon nanoparticles for sonodynamic therapy applications. Appl Phys Lett. 2015;107:123107. [Google Scholar]
- [111].Ninomiya K, Noda K, Ogino C, Kuroda S, Shimizu N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: its application to targeted sonodynamic therapy. Ultrason Sonochem. 2014;21:289–294. doi: 10.1016/j.ultsonch.2013.05.005. [DOI] [PubMed] [Google Scholar]
- [112].Abdulla-Al-Mamun M, Kusumoto Y, Zannat T, Islam MS. Synergistic cell-killing by photocatalytic and plasmonic photothermal effects of Ag@TiO2 core–shell composite nanoclusters against human epithelial carcinoma (HeLa) cells. Appl Catal A. 2011;398:134–142. [Google Scholar]
- [113].Devanand Venkatasubbu G, Ramasamy S, Ramakrishnan V, Kumar J. Folate targeted PEGylated titanium dioxide nanoparticles as a nanocarrier for targeted paclitaxel drug delivery. Adv Powder Technol. 2013;24:947–954. [Google Scholar]
- [114].Ninomiya K, Fukuda A, Ogino C, Shimizu N. Targeted sonocatalytic cancer cell injury using avidin-conjugated titanium dioxide nanoparticles. Ultrason Sonochem. 2014;21:1624–1628. doi: 10.1016/j.ultsonch.2014.03.010. [DOI] [PubMed] [Google Scholar]
- [115].Ninomiya K, Ogino C, Oshima S, Sonoke S, Kuroda S-I, Shimizu N. Targeted sonodynamic therapy using protein-modified TiO2 nanoparticles. Ultrason Sonochem. 2012;19:607–614. doi: 10.1016/j.ultsonch.2011.09.009. [DOI] [PubMed] [Google Scholar]
- [116].Ebrahimi Fard A, Zarepour A, Zarrabi A, Shanei A, Salehi H. Synergistic effect of the combination of triethylene-glycol modified Fe3O4 nanoparticles and ultrasound wave on MCF-7 cells. J Magn Magn Mater. 2015;394:44–49. [Google Scholar]
- [117].Varchi G, Foglietta F, Canaparo R, Ballestri M, Arena F, Sotgiu G, Guerrini A, Nanni C, Cicoria G, Cravotto G, Fanti S, et al. Engineered porphyrin loaded core-shell nanoparticles for selective sonodynamic anticancer treatment. Nanomedicine. 2015;10:3483–3494. doi: 10.2217/nnm.15.150. [DOI] [PubMed] [Google Scholar]
- [118].Mišík V, Riesz P. Free radical intermediates in sonodynamic therapy. Annal N Y Acad Sci. 2000;899:335–348. doi: 10.1111/j.1749-6632.2000.tb06198.x. [DOI] [PubMed] [Google Scholar]
- [119].Apfel RE. The role of impurities in cavitation-threshold determination. J Acoust Soc Am. 1970;48:1179–1186. [Google Scholar]
- [120].Yildirim A, Chattaraj R, Blum NT, Goldscheitter GM, Goodwin AP. Stable encapsulation of air in mesoporous silica nanoparticles: fluorocarbon-free nanoscale ultrasound contrast agents. Adv Healthcare Mater. 2016;5:1290–1298. doi: 10.1002/adhm.201600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhao Y, Zhu Y. Synergistic cytotoxicity of low-energy ultrasound and innovative mesoporous silica-based sensitive nanoagents. J Mater Sci. 2014;49:3665–3673. [Google Scholar]
- [122].Kwan JJ, Graham S, Coussios CC. Inertial cavitation at the nanoscale. Proc Mtgs Acoust. 2013;19 075031. [Google Scholar]
- [123].Kwan JJ, Graham S, Myers R, Carlisle R, Stride E, Coussios CC. Ultrasound-induced inertial cavitation from gas-stabilizing nanoparticles. Phys Rev E Stat Nonlin Soft Matter Phys. 2015;92:023019. doi: 10.1103/PhysRevE.92.023019. [DOI] [PubMed] [Google Scholar]
- [124].Yildirim A, Chattaraj R, Blum NT, Goodwin AP. Understanding acoustic cavitation initiation by porous nanoparticles: toward nanoscale agents for ultrasound imaging and therapy. Chem Mater. 2016;28:5962–5972. doi: 10.1021/acs.chemmater.6b02634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kwan JJ, Myers R, Coviello CM, Graham SM, Shah AR, Stride E, Carlisle RC, Coussios CC. Ultrasound-propelled nanocups for drug delivery. Small. 2015;11:5305–5314. doi: 10.1002/smll.201501322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhao Y, Zhu Y, Fu J, Wang L. Effective cancer cell killing by hydrophobic nanovoid-enhanced cavitation under safe low-energy ultrasound. Chem Asian J. 2014;9:790–796. doi: 10.1002/asia.201301333. [DOI] [PubMed] [Google Scholar]
- [127].Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Li Y, Zhou Q, Deng Z, Pan M, Liu X, Wu J, Yan F, Zheng H. IR-780 Dye as a Sonosensitizer for Sonodynamic Therapy of Breast Tumor. Sci Rep. 2016;6 doi: 10.1038/srep25968. 25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yumita N, Iwase Y, Nishi K, Ikeda T, Umemura S-I, Sakata I, Momose Y. Sonodynamically induced cell damage and membrane lipid peroxidation by novel porphyrin derivative, DCPH-P-Na(I) Anticancer Res. 2010;30:2241–2246. [PubMed] [Google Scholar]
- [130].Hiraoka W, Honda H, Feril LB, Kudo N, Kondo T. Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing. Ultrason Sonochem. 2006;13:535–542. doi: 10.1016/j.ultsonch.2005.10.001. [DOI] [PubMed] [Google Scholar]
- [131].Hilgenfeldt S, Grossmann S, Lohse D. A simple explanation of light emission in sonoluminescence. Nature. 1999;398:402–405. [Google Scholar]
- [132].Umemura S, Yumita N, Nishigaki R, Umemura K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res. 1990;81:962–966. doi: 10.1111/j.1349-7006.1990.tb02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sazgarnia A, Shanei A, Eshghi H, Hassanzadeh-Khayyat M, Esmaily H, Shanei MM. Detection of sonoluminescence signals in a gel phantom in the presence of Protoporphyrin IX conjugated to gold nanoparticles. Ultrasonics. 2013;53:29–35. doi: 10.1016/j.ultras.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [134].Melero J, Martínez F, Molina R, Segura Y. Handbook on Applications of Ultrasound. CRC Press; 2011. Role of Heterogeneous Catalysis in the Sonocatalytic Degradation of Organic Pollutants in Wastewater. [Google Scholar]
- [135].Deepagan VG, You DG, Um W, Ko H, Kwon S, Choi KY, Yi GR, Lee JY, Lee DS, Kim K, Kwon IC, et al. Long-circulating Au-TiO2 nanocomposite as a sonosensitizer for ROS-mediated eradication of cancer. Nano Lett. 2016;16:6257–6264. doi: 10.1021/acs.nanolett.6b02547. [DOI] [PubMed] [Google Scholar]
- [136].Dai C, Zhang S, Liu Z, Wu R, Chen Y. Two-dimensional graphene augments nanosonosensitized sonocatalytic tumor eradication. ACS Nano. 2017;11:9467–9480. doi: 10.1021/acsnano.7b05215. [DOI] [PubMed] [Google Scholar]
- [137].Miyoshi N, Misík V, Fukuda M, Riesz P. Effect of gallium-porphyrin analogue ATX-70 on nitroxide formation from a cyclic secondary amine by ultrasound: on the mechanism of sonodynamic activation. Radiat Res. 1995;143:194–202. [PubMed] [Google Scholar]
- [138].Hachimine K, Shibaguchi H, Kuroki M, Yamada H, Kinugasa T, Nakae Y, Asano R, Sakata I, Yamashita Y, Shirakusa T, Kuroki M. Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-P-Na(I), which is devoid of photosensitivity. Cancer Sci. 2007;98:916–920. doi: 10.1111/j.1349-7006.2007.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Saravanakumar G, Kim J, Kim WJ. Reactive-oxygen-species-responsive drug delivery systems: promises and challenges. Adv Sci. 2017;4:1600124. doi: 10.1002/advs.201600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Pu H-L, Chiang W-L, Maiti B, Liao Z-X, Ho Y-C, Shim MS, Chuang E-Y, Xia Y, Sung H-W. Nanoparticles with Dual Responses to Oxidative Stress and Reduced pH for Drug Release and Anti-inflammatory Applications. ACS Nano. 2014;8:1213–1221. doi: 10.1021/nn4058787. [DOI] [PubMed] [Google Scholar]
- [141].Cravotto G, Gaudino EC, Cintas P. On the mechanochemical activation by ultrasound. Chem Soc Rev. 2013;42:7521–7534. doi: 10.1039/c2cs35456j. [DOI] [PubMed] [Google Scholar]
- [142].Skorb EV, Möhwald H, Andreeva DV. Effect of cavitation bubble collapse on the modification of solids: crystallization aspects. Langmuir. 2016;32:11072–11085. doi: 10.1021/acs.langmuir.6b02842. [DOI] [PubMed] [Google Scholar]
- [143].Sun X, Xu H, Shen J, Guo S, Shi S, Dan J, Tian F, Tian Y, Tian Y. Real-time detection of intracellular reactive oxygen species and mitochondrial membrane potential in THP-1 macrophages during ultrasonic irradiation for optimal sonodynamic therapy. Ultrason Sonochem. 2015;22:7–14. doi: 10.1016/j.ultsonch.2014.06.016. [DOI] [PubMed] [Google Scholar]
- [144].Tsuru H, Shibaguchi H, Kuroki M, Yamashita Y, Kuroki M. Tumor growth inhibition by sonodynamic therapy using a novel sonosensitizer. Free Radical Biol Med. 2012;53:464–472. doi: 10.1016/j.freeradbiomed.2012.04.025. [DOI] [PubMed] [Google Scholar]
- [145].Jin H, Zhong X, Wang Z, Huang X, Ye H, Ma S, Chen Y, Cai J. Sonodynamic effects of hematoporphyrin monomethyl ether on CNE-2 cells detected by atomic force microscopy. J Cell Biochem. 2011;112:169–178. doi: 10.1002/jcb.22912. [DOI] [PubMed] [Google Scholar]
- [146].Güvener N, Appold L, de Lorenzi F, Golombek SK, Rizzo LY, Lammers T, Kiessling F. Recent advances in ultrasound-based diagnosis and therapy with micro- and nanometer-sized formulations. Methods. 2017;130:4–13. doi: 10.1016/j.ymeth.2017.05.018. [DOI] [PubMed] [Google Scholar]
- [147].Kotopoulis S, Dimcevski G, Gilja OH, Hoem D, Postema M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Med Phys. 2013;40:072902. doi: 10.1118/1.4808149. [DOI] [PubMed] [Google Scholar]
- [148].Dimcevski G, Kotopoulis S, Bjanes T, Hoem D, Schjott J, Gjertsen BT, Biermann M, Molven A, Sorbye H, McCormack E, Postema M, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Controlled Release. 2016;243:172–181. doi: 10.1016/j.jconrel.2016.10.007. [DOI] [PubMed] [Google Scholar]
- [149].Trendowski M. Using the promise of sonodynamic therapy in the clinical setting against disseminated cancers. Chemother Res Pract. 2015;2015:316015. doi: 10.1155/2015/316015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Feril LB, Jr, Kondo T, Cui ZG, Tabuchi Y, Zhao QL, Ando H, Misaki T, Yoshikawa H, Umemura S. Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett. 2005;221:145–152. doi: 10.1016/j.canlet.2004.08.034. [DOI] [PubMed] [Google Scholar]
- [151].Lagneaux L, de Meulenaer EC, Delforge A, Dejeneffe M, Massy M, Moerman C, Hannecart B, Canivet Y, Lepeltier MF, Bron D. Ultrasonic low-energy treatment: a novel approach to induce apoptosis in human leukemic cells. Exp Hematol. 2002;30:1293–1301. doi: 10.1016/s0301-472x(02)00920-7. [DOI] [PubMed] [Google Scholar]
- [152].Feril LB, Jr, Kondo T, Zhao QL, Ogawa R. Enhancement of hyperthermia-induced apoptosis by non-thermal effects of ultrasound. Cancer Lett. 2002;178:63–70. doi: 10.1016/s0304-3835(01)00826-6. [DOI] [PubMed] [Google Scholar]
- [153].Honda H, Zhao QL, Kondo T. Effects of dissolved gases and an echo contrast agent on apoptosis induced by ultrasound and its mechanism via the mitochondria-caspase pathway. Ultrasound Med Biol. 2002;28:673–682. doi: 10.1016/s0301-5629(02)00509-4. [DOI] [PubMed] [Google Scholar]
- [154].Feng Y, Tian ZM, Wan MX, Zheng ZB. Low intensity ultrasound-induced apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2008;14:4873–4879. doi: 10.3748/wjg.14.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Feng Y, Tian Z, Wan M. Bioeffects of low-intensity ultrasound in vitro: apoptosis, protein profile alteration, and potential molecular mechanism. J Ultrasound Med. 2010;29:963–974. doi: 10.7863/jum.2010.29.6.963. [DOI] [PubMed] [Google Scholar]
- [156].Honda H, Kondo T, Zhao QL, Feril LB, Jr, Kitagawa H. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med Biol. 2004;30:683–692. doi: 10.1016/j.ultrasmedbio.2004.02.008. [DOI] [PubMed] [Google Scholar]
- [157].Hutcheson JD, Schlicher RK, Hicks HK, Prausnitz MR. Saving cells from ultrasound-induced apoptosis: quantification of cell death and uptake following sonication and effects of targeted calcium chelation. Ultrasound Med Biol. 2010;36:1008–1021. doi: 10.1016/j.ultrasmedbio.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Baker K, Robertson V, Duck F. A review of therapeutic ultrasound: biophysical effects. Phys Ther. 2001;81:1351–1358. [PubMed] [Google Scholar]
- [159].Barnett S. Ultrasound Biological Effects and Potential Hazards. In: Roy Williams A, editor. J Clin Ultrasound. Vol. 13. Academic Press; New York: 1985. p. 73. [Google Scholar]
- [160].Doan N, Reher P, Meghji S, Harris M. In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes. J Oral Maxillofac Surg. 1999;57:409–419. doi: 10.1016/s0278-2391(99)90281-1. [DOI] [PubMed] [Google Scholar]
- [161].Whitney NP, Lamb AC, Louw TM, Subramanian A. Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes. Ultrasound Med Biol. 2012;38:1734–1743. doi: 10.1016/j.ultrasmedbio.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Johns LD. Nonthermal effects of therapeutic ultrasound: the frequency resonance hypothesis. J Athl Train. 2002;37:293–299. [PMC free article] [PubMed] [Google Scholar]
- [163].Ling L, Wei T, He L, Wang Y, Wang Y, Feng X, Zhang W, Xiong Z. Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif. 2017;50 doi: 10.1111/cpr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Duco W, Grosso V, Zaccari D, Soltermann AT. Generation of ROS mediated by mechanical waves (ultrasound) and its possible applications. Methods. 2016;109:141–148. doi: 10.1016/j.ymeth.2016.07.015. [DOI] [PubMed] [Google Scholar]
- [165].Soenen SJ, Rivera-Gil P, Montenegro JM, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. 2011;6:446–465. [Google Scholar]
- [166].Ivask A, Titma T, Visnapuu M, Vija H, Kakinen A, Sihtmae M, Pokhrel S, Madler L, Heinlaan M, Kisand V, Shimmo R, et al. Toxicity of 11 Metal Oxide Nanoparticles to Three Mammalian Cell Types In Vitro. Curr Top Med Chem. 2015;15:1914–1929. doi: 10.2174/1568026615666150506150109. [DOI] [PubMed] [Google Scholar]
- [167].Shin S, Song I, Um S. Role of physicochemical properties in nanoparticle Toxicity. Nanomaterials. 2015;5:1351. doi: 10.3390/nano5031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Saliani M, Jalal R, Goharshadi EK. Mechanism of oxidative stress involved in the toxicity of ZnO nanoparticles against eukaryotic cells. Nanomed J. 2016;3:1–14. [Google Scholar]
- [169].Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- [170].Chen KL, Bothun GD. Nanoparticles meet cell membranes: probing nonspecific interactions using model membranes. Environ Sci Technol. 2014;48:873–880. doi: 10.1021/es403864v. [DOI] [PubMed] [Google Scholar]
- [171].Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:2699–2711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- [172].Stankic S, Suman S, Haque F, Vidic J. Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J Nanobiotechnol. 2016;14:73. doi: 10.1186/s12951-016-0225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Mirhosseini M. Synergistic antibacterial effect of metal oxid nanoparticles and ultrasound stimulation. J Biol Today’s World. 2015;4:138–144. [Google Scholar]
- [174].Bisht G, Rayamajhi S. ZnO nanoparticles: a promising anticancer agent. Nanobiomedicine. 2016;3:9. doi: 10.5772/63437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomed. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kaneko OF, Willmann JK. Ultrasound for molecular imaging and therapy in cancer. Quant Imaging Med Surg. 2012;2:87–97. doi: 10.3978/j.issn.2223-4292.2012.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Lentacker I, De Smedt SC, Sanders NN. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter. 2009;5:2161–2170. [Google Scholar]
- [178].Dimcevski G, Kotopoulis S, Bjånes T, Hoem D, Schjøtt J, Gjertsen BT, Biermann M, Molven A, Sorbye H, McCormack E, Postema M, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Controlled Release. 2016;243:172–181. doi: 10.1016/j.jconrel.2016.10.007. [DOI] [PubMed] [Google Scholar]
- [179].Blood-Brain Barrier Disruption Using Transcranial MRI-Guided FocusedUltrasound. ClinicalTrials.gov. 2015 < https://clinicaltrials.gov/ct2/show/study/NCT02343991?term=NCT02343991&rank=1 >. [Google Scholar]
- [180].Mullin LB, Phillips LC, Dayton PA. Nanoparticle Delivery Enhancement With Acoustically Activated Microbubbles. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:65–77. doi: 10.1109/TUFFC.2013.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Celsion Corporation Clinical trials. 2017 < http://www.celsion.com/trials.cfm >.
- [182].Xiaohuai W, Lewis TJ, Mitchell D. The tumoricidal effect of sonodynamic therapy (SDT) on S-180 sarcoma in mice. Integr Cancer Ther. 2008;7:96–102. doi: 10.1177/1534735408319065. [DOI] [PubMed] [Google Scholar]
- [183].Wang XJ, Mitchell D, Lewis TJ. Primary clinical use of sonodynamic therapy (SDT) for advanced breast cancer. J Clin Oncol. 2008;26:12029. [Google Scholar]
- [184].Wang X, Zhang W, Xu Z, Luo Y, Mitchell D, Moss RW. Sonodynamic and photodynamic therapy in advanced breast Carcinoma: a report of 3 cases. Integr Cancer Ther. 2009;8:283–287. doi: 10.1177/1534735409343693. [DOI] [PubMed] [Google Scholar]
- [185].Li LQ, Wang X, Zhang IW, Mitchell D. Primary clinical use of the sono-photo-dynamic therapy for advanced esophagocadiac and gastric adenocarcinoma. J Clin Oncol. 2014;32 abstract e15024. [Google Scholar]
- [186].Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. Adv Exp Med Biol. 2016;880:83–95. doi: 10.1007/978-3-319-22536-4_5. [DOI] [PubMed] [Google Scholar]
- [187].Marinova M, Rauch M, Mucke M, Rolke R, Gonzalez-Carmona MA, Henseler J, Cuhls H, Radbruch L, Strassburg CP, Zhang L, Schild HH, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26:4047–4056. doi: 10.1007/s00330-016-4239-0. [DOI] [PubMed] [Google Scholar]
- [188].Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol: WJG. 2014;20:16480–16488. doi: 10.3748/wjg.v20.i44.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Maloney E, Khokhlova T, Pillarisetty VG, Schade GR, Repasky EA, Wang Y-N, Giuliani L, Spring M, Hwang JH. Focused ultrasound for immuno-adjuvant treatment of pancreatic cancer: an emerging clinical paradigm in the era of personalized oncotherapy. Int Rev Immunol. 2017;36:1–14. doi: 10.1080/08830185.2017.1363199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lammertink BH, Bos C, Deckers R, Storm G, Moonen CT, Escoffre JM. Sonochemotherapy: from bench to bedside. Front Pharmacol. 2015;6:138. doi: 10.3389/fphar.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Yamaguchi S, Kobayashi H, Narita T, Kanehira K, Sonezaki S, Kudo N, Kubota Y, Terasaka S, Houkin K. Sonodynamic therapy using water-dispersed TiO2-polyethylene glycol compound on glioma cells: comparison of cytotoxic mechanism with photodynamic therapy. Ultrason Sonochem. 2011;18:1197–1204. doi: 10.1016/j.ultsonch.2010.12.017. [DOI] [PubMed] [Google Scholar]
- [192].Miele E, Spinelli GP, Miele E, Di Fabrizio E, Ferretti E, Tomao S, Gulino A. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int J Nanomed. 2012;7:3637–3657. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Sahakyan N, Haddad A, Richardson S, Forcha-Etieundem V, Christopher L, Alharbi H, Campbell R. Personalized nanoparticles for cancer therapy: a call for greater precision. Anti-cancer Agents Med Chem. 2017;17:1033–1039. doi: 10.2174/1871520617666170102150730. [DOI] [PubMed] [Google Scholar]
- [194].Yaari Z, da Silva D, Zinger A, Goldman E, Kajal A, Tshuva R, Barak E, Dahan N, Hershkovitz D, Goldfeder M, Roitman JS, et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat Commun. 2016;7 doi: 10.1038/ncomms13325. 13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Fruscella M, Ponzetto A, Crema A, Carloni G. The extraordinary progress in very early cancer diagnosis and personalized therapy: the role of oncomarkers and nanotechnology. J Nanotechnol. 2016;2016:18. [Google Scholar]
- [196].Moller K, Muller K, Engelke H, Brauchle C, Wagner E, Bein T. Highly efficient siRNA delivery from core-shell mesoporous silica nanoparticles with multifunctional polymer caps. Nanoscale. 2016;8:4007–4019. doi: 10.1039/c5nr06246b. [DOI] [PubMed] [Google Scholar]
- [197].Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24:1159–1166. doi: 10.1016/j.copbio.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Harada Y, Ogawa K, Irie Y, Endo H, Feril LB, Jr, Uemura T, Tachibana K. Ultrasound activation of TiO2 in melanoma tumors. J Controlled Release. 2011;149:190–195. doi: 10.1016/j.jconrel.2010.10.012. [DOI] [PubMed] [Google Scholar]
- [199].You DG, Deepagan VG, Um W, Jeon S, Son S, Chang H, Yoon HI, Cho YW, Swierczewska M, Lee S, Pomper MG, et al. ROS-generating TiO2 nanoparticles for non-invasive sonodynamic therapy of cancer. Sci Rep. 2016;6 doi: 10.1038/srep23200. 23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Moosavi Nejad S, Takahashi H, Hosseini H, Watanabe A, Endo H, Narihira K, Kikuta T, Tachibana K. Acute effects of sono-activated photocatalytic titanium dioxide nanoparticles on oral squamous cell carcinoma. Ultrason Sonochem. 2016;32:95–101. doi: 10.1016/j.ultsonch.2016.02.026. [DOI] [PubMed] [Google Scholar]
- [201].Leighton TG. The Acoustic Bubble. first ed. Academic Press; 1997. [Google Scholar]